Abstract
Background
Electrocorticography (ECoG) language mapping is often performed extraoperatively, frequently involves offline processing, and relationships with direct cortical stimulation (DCS) remain variable. We sought to determine the feasibility and preliminary utility of an intraoperative language mapping approach guided by real-time visualization of electrocorticograms.
Methods
A patient with astrocytoma underwent awake craniotomy with intraoperative language mapping, utilizing a dual iPad stimulus presentation system coupled to a real-time neural signal processing platform capable of both ECoG recording and delivery of DCS. Gamma band modulations in response to 4 language tasks at each electrode were visualized in real-time. Next, DCS was conducted for each neighboring electrode pair during language tasks.
Results
All language tasks resulted in strongest heat map activation at an electrode pair in the anterior to mid superior temporal gyrus. Consistent speech arrest during DCS was observed for Object and Action naming tasks at these same electrodes, indicating good correspondence with ECoG heat map recordings. This region corresponded well with posterior language representation via preoperative functional MRI.
Conclusions
Intraoperative real-time visualization of language task-based ECoG gamma band modulation is feasible and may help identify targets for DCS. If validated, this may improve the efficiency and accuracy of intraoperative language mapping.
Keywords: brain mapping, case report, electrocorticography, glioma, language
Modern brain mapping techniques aim to mitigate risk of surgically-acquired cognitive-linguistic deficits in patients undergoing resection of lesions within or near suspected eloquent cortex.1 Despite this, a sizable proportion (~70%) of patients with lesions involving language-eloquent regions exhibit postoperative dysphasia.2 While acquired aphasia is often mild and may be transient in some patients, the importance of preserving cognitive-linguistic functioning is underscored by the known association between neurocognitive impairment and reduced quality of life, functional independence, and even survival.3,4
Awake craniotomy with intraoperative direct cortical stimulation (DCS) is considered the gold standard for language mapping.1 Task-based functional MRI (fMRI) is increasingly utilized as a noninvasive preoperative mapping technique, helping identify potentially eloquent cortex for further interrogation via intraoperative DCS. However, previous studies demonstrated only modest accuracy of fMRI in localizing DCS confirmed language-eloquent cortex, especially regarding specificity (~55%).5 As such, fMRI may best serve as an informative adjunct to DCS rather than supplanting the method. Notably, the accuracy of fMRI mapping can be greatly improved when combined with other mapping methods such as transcranial magnetic stimulation(TMS).6 While electrocorticography (ECoG) represents yet another brain mapping technique, it remains to be determined whether this approach better corresponds to DCS than others, and whether it incrementally adds to existing approaches to neurosurgical planning.
ECoG is a form of intracranial electroencephalography (EEG), allowing the recording of event-related dynamics of brain oscillations across a variety of frequency ranges. High frequency (gamma) modulations appear more related to the timing and localization of functional brain activity as compared to lower frequency oscillations (e.g., alpha or beta).7 Using gamma band recordings directly from the cortical surface, the activity of neuronal populations elicited by specific tasks of interest can be visualized as heat maps (electrocorticograms) with excellent signal-to-noise ratio and spatial resolution. Accordingly, task-based ECoG may be particularly useful for intraoperative language mapping by localizing gamma band activations associated with specific linguistic functions, potentially indicating eloquence. To date, ECoG language mapping has most frequently been employed extraoperatively in epilepsy patients.8 In addition, ECoG visualization is often only available post hoc, limiting its usefulness for mapping in real-time during awake craniotomy. It also remains unclear how well ECoG-based methods correspond with DCS results and other adjunctive approaches to language mapping across various linguistic functions.
We recently initiated a prospective investigation into the feasibility and utility of an innovative ECoG-based approach to intraoperative cortical language mapping during awake craniotomy. The approach utilizes ECoG with a pipeline enabling real-time visualization of gamma band electrocorticograms during administration of diverse language tasks in the intraoperative setting. The same ECoG grid is also employed for DCS with cortical placement unchanged, allowing precise correspondence between recording and stimulation results. We expect areas of peak gamma band activity will correspond to disruption of function during DCS (confirming eloquence), though some variability is likely according to differences in specific tasks and regions mapped. A secondary aim of the project is to incorporate other adjunctive approaches to language mapping, including fMRI and TMS, with the hope that convergence of results across modalities will enable even better prediction of eloquence as determined by DCS.
Ultimately, we believe this work will provide significant insight into the neurofunctional underpinnings of linguistic neural processes, while also improving upon standard of care brain mapping for patients with brain tumors. It is hoped that gamma band hot spots convey high probability of eloquence and reliably guide identification of target regions for DCS. With validation, utilization of ECoG mapping could narrow regions of interest for DCS, decrease seizure risk associated with more extensive stimulation, and potentially improve efficiency of brain mapping and awake craniotomy. The following describes an initial experience with this approach for a patient with astrocytoma near suspected language-eloquent cortex.
Methods
Participant
An adult right-handed (Edinburgh Handedness Inventory = +70) patient was recruited to a prospective study investigating the utility of task-based ECoG intraoperative language mapping within the context of awake glioma resection. Regarding relevant medical history, while living abroad the patient developed episodes of nonspecific visual anomalies in the right field, slurred speech, and confusion. The patient presented to a medical provider and was prescribed a triptan for suspected migraines, which provided minimal alleviation of symptoms. Symptoms progressed to include episodic impairment in spelling and reading, which were believed to be partial seizures and the patient was started on levetiracetam, without recurrence of episodes. Subsequent neuroimaging revealed a large nonenhancing mass in the posterior left temporo-occipital area. Biopsy was performed at the outside institution, with pathology interpreted as WHO grade II IDH-mutant astrocytoma. Upon returning to his native country, he presented to our institution for further care and was considered a good candidate for surgical resection and participation as the first patient on our ECoG language mapping study. The patient provided written informed consent for participation in the study which was approved by the institutional review board (protocol PA15-0595).
Preoperative structural MRI is presented in online supplemental material (see Figure S1, top). The patient underwent standard of care preoperative neuropsychological evaluation to determine the feasibility of participation in pre- and intraoperative language mapping. Testing revealed severe verbal learning and memory impairment, mildly impaired visuospatial learning, moderate dysnomia, and mildly reduced phonemic and semantic fluency and mental flexibility. Despite noted deficits, he was conversationally fluent and deemed at least minimally capable of participating in all language mapping procedures. In addition, deficits in verbally mediated functions identified on neuropsychological testing increased suspicion that the tumor potentially involved or impacted surrounding language-eloquent structures. Complete neuropsychological test results are presented in online Supplemental Material Table S1.
Procedure
Preoperative task-based fMRI at 3T was performed utilizing block design covert word generation (ie, lexical and semantic fluency) and sentence completion paradigms processed using DynaSuite Neuro software (Phillips). See Black and colleagues for description of analogous paradigms.9 Resting-state fMRI was also completed and processed with in-house software to detect the posterior language network via seeding of anterior language activation obtained from task-based fMRI.10 Preoperative navigated TMS language mapping was planned but the patient was unavailable for the study.
Intraoperative language mapping utilized a dual iPad stimulus presentation system (NeuroMapper11) as displayed in online Supplemental Material Figure S2. The patient was baselined on Object, Action, Auditory, and Written description naming tasks per protocol. For Object naming, the patient was required to name pictured objects (eg, line drawing of a “drum”) from the Snodgrass and Vanderwart12 set of stimuli frequently used in brain mapping research and practice.13 Action naming involved naming illustrated verbs (eg, picture of a girl “throwing” a ball) from the Object and Action Naming Battery.14 Auditory naming stimuli were drawn from published standardized descriptive naming stimuli15 and required naming objects described aurally (eg, respond with “crown” to hearing “what a king wears on his head”). Written naming was similar to the Auditory task but with written descriptions presented visually to be read aloud by the patient. Despite moderate dysnomia identified on preoperative neuropsychological testing (Boston Naming Test demographically adjusted z-score = −2.00), a large set of reliable baseline naming stimuli were obtained for all NeuroMapper language mapping tasks prior to surgery.
For surgery, the patient was initially placed under general anesthesia and a craniotomy was performed. After opening the dura, a 32-channel ECoG grid (4 × 8 with 1 cm pitch; Ad-Tech Medical Instrument Co., WI, USA) was placed covering the majority of the left temporal cortex, including anterior aspects under the dura but unexposed by the craniotomy (online Supplemental Material Figure S3). The anesthetized patient was then awakened by the neuro-anesthesiologist. Once the patient was fully awake, alert, and responding at baseline level on the NeuroMapper language tasks, mapping procedures were initiated. Baseline ECoG did not reveal any epileptiform discharges in the awake patient.
For intraoperative mapping, NeuroMapper was coupled to a portable real-time neural signal processing system capable of both recording ECoG cortical activity and delivering DCS in a closed-loop fashion (see Figure 1). ECoG recording and DCS were synchronized with stimulus presentation via a photosensor programmed with an Arduino Teensy microcontroller attached to NeuroMapper. For neural recording, data were obtained from the ECoG grid with a multichannel bio signal amplifier (256 channels; gHIamp, g Tec medical engineering GmbH, Graz, Austria) at a sampling frequency of 2.4 kHz and 24 bit A/D resolution. A 1 × 4 electrode strip was flipped and placed under the dura as reference and ground. The neural data were acquired and visualized using Simulink/MATLAB (MathWorks, Natick, MA, USA) and gHIsys block sets (g.Tec medical engineering GmbH, Graz Austria) and presented on a large monitor. The physical layout of the hardware and output display in the intraoperative environment are depicted in Figure 2.
Figure 1.
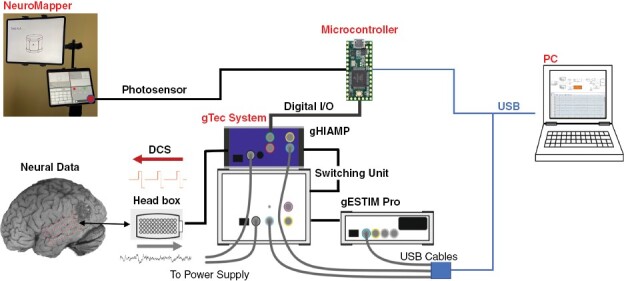
ECoG Language Mapping Schema. NeuroMapper and ECoG processing/stimulation system (g Tec; GmbH Austria).
Figure 2.
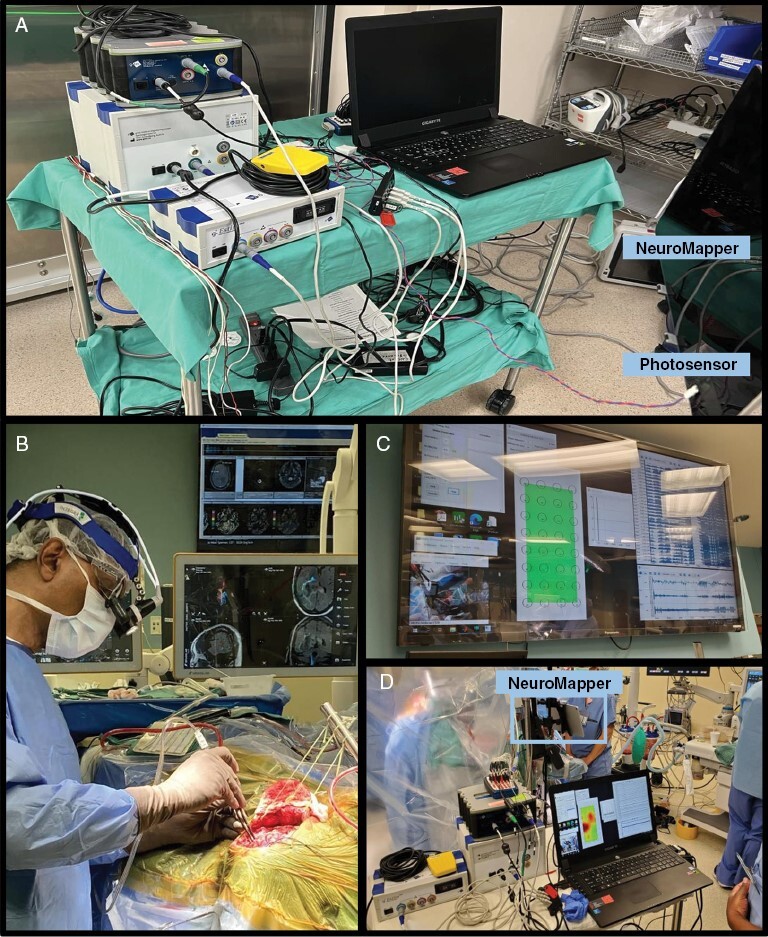
Physical Layout of the Intraoperative Environment. Intraoperative system layout: a) hardware configuration of the ECoG processing/stimulation system; b) sterile field during surgery; c) real-time electrocorticogram and raw waveform display in the surgeon’s field of view; d) mapping system in use during gamma band recording and real-time processing.
ECoG oscillatory cortical activity was recorded during administration of each language task, with around 30 trials administered per task to ensure reliability of the recordings. Gamma frequency band (50–100 Hz) modulations in response to individual trials at each electrode were processed in real-time and visualized as heat maps via custom scripts. While cognitive tasks induce neural electrical activity modulations across various frequency bands (eg, alpha, 8–12 Hz; beta, 12–30 Hz; gamma, >30 Hz), we selected high gamma band activity due to strong associations with localized neural spiking, supporting particular relevance to localizationist approaches to task-based brain mapping.7
Following ECoG recording and real-time visualization, bipolar DCS through the grid was conducted for each neighboring electrode pair for 2 s duration during administration of language tasks. Target electrode pairs were identified by the neurosurgeon and input into the computer interface by a technician. As depicted in Figure 1, stimulation was triggered through NeuroMapper via the photosensor coupled to the bi-phasic cortical stimulator (gEstim-pro) and a switching unit enabling shift from recording from an electrode contact to stimulating the cortical surface with that electrode contact (g.Estim PRO and Switching Unit, g.tec medical engineering GmbH, Graz Austria). Patient responses during stimulation were monitored for errors (e.g, phonemic and semantic) and overt speech arrest. A positive DCS site (ie, a hit) was recorded when error and/or speech arrest occurred at least twice at that location. A neurophysiologist monitored raw electrophysiological waveforms for epileptiform after-discharges during stimulation. Stimulation intensity was initially set to 4 mA for the first pass across all paradigms. This was followed by a second pass at 6 mA for all paradigms. A few after-discharges were recorded during stimulation at the higher intensity, which were quickly alleviated with cold irrigation and patient performance returned to baseline level. Language disruption was not recorded as error or speech arrest when after-discharges occurred following stimulation.
Although individual trials for each task were visualized in real-time in the operating environment, we also summarized results across all recording trials (~30) for each task as aggregate maps processed offline to facilitate post hoc comparison of electrocorticograms by task. Neural data were processed using MATLAB 2018b (MathWorks, Natick, MA, USA). After visually examining ECoG recordings to exclude corrupted trials and artifacts, power line noise at 60 Hz and its harmonics were removed with second-order infinite impulse response notch filters. Next, data segments around stimulus onsets were extracted (−1 to 3 s) and short-time Fourier transform was applied to each channel for every trial. The resulting time–frequency maps were averaged across trials and normalized using a baseline power of −1 to 0 s before stimulation onset. Finally, the averaged spectrograms were transformed into a decibel (dB) scale, providing centered spectrograms for focused investigation of stimulus responses.
Results
While some trials showed differing robustness of activation, real-time electrocorticograms visualized on an individual trial basis showed a similar localizations of peak gamma band activity as compared to the aggregate maps processed offline. See Figure 3 for depiction of aggregate electrocorticograms by task. Electrocorticograms differed somewhat across the naming tasks, though all tasks resulted in strongest activation at electrode 12 located in the anterior to mid superior temporal gyrus. During DCS, consistent speech arrest was observed with Object and Action naming tasks when stimulating electrode pair 11–12 (at 6 mA only), indicating good correspondence with ECoG gamma band heat map recordings for these tasks (Figure 3). The same region was within the vicinity of posterior language network representation on task-based and resting-state fMRI (online Supplemental Material Figure S4). Given identification of reliable positive DCS sites at 6 mA and elicitation of some after-discharges during trials, stimulation was not conducted at a higher intensity.
Figure 3.
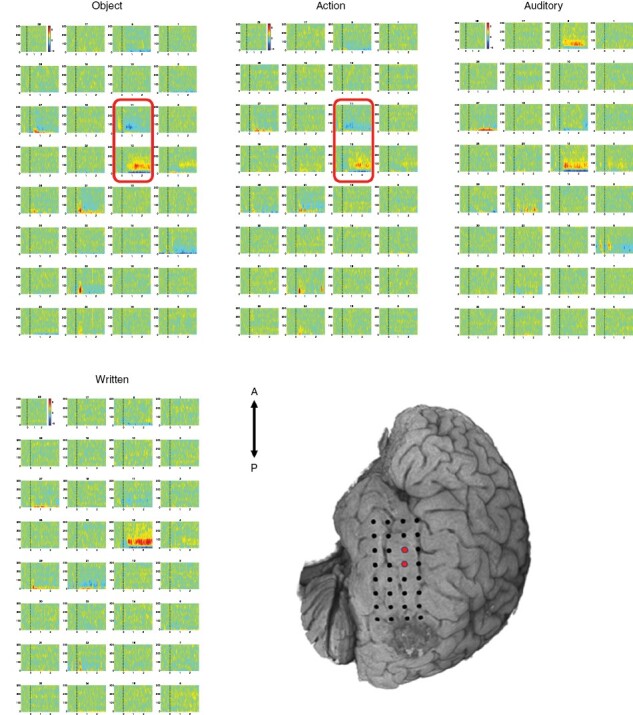
Electrocorticograms and DCS Points. ECoG gamma band heatmaps for each language task. Red rectangles on electrocorticograms and dots on the cortical surface represent bipolar pairs with speech arrest during stimulation.
Upon initiation of resection, boundaries were identified sparing the positive DCS site. The previous corticectomy was observed and the cavity was filled with hemostatic agents, which were gently removed. An approach was taken inferior to the previous corticectomy below the vein of Labbe. During the resection, the patient was monitored continuously with conversation and periodic naming. The patient did well during the awake portion of the resection though speech became increasingly variable as the resection proceeded deeper, appearing qualitatively slowed with occasional phonemic errors during conversation noted. No seizure activity was recorded during resection. Ultimately, subtotal resection of a large portion of the mass was achieved as indicated on postoperative imaging (online Supplemental Material Figure S1). Comparison of pre- to postoperative volumetrics indicated resection of 90.0% T1 hypointense (pre, 60.6 cm3; post 6.04 cm3) and 74.3% FLAIR hyperintense lesion (pre, 74.0 cm3; post 19.04 cm3). Pathology indicated WHO grade 4 IDH-mutant astrocytoma.
Discussion
Intraoperative real-time visualization of task-based ECoG gamma band modulation is feasible and may help identify targets for DCS, potentially improving the efficiency of intraoperative mapping. This case is consistent with prior preliminary work involving somewhat similar ECoG mapping methods, both extra- and intraoperatively.8,16 However, most work to date has involved single-task recordings and only offline post hoc signal processing. Our case demonstrates the feasibility of using ECoG mapping intraoperatively via real-time processing of gamma band oscillatory data. Importantly, the results of gamma band peak localization were highly consistent with DCS. This lends support to the small series reported by Ogawa et al. that indicated a sensitivity and specificity around 90% for ECoG involving a similar, though less comprehensive, approach to intraoperative language mapping.17
While others have reported more limited correspondence between ECoG language mapping and DCS,18 it is believed that methodological variation is a likely contributor. Among existing work, mapping is frequently limited to single expressive or passive listening tasks, often involving long sessions (sometimes hours) for data acquisition and processing. Furthermore, tasks used for ECoG recording (eg, word reading) do not always correspond well with the functions interrogated by DCS (eg, object naming, counting). It is possible that by sampling a diversity of language functions in a standardized fashion across modalities, more reliable correspondence between ECoG recording and DCS may be observed. Close correspondence of ECoG results with noninvasive preoperative mapping techniques (eg, fMRI), such as in the present case, may also increase confidence regarding the eloquence of structures under consideration.
This case specifically focused on gamma band activity due to its known association with higher-level cognitive abilities.19 However, incorporation of other frequencies and consideration of regional suppression of activity may also convey important information regarding language localization, potentially further improving correspondence with DCS. Future work is needed to identify optimal ECoG acquisition and processing methods and language mapping task selection within large samples with diverse lesion locations and mapping regions of interest. As this study continues to accrue, we hope to confirm the preliminary utility of our mapping approach while identifying the methodological aspects most critical for accuracy and expediency.
Although localization of the strongest gamma band activations was similar across naming tasks, considerable variation was noted in the more general activation patterns, as expected given the differing behavioral neuroanatomy known to underlie the diverse language functions interrogated.20 It should be noted that the DCS duration for Auditory and Written descriptive naming tasks did not cover the entire duration of stimulus presentation, which might have contributed to the lack of speech arrest during these tasks. In addition, despite the current consensus that DCS is the gold standard for determination of eloquence, the functional relevance of secondary or tertiary gamma band hot spots (regardless of correspondence with DCS) remains an open question. Accordingly, future work should incorporate important patient outcomes to better understand the functional relevancy of mapping results across all modalities.
Unfortunately, the patient did not have postoperative neuropsychological evaluation proximal to the surgery, limiting ability to understand how cognitive-linguistic outcome might relate to resection (or sparing) of ECoG and fMRI mapping results. While the primary aim of the prospective study pertains to the feasibility of the ECoG mapping approach and correspondence of task-based gamma band modulations with DCS results, we plan to also acquire neuropsychological outcomes per standard of care when feasible to better address such questions. Nonetheless, the patient did exhibit some intraoperative decline in conversational fluency during aspiration of the lesion toward the end of the awake portion of surgery, despite sparing of eloquent cortex identified via DCS mapping. We believe observed changes during surgery likely pertain to white matter manipulation or tissue resection. Although subcortical DCS mapping was not performed in this case, and this project centers specifically around cortical mapping at this stage of investigation, we plan to later explore the feasibility of using a similar ECoG grid approach to subcortical DCS mapping.
An advantage of the electrode grid approach for stimulation delivery, whether to cortical or subcortical structures, is potential for improved consistency in stimulation localization over repeated trials and programmed stimulation duration, limiting possible variation attributable to human error when using manual bipolar or monopolar probes. In addition, even if employing a probe for DCS delivery, simultaneous ECoG recording may uncover alterations in electrocorticograms on the cortical surface during subcortical stimulation. This could aid identification of subcortical structures involved in networks associated with eloquent cortical areas in real-time. Another consideration worth investigating is how convergence of mapping results across methods may inform localization of eloquence. For instance, it may be the case that resection of gamma band hot spots converging with fMRI BOLD signal or TMS results increases probability of postoperative cognitive-linguistic deterioration regardless of structures being “confirmed” eloquent via DCS. With empirical support and validation, such results would challenge the common assumption that DCS results represent the ground truth for eloquence.
While preliminary, our work demonstrates that relatively rapid ECoG gamma band data acquisition and processing in the mapping of diverse language functions is feasible, and at least initial evidence suggests, potentially useful in aiding identification of language-eloquent cortex. This may represent a first step toward advancing brain mapping methodology and leading to exploration of other novel uses of ECoG in the intraoperative environment, with the ultimate aim of improving the cognitive-linguistic outcomes for patients undergoing awake craniotomy for resection of glioma.
Supplementary Material
Acknowledgments
This work was presented at the 27th Annual Scientific Meeting of the Society for Neuro-Oncology, Tampa, Florida, USA, November 18, 2022.
Contributor Information
Kyle R Noll, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Priscella Asman, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Israt Tasnim, Department of Biomedical Engineering, University of Houston, Houston, Texas, USA.
Matthew Hall, Department of Biomedical Engineering, University of Houston, Houston, Texas, USA.
Katherine Connelly, Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Chandra Swamy, Department of Biomedical Engineering, University of Houston, Houston, Texas, USA.
Chibawanye Ene, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Sudhakar Tummala, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Roxana M Grasu, Department of Anesthesiology and Perioperative Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Ho-Ling Liu, Department of Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Vinodh A Kumar, Department of Neuroradiology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Matthew Muir, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Sarah Prinsloo, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA; Department of Palliative, Rehabilitation and Integrative Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Hayley Michener, Department of Palliative, Rehabilitation and Integrative Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Jeffrey S Wefel, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA; Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Nuri F Ince, Department of Biomedical Engineering, University of Houston, Houston, Texas, USA.
Sujit S Prabhu, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Funding
This work was supported by funding from the National Cancer Institute of the National Institutes of Health under award number P30 CA016672, National Science Foundation under grant number 2124705 (N.F.I.), the National Cancer Institute of the National Institutes of Health under award number R01 CA258788 (H.L.), and the University Cancer Foundation and the Duncan Family Institute for Cancer Prevention and Risk Assessment (K.R.N.).
Conflict of interest statement
None to disclose.
References
- 1. Tharin S, Golby A.. Functional brain mapping and its applications to neurosurgery. Neurosurgery. 2007;60(4):185–201; discussion 201. [DOI] [PubMed] [Google Scholar]
- 2. Wilson SM, Lam D, Babiak MC, et al. Transient aphasias after left hemisphere respective surgery. J Neurosurg. 2015;123(3):581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGirt MJ, Mukherjee D, Chaichana KL, et al. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65(3):463–9; discussion 469. [DOI] [PubMed] [Google Scholar]
- 4. Noll KR, Sullaway CM, Wefel JS.. Depressive symptoms and executive function in relation to survival in patients with glioblastoma. J Neurooncol. 2019;142(1):183–191. [DOI] [PubMed] [Google Scholar]
- 5. Weng HH, Noll KR, Johnson JM, et al. Accuracy of presurgical functional MR imaging for language mapping of brain tumors: a systematic review and meta-analysis. Radiology. 2018;286(2):512–523. [DOI] [PubMed] [Google Scholar]
- 6. Ille S, Sollmann N, Hauck T, et al. Combined noninvasive language mapping by navigated transcranial magnetic stimulation and functional MRI and its comparison with direct cortical stimulation. J Neurosurg. 2015;123(1):212–225. [DOI] [PubMed] [Google Scholar]
- 7. Crone NE, Sinai A, Korzeniewska A.. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–295. [DOI] [PubMed] [Google Scholar]
- 8. Cervenka MC, Corines J, Boatman-Reich DF, et al. Electrocorticographic functional mapping identifies human cortex critical for auditory and visual naming. Neuroimage. 2013;69:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Black DF, Vachha B, Mian A, et al. American society of functional neuroradiology–recommended fMRI paradigm algorithms for presurgical language assessment. Am J Neuroradiol 2017;38(10):E65–E73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hsu AL, Hou P, Johnson JM, et al. IClinfMRI software for integrating functional MRI techniques in presurgical mapping and clinical studies. Front Neuroinform 2018;12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabsevitz DS, Middlebrooks EH, Tatum W, et al. Examining the function of the visual word form area with stereo EEG electrical stimulation: a case report of pure alexia. Cortex. 2020;129:112–118. [DOI] [PubMed] [Google Scholar]
- 12. Snodgrass JG, Vanderwart MA.. standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol: Learn Mem 1980;6(2):174–215. [DOI] [PubMed] [Google Scholar]
- 13. Gisbert-Muñoz S, Quiñones I, Amoruso L, et al. MULTIMAP: multilingual picture naming test for mapping eloquent areas during awake surgeries. Behav Res Methods. 2021;53(2):918–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masterson J, Druks J.. Description of a set of 164 nouns and 102 verbs matched for printed word frequency, familiarity and age-of-acquisition. J Neurolinguistics. 1998;11(4):331–354. [Google Scholar]
- 15. Hammeke TA, Kortenkamp SJ, Binder JR.. Normative data on 372 stimuli for descriptive naming. Epilepsy Res. 2005;66(1-3):45–57. [DOI] [PubMed] [Google Scholar]
- 16. Swift JR, Coon WG, Guger C, et al. Passive functional mapping of receptive language areas using electrocorticographic signals. Clin Neurophysiol. 2018;129(12):2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ogawa H, Kamada K, Kapeller C, et al. Clinical impact and implication of real-time oscillation analysis for language mapping. World Neurosurg. 2017;97:123–131. [DOI] [PubMed] [Google Scholar]
- 18. Bauer PR, Vansteensel MJ, Bleichner MG, et al. Mismatch between electrocortical stimulation and electrocorticography frequency mapping of language. Brain Stimul. 2013;6(4):524–531. [DOI] [PubMed] [Google Scholar]
- 19. Bosman CA, Lansink CS, Pennartz CM.. Functions of gamma‐band synchronization in cognition: from single circuits to functional diversity across cortical and subcortical systems. Eur J Neurosci. 2014;39:1982–1999. [DOI] [PubMed] [Google Scholar]
- 20. Hickok G. The functional neuroanatomy of language. Phys Life Rev. 2009;6(3):121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


