Abstract
Monocyte-dependent as well as direct inhibitory effects of antimalarial antibodies point toward antigens accessible at the time of merozoite release as targets for biologically active antibodies capable of mediating protection against Plasmodium falciparum. The glutamate-rich protein (GLURP), being an antigen associated with mature schizont-infected erythrocytes, was therefore the object of the present investigation, in which we analyzed whether anti-GLURP antibodies can either interfere directly with merozoite invasion or act indirectly by promoting a monocyte-dependent growth inhibition, antibody-dependent cellular inhibition. GLURP-specific human immunoglobulin G (IgG) antibodies, from pooled IgG of healthy Liberian adults who were clinically immune to malaria, were purified by affinity chromatography on columns containing R0 (N-terminal nonrepetitive region of GLURP) or R2 (C-terminal repetitive region of GLURP) recombinant protein or synthetic peptides as ligands. Analysis of the pattern of reactivity of highly purified anti-GLURP antibodies led to the definition of at least four B-cell epitopes. One epitope was specific for R0, two were specific for R2, and the fourth displayed cross-reactivity between R0 and R2. None of the purified IgG antibodies had direct invasion-inhibitory effects, even at high concentrations. In contrast, when allowed to cooperate with monocytes, all anti-GLURP IgG preparations mediated a strong monocyte-dependent parasite growth inhibition in a dose-dependent manner.
Epidemiological surveys performed in areas of intense malaria transmission have consistently shown that individuals who are continuously exposed to repeated malaria infection gradually develop clinical immunity (14, 20, 29). This acquired immunity is strong, although incomplete, and is nonsterilizing (3, 25, 26). Experiments with antibodies purified from the sera of African adults who were clinically immune to malaria and given by passive transfer to susceptible children have established that immunoglobulin G (IgG) is at least a main component of defense against the asexual blood stage of Plasmodium falciparum (5, 9, 11). Recent passive transfer experiments have enabled us to acquire clinically demonstrated protective antibodies from the donor and nonprotective antibodies from the recipients. These sets of antibodies were used to assess the extent to which the in vitro data correlated with the in vivo results for each recipient isolate (5). Results from these in vitro studies suggested that clinically protective antibodies had little direct effect on merozoite invasion, but that they could act in conjunction with blood monocytes to contain parasite multiplication. This mechanism was called antibody-dependent cellular inhibition (ADCI) (5, 17, 19).
The assay provides a screen to select molecules which may be targeted by clinically effective antibodies. Further experiments have indicated that antibody-monocyte cooperation in parasite inhibition is mediated not through parasite opsonization but rather through indirect effects. These activities were mediated by soluble monocyte-derived substances whose release was triggered through monocyte interaction with cytophilic antibodies bound to merozoite antigens (7). A critical role for merozoite surface molecules in this mechanism is also supported by the identification of Msp3, a new molecule from the merozoite surface, when an expression library was screened by ADCI (23).
Based on the published immunoepidemiological data for the glutamate-rich protein (GLURP) (4, 12, 13, 15) and the report that this molecule is located on the surface of the merozoite (2), we chose to investigate the potential of affinity purified anti-GLURP human IgG in assays of direct parasite inhibition and to compare this with activity in ADCI assays.
MATERIALS AND METHODS
Antigens.
The two recombinant GLURP fragments, GLURP94-489 (R0), and GLURP705-1178 (R2), were purified as previously described (27). The five peptides, GL8 (GGPKLRGNVTSNIKFPSDNKGK [amino acids {aa} 36 to 57]), GL6 (KQNSQIPSLDLKEPTNEDI [aa 309 to 327]), GL9 (PNFVDSQPNPQEPVEPSFVKIEKVPSEEN [aa 732 to 760]), GL5 (EFKEINEDDKSAHIQHEIVEVEEILPEDD [aa 853 to 881]), and GL7 (KNKKKSSFITYISTKKFK [aa 1215 to 1232]) correspond to repetitive as well as nonrepetitive regions of GLURP. The peptides MSP-3b and RESA have been described previously (23). Synthetic peptides were produced according to standard peptide synthesis procedures (22). Cleavage and deblocking used trifluoromethanesulfonic acid followed by an ether wash.
Immunoprecipitation.
Metabolic labelling and immunoprecipitation of P. falciparum polypeptides were performed as described previously (27). Immunoprecipitations were performed as follows. Affinity-purified human anti-R0 and anti-R2 antibodies (fractions 1 and 2) were added to 0.5 ml of whole-cell lysate or to 250 μl of culture supernatant plus 250 μl of 2× radioimmunoprecipitation assay (RIPA) buffer (1× RIPA buffer is 50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, and 0.1% sodium dodecyl sulfate [SDS]), and the mixtures were incubated overnight at 4°C with rotation. Seventy-five microliters of a 50% suspension of protein A-Sepharose beads (Pharmacia, Uppsala, Sweden) bound to rabbit anti-human IgG, IgA, and IgM (Dako, Glostrup, Denmark) in RIPA buffer was added to each immunoprecipitation solution, and the mixture was incubated with rotation for 4 h at 4°C. The beads were washed several times with RIPA buffer and resuspended in 50 μl of Laemmli sample buffer. The immunoprecipitates were separated by electrophoresis on SDS–7.5% polyacrylamide gels and subsequently treated with Amplify (Amersham) prior to exposure to X-ray film.
Enzyme-linked immunosorbent assay (ELISA).
Microtiter plates (Maxisorb; Nunc, Roskilde, Denmark) were coated with purified recombinant molecules as previously described (28), blocked with 2.5% (wt/vol) milk powder in phosphate-buffered saline (PBS)–0.05% Tween 20 (PBST) for 2 h, and reacted with sera diluted 1/100 in 1.25% (wt/vol) milk powder in PBST for 1 h. The secondary antibody was a peroxidase-conjugated rabbit anti-human IgG (Dako) diluted 1/1,000 in 1.25% (wt/vol) milk powder in PBST. After 1 h of incubation, the reactions were revealed by using o-phenylenediamine and H2O2 in citrate buffer (Sigma, St. Louis, Mo.) for 10 min and read at 450 nm in a Multiscan MCC340 plate reader.
ELISA with synthetic peptides was performed essentially as described above, with the exception that microtiter wells were coated overnight at 4°C with 0.25 μg of peptide per well in PBS. This coating buffer was chosen as most effective based on a series of experiments utilizing other buffer systems such as carbonate buffer, borate buffer, and Tris-buffered saline.
For IgG subclass detection, monoclonal mouse anti-human subclasses IgG1 to IgG4 (clones NL16 [Boehringer], HP6002 [Sigma], Zg4 [Immunotech], and RJ4 [Immunotech]) were used as secondary antibodies in duplicate wells in dilutions of 1:2,000, 1:10,000, 1:10,000, and 1:1,000, respectively, followed by incubation with alkaline phosphatase-conjugated rabbit anti-mouse IgG (diluted 1/30,000; Promega) as described previously (6). Plates were washed extensively with PBST between each incubation step.
Competitive ELISA.
The specificity of the antibody in fractions 1 and 3 was analyzed by competition ELISA. To this end, the recombinant proteins R0 and R2 were added at concentrations ranging from 0 to 1 μM (as shown in Fig. 2) to fractions 1 and 2, each diluted 640-fold in 1.25% (wt/vol) milk powder in PBST. The mixtures were incubated overnight at 4°C, and subsequently the competitive effect on the binding to either R0 or R2 was determined in ELISAs as described above.
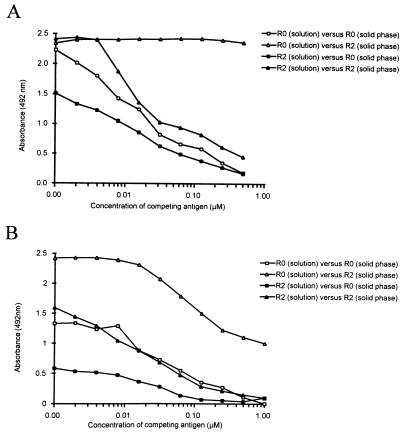
FIG. 2.
Analysis of antibody specificities in fraction 1 (A) and fraction 2 (B). The fractions were each diluted 640-fold and preincubated with the recombinant antigens R0 (open symbols) of R2 (filled symbols) at the indicated concentration before addition to wells coated with R0 (squares) or R2 (triangles). The key indicates the competing antigen (solution) and the solid-phase antigen (ELISA) used for each curve. Please note that the curves do not originate from the same point on the ordinate since competing antigen was already added at this point.
Affinity purification of human IgG by using recombinant proteins.
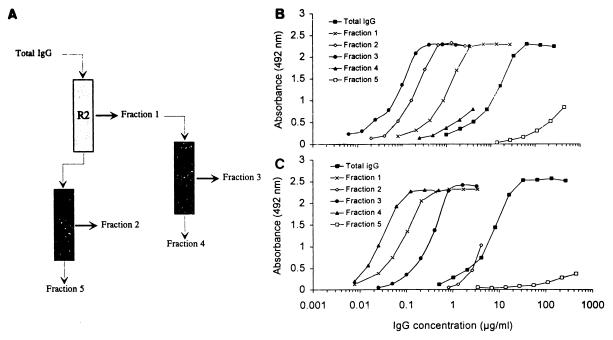
GLURP-specific IgG was purified from pooled total IgG obtained from eight healthy adult blood donors living in Liberia, where malaria is holoendemic and the transmission is intense year round. At the time of blood collection, microscopic examination of thick blood films showed no circulating parasites. The recent medical history, if any, was recorded by one of us. Persons who were medicated or had a recent history of febrile illness were excluded. Very low grade parasitemia without clinical symptoms was an exclusion criterion. Affinity chromatography was done with R0 and R2 recombinant proteins immobilized on a solid matrix (Fig. 1) as previously described (16). Briefly, 20 mg of purified recombinant protein was coupled to 1 g of cyanogen bromide-activated Sepharose 4B (Pharmacia) according to the manufacturer’s instructions. Total IgG (13 g) in column buffer (70 mM Tris [pH 8.6], 20 mM barbiturate, 0.5 M NaCl, 15 mM NaN3) was applied to the R2 column, and the run-through was then passed over the R0 column. The columns were washed extensively with column buffer. Bound IgG was eluted with 20 mM Tris (pH 8.6)–20 mM barbiturate–3 M potassium cyanide and immediately dialyzed extensively against the column buffer. The eluate from the R2 column was further purified by passage over the R0 column. All eluates were concentrated in dialysis bags covered with polyethylene glycol and subsequently dialyzed against PBS.
FIG. 1.
Purification and reactivity of anti-R0 and anti-R2 antibodies in ELISAs. (A) Schematic representation of the purification of anti-R0 and anti-R2 antibodies. Numbers represent IgG fractions listed in Table 1. Run-through and eluates are indicated by thin and thick arrows, respectively. (B and C) Results obtained with different fractions in ELISA. (B) Binding of IgG fractions to R0; (C) binding of IgG fractions to R2-coated plates. IgG concentration is plotted on a semilogarithmic scale.
Selection of peptides and affinity purification of human IgG by using the peptides GL5 and GL9.
A set of five synthetic peptides chosen within the R0 and R2 regions of GLURP was tested in ELISA using 20 sera obtained from immune adults in the Ivory Coast. Two peptides GL5 and GL9 were strongly reactive (mean) optical density [OD] ratios [OD test/mean OD + 3 standard deviations of five controls]), 12.08 ± 5 and 2.9 ± 3.4, respectively) when peptides GL6, GL7, and GL8 were negative (mean OD ratios, 0.85 ± 0.3, 0.73 ± 0.2, and 0.77 ± 0.2, respectively). Microtiter plates were coated with GL5 or GL9 (10 μg/ml) and incubated with three of the strongest-reacting immune sera (HIS5, HIS18, and HIS35). The peptides RESA [H-(EENVEHDA)2-(EENV)2-OH; Bachem, Bübendorf, Switzerland) and MSP-3b (AKEASSYDYILGWEFGGGVPEHKKEEN), employed as controls, were used to coat microtiters plates at the same final concentration and incubated with the same three immune sera. Antibodies bound to the plates after washing were eluted with 0.2 M glycine (pH 2.5), neutralized, and dialyzed as previously described (8). The concentration of eluted antibodies was indirectly estimated by immunofluoresence assay (IFA) and/or by ELISA. The stock solution of anti-GL5 titrated at 1/25, the stock solution of anti-GL9 titrated at 1/32, the stock solution of anti-RESA titrated at 1/64, and the stock solution of anti-MSP-3b titrated at 1/32.
Parasite isolates and culture.
Two African isolates (NF 54 and FCIP 150) and one Thai isolate (T23) were cultured in O+ human erythrocytes in RPMI–10% Albumax (Gibco) as previously described (23).
Direct merozoite invasion inhibition assays.
The effects of purified IgG at 135 μg/ml upon P. falciparum were determined in 48-h cultures of P. falciparum starting with a majority of schizont-stage-infected erythrocytes obtained by Plasmagel (Roger Bellon, Neuilly sur Seine, France) (24). Only cultures with a multiplication rate of at least six times per 48 h were used in assays. The parasites were added in 96-well plates adjusted to 0.3 to 0.5% and at 2.5% hematocrit in 200 μl of complete RPMI 1640 medium. Thin smears were done from each duplicate well after 48 h of incubation in a candle jar at 37°C. Parasitemia was assessed under 1,000× power by counting the presence of parasites over 20,000 erythrocytes in different locations. Test IgG or control IgG obtained from a pool of healthy French donors who had never been exposed to malaria was added to parasite cultures to a final concentration of 200 μg/ml.
ADCI assay.
ADCI assays were performed as described previously (5, 17). The four affinity-purified IgG fractions 1 to 4 were concentrated in Centricon concentrators (Amicon, Inc., Lexington, Mass.) to 1.88, 0.87, 0.65, and 0.79 mg/ml, respectively, and used at the indicated concentrations. After cultivation for 72 h, parasitemia was determined on Giemsa-stained thin smears from each well by microscopic examination of more than 20,000 erythrocytes. All readings of parasitemia were made under blind conditions (the microscopists were not awared of the coding of the slides). Monocyte-dependent parasite killing is expressed as the specific growth inhibition index (SGI): SGI = 1 − [(percentage of parasitemia with monocytes and test IgG/percentage of parasitemia with test IgG)/(percentage of parasitemia with monocytes and normal IgG/percentage of parasitemia with normal IgG)] × 100.
IFA.
IFAs were performed on air-dried, acetone-fixed, thin smears of P. falciparum asexual blood stages as described (10). The IFA endpoint titer is defined as the highest antibody dilution which results in a visible staining of the parasites. For IFA competition assays, the IgG fractions were incubated with the antigen at the indicated concentration for 2 h at room temperature before IFA.
RESULTS
Characterization of anti-R0 and anti-R2 antibodies.
Total IgG was isolated from a pool of plasma obtained from healthy Liberian adults who were clinically immune to malaria with high anti-GLURP antibody titers (28). The IgG was fractionated by affinity chromatography on recombinant GLURP fragments R2 and R0 (Fig. 1A). A total of 13 g of IgG starting material was applied sequentially to the R2 and R0 columns. The eluted material consisted of 8.1 and 2.24 mg of anti-R2 IgG and anti-R0 IgG, respectively (Fig. 1 and Table 1, fractions 1 and 2). The antibodies eluted from the R0 column (fraction 2) were specific for R0 as determined by ELISA (Fig. 1B), while the antibodies eluted from the R2 column (fraction 1) reacted with R2 and to a lesser extent with R0 (Fig. 1B and C). Both fractions were negative on control peptides MSP-3b and RESA. To determine if the anti-R0 reactivity in fraction 1 is caused by antibodies displaying cross-reactivity between R2 and R0, competition experiments were performed. Each of the two recombinant GLURP antigens, R0 and R2, was added to fraction 1, and the competing effect on the binding of antibodies to R0- or R2-coated ELISA plates was determined. Addition of either of the two recombinant proteins inhibited the anti-R0 antibody reactivity, while the addition of R0 antigen had no effect on the antibody binding to R2-coated plates (Fig. 2A). Therefore, these results indicate that fraction 1 contains at least two antibody populations, one which binds to both R0 and R2 and a second which is specific for R2. From the data presented in Fig. 2A, it appears that added recombinant R2 inhibits the anti-R2 reacting specificity, while added R0 does not. In contrast, the addition of both R2 and R0 inhibits the antibody binding to R0-coated plates. These results indicate that at least two antibody populations are present in fraction 1, one which is specific for R2 and another which binds to both R0 and R2.
TABLE 1.
Protein concentrations, yields, and IFA titers in total IgG and in the five fractions obtained by affinity chromatography (see Fig. 1)
| Fraction | Vol (ml) | Protein concn (mg/ml) | Total protein (mg) | Yield (%) | IFA titera |
|---|---|---|---|---|---|
| Total IgG | 500 | 26 | 13,000 | 100 | 48,000 |
| Fraction | |||||
| 1 | 15 | 0.54 | 8.1 | 0.06 | 100 |
| 2 | 8 | 0.28 | 2.24 | 0.02 | 600 |
| 3 | 20 | 0.19 | 3.8 | 0.03 | 300 |
| 4 | 10 | 0.24 | 2.4 | 0.02 | >600 |
| 5 | 5,875 | 2.0 | 11,750 | 90 | >4,800 |
Against schizont stage of P. falciparum NF54.
To separate the two populations, fraction 1 was applied to the column containing R0 as ligand (Fig. 1A), yielding 3.8 mg of IgG in the eluate and 2.4 mg of IgG in the run-through (Table 1, fractions 3 and 4). Antibodies in fraction 4 were specific for R2 as determined by ELISA, while antibodies in fraction 3 were reactive with both R0 and R2 (Fig. 1B and C). This cross-reactivity in fraction 3 was further analyzed by competition ELISA (Fig. 2B) where both recombinant proteins inhibited antibody binding to the homologous as well as the heterologous sequences.
Reactivity of anti-GLURP antibody fractions with authentic GLURP.
Several lines of evidence indicate that the anti-GLURP antibody fractions specifically bind to native GLURP. First, immunoprecipitation of parasite-derived polypeptides demonstrated that antibody fractions 1 and 2 both immunoprecipitated a polypeptide of approximately 220,000 Da, the apparent molecular mass previously found for GLURP in SDS-polyacrylamide gel electrophoresis (Fig. 3). Control for immunoprecipitation used total IgG obtained from healthy blood donors not previously exposed to malaria. Second, all antibody preparations reacted strongly with parasite proteins in IFA, with endpoint titers ranging from 1:48,000 to 1:300 (Table 2). This binding was specific since it could be reversed by competition with either the R0 or R2 recombinant (Table 2).
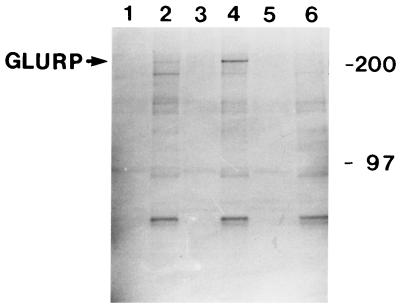
FIG. 3.
Immunoprecipitation analysis of P. falciparum proteins. Parasites were grown and labelled as previously described (21). Labelled polypeptides were subjected to immunoprecipitation with fraction 2 (lane 1, culture supernatant; lane 2, cell pellet), fraction 1 (lane 3, culture supernatant; lane 4, cell pellet), or purified IgG from Danish donors never exposed to malaria (lane 5, culture supernatant; lane 6, cell pellet). The sizes (in kilodaltons) of molecular mass markers are indicated on the right.
TABLE 2.
Competition IFAa
| Antibody | Antigen | Antigen concn (μg/ml) resulting in:
|
||
|---|---|---|---|---|
| 50% inhibition | 100% inhibition | No inhibition | ||
| Fraction 2 | R0 | 10 | >100 | |
| Fraction 3 | R0 | ND | 0.1 | |
| Fraction 4 | R0 | ND | ND | 100 |
| Fraction 2 | R2 | ND | ND | 100 |
| Fraction 3 | R2 | <0.1 | 1 | |
| Fraction 4 | R2 | 0.1 | 10 | |
Soluble antigen was added to the three fractions at an antibody concentration which was fourfold higher than the IFA endpoint titer. ND, not determined.
Characterization of antipeptide antibodies.
To further localize B-cell epitopes of GLURP, five peptides covering repetitive as well as nonrepetitive amino acid sequences were studied in ELISA, using four coating conditions. Only two peptides, GL5 and GL9, corresponding to amino acid sequences covered by the R2 recombinant protein were found to be reactive with sera from clinically immune adults living in an area of the Ivory Coast where malaria is endemic. Human IgG antibodies were affinity purified on ELISA plates coated with GL5 or GL9 as previously described (8). The antibodies purified by using either peptide did not bind to the other peptide, demonstrating that these two peptides define distinct epitopes (data not shown). Both antibody preparations reacted with recombinant R2 but not with R0, suggesting that none of the epitopes defined by peptides GL5 and GL9 correspond to the R2 cross-reactive epitope described above. This conclusion was supported by the observation that fraction 4 reacted strongly with GL5, while fraction 3 reacted only weakly with this peptide (data not shown). GL9 was not recognized by any of the affinity-purified antirecombinant antibody fractions. The anti-GL5 and anti-GL9 antibody preparations also reacted with the authentic GLURP molecule as determined by immunoblotting of blood-stage parasite proteins and by IFA (data not shown).
Direct effect of anti-GLURP antibodies on merozoite invasion.
None of the antibodies prepared proved to exert an inhibitory effect on merozoite invasion at all concentrations tested. Results obtained with two different preparations of each of the three antirecombinant and the two antipeptide antibodies did not reveal any significant inhibitory effect with antibody concentrations of up to 135 μg/ml. Thus, none of the 10 antibody solutions tested in duplicate each time on two different culture strains was effective in the direct assay. In view of this result, the second preparation of each of the various antibodies was studied the same day by Western blotting and IFA in parallel with invasion inhibition assays. Despite their strong reactivity with parasite proteins, by both Western blotting and IFA, results of the second study confirmed those of the first.
Effect of anti-GLURP antibodies in cooperation with blood monocytes on parasite growth in vitro.
In vitro ADCI experiments were performed to assess whether there were cooperative effects of human anti-GLURP antibodies with normal blood monocytes. In one experiment, human anti-R2 (fraction 1) and anti-R0 (fraction 2) antibody preparations, purified as described for Fig. 1, were analyzed by using African and European IgG preparations as positive and negative controls (Fig. 4A). Both fractions 1 and 2 proved to be effective in ADCI. However, the degree of inhibition of parasite growth was higher for anti-R0 antibodies, despite the fact that anti-R2 antibodies had the highest affinity for GLURP as measured by adding increasing concentrations of ammonium thiocyanate to both preparations and testing the effect in IFA on mature schizonts (data not shown). Affinity-purified antipeptide human antibodies also proved consistently effective in three successive assays (Fig. 4A). Using the fraction 1 and fraction 2 preparations, we evaluated the parasite inhibition over a wide range of antibody concentrations (Fig. 4B). The results showed a clear dose-dependent effect, and again the inhibitory effect of anti-R0 antibodies was more pronounced than that of the anti-R2 antibodies.
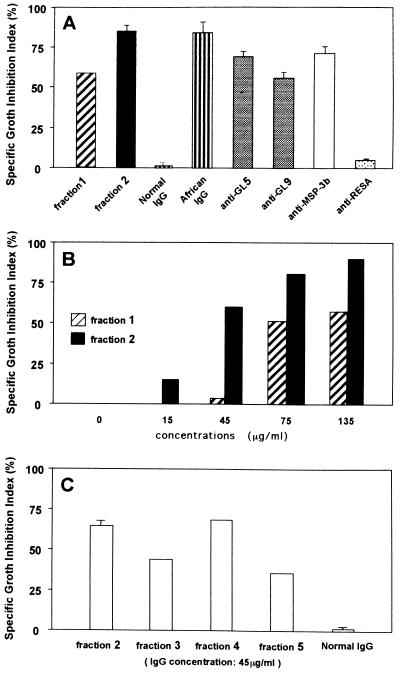
FIG. 4.
ADCI assay. (A) Shown are the means of the SGI (calculated as described in Materials and Methods) obtained with the following antibodies: fraction 1 (135 μg/ml) (number of independent experiments [n = 1) and fraction 2 (135 μg/ml) (n = 3); IgG (200 μg/ml) from normal French blood donors (n = 2), IgG (200 μg/ml) from hyperimmune African blood donors (n = 6), and a 1/10 solution in RPMI 1640 of the antibodies affinity purified on peptides GL5 (n = 3) and GL9 (n = 3) as described in Materials and Methods. Human affinity-purified antibodies on MSP-3b (n = 3) and RESA (n = 3), diluted 1/10 in RPMI 1640, were included as positive and negative controls, respectively (23). (B) Dose dependency of anti-GLURP antibodies in ADCI experiments. Shown is the SGI plotted versus the IgG concentration for fraction 1 (n = 3) and fraction 2 (n = 2). (C) Comparison of ADCI effects of the fractions 2 to 5. Shown are the means of the SGI of purified IgG adjusted to 45 μg/ml (fractions 2, 3, and 4) and 200 μg/ml (fraction 5 and normal French IgG). The experiments with fraction 2 and normal French IgG were performed twice.
In a second set of experiment using a separate antibody preparation, the ADCI assays were carried out after fraction 1 had been further fractionated into fraction 3, cross-reactive with both R0 and R2, and fraction 4, specific for the repetitive domain. All fractions obtained in this experiment were able to exert an ADCI effect (Fig. 4C). Thus, all anti-GLURP antibody preparations showed a consistent monocyte-dependent inhibition of parasite growth, whereas matched controls using antibodies alone without monocytes were all ineffective.
Distribution of Ig subclass levels.
The distribution of IgG subclass levels in total IgG and in fractions 2, 3, and 4 (Table 3) suggests that the proportion of cytophilic antibody (i.e., IgG1 and IgG3) relative to noncytophilic antibody (IgG2 and IgG4) could explain the stronger effect of anti-R0 (fraction 2) antibodies than of anti-R2 (fraction 4) antibodies.
TABLE 3.
Immunoglobulin subclass concentrations in total IgG and in fractions 2, 3, and 4
| Fraction | Concn (Ua)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IgG1
|
IgG2
|
IgG3
|
IgG4
|
|||||
| R0 | R2 | R0 | R2 | R0 | R2 | R0 | R2 | |
| Total IgG | 25 | 57 | 4.5 | 12 | 20 | 16 | 1.5 | 1.65 |
| Fraction | ||||||||
| 2 | 14 | 0 | 13 | 0 | 12 | 0 | 3.0 | 0 |
| 3 | 7.5 | 9 | 1.2 | 1.8 | 2.4 | 3.9 | 2.3 | 0.8 |
| 4 | 0 | 180 | 0 | 36 | 0 | 48 | 0 | 1.8 |
OD value transformed into arbitrary units, using a World Health Organization standard serum as the reference serum.
DISCUSSION
Clinical and epidemiological observations of human P. falciparum infections suggest that there exists a series of states of acquired protection. One can distinguish at least four stages: (i) the ability to control neurological complications, which is acquired rapidly; (ii) antidisease or antitoxic immunity which becomes progressively evident through childhood; (iii) isolate- or strain-specific antiparasite immunity; and (iv) strain-independent antiparasite immunity, or premunition (11). We have chosen to focus on the latter since it seems to represent the most effective immune resistance that humans have been shown to develop against the pathogenic asexual erythrocytic stage (20, 29). Of the various in vitro correlates of immunity which have been proposed to explain the role of antibody, such as inhibition of merozoite invasion, ADCI, inhibition of rosetting, inhibition of cytoadherence, and neutralization of toxic products inducing tumor necrosis factor, the first two are likely to have the most immediate effect on antiparasite immunity, i.e., the containment of parasite multiplication.
In this study, we investigated the potential involvement of human anti-GLURP antibodies, using an in vitro assay found to correlate with the premunition state. We demonstrated that highly purified antibodies had no significant direct inhibitory effect on merozoite invasion but were able to promote a strong monocyte-dependent inhibition of P. falciparum growth. These results are similar to those previously obtained with antibodies directed to another merozoite surface antigen, MSP3 (21, 23). The present study, therefore, lends support to our previous finding that (i) merozoite-associated molecules are important targets and (ii) indirect mechanisms such as ADCI probably are important in naturally acquired immunity (4). The results bring further indication in favor of evaluating the GLURP molecule for vaccine purposes (5). These results also suggest that in the perspective of reproducing the natural type of immunity called premunition, GLURP deserves to be evaluated for vaccine purposes.
In this investigation, we have chosen to study GLURP-specific antibodies purified from sera of clinically protected individuals rather than anti-GLURP antibodies obtained from immunized animals, in order to assess the potential role of various antibody specificities in naturally acquired protection. Considerable care was taken to achieve a high degree of purity during the affinity process and to assess the true specificity of the antibodies. Antibodies purified on either large recombinant or short synthetic peptides were all found after extensive analysis to react in a specific manner both with artificial (i.e., recombinant) and with native GLURP as assessed by IFA, Western blotting, and immunoprecipitation. Thus, GLURP was highly likely to be the sole target of the antibodies found effective in ADCI.
The pattern of reactivity of the different affinity-purified antibodies with the respective GLURP-derived antigens led to the identification of several distinct B-cell epitopes within GLURP. The peptides in GL5 and GL9 each define at least one B-cell epitope, since (i) antibodies to each peptide reacted only with the homologous sequence; (ii) both antibody preparations reacted with the R2 recombinant protein, which contains the repeats included in GL5 and in addition the nonrepetitive region included in GL9; (iii) neither antibody preparation reacted with recombinant R0, which does not contain the sequences; and (iv) conversely, anti-R0 (fraction 2) did not react with either of the two peptides. Comparison of the results obtained with fractions 2 and 3 suggested that the R0 region contains at least two B-cell epitopes, one displaying cross-reactivity with R2, but distinct from sequences GL5 and GL9, and another specific only for R0. In summary, the results provide evidence for the existence of (i) two distinct B-cell epitopes within R0, (ii) one B-cell epitope defined by the repeats contained in R2, (iii) one B-cell epitope contained in the R2 recombinant outside the repeat region (GL9), and (iv) a third epitope also contained in R2 and distinct from the repeat and from GL9 but cross-reactive with an epitope in R0. In addition, we have previously produced a mouse monoclonal antibody which defines a rodent B-cell epitope in the R1 region of GLURP and which, under our experimental conditions, did not react with R0 or R2. This would bring to five, and potentially six, the number of distinct B-cell epitopes in GLURP. Further epitope mapping will be needed to delineate more precisely the different regions recognized by human antibodies particularly within the R0 region.
The potential importance of the B-cell epitopes identified in the present study was demonstrated by the fact that antibodies to all of these epitopes were found able to promote a monocyte-dependent inhibition of parasite growth. This finding suggests that whatever the native conformation of GLURP and whatever the localized schizont/merozoite distribution of the molecule, the epitopes that we have defined are accessible to antibodies at some critical point during parasite development. Our data also suggest that repeat and nonrepeat epitopes in GLURP may not be equally valuable targets for antibodies effective in ADCI. Adjusted to similar antibody concentrations, the various affinity-purified antibodies displayed different reactivities with recombinant or synthetic peptides in ELISA and with native GLURP protein in IFA and also different ADCI-promoting activities in bioassays. At the same antibody concentration, or when adjusted to the same IFA endpoint titer on native protein, anti-R0 antibodies consistently exerted a greater ADCI effect than anti-R2 antibodies. Similarly, when considering the endpoint titer by IFA, anti-GL9, directed to a nonrepeat portion of the R2 recombinant, exerted a greater effect in ADCI than anti-GL5 directed to the repeats themselves.
In summary, our results suggest that GLURP epitopes defined by nonrepetitive sequences may be more effective as targets of ADCI antibodies and should therefore be preferred in future vaccine formulations. Furthermore, repetitive amino acid sequences from malarial antigens have been thought to act as a “smoke screen” to the immune system (1) or even to have an immunosuppressive effect (18). However, two different studies have also suggested that it may prove difficult to induce high-titer antibodies to the nonrepetitive GLURP epitopes. First, we have found a markedly higher prevalence of antibodies to R2 than to R0 in an area of Liberia where malaria is endemic (28), and second, results of immunization of mice and rabbits with either full-length GLURP or the R0, R1, or R2 recombinant protein absorbed on alum clearly showed that R2 was consistently more immunogenic in these species than either R1 or R0 (27, 28). However, by using the more powerful Freund’s complete adjuvant, high-titer antibodies to the nonrepetitive epitopes could be induced (28).
For vaccine development purposes, the question therefore arises as to whether epitopes that are good targets for ADCI-effective antibodies, but are suboptimally immunogenic, are to be preferred over epitopes which may require higher antibody concentrations to reach the same ADCI effect but are more immunogenic. Results of ongoing studies aimed at investigating the potential polymorphism of these regions may in part answer this question. Available preliminary data point to R0 as a well-conserved region. To define a vaccine strategy, a more detailed comparative analysis of the following three criteria will be needed: sequence conservation, immunogenicity, and antigenicity in terms of the biological effect in ADCI of the corresponding antibodies.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank I. Rosenstadt and M. Paulli Andersen for technical assistance.
This work was supported by the Scientific and Technological Cooperation with the Developing Countries program of the Commission of the European Communities contract CT 950021 and by the Research Center for Medical Biotechnology under the Danish Biotechnology Research and Development program. Soe Soe was supported by a fellowship from the RSWG committee of the UND/WB/WHO-tdr.
REFERENCES
- 1.Anders R F. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986;8:529–539. doi: 10.1111/j.1365-3024.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 2.Borre M B, Dziegiel M, Hogh B, Petersen E, Rieneck K, Riley E, Meis J F, Aikawa M, Nakamura K, Harada M, Wind A, Jakobsen P H, Cowland J, Jepsen S, Axelsen N H, Vuust J. Primary structure and localization of a conserved immunogenic Plasmodium falciparum glutamate rich protein (GLURP) expressed in both the preerythrocytic and erythrocytic stages of the vertebrate life cycle. Mol Biochem Parasitol. 1991;49:119–132. doi: 10.1016/0166-6851(91)90135-s. [DOI] [PubMed] [Google Scholar]
- 3.Bottius E, Guanzirolli A, Trape J-F, Rogier C, Konate L, Druilhe P. Malaria: even more chronic in nature than previously thought; evidence for submicroscopic parasitaemia detectable by polymerase chain reaction. Trans R Soc Trop Med Hyg. 1996;90:15–19. doi: 10.1016/s0035-9203(96)90463-0. [DOI] [PubMed] [Google Scholar]
- 4.Boudin C, Chumpitazi B, Dziegiel M, Peyron F, Picot S, Hogh B, Ambroise-Thomas P. Possible role of specific immunoglobulin M antibodies to Plasmodium falciparum antigens in immunoprotection of humans living in hyperendemic area, Burkina Faso. J Clin Microbiol. 1993;31:636–641. doi: 10.1128/jcm.31.3.636-641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun H, Attanah P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouharoun-Tayoun H, Druilhe P. P. falciparum malaria: evidence for an isotype imbalance which may be responsible for the delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahimi K, Pérignon J-L, Bossus M, Gras H, Tartar A, Druilhe P. Fast immunopurification of small amounts of specific antibodies on peptides bound to ELISA plates. J Immunol Methods. 1993;162:69–75. doi: 10.1016/0022-1759(93)90408-y. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, McGregor A, Carrington S. Gamma globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 10.Druilhe P, Khusmith S. Epidemiological correlation between levels of antibodies promoting merozoite phagocytosis of Plasmodium falciparum and malaria-immune status. Infect Immun. 1987;55:888–891. doi: 10.1128/iai.55.4.888-891.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Druilhe P, Pérignon J L. Mechanisms of defense against P. falciparum asexual blood stages in humans. Immunol Lett. 1994;41:115–120. doi: 10.1016/0165-2478(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Dziegiel M, Borre M B, Jepsen S, Högh B, Petersen E, Vuust J. Recombinant Plasmodium falciparum glutamate rich protein; purification and use in enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1991;44:306–313. doi: 10.4269/ajtmh.1991.44.306. [DOI] [PubMed] [Google Scholar]
- 13.Dziegiel M, Rowe P, Bennett S, Allen S J, Olerup O, Gottschau A, Borre M, Riley E M. Immunoglobulin M and G antibody responses to Plasmodium falciparum glutamate-rich protein: correlation with clinical immunity in Gambian children. Infect Immun. 1993;61:103–108. doi: 10.1128/iai.61.1.103-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman S L, Wistar R J, Ballou W R, Hollingdale M R, Wirtz R A, Schneider I, Marwoto H A, Hockmeyer W T. Immunity to malaria and naturally acquired antibodies to the circumsporozoite protein of Plasmodium falciparum. N Engl J Med. 1986;315:601–606. doi: 10.1056/NEJM198609043151001. [DOI] [PubMed] [Google Scholar]
- 15.Hogh B, Petersen E, Dziegiel M, David K H A, Borre M, Holm A, Vuvst J, Jepsen S. Antibodies to a recombinant glutamate-rich Plasmodium falciparum protein: evidence for protection of individuals living in a holoendemic area of Liberia. Am J Trop Med Hyg. 1992;46:307–313. doi: 10.4269/ajtmh.1992.46.307. [DOI] [PubMed] [Google Scholar]
- 16.Jepsen S. Inhibition of in vitro growth of Plasmodium falciparum by purified antimalarial human IgG antibodies. Isolation of target antigens from culture supernatant. Scand J Immunol. 1983;18:567–561. doi: 10.1111/j.1365-3083.1983.tb00893.x. [DOI] [PubMed] [Google Scholar]
- 17.Khusmith S, Druilhe P. Cooperation between antibodies and monocytes that inhibit in vitro proliferation of Plasmodium falciparum. Infect Immun. 1983;41:219–223. doi: 10.1128/iai.41.1.219-223.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Londono J A, Gras-Masse H, Dubeaux C, Tartar A, Druilhe P. Secondary structure and immunogenicity of hybrid synthetic peptides derived from two Plasmodium falciparum pre-erythrocytic antigens. J Immunol. 1990;145:1557–1563. [PubMed] [Google Scholar]
- 19.Lunel F, Druilhe P. Effector cells involved in nonspecific and antibody-dependent mechanisms directed against Plasmodium falciparum blood stages in vitro. Infect Immun. 1989;57:2043–2049. doi: 10.1128/iai.57.7.2043-2049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh K. Malaria—a neglected disease. Parasitology. 1992;104:S53–S69. doi: 10.1017/s0031182000075247. [DOI] [PubMed] [Google Scholar]
- 21.McColl D J, Silva A, Foley M, Kun J F, Favaloro J M, Thomson J K, Marshall V M, Coppel R L, Kemp D J, Anders R F. Molecular variation in a novel polymorphic antigen associated with Plasmodium falciparum merozoites. Mol Biochem Parasitol. 1994;68:53–67. doi: 10.1016/0166-6851(94)00149-9. [DOI] [PubMed] [Google Scholar]
- 22.Merrifield R B. Solid-phase peptide synthesis. The synthesis of a tetrapeptide. J Am Chem Soc. 1963;85:2149–2152. [Google Scholar]
- 23.Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, Bottius E, Kaidoh T, Aikawa M, Filgueira M-C, Tartar A, Druilhe P. MSP-3: a malaria protein inducing antibodies which promote Plasmodium falciparum killing in cooperation with blood monocytes. Blood. 1994;84:1594–1602. [PubMed] [Google Scholar]
- 24.Reese, R. T., S. G. Langreth, and W. Trager. 1979. Isolation of stages of the human parasite Plasmodium falciparum from culture and from animal blood. Bull. W. H. O. 53–67. [PMC free article] [PubMed]
- 25.Roper C, Elhassan I M, Hviid L, Giha H, Richardson W, Babiker H, Satti G, Theander T G, Arnot D E. Detection of very low level plasmodium falciparum infections using the nested polymerase chain reaction and a reassessment of the epidemiology of unstable malaria in Sudan. Am J Trop Med Hyg. 1996;54:325–331. doi: 10.4269/ajtmh.1996.54.325. [DOI] [PubMed] [Google Scholar]
- 26.Sergent E D, Parrot L. L’immunité, la prémunition et la résistance innée. Arch Inst Pasteur Alger. 1935;23:279–319. [Google Scholar]
- 27.Theisen M, Cox G, Hogh B, Jepsen S, Vuust J. Immunogenicity of the Plasmodium falciparum glutamate-rich protein expressed by vaccinia virus. Infect Immun. 1994;62:3270–3275. doi: 10.1128/iai.62.8.3270-3275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theisen M, Vuust J, Gottschau A, Jepsen S, Hogh B. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin Diagn Lab Immunol. 1995;2:30–34. doi: 10.1128/cdli.2.1.30-34.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trape J-F, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, Legros F, Badji A, Ndiaye G, Ndiaye P, Brahimi K, Faye O, Druilhe P, Pereira da Silva L. The Dielmo project. A longitudinal study of natural malaria infection in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–137. doi: 10.4269/ajtmh.1994.51.123. [DOI] [PubMed] [Google Scholar]