Abstract
Purpose
To report the retrospectively-based, clinical diagnostic findings for the horizontal, distance, fusional facility (DFF) test in the non-TBI (traumatic brain inury), ABI (acquired brain injury) population.
Methods
The DFF test (4 pd base-out/2 pd base-in) was assessed and compared retrospectively in the first author's optometric practice in three clinical populations: (1) post-mTBI, visually-symptomatic (n = 52), (2) post-ABI, non-mTBI, visually-symptomatic (n = 34), and (3) visually-normal, visually asymptomatic (n = 44).
Results
The DFF values in each group were significantly different from each other (p < 0.05). The mean non-TBI, ABI group value was significantly lower than found in the mTBI group, and both were significantly lower than the mean found in the normal cohort (p < 0.05). There was a significant reduction in DFF with increased age (p < 0.001). ROC values for the AUC ranged from excellent to acceptable (0.94–0.74).
Conclusion
The DFF test is a new and useful way to assess horizontal, distance, dynamic, fusional facility in those with presumed non-mTBI, ABI neurological conditions to assist in its diagnosis.
Keywords: Distance horizontal fusional facility (DFF), Vergence, Acquired brain injury, Neuro-optometry, Diagnosis
Introduction
The ability to perform vergence eye movements in a time-optimal manner to targets at different distances is critical for visual efficiency and visual comfort.1, 2, 3, 4 If dynamically-slowed, both aspects may be compromised, which could adversely affect one's vocational and avocational goals, and hence quality of life.1, 2, 3 In addition, slowed vergence responsivity may produce visual symptomatology, such as intermittent diplopia, skipping of lines during reading, and transient blur.1, 2, 3
Moreover, testing of the aforementioned fusional vergence facility (i.e., overall dynamic temporal responsivity) is of clinical diagnostic value, both at near4 and more recently at distance.5 In this more recent report,5 it was demonstrated that this new test of distance vergence facility, the Tannen Flipper Test (TFT; total 4 prism diopters base-out/2 prism diopters base-in), could significantly differentiate between those with concussion/mild traumatic brain injury (C/mTBI) and a matched, visually-normal, non-concussed cohort. Thus, it was proposed to represent a new, non-invasive, visual biomarker for concussion.5
In our pilot studies, it was found that the static, distance horizontal fusional vergence range was frequently reduced both in the concussion/mTBI and general acquired brain injury (ABI) populations. This led us to investigate its dynamic counterpart, similar to what has been established diagnostically for years, at near.4 Both the final prism values and distance test target were determined empirically over a two-year period to obtain the best protocol conditions for the highest diagnostic yield in both groups.
In the present study of a retrospective nature, diagnostic use of the TFT has now been expanded to include the non-C/mTBI, ABI population. This included those with more general ABI, such as cerebrovascular accident/stroke, neurodegenerative disease, brain infection, and post-neurosurgery.
Materials and methods
In this retrospective study, a record review was performed from 3/1/2018 to 3/1/2021 in the private practice of the first author that included all consecutive patients having the diagnosis of mTBI or ABI (non-TBI). All patients had a full, optometric vision examination, namely refractive, binocular, and ocular health components including visual fields performed in the first author's practice.6 The average refractive status of the entire group (n = 130) was sphere =−0.60D (ranging from +3.50 to −6.75, SD +/−1.17) and cylinder =−0.38D (ranging from 0.00 to −2.50, SD +/−0.37). There were three categories of patients tested, namely those: (1) optometrically-diagnosed as visually-normal and visually-asymptomatic based on case history and clinical findings using conventional diagnostic criteria (n = 44, average age = 41 years, age range = 11–68 years, SD +/−19 years); (2) medically-diagnosed (e.g., a neurologist) as C/mTBI and visually-symptomatic using conventional diagnostic criteria (e.g., intermittent diplopia, eye tracking problems) (n = 52, average age = 28 years, age range = 11–60 years, SD +/−16 years); and (3) medically-diagnosed (e.g., a neurologist) as non-C/mTBI, acquired brain injury (ABI) and visually-symptomatic using conventional diagnostic criteria (e.g., diplopia, eye tracking problems) (n = 34, average age = 59 years, age range = 19–86 years, SD +/−17 years). This last group suffered from internal neurological insult, such as cerebrovascular accident, brain tumour, post-neurosurgery, neurogenerative disease, brain infection, and multiple sclerosis. None in this last group had a history of a C/mTBI.
The Tannen Flipper Test (TFT-available at www.Bernell.com) was used to measure the horizontal, distance, dynamic fusional facility (DFF) of each patient.5,7 The TFT consists of prism values (total) of 4 base-out and 2 base-in (Fig. 1). During testing, the patient binocularly viewed a single, 20/30 Snellen letter at a distance of 20 ft (6 m) positioned along the midline in primary position, with their distance refraction in place. They were instructed to make as many alternations between the pair of prisms as rapidly as possible, once the target was single and in focus. This was followed by a short demonstration and brief practice. Then the actual testing commenced. Fusion on both the base-out and base-in prism flipper sides constituted one full cycle. The process was continued for one continuous minute, and the number of completed cycles per minute (cpm) was recorded.
Fig. 1.
The TFT flippers.
Results
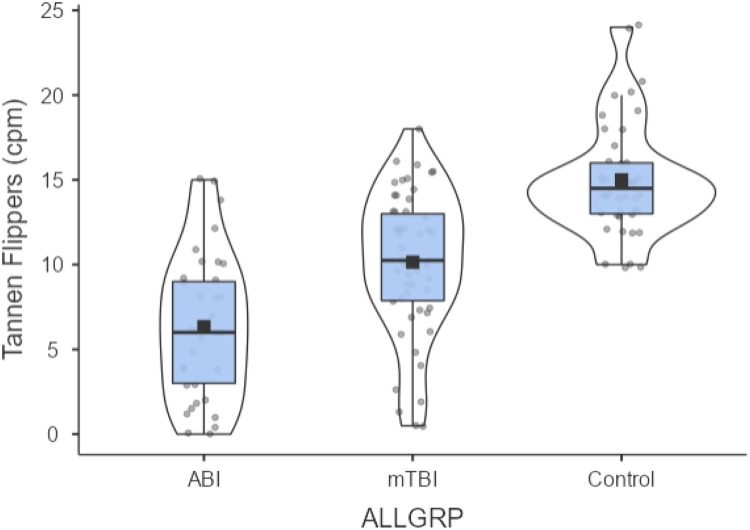
Table 1 presents the TFT results (mean and standard deviation) for the three diagnostic groups. A one-way ANOVA was performed: [f(2, 126) = 41.996, p < 0.001)]. There were one or more significant differences found between the group comparisons. Thus, this was followed by the post-hoc LSD test which corrected for multiple comparisons. The groups were significantly different from each other (all p < 0.05, see Table 1). These differences were still significant when controlled for age {ANCOVA, [f(1126 = 6.287, p = 0.013]}. Lastly, there was a small but significant reduction in the TFT value with increased age [Pearson correlation, two-tailed, r= −0.293, p < 0.001]. The TFT individual and group data are graphically presented in Fig. 2.
Table 1.
TFT findings.
| Normal | C/mTBI | ABI | |
|---|---|---|---|
| Mean DFFa | 15.0 | 10.1 | 6.3 |
| Std dev. | 3.3 | 4.2 | 4.3 |
Unit is cycles per minute, cpm.
Fig. 2.
TFT for all three populations using a density plot where the width represents the estimated frequency. Black square is the median. Upper and lower parts of grey box are the first and third quartiles. Individual subject data points are in grey circles.
In addition, an ROC analysis was performed (Table 2). The area under the curve (AUC) values ranged from excellent to acceptable, all with p-values <0.001.
Table 2.
ROC analyses for TFT.
| Comparison | AUCa | Std. error | p value | Lower bound | Upper bound |
|---|---|---|---|---|---|
| mTBI V Con | 0.81 | 0.043 | < 0.001 | 0.729 | 0.987 |
| ABI V Con | 0.94 | 0.028 | < 0.001 | 0.886 | 0.967 |
| mTBI V ABI | 0.74 | 0.055 | < 0.001 | 0.632 | 0.848 |
Note. AUC values of 0.7–0.79 are acceptable, 0.8–0.89 are good, and 0.9–1.0 are excellent.
Discussion
The present findings demonstrate for the first time a clinically-based, dynamic, horizontal, vergence dysfunction at distance in those manifesting a wide range of neurological conditions falling within the non-C/mTBI, ABI diagnostic domain. This new information extends our earlier work in the C/mTBI population.5 Furthermore, the results reveal for the first time that distance vergence facility is not only significantly worse in the ABI group than in the normal cohort, but also significantly worse than found in the C/mTBI group. Thus, this simple and rapid test provides new insights into the oculomotor system of the general ABI population that deserves further exploration, both clinically and in the laboratory (e.g., with objective recording of vergence1, 2, 3).
The present findings demonstrated that the DFF test could discriminate between each of the three population inter-comparisons, and hence reflects a testament to its robustness and clinical utility. The test was best at discriminating between those with ABI versus the controls, which was the primary objective of the investigation. It was least robust, but still acceptable per the ROC analysis, for discriminating between those with mTBI and ABI. This seems reasonable, as these two populations are most similar and closely related in terms of expected oculomotor/vergence problems, as both have brain damage and related oculomotor visual dysfunction.
Why might the ABI findings be significantly worse than those found in the C/mTBI cohort? There are at least three possibilities. First, one might speculate that most/all ABI neurological conditions, as per the current study, have more severe adverse impact on overall brain dysfunction than in those with C/mTBI, especially affecting vergence midbrain control.8 Second, the C/mTBI cohort was significantly younger than in those with ABI (i.e., mean 27.5 vs. 58.7 years). However, age was accounted for in the comparative statistical analyses. Third, and related to the above, those with ABI were typically affected for many more years than in those with C/mTBI which was rarely more than one year. Hence, there was a much longer time frame for neural degeneration and correlated dysfunction to progress and manifest itself, as well as any normal age-related effects. However, additional studies are needed, perhaps using the objective and quantitative visual-evoked potential to assess for binocular summation effects, and brain imaging techniques to assess for sites of neural insult/recovery, as well as other clinical and laboratory tools (e.g., objective recording of vergence tracking) to answer fully this important question.9
Related to the above, the present findings encourage more global testing in the research laboratory in these populations. For example, in earlier studies in those with C/mTBI,1, 2, 3 it was demonstrated objectively that overall near vergence dynamics were considerably slowed, with consistently reduced peak velocity, which suggested neurological midbrain insult.8 This should be tested objectively for distance vergence dynamics. Furthermore, with simple neuro-optometric rehabilitation (e.g., prism flippers at near), the laboratory-based vergence dynamics significantly improved,1, 2, 3 and moreover correlated with the related clinical vergence findings and reduced visual symptoms.2 Similar training and testing should be performed in the future in the general ABI population to determine clinical efficacy, as well as corroboration with the related objective vergence dynamics, at both distance and near. This could be in the form of either a retrospective study or a randomized clinical trial.
There was one study limitation. Intrasession repeatability was not performed.
Lastly, it would be important to develop a full clinical profile of vergence, and its interaction with accommodation, in the ABI population. This should include distance and near horizontal vergence facility, distance and near horizontal and vertical phoria and vergence ranges, positive and negative relative accommodation, the amplitude of accommodation and vergence, near accommodative facility, and the accommodation-to-convergence ratio (i.e., the ACA ratio), and in some cases include the objective analogs. With this comprehensive oculomotor profile, the ABI patient would be more broadly understood functionally and indirectly neurologically.1, 2, 3,8 Such full information would have a positive impact on future diagnostic and therapeutic aspects in the ABI population in general. This remains virgin territory for the contemporary neuro-optometrist and others (e.g., clinical vision scientists, neurologists) to explore.
Conflicts of interest
Dr. Barry Tannen has a financial interest in the Tannen Flipper Test.
Funding
None.
References
- 1.Thiagarajan P., Ciuffreda K.J. Effect of oculomotor rehabilitation on vergence responsivity in mild traumatic brain injury. J Rehabil Res Dev. 2013;50:1223–1240. doi: 10.1682/JRRD.2012.12.0235. [DOI] [PubMed] [Google Scholar]
- 2.Thiagarajan P., Ciuffreda K.J. Accommodative and vergence dysfunctions in mTBI: treatment effects and system correlations. Optom Vis Perf. 2014;2:539–554. [Google Scholar]
- 3.Ciuffreda K.J., Thiagarajan P. Objectively-based vergence and accommodative dynamics in mild traumatic brain injury (mTBI): a mini-review. Vision Res. 2022;191 doi: 10.1016/j.visres.2021.107967. [DOI] [PubMed] [Google Scholar]
- 4.Scheiman M., Wick B. 4th ed. Walters Kluwer/Lippincott Williams; New York: 2014. Clinical Management of Binocular Vision. [Google Scholar]
- 5.Tannen B., Rogers J., Ciuffreda K.J., Lyon E., Shelley-Tremblay J. Distance horizontal fusional facility: a proposed new test for concussion patients. Vis Dev Rehabil. 2016;2:170–175. [Google Scholar]
- 6.Benjamin W.J., editor. Borish's Clinical Refraction. St. Louis; Butterworth-Heinemann: 2006. [Google Scholar]
- 7.Tannen B., John J., Ciuffreda K.J., Shelley-Tremblay J. Assessment of three clinical tests for evaluation of concussion/mild traumatic brain injury. Vis Dev Rehabil. 2021;7:43–49. [Google Scholar]
- 8.Rucker J.C., Buettner-Ennever J.A., Straumann D., Cohen B. Case studies in neuroscience: instability of the visual near triad in traumatic brain injury–evidence for a putative convergence integrator. J Neurophysiol. 2019;122:1254–1263. doi: 10.1152/jn.00861.2018. [DOI] [PubMed] [Google Scholar]
- 9.Ciuffreda K.J., Tannen B., Rutner D., Yadav N.K., Suter P.S. Objective vision-based testing in mild traumatic brain injury: a bibliography. Vis Dev Rehabil. 2023;9(2):119–124. [Google Scholar]