Abstract
The immune system plays a key role in the development and progression of numerous diseases such as chronic wounds, autoimmune diseases, and various forms of cancer. Hence, controlling the behavior of immune cells has emerged as a promising approach for treating these diseases. Current modalities for immunomodulation focus on chemical based approaches, which while effective have the limitations of nonspecific systemic side effects or requiring invasive delivery approaches to reduce the systemic side effects. Recent advances have unraveled the significance of electrical stimulation as an attractive noninvasive approach to modulate immune cell phenotype and activity. This review provides insights on electrical stimulation strategies employed for regulating the behavior of macrophages, T and B cells, and neutrophils. For obtaining a better understanding, two major types of electrical stimulation sources, conventional and self-powered sources, that have been used for immunomodulation are extensively discussed. Next, the strategies of electrical stimulation that may be applied to cells in vitro and in vivo are discussed, with a focus on conventional and stimuli-responsive self-powered sources. A description of how these strategies influence the polarization, phagocytosis, migration, and differentiation of immune cells is also provided. Finally, recent developments in the use of highly localized and efficient platforms for electrical stimulation based immunomodulation are also highlighted.
1. Introduction
All cells have a membrane potential that is regulated by ion channels. Traditionally, altering ion concentrations in the extracellular milieu was used as a method to tune a cell’s potential, but more recently, there is a growing appreciation of the fact that the membrane potential may be tuned through external electrical signals/fields, and this approach is termed “electrical stimulation” (ES).1−5 ES based alterations in a cell’s potential may be used to modulate that cell’s function. Indeed, ES has been extensively used to accelerate the physiological processes associated with wound healing and for bone, muscle, and nerve regeneration.6−11 But it is believed that electrical modulation of cellular function is not limited to these cell types and is likely applicable to immune cells too. The goal of this review is to present a comprehensive summary of the use of electrical stimulation for modulating immune cell behavior for applications ranging from wound healing to cancer therapy.
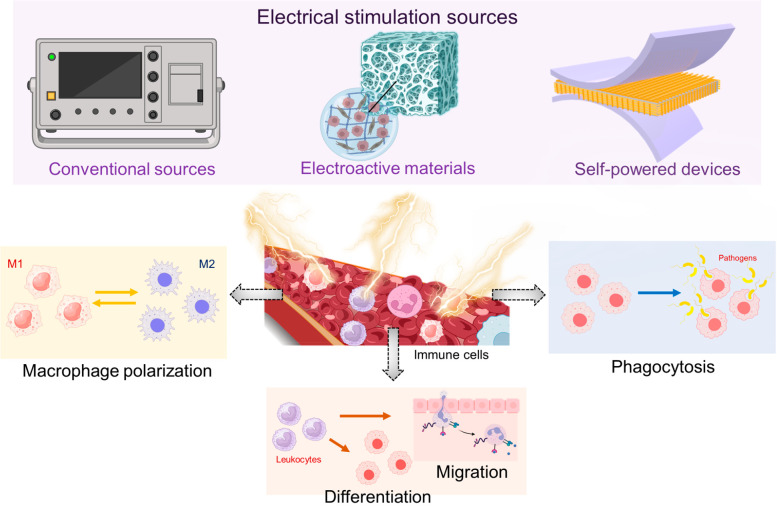
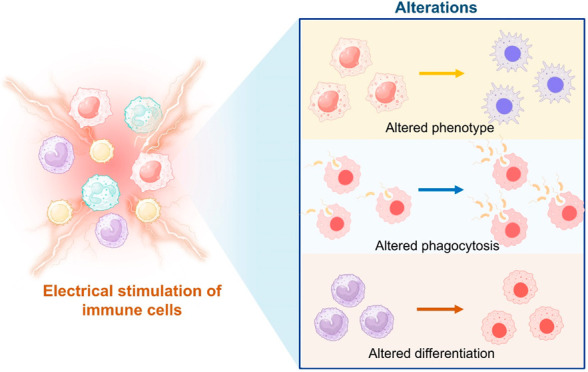
Immune cells possess electrical characteristics just like other cells in the human body.12−17 Traditionally, it was thought that immune cell functions were solely regulated by a plethora of biochemical signals that they receive from their environment.18−21 However, in recent years, it has been suggested that physical signals present in the cellular microenvironments are also key determinants of immune cell activities. One such potentially important physical cue is the electric field.14,22,23 This review exclusively highlights the effect of various external ES approaches on immune cell activity such as polarization, migration, differentiation, and phagocytosis (Figure 1). Further, this paper aims to provide an extensive evaluation of the advancements, potential applications, challenges, and future perspectives of ES based approaches for immunomodulation.
Figure 1.
Schematic representation of different electrical stimulation strategies employed for immunomodulation and how they might affect the processes of immune cells. Created with BioRender.com.
2. Types of Electrical Stimulation Sources for Immunomodulation
2.1. Electrical Stimulation via Conventional Sources
External electrical fields, referred to as electrical stimulation (ES), when applied in a controlled manner have been shown to regulate cellular functions. For example, ES has shown promising results in wound healing, muscle regeneration, nerve regeneration, and cancer therapy.14−17 Numerous studies have also shown that ES can promote nerve regeneration by enhancing the axon outgrowth across the injury site, increasing the nerve fiber density, as well as by improving the expression of neurotrophic factors for functional recovery. The regeneration is typically mediated by influencing the ionic influx and membrane potential, which in turn affects the intracellular signal transduction.18−22 Like nerve cells, immune cells are also influenced by the application of external electric fields. It has been reported that continuous as well as pulsed electric fields can influence immune cells such as macrophages by promoting their polarization, cytokine secretion profiles, phagocytic behavior, migration, and differentiation, summarized in Table 1. In addition to macrophages, electric fields are also known to influence other immune cells such as T and B lymphocytes by modulating their membrane capacities and conductivities which further influence their proliferation, migration, and cytokine profiles.23,24 On the basis of the directionality, two sources of electrical stimulation, namely direct current (DC) and alternating current (AC), have been extensively employed for immunomodulation.25,26 In addition, unidirectional and bidirectional pulsed currents (PCs) which can generate current pulses of around 1 s have been used for immunomodulation owing to lesser electrothermal side effects compared to DC.27,28
Table 1. Summary of Conventional ES Techniques Used for Immunomodulation.
| electrical device | electrode material | stimulus type | electric field strength (mV/mm) | immune cell model | effect on immune cells | applications | ref |
|---|---|---|---|---|---|---|---|
| oscilloscope power | – | AC | 58 | THP-1 | M2 polarization | wound healing | (40) |
| DC power supply | copper | DC | 53 | THP-1 | M2 polarization | wound healing | (41) |
| DC generator | platinum | DC | 100 | J774A.1 macrophages | M2 polarization | osteogenesis | (42) |
| square wave pulse electroporator | aluminum | DC | 15 × 104 | THP-1, RAW 264.7 macrophages | M1 polarization | cancer therapy | (44) |
| DC power supply | platinum | DC | 192 | human PBMCs | migration | wound healing | (48) |
| DC power supply | platinum | DC | 700 | human PBMCs | migration | wound healing | (49) |
| sinusoidal wave function generator | silver chloride | DC | 200 | human primary macrophages | migration | wound healing | (50) |
| DC power supply | silver chloride | DC | 3000–15000 | human primary macrophages | phagocytosis | wound healing | (57) |
| nanosecond pulse power generator | stainless wire | AC | 4 × 105 | THP-1 | phagocytosis | cancer therapy | (55) |
| square wave pulse electroporator | stainless steel | DC | 2.1 × 105 | mouse B cell lymphoblast, J774A.1 macrophages | phagocytosis | cancer therapy | (59) |
2.1.1. Macrophage Polarization
Macrophages are an important class of multifunctional immune cells that play key roles in tissue repair, host defense, and homeostasis. Macrophages possess the ability to alter their functionality in response to the cues from their microenvironment, which ultimately leads to their polarization toward functional phenotypes.6,29−31 Typically, there are two functional phenotypes: M1 macrophages which are classically activated and exhibit a pro-inflammatory response and M2 macrophages that play a role in the anti-inflammatory or wound-healing response.32,33 While it is well-established that biochemical signals, such as bacterial components (such as lipopolysaccharide) or cytokines such as IL-1β or IL-4, result in the polarization of macrophages toward M1 or M2 phenotype, it is now becoming apparent that electrical fields may also induce such polarization.6,32,34
Several studies have reported that macrophages are responsive to endogenous electric fields, suggesting that ES may be an effective technique for modulating macrophage polarization.33,35 ES with different voltages, currents, frequencies, and waveforms has been utilized to induce macrophage polarization. Especially for wound repair, the transition from the inflammatory phase to the remodeling phase is determined by the suboptimal polarization of the macrophages which can be controllably tuned under ES application.36−38 In 1983, Mccann et al. first reported that macrophages could be polarized to the M2 phenotype when subjected to an electric field of 12.7–30.5 V/s from a conventional DC source for 2 h using glass microelectrodes.39 A gradual increase in intracellular Ca2+ ion was observed after ∼2 min of ES with repetitive Ca2+ dependent peaks which was related to the M2 polarization.
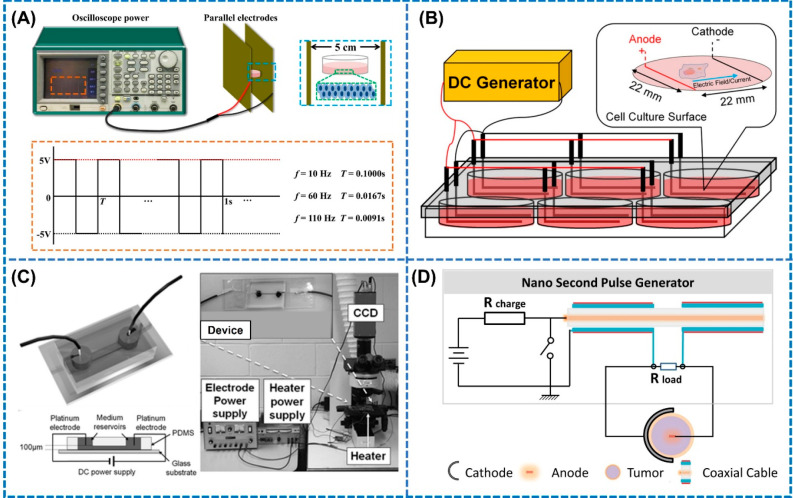
In 2022, Jia et al. also utilized AC electric fields with capacitive characteristics for modulating the phenotype of macrophages in a self-designed system.40 As shown in Figure 2A, cultured cells in Petri dishes were placed between two parallel electrodes connected to the oscilloscope power for generating homogeneous electric fields between the electrodes. The frequency of the electric field was adjusted by use of an AC/DC module. Interestingly, it was observed that the frequency of the applied electric fields significantly influenced the macrophage polarization from M1 to M2, with 10 and 60 Hz increasing the expression of CD206 markers which validated the polarization.40 However, direct application of ES to the cells or tissues using percutaneous electrodes is at times associated with underlying challenges including microbial contamination and secondary damages due to heat generation. To combat this limitation, Xu et al. in 2022 proposed a noncontact electrical stimulation device based on capacitive coupling to study its influence on the macrophage behavior during the tissue repair process.41 During the experiments, cells were placed between the two parallel copper electrode plates separated by 10 cm, which were connected to the DC power supply for supplying the ES in the longitudinal section. Under an electric field strength of 25 mV/mm, no obvious change in expression of CCR7 (M1 marker) and CD206 (M2 marker) was observed.41 However, when the electric field was increased to 53 mV/mm, the expressions of CCR7 and CD206 were significantly down- and upregulated, respectively. Moreover, the number ratio of M2/M1 macrophages also increased, which clearly suggests that noncontact ES of 53 mV/mm could successfully modulate the polarization of macrophages toward M2 phenotypes, thereby improving the immune response during the wound healing process.
Figure 2.
Different modules for conventional electrical sources used for immunomodulation. (A) AC electric fields with capacitive characteristics applied to cultured cells via platinum electrodes at different frequencies for promoting M2 polarization. Reproduced with permission from ref (40). Copyright 2022 Elsevier. (B) DC electric fields applied using L-shaped platinum electrodes to macrophages for modulating their phenotype toward M2. Reproduced with permission from ref (42). Copyright 2019 Wiley-VCH. (C) PDMS microfluidic devices incorporated with platinum electrodes connected to DC power supply for providing microcurrents to T cells in the microchannel. (i) Cell culture medium, (ii) platinum electrodes, (iii) PDMS, and (iv) glass substrate. Reproduced with permission from ref (49). Copyright 2011 Royal Society of Chemistry. (D) Setup for the nanosecond pulse generator for generating high electric fields across tumor cells for enhancing phagocytosis by macrophages. Reproduced with permission from ref (55). Copyright 2014 Elsevier.
The responses of macrophages under ES have also been investigated in preosteoblasts to study its role in bone regeneration. Srirussamee et al. in 2019 studied the effect of ES on the response of macrophages (J774A.1) in promoting bone regeneration by stimulating the cells with a DC voltage and current of 2.2 V and 70 μA, respectively.42 The electric field was applied to the macrophages with L-shaped platinum electrodes arranged in a parallel circuit in a six-well plate connected to a commercial DC generator (Figure 2B). The results from their study showed the increased expression of Spp1 mRNA, representing anti-inflammatory M2 phenotype, under direct ES treatment for 2 h, hence verifying that macrophages are indeed responsive to electrical cues. Similarly, irreversible electroporation (IRE), which generates extremely high electric fields across cells, has been shown to be useful in stimulating macrophages, with applications in cancer therapy.43,44 For instance, He et al. in 2021 explored the use of IRE to instigate an antitumor immune response for treating pancreatic ductal adenocarcinoma (PDAC).44 IRE induced macrophage polarization was evaluated by placing a combination of tumor and immune cells between aluminum plate electrodes and subjecting them an electric field strength, frequency, and pulse duration of 500–1500 V, 1 Hz, and 100 μs, respectively. The expression of mRNA levels of M1 markers including CCL2, IL-1β, and TNF-α elevated with the increase in the electric field strength from 500 to 1500 V/cm, whereas the mRNA levels of M2 markers did not show a continuous increase. The authors suggested that these changes could result from electroporation associated damage to tumor cells that caused the release of damage associated molecular patterns (DAMPs) such as HMGB1, which stimulated the M1 macrophage polarization by triggering the MAPK–p38 signaling pathway.
2.1.2. Proliferation, Migration, and Differentiation
Cell migration is crucial for numerous physiological processes including wound healing, bone regeneration, nerve regeneration, and cancer metastasis.6,17,45−47 An applied low intensity electric field can initiate directional cell migration or electrotaxis of cells.43 Based on their polarity, some cells such as fibroblasts, keratinocytes, etc. migrate toward the negatively charged terminal, whereas endothelial cells migrate toward the positively charged terminal.46,47 Recently, electrical stimulation has also been reported to promote the migration of immune cells including neutrophils, lymphocytes, and macrophages.34,45 Lin et al. in 2008 successfully demonstrated the DC electric field directed the migration of lymphocytes toward the negatively charged terminal.48 Cells were seeded onto a microfluidic device, and two platinum electrodes were placed in parallel orientation to the cell medium which was later connected to a DC power supply. Although all circulating lymphocytes displayed electrotactic migration, the migration of the T cells was highly directional and was comparable to the widely used chemotaxis assays. In vivo results showed that the T cells migrated randomly in the absence of electric fields; however, when an electric field of 0.2–0.5 V/mm was applied to the mice by placing the electrodes in the peripheral ear tissue, the cells migrated 100 μm directionally toward the cathode.48
To further improve the migration rate of lymphocytes, microfluidic devices were integrated with an on-chip platinum electrode array for highly controllable stimulation of lymphocytes.49 Li et al. in 201149 reported a PDMS based microfluidic device with two platinum electrodes inserted into the medium reservoir for supplying the electric fields to the microchannel through a DC power supply to stimulate T cells, as shown in Figure 2C. Employing such an advanced electrode orientation, the migration of T cells displayed significantly better motility and directionality under an applied DC electric field of 7 V/cm compared to the T cells subjected to chemotactic gradients (100 nM CCL19). This study highlights that lymphocytes do migrate in response to electric field lines, which the authors attributed to the prevalence of higher membrane potential at the cathode that causes the influx of Ca2+ ions by activation of the Erk1/2 and Akt signaling pathway. Utilization of DC electric fields for immune cell migration, however, is often associated with cell death and detachment from the substrates owing to its high intensity. Oscillatory electrical signals are advantageous for cell migration without causing cell death because they do not maintain a continuous potential difference across the cell medium. In 2000, Cho et al. reported the directional migration of macrophages in a direction perpendicular to the electric field at a velocity of (5.2 ± 0.4) × 10–2 μm/mm when exposed to an oscillatory electric field of 2 V/cm at a frequency of 1 Hz.50 Moreover, a change in the surface morphology to an elongated shape from the rounded ones was also observed when they were treated with an oscillating electric field. Unlike the static electric fields, the oscillatory electric fields did not cause any changes in the influx of Ca2+, instead resulting in the rearrangement and redistribution of the microfilament structures and cell surface receptors, respectively, leading to cell movement. Further, Arnold et al. in 2019 showed that human T cells migrate toward the cathode under electric fields with directedness. The applied electric filed enhanced the migration of T cells by 3-fold under 50 mV/mm EF, which further increased to 6-fold under 150 mV/mm EF.51 These findings suggest that the electric field can help position T cells in the wound sites where epithelium is damaged in order to aid in the healing process. Moreover, the stimulation of T lymphocytes by electric fields reduced the secretion of IL-2 cytokine which impaired their proliferation after 18 h of exposure by attenuating the STAT3 signaling pathways for anticancer therapy.51 On the other hand, Zhang et al. in 2019 reported that the use of nanosecond pulsed EF increased the number of T lymphocytes in the spleen of malignant mice which activated their immune system to attack the tumor. An EF of 30 kV/cm applied in 100 ns increased the killer T cells such as CD3+CD4+ T cells and CD3+CD8+ T cells, whereas the regulatory T cells decreased in number for improving the cancer metastasis.52
In addition to migration, differentiation of immune cells could also be modulated by an electrical stimulus.40,53,54 For instance, AC, which induce bidirectional flow of currents, have been used to promote the differentiation of circulating monocytes into macrophages.40,54 Jia et al. in 2022 showed that AC EF of 58 mV/mm can significantly enhance the efficiency of PMA induced differentiation of THP-1 cells into macrophages by ∼50% compared to the control groups.40 Moreover, the macrophage viability decreased with the increase in the frequency of AC EF with the highest viability of 1.6 obtained at a frequency of 10 Hz which further decreased when the frequency was escalated to 110 Hz, signifying that the frequency of the electric field is an important parameter governing the differentiation of immune cells.
2.1.3. Phagocytosis
Phagocytosis is a key event of the innate immune system, which is comprised of multiple cellular processes involving recognition of the target cells, cellular engulfment, and lysosomal digestion. Typically, resting macrophages are associated with low levels of phagocytic activity, whereas their physiological activation by electric fields leads to increased levels of phagocytosis.55 Hoare et al. reported that macrophages exposed to electric fields of varying intensities increased the phagocytic uptake against different targets such as carboxylate microspheres, apoptotic neutrophils, and pathogens such as Candida albicans.6 Electric-field-dependent phagocytic behavior of macrophages against carboxylate microspheres was observed with phagocytic index (PI) enhancement of 4, 7, and 23% when they were exposed to electric field strengths of 50, 100 and 150 mV/mm, respectively. In addition, high EF of 150 mV/mm significantly boosted the clearance efficiency of C. albicans and apoptotic neutrophils by 16.9 and 35.5%, respectively. It has been suggested that the electrical stimulation could induce the clustering of mannose and scavenger receptors on the cell surface, which causes the lowering of the threshold to trigger phagocytosis. Studies have also speculated that electrical stimulation regulates the expression of transient receptor potential (TRP) ion channels in macrophages, which controls macrophage functions such as phagocytosis.56,57 At the molecular level, electrical stimulation activates the PI3K and ERK signaling pathway causing intercellular influx of Ca2+, resulting in enhanced phagocytic efficiency.
To increase phagocytosis in vivo, among the various types of electric fields that may be provided, pulsed electric fields have displayed promising advantages for tissue regeneration and cancer therapy. Particularly nanosecond pulsed electric stimulation, which can generate high voltage electric fields in short durations in tumor microenvironments to change the properties of the plasma membrane and result in apoptotic cell death, has been widely reported for therapy by driving phagocytosis. In a typical setup displayed in Figure 2D, a pair of electrodes with the cathode placed on the tumor periphery and the anode placed within the tumor is used to deliver the nanosecond electric fields to the subcutaneous tumors, which forms semicircular electric fields between the electrodes.55 For instance, Ogura et al. reported that nanosecond electric field treatment with acharging voltage of 3.2 kV and a number of pulses in the range 9000–15000 progressed the apoptosis of hepatocellular carcinoma (HCC) cells from early stage to later stage, leading to enhanced engulfment by macrophages.58 In another study, the application of 18 electric pulses of 40 kV/cm for 100 ns improved the phagocytosis of HCC cells by THP1 macrophage cells, as evidenced by the flow cytometry results. The authors suggested that the electric field instigated the externalization of phosphatidylserine (PS) on the cell membrane, which is vital for phagocytic clearance of target cells. In addition, electric fields also contributed to enhancing the phagocytosis of B cells by macrophages in apoptotic cellular microenvironments. Tekle et al. in 2008 showed that when an electric field strength of 2.1 kV/cm was applied for 200 μs, it electroporated the B cell membrane to cause PS externalization, which was recognized by macrophages for apoptotic clearance.59 The macrophage uptake percentage was 30% for electroporated B cells compared to only 4% for control (untreated) cells. The authors elucidated two possible pathways for explaining the enhanced phagocytosis in the presence of electric fields. First, the pathway suggested that electric fields could drive the PS diffusion along the membrane pores, causing lipid inversion. In addition, electric fields could also lead to bleb formation which initiates the breakdown of the inner and outer lipid membranes due to bleb curvature induced lipid packing, resulting in PS externalization.
2.2. Electrical Stimulation via Electroactive Materials and Self-Powered Devices
Although electrical stimulation has been successfully applied for several clinical applications, all of them are based on the utilization of conventional AC or DC sources, which require extracorporeal power supplies to operate. Apart from the power requirement, low portability, high operational risk, and low flexibility of the devices, it necessitates patient hospitalization.25,60 To resolve these constraints, considerable efforts have been made to develop smart platforms based on electroactive materials that have intrinsic electroactive properties, which can alter bioelectric fields and hence promote cell migration, differentiation, and polarization (Table 2). Typically, electroactive materials are conductive materials, which act as an assisted bioelectricity conductor by providing a conductive pathway to the cell’s intrinsic bioelectricity.61−63 Owing to their excellent conductivity in the range 10–2–10–4 S/cm, biomaterials based on 3D hydrogels and graphene have emerged as promising candidates for direct electrical stimulation of immune cells.61−64
Table 2. Summary of Electroactive Materials and Devices Used for Immunomodulation.
| electroactive materials/devices | ES mechanism | electrical parameters | immune cell model | effect on immune cells | applications | ref |
|---|---|---|---|---|---|---|
| GelMA–PPy hydrogel | electrical conductivity | conductivity: 1.3 × 10–4 S/cm | RAW 264.7 macrophages | M2 polarization | osteogenesis | (61) |
| BaTiO3/PLLA nanofibers | piezoelectric | output voltage: 50.33 mV | RAW 264.7 macrophages | M2 polarization | osteogenesis | (66) |
| BaTiO3/β-TCP ceramics | piezoelectric | output voltage: 590 mV; current: 300 nA | RAW 264.7 macrophages | M2 polarization | osteogenesis | (69) |
| bioinspired PHBV/PHA/PBT polymer matrix | piezoelectric | output voltage: 11.2 V; current: 4.3 μA | RAW 264.7 macrophages | M2 polarization | osteogenesis | (71) |
| MXene/silk fibroin nanocomposite | piezoelectric | output voltage: 100 mV | RAW 264.7 macrophages | M2 polarization | osteogenesis | (82) |
| BaTiO3/Ti6Al4V scaffold | piezoelectric/ultrasound | current: 6.7 μA | RAW 264.7 macrophages | M2 polarization | osteogenesis | (83) |
| P(VDF–TrFE) film | piezoelectric | output voltage: 500–1500 mV | BMDMs | M2 polarization | osteogenesis | (87) |
| TiO2 nanotubes–PVDF | piezoelectric | output voltage: 2 V; current: 10 nA | RAW 264.7 macrophages | M2 polarization, phagocytosis | osteogenesis | (97) |
| GDFE hydrogel | electrical conductivity | conductivity: 4 × 10–4 S/cm | RAW 264.7 macrophages | M2 polarization | wound healing | (63) |
| P(VDF–TrFE) | piezoelectric | output voltage: 2.3 V | BMDMs | M2 polarization | wound healing | (80) |
| BaTiO3 nanorobots | piezoelectric/ultrasound | output voltage: 35 mV | RAW 264.7 macrophages | M2 polarization, phagocytosis | rheumatoid arthritis | (86) |
| Fe/BiOCl NSs | piezoelectric | output voltage: 23.7 mV | mouse primary macrophages | M2 polarization, phagocytosis | rheumatoid arthritis | (85) |
| β-PVDF film | piezoelectric/ultrasound | output voltage: 10–100 mV | THP-1 | M1 polarization | cancer therapy | (88) |
| Cu/PET/PTFE | triboelectric nanogenerators (TENG) | output voltage: 4 V; current: 28 nA | THP-1 | M2 polarization | wound healing | (90) |
| Bi2Fe4O9 nanosheets | pyroelectric | output voltage: 7 mV; current: 7 μA | J774A.1 macrophages | M1 polarization | cancer therapy | (71) |
In addition, self-powered systems which possess the ability to harvest energy from the environment and generate electrical signals have shown tremendous progress for immunomodulation in recent years due to their excellent biocompatibility, high output generation, wide variety of raw material selection, and miniaturization. Among all energy sources, mechanical energy is widely available in our daily lives either from human motions such as walking and running or from nature such as water flow, vibrations, and so on. To harvest these mechanical energies, self-powered systems based on piezoelectric materials have been developed, which can generate electric fields in response to small mechanical deformations such as cell movements, body motions, or external stimulation, to regulate the membrane voltage and ion channel activity of immune cells.65−71 Piezoelectric materials are noncentrosymmetric in nature, which undergo deformation when subjected to external force and lead to piezoelectric polarization, resulting in electric charge generation across the edges. Electrical stimulation based on piezoelectric materials such as poly-l-lactic acid (PLLA), barium titanate (BaTiO3), polyvinylidene fluoride (PVDF), etc. have emerged as effective immunomodulatory strategies for controlling tissue regeneration.68,70 Although a few piezoelectric materials are biologically active and can directly influence the immune cell behavior, in section 2.2.1, we discuss examples of piezoelectric materials that demonstrate electrically driven immunomodulation which are also summarized in Table 2. Another form of self-powered devices which can harness mechanical energy are triboelectric nanogenerators (TENG). TENG can generate electrical energy from friction between two triboelectric layers or even from biomechanical motions such as walking and running. Based on the synergistic principle of contact separation and electrostatic induction, TENG can generate an electric field, which is known to influence the immune cell behavior, especially macrophage polarization. Temperature is another stimulus which can activate the self-powered devices and convert it into electricity for modulating the immune cell microenvironments.71,73 In particular, self-powered pyroelectric devices can generate electrical fields due to small temperature changes, which lead to electron–hole pair separations for catalytic reactions, which are conducive for immunomodulation, especially for cancer therapy.72,73
2.2.1. Macrophage Polarization
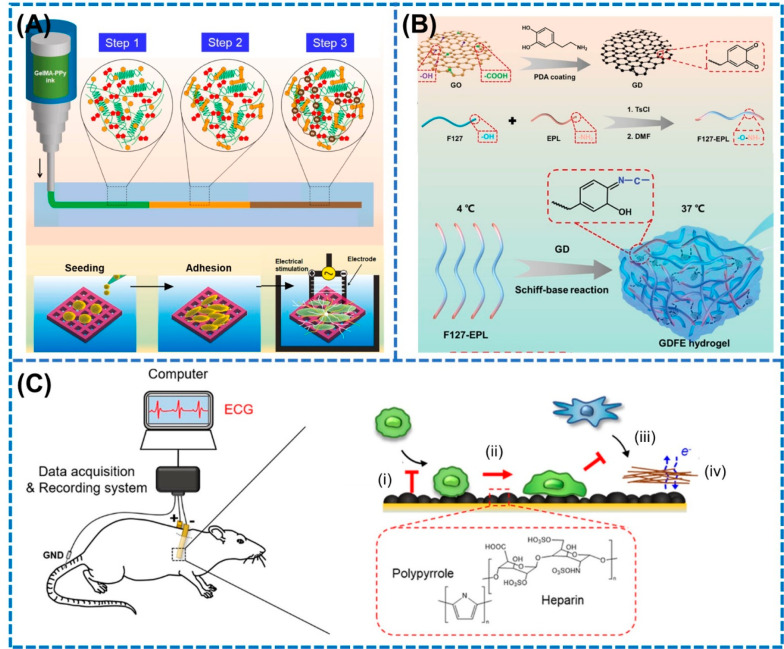
Electroactive biomaterials have garnered immense attention for their exogenous electrical stimulation ability. Among them, graphene based scaffolds have been widely used for tissue engineering applications due to their excellent biocompatibility, flexibility, simple functionalization, and unique physiochemical properties.64,74 For instance, Tu et al. in 2021 prepared injectable and self-healing polydopamine-modified GO scaffolds by mixing polydopamine-modified GO scaffolds with polymer F127-EPL solution through hydrophobic–hydrophilic interactions which produced a highly conductive polymer scaffold with conductivity of 4 × 10–4 S/cm for directly stimulating the macrophage toward M2 phenotype to facilitate the healing of diabetic wounds (Figure 3A).63 The M2 polarization was validated by the significant upregulation of the markers CD163, CD206, IL-10, and Arg-1, whereas the expression of M1 markers such as IL-1, iNOS, and TNF-α were remarkably decreased. Further, in vivo studies revealed that M2a and M2c macrophages decreased and increased, respectively, during days 7–14 of the healing period, suggesting that M2 polarization is indeed responsible for the anti-inflammatory effect during diabetic wound recovery. Electrical stimulation based on other hydrogels such as polypyrrole-grafted gelatin methacryloyl (GelMA–PPy) have also been utilized for their macrophage polarization potential for osteogenesis.61 Dutta et al. in 2023 fabricated a 3D printed electroactive GelMA–PPy hydrogel scaffold with a high conductivity of 135 × 10–4 S/cm which generated a microcurrent of 250 mV for stimulating the macrophage toward the M2 phenotype, as shown in Figure 3B.61
Figure 3.
Electroactive materials and electrodes for generating exogenous electric fields for promoting macrophage polarization. (A) Synthesis method of electrically active GDFE hydrogel with high conductivity for promoting M2 polarization. Steps 1–3 correspond to physical, photo, and ionic cross-linking steps, respectively. Reproduced with permission from ref (63). Copyright 2021 Wiley-VCH. (B) Three-dimensional printing of triply cross-linked gelatin–polypyrrole hydrogel for promoting M2 polarization for osteogenesis. Reproduced with permission from ref (61). Copyright 2023 Elsevier. (C) Implantable bioelectrodes coated with heparin-doped polypyrrole (PPy/Hep) for altering the macrophage toward anti-inflammatory M2 phenotype. (i) Recruitment of macrophages, (ii) M1 suppression, (iii) decreasing of scarring, and (iv) signaling process. Reproduced from ref (76). Copyright 2022 American Chemical Society.
For muscle regeneration, electroactive hydrogels and nanocomposite scaffolds have been reported to induce the M1 to M2 polarization by promoting the nuclear translocation of STAT6 which elevated the expression of gene related anti-inflammatory cytokines, as detected by the significant expression of CD163.62,63,75 Apart from electroactive materials, bioelectrodes prepared by functionalizing conductive polymers on electrode surfaces have also been employed for applying ES to macrophages for modulating its phenotypes. In this regard, Lee et al. in 2022 fabricated biocompatible heparin-doped polypyrrole (PPy/Hep) electrodes, which were implanted subcutaneously to evaluate their in vivo macrophage polarization behavior (Figure 3C).76 The authors observed a decrease in the iNOS and Arg-1 expression with the increase in the surface roughness of the electrode surfaces. Moreover, the RNA profile of iNOS decreased significantly compared to Arg1 with the increase in the electrode roughness, causing a decrease in the iNOS/Arg-1 ratios.76 In addition to the lower impedance characteristics, the hydrophilic surface area of high roughness electrodes contributed toward the downregulation of the pro-inflammatory genes leading to M2 polarization.
Due to their high biocompatibility and biodegradability, piezoelectric polymers such as PVDF–TrFE and poly(hydroxybutyrate-co-valerate) (PHBV) have shown promising results in transitioning the phenotypes of macrophages for the systematic repair of wounds in a self-powered manner.70,71,77−81 The modulation of macrophage behavior is typically dependent on the material surface properties such as surface potential, surface roughness, and hydrophobicity.70,81 Particularly for piezoelectric materials, the dynamic surface potential generated at the cell–material interface is known to influence the cell behavior significantly.79,80 For instance, Zhou et al. in 2023 studied the influence of differing surface potential generation from piezoelectric PVDF–TrFE films on macrophage polarization for accelerated wound recovery.79 The as-prepared PVDF–TrFE films were first polarized with electric field intensities of 50 V/μm (low polarized) and 100 V/μm (high polarized), which generated surface potentials of 19 ± 13 and 232 ± 7 mV, respectively, and macrophages were seeded on their surfaces. It was observed that the high surface potential PVDF–TrFE films promoted the polarization of both M1 and M2 macrophages, as evidenced by the enhanced expression of CD206, IL-10 and TNF-α, IL-1β, respectively. On the other hand, unpolarized PVDF–TrFE films did not have any effect on the phenotypic alteration of macrophages, suggesting that a high surface potential of piezoelectric materials is crucial for macrophage polarization which could facilitate the transition from the inflammatory to the proliferative phase during wound healing.79
Relying on the fact that piezoelectric cues are inherent to bones, biocompatible piezoelectric polymers, ceramics, and scaffolds have been widely explored for providing a favorable microenvironment for bone regeneration.82,83 Various studies have reported that surface potential mediated piezoelectric charge generation can play a vital role in polarizing the macrophage phenotypes for aiding bone repair. For instance, a significant upregulation of M2 markers and genes was observed with the increase in the charge density on PVDF–TrFE films, suggesting that enhanced piezoelectricity could lead to M2 polarization.83 The presence of surface charges upregulated the expression of the integrin related signaling pathway and potassium ion channels which promoted the macrophage polarization. The transition toward a M2 phenotype could be related to the higher expression of integrin α5β1 compared to αMβ2.83 Moreover, an enhanced immunomodulatory effect was observed with positively charged piezoelectric materials compared to their negative counterparts. Mao et al.69 in 2022 showed that positive charges on BaTiO3/β-TCP (BTCP) favored the transition of macrophages toward the M2 phenotype which reduced the local inflammatory response and facilitated osteogenesis. BTCP+ and BTCP– were developed by orienting the polarization in the opposite direction, and their surface potentials were measured to be +107 and −175 mV, respectively.69 The output current and voltage of the as-prepared BTCP films were 300 nA and 590 mV, respectively, which were like the endogenous electric fields. Cytokine generation profiles showed enhanced upregulation of M2 markers such as IL-10 and CD206 when macrophages were seeded on BTCP+, suggesting that positive charges are conducive for regulating macrophage behavior.
Besides, improving the electroactivity of piezoelectric materials by fabricating composite materials has also been shown to improve the M2 polarization for inducing the anti-inflammatory effect during bone regeneration. Although intrinsic electrical stimulation from piezoelectric materials has displayed promising results in ameliorating macrophage polarization, the electric field generated is not sufficient in inducing in vivo macrophage polarization. Therefore, to intensify the electric field generation especially for in vivo models, piezoelectric materials are stimulated with external sources such as ultrasound, cyclic loading, and other means to convert the generated mechanical energy into electrical energy for modulating immune cell behavior. Wu et al. in 2022 reported improved M2 polarization when piezoelectric BaTiO3/Ti6Al4V (BT/Ti) scaffolds were subcutaneously implanted and were stimulated by low intensity pulsed ultrasound (US; frequency 1.5 MHz and pulse strength 30 mW/cm2), which generated a current of 6.8 μA in tissues.83 In vivo results showed the BT/Ti scaffolds under US stimulation enhanced the CD206 levels in tissues by 2-fold compared to the only BT/Ti scaffold groups, suggesting that electrical stimuli are essential for M2 polarization rather than the untreated piezoelectric BT/Ti scaffolds. The authors speculated that macrophages could sense the electric fields generated by the ultrasound activated BT/Ti scaffolds and employ the energy for ATP synthesis and oxidative phosphorylation by inhibiting the MAPK/JNK signaling pathway. In another study, 3D multifunctional piezoelectric material TiO2 nanotube functionalized PVDF layer was directly coated on the surface of titanium implants to treat the implant related inflammation for preventing osteogenic impairment. The in vitro piezoelectric stimulation generated an output voltage and current of 2 V and 20 nA/cm2, which repolarized the macrophages from the M1 to M2 phenotype due to the generation of piezoelectric potential, which triggered the hyperpolarization of macrophages by influencing the NF-κB and MAPK signaling pathways. Results from in vivo subcutaneous and femoral condyle implantation models revealed reduced expression of iNOS and increased expression of CD206 cells, thus confirming the piezoelectric generated electric field mediated macrophage polarization from M1 to M2. Similarly, Wu et al. in 2023 prepared the self-powered piezoelectric nanogenerator (PENG) based on PDA-modified BaTiO3 nanoparticles into a chitosan/gelatin (Cs/Gel) matrix which generated 0.8 V under 1 kPa pressure and resulted in M2 polarization for enhancing bone regeneration.84
In addition, ultrasound activated piezoelectricity generation has also been utilized to tune macrophage behavior for the treatment of inflammatory diseases such as rheumatoid arthritis.85,86 For instance, Li et al. in 2023 stimulated piezoelectric nanosheets, Fe/BiOCl NSs, using ultrasound in an in vivo articular inflammation model to study its effect on macrophage polarization.85 From the results, it was observed that CD80+ macrophages decreased by 12.3% and CD206+ macrophages increased by 3.5%, suggesting a successful M2 polarization. Upon US stimulation, Fe/BiOCl NSs generated the electrons which led to the consumption of H+ in the mitochondrial membrane causing the depolarization of the mitochondrial membrane potential, hence induing mitophagy which contributes to the anti-inflammatory effect by polarizing the macrophages to the M2 phenotype. In another study, Jiang et al. in 2023 developed piezoelectric BTO nanoparticles which can be activated by ultrasound for generating electrical stimulation for reprogramming the macrophage phenotype toward M2 for improving the pro-inflammatory conditions for the treatment of rheumatoid arthritis (RA).86 The obtained results showed that electrical stimulation resulted in decreases in the levels of FAP-α and TNF-α which altered the macrophage phenotype from M1 to M2.
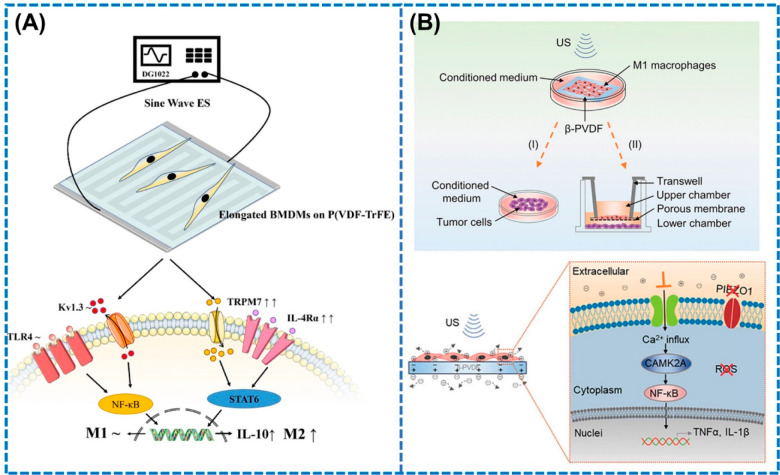
Although the mechanism behind the M2 polarizing ability of piezoelectric materials is not well-defined, a few studies in the recent years have suggested the synergistic roles of electrical stimulation and morphology of piezoelectric materials for driving M2 polarization. Gu et al. in 2023 reported that electric fields upregulate the IL-4Rα and TLR4 receptors which activate the ion channels such as TRPM7, triggering both M1 and M2 polarization.87 Nevertheless, the specific microstripe-like morphology of the piezoelectric material modulated the actin rearrangement and resulted in macrophage elongation, thereby increasing the expression of IL-4Rα receptor which ultimately altered the macrophages toward M2, as shown in Figure 4A. Piezoelectric based electric fields have been reported to regulate not only M2 but also M1 polarization, which is vital for inhibiting tumor growth.88,89 Localized electric fields were generated by activating β-PVDF film by ultrasound (90 W, 80 kHz) which selectively enhanced the expression of pro-inflammatory cytokines TNF-α and IL-1β, leading to M1 polarization, which were 3-fold and 2-fold higher than the untreated β-PVDF film, respectively.88 The authors suggested that the generated piezoelectric potential enabled the Ca2+ influx through the voltage-gated ion channels and established the Ca2+–CAMK2A–NF-κB axis which significantly promoted the secretion of pro-inflammatory cytokines for inducing cytocidal effects on tumor cells, causing M1 polarization (Figure 4B).
Figure 4.
Potential mechanisms of macrophage polarization driven by piezoelectric materials. (A) Synergistic effects of electrical stimulation and material morphology for promoting M2 polarization. Reproduced from ref (87). Copyright 2023 American Chemical Society. (B) Electrical stimulation influences influx of Ca2+ ions for driving the translocation of NF-κB which causes M1 polarization. Reproduced with permission from ref (88). Copyright 2021 Wiley.
Recently, electric fields generated from mechanical force have also been used to activate self-powered TENG for altering the macrophage phenotype to promote wound healing. Luo et al. in 2023 fabricated an electroactive dressing integrated with PTFE/Al based TENG generated electric fields in the range of which upregulated the expression of CD206 marker by ∼15% compared to the unstimulated groups, thereby promoting M2 polarization.90 In another study, Qian et al. in 2023 developed a microneedle based TENG platform for initiating immunomodulation for the treatment of autoimmune disease psoriasis. The electrical field caused an increase in the levels of Arg-1 (M2) and a decrease in iNOS (M1) markers in psoriatic skin, resulting in macrophage polarization by regulating the intracellular calcium signaling.27 In addition to the mechanical stimulus, temperature stimuli responsive self-powered pyroelectric materials have shown promising results in modulating the macrophage phenotype for antitumor immunity.71,74 Novel immunomodulation strategies based on cold temperature treatment of pyroelectric materials such as Bi2Fe4O9 nanosheets (NSs) and black phosphorus in the range 4–37 °C have been used to repolarize the macrophages from the M2 to the M1 phenotype for suppressing tumor metastasis.71 The obtained results suggested that cold temperature based pyroelectric treatment induced M1 polarization by influencing the ROS generation through the IKK/NF-kβ signaling pathways.
2.2.2. Migration and Differentiation
Recent studies have highlighted that external electrical fields could provide directional signals for stimulating the migration of immune cells including macrophages and neutrophils. Biomaterials such as ZnO nanorods (ZnO NRs)and hydroxyapatite nanocrystals, which possess the inherent capacity to generate electric signals or produce them under an external stimulus such as ultrasound have been known to influence cell migration by converting mechanical stresses into electricity.66,68,91−93 Upon application of mechanical force, surface charges are developed on piezoelectric materials, which causes the accumulation of charged biomacromolecules via electrostatic interactions, thereby inducing cellular responses including the migration of immune cells. For wound healing applications, Bhang et al. prepared a piezoelectric dermal patch functionalized with ZnO NRs, which remarkably promoted migration and differentiation of macrophages for enhanced wound recovery due to the generation of piezoelectric potentials in the range 300–900 mV across the wound bed which further activated various electric field induced intracellular signaling pathways.92
Piezoelectric materials have also been reported to stimulate the motility of macrophages and promote long-distance displacements by triggering the opening of ion channels. For instance, Murillo et al. in 2017 developed a PENG based on 2D ZnO nanosheets for improving the motility of macrophages.93 When cells were cultured on the surface of the PENG, the electromechanical interactions between the ZnO NRs and cells generated the local electric fields which triggered the motility of macrophages that was around 18% higher than that of the control group. Intracellular studies revealed that Ca2+ influx increased for ∼64% of the cells which were subjected to the electric fields generated by the PENG in contrast to only ∼9% of cells belonging to the control group, thereby suggesting that the intracellular Ca2+ transients are indeed responsible for macrophage motility. Furthermore, ultrasonic irradiation of piezoelectric materials has also shown excellent efficiency in regulating the migration of immune cells. For instance, BaTiO3 nanoparticles under US irradiation (1.5 W cm–2, 1 MHz) facilitated the migration of macrophages for promoting wound repair by generating the surface charge carriers induced by the electron–hole pair separation due to the mechanical deformations.83
2.2.3. Phagocytosis
Exogeneous electrical stimulation has also been reported to enhance the phagocytic ability of immune cells. Piezoelectric materials have been shown to influence the phagocytosis of variety targets such as bacteria, biofilms, cancer cells, and apoptotic debris by macrophages.90,94−99 For instance, Li et al. in 2023 have studied the effect of piezoelectricity on phagocytic potential of macrophages in immunosuppressive microenvironments generated by deep-tissue implant related bacterial infections.94 The authors developed a piezoelectric coating of barium titanate (BTO) nanostructures on Ti implants which was activated by ultrasound (1 MHz, 2 W cm–2) to generate the piezoelectric potentials, and its effects on the phagocytosis of Staphylococcus aureus bacteria as well as biofilms were analyzed.94 Under US treatment, macrophages on piezoelectric BTO displayed the highest phagocytosis rate compared to bare Ti and BTO surfaces, which was confirmed by the increase in the green intensity of the phagocytosed S. aureus bacteria inside the cells. The phagocytotic efficiency under US irradiation was attributed to the synergistic upregulation of differentiated expressed genes (DEGs) such as Adgrb1 and Treml4 in metabolism related signaling pathways as well as the activation of FcγR receptors, which amplified the innate immunity against S. aureus. Therefore, the electrical fields generated on the activated macrophages at the piezoelectric surface under US treatment facilitated a pro-inflammatory response by triggering the PI3K–AKT2 and MAPK pathways which further contributed to the phagocytosis behavior against S. aureus. Recently, electrical stimulation has also been reported to promote macrophage phagocytosis for relieving the progression of rheumatoid arthritis (RA) by targeting the tissue resident macrophage (TRM) receptors. Jiang et al. in 2023 employed ultrasound to trigger the piezoelectric tetragonal BaTiO3 (BTO) for generating electric fields to stimulate the TRM barrier across the joints in a noninvasive manner.86 The ultrasound treatment generated negative charges on the surface of BTO which caused the binding between TREM2 TRM and BTO, patching the immunological barrier. In another study, electric fields generated by 3D printed piezoelectric hydrogels consisting of ZnO nanoparticle modified PVDF/sodium alginate (SA) increased the expression of TNF-α for promoting neutrophil phagocytosis.95 Apart from piezoelectric stimulation, a pulsed direct current of 9 μA generated by TENG initiated the phagocytosis of dead cancer cells by antigen-presenting cells, confirmed by the enhanced expression of calreticulin (chaperone protein).96 This electrically enhanced phagocytic behavior contributed to the release of DAMPs, which activated the T cell mediated adaptive immune responses for inhibiting the tumor growth. Moreover, electric fields generated by pyroelectric materials such as Janus BaTiO3 NPs have also been reported to modulate the phagocytosis of cancer cells.99
3. Electrical Stimulation Combined with Drug Therapy
To further improve the efficacy of electrical stimulation for immunomodulation, ES has been combined with chemical drugs for adapting to clinical scenarios. Typically, this combinatorial therapy is used for immunomodulation as a single-step or two-step approach. Owing to its highly localized effect, ES is used to release drugs to targeted sites for inducing immunomodulation, which constitutes the single-step process. Electrical parameters such as field strength, pulse type, polarity, time, and frequency significantly influence the efficiency of drug release which can be optimized for controllable release. These electrically stimulative drug release platforms are based on electroactive hydrogels which trigger the release in the presence of electric fields. For instance, Naficy et al. prepared the biopolymeric chitosan and single walled carbon nanotube (CNT/SWNT) composite films loaded with dexamethasone (DEX) which was stimulated by a negative potential.100 DEX is a negatively charged anti-inflammatory drug for reducing cerebral edema. Interestingly, it was observed that the polarity of the electric potential influenced the rate of drug release. In the presence of a negative potential of −0.8 V, the rate of DEX release was significantly faster compared to the positive potential of +0.15 V, which could only release 30% of the drug, and its performance was similar to hose of the unstimulated groups. The authors suggested that the electrostatic interactions between the SWNT and DEX was mainly responsible for accelerating and retarding the DEX release.100 In another study, Wu et al. in 2023 fabricated a porous network structure of polypyrrole/DEX (PPy/DEX) composite films functionalized with extracellular matrix (ECM) which was electrically stimulated with a negative pulse of −2 V at 1 Hz frequency for 60 min to controllably release the DEX, which further promoted the secretion of anti-inflammatory factors and inhibited the pro-inflammatory cytokines supporting osteogenic environments for bone regeneration.101 When the film was subjected to negative voltage, PPy became neutralized due to the electron absorption, thereby reducing its electrostatic attraction to the drug, and as a result, the drug was released to the targeted site owing to its hydrophilic nature leading to electric-field-controlled drug release.
Recently, self-powered ES controlled drug delivery systems have gained immense attention due to their ability to develop into a completely wearable module. Qian et al. in 2023 developed a self-powered microneedle based drug delivery platform which can generate electric fields from TENG for simultaneously releasing multiple drugs such as tazarotene (TAZ) and betamethasone (BM) for immunomodulation in order to treat psoriasis.27 In contrast to the reported systems, this self-powered device produced pulsed electrons from TENG directly onto the tissues for increasing the penetration of the drugs which initiated immunomodulation by activating the Ca2+ signaling pathway. The obtained results showed that a TENG output of 1 V resulted in complete release of the drug within 2 h which inhibited the levels of macrophages and T cells and resulted in a high inhibition efficiency closer to that of normal skin, whereas the only ES group and drug group still had 4.8 and 3.8% cells, respectively, hence validating the efficiency of the combined therapy for alleviating skin inflammation.27 In addition, ES modalities are also used to deliver next-generation drugs such as short interfering RNAs (siRNAs) which are becoming popular due to the increasing problem of drug resistance with conventional drugs. For instance, a self-assembled tannic acid–siRNA–PVA hydrogel matrix was prepared and subjected to electric stimulation (0.05 mA, 1 Hz) for 30 min, which released the siRNA from the matrix and caused cellular endocytosis of the hydrogel. This combinatorial therapy reduced the MMP-9 levels and other pro-inflammatory cytokines, thus promoting macrophage polarization.102
On the other hand, in tumor microenvironments, electric fields are first applied to permeabilize the cells, followed by drug administration into the tumor site which can enter the target cells and cause immunomodulation, thereby resulting in a two-step process. Although cell delivery based immunotherapies are becoming increasing popular for cancer treatment, they are associated with limitations in terms of scale-up potential and preservation of functional surface proteins on cell membranes.103 Moreover, it is difficult to control the characteristics of cell carriers with external stimuli for the controllable release of drugs. Therefore, nanoparticle based drug delivery vehicles which can respond easily to an electrical stimulus owing to their surface charges have emerged as promising alternatives for combinatorial therapy.103 In the presence of electrical fields, these nanoparticle drug delivery systems can release the drugs controllably and lead to improved tumor penetration owing to their enhanced permeability and retention effects.104 For instance, Yu et al.103 in 2020 prepared iron oxide nanoparticles loaded with indoleamine 2,3-dioxygenase (IDO) inhibitors which released the drug under an electric field of 2000 V/cm at the tumor site for irreversible electroporation (IRE) based cell killing and immunomodulation by increasing the infiltration of tumor-infiltrating T cells (CD3+).105
4. Conclusions and Future Perspectives
Immune cells are key players in numerous physiological processes that are critical for disease prevention. Therefore, controlling the behavior of immune cell subsets has emerged as an important strategy in therapeutic applications for wound healing, cancer therapy, and bone regeneration, among many other diseases. Numerous methods to modulate immune responses have been developed, and electrical stimulation based modulation techniques are likely to be noninvasive and hence possibly the easiest to translate. Herein, we have reviewed the different strategies for electrically stimulating immune cells, and their effects on different cellular processes such as polarization, phagocytosis, migration, and differentiation has been elucidated. Studies have shown that immune cells including the macrophages, T cells, and B cells are electrically excitable in nature due to the presence of specific ion channels, which tune their membrane potentials in the presence of endogenous and exogenous electric fields. A systematic literature survey has revealed that stimulation of macrophages by conventional DC sources has been successful in altering the phenotypes of macrophages. In addition, different electrical parameters such as the intensity of the electric fields, frequency, and treatment time are critical in regulating macrophage polarization, although these have not been assessed systematically. For instance, application of lower voltage appears to polarize the macrophages toward an anti-inflammatory phenotype, which is desirable for wound healing and bone regeneration. On the other hand, higher voltages altered macrophages toward a M1 phenotype, which could be used for anticancer applications. In addition, electric fields influenced the migration rate of distinct immune cells with DC electric fields and oscillating electric fields, enhancing the migration of lymphocytes and macrophages, respectively. Separately, pulsed electric fields alleviated the phagocytic uptake of apoptotic debris and pathogens by immune cells, specifically macrophages, by causing either lipid inversion or bleb formation.
To address the limitations such as the requirement of expensive instrumentation with a power supply, low portability, and high operational cost associated with the conventional electrical stimulation sources, researchers have started to utilize smart platforms based on electroactive materials, bioelectrodes, and piezoelectric materials. Highly conductive materials such as graphene oxide and polypyrrole-grafted gelatin methacryloyl (GelMA–PPy) hydrogels have shown a significant ability to promote M2 polarization for wound healing and bone regeneration. Owing to their ability to self-generate electric fields under mechanical stimuli, piezoelectric materials such as PVDF–TrFE, PHBV, and BaTiO3 have demonstrated macrophage polarization toward M1 as well as M2 phenotypes either by self-stimulation or under ultrasound stimulus. Moreover, inorganic piezoelectric nanomaterials including BaTiO3 and ZnO modulated other cellular processes such as phagocytosis and migration of immune cells by causing electron–hole pair separations under electrical stimulus. Although several studies have reported the successful use of electrical stimulation for modulating the immune cell responses, there are still a few bottlenecks associated with this approach. First, the electrodes used for supplying the electrical stimulus to the immune cells are metal based, which increases the in vivo toxicity issues. Hence, designing biocompatible and biodegradable electrodes for electrical stimulation is highly desired for improving the reliability of such systems. Second, the stimulation sources used currently are incorporated with several electrical components such as rectifiers and capacitors, which increase the size and complexity of the devices. Hence, miniaturizing the system size is also necessary for practical applications. Third, the efficiencies of the electrical stimulation systems might vary among different individuals, which would require fabricating sophisticated control circuits in the future for realizing the preciseness of electrical stimulation. In addition, the mechanism by which electrical stimulation influences the functionality of the immune cells remains unclear. Various studies have speculated that electric fields activate numerous signaling pathways including NF-κB and STAT1, MAPK–p38, and Erk1/2 and Akt; nevertheless the experimental studies validating such hypotheses are missing. Therefore, detailed investigations emphasizing the underlying mechanisms at the cellular levels should be carried out to obtain better understanding of ES for immunomodulation and improving its therapeutic efficiency.
Further optimization in electrical parameters such as voltage, frequency, waveform type, and directionality is also desirable in the future for acquiring target-specific immunomodulation depending on the immune cell types. Moreover, the safe range of electrical stimulation for immunomodulation is not very well-defined for animal as well as human studies. For the first generation of electrical stimulation devices approved by the FDA for nerve and brain stimulation, the safety was determined in terms of charge density. For instance, 30 μC/cm2 was approved for brain stimulation, which was based on damage thresholds in animal studies. However, later it was suggested that parameters such as electric field strength, charge density, electrode surface area, stimulation waveform, pulse frequency, duty cycle, time requirements, and type of tissue to be stimulated (thickness and conductivity) play important roles in determining the safe range of electrical stimulation not only for immunomodulation but also for overall human use. For a few cell types, these parameters have been optimized individually such that they will not cause any damage to the target as well as to the surrounding tissues. Pulsed electric fields in the range 50–400 mV/mm have been reported to be safe for human endothelial cells, whereas for the muscle cells a 20 mA pulsed current in the frequency range 10–50 Hz has been denoted safe. Only a few studies have indicated the pulsed waveform and current below 0.5 mA is safe for stimulation irrespective of the tissue type and without causing any side effects; however, its effectiveness for immunomodulation is yet to be determined. In addition to the electric field strength, frequency is an important parameter for consideration when assessing the safety of electrical stimulation devices for immunomodulation. A lower frequency of 1 Hz can stimulate cells for their growth through membrane depolarization, whereas frequencies higher than megahertz might cause tissue heating and damage. As a result, it can be anticipated that the safe range of electrical stimulation varies based on the cumulative combinations of electrical parameters used and type of tissue to be stimulated. Nevertheless, given the fact that all these parameters are not well-studied and their role in tissue damage is still unknown, it becomes quite difficult to define an explicit range of safe electric field strength. Therefore, it is imperative to conduct in-depth studies to analyze the effects of different electrical parameters on immune cell types in order to determine the safe range which will ease its clinical translation.
Further, the sources of ES for immunomodulation are mostly based on electroactive polymer materials, which electrically stimulate immune cells by cell–material interactions at the interface. However, these materials are typically insoluble in water, are highly cytotoxic, have low surface wettability, and possess high impedance, which limit the attachment of the cells to the material surface, and as a result the immunomodulation effect decreases. Therefore, there is also a need to develop device based platforms which can directly supply the electric fields to the immune cells instead of relying on the cell–material interactions for modulating the cellular processes such that they can increase the therapeutic efficiency and improve the controllability for localized stimulation. In this regard, fabrication of stimuli-responsive nanogenerator devices such as triboelectric nanogenerators (TENG), piezoelectric nanogenerators (PENG), and pyroelectric nanogenerators (PyNG) which can generate electric fields upon self-activation by a ubiquitous stimulus, such as mechanical force and temperature, can open promising directions for future studies in immunomodulation for regenerative medicine and anticancer therapy.
Acknowledgments
This work was supported by the Institute of Eminence IoE-IISc-788 from the Indian Institute of Science to S.R.B. and by the Dr. Vijaya and Dr. Rajagopal Rao Biomedical Research fund to S.J.
The authors declare no competing financial interest.
References
- Schofield Z.; Meloni G. N.; Tran P.; Zerfass C.; Sena G.; Hayashi Y.; Grant M.; Contera S. A.; Minteer S. D.; Kim M.; Prindle A.; Rocha P.; Djamgoz M. B. A.; Pilizota T.; Unwin P. R.; Asally M.; Soyer O. S. Bioelectrical Understanding and Engineering of Cell Biology. J R Soc Interface. 2020, 17 (166), 20200013 10.1098/rsif.2020.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Bioelectric Mechanisms in Regeneration: Unique Aspects and Future Perspectives. Semin Cell Dev Biol 2009, 20 (5), 543–556. 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsmans M.; Clauss S.; Xiao L.; Aguirre A. D.; King K. R.; Hanley A.; Hucker W. J.; Wülfers E. M.; Seemann G.; Courties G.; Iwamoto Y.; Sun Y.; Savol A. J.; Sager H. B.; Lavine K. J.; Fishbein G. A.; Capen D. E.; Da Silva N.; Miquerol L.; Wakimoto H.; Seidman C. E.; Seidman J. G.; Sadreyev R. I.; Naxerova K.; Mitchell R. N.; Brown D.; Libby P.; Weissleder R.; Swirski F. K.; Kohl P.; Vinegoni C.; Milan D. J.; Ellinor P. T.; Nahrendorf M. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169 (3), 510–522.e20. 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Liu Q.; Li Z.; Tang S.; An Q.; Fan D.; Xiang Y.; Wu X.; Jin Z.; Ding J.; Hu Y.; Du Q.; Xu J.; Xie R. The Role of Ion Channels in Immune-Related Diseases. Prog. Biophys. Mol. Biol. 2023, 177, 129–140. 10.1016/j.pbiomolbio.2022.11.003. [DOI] [PubMed] [Google Scholar]
- Horn R. How Ion Channels Sense Membrane Potential. Proc Natl Acad Sc. 2005, 102 (14), 4929–493. 10.1073/pnas.0501640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J. I.; Rajnicek A. M.; McCaig C. D.; Barker R. N.; Wilson H. M. Electric Fields Are Novel Determinants of Human Macrophage Functions. J. Leukocyte Biol. 2016, 99 (6), 1141. 10.1189/jlb.3A0815-390R. [DOI] [PubMed] [Google Scholar]
- Lewis R. S.; Cahalan M. D. The Plasticity of ion Channels: Parallels between the Nervous and Immune Systems. Trends Neurosci. 1988, 11 (5), 214–218. 10.1016/0166-2236(88)90129-4. [DOI] [PubMed] [Google Scholar]
- Feske S.; Concepcion A. R.; Coetzee W. A. Eye on Ion Channels in Immune Cells. Sci. Signal. 2019, 12 (572), eaaw8014 10.1126/scisignal.aaw8014. [DOI] [PubMed] [Google Scholar]
- Da Silva L. P.; Kundu S. C.; Reis R. L.; Correlo V. M. Electric Phenomenon: A Disregarded Tool in Tissue Engineering and Regenerative Medicine. Trends Biotechnol. 2020, 38 (1), 24–49. 10.1016/j.tibtech.2019.07.002. [DOI] [PubMed] [Google Scholar]
- Chen C.; Bai X.; Ding Y.; Lee I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. 10.1186/s40824-019-0176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamampita C.; Trautmann A. Ionic Channels in Murine Macrophages. J. Cell Biol. 1987, 105 (2), 761–769. 10.1083/jcb.105.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand B.; Chou I. N. Microtubules and Microtubule-Associated Proteins in Resting and Mitogenically Activated Normal Human Peripheral Blood T Cells. J. Biol. Chem. 1992, 267 (15), 10716–10722. 10.1016/S0021-9258(19)50077-1. [DOI] [PubMed] [Google Scholar]
- Bregestovski P.; Redkozubov A.; Alexeev A. Elevation of Intracellular Calcium Reduces Voltage-Dependent Potassium Conductance in Human T Cells. Nature 1986, 319 (6056), 776–778. 10.1038/319776a0. [DOI] [PubMed] [Google Scholar]
- Das R.; Langou S.; Le T. T.; Prasad P.; Lin F.; Nguyen T. D. Electrical Stimulation for Immune Modulation in Cancer Treatments. Front. Bioeng. Biotechnol. 2022, 9, 795300 10.3389/fbioe.2021.795300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conta G.; Libanori A.; Tat T.; Chen G.; Chen J. Triboelectric Nanogenerators for Therapeutic Electrical Stimulation. Adv. Mater. 2021, 33, 2007502 10.1002/adma.202007502. [DOI] [PubMed] [Google Scholar]
- Zhao S.; Mehta A. S.; Zhao M. Biomedical Applications of Electrical Stimulation. Cell. Mol. Life Sci. 2020, 77 (14), 2681–2699. 10.1007/s00018-019-03446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. P.Applied Bioelectricity: From Electrical Stimulation to Electropathology; Springer Science & Business Media: 1998; p 371. [Google Scholar]
- Gordon T.; Brushart T. M.; Chan K. M. Augmenting Nerve Regeneration with Electrical Stimulation. Neurol Res. 2008, 30 (10), 1012–1022. 10.1179/174313208X362488. [DOI] [PubMed] [Google Scholar]
- Koppes A. N.; Keating K. W.; McGregor A. L.; Koppes R. A.; Kearns K. R.; Ziemba A. M.; McKay C. A.; Zuidema J. M.; Rivet C. J.; Gilbert R. J.; Thompson D. M. Robust Neurite Extension Following Exogenous Electrical Stimulation within Single Walled Carbon Nanotube-Composite Hydrogels. Acta Biomater. 2016, 39, 34–43. 10.1016/j.actbio.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.; Zhang Y.; Lu L.; Hu X.; Luo Z. Electrical Stimulation Accelerates Nerve Regeneration and Functional Recovery in Delayed Peripheral Nerve Injury in Rats. Eur. J. Neurosci. 2013, 38 (12), 3691–3701. 10.1111/ejn.12370. [DOI] [PubMed] [Google Scholar]
- Willand M. P.; Nguyen M.-A.; Borschel G. H.; Gordon T. Electrical Stimulation to Promote Peripheral Nerve Regeneration. Neurorehabil Neural Repair. 2016, 30 (5), 490–496. 10.1177/1545968315604399. [DOI] [PubMed] [Google Scholar]
- Gordon T. Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans. Neurotherapeutics 2016, 13 (2), 295–310. 10.1007/s13311-015-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernier P. T.; Li A.; Marcu L.; Craft C. M.; Gundersen M. A. Ultrashort pulsed electric fields induce membrane phospholipid translocation and caspase activation: differential sensitivities of Jurkat T lymphoblasts and rat glioma C6 cells. IEEE Trans. Dielectr. Electr. Insul. 2003, 10 (5), 795–809. 10.1109/TDEI.2003.1237329. [DOI] [Google Scholar]
- Kotnik T.; Miklavčič D.; Slivnik T. Time course of transmembrane voltage induced by time-varying electric fields—a method for theoretical analysis and its application. Bioelectrochem Bioenerg. 1998, 45 (1), 3–16. 10.1016/S0302-4598(97)00093-7. [DOI] [Google Scholar]
- Luo R.; Dai J.; Zhang J.; Li Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Healthc. Mater. 2021, 10 (16), 2100557 10.1002/adhm.202100557. [DOI] [PubMed] [Google Scholar]
- Thakral G.; LaFontaine J.; Najafi B.; Talal T. K.; Kim P.; Lavery L. A. Electrical Stimulation to Accelerate Wound Healing. Diabet Foot Ankle. 2013, 4 (1), 22081. 10.3402/dfa.v4i0.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L.; Jin F.; Wei Z.; Li T.; Sun Z.; Lai C.; Ma J.; Xiong R.; Ma X.; Wang F.; Sun F.; Zheng W.; Dong W.; Sun K.; Wang T.; Feng Z.-Q. Wearable, Self-powered, Drug-Loaded Electronic Microneedles for Accelerated Tissue Repair of Inflammatory Skin Disorders. Adv. Funct. Mater. 2023, 33, 2209407 10.1002/adfm.202209407. [DOI] [Google Scholar]
- Cheah Y. J.; Buyong M. R.; Mohd Yunus M. H. Wound Healing with Electrical Stimulation Technologies: A Review. Polymers 2021, 13 (21), 3790. 10.3390/polym13213790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. J.; Wynn T. A. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011, 11 (11), 723–737. 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns K. R.; Thompson D. M.. Macrophage Response to Electrical Stimulation. In 41st Annual Northeast Biomedical Engineering Conference, NEBEC 2015; Institute of Electrical and Electronics Engineers Inc.: 2015. [Google Scholar]
- Sridharan R.; Cameron A. R.; Kelly D. J.; Kearney C. J.; O’Brien F. J. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today. 2015, 18 (6), 313–325. 10.1016/j.mattod.2015.01.019. [DOI] [Google Scholar]
- Li J.; Jiang X.; Li H.; Gelinsky M.; Gu Z. Tailoring Materials for Modulation of Macrophage Fate. Adv. Mater. 2021, 33 (12), 2004172 10.1002/adma.202004172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79 (1), 541–566. 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Xu H.; Chen M; et al. CCL24/CCR3 axis plays a central role in angiotensin II–induced heart failure by stimulating M2 macrophage polarization and fibroblast activation. Cell Biol Toxicol. 2023, 39, 1413–1431. 10.1007/s10565-022-09767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B.; Gu Y.; Pu J.; Reid B.; Zhao Z.; Zhao M. Application of Direct Current Electric Fields to Cells and Tissues in Vitro and Modulation of Wound Electric Field in Vivo. Nat Protoc. 2007, 2 (6), 1479–1489. 10.1038/nprot.2007.205. [DOI] [PubMed] [Google Scholar]
- Oliveira K. M. C.; Barker J. H.; Berezikov E.; et al. Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model. Sci. Rep. 2019, 9, 11433. 10.1038/s41598-019-47389-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D. J.; McLaughlin K. A.; Levin M. Bioelectric Controls of Cell Proliferation: Ion Channels, Membrane Voltage and the Cell Cycle. Cell Cycle. 2009, 8, 3527–3536. 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Levin M.; Kaplan D. L. Bioelectric Modulation of Macrophage Polarization. Sci. Rep. 2016, 6, 21044. 10.1038/srep21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccann F. V.; Cole J. J.; Guyre P. M.; Russell J. A. G. Action Potentials in Macrophages Derived from Human Monocytes. Science 1983, 219 (4587), 991–993. 10.1126/science.6823563. [DOI] [PubMed] [Google Scholar]
- Jia Y.; Xu J.; Shi Q.; Zheng L.; Liu M.; Wang M.; Li P.; Fan Y. Study on the Effects of Alternating Capacitive Electric Fields with Different Frequencies on Promoting Wound Healing. Med Nov Technol Devices. 2022, 16, 100142 10.1016/j.medntd.2022.100142. [DOI] [Google Scholar]
- Xu J.; Jia Y.; Huang W.; Shi Q.; Sun X.; Zheng L.; Wang M.; Li P.; Fan Y. Non-Contact Electrical Stimulation as an Effective Means to Promote Wound Healing. Bioelectrochemistry 2022, 146, 108108 10.1016/j.bioelechem.2022.108108. [DOI] [PubMed] [Google Scholar]
- Srirussamee K.; Mobini S.; Cassidy N. J.; Cartmell S. H. Direct Electrical Stimulation Enhances Osteogenesis by Inducing Bmp2 and Spp1 Expressions from Macrophages and Preosteoblasts. Biotechnol. Bioeng. 2019, 116 (12), 3421–3432. 10.1002/bit.27142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel-White N.; Martin R. C. G.; Li Y.; Brock R. M.; Allen I. C.; Davalos R. V. Real-Time Prediction of Patient Immune Cell Modulation during Irreversible Electroporation Therapy. Sci. Rep. 2019, 9 (1), 17739. 10.1038/s41598-019-53974-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; Sun S.; Zhang Y.; Xie F.; Li S. The Role of Irreversible Electroporation in Promoting M1 Macrophage Polarization via Regulating the HMGB1-RAGE-MAPK Axis in Pancreatic Cancer. Oncoimmunology 2021, 10 (1), 1897295. 10.1080/2162402X.2021.1897295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian A.; Syed F.; Perry D.; Balamurugan V.; Colthurst J.; Chaudhry I. H.; Bayat A. Acceleration of Cutaneous Healing by Electrical Stimulation: Degenerate Electrical Waveform down-Regulates Inflammation, up-Regulates Angiogenesis and Advances Remodeling in Temporal Punch Biopsies in a Human Volunteer Study. Wound Repair and Regeneration 2011, 19 (6), 693–708. 10.1111/j.1524-475X.2011.00736.x. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M.; Webb D. J.; Horwitz A. R. Cell Migration at a Glance. J Cell Sci 2005, 118 (21), 4917–4919. 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- Jain S.; Cachoux V. M. L.; Narayana G. H. N. S.; de Beco S.; D’Alessandro J.; Cellerin V.; Chen T.; Heuzé M. L.; Marcq P.; Mège R.-M.; Kabla A. J.; Lim C. T.; Ladoux B. The Role of Single-Cell Mechanical Behaviour and Polarity in Driving Collective Cell Migration. Nat Phys 2020, 16 (7), 802–809. 10.1038/s41567-020-0875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F.; Baldessari F.; Gyenge C. C.; Sato T.; Chambers R. D.; Santiago J. G.; Butcher E. C. Lymphocyte Electrotaxis In Vitro and In Vivo. J. Immunol. 2008, 181, 2465. 10.4049/jimmunol.181.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Nandagopal S.; Wu D.; Romanuik S. F.; Paul K.; Thomson D. J.; Lin F. Activated T Lymphocytes Migrate toward the Cathode of DC Electric Fields in Microfluidic Devices. Lab Chip 2011, 11 (7), 1298–1304. 10.1039/c0lc00371a. [DOI] [PubMed] [Google Scholar]
- Cho M. R.; Thatte H. S.; Lee R. C.; Golan D. E. Integrin-Dependent Human Macrophage Migration Induced by Oscillatory Electrical Stimulation. Ann. Biomed. Eng. 2000, 28, 234. 10.1114/1.263. [DOI] [PubMed] [Google Scholar]
- Arnold C. E.; Rajnicek A. M.; Hoare J. I.; et al. Physiological strength electric fields modulate human T cell activation and polarisation. Sci Rep. 2019, 9, 17604. 10.1038/s41598-019-53898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Zhang Y.; Chen J.; Wu Y.; Zhang J.; Wang J. Nanosecond pulsed electric field inhibits malignant melanoma growth by inducing the change of systemic immunity. Med Oral Patol Oral Cir Bucal. 2019, 24 (4), e555–e561. 10.4317/medoral.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrivikraman G.; Boda S. M.; Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. 10.1016/j.biomaterials.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Todd I.; Clothier R. H.; Huggins M. L.; et al. Electrical Stimulation of Transforming Growth Factor-β1 Secretion by Human Dermal Fibroblasts and the U937 Human Monocytic Cell Line. Altern. Lab. Anim. 2001, 29 (6), 693–701. 10.1177/026119290102900611. [DOI] [PubMed] [Google Scholar]
- Yin S.; Chen X.; Hu C.; Zhang X.; Hu Z.; Yu J.; Feng X.; Jiang K.; Ye S.; Shen K.; Xie H.; Zhou L.; James Swanson R.; Zheng S. Nanosecond Pulsed Electric Field (NsPEF) Treatment for Hepatocellular Carcinoma: A Novel Locoregional Ablation Decreasing Lung Metastasis. Cancer Lett 2014, 346 (2), 285–291. 10.1016/j.canlet.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Katoh K. Effects of Electrical Stimulation of the Cell: Wound Healing, Cell Proliferation, Apoptosis, and Signal Transduction. Med. Sci. (Basel) 2023, 11 (1), 11. 10.3390/medsci11010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smani T.; Shapovalov G.; Skryma R.; Prevarskaya N.; Rosado J. Functional and physiopathological implications of TRP channels. Biochim. Biophys. Acta - Mol. Cell Res. 2015, 1853 (8), 1772–1782. 10.1016/j.bbamcr.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Ogura S.; Matsubara S.; Kuniyasu S.; Teranishi K.; Uto Y.; Shimomura N.. Study of Effects of Nanosecond Pulsed Electric Fields on Cancer Cell by Using in Vitro and Ex Vivo Assay. In IEEE International Power Modulator and High Voltage Conference, IPMHVC 2016; Institute of Electrical and Electronics Engineers Inc.: 2017; pp 377–381. [Google Scholar]
- Tekle E.; Wolfe M. D.; Oubrahim H.; Chock P. B. Phagocytic Clearance of Electric Field Induced “apoptosis-Mimetic” Cells. Biochem. Biophys. Res. Commun. 2008, 376 (2), 256–260. 10.1016/j.bbrc.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman S. R.; Chan S.-W.; Kao F.-C.; Ho H.-Y.; Khan I.; Pal A.; Huang C.-C.; Lin Z.-H. A Self-Powered Multifunctional Dressing for Active Infection Prevention and Accelerated Wound Healing. Sci. Adv. 2023, 9 (4), eadc8758 10.1126/sciadv.adc8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S. D.; Ganguly K.; Randhawa A.; Patil T. V.; Patel D. K.; Lim K. T. Electrically Stimulated 3D Bioprinting of Gelatin-Polypyrrole Hydrogel with Dynamic Semi-IPN Network Induces Osteogenesis via Collective Signaling and Immunopolarization. Biomaterials 2023, 294, 121999. 10.1016/j.biomaterials.2023.121999. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wei X.; Wang L.; Qian Z.; Liu H.; Fan Y. Quercetin-Based Composite Hydrogel Promotes Muscle Tissue Regeneration through Macrophage Polarization and Oxidative Stress Attenuation. Compos. B: Eng. 2022, 247, 110311. 10.1016/j.compositesb.2022.110311. [DOI] [Google Scholar]
- Tu Z.; Chen M.; Wang M.; Shao Z.; Jiang X.; Wang K.; Yao Z.; Yang S.; Zhang X.; Gao W.; Lin C.; Lei B.; Mao C. Engineering Bioactive M2 Macrophage-Polarized Anti-Inflammatory, Antioxidant, and Antibacterial Scaffolds for Rapid Angiogenesis and Diabetic Wound Repair. Adv. Funct. Mater. 2021, 31 (30), 2100924 10.1002/adfm.202100924. [DOI] [Google Scholar]
- Dong C.; Qiao F.; Hou W.; Yang L.; Lv Y. Graphene-Based Conductive Fibrous Scaffold Boosts Sciatic Nerve Regeneration and Functional Recovery upon Electrical Stimulation. Appl. Mater. Today 2020, 21, 100870. 10.1016/j.apmt.2020.100870. [DOI] [Google Scholar]
- Dai X.; Heng B. C.; Bai Y.; You F.; Sun X.; Li Y.; Tang Z.; Xu M.; Zhang X.; Deng X. Restoration of Electrical Microenvironment Enhances Bone Regeneration under Diabetic Conditions by Modulating Macrophage Polarization. Bioact Mater 2021, 6 (7), 2029–2038. 10.1016/j.bioactmat.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T.; Yu Y.; Pang Y.; Zhang D.; Wang Y.; Zhao H.; Zhang X.; Leng H.; Yang X.; Cai Q. Improving Bone Regeneration with Composites Consisting of Piezoelectric Poly(L-Lactide) and Piezoelectric Calcium/Manganese Co-Doped Barium Titanate Nanofibers. Compos. B: Eng. 2022, 234, 109734. 10.1016/j.compositesb.2022.109734. [DOI] [Google Scholar]
- Liu H.; Shi Y.; Zhu Y.; Wu P.; Deng Z.; Dong Q.; Wu M.; Cai L. Bioinspired Piezoelectric Periosteum to Augment Bone Regeneration via Synergistic Immunomodulation and Osteogenesis. ACS Appl Mater Interfaces 2023, 15 (9), 12273–12293. 10.1021/acsami.2c19767. [DOI] [PubMed] [Google Scholar]
- Wang L.; Pang Y.; Tang Y.; Wang X.; Zhang D.; Zhang X.; Yu Y.; Yang X.; Cai Q. A Biomimetic Piezoelectric Scaffold with Sustained Mg2+ Release Promotes Neurogenic and Angiogenic Differentiation for Enhanced Bone Regeneration. Bioact Mater 2023, 25, 399–414. 10.1016/j.bioactmat.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L.; Bai L.; Wang X.; Chen X.; Zhang D.; Chen F.; Liu C. Enhanced Cell Osteogenesis and Osteoimmunology Regulated by Piezoelectric Biomaterials with Controllable Surface Potential and Charges. ACS Appl Mater Interfaces 2022, 14 (39), 44111–44124. 10.1021/acsami.2c11131. [DOI] [PubMed] [Google Scholar]
- Zhu P.; Lai C.; Cheng M.; He Y.; Xu Y.; Chen J.; Zhou Z.; Li P.; Xu S. Differently Charged P (VDF-TrFE) Membranes Influence Osteogenesis Through Differential Immunomodulatory Function of Macrophages. Front. Mater. 2022, 8, 790753. 10.3389/fmats.2021.790753. [DOI] [Google Scholar]
- Goonoo N.; Gimié F.; Ait-Arsa I.; Cordonin C.; Andries J.; Jhurry D.; Bhaw-Luximon A. Piezoelectric Core-Shell PHBV/PDX Blend Scaffolds for Reduced Superficial Wound Contraction and Scarless Tissue Regeneration. Biomater Sci 2021, 9 (15), 5259–5274. 10.1039/D1BM00379H. [DOI] [PubMed] [Google Scholar]
- Zou Y.; Jin B.; Li H.; Wu X.; Liu Y.; Zhao H.; Zhong D.; Wang L.; Chen W.; Wen M.; Liu Y. N. Cold Nanozyme for Precise Enzymatic Antitumor Immunity. ACS Nano 2022, 16 (12), 21491–21504. 10.1021/acsnano.2c10057. [DOI] [PubMed] [Google Scholar]
- Wu X.; Wen M.; Zou Y.; Gao X.; Wei C.; Liu R.; Li J.; Wang L.; Li X.; Liu Y. N.; Chen W. Cold-Catalytic Antitumor Immunity with Pyroelectric Black Phosphorus Nanosheets. Chem Sci 2022, 13 (23), 6842–6851. 10.1039/D2SC01894B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C.; Qiao F.; Hou W.; Yang L.; Lv Y. Graphene-Based Conductive Fibrous Scaffold Boosts Sciatic Nerve Regeneration and Functional Recovery upon Electrical Stimulation. Appl Mater Today 2020, 21, 100870 10.1016/j.apmt.2020.100870. [DOI] [Google Scholar]
- Zheng H.; Cheng F.; Guo D.; He X.; Zhou L.; Zhang Q. Nanoenzyme-Reinforced Multifunctional Scaffold Based on Ti3C2Tx MXene Nanosheets for Promoting Structure–Functional Skeletal Muscle Regeneration via Electroactivity and Microenvironment Management. Nano Letters 2023, 23 (16), 7379–7388. 10.1021/acs.nanolett.3c01784. [DOI] [PubMed] [Google Scholar]
- Lee S.; Park J.; Kim S.; Ok J.; Yoo J. Il; Kim Y. S.; Ahn Y.; Kim T. Il; Ko H. C.; Lee J. Y. High-Performance Implantable Bioelectrodes with Immunocompatible Topography for Modulation of Macrophage Responses. ACS Nano 2022, 16 (5), 7471–7485. 10.1021/acsnano.1c10506. [DOI] [PubMed] [Google Scholar]
- Tang J.; Zhang Y.; Fang W.; Man Y.; Zhang J.; Zhao Q.; Lei X.; Chen J.; Li J.; Li Y.; Zuo Y. Electrophysiological microenvironment and site-specific cell behaviors regulated by fibrous aniline trimer-based polyurethanes in bone progressive regeneration. Chem. Eng. J. 2023, 460, 141630 10.1016/j.cej.2023.141630. [DOI] [Google Scholar]
- Li J.; Fan J.; Gao Y.; Huang S.; Huang D.; Li J.; Wang X.; Santos H. A.; Shen P.; Xia B. Porous Silicon Nanocarriers Boost the Immunomodulation of Mitochondria-Targeted Bovine Serum Albumins on Macrophage Polarization. ACS Nano 2023, 17 (2), 1036–1053. 10.1021/acsnano.2c07439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Wang J.; Zhang J.; Duan X.; Lin W.; Cheng K.; Weng W.; Chen Z. Polarized P(VDF-TrFE) Film Promotes Skin Wound Healing through Controllable Surface Potential. Colloids Surf B Biointerfaces 2023, 221, 112980 10.1016/j.colsurfb.2022.112980. [DOI] [PubMed] [Google Scholar]
- Wang Z.; He X.; Tang B.; Chen X.; Dong L.; Cheng K.; Weng W. Polarization Behavior of Bone Marrow-Derived Macrophages on Charged P(VDF-TrFE) Coatings. Biomater Sci 2021, 9 (3), 874–881. 10.1039/D0BM01604G. [DOI] [PubMed] [Google Scholar]
- Heng B. C.; Bai Y.; Li X.; Lim L. W.; Li W.; Ge Z.; Zhang X.; Deng X. Electroactive Biomaterials for Facilitating Bone Defect Repair under Pathological Conditions. Adv. Sci. 2023, 10, 2204502 10.1002/advs.202204502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z. C.; Lu J. Q.; Zhang T. W.; Liang H. F.; Yuan H.; Su D. H.; Ding W.; Lian R. X.; Ge Y. X.; Liang B.; Dong J.; Zhou X. G.; Jiang L. B. Piezoresistive MXene/Silk Fibroin Nanocomposite Hydrogel for Accelerating Bone Regeneration by Re-Establishing Electrical Microenvironment. Bioact. Mater. 2023, 22, 1–17. 10.1016/j.bioactmat.2022.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Dong H.; Tang Z.; Chen Y.; Liu Y.; Wang M.; Wei X.; Wang N.; Bao S.; Yu D.; Wu Z.; Yang Z.; Li X.; Guo Z.; Shi L. Electrical Stimulation of Piezoelectric BaTiO3 Coated Ti6Al4V Scaffolds Promotes Anti-Inflammatory Polarization of Macrophages and Bone Repair via MAPK/JNK Inhibition and OXPHOS Activation. Biomaterials 2023, 293, 121990 10.1016/j.biomaterials.2022.121990. [DOI] [PubMed] [Google Scholar]
- Wu P.; Shen L.; Liu H. F.; et al. The marriage of immunomodulatory, angiogenic, and osteogenic capabilities in a piezoelectric hydrogel tissue engineering scaffold for military medicine. Military Med Res. 2023, 10, 35. 10.1186/s40779-023-00469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.; Yang C.; Guo M.; Wang S.; Peng W.; Guo Q.; Ming D.; Teng Y.; Zheng B. Ultrasound-Remote Selected Activation Mitophagy for Precise Treatment of Rheumatoid Arthritis by Two-Dimensional Piezoelectric Nanosheets. ACS Nano 2023, 17 (1), 621–635. 10.1021/acsnano.2c09834. [DOI] [PubMed] [Google Scholar]
- Jiang L.; Wang Y.; Liu C.; Xu N.; Li W.; Wang L.; Wu Y.; Wang J.; He Z.; Sun F.; Zhao L.; Wu Q.; Wang X.; Yuan H.; Wang X.; Sun X. Ultrasound-Driven BaTiO3 Nanorobots Patching Immunologic Barrier to Cure Chronic Rheumatoid Arthritis. J. Adv. Ceram. 2023, 12 (5), 1105–1117. 10.26599/JAC.2023.9220730. [DOI] [Google Scholar]
- Gu J.; Wu C.; He X.; Chen X.; Dong L.; Weng W.; Cheng K.; Wang D.; Chen Z. Enhanced M2 Polarization of Oriented Macrophages on the P(VDF-TrFE) Film by Coupling with Electrical Stimulation. ACS Biomater Sci Eng. 2023, 9 (5), 2615–2624. 10.1021/acsbiomaterials.2c01551. [DOI] [PubMed] [Google Scholar]
- Kong Y.; Liu F.; Ma B.; Duan J.; Yuan W.; Sang Y.; Han L.; Wang S.; Liu H. Wireless Localized Electrical Stimulation Generated by an Ultrasound-Driven Piezoelectric Discharge Regulates Proinflammatory Macrophage Polarization. Adv. Science. 2021, 8 (13), 2100962 10.1002/advs.202100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Xiao C.; Zhan L.; Zhang Z.; Xing J.; Zhai J.; Zhou Z.; Tan G.; Piao J.; Zhou Y.; Qi S.; Wang Z.; Yu P.; Ning C. Wireless Electrical Stimulation at the Nanoscale Interface Induces Tumor Vascular Normalization. Bioact. Mater. 2022, 18, 399–408. 10.1016/j.bioactmat.2022.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R.; Liang Y.; Yang J.; Feng H.; Chen Y.; Jiang X.; Zhang Z.; Liu J.; Bai Y.; Xue J.; Chao S.; Xi Y.; Liu X.; Wang E.; Luo D.; Li Z.; Zhang J. Reshaping the Endogenous Electric Field to Boost Wound Repair via Electrogenerative Dressing. Adv. Mater. 2023, 35, 2208395 10.1002/adma.202208395. [DOI] [PubMed] [Google Scholar]
- Dai X.; Heng B. C.; Bai Y.; You F.; Sun X.; Li Y.; Tang Z.; Xu M.; Zhang X.; Deng X. Restoration of Electrical Microenvironment Enhances Bone Regeneration under Diabetic Conditions by Modulating Macrophage Polarization. Bioact. Mater. 2021, 6 (7), 2029–2038. 10.1016/j.bioactmat.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhang S. H.; Jang W. S.; Han J.; Yoon J. K.; La W. G.; Lee E.; Kim Y. S.; Shin J. Y.; Lee T. J.; Baik H. K.; Kim B. S. Zinc Oxide Nanorod-Based Piezoelectric Dermal Patch for Wound Healing. Adv Funct Mater 2017, 27 (1), 1603497 10.1002/adfm.201603497. [DOI] [Google Scholar]
- Murillo G.; Blanquer A.; Vargas-Estevez C.; Barrios L.; Ibáñez E.; Nogués C.; Esteve J. Electromechanical Nanogenerator–Cell Interaction Modulates Cell Activity. Adv. Mater. 2017, 29 (24), 1605048 10.1002/adma.201605048. [DOI] [PubMed] [Google Scholar]
- Li K.; Xu W.; Chen Y.; Liu X.; Shen L.; Feng J.; Zhao W. W.; Wang W.; Wu J.; Ma B.; Ge S.; Liu H.; Li J. Piezoelectric Nanostructured Surface for Ultrasound-Driven Immunoregulation to Rescue Titanium Implant Infection. Adv. Funct. Mater. 2023, 33, 2214522 10.1002/adfm.202214522. [DOI] [Google Scholar]
- Liang J.; Zeng H.; Qiao L.; Jiang H.; Ye Q.; Wang Z.; Liu B.; Fan Z. 3D Printed Piezoelectric Wound Dressing with Dual Piezoelectric Response Models for Scar-Prevention Wound Healing. ACS Appl Mater Interfaces. 2022, 14 (27), 30507–30522. 10.1021/acsami.2c04168. [DOI] [PubMed] [Google Scholar]
- Li H.; Chen C.; Wang Z.; Huang Y.; He G.; Liu Y.; Jiang P.; Wang Z. L. Triboelectric Immunotherapy Using Electrostatic-Breakdown Induced Direct-Current. Mater Today. 2023, 64, 40–51. 10.1016/j.mattod.2023.02.026. [DOI] [Google Scholar]
- Sun L.; Chen X.; Ma K.; Chen R.; Mao Y.; Chao R.; Wang H.; Yu B.; Wang J.; Zhang S. Novel Titanium Implant: A 3D Multifunction Architecture with Charge-Trapping and Piezoelectric Self-Stimulation. Adv. Healthc. Mater. 2023, 12 (11), 2202620 10.1002/adhm.202202620. [DOI] [PubMed] [Google Scholar]
- Kim J. I.; Hwang T. I.; Lee J. C.; Park C. H.; Kim C. S. Regulating Electrical Cue and Mechanotransduction in Topological Gradient Structure Modulated Piezoelectric Scaffolds to Predict Neural Cell Response. Adv. Funct. Mater. 2020, 30 (3), 1907330 10.1002/adfm.201907330. [DOI] [Google Scholar]
- Wei J.; Liu Y.; Li Y.; Zhang Z.; Meng J.; Xie S.; Li X. Photothermal Propelling and Pyroelectric Potential-Promoted Cell Internalization of Janus Nanoparticles and Pyroelectrodynamic Tumor Therapy. Adv. Healthc. Mater. 2023, 12, 2300338 10.1002/adhm.202300338. [DOI] [PubMed] [Google Scholar]
- Naficy S.; Razal J. M.; Spinks G. M.; Wallace G. G. Modulated Release of Dexamethasone from Chitosan–Carbon Nanotube Films. Sens Actuators A Phys. 2009, 155 (1), 120–124. 10.1016/j.sna.2009.07.021. [DOI] [Google Scholar]
- Wu C.; He X.; Zhu Y.; Weng W.; Cheng K.; Wang D.; Chen Z. Electrochemical Deposition of Ppy/Dex/ECM Coatings and Their Regulation on Cellular Responses through Electrical Controlled Drug Release. Colloids Surf B Biointerfaces 2023, 222, 113016 10.1016/j.colsurfb.2022.113016. [DOI] [PubMed] [Google Scholar]
- Lei H.; Fan D. A Combination Therapy Using Electrical Stimulation and Adaptive, Conductive Hydrogels Loaded with Self-Assembled Nanogels Incorporating Short Interfering RNA Promotes the Repair of Diabetic Chronic Wounds. Advanced Science 2022, 9 (30), 2201425 10.1002/advs.202201425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B.; Zhang W.; Kwak K.; Choi H.; Kim D.-H. Electric Pulse Responsive Magnetic Nanoclusters Loaded with Indoleamine 2,3-Dioxygenase Inhibitor for Synergistic Immuno-Ablation Cancer Therapy. ACS Appl Mater Interfaces 2020, 12 (49), 54415–54425. 10.1021/acsami.0c15679. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Han Y.; Sun Q.; Li Z. Harnessing Anti-Tumor and Tumor-Tropism Functions of Macrophages via Nanotechnology for Tumor Immunotherapy. Exploration 2022, 2 (3), 20210166 10.1002/EXP.20210166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.; Wang Y.; Hu Q. Recent Advances in Overcoming Barriers to Cell-Based Delivery Systems for Cancer Immunotherapy. Exploration 2022, 2 (3), 20210106 10.1002/EXP.20210106. [DOI] [PMC free article] [PubMed] [Google Scholar]