Abstract
Background
Multimorbidity is increasing among adults in the United States. Yet limited research has examined multimorbidity clusters in persons aged 50 years and older with and without a history of cancer. An increased understanding of multimorbidity clusters may improve the cancer survivorship experience for survivors with multimorbidity.
Methods
We identified 7580 adults aged 50 years and older with 2 or more diseases—including 811 adults with a history of primary breast, colorectal, cervical, prostate, or lung cancer—from the 2018 National Health Interview Survey. Exploratory factor analysis identified clusters of multimorbidity among cancer survivors and individuals without a history of cancer (controls). Frequency tables and chi-square tests were performed to determine overall differences in sociodemographic characteristics, health-related characteristics, and multimorbidity between groups.
Results
Cancer survivors reported a higher prevalence of having 4 or more diseases compared to controls (57% and 38%, respectively). Our analysis identified 6 clusters for cancer survivors and 4 clusters for controls. Three clusters (pulmonary, cardiac, and liver) included the same diseases for cancer survivors and controls.
Conclusions
Diseases clustered differently across adults ≥ 50 years of age with and without a history of cancer. Findings from this study may be used to inform clinical care, increase the development and dissemination of multilevel public health interventions, escalate system improvements, and initiate innovative policy reform.
Keywords: Cancer survivors, Multiple chronic conditions, Multimorbidity, Multimorbidity clusters, Cancer health disparities, Factor analysis
Background
Multimorbidity is commonly defined as the presence of 2 or more simultaneous diseases in an individual [1]. Multimorbidity is a considerable public health concern due to its impact on health-related quality of life (HRQOL) [2, 3], health care utilization [4], cost of care [4], and mortality rates [5]. The prevalence of multimorbidity in the US adult population has increased from 21.8% in 2001 to 27% in 2018 [6], and it is projected to increase to 50% by 2030 [7]. An aging US population [8] may contribute to this increased multimorbidity prevalence. The percentage of the US population aged 65 and older rose from 12.4% in 2000 to 15.2% in 2016, and it is projected to increase to 21% of the total US population by 2030 [8]. The increase in multimorbidity is also related to the rising number of incident cases of cancer [9] and cancer prevalence [10].
In 2010, to drive changes in care delivery and increase research on multimorbidity, the US Department of Health and Human Services (HHS) developed the Strategic Framework on Multiple Chronic Conditions [11]. The framework called for greater understanding of combinations of diseases to inform prevention and management strategies and to improve health and QOL among populations with multimorbidity [11]. However, literature on the prevalence and impact of multimorbidity combinations is limited. There is heterogeneity in (a) terms used (multiple chronic conditions [12], comorbidity [1], and multimorbidity [1]), (b) measurement indices [13], and (c) methods of analysis [14], making comparisons across studies difficult. Nevertheless, studies [15, 16] have shown that diseases co-occur in individuals at rates higher than what would be expected by mere chance. Therefore, it has been suggested that studies examine disease clusters in patients with multimorbidity [17, 18].
The characterization of multimorbidity clusters may provide greater insights about: projected patient outcomes; ways to reduce disease progression; medication or behavioral intervention protocols to enhance patient health and well-being; or community or system changes to reduce risks for poor outcomes, suboptimal disease management, or additional disease diagnoses. Further, since many studies limit their examination to individual diseases or report on the numerical count of diseases, exploring multimorbidity clusters at a population level may provide added information about the health status of the US population. As a result, cluster analysis has emerged as a useful method of understanding the patterns and distribution of multimorbidity [17].
Current US research on multimorbidity clusters has focused on multimorbidity in subpopulations, such as American Indian [19], African American men [20], homeless veterans, and adults aged 65 and older [21, 22]. Kenzik et al. used population-based survey data to assess multimorbidity clusters in cancer survivors aged 65 and older [23]. This study found that multimorbidity clusters were associated with worse functional impairment than multiple unclustered diseases [23]. However, this study did not include a control group of adults without cancer, adults aged 50–64 years, or adults older than 80 years of age. Our study aimed to fill gaps in the current literature by assessing multimorbidity clusters in adults 50 years of age and above, with and without a history of cancer.
Methods
Data source
The National Health Interview Survey (NHIS) (https://www.cdc.gov/nchs/nhis/) is a cross-sectional household survey of the US civilian, noninstitutionalized population conducted annually by the National Center for Health Statistics (NCHS) [24]. NHIS collected data on demographics, health-related characteristics, and multimorbidity. In 2018, the final response rate for the sample adult component was 53.1% [24]. More detail about how the sample adult component was selected can be found in the NCHS 2018 Survey Description [24].
Measures
Participants and cancer characteristics
Males and females aged ≥ 50 years without a history of cancer were the control group, hereafter called controls. Males and females aged ≥ 50 years with a history of breast, colorectal, cervical, prostate, or lung cancer were included in the cancer survivor group. These cancer types were selected because they are the most commonly diagnosed in the United States (https://gis.cdc.gov/Cancer/USCS/?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcancer%2Fdataviz%2Findex.htm#/AtAGlance/), with associated routine, population-level preventive screenings for average risk individuals unanimously recommended by professional and guidance organizations and widely covered by insurance [10]. These cancer types are particularly important for our research question given that screenings for these cancers begin around ages 40–50 for average risk individuals (except cervical cancer screening). Therefore, there is a greater likelihood of initial diagnosis of these cancers between the ages of 50–65, which may impact the health trajectory and clustering of multimorbidities in adults with and without cancer across age groups and inform policy and practice recommendations related to these preventable cancers. Cancer survivors were individuals who responded “yes” to the question, “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?” and who self-reported the type as breast, colorectal, cervical, prostate, or lung. Cancer survivors diagnosed before the age of 21 or those who reported multiple cancers were excluded due to differences in treatment exposure and survivorship experience that may impact multimorbidity [25, 26]. Information about time since cancer diagnosis (< 2 years, 2–5 years, > 5 years) and age at diagnosis (< 50, 50–64, 65–74, 75–84, 85 +) was also collected.
Multimorbidity
We examined 14 diseases in both cancer survivors and controls. Only diseases assessed using the “Have you ever been told” question stem were included in the cluster analysis to minimize variability in multimorbidity regarding recency of diagnosis. Participants self-reported having ever been told by a doctor or other health professional that they had any of the following: hypertension, coronary heart disease, angina pectoris or heart condition/disease, heart attack, stroke, emphysema, asthma, ulcer, diabetes, liver condition, arthritis, high cholesterol, chronic obstructive pulmonary disease (COPD), and hepatitis. A composite multimorbidity count variable was created that summed the number of diseases reported (including cancer) by each participant.
Demographic characteristics
We examined several demographic characteristics including age (50–64, 65–74, 75–84, 85 +), sex (male or female), marital status (married, divorced/widowed/separated, never married/unmarried couple), highest education level (< high school, high school graduate/GED, some college, college graduate or higher), and family income (< $35,000, $35,000–$49,999, $50,000–$74,999, $75,000–$99,999, $100,000 +). The sample included persons from non-Hispanic White, non-Hispanic Black/African American, Hispanic, and additional racial and ethnic minority groups. Due to insufficient sample sizes, American Indian/Alaska Native (AIAN), Asian, or multiple race respondents were combined in the additional racial and ethnic minorities group.
Health-related characteristics
Health-related risk behaviors (e.g., smoking, alcohol use, and physical activity) were assessed based on self-reported responses. We used the NCHS recode to classify smoking status. Smoking status was defined as never (smoked < 100 cigarettes in lifetime and no longer currently smokes); current (smoked ≥ 100 cigarettes in lifetime and currently smokes); or former (previously smoked ≥ 100 cigarettes in lifetime but no longer currently smokes) (https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm). We used the NCHS alcohol consumption classification as well: abstainer (< 12 drinks in lifetime); former drinker (at least 1 drink in any year but no drinks in the past year); infrequent drinking (1–11 drinks in the past year); light drinking (< 3 drinks per week); moderate drinking (3–14 drinks per week); and heavy drinking (> 14 drinks per week) (https://www.cdc.gov/nchs/nhis/alcohol/alcohol_glossary.htm). For this analysis, we classified alcohol consumption using the terms: abstainer, former drinker, infrequent/light current drinker, and moderate/heavy current drinker— a stratification also used in previous studies [27]. The physical activity variable was defined using the 2008 HHS minimum physical activity recommendation (https://health.gov/sites/default/files/2019-09/paguide.pdf) (the recommendation of record at the time of survey administration)—weekly totals of 150 min of moderate-intensity physical activity or 75 min of vigorous-intensity physical activity. We classified physical activity as: no activity; some activity (< 150 min of moderate-intensity physical activity weekly or < 75 min of vigorous-intensity activity weekly); and met or exceeded (≥ 150 min of moderate-intensity physical activity weekly or ≥ 75 min of vigorous-intensity activity weekly). We also used self-reported status of health (excellent/very good, good, fair/poor) and obesity (body mass index [BMI] ≥ 30 kg/m2).
Analysis
We restricted the analysis to participants with 2 or more diseases (including cancer) and conducted an exploratory factor analysis for cancer survivors and controls. Given that this was intended to be an exploratory analysis of disease clusters and differences in clusters found in adults with cancer compared to controls focused on diseases and cancer status, and not to make nationally representative estimates of these conditions, we treated the NHIS data as a convenience sample; all analyses are unweighted and did not account for the complex survey factors. The cluster analysis used an orthogonal varimax rotation. Factors were extracted with eigenvalues > 1 and retained after rotation if the variance explained was > 5%. Items with a moderate to high loading of at least 0.3 on any factor were retained for the corresponding factor [28]. Items could potentially load on multiple factors.
Frequencies of sociodemographic characteristics, health-related characteristics, and chronic conditions were calculated for individuals within the derived clusters for cancer survivors and controls. Membership in a cluster required having any of the diseases defined by the cluster. All variables were examined using frequency tables, and chi-square tests were performed to determine overall differences in cancer survivors compared to controls. For this analysis, p values < 0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Study sample
Table 1 summarizes sample characteristics by cancer status. Significant differences were observed in age, sex, race and ethnicity, and smoking status between cancer survivors and controls. Controls comprised more 50–64-year-old adults (43.0%) compared to cancer survivors (25.4%). Conversely, cancer survivors had almost twice the percentage of adults aged 85 + (12.3%) compared to controls (6.4%). A greater percentage of cancer survivors were widowed/divorced/separated adults (50.2%) compared to controls (42.8%). More cancer survivors were college graduates or had some college compared to controls (60.2% vs 55.8%).
Table 1.
Characteristics of Persons with Multimorbidity, NHIS 2018 (n = 7580)
|
Cancer Survivors n (%) |
Controls n (%) | p value | |
|---|---|---|---|
| Age Group | n = 811 | n = 6769 | < .0001 |
| 50–64 | 206 (25.4) | 2907 (43.0) | |
| 65–74 | 295 (36.4) | 2277 (33.6) | |
| 75–84 | 210 (25.9) | 1152 (17.0) | |
| 85 + | 100 (12.3) | 433 (6.40) | |
| Sex | < .0001 | ||
| Male | 304 (37.5) | 3058 (45.2) | |
| Female | 507 (62.5) | 3711 (54.8) | |
| Race/Ethnicity | 0.0002 | ||
| Non-Hispanic White | 613 (75.6) | 4854 (71.7) | |
| Non-Hispanic Black | 112 (13.8) | 850 (12.6) | |
| Hispanic | 37 (4.56) | 607 (8.97) | |
| People from additional racial and ethnic minorities groupa | 49 (6.04) | 458 (6.77) | |
| Marital Status | < .0001 | ||
| Married | 340 (41.9) | 3011 (44.6) | |
| Widowed/Divorced/Separated | 407 (50.2) | 2889 (42.8) | |
| Never married/Unmarried couple | 64 (7.89) | 857 (12.7) | |
| Highest Education | 0.03 | ||
| < High school | 96 (11.9) | 1023 (15.2) | |
| High school graduate | 225 (27.9) | 1953 (29.0) | |
| Some college | 244 (30.3) | 1977 (29.4) | |
| ≥ College graduate | 241 (29.9) | 1781 (26.4) | |
| Family Income | 0.439 | ||
| < $35,000 | 320 (39.5) | 2773 (41.0) | |
| $35,000–$49,999 | 103 (12.7) | 884 (13.1) | |
| $50,000–$74,999 | 155 (19.1) | 1149 (17.0) | |
| $75,000–$99,999 | 87 (10.7) | 658 (9.72) | |
| $100,000 + | 146 (18.0) | 1305 (19.3) | |
| Self-Rated Health | 0.551 | ||
| Excellent/Very good | 320 (39.5) | 2563 (37.9) | |
| Good | 272 (33.6) | 2395 (35.4) | |
| Fair/Poor | 218 (26.9) | 1808 (26.7) | |
| Smoking Status | < .0001 | ||
| Never smoker | 425 (52.5) | 3384 (50.2) | |
| Current smoker | 72 (8.89) | 985 (14.6) | |
| Former smoker | 313 (38.6) | 2377 (35.2) | |
| Alcohol Use | 0.681 | ||
| Never drinker | 165 (20.5) | 1273 (19.1) | |
| Former drinker | 189 (23.5) | 1619 (24.3) | |
| Current drinker—infrequent/light | 308 (38.4) | 2524 (37.9) | |
| Current drinker—moderate/heavy | 141 (17.6) | 1247 (18.7) | |
| Physical Activity (in past week) | 0.519 | ||
| No activity | 310 (39.3) | 2488 (37.8) | |
| Some activity: < 150 min moderate or < 75 min vigorous | 179 (22.7) | 1608 (24.4) | |
| Meets/exceeds activity noted above | 300 (38.0) | 2482 (37.7) | |
| BMI | < .0001 | ||
| Obesity (≥ 30 kg/m2) | 254 (32.0) | 2603 (39.9) | |
| Cancer Type | |||
| Prostate | 242 (29.8) | ||
| Breast | 386 (47.6) | ||
| Colon/rectal | 91 (11.2) | ||
| Lung | 45 (5.55) | ||
| Cervical | 47 (5.79) | ||
| Time Since Diagnosis | |||
| Immediate (< 2 years) | 107 (13.2) | ||
| Short-term (2–5 years) | 192 (23.7) | ||
| Long-term (> 5 years) | 512 (63.1) | ||
| Age at Diagnosis | |||
| < 50 | 147 (18.1) | ||
| 50–64 | 358 (44.1) | ||
| 65–74 | 215 (26.5) | ||
| 75–84 | 76 (9.37) | ||
| 85 + | 15 (1.85) |
aPeople from additional racial and ethnic minorities group includes American Indian persons, Alaska Native persons, Asian persons, persons of multiple races, and persons of other races not releasable
Fewer cancer survivors were current smokers (8.9%) and obese (32.0%) compared to controls (14.6% and 39.9%, respectively). There were no significant differences in distribution of self-rated health status, family income, alcohol use, or physical activity between cancer survivors and controls. The majority of cancer survivors were diagnosed more than 5 years before (63.1%). The survivor sample comprised primarily former breast cancer (47.6%) patients, and most survivors had been diagnosed at ages 50–64 (44.1%).
Prevalence of multimorbidity
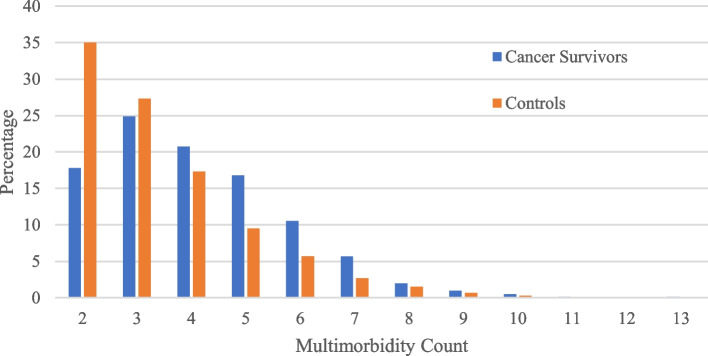
Table 2 summarizes disease prevalence in the sample stratified by cancer status. Controls reported a significantly higher prevalence of hypertension, heart attack, diabetes, arthritis, and high cholesterol compared to cancer survivors, whereas cancer survivors reported higher prevalence of emphysema. There was a significant difference in the distribution of the number of multimorbidities between cancer survivors and controls (Table 2). Cancer survivors had higher prevalence of reporting 4 or more diseases compared to controls (Fig. 1), whereas Table 2 reveals that controls more frequently reported multimorbidity counts of 2–3.
Table 2.
Prevalence of multimorbidity in persons with at least 2 diseases, NHIS 2018 (n = 7580)
| Cancer Survivors n (%) |
Controls n (%) |
p value | |
|---|---|---|---|
| Individual Diseases | n = 811 | n = 6769 | |
| Hypertension | 569 (70.3) | 5097 (75.3) | 0.002 |
| Coronary heart disease | 114 (14.1) | 1011 (15.0) | 0.497 |
| Heart attack (myocardial infarction) | 58 (7.16) | 698 (10.3) | 0.004 |
| Angina/other heart condition | 185 (22.8) | 1557 (23.1) | 0.886 |
| Stroke | 75 (9.27) | 641 (9.48) | 0.849 |
| Emphysema | 53 (6.56) | 324 (4.79) | 0.029 |
| Asthma | 144 (17.8) | 1228 (18.1) | 0.828 |
| Ulcer | 103 (12.7) | 866 (12.8) | 0.938 |
| Diabetes | 167 (20.6) | 1875 (27.7) | < .0001 |
| Liver disease | 19 (2.38) | 218 (3.26) | 0.181 |
| Arthritis | 440 (54.4) | 4059 (60.0) | 0.002 |
| High cholesterol | 472 (58.5) | 4536 (67.2) | < .0001 |
| COPD | 98 (12.1) | 751 (11.1) | 0.392 |
| Hepatitis | 34 (4.24) | 365 (5.47) | 0.143 |
| Multimorbidity Count | |||
| 2–3 | 346 (42.7) | 4220 (62.3) | < .0001 |
| 4–5 | 304 (37.5) | 1811 (26.8) | |
| 6 + | 161 (19.8) | 738 (10.9) | |
Fig. 1.
Percentages of multimorbidity count by cancer status
Characteristics of multimorbidity clusters
The cluster analysis yielded 6 clusters for cancer survivors and 4 clusters for controls. Only clusters that matched across both groups were named. These included the pulmonary cluster, with 3 conditions: COPD, emphysema, and asthma; the cardiac cluster, with 3 conditions: coronary heart disease, heart attack, and angina/other heart condition; and the liver cluster, with 2 conditions: hepatitis and liver disease. Additionally, there were 3 unmatched clusters for cancer survivors and 1 unmatched cluster for the controls.
Clusters occurring in both cancer survivors and controls were compared in Table 3. In both the pulmonary cluster and the cardiac cluster, there were significant differences between cancer survivors and controls in age group, sex, marital status, smoking status, and multimorbidity count. Significant differences in race/ethnicity were observed in the cardiac cluster only. In the liver cluster, there were significant differences in age group and multimorbidity count between cancer survivors and controls.
Table 3.
Characteristics of cluster membership for individuals with 2 or more diseases
| Pulmonary Cluster | Cardiac Cluster | Liver Cluster | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer Survivors n (%) |
Controls n (%) |
p value | Cancer Survivors n (%) |
Controls n (%) |
p value | Cancer Survivors n (%) |
Controls n (%) |
p value | |
| Age Group | n = 213 | n = 1788 | 0.001 | n = 247 | n = 2193 | < .001 | n = 47 | n = 493 | < .001 |
| 50–64 | 70 (32.9) | 854 (47.8) | 35 (14.2) | 753 (34.3) | 12 (25.5) | 245 (49.7) | |||
| 65–74 | 81 (38.0) | 562 (31.4) | 85 (34.4) | 746 (34.0) | 17 (36.2) | 183 (37.1) | |||
| 75–84 | 46 (21.6) | 271 (15.2) | 83 (33.6) | 478 (21.8) | 14 (29.8) | 51 (10.3) | |||
| 85 + | 16 (7.51) | 101 (5.65) | 44 (17.8) | 216 (9.85) | 4 (8.51) | 14 (2.84) | |||
| Sex | 0.018 | 0.009 | 0.805 | ||||||
| Male | 70 (32.9) | 738 (41.3) | 107 (43.3) | 1143 (52.1) | 24 (51.1) | 261 (52.9) | |||
| Female | 143 (67.1) | 1050 (58.7) | 140 (56.7) | 1050 (47.9) | 23 (48.9) | 232 (47.1) | |||
| Race/Ethnicity | 0.123 | 0.004 | 0.326 | ||||||
|
Non-Hispanic White |
162 (76.1) | 1295 (72.4) | 184 (74.5) | 1641 (74.8) | 35 (74.5) | 339 (68.8) | |||
| Non-Hispanic Black | 28 (13.2) | 229 (12.8) | 39 (15.8) | 239 (10.9) | 6 (12.8) | 57 (11.6) | |||
| Hispanic | 8 (3.76) | 151 (8.45) | 6 (2.43) | 169 (7.71) | 5 (10.6) | 52 (10.5) | |||
| People from additional racial and ethnic minorities group | 15 (7.04) | 113 (6.32) | 18 (7.29) | 144 (6.57) | 1 (2.13) | 45 (9.13) | |||
| Marital Status | 0.009 | 0.003 | 0.187 | ||||||
| Married | 89 (41.8) | 695 (39.0) | 90 (36.4) | 943 (43.1) | 21 (44.7) | 200 (40.7) | |||
|
Widowed/ Divorced/Separated |
109 (51.2) | 825 (46.3) | 141 (57.1) | 1009 (46.1) | 23 (48.9) | 210 (42.8) | |||
|
Never married/ Unmarried couple |
15 (7.04) | 262 (14.7) | 16 (6.48) | 235 (10.8) | 3 (6.38) | 81 (16.5) | |||
| Highest Education | 0.626 | 0.284 | 0.128 | ||||||
| < High school | 33 (15.6) | 302 (17.0) | 30 (12.2) | 356 (16.3) | 2 (4.26) | 78 (15.9) | |||
|
High school graduate |
56 (26.4) | 505 (28.4) | 67 (27.2) | 626 (28.7) | 11 (23.4) | 127 (25.9) | |||
| Some college | 77 (36.3) | 566 (31.9) | 81 (32.9) | 645 (29.6) | 20 (42.6) | 157 (32.0) | |||
| ≥ College graduate | 46 (21.7) | 403 (22.7) | 68 (27.6) | 554 (25.4) | 14 (29.8) | 128 (26.1) | |||
| Family Income | 0.612 | 0.192 | 0.355 | ||||||
| < $35,000 | 106 (49.8) | 830 (46.4) | 100 (40.5) | 979 (44.6) | 19 (40.4) | 244 (49.5) | |||
| $35,000–$49,999 | 21 (9.86) | 229 (12.8) | 38 (15.4) | 275 (12.5) | 8 (17.0) | 54 (11.0) | |||
| $50,000–$74,999 | 29 (13.6) | 277 (15.5) | 51 (20.7) | 361 (16.5) | 5 (10.6) | 74 (15.0) | |||
| $75,000–$99,999 | 18 (8.45) | 159 (8.89) | 24 (9.72) | 203 (9.26) | 6 (12.8) | 36 (7.30) | |||
| $100,000 + | 39 (18.3) | 293 (16.4) | 34 (13.8) | 375 (17.1) | 9 (19.2) | 85 (17.2) | |||
| Self-Rated Health | 0.785 | 0.397 | 0.238 | ||||||
|
Excellent/Very good |
59 (27.7) | 505 (28.2) | 67 (27.2) | 651 (29.7) | 15 (32.6) | 162 (32.9) | |||
| Good | 78 (36.6) | 613 (34.3) | 95 (38.6) | 752 (34.3) | 19 (41.3) | 150 (30.5) | |||
| Fair/Poor | 76 (35.7) | 670 (37.5) | 84 (34.2) | 789 (63.0) | 12 (26.1) | 180 (36.6) | |||
| Smoking Status | 0.008 | 0.003 | 0.222 | ||||||
| Never smoker | 88 (41.3) | 707 (39.7) | 115 (46.6) | 992 (45.4) | 18 (38.3) | 193 (39.2) | |||
| Current smoker | 28 (13.2) | 390 (21.9) | 17 (6.88) | 317 (14.5) | 5 (10.6) | 98 (19.9) | |||
| Former smoker | 97 (45.5) | 684 (38.4) | 115 (46.6) | 874 (40.0) | 24 (51.1) | 201 (40.9) | |||
| Alcohol Use | 0.088 | 0.475 | 0.484 | ||||||
| Never drinker | 43 (20.4) | 272 (15.5) | 55 (22.5) | 417 (19.4) | 7 (14.9) | 79 (16.3) | |||
| Former drinker | 68 (32.2) | 497 (28.4) | 72 (29.5) | 622 (28.9) | 11 (23.4) | 136 (28.0) | |||
|
Current drinker- infrequent/light |
69 (32.7) | 655 (37.4) | 75 (30.7) | 759 (35.2) | 22 (46.8) | 173 (35.6) | |||
|
Current drinker- moderate/heavy |
31 (14.7) | 328 (18.7) | 42 (17.2) | 355 (16.5) | 7 (14.9) | 98 (20.2) | |||
| Physical Activity | 0.294 | 0.952 | 0.685 | ||||||
| No activity | 100 (48.3) | 755 (43.4) | 107 (44.0) | 945 (44.2) | 19 (41.3) | 199 (41.9) | |||
| Some activity | 40 (19.3) | 409 (23.5) | 57 (23.5) | 483 (22.6) | 8 (17.4) | 105 (22.1) | |||
| Meets/exceeds | 67 (32.4) | 575 (33.1) | 79 (32.5) | 708 (33.2) | 19 (41.3) | 171 (36.0) | |||
| Obesity | 0.596 | 0.062 | 0.866 | ||||||
| BMI (≥ 30 kg/m2) | 82 (39.0) | 704 (40.9) | 82 (34.0) | 861 (40.2) | 17 (36.2) | 180 (37.4) | |||
| Multimorbidity Count | < .0001 | < .0001 | 0.0001 | ||||||
| 2–3 | 43 (20.2) | 698 (39.0) | 24 (9.72) | 648 (29.6) | 4 (8.51) | 195 (39.6) | |||
| 4–5 | 83 (39.0) | 637 (35.6) | 107 (43.3) | 911 (41.5) | 22 (46.8) | 169 (34.3) | |||
| 6 + | 87 (40.8) | 453 (25.3) | 116 (47.0) | 634 (28.9) | 21 (44.7) | 129 (26.2) | |||
Table 4 displays characteristics of clusters that did not match across cancer survivor and control groups. There were 3 unmatched clusters for cancer survivors. Unmatched cluster 1 for cancer survivors contained 3 conditions: hypertension, high cholesterol, and diabetes. Unmatched cluster 2 for cancer survivors contained 2 conditions: stroke and arthritis. And unmatched cluster 3 for cancer survivors contained 2 conditions: high cholesterol and ulcer. There was 1 unmatched cluster for controls, which contained 6 conditions: hypertension, high cholesterol, arthritis, asthma, diabetes, and ulcer. Further analyses could not be conducted on these groups due to a lack of comparison group. Demographic composition of each unmatched cluster was similar to demographics for cancer survivor and control groups, as seen in Table 1.
Table 4.
Characteristics of cluster membership for individuals with 2 or more diseases
| Cancer Unmatched Cluster 1 n (%) | Cancer Unmatched Cluster 2 n (%) |
Cancer Unmatched Cluster 3 n (%) |
Control Unmatched Cluster n (%) |
|
|---|---|---|---|---|
| Hypertension, High Cholesterol, Diabetes | Stroke, Arthritis | High Cholesterol, Ulcer | Hypertension, High Cholesterol, Arthritis, Asthma, Diabetes, Ulcer | |
| Age Group | n = 706 | n = 462 | n = 510 | n = 6706 |
| 50–64 | 164 (23.2) | 100 (21.6) | 120 (23.5) | 2887 (43.1) |
| 65–74 | 269 (38.1) | 165 (35.7) | 193 (37.8) | 2254 (33.6) |
| 75–84 | 190 (26.9) | 126 (27.3) | 135 (26.5) | 1139 (17.0) |
| 85 + | 83 (11.8) | 71 (15.4) | 62 (12.2) | 426 (6.35) |
| Sex | ||||
| Male | 274 (38.8) | 170 (36.8) | 200 (39.2) | 3018 (45.0) |
| Female | 432 (61.2) | 292 (63.2) | 310 (60.8) | 3688 (55.0) |
| Race/Ethnicity | ||||
| Non-Hispanic White | 523 (74.1) | 362 (78.4) | 385 (75.5) | 4804 (71.6) |
| Non-Hispanic Black | 108 (15.3) | 49 (10.6) | 74 (14.5) | 845 (12.6) |
| Hispanic | 35 (4.96) | 18 (3.90) | 21 (4.12) | 452 (6.74) |
| People from additional racial and ethnic minorities group | 40 (5.67) | 33 (7.14) | 30 (5.88) | 605 (9.02) |
| Marital Status | ||||
| Married | 294 (41.6) | 189 (40.9) | 211 (41.4) | 2990 (44.7) |
| Widowed/Divorced/ Separated | 354 (50.1) | 241 (52.2) | 257 (50.4) | 2856 (42.7) |
| Never married/Unmarried couple | 58 (8.22) | 32 (6.93) | 42 (8.24) | 848 (12.7) |
| Highest Education | ||||
| < High school | 86 (12.3) | 61 (13.3) | 64 (12.6) | 1013 (15.2) |
| High school graduate | 195 (27.8) | 121 (26.4) | 137 (27.0) | 1931 (28.9) |
| Some college | 211 (30.1) | 147 (32.0) | 164 (32.4) | 1958 (29.4) |
| ≥ College graduate | 209 (29.8) | 130 (28.3) | 142 (28.0) | 1770 (26.5) |
| Family Income | ||||
| < $35,000 | 277 (39.2) | 195 (42.2) | 194 (38.0) | 2742 (40.9) |
| $35,000–$49,999 | 90 (12.8) | 51 (11.0) | 74 (14.5) | 873 (13.0) |
| $50,000–$74,999 | 146 (20.7) | 97 (21.0) | 111 (21.8) | 1143 (17.0) |
| $75,000–$99,999 | 75 (10.6) | 48 (10.4) | 50 (9.80) | 654 (9.75) |
| $100,000 + | 118 (16.7) | 71 (15.4) | 81 (15.9) | 1294 (19.3) |
| Self-Rated Health | ||||
| Excellent/Very good | 277 (39.3) | 152 (32.9) | 195 (38.3) | 2538 (37.9) |
| Good | 238 (33.8) | 168 (36.4) | 168 (33.0) | 2379 (35.5) |
| Fair/Poor | 190 (27.0) | 142 (30.7) | 146 (28.7) | 1786 (26.6) |
| Smoking Status | ||||
| Never smoker | 373 (52.9) | 237 (51.4) | 256 (50.3) | 3365 (50.4) |
| Current smoker | 60 (8.51) | 48 (10.4) | 51 (10.0) | 969 (14.5) |
| Former smoker | 272 (38.6) | 176 (38.2) | 202 (39.7) | 2349 (35.1) |
| Alcohol Use | ||||
| Never drinker | 148 (21.2) | 96 (21.0) | 97 (19.2) | 1262 (19.1) |
| Former drinker | 169 (24.2) | 109 (23.9) | 128 (25.4) | 1599 (24.2) |
| Current drinker—infrequent/light | 266 (38.0) | 180 (39.4) | 190 (37.6) | 2508 (38.0) |
| Current drinker—moderate/heavy | 116 (16.6) | 72 (15.7) | 90 (17.8) | 1234 (18.7) |
| Physical Activity | ||||
| No activity | 278 (40.3) | 198 (44.1) | 196 (39.5) | 2458 (37.7) |
| Some activity | 158 (22.9) | 105 (23.4) | 112 (22.6) | 1594 (24.5) |
| Meets/exceeds | 254 (36.8) | 146 (32.5) | 188 (37.9) | 2464 (37.8) |
| Obesity | ||||
| BMI (≥ 30 kg/m2) | 233 (33.8) | 160 (35.1) | 167 (33.4) | 2597 (40.1) |
| Multimorbidity Count | ||||
| 2–3 | 264 (37.4) | 123 (26.6) | 140 (27.5) | 4162 (62.1) |
| 4–5 | 283 (40.1) | 200 (43.3) | 230 (45.1) | 1807 (26.9) |
| 6 + | 159 (22.5) | 139 (30.1) | 140 (27.4) | 737 (11.0) |
| Cancer Type | ||||
| Prostate | 222 (31.4) | 138 (29.9) | 161 (31.6) | |
| Breast | 337 (47.7) | 215 (46.5) | 234 (45.9) | |
| Colon/rectal | 77 (10.9) | 57 (12.3) | 55 (10.8) | |
| Lung | 37 (5.24) | 21 (4.55) | 33 (6.47) | |
| Cervical | 33 (4.67) | 31 (6.71) | 27 (5.29) | |
| Time Since Diagnosis | ||||
| Immediate (< 2 years) | 91 (12.9) | 58 (12.5) | 69 (13.5) | |
| Short-term (2–5 years) | 173 (24.5) | 96 (20.8) | 122 (23.9) | |
| Long-term (> 5 years) | 442 (62.6) | 308 (66.7) | 319 (62.6) | |
| Age at Diagnosis | ||||
| < 50 | 114 (16.1) | 86 (18.6) | 86 (16.9) | |
| 50–64 | 321 (45.5) | 185 (40.0) | 237 (46.5) | |
| 65–74 | 189 (26.8) | 136 (29.4) | 138 (27.1) | |
| 75–84 | 69 (9.77) | 43 (9.31) | 38 (7.45) | |
| 85 + | 13 (1.84) | 12 (2.60) | 11 (2.16) | |
Figure 1 demonstrates the percentage of adults with 2–3 diseases is higher for controls compared to cancer survivors. However, the percentage of adults with 4–10 diseases is higher for cancer survivors compared to controls. Table 5 demonstrates that a higher proportion of cancer survivors age 85 + had 2–3, 4–5, or 6 + diseases (10.7%, 11.8%, and 16.8%, respectively), compared to adults 85 + in the control group (5.57%, 7.56%, and 8.27%, respectively). Notably, in the age 85 + groups, there were twice as many cancer survivors with 6 + diseases (16.8%) compared to controls (8.27%).
Table 5.
Number of diseases by age groups and cancer status
| Number of Diseases n (%) | ||||
|---|---|---|---|---|
| 2–3 | 4–5 | 6 + | p value | |
| Cancer Survivors | 0.0001 | |||
| 50–64 | 117 (33.8) | 62 (20.4) | 27 (16.8) | |
| 65–74 | 117 (33.8) | 113 (37.2) | 65 (40.4) | |
| 75–84 | 75 (21.7) | 93 (30.6) | 42 (26.1) | |
| 85 + | 37 (10.7) | 36 (11.8) | 27 (16.8) | |
| Controls | < .0001 | |||
| 50–64 | 2006 (47.5) | 662 (36.5) | 239 (32.4) | |
| 65–74 | 1340 (31.8) | 651 (36.0) | 286 (38.7) | |
| 75–84 | 639 (15.1) | 361 (19.9) | 152 (20.6) | |
| 85 + | 235 (5.57) | 137 (7.56) | 61 (8.27) | |
Discussion
This study assessed multimorbidity clusters in adults 50 years of age and older with and without a history of cancer. Our study demonstrates that cancer survivors bear a greater burden of co-occurring conditions as the average multimorbidity count is higher in cancer survivors compared to controls. Multimorbidity was defined as having at least 2 diseases, including cancer, because the co-occurrence of at least 1 disease in addition to cancer can impact health outcomes [3]. However, the difference in multimorbidity counts between cancer survivors and controls is not likely explained solely by the inclusion of cancer as a disease, because the discrepancy between multimorbidity counts most frequently reported by each group differed by more than 1 disease. Specifically, controls reported multimorbidity counts of 2–3 significantly more often than cancer survivors, whereas cancer survivors more often reported multimorbidity counts of 4–5 and 6 + . Higher multimorbidity count is associated with increased care utilization and lower HRQOL [29], functional limitations and geriatric syndromes [30], and risk of care dependence [31].
The clusters identified in our study varied by cancer status. Cardiac, pulmonary, and liver clusters emerged across both cancer survivors and controls, but other clusters were observed only among survivors or among controls. The most reported multimorbidity clusters in the literature, particularly among adults 50 and older, include cardiac, musculoskeletal/arthritis, and mental health clusters, with pulmonary and gastrointestinal disorders often included in the mental health cluster [16]. Other studies identified a cardiopulmonary cluster [20, 32], or a pulmonary cluster with other conditions such as osteoporosis and depression [33]. However, findings are dependent on how multimorbidity is defined [2] and the conditions included in the analysis [34]. For example, NHIS 2018 includes arthritis, rheumatoid arthritis, gout, lupus, and fibromyalgia in its arthritis question. However, the Surveillance, Epidemiology, and End Results cancer registry and Medicare Health Outcomes Survey (SEER-MHOS) asks if the patient has ever had arthritis of the hip/knee or hand/wrist [35]. Additionally, in this study, we determined frequency in each cluster by requiring that the individual have at least 1 condition from that cluster, unlike Kenzik et al. [23], which required that people have the majority of conditions in each cluster. Restricting cluster inclusion to those with the majority of conditions in a cluster may bias toward individuals with higher multimorbidity counts and people with more severe disease in that cluster (cardiovascular, pulmonary, metabolic, etc.)—which would be more restrictive and less representative of the general population. Since few studies have analyzed multimorbidity clustering across adults with and without cancer, diseases that cluster differently across both groups may be an important area of future exploration.
Interestingly, in all matched clusters comparing cancer survivors and controls (Table 3), controls consistently feature a higher proportion of individuals in the 50–64 age group. There are several possible explanations for this overrepresentation in multimorbidity clusters at a relatively younger age. Prior literature has found that middle-aged adults, typically considered those between the ages of 40 and 65, experience a significant increase in multimorbidity with age [36] until about age 75, where the number of multimorbid conditions will plateau [37]. However, in the aforementioned systematic review, the majority of studies only included adults up to age 80 years old[37]. Furthermore, many of these studies have been conducted in non-U.S. populations, with significant differences in social risks and needs impacting their populations across the lifespan. Additionally, the lack of data collected in national surveys, such as NHIS, related to time since diagnosis, severity, or treatment of self-reported diseases makes it difficult to explain whether these differences are due to treatment or resolution of disease in older populations. Finally, recall bias may play a role in these differences as diagnoses that occurred earlier in life may be underreported and diagnoses made later in life may be overreported, especially when focused on self-report data from older adults in a cross-sectional study.
Strengths
The strengths of this study include: first, the use of NHIS data, which is drawn from the US population and includes the fee-for-service Medicare population. Second, analyses of multimorbidity clusters in older adults typically restrict samples to adults aged 65 and older. However, the majority of cancer diagnoses in our population-based sample occurred at ages 50–64, so inclusion of this age group in our analysis was imperative. Multimorbidity has a significant impact on HRQOL [38] and health care expenditures [30] in this age group. Additionally, insurance coverage is not guaranteed for adults age 50–64, which may contribute to age-related disparities in access to care. Therefore, this is a critical age group in which to focus both cancer and noncancer related preventive measures, to improve multimorbidity-related outcomes among older age groups. Third, although we were unable to include all racial/ethnic groups, the inclusion of non-Hispanic Black persons and Hispanic persons increases our understanding of differences in multimorbidity clusters, cancer diagnoses, and sociodemographic factors across race and ethnicity. Fourth, prior studies of multimorbidity clusters include cancer as a multimorbidity [22], analyze symptom clusters in relation to a specific cancer type [39], or do not include a noncancer comparison group [23]. However, significant differences between the cancer survivor and noncancer groups in our study demonstrate that cancer diagnoses are associated with higher multimorbidity overall, as well as certain multimorbidity clusters per demographic factors such as age, sex, and race/ethnicity. Finally, our analysis includes multimorbidity clusters rather than simple counts, dyads, or triads, because multimorbidity counts do not demonstrate which specific combinations of diseases are associated with health care utilization [22, 40], disability [41], or complexity [40]. Therefore, although more difficult to analyze and interpret, multimorbidity clusters can inform more focused, economical, and effective prevention strategies.
Limitations
The results of a multimorbidity cluster analysis depend on how multimorbidity is defined [2] and the conditions included in the analysis [34]. Due to the lack of consistency in definitions, data sources, and methodology, Goodman et al. [42] proposed a list of 20 diseases to include in future multimorbidity studies. However, we could not utilize this proposed list because not all diseases were assessed directly or used the same question stem in the NHIS 2018 survey. Only conditions assessed using the “Have you ever been told” question stems were included in the cluster analysis, to minimize variability in multimorbidity related to recency of diagnosis. As a result, our analysis did not include the following recommended diseases: autism, chronic kidney disease, dementia, depression, HIV/AIDS, osteoporosis, schizophrenia, or substance use disorder [42]. The exclusion of mental health conditions is a significant limitation given their prevalence in the US and the association between mental health disorders, multimorbidity, and increased incidence of common diseases [43], hospital length of stay [44], and risk of care dependence [31]. Moreover, for individual diseases, NHIS does not include questions about disease severity, management, treatment methods, age at diagnosis, or resolution. Diseases in NHIS are also self-reported, and there is evidence of misalignment between self-reported multimorbidity count and multimorbidity count from other data sources, such as insurance claims, electronic health records, and reports from providers [45, 46].
Of note, we use the term multimorbidity as defined by the presence of 2 or more simultaneous diseases, rather than chronic conditions, in an individual. We also discuss individual disease or diseases, instead of the more commonly used terms—chronic conditions or multiple chronic conditions—because our and other common data sources in the literature do not include temporality of disease diagnosis, treatment, or resolution. Therefore, the chronicity of an individual’s disease is unknown. Furthermore, although the time course for many of the commonly discussed diseases is typically over months and years rather than days or weeks, disease severity or certain treatments can produce transient changes in liver function, kidney function, and blood sugar regulation, to name a few [10, 47].
Additionally, temporal relationships between cancer diagnosis and development of other diseases were difficult to assess due to the lack of information regarding age at diagnosis, severity, and resolution of diseases. Cancer-specific temporal information—including onset of chemotherapy or radiation, severity/staging of cancer, remission, recurrence, and metastasis—is important to understanding the association between multimorbidity and cancer diagnosis [3]. Some of these variables (metastasis, current treatment, and recurrence) were included in the Cancer Supplement in previous years but were not included in the 2018 NHIS survey. Our study excluded: survivors of childhood cancer (defined as those diagnosed with cancer before age of 21); adults reporting multiple cancers; or adults diagnosed with a cancer other than those identified for this analysis. Additional studies can examine multimorbidity clusters in these groups, as different patterns may emerge.
Our study aimed to include several demographic characteristics, including the “oldest old” population and BMI. However, in NHIS, all adults over the age of 85 are coded as 85 (https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2018/samadult_layout.pdf). Therefore, age cannot be used as a continuous variable above the age of 85, which is the age group with the most rapidly increasing incidence of cancer [47]. Additionally, NHIS does not sample institutionalized individuals, such as those in nursing home or other long-term care facilities, which disproportionately impacts the “oldest old” population in the United States. Similarly, BMI was not calculated for all participants because the lowest and highest heights and weights are considered extreme categories and are not included in the data set (https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2018/samadult_layout.pdf). This limitation impacted our ability to provide insight on the oldest old (85 +) population, which is often absent in existing literature. Furthermore, it limited our ability to discuss cachectic and underweight older adults, which can often impact their health, especially among those with cancer [48].
Additional variables associated with multimorbidity clusters in adults older than 65 with and without cancer, such as HRQOL, were not included in this study. The Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaire (https://www.healthmeasures.net/explore-measurement-systems/promis), which measures HRQOL, was previously administered in the Cancer Supplement of NHIS but was not included the 2018 survey. These limitations demonstrate that existing data sources were not created for this type of complex research; thus, it may contribute to the scarcity of cluster analysis and multimorbidity research, especially at a population level.
Finally, our study aimed to understand the differences in disease clusters among adults with cancer and controls. As one of the first studies to use NHIS data for a disease cluster analysis not in relation to a primary diagnosis or within a subpopulation of the US, it was important to analyze diseases in relationship to cancer status, not geographic or demographic distribution. Therefore, the analysis was conducted using data without survey weights. Thus, our results are not generalizable, or nationally representative, and do not account for biases incurred in the sampling process, such as non-response and social desirability bias. Additionally, recall period bias may lead to underreporting of diseases diagnosed at younger ages and overreporting of diseases diagnosed at older ages, especially when focused on self-report data from older adults in a cross-sectional study. Future studies can compare self-report data to other sources of data, such as electronic medical record or claims data, to investigate the impact of recall period bias on self-report of diseases in surveys such as NHIS. However, this was out of the scope of our research.
Conclusions
Our study aimed to fill gaps in the current literature by assessing multimorbidity clusters in adults 50 years of age and above, with and without a history of cancer. We demonstrated that cancer survivors reported a higher prevalence of having 4 or more diseases compared to controls (57% and 38%, respectively). Furthermore, our analysis identified 6 clusters for cancer survivors and 4 clusters for controls. Three clusters (pulmonary, cardiac, and liver) included the same diseases for cancer survivors and controls. These findings are particularly important given that current clinical trials, guidelines, care management strategies, and health policies overwhelmingly focus on single diseases. Yet diseases not viewed as an individual’s primary disease are often undertreated [16], which can lead to worse health outcomes in individuals with multimorbidity, particularly adults older than age 65 [49] and cancer survivors with other diseases [47].
Identifying patients at risk for multimorbidity clusters may prevent the development of further conditions within a cluster, or conditions that overlap with other clusters. Early identification of these at-risk patients may reduce health care utilization [22], reduce polypharmacy and/or drug interactions [50], and improve case management strategies [48]. Furthermore, tertiary prevention of conditions within multimorbidity clusters has been shown to improve HRQOL among cancer survivors [3] and may improve coordinated care for older cancer survivors [51].
Despite the public health implications of multimorbidity, the aforementioned limitations, which are not unique to our design or data source, may explain why there are so few population-based multimorbidity cluster studies evaluating differences in cancer and noncancer groups in the US. Additional cancer and noncancer related temporal, severity, treatment, and demographic data may allow researchers to further examine the impact of multimorbidity in cancer survivors. If researchers can develop a standard list of diseases (similar to that proposed by Goodman et al. [42]), with an identical question stem format included across national surveys, we could potentially improve comparability of results from multimorbidity studies and identification of health disparities. This standard list could include not only the most prevalent diseases, but also diseases from each organ system—similar to the “Review of Systems” typically performed by physicians—to ensure inclusion of all possible factors contributing to clusters, which would be helpful given the exploratory nature of cluster analysis [18] and the variability of clusters identified based on conditions included [16]. By improving multimorbidity definitions and measurement, and engaging in additional research on multimorbidity clusters, we may have an enhanced understanding about the health status of persons aged 50 and older in the US and may inform multilevel public health action to reduce the burden of multimorbidity.
Acknowledgements
Dr. Plasencia’s role as an author of this manuscript was initially supported by her appointment to the Epidemiology Elective Program at the Centers for Disease Control and Prevention (CDC) while attending Loyola University Chicago Stritch School of Medicine.
Disclaimer
All authors have read and approved the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations
- HRQOL
Health-related quality of life
- HHS
Health & Human Services
- NHIS
National Health Interview Survey
- NCHS
National Center for Health Statistics
- COPD
Chronic obstructive pulmonary disease
- AIAN
American Indian/Alaskan Native
- BMI
Body mass index
- SEER-MHOS
Surveillance, Epidemiology, and End Results-Medicare Health Outcomes Survey
- PROMIS
Patient Reported Outcomes Measurement Information System
Authors’ contributions
GP designed the study, interpreted the results, and was a major contributor in writing the manuscript. SG designed the study, analyzed the data, prepared the tables and figures, interpreted the results, and revised the manuscript. IH designed the study, interpreted the results, and revised the manuscript. JLS designed the study, interpreted the results, revised the manuscript, and secured final approval for submission of manuscript.
Authors’ informations
Gabriela Plasencia, MD, MAS, is a family medicine physician scientist working to improve health equity among marginalized populations through research, policy, and clinical care with particular focus on systems changes. She is the current Health Equity and Primary Care Policy Fellow through Duke-Margolis Center for Health Policy, a Postdoctoral fellow with the National Clinician Scholars Program, and Clinical Associate Faculty at Duke Department of Family Medicine and Community Health. She has a Masters in Applied Science in Population Health Management and has used this training to lead community-engaged research to map systems, their assets, and evaluate the impact multi-sector coalitions have on improving community level outcomes (e.g., community capacity and coordination).
Funding
Dr. Plasencia is currently supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number TL1 TR002555.
Availability of data and materials
The data sets used and/or analyzed during the current study are publicly available at https://www.cdc.gov/nchs/nhis/nhis_2018_data_release.htm.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity. European J General Pract. 2009;2(2):65–70. doi: 10.3109/13814789609162146. [DOI] [Google Scholar]
- 2.Wang L, Palmer AJ, Cocker F, Sanderson K. Multimorbidity and health-related quality of life (HRQoL) in a nationally representative population sample: implications of count versus cluster method for defining multimorbidity on HRQoL. Health Qual Life Outcomes. 2017;15(1):7. doi: 10.1186/s12955-016-0580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith AW, Reeve BB, Bellizzi KM, Harlan LC, Klabunde CN, Amsellem M, Bierman AS, Hays RD. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29(4):41–56. [PMC free article] [PubMed] [Google Scholar]
- 4.Lehnert T, Heider D, Leicht H, Heinrich S, Corrieri S, Luppa M, Riedel-Heller S, Konig HH. Review: health care utilization and costs of elderly persons with multiple chronic conditions. Med Care Res Rev. 2011;68(4):387–420. doi: 10.1177/1077558711399580. [DOI] [PubMed] [Google Scholar]
- 5.Nunes BP, Flores TR, Mielke GI, Thume E, Facchini LA. Multimorbidity and mortality in older adults: A systematic review and meta-analysis. Arch Gerontol Geriatr. 2016;67:130–138. doi: 10.1016/j.archger.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Boersma P, Black LI, Ward BW. Prevalence of multiple chronic conditions among US adults, 2018. Prev Chronic Dis. 2020;17:E106. doi: 10.5888/pcd17.200130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson G: Chronic Care: Making the Case for Ongoing Care. In.: Robert Wood Johnson Foundation; 2010.
- 8.Vespa J, Armstrong DM, Medina L: Demographic Turning Points for the United States: Population Projections for to 2060. Current Population Reports. 2020;2018(March):P25–1144. [Google Scholar]
- 9.Weir HK, Stewart SL, Allemani C, White MC, Thomas CC, White A, Coleman MP. Group CW: Population-based cancer survival (2001 to 2009) in the United States: Findings from the CONCORD-2 study. Cancer. 2017;123(24):4963–4968. doi: 10.1002/cncr.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, Forsythe L, Scoppa S, Hachey M, Rowland JH. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol, Biomarker Prevent: A Publicat American Assoc Cancer Re, Cospons American Soc Prevent Oncol. 2013;22(4):561–570. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services: Multiple chronic conditions—a strategic framework: optimum health and quality of life for individuals with multiple chronic conditions. In: Washington, DC. 2010.
- 12.Parekh AK, Goodman RA. The HHS Strategic Framework on multiple chronic conditions: genesis and focus on research. J comorbidity. 2013;3:22–29. doi: 10.15256/joc.2013.3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases–a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66(3):301–311. doi: 10.1093/gerona/glq208. [DOI] [PubMed] [Google Scholar]
- 14.Ng SK, Tawiah R, Sawyer M, Scuffham P. Patterns of multimorbid health conditions: a systematic review of analytical methods and comparison analysis. Int J Epidemiol. 2018;47(5):1687–1704. doi: 10.1093/ije/dyy134. [DOI] [PubMed] [Google Scholar]
- 15.Verbrugge LM, Lepkowski JM, Imanaka Y. Comorbidity and its impact on disability. Milbank Q. 1989;67(3–4):450–484. doi: 10.2307/3350223. [DOI] [PubMed] [Google Scholar]
- 16.Prados-Torres A, Calderon-Larranaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67(3):254–266. doi: 10.1016/j.jclinepi.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Marengoni A, Fratiglioni L. Disease clusters in older adults: rationale and need for investigation. J Am Geriatr Soc. 2011;59(12):2395–2396. doi: 10.1111/j.1532-5415.2011.03687.x. [DOI] [PubMed] [Google Scholar]
- 18.Whitty CJM, Watt FM. Map clusters of diseases to tackle multimorbidity. Nature. 2020;579(7800):494–496. doi: 10.1038/d41586-020-00837-4. [DOI] [PubMed] [Google Scholar]
- 19.John R, Kerby DS, Hennessy CH. Patterns and impact of comorbidity and multimorbidity among community-resident American Indian elders. Gerontologist. 2003;43(5):649–660. doi: 10.1093/geront/43.5.649. [DOI] [PubMed] [Google Scholar]
- 20.Clay OJ, Perkins M, Wallace G, Crowe M, Sawyer P, Brown CJ. Associations of multimorbid medical conditions and health-related quality of life among older african American Men. J Gerontol B Psychol Sci Soc Sci. 2018;73(2):258–266. doi: 10.1093/geronb/gbx090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng DD, Loewenstein DA, Christ SL, Feaster DJ, Lam BL, McCollister KE, Curiel-Cid RE, Lee DJ. Multimorbidity patterns and their relationship to mortality in the US older adult population. PLoS ONE. 2021;16(1):e0245053. doi: 10.1371/journal.pone.0245053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajat C, Siegal Y, Adler-Waxman A. Clustering and healthcare costs with multiple chronic conditions in a US study. Front Public Health. 2020;8:607528. doi: 10.3389/fpubh.2020.607528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenzik KM, Kent EE, Martin MY, Bhatia S, Pisu M. Chronic condition clusters and functional impairment in older cancer survivors: a population-based study. J Cancer Surviv: Res Pract. 2016;10(6):1096–1103. doi: 10.1007/s11764-016-0553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics: Survey Description. In: National Health Interview Survey, 2018. Hyattsville, Maryland; 2019.
- 25.Holmes HM, Nguyen HT, Nayak P, Oh JH, Escalante CP, Elting LS. Chronic conditions and health status in older cancer survivors. Eur J Intern Med. 2014;25(4):374–378. doi: 10.1016/j.ejim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Kopp LM, Gupta P, Pelayo-Katsanis L, Wittman B, Katsanis E. Late effects in adult survivors of pediatric cancer: a guide for the primary care physician. Am J Med. 2012;125(7):636–641. doi: 10.1016/j.amjmed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Atere-Roberts J, Gray SC, Hall IJ, Smith JL. Racial and ethnic disparities in health status, chronic conditions, and behavioral risk factors among prostate cancer survivors, United States, 2015. Prev Chronic Dis. 2021;18:E39. doi: 10.5888/pcd18.200523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello AB, Osborne J. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10(1):7. [Google Scholar]
- 29.Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10(2):134–141. doi: 10.1370/afm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koroukian SM, Schiltz NK, Warner DF, Sun J, Stange KC, Given CW, Dor A. Multimorbidity: constellations of conditions across subgroups of midlife and older individuals, and related Medicare expenditures. J comorbidity. 2017;7(1):33–43. doi: 10.15256/joc.2017.7.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao J, Chua KC, Prina M, Prince M. Multimorbidity and care dependence in older adults: a longitudinal analysis of findings from the 10/66 study. BMC Public Health. 2019;19(1):585. doi: 10.1186/s12889-019-6961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57(2):225–230. doi: 10.1111/j.1532-5415.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- 33.Islam MM, Valderas JM, Yen L, Dawda P, Jowsey T, McRae IS. Multimorbidity and comorbidity of chronic diseases among the senior Australians: prevalence and patterns. PLoS ONE. 2014;9(1):e83783. doi: 10.1371/journal.pone.0083783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roso-Llorach A, Violan C, Foguet-Boreu Q, Rodriguez-Blanco T, Pons-Vigues M, Pujol-Ribera E, Valderas JM. Comparative analysis of methods for identifying multimorbidity patterns: a study of ‘real-world’ data. BMJ Open. 2018;8(3):e018986. doi: 10.1136/bmjopen-2017-018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.SEER-MHOS Data Dictionary [https://healthcaredelivery.cancer.gov/seer-mhos/aboutdata/dictionary.html]
- 36.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43.10.1016/S0140-6736(12)60240-2. [DOI] [PubMed]
- 37.Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10(2):142–151. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanesarajah J, Waller M, Whitty JA, Mishra GD. Multimorbidity and quality of life at mid-life: a systematic review of general population studies. Maturitas. 2018;109:53–62. doi: 10.1016/j.maturitas.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Hershey DS, Pierce SJ. Examining patterns of multivariate, longitudinal symptom experiences among older adults with type 2 diabetes and cancer via cluster analysis. Eur J Oncol Nurs. 2015;19(6):716–723. doi: 10.1016/j.ejon.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Juul-Larsen HG, Christensen LD, Bandholm T, Andersen O, Kallemose T, Jorgensen LM, Petersen J. Patterns of multimorbidity and differences in healthcare utilization and complexity among acutely hospitalized medical patients (>/=65 Years) - a latent class approach. Clin Epidemiol. 2020;12:245–259. doi: 10.2147/CLEP.S226586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacob ME, Ni P, Driver J, Leritz E, Leveille SG, Jette AM, Bean JF. Burden and patterns of multimorbidity: impact on disablement in older adults. Am J Phys Med Rehabil. 2020;99(5):359–365. doi: 10.1097/PHM.0000000000001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birk JL, Kronish IM, Moise N, Falzon L, Yoon S, Davidson KW. Depression and multimorbidity: Considering temporal characteristics of the associations between depression and multiple chronic diseases. Health Psych: Official J Divis Health Psychol, American Psycholog Assoc. 2019;38(9):802–811. doi: 10.1037/hea0000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beeler PE, Cheetham M, Held U, Battegay E. Depression is independently associated with increased length of stay and readmissions in multimorbid inpatients. Eur J Intern Med. 2020;73:59–66. doi: 10.1016/j.ejim.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Gruneir A, Griffith LE, Fisher K, Perez R, Favotto L, Patterson C, Markle-Reid M, Ploeg J, Upshur R. Measuring multimorbidity series. An overlooked complexity - Comparison of self-report vs administrative data in community-living adults: Paper 3. Agreement across data sources and implications for estimating associations with health service use. J Clinic Epidemiol. 2020;124:173–182. doi: 10.1016/j.jclinepi.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Hansen H, Schafer I, Schon G, Riedel-Heller S, Gensichen J, Weyerer S, Petersen JJ, Konig HH, Bickel H, Fuchs A, et al. Agreement between self-reported and general practitioner-reported chronic conditions among multimorbid patients in primary care - results of the MultiCare Cohort Study. BMC Fam Pract. 2014;15:39. doi: 10.1186/1471-2296-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer epidemiol, Biomark Prevent: A Public American Assoc Cancer Res, Cospons American Soc Prevent Oncol. 2016;25(7):1029–36. [DOI] [PMC free article] [PubMed]
- 48.Bretos-Azcona PE, Sanchez-Iriso E, Cabases Hita JM. Tailoring integrated care services for high-risk patients with multiple chronic conditions: a risk stratification approach using cluster analysis. BMC Health Serv Res. 2020;20(1):806. doi: 10.1186/s12913-020-05668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guthrie B, Payne K, Alderson P, McMurdo ME, Mercer SW. Adapting clinical guidelines to take account of multimorbidity. BMJ (Clinical research ed) 2012;345:e6341. doi: 10.1136/bmj.e6341. [DOI] [PubMed] [Google Scholar]
- 50.Guisado-Clavero M, Violan C, Lopez-Jimenez T, Roso-Llorach A, Pons-Vigues M, Munoz MA, Foguet-Boreu Q. Medication patterns in older adults with multimorbidity: a cluster analysis of primary care patients. BMC Fam Pract. 2019;20(1):82. doi: 10.1186/s12875-019-0969-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee Smith J, Hall IJ. Advancing Health Equity in Cancer Survivorship: Opportunities for Public Health. Am J Prev Med. 2015;49(6 5):S477–482. doi: 10.1016/j.amepre.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are publicly available at https://www.cdc.gov/nchs/nhis/nhis_2018_data_release.htm.