Abstract
Patients with inflammatory bowel disease (IBD), both Crohn's disease and ulcerative colitis, frequently experience venous thromboembolism (VTE), a potentially fatal consequence. The pathophysiological mechanisms contributing to VTE include inflammation, modifications in coagulation factors, endothelial dysfunction, and platelet activation. Numerous pro-inflammatory cytokines and markers, such as tumor necrosis factor-alpha and interleukin-6, have a significant impact on the thrombotic cascade. Patients with IBD are more likely to suffer VTE for a variety of causes. Exacerbations of preexisting conditions, admission to the hospital, surgical intervention, immobilization, corticosteroid usage, central venous catheterization, and hereditary susceptibility all fit into this category. The mainstay of therapy for VTE in IBD patients includes anticoagulation that is individualized for each patient depending on the thrombosis site, severity, bleeding risk, and interaction with other drugs. In some high-risk IBD patients, such as those having major surgery or hospitalized with severe flare, preventive anticoagulation may play a role. However, the acceptance rate for this recommendation is low. Additionally, there is a subset of patients who would require extended thromboprophylaxis. The majority of the studies that looked into this question consisted of patients in the surgical setting. Emerging data suggest that risk factors other than surgery can also dictate the duration of anticoagulation. While extending anticoagulation in all patients may help reduce VTE-related mortality, identifying these risk factors is important. Hence, the decision to initiate prophylaxis should be individualized, considering the overall thrombotic and bleeding risks. This review explores the relationship between IBD and VTE, including risk factors, epidemiology, and prevention. A multifactorial approach involving aggressive management of underlying inflammation, identification of modifiable risk factors, and judicious use of anticoagulant therapy is essential for reducing the burden of VTE in this vulnerable population.
How to cite this article
Harindranath S, Varghese J, Afzalpurkar S, et al. Standard and Extended Thromboprophylaxis in Patients with Inflammatory Bowel Disease: A Literature Review. Euroasian J Hepato-Gastroenterol 2023;13(2):133–141.
Keywords: Crohn's disease, Inflammatory bowel disease, Ulcerative colitis, Venous thromboembolism
Introduction
Inflammatory bowel disease (IBD), both ulcerative colitis (UC) and Crohn's disease (CD), affects a significant proportion of the global population. Systemic thromboembolic events are reported to occur in 1–7.7% of the cases in clinical studies, while in postmortem studies, the reported incidence is 39–41%.1,2 Of these, the most concerning is venous thromboembolism (VTE) due to its high morbidity and mortality rate.3–5 These include deep venous thrombosis (DVT), pulmonary embolism (PE), and splanchnic vein thrombosis (SVT). Although the institution of thromboprophylaxis can improve the outcomes, the acceptance rate for these regimens is unacceptably low for various reasons, the primary among which is the risk of bleeding. Also, the question of the duration of thromboprophylaxis remains unanswered.
This is an important question to answer, as continued anticoagulation is not without risks and is expensive to maintain. This article will discuss the epidemiology, risk factors, and prevention strategies for VTE in persons with IBD. Understanding this relationship is crucial for clinicians to provide optimal care for patients with IBD and reduce the risk of VTE-related morbidity and mortality.
Epidemiology
People with IBD are at a 2–3-fold increased risk of developing VTE compared with the general population.4–6 Fumery et al., in a meta-analysis, found that individuals with IBD had an increased risk of DVT and PE, with an overall RR of 1.96. [95% confidence interval (CI), 1.67–2.30].4 Due to the significant variability of the included studies, the Canadian consensus recommendations only extrapolated data from three of the available studies, which reported a 2.85-fold higher incidence of VTE (95% CI: 1.86–4.34) in patients with IBD.5 However, most of these studies were undertaken in a predominantly Western population. Data on VTE in IBD, particularly in the Asian population, is scarce. A prospective study by Ando et al. showed the incidence to be 16.7% in Japanese patients.6 An earlier study by Sonoda et al. estimated the incidence to be 17%, and most were diagnosed within 2 weeks of admission.7
Compared with the general population, thromboembolic events occur at an earlier age in patients with IBD.8,9 The highest risk for developing a VTE is concurrent disease activity. While individuals with IBD were found to be at an elevated risk of VTE even during clinical remission [Hazard ratio (HR) of 2.1], their risk of VTE was highest during a flare, as determined by Grainge et al. (HR of 8.4 compared with controls).10 IBD patients have a procoagulant propensity, as shown by this finding. This was validated in research by Han et al., who used thromboelastography (TEG) to show that individuals with severe acute UC were hypercoagulable. Platelet counts, fibrinogen levels, D-dimer levels, alpha angle, maximum amplitude (MA), and the TEG index were all greater, but the R and K values were lower. In addition, anticoagulation was able to reverse these findings.11 However, the point to be noted is that VTE is a specific feature of IBD, unlike other chronic inflammatory conditions like rheumatoid arthritis and celiac disease, where the risk remains very low. VTE's etiopathogenesis in IBD is depicted in Figure 1.
Fig. 1.
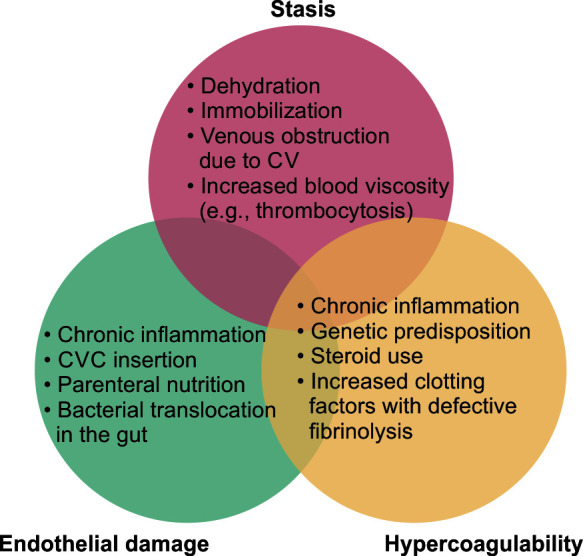
Inflammatory bowel illness and the development of venous thromboembolism. CVC: central venous catheter
Pathophysiology
Thrombosis originates from an imbalance between the prothrombotic and antithrombotic systems, which may result from a single or several processes, but Virchow's triad highlights the three most common ones: vascular stasis, endothelial damage, and hypercoagulability. Multiple factors, both genetic and environmental, have been implicated in the development of thrombosis in IBD. Inflammation, advanced age, surgery, prolonged immobilization, central venous catheters, fluid deprivation, steroid therapy, smoking, and oral contraceptives are all risk factors for thromboembolism in patients with IBD. IBD patients face a higher risk of thromboembolism, but this risk is not solely attributable to these factors.12 Arguments have been made about the relationship between IBD and inherited thrombophilia. Hence, an increasing number of risk factors compound the risk of thrombosis.
Inflammation is thought to have a significant role in the development of thrombosis. Prothrombotic factors, including many acute phase reactants, are elevated in the plasma, whereas natural anticoagulants are depleted. Fibrinolytic activity is decreased, which is a second way in which inflammation is harmful. Third, the blood levels of endothelial damage markers, such as von Willebrand factor (vWF), endothelium protein C receptor (EPCR), and thrombomodulin are increased in people with the condition. This results in an overall prothrombotic milieu. It is debatable whether this vasculopathy in IBD is a cause or result of intestinal inflammation.13,14 Finally, inflammation results in quantitative as well as qualitative platelet dysfunction, which contributes to a thrombophilic state, as discussed below.
Association with Inherited Thrombophilic Conditions
The risk and association of inherited thrombophilias and its ramifications on patient management in patients with IBD are debatable. Testing for inherited thrombophilias is not widely available and is expensive. But on a case-to-case basis, it can potentially change disease management. There may be inherited thrombophilias detected in up to a third of people with IBD.15 Those with IBD and VTE are more likely to carry the Factor V Leiden mutation compared with those without thrombosis.16 Two meta-analyses17,18 show that patients with IBD who have a mutation in Factor V have an increased risk of thrombosis. The prothrombin G20210A mutation has also been linked to an increased risk of thrombosis in patients with IBD.19 The attribution of MTHFR polymorphism to the pathophysiology of VTE is controversial. There have been significant differences in the occurrence of these genetic mutations between studies,19,20 mainly as a result of the small sample sizes in most of the studies and geographical variation. Hence, the current guidelines do not recommend routine testing for prothrombotic conditions in IBD.
Naito et al. conducted a study to determine the hereditary predisposition to VTE in IBD patients. Individuals at high risk of VTE were identified using whole-exome sequencing (WES) and whole-genome genotyping based on a combination of monogenic and polygenic risk factors. Genetic risk factors were found in 1 out of each 7 patients, which increased an individual's probability of developing a VTE by a factor of 2.5 compared with those without hereditary risk factors. Patients with a higher hereditary risk should take preventative measures seriously, and medications like JAK inhibitors should be cautiously used in these cases. Also, this study demonstrated a greater severity and involvement of multiple sites in the case of a VTE. Although the panel used for testing was extensive, not all variants of polygenic risk genes could be included.21 It is currently not advisable to advocate for widespread thrombophilia testing solely based on this study. Therefore, more extensive research is needed to standardize testing for these mutations in patients with IBD and VTE.
The Role of Platelets in Thrombosis in IBD
Patients with IBD have measurable and discernible changes in platelet quantity and quality. Many chronic inflammatory disorders also exhibit thrombocytosis; hence, it is now known that this is only a non-specific sign of inflammation. Studies have shown that enhanced thrombopoiesis owing to higher plasma levels of thrombopoietin and IL-6 may make thrombocytosis in the context of IBD distinct in pathogenesis.22 Some studies have indicated that this reactive rise in platelet levels might remain even after intestinal resection and that it is correlated with disease severity.23 Also, platelets in IBD have been found to be in a persistent activated state and are more reactive and sensitive to activation. During active disease, there is an increased tendency to form platelet agglomerates, thereby increasing the risk of thrombosis. In vitro investigations have shown that nearly 30% of individuals with IBD had platelet aggregation.2 Since this is not seen in other chronic inflammatory conditions,24 it is possible that it is exclusive to people with IBD. Activation indicators, such as P-selectin, GP-53, thromboglobulin, and CD-40 ligands are upregulated on platelets from patients with IBD.12,25 Elucidation of these mechanistic pathways may provide insight into therapeutic options to prevent thrombosis in IBD.
Risks Associated with Disease Activity
The risk of VTE increases concurrently with increasing disease severity. Colonic involvement in CD and severe flare in UC both enhance the risk. Grainge et al. observed in a retrospective analysis that individuals experiencing an IBD flare were more likely to have a VTE than those who were in remission.10 According to a study of pregnant women, those who were experiencing active flares had a greater chance of developing VTE.26 The severity and extent of IBD both affect VTE risk. According to Solem et al., those with VTE showed significant involvement, with major surfaces involved in 79% of CD patients and the pancolonic region in 76% of UC patients.15
Certain medications administered during hospital stay increase the risk of VTE. Steroids used to treat IBD have several anti-inflammatory effects in addition to raising plasma fibrinogen levels, decreasing tissue plasminogen activity, and producing less prostacyclin,27 which increases the risk of thrombosis. The use of corticosteroids before admission is an independent predictor of hospitalization. Ngyuen et al. analyzed the relation of steroid use with postoperative VTE and reported that in both CD and UC, preoperative steroid use was linked to an elevated incidence of VTE (OR, 1.66 and 2.66, respectively).28
Risk of Thrombosis with JAK Inhibitors
Janus kinase (JAK) inhibitors, initially developed and used for rheumatological disorders, have recently become an indispensable part of the armamentarium against IBD. Small pharmacological inhibitors of the JAK-STAT pathway, such as JAK1, JAK2, JAK3, and TYK2, alter immune regulatory responses by interfering with cytokine signaling and preventing the activation of one or more intracellular tyrosine kinases. These drugs increase the risk of arterial and venous thrombosis in rheumatoid arthritis patients.29,30 The JAK 1 and 3 inhibitor tofacitinib is now FDA-approved for the management of moderate to severe UC. Tofacitinib at a dose of 20 mg daily and TNF blockers have been linked to an increased risk of VTE in recent studies, with mortality being higher in tofacitinib than TNF blockers. However, it should be highlighted that the majority of the study's participants were patients with rheumatoid arthritis, a condition for which tofacitinib is also a treatment option. In addition, the majority of patients being older than 50 years old was a serious risk factor for cardiovascular disease. Patients who were taking a 10 mg twice-daily dosage were disproportionately likely to get a VTE. Tofacitinib should be used with caution, particularly in elderly patients with one or more risk factors for VTE. Due to these considerations, the FDA has mandated a black box warning against the use of these medications in the treatment of IBD.31
At a dosage of 10 mg BID, one patient got DVT, and four experienced PE in a post hoc analysis of tofacitinib's effect on UC.32 However, more clinical research is needed because of the study's limited drug exposure and sample size. Zhang et al. found that JAK inhibitors did not enhance the risk of VTE compared with placebo or TNF-alpha inhibitors [the pooled risk ratio of JAK inhibitors versus placebo was 0.72 (95% CI 0.33, 1.55)] and pooled risk ratio of JAK inhibitors vs TNF-alpha inhibitors was 0.94 (95% CI 0.33, 2.69)]. A higher tofacitinib dose was associated with a higher VTE incidence compared with a lower dose,33 as was seen in a subgroup analysis. However, recent literature paints a different picture. A recent meta-analysis of randomized controlled trials found that patients who took JAK inhibitors were no more likely to experience a VTE than those who took a placebo.6,34–42 Considerations unique to each patient, such as their age, risk of cardiovascular disease, and VTE risk, must be considered while weighing the potential advantages and hazards of JAK inhibitor therapy.
Risk of In-hospital Venous Thromboembolism
In addition to the already known risk factors, such as younger age, pregnancy, and inherited thrombophilia, hospitalization also increases the risk of VTE. In a study by Grainge et al., hospitalized IBD patients, regardless of disease extent, had a higher risk of developing VTE.10 Similar results were shown by Nguyen et al. in their nationwide study, where hospitalized IBD patients had a higher rate of VTE and VTE-associated mortality as compared with hospitalized patients without IBD.28 There is also data to suggest that there is a higher chance of asymptomatic VTE in hospitalized patients with IBD.9 The risk remains high even if the disease is in clinical remission. Compared with patients without IBD, hospitalized IBD patients had a greater incidence of VTE (all patients: 2.1; 95% CI: 1.4–3.2; patients in remission: 1.7; 95% CI: 1.1–2.9), according to a UK cohort study.10 A venous catheter for parenteral feeding, immobility, and fluid loss from diarrhea are other variables that may raise the risk of VTE during hospitalization. We summarize the results of various studies on the parameters that influence the risk of VTE in IBD in Table 1.
Table 1.
Summary of the risk factors associated with in-hospital venous thromboembolism in patients with inflammatory bowel disease
| Studies | Predictors of VTE |
|---|---|
| Nguyen 200828 | • Age (OR 2.01) • Comorbidities (OR 2.5) • Ulcerative colitis (OR 1.85) • IBD-related surgery (OR 4.80) |
| Novacek 201035 | • Male gender (OR 3.0) • Age (OR 1.03) |
| Ra 201336 | • Surgery (OR 3.82) • Hospitalization (OR 2.4) |
| Tinsley 201337 | • Additional VTE risk factor (OR 2.46) • Surgery (OR 12.03) • Extensive colitis (OR 2.26) |
| Ananthakrishnan 201438 | • IBD-related hospitalization (HR 1.72) • Comorbidities (HR 1.3) • Systemic steroids (HR 1.71) • Low serum albumin (HR 0.66) |
| Brady 201739 | • Stoma creation (OR 1.95) • Pouch construction (OR 2.66) • Preoperative steroid use (OR 1.57) • Longer length of hospital stay (OR 1.89) |
| Benlice 201840 | • Age (OR 1.01) • Steroid use (OR 1.56) • Bleeding disorders (OR 2.5) • Open surgery (OR 4.32) • Hypertension (OR 1.23) • Longer operative time (OR 1.17) • Preoperative hospitalization (OR 2.37-3.59) |
| Ando 20186 | • Indwelling venous catheter • Low total protein • Low activated partial thromboplastin clotting time • High FDP (fibrin degradation products) values |
| Kadourrah 201941 | • Smoking • Males • Steroid use • History of VTE • GI bleeding • Chronic kidney disease |
| Faye 201942 | • Prior VTE (OR 2.4–3.48) • Length of hospital stay (OR 1.01–1.02) • Comorbidities (OR 2.11–3.12) • Sigmoidoscopy or colonoscopy at baseline (OR1.12–1.15) • Age ≥18 Years (OR 1.56–3.06) • Clostridium difficile infection (OR 1.9) |
IBD, inflammatory bowel disease; VTE, venous thromboembolism; OR, odds ratio
Risk of Venous Thromboembolism after Discharge
Assessing the risk of VTE after discharge is an area where there is a significant dearth of information at present. Although the risk has been well explained in patients undergoing colorectal surgery, other factors have not been studied adequately.38 Also, no RCTs have analyzed these patients’ outcomes and benefits of extended thromboprophylaxis. Patients who have had abdominal or pelvic surgery are more likely to develop VTE.43,44
Data on the etiology of VTE outside of surgical settings following hospital discharge is limited. Among the 872,122 patients with IBD who had index hospitalizations analyzed by Faye et al., 1160 (0.13%) were readmitted with an incident of VTE. The rate of readmissions peaked during the first 10 days after discharge and remained somewhat high for the subsequent 60 days, with similar incidence in UC and CD. Readmission for VTE was found to be more likely among patients over the age of 65, those with multiple comorbidities, those who had previously been diagnosed with VTE, those who had undergone a flexible sigmoidoscopy or endoscopy during the index hospitalization, Clostridium difficile infection, and those who had been discharged to a nursing or intermediate care facility. Female patients, those who had self-pay insurance rather than private insurance and had surgery on an organ other than the intestines during their first hospitalization, had reduced rates of VTE. A higher risk of VTE was also observed after therapy-related transfers to nursing homes and other long-term care facilities. The authors recommended that patients at high risk of thrombosis should continue their anticoagulants even after discharge.42
According to some recent studies, the incidence of post-discharge VTE ranges from 0.13% at 90 days to 3% at 180 days.44,45 This variability in rates is most likely attributed to differences in study designs and timing of assessment for VTE. The highest rates are usually seen in studies from tertiary referral centers, which are most likely to capture a majority of the VTE events. The rising awareness of VTE risk in IBD may also explain the recent increase in VTE cases reported after discharge. Moreover, hospitalization is often reserved for patients with multiple comorbidities, which may increase their risk of VTE. Table 2 summarizes the findings from studies on the factors that predict VTE after discharge. Figure 2 shows reported sites of venous thrombosis in patients with IBD.
Table 2.
Summary of risk factors associated with venous thromboembolism after discharge in hospitalized patients with IBD
| Surgical risk factors | Nonsurgical risk factors |
|---|---|
| • Stoma creation • Pouch construction • Pelvic surgery • Surgery for enterocutaneous fistula • Longer operative time • Preoperative blood transfusion • Preoperative steroid use |
• IBD-related hospitalization • Associated comorbidities • Low serum albumin • Longer length of hospital stay • Prior venous thromboembolism • Sigmoidoscopy or colonoscopy at baseline • Older age • Clostridium difficile infection • Ulcerative colitis |
Fig. 2.
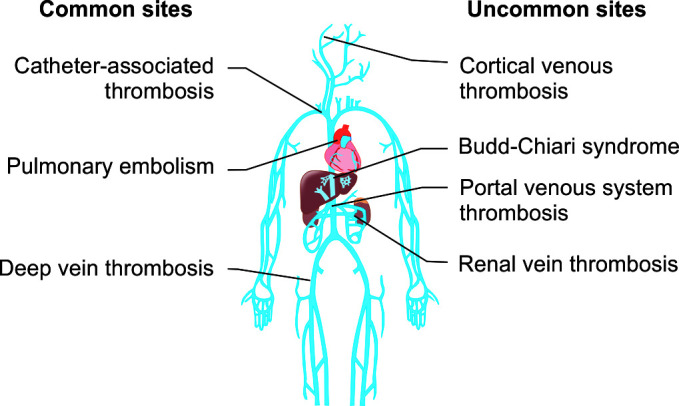
IBD patients’ most common sites for venous thrombosis
Current Practice of Thromboprophylaxis
As VTE in IBD is multifactorial, addressing a number of acquired variables may aid in lowering its prevalence. Some of these measures include adequate hydration, vitamins B6 and B12 supplementation to lower homocysteine levels, using graded compression stockings or pneumatic devices, and post-surgery early immobilization. Present evidence now supports the use of anticoagulation in people with IBD to prevent VTE.46,47 Clinical studies of VTE preventive medications in hospitalized patients, have shown that LMWH lowers the risk of VTE and death.46 Although intermittent pneumatic compression devices and stockings, which are mechanical thromboprophylaxis techniques, have been included to standard VTE practice recommendations, no research have clearly demonstrated that using these modalities will eventually lower the risk of mortality and/or PE.
In light of current evidence and demonstrations of the safety and effectiveness of pharmacological thromboprophylaxis methods, several society guidelines, including those of ACG, ACCP, BSG, and ECCO guidelines, recommend thromboprophylactic measures in selected patients with UC or CD. Despite these recommendations, routine use of these measures has not been universally adopted. In a survey, although 80% of respondents were aware that patients with IBD had an increased risk of thrombosis, 30–40% were unaware of the most recent practice recommendations for thromboprophylaxis. This was reflected even in the group that caters to a larger proportion of IBD patients in their practice.37 In another study by Dwyer et al., thromboprophylaxis measures were underutilized in more than half of IBD patients admitted with one or more VTE events.48 This study demonstrated that VTE-related events were a potentially preventable cause of significant morbidity, mortality, and in-hospital stay.
Concerns regarding safety, especially bleeding risk linked with the use of anticoagulants in these individuals, maybe the second reason for the lack of mainstream adoption. However, there is no data to support this concern. A retrospective analysis of 974 patients with IBD found that the use of pharmacological prophylaxis at admission was high (80%), with low rates of major and minor bleeding, which was similar to those who did not receive thromboprophylaxis.36 There was also no increase in the risk of serious bleeding following surgery when these drugs were used. There were no statistically significant increases in the incidence of serious bleeding among patients receiving heparin prophylaxis in 7 of 8 clinical studies combined.46 Therefore, anticoagulation should be used in these individuals according to the available data. A recent promising shift in the pattern of VTE use rates has occurred. In their study, Ra et al. found an average thromboprophylaxis usage rate of 80%, but surgical services reported higher rates (96%). Lower rates of 68% and 50%, respectively, were found for general medical and gastrointestinal services.36
Apart from the known risk factors related to IBD, such as hospitalization and disease activity, other factors should also be considered before deciding on prophylactic anticoagulation. Obesity, oral contraceptive use, inherited causes of thrombophilia, smoking, inactivity, and the presence of venous catheters are all risk factors for VTE. Since surgery is an established risk factor for both acute and chronic VTE events in their natural history, thromboprophylaxis should be routinely utilized in this group of patients. Patients’ records in a surgical database were analyzed retrospectively, and it was determined that several measures had the potential to lower postoperative VTE rates. These include reducing the need for emergency surgery by doing early surgery, using shorter anesthesia durations, as well as correcting coagulopathy and/or anemia beforehand, improving nutritional condition, and using limiting steroid use.49
In-hospital Thromboprophylaxis
The current practice of thromboprophylaxis extends to IBD patients who require hospitalization. This is endorsed by all standard guidelines.5,47,50 Clinical intuition dictates that IBD flare alone, as a thrombosis risk factor, be the only cause of admission. Hospitalization, however, has been shown to raise the incidence of VTE regardless of the underlying medical indication. Grainge et al. found that inpatients with IBD had a greater probability of having VTE than those without IBD and clinical remission did not reduce the risk (HR 1.7, 95% CI 1.1–2.9; p = 0.03).10 The risk of post-discharge VTE was shown to be greatly reduced when thromboprophylaxis was used (HR 0.46, 95% CI 0.22–0.97).38
It is important to discuss the methods by which thromboprophylaxis reduces VTE risk. First, the advantages of in-hospital thromboprophylaxis may be explained by the possibility that some of the thrombosis events discovered in the studies were already present during hospitalization and became apparent only after the patient was discharged. A small number of studies have shown that unfractionated heparin (UFH) can improve clinical activity in patients with IBD.51,52 Heparin's anti-inflammatory properties may help to attenuate the significant inflammatory burden in these individuals. Also, since these mechanisms are short-lived, the protective effects are seen to be the strongest during the first 1–3 months post-discharge.
As data suggest that pharmacological measures are more successful in preventing PE and symptomatic VTE, standard guidelines advocate pharmacological over mechanical thromboprophylaxis.53,54 Studies have indicated that LMWH and fondaparinux are more effective blood thinners than UFH. Heparin-induced thrombocytopenia (HIT), PE, and severe bleeding were all reduced when LMWH was used.55 Another advantage of LMWH is once-a-day dosing. Fondaparinux was found to have lower mortality, PE, DVT, and severe bleeding rate in studies that indirectly compared it to LMWH.56 For the prevention of VTE in hospitalized patients, innovative direct-acting oral anticoagulants (DOACs) have not been proven to provide a therapeutic benefit over LMWH.57–59 Additionally, DOACs have been linked to an increased risk of severe bleeding. This shows that there is no sufficient evidence to recommend DOACs for IBD patients currently.
Extended Thromboprophylaxis
Most risk factors for VTE in IBD continue to be present even after patients are discharged from the hospital. Extending the period of thromboprophylaxis is currently not the norm for IBD patients. The duration of anticoagulation is also a matter of some debate. This may be due to the competing risks of recurrent VTE and the potential risk of major bleeding while on thromboprophylaxis. While the majority of studies on VTE risk are conducted in postoperative settings, some in the non-operative scenario suggest that it may persist even after patients are discharged.60,61 These people might benefit more from a longer course of thromboprophylaxis. Long-term thromboprophylaxis medication is very beneficial for patients at high risk of recurrent VTE, as demonstrated by Faye et al.42 However, how long treatment should last is still up for debate. The authors suggested that anticoagulation should be continued for at least 3 months after discharge, as the risk was still higher up to 90 days after discharge in their study. Patients with IBD who underwent elective surgery had 30-day total and post-discharge VTE rates of 2% and 1%, respectively, according to a retrospective cohort study.60 A similar elevated risk for VTE was identified by Chu et al. in a study following up patients for 6 weeks following surgery.61
There are limited studies that looked into the question of the cost-effectiveness of this strategy. Prolonged thromboprophylaxis, defined as continuing enoxaparin for 28 days after discharge, was more expensive than standard therapy but yielded higher QALYs and a superior cost-effectiveness ratio, according to a study conducted in Canada.62 Using Markov decision analysis models, Nguyen et al. examined the effects of prolonged versus time-listed anticoagulation and found that while the former produced slightly higher QALYs (4.40 vs 4.38), the latter produced greater expenses and a worse cost-effectiveness ratio.63 Leeds et al. observed that this technique was not cost-effective for Crohn's disease patients when the cumulative incidence of post-hospital VTE was less than 4.9%.64 To get a definitive answer to this, larger studies need to be conducted.
From the patient's perspective, it is of the utmost significance to identify those at high risk of VTE and to continue thromboprophylaxis for an additional 4–8 weeks following discharge. A risk assessment model was created as a result of McCurdy et al. in a study of 2161 IBD-related admissions. Age >45, many hospitalizations, ICU admission, length of stay >7 days, and the presence of a central catheter were among the features listed. Patients with one or more of these risk factors were shown to benefit most from prolonged thromboprophylaxis, reducing the probability of post-discharge VTE by as much as 92%. However, this needs further prospective validation, and at the moment, clinicians have to rely on clinical intuition to determine which patients require extended thromboprophylaxis. However, in view of the increased understanding and obvious hazard of post-discharge VTE, new consensus recommendations encourage a longer time of prophylaxis following release in patients with significant risk factors for VTE.65
Table 3 summarizes studies contrasting the risk of VTE with in-hospital thromboprophylaxis against extended thromboprophylaxis. Flowchart 1 shows the patient selection for thromboprophylaxis.
Table 3.
Summary of studies on the incidence of VTE after hospital discharge among IBD patients
| VTE risk | Recommendation | ||||||
|---|---|---|---|---|---|---|---|
| Author, year | Patients | 30 days | 90 days | 180 days | 360 days | In-hospital TP | Extended TP |
| Ananthakrishnan 2014 | 11,058 | 3.7/1000 p-d | 5.4/1000 p-d | 9.4/1000 p-d | NA | Yes | NA |
| Brady 2017 | 7,078 | 2.1% | 3.3% | NA | NA | Yes | NA |
| Benlice 2018 | 24,182 | 1.0% | NA | NA | NA | Yes | NA |
| Chu 2018 | 23,046 | 12.3/1000 p-y | NA | NA | NA | Yes | NA |
| McCurdy 2019 | 81,900 | 0.6% | 0.9% | 3.1% | 1.6% | Yes | Yes |
| Faye 2019 | 87,2122 | NA | 0.12% | 0.13% | NA | Yes | Yes |
| Levatorvsky 2020 | 2,405 | NA | NA | NA | NA | Yes | Yes |
Flowchart 1.
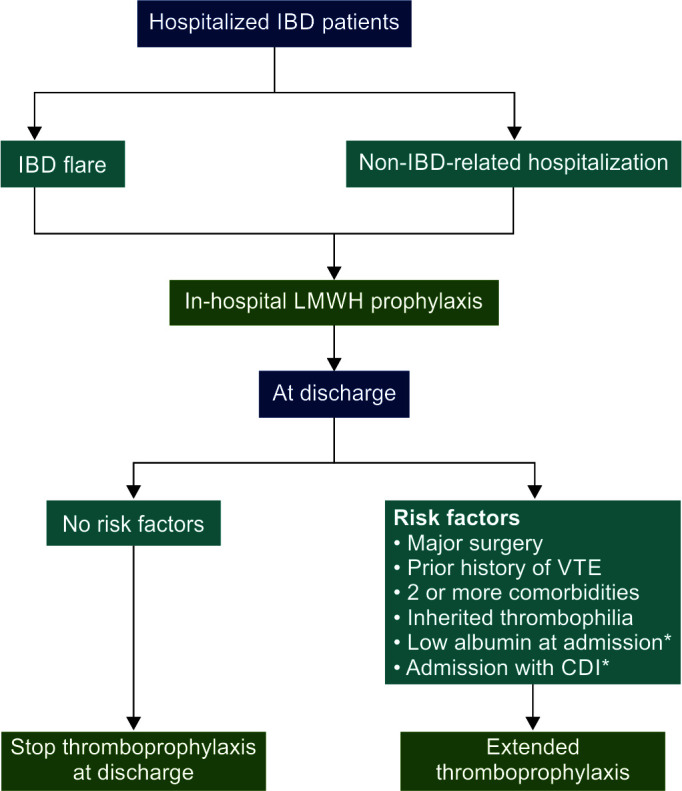
Patients with inflammatory bowel illness must be carefully selected for in-hospital and long-term thromboprophylaxis
*Indicates limited data
Risk of Bleeding with Thromboprophylaxis
Most patients admitted with IBD flare present with episodes of bloody diarrhea, making it challenging for the physician to start anticoagulation as it goes against clinical intuition. Since the risk of bleeding was not significantly higher than in the general population, Ra et al. determined that thromboprophylaxis was safe for this patient cohort.36 Kaddourah et al. found in another retrospective analysis that VTE prophylaxis was safer and that the decline in hemoglobin throughout the admission period was not different from those who were not put on prophylaxis. They found that a low utilization rate of VTE prophylaxis and a history of GI bleeding or active hematochezia were dominant factors in deciding whether to start pharmacological prophylaxis.41 Recent research, however, by Sultan et al. showed that the need for packed RBC transfusions was increased among IBD patients treated with pharmacological thromboprophylaxis approaches during a flare.66
This finding was contrary to all previous studies that demonstrated no major increase in risk of major or minor bleeding. The authors noted that all previous studies were retrospective, so only the most severe cases of bleeding, such as cerebral or retroperitoneal bleeding, would have been recorded. There would have been a lack of clarity around the definition of other mild bleeding occurrences, which would have relied on subjective criteria and patient reporting. The authors could not provide sufficient evidence to conclude that the bleeding was caused by anticoagulation alone and not by high levels of disease activity. Using anticoagulants increased the risk of major bleeding, according to a second retrospective analysis of 107 persons with IBD (2.6 per 100 patient-years vs 0.9 per 100 patient-years). Patients diagnosed with UC who had previously had anticoagulation were at a higher risk for experiencing a major bleeding episode.67 However, the significant recurrence incidence of VTE in these individuals more than makes up for this risk. So, although this caveat should be kept in mind, it should not deter the clinician from prescribing thromboprophylaxis, and multiple studies have demonstrated that its benefits outweigh the risks. The safety of VTE prophylaxis has also been demonstrated in children as well”. In a study of 200 children with UC, Story et al. discovered that in the subgroup of children with acute severe UC, use of enoxaparin during the first week of admission was not associated with a greater decline in hemoglobin. Also, there was no increased RBC transfusion requirement in this group as well.68
Conclusion
The risk of VTE is at least two to three times higher in IBD patients than that in the general population. This risk is increased for inpatients and outpatients, even while the disease is in remission. Several risk factors, such as immobility and extended bed rest, including hospitalization, raise the absolute risk of VTE. Due to its high morbidity and death rate, VTE prevention remains a priority. VTE can be prevented by addressing modifiable risk factors and giving pharmacologic as well as mechanical prophylactic measures. Considering the high thrombotic risk, thromboprophylaxis is underused in IBD patients due to fears for the safety of the treatment and a lack of awareness of the issue. In addition, there is currently no general agreement on which patients should get prolonged thromboprophylaxis and for how long. There is still a need for large-scale research to investigate the safety, efficacy, and cost-effectiveness of extending thromboprophylaxis in chosen individuals in order to decrease preventable VTE in IBD patients.
Orcid
Jijo Varghese https://orcid.org/0000-0002-9794-9188
Suprabhat Giri https://orcid.org/0000-0002-9626-5243
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Talbot RW, Heppell J, Dozois RR, et al. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61(2):140–145. doi: 10.1016/s0025-6196(12)65200-8. [DOI] [PubMed] [Google Scholar]
- 2.Webberley MJ, Hart MT, Melikian V. Thromboembolism in inflammatory bowel disease: Role of platelets. Gut. 1993;34(2):247–251. doi: 10.1136/gut.34.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuhara H, Steinmaus C, Corley D, et al. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37(10):953–962. doi: 10.1111/apt.12294. [DOI] [PubMed] [Google Scholar]
- 4.Fumery M, Xiaocang C, Dauchet L, et al. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: A meta-analysis of observational studies. J Crohns Colitis. 2014;8(6):469–479. doi: 10.1016/j.crohns.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen GC, Bernstein CN, Bitton A, et al. Consensus Statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146(3):835–848.e6. doi: 10.1053/j.gastro.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 6.Ando K, Fujiya M, Nomura Y, et al. The incidence and risk factors of venous thromboembolism in patients with inflammatory bowel disease: a prospective multicenter cohort study. Digestion. 2019;100(4):229–237. doi: 10.1159/000495289. [DOI] [PubMed] [Google Scholar]
- 7.Sonoda K, Ikeda S, Mizuta Y, et al. Evaluation of venous thromboembolism and coagulation-fibrinolysis markers in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2004;39(10):948–954. doi: 10.1007/s00535-004-1426-6. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein CN, Blanchard JF, Houston DS, et al. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: A population-based cohort study. Thromb Haemost. 2001;85:430–434. 11307809 [PubMed] [Google Scholar]
- 9.Kappelmann MD, Horvath-Puho E, Sandler RS, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: A population-based nationwide study. Gut. 2011;60(7):937–943. doi: 10.1136/gut.2010.228585. [DOI] [PubMed] [Google Scholar]
- 10.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375(9715):657–663. doi: 10.1016/S0140-6736(09)61963-2. [DOI] [PubMed] [Google Scholar]
- 11.Han Y, Zhao J, Huang H, et al. Characteristics of hemorheology in patients with acute severe ulcerative colitis and the clinical study of rivaroxaban anticoagulant therapy. Hepat Mon. 2019;19:e92536. doi: 10.5812/hepatmon.92536. [DOI] [Google Scholar]
- 12.Danese S, Papa A, Saibeni S, et al. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am J Gastroenterol. 2007;102(1):174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 13.Boehme MW, Autschbach F, Zuna I, et al. Elevated serum levels and reduced immunohistochemical expression of thrombomodulin in active ulcerative colitis. Gastroenterology. 1997;113(1):107–117. doi: 10.1016/s0016-5085(97)70086-6. [DOI] [PubMed] [Google Scholar]
- 14.Meucci G, Pareti F, Vecchi M, et al. Serum von Willebrand factor levels in patients with inflammatory bowel disease are related to systemic inflammation. Scand J Gastroenterol. 1999;34(3):287–290. doi: 10.1080/00365529950173708. [DOI] [PubMed] [Google Scholar]
- 15.Solem CA, Loftus EV, Tremaine WJ, et al. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. 2004;99(1):97–101. doi: 10.1046/j.1572-0241.2003.04026.x. [DOI] [PubMed] [Google Scholar]
- 16.Koutroubakis IE, Sfiridaki A, Tsiolakidou G, et al. Genetic risk factors in patients with inflammatory bowel disease and vascular complications: case-control study. Inflamm Bowel Dis. 2007;13(4):410–415. doi: 10.1002/ibd.20076. [DOI] [PubMed] [Google Scholar]
- 17.Zhong M, Dong XW, Zheng Q, et al. Factor V Leiden and thrombosis in patients with inflammatory bowel disease (IBD): A meta-analysis. Thromb Res. 2011;2011;128128(5):403–409. 403–409. doi: 10.1016/j.thromres.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Liang J, Wu S, Feng B, et al. Factor V Leiden and inflammatory bowel disease: a systematic review and meta-analysis. J Gastroenterol. 2011;46(10):1158–1166. doi: 10.1007/s00535-011-0441-7. [DOI] [PubMed] [Google Scholar]
- 19.Turri D, Rosselli M, Simioni P, et al. Factor V Leiden and prothrombin gene mutation in inflammatory bowel disease in a Mediterranean area. Dig Liver Dis. 2001;33:559–562. doi: 10.1016/s1590-8658(01)80107-9. [DOI] [PubMed] [Google Scholar]
- 20.Larsen TB, Nielsen JN, Fredholm L, et al. Hyperhomocysteinemia, coagulation pathway activation and thrombophilia in patients with inflammatory bowel disease. Scand J Gastroenterol. 2002;2002;3737(1):62–67. 62–67. doi: 10.1080/003655202753387374. [DOI] [PubMed] [Google Scholar]
- 21.Naito T, Botwin GJ, Haritunians T, et al. Prevalence and effect of genetic risk of thromboembolic disease in inflammatory bowel disease. Gastroenterology. 2021;160(3):771–780.e4. doi: 10.1053/j.gastro.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heits F, Stahl M, Ludwig D, et al. Elevated serum thrombopoietin and interleukin-6 concentrations in thrombocytosis associated with inflammatory bowel disease. J Interferon Cytokine Res. 1999;19(7):757–760. doi: 10.1089/107999099313604. [DOI] [PubMed] [Google Scholar]
- 23.Chiarantini E, Valanzano R, Liotta AA, et al. Persistence of hemostatic alterations in patients affected by Crohn's disease after bowel surgery. Thromb Res. 1997;87(6):539–546. doi: 10.1016/s0049-3848(97)00183-7. [DOI] [PubMed] [Google Scholar]
- 24.Collins CE, Cahill MR, Newland AC, et al. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994;106(4):840–845. doi: 10.1016/0016-5085(94)90741-2. [DOI] [PubMed] [Google Scholar]
- 25.Danese S, Fiocchi C. Platelet activation and the CD40/CD40L pathway: mechanisms and implications for human disease. Crit Rev Immunol. 2005;25(2):103–121. doi: 10.1615/critrevimmunol.v25.i2.20. [DOI] [PubMed] [Google Scholar]
- 26.Kim YH, Pfaller B, Marson A, et al. The risk of venous thromboembolism in women with inflammatory bowel disease during pregnancy and the postpartum period: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98(38):e17309. doi: 10.1097/MD.0000000000017309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maxwell SR, Moots RJ, Kendall MJ. Corticosteroids: do they damage the cardiovascular system? Postgrad Med J. 1994;70(830):863–870. doi: 10.1136/pgmj.70.830.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103(9):2272–2280. doi: 10.1111/j.1572-0241.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- 29.Ytterberg, S R, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 30.Curtis JR, Yamaoka K, Chen YH, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: Results from the open-label, randomised controlled ORAL surveillance trial. Ann. Rheumatic Dis. 2023;82(3):331–343. doi: 10.1136/ard-2022-222543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Communication, FDS FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR); FDA Drug Safety Communication; 2019 [Google Scholar]
- 32.Sandborn WJ, Panés J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther. 2019;50(10):1068–1076. doi: 10.1111/apt.15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Li W, Gong M, et al. Risk of venous thromboembolism with Janus kinase inhibitors in inflammatory immune diseases: A systematic review and meta-analysis. Front Pharmacol. 2023;14:1189389. doi: 10.3389/fphar.2023.1189389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campanaro F, Zaffaroni A, Cacioppo E, et al. Venous and arterial thromboembolic risk of JAK inhibitors: A systematic review with meta-analysis. Rheumatology (Oxford). 2023;62(10):3245–3255. doi: 10.1093/rheumatology/kead211. [DOI] [PubMed] [Google Scholar]
- 35.Novacek G, Weltermann A, Sobala A, et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139(3):779–787.e1. doi: 10.1053/j.gastro.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Ra G, Thanabalan R, Ratneswaran S, et al. Predictors and safety of venous thromboembolism prophylaxis among hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2013;7(10):e479–e485. doi: 10.1016/j.crohns.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Tinsley A, Naymagon S, Trindade AJ, et al. A survey of current practice of venous thromboembolism prophylaxis in hospitalized inflammatory bowel disease patients in the United States. J Clin Gastroenterol. 2013;47(1):e1–e6. doi: 10.1097/MCG.0b013e31824c0dea. [DOI] [PubMed] [Google Scholar]
- 38.Ananthakrishnan AN, Cagan A, Gainer VS, et al. Thromboprophylaxis is associated with reduced post-hospitalization venous thromboembolic events in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12(11):1905–1910. doi: 10.1016/j.cgh.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brady MT, Patts GJ, Rosen A, et al. Postoperative venous thromboembolism in patients undergoing abdominal surgery for IBD: A common but rarely addressed problem. Dis Colon Rectum. 2017;60(1):61–67. doi: 10.1097/DCR.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 40.Benlice C, Holubar SD, Gorgun E, et al. Extended venous thromboembolism prophylaxis after elective surgery for IBD patients: nomogram-based risk assessment and prediction from nationwide cohort. Dis Colon Rectum. 2018;61(10):1170–1179. doi: 10.1097/DCR.0000000000001189. [DOI] [PubMed] [Google Scholar]
- 41.Kaddourah O, Numan L, Jeepalyam S, et al. Venous thromboembolism prophylaxis in inflammatory bowel disease flare-ups. Ann Gastroenterol. 2019;32(6):578–583. doi: 10.20524/aog.2019.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faye AS, Wen T, Ananthakrishnan AN, et al. Acute venous thromboembolism risk highest within 60 days after discharge from the hospital in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020;18(5):1133–1141.e3. doi: 10.1016/j.cgh.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKechnie T, Wang J, Springer JE, et al. Extended thromboprophylaxis following colorectal surgery in patients with inflammatory bowel disease: a comprehensive systematic clinical review. Colorectal Dis. 2020;22(6):663–678. doi: 10.1111/codi.14853. [DOI] [PubMed] [Google Scholar]
- 44.Stubbs JM, Assareh H, Curnow J, et al. Incidence of in-hospital and post-discharge diagnosed hospital-associated venous thromboembolism using linked administrative data. Intern Med J. 2018;48(2):157–165. doi: 10.1111/imj.13679. [DOI] [PubMed] [Google Scholar]
- 45.Ambra N, Mohammad OH, Naushad VA, et al. Venous thromboembolism among hospitalized patients: incidence and adequacy of thromboprophylaxis – A retrospective study. Vasc Health Risk Manag. 2022;18:575–587. doi: 10.2147/VHRM.S370344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen J, Ran ZH, Tong JL, et al. Meta-analysis: the utility and safety of heparin in the treatment of active ulcerative colitis. Aliment Pharmacol Ther. 2007;26(5):653–663. doi: 10.1111/j.1365-2036.2007.03418.x. [DOI] [PubMed] [Google Scholar]
- 47.Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198–3225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dwyer JP, Javed A, Hair CS, et al. Venous thromboembolism and underutilisation of anticoagulant thromboprophylaxis in hospitalized patients with inflammatory bowel disease. Intern Med J. 2014;44(8):779–784. doi: 10.1111/imj.12488. [DOI] [PubMed] [Google Scholar]
- 49.Wallaert JB, De Martino RR, Marsicovetere PS, et al. Venous thromboembolism after surgery for inflammatory bowel disease: Are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum. 2012;55(11):1138–1144. doi: 10.1097/DCR.0b013e3182698f60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olivera PA, Zuily S, Kotze PG, et al. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(12):857–873. doi: 10.1038/s41575-021-00492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carty E, Rampton DS. Evaluation of new therapies for inflammatory bowel disease. Br J Clin Pharmacol. 2003;56(4):351–361. doi: 10.1046/j.1365-2125.2003.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ang YS, Mahmud N, White B, et al. Randomized comparison of unfractionated heparin with corticosteroids in severe active inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14(8):1015–1022. doi: 10.1046/j.1365-2036.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- 53.Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th edition, American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60(5):571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 55.Junqueira DR, Zorzela LM, Perini E. Unfractionated heparin versus low-molecular weight heparins for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2017;4:CD007557. doi: 10.1002/14651858.CD007557.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar A, Talwar A, Farley JF, et al. Fondaparinux sodium compared with low-molecular-weight heparins for perioperative surgical thromboprophylaxis: a systematic review and meta-analysis. J Am Heart Assoc. 2019;8(10):e012184. doi: 10.1161/JAHA.119.012184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365(23):2167–2177. doi: 10.1056/NEJMoa1110899. [DOI] [PubMed] [Google Scholar]
- 58.Riva N, Ageno W. Direct oral anticoagulants for unusual-site venous thromboembolism. Res Pract Thromb Haemost. 2021;5:265–277. doi: 10.1002/rth2.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neumann I, Izcovich A, Zhang Y, et al. DOACs vs LMWHs in hospitalized medical patients: a systematic review and meta-analysis that informed 2018 ASH guidelines. Blood Adv. 2020;4(7):1512–1517. doi: 10.1182/bloodadvances.2019000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tøttrup A, Erichsen R, Sværke C, et al. Thirty-day mortality after elective and emergency total colectomy in Danish patients with inflammatory bowel disease: A population-based nationwide cohort study. BMJ Open. 2012;2:e000823. doi: 10.1136/bmjopen-2012-000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chu TPC, Grainge MJ, Card TR. The risk of venous thromboembolism during and after hospitalization in patients with inflammatory bowel disease activity. Aliment Pharmacol Ther. 2018;48(10):1099–1108. doi: 10.1111/apt.15010. [DOI] [PubMed] [Google Scholar]
- 62.Trepanier M, Alhassan N, Sabapathy CA, et al. Cost-effectiveness of extended thromboprophylaxis in patients undergoing colorectal surgery from a Canadian Health Care System perspective. Dis Colon Rectum. 2019;62(11):1381–1389. doi: 10.1097/DCR.0000000000001438. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen GC, Bernstein CN. Duration of anticoagulation for the management of venous thromboembolism in inflammatory bowel disease: a decision analysis. Am J Gastroenterol. 2013;108(9):1486–1495. doi: 10.1038/ajg.2013.220. [DOI] [PubMed] [Google Scholar]
- 64.Leeds IL, Dibrito SR, Canner JK, et al. Cost-benefit limitations of extended, outpatient venous thromboembolism prophylaxis following surgery for Crohn's disease. Dis Colon Rectum. 2019;62(11):1371–1380. doi: 10.1097/DCR.0000000000001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCurdy JD, Israel A, Hasan M, et al. A clinical predictive model for post-hospitalization venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49(12):1493–1501. doi: 10.1111/apt.15286. [DOI] [PubMed] [Google Scholar]
- 66.Sultan K, Shah D, Bhorania K, et al. Increased transfusion requirements with pharmacologic thromboembolism prophylaxis during inflammatory bowel disease exacerbation. Dig Dis Sci. 2019;64(11):3256–3262. doi: 10.1007/s10620-019-05650-2. [DOI] [PubMed] [Google Scholar]
- 67.Scharrer S, Primas C, Eichinger S, et al. Austrian IBD Study Group. Inflammatory bowel disease and risk of major bleeding during anticoagulation for venous thromboembolism. Inflamm Bowel Dis. 2021;27(11):1773–1783. doi: 10.1093/ibd/izaa337. [DOI] [PubMed] [Google Scholar]
- 68.Story E, Bijelic V, Penney C, et al. Safety of venous thromboprophylaxis with low-molecular-weight heparin in children with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2021;73(5):604–609. doi: 10.1097/MPG.0000000000003231. [DOI] [PubMed] [Google Scholar]


