Abstract
Acanthamoeba keratitis is a chronic inflammatory disease of the cornea which is highly resistant to many antimicrobial agents. The pathogenic mechanisms of this disease are poorly understood. However, it is believed that the initial phases in the pathogenesis of Acanthamoeba keratitis involve parasite binding and lysis of the corneal epithelium. These processes were examined in vitro, using Acanthamoeba castellanii trophozoites. Parasites readily adhered to Chinese hamster corneal epithelial cells in vitro; however, parasite binding was strongly inhibited by mannose but not by lactose. Although mannose prevented trophozoite binding, it did not affect cytolysis of corneal epithelial cells. Moreover, mannose treatment induced trophozoites to release cytolytic factors that lysed corneal epithelial cells in vitro. These factors were uniquely induced by mannose because supernatants collected from either untreated trophozoites or trophozoites treated with other sugars failed to lyse corneal cells. The soluble factors were size fractionated in centrifugal concentrators and found to be ≥100 kDa. Treatment of the supernatants with the serine protease inhibitor phenylmethylsulfonyl fluoride inhibited most, but not all, of the cytopathic activity. These data suggest that the binding of Acanthamoeba to mannosylated proteins on the corneal epithelium may exacerbate the pathogenic cascade by initiating the release of cytolytic factors.
Acanthamoeba spp. are protozoal parasites capable of infecting the skin, brain, and eye (10, 15, 17, 31, 32, 37). Corneal inflammation produced by Acanthamoeba was first recognized in 1973 and has since been intimately associated with contact lens wear (15, 31). Often the disease displays a ring-like neutrophilic stromal infiltrate with an overlying epithelial ulcer. The epithelium often undergoes a recurrent cycle of healing and breakdown during the progression of the disease. Topical or systemic treatment with antibiotics, antifungals, corticosteroids, and antivirals is often ineffectual (2). Typical treatment consists of around-the-clock hourly topical treatments with propamidine isothionate, polyhexamethylene biguanide, neomycin, or chlorhexidine, alone or in combination. This therapeutic regimen may continue for weeks. Many patients receive therapeutic corneal transplants, which can be reinfected by quiescent parasites residing in the periphery of the cornea.
Parasite binding to the corneal epithelium is believed to be an important first step in the infectious cascade of Acanthamoeba keratitis. We have shown that adherence of Acanthamoeba to corneal buttons in vitro varies among mammalian species and correlates with susceptibility to experimental Acanthamoeba keratitis (14, 19, 35). Parasitic infections, such as Acanthamoeba keratitis, often occur in a sequential manner and are initiated by the pathogen’s adherence to host cells. Bacteria, fungi, and amoebae have been shown to bind to epithelial cells via lectin-glycoprotein interactions (5, 6, 11, 18, 20–22, 26, 27, 40). The cell surface of Pseudomonas aeruginosa is decorated with lectins which bind surface glycoproteins of the epithelium to be invaded (30, 39). Entamoeba histolytica also utilizes glycoproteins as receptor ligands for adherence to the gastrointestinal epithelium (6, 16, 25–29). Binding of Acanthamoeba polyphaga and A. castellanii to corneal epithelial cells in culture is inhibited by mannose (18, 40). Subsequent studies have indicated that the binding of A. castellanii to corneal epithelial cells is mediated by a 136-kDa mannose-binding protein on the trophozoite cell membrane (40).
The pathophysiology of Acanthamoeba keratitis is poorly understood. Several studies have demonstrated that Acanthamoeba trophozoites can induce either cytolysis or apoptosis of target cells in vitro (1, 7, 24, 33, 34). Pathogenic Acanthamoeba trophozoites produce a variety of proteases which are believed to facilitate parasite penetration into the corneal stroma (9). Once in the stroma, Acanthamoeba trophozoites secrete collagenolytic enzymes which contribute to the dissolution of the stromal matrix (13).
This study was undertaken to examine the cytopathic mechanisms employed by Acanthamoeba during the initial phase of ocular infection. We tested the hypothesis that blocking parasite binding to corneal epithelial cells with mannose would prevent parasite-mediated cytolysis and invasion of the corneal stroma. The results, however, indicate that although mannose blocks parasite binding, it also facilitates the release of cytolytic factors which kill corneal epithelial cells.
MATERIALS AND METHODS
Parasites and cell lines.
A. castellanii ATCC 30868, originally isolated from a human cornea, was obtained from the American Type Culture Collection, Rockville, Md. Parasites were grown as axenic cultures in peptone-yeast extract-glucose (PYG) at 35°C with constant agitation (36).
Chinese hamster corneal epithelial (CHCE) cells were immortalized with human papillomavirus E6 and E7 genes as previously described (38) and cultured in minimal essential medium (MEM; JRH Biosciences, Lenexa, Kans.) containing 1% l-glutamine (BioWhittaker, Walkersville, Md.), 1% penicillin, streptomycin, and amphotericin B (Fungizone; BioWhittaker), 1% sodium pyruvate (BioWhittaker), and 10% fetal calf serum (FCS; HyClone Laboratories, Logan, Utah) (complete MEM). A. castellanii trophozoites neither proliferate nor encyst when incubated in complete MEM. Thus, complete MEM serves as a maintenance medium for A. castellanii trophozoites.
Adherence assay.
A. castellanii trophozoites were metabolically labeled by culturing 5 × 106 parasites/ml in PYG containing 100 μCi of [35S]methionine and [35S]cysteine (Amersham Life Science, Arlington Heights, Ill.) overnight at 35°C. Radiolabeled trophozoites were washed three times with complete MEM and resuspended in complete MEM. The radiolabeled parasites were added to 96-well plates containing confluent monolayers of CHCE cells at 105 parasites/well. Adherence of Acanthamoeba trophozoites to CHCE cells was measured after 2 h of incubation in the presence of either 100 mM mannose or 100 mM lactose at 35°C. The wells were washed three times with Hanks balanced salt solution (BioWhittaker) followed by solubilization of the remaining contents with 0.1 ml of 5% sodium dodecyl sulfate. The fluid was transferred to scintillation vials containing 2 ml of Ready-Gel scintillation cocktail (Becton Dickinson, Fullerton, Calif.), and counts were measured in a Beckman LS3801 liquid scintillation counter. Percent adherence was calculated relative to the total number of counts placed in the well.
Assay for CPE.
Acanthamoeba trophozoites were washed three times in MEM and resuspended in either complete MEM with 5% FCS or complete MEM without FCS at a concentration of 2.5 × 106 trophozoites/ml. Parasites (2.5 × 105/well) were added to 96-well plates with or without confluent monolayers of CHCE cells and incubated 48 h at 35°C. Control wells contained confluent monolayers of CHCE cells without trophozoites. Following incubation, all wells were washed three times with MEM and stained with Giemsa stain. The remaining contents were solubilized in 0.1 ml of 5% sodium dodecyl sulfate in phosphate-buffered saline (pH 7.2) and transferred to a new 96-well plate, and the optical density (OD) was read at 590 nm in a Molecular Devices (Menlo Park, Calif.) Microplate Reader. Percent cytopathic effect (CPE) was calculated according to the following formula: % CPE = 100 − [(OD of experimental well − OD of trophozoites alone or OD of supernatant alone/OD of CHCE cells alone) × 100].
Acanthamoeba supernatants.
For assays testing the cytolytic capability of trophozoite supernatants, 107 trophozoites were grown in complete MEM containing 5% FCS and either 50, 100, or 200 mM methyl-α-d-mannopyranoside or 50, 100, or 200 mM control sugars lactose and galactose (Sigma Chemical Co., St. Louis, Mo.). Following 48 h of incubation at 35°C, cultures were centrifuged at 250 × g in an IEC Centra 4B centrifuge (International Equipment Co., Needham Heights, Mass.), filter sterilized through a 0.45-μm-pore-size syringe filter (Millipore Corp., Bedford, Mass.), and vacuum concentrated to one-fourth the initial volume. Concentrated supernatants were dialyzed overnight in MEM with 5% FCS, collected, and stored at −80°C until used. Supernatants were tested for cytolytic activity as described above by adding 0.2 ml of concentrated supernatant to each well in 96-well plates containing confluent monolayers of CHCE cells. Initial dose-response studies indicated that maximal CPE was produced with 100 mM methyl-α-d-mannopyranoside and insignificant CPE was found with any of the concentrations of the control sugars (data not shown). Therefore, CPE assays were performed with supernatants collected from trophozoites treated with 100 mM methyl-α-d-mannopyranoside.
Fractionation of supernatants.
Supernatants were fractionated by using microcentrifugal concentrators with membranes having a molecular mass cutoff approximating 30 or 100 kDa (Pall-Filtron, Northborough, Mass.). One milliliter of vacuum-concentrated supernatant was added to the centrifugal concentrator followed by centrifugation at 1,900 × g in a Sorvall RC5C floor centrifuge (Dupont, Newtown, Conn.) for 1 h. Supernatants were removed from the top chamber, and the volume was adjusted to the starting volume or removed from the bottom chamber and used directly in the CPE assays. Inhibition of the cytolytic factors was carried out by addition of 10 μl of phenylmethylsulfonyl fluoride (PMSF; 10 mg/ml; Sigma) for 30 min prior to use in the assay.
Statistics.
All statistical analyses were performed by using an unpaired Student’s t test.
RESULTS
Effect of mannose on trophozoite binding and cytolysis of corneal epithelial cells.
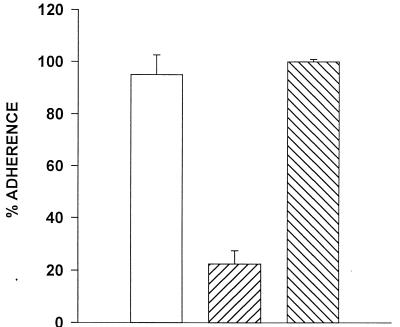
We and others have previously demonstrated that Acanthamoeba trophozoites bind to corneal epithelial cells from a variety of mammalian species (18, 19, 22, 40). The capacity of Acanthamoeba trophozoites to bind to immortalized CHCE cells was confirmed using radiolabeled trophozoites in a 2-h incubation period. Trophozoites bound equally to CHCE cells in medium alone and in medium containing 100 mM lactose (Fig. 1). By contrast, binding of trophozoites to CHCE cells in the presence of 100 mM mannose was reduced by 80% compared to the medium control.
FIG. 1.
Effect of mannose on Acanthamoeba binding to CHCE cells. Adherence of radiolabeled A. castellanii trophozoites to CHCE cells was examined after 2 h of incubation in the presence of either 100 mM mannose (▨) or 100 mM lactose (▧). Percent adherence is calculated by dividing the counts of radiolabel recovered after washing monolayers with Hanks balanced salt solution by the total counts of the initial inoculum. □, medium control.
Binding to the corneal epithelium is the first step in the pathogenic cascade of Acanthamoeba keratitis and is followed by cytolysis of the corneal epithelial cells. We next determined if the inhibition of binding by mannose affected parasite-mediated cytolysis of CHCE cells. Trophozoites were placed on CHCE monolayers for 48 h. Following the incubation, the remaining epithelial cells were stained to assess the CPE. Although mannose strongly inhibited trophozoite binding to the corneal epithelial cells, it did not significantly inhibit cytolysis in one experiment (Fig. 2A) and prevented cytolysis by only 15% in a second assay (Fig. 2B). Moreover, the presence of FCS in the culture medium did not affect CPE, as there was no difference between assays performed with medium containing 5% FCS and those without FCS (P > 0.05; data not shown). Since the CHCE cells are normally cultured in the presence of FCS, and FCS did not appear to alter CPE, we included 5% FCS in all subsequent experiments.
FIG. 2.
Effect of mannose on CPE mediated by A. castellanii trophozoites. CHCE cell monolayers were incubated for 48 h with trophozoites in MEM with (▨) or without (□) 100 mM mannose (A) or with 100 mM lactose (▧) (B). Monolayers were washed and stained with Giemsa stain, and CPE was assessed spectrophotometrically.
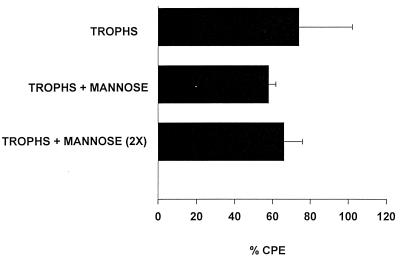
The finding that mannose strongly inhibited adherence of Acanthamoeba trophozoites to the CHCE cells yet did not impair CPE came as a surprise. Two fundamental explanations might account for this unexpected result. It is possible that trophozoite binding to corneal epithelial cells was inhibited throughout the entire 48-h incubation period, but the presence of mannose induced the release of soluble cytolytic factors which killed the corneal epithelial cells. Alternatively, mannose might have been degraded or metabolized during the 48-h incubation and was not available to inhibit the binding of trophozoites during the entire test period. To test the hypothesis that mannose was depleted, trophozoites were incubated with CHCE cells and mannose. After 24 h, the mannose-containing medium was replaced with fresh medium containing 100 mM mannose. The results show that addition of medium containing fresh mannose did not affect trophozoite-mediated cytolysis (Fig. 3).
FIG. 3.
Mannose reconstitution and Acanthamoeba-mediated CPE. CHCE cells were incubated with A. castellanii trophozoites (trophs) in the presence or absence of 100 mM mannose. After 24 h of incubation, the culture medium in one group was removed and replaced with fresh medium containing 100 mM mannose [trophs + mannose (2X)]. The negative control consisted of monolayers cultured in medium without trophozoites. Monolayers were washed and stained with Giemsa stain, and CPE was assessed spectrophotometrically. The differences between the experimental groups were insignificant (P > 0.05).
Mannose-induced secretion of cytolytic factors by Acanthamoeba trophozoites.
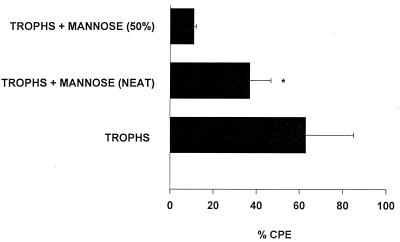
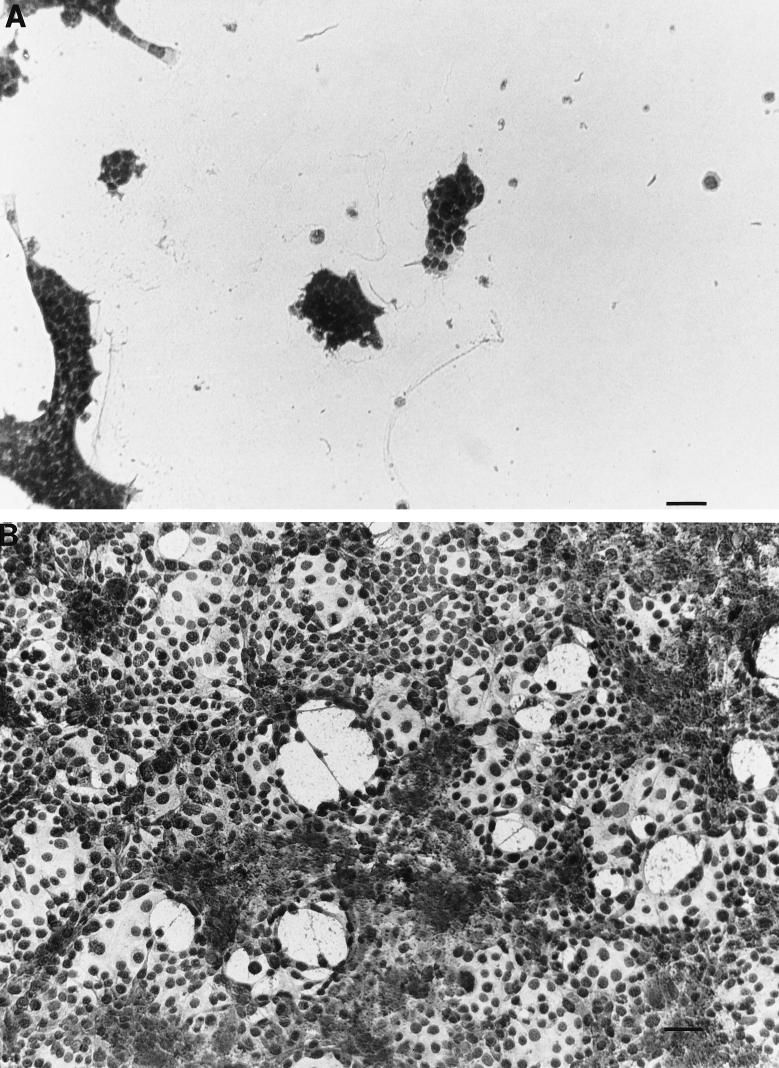
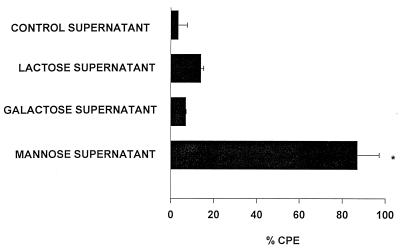
The failure of fresh mannose to significantly impede trophozoite-mediated CPE supported an alternative hypothesis that mannose induced Acanthamoeba trophozoites to secrete soluble cytolytic factors. To test this hypothesis, supernatants were collected from trophozoites grown in medium containing 100 mM mannose and tested directly for cytolytic activity. The results shown in Fig. 4 demonstrate that mannose induced trophozoites to release soluble factors that lysed approximately one-third of the CHCE cells compared to the medium control. To more definitively demonstrate the presence of cytopathic factors, supernatants from mannose-treated trophozoites were compared to supernatants from trophozoites treated with two control sugars, lactose and galactose. For these experiments, all of the supernatants were vacuum concentrated fourfold and dialyzed against a 10-kDa-cutoff membrane before use in the CPE assays. As in the previous experiment, supernatants from trophozoites grown in mannose produced significant lysis of CHCE cells (Fig. 5). By contrast, supernatants from trophozoites cultured in either complete MEM or complete MEM containing either galactose or lactose did not produce significant CPE (Fig. 6). Likewise, fresh medium containing 100 mM mannose did not affect CHCE cell viability (data not shown).
FIG. 4.
Cytolytic activity of supernatants from A. castellanii trophozoites stimulated with mannose. Trophozoites were incubated with 100 mM mannose for 48 h at 35°C. Supernatants were collected, centrifuged, filter sterilized, dialyzed, and added to CHCE cell monolayers. CPE was assessed spectrophotometrically after 48 h. Positive controls consisted of CHCE cell monolayers incubated with 2.5 × 106 trophozoites/ml (trophs). Negative controls consisted of monolayers incubated in medium alone and, by definition, had no CPE (data not shown). ∗, the difference between the undiluted mannose supernatant group (neat) and the medium control (0% CPE; not shown) was significant (P = 0.0078). Trophs + mannose (50%), mannose supernatant diluted 1:2 in complete MEM. Results are expressed as mean ± standard deviation.
FIG. 5.
Effects of supernatants from mannose-treated A. castellanii trophozoites on CHCE cell monolayers. Trophozoites were incubated with 100 mM mannose for 48 h at 35°C. Supernatants were collected, centrifuged, filter sterilized, concentrated fourfold, and dialyzed against a 10-kDa-cutoff membrane. The concentrated supernatants were added to CHCE cell monolayers and incubated at 35°C for 48 h (A). MEM containing 100 mM mannose, but not exposed to trophozoites, was similarly treated and added to monolayers (B). Monolayers were photographed 48 h later. Bar = 100 μm.
FIG. 6.
Effects of lactose, galactose, and mannose on the release of cytolytic factors by A. castellanii trophozoites. Trophozoites were incubated with complete MEM or complete MEM containing 100 mM mannose, 100 mM lactose, or 100 mM galactose for 48 h at 35°C. Supernatants were collected, centrifuged, filter sterilized, concentrated fourfold, dialyzed against a 10-kDa-cutoff membrane, and added to CHCE cell monolayers. Negative controls consisted of monolayers incubated in medium alone (0% CPE; not shown). CPE was assessed spectrophotometrically 48 h later. Results are expressed as mean ± standard deviation. ∗, the mannose treatment group was significantly different from each of the other groups (P = 0.0001). The lactose and galactose groups were not significantly different from the complete MEM control supernatant (P > 0.05).
Characterization of Acanthamoeba cytopathic factors.
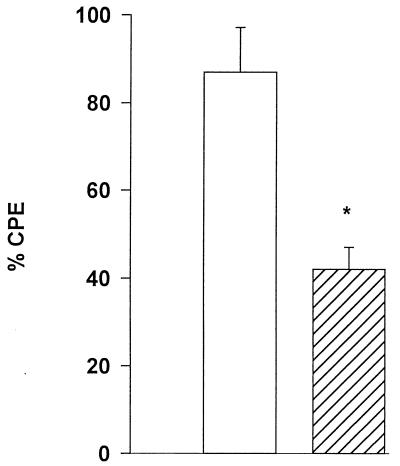
To characterize the nature of the cytopathic factors produced by Acanthamoeba trophozoites, culture supernatants were collected and concentrated as described above. Since other pathogenic amoebae produce proteases which contribute to cytopathology, we considered the possibility that the soluble factors induced by mannose were also proteases. Concentrated supernatants from mannose-treated trophozoite cultures were treated with trypsin to determine if the cytolytic factors were proteinaceous. However, trypsin itself was toxic to CHCE cells and we were unable to distinguish between the toxic effects of residual trypsin and the soluble mannose-induced factors. As an alternative approach, supernatants were treated with the protease inhibitor PMSF to determine if the cytolytic factor was a serine protease. Supernatants were mixed with 10 μg of PMSF per ml 30 min prior to addition to CHCE cells. Figure 7 shows that this treatment clearly inhibited the function of the cytolytic factors and indicates that at least one of the factors is a serine protease.
FIG. 7.
Effect of a serine protease inhibitor on Acanthamoeba-derived cytolytic factors. Trophozoites were incubated with 100 mM mannose for 48 h at 35°C. Supernatants were collected, centrifuged, filter sterilized, concentrated fourfold, and dialyzed against a 10-kDa-cutoff membrane. Supernatants were mixed with 10 μg of PMSF per ml 30 min prior to addition to CHCE cell monolayers. CPE was assessed spectrophotometrically after 48 h. Negative controls consisted of monolayers incubated in medium alone (0% CPE). Results are expressed as mean ± standard deviation. ∗, the difference between the two groups was significant (P = 0.04). □, mannose supernatant; ▨, mannose supernatant + PMSF.
Having demonstrated that one of the factors is a serine protease, experiments were conducted to size the protein. Supernatants were collected and applied to microcentrifugal concentrators with membranes having a molecular mass cutoff approximating 30 or 100 kDa. Both the upper and lower fractions were tested for the ability to lyse CHCE cells. Supernatants taken from fractions above 30 and 100 kDa demonstrated CPE equal to that of unfractionated supernatants (Fig. 8). This result indicated that the cytolytic serine protease was ≥100 kDa.
FIG. 8.
Molecular weight estimation of Acanthamoeba-derived cytolytic factors. Trophozoites were incubated with 100 mM mannose for 48 h at 35°C. Supernatants were collected, centrifuged, filter sterilized, and size fractionated with centrifugal concentrators having a molecular mass cutoff of either 30 or 100 kDa. Fractions were added to CHCE cell monolayers, and CPE was assessed spectrophotometrically. Results are expressed as mean ± standard deviation.
DISCUSSION
It is generally accepted that the pathogenesis of Acanthamoeba keratitis is a sequential process beginning with adhesion to the corneal epithelium, followed by invasion of the corneal stroma and the subsequent elaboration of collagenolytic enzymes (13). Binding of Acanthamoeba trophozoites to corneal epithelial cells and corneal buttons in vitro correlates with pathogenicity in vivo (14, 19, 35). Acanthamoeba trophozoites express a mannose-binding receptor which facilitates adhesion to corneal epithelial cells in vitro (40). Moreover, parasite binding is effectively inhibited by free mannose but not by galactose, lactose, or fucose (40). Since cytolysis of target cells by other extracellular protozoal parasites, such as E. histolytica, is contact dependent (16), we anticipated that inhibiting Acanthamoeba binding to corneal epithelial cells with mannose would result in greatly diminished cytolysis. However, this was not the case. Although mannose inhibited trophozoite binding by 80%, it did not prevent the cytolysis of corneal cells over a prolonged incubation period (i.e., 48 h). The trivial explanation that mannose was either degraded or metabolized during the in vitro incubation period was ruled out by replacing the culture medium with fresh mannose-containing medium midway through the assay.
We are attracted to the hypothesis that the mannose receptor on the cell membrane of A. castellanii functions as a cell adhesion molecule when the parasite encounters glycoproteins on the cell membranes of eukaryotic cells such as corneal epithelial cells. However, since Acanthamoeba spp. normally occur as free-living organisms and derive their nutrition by ingestion of bacteria, the most likely primary function of the mannose receptor is for the phagocytosis and digestion of bacteria. Allen and Dawidowicz (3, 4) have shown that treatment of A. castellanii with mannosylated glycoproteins promotes adhesion to mannose-containing yeast particles and induces the generation of second-messenger molecules within the trophozoite. The induction of second-messenger molecules by engagement of the mannose receptor is believed to be an important step in the phagocytic process leading to the secretion of lysosomal enzymes (4). The present results suggest that in the case of eukaryotic target cells, the mannose receptor on A. castellanii serves initially as an adhesion molecule and facilitates contact-dependent cytolysis. However, engagement of the mannose receptor induces the release of a serine protease which mediates contact-independent cytolysis of corneal epithelial cells. The latter process is consistent with the known function of the mannose receptor in initiating the synthesis of second-messenger molecules.
Pathogenic amoebae elaborate a variety of cytolytic molecules which might be elicited by engagement of the mannose receptor. Hadas and Mazur (12) examined eight species of Acanthamoeba and detected a 35-kDa serine proteinase and a 65-kDa cysteine protease. However, both of these proteinases are significantly smaller than the serine protease secreted by mannose-treated A. castellanii trophozoites. E. histolytica produces 5- and 14-kDa protein complexes, called amoebapores, which form ion channels leading to the osmotic lysis of eukaryotic cells (8). However, the Acanthamoeba-derived cytolytic factors induced by mannose have a molecular mass in excess of 100 kDa and thus are too large to be amoebapores.
The present results imply that the adherence of trophozoites to corneal epithelial cells is essential for initiating the cytolytic machinery of Acanthamoeba but is unnecessary once the mannose receptor is engaged. We are aware of a report of a study by Panjwani et al. (23) in which Acanthamoeba-mediated cytolysis of rabbit corneal epithelial cells was inhibited by free mannose. However, in that assay, CPE was evaluated within 24 h of incubation, while we examined CPE after 48 h. We suspect that the mannosylated proteins on the corneal epithelium serve initially as adhesion molecules to promote parasite binding and act later as inducers of the cytolytic machinery of Acanthamoeba by activating second-messenger molecules. Free mannose effectively prevents parasite binding to corneal epithelial cells and as a result prevents the initial activation of trophozoites by mannosylated proteins. Under these conditions, free mannose inhibits contact-dependent cytolysis of corneal epithelial cells. However, the results reported here suggest that prolonged exposure to free mannose eventually induces second-messenger molecules and the release of cytolytic molecules which mediate contact-independent cytolysis. Therefore, we propose that Acanthamoeba trophozoites are capable of mediating both contact-dependent and contact-independent CPE. The mannose receptor appears to be crucial for both of these processes.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant EY09756 and an unrestricted grant from Research to Prevent Blindness, Inc., New York, N.Y.
We thank Jerry Shay for assistance in transforming the CHCE cells.
REFERENCES
- 1.Alizadeh H, Pidherney M S, McCulley J P, Niederkorn J Y. Apoptosis as a mechanism of cytolysis of tumor cells by a pathogenic free-living amoeba. Infect Immun. 1994;62:1298–1303. doi: 10.1128/iai.62.4.1298-1303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alizadeh H, Niederkorn J Y, McCulley J P. Acanthamoeba keratitis. In: Pepose J S, Holland G N, Wilhelmus K R, editors. Ocular infection and immunity. St. Louis, Mo: Mosby; 1996. pp. 1062–1071. [Google Scholar]
- 3.Allen P G, Dawidowicz E A. Phagocytosis in Acanthamoeba. I. A mannose receptor is responsible for the binding and phagocytosis of yeast. J Cell Physiol. 1990;145:508–513. doi: 10.1002/jcp.1041450317. [DOI] [PubMed] [Google Scholar]
- 4.Allen P G, Dawidowicz E A. Phagocytosis in Acanthamoeba. II. Soluble and insoluble mannose-rich ligands stimulate phosphoinositide metabolism. J Cell Physiol. 1990;145:514–521. doi: 10.1002/jcp.1041450318. [DOI] [PubMed] [Google Scholar]
- 5.Blaylock W K, Yue B Y, Robin J B. The use of concanavalin A to competitively inhibit Pseudomonas aeruginosa adherence to rabbit corneal epithelium. CLAO J. 1990;16:223–227. [PubMed] [Google Scholar]
- 6.Chadee K, Petri W A, Jr, Innes D J, Ravdin J I. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest. 1987;80:1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cursons R T, Brown T J, Keys E A. Virulence of pathogenic free-living amebae. J Parasitol. 1978;64:744–745. [PubMed] [Google Scholar]
- 8.Dandekar T, Leippe M. Molecular modeling of amoebapore and NK-lysin: a four-alpha-helix bundle motif of cytolytic peptides from distantly related organisms. Folding and Design. 1997;2:47–52. doi: 10.1016/s1359-0278(97)00005-9. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante A, Bates E J. Elastase in the pathogenic free-living amoebae Naegleria and Acanthamoeba spp. Infect Immun. 1988;56:3320–3321. doi: 10.1128/iai.56.12.3320-3321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garner A. Pathogenesis of acanthamoebic keratitis: hypothesis based on a histological analysis of 30 cases. Br J Ophthalmol. 1993;77:366–370. doi: 10.1136/bjo.77.6.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gesztesi J L, Puccia R, Travassos L R, Vicentini A P, de Moraes J Z, Franco M F, Lopes J D. Monoclonal antibodies against the 43,000 Da glycoprotein from Paracoccidiodes brasiliensis modulate laminin-mediated fungal adhesion to epithelial cells and pathogenesis. Hybridoma. 1996;15:415–422. doi: 10.1089/hyb.1996.15.415. [DOI] [PubMed] [Google Scholar]
- 12.Hadas E, Mazur T. Biochemical markers of pathogenicity and virulence of Acanthamoeba sp. strains. Parasitol Res. 1993;79:696–698. doi: 10.1007/BF00932513. [DOI] [PubMed] [Google Scholar]
- 13.He Y G, Niederkorn J Y, McCulley J P, Stewart G L, Meyer D R, Silvany R. In vivo and in vitro collagenolytic activity of Acanthamoeba castellanii. Invest Ophthalmol Visual Sci. 1990;31:2235–2240. [PubMed] [Google Scholar]
- 14.He Y G, McCulley J P, Alizadeh H, Pidherney M, Mellon J, Ubelaker J E, Stewart G L, Silvany R E, Niederkorn J Y. A pig model of Acanthamoeba keratitis: transmission via contaminated contact lenses. Invest Ophthalmol Visual Sci. 1992;33:126–133. [PubMed] [Google Scholar]
- 15.Jones D B, Visvesvara G S, Robinson N M. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc UK. 1975;95:221–232. [PubMed] [Google Scholar]
- 16.McCoy J J, Mann B J, Petri W A., Jr Adherence and cytotoxicity of Entamoeba histolytica or how lectins let parasites stick around. Infect Immun. 1994;62:3045–3050. doi: 10.1128/iai.62.8.3045-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCulley J P, Alizadeh H, Niederkorn J Y. Acanthamoeba keratitis. CLAO J. 1995;21:73–76. [PubMed] [Google Scholar]
- 18.Morton L D, McLaughlin G L, Whiteley H E. Effects of temperature, amebic strain, and carbohydrates on Acanthamoeba adherence to corneal epithelium in vitro. Infect Immun. 1991;59:3819–3822. doi: 10.1128/iai.59.10.3819-3822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niederkorn J Y, Ubelaker J E, McCulley J P, Stewart G L, Meyer D R, Mellon J A, Silvany R E, He Y G, Pidherney M, Martin J H, Alizadeh H. Susceptibility of corneas from various animal species to in vitro binding and invasion by Acanthamoeba castellanii. Invest Ophthalmol Visual Sci. 1992;33:104–112. [PubMed] [Google Scholar]
- 20.Panjwani N, Zaidi T S, Gigstad J E, Jungalwala F B, Barza M, Baum J. Binding of Pseudomonas aeruginosa to neutral glycosphingolipids of rabbit corneal epithelium. Infect Immun. 1990;58:114–118. doi: 10.1128/iai.58.1.114-118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panjwani N, Clark B, Cohen M, Barza M, Baum J. Differential binding of P. aeruginosa and S. aureus to corneal epithelium in culture. Invest Ophthalmol Visual Sci. 1990;31:696–701. [PubMed] [Google Scholar]
- 22.Panjwani N, Zhao Z, Baum J, Pereira M, Zaidi T. Acanthamoebae bind to glycolipids of rabbit corneal epithelium. Infect Immun. 1992;60:3460–3463. doi: 10.1128/iai.60.8.3460-3463.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panjwani N, Yang Z, Cao Z. Pathogenesis of Acanthamoeba keratitis: carbohydrate-mediated host parasite interactions. Invest Ophthamol Visual Sci. 1997;38:S229. doi: 10.1128/iai.65.2.439-445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pidherney M S, Alizadeh H, Stewart G L, McCulley J P, Niederkorn J Y. In vitro and in vivo tumoricidal properties of pathogenic/free-living amoeba. Cancer Lett. 1993;72:91–98. doi: 10.1016/0304-3835(93)90016-3. [DOI] [PubMed] [Google Scholar]
- 25.Ravdin J I, Croft B Y, Guerrant R L. Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med. 1980;152:377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravdin J I, John J E, Johnston L I, Innes D J, Guerrant R L. Adherence of Entamoeba histolytica trophozoites to rat and human colonic mucosa. Infect Immun. 1980;48:292–297. doi: 10.1128/iai.48.2.292-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravdin J I, Stanley P, Murphy C F, Petri W A., Jr Characterization of cell surface carbohydrate receptors for Entamoeba histolytica adherence lectin. Infect Immun. 1989;57:2179–2186. doi: 10.1128/iai.57.7.2179-2186.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravdin J I. Entamoeba histolytica: from adherence to enteropathy. J Infect Dis. 1989;159:420–429. doi: 10.1093/infdis/159.3.420. [DOI] [PubMed] [Google Scholar]
- 29.Ravdin J I, Shain D C, Kelsall B L. Antigenicity, immunogenicity and vaccine efficacy of the galactose-specific adherence protein of Entamoeba histolytica. Vaccine. 1993;11:241–246. doi: 10.1016/0264-410x(93)90024-r. [DOI] [PubMed] [Google Scholar]
- 30.Rudner X L, Zheng Z, Berk R S, Irvin R T, Hazlett L D. Corneal epithelial glycoproteins exhibit Pseudomonas aeruginosa pilus binding activity. Invest Ophthalmol Visual Sci. 1992;33:2185–2193. [PubMed] [Google Scholar]
- 31.Stehr-Green J K, Bailey T M, Visvesvara G S. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthamol. 1989;107:331–336. doi: 10.1016/0002-9394(89)90654-5. [DOI] [PubMed] [Google Scholar]
- 32.Tan B, Weldon-Linne C M, Rhone D P, Penning C L, Visvesvara G S. Acanthamoeba infection presenting as skin lesions in patients with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1993;117:1043–1046. [PubMed] [Google Scholar]
- 33.Taylor W M, Pidherney M S, Alizadeh H, Niederkorn J Y. In vitro characterization of Acanthamoeba castellanii cytopathic effect. J Parasitol. 1995;81:603–609. [PubMed] [Google Scholar]
- 34.van Klink F, Alizadeh H, Stewart G L, Pidherney M S, Silvany R E, He Y G, McCulley J P, Niederkorn J Y. Characterization and pathogenic potential of a soil isolate and an ocular isolate of Acanthamoeba castellanii in relation to Acanthamoeba keratitis. Curr Eye Res. 1992;12:1207–1220. doi: 10.3109/02713689208999546. [DOI] [PubMed] [Google Scholar]
- 35.van Klink F, Alizadeh H, He Y G, Mellon J A, Silvany R E, McCulley J P, Niederkorn J Y. Chinese hamster model of Acanthamoeba keratitis: role of contact lenses, trauma, and Langerhans cells. Invest Ophthalmol Visual Sci. 1993;34:1937–1944. [PubMed] [Google Scholar]
- 36.Visvesvara G S, Mirra S S, Brandt F H, Moss D M, Mathews H M, Martinez A J. Isolation of two strains of Acanthamoeba castellanii from human tissue and their pathogenicity and isoenzyme profiles. J Clin Microbiol. 1983;18:1405–1412. doi: 10.1128/jcm.18.6.1405-1412.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visvesvara G S, Stehr-Green J K. Epidemiology of free-living ameba infections. J Protozool. 1990;37:25s–33s. doi: 10.1111/j.1550-7408.1990.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 38.Wilson S E, Weng J, Blair S, He Y G, Lloyd S. Expression of E6/E7 or SV40 large T antigen-coding oncogenes in human corneal endothelial cells indicates regulated high-proliferative capacity. Invest Ophthalmol Visual Sci. 1995;36:32–40. [PubMed] [Google Scholar]
- 39.Wu X, Gupta S K, Hazlett L D. Characterization of P. aeruginosa pili binding human corneal epithelial proteins. Curr Eye Res. 1995;14:969–977. doi: 10.3109/02713689508995137. [DOI] [PubMed] [Google Scholar]
- 40.Yang Z T, Cao Z Y, Panjwani N. Pathogenesis of Acanthamoeba keratitis–carbohydrate-mediated host-parasite interactions. Infect Immun. 1997;65:439–445. doi: 10.1128/iai.65.2.439-445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]