Abstract
Background
Women are overrepresented among individuals with post-acute sequelae of SARS-CoV-2 infection (PASC). Biological (sex) as well as sociocultural (gender) differences between women and men might account for this imbalance, yet their impact on PASC is unknown.
Aim
We assessed the impact of sex and gender on PASC in a Swiss population.
Method
Our multicentre prospective cohort study included 2,856 (46% women, mean age 44.2 ± 16.8 years) outpatients and hospitalised patients with PCR-confirmed SARS-CoV-2 infection.
Results
Among those who remained outpatients during their first infection, women reported persisting symptoms more often than men (40.5% vs 25.5% of men; p < 0.001). This sex difference was absent in hospitalised patients. In a crude analysis, both female biological sex (RR = 1.59; 95% CI: 1.41–1.79; p < 0.001) and a score summarising gendered sociocultural variables (RR = 1.05; 95% CI: 1.03–1.07; p < 0.001) were significantly associated with PASC. Following multivariable adjustment, biological female sex (RR = 0.96; 95% CI: 0.74–1.25; p = 0.763) was outperformed by feminine gender-related factors such as a higher stress level (RR = 1.04; 95% CI: 1.01–1.06; p = 0.003), lower education (RR = 1.16; 95% CI: 1.03–1.30; p = 0.011), being female and living alone (RR = 1.91; 95% CI: 1.29–2.83; p = 0.001) or being male and earning the highest income in the household (RR = 0.76; 95% CI: 0.60–0.97; p = 0.030).
Conclusion
Specific sociocultural parameters that differ in prevalence between women and men, or imply a unique risk for women, are predictors of PASC and may explain, at least in part, the higher incidence of PASC in women. Once patients are hospitalised during acute infection, sex differences in PASC are no longer evident.
Keywords: SARS-CoV-2; Gender; Sex; Women; Post-COVID-19, Long-COVID, Long-Haulers
Key public health message.
What did you want to address in this study?
Women are more often affected by post-COVID symptoms than men. We sought to assess whether biological (sex) or sociocultural (gender) differences between women and men account for this imbalance.
What have we learnt from this study?
Sociocultural parameters that differ between women and men are risk predictors of post-COVID symptoms and may explain the female propensity towards a higher risk of those.
What are the implications of your findings for public health?
Currently, international guidelines suggest an approach to treat post-COVID effects based on symptoms, however, our data imply that a tailored gender-sensitive approach of healthcare services may be required to support the needs of affected individuals.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can cause a prolonged disease course beyond acute COVID-19 [1]. The clinical presentation of these post-acute sequelae of SARS-CoV-2 infection (PASC) includes a variety of fluctuating and unpredictable somatic symptoms persisting even beyond 12 months after initial infection, thereby creating a rising healthcare and economic burden [2-4]. In fact, recent data from the United States (US) indicate a considerable impact of PASC on the labour market, with 2–4 million individuals (out of 16 million working-age Americans affected by PASC) being on sick leave [5], while data from the UK showed an increase of 0.5 million people being out of the labour market because of long-term sickness from 2019 to 2022 [6]. Worldwide, at least 65 million individuals are estimated to have PASC, with a daily increase in cases [7]. Despite substantial efforts to identify pathophysiological mechanisms and risk factors of PASC, current diagnostic and treatment options are insufficient in dealing with this condition.
Although mortality and morbidity, such as intensive care unit admission, from acute COVID-19 infection is substantially lower in women than men, women are overrepresented among patients with PASC [8-13]. Accordingly, factors increasing the risk of severe acute COVID-19, such as advanced age or male sex, do not also increase the risk of PASC [14]. The causes for the differential sex and gender distribution in acute vs chronic COVID-19 remain enigmatic. Factors beyond innate sex, such as sociocultural gender, have been widely ignored in analysing the causes of sex and gender disparities in COVID-19 outcomes [15], an omission that has been criticised by several institutions including the Canadian Institutes of Health Research and the European Parliament [16,17].
We sought to assess the impact of social context, gender and behaviours in addition to biological data on PASC in a large and well-characterised multi-centre cohort in Switzerland comprising both hospitalised patients and outpatients with confirmed SARS-CoV-2 infection.
Methods
Study design and procedures
Our study is based on data from patients of the Swiss COGEN cohort study, a prospective, observational cohort of individuals who were diagnosed with PCR-confirmed SARS-CoV-2 infection between February and December 2020 at one of four Swiss study sites. Eligible patients were adults aged ≥ 18 years at follow-up who survived acute COVID-19 infection, residing in Switzerland during primary SARS-CoV-2 infection, fluent in German, English, French or Italian and able to provide written informed consent. After a minimum follow-up time of 12 weeks (based on current definitions of PASC [1]), each participant was contacted by telephone and asked to complete a questionnaire either by phone, email or on paper. Of 5,938 patients, 3,005 individuals (patients directly (n = 2,996) or their legal representatives (n = 9)) completed the questionnaire after giving informed consent; we append a flowchart of patient selection in Supplementary Figure S1. We obtained clinical data and laboratory data from electronic medical records containing information about demographic characteristics (age, sex), cardiovascular risk factors (including diabetes mellitus, hypertension, dyslipidaemia, family history of coronary artery disease, smoking, and obesity), symptoms and date of symptom onset, medication, pre-existing comorbidities, data on weight and height, and disease severity of COVID-19 classified according to symptoms and necessity of in-hospital (normal ward, intermediate or intensive care) treatment. Data on vital signs, respiratory parameters and organ support measures were gathered within the first 24 h of and during hospitalisation (worst value/highest level of organ support). We analysed stored blood samples available from patients who consented to provide blood samples for biobank storage for circulating hormone levels (testosterone, oestradiol, progesterone and cortisol). These blood samples were drawn from hospitalised patients at the first day of hospitalisation.
Assessment of gender
Gender consists of four interrelated dimensions (definition provided by the World Health Organization [18] and the Women Health Research Network of the Canadian Institute of Health Research [19]) encompassing gender roles (e.g. child care), gender identity (a personal conception of oneself as man or woman), ‘gender relationships’ (e.g. social support), and ‘institutionalised gender’ (e.g. education level, personal income). Currently, there is no academic consensus on how to define the construct ‘gender’ as different approaches to operationalise gender have been proposed [20]. Consequently, there is no gold standard for a measure of gender. However, Pelletier et al. have previously introduced and validated a methodological approach where a composite gender score as a continuous variable between zero (behaviours typically ascribed to men) to 100 (behaviours typically ascribed to women) was applied to measure the effect of gender on health outcomes [21]. Their gender score represents a summary of multiple variables comprising the four dimensions of gender and, hence, a pragmatic instrument to measure gender. The fact that the score considers gender as a bipolar, one-dimensional continuum offers a methodological advantage over more complex instruments, as it allows to include only one variable in statistical models as opposed to multiple single variables, which may lower statistical power and make the interpretation of results more difficult. The gender score is based on the short version of a questionnaire (appended in Supplementary Figure S2), which comprises a number of gender-related items including employment status, perceived social standing, housework responsibility status, education level, social support, domestic stress level as well as the Bem sex-role inventory, a measure used to assess gender roles [21-23]. Given that gender variables are time- and context-sensitive, we chose this instrument as it has been applied and validated in Switzerland in recent years [21,23]. The gender-related variables were included in a previously described logistic regression model using biological sex as the dependent variable [21]. The identified gender-related variables served as predictors to estimate the ‘probability of an individual being a woman’ which was named gender score. The mean gender score in our study population was 46.2 ± 25.9. As gender and sex usually overlap [24], the gender score and biological sex were correlated in our study (Pearson r = 0.52; 95% CI: 0.49–0.55; p < 0.001), which is consistent with data (r = 0.62) reported previously by Pelletier et al. [21]
Statistical analyses
We defined the primary outcome measure of our analysis as the persistence of at least one COVID-19-related somatic symptom for at least 12 weeks after their first infection [1]. We performed biological sex comparisons using an independent t-test, a Mann–Whitney U test, or a chi-squared test, as appropriate. We applied a Cox proportional hazards regression with a robust variance estimator to estimate risk ratios (RR) by setting the follow-up time to a constant value to all subjects with a backward selection method to explore an association between factors and PASC [25]. A p value of less than 0.05 was used to select variables from univariate testing, and important variables such as age, sex and gender score were forced to remain in the model. Considering the multicollinearity between the gender score and the gender-sensitive sociocultural variables that were acquired to construct the gender score, we performed the multivariable analysis separately on two models. Model 1 included age, sex, gender score and other factors; Model 2 included age, sex, single features of the gender score (sociocultural variables) and other factors. After the final model was developed, we tested the interaction between sex and other variables in the model. If no interaction was presented, this term was removed from the model. We considered the Akaike information criterion (AIC) when comparing the model selection. Missing data in sociocultural and economic variables were less than 3% and can be assumed missing completely at random, therefore imputation was not performed in this study. Statistical testing was done within an exploratory framework at a two-sided significance level of α = 0.05. We performed all statistical tests using Stata IC15 (StataCorp, 2017, College Station, US).
Results
Patient baseline characteristics
The final study cohort comprised 2,856 individuals (1,307 (45.8%) female study participants and 1,549 (54.2%) male study participants, Table 1) and was stratified by biological sex and severity of acute illness (outpatients and hospitalised patients). The mean age (± standard deviation (SD)) of the overall study sample was 44.2 ± 16.8 years. Females were younger than males (42.7 ± 16.4 vs 45.4 ± 17.0 years) and had a lower body mass index (24.5 ± 5.2 vs 26.3 ± 4.4 kg/m2). The average number of cardiovascular risk factors was lower in females as compared with males. Accordingly, females less often had pre-existing cardiovascular disease than males (6.0% vs 13.7%), while females more often had mental disease (5.0% vs 3.4%) or autoimmune disorders (8.5% vs 5.0%). The average number of reported symptoms during primary infection was higher in females than males (5.3 ± 2.3 vs 4.4 ± 2.2; p < 0.001), with females reporting anosmia/dysosmia, ageusia/dysgeusia, gastrointestinal symptoms, dyspnoea or fatigue more often, while males more often presented with fever (Table 1). Notably, mean age, the frequency of comorbidities, cardiovascular risk factors and medications was higher in inpatients than outpatients (Table 1).
Table 1. Baseline characteristics of the study population. Stratification by biological sex and severity of acute illness (outpatients and hospitalised patients), Switzerland, February–December 2020 (n = 2,856).
| Overall | Outpatients | Inpatients | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n = 2,856 |
Male n = 1,549 |
Female n = 1,307 |
Male n = 1,201 |
Female n = 1,130 |
Male n = 348 |
Female n = 177 |
|||||||||||
| Distribution | Mean | SD | Mean | SD | Mean | SD | p value | Mean | SD | Mean | SD | p value | Mean | SD | Mean | SD | p value |
| Age (years) | 44.2 | 16.8 | 45.4 | 17.0 | 42.7 | 16.4 | <0.001 | 40.6 | 14.4 | 40.0 | 14.6 | 0.33 | 62.3 | 14.4 | 59.8 | 17.1 | 0.086 |
| BMI (kg/m2) | 25.5 | 4.8 | 26.3 | 4.4 | 24.5 | 5.2 | <0.001 | 25.8 | 4.1 | 23.9 | 4.7 | <0.001 | 28.0 | 4.7 | 28.5 | 6.3 | 0.35 |
| Number of CVRFs | 0.7 | 1.0 | 0.8 | 1.1 | 0.5 | 0.9 | <0.001 | 0.5 | 0.9 | 0.4 | 0.7 | <0.001 | 1.8 | 1.3 | 1.4 | 1.3 | 0.003 |
| Frequency | n | % | n | % | n | % | p value | n | % | n | % | p value | n | % | n | % | p value |
| Cardiovascular risk factors | |||||||||||||||||
| Hypertension | 601 | 21.0 | 410 | 26.5 | 191 | 14.6 | <0.001 | 185 | 15.4 | 102 | 9.0 | <0.001 | 225 | 64.7 | 89 | 50.3 | 0.001 |
| Dyslipidaemia | 295 | 10.3 | 213 | 13.8 | 82 | 6.3 | <0.001 | 85 | 7.1 | 34 | 3.0 | <0.001 | 128 | 36.8 | 48 | 27.1 | 0.027 |
| Diabetes mellitus | 214 | 7.5 | 158 | 10.2 | 56 | 4.3 | <0.001 | 58 | 4.8 | 21 | 1.9 | <0.001 | 100 | 28.7 | 35 | 19.8 | 0.026 |
| Family history of CAD | 53 | 1.9 | 36 | 2.3 | 17 | 1.3 | 0.043 | 10 | 0.8 | 10 | 0.9 | 0.89 | 26 | 7.5 | 7 | 4.0 | 0.12 |
| Present smoking | 210 | 7.4 | 124 | 8.0 | 86 | 6.6 | 0.15 | 102 | 8.5 | 78 | 6.9 | 0.15 | 22 | 6.3 | 8 | 4.5 | 0.40 |
| Obesity (BMI ≥ 30 kg/m2) | 526 | 18.4 | 306 | 19.8 | 220 | 16.8 | 0.045 | 182 | 15.2 | 153 | 13.5 | 0.27 | 124 | 35.6 | 67 | 37.9 | 0.62 |
| Pre-existing conditions | |||||||||||||||||
| Mental disorder | 119 | 4.2 | 53 | 3.4 | 66 | 5.0 | 0.030 | 28 | 2.3 | 30 | 2.7 | 0.62 | 25 | 7.2 | 36 | 20.3 | <0.001 |
| Autoimmune/rheumatoid disease | 189 | 6.6 | 78 | 5.0 | 111 | 8.5 | <0.001 | 35 | 2.9 | 75 | 6.6 | <0.001 | 43 | 12.4 | 36 | 20.3 | 0.016 |
| Chronic pulmonary disease | 285 | 10.0 | 147 | 9.5 | 138 | 10.6 | 0.34 | 77 | 6.4 | 101 | 8.9 | 0.022 | 70 | 20.1 | 37 | 20.9 | 0.83 |
| Neurological disease | 142 | 5.0 | 73 | 4.7 | 69 | 5.3 | 0.49 | 23 | 1.9 | 41 | 3.6 | 0.011 | 50 | 14.4 | 28 | 15.8 | 0.66 |
| Cancer | 133 | 4.7 | 82 | 5.3 | 51 | 3.9 | 0.079 | 31 | 2.6 | 27 | 2.4 | 0.77 | 51 | 14.7 | 24 | 13.6 | 0.73 |
| Cardiovascular disease | 291 | 10.2 | 212 | 13.7 | 79 | 6.0 | <0.001 | 79 | 6.6 | 41 | 3.6 | 0.001 | 133 | 38.2 | 38 | 21.5 | <0.001 |
| Medications before COVID-19 | |||||||||||||||||
| Cardiovascular drugs | 531 | 18.6 | 361 | 23.3 | 170 | 13.0 | <0.001 | 154 | 12.8 | 91 | 8.1 | <0.001 | 207 | 59.5 | 79 | 44.6 | 0.001 |
| Asthma treatment | 123 | 4.3 | 62 | 4.0 | 61 | 4.7 | 0.38 | 33 | 2.7 | 44 | 3.9 | 0.12 | 29 | 8.3 | 17 | 9.6 | 0.63 |
| Analgesics | 298 | 10.4 | 124 | 8.0 | 174 | 13.3 | <0.001 | 71 | 5.9 | 128 | 11.3 | <0.001 | 53 | 15.2 | 46 | 26.0 | 0.003 |
| Immunosuppressive medication | 69 | 2.4 | 33 | 2.1 | 36 | 2.8 | 0.28 | 13 | 1.1 | 23 | 2.0 | 0.062 | 20 | 5.7 | 13 | 7.3 | 0.48 |
| Antidepressants | 73 | 2.6 | 28 | 1.8 | 45 | 3.4 | 0.006 | 21 | 1.7 | 32 | 2.8 | 0.080 | 7 | 2.0 | 13 | 7.3 | 0.003 |
| Thyroid hormones | 65 | 2.3 | 8 | 0.5 | 57 | 4.4 | <0.001 | 7 | 0.6 | 48 | 4.2 | <0.001 | 1 | 0.3 | 9 | 5.1 | <0.001 |
| Antiandrogens | 11 | 0.4 | 11 | 0.7 | 0 | 0.0 | 0.002 | 8 | 0.7 | 0 | 0.0 | 0.006 | 3 | 0.9 | 0 | 0.0 | 0.22 |
| Symptoms at first infection | |||||||||||||||||
| Anosmia/dysosmia | 1,523 | 53.3 | 729 | 47.1 | 794 | 60.7 | <0.001 | 607 | 50.5 | 717 | 63.5 | <0.001 | 122 | 35.1 | 77 | 43.5 | 0.059 |
| Fever | 1,515 | 53.0 | 872 | 56.3 | 643 | 49.2 | <0.001 | 636 | 53.0 | 530 | 46.9 | 0.003 | 236 | 67.8 | 113 | 63.8 | 0.36 |
| Gastrointestinal symptoms | 556 | 19.5 | 235 | 15.2 | 321 | 24.6 | <0.001 | 143 | 11.9 | 256 | 22.7 | <0.001 | 92 | 26.4 | 65 | 36.7 | 0.015 |
| Dyspnoea | 709 | 24.8 | 345 | 22.3 | 364 | 27.9 | <0.001 | 171 | 14.2 | 270 | 23.9 | <0.001 | 174 | 50.0 | 94 | 53.1 | 0.50 |
| Cough | 1,362 | 47.7 | 745 | 48.1 | 617 | 47.2 | 0.64 | 558 | 46.5 | 511 | 45.2 | 0.55 | 187 | 53.7 | 106 | 59.9 | 0.18 |
| Fatigue | 2,076 | 72.7 | 1,055 | 68.1 | 1,021 | 78.1 | <0.001 | 813 | 67.7 | 886 | 78.4 | <0.001 | 242 | 69.5 | 135 | 76.3 | 0.11 |
| Ageusia/dysgeusia | 1,490 | 52.2 | 712 | 46.0 | 778 | 59.5 | <0.001 | 576 | 48.0 | 692 | 61.2 | <0.001 | 136 | 39.1 | 86 | 48.6 | 0.037 |
BMI: body mass index; CAD: coronary artery disease; CVRF: cardiovascular risk factors; SD: standard deviation.
Socioeconomic characteristics
Gender differences in education level were most pronounced in hospitalised patients, with men in general having obtained a higher educational qualification than women (Table 2). In general, women were more often single parents than men (10.4% vs 5.8%), were more often divorced/separated (10.7% vs 7.9%) or widowed (3.4% vs 1.9%) and lived less often in a partnership than men (66.3% vs 69.1%). Men earned more often the highest income in the household (50.8% vs 19.4%), while women were more often the main person responsible for household work (39.3% vs 15.8%), had a higher responsibility for childcare/care of family members than men (score 0–6: 1.9 ± 2.3 vs 1.7 ± 2.1) and reported a higher stress level at home than men (score 0–10: 3.7 ± 2.3 vs 3.1 ± 2.0). Women more often lived alone than men (21.1% vs 18.4%). The Bem score, a measure used to assess masculine gender roles [22], was significantly higher in men than in women (5.0 ± 1.0 vs 4.9 ± 0.9). As expected, the summary gender score (0–100 with 100 being behaviours typically ascribed to women), containing all the above variables, was significantly higher in women than in men (60.8 ± 23.0 vs 33.7 ± 21.4, Table 2).
Table 2. Socioeconomic characteristics of the study population. Stratification by biological sex and severity of acute illness (outpatients and hospitalised patients), Switzerland, February–December 2020 (n = 2,856).
| Sociocultural-and economic variablesa | Overall | Outpatient | Inpatients | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n = 2,856 |
Male n = 1,549 |
Female n = 1,307 |
p value | Male n = 1,201 |
Female n = 1,130 |
p value | Male n = 348 |
Female n = 177 |
p value | ||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||||
| Healthcare worker | |||||||||||||||||
| Yes | 564 | 19.9 | 176 | 11.4 | 388 | 29.9 | <0.001 | 155 | 12.9 | 358 | 31.9 | <0.001 | 21 | 6.1 | 30 | 17.0 | <0.001 |
| No | 2,277 | 80.1 | 1,367 | 88.6 | 910 | 70.1 | 1,043 | 87.1 | 764 | 68.1 | 324 | 93.9 | 146 | 83.0 | |||
| Education | |||||||||||||||||
| No education qualification | 181 | 6.4 | 97 | 6.3 | 84 | 6.4 | 0.13 | 60 | 5.0 | 47 | 4.2 | 0.041 | 37 | 10.8 | 37 | 21.1 | <0.001 |
| Primary education | 197 | 6.9 | 91 | 5.9 | 106 | 8.1 | 58 | 4.9 | 79 | 7.0 | 33 | 9.6 | 27 | 15.4 | |||
| Secondary education/vocational degree | 1,126 | 39.7 | 610 | 39.7 | 516 | 39.6 | 428 | 35.9 | 435 | 38.6 | 182 | 52.9 | 81 | 46.3 | |||
| University or technical college degree | 1,335 | 47.0 | 738 | 48.0 | 597 | 45.8 | 646 | 54.2 | 567 | 50.3 | 92 | 26.7 | 30 | 17.1 | |||
| Marital status | |||||||||||||||||
| Married/partnership | 1,924 | 67.8 | 1,061 | 69.1 | 863 | 66.3 | 0.003 | 800 | 67.1 | 751 | 66.7 | 0.011 | 261 | 76.1 | 112 | 63.6 | <0.001 |
| Divorced/separated | 260 | 9.2 | 121 | 7.9 | 139 | 10.7 | 85 | 7.1 | 116 | 10.3 | 36 | 10.5 | 23 | 13.1 | |||
| Single | 580 | 20.4 | 324 | 21.1 | 256 | 19.7 | 295 | 24.7 | 241 | 21.4 | 29 | 8.5 | 15 | 8.5 | |||
| Widowed | 73 | 2.6 | 29 | 1.9 | 44 | 3.4 | 12 | 1.0 | 18 | 1.6 | 17 | 5.0 | 26 | 14.8 | |||
| Parenthood | |||||||||||||||||
| Two-parent family | 1,648 | 58.3 | 957 | 62.5 | 691 | 53.4 | <0.001 | 690 | 57.9 | 579 | 51.4 | <0.001 | 267 | 78.8 | 112 | 66.3 | 0.006 |
| Single-parent family | 224 | 7.9 | 89 | 5.8 | 135 | 10.4 | 67 | 5.6 | 113 | 10.0 | 22 | 6.5 | 22 | 13.0 | |||
| No children | 954 | 33.8 | 485 | 31.7 | 469 | 36.2 | 435 | 36.5 | 434 | 38.5 | 50 | 14.7 | 35 | 20.7 | |||
| Income | |||||||||||||||||
| Earns highest income in household | 1,028 | 36.4 | 775 | 50.8 | 253 | 19.4 | <0.001 | 584 | 49.0 | 227 | 20.1 | <0.001 | 191 | 57.4 | 26 | 14.9 | <0.001 |
| Earns lowest income in household | 804 | 28.5 | 247 | 16.2 | 557 | 42.8 | 208 | 17.4 | 470 | 41.7 | 39 | 11.7 | 87 | 50.0 | |||
| Equal between partners | 439 | 15.5 | 222 | 14.6 | 217 | 16.7 | 164 | 13.8 | 188 | 16.7 | 58 | 17.4 | 29 | 16.7 | |||
| Lives alone | 555 | 19.6 | 281 | 18.4 | 274 | 21.1 | 236 | 19.8 | 242 | 21.5 | 45 | 13.5 | 32 | 18.4 | |||
| Main person responsible for household work | |||||||||||||||||
| No | 681 | 24.1 | 515 | 33.7 | 166 | 12.8 | <0.001 | 372 | 31.2 | 145 | 12.9 | <0.001 | 143 | 42.3 | 21 | 12.0 | <0.001 |
| Yes | 751 | 26.6 | 241 | 15.8 | 510 | 39.3 | 196 | 16.5 | 433 | 38.6 | 45 | 13.3 | 77 | 44.0 | |||
| Equal distribution between partners | 952 | 33.7 | 550 | 36.0 | 402 | 31.0 | 435 | 36.5 | 352 | 31.3 | 115 | 34.0 | 50 | 28.6 | |||
| Single household | 443 | 15.7 | 223 | 14.6 | 220 | 16.9 | 188 | 15.8 | 193 | 17.2 | 35 | 10.4 | 27 | 15.4 | |||
| Other gender variables | Mean | SD | Mean | SD | Mean | SD | p value | Mean | SD | Mean | SD | p value | Mean | SD | Mean | SD | p value |
| Main responsibility for childcare/care of family members (score 0–6) | 1.8 | 2.2 | 1.7 | 2.1 | 1.9 | 2.3 | 0.004 | 1.7 | 2.1 | 1.9 | 2.3 | 0.056 | 1.6 | 2.0 | 2.2 | 2.2 | 0.004 |
| Average domestic stress level (score 0–10) | 3.4 | 2.2 | 3.1 | 2.0 | 3.7 | 2.3 | <0.001 | 3.1 | 2.0 | 3.7 | 2.3 | <0.001 | 2.8 | 2.0 | 3.8 | 2.5 | <0.001 |
| Bem score [22] | 4.9 | 1.0 | 5.0 | 1.0 | 4.9 | 0.9 | <0.001 | 5.0 | 0.9 | 4.9 | 0.9 | 0.003 | 4.9 | 1.1 | 4.6 | 1.2 | 0.007 |
| Gender score (0 = masculine, 100 = feminine) [23] | 46.2 | 25.9 | 33.7 | 21.4 | 60.8 | 23.0 | <0.001 | 34.7 | 21.2 | 60.2 | 23.1 | <0.001 | 29.8 | 22.1 | 65.0 | 21.9 | <0.001 |
Bem score: measure used to assess masculine gender roles; SD: standard deviation.
a Sociocultural-and economic variables have missing data between 0.6% and 2.3%, and the missing data in the gender score was 10%.
Acute disease characteristics in hospitalised individuals
Among hospitalised patients (n = 525 (18.4% of total study population), n = 177 (33.7%) females), routine laboratory markers of inflammation including C-reactive protein level, procalcitonin, neutrophil:lymphocyte ratio and ferritin levels were all higher in males than in females during primary infection (Table 3). Similarly, males had higher levels of indicators of organ injury such as creatinine, liver transaminases, cardiac biomarkers and lactate levels than females, and experienced more often than females respiratory, renal, thromboembolic or neurological complications (Table 3). Notably, males obtained more often anti-inflammatory or antiviral treatment such as corticosteroids (41.7% vs 32.2%; p = 0.035) or remdesivir (25.9% vs 18.1%; p = 0.046, Table 3).
Table 3. Acute disease characteristics in hospitalised individuals. Stratification by biological sex, Switzerland, February–December 2020 (n = 525) .
| Total n = 525 |
Male n = 348 |
Female n = 177 |
|||||
|---|---|---|---|---|---|---|---|
| Clinical parameters at first day in-hospital | Mean | SD | Mean | SD | Mean | SD | p value |
| CURB-65a | 1.7 | 1.3 | 1.7 | 1.3 | 1.6 | 1.3 | 0.26 |
| MAP (mmHg) | 79.6 | 14.7 | 79.8 | 14.7 | 79.3 | 14.8 | 0.73 |
| Heart rate (beats/minute) | 90.0 | 24.4 | 90.2 | 24.4 | 89.5 | 24.4 | 0.74 |
| Respiratory rate (breaths/minute) | 25.5 | 8.1 | 25.7 | 8.6 | 25.2 | 7.2 | 0.49 |
| P/F ratio (mmHg) | 277.5 | 115.1 | 269.3 | 114.3 | 294.1 | 115.4 | 0.021 |
| Oxygen saturation (SpO2) (%) | 90.4 | 5.8 | 90.1 | 5.8 | 91.1 | 5.8 | 0.073 |
| Body temperature (°C) | 38.0 | 1.0 | 38.0 | 1.0 | 37.8 | 0.9 | 0.005 |
| Disease course of acute COVID-19 | n | % | n | % | n | % | p value |
| Respiratory complications | 412 | 78.5 | 285 | 81.9 | 127 | 71.8 | 0.008 |
| Invasive ventilation | 123 | 23.4 | 89 | 25.6 | 34 | 19.2 | 0.10 |
| Haemodynamic support | 126 | 24.0 | 92 | 26.4 | 34 | 19.2 | 0.067 |
| Cardiac complications | 70 | 13.3 | 50 | 14.4 | 20 | 11.3 | 0.33 |
| Renal complications | 93 | 17.7 | 70 | 20.1 | 23 | 13.0 | 0.043 |
| Thromboembolic complications | 50 | 9.5 | 41 | 11.8 | 9 | 5.1 | 0.013 |
| Neurological complications | 84 | 16.0 | 66 | 19.0 | 18 | 10.2 | 0.009 |
| Medical treatment of acute COVID-19 | n | % | n | % | n | % | p value |
| Corticosteroids | 202 | 38.5 | 145 | 41.7 | 57 | 32.2 | 0.035 |
| Ritonavir/lopinavir | 77 | 14.7 | 54 | 15.5 | 23 | 13.0 | 0.44 |
| Remdesivir | 122 | 23.2 | 90 | 25.9 | 32 | 18.1 | 0.046 |
| Tocilizumab | 43 | 8.2 | 33 | 9.5 | 10 | 5.6 | 0.13 |
| Chloroquine/hydroxychloroquine | 122 | 23.2 | 85 | 24.4 | 37 | 20.9 | 0.37 |
| Slow onset of acute COVID-19 | 244 | 46.5 | 155 | 44.5 | 89 | 50.3 | 0.21 |
| Laboratory results at first day in-hospital | Median | IQR | Median | IQR | Median | IQR | p value |
| Leucocytes (G/L) | 6.7 | 5–9 | 6.7 | 5–9 | 6.6 | 5–9 | 0.70 |
| Lymphocytes (%) | 15.2 | 9–23 | 13.8 | 8–21 | 17.8 | 11–27 | <0.001 |
| Neutrophils (%) | 75.0 | 66–83 | 76.3 | 68–84 | 72.1 | 61–81 | 0.003 |
| Ratio neutrophils:lymphocytes | 5.0 | 2.9–9.6 | 5.6 | 3–10 | 4.2 | 2–8 | 0.002 |
| CRP (mg/L) | 58.0 | 21–123 | 62.0 | 24–126 | 43.2 | 16–115 | 0.020 |
| Procalcitonin (µg/L) | 0.1 | 0.1–0.3 | 0.1 | 0.1–0.3 | 0.1 | 0.1–0.2 | <0.001 |
| Haemoglobin (lowest value) (g/L) | 133.0 | 119–144 | 135.5 | 124–148 | 127.0 | 116–138 | <0.001 |
| Fibrinogen (g/L) | 198.5 | 149–266 | 190.0 | 138–252 | 217.0 | 165–276 | <0.001 |
| ALAT (U/L) | 33.0 | 23–52 | 35.5 | 25–54 | 29.5 | 19–45 | <0.001 |
| ASAT (U/L) | 40.0 | 28–56 | 42.0 | 29–60 | 36.0 | 26–49 | 0.004 |
| Bilirubin (µmol/L) | 8.0 | 6–11 | 8.7 | 7–12 | 7.2 | 4–10 | <0.001 |
| Creatinine (µmol/L) | 82.0 | 67–103 | 88.0 | 75–108 | 68.0 | 57–86 | <0.001 |
| Fibrinogen (g/L) | 4.9 | 3.8–6.1 | 4.8 | 4–6 | 5.2 | 4–7 | 0.33 |
| Troponin-T (ng/L) | 12.0 | 7.0–24.0 | 13.0 | 8–26 | 10.0 | 5–22 | 0.007 |
| Creatine kinase (U/L) | 86.5 | 52–178 | 110.5 | 61–203 | 61.5 | 44–119 | <0.001 |
| Lactate dehydrogenase (U/L) | 346.0 | 259–472 | 361.0 | 268–505 | 318.0 | 245–454 | 0.017 |
| Hormone levelsb | n = 256 | n = 157 | n = 99 | p value | |||
| Mean | SD | Mean | SD | Mean | SD | ||
| Cortisol (nmol/L) | 422.9 | 293.9 | 422.1 | 303.7 | 424.2 | 279.3 | 0.95 |
| Oestradiol (E2) (pmol/L) | 126.2 | 146.6 | 107.0 | 80.4 | 157.3 | 211.4 | 0.009 |
| Testosterone (nmol/L) | 4.0 | 5.1 | 5.9 | 5.6 | 0.9 | 1.3 | <0.001 |
| Progesterone (ng/mL) | 0.8 | 1.3 | 0.8 | 1.3 | 0.8 | 1.3 | 0.94 |
| Ratio testosterone:oestradiol | 4.8 | 5.7 | 6.8 | 6.2 | 1.1 | 1.2 | <0.001 |
ALAT: alanine aminotransferase; ASAT: aspartate aminotransferase; CRP: C-reactive protein; IQR: interquartile range; MAP: mean arterial pressure; P/F ratio: PaO2/FiO2 ratio; SD: standard deviation.
a CURB-65: confusion, urea nitrogen, respiratory rate, blood pressure, ≥ 65 years.
b Assessed only in patients who agreed to provide samples for hormone measurement.
Hormone levels
Among patients who agreed to provide blood samples for hormone measurements (n = 256, n = 99 (38.7%) females), cortisol or progesterone levels did not differ significantly between females and males, while the testosterone:oestradiol ratio was significantly higher in males as compared with females (p < 0.001) (Table 3).
Post-acute sequelae of SARS-CoV-2 infection: prevalence and type of symptoms
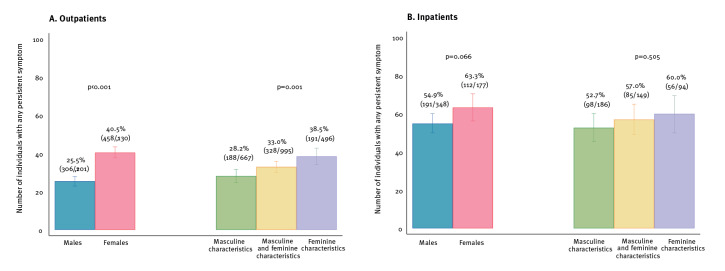
During a mean follow-up time of 203 ± 76 days (males: 200 ± 76 days vs females: 206 ± 77 days) 1,067 (37.4%) individuals reported at least one somatic symptom that persisted beyond 12 weeks following primary infection. The prevalence of PASC was higher in hospitalised patients than in outpatients (57.7% vs 32.8%; p < 0.001). Among outpatients, females reported more often than males at least one persistent somatic symptom (40.5% vs 25.5%; p < 0.001). Similarly, the prevalence of PASC was higher in outpatients scoring within the highest tertile of the gender score (= feminine characteristics) as compared with lower tertiles (p = 0.001) (Figure 1A). However, these significant sex and gender differences were no longer evident in patients who had been hospitalised during primary infection (Figure 1B).
Figure 1.
Percentage of patients reporting any persisting symptom following acute COVID-19 disease, either mild (A) or moderate/severe (B), Switzerland, June 2020–June 2021 (n = 2,856)
Data are stratified by sex as a binary variable (left) or tertiles of gender (right). Data are presented as incidence rates and 95% confidence interval.
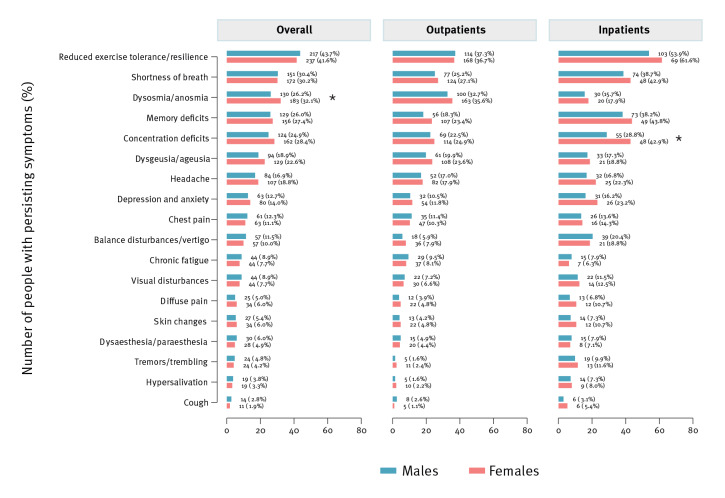
The most frequently reported PASC symptom was reduced exercise tolerance and resilience in both sexes, which was reported by 43.7% of males and 41.6% of females (p = 0.49), followed by shortness of breath (30.4% of males and 30.2% of females; p = 0.94) and dysosmia/anosmia (26.2% of males and 32.1% of females; p = 0.033, Figure 2). No significant sex difference in the type of PASC symptoms, except for dysosmia/anosmia, was seen in the overall cohort and outpatients. Concentration deficits were more often reported by female inpatients as compared with male inpatients (42.9% vs 28.8%; p = 0.013, Figure 2).
Figure 2.
Persistent symptoms reported at follow-up stratified by sex and symptom, Switzerland, June 2020–June 2021 (n = 2,856)
Data are presented as percentage of patients reporting persistent symptoms.
Predictors of post-acute sequelae of SARS-CoV-2 infection: sex vs gender
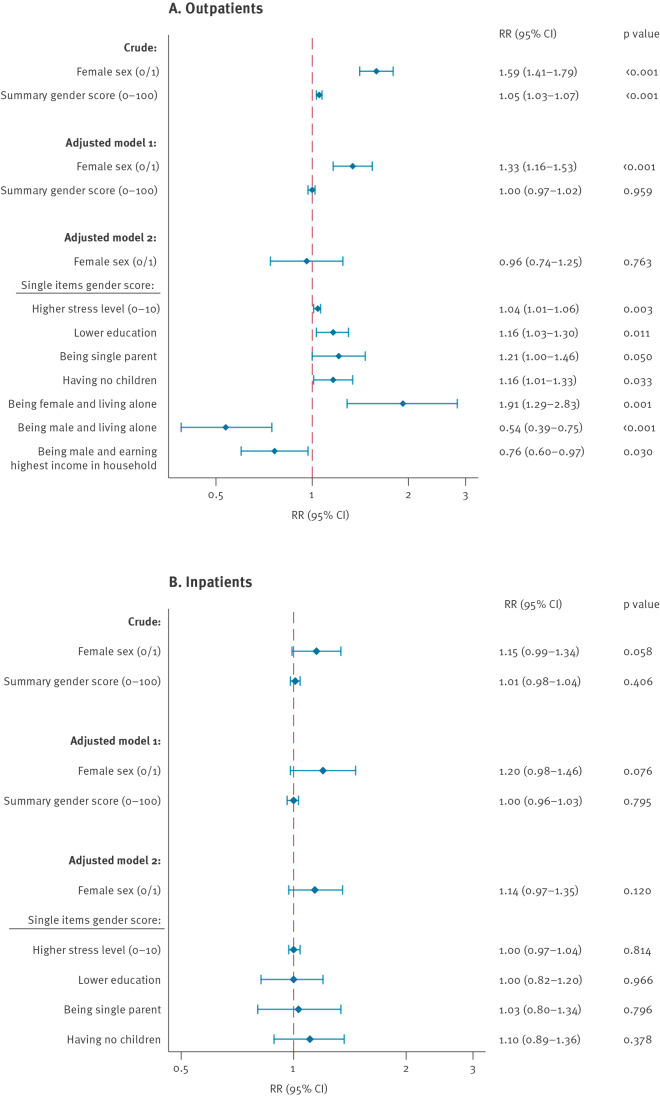
In a crude analysis, both female sex (RR = 1.59; 95% CI: 1.41–1.79; p < 0.001) and the gender score (RR = 1.05; 95% CI: 1.03–1.07; p < 0.001) were significantly associated with PASC in outpatients (Figure 3A). In outpatients, following multivariable adjustment, biological female sex (RR = 1.33; 95% CI: 1.16–1.53; p < 0.001), but not the gender score, remained a significant predictor of PASC (Model 1, Figure 3A). However, when the single features, instead of the summary gender score, were introduced into the model, biological sex (RR = 0.96; 95% CI: 0.74–1.25; p = 0.763) was outperformed by gender-related factors such as a higher stress level (RR = 1.04; 95% CI: 1.01–1.06; p = 0.003), lower education (RR = 1.16; 95% CI: 1.03–1.30; p = 0.011), being female and living alone (RR = 1.91; 95% CI: 1.29–2.83; p = 0.001) or having no children (RR = 1.16; 95% CI: 1.01–1.33; p = 0.033, Model 2, Figure 3A). In addition, we observed a trend towards a higher risk for PASC for being single parent (RR = 1.21; 95% CI: 1.00–1.46; p = 0.050). Conversely, being male and living alone (RR = 0.54; 95% CI: 0.39–0.75; p < 0.001) or earning the highest income in the household (RR = 0.76; 95% CI:0.60–0.97; p = 0.030) were independently associated with a lower risk of PASC (Figure 3A).
Figure 3.
Risk and protective factors associated with any persistent symptom following SARS-CoV-2 infection in outpatients (A) and hospitalised patients (B), Switzerland, February 2020–June 2021 (n = 2,856)
CI: confidence interval; RR: risk ratio.
Forest plots derived from Cox proportional hazards regression models depicting risk ratios and 95% confidence intervals. For the gender score the risk per unit changes by 10 points.
In hospitalised patients, neither sex nor gender were associated with the occurrence of PASC (Figure 3B). The full models for both outpatients and inpatients are provided in Supplementary Tables S3 and S4. Notably, despite being independently associated with the occurrence of PASC, the RRs presented in the outpatient model were relatively small, except for the interaction variable ‘being female and living alone’ (RR = 1.91). In addition, neither age nor sex hormone levels or their ratios were significant predictors of PASC in our models (Supplementary Table S4). Finally, there was no association between hormone intake/replacement (hormonal contraception in 278 women, postmenopausal hormone replacement in 35 women, fertility treatments in nine women, regular testosterone intake in 22 men, daily intake of phytoestrogens in 69 women and 33 men) or hormone deprivation therapies (anti-oestrogen therapy for breast/gynaecological cancer in 17 women, anti-androgenic treatment for prostate cancer in five men) and PASC in our study (data not shown).
Discussion
Our study reports that, unlike biological sex, sociocultural parameters, that differ in prevalence between women and men, were risk predictors of PASC and may explain, at least in part, the female propensity towards a higher risk of PASC, despite their lower risk of severe acute illness. Independent risk predictors of PASC in both sexes comprise a higher stress level, lower education, being a single parent or having no children. We identified ‘living alone’ as a PASC predictor unique to women, while earning the highest income in the household was a protective factor unique to men. We also demonstrate that, once patients were hospitalised during SARS-CoV-2 infection, sex differences in the incidence of PASC were no longer evident.
Our study adds to increasing evidence indicating that COVID-19 sex disparities cannot solely be explained by sex-specific biological mechanisms and are also explained by gendered patterns in contextual factors [26]. In line with this notion, age-related changes in hormone status, hormone replacement or hormone deprivation therapies were not associated with PASC in either sex in our study, although it has recently been proposed that the symptoms of PASC may overlap with those of perimenopause [27]. Similarly, psychosocial and behavioural factors and their interaction with sex (e.g. living alone and being female) remained among the strongest predictors of PASC, even when a large amount of biological variables derived from our well-characterised study cohort were included in the model. Consistent with our conclusion, increasing evidence suggests that substantial variation in the magnitude and direction of COVID-19 sex disparities exists across geographical localities, among racial and ethnic groups and over time, all of which indicate that the analysis of gendered contextual factors might offer important insights into outcomes and should be considered alongside sex differences as COVID-19 research moves forward [26,28,29].
In our study population the reported domestic stress level was significantly higher in women than in men. This observation is consistent with previous reports indicating that the burden of psychosocial stress has increased more in women than in men during the pandemic. Indeed, women have been disproportionately affected by imposed quarantine and lockdown measures given that typical feminine roles such as parenting, home-schooling and other caring duties are still predominantly assumed by women [30]. Isolation at home measures along with financial and security concerns can put an additional strain on women, who, more often than men, led single parent families, lived alone or had a lower education level in our study. Although lower education or increased domestic stress were significant predictors of PASC in both men and women in our study, the higher prevalence of these risk factors in the female study population might mirror not only the vulnerable socioeconomic positions of women during the pandemic but also highlights that their ability to return to work might be further impeded by the chronicity of symptoms of PASC. However, while the link between these variables and the occurrence of PASC provides important information, it is notable that sociocultural gender consists of intertwined dimensions [31]. Hence, it has to be taken into account that single variables of the gender score cannot reflect the multiple dimensions provided by the gender score and might be seen as simple sociodemographic variables.
The reasons for the differential impact of sex and gender in outpatients vs hospitalised patients can only be hypothesised, but might be attributed to the substantial differences in baseline characteristics between hospitalised patients and outpatients. In fact, compared with outpatients, hospitalised patients were significantly older (61.5 ± 15.4 years vs 40.3 ± 14.5 years in outpatients) and had more frequently comorbidities and cardiovascular risk factors. As gender roles and attributes largely depend on age and generation, the age difference between in- and outpatients might have impacted the association between gender-related factors and study outcomes. The high prevalence of comorbidities was seen in both female and male hospitalised patients resulting in a more homogenous study population as compared with outpatients, where sex differences were more obvious. Accordingly, variables such as invasive ventilation during acute illness, known hypertension, or specific symptoms at presentation for acute COVID-19 were all better predictors for PASC in hospitalised patients than sex or gender and might drive their risk for long-term consequences or the disease. Our differential results in hospitalised vs outpatients also support the hypothesis that PASC in hospitalised individuals might have different aetiologies than PASC in outpatients and may resemble post-hospital-syndrome or post-intensive-care-syndrome (PICS). Nevertheless, it is notable that the vast majority of PASC patients were younger (mean age: 47 years) and were only mildly ill during their first infection, thereby imposing a substantial burden on healthcare systems and economies.
The strength of our analysis consists of its near-complete, geographically defined cohort with availability of more than 200 clinical, laboratory, socioeconomic and psychosocial variables, the capture of a wide spectrum of post-COVID-19 symptoms, the availability of SARS-CoV-2 swab test results in all study participants and the multicentre design permitting to include both outpatients with mild disease as well as hospitalised patients. Data characterising primary infection were collected during ambulatory visits or hospitalisation, thereby minimising recall bias. However, our study also has several limitations related to its cross-sectional and observational design. Firstly, although the variables in our study covered many aspects of sex- and gender-specific demographic, behavioural and contextual characteristics, residual confounding due to unmeasured parameters in our dataset is possible. Secondly, self-selection or other biases may have occurred if individuals who are more concerned with their health were more likely to participate. Thirdly, our study was conducted in Switzerland, a high-income country with a high gender equality index [32]. Given that gender-related characteristics are culturally sensitive, our observations may not be extrapolated to other societies and geographical regions. Similarly, the Swiss population is ca 95% white, with only very small minority groups. Data derived from this ethnically and racially homogenous population allows for a focused consideration of gender in COVID-19 outcomes, however, at the expense of more complex interactions of other factors with disease outcomes. Fourthly, recent studies indicate that vaccination might protect from PASC [4]. As vaccination started after recruitment was completed, we were unable to address this issue. Finally, our study does not allow to assess the impact of SARS-CoV-2 variants on the epidemiology and severity of PASC as this information was not collected.
Conclusion
Taken together, while we did not observe major sex and gender differences in PASC symptom presentation, the incidence of PASC was substantially higher in women than in men in our Swiss cohort of almost 3,000 patients. Although biological variables may have a role in explaining sex disparities in acute COVID-19 illness, our study suggests an impact of societally constructed characteristics, and their interaction with biological sex, in producing sex and gender differences in PASC. Currently, a symptom-specific approach to treat PASC is recommended, however, our data imply that a tailored gender-sensitive approach of healthcare services may be required to support the needs of individuals affected by PASC. Indeed, many predictor variables of PASC identified in the present study are targets of interventions aiming at stress coping and social support. Also, the reported PASC risk factors can be easily identified at an early stage of disease by taking a thorough patient history without additional blood sampling or extensive diagnostic testing. Further research will be needed to determine if interventions targeted at these factors could improve outcomes. Finally, the fact that single features of the gender score, but not the summary gender score itself, were major predictors of PASC was surprising as the gender score was designed to provide a more holistic view of the impact of gender than single variables. Potential explanations for our finding comprise the fact that our study population was younger than the standard populations in which the gender score was validated, as well as the impact of differential environmental and health-related factors operating during a major pandemic on our study endpoints. Hence, our study emphasises the need to further advance instruments for the operationalisation of gender which may require adaptation to specific societies, age groups and disease conditions. Accordingly, we will use the data collected during this study to further optimise the gender score for contemporary research questions in future projects in Switzerland.
Ethical statement
The study complies with the Declaration of Helsinki and its later amendments, and the research protocol was approved by the responsible ethics committee of the Canton of Basel (EKNZ, ethics approval #2020-01311). Written informed consent was obtained from all patients or their legally authorised representative, as appropriate.
Funding statement
This work was supported by the Swiss National Science Foundation (Project #196140, to CG, CEG, and VRZ), the LOOP Zurich (CG, VRZ, UH), an unrestricted research grant from the intensive care unit research foundation of the University Hospital Basel (CEG), the Swiss National Science Foundation Starting #211422 (SDB), the University of Zurich CRPP precision medicine for bacterial infections, and a grant from University Hospital Zurich (USZ) Foundation USZF270808 (SDB, ASZ).
Data availability
Based on the Business Administration System for Ethics Committees (BASEC) ethics approval, the non-anonymised raw data cannot be shared publicly. However, anonymised data that underlie the results reported in this article will become available to interested parties for non-commercial reasons, after the publication upon reasonable requests made to the corresponding author. Data requestors will need to sign a data access agreement.
Acknowledgements
We would like to thank the staff of the Department of Nuclear Medicine of the University Hospital Zurich and the Intensive Care Units of the University Hospital Basel and the University Hospital Zurich for their excellent work and dedication to this study. The authors thank Christian Schindler, PhD, biostatistician at the Swiss Tropical and Public Health Institute Basel, Switzerland, for providing expert advice to the statistical analysis and for critically reviewing the manuscript. And last, we thank all the study patients for their valuable time and commitment to the Swiss COGEN Cohort.
Supplementary Data
Conflict of interest: CG has received research grants and speaker’s fees from the Novartis Foundation, from Sanofi Genzyme, Switzerland, and Bayer Pharmaceuticals outside of the submitted work. The University Hospital Zurich (CG, RRB, APP, MM, PAK) holds a research contract with GE Healthcare outside of the submitted work. AM has received research grants from Bayer Pharmaceuticals outside of the submitted work. JCS and TS reports (full departmental disclosure) grants from Orion Pharma, Abbott Nutrition International, B. Braun Medical AG, CSEM AG, Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, Nestle, Pierre Fabre Pharma AG, Pfizer, Bard Medica S.A., Abbott AG, Anandic Medical Systems, Pan Gas AG Healthcare, Bracco, Hamilton Medical AG, Fresenius Kabi, Getinge Group Maquet AG, Dräger AG, Teleflex Medical GmbH, Glaxo Smith Kline, Merck Sharp and Dohme AG, Eli Lilly and Company, Baxter, Astellas, Astra Zeneca, CSL Behring, Novartis, Covidien, Nycomed, and Phagenesis, outside of the submitted work. The money went into departmental funds, no personal financial gain applies. All authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Authors’ contributions: CG, CEG and VRZ conceptualised and designed the Swiss COGEN study. CEG, CS, SB and KPB coordinated the study. CEG, PO, SB, AM and NZ prepared the study data. AT, UH, MD, CEG, SB, BG and CG have verified the underlying data, PG and AT performed the statistical analysis and prepared tables and figures. CG, CEG and CS wrote the first manuscript draft. VRZ, VT, PAK, RRB, MM, APP, RAS, ASZ, JHB, AF, MS, HP, JCS, RT, GMK, JB, RvK and STS contributed to interpretation of the results and critical revision of the manuscript. SB, KPB, CEG, CG, SDB, CA, DP THIS and BW implemented and coordinated the recruitment of study patients and biobank samples. THOS, JT, DAH, PDWG, JCS, CH, MP, DFS, LM, MCG, ASZ, AH, MG, NM, AR, FF, AT, JB and AP contributed to the enrolment of study patients and data collection. All authors approved the final manuscript. CG is the guarantor for the study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
References
- 1.National Institute for Health and Care Excellence (NICE). COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: NICE; 2022. Available from: https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742
- 2.Reuters. Long COVID could become Finland's largest chronic disease. Reuters; 2022. Available from: https://www.reuters.com/article/us-health-coronavirus-finland-long-covid-idUSKBN2JH14W
- 3. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259-64. 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 4. Tsampasian V, Elghazaly H, Chattopadhyay R, Debski M, Naing TKP, Garg P, et al. Risk factors associated with post−COVID-19 condition: a systematic review and meta-analysis. JAMA Intern Med. 2023;183(6):566-80. 10.1001/jamainternmed.2023.0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bach K. New data shows long Covid is keeping as many as 4 million people out of work. Washington: Brookings Institution; 2022. Available from: https://www.brookings.edu/research/new-data-shows-long-covid-is-keeping-as-many-as-4-million-people-out-of-work
- 6.Office for National Statistics. Half a million more people are out of the labour force because of long-term sickness. Newport: Office for National Statistics; 2022. Available from: https://www.ons.gov.uk/employmentandlabourmarket/peoplenotinwork/economicinactivity/articles/halfamillionmorepeopleareoutofthelabourforcebecauseoflongtermsickness/2022-11-10
- 7. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133-46. 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626-31. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2023;401(10393):e21-33. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayoubkhani D, Pawelek P. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 6 January 2022. Newport: Office for National Statistics; 2022. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/6january2022
- 11. Kozak R, Armstrong SM, Salvant E, Ritzker C, Feld J, Biondi MJ, et al. Recognition of long-COVID-19 patients in a Canadian tertiary hospital setting: a retrospective analysis of their clinical and laboratory characteristics. Pathogens. 2021;10(10):1246. 10.3390/pathogens10101246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández-de-Las-Peñas C, Martín-Guerrero JD, Pellicer-Valero ÓJ, Navarro-Pardo E, Gómez-Mayordomo V, Cuadrado ML, et al. Female sex is a risk factor associated with long-term post-COVID related-symptoms but not with COVID-19 symptoms: The LONG-COVID-EXP-CM Multicenter Study. J Clin Med. 2022;11(2):413. 10.3390/jcm11020413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Sex, Gender and COVID-19 Project. The COVID-19 sex-disaggregated data tracker. Cambridge: Global Health 50/50. [Accessed: 24 Aug 2023]. Available from: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker
- 14. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374(1648):n1648. 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- 15. Lott N, Gebhard CE, Bengs S, Haider A, Kuster GM, Regitz-Zagrosek V, et al. Sex hormones in SARS-CoV-2 susceptibility: key players or confounders? Nat Rev Endocrinol. 2023;19(4):217-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regner E. "Gender should be taken into account in clinical trials for anti-COVID-19 treatments”. Press release. Brussels: European Parliament; 2021. Available from: https://www.europarl.europa.eu/news/en/press-room/20210706IPR07855/gender-should-be-taken-into-account-in-clinical-trials-for-anti-covid-treatments [Google Scholar]
- 17. Tadiri CP, Gisinger T, Kautzy-Willer A, Kublickiene K, Herrero MT, Raparelli V, et al. The influence of sex and gender domains on COVID-19 cases and mortality. CMAJ. 2020;192(36):E1041-5. 10.1503/cmaj.200971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manandhar M, Hawkes S, Buse K, Nosrati E, Magar V. Gender, health and the 2030 agenda for sustainable development. Bull World Health Organ. 2018;96(9):644-53. 10.2471/BLT.18.211607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson JL, Greaves L, Repta R. Better science with sex and gender: Facilitating the use of a sex and gender-based analysis in health research. Int J Equity Health. 2009;8(1):14. 10.1186/1475-9276-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nielsen MW, Stefanick ML, Peragine D, Neilands TB, Ioannidis JPA, Pilote L, et al. Gender-related variables for health research. Biol Sex Differ. 2021;12(1):23. 10.1186/s13293-021-00366-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pelletier R, Ditto B, Pilote L. A composite measure of gender and its association with risk factors in patients with premature acute coronary syndrome. Psychosom Med. 2015;77(5):517-26. 10.1097/PSY.0000000000000186 [DOI] [PubMed] [Google Scholar]
- 22. Bem SL. The measurement of psychological androgyny. J Consult Clin Psychol. 1974;42(2):155-62. 10.1037/h0036215 [DOI] [PubMed] [Google Scholar]
- 23. Pelletier R, Khan NA, Cox J, Daskalopoulou SS, Eisenberg MJ, Bacon SL, et al. Sex versus gender-related characteristics: Which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol. 2016;67(2):127-35. 10.1016/j.jacc.2015.10.067 [DOI] [PubMed] [Google Scholar]
- 24. Döring N. Zur Operationalisierung von Geschlecht im Fragebogen: Probleme und Lösungsansätze aus Sicht von Mess-, Umfrage-, Gender-und Queer-Theorie. GENDER–Zeitschrift für Geschlecht. [On the operationalization of gender in questionnaires: Problems and suggested solutions from a perspective of measuring, survey, gender and queer theory. GENDER magazine for gender]. Kultur und Gesellschaft. 2013;5(2):94-113. German. [Google Scholar]
- 25. Cummings P. Methods for estimating adjusted risk ratios. Stata J. 2009;9(2):175-96. 10.1177/1536867X0900900201 [DOI] [Google Scholar]
- 26. Danielsen AC, Lee KM, Boulicault M, Rushovich T, Gompers A, Tarrant A, et al. Sex disparities in COVID-19 outcomes in the United States: Quantifying and contextualizing variation. Soc Sci Med. 2022;294:114716. 10.1016/j.socscimed.2022.114716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart S, Newson L, Briggs TA, Grammatopoulos D, Young L, Gill P. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Health Eur. 2021;11:100242. 10.1016/j.lanepe.2021.100242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dehingia N, Raj A. Sex differences in COVID-19 case fatality: do we know enough? Lancet Glob Health. 2021;9(1):e14-5. 10.1016/S2214-109X(20)30464-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Islam N, Khunti K, Dambha-Miller H, Kawachi I, Marmot M. COVID-19 mortality: a complex interplay of sex, gender and ethnicity. Eur J Public Health. 2020;30(5):847-8. 10.1093/eurpub/ckaa150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wenham C, Smith J, Morgan R, Gender and COVID-19 Working Group . COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395(10227):846-8. 10.1016/S0140-6736(20)30526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horstmann S, Schmechel C, Palm K, Oertelt-Prigione S, Bolte G. The operationalisation of sex and gender in quantitative health-related research: a scoping review. Int J Environ Res Public Health. 2022;19(12):7493. 10.3390/ijerph19127493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.United Nations Development Programme (UNDP). Gender Inequality Index (GII). New York: UNDP. [Accessed: 14 Dec 2023]. Available from: https://hdr.undp.org/data-center/thematic-composite-indices/gender-inequality-index#/indicies/GII
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.