Abstract
Adsorption heat conversion systems can provide heating and cooling across time and space in a more environmentally friendly way. Porous materials are potential candidates for water-based adsorption thermal conversion, in which a metal–organic framework (MOF) has a larger specific surface area and porosity than other porous matrices. However, many MOFs with high saturated adsorption capacity have great deficiencies in performance at low water vapor partial pressure, which hinder their application in adsorption thermal conversion. To improve the water vapor adsorption performance of MIL-101 (Cr), different contents of magnesium chloride, lithium chloride, and lanthanum chloride are mixed into MIL-101 (Cr) by an impregnation method. The properties and structures of the materials are characterized by XRD, SEM, nitrogen adsorption tests, water vapor adsorption tests, TG, FTIR, and so on. The results show that the saturated water vapor adsorption capacity of the sample impregnated with salt increases by 1.5–2.3 times, up to 2.24 g/g, compared with that of the unimpregnated sample. When the partial pressure of water vapor is 0.3, the adsorption capacity increases by 5.3–7.5 times and reaches 0.68 g/g at most. The maximum heat storage density of impregnated samples can be increased by 866 J/g. Impregnated MgCl2 can greatly improve the adsorption and thermal conversion performance of MOF, and impregnated MgCl2 and the proper amount of LiCl can further improve the performance of the material system. Our experiments show that the composite impregnation of magnesium chloride and the proper amount of lithium chloride can improve the application performance of the MOF materials in the adsorption thermal conversion process.
1. Introduction
The energy problem is one of the important challenges facing today’s society. Energy storage and utilization across time and space are important methods to solve this problem, and it is also an urgent problem to be further solved at present. Heat storage is a more economical and efficient method of energy storage than electricity storage. Heat storage is divided into sensible heat storage, latent heat storage, and chemical reaction heat storage.1 Chemical reaction thermal storage has the characteristics of high-energy storage density and a wide temperature range.2 The adsorption heat pump is widely used in chemical reaction heat storage. It is an exothermic/endothermic system that utilizes adsorption/desorption between adsorbents and adsorbates and has been applied in industrial waste heat recovery,3 seasonal heat storage,4 refrigeration,5 and so on. According to the adsorption process, adsorption heat pumps can be divided into three categories:6 physical adsorption, chemical adsorption, and mixed adsorption. Ammonia,7 acetone,8 water vapor,9 etc., are commonly used adsorbents in adsorption heat pumps. Because water is easily available and harmless, more adsorption heat pump systems choose water vapor as the adsorbate material. The commonly used adsorbents are mainly various porous materials, such as zeolite,10 expanded vermiculite,11 kaolin,12 diatomite,13 microporous aluminum phosphate,14 and so on. They physically adsorb with water at lower temperatures and release heat, while they store heat by dehydration and heat absorption at higher temperatures.1
MOFs are a new kind of porous material developed in recent years. Compared with the porous materials mentioned above, MOF materials have the characteristics of large specific surface area, uniform pore structure distribution, and more micropore distribution, so they have better adsorption performance. MIL-101,15 MIL-125,16 MOF-505,17 UIO-66,18 and MOF-80119 have been widely studied and paid attention to as adsorbent materials. Because of their excellent adsorption performance, MOFs have broad application prospects in adsorption thermal conversion. Harry Kummer47 and others studied HKUST-1 and MIL-101 (Cr) as adsorption refrigerants and found that these materials have excellent adsorption properties. Jeremias et al.45 studied the application of metal–organic framework UiO-66 and MIL-101 in adsorption thermal conversion. Henninger and others46 studied ISE-1 and thought that this MOF could be used as an adsorbent for low-temperature heating and cooling applications. MIL-101 is a promising material for adsorption heat conversion because of its large specific surface area, high saturated water vapor adsorption capacity, and good water stability. Ehrenmann et al.22 studied the performance of MIL-101 (Cr) as an adsorbent in an adsorption heat conversion system and found that its saturated adsorption capacity of water vapor can reach 1 g/g and its adsorption/desorption heat density can reach 2.557 kJ/g and 2.620 kJ/g. The disadvantage of MIL-101 (Cr) is that its water vapor adsorption capacity is not high at a low vapor pressure. Anupam Khutia et al.23 prepared four kinds of nitro- or amino-functionalized MIL-101 (Cr). Although the saturated adsorption capacity of water vapor was increased, the adsorption capacity for a low water vapor partial pressure was hardly improved. George Akiyama et al.17 prepared MIL-101 (Cr) with HCl, H2SO4, and HNO3 instead of HF, which slightly increased the adsorption heat of the material but reduced the saturated adsorption capacity of MIL-101 (Cr) by 0.1 g/g. Existing studies have shown that the effect of increasing adsorption performance by changing the structure of MOF itself is limited, and it is difficult to improve the adsorption performance of materials under low water vapor partial pressure. Therefore, to further improve the water vapor adsorption performance and adsorption heat conversion performance of MIL-101 (Cr), it is imperative to prepare a composite system by compounding MIL-101 (Cr) with other adsorption heat conversion materials such as hydrate salt.2
Salt is a widely studied adsorbent material because of its low price and wide application temperature range. It can be used as adsorption heat conversion material at high temperatures,23 medium temperatures,24 and low temperatures.25 Because the hydration/dehydration temperature of salt is almost the same as the adsorption/desorption temperature of the porous matrix, it is an effective means of preparing a composite system of salt and porous materials by impregnation. Yu et al.28 prepared a consolidated composite matrix composed of activated carbon, LiCl, expanded graphite, and silica solution. Under the operating conditions of 85 and 40 °C, the heat storage efficiency of the system is 93% and the heat storage density is 874 kJ/kg. Emilie Courbon et al.27 prepared silica gel loaded with CaCl2 by continuous impregnation/drying, and its energy storage density reached 350 kWh/m3. Vincenza Brancato et al.30 prepared silicone foam filled with MgSO4·7H2O. The results show that a good relationship seems to be established between foam and salt, and the dehydration/hydration ability of salt is not hindered by the foaming process. Hanane Ait Ousaleh et al.48 combined MgSO4 and MgCl2 into a graphene matrix for medium and low-temperature heat storage. MgSO4 and MgCl2 composites achieved excellent energy storage densities of 1194.3 and 890 J/g, respectively. These studies show that the composite of porous materials and hydrated salts can significantly improve the heat storage capacity and adsorption capacity of a porous matrix at low temperatures.3
Similar to other porous materials, it is also an important method to improve the heat storage and adsorption properties of MOF materials by impregnation and other methods. Luis Garzón-Tovar et al.32 prepared the mixture of CaCl2 and UIO-66 by spray drying continuous flow method. The material has a heat storage density of 367 kJ kg–1, and the composite is almost no deliquescence. Emilie Courbon et al.29 doped LiCl into UIO-66 by the impregnation method, the saturated adsorption capacity of the composite reached 2.15 g/g and the heat storage density reached 900 kJ/kg. All of the above studies show that it is an effective means to combine MOF and salt to improve the water vapor adsorption performance and adsorption heat conversion performance of MOF materials. The dual-solvent method is an impregnation method developed from ordinary impregnation methods. Hanane Ait Ousaleh29 and others prepared graphene containing MgSO4 and MgCl2 by dual-solvent method. Compared with the double solvent method, the traditional impregnation method has the advantages of simple operation and is suitable for large-scale industrial production. Therefore, we choose the impregnation method to obtain samples with a morphology closer to the practical application background. MIL-101 (Cr) can adsorb water vapor at room temperature, while salt can hydrate at room temperature. The working ranges of the two materials are similar. Salt has a better affinity with water at low partial pressure, and it is also expected to improve the water vapor adsorption performance of the MOF at low water pressure. The large pore volume of MIL-101(Cr) can accommodate more salt, which can greatly improve the water absorption. Therefore, we combine MIL-101 (Cr) with salt to improve the water vapor adsorption capacity and saturated water vapor adsorption capacity of MIL-101 (Cr) at low water vapor partial pressure and at the same time increase the heat storage density of MIL-101 (Cr). We chose MgCl2, a salt with high heat storage density, low cost, and easy to obtain, to compound with MIL-101 (Cr). N’Tsoukpoe et al.6 evaluated the applicability of 125 hydrated salts, among which LiCl and LaCl3 have higher heat storage density than other salts. The article mentioned that the thermal storage density of MgCl2 can reach 1252.8 kJ/g, LaCl3 has a thermal storage density of 957.6 J/g, and LiCl has a thermal storage density of 1029.6 J/g, which is higher than other chloride salts. So we also attempted to impregnate LiCl or LaCl3 at the same time as MgCl2, exploring the effect of double salt impregnation. We also tried to impregnate another salt while impregnating MgCl2, to further improve the water vapor adsorption capacity and adsorption heat conversion performance of the material system.
2. Materials and Methods
2.1. Materials
The chemicals used in the experiment included terephthalic acid (99%), chromium nitrate (99%), N,N-dimethylformamide (DMF, 99.5%), hydrofluoric acid (40%), lithium chloride (99%), anhydrous magnesium chloride (99%), lanthanum chloride heptahydrate (99.99%), and anhydrous ethanol (99.7%), all of which are of commercial quality and can be used directly without further purification. All of the above chemicals were purchased from Beijing Honghu United Chemical Products Co., Ltd. Beijing, China.
2.2. Preparation of MIL-101(Cr) Composite MgCl2, LiCl/LaCl3 System
First, we prepared undoped MIL-101 (Cr)32,33 according to the preparation method of Yan and others’ published work and then impregnated the obtained products in solutions containing different amounts of salts. Each solution was obtained by mixing and stirring weighed salt and 20 mL of water, and the salt content in the solution is listed in Table 1. We hope that the salt concentration of the impregnation solution is high, so that as much salt can enter or adhere to MIL-101 (Cr) as possible, and it is necessary to ensure that it will not precipitate. Therefore, we chose 5 g salt with 20 mL water to prepare a salt solution with a mass concentration of 20%. After the salt solution was prepared, a 0.2 g MIL-101 (Cr) sample was added to each solution and stirred at the same time, so that it was uniformly mixed to obtain a uniform green suspension. After sealing the beaker and standing for 24 h, centrifuge and remove the supernatant and dry it in a 90 °C oven for 11 h. After drying, the sample was ground to obtain a green powder sample. We numbered the samples according to different preparation conditions, and the specific numbering is shown in Table 1.
Table 1. Sample Number (Based on Salt Content in Impregnation Solution).
| sample number | MgCl2 mass (g) | LiCl mass (g) | LaCl3·7H2O mass (g) | molar ratio |
|---|---|---|---|---|
| 1 # | 0 | 0 | 0 | |
| 2 # | 5.00 | 0 | 0 | |
| 3 # | 5.00 | 0.56 | 0 | 1:0.25:0 |
| 4 # | 5.00 | 0.74 | 0 | 1:0.33:0 |
| 5 # | 5.00 | 1.11 | 0 | 1:0.5:0 |
| 6 # | 5.00 | 0 | 4.88 | 1:0: 0.25 |
| 7 # | 5.00 | 0 | 6.50 | 1:0: 0.33 |
| 8 # | 5.00 | 0 | 9.75 | 1:0: 0.5 |
3. Results and Discussion
3.1. XRD Patterns of Samples
We use an X-ray diffractometer (XRD, Rigaku D/max2500X) to scan the diffraction spectrum of the sample, in which the X-ray source is Cu-Ka ray, the scanning step is 0.01, and the conventional 2 θ is used/θ (5–50). The X-ray diffraction pattern of the sample is shown in Figure 1. It can be seen from the figure that the XRD curve of the 1# sample is the same as the characteristic curve of MIL-101 (Cr) in the literature, and its characteristic peak corresponds to the simulation results, indicating that the 1# sample is pure MIL-101 (Cr). After impregnation, only part of the characteristic peaks of MIL-101 (Cr) were retained, and the intensity of the peaks was weakened. This shows that salt impregnation leads to the change of pore structure and properties or the disorder of crystal structure.34 The XRD images of all impregnated samples contain characteristic peaks of MgCl2,35 which indicates that the MgCl2 crystal is attached to the surface or between particles of MIL-101 (Cr). There is no characteristic peak of LiCl in the samples impregnated with LiCl and MgCl2, so we cannot judge the existence state of LiCl by XRD. The peak of LaCl3 is also detected in the samples impregnated with LaCl and MgCl2 at the same time, which indicated that LaCl3 crystals also existed on the surface or between particles of the samples impregnated with LaCl3.4 The main peak in the range 22–26 is MgCl2·6(H2O). This may be due to the slight deliquescence of salt on the surface of the sample. Therefore, this peak can be found in samples 2–8. MgCl2 and LiCl exist simultaneously in samples 3, 4, and 5, while MgCl2 and LaCl3 exist simultaneously in samples 6, 7, and 8. We believe that the presence of lithium chloride enhances the deliquescence of samples, thus enhancing the peak of magnesium chloride hexahydrate. However, the crystal size of lanthanum chloride is larger, which may weaken the deliquescence of samples.
Figure 1.
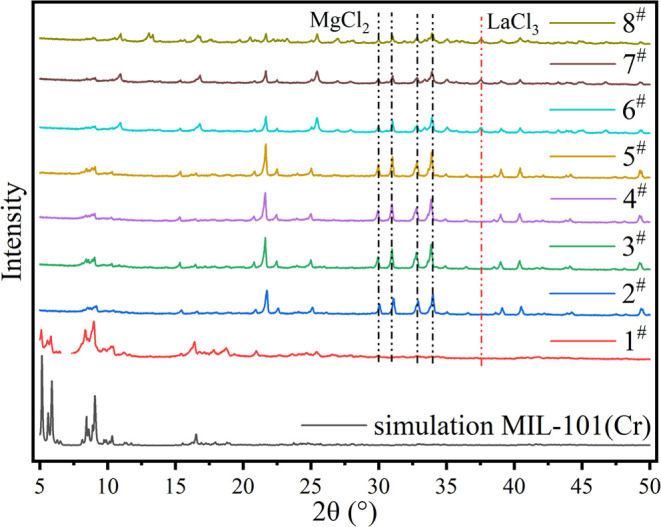
XRD patterns of samples.
3.2. SEM Images of the Samples MIL-101 (Cr) and MIL-101 (Cr, Mn)
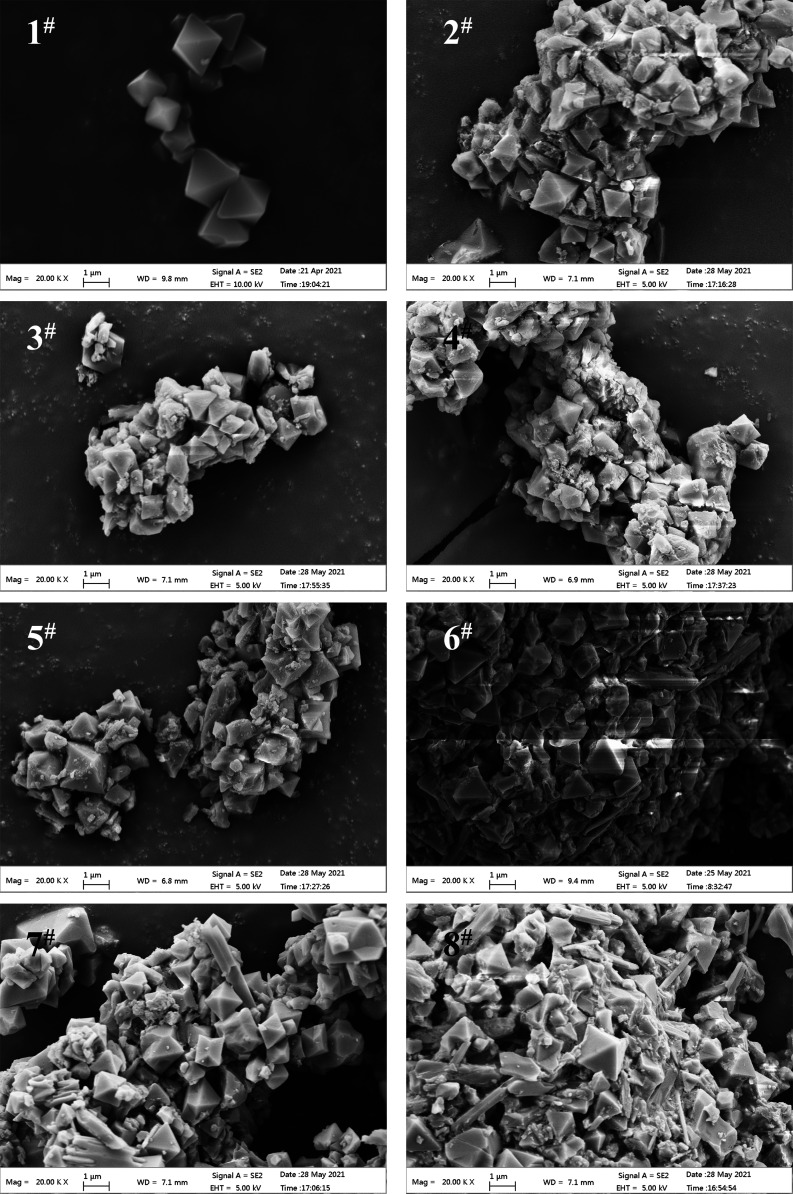
We used scanning electron microscopy (FESEM, Zeiss supra 55) to characterize and analyze the microscopic particle morphology of the prepared samples. Before the observation, we sprayed gold onto the samples. Figure 2 shows SEM images of eight samples. As can be seen from Figure 2, the particles of MIL-101(Cr) have a regular octahedral structure and a smooth surface. After impregnation, the sample still basically keeps an octahedral structure, but burrs and pits appear on the surface. This is also the reason for the weakening of the MIL-101 (Cr) characteristic peak in the XRD results of samples. It can be observed that most of the particles condense with salt, and the attached salt can also be observed on the scattered particle surface, which is consistent with the results of XRD.
Figure 2.
SEM images of the samples.
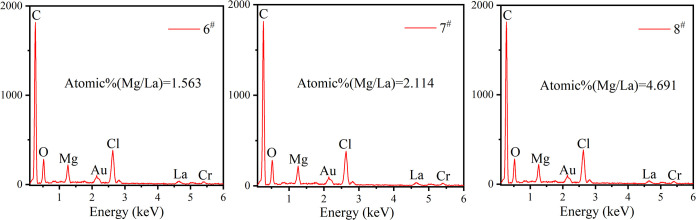
At the same time as morphology characterization, we carried out a microelemental analysis to observe the proportion and distribution of each element in the sample. Although the impregnation method is suitable for large-scale industrial production and the method is simple, the samples obtained by it may have uneven ion distribution. Therefore, we hope to determine whether the relative content of metal ions in the sample is basically consistent with that in the impregnation solution by the EDX test. Because EDS cannot detect the signal of the Li element, we only carried out relevant tests on samples 6–8. Figure 3 shows the EDS spectra of samples 6–8. The impregnation solution of samples 6–8 is a mixed solution of MgCl2 and LaCl3. Mg, Cl, and La elements can be detected in the energy spectrum. The atomic ratio of Mg to La in samples 6–8 increased with the increase of the molar ratio of Mg to La in the impregnation solution, which indicated that the salt content in the impregnation sample was directly related to the salt concentration in the impregnation solution.
Figure 3.
EDS spectra of the samples.
3.3. FTIR Spectra and the Raman Spectra of the Samples MIL-101 (Cr, Mn) and MIL-101 (Cr)
We used FTIR (Lambda FTIR-7600) to test the mid-infrared absorption spectrum of the sample. KBr was used as the background in the test. The spectral resolution is 2 cm–1, and the scanning range is 400–4000 cm–1. All samples showed a pattern similar to MIL-101 (Cr), which is consistent with that reported in the literature.36 MIL-101 (Cr) has a moderate absorption peak at the wave number of 587 cm–1, which is the stretching vibration peak of (Cr–O).37 The peaks of samples impregnated with the salt move to a high wave number, and the peak width becomes larger. This indicates that the chemical environment of the Cr–O bond in the impregnated samples may change. Compared with the unimpregnated sample, the impregnated sample has a new characteristic peak at 2276 cm–1, which is the characteristic peak of MgCl2.38 This indicates the presence of MgCl2 in the impregnated sample. A new peak appeared in the impregnated sample at 3242 cm–1, which, like the peak at 3423 cm–1, is caused by the stretching vibration of the H–O bond.39 Compared with MIL-101 (Cr), the impregnated sample increased the way of absorbing water vapor, and the connection mode between adsorbed water and the sample has increased. After impregnation, there are small peaks at 2925 and 2853 cm–1, which may be the peaks of liquid water molecules.39 This shows that MIL-101 (Cr) is deliquescent more easily by salt impregnation than before impregnation (Figure 4).
Figure 4.
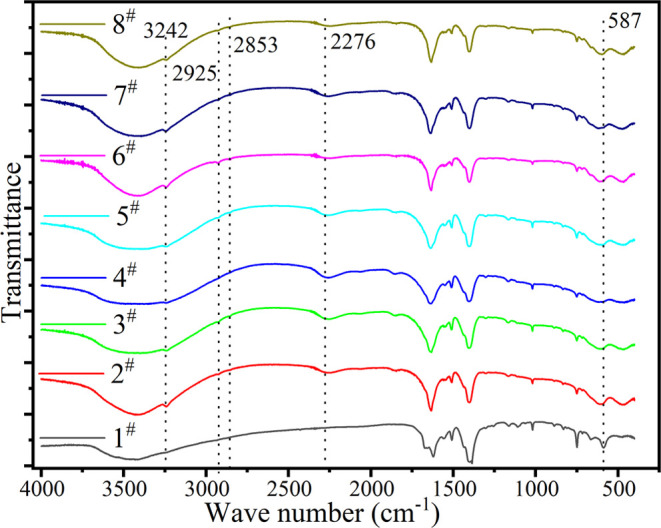
FTIR spectra of MIL-101 (Cr) and MIL-101 (Cr, Mn).
3.4. N2 Adsorption Isotherms of the Samples
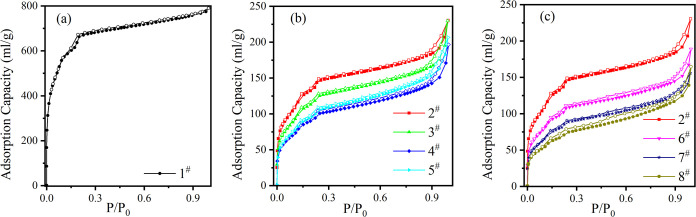
The sample was degassed at 120 °C for 6 h by using the vapor adsorption and specific surface pore size analyzer (BSD-PMV), and then the nitrogen adsorption curve of the sample was measured at 77.3 K constant temperature, and then the specific surface area and pore size distribution of the sample were obtained. Figure 5 shows the nitrogen adsorption curve of the sample. It can be seen from the figure that the nitrogen adsorption curve of MIL-101 (Cr) is a type I curve, and no obvious hysteresis loop is observed. The nitrogen adsorption curve of the sample impregnated with salt solution changed to a type II curve, and an H3-type hysteresis loop appeared. The change of the hysteresis loop of the nitrogen adsorption curve of impregnated samples shows that the proportion of mesopores in samples increases after impregnating salt solution, and the pore distribution is more dispersed than that without impregnation. Table 2 lists the BET-specific surface area and BJH average pore size of each sample. It can be seen from the table that the BET-specific surface area of the impregnated sample decreases greatly and the average pore size increases slightly, which also shows that the mesopore ratio of the impregnated sample increases.
Figure 5.
N2 isotherms of unimpregnated (a), impregnated magnesium chloride, lithium chloride (b), and impregnated magnesium chloride, green lanthanum (c).
Table 2. Variable Specific Surface Areas of the Samples.
| adsorbent | BET-specific surface area (m2/g) | the average pore diameter of BJH desorption (nm) |
|---|---|---|
| 1 # | 2119.7 | 2.4 |
| 2 # | 479.1 | 2.9 |
| 3 # | 416.3 | 3.4 |
| 4 # | 334.0 | 3.4 |
| 5 # | 349.6 | 3.5 |
| 6 # | 355.7 | 3.1 |
| 7 # | 292.5 | 3.2 |
| 8 # | 250.9 | 3.6 |
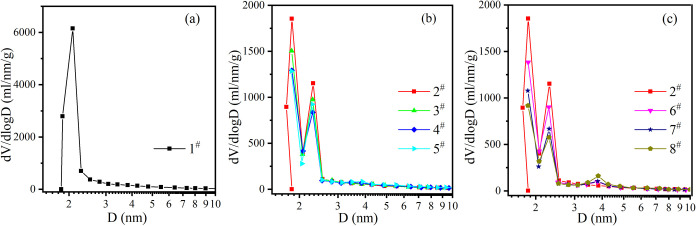
Figure 6 shows the pore size distribution of the sample. It can be found from the figure that the pores of MIL-101 (Cr) are distributed at 2 nm, while the impregnated samples have a mainly distributed pore size around 2 nm, which leads to the increase of pore ratio in the samples and then leads to the change of hysteresis loop in the nitrogen adsorption curve. The three chloride salts used in the experiment do not exist as separate ions in an aqueous solution, The Cl–-H2O cluster size is about 0.28–0.32 nm,40 the La3+-H2O cluster size is about 0.39 nm,41 and the Mg2+-H2O cluster size and the Li+-H2O cluster size are about 0.2 nm,42 which are all smaller than the 2 nm pore size of the sample without impregnation. Therefore, the pore size of MIL-101 (Cr) may be filled when the specific surface area of the sample is greatly reduced. The pore size distribution of samples impregnated with La increases to a small peak near 4 nm. Combined with the results of SEM and XRD, 4 nm pores may be formed between salts attached to the surface of MOF.5
Figure 6.
Pore size distribution of unimpregnated (a), impregnated magnesium chloride, lithium chloride (b), and impregnated magnesium chloride, green lanthanum (c).
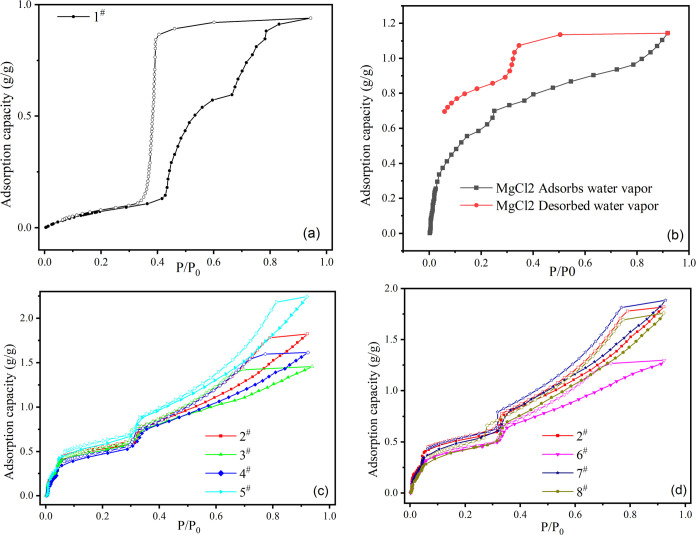
3.5. Water Vapor Isotherms of the Samples
Steam adsorption and a specific surface pore size analyzer (BSD-PMV) were used to measure the water vapor adsorption curve of the sample at 25 °C. Before the test, the sample was degassed at 120 °C for 6 h. After degassing, we put the sample in the instrument, set the water vapor adsorption temperature (25 °C), and input the saturated vapor pressure at this temperature. The instrument will automatically test the adsorption capacity of samples under different vapor pressures, so we need to wait for the instrument to finish the test. Figure 6 shows the water vapor adsorption curve of the sample. For unimpregnated samples, only MIL-101 (Cr) plays an adsorption role; therefore, the whole adsorption process is physical adsorption. For impregnated samples, MIL-101 (Cr) and impregnated salt are involved in the adsorption process; therefore, water vapor adsorption can be divided into two parts: physical adsorption and chemical adsorption. It can be seen from Figure 7 that the saturated water vapor adsorption capacity of the sample impregnated with salt is higher than that of pure MIL-101 (Cr). The saturated adsorption capacity of each sample is given in Table 3. In Figure 6, when P/P0 < 0.1, all impregnated samples have a sudden rising curve, which is mainly due to the hydration reaction between impregnated salt and water vapor. The desorption curve of the impregnated sample terminates at the highest part of this rising curve, which also shows that the adsorption process is mainly participated by salt, and the sample is chemisorbed at this stage, so it is difficult to desorb by physical means. Figure 6b shows the water vapor adsorption curve of MgCl2. The water vapor adsorption isotherms of impregnated samples are very similar to those of pure salts, and the steps correspond to the formation of crystalline hydrates at low P/P0 and then the absorption of water by deliquescent salts at higher P/P0 due to deliquescence and absorption of salt hydrates, which indicates that most of the water adsorbed by impregnated samples comes from impregnated salts.43,44 When we prepare samples, the amount of magnesium chloride in the impregnation solution used for each sample is the same, but different amounts of the second salt are added. Therefore, the relative content of MgCl2 in the impregnation solution of samples 3 and 4 will be lower than that of the impregnation solution of sample 2, and the content of MgCl2 in these two samples is higher than that of LiCl. For the surface of the sample, the number of water molecules that lithium chloride can bind to is lower than that of magnesium chloride, and the hydrates formed at this time may block the pores of the MIL, preventing more water vapor from entering the pores or coming into contact with more salts. Therefore, the adsorption capacity of samples 3 and 4 is lower than that of sample 2. The significant increase in the adsorption capacity of sample 5 may be because the amount of salt in the impregnation solution of sample 5 is the highest compared with samples 3 and 4, and then, there are more salts on the surface. For sample 6, compared with sample 2, part of MgCl2 was replaced by LaCl3 with a larger molecular diameter, which led to the decrease of the total amount of salts on the surface of the sample that could participate in hydration, and the formed hydrate also led to the blockage of the pores of MIL-101. According to our current experimental results, we think that the further increase of LiCl content may contribute to the improvement of adsorption capacity. When P/P0 > 0.8, the adsorption capacity of MIL-101 (Cr) for water vapor hardly increased, but the adsorption capacity of impregnated samples still increased at this stage. This shows that when P/P0 > 0.8, MIL almost no longer adsorbs water vapor, and the increase of adsorption capacity in impregnated samples is due to the adsorption of water vapor by impregnated salts. Gordeeva18 et al. studied the process of water adsorption by salt. Their research showed that hydrated salt adsorbed water vapor to form crystal hydrate at low P/P0, and hydrated salt continued to adsorb water vapor at high P/P0 and then deliquesced to form salt aqueous solution. For our sample, according to the results of pore size analysis, the volume of adsorbed water vapor has far exceeded the specific pore volume of the composite material. Therefore, we think that most of the adsorbed water after impregnating the sample P/P0 > 0.8 comes from the excessive water vapor adsorbed by salt, which leads to deliquescence and saline solution. All samples have obvious hysteresis loops at 0.4 < P/P0 < 0.8, which indicates that the samples adsorb water vapor by capillary action at this stage.45 The hysteresis loop of the impregnated sample is much reduced. According to the experimental results of nitrogen adsorption, the average pore size of the impregnated sample increases, which may lead to the decrease of the resistance caused by the capillary effect in the water vapor desorption stage, resulting in the decrease in the hysteresis loop. The decrease in the hysteresis loop may also be due to the decrease in pores and the increase in adsorption capacity, so it seems that the hysteresis loop caused by pore water absorption also becomes smaller. Compared with the samples impregnated with different impregnating solutions, the saturated adsorption capacity of the samples impregnated with MgCl2 and LaCl3 did not increase significantly compared with the samples impregnated with MgCl2 alone. However, the increase in saturated adsorption capacity was greatest for the samples immersed in the most LiCl, suggesting that further addition of LiCl after addition of MgCl2 is a more effective means than further addition of LaCl3.6
Figure 7.
Water vapor isotherms of unimpregnated (a), water vapor isotherms of MgCl2 (b), impregnated magnesium chloride, lithium chloride (c), and impregnated magnesium chloride, green lanthanum (d).
Table 3. Maximum Adsorption Capacity and the α Value of Samples.
| adsorbent | saturated adsorption capacity (g/g) | adsorption capacity at P/P0 = 0.3 (g/g) | α |
|---|---|---|---|
| 1 # | 0.94 | 0.09 | 0.51 |
| 2 # | 1.82 | 0.58 | 0.46 |
| 3 # | 1.46 | 0.59 | 0.34 |
| 4 # | 1.61 | 0.55 | 0.40 |
| 5 # | 2.24 | 0.68 | 0.49 |
| 6 # | 1.30 | 0.48 | 0.36 |
| 7 # | 1.88 | 0.59 | 0.46 |
| 8 # | 1.76 | 0.49 | 0.48 |
According to Canivet, the change of α can be used to characterize the adsorption capacity of water vapor under the low partial pressure of materials. α is the ratio of pressure to saturated vapor pressure at room temperature when the adsorbed substance reaches half of the maximum adsorption capacity (P/P0). When α is less than 0.05, this indicates that the material has strong water vapor adsorption capacity at low partial pressure. When α is greater than 0.45, the water vapor adsorption ability of the material is weak under low partial pressure.36 The smaller the α, the stronger the adsorption capacity of the material at lower water vapor partial pressure. Table 3 lists the adsorption capacity and α value of the sample at a partial pressure of 0.3. It can be seen from the table that the α value of the samples impregnated with salt solution has been improved to some extent, and the water vapor adsorption capacity of the impregnated samples under lower vapor pressure has also been improved. For samples impregnated with the same salt solution, the lower the concentration of the salt solution, the smaller the value of α after impregnation, indicating that the material is relatively hydrophilic. The sample with the largest LiCl content has the highest water vapor adsorption capacity and the largest saturated adsorption capacity at low water pressure, while the addition of LaCl3 has no obvious improvement compared with the impregnation of MgCl2.7
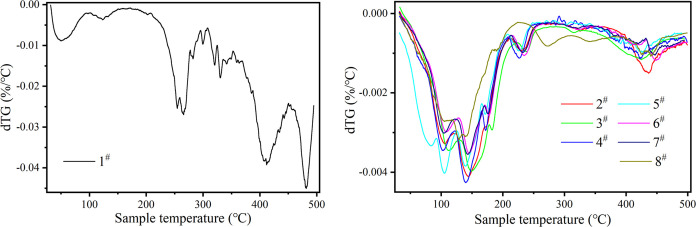
3.6. TG Curves of the Samples
Samples subjected to water vapor adsorption–desorption experiments were placed in a synchronous thermogravimetric analyzer (TGA, PerkinElmer STA8000), and their mass change curves were measured at a temperature range of 30–500 °C and a heating rate of 5 °C/min, as shown in Figure 8. As can be seen from Figure 8, all of the impregnated samples can observe a significant decline in quality before 200 °C. At 200 °C, the remaining mass of each sample accounts for the initial mass, as shown in Table 4, and the mass of impregnated samples decreases by 30–40%. For impregnated samples, the hydrated salt in the sample removes the water bound by adsorption at this temperature. After 200 °C, the quality change of impregnated samples tends to be stable, while that of unimpregnated samples changes greatly. Compared with 200 °C, the final mass of unimpregnated samples decreased by 56.1%, and that of impregnated samples decreased by about 30%. Figure 8 shows a DTG curve of the sample during heating. For unimpregnated samples, the peak at about 50 °C represents the desorption of adsorbed water on the samples. The peak between 200 and 350 °C represents the decomposition of free organic ligands and the OH/F group and the decomposition of terephthalic acid. Peaks above 400 °C represent the decomposition of the organic ligand chain of the sample itself. For the impregnated sample, the DTG peak at 200 °C is less than that of the unimpregnated sample, which may be attributed to the salt impregnated in MOF.
Figure 8.
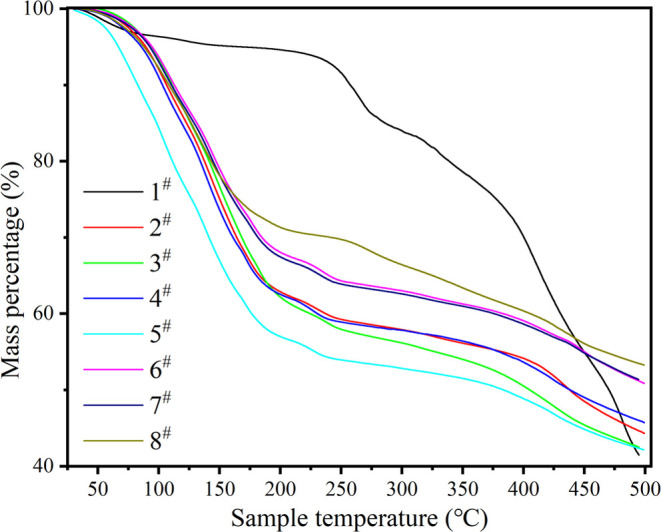
Thermogravimetry of samples.
Table 4. Remaining Mass of Samples Heated to Different Temperatures (Expressed in Percentage).
| adsorbent | 200 °C (%) | 500 °C (%) |
|---|---|---|
| 1 # | 94.6 | 41.5 |
| 2 # | 62.8 | 44.2 |
| 3 # | 62.1 | 42.4 |
| 4 # | 62.5 | 47.0 |
| 5 # | 56.9 | 42.5 |
| 6 # | 68.0 | 50.9 |
| 7 # | 67.4 | 51.3 |
| 8 # | 71.3 | 53.1 |
The heat flux curve during the heating process of the sample is shown in Figure 9. It can be seen from the figure that the heat flux peaks of samples after impregnation are mainly distributed before 200 °C, 200–250 °C, and after 400 °C. The peak before 200 °C comes from the dehydration and endothermic process of the salt. The peak shape of the heat flux curve of DTG also corresponds to the main peak position of DTG, which indicates that the main mass change of samples also appears at the corresponding temperature during dehydration and decomposition. From this heat flow curve, we calculated the heat storage density of each sample, which is listed in Table 5. Because the samples we used for thermogravimetric experiments have undergone the water vapor adsorption–desorption test, the water contained in the thermogravimetric samples is only the water adsorbed in the chemical adsorption process. Therefore, the heat storage density calculated from this heat flow curve is only the heat storage density in chemisorption. It can be seen from the table that the increased chemisorption heat storage density of impregnated samples is about 800 J/g, and the chemical heat storage density of double salt components is not improved compared with that of single salt components. The increased thermal storage density of two-component salt-impregnated samples impregnated with LiCl and MgCl2 is obviously higher than that of single salt-impregnated samples impregnated with MgCl2, but the increased thermal storage density of two-component salt-impregnated samples impregnated with LaCl3 and MgCl2 is almost unchanged or even decreased compared with single salt impregnated samples impregnated with MgCl2 (Figure 10).8
Figure 9.
Differential thermogravimetry curve of samples.
Table 5. Heat Storage Density during Sample Dehydration (<200 °C).
| sample number | 2 # | 3 # | 4 # | 5 # | 6 # | 7 # | 8 # |
|---|---|---|---|---|---|---|---|
| ΔH (J/g) | 818.9 | 810.1 | 866.1 | 817.5 | 712.5 | 707.6 | 820.8 |
Figure 10.
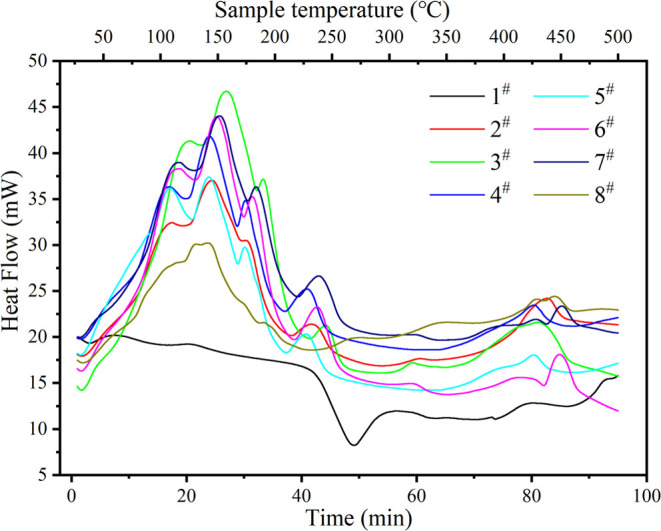
Differential thermogravimetry curve of samples.
4. Conclusions
The water vapor adsorption and heat storage properties of the MIL-101 (Cr) complex salt system were studied by an impregnation method with different concentrations of MgCl2, LiCl, and LaCl3 doped into MIL-101 (Cr). Generally speaking, the addition of salt makes the saturated adsorption capacity of the system greatly increased compared with pure MIL-101 (Cr), which makes the saturated adsorption capacity more than doubled and makes the system more compatible with water vapor. At 25 °C and P/P0 = 0.3, the water vapor adsorption capacity increased by up to five times to 0.68 g/g, which greatly increased the service performance of the system in a low water vapor partial pressure environment. Our experimental results show that compared with MgCl2–LaCl3 composite impregnation, MgCl2–LiCl composite impregnation can improve the water vapor adsorption capacity of the system more significantly. Compared with single-component impregnation, multicomponent impregnation can improve the water vapor adsorption capacity of samples under low water pressure, but it will make the materials relatively hydrophobic. The results of TGA showed that the thermal storage density of the impregnated samples increased obviously, and the chemical thermal storage density of the impregnated samples with MgCl2 and the proper amount of LiCl increased the most, which could increase by 866 kJ/mol. Nitrogen adsorption experiments show that impregnation can reshape the pore structure of MOF materials and increase the mesoporous content in the system. The results of XRD and FTIR showed that the impregnation did not change the structure of MIL-101 (Cr), and it increased the adsorption mode of water vapor. Our experiments show that the sample containing both MgCl2 and LiCl in the impregnation solution has the highest water vapor adsorption capacity, saturated water vapor adsorption capacity, and heat storage density under low partial pressure. This composite material greatly improves the adsorption capacity and heat storage density of MIL-101 (Cr) under low water vapor partial pressure, and its preparation method is simple, which is suitable for large-scale industrial production and is expected to become a candidate material for low-temperature heat storage.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51836009).
Data Availability Statement
The data that support this study will be shared upon reasonable request by the corresponding author.
The authors declare no competing financial interest.
References
- André L.; Abanades S.; Flamant G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renewable Sustainable Energy Rev. 2016, 64, 703–715. 10.1016/j.rser.2016.06.043. [DOI] [Google Scholar]
- Yan J.; Zhao C. Y. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage. Appl. Energy 2016, 175, 277–284. 10.1016/j.apenergy.2016.05.038. [DOI] [Google Scholar]
- Kim Y.; Kim N.; Kim T. S.; Park G. J.; Kwon Y.; Yu H. K. Mg(OH)2 nano-sheet decorated MgO micro-beams by electron beam irradiation for thermochemical heat storage. Ceram. Int. 2019, 45 (15), 18908–18913. 10.1016/j.ceramint.2019.06.126. [DOI] [Google Scholar]
- Zhong Y.; Critoph R. E.; Thorpe R. N.; Tamainot-Telto Z.; Aristov Y. I. Isothermal sorption characteristics of the BaCl2–NH3 pair in a vermiculite host matrix. Appl. Therm. Eng. 2007, 27 (14–15), 2455–2462. 10.1016/j.applthermaleng.2007.02.011. [DOI] [Google Scholar]
- Cosquillo Mejia A.; Afflerbach S.; Linder M.; Schmidt M. Experimental analysis of encapsulated CaO/Ca(OH)2 granules as thermochemical storage in a novel moving bed reactor. Appl. Therm. Eng. 2020, 169, 114961 10.1016/j.applthermaleng.2020.114961. [DOI] [Google Scholar]
- N’Tsoukpoe K. E.; Kuznik F. A reality check on long-term thermochemical heat storage for household applications. Renewable Sustainable Energy Rev. 2021, 139, 110683 10.1016/j.rser.2020.110683. [DOI] [Google Scholar]
- Li M.; Lin Z.; Sun Y.; Wu F.; Xu T.; Wu H.; Zhou X.; Wang D.; Liu Y. Preparation and characterizations of a novel temperature-tuned phase change material based on sodium acetate trihydrate for improved performance of heat pump systems. Renewable Energy 2020, 157, 670–677. 10.1016/j.renene.2020.05.061. [DOI] [Google Scholar]
- N’Tsoukpoe K. E.; Schmidt T.; Rammelberg H. U.; Watts B. A.; Ruck W. K. L. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage. Appl. Energy 2014, 124, 1–16. 10.1016/j.apenergy.2014.02.053. [DOI] [Google Scholar]
- Meesenburg W.; Markussen W. B.; Ommen T.; Elmegaard B. Optimizing control of two-stage ammonia heat pump for fast regulation of power uptake. Appl. Energy 2020, 271, 115126 10.1016/j.apenergy.2020.115126. [DOI] [Google Scholar]
- Guo J.; Huai X.; Xu M. Study on Isopropanol–Acetone–Hydrogen chemical heat pump of storage type. Sol. Energy 2014, 110, 684–690. 10.1016/j.solener.2014.09.038. [DOI] [Google Scholar]
- Li S.; Huang H.; Yang X.; Bai Y.; Li J.; Kobayashi N.; Kubota M. Hydrophilic substance assisted low temperature LiOH·H2O based composite thermochemical materials for thermal energy storage. Appl. Therm. Eng. 2018, 128, 706–711. 10.1016/j.applthermaleng.2017.09.050. [DOI] [Google Scholar]
- Myat A.; Kim Choon N.; Thu K.; Kim Y.-D. Experimental investigation on the optimal performance of Zeolite–water adsorption chiller. Appl. Energy 2013, 102, 582–590. 10.1016/j.apenergy.2012.08.005. [DOI] [Google Scholar]
- Thao Ho T. T.; Zimmermann T.; Caseri W. R.; Smith P. Liquid ammonia treatment of (cationic) nanofibrillated cellulose/vermiculite composites. J. Polym. Sci., Part B: Polym. Phys. 2013, 51 (8), 638–648. 10.1002/polb.23241. [DOI] [Google Scholar]
- Shrungi M.; Goswami A.; Bajpai J.; Bajpai A. K. Designing kaolin-reinforced bionanocomposites of poly(vinyl alcohol)/gelatin and study of their mechanical and water vapor transmission behavior. Polym. Bull. 2019, 76 (11), 5791–5811. 10.1007/s00289-019-02684-4. [DOI] [Google Scholar]
- Lin K.-L.; Lee T.-C.; Chang J.-C.; Lan J.-Y. Water absorption and retention of porous ceramics cosintered from waste diatomite and catalyst. Environ. Prog. Sustainable Energy 2013, 32 (3), 640–648. 10.1002/ep.11675. [DOI] [Google Scholar]
- Krajnc A.; Varlec J.; Mazaj M.; Ristić A.; Logar N. Z.; Mali G. Superior Performance of Microporous Aluminophosphate with LTA Topology in Solar-Energy Storage and Heat Reallocation. Adv. Energy Mater. 2017, 7 (11), 1601815 10.1002/aenm.201601815. [DOI] [Google Scholar]
- Akiyama G.; Matsuda R.; Sato H.; Hori A.; Takata M.; Kitagawa S. Effect of functional groups in MIL-101 on water sorption behavior. Microporous Mesoporous Mater. 2012, 157, 89–93. 10.1016/j.micromeso.2012.01.015. [DOI] [Google Scholar]
- Solovyeva M. V.; Aristov Y. I.; Gordeeva L. G. NH 2 -MIL-125 as promising adsorbent for adsorptive cooling: Water adsorption dynamics. Appl. Therm. Eng. 2017, 116, 541–548. 10.1016/j.applthermaleng.2017.01.080. [DOI] [Google Scholar]
- Hu Y.; Xiang S.; Zhang W.; Zhang Z.; Wang L.; Bai J.; Chen B. A new MOF-505 analog exhibiting high acetylene storage. Chem. Commun. 2009, (48), 7551–7553. 10.1039/b917046d. [DOI] [PubMed] [Google Scholar]
- Ehrenmann J.; Henninger S. K.; Janiak C. Water Adsorption Characteristics of MIL-101 for Heat-Transformation Applications of MOFs. Eur. J. Inorg. Chem. 2011, 2011 (4), 471–474. 10.1002/ejic.201001156. [DOI] [Google Scholar]
- Khutia A.; Rammelberg H. U.; Schmidt T.; Henninger S.; Janiak C. Water Sorption Cycle Measurements on Functionalized MIL-101Cr for Heat Transformation Application. Chem. Mater. 2013, 25 (5), 790–798. 10.1021/cm304055k. [DOI] [Google Scholar]
- Akiyama G.; Matsuda R.; Kitagawa S. Highly Porous and Stable Coordination Polymers as Water Sorption Materials. Chem. Lett. 2010, 39 (4), 360–361. 10.1246/cl.2010.360. [DOI] [Google Scholar]
- Wu Y.-t.; Ren N.; Wang T.; Ma C.-f. Experimental study on optimized composition of mixed carbonate salt for sensible heat storage in solar thermal power plant. Sol. Energy 2011, 85 (9), 1957–1966. 10.1016/j.solener.2011.05.004. [DOI] [Google Scholar]
- Donkers P. A. J.; Pel L.; Adan O. C. G. Experimental studies for the cyclability of salt hydrates for thermochemical heat storage. J. Energy Storage 2016, 5, 25–32. 10.1016/j.est.2015.11.005. [DOI] [Google Scholar]
- Yu N.; Wang R. Z.; Wang L. W. Theoretical and experimental investigation of a closed sorption thermal storage prototype using LiCl/water. Energy 2015, 93, 1523–1534. 10.1016/j.energy.2015.10.001. [DOI] [Google Scholar]
- Courbon E.; D’Ans P.; Permyakova A.; Skrylnyk O.; Steunou N.; Degrez M.; Frère M. Further improvement of the synthesis of silica gel and CaCl2 composites: Enhancement of energy storage density and stability over cycles for solar heat storage coupled with space heating applications. Sol. Energy 2017, 157, 532–541. 10.1016/j.solener.2017.08.034. [DOI] [Google Scholar]
- Brancato V.; Calabrese L.; Palomba V.; Frazzica A.; Fullana-Puig M.; Solé A.; Cabeza L. F. MgSO4·7H2O filled macro cellular foams: An innovative composite sorbent for thermo-chemical energy storage applications for solar buildings. Sol. Energy 2018, 173, 1278–1286. 10.1016/j.solener.2018.08.075. [DOI] [Google Scholar]
- Garzón-Tovar L.; Pérez-Carvajal J.; Imaz I.; Maspoch D. Composite Salt in Porous Metal-Organic Frameworks for Adsorption Heat Transformation. Adv. Funct. Mater. 2017, 27 (21), 1606424 10.1002/adfm.201606424. [DOI] [Google Scholar]
- Sun Y.; Spieß A.; Jansen C.; Nuhnen A.; Gökpinar S.; Wiedey R.; Ernst S.-J.; Janiak C. Tunable LiCl@UiO-66 composites for water sorption-based heat transformation applications. J. Mater. Chem. A 2020, 8 (26), 13364–13375. 10.1039/D0TA03442H. [DOI] [Google Scholar]
- Yan J.; Yu Y.; Ma C.; Xiao J.; Xia Q.; Li Y.; Li Z. Adsorption isotherms and kinetics of water vapor on novel adsorbents MIL-101(Cr)@GO with super-high capacity. Appl. Therm. Eng. 2015, 84, 118–125. 10.1016/j.applthermaleng.2015.03.040. [DOI] [Google Scholar]
- Oh J. S.; Shim W. G.; Lee J. W.; Kim J. H.; Moon H.; Seo G. Adsorption Equilibrium of Water Vapor on Mesoporous Materials. J. Chem. Eng. Data 2003, 48 (6), 1458–1462. 10.1021/je0301390. [DOI] [Google Scholar]
- de Lange M. F.; Verouden K. J.; Vlugt T. J.; Gascon J.; Kapteijn F. Adsorption-Driven Heat Pumps: The Potential of Metal-Organic Frameworks. Chem. Rev. 2015, 115 (22), 12205–12250. 10.1021/acs.chemrev.5b00059. [DOI] [PubMed] [Google Scholar]
- Jeremias F.; Khutia A.; Henninger S. K.; Janiak C. MIL-100(Al, Fe) as water adsorbents for heat transformation purposes—a promising application. J. Mater. Chem. 2012, 22 (20), 10148–10151. 10.1039/C2JM15615F. [DOI] [Google Scholar]
- Zhao Y. J.; Wang R. Z.; Zhang Y. N.; Yu N. Development of SrBr2 composite sorbents for a sorption thermal energy storage system to store low-temperature heat. Energy 2016, 115, 129–139. 10.1016/j.energy.2016.09.013. [DOI] [Google Scholar]
- Ferey G.; Mellot-Draznieks C.; Serre C.; Millange F.; Dutour J.; Surble S.; Margiolaki I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309 (5743), 2040–2042. 10.1126/science.1116275. [DOI] [PubMed] [Google Scholar]
- Whiting G. T.; Grondin D.; Stosic D.; Bennici S.; Auroux A. Zeolite–MgCl2 composites as potential long-term heat storage materials: Influence of zeolite properties on heats of water sorption. Sol. Energy Mater. Sol. Cells 2014, 128, 289–295. 10.1016/j.solmat.2014.05.016. [DOI] [Google Scholar]
- Balula S. S.; Granadeiro C. M.; Barbosa A. D. S.; Santos I. C. M. S.; Cunha-Silva L. Multifunctional catalyst based on sandwich-type polyoxotungstate and MIL-101 for liquid phase oxidations. Catal. Today 2013, 210, 142–148. 10.1016/j.cattod.2012.12.003. [DOI] [Google Scholar]
- Ren J.; Dyosiba X.; Musyoka N. M.; Langmi H. W.; North B. C.; Mathe M.; Onyango M. S. Green synthesis of chromium-based metal-organic framework (Cr-MOF) from waste polyethylene terephthalate (PET) bottles for hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41 (40), 18141–18146. 10.1016/j.ijhydene.2016.08.040. [DOI] [Google Scholar]
- Zhang D.; Zhang K.; Hu X.; He Q.; Yan J.; Xue Y. Cadmium removal by MgCl2 modified biochar derived from crayfish shell waste: Batch adsorption, response surface analysis and fixed bed filtration. J. Hazard Mater. 2021, 408, 124860 10.1016/j.jhazmat.2020.124860. [DOI] [PubMed] [Google Scholar]
- Yang X.; Shen L.; Yang Q.; Wang K.; Wang C. Study on the synthesis and thermal properties of magnesium chloride hexahydrate–magnesium sulfate heptahydrate–activated carbon phase change heat storage materials. Appl. Phys. A: Mater. Sci. Process. 2021, 127 (8), 578 10.1007/s00339-021-04675-7. [DOI] [Google Scholar]
- Jeremias F.; Lozan V.; Henninger S. K.; Janiak C. Programming MOFs for water sorption: amino-functionalized MIL-125 and UiO-66 for heat transformation and heat storage applications. Dalton Trans. 2013, 42 (45), 15967–15973. 10.1039/c3dt51471d. [DOI] [PubMed] [Google Scholar]
- Stefan K.; Henninger H. A. H.; Janiak C. MOFs as Adsorbents for Low Temperature Heating and Cooling Applications. J. Am. Chem. Soc. 2009, 131 (8), 2776–2777. 10.1021/ja808444z. [DOI] [PubMed] [Google Scholar]
- Kummer H.; Baumgartner M.; Hügenell P.; Fröhlich D.; Henninger S. K.; Gläser R. Thermally driven reflection by metal adsorption on coatings of HKUST-1 and MIL-101 (Cr). Appl. Therm. Eng. 2017, 117, 689–697. 10.1016/j.applthermaleng.2016.11.026. [DOI] [Google Scholar]
- Ait Ousaleh H.; Sair S.; Zaki A.; Faik A.; Mirena Igartua J.; El Bouari A. Double hydrates salt as sustainable thermochemical energy storage materials: Evaluation of dehydration behavior and structural phase transition reversibility. Sol. Energy 2020, 201, 846–856. 10.1016/j.solener.2020.03.067. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support this study will be shared upon reasonable request by the corresponding author.