Abstract
Tissue engineering is an emerging technological field that aims to restore and replace human tissues. A significant number of individuals require bone replacement annually as a result of skeletal abnormalities or accidents. In recent decades, notable progress has been made in the field of biomedical research, specifically in the realm of sophisticated and biocompatible materials. The purpose of these biomaterials is to facilitate bone tissue regeneration. Carbon nanomaterial-based scaffolds are particularly notable due to their accessibility, mechanical durability, and biofunctionality. The scaffolds exhibit the capacity to enhance cellular proliferation, mitigate cell damage, induce bone tissue growth, and maintain biological compatibility. Therefore, they play a crucial role in the development of the bone matrix and the necessary cellular interactions required for bone tissue restoration. The attachment, growth, and specialization of osteogenic stem cells on biomaterial scaffolds play critical roles in bone tissue engineering. The optimal biomaterial should facilitate the development of bone tissue in a manner that closely resembles that of human bone. This comprehensive review encompasses the examination of graphene oxide (GO), carbon nanotubes (CNTs), fullerenes, carbon dots (CDs), nanodiamonds, and their respective derivatives. The biomaterial frameworks possess the ability to replicate the intricate characteristics of the bone microenvironment, thereby rendering them suitable for utilization in tissue engineering endeavors.
1. Introduction
Bone cells are vital to the body and need a well-designed framework to regenerate synthetic biological bone.1 Repairing or replacing damaged bones is crucial (Figure 1).2 In scaffold development, tissue engineering and nanotechnology may replace conventional healing methods.3 This multidisciplinary approach may improve bone damage and dysfunction treatments, improving patient outcomes.4 However, more research is needed to determine this strategy’s pros and cons. Long-term studies are needed to assess these treatments’ safety, efficacy, and cost-effectiveness in humans.5 Therefore, this approach is promising for future research due to its potential benefits.
Figure 1.
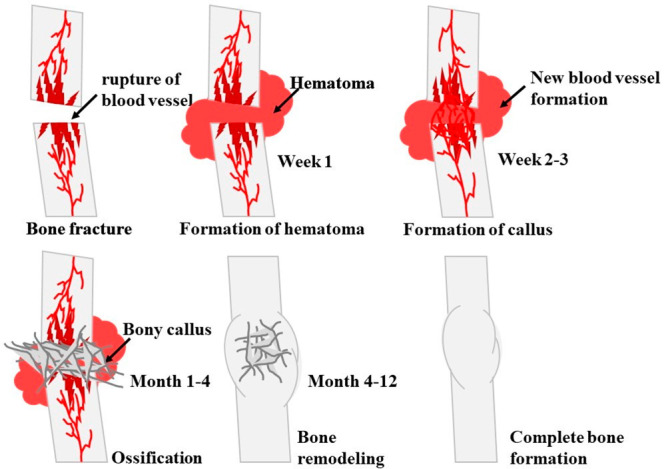
Complete bone healing process of a fractured hard tissue.
Tissue engineering addresses the organ shortage by creating artificial organs from biological structures.6 Tissue engineering integrates biology, engineering, and medicine to create new restorative tissue or organ constructs.7 To replace injured tissues, scaffolds, cells, growth factors, and nanomaterials are used to fabricate tissue structures.8 In tissue engineering, scaffolds made of natural or synthetic materials should not cause inflammation.9 They should be biodegradable and provide mechanical support to guide new tissue growth after implantation to regulate the tissue functions. The scaffold material also depends on the tissue construct and its use.10
Graphene and functionalized graphene are being studied in bone biomaterials, and their interactions with bone-healing proteins must be understood.11 Biomedical applications for graphene and its derivatives are promising due to their mechanical, electrical, and optical properties.12 Adding functional groups to graphene improves its properties and suitability for certain applications.13 The interactions between these materials and bone-healing proteins are unclear. This knowledge gap hinders bone healing biomaterial development.
Surface functionalization, which involves the attachment of molecules or groups to graphene surfaces to enhance interactions with cells and tissues, offers a potential solution to this issue.14 Bioactive molecules like growth factors or peptides can boost bone cell activity and healing.15 Researchers are also using 3D printing to create customized scaffolds that mimic natural bone tissue and support new bone growth.16 Biomaterials research is promising for improving bone healing and addressing complex fractures and bone defects. Understand how different materials interact with biological systems to develop more effective therapies that speed healing and improve patient outcomes.17 Therefore, more research is needed to understand the mechanisms and improve graphene-based biomaterials for bone regeneration. Understanding these processes may help develop biomaterials that speed up bone healing and improve patient outcomes and quality of life.18
2. Carbon-Based (Carbon Nanotubes) Materials for Bone Tissue Engineering
Several studies are using carbon-based scaffolds for bone regeneration in bone tissue engineering, a crucial field.19 Carbon nanomaterials, including metallic and nonmetallic nanoparticles, are essential in hard tissue engineering, and nanocarbon materials are used in many biomedical applications.20 Due to their 10–100 nm measurements, fullerenes, graphene, and carbon nanotubes (CNTs) have fascinated researchers.21 These nanostructures’ unique architectures and properties make them excellent regenerative medicine materials.22
Due to their unique physical and chemical properties, carbon nanotubes may be used in drug delivery and cancer therapy.23 Carbon nanoparticles have also been used as structural strengthening agents to improve the mechanical properties of bone-based scaffolds, allowing better shape and size control and more complex tissue structures. These scaffolds are better for tissue regenerative applications because their mechanical properties improve cell growth and proliferation.24 Carbon nanostructures as strengthening agents could also lead to new biomedical materials with improved physical, chemical, and biological properties.25 These innovations could revolutionize healthcare and improve scaffold performance with further research.26
Due to their excellent mechanical properties and potential to improve long-established medical techniques, carbon nanomaterials have become popular biotherapeutics for bone tissue engineering. Their flexibility and large surface area make them ideal for scaffold development, making them biomedicine’s most valuable asset.27 The scaffolds use single-walled, multiwalled, or ultrashort carbon nanotubes (CNTs).28 Polymeric bone-based scaffolds were made from ultrashort single-walled carbon nanotubes (US-SWCNTs) and biodegradable polymers.29 These scaffolds were tested with defective bone tissue in a rabbit model and found that the scaffold’s surface chemical composition affected bone cell behavior and survival in the bone microenvironment.30 Gao et al.31 found that CNT structures with improper surface chemical composition did not induce osteogenic cell development. Thus, maintaining molecular equilibrium for bone cell proliferation and maturation is difficult.32 A better understanding of CNT synthesis processes allows for precise bone scaffold surface molecular composition.33 All carbon nanomaterials have at least one functional property.102 Electrical conductivity, thermal stability, and high surface area-to-volume ratio make these nanomaterials useful in electronics, energy storage, environmental remediation, and biomedical engineering.34 These nanostructured materials are particularly good for bone tissue engineering due to their mechanical properties, cytotoxicity, biocompatibility, antibacterial effect, and pro-angiogenic efficacy.35 Due to their biocompatibility and osteoconductive properties, carbon nanomaterials have been extensively studied for bone implant development. Their high surface area allowed controlled release of drugs and growth factors, making them promising candidates for targeted drug delivery.36 Due to their advantages, they have been extensively researched for bone tissue engineering applications such as strengthening matrix materials or composites.37 Developing orthopedic implants that support cell attachment, proliferation, differentiation, and migration with carbon nanomaterials is promising for regenerative medicine.38 Nevertheless, it is crucial to acknowledge and tackle the concerns related to the cytotoxicity of these substances, which can be observed in both their pure forms and when combined with other materials. Multiple studies have documented the possible harmful effects of CNTs, highlighting the significance of comprehensively understanding their ability to interact with living organisms.107,108 Within the realm of bone tissue engineering, CNTs possess distinctive characteristics, including exceptional mechanical robustness and a substantial surface area-to-volume ratio, rendering them highly advantageous for the advancement of scaffolds. In order to maximize their capabilities while minimizing the risk of cell damage, scientists have directed their attention toward altering the surface properties and achieving precise regulation of the molecular makeup. These strategies seek to achieve a harmonious equilibrium between the mechanical benefits of scaffolds made from CNTs and their influence on the behavior and viability of cells in the bone microenvironment. The advantages of CNTs go beyond their mechanical properties. In addition, they demonstrate electrical conductivity, thermal stability, and the capacity for controlled release of drugs and growth factors. These characteristics make them highly promising options for precise drug delivery in the field of bone tissue engineering. Consequently, there is a significant amount of research being conducted on CNTs to explore their potential in creating orthopedic implants that facilitate the attachment, growth, specialization, and movement of cells. This research contributes to the advancement of regenerative medicine. To summarize, although the potential toxicity of CNTs is a legitimate concern, continuous research and the implementation of surface modification techniques are actively tackling these problems. As a result, CNTs are able to play a pivotal role in the progress of bone tissue engineering and regenerative medicine.107,108
3. Graphene and Graphene Oxide
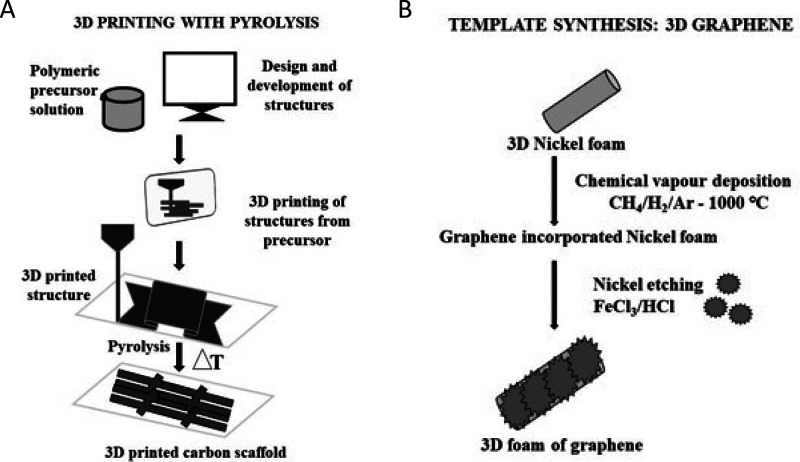
In addition to carbon nanotubes, graphene oxide has shown promise in tissue restoration.31 This material outperforms CNTs in conductivity, surface area-to-volume ratio, and purification speed.39 The thinnest material is graphene at one atom. It is a strong, flexible, and transparent monolayer carbon allotrope with sp2-hybridized carbon atoms in a hexagonal lattice.40 Due to its large surface area, high electrical and thermal conductivity, and excellent mechanical properties, the material is attractive for energy storage devices like batteries and super capacitors.41 Graphene, a crystalline form of carbon, is used to make graphite, charcoal, nanotubes, and fullerenes.42 Material molecular structure is influenced by chemical stability, thermal conductivity, electrical conductivity, and unique optical properties.43 Due to high cost and difficulty controlling the structure and properties, large-scale production remains difficult. Physico-chemical processing can also reconstruct graphene sheets into single and multilayered graphene or graphene oxide sheets.44 Graphite and oxidizing agents react to form graphene oxide (GO) sheets.45 Carbon, oxygen, and hydrogen in graphene oxide matrices with better hydrophilicity, dispersibility, large surface area, and mechanical strength give sheets structure and make them useful in various industries.46 Their unique properties make GO-based materials suitable for organ-specific tissue engineering, biosensor development, and personalized medicine.47,48 Functionalizing GO with organic molecules improves its solubility and dispersibility in various solvents.49 GO-based materials can be doped with elements to change their conductivity and bandgap for energy transfer applications, improving their performance in energy storage, catalysis, and biosensing.50 They can also be modified with functional groups to improve compatibility with other materials and add chemical functionalities for targeted drug delivery and other biomedical applications.51 When developed as 3D substrates (Figure 2A) or 3D foams (Figure 2B), mineral-based materials reduce graphene biomaterial toxicity.52 Bioactive scaffolds with surface chemical modification, tolerable cytotoxicity, and graphene nanocomposites’ bioresorbability have shown promise in stem cell differentiation and tissue regeneration.53
Figure 2.
(A) Fabrication process using lithography and pyrolysis for the production of a 3D printed carbon scaffold. (B) The process for carbon-based scaffold fabrication using chemical vapor deposition and etching on nickel foam to form a 3D graphene foam.
Nanomedicine and dentistry are rapidly adopting graphene-based synthetic nanoparticles. Graphene and its derivatives can be used as hybrid scaffolds in regenerative dentistry because they can be functionalized with many bioactive compounds.54 Graphene and its derivatives have shown promise in titanium dental implants, bone regeneration membranes, resins, cements, and adhesives.55 Biomedicine has recently focused on graphene for coating implants and making biosensors. Kalbacova et al. examined the early behavior of a human osteoblastic cell line after 2–48 h of incubation with graphene sheets treated with hydrogen or oxygen.98 In graphene substrates, cell attachment and proliferation were unexpectedly affected. However, hydrogen-treated (1-LG) single-layer graphene (hydrophobic surface) promoted cell proliferation, while oxygen-treated (1-LG-O) single-layer graphene (hydrophilic surface) elicited a cell response comparable to tissue-culture plastic for cell cultivation. Recent nanomaterial research has focused on graphene-based materials (GBMs). Nanomedicine is growing rapidly. The immune system is essential for most nanomedicines and nanomedicine GBMs. Innovative, effective diagnostic and treatment strategies require an understanding of the complex relationships among GBMs, immune cells, and immunological components. A cutting-edge nanomedicine tool was created by manipulating GBM toxicity and protein conjugation against the immune system. It was difficult to study its effects on immune cells, its use as an immunobiosensor, and tumor targeting antibody adhesion during fabrication. Due to recent advances in graphene research, immune-conjugates with specific immunological properties may pass preclinical testing and find applications in nanomedicine soon.56
AFM tip-induced local oxidation and an external source meter were used to create nanogrids on the CVD-grown graphene. Jiang and team used the lateral mode of AFM to characterize the friction and adhesion properties of graphene with nanogrids. They found that the friction force on the nanogrid borders was greater than that on the normal area, while the adhesion force decreased slightly due to the surface roughness. These findings affected nanoscale device and system development. Nanogrid borders’ increased friction force of nanogrid borders could be used to make microscale motors or other mechanical components. The decrease in adhesion force could also be used to create nonsticking surfaces. However, more research is needed to understand the mechanisms and optimize these properties for specific applications. The study illuminated graphene’s nanoscale behavior and opened up exciting new research avenues.57 The prevalence of bone abnormalities and injuries has increased the use of biomaterials in medicine. Kandiah et al. synthesized biomaterials from titania–graphene nanocomposites (TGS) using an in situ sol–gel.99 Due to TiO2 sphere intercalation, TGS nanocomposites formed a sheet with a spherical shape. The mesoporosity and swelling of the nanocomposites increased cell attachment and prevented nanoparticle migration and aggregation in the host. In addition, biological evaluation of TGS nanocomposites in artificial body fluid and human cell lines (osteoblastic cells-MG63) showed that TG2 (2:2) and TG4 (2:4) samples are better for in vivo bone repair. Regenerative medicine has made great strides with human mesenchymal stem cells (hMSCs), but MSC proliferation and differentiation are still difficult to control. Recent cell culture mediums include graphene. Zhang et al. showed that mineralized 3D graphene (3DG) scaffolds grow hMSCs.106 Mineralization in 10 times concentrated simulated bodily fluid (10SBF) containing 10 mM HCO–3 produced 3D graphene (HA-3DG) scaffolds with nanostructured hydroxyapatite (HA) particles. Compared to 2D graphene sheets, HA-3DG scaffolds had better roughness and cell proliferation. Western blotting showed that mineralized 3DG scaffolds differentiated osteoblasts. The mineralized 3DG scaffold was found to be effective for hMSC culture and bone repair. Surface alteration yields implants with better osseointegration in comparison to other scaffolding materials.
The biological properties of silanized graphene oxide must be studied for biomedical applications. Vuppaladadium et al. studied silanized graphene oxide (SiGO) fabrication for toxicity, immunogenicity, and various biological properties like osteogenicity. Infrared spectroscopy and elemental mapping confirmed that graphene oxide (GO) was silanized with the ubiquitous silanizing chemical 3-aminopropyltriethoxysilane (APTES) without any morphological changes. In vitro cytotoxicity of SiGO was lower in primary human dermal fibroblasts, murine embryonic fibroblasts, and human osteosarcoma cell lines. In vitro immunological analysis showed that these SiGO activated fewer macrophages. Furthermore, human mesenchymal stem cell osteogenic differentiation profiling showed that SiGO is less osteogenic than GO. In a mouse model of acute toxicity, GO was hepatotoxic at experimental concentrations but SiGO was not. SiGO was found to be more biocompatible and osteogenic than GO.58
4. Graphene Oxide Synthesis Approaches
Most research on GO synthesis and coating uses chemical vapor deposition. A simple cell assembly method for osteoblast-like structures was developed by using suspended GO synthesized from graphite powder, H2SO4, and KMnO4. The cell line with some osteoblastic traits, the human cancer cell line saos-2, and normal MSCs were tested for GO toxicity. After transforming mesenchymal stem cells (MSCs) into osteoblast cells in suspended GO, scanning electron microscopy and real-time polymerase chain reaction examined their cellular attachment and gene expression. The osteogenic media with suspended GO showed dose-dependent toxicity. Osteopontin, osteocalcin, and connexin were upregulated. GO can increase the number of cell layers and create a multilayer shape in osteoblast cells, making this technology a promising bone tissue engineering strategy.59 To seriously explore graphene for biomedical applications, one must understand how the graphene–substrate affects graphene-cell interaction. Graphene films on various substrates were tested for biocompatibility by using osteoblasts.
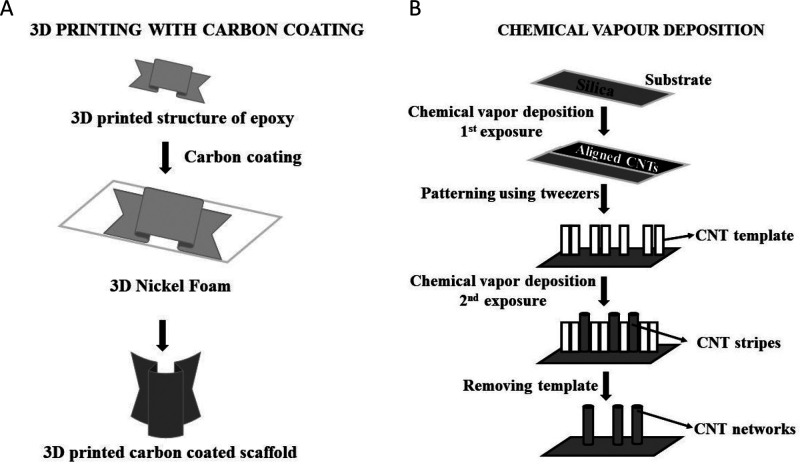
Methane and hydrogen precursors were used to synthesize graphene on a copper substrate using chemical vapor deposition (Figure 3A). The study found that graphene films on various substrates had good cell attachment quality and thickness. The results showed that graphene is safe for osteoblasts and that graphene-coated substrates have better cell adhesion. This study found that graphene coating bone implants may improve osteoblast adhesion and proliferation.60 Due to their antimicrobial properties and affinity for functional groups, graphene oxide nanoribbons are promising biomedical nanomaterials. Functionalized nanoribbons increase human osteoblast cell proliferation. The study found that high drug concentrations inhibited Gram-positive and Gram-negative bacteria. Graphene nanoribbons were grown by treating multiwalled carbon nanotubes with oxygen plasma and chemical vapor deposition after analyzing their synthesis, characterization, morphology, and composition (Figure 3B).
Figure 3.
(A) Method for the development of a 3D printed carbon coated scaffold using diamond-like carbon (DLC) coating on a 3D printed epoxy scaffold. (B) Fabrication methodology to produce CNT networks using chemical vapor deposition.
Characterization showed that these ribbons were structurally sound and uniform. The nanoribbons’ effects on biological systems were assessed through cell and protein interactions, revealing low toxicity and excellent biocompatibility at dosage levels of 10, 100, 200, and 300 μg/mL. The drug did not have cytotoxic effects or affect bone healing mRNA gene expression at concentrations of up to 100 μg/mL. Incubation with 100 μg/mL nanoribbon caused approximately 50% bacterial mortality in both Gram-positive and Gram-negative bacteria (S. aureus and E. coli). The results showed better bactericidal impact and no adverse effects on bone repair, so the material can be used for bone regeneration.61
Xie et al. created a simple and flexible colloidal chemistry method for synthesizing these structures. Citrate-stabilized hydroxyapatite (HAp) nanoparticles were suspended with GO in water to form a graphite-like hybrid structure in 3D. The hydrothermal colloidal solution increased the graphene–graphene oxide ratio and distributed HAp nanoparticles homogeneously on graphene oxide sheets (Figure 4A). These results show that HAp/GO hybrid sheets with graphene oxide have anti-inflammatory effects that may improve cell adhesion, cyto-compatibility, and biocompatibility. The high surface area of the bone extracellular matrix mimicked by HAp/GO hybrid sheets enhanced osteogenic differentiation on hMSCs and provided a favorable environment for the intrinsic cell signaling needed for bone tissue regeneration.77
Figure 4.
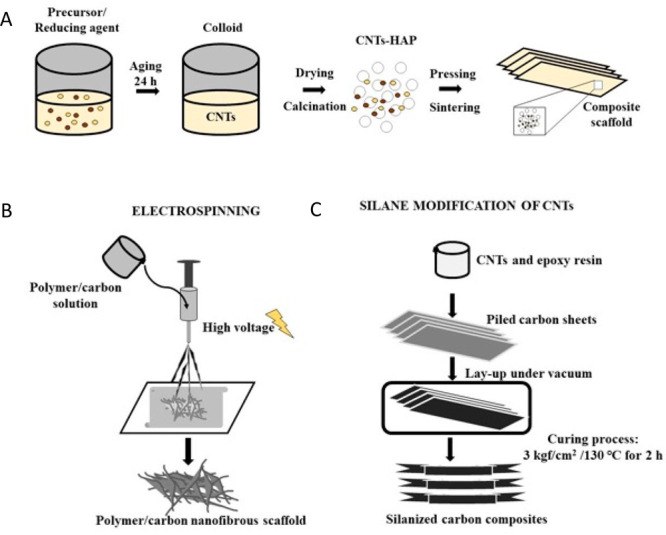
(A) Sol–gel in situ modification of carbon nanotubes (CNTs) with hydroxyapatite (HAP) to design a bulk composite using colloidal chemistry. (B) Fabrication of a carbon nanofibrous scaffold using the top-down nanotechnological electrospinning technique. (C) Process for the design of a silanized carbon composite scaffold using CNTs and epoxy resin.
The demand for cell-co-polymer composite bone tissue implants is constant. This study investigated how a nanostructured biocomposite could improve human induced pluripotent stem cell (iPSC)-based osteogenic development. PVDF-GO nanofibers were electrospun (Figure 4B) and tested for properties using a scanning electron microscope, tensile tests, and viability assays. We then divided the iPSCs into PVDF, PVDF-GO, and a control set of tissue culture plates to study their osteogenic differentiation. PVDF-GO-grown iPSCs had higher calcium and alkaline phosphatase activities than those grown on other substrates. PVDF-GO-grown iPSCs had higher Runx2, osteocalcin, and osteonectin gene expression than PVDF and control. After measuring osteocalcin and osteopontin protein expression, PVDF-GO nanofibers had higher osteoinductivity than PVDF nanofibers. Thus, PVDF-GO nanofibers have high osteo-inductive potential and are a promising bioimplant for bone tissue creation when combined with iPSCs.62
The mesoporous bioactive glass (MBG)-GO scaffold for skull bone recovery in a rat cranial defect model showed new bone formation and vascularization. Its improved mechanical properties allowed it to withstand stress and strain and accelerate osteoblast proliferation and differentiation, resulting in new cranial bone tissue. By release of calcium and phosphate ions into the environment to mineralize bone, the MBG-GO scaffold helped rats recover from cranial abnormalities. The MBG-GO scaffold promotes endothelial, smooth muscle, and fibroblast growth to increase angiogenesis, which is essential for tissue repair and regeneration.63 Liu et al. developed an osteoinductive extracellular matrix (OiECM) in GO-collagen hybrids to demonstrate the biocompatibility of collagen and GO by creating a scaffold similar to the BMSC extracellular matrix. After 21 days of differentiation, BMSC formed the OiECM-GO-Col scaffold by encasing it in GO-collagen hybrids. Due to its high mechanical strength and bioactivity, the OiECM-GO-Col scaffolds outperformed the control groups in a 5 mm rat cranial defect model for new bone development and host tissue integration. The porous scaffold transported nutrients and oxygen, which stimulated cell growth and differentiation, making the OiECM-GO-Col scaffold a promising biomaterial for bone tissue engineering.64
Another study examined how ultrasonicated graphene oxide (UGO) could speed bone fracture and skin wound healing. Ultrasonication disperses particles to increase the zeta potential of a GO solution, but UGO has good water dispersion, despite having fewer oxygen-containing groups. The well-dispersed UGO suspension enhances cell adhesion and proliferation in human endothelial (EA.hy 926) cells, human fetal osteoblast (hFOB) cells, and mouse embryonic fibroblasts. UGO’s osteogenic and cell-proliferative effects suggest they can be used to create bone tissue engineering scaffolds. UGO is promising for drug delivery due to its water dispersion in biological fluids. In an in vitro skin scratch test and an in vivo rat excisional skin defect model, a 1% UGO suspension improved wound healing. According to Hussein et al.,65 UGO may aid tissue regeneration by providing an optimal environment for bone and skin healing cells. Shin et al. developed a bioactive surface modification system for implant contact using reduced graphene oxide (rGO)-coated sandblasted, large-grit, and acid-etched (SLA) Ti. This SLA Ti (ST control) implant with reduced graphene oxide [rGO] and recombinant human bone morphogenetic protein-2 [rhBMP-2] helped animal models regenerate bone tissue. As shown by increased apatite formation and osteoblast cell adhesion and proliferation, the rGO coating significantly improved the bioactivity of the SLA titanium surface. In vitro, the rGO coating showed good biocompatibility and stability, suggesting orthopedic and dental implant applications. In in vitro and in vivo studies, rGO-coated ST (R-ST) had significantly higher cell attachment and proliferation, alkaline phosphatase activity, matrix mineralization, osteogenesis-related gene and protein expression, and osseointegration examinations than the control (ST), BI-ST, and BT-ST groups. This study suggests developing bioactive surfaces on metallic implants to improve clinical performance and patient outcomes.66 The synthesis of hollow TiO2 microspheres using chitosan-gelatin (CG) microspheres as unique sacrificial templates revealed a hollow structure with a thin shell. Drug delivery can be improved by loading hollow microspheres with pharmaceuticals and delivering them to specific cells or tissues. Due to their porosity and high specific surface area, hollow microspheres are promising catalysts. The physicochemical analysis of these hollow microspheres revealed a spherical structure with a diameter of 50–300 μm and a shell thickness of 500–600 nm. In vitro cell culture studies showed that fibroblast L929 cells attached well to TiO2 hollow microspheres and proliferated with culture time, making them a biocompatible cell carrier.67
Current bone regenerating material design includes adding silica. By combining 3-aminopropyltriethoxysilane (APTES), graphene oxides (GOs), and water, RGO-aminosilica hybrid nanosheets with improved osteocompatibility were created. Adding APTES to the mixture hydrolyzed, condensed it, and converted GO to RGO. APTES suppressed GO to RGO conversion and hybrid nanosheets delivered silicon ions sustainably (Figure 4C). Hybrid nanosheets were tested in vitro with osteoblast MC3T3-E1 cells for osteocompatibility. A quantitative analysis using a water-soluble tetrazolium salt assay showed that the hybrid nanosheets were nontoxic and biocompatible. Quantitative alkaline phosphatase analysis showed that hybrid nanosheets improved osteoblast differentiation over GO nanosheets. An immunochemical study added qualitative evidence that hybrid nanosheets increased osteopontin synthesis, a marker for osteoblast development. The hybrid nanosheets may be useful for bone regeneration.68,69 It is well-known that graphene compounds are useful in medicine, especially stem cell treatments. Microsized (MGO) and nanosized (NGO) graphene oxide (GO) sheets were tested on hADMSC development. The drop casting method was used to cover glass substrates with MGO and NGO sheets (1–10 μm and 100–300 nm lateral diameters, respectively). At all cell densities, hADMSCs cultured on MGO-coated substrates proliferated faster than their NGO counterparts. After 4 weeks of differentiation under the same culture conditions, MGO-coated hADMSCs have much higher osteogenesis in comparison to NGO-coated ones. Following 21–28 days of differentiation, hADMSCs showed increased osteogenic differentiation and osteoblast calcification. These findings suggested that graphene size regulated hADMSCs osteogenesis, which could lead to new stem cell research and treatment applications.70 Cell survival, proliferation, and differentiation in microfluidic cell-based experiments require strong cell–substrate adhesion. Polydimethylsiloxane (PDMS) is a popular microfluidic polymer, but its cell–substrate interactions prevent its use in cell-based tests.
Previous studies showed chemically modifying, plasma-treating, and protein-coating PDMS functionalized surfaces. These methods are reversible and time-consuming and may create cell aggregates, making them inefficient and environmentally harmful. To meet these needs, biocompatible nanomaterial-doped PDMS controls the cell-surface interaction. PDMS has been paired with gold nanowires (AuNW), superparamagnetic iron oxide nanoparticles (SPION), graphene oxide sheets (GO), and graphene quantum dots (GQD) as microfluidic biomaterial. The Young’s modulus, surface roughness, and nanotopology of nanostructured substrates have been examined using atomic force microscopy (AFM). In cyto-compatibility testing with human amniotic mesenchymal stem cells (hAMSCs), nanostructured PDMS composites had similar cell viabilities. It has been shown that microfluidic devices increase the level of osteogenic stem cell differentiation. Nanomaterials also increased the surface roughness. The results also showed that after 14 days SPION and AuNWs-PDMS scaffolds had higher cell surface marker expression and calcification than pristine PDMS. Nanostructured composites show promise in stem cell research and therapeutics.71
5. Biocompatibility and Cellular Interaction of Graphene Oxide with Bone Cells
The design and improvement of novel materials depend on biocompatibility for implants and tissue engineering scaffolds. GO has cytotoxicity, but its toxic manifestation is concentration-dependent and correlates with GO shape according to more studies. Wang et al. cocultured human fibroblasts with three GO concentration gradients and found no toxicity below 20 μg/mL, but cytotoxicity above 50 μg/mL. Further in vivo studies in mice showed no significant toxicity in the low dose group, but the high dose group showed chronic toxicity in the lung, liver, and kidney, causing mortality and lung granuloma. In mammalian cells, microsized GO had higher cytotoxicity than nanosized GO due to faster sedimentation and the formation of compact GO aggregates on top of adherent cells in the wells, inhibiting cell growth. Amazingly, GO autodegrades in water. After prolonged water exposure, C–C bond cleavage reduces GO lattice sizes, resulting in a structure similar to that of humid acid, a benign degradation product of all organic matter. GO has moderate biocompatibility and can be cleared in vivo in months.
Lee et al. found that rGO-hydroxyapatite nanocomposites could increase MC3T3-E1 preosteoblast osteogenic differentiation and bone cell formation. They found that rGO/HAp nanocomposites stimulated MC3T3-E1 preosteoblast osteogenic differentiation to form new bone cells. The unique properties of rGO and HAp may create a microenvironment that promotes bone cell differentiation and proliferation. Although less cytotoxic, these nanocomposites are biocompatible and could be used in bone tissue engineering.47,48 Nanoformulations of graphene oxide (GO) and its functionalized derivatives have garnered attention for drug administration, tissue engineering, and photothermal cancer therapy, but their biocompatibility has not been studied. The study found that PEG functionalized with fluorescein isothiocyanate (FITC) GO nanosheets promoted MC3T3-E1 preosteoblast proliferation and differentiation. After 12 days of nanosheet (40 g/mL) absorption, preosteoblasts developed osteoblasts with normal alkaline phosphatase levels and matrix mineralization. Thus, internalized PEG-GO nanosheets may be useful for biomedical applications in local hyperthermia for bone cancer treatment.72
For bone tissue regeneration, capsaicin was biologically adsorbed with reduced graphene oxide (RGO). Capsaicin was decorated on graphene to prevent agglomeration. Capsaicin-functionalized RGO promoted osteoblastic differentiation and proliferation to support bone cell growth and new bone formation. The anti-inflammatory properties of capsaicin can also reduce bone tissue inflammation. Capsaicin and graphene-based materials may be used to create advanced biomaterials for bone tissue engineering with better mechanical properties than unmodified RGO. Due to their chemical and physical properties, graphene-based materials are promising for bone tissue engineering, medication delivery, biosensing, and energy storage.73 Molecular dynamics was used to study BMP-2 interactions with graphene, reduced graphene oxide, graphene oxide, and nitrogen functionalized graphene. Increasing surface polarity with oxygen or nitrogen atoms improved BMP-2’s interaction with altered surfaces. Graphene oxide had the highest BMP-2 adsorption energy due to its undulated molecular surface. Graphene oxide may interact with this protein’s wrist and knuckle epitopes, strengthening binding. Graphene oxide promotes osteogenic cell adhesion and proliferation, making it promising for bone tissue engineering. Graphene oxide-based bone biomaterials with added functions also interacted better with bone repair proteins.74 Graphene oxide is biocompatible and is ideal for stem cell growth. GO was treated with polydopamine (PDA) to improve its deposition on polystyrene (PT) for tissue culture. Then, pluripotent embryonic stem cells (ESCs) were tested for the osteogenic performance of the PDA/GO composite. Microscopy and X-ray photoelectron spectroscopy showed better surface chemistry of PDA/GO-coated PT. It promotes ESC proliferation on a PDA/GO composite-coated surface with good cell viability. Alkaline phosphatase activity, intracellular calcium levels, matrix mineralization, and osteogenic factor mRNA and protein levels showed that ESCs cultured on the PDA/GO substrate had a higher osteogenic potential than those cultured on an uncoated control surface. Compared to control groups, ESCs grown on the PDA/GO substrate showed higher levels of integrin α5, β1, and BMPR types I and II. In PDA/GO-treated ESCs, MAPKs, ERK1/2, p38, and JNK phosphorylation was significant, and BMP signal transduction through SMAD1/5/8 phosphorylation was enhanced compared to control cells. The PDA/GO substrate also processed the nuclear translocation of SMAD1/5/8 in cells and suppressed osteogenic differentiation of ESCs by blocking integrin 5/1, mitogen-activated protein kinase, or p38. These findings showed that the PDA/GO composite activated integrin 5/1, MAPK, and BMPR/SMAD signaling pathways to promote ESC osteogenic differentiation.75
Another study examined how low-oxygen graphene (LOG) nanoparticles stimulated adult mesenchymal stem cell osteogenesis. Research showed that 0.1 mg/mL LOG nanoparticles kept adult goat MSCs alive in hypoxia. They also increased runx2 and osteocalcin expression in MSCs, indicating osteogenic differentiation. MSCs and LOG nanoparticles enclosed in a 3D scaffold had defined cell morphology and MSC adhesion, as shown by scanning electron microscopy. For bone tissue engineering, xenogenic LOG and MSCs on a 3D scaffold in a rat unicortical tibial bone defect site increased active bone growth and mineralization.76 Nanocomposites with carboxylated graphene oxide (GO–COOH) sheets and zinc oxide (ZnO) nanoparticles (NPs) were prepared and tested for bone tissue engineering. ZnO/GO–COOH nanocomposites were synthesized by carboxylating graphene oxide (GO) and nucleating ZnO on the GO–COOH sheets. GO–COOH sheets were evenly decorated with 12 nm ZnO nanoparticles to make nanocomposites. ZnO/GO–COOH nanocomposites upregulated osteogenic-related genes (ALP, OCN, and Runx2) in MG63 osteoblast-like cells, increasing ALP activity, osteocalcin production, and extracellular matrix mineralization compared with GO–COOH and the control group. Antibacterial ZnO/GO–COOH nanocomposites inhibited Streptococcus mutans. Thus, these nanocomposites showed great promise as novel biomaterials for bone tissue engineering with improved osteogenic activity and antibacterial activity.68,69
6. Graphene Oxide as a Single Standing Material for Bone Regeneration
2D carbon-based materials graphene, graphene oxide (GO), and reduced graphene oxide (rGO) have excellent physical, chemical, and biological properties. Strong graphene sheets have a large specific surface area. Additionally, they alter stem cell development and improve biomaterial function. Recent advances using graphene and its derivatives to improve biomaterial properties and bioactivity are challenging. Before these carbon-based polymers can be used clinically, biosafety issues must be addressed. Graphene can be modified with bioactive compounds to make dental and medical biomaterials. Graphene modification of composites often improves bioactivity and physicomechanical properties. Composites treated with graphene oxide showed promise for delivering bioactive molecules, such as growth factors and other therapeutics. Thus, 2D graphene and graphene oxide can improve the biomaterial’s physical, chemical, and mechanical properties and transport drugs to substrates and scaffolds for cell-based tissue engineering.77 Over the past decade, graphene and its derivatives have been extensively studied in materials science and nanotechnology. Since nanomedicine can detect and treat anomalies early, graphene is now being touted as a potential lifesaver. Functionalization processes have improved graphene oxide, making it a better nanosystem for biosensing, drug transport, gene therapy, bioimaging, phototherapy, and hybrid theranostics. Due to its electrically programmable surface chemistry, larger surface-to-volume ratio, simple functionalization capacity, and mechanical robustness, graphene oxide is popular in tissue engineering for cardiac, nerve, bone, skin, and stem cell applications. Recent advances in graphene oxide-based nanotheranostics and tissue engineering show that modern medical ideas can effectively treat a wide range of disorders.
Recent advances in stem cell research and nanotechnology have transformed tissue engineering and regenerative medicine. Thus, precise and repeatable stem cell fate and lineage determination regulation are increasingly essential for stem cell-based technologies. Intensive research has focused on materials that mimic stem cells’ physiological milieu to guide cell differentiation. In this regard, 2-dimensional materials like graphene and its analogues are intriguing. Due to their high specific surface area, chemical stability, biocompatibility, and functionalization flexibility, graphene-based nanomaterials improve stem cell growth, proliferation, and differentiation. Based on stem cell regulation, biocompatibility, biodistribution, and biodegradability, graphene oxide nanomaterials promoted stem cell growth and differentiation.78 Due to its unique physicochemical, electrical, and biological properties, graphene oxide is being studied for multidisciplinary applications. The faster advancements of graphene oxide-based biofunctional nanostructures make them more important for bridging the gap between graphene’s basic benefits and other hybridized biomaterials. Recent developments in graphene biofunctional nanostructures like graphene-inorganic nanohybrids, functional nanoinks, nacre-like layered composites, macroporous scaffolds, 3D printed microlattices, and injectable hydrogels for biological and cellular interfaces include broad synthesis protocols to fine-tune physiological stability, stimuli responsiveness, and biofunctional properties. At their interfaces, these nanostructures’ biocompatibility and biointeractions with viruses, bacteria, and stem cells are highlighted for nanomedicine, tissue regeneration, and biosensor applications.79 Recent innovations based on graphene materials’ biological interactions have illuminated graphene oxide-containing composites’ biomedical uses. Due to its mechanical properties and low thermal expansion, graphene oxide is widely used to reinforce biocomposites. Bone grafts reinforced with calcium silicate and graphene oxide had uniform graphene distribution and increased young’s modulus. In simulated body fluid, graphene oxide composites formed bone-like apatite. SEM analysis of the composites showed an apatite layer similar to bone that supported mesenchymal stem cell growth in vitro and osteogenesis-related protein production, such as alkaline phosphatase, osteopontin, and osteocalcin. Increasing graphene oxide in composites also greatly increased the cell production of angiogenesis-related vascular endothelial growth factor and ang-1 proteins. These findings suggest graphene oxide-containing calcium silicate bone grafts may be useful in bone tissue engineering.80
For tissue regeneration, bone tissue engineering scaffolds must be biocompatible, mechanically stable, and osteo-inductive. Electrospun nanofibers with extracellular matrix structures are effective scaffolds for bone tissue regeneration. Due to their nanotechnological properties, these nanofibers have gained popularity in recent years. To improve hMSC osteogenic differentiation and biomineralization, electrospun cellulose acetate nanofibrous scaffolds with graphene oxide were made. As GO concentration increased, fiber diameter decreased. Most importantly, hybrid nanofibers’ mechanical strength, biocompatibility, and extracellular matrix mimicking structure increased hMSC adhesion and proliferation on scaffolds. GO-doped nanofibers biomineralized better in simulated bodily fluid because they had more calcium phosphate binding nucleation sites. Alkaline phosphatase activity increased due to enhanced biomineralization on these nanofibers, promoting hMSC osteogenic differentiation. Cellulose acetate GO nanofibrous scaffolds may be useful in bone tissue engineering in regenerative medicine.81 Congenital abnormalities, trauma, and injuries have made bone deterioration a global epidemic. Purohit et al. assembled a nanocomposite scaffold of graphene oxide, gelatin, and alginate with different GO concentrations to test its performance. The compressive strength of gelatin-alginate (GA) scaffolds with and without GO showed that hydrophilic nanocomposite scaffolds with high swelling rates were significantly improved. Cell adhesion and proliferation studies showed improved activity when osteoblastic cells were seeded onto nanocomposite scaffolds in vitro compared with GA scaffolds, suggesting that GO may improve scaffold characteristics and bone regeneration. Scaffolds seeded with mesenchymal stem cells showed increased Runx2 and Osteocalcin expression and alkaline phosphatase activity, suggesting that they are osteo-inductive. Thus, the nanocomposite scaffold may be useful in bone tissue engineering.82
7. Graphene Oxide Incorporated Scaffolds
Biomedicine may benefit from graphene oxide (GO), a nanomaterial with intriguing electrical and mechanical properties and a tiny two-dimensional form. However, GO’s biocompatibility and cell response are unknown. Precultured Saos-2 osteoblasts, MC3T3-E1 preosteoblasts, and RAW-264.7 macrophages were used to identify key cell markers for in vitro biocompatibility testing of graphene oxide nanosheets (GOs) decorated with 1-arm (1-GOs) and 6-arm (6-GOs) polyethylene glycol-amine. After internalization, GO nanosheets were localized on F-actin filaments, altering the cell cycle, apoptosis, and oxidative stress. GOs’ synergistic effect should be considered in photothermal cancer treatment. However, another study synthesized graphene oxide with a fibrin coating to improve the surface functionality. Using the osteoblast-like cell line MG-63 and the normal cell line NIH 3T3, fibrin coated graphene oxide (FGO) was characterized physiochemically and assessed for osteo-inductive potential. Transmission electron microscopy revealed cubical FGO, and scanning electron microscopy showed spherical GO nanoparticles coated with fibrin. In vitro tests showed higher alkaline phosphatase and calcium ion release, confirming FGO’s osteo-inductive properties. To prove that FGO is biocompatible, MTT was used to boost bone cell growth. Thus, FGO may be a scaffold for bone tissue reconstruction.83
Bone marrow mesenchymal stem cells produce preosteoblasts, which develop and repair bone. Cells with a high osteogenic lineage differentiation potential may be useful in bone tissue engineering and regenerative medicine. To effectively use osteoprogenitor cells in bone regeneration, biochemical, physical, or pharmacological variables that precisely regulate osteogenic differentiation must be identified. Thus, the nanocomposite with reduced graphene oxide (rGO) and hydroxyapatite (HAp) synergistically promoted preosteoblast osteogenic differentiation. Alkaline phosphatase activity and calcium and phosphate mineralization as early and late stage markers of osteogenic differentiation were increased by rGO/HAp composites without inhibiting MC3T3-E1 preosteoblast proliferation. This was because graphene-based composite materials can stimulate spontaneous osteogenesis without osteogenic stimuli. Thus, rGO/HAp hybrid composites may be biocompatible, transferable, and implantable bone regeneration scaffolds. The effects of biphasic calcium phosphate (BCP) covered in reduced graphene oxide (rGO) on bone regeneration were examined. Different amounts of rGO and BCP were mixed to make an rGO-coated bone graft material. A reference electrode with a surface charge of −14.43 mV and rGO-coated BCP made a static electrostatic contact. Higher doses drastically reduced cell viability, and BCPs coated with rGO transplanted into rats’ calvarial defects showed new bone development under micro-CT and histological examination. Treatment group bone formation was much higher than the control group bone formation. Histological analysis showed that rGO groups formed more new bone than controls. Thus, the composite concentration was crucial to the rGO-coated BCP’s osteogenesis promotion.84
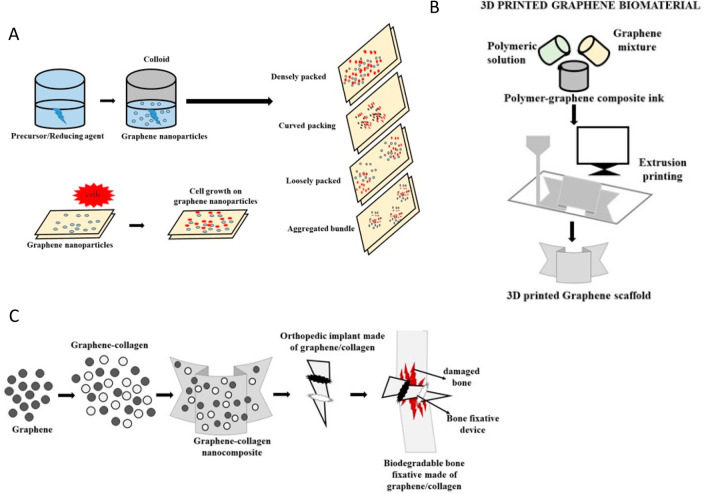
Protein adsorption occurs quickly after the biomedical device implantation. Thus, the biomedical device’s character controls biological activities, and protein adsorption strongly influences it. Depan and colleagues explained the relationship between protein adsorption and biological activity (osteoblast function) on chitosan scaffolds nanostructured with graphene oxide and a single-walled carbon nanohorn. The cell–substrate interactions of nanostructured carbon-modified and unmodified chitosan were compared to determine the protein adsorption imprint. According to the amount and shape of the protein adsorbed on scaffolds, nanostructured carbon improved biological processes. Due to protein adsorption and nanostructured carbon synergy, nanostructured carbon-modified scaffolds enhanced biological processes such as cell attachment, proliferation, and survival. Nanostructured carbon-modified scaffolds improved protein adsorption, bioactivity, and biological function.85 In vitro cytotoxicity and cell differentiation tests were performed on graphene oxide (GO) by using dental pulp stem cells (DPSC). GO was chemically exfoliated from graphite using Hummer’s process and showed cell attachment and proliferation after 5 days. AFM showed that the GO sample’s surface was rougher and GO deposition was random, which could improve cell adhesion. The gene expression studies showed that DPSCs could adhere and proliferate on a GO-based substrate, which increased the expression of many mineral-producing genes (Figure 5A). The findings opened the door to studying the benefits of adding GO to dental materials alone and in combination.86
Figure 5.
(A) 3D printing method of fabricating a graphene scaffold. (B) The cell adhesion and proliferating property of cells on exposure to graphene nanoparticles upon its cell assembling characteristics. (C) Design and development of a bone fixative using graphene and collagen for bone tissue engineering.
Tissue engineers who grow cells on biomaterial scaffolds must understand the form dimensionality (1D vs 2D vs 3D) of cell–scaffold interactions, which increases interest. Using physically orthogonal measurements to fluorescent confocal laser scanning microscopy, the team validated five statistical and three geometric contact models to select one from each class. Cell–scaffold contact measurements on spun coat, big microfiber, and medium microfiber scaffolds showed surface roughness 2 to 8 times less than CLSM resolution, proving the validity of a planar geometrical model for spun coat scaffolds. Multiview 2D scanning electron microscopy images verified a cylindrical fiber scaffold model, and SEM and CLSM fiber diameter comparisons determined segmentation error. This explained the use of statistical intensity and geometrical shape models to represent cell–scaffold contact sites, methods for validating 3D geometrical contact models, and a way to visually verify hundreds of 3D measurements.87 Since collagen (Col) type I is the main extracellular matrix protein in bones, bone tissue engineering research has focused on it. It cannot bear a load due to its poor mechanical properties. Norahan and team explain that graphene oxide (GO) covers freeze-dried Col scaffolds by covalently bonding amine Col with graphene carboxyl groups. The synthesized scaffolds had a porous structure under scanning electron microscopy and a higher compressive modulus after the GO coating. In an MTT assay, human bone marrow mesenchymal stem cells showed better cyto-compatibility and differentiated into osteoblasts without an osteogenic differentiation medium after week 2. This study found that GO and its reduced form were promising bone replacements for orthopedic and dentistry procedures.88
Nanosystems help immune and skeletal cells cooperate during bone regeneration, a multistep process. Activated monocytes may signal mesenchymal stem cells to promote osteogenesis. Monocyte stimulation may promote bone repair. Thus, the biocompatible nanomaterial with maGO-CaP (monocyte activator GO complexed with CaP) combined graphene oxide (GO)’s immune-stimulating properties with CaP’s osteoinductive potential. Increased osteogenic marker expression showed that Wnt and BMP signaling pathways were stimulated to provide Oncostatin M, a monocyte-made pro-osteogenic factor for immune activation. In mice, maGO-CaP injected into the tibia increased bone growth, indicating that it can stimulate monocytes in both in vitro and in vivo conditions to boost osteogenesis.89 Graphene and its derivatives are well-known osteoblastic stem/progenitor cell differentiation stimulators. The effects of graphene concentration, size, type, dimension, hydrophilicity, functionalization, and composition on bone regeneration have been debated in the scientific literature. They affect cell proliferation, viability, adhesion, and osteogenic differentiation. The reviews found graphene, graphene oxide (GO), and reduced graphene oxide (RGO) to be safe for most cell types at concentrations up to 50 g/mL for graphene and GO and 60 g/mL for RGO. However, cell types greatly affect concentrations. Cell viability was reduced for graphene with 5 m GO and 1 m RGO lateral dimensions. 3D graphene may also promote cell–cell contact, migration, and proliferation (Figure 5B). When integrated with graphene and its derivatives, metals, polymers, and minerals improve the mechanical properties and bioactivity. Finally, graphene and its derivatives increase surface roughness and porosity, which promotes cell adhesion and differentiation.90
With no chemical stimulus, graphene can stimulate osteogenesis. Many mechanisms are still unknown. A study examined the role of the integrin/FAK mechanotransduction axis in graphene-induced osteoblast differentiation and whether graphene scaffolds can sustain osteogenesis in vivo. In immune-compromised mice, MSC-impregnated graphene scaffolds were fixed. Another method involved seeding MSCs onto PDMS substrates covered with a monomolecular graphene layer to express runx2, osteocalcin, and osteopontin. MSCs were cultured on uncoated PDMS without mechanotransduction inhibitors (echistatin, Y27632, or DMH1). The MSC-impregnated graphene scaffolds showed favorable immune expression of bone-related markers without osteogenic inducers. The expression levels of osteogenic and integrin/FAK proteins were higher in MSCs seeded onto graphene-coated PDMS substrates than in PDMS alone in vitro regardless of substrate stiffness. By activating the integrin-dependent kinase (FAK) axis in response to mechanical stress, graphene promoted bone growth.79 The unusual properties of graphene oxide (GO), a monolayer of carbon, have led to extensive studies of its use in tissue engineering platforms. Preclinical studies examined the GO scaffolds’ ability to stimulate bone formation after tooth extraction in canine models. A collagen sponge scaffold was coated with 0.1 and 1 g/mL of GO to make GO scaffolds. SEM images showed a networked GO on the collagen scaffold. GO improved the material strength, enzyme resistance, calcium and protein adsorption, and osteoblastic MC3T3-E1 cell proliferation. The subcutaneous tissue response in rats showed that the 1 g/mL GO scaffold increased cellular ingrowth, indicating biocompatibility. ED2-positive (M2) macrophages and blood vessels infiltrated the GO scaffold extensively. Dog bone development was improved by the use of 1 g/mL of GO scaffolds. These findings showed that GO is biocompatible and can form bones as a scaffold.91
Simulating bone tissues with layered structures is difficult because there are no methods for assembling osteoblast cell types into bone structures (Figure 5C). Due to their unique properties, graphene and graphene-based nanostructures like graphene oxide (GO) have garnered attention in biomedical and technical fields. Carbon-based implants are suitable due to their mechanical and biocompatibility. Carbon-based interfaces for tissue scaffolds and medical implants are widely used. Optical, X-ray, and atomic force microscopy test graphene orientation, crystallinity, and surface contact. Samples with and without a favored carbon orientation were made to examine the effect. The cellular responses were observed using fluorescence, confocal, and environmental scanning microscopy. Unidirectional carbon efficiently promoted cell adhesion, proliferation, and extension. Due to increased crystallinity, cells aligned parallel to the fiber axis and multiplied, but oxygen or other functional groups disrupted cell-graphene surface contact, preventing further cell proliferation. Thus, crystallite size, graphene orientation, and carbon graphitization affect osteoblast attachment and development.92 Poly(lactic acid)/graphene oxide (PLA/GO) nanocomposites were 3D-printed beyond the porous network with typical dimensions of 300 nm to optimize the surface roughness and hydrophilicity. Nanocomposites helped osteogenic cell adhesion, proliferation, and differentiation without changing the transition temperature. The scaffolds with low polymer crystallinity and high mechanical strength may promote cell differentiation and bone development, while adding GO may improve surface roughness and hydrophilicity, which would boost cell adhesion and proliferation.93 Graphene oxide (GO) and its derivatives are being studied for bone-based biomaterial modification. By coating pure titanium with GO using dopamine, the unanswered question of how GO coatings affect immune regulation and osteogenesis was addressed. Experimental results showed that GO coatings increased osteoblast proliferation and differentiation, promoting bone regeneration.95−106 The GO coatings did not elicit an immune response, indicating biocompatibility with orthopedic implants. In human mesenchymal stem cells (hMSCs), this coating increased osteogenic gene expression, extracellular matrix mineralization, macrophage polarization, and inflammatory cytokine production via the Toll-like receptor pathway. In physiological conditions, titanium-GO coatings activated macrophages, causing moderate inflammation and a pro-osteogenic milieu with elevated TGF-1 and oncostatina M gene expression. Titanium-GO also reduced proinflammatory cytokines, suppressing inflammation. Thus, titanium-coated GO surfaces had immunomodulatory effects on osteogenesis, suggesting that GO may be a viable material for bone scaffolds and implants.94,95 Graphene oxide has emerged as a promising material in the field of bone tissue engineering. Several studies have explored its potential in enhancing osteogenesis and addressing various challenges in orthopedic applications.107 They conducted research on the chemical functionalization of graphene to promote stem cell osteogenesis and inhibit biofilm formation on polymer composites, demonstrating its potential for orthopedic applications. This work provides valuable insights into the functionalization of graphene for bone tissue engineering.107 A subsequent study showed graphene oxide to create chemically cross-linking-free alginate-chitosan-collagen scaffolds, further demonstrating the versatility of graphene in designing scaffolds for bone tissue engineering.108 The impact of 3D scaffolds on cellular responses to graphene in polymer composites was investigated, shedding light on how scaffold design influences the interaction between cells and graphene in orthopedic applications.107 More recent research by Joy et al. explores the incorporation of graphene oxide into nanocomposites and highlights its potential in various biomedical applications, including polycaprolactone nanocomposites and strontium nanohybrids.108 Other studies have investigated the synthesis of materials such as hydroxyapatite/agarose powders for bone filler and drug delivery applications, further extending the potential applications of graphene-based materials in bone tissue engineering.108 Overall, these studies collectively contribute to our understanding of how graphene oxide can be harnessed to advance the field of bone tissue engineering, offering potential solutions for orthopedic applications and regenerative medicine.
8. Future Perspectives and Conclusion
Tissue engineering is gaining momentum as a viable substitute for conventional methods in bone regeneration and replacement therapies within the field of contemporary medicine. The increase in interest is fueled by the rising need for tissue-engineered bone structures, which is driven by the scarcity of appropriate autograft and allograft materials. Researchers are diligently working to create novel solutions that not only accelerate the bone healing process but also provide fresh insights into our approach to bone regeneration. Scaffolds play a crucial role in tissue engineering and are considered to be essential components of this paradigm shift. Scaffolds are essential in tissue engineering, as they provide the necessary framework for cell proliferation and the growth of new tissue, serving as structural cornerstones.
This study focuses on investigating different materials derived from graphene and their potential use in constructing scaffolds for bone tissue engineering. The latest research has shed light on how to effectively utilize graphene’s capabilities by integrating it into scaffold materials. Research has demonstrated that the inclusion of this substance enhances the biological properties of scaffolds, primarily by promoting important processes such as cell attachment, growth, and bone development through modifications made to the surface.
Scaffolding materials in the field of bone tissue engineering have a dual function. They have the ability to either stimulate bone growth from nearby tissue or serve as channels for the transfer of transplanted bone cells or other bioactive substances. This fundamental differentiation gives rise to two main classifications of scaffolds: injectable scaffolds and those that require surgical implantation. The selection of a scaffold is contingent on the particular needs of the patient and the characteristics of the bone injury or deficiency. Irrespective of the type employed, these scaffolds are crucial for the achievement of bone tissue engineering.
Graphene-based nanomaterials are highly valuable in the fields of tissue engineering and regenerative medicine. Their notable impact is particularly apparent in the growth, spread, and specialization of stem cells, offering substantial progress in the field of tissue regeneration. The motivation for this research stems from the remarkable characteristics of GO. GO possesses remarkable specificity, outstanding chemical stability, compatibility with biological systems, and the ability to be functionalized, which position it as an excellent choice for a wide range of applications.
Although the potential of graphene oxide in bone tissue engineering is promising, additional investigations are necessary. Assessing its influence on the durability of bonding and the fusion of bone is of utmost importance, particularly within the realm of animal testing. The empirical evidence will have a substantial impact on our further comprehension of the mechanical and chemical factors that facilitate osteogenesis. In order to fully exploit the capabilities of graphene oxide, it is imperative to conduct thorough research to confirm its safety and effectiveness, thereby establishing its status as a highly promising material for scaffolding in the next generation.
To summarize, tissue engineering presents a hopeful pathway for the progress of bone regeneration and replacement treatments. Scaffolds play a crucial role in promoting cell proliferation and serving as structural frameworks for tissue growth, making them essential in this field. The incorporation of graphene into scaffold materials presents novel opportunities, enhancing their biological attributes. Graphene oxide possesses highly promising characteristics that distinguish it as an exceptional candidate. However, continuous research and experimentation are imperative in order to fully exploit its capabilities. By conducting additional investigations, we can narrow the divide between theoretical concepts and their practical implementation, thereby advancing us toward a forthcoming era of bone tissue engineering.
Acknowledgments
This work was supported by the Department of Science and Technology, Science and Engineering Research Board, Research Scientist scheme, Government of India for the research Grant to S. Vimalraj (Grant no. BS/SRS/2022-23/109/LS). The authors are also grateful for the financial support from IITM-ISRO JPC (SP22231133AMISRO009000) and IIT Madras through the Institutions of Eminence (IoE) Scheme (Grant no. SP2221234CPETWOCSMHOC), the Ministry of Education, Government of India.
Author Contributions
S.V.: Conceptualization, Methodology, Writing—Original draft preparation, Supervision, Validation. D.V.: Writing—Original draft preparation. S.S. and S.S.: Draft preparation.
The authors declare no competing financial interest.
References
- Lopes D.; Martins-Cruz C.; Oliveira M. B.; Mano J. F. Bone Physiology as Inspiration for Tissue Regenerative Therapies. Biomaterials 2018, 185, 240–275. 10.1016/j.biomaterials.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. F.; Su T.; Yang M.; Li C. J.; Guo Q.; Xiao Y.; Huang Y.; Liu Y.; Luo X. H. The Role of Autophagy in Bone Homeostasis. J. Cell. Physiol. 2021, 236 (6), 4152–4173. 10.1002/jcp.30111. [DOI] [PubMed] [Google Scholar]
- McGovern J. A.; Griffin M.; Hutmacher D. W. Animal Models for Bone Tissue Engineering and Modelling Disease. Dis. Model. Mech. 2018, 11 (4), dmm033084. 10.1242/dmm.033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannigan K.; Griffin M. An Update into the Application of Nanotechnology in Bone Healing. Open Orthop. J. 2016, 10, 808–823. 10.2174/1874325001610010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garimella R.; Eltorai A. E. Nanotechnology in Orthopedics. J. Orthop. 2017, 14 (1), 30–33. 10.1016/j.jor.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz E.; Zimmermann E. A.; Lee J. S.; Wegst U. G.; Tomsia A. P. Perspectives on the Role of Nanotechnology in Bone Tissue Engineering. Dent. Mater. 2013, 29 (1), 103–115. 10.1016/j.dental.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. R.; Hudson P. W.; Ponce B. A.; Rajaram Manoharan S. R. Nanotechnology in Orthopedics: A Clinically Oriented Review. B.M.C. Musculoskelet. Disord. 2018, 19 (1), 67. 10.1186/s12891-018-1990-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati K.; Maher S.; Findlay D. M.; Losic D. Titania Nanotubes for Orchestrating Osteogenesis at the Bone-Implant Interface. Nanomedicine (Lond) 2016, 11 (14), 1847–1864. 10.2217/nnm-2016-0169. [DOI] [PubMed] [Google Scholar]
- Gavaskar A.; Rojas D.; Videla F. Nanotechnology: The Scope and Potential Applications in Orthopedic Surgery. Eur. J. Orthop. Surg. Traumatol. 2018, 28 (7), 1257–1260. 10.1007/s00590-018-2193-z. [DOI] [PubMed] [Google Scholar]
- Savvidou O. D.; Bolia I. K.; Chloros G. D.; Goumenos S. D.; Sakellariou V. I.; Galanis E. C.; Papagelopoulos P. J. Applied Nanotechnology and Nanoscience in Orthopedic Oncology. Orthopedics 2016, 39 (5), 280–286. 10.3928/01477447-20160823-03. [DOI] [PubMed] [Google Scholar]
- Yue K.; Trujillo-de Santiago G.; Alvarez M. M.; Tamayol A.; Annabi N.; Khademhosseini A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasadh S.; Suresh S.; Wong R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials (Basel) 2018, 11 (8), 1430. 10.3390/ma11081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. C.; Chang T. K.; Yeh S. T.; Lin T. C.; Lin H. S.; Chen C. H.; Huang C. H.; Huang C. H. Evaluation of Graphene-Derived Bone Scaffold Exposure to the Calvarial bone_in-vitro and In-Vivo Studies. Nanotoxicology 2022, 16 (1), 1–15. 10.1080/17435390.2022.2027036. [DOI] [PubMed] [Google Scholar]
- Wang W.; Chen J. X.; Hou Y.; Bartolo P.; Chiang W. H. Investigations of Graphene and Nitrogen-Doped Graphene Enhanced Polycaprolactone 3D Scaffolds for Bone Tissue Engineering. Nanomaterials (Basel) 2021, 11 (4), 929. 10.3390/nano11040929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini F. S.; Nair L. S.; Laurencin C. T. Inductive Materials for Regenerative Engineering. J. Dent. Res. 2021, 100 (10), 1011–1019. 10.1177/00220345211010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X.; Wang T.; Guo S. Applications of 3D Printed Bone Tissue Engineering Scaffolds in the Stem Cell Field. Regen. Ther. 2021, 16, 63–72. 10.1016/j.reth.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey N.; Bentini R.; Islam I.; Cao T.; Castro Neto A. H.; Rosa V. Graphene: A Versatile Carbon-Based Material for Bone Tissue Engineering. Stem Cells Int. 2015, 2015, 804213. 10.1155/2015/804213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y. W.; Liu X. L.; Yu D. G.; Zhu Z. A.; Ke Q. F.; Mao Y. Q.; et al. Graphene-Modified CePO4 Nanorods Effectively Treat Breast Cancer-Induced Bone Metastases and Regulate Macrophage Polarization to Improve Osteo-Inductive Ability. J. Nanobiotechnology 2021, 19, 11. 10.1186/s12951-020-00753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Correction to: Graphene-modified CePO4 nanorods effectively treat breast cancer-induced bone metastases and regulate macrophage polarization to improve osteo-inductive ability. J. Nanobiotechnology 202119 ( (1), ), 91. 10.1186/s12951-021-00829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata D.; Horovistiz A. L.; Branco I.; Ferro M.; Ferreira N. M.; Belmonte M.; Lopes M. A.; Silva R. F.; Oliveira F. J. Carbon Nanotube-Based Bioceramic Grafts for Electrotherapy of Bone. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 360–368. 10.1016/j.msec.2013.09.028. [DOI] [PubMed] [Google Scholar]
- Gerhard E. M.; Wang W.; Li C.; Guo J.; Ozbolat I. T.; Rahn K. M.; Armstrong A. D.; Xia J.; Qian G.; Yang J. Design Strategies and Applications of Nacre-Based Biomaterials. Acta Biomater. 2017, 54, 21–34. 10.1016/j.actbio.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Raucci M. G.; Alvarez-Perez M.; Giugliano D.; Zeppetelli S.; Ambrosio L. Properties of Carbon Nanotube-Dispersed Sr-Hydroxyapatite Injectable Material for Bone Defects. Regen. Biomater. 2016, 3 (1), 13–23. 10.1093/rb/rbv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K.; Acri T.; Geary S.; Salem A. K. Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine < Sup/>. Tissue Eng. Part A 2017, 23 (19–20), 1169–1180. 10.1089/ten.tea.2016.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.; Zhang K.; Zhao L.; Li C.; Bu W.; Shen Y.; Gu Z.; Chang B.; Zheng C.; Lin C.; Sun H.; Yang B. Aspirin-Based Carbon Dots, a Good Biocompatibility of Material Applied for Bioimaging and Anti-inflammation. A.C.S. Appl. Mater. Interfaces 2016, 8 (48), 32706–32716. 10.1021/acsami.6b12252. [DOI] [PubMed] [Google Scholar]
- Kang E. S.; Kim D. S.; Suhito I. R.; Choo S. S.; Kim S. J.; Song I.; Kim T. H. Guiding Osteogenesis of Mesenchymal Stem Cells Using Carbon-Based Nanomaterials. Nano Converg. 2017, 4 (1), 2. 10.1186/s40580-017-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubun K.; Matsumura S.; Yudasaka M.; Iijima S.; Shiba K. Immobilization of a Carbon Nanomaterial-Based Localized Drug-Release System Using a Bispecific Material-Binding Peptide. Int. J. Nanomedicine 2018, 13, 1643–1652. 10.2147/IJN.S155913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu T. K.; Kang R. H.; Jeong K. Y.; Jun D. R.; Koh J. M.; Kim D.; Bae S. K.; Choi S. W. Bone-Targeted Delivery of Nanodiamond-Based Drug Carriers Conjugated with Alendronate for Potential Osteoporosis Treatment. J. Controlled Release 2016, 232, 152–160. 10.1016/j.jconrel.2016.04.025. [DOI] [PubMed] [Google Scholar]
- No Y. J.; Roohani-Esfahani S.-i.; Zreiqat H. Nanomaterials: The Next Step in Injectable Bone Cements. Nanomedicine (Lond) 2014, 9 (11), 1745–1764. 10.2217/nnm.14.109. [DOI] [PubMed] [Google Scholar]
- Li H.; He D.; Xiao X.; Yu G.; Hu G.; Zhang W.; Wen X.; Lin Y.; Li X.; Lin H.; Diao Y.; Tang Y. Nitrogen-Doped Multiwalled Carbon Nanotubes Enhance Bone Remodeling Through Immunomodulatory Functions. A.C.S. Appl. Mater. Interfaces 2021, 13 (21), 25290–25305. 10.1021/acsami.1c05437. [DOI] [PubMed] [Google Scholar]
- Martins-Júnior P. A.; Alcântara C. E.; Resende R. R.; Ferreira A. J. Carbon Nanotubes: Directions and Perspectives in Oral Regenerative Medicine. J. Dent. Res. 2013, 92 (7), 575–583. 10.1177/0022034513490957. [DOI] [PubMed] [Google Scholar]
- Andrade V. B.; Sá M. A.; Mendes R. M.; Martins-Júnior P. A.; Silva G. A. B.; Sousa B. R.; Caliari M. V.; Ávila E. S.; Ladeira L. O.; Resende R. R.; Ferreira A. J. Enhancement of Bone Healing by Local Administration of Carbon Nanotubes Functionalized with Sodium Hyaluronate in Rat Tibiae. Cells Tissues Organs 2017, 204 (3–4), 137–149. 10.1159/000453030. [DOI] [PubMed] [Google Scholar]
- Gao C.; Feng P.; Peng S.; Shuai C. Carbon Nanotube, Graphene and Boron Nitride Nanotube Reinforced Bioactive Ceramics for Bone Repair. Acta Biomater. 2017, 61, 1–20. 10.1016/j.actbio.2017.05.020. [DOI] [PubMed] [Google Scholar]
- Sá M. A.; Ribeiro H. J.; Valverde T. M.; Sousa B. R.; Martins-Júnior P. A.; Mendes R. M.; Ladeira L. O.; Resende R. R.; Kitten G. T.; Ferreira A. J. Single-Walled Carbon Nanotubes Functionalized with Sodium Hyaluronate Enhance Bone Mineralization. Braz. J. Med. Biol. Res. 2016, 49 (2), e4888 10.1590/1414-431x20154888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z.; Feng X.; Cao G.; She Z.; Tan R.; Aifantis K. E.; Zhang R.; Li X. The Effect of Carbon Nanotubes on Osteogenic Functions of Adipose-Derived Mesenchymal Stem Cells In Vitro and Bone Formation In Vivo Compared with That of Nano-Hydroxyapatite and the Possible Mechanism. Bioact. Mater. 2021, 6 (2), 333–345. 10.1016/j.bioactmat.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S.; Hirata E.; Sakairi M.; Miyako E.; Takano Y.; Ushijima N.; Yudasaka M.; Iijima S.; Yokoyama A. Carbon Nanohorn Coating by Electrodeposition Accelerate Bone Formation on Titanium Implant. Artif. Cells Nanomed. Biotechnol. 2021, 49 (1), 20–29. 10.1080/21691401.2020.1865388. [DOI] [PubMed] [Google Scholar]
- De Carvalho J. O.; de Carvalho Oliveira F.; Freitas S. A. P.; Soares L. M.; de Cássia Barros Lima R.; de Sousa Gonçalves L.; Webster T. J.; Marciano F. R.; Lobo A. O. Carbon Nanomaterials for Treating Osteoporotic Vertebral Fractures. Curr. Osteoporos. Rep. 2018, 16 (5), 626–634. 10.1007/s11914-018-0476-2. [DOI] [PubMed] [Google Scholar]
- Shrestha S.; Shrestha B. K.; Ko S. W.; Kandel R.; Park C. H.; Kim C. S. Engineered Cellular Microenvironments from Functionalized Multiwalled Carbon Nanotubes Integrating Zein/Chitosan @Polyurethane for Bone Cell Regeneration. Carbohydr. Polym. 2021, 251, 117035. 10.1016/j.carbpol.2020.117035. [DOI] [PubMed] [Google Scholar]
- Lalwani G.; Henslee A. M.; Farshid B.; Parmar P.; Lin L.; Qin Y. X.; Kasper F. K.; Mikos A. G.; Sitharaman B. Tungsten Disulfide Nanotubes Reinforced Biodegradable Polymers for Bone Tissue Engineering. Acta Biomater. 2013, 9 (9), 8365–8373. 10.1016/j.actbio.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z.; Yang C.; Jiao J.; Li X.; Zhu D.; Yang Y.; Yang J.; Che Y.; Lu Y.; Feng X. Rhein and Polydimethylsiloxane Functionalized Carbon/Carbon Composites as Prosthetic Implants for Bone Repair Applications. Biomed. Mater. 2017, 12 (4), 045004. 10.1088/1748-605X/aa6e27. [DOI] [PubMed] [Google Scholar]
- Aslam Khan M. U.; Haider A.; Abd Razak S. I.; Abdul Kadir M. R.; Haider S.; Shah S. A.; Hasan A.; Khan R.; Khan S. D.; Shakir I. Arabinoxylan/Graphene-Oxide/nHAp-NPs/PVA Bionano Composite Scaffolds for Fractured Bone Healing. J. Tissue Eng. Regen. Med. 2021, 15 (4), 322–335. 10.1002/term.3168. [DOI] [PubMed] [Google Scholar]
- Phan L. M. T.; Vo T. A. T.; Hoang T. X.; Cho S. Graphene Integrated Hydrogels Based Biomaterials in Photothermal Biomedicine. Nanomaterials (Basel) 2021, 11 (4), 906. 10.3390/nano11040906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Xiong P.; Yan F.; Li S.; Ren C.; Yin Z.; Li A.; Li H.; Ji X.; Zheng Y.; Cheng Y. An Overview of Graphene-Based Hydroxyapatite Composites for Orthopedic Applications. Bioact. Mater. 2018, 3 (1), 1–18. 10.1016/j.bioactmat.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menaa F.; Abdelghani A.; Menaa B. Graphene Nanomaterials as Biocompatible and Conductive Scaffolds for Stem Cells: Impact for Tissue Engineering and Regenerative Medicine. J. Tissue Eng. Regen. Med. 2015, 9 (12), 1321–1338. 10.1002/term.1910. [DOI] [PubMed] [Google Scholar]
- Jiao D.; Zheng A.; Liu Y.; Zhang X.; Wang X.; Wu J.; She W.; Lv K.; Cao L.; Jiang X. Bidirectional Differentiation of BMSCs Induced by a Biomimetic Procallus Based on a Gelatin-Reduced Graphene Oxide Reinforced Hydrogel for Rapid Bone Regeneration. Bioact. Mater. 2021, 6 (7), 2011–2028. 10.1016/j.bioactmat.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliormanlı A. M.; Türk M.; Atmaca H. Response of Mouse Bone Marrow Mesenchymal Stem Cells to Graphene-Containing Grid-Like Bioactive Glass Scaffolds Produced by Robocasting. J. Biomater. Appl. 2018, 33 (4), 488–500. 10.1177/0885328218799610. [DOI] [PubMed] [Google Scholar]
- Holt B. D.; Wright Z. M.; Arnold A. M.; Sydlik S. A. Graphene Oxide as a Scaffold for Bone Regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 10.1002/wnan.1437. [DOI] [PubMed] [Google Scholar]
- Dalgic A. D.; Alshemary A. Z.; Tezcaner A.; Keskin D.; Evis Z. Silicate-Doped Nano-Hydroxyapatite/Graphene Oxide Composite Reinforced Fibrous Scaffolds for Bone Tissue Engineering. J. Biomater. Appl. 2018, 32 (10), 1392–1405. 10.1177/0885328218763665. [DOI] [PubMed] [Google Scholar]
- Lee J. H.; Shin Y. C.; Lee S. M.; Jin O. S.; Kang S. H.; Hong S. W.; Jeong C. M.; Huh J. B.; Han D. W. Enhanced Osteogenesis by Reduced Graphene Oxide/Hydroxyapatite Nanocomposites. Sci. Rep. 2015, 5, 18833. 10.1038/srep18833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. C.; Lim C. H.; Kenry S. C.; Su C.; Loh K. P.; Lim C. T. Cell-Assembled Graphene Biocomposite for Enhanced Chondrogenic Differentiation. Small 2015, 11 (8), 963–969. 10.1002/smll.201401635. [DOI] [PubMed] [Google Scholar]
- Siddiqui H. A.; Pickering K. L.; Mucalo M. R. A Review on the Use of Hydroxyapatite-Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes. Materials (Basel) 2018, 11 (10), 1813. 10.3390/ma11101813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlanda A.; Walejewska E.; Kowiorski K.; Heljak M.; Swieszkowski W.; Lipińska L. Investigation into Morphological and Electromechanical Surface Properties of Reduced-Graphene-Oxide-Loaded Composite Fibers for Bone Tissue Engineering Applications: A Comprehensive Nanoscale Study Using Atomic Force Microscopy Approach. Micron 2021, 146, 103072. 10.1016/j.micron.2021.103072. [DOI] [PubMed] [Google Scholar]
- Demir-Oguz Ö.; Ege D. Effect of Zoledronic Acid and Graphene Oxide on the Physical and In Vitro Properties of Injectable Bone Substitutes. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111758. 10.1016/j.msec.2020.111758. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Hong X.; Wang J.; Feng L.; Fan T.; Guo R.; Zhang H. 2D Nanomaterials for Tissue Engineering and Regenerative Nanomedicines: Recent Advances and Future Challenges. Adv. Healthc. Mater. 2021, 10 (7), e2001743 10.1002/adhm.202001743. [DOI] [PubMed] [Google Scholar]
- Scarano A.; Orsini T.; Di Carlo F.; Valbonetti L.; Lorusso F. Graphene-Doped Poly (Methyl-Methacrylate) (Pmma) Implants: A Micro-CT and Histomorphometrical Study in Rabbits. Int. J. Mol. Sci. 2021, 22 (3), 1441. 10.3390/ijms22031441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.; Parekh S. H. Molecular Control of Interfacial Fibronectin Structure on Graphene Oxide Steers Cell Fate. A.C.S. Appl. Mater. Interfaces 2021, 13 (2), 2346–2359. 10.1021/acsami.0c21042. [DOI] [PubMed] [Google Scholar]
- Tahriri M.; Del Monico M.; Moghanian A.; Tavakkoli Yaraki M.; Torres R.; Yadegari A.; Tayebi L. Graphene and Its Derivatives: Opportunities and Challenges in Dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 171–185. 10.1016/j.msec.2019.04.051. [DOI] [PubMed] [Google Scholar]
- Orecchioni M.; Ménard-Moyon C.; Delogu L. G.; Bianco A. Graphene and the Immune System: Challenges and Potentiality. Adv. Drug Delivery Rev. 2016, 105 (B), 163–175. 10.1016/j.addr.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Sun Y.; Song J. Fabrication and Tribological Properties of Nanogrids on CVD-Grown Graphene. Micron 2017, 97, 29–34. 10.1016/j.micron.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Vuppaladadium S. S. R.; Agarwal T.; Kulanthaivel S.; Mohanty B.; Barik C. S.; Maiti T. K.; Pal S.; Pal K.; Banerjee I. Silanization Improves Biocompatibility of Graphene Oxide. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110647. 10.1016/j.msec.2020.110647. [DOI] [PubMed] [Google Scholar]
- Foroutan T.; Nazemi N.; Tavana M.; Kassaee M. Z.; Motamedi E.; Sonieshargh S.; Zare Zardini H. Suspended Graphene Oxide Nanoparticle for Accelerated Multilayer Osteoblast Attachment. J. Biomed. Mater. Res., Part A 2018, 106 (1), 293–303. 10.1002/jbm.a.36231. [DOI] [PubMed] [Google Scholar]
- Aryaei A.; Jayatissa A. H.; Jayasuriya A. C. The Effect of Graphene Substrate on Osteoblast Cell Adhesion and Proliferation. J. Biomed. Mater. Res., Part A 2014, 102 (9), 3282–3290. 10.1002/jbm.a.34993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci R.; Leite N. C. S.; da-Silva N. S.; Pacheco-Soares C.; Canevari R. A.; Marciano F. R.; Webster T. J.; Lobo A. O. Graphene Oxide Nanoribbons as Nanomaterial for Bone Regeneration: Effects on Cytotoxicity, Gene Expression and Bactericidal Effect. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 341–348. 10.1016/j.msec.2017.03.278. [DOI] [PubMed] [Google Scholar]
- Saburi E.; Islami M.; Hosseinzadeh S.; Moghadam A. S.; Mansour R. N.; Azadian E.; Joneidi Z.; Nikpoor A. R.; Ghadiani M. H.; Khodaii Z.; Ardeshirylajimi A. In Vitro Osteogenic Differentiation Potential of the Human Induced Pluripotent Stem Cells Augments When Grown on Graphene Oxide-Modified Nanofibers. Gene 2019, 696, 72–79. 10.1016/j.gene.2019.02.028. [DOI] [PubMed] [Google Scholar]
- Wang W.; Liu Y.; Yang C.; Qi X.; Li S.; Liu C.; Li X. Mesoporous Bioactive Glass Combined with Graphene Oxide Scaffolds for Bone Repair. Int. J. Biol. Sci. 2019, 15 (10), 2156–2169. 10.7150/ijbs.35670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Mou S.; Zhou C.; Guo L.; Zhong A.; Yang J.; Yuan Q.; Wang J.; Sun J.; Wang Z. Off-the-Shelf Biomimetic Graphene Oxide-Collagen Hybrid Scaffolds Wrapped with Osteoinductive Extracellular Matrix for the Repair of Cranial Defects in Rats. A.C.S. Appl. Mater. Interfaces 2018, 10 (49), 42948–42958. 10.1021/acsami.8b11071. [DOI] [PubMed] [Google Scholar]
- Hussein K. H.; Abdelhamid H. N.; Zou X.; Woo H. M. Ultrasonicated Graphene Oxide Enhances Bone and Skin Wound Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 484–492. 10.1016/j.msec.2018.09.051. [DOI] [PubMed] [Google Scholar]
- Shin Y. C.; Bae J. H.; Lee J. H.; Raja I. S.; Kang M. S.; Kim B.; Hong S. W.; Huh J. B.; Han D. W. Enhanced Osseointegration of Dental Implants with Reduced Graphene Oxide Coating. Biomater. Res. 2022, 26 (1), 11. 10.1186/s40824-022-00257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Zhang J.; Li S.; Chen S.; Guo R.; Ikoma T.; Li X.; Chen W. Novel Template Synthesis, Microstructure, and In Vitro Biocompatibility of titania Hollow Microspheres. Mater. Lett. 2022, 327, 133058. 10.1016/j.matlet.2022.133058. [DOI] [Google Scholar]
- Chen J.; Zhang X.; Cai H.; Chen Z.; Wang T.; Jia L.; Wang J.; Wan Q.; Pei X. Osteogenic Activity and Antibacterial Effect of Zinc Oxide/Carboxylated Graphene Oxide Nanocomposites: Preparation and In Vitro Evaluation. Colloids Surf. B Biointerfaces 2016, 147, 397–407. 10.1016/j.colsurfb.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Chen S.; Du X.; Jia L.; Chang H.; Ikoma T.; Hanagata N. Synthesis and Osteo-Compatibility of Novel Reduced Graphene Oxide-Aminosilica Hybrid Nanosheets. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 251–256. 10.1016/j.msec.2015.12.056. [DOI] [PubMed] [Google Scholar]
- Kang E. S.; Song I.; Kim D. S.; Lee U.; Kim J. K.; Son H.; Min J.; Kim T. H. Size-Dependent Effects of Graphene Oxide on the Osteogenesis of Human Adipose-Derived Mesenchymal Stem Cells. Colloids Surf. B Biointerfaces 2018, 169, 20–29. 10.1016/j.colsurfb.2018.04.053. [DOI] [PubMed] [Google Scholar]
- Hashemzadeh H.; Allahverdi A.; Sedghi M.; Vaezi Z.; Tohidi Moghadam T.; Rothbauer M.; Fischer M. B.; Ertl P.; Naderi-Manesh H. PDMS Nano-modified Scaffolds for Improvement of Stem Cells Proliferation and Differentiation in Microfluidic Platform. Nanomaterials (Basel) 2020, 10 (4), 668. 10.3390/nano10040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicuéndez M.; Silva V. S.; Hortigüela M. J.; Matesanz M. C.; Vila M.; Portolés M. T. MC3T3-E1 Pre-osteoblast Response and Differentiation After Graphene Oxide Nanosheet Uptake. Colloids Surf. B Biointerfaces 2017, 158, 33–40. 10.1016/j.colsurfb.2017.06.019. [DOI] [PubMed] [Google Scholar]
- Zhai L.; Li L.; Zhang Q. Fabrication of Capsaicin Functionalized Reduced Graphene Oxide and Its Effect on Proliferation and Differentiation of Osteoblasts. Environ. Toxicol. Pharmacol. 2018, 57, 41–45. 10.1016/j.etap.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Wang M.; Wang K.; Sun Y.; Zhang H.; Lu X.; Duan K. Molecular Mechanisms of Interactions Between BMP-2 and Graphene: Effects of Functional Groups and Microscopic Morphology. Appl. Surf. Sci. 2020, 525, 146636. 10.1016/j.apsusc.2020.146636. [DOI] [Google Scholar]
- Shim N. Y.; Heo J. S. Performance of the Polydopamine-Graphene Oxide Composite Substrate in the Osteogenic Differentiation of Mouse Embryonic Stem Cells. Int. J. Mol. Sci. 2021, 22 (14), 7323. 10.3390/ijms22147323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhenany H.; Bourdo S.; Hecht S.; Donnell R.; Gerard D.; Abdelwahed R.; Lafont A.; Alghazali K.; Watanabe F.; Biris A. S.; Anderson D.; Dhar M. Graphene Nanoparticles as Osteoinductive and Osteoconductive Platform for Stem Cell and Bone Regeneration. Nanomedicine 2017, 13 (7), 2117–2126. 10.1016/j.nano.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Xie H.; Cao T.; Rodríguez-Lozano F. J.; Luong-Van E. K.; Rosa V. Graphene for the Development of the Next-Generation of Biocomposites for Dental and Medical Applications. Dent. Mater. 2017, 33 (7), 765–774. 10.1016/j.dental.2017.04.008. [DOI] [PubMed] [Google Scholar]
- Kenry L. W. C.; Lee W. C.; Loh K. P.; Lim C. T. When Stem Cells Meet Graphene: Opportunities and Challenges in Regenerative Medicine. Biomaterials 2018, 155, 236–250. 10.1016/j.biomaterials.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Xie H.; Cao T.; Franco-Obregón A.; Rosa V. Graphene-Induced Osteogenic Differentiation Is Mediated by the Integrin/FAK Axis. Int. J. Mol. Sci. 2019, 20 (3), 574. 10.3390/ijms20030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie M. Y.; Chiang W. H.; Chen I. P.; Liu W. Y.; Chen Y. W. Synergistic Acceleration in the Osteogenic and Angiogenic Differentiation of Human Mesenchymal Stem Cells by Calcium Silicate-Graphene Composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 726–735. 10.1016/j.msec.2016.12.071. [DOI] [PubMed] [Google Scholar]; Erratum in: Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1308–1309. 10.1016/j.msec.2019.01.059. [DOI] [PubMed] [Google Scholar]
- Liu X.; Shen H.; Song S.; Chen W.; Zhang Z. Accelerated Biomineralization of Graphene Oxide - Incorporated Cellulose Acetate Nanofibrous Scaffolds for Mesenchymal Stem Cell Osteogenesis. Colloids Surf. B Biointerfaces 2017, 159, 251–258. 10.1016/j.colsurfb.2017.07.078. [DOI] [PubMed] [Google Scholar]
- Purohit S. D.; Bhaskar R.; Singh H.; Yadav I.; Gupta M. K.; Mishra N. C. Development of a Nanocomposite Scaffold of Gelatin-Alginate-Graphene Oxide for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 133, 592–602. 10.1016/j.ijbiomac.2019.04.113. [DOI] [PubMed] [Google Scholar]
- Deepachitra R.; Chamundeeswari M.; Santhosh Kumar B.; Krithiga G.; Prabu P.; Pandima Devi M.; Sastry T. P. Osteo Mineralization of Fibrin-Decorated Graphene Oxide. Carbon 2013, 56, 64–76. 10.1016/j.carbon.2012.12.070. [DOI] [Google Scholar]
- Kim J. W.; Shin Y. C.; Lee J. J.; Bae E. B.; Jeon Y. C.; Jeong C. M.; Yun M. J.; Lee S. H.; Han D. W.; Huh J. B. The Effect of Reduced Graphene Oxide-Coated Biphasic Calcium Phosphate Bone Graft Material on Osteogenesis. Int. J. Mol. Sci. 2017, 18 (8), 1725. 10.3390/ijms18081725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depan D.; Misra R. D. The Interplay Between Nanostructured Carbon-Grafted Chitosan Scaffolds and Protein Adsorption on the Cellular Response of Osteoblasts: Structure-Function Property Relationship. Acta Biomater. 2013, 9 (4), 6084–6094. 10.1016/j.actbio.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Rosa V.; Xie H.; Dubey N.; Madanagopal T. T.; Rajan S. S.; Morin J. L.; Islam I.; Castro Neto A. H. Graphene Oxide-Based Substrate: Physical and Surface Characterization, Cytocompatibility and Differentiation Potential of Dental Pulp Stem Cells. Dent. Mater. 2016, 32 (8), 1019–1025. 10.1016/j.dental.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Bajcsy P.; Yoon S.; Florczyk S. J.; Hotaling N. A.; Simon M.; Szczypinski P. M.; Schaub N. J.; Simon C. G.; Brady M.; Sriram R. D. Modeling, Validation and Verification of Three-Dimensional Cell-Scaffold Contacts from Terabyte-Sized Images. B.M.C. Bioinformatics 2017, 18 (1), 526. 10.1186/s12859-017-1928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norahan M. H.; Amroon M.; Ghahremanzadeh R.; Rabiee N.; Baheiraei N. Reduced Graphene Oxide: Osteogenic Potential for Bone Tissue Engineering. I.E.T. Nanobiotechnol. 2019, 13 (7), 720–725. 10.1049/iet-nbt.2019.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoni V.; Reina G.; Orecchioni M.; Furesi G.; Thiele S.; Gardin C.; Zavan B.; Cuniberti G.; Bianco A.; Rauner M.; Delogu L. G. Stimulation of Bone Formation by Monocyte-Activator Functionalized Graphene Oxide In Vivo. Nanoscale 2019, 11 (41), 19408–19421. 10.1039/C9NR03975A. [DOI] [PubMed] [Google Scholar]
- Mohammadrezaei D.; Golzar H.; Rezai Rad M.; Omidi M.; Rashedi H.; Yazdian F.; Khojasteh A.; Tayebi L. In Vitro Effect of Graphene Structures as an Osteoinductive Factor in Bone Tissue Engineering: A Systematic Review. J. Biomed. Mater. Res., Part A 2018, 106 (8), 2284–2343. 10.1002/jbm.a.36422. [DOI] [PubMed] [Google Scholar]
- Nishida E.; Miyaji H.; Kato A.; Takita H.; Iwanaga T.; Momose T.; Ogawa K.; Murakami S.; Sugaya T.; Kawanami M. Graphene Oxide Scaffold Accelerates Cellular Proliferative Response and Alveolar Bone Healing of Tooth Extraction Socket. Int. J. Nanomedicine 2016, 11, 2265–2277. 10.2147/IJN.S104778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki J. S.; Lafdi K.; Tsonis P. A. A Novel Approach to Control Growth, Orientation, and Shape of Human Osteoblasts. Tissue Eng. Part A 2008, 14 (2), 255–265. 10.1089/tea.2007.0169. [DOI] [PubMed] [Google Scholar]
- Guo W.; Yang Y.; Liu C.; Bu W.; Guo F.; Li J.; Wang E.; Peng Z.; Mai H.; You H.; Long Y. 3D Printed TPMS Structural PLA/GO Scaffold: Process Parameter Optimization, Porous Structure, Mechanical and Biological Properties. J. Mech. Behav. Biomed. Mater. 2023, 142, 105848. 10.1016/j.jmbbm.2023.105848. [DOI] [PubMed] [Google Scholar]
- Su J.; Du Z.; Xiao L.; Wei F.; Yang Y.; Li M.; Qiu Y.; Liu J.; Chen J.; Xiao Y. Graphene Oxide Coated Titanium Surfaces with Osteoimmunomodulatory Role to Enhance Osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110983. 10.1016/j.msec.2020.110983. [DOI] [PubMed] [Google Scholar]
- Shadjou N.; Hasanzadeh M.; Khalilzadeh B. Graphene Based Scaffolds on Bone Tissue Engineering. Bioengineered 2018, 9 (1), 38–47. 10.1080/21655979.2017.1373539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha Bai R.; Ninan N.; Muthoosamy K.; Manickam S. Graphene: A Versatile Platform for Nanotheranostics and Tissue Engineering. Prog. Mater. Sci. 2018, 91, 24–69. 10.1016/j.pmatsci.2017.08.004. [DOI] [Google Scholar]
- Gogoi S.; Maji S.; Mishra D.; Devi K. S.; Maiti T. K.; Karak N. Nano-bio Engineered Carbon Dot-Peptide Functionalized Water Dispersible Hyperbranched Polyurethane for Bone Tissue Regeneration. Macromol. Biosci. 2017, 10.1002/mabi.201600271. [DOI] [PubMed] [Google Scholar]
- Kalbacova M. H.; Verdanova M.; Broz A.; Vetushka A.; Fejfar A.; Kalbac M. Modulated Surface of Single-Layer Graphene Controls Cell Behavior. Carbon 2014, 72, 207–214. 10.1016/j.carbon.2014.02.004. [DOI] [Google Scholar]
- Kandiah K.; Muthusamy P.; Mohan S.; Venkatachalam R. TiO2-Graphene Nanocomposites for Enhanced Osteocalcin Induction. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 252–262. 10.1016/j.msec.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Matesanz M. C.; Vila M.; Feito M. J.; Linares J.; Gonçalves G.; Vallet-Regi M.; Marques P. A.; Portolés M. T. The Effects of Graphene Oxide Nanosheets Localized on F-Actin Filaments on Cell-Cycle Alterations. Biomaterials 2013, 34 (5), 1562–1569. 10.1016/j.biomaterials.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Nie C.; Ma L.; Li S.; Fan X.; Yang Y.; Cheng C.; Zhao W.; Zhao C. Recent Progresses in Graphene Based Bio-Functional Nanostructures for Advanced Biological and Cellular Interfaces. Nano Today 2019, 26, 57–97. 10.1016/j.nantod.2019.03.003. [DOI] [Google Scholar]
- Oyefusi A.; Olanipekun O.; Neelgund G. M.; Peterson D.; Stone J. M.; Williams E.; Carson L.; Regisford G.; Oki A. Hydroxyapatite Grafted Carbon Nanotubes and Graphene Nanosheets: Promising Bone Implant Materials. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 410–416. 10.1016/j.saa.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros M.; Momin M.; Verma G. Design Strategies and Evolving Role of Biomaterial Assisted Treatment of Osteosarcoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111875. 10.1016/j.msec.2021.111875. [DOI] [PubMed] [Google Scholar]
- Shin Y. C.; Lee J. H.; Jin O. S.; Kang S. H.; Hong S. W.; Kim B.; Park J.; Han D. Synergistic Effects of Reduced Graphene Oxide and Hydroxyapatite on Osteogenic Differentiation of MC3T3-E1 Preosteoblasts. Carbon 2015, 95, 1051–1060. 10.1016/j.carbon.2015.09.028. [DOI] [Google Scholar]
- Xie X.; Hu K.; Fang D.; Shang L.; Tran S. D.; Cerruti M. Graphene and Hydroxyapatite Self-Assemble into Homogeneous, Free Standing Nanocomposite Hydrogels for Bone Tissue Engineering. Nanoscale 2015, 7 (17), 7992–8002. 10.1039/C5NR01107H. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Li N.; Li K.; Gao R.; Gu W.; Wu C.; Su R.; Liu L.; Zhang Q.; Liu J. Enhanced Proliferation and Osteogenic Differentiation of Human Mesenchymal Stem Cells on Biomineralized Three-Dimensional Graphene Foams. Carbon 2016, 105, 233–243. 10.1016/j.carbon.2016.04.027. [DOI] [Google Scholar]
- Kumar S.; Raj S.; Kolanthai E.; Sood A. K.; Sampath S.; Chatterjee K. Chemical Functionalization of Graphene to Augment Stem Cell Osteogenesis and Inhibit Biofilm Formation on Polymer Composites for Orthopedic Applications. A.C.S. Appl. Mater. Interfaces 2015, 7 (5), 3237–3252. 10.1021/am5079732. [DOI] [PubMed] [Google Scholar]
- Joy A.; Unnikrishnan G.; Megha M.; Haris M.; Thomas J.; Kolanthai E.; Senthilkumar M. Hybrid Gold/Graphene Oxide Reinforced Polycaprolactone Nanocomposite for Biomedical Applications. Surf. Interfaces 2023, 40, 103000. 10.1016/j.surfin.2023.103000. [DOI] [Google Scholar]