Abstract
Diverse gram-negative bacterial cells communicate with each other by using diffusible N-acyl homoserine lactone (AHL) signal molecules to coordinate gene expression with cell population density. Accumulation of AHLs above a threshold concentration renders the population “quorate,” and the appropriate target gene is activated. In pathogenic bacteria, such as Pseudomonas aeruginosa, AHL-mediated quorum sensing is involved in the regulation of multiple virulence determinants. We therefore sought to determine whether the immune system is capable of responding to these bacterial signal molecules. Consequently the immunomodulatory properties of the AHLs N-(3-oxododecanoyl)-l-homoserine lactone (OdDHL) and N-(3-oxohexanoyl)-l-homoserine lactone (OHHL) were evaluated in murine and human leukocyte immunoassays in vitro. OdDHL, but not OHHL, inhibited lymphocyte proliferation and tumor necrosis factor alpha production by lipopolysaccharide-stimulated macrophages. Furthermore, OdDHL simultaneously and potently down-regulated the production of IL-12, a Th-1-supportive cytokine. At high concentrations (>7 × 10−5 M) OdDHL inhibited antibody production by keyhole limpet hemocyanin-stimulated spleen cells, but at lower concentrations (<7 × 10−5 M), antibody production was stimulated, apparently by increasing the proportion of the immunoglobulin G1 (IgG1) isotype. OdDHL also promoted IgE production by interleukin-4-stimulated human peripheral blood mononuclear cells. These data indicate that OdDHL may influence the Th-1–Th-2 balance in the infected host and suggest that, in addition to regulating the expression of virulence determinants, OdDHL may contribute to the pathogenesis of P. aeruginosa infections by functioning as a virulence determinant per se.
Intracellular signalling mechanisms are employed by microorganisms to sense, integrate, and process information from their surroundings. However, the ability of bacterial cells to communicate intercellularly has only been recognized relatively recently. Quorum sensing is a bacterial intercellular communication device for controlling gene expression in response to population density (10, 26, 29). Quorum-sensing systems generally consist of two components, a small diffusible signal molecule and a transcriptional activator protein. In gram-negative bacteria, the quorum-sensing signal molecule is usually an N-acyl homoserine lactone (AHL), which directly binds to and activates a transcriptional regulator protein (a member of the LuxR family) such that the AHL-regulator complex stimulates expression of the target gene(s) (10, 26, 29).
AHLs were originally identified in marine bacteria, where they were discovered to play a pivotal role in the regulation of bioluminescence in Photobacterium (Vibrio) fischeri (9). In P. fischeri N-(3-oxohexanoyl)-l-homoserine lactone (OHHL), synthesized by the luxI gene product, activates the P. fischeri lux operon via the AHL-responsive transcriptional activator protein, LuxR (17). Analogous regulatory systems have now been described in various gram-negative bacteria, including a number of animal and plant pathogens (2, 14, 20, 28, 30). In addition, a family of AHLs, which differ primarily in either the presence or absence of an acyl chain C-3 substituent (oxo or hydroxy) or the length of the N-acyl side chain, has been chemically characterized (2, 22, 23, 30, 35). For example, in Pseudomonas aeruginosa, an opportunistic pathogen of immunocompromised individuals, two major [N-(3-oxododecanoyl)-l-homoserine lactone (OdDHL) and N-butanoyl-l-homoserine lactone (BHL)] and two minor [N-(3-oxohexanoyl)-l-homoserine lactone (OHHL) and N-hexanoyl-l-homoserine lactone (HHL)] AHLs have been identified (22, 23, 35) and shown to be involved in regulating the expression of multiple exogenous virulence determinants (14, 15, 20, 22, 23, 35). OHHL is also produced by other gram-negative opportunistic pathogens belonging to the genera Serratia, Enterobacter, and Yersinia (2, 28, 30), although the contribution of quorum sensing to the pathogenicity of these microorganisms has yet to be established.
Since diverse gram-negative bacteria communicate by using AHL signal molecules and since AHLs appear to readily diffuse across cell membranes (10), it is conceivable that these bacterial signal molecules per se may influence the outcome of an infection by modulating the host immune response. Such a finding would implicate AHLs as virulence determinants in their own right and perhaps provide an explanation for the difficulties encountered in identifying quorum-sensing-dependent phenotypes in bacteria such as Yersinia (30). In P. aeruginosa, the signal molecule OdDHL has been reported by some (8) but not others (19) to stimulate dose-dependent interleukin-8 (IL-8) production by respiratory epithelial cells, albeit at higher levels (30 to 100 μM) than are normally encountered in laboratory media (approximately 5 μM [23]). These data provide preliminary evidence for a direct contribution of AHLs to the infection process.
In the present study we sought to determine whether a short-chain C-6 AHL, OHHL, and a C-12, long-chain AHL, OdDHL, were capable of modulating immune responses. A number of assays were chosen, on the basis that they represented a range of in vitro immunological phenomena likely to be encountered by the pathogen in vivo. The data generated with these assays demonstrate that OdDHL, depending on the concentration, either stimulates or reduces antibody production by antigen-stimulated keyhole limpet hemocyanin (KLH)-primed spleen cells, stimulates immunoglobulin E (IgE) secretion by human B cells, and inhibits IL-12 and tumor necrosis factor alpha (TNF-α) production by adherent peritoneal macrophages. Antimitogenic effects were also observed. OHHL was not significantly active in any of the in vitro systems used, apart from its effects against protein tyrosine kinase (PTK) activity at high doses.
MATERIALS AND METHODS
Cell lines.
The human Jurkat E6.1 T-cell leukemic cell line was obtained from the European Collection of Animal Cell Cultures (Department of Animal Resources, Centre for Applied Microbiology, Porton Down, United Kingdom). Culture of human peripheral blood mononuclear cells (PBMC.) was carried out with Iscove’s modified Dulbecco’s medium (IMDM) (JRH Biosciences, Lenexa, Kans.) supplemented with 10% (vol/vol) fetal calf serum (FCS), 2 mM glutamine, 500 U of penicillin/ml, and 50 μg of streptomycin (Seralab, Crawley Down, West Sussex, United Kingdom)/ml as described by Wheeler et al. (33).
Synthesis of AHLs.
The general method described by Chhabra et al. (5) for the synthesis of a series of AHLs was used to produce OdDHL and OHHL. Each compound was purified to homogeneity by preparative high-performance liquid chromatography, and its structure was confirmed by mass spectrometry and proton nuclear magnetic resonance spectroscopy as described before (5, 35).
Cytotoxicity.
The cytotoxicity of OdDHL and OHHL was assessed by determining lactate dehydrogenase (LDH) release (7) from 105 human Jurkat E6.1 leukemic T cells. OdDHL and OHHL at concentrations of 0.1 M were dissolved in dimethyl sulfoxide and diluted in complete cell culture medium (CTCM) consisting of RPMI 1640 medium with 5% (vol/vol) FCS, 2 mM l-glutamine, and 5 × 10−5 M 2-mercaptoethanol. For the cytotoxicity assay, the two AHLs were evaluated at final concentrations of 10−4, 10−5, 10−6, and 10−7 M in a final volume of 250 μl of CTCM. After incubation for 24 h at 37°C in 5% CO2-air, LDH levels in the supernatants were measured biochemically by an in vitro toxicity assay kit (Tox-7; Sigma). Results were expressed as changes in optical density (OD) compared to a 100% lysis control.
ConA mitogen-stimulated proliferation of murine spleen cells.
The concanavalin A (ConA) assay was used to assess the effect of AHLs on T-cell activation and proliferation. Proliferation was assessed by the incorporation of [3H]thymidine into DNA. Eight-week-old female BALB/c mice were obtained from Harlan (Bicester, Oxon, United Kingdom) and given food and water ad libitum. Spleen cell suspensions were prepared by removing the spleens and placing them into Hanks’ balanced salt solution (HBSS). The spleens were chopped with scissors and forced through a Falcon 70-μm-pore-size nylon cell strainer (2350; Becton Dickinson, Franklin Lakes, N.J.) with the plunger from a 5-ml syringe to produce a single cell suspension. The cells were pelleted by centrifugation, and erythrocytes were lysed with 0.017 M Tris–0.144 M ammonium chloride, pH 7.2. Leukocytes were washed twice with HBSS and resuspended in CTCM. OdDHL and OHHL were tested at concentrations of 10−4, 10−5, 10−6, and 10−7 M in a final volume of 250 μl of CTCM, containing ConA at 3 μg/ml and 5 × 105 spleen cells, by the method of Tomkins et al. (31). Following incubation for 48 h at 37°C in 5% CO2-air, 20 μl of [3H]thymidine in CTCM (1 μCi/well, 5 Ci/mmol) was added and the mixture was incubated for a further 24 h. Cells were harvested onto fiberglass filters with a Packard filtermate 196 harvester. After the addition of 20 μl of Microscint-O to each well the filters were counted with the Packard Topcount scintillation counter.
Tyrosine kinase activity of the T-lymphocyte activation PTKs p59fyn and p56lck.
To assess their effects on the activity of these key enzymes, p59fyn and p56lck, OdDHL and OHHL were examined at final concentrations of 10−4, 10−5, 10−6, and 10−7 M with the Boehringer Mannheim tyrosine kinase assay kit and purified bovine Lck and Fyn kinases from Upstate Biotechnology, Lake Placid, N.Y., at 500 U/ml. The substrate used in each assay was biotin-KVEKIGEGTYGVVYK-amide (PKS-1), which corresponds to amino acids 6 to 20 of the cell division kinase p34cdc2 (4). Briefly, enzyme was mixed with PKS-1 substrate with or without AHL, and after a short incubation the substrate was removed by incubating the mixture in 96-well plates coated with streptavidin. Phosphorylated substrate was detected by enzyme-linked immunosorbent assay (ELISA) with a monoclonal antibody specific to phosphotyrosine.
TNF-α and IL-12 production by LPS-stimulated adherent murine peritoneal macrophages.
Peritoneal exudate cells (PECs) were prepared by injecting 8-week-old female BALB/c mice intraperitoneally with 0.5 ml of 2.9% thioglycollate in water (31). Three days later, the animals were killed and the PECs were removed by peritoneal lavage with 5 ml of RPMI 1640 medium. Cells were washed twice and resuspended in CTCM (equivalent to 5 ml of CTCM per mouse) before being placed into 5-cm-diameter petri dishes (5 ml per dish). After 1 h at 37°C, the medium and the loose cells were aspirated and discarded and adherent cells were detached by incubation in a 5 mM solution of glucose in phosphate-buffered saline (PBS) (5 ml) for 30 min at 37°C. Detached macrophages were washed twice with CTCM. OdDHL and OHHL were evaluated in this assay at concentrations of 10−4, 10−5, 10−6, and 10−7 M in a final volume of 200 μl of CTCM containing 5 × 105 PECs. After 24 h of incubation at 37°C in 5% CO2-air, 50 μl of lipopolysaccharide (LPS) (Escherichia coli 0127:B8) was added to a final concentration of 1 μg/ml and the mixture was incubated for a further 6 h. TNF-α levels of the cell culture supernatants were determined by ELISA. Briefly, ELISA plates (Becton Dickinson) were coated with 50 μl of a 1-μg/ml solution of monoclonal hamster anti-murine TNF-α (Genzyme, West Malling, United Kingdom) in 0.1 M carbonate (pH 8.2) overnight at 4°C. After being washed three times with PBS containing 0.5% (vol/vol) Tween 20 (PBS-Tween), the plates were blocked with 10% (vol/vol) sheep serum in PBS-Tween at 37°C for 30 min. After the blocking solution was aspirated, 100-μl samples were added, incubated at 37°C for 1 h, and washed and 100 μl of a second antibody, polyclonal rabbit anti-murine TNF-α (Genzyme) was added at a dilution of 1:1,000 in 100 μl of PBS-Tween. After 2 h of incubation at 37°C, bound polyclonal antibody was detected with 100 μl of a 1:500 dilution of sheep anti-rabbit IgG peroxidase-conjugated antibody (Binding Site, Birmingham, United Kingdom) and visualized by addition of 0.2 mg of tetramethyl benzidine substrate (Sigma)/ml in 0.1 M citrate-phosphate buffer (pH5.0). Total IL-12 levels were similarly measured by ELISA with the Genzyme Intertest ELISA kit (80-4223-01).
KLH antibody production (KAP) model of a secondary antibody response.
OdDHL and OHHL were tested at final concentrations of 10−4 to 10−7 M in a final volume of 250 μl of CTCM in the assay described by Pritchard et al. (24). Specific anti-KLH antibodies in cell culture supernatants were assayed by ELISA. Immunoglobulin isotype levels were measured by ELISA by the same technique but with isotype-specific peroxidase-conjugated antibodies for murine IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3 (Binding Site) diluted 1:500 in PBS-Tween.
IgE production by IL-4-stimulated human PBMCs.
The human peripheral blood system for IgE synthesis was established as described by Wheeler et al. (33). Briefly, PBMCs (3 × 105/well; 20 μl/well) were cultured in IMDM–10% FCS containing IL-4 (200 U/ml) and transferrin (50 μg/ml). The cells were cultured in U-shaped microtiter wells in a 5% CO2 incubator at 37°C for 17 days. After 17 days of culture, supernatants were harvested and tested for IgE production by ELISA with a Milenia IgE kinetic enzyme immunoassay kit (Diagnostic Products Corp., Caernarfon, United Kingdom).
Statistical analysis of the data.
One-way analysis of variance followed by Dunnett’s test was used for all analyses except the IgE and KAP isotyping, which used Student’s t test.
RESULTS
Cytotoxicity.
It was necessary to assess the cytotoxic effects of the AHLs before further in vitro tests were carried out. LDH release by human Jurkat E6.1 cells is a good model for this effect, as the cells are constitutively activated human T cells and, unlike quiescent T cells, are susceptible to inhibitors of activation. Table 1 shows that neither OdDHL nor OHHL were toxic in this assay.
TABLE 1.
Results of in vitro testing by LDH release from Jurkat E6.1 cellsa
| Compound | Concn (mM) | No. of replicates | OD
|
||
|---|---|---|---|---|---|
| Mean | SEM | % Change | |||
| OHHL | 0.1 | 4 | 0.08 | 0.001 | 0.4 |
| 0.01 | 4 | 0.08 | 0.003 | 0.3 | |
| 0.001 | 4 | 0.08 | 0.001 | −1.0 | |
| 0.0001 | 4 | 0.08 | 0.002 | −0.1 | |
| OdDHL | 0.1 | 4 | 0.12 | 0.004 | −7.8 |
| 0.01 | 4 | 0.08 | 0.003 | −0.4 | |
| 0.001 | 4 | 0.08 | 0.001 | 0 | |
| 0.0001 | 4 | 0.08 | 0.001 | 0.4 | |
| DMSO | 0.1b | 4 | 0.08 | 0.001 | −0.8 |
| 0.01b | 4 | 0.08 | 0.001 | 0 | |
| 0.001b | 4 | 0.08 | 0.001 | −0.5 | |
| 0.0001b | 4 | 0.08 | 0.001 | 0.1 | |
| Cells alone | 8 | 0.08 | 0.001 | 0 | |
| Lysis control | 8 | 0.58 | 0.019 | −100** | |
Statistical analysis by one-way analysis of variance of OD data, followed by Dunnett’s test. **, P < 0.01.
Dimethyl sulfoxide (DMSO) control diluted the same as equivalent compound concentrations. No significance was noted with the small changes in calculated viability.
ConA mitogen-stimulated proliferation of murine spleen cells.
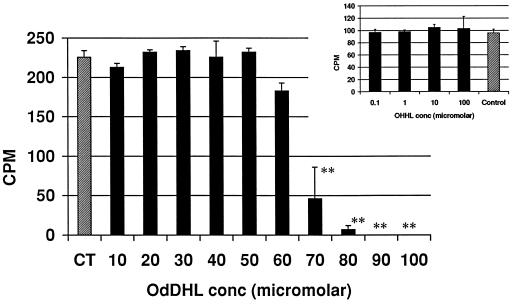
The ConA assay was used to assess the effect of OdDHL on T-cell activation and proliferation. Proliferation was assessed by the incorporation of [3H]thymidine into DNA. Figure 1 shows the influence of OdDHL on [3H]thymidine uptake at concentrations from 1 × 10−5 to 10 × 10−5 M in both assays, with a 50% inhibitory concentration (IC50; i.e., the concentration of OdDHL causing a 50% reduction in [3H]thymidine uptake) of 6.5 × 10−5 M. OHHL did not inhibit the uptake of [3H]thymidine in this assay (Fig. 1).
FIG. 1.
Influence of OHHL and OdDHL on ConA mitogen-driven proliferation of murine spleen cells (+1 standard deviation). OdDHL inhibited [3H]thymidine uptake at concentrations over 5 × 10−5 M in two assays. OHHL (Inset) had no effect on the uptake of [3H]thymidine. DMSO at concentrations equivalent to those in AHL-containing samples had no effect on the assay (data not shown). Counts per minute (CPM) are in thousands. ∗∗, P < 0.01.
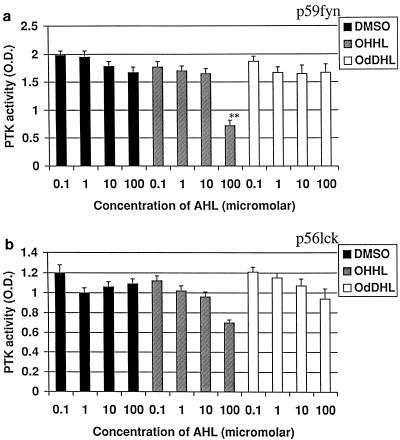
Tyrosine kinase activity of the T-lymphocyte activation PTKs p59fyn and p56lck.
p59fyn and p56lck are key enzymes in the initial activation of T lymphocytes because they are coupled to the T-cell receptor complex and as such are essential in linking the coupling of the T-cell receptor following the engagement of antigen with intracellular signalling. The effects of OdDHL and OHHL on the activities of these key enzymes are shown in Fig. 2. OHHL inhibited phosphorylation of PKS-1 substrate by p59fyn at 10−4 M and weakly inhibited the activity of p56lck. OdDHL had no effect on the activity of either enzyme.
FIG. 2.
Influence of OHHL and OdDHL on PTK activity. OHHL at 10−4 M inhibited phosphorylation of PKS-1 substrate by both p59fyn (a) and p56lck (b). OdDHL had no effect. ∗∗, P < 0.01. Error bars indicate standard deviations.
TNF-α and IL-12 production by LPS-stimulated adherent murine peritoneal macrophages.
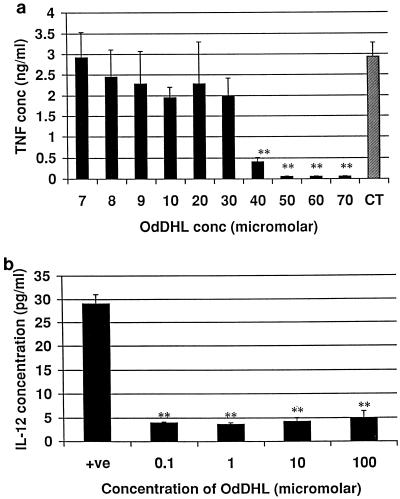
Macrophages produce cytokines which are capable of influencing T cells, e.g., IL-12 and TNF-α. By stimulating macrophages with LPS to produce IL-12 and TNF-α, we determined the ability of OdDHL and OHHL to create an environment which would influence T-cell development. The influence of OdDHL on TNF-α production is shown in Fig. 3a. OdDHL inhibited TNF-α production at concentrations greater than 3 × 10−5 M. This inhibition is statistically significant (P < 0.01). An IC50 of 3.35 × 10−5 M was calculated. In contrast, OHHL did not inhibit the release of TNF-α from murine macrophages (data not shown). OdDHL also significantly (P < 0.01) reduced total IL-12 production by LPS-stimulated macrophages compared to that by positive control cells (Fig. 3b).
FIG. 3.
Influence of OHHL and OdDHL on TNF-α and IL-12 production by LPS-stimulated adherent murine peritoneal macrophages. (a) OdDHL inhibited TNF-α release at 3.35 × 10−5 M. OHHL did not inhibit the production of TNF-α. CT, control level of TNF-α release. (b) OdDHL also inhibited IL-12 production by LPS-stimulated macrophages at all concentrations tested. DMSO at equivalent concentrations to those in AHL-containing samples had no effect on the assay (data not shown). Error bars indicate standard deviations. +ve, positive control (no OdDHL added). ∗∗, P < 0.01.
KAP model of a secondary antibody response.
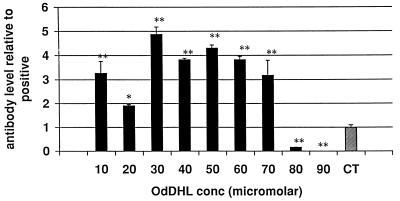
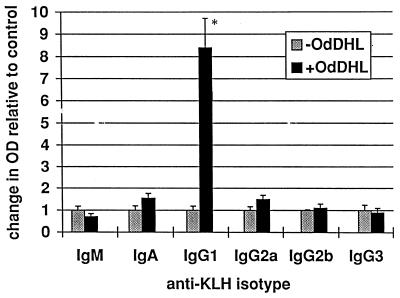
The KAP model of a secondary antibody response in vitro was used to assess the effects of AHLs on a more complete and functionally relevant immune response. Measurement of the relative proportions of the isotypes of the antibodies produced can also give an indication of the way in which the response is being affected. OdDHL inhibited the production of total specific antibody by antigen-stimulated KLH-primed murine spleen cells at a concentration of 7 × 10−5 M in one assay (P < 0.01) and 8 × 10−5 M in a second assay (P < 0.01) (Fig. 4). When individual isotypes were analyzed, OdDHL, at 5 × 10−5 M, caused a significant increase in IgG1 levels (Fig. 5) whereas IgA, IgM, IgG2a, IgG2b, and IgG3 levels were not significantly affected. OHHL exerted no effect on antibody levels in the KAP assay (data not shown).
FIG. 4.
Influence of OHHL and OdDHL on specific antibody production in the KAP model of a secondary antibody response. OdDHL inhibited the production of specific antibody by antigen-stimulated KLH-primed murine spleen cells at a concentration of 8 × 10−5 M (P < 0.01). Antibody production was significantly enhanced at lower concentrations of the compound. ∗, P < 0.05; ∗∗, P < 0.01. Error bars indicate standard deviations. The value of 1 represents the positive control level (CT). DMSO at equivalent concentrations to those in AHL-containing samples had no effect on the assay (data not shown).
FIG. 5.
Influence of OdDHL on IgG1 production in the KAP model of a secondary antibody response. OdDHL, at 5 × 10−5 M, caused a significant increase in IgG1. ∗, P < 0.05. DMSO at equivalent concentrations to those in AHL-containing samples had no effect on the assay (data not shown). Error bars indicate standard deviations.
IgE production by IL-4-stimulated human PBMCs.
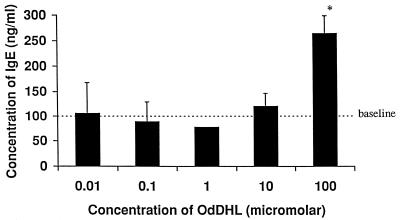
The IgE synthesis assay was used to assess activity in a human system and to further elucidate a possible mode of action. Although the system is IL-4 driven, costimulation by T cells is also required. Figure 6 shows that OdDHL at 10−4 M caused a significant increase in the level of IgE production, mirroring the effects seen in the KAP assay. This provides further evidence that OdDHL has the ability to support a Th-2 response and, in addition, shows that the compound is active against human lymphocyte populations.
FIG. 6.
Influence of OdDHL on IgE production by IL-4-stimulated human PBMCs. OdDHL caused an increase in IgE levels at 10−4 M. ∗, P < 0.05. DMSO at equivalent concentrations to those in AHL-containing samples had no effect on the assay (data not shown). Error bars indicate standard deviations.
DISCUSSION
P. aeruginosa is an opportunistic pathogen of immunocompromised individuals, an observation which implies that it is generally susceptible to immune attack. In experimental infection models employing inbred mouse strains, the host response to P. aeruginosa infection is highly variable (27). Some strains (e.g., BALB/c) are resistant while others (e.g., C57/B16) are highly susceptible. Comparative studies of these mouse strains have indicated that T-cell responses constitute an important component of the host immune defense against P. aeruginosa (27). For example, BALB/c mice are resistant to the establishment of chronic pulmonary P. aeruginosa infection and mount significantly enhanced T-cell responses to the mitogen ConA compared with that of the susceptible mouse strain C57/B16 (27). Suppression of T-cell activity is therefore likely to be advantageous to P. aeruginosa.
The data presented in this paper indicate that the quorum-sensing signal molecule OdDHL, which regulates a number of essential P. aeruginosa virulence determinants (20, 22), can itself exert a significant immunomodulatory effect on mammalian immune responses in vitro. OdDHL, but not the related AHL, OHHL, significantly reduced the ability of lymphocytes to respond to ConA (IC50, 6.5 × 10−5 M). Although assays involving mitogens do not directly correlate with physiological activities of cells, they do provide a first indication of the pharmacological activity of test compounds. ConA stimulates T cells independently of accessory cells and activates via the T-cell receptor and other cell surface receptors. This activation would certainly involve, for example, PTKs, phospholipase C, and phosphatidylinositol bisphosphate hydrolysis (31). Given that ConA signals via PTK, we investigated the effects of AHLs on the in vitro enzymatic activities of p56lck and p59fyn. Since OdDHL did not inhibit PTK activity, it presumably acts at a downstream level, although inhibition of interaction with the T-cell receptor or of autophosphorylation, cannot be ruled out. In this context it will also be important to evaluate the interaction of OdDHL with other important PTKs, such as ZAP-70, p120fak, and PI3 kinase. It is equally conceivable that OdDHL acts as, or activates, a transcription factor in T cells. Clearly, further work is required to elucidate the mechanism of action of OdDHL in inhibiting ConA-driven proliferation of murine spleen cells. Although AHLs have to penetrate both inner and outer membranes to reach their transcriptional activator target (LuxR homolog) in bacteria, it is not known whether OdDHL, for example, is capable of penetrating the eukaryotic cell membrane. Determining the permeability of leukocytes to AHLs will be essential for determining their mode of action. The fact that OHHL inhibited the tyrosine kinase p59fyn without affecting the immunological assay described further supports our belief that OdDHL is not acting via the inhibition of tyrosine kinase activity.
Inbred mice which are refractory to P. aeruginosa lung infection mount an earlier and more severe local inflammatory response and exhibit a more efficient clearance of bacteria than their susceptible counterparts (18). Susceptible mice have a defect in TNF-α production, implying an important role for TNF-α in protecting against pseudomonas infection. In fact, the introduction of recombinant TNF-α into the airways significantly increased bacterial clearance by enhancing neutrophil phagocytosis (3). Several P. aeruginosa surface polymers, including LPS, are potent inducers of TNF-α (18), which exhibits a number of pleiotropic effects on the immune response, including T-cell and phagocyte activation and acute phase protein synthesis (32). It would therefore be advantageous for P. aeruginosa to be able to interfere with LPS-induced TNF-α production. Our in vitro data suggest that OdDHL could conceivably function in this manner, since OdDHL suppressed TNF-α production by murine peritoneal macrophages, with an IC50 of around 34 μM. Stationary-phase in vitro cultures of P. aeruginosa have been reported to contain around 5 μM OdDHL (23). Whether such concentrations of OdDHL are achieved locally in host tissues during infection has yet to be established. Also the mechanism of inhibition by OdDHL of TNF-α production is unlikely to be similar to that recorded for bactericidal/permeability-increasing factor, which apparently interferes with the binding of TNF-α-inducing bacterial products (13). OdDHL was also shown to reduce the production of IL-12, a cytokine which directs the generation of a Th-1 response via the induction of gamma interferon secretion, by LPS-stimulated macrophages. This effect would further support the generation of a Th-2-like, probacterial environment and further reduce TNF-α production.
P. aeruginosa AHLs regulate a diverse range of exoproducts, many of which contribute to the virulence of this opportunistic pathogen (21, 34). These include elastase, alkaline protease, exotoxin A, lipase, and phospholipase C, exoproducts which are capable of modifying host target cells and interfering with immune clearance mechanisms (1). Numerous reports have demonstrated the presence of antibodies to these virulence determinants following P. aeruginosa infection (11); in cystic fibrosis patients, there is good correlation between the severity of lung disease and P. aeruginosa antibody titers (12). Antibodies against specific virulence determinants, such as elastase (which is capable of degrading immunoglobulins and serum complement factors [1, 6]) are therefore likely to play an important role in protecting host tissues, as protection against P. aeruginosa is associated with opsonization as well as with cell-mediated responses (6).
Consequently, the ability to suppress the production of protective antibody would confer a selective advantage on the pathogen. Our KAP assay data support this possibility, since we observed suppression of antibody production at OdDHL concentrations of around 70 to 80 μM, although at lower concentrations, antibody production was enhanced. In the latter case, it may be important that the immunoglobulin isotype promoted by high doses of OdDHL was IgG1, which is indicative in mice of a Th-2 response (25). Th-2 responses counterregulate antibacterial Th-1 responses, which support cell-mediated immunity. The apparent ability of OdDHL to support a Th-2 response may therefore represent a significant bacterial defense mechanism. This data is further supported by the ability of OdDHL to suppress IL-12 synthesis and to stimulate IgE synthesis by human leukocytes (another Th-2 response [33]). Given that OdDHL has a significant effect against human leukocytes, it is now important to assess the effects of OdDHL against TNF-α and IL-12 production in human monocyte culture systems similar to those described by Jahr et al. (13). The differences in activity of OdDHL in human and murine systems can be explained in several ways. Firstly, the species difference: the responses of human and murine cells are not likely to be identical due to their inherent variation. Secondly, very different cell types were involved: the human studies used peripheral blood leukocytes, whereas the murine system used spleen cells, two very distinct populations. Thirdly, the human assay is not antigen driven and is dependent upon chronic stimulation by high concentrations of exogenous IL-4, whereas the murine system is more representative of the physiological process.
In conclusion, the data presented in this paper not only suggest that OdDHL functions to regulate bacterial virulence gene expression through cell-cell communication but also that OdDHL, by virtue of its immunomodulatory properties, may be a virulence determinant per se. During the early stages of acute infection, for example, in cystic fibrosis patients, a scenario can be envisaged where P. aeruginosa LPS induces mucin production by epithelial cells (16). The mucin physically enmeshes the organism, providing a degree of protection. Secondary protection against the cytokine-inducing properties of LPS is then perhaps provided by OdDHL (Fig. 7), which down-regulates the production of IL-12, a cytokine which supports the bactericidal Th-1 milieu, and TNF-α, a pleiotropic and potentially host-protective cytokine.
FIG. 7.
Influence of OdDHL on the immune system. The mammalian T-helper (Th) cell response can be subdivided into two distinct arms, Th-1 and Th-2; the development of T cells along these distinct lineages is stimulus dependent and results in the release of cytokines characteristic of each lineage. (a) bacterial pathogens induce a Th-1 response, characterized by the secretion of IL-12 and gamma interferon. This developmental pathway results in the generation of activated macrophage populations, which are bactericidal and proinflammatory. (b) TNF-α is a key player in the establishment of this inflammatory process. It causes neutrophil activation, adhesion and extravasation, and the secretion of further inflammatory cytokines and chemokines (IL-6 and IL-8). Conversely, environmental allergens and helminth parasites induce a Th-1 contrasuppressive Th-2 response, characterized by the secretion of IL-4, IL-5, and IL-13, which induce a state of immediate hypersensitivity. Given the demarcation of these developmental pathways into antibacterial and anthelminthic, it would be of evolutionary advantage to bacterial pathogens to suppress the Th-1 response. This would in turn be supportive of the Th-2 response. It can be envisaged that OdDHL, by influencing both T-cell and macrophage functions at the points indicated in panel a, modulates host inflammatory responses to a degree which may promote the growth and survival of the pathogen. Compounds with this type of immunological activity could have applications in disease states, such as toxic shock syndrome and cerebral malaria, which have a significant TNF-α component.
ACKNOWLEDGMENTS
We thank Siri Ram Chhabra for the synthesis of OHHL and OdDHL. Figure 7 was drawn by Mari Nowell.
We thank Knoll Pharmaceuticals for providing financial support for the project.
REFERENCES
- 1.Bainbridge T, Fick R D. Functional importance of cystic fibrosis immunoglobulin fragments generated by Pseudomonas aeruginosa elastase. J Lab Clin Med. 1989;114:728–733. [PubMed] [Google Scholar]
- 2.Bainton N J, Bycroft B W, Chhabra S R, Stead P, Gledhill L, Hill L P J, Rees C E D, Winson M K, Salmond G P C, Stewart G S A B, Williams P. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene. 1992;116:87–91. doi: 10.1016/0378-1119(92)90633-z. [DOI] [PubMed] [Google Scholar]
- 3.Buret A, Dunkley M L, Pang G, Clancy R L, Cripps A W. Pulmonary immunity to Pseudomonas aeruginosa in intestinally immunized rats: roles of alveolar macrophages, tumor necrosis factor alpha, and interleukin-1α. Infect Immun. 1994;62:5335–5343. doi: 10.1128/iai.62.12.5335-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng H C, Nishio H, Hatase O, Ralph S, Wang J H. A synthetic peptide derived from p34 cdc 2 is a specific and efficient substrate of src-family tyrosine kinases. J Biol Chem. 1992;267:9248–9256. [PubMed] [Google Scholar]
- 5.Chhabra S R, Stead P, Bainton N J, Salmond G P C, Stewart G S A B, Williams P, Bycroft B W. Autoregulation of carbapenem biosynthesis in Erwinia carotovora ATCC 39048 by analogues of N-(3-oxohexanoyl)-l-homoserine lactone. J Antibiot. 1993;46:441–454. doi: 10.7164/antibiotics.46.441. [DOI] [PubMed] [Google Scholar]
- 6.Cripps A N, Dunkley M L, Clancy R L, Kyd J. Pulmonary immunity to Pseudomonas aeruginosa. Immunol Cell Biol. 1995;73:418–424. doi: 10.1038/icb.1995.65. [DOI] [PubMed] [Google Scholar]
- 7.Decker T, Lohmann-Matthews M L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumour necrosis factor (TNF) activity. J Immunol Methods. 1988;15:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 8.Dimango E, Zar H J, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest. 1995;5:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberhard A, Burlingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer N J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 10.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galloway D R. Pseudomonas aeruginosa elastase and elastolysis revisited: recent developments. Mol Microbiol. 1991;5:2315–2321. doi: 10.1111/j.1365-2958.1991.tb02076.x. [DOI] [PubMed] [Google Scholar]
- 12.Jaffar-Bandjee M C, Lazdunski A, Bally M, Carrère J, Chazalette J P, Galabert C. Production of elastase, exotoxin A, and alkaline protease in sputa during pulmonary exacerbation of cystic fibrosis in patients chronically infected by Pseudomonas aeruginosa. J Clin Microbiol. 1995;33:924–929. doi: 10.1128/jcm.33.4.924-929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahr T G, Ryan L, Sundan A, Lichenstein H S, Skjåk-Bræk G, Espevik T. Induction of tumor necrosis factor production from monocytes stimulated with mannuronic acid polymers and involvement of lipopolysaccharide-binding protein, CD14, and bactericidal/permeability-increasing factor. Infect Immun. 1997;65:89–94. doi: 10.1128/iai.65.1.89-94.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones S, Yu B, Bainton N J, Birdsall M, Bycroft B W, Chhabra S R, Cox A J R, Golby P, Reeves P J, Stephens S, Winson M K, Salmond G P C, Stewart G S A B, Williams P. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latifi A, Winson M K, Foglino M, Bycroft B W, Stewart G S A B, Lazdunski A, Williams P. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PA01. Mol Microbiol. 1995;17:333–343. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x. [DOI] [PubMed] [Google Scholar]
- 16.Li J-D, Dohrman A F, Gallup M, Miyata S, Gum J R, Kini Y S, Nadel J A, Prince A, Basbaum C B. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci USA. 1997;94:967–972. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meighen E A. Genetics of bacterial bioluminescence. Annu Rev Genet. 1994;28:117–139. doi: 10.1146/annurev.ge.28.120194.001001. [DOI] [PubMed] [Google Scholar]
- 18.Morissette C, Francoeur C, Darmond-Zwaig C, Gervais F. Lung phagocyte bactericidal function in strains of mice resistant and susceptible to Pseudomonas aeruginosa. Infect Immun. 1996;64:4984–4992. doi: 10.1128/iai.64.12.4984-4992.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palfreyman R W, Watson M L, Eden C, Smith A W. Induction of biologically active interleukin-8 from lung epithelial cells by Burkholderia (Pseudomonas) cepacia products. Infect Immun. 1997;65:617–622. doi: 10.1128/iai.65.2.617-622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 21.Passador L, Iglewski B H. Quorum sensing and virulence gene regulation in Pseudomonas aeruginosa. In: Roth J A, Bolin C A, Brogden K A, Minion F C, Wannemuehler M J, editors. Virulence mechanisms of bacterial pathogens. 2nd ed. Washington, D.C: ASM Press; 1995. pp. 65–78. [Google Scholar]
- 22.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of the Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson J P, Passador L, Iglewski B H, Greenberg E P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritchard D I, Lawrence C E, Appleby P, Gibb I A, Glover K. Immunosuppressive proteins secreted by the gastrointestinal nematode parasite Heligmosomoides polygyrus. Int J Parasitol. 1994;24:495–500. doi: 10.1016/0020-7519(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 25.Pritchard, D. I., and R. Moqbel. IgE, helminth infection, and the benefits of an allergic response. Is allergy a hangover from parasitism? Parasitology, in press.
- 26.Salmond G P C, Bycroft B W, Stewart G S A B, Williams P. The bacterial enigma: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson M M, Kondratieva T K, Apt A S, Tam M F, Skameme E. In vitro and in vivo T cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin Exp Immunol. 1996;99:98–105. doi: 10.1111/j.1365-2249.1995.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swift S, Winson M K, Chan P F, Bainton N J, Birdsall M, Reeves P J, Rees C E D, Chhabra S R, Hill P J, Throup J P, Bycroft B W, Salmond G P C, Williams P, Stewart G S A B. A novel strategy for the isolation of luxI homologues: evidence for the widespread distribution of a LuxR:LuxI superfamily in enteric bacteria. Mol Microbiol. 1993;10:511–520. doi: 10.1111/j.1365-2958.1993.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 29.Swift S, Throup J P, Salmond G P C, Williams P, Stewart G S A B. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem Sci. 1996;21:214–219. [PubMed] [Google Scholar]
- 30.Throup J P, Camara M, Briggs G S, Winson M K, Chhabra S R, Bycroft B W, Williams P, Stewart G S A B. Characterisation of the yenI/yenR locus from Yersinia enterocolitica mediating the synthesis of two N-acyl-homoserine lactone signal molecules. Mol Microbiol. 1995;17:345–356. doi: 10.1111/j.1365-2958.1995.mmi_17020345.x. [DOI] [PubMed] [Google Scholar]
- 31.Tomkins P T, Cooper K L, Titchmarsh S A, Appleby P, Webber D D. BTS 71 412: in vitro profile of a novel pyrazolinone immunosuppressant. Int J Immunopharmacol. 1995;17:357–366. doi: 10.1016/0192-0561(95)00024-v. [DOI] [PubMed] [Google Scholar]
- 32.Vassalli P. The pathophysiology of tumour necrosis factors. Annu Rev Immunol. 1992;10:411. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 33.Wheeler D J, Robins R A, Pritchard D I, Bundick R V, Shakib F. Peripheral blood based T cell containing and T cell-depleted culture systems for human IgE synthesis: the role of T cells. Clin Exp Allergy. 1996;26:28–35. doi: 10.1111/j.1365-2222.1996.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 34.Williams P, Stewart G S A B, Camara M, Winson M K, Chhabra S R, Salmond G P, Bycroft B W. Signal transduction through quorum sensing in Pseudomonas aeruginosa. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Pseudomonas: molecular biology and biotechnology. Washington, D.C: American Society for Microbiology; 1996. pp. 195–206. [Google Scholar]
- 35.Winson M K, Camara M, Latifi A, Foglino M, Chhabra S R, Daykin M, Bally M, Chapon V, Salmond G P C, Bycroft B W, Lazdunski A, Stewart G S A B, Williams P. Multiple N-acyl-l-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]