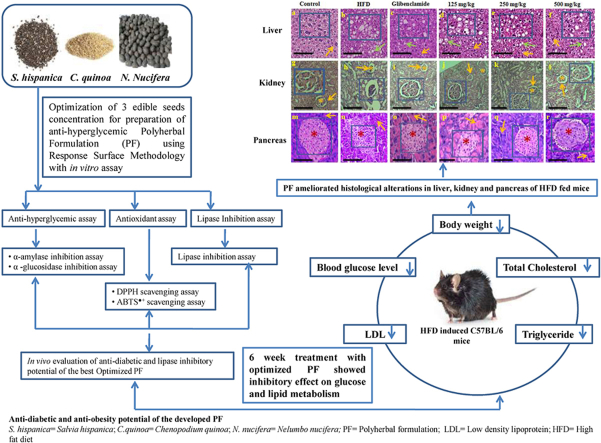
Abstract
Background and aim
The seeds of Nelumbo nucifera, Chenopodium quinoa and Salvia hispanica are known as super foods due to their various therapeutic properties. The present study aimed to develop an optimized polyherbal formulation from edible seeds aqueous extract and to evaluate its anti-diabetic and lipase inhibitory effect on diet-induced obese diabetic mice.
Experimental procedure
Response surface methodology based various formulations were evaluated for their potent anti-diabetic, lipase-inhibitory and antioxidant activities. Acute toxicity of the best optimized formulation was conducted. The mice were fed a high fat diet for 10 weeks resulting in hyperglycemia and obesity. Oral tolerance tests (sucrose, starch and lipid) of the formulation were performed. The mice were supplemented with different doses (125, 250 and 500 mg/kg) of the formulation for 6 weeks. The body weight and blood glucose level were monitored on a weekly basis. Finally, histological alterations and lipid profiles were analysed.
Results and conclusion
The formulation containing equal concentration (1.5 mg/ml) of each seed extract showed maximum bioactivities. The formulation was found to be safe during toxicity assay. The tolerance tests supported the anti-diabetic and anti-obesity effect. Higher dose (500 mg/kg) of the formulation significantly (p < 0.01) lowered elevated fasting blood glucose, lipid indices and ameliorated the histological alterations in liver, kidney and pancreas caused by high fat diet. We demonstrated for the first time that the developed aqueous extract optimized formulation possess anti-diabetic and anti-obesity potential and thus could be used as adjuvant therapy for holistic management of type 2 diabetes mellitus.
Keywords: Anti-diabetic activity, Anti-obesity activity, Chenopodium quinoa, Nelumbo nucifera, Optimized polyherbal formulation, Salvia hispanica
Graphical abstract
Highlights
-
•
An optimized anti-hyperglycemic PF was developed using RSM.
-
•
Optimized PF showed potent in vitro bioactivities.
-
•
PF attenuated hyperglycemia and hyperlipidemia in DIO mice.
-
•
PF improved histological alterations caused by high fat diet.
-
•
The PF could be a functional food candidate against type 2 diabetes mellitus and obesity.
List of Abbreviations
- T2DM –
Type 2 diabetes mellitus
- N. nucifera –
Nelumbo nucifera
- C. quinoa –
Chenopodium quinoa
- S.hispanica –
Salvia hispanica
- HFD –
High fat diet
- PF –
Polyherbal formulation
- RSM –
Response Surface Methodology
- DOE –
Design of Experiment
- CCD –
Central Composite Design
- ANOVA –
Analysis of Variance
- DPPH
2,2-diphenylpicrylhydrazyl
- ABTS●+
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- CPCSEA
Committee for Purpose of Control and supervision of Experiments on Animals
- OECD –
Organization for Economic Cooperation and Development
- CMC –
Carboxymethyl cellulose
- b.w. –
Body weight
- DIO –
Diet induced obesity
- OSTT –
Oral sucrose tolerance test
- OStTT –
oral starch tolerance test
- OLTT –
Oral lipid tolerance test
- TG
Triglyceride
- LDL –
Low-density lipoprotein
- HDL –
High-density lipoprotein
- TPC –
Total Phenolic Content
- TFC –
Total Flavonoid Content
- GAE –
Gallic Acid Equivalent
- QE –
Quercetin Equivalent
- mg
milligram
- Kg
Kilogram
- H&E −
Hematoxylin and Eosin
- 3D –
3 Dimensional
1. Introduction
Diabetes mellitus is a chronic metabolic disorder caused by a deficiency in insulin secretion, insulin action or combination of both which results in persistent hyperglycemia due to an alteration in carbohydrate, fat and protein metabolism.1,2 Altered lipid metabolism is related to insulin resistance which aggravates metabolic syndrome and hyperlipidemia in the progression of type 2 diabetes mellitus (T2DM).3 Additionally consumption of carbohydrate and fat-enriched foods results in hyperglycemia and oxidative stress by excess formation of reactive oxygen species.4
One of the therapeutic approaches to decrease postprandial hyperglycemia and hyperlipidemia is to delay digestion of carbohydrates and fats through inhibition of α-amylase, α-glucosidase and lipase enzyme thus diminishing postprandial blood glucose levels, preventing obesity, neutralizing weight gain and slow down the progression of T2DM.5
Several carbohydrate-hydrolyzing and pancreatic lipase inhibiting drugs are available. However long-term pharmacotherapy to achieve normal blood glucose levels and body weight along with suitable tolerability and safety remained an insurmountable challenge.6 Hence, the search for more effective and safer anti-hyperglycemic agents with added anti-hyperlipidemic and antioxidant potential of natural origin is continuing as these are considered safe compared to synthetic drugs.7
Edible seeds can be an alternative therapeutic strategy for T2DM management. Nelumbo nucifera (lotus) are flowering perennial aquatic plant that has been used in Ayurvedic and Traditional Chinese medicine.8 The seeds exhibit various phytochemicals which contribute to their antioxidant, anti-hyperglycemic and lipase inhibitory properties.9,10
Salvia hispanica (Chia) seeds are used as medicine, food and energizer.11 The seeds have been reported to exhibit inhibitory potential towards α-amylase, α-glucosidase and lipase enzyme along with antioxidant properties.9,12 The administration of chia seeds reversed dyslipidemia, and hyperglycemia in sucrose rich diet fed rats and in T2DM patients.13,14
Chenopodium quinoa (quinoa) is called “nutritional gold”, “super grain” and “future food” and has been reported to exhibit anti-hyperglycemic and antioxidant activity.15,16 The seeds have been reported to improve glycolipid metabolism in high-fat diet (HFD) fed mice and lower triglyceride level in human subjects.17,18
T2DM is a multi-factorial metabolic disorder and therefore a combination of edible seeds as polyherbal formulation (PF) over single edible seed may provide multimodal therapeutic benefits due to their synergistic effect.19 This synergistic effect enhances the therapeutic outcomes by reducing side effects and lowering the dosage to reach threshold.20
Most of the anti-diabetic PF consists of traditional herbal ratios.21 The present study, however, used response surface methodology (RSM) to develop a new optimum PF for the holistic management of T2DM. For this purpose, RSM based different concentrations of 3 seeds in the form of a PF was screened by in vitro anti-hyperglycemic, lipase inhibitory and antioxidant assays and the optimum PF were selected to determine its anti-diabetic and anti-obesity effect in a mouse model of obesity (C57BL/6). To the best of our knowledge, this is the first study reporting the in vitro and in vivo anti-diabesity effect of a PF aqueous extract which was developed from optimized ratio of edible seeds.
2. Materials and methods
2.1. Collection of seeds
The seeds were purchased from local shops of Bangalore and its authentication was performed at Regional Ayurveda Research Institute for Metabolic Disorders (RARIMD). The accession numbers RRCBI-10394, RRCBI- mus189 and RRCBI- mus213 were assigned to N. nucifera, S. hispanica and C. quinoa respectively.
2.2. Preparation of seed extract
The seeds were dried and ground to a fine powder and this powder was extracted with water using soxhlet apparatus. The extracts were filtered through Whatman No.1 filter paper. The filtrates were collected and concentrated under reduced pressure using a rotary evaporator. The semi-dried residues were then frozen in a refrigerator overnight and then dried using a lyophilizer to remove the entire aqueous residue.22 This seed extract was kept in a desiccator until used for the experiment.
2.3. Development of PF using design of experiment (DOE)
Design expert software was used to develop the PF. RSM based central composite design (CCD) of the software was used to find optimum conditions for developing the best anti-hyperglycemic PF with added lipase inhibitory and antioxidant potential. 3 independent variables were chosen as: S. hispanica concentration: (X1); C. quinoa concentration: (X2); N. nucifera concentration: (X3). These 3 variables were studied at 5 different levels coded as –α, −1, 0, +1 and + α. α was calculated as = (23)1/4 = 2.34 and the value is ±1.68179 for the experiment.23 The responses were considered as dependent variables and these were Pancreatic ɑ-amylase: (Y1); ɑ-glucosidase: (Y2); Lipase: (Y3); DPPH: (Y4); ABTS●+: (Y5).
CCD suggested 20 experimental runs including 6 at central points, 6 at axial, and 8 at factorial points. The total experimental run was calculated by equation (1) 24:
| (1) |
where n represents the number of independent variables (factors), nc represents the number of center points and N represents total experimental runs.
The experimental data obtained from the CCD model were subjected to multiple regression analysis and was expressed by quadratic polynomial model, as shown below in Eq. (2) 25:
| (2) |
where, 0 represents the coefficient constant, i, ij, and ii represents the regression coefficients for the linear, quadratic and interaction terms, respectively, and Xi and Xj represents the independent variables, E is random error. The coded and uncoded levels of CCD have been shown in Table 1.
Table 1.
Three-independent variable, five-level CCD and experimental data for response variables.
| Coded and real variable levels |
Tested bioactivities in percentage (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Run | Factor 1 S.hispanica (mg/ml) | Factor 2 C.quinoa (mg/ml) | Factor 3 N.nucifera (mg/ml) | Response 1 pancreatic α-amylase inhibition | Response 2 α-glucosidase inhibition | Response 3 Lipase inhibition | Response 4 DPPH Scavenging | Response 5 ABTS●+ Scavenging |
| 1 | 1.68179 (2.34) | 0 (1.5) | 0 (1.5) | 51.34 | 55.55 | 53.89 | 72.52 | 66.52 |
| 2 | −1 (1) | 1 (2) | −1 (1) | 59.45 | 61.93 | 51.46 | 61.75 | 55.75 |
| 3 | 0 (1.5) | 0 (1.5) | 0 (1.5) | 72.29 | 71.87 | 60.49 | 72.67 | 63.56 |
| 4 | 0 (1.5) | 0 (1.5) | −1.68179 (0.65) | 50.30 | 54.20 | 52.49 | 62.47 | 51.77 |
| 5 | −1 (1) | −1 (1) | −1 (1) | 33.58 | 62.61 | 57.58 | 56.40 | 48.95 |
| 6 | 1 (2) | 1 (2) | 1 (2) | 49.65 | 55.89 | 53.52 | 71.51 | 63.20 |
| 7 | 0 (1.5) | 0 (1.5) | 0 (1.5) | 74.73 | 70.49 | 57.58 | 70.86 | 65.29 |
| 8 | 0 (1.5) | 1.68179 (2.34) | 0 (1.5) | 58.46 | 60.17 | 52.43 | 68.84 | 59.94 |
| 9 | 0 (1.5) | 0 (1.5) | 0 (1.5) | 75.07 | 71.25 | 59.75 | 72.67 | 62.11 |
| 10 | 0 (1.5) | 0 (1.5) | 1.68179 (2.34) | 48.06 | 62.69 | 52.43 | 71.51 | 55.82 |
| 11 | 0 (1.5) | −1.68179 (0.65) | 0 (1.5) | 41.54 | 60.24 | 56.32 | 64.50 | 44.32 |
| 12 | 0 (1.5) | 0 (1.5) | 0 (1.5) | 76.57 | 73.62 | 60.26 | 69.63 | 66.38 |
| 13 | 1 (2) | −1 (1) | −1 (1) | 46.67 | 57.95 | 58.95 | 67.32 | 45.99 |
| 14 | 1 (2) | 1 (2) | −1 (1) | 52.79 | 54.43 | 52.72 | 64.86 | 51.63 |
| 15 | −1.68179 (0.65) | 0 (1.5) | 0 (1.5) | 42.19 | 66.36 | 50.49 | 62.26 | 47.14 |
| 16 | −1 (1) | −1 (1) | 1 (2) | 30.55 | 61.31 | 54.2 | 64.64 | 43.17 |
| 17 | 1 (2) | −1 (1) | 1 (2) | 48.96 | 56.27 | 56.15 | 68.26 | 51.48 |
| 18 | 0 (1.5) | 0 (1.5) | 0 (1.5) | 71.39 | 71.64 | 60.26 | 70.86 | 64.14 |
| 19 | −1 (1) | 1 (2) | 1 (2) | 39.75 | 61.31 | 50.94 | 68.35 | 53.43 |
| 20 | 0 (1.5) | 0 (1.5) | 0 (1.5) | 73.73 | 73.39 | 59.86 | 71.66 | 67.39 |
∗α = 1.68179 (star or axial point for CCD in the case of three independent variables).
∗Numbers in bracket denotes the concentration of seed extracts (mg/ml) in each run.
Seed combination of polyherbal formulation showing maximum bioactivities has been highlighted.
Statistical analysis using RSM employing CCD, data analysis, quadratic model building and generation of response surface curves was conducted using Design-Expert software (version 11.0, Stat-Ease Inc. Minneapolis, USA). The adequacy of the model was evaluated using the analysis of variance (ANOVA) of Design-Expert software.
2.4. Combining seed extract for PF development
The seeds extracts were combined to prepare PF according to the combinations suggested by CCD in the form of 20 runs and screened for in vitro bioactivities (Table 1).
2.5. Anti-hyperglycemic, lipase inhibitory and antioxidant assay
The in vitro anti-hyperglycemic, lipase inhibition and antioxidant activity were performed according to our earlier reported protocol.9 For the anti-hyperglycemic assay (pancreatic α-amylase, α-glucosidase inhibition assay), acarbose was used as a standard. Orlistat was used as a standard for lipase inhibition assay whereas ascorbic acid was the standard for antioxidant assay (DPPH and ABTS●+ scavenging).
The percentage (%) of enzyme inhibition and radical scavenging activity was calculated using following formula:
where A control is the absorbance of the control reaction and A sample is the absorbance of the sample.
The optimum PF showing best bioactivity was screened for presence of phytochemicals qualitatively and quantitatively using our earlier reported method.9
2.6. Validation of the model
For validation, the response of best activity showing PF was again determined under the optimized conditions. The maximum bioactivity was shown by the formulation containing equal concentration (1.5 mg/ml) of the edible seeds. The optimum formulation showed 76.57%, 73.39%, 60.49%, 72.67% and 67.39% of α-amylase, α-glucosidase, lipase and DPPH and ABTS●+ scavenging activity respectively. The experiment was conducted in triplicate with the specified concentration of seeds in developed PF to verify the results of RSM model. The obtained results were found to be 76.02%, 72.99%, 59.49%, 71.00% and 65.39% of α-amylase, α-glucosidase, lipase and DPPH and ABTS●+ scavenging activities respectively. The model was verified because the experimental values were found to be closer to the predicted values. Thus, the model was found useful in predicting the optimal concentration of seeds in PF with enhanced therapeutic properties for holistic management of T2DM.26
2.7. Confirmation of the anti-diabetic property of the developed PF using in vivo study
2.7.1. Preparation of PF
The best optimized PF (containing 1:1:1 ratio of each seed) obtained from RSM study was subjected to in vivo study to confirm its anti-diabetic and anti-obesity potential. The PF aqueous extract was prepared according to the above mentioned protocol (section 2.2).
2.8. Experimental animals
Male mice (C57BL/6) aging 6 weeks with a weight of 22 ± 3 g were obtained from In Vivo Biosciences, Bangalore, India. A total of 36 mice were divided into six groups (n = 6) and were acclimatized to the laboratory conditions for a week before initiating the experiment. All animals had free access to food and water and were housed in polypropylene cages, maintained under controlled temperature (23 ± 1 °C), humidity (60 ± 10%) and 12 h light-dark cycles. All the experimental protocols were in agreement with the Committee for Purpose of Control and supervision of Experiments on Animals (CPCSEA) guidelines, and approved by In Vivo Biosciences (Approval No. 1165/PO/RcBiBt-S/NRc-L/08/CPCSEA, Proposal number 62/2018).
2.9. Acute toxicity studies
Acute toxicity test of the PF was carried out on normal C57BL/6 mice according to the Organization for Economic Cooperation and Development (OECD) guidelines 423 (OECD 2001).27 The study was performed at In Vivo Biosciences, Bangalore. Mice were divided into 6 groups (n = 6 mice). Mice fasted for 16 h before starting the experiment. Mice were supplemented with different doses (250, 500, 1000 and 2000 mg/kg of body weight (b.w.)) of PF extract via oral gavage. The control group received 10 ml/kg b. w. of 1% carboxymethyl cellulose (CMC) gel. Food was provided to animals after 1–2 h of dosing. Animals were observed closely for signs of toxicity, physical and behavioral change for 6 h and then regularly for 14 days. The test showed that a maximum dose of 2000 mg/kg b. w. was safe for animals.
Based on the results obtained in acute toxicity study, the extract doses to be administered were determined and the volume of administration was 1 ml/100 g of b. w. of the mice. In order to achieve a better therapeutic dose, 3 different doses of the PF i.e., low (125 mg/kg), middle (250 mg/kg) and high dose (500 mg/kg) were studied.
2.10. Development of diet-induced obese (DIO) mice model
According to the groups assigned, the mice were fed with a chow diet (10% Kcal fat; D12450B; Research Diets Inc.) and HFD (60% kcal from fat; D12492; Research Diets Inc.) for 10 weeks. After 10 weeks, the mice attained the hyperglycemic state which was confirmed by fasting glucose levels.
2.11. Oral tolerance tests
To identify one of the probable mechanisms by which PF exerts its anti-hyperglycemic activity, α-glucosidase inhibitory assay (with sucrose and starch) were performed following standard protocol.28 Sucrose and starch tolerance tests were performed on overnight fasted (16 h) DIO mice. The animals were kept on fasting for 16 h before experiment but had access to water ad libitum. Mice were divided into 5 groups (n = 6) and administrated with the following treatments: Group I (Control) was given 1% CMC (10 ml/kg body weight (b.w.)). Group II was treated with Glibenclamide. Group III – V received PF extract at different doses (low, middle and high) for oral sucrose tolerance test (OSTT) and oral starch tolerance test (OStTT) respectively. 30 min after dosing, the mice were loaded with sucrose (4 g/kg b. w.) for OSTT and corn starch (3 g/kg b. w.) for OstTT using an oral gavage. Blood was collected from tip of tail vein at 0, 0.5, 1 and 2 h. Blood glucose concentration was determined by Accu-check glucometer (Roche Diagnostics).
2.12. Oral lipid tolerance test
Oral lipid tolerance test (OLTT) was conducted as per standard protocol.29 The grouping of the animals was similar to OSTT and OStTT. Group I received 1% CMC (10 ml/kg body weight (b.w.)) and group II was administered with orlistat (50 mg/kg). Remaining groups received low, middle and high dose of PF extract. 30 min after dosing, the mice were loaded with corn oil emulsion (5 ml/kg). The composition of oil emulsion was a combination of cholic acid (80 mg), saline (6 ml), corn oil (6 ml) and cholesteryl oleate (2 mg). Blood was collected from tip of tail vein at 0, 1.5, 3 and 4 h. Level of plasma triglyceride (TG) was measured using Accutrend Plus system (Roche).
2.13. Grouping and dosing of animals for the anti-diabetic study
The mice were divided into 6 groups (n = 6). Group I (normal mice) was used as a negative control receiving vehicle (1% CMC). Group II (diabetic obese mice) was used as diabetic control which did not receive any treatment but vehicle (1% CMC). Group III (positive control) received a standard drug glibenclamide (25 mg/kg/b. w.). Group IV-VI received different doses of PF extract (low, middle and high). The mice were supplemented with PF once daily for 6 weeks. Furthermore, in the normal group mice were fed with a normal diet and the remaining 5 groups were kept on HFD while receiving PF throughout the study (i.e., for 6 weeks).
2.14. Anti-diabetic activity of PF extract
According to the above described groups, the mice were treated with vehicle (1% CMC), glibenclamide and selected doses of PF extracts. Fasting blood glucose levels of mice groups were measured just before starting the treatment (week 0), and then on a weekly basis following overnight fasting for 16 h throughout the study.30 For blood glucose measurements, blood was collected from the tip of the tail of overnight fasted mice and measured using Accu-check glucometer (Roche Diagnostics).
2.15. Body weight determination
The body weight of mice was documented before treatment (week 0) and then throughout the treatment period (till week 6) using an electronic balance.
2.16. Serum lipid profile of DIO mice
To evaluate the effect of PF extract on serum lipid level of DIO mice, blood was drawn by cardiac puncture from overnight fasted (16 h) diabetic mice. Blood was collected in microfuge tube and allowed to stand for 30 min at room temperature. This sample was then centrifuged at 10,000 rpm for 10 min. Supernatant was separated from pellet to prepare serum samples to determine the serum level of total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL).31
2.17. Histological studies
To investigate the effect of PF extract on HFD-mediated histological changes, vital organs (kidney, liver and pancreas) were excised and stored in cold 10% formalin containing phosphate-buffered saline for at least 24 h. Tissues were embedded in paraffin wax, then 4 μm sections were stained with hematoxylin and eosin (H&E). Samples of tissues were observed using inverted microscope (Nikon Eclipse TE2000-5, Japan) at a magnification of 100 × 32,33
2.18. Statistical analysis
Experimental values were expressed as Mean ± SD (n = 6). Statistical analysis was performed using one way analysis of variance followed by Dunnett's test in Graph pad prism 6.0. The p-value was considered statistically significant at < 0.001.
3. Results and discussion
3.1. DOE
The present study used Design Expert Software (Version 11) for the development of PF. The concentration of seed extract in PF was coded at 5 different levels (Table 1). The effect of 3 independent variables on 5 responses of interest was investigated using RSM and the results of 20 runs using CCD were presented (Table 1). The results of CCD were fitted to a full quadratic second-order polynomial equation by applying multiple regression analysis and the estimated quadratic polynomial equation in terms of coded factors is shown below:
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
where,
A = Concentration of S. hispanica; B= Concentration of C. quinoa; C= Concentration of N. nucifera. In polynomial equation, a negative sign represents an antagonistic effect of the variables whereas positive sign represents a synergistic effect of the variables.34
The fitness of the second-order polynomial model was evaluated by ANOVA using Fisher's statistical analysis (F-value), coefficients of determination (R2), probability values (P) and lack-of-fit value.35 In the case of evaluated bioactivities, the model showed a larger F-value and p-value less than 0.05 thus the model was considered to be significant. Lack of fit (p-value of >0.05) was insignificant for all the tested bioactivities, implying an overall good model fitting. R2 value was closer to 1 for all the evaluated models showing the statistical significance of the model. Table 2 shows the data obtained from the ANOVA for the response surface reduced quadratic model.
Table 2.
Analysis of variance for response surface quadratic model.
| α-amylase | |||||
|---|---|---|---|---|---|
| Source | Sum of squares | Degrees of freedom | Mean of square | F value | P value |
| Model | 3992.58 | 9 | 443.62 | 61.63 | <0.0001 (Significant) |
| Residual | 71.98 | 10 | 7.20 | ||
| Lack of fit | 53.89 | 5 | 10.78 | 2.98 | 0.1280 (not significant) |
| Pure Error | 18.08 | 5 | 3.62 | ||
| Total | 4064.55 | 19 | |||
| R2 |
0.982 |
||||
|
α-glucosidase | |||||
|
Source |
Sum of squares |
Degrees of freedom |
Mean of square |
F value |
P value |
| Model | 823.77 | 9 | 91.53 | 25.63 | <0.0001 (Significant) |
| Residual | 35.71 | 10 | 3.57 | ||
| Lack of fit | 28.17 | 5 | 5.63 | 3.74 | 0.0870 (not significant) |
| Pure Error | 7.53 | 5 | 1.51 | ||
| Total | 859.48 | 19 | |||
| R2 |
0.958 |
||||
|
Lipase | |||||
|
Source |
Sum of squares |
Degrees of freedom |
Mean of square |
F value |
P value |
| Model | 212.27 | 9 | 23.59 | 10.45 | 0.0005 (Significant) |
| Residual | 22.57 | 10 | 2.26 | ||
| Lack of fit | 16.80 | 5 | 3.36 | 2.91 | 0.1331 (not significant) |
| Pure Error | 5.77 | 5 | 1.15 | ||
| Total | 234.84 | 19 | |||
| R2 |
0.903 |
||||
|
DPPH | |||||
|
Source |
Sum of squares |
Degrees of freedom |
Mean of square |
F value |
P value |
| Model | 370.48 | 9 | 41.16 | 20.14 | <0.0001 (Significant) |
| Residual | 20.44 | 10 | 2.04 | ||
| Lack of fit | 13.43 | 5 | 2.69 | 1.92 | 0.2463 (not significant) |
| Pure Error | 7.01 | 5 | 1.40 | ||
| Total | 390.92 | 19 | |||
| R2 |
0.947 |
||||
|
ABTS | |||||
|
Source |
Sum of squares |
Degrees of freedom |
Mean of square |
F value |
P value |
| Model | 1157.58 | 9 | 128.62 | 13.49 | 0.0002 (Significant) |
| Residual | 95.35 | 10 | 9.54 | ||
| Lack of fit | 76.70 | 5 | 15.34 | 4.11 | 0.0734 (not significant) |
| Pure Error | 18.65 | 5 | 3.73 | ||
| Total | 1252.93 | 19 | |||
| R2 | 0.923 | ||||
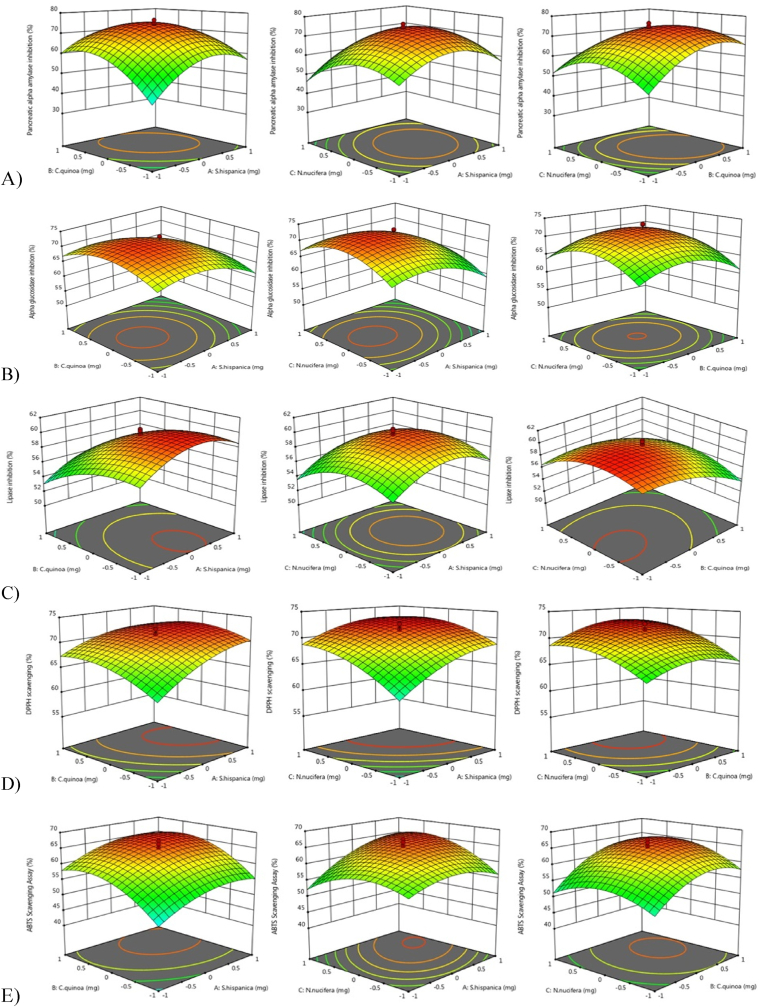
A 3D response surface plot was used to visualize the mutual interaction of two variables and their effect on dependent variables while keeping the third variable constant at the central level. It is evident in the figures that equal ratio of seeds (i.e., 1:1:1 or 1.5 mg/ml) of each seed extract showed maximum bioactivity compared to other seed combinations (Fig. 1). Earlier studies have utilized RSM to achieve a PF with phyto-synergistic action against carbohydrate hydrolyzing enzymes and antioxidants. The results of those studies suggested that RSM is a useful tool in obtaining best herb combination and multi-response optimization for development of an anti-hyperglycemic PF with multi-faceted benefits.21,36 The results of the present study are in accordance with the above mentioned studies. The results of the best activity showing seed combination were validated in order to verify the results of the RSM model.
Fig. 1.
Response surface plots (3D) showing the mutual interactions of (A) S. hispanica and C. quinoa (B) S. hispanica and N. nucifera and (C) C. quinoa and N. nucifera on A) α-amylase, B) α-glucosidase, C) lipase inhibition and D) DPPH and E) ABTS●+ scavenging.
Qualitative screening of optimized PF showed the presence of phenol, flavonoid, tannin, glycosides and phytosterols whereas, quantitative analysis showed appreciable amount of TPC (0.918 μg.GAE/mg) and TFC (0.738 μg. QE/mg) (Appendix Table 1). After deciding the optimum PF, we investigated the anti-diabetic effect of the PF on HFD fed mice.
3.2. Animal study
3.2.1. Acute toxicity studies
PF aqueous extract was safe up to a dose of 2000 mg/kg b. w. in the observation period of 14 days.
3.2.2. Development of diet-induced obese (DIO) C57BL/6J mice
HFD supplementation to DIO models has been considered to mimic human obesity.37 In present study, supplementation of HFD for 10 weeks resulted in increased body weight (2 fold) of mice compared to normal diet-fed mice.
3.2.3. Oral tolerance test
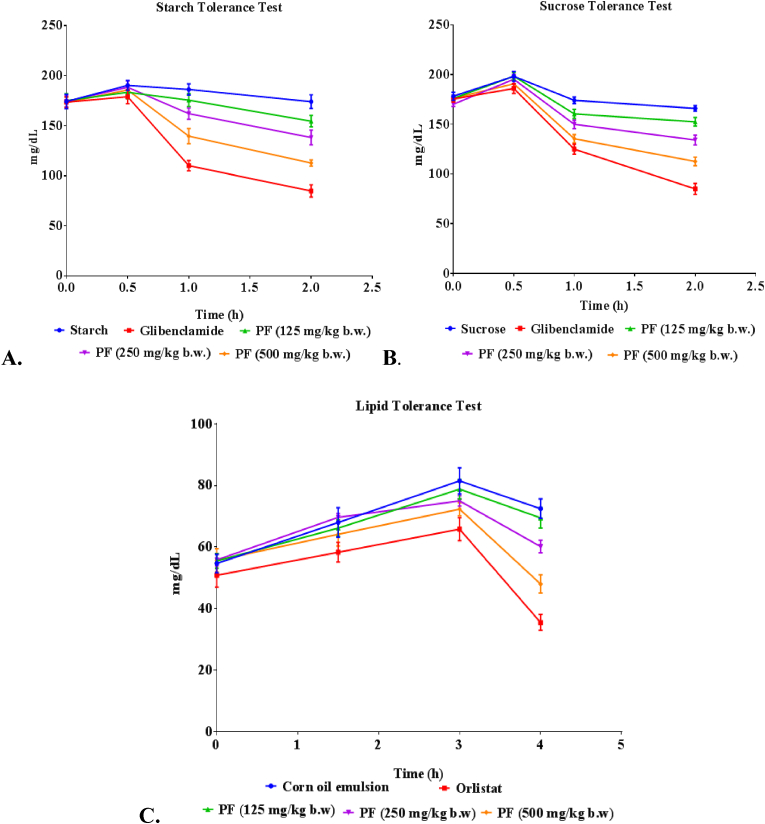
The oral tolerance test is performed to study the acute effects of extract in diabetic animals and is based on the body's ability to utilize sucrose and starch load administered orally.38 In both of these tests, the control group showed significant elevation in their blood glucose level due to the inability of the system to utilize glucose. At 2 h, the anti-hyperglycemic potential of PF extracts was observed (Fig. 2). The observed in vitro α-glucosidase inhibitory effects of the optimized PF were replicated in the starch and sucrose tolerance tests.
Fig. 2.
Effect of PF on (A) oral starch tolerance and (B) oral sucrose tolerance over 2 h and C. oral lipid tolerance over a period of 4 h in DIO mice. Values were expressed as mean ± SEM (n = 6); compared with the control. PF= Polyherbal formulation.
3.2.4. Oral lipid tolerance test
OLTT assay can be used to access information about pancreatic and intestinal lipid absorption when investigating the suppressive effects of an herbal formulation on postprandial hypertriglyceridemia.39 Present study indicated that the postprandial serum TG levels had an upward trend until 2 h after administration of the corn oil emulsion (Fig. 2). Studies have shown that the plasma TG levels reached to maximum level 2–3 h after the administration of fat emulsions,40 which is similar to the timeframe observed in this study. PF supplementation decreased serum TG level which is a useful finding as there are rarely any PF studied for lipase inhibitory potential using OLTT.
3.2.5. Glucose measurements
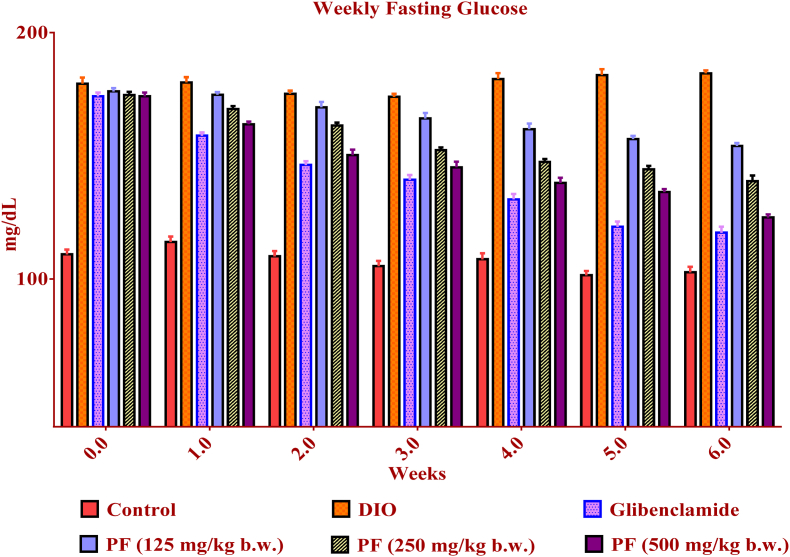
In the present study, the glycemic level decreased in the diabetic group receiving PF extract. The blood glucose level of the control group was stable throughout the experiment (Fig. 3). The data obtained were comparable to the standard (glibenclamide) used in the study. The observed glucose suppressive potential of PF extract could be due to the water solubility properties of the phenolic compounds which could enhance their interactions with target enzymes within the hydrophilic environment of the gastrointestinal tract as reported in earlier studies.41
Fig. 3.
Effect of PF on blood glucose level (mg/dL) in DIO mice. ∗ Values are expressed as mean ± SEM (n = 6); compared with the control. Data were analysed with One way ANOVA followed by post hoc analysis (Dunnets multiple comparison test). Values are statistically significant at p < 0.001. PF= Polyherbal formulation.
3.2.6. Body weight
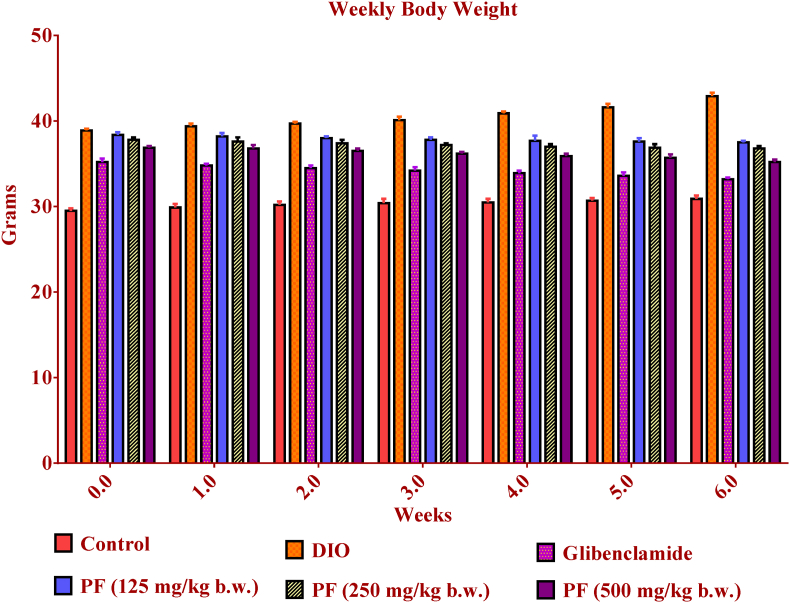
The consumption of HFD contributes to weight gain through the reduction of satiety signals.42 Our study demonstrated that the body weight significantly increased in mice supplemented with HFD when compared to the mice of the normal control group throughout the experimental period. However, the weight gain tendency was slowed down in the mice group treated with PF (Fig. 4). The decrease in weight upon PF treatment could be attributed to its potential to increase satiety signals that mediate the reduction of food intake; decreases body weight and increases energy expenditure.42
Fig. 4.
Effect of PF treatment on body weight of mice during 6 weeks. ∗ Values are expressed as mean ± SEM (n = 6); compared with the control. Data were analysed with One way ANOVA followed by post hoc analysis (Dunnets multiple comparison test). Values are statistically significant at p < 0.001. PF= Polyherbal formulation.
3.2.7. Serum lipid profile
The majority of T2DM patients exhibit dyslipidemia which is important in mediating the cardiovascular risk in T2DM.43 Therefore, regulation of lipid metabolism should be considered as an important criterion along with glucose-regulating function in T2DM patients.44
The administration of PF decreased levels of TC, TG, and LDL in treated mice. PF (500 mg/kg) and glibenclamide supplemented group showed a significant decrease in the changes induced by HFD, whereas lower dose of PF (125 mg/kg) did not exhibit such ameliorative actions (Table 3). However, plasma high-density lipoprotein cholesterol (HDL-c) levels were not significantly different among these treated groups. The observed reduction of lipid profiles might be attributed to the PF extract's phytochemicals in augmentation of satiety, suppression of absorption and digestion of dietary lipids.42
Table 3.
The effects of PF on lipid profile of DIO mice after 6 weeks of treatment.
| Groups | HDL (mg/dL) | LDL (mg/dL) | TG (mg/dL) | TC (mg/dL) |
|---|---|---|---|---|
| Normal Control | 53.40 ± 0.41 | 36.46 ± 0.95 | 95 ± 0.73 | 143.1 ± 0.9 |
| Diabetic control | 25.80 ± 0.46 | 81.78 ± 0.80 | 135.7 ± 1.1 | 259.6 ± 3.7 |
| Glibenclamide | 38.70 ± 0.66 | 80.83 ± 0.94 | 134 ± 0.93 | 262.3 ± 2.0 |
| PF (125 mg/kg.bw) | 24.85 ± 0.48 | 81.61 ± 0.40 | 115.33 ± 2.39 | 233.0 ± 3.0 |
| PF (250 mg/kg.bw) | 24.15 ± 0.68 | 80.29 ± 0.52 | 112.67 ± 2.16 | 218.6 ± 0.8 |
| PF (500 mg/kg.bw) | 32.50 ± 0.80 | 80.22 ± 0.62 | 115.67 ± 1.89 | 211.1 ± 1.4 |
∗ Values represent mean ± SEM (n = 6); PF= Polyherbal formulation; HDL= High density lipoprotein; LDL = Low density lipoprotein, TG = triglyceride; TC = Total cholesterol. Values are statistically significant at p < 0.001 (one-way ANOVA followed by Dunnett's test).
3.2.8. Histological studies
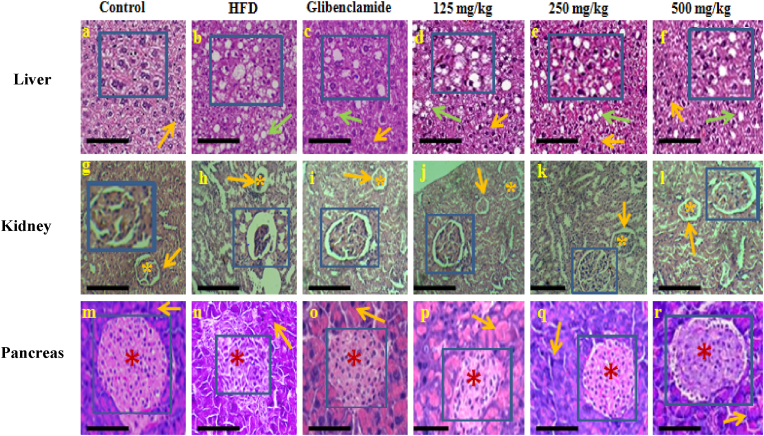
3.2.8.1. Liver: The imbalance between lipid deposition and removal results in hepatic lipid accumulation, which is related to increased hepatic lipogenesis leading to hepatic steatosis and metabolic syndrome.45 We assessed whether PF supplementation affects hepatic morphology and fat accumulation in DIO mice. The results of study indicated that the higher dose of PF suppressed the development of fatty liver and improvement in histological profiles of liver (Fig. 5). However, the lower dose of PF (125 mg/kg b. wt.) did not showed a reduction in lipid accumulation in liver. Similarly, lower dose (125 mg/kg b. wt) of a PF named GGEx18 (Laminaria japonica, Rheum palmatum and Ephedra sinica)) did not show any significant changes in the tissue architecture of the liver of HFD-fed mice. Although a higher dose (250 and 500 mg/kg b. wt.) was found to be effective in the prevention of hepatic steatosis, hyperlipidemia and thus, suppressing lipogenesis in HFD-fed C57BL/6 mice.46 This potential of PF is noteworthy as glucose and lipid metabolism is regulated by the liver.43
Fig. 5.
Histological examination of liver, kidney and pancreatic tissue (magnification × 100) after hematoxylin and eosin staining. Liver: Normal hepatocytes in control, glibenclamide and PF treated groups (low, middle, and high) are indicated by orange arrow. Whereas lipid droplets in HFD group, glibenclamide and PF treated groups are indicated by green arrow. HFD= High Fat Diet; PF= Polyherbal Formulation. Kidney: Normal glomerulus is shown by asterisk mark in control, glibenclamide and PF treated groups (low, middle, and high). Increased bowman's space in HFD group is shown by orange arrows. Pancreas: Arrows indicates acini in all studied groups, whereas asterisk mark indicates islet of langerhans in pancreas of control, glibenclamide and PF treated groups (125, 250, 500 mg/kg). The large view of changes has been shown with the help of inset (scale bar = 20 μm). A).
3.2.8.2. Kidney: HFD consumption results in altered lipid metabolism in the kidney leading to renal damage including glomerulosclerosis, albuminuria and increased oxidative stress ultimately causing T2DM and its complications.47
In present study, the control group showed normal histology of the kidney. However, in HFD-fed mice group, histological alteration was observed in the kidney with increased bowman's space, mild swelling of glomerulus apart from this no other significant changes were found. Administration of high dose of PF resulted in alleviation of glomerulus swelling and also restoration of normal architecture of glomerulus (Fig. 5). It was observed that C57BL/6 did not develop significant pathological changes in the kidney within the time frame examined in our study.
Similar findings were reported for a potent anti-obesity formulation named. Supplementation of 18KHT01 ameliorated histological alterations of the kidney including mild infiltration of macrophages and enlargement of Bowman's space of glomeruli in HFD-fed C57BL/6 male mice.48 Moreover, the kidneys did not show any significant changes when fed with HFD (45% kcal) for 12 weeks.49 Whereas, HFD fed C57BL/6 mice showed a significant increase in glomerular size on 12 weeks which was worsened at week 16.50 The observations of above mentioned studies and present study indicates that the length of the modelling and diet composition may be the key to induce meaningful renal changes caused by obesity and insulin resistance.47,51
3.2.8.3. Pancreas: Long-term (16 weeks) supplementation of HFD to C57BL/6 mice has been reported to exhibit impaired glucose tolerance, and insulin resistance leading to β cell dysfunction.52 Moreover, obese humans with impaired fasting glucose and T2DM have shown less β-cell volume due to increased β-cell apoptosis when compared with non-diabetic obese and lean control.53
In support of earlier findings, in present study an irregular shape of pancreatic islets was observed with decreased beta cell mass in DIO mice. However, the mice group receiving higher dose of PF showed normal pancreatic architecture with normal beta cell mass compared to DIO mice (Fig. 5). Besides this, no significant pathological changes were observed in HFD fed mice group. Studies have demonstrated that a functional rather than a morphological abnormality appears deto underlie HFD induced beta cell dysfunction.54 Moreover, different experimental conditions, time length utilizeAppendix A. Supplementary data
d in model development and diet composition may also have an influence on the outcome of experiments and results of our study support these earlier observations.55
4. Conclusion
In conclusion, present study showed that RSM can be utilized for achieving an accurate model for optimization of ingredients of a PF. The optimum PF (containing 1.5 mg/ml or 1:1:1 ratio of each seed) exhibited potent in vitro anti-hyperglycemic, lipase inhibitory and antioxidant activities. The developed PF was proven to be effective in controlling hyperglycemia and hyperlipidemia while improving histological alterations. The results of the present study, therefore, affirm the usage of these edible seeds as an alternative therapy for the management of diabesity and associated complications. However, further identification of the phytochemicals responsible for this activity is required.
Author contributions
MM: Conceptualised and supervised the work, edited and reviewed the manuscript; Tanisha: collected the samples, developed the formulation and perform in vitro experiments and in vivo experiments, and wrote the manuscript. SV: helped Tanisha in performing animal study. The authors have read and approved the submission of the manuscript.
Funding
This work was supported by University Grants Commission [Grant number- F1-17.1/2013-14/RGNF-2013-14-SCBIH-36937] in the form of fellowship to Ms. Tanisha.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by Jain (Deemed-to-be University), Bangalore, India. The authors are grateful to Regional Ayurveda Research Institute for Metabolic Disorders, Bangalore for authentication of edible seeds. The authors are also grateful to In Vivo Biosciences, Bangalore, India for providing the animal facility to conduct the experiment. The authors express special thanks to Dr. Sunil S. More (Dean, School of Basic and Applied Sciences, Dayanand Sagar University, Bangalore) for his kind support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2023.07.002.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Vinodhini S., Rajeswari V.-D. Exploring the antidiabetic and anti-obesity properties of Samanea saman through in vitro and in vivo approaches. J Cell Biochem. 2018;2018:1–11. doi: 10.1002/jcb.27385. [DOI] [PubMed] [Google Scholar]
- 2.Sanches J.-M., Zhao L.-N., Wollheim C.-B., Kaldis P. Pathophysiology of type 2 diabetes and the impact of altered metabolic interorgan crosstalk. FEBS J. 2021;290:620–648. doi: 10.1111/febs.16306. [DOI] [PubMed] [Google Scholar]
- 3.Esquivel-Gutiérrez E.R., Manzo-Avalos S., Peña-Montes D.J., Saavedra-Molina A., Morreeuw Z.P., Reyes A.G. Hypolipidemic and antioxidant effects of Guishe extract from Agave lechuguilla, a Mexican plant with biotechnological potential, on streptozotocin-induced diabetic male rats. Plants. 2021;10:1–12. doi: 10.3390/plants10112492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sompong W., Muangngam N., Kongpatpharnich A., et al. The inhibitory act ivity of herbal medicines on the keys enzymes and steps related to carbohydrate and lipid digestion. BMC Compl Alternative Med. 2016;16:1–9. doi: 10.1186/s12906-016-1424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spinola V., Castilho P.C. Assessing the in vitro inhibitory effects on key enzymes linked to type-2 diabetes and obesity and protein glycation by phenolic compounds of Lauraceae plant species endemic to the Laurisilva forest. Molecules. 2021;26:1–11. doi: 10.3390/molecules26072023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller T.D., Bluher M., Tschop M.H., DiMarchi R.D. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21:201–223. doi: 10.1038/s41573-021-00337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair R.V.R., Varma K., Paul B., Amalraj A., Kuttapan S. Evaluation of the anti-hyperglycemic and antioxidant activities of a novel phytochemical formulation. Phytomed Plus. 2021;2021:1–7. doi: 10.1016/j.phyplu.2021.100093. [DOI] [Google Scholar]
- 8.Temviriyanukul P., Sritalahareuthai V., Promyos N., et al. The effect of sacred Lotus (Nelumbo nucifera) and its mixtures on phenolic profiles, antioxidant activities, and inhibitions of the key enzymes relevant to alzheimer's disease. Molecules. 2020;25:1–18. doi: 10.3390/molecules25163713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanisha Majumdar M. Evaluation of in vitro anti-hyperglycemic, anti-lipase and antioxidant activities of Salvia hispanica and Chenopodium quinoa seeds. Int J Biol Pharm Allied Sci. 2018;7:302–312. [Google Scholar]
- 10.Wang Z., Hu J., Hamzah S.S., et al. N-Butanol extract of lotus seeds exerts antiobesity effects in 3T3-L1 preadipocytes and high-fat diet-fed mice via activating adenosine monophosphate activated protein kinase. J Agric Food Chem. 2019;67:1092–1103. doi: 10.1021/acs.jafc.8b05281. [DOI] [PubMed] [Google Scholar]
- 11.Hernández-Pérez T., Valverde M.E., Orona-Tamayo D., Paredes-Lopez O. Chia (Salvia hispanica): nutraceutical properties and therapeutic applications. Proceedings. 2019;53:1–5. doi: 10.3390/proceedings2020053017. [DOI] [Google Scholar]
- 12.Rahman J.M., de Camargo A.C., Shahidi F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J Funct Foods. 2017;35:622–634. doi: 10.1016/j.jff.2017.06.044. [DOI] [Google Scholar]
- 13.Joubert M.B.V., Ingaramo P., Oliva M.E., D'Alessandro M.E. Salvia hispanica L. (chia) seed ameliorates liver injury and oxidative stress by modulating NrF2 and NFκB expression in sucrose-rich diet-fed rats. Food Funct. 2022;13:7333–7345. doi: 10.1039/D2FO00642A. [DOI] [PubMed] [Google Scholar]
- 14.Lovato A.D.C., Corgozinho M.L.M.V., Alves L.V., et al. Effect of the use of Chia (Salvia Hispanica L.) seeds on antioxidant status and anthropometric parameters in obese, type 2 diabetics and/or hypertensive patients. Res Soc Dev. 2022;11:1–10. doi: 10.33448/rsd-v11i4.27432. [DOI] [Google Scholar]
- 15.Tan M., Chang S., Liu J., et al. Physicochemical properties, antioxidant and antidiabetic activities of polysaccharides from quinoa (Chenopodium quinoaWilld.) seeds. Molecules. 2020;25:1–18. doi: 10.3390/molecules25173840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X., He X., Sun J., Wang Z. Phytochemical composition, antioxidant activity, α-glucosidase and acetylcholinesterase inhibitory activity of quinoa extract and its fractions. Molecules. 2022;27:1–18. doi: 10.3390/molecules27082420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro-Perez D., Radcliffe J., Tierney A., Jois M. Quinoa seed lowers serum triglycerides in overweight and obese subjects: a dose-response randomized controlled clinical trial. Curr Dev Nutr. 2017;1:1–9. doi: 10.3945/cdn.117.001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An T., Liu J.-X., Yang X.-Y., Lv B-h, Wu Y.-X. Supplementation of quinoa regulates glycolipid metabolism and endoplasmic reticulum stress in the high-fat diet-induced female obese mice. Nutr Metab. 2021;18:1–11. doi: 10.1186/s12986-021-00622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De B., Bhandari K., Katakam P., Goswami T.K. Development of a standardized combined plant extract containing nutraceutical formulation ameliorating metabolic syndrome components. SN Appl Sci. 2019;1:1–12. doi: 10.1007/s42452-019-1518-9. [DOI] [Google Scholar]
- 20.Hsu C.-Y., Lin G.-M., Chang S.-T. Hypoglycemic activity of extracts of Chamaecyparis obtuse var. Formosana leaf in rats with hyperglycemia induced by high-fat diets and streptozotocin. J Trad Complement Med. 2020;10:389–395. doi: 10.1016/j.jtcme.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H.-J., Chiang B.-H. Anti-diabetic effect of a traditional Chinese medicine formula. Food Funct. 2012;3:1161–1169. doi: 10.1039/C2FO30139C. [DOI] [PubMed] [Google Scholar]
- 22.Yarrappagaari S., Thopireddy L., Cheemanapalli S., Narala V.-R.-R., Mohan K.-C., Saddala R.-R. Phytochemical analysis of Cleome viscosa active polyphenolic compounds possessing antidiabetic activity. Pharmacogn Res. 2022;14:195–203. doi: 10.5530/pres.14.2.28. [DOI] [Google Scholar]
- 23.Feng J., Zhang J., Zhang J., et al. Enhanced methane production of vinegar residue by response surface methodology. Amb Express. 2017;7:1–8. doi: 10.1186/s13568-017-0392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayuo J., Abukari M.A., Pelig-Ba K.B. Optimization using central composite design (CCD) of response surface methodology (RSM) for biosorption of hexavalent chromium from aqueous media. Appl Water Sci. 2020;10:1–12. doi: 10.1007/s13201-020-01213-3. [DOI] [Google Scholar]
- 25.Behera S.K., Meena H., Chakraborty S., Meikap B.C. Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int J Min Sci Technol. 2018;28:621–629. doi: 10.1016/j.ijmst.2018.04.014. [DOI] [Google Scholar]
- 26.Bacha E.-G. Response surface methodology modelling, experimental validation and optimization of acid hydrolysis process parameters for nanocellulose extraction. S Afr J Chem Eng. 2022;40:176–185. doi: 10.1016/j.sajce.2022.03.003. [DOI] [Google Scholar]
- 27.Organization for Economic Co-operation and Development (OECD) The OECD Guideline for Testing of Chemicals, OECD; Rome: 2001. Test No. 423: Acute Oral Toxicity-Acute Toxic Class Method. 2001. [Google Scholar]
- 28.Jurgonski A., Billing-Marczak K., Juskiewicz J., Krotkiewski M. Formulation of a mixture of plant extracts for attenuating postprandial glycemia and diet-induced disorders in rats. Molecules. 2019;24:1–12. doi: 10.3390/molecules24203669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim M.-O., Seo J.-H., Kwon E.-B., et al. Aceriphyllum rossii extract exerts lipid lowering action in both normal and hyperlipidemic mice. Nat Prod Commun. 2018;13:471–474. doi: 10.1177/1934578X1801300423. [DOI] [Google Scholar]
- 30.Belayneh Y.-M., Birru E.-M. Antidiabetic activities of hydromethanolic leaf extract of calpurnia aurea (ait.) benth. subspecies aurea (fabaceae) in mice. Evid Based Complement Alternat Med. 2018;2018:1–9. doi: 10.1155/2018/3509073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asokan S.-M., Wang T., Su W.-T., Lin W.-T. Antidiabetic effects of a short peptide of potato protein hydrolysate in STZ-induced diabetic mice. Nutrients. 2019;11:1–13. doi: 10.3390/nu11040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho Y.-R., Lee J.-A., Kim Y.-Y., Kkang J.-S., Lee J.-H., Ahn E.-K. Anti-obesity effects of Clausena excavate in high-fat diet-induced obese mice. Biomed Pharmacother. 2018;99:253–260. doi: 10.1016/j.biopha.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 33.Koh Y.-M., Jang S.-W., Ahn T.-W. Anti-obesity effect of Yangkyuksanwhatang in hig-fat diet-induced obese mice. BMC Compl Alternative Med. 2019;19:1–12. doi: 10.1186/s12906-019-2669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ani J.-U., Okoro U.-C., Aneke L.-E., et al. Application of response surface methodology for optimization of dissolved solids adsorption by activated coal. Appl Water Sci. 2019;9:1–11. doi: 10.1007/s13201-019-0943-7. [DOI] [Google Scholar]
- 35.Zhou Q., Ding L., Zhu Y., Zhong M., Yang C. Process parameters optimization of gallic acid removal from water by MIEX resin based on response surface methodology. Processes. 2020;8:1–11. doi: 10.3390/pr8030273. [DOI] [Google Scholar]
- 36.De B., Bhandari K., Singla R.K., et al. Chemometrics optimized extraction procedures, phyto-synergistic blending, and in vitro screening of natural enzyme inhibitors among leaves of Tulsi, Banyan, and Jamun. Phcog Mag. 2015;12:1–12. doi: 10.4103/0973-1296.172956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preguica I., Alves A., Nunes S., et al. Diet-induced rodent models of obesity-related metabolic disorders—a guide to a translational perspective. Obes Rev. 2020;2020:1–29. doi: 10.1111/obr.13081. [DOI] [PubMed] [Google Scholar]
- 38.Ramesh C., Prameela R.A. In vivo and in vitro evaluation of Tephrosia calophyll for anti-diabetic properties. Int J Pharm Pharmaceut Sci. 2018;10:138–144. doi: 10.22159/ijpps.2018v10i6.25551. [DOI] [Google Scholar]
- 39.Kim M.O., Seo C.H., Kwon E.B., et al. Aceriphyllum rossii exerts lipid-lowering action in both normal and hyperlipidemic mice. Nat Prod Commun. 2018;13:471–474. doi: 10.1177/1934578X1801300423. [DOI] [Google Scholar]
- 40.Ochiai M. Evaluating the appropriate oral lipid tolerance test model for investigating plasma triglyceride elevation in mice. PLoS One. 2020;15:1–17. doi: 10.1371/journal.pone.0235875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sultana R., Alashi A.M., Islam K., Saifullah Md, Haque C.E., Aluko R.E. Inhibitory activities of polyphenolic extracts of Bangladeshi vegetables against α-amylase, α-glucosidase, pancreatic lipase, renin, and angiotensin-converting enzyme. Foods. 2020;9:1–13. doi: 10.3390/foods9070844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arika W.M., Kibiti C.M., Njagi J.M., Ngugi M.P. Anti-obesity effects of dichloromethane leaf extract of Gnidia glauca in high fat diet-induced obese rats. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02800. 1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parhofer G.K. Interaction between glucose and lipid metabolism: more than diabetic dyslipidemia. Diabetes Metab J. 2015;39:353–362. doi: 10.4093/dmj.2015.39.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song X., Dong H., Zang Z., et al. Kudzu resistant starch: an effective regulator of type 2 diabetes mellitus. Oxid Med Cell Longev. 2021;2021:1–15. doi: 10.1155/2021/4448048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park Y.-J., Lee G.-S., Cheon S.-Y., Cha Y.-Y., An H.-J. The anti-obesity effects of Tongbi-San in a high-fat diet-induced obese mouse model. BMC Compl Alternative Med. 2019;19:1–14. doi: 10.1186/s12906-018-2420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin S.S., Yoon M. The herbal composition GGEx18 from Laminaria japonica, Rheum palmatum, and Ephedra sinica inhibits high-fat diet-induced hepatic steatosis via hepatic PPARα activation. Pharm Biol. 2012;50:1261–1268. doi: 10.3109/13880209.2012.666982. [DOI] [PubMed] [Google Scholar]
- 47.Glastras S.J., Chen H., Teh R., et al. Mouse models of diabetes, obesity and related kidney disease. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0162131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandeya P.R., Lamichhane R., Lamichhane G., et al. 18KHT01, a potent anti-obesity polyherbal formulation. Front Pharmacol. 2021;12:1–19. doi: 10.3389/fphar.2021.807081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wicks S.E., Nguyen T.-T., Breaux C., Kruger C., Stadler K. Diet-induced obesity and kidney disease – in search of a susceptible mouse model. Biochimie. 2016;124:65–73. doi: 10.1016/j.biochi.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y., Ge X., Li X., et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020;11:1–14. doi: 10.1038/s41419-020-03122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brus J.E., Quan D.L., Wiley K.J., et al. Diet significantly influences the immunopathology and severity of kidney injury in male C57Bl/6J mice in a model dependent manner. Nutrients. 2021;13:1–18. doi: 10.3390/nu13051521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fraulob J.C., Ogg-Diamantino R., Fernandes-Santos C., Aguila M.B., Mandarim-de-Lacerda C.A. A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 2010;46:212–223. doi: 10.3164/jcbn.09-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. β-cell deficit and increased β -Cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 54.Hull R.L., Kodama K., Utzschneider K.M., Carr D.B., Prigeon R.L., Kahn S.E. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia. 2005;48:1350–1358. doi: 10.1007/s00125-005-1772-9. [DOI] [PubMed] [Google Scholar]
- 55.Attane C., Peyot M.-L., Lussier R., et al. Differential insulin secretion of high-fat diet-fed C57BL/6NN and C57BL/6NJ mice: implications of mixed genetic background in metabolic studies. PLoS One. 2016;11:1–11. doi: 10.1371/journal.pone.0159165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.