Abstract
Background
We report our experience with using a ventriculoperitoneal shunt (VPS) with an on–off valve and in-line Ommaya reservoir for the treatment of hydrocephalus or intracranial hypertension in patients with leptomeningeal disease (LMD). Our goal was to determine whether control of intracranial pressure elevation combined with intrathecal (IT) chemotherapy would extend patient survival.
Methods
In this IRB-approved retrospective study, we reviewed 58 cases of adult patients with LMD from solid cancers who received a VPS with a reservoir and an on–off valve at M D Anderson Cancer Center from November 1996 through December 2021. Primary tumors were most often melanoma (n = 19) or breast carcinoma (n = 20). Hydrocephalus was diagnosed by clinical symptoms and findings on magnetic resonance imaging (MRI), and LMD by MRI or cerebrospinal fluid analysis. Differences in overall survival (OS) were assessed with standard statistical techniques.
Results
Patients who received a VPS and more than 3 IT chemotherapy sessions survived longer (n = 26; OS time from implantation 11.7 ± 3.6 months) than those who received an occludable shunt but no IT chemotherapy (n = 24; OS time from implantation 2.8 ± 0.7 months, P < .018). Peritoneal seeding appeared after shunt insertion in only two patients (3%).
Conclusions
This is the largest series reported to date of patients with LMD who had had shunts with on–off valves placed to relieve symptoms of intracranial hypertension. Use of IT chemotherapy and control of hydrocephalus via such shunts was associated with improved survival.
Keywords: carcinomatous meningitis, intracranial hypertension, leptomeningeal carcinomatosis, on–off valve, ventriculoperitoneal shunt
Leptomeningeal dissemination of tumor cells (LMD) is a highly morbid complication of cancer.1–3 Left untreated, survival for patients with LMD can be as short as 4–6 weeks, but even with aggressive treatment historically survival is only 4–6 months.4 LMD can impede cerebral and cranial nerve function5,6 and cerebrospinal fluid (CSF) circulation.1,7 The current diagnostic “gold standard” for diagnosis of LMD consists of cytology/flow cytometry analysis of CSF samples and postcontrast magnetic resonance imaging (MRI) of the entire neuraxis.8–10
According to EANO/ESMO criteria, the diagnosis of LMD can be verified (or excluded) only with cytologic or histologic findings, and is considered either type I (ie, CSF cytology positive for LMD) or type II (equivocal meaning “suspicious” or “atypical” cells in the CSF, with diagnosis of LMD made by radiological findings),11 and survival is shorter among patients with type I LMD. Multivariable analysis indicated that administration of either intrathecal (IT) or systemic pharmacotherapy was associated with improved outcome in type I LMD, but not in type II LMD.11
Hydrocephalus is a known sequela of LMD and is an independent risk factor for poor survival.12 Efforts have been undertaken to mitigate hydrocephalus and reduce the concomitant increase in intracranial pressure (ICP), but CSF flow studies have had varying design and results.13–15 Moreover, it is difficult to predict when ICP meets the intracranial compliance limit (and thus when patients will develop symptoms of hydrocephalus).
Although prior studies have demonstrated that placing a ventriculoperitoneal shunt (VPS) can have palliative effects in LMD-associated intracranial hypertension,16,17 little has been reported regarding outcomes after placement of a VPS with an Ommaya reservoir and an “on–off” switch that allows reversible occlusion.18,19 Distribution of IT chemotherapy via earlier VPS devices that did not include reversible occlusion was somewhat restricted in patients with shunted hydrocephalus, because the drug was siphoned from the ventricular system into the peritoneal cavity, resulting in underdosage.19 On the other hand, use of a reservoir (without a VPS) can lead to increased ICP and hydrocephalus, which would require replacing the reservoir with a VPS device and discontinuing IT chemotherapy.20 When Ommaya reservoirs are used in combination with on-off shunt systems, the valve can be closed to allow IT therapy and then reopened to relieve hydrocephalus between the IT therapy sessions (Figure 1).
Figure 1.
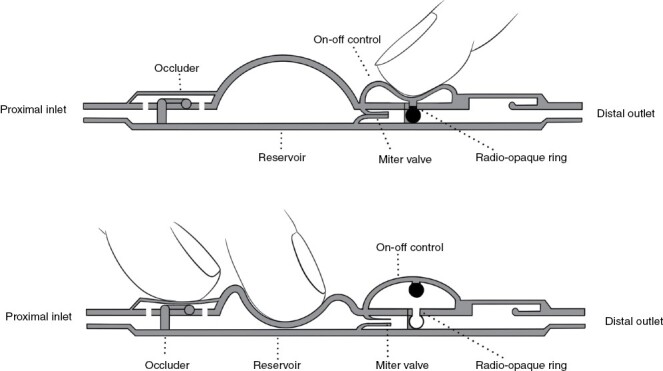
Schematic of an on-off shunt valve with a reservoir. To turn off the valve, the operator compresses the distal dome (upper panel), thereby pushing a small globe at the end of a post into a tailored receptacle. The system then stays closed until the operator sequentially pushes the proximal portion of the valve (the occluder) and then depresses the reservoir. The occluder prevents backflow of pressure into the Ommaya reservoir (which is attached to the proximal inlet) and forces any pressure created by compression of the reservoir through the miter valve, where it forces that valve back into the open position and returns the on–off control to the “open” position (lower panel).
The utility of an on–off valve VPS/Ommaya system remains somewhat controversial because of observed malfunctions and concerns about drug distribution in shunt-dependent patients. Here, we report our experience with placing a VPS with a reservoir and an on–off valve for treating LMD-associated hydrocephalus, including surgical technique, safety, and survival outcomes.
Methods
Study Design
This retrospective case series analysis was approved by the institutional review board of The University of Texas M D Anderson Cancer Center. Patients were identified from a prospectively maintained institutional database as having received a VPS/Ommaya system with an on–off valve at M D Anderson from November 1, 1996 through December 31, 2021. Patients without clinical or radiographic follow-up were excluded. Data collected included patient characteristics (ie, age, sex, ventriculomegaly, CSF cytology, performance status, and cancer histology), presenting and resolved symptoms and signs, and any related complications. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria were followed.
Statistical Analysis
Cox regression and log-rank analyses were used to identify factors associated with overall survival. Student’s t tests were used to compare continuous variables, and chi-square analysis to compare categorical variables. P values of ≤ .05 were considered to indicate statistically significant differences. GraphPad Prism Statistical Software (version 8.0.0) was used for statistical analysis.
Operative Techniques
Reservoir placement and shunt positioning.
All of our patients required indwelling ventricular access to enable delivery of IT therapy. Burr holes were preferentially placed in the frontal position 2.5–3 cm lateral to the midline and just anterior to the coronal suture (Figure 2). A convertible Ommaya reservoir with side arm specifically designed to be placed directly over the burr hole, was then inserted. We recommend avoiding the retro-auricular or occipital regions for shunt insertion, to minimize the risk of the patient inadvertently switching the valve to the “off” position upon lying down.
Figure 2.
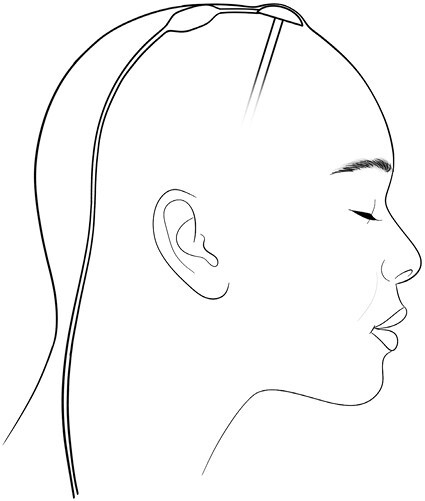
Ommaya reservoir with “on–off” shunt valve. A convertible Ommaya reservoir with a side port aimed posteriorly is placed at or slightly anterior to the coronal suture in the frontal area. The side port connects to the proximal end of the shunt valve, which must be placed high on the patient’s head to avoid accidental occlusion of CSF flow. The distal tubing leading from the valve towards its terminus in the peritoneal cavity is tunneled under the retroauricular area of the scalp. The shunt valve is usually rotated slightly to facilitate passing the peritoneal tubing, but it must not be placed on the side of the patient’s head.
Conversion from previous Ommaya device to an Ommaya with in-series on–off valve.
A VP shunt was placed in all patients with the aim to continue or initiate ongoing intrathecal chemotherapy. These patients were patients who had imaging findings and/or symptoms that was concerning for hydrocephalus. After verifying the patency of the Ommaya reservoir catheter, we passed a standard distal peritoneal shunt catheter from the cranial subgaleal space to the abdominal site, where the peritoneum had been exposed. This distal catheter was then left in situ. An on–off Heyer–Schulte valve was then attached in series with the shunt catheter system at the cranial end, such that the distal end of the reservoir would drain to the abdominal intraperitoneal space. After the proximal end was connected to the side-arm outlet of the Ommaya reservoir (already in place) and secured to the pericranium, all critical connections were secured with 2-0 silk sutures, hemostasis was assured, and patency of the shunt system was verified. The Ommaya reservoir/shunt valve combination is shown in Figure 2.
Results
We identified 58 patients with LMD who underwent insertion of an Ommaya reservoir, with or without a VPS with an on–off valve (Table 1). Slightly more patients were female (59% vs 41% male); in 54 patients (93%), the diagnosis of LMD had been confirmed by the presence of malignant cells in the CSF; diagnosis in the other 7% was by the presence of linear enhancement of sulci or cranial nerves on brain MRI, with or without linear enhancement on the surface of the spinal cord or nerve roots on spinal MRI; 51 patients (88%) had positive findings on both CSF analysis and brain or spinal MRI. The opening pressure was measured by lumbar puncture before VPS placement, or, in 19 patients, during insertion of the Ommaya reservoir (median, 33.4 cm H2O; range, 27–75 cm H2O). Ventriculomegaly was seen on preoperative MRI in 34 patients (57%), but all 58 patients had clinical signs or symptoms of high ICP. Sixteen patients (28%) initially had only an Ommaya reservoir placed, which was later converted to an on–off valve VPS/Ommaya system upon recognition of hydrocephalus or symptomatic elevated ICP. The mean time to conversion in that subgroup was 93 days (range 2–292 days). Median overall survival times were as follows: 56.7 months from the cancer diagnosis; 12.7 months from the LMD diagnosis; and 8.9 months after the VPS placement (Table 1).
Table 1.
Patient Characteristics
| Value or No | ||||
|---|---|---|---|---|
| Characteristic | All Patients | No IT Chemo | ≥3 IT Chemo | P Value |
| No. of patients | 58 | 24 | 26 | |
| Median age at diagnosis (yrs ± SD) | 42.8 ± 12.5 | 41.8 ± 12.9 | 43.2 ± 13.2 | n.s. |
| Sex | ||||
| Female | 34 (59%) | 17 (71%) | 14 (54%) | |
| Male | 24 (41%) | 7 (29%) | 12 (46%) | |
| Primary cancer histology | ||||
| Melanoma | 9 (38%) | 9 (35%) | n.s. | |
| Breast cancer | 8 (33%) | 10 (38% | n.s. | |
| Ductal | 5 (21.0%) | 8 (31%) | n.s. | |
| Lobar | 3 (13%) | 2 (8%) | n.s. | |
| Ventriculomegaly present | 34 (59%) | 9 (38%) | 15 (58%) | n.s. |
| Positive CSF cytology | 54 (93%) | 20 (83%) | 16 (62%) | n.s. |
| Median opening pressure, cm H2O ± SD | 33.4 ± 11.7 | 36.8 ± 14.5 | 35.2 ± 10.5 | n.s. |
| Karnofsky Performance Status Score | 77.1 ± 12.7 | 83.9 ± 13.0 | n.s. | |
| Radiation therapy administered | 9 (38%) | 12 (46%) | n.s. | |
| Systemic progression at time of procedure | 4 (17%) | 6 (23%) | n.s. | |
| Median time from cancer dx to LMD diagnosis (mo ± SD) | 41.0 ± 32.0 | 50.2 ± 70.0 | n.s. | |
| Median time from LMD dx to shunt procedure (mo ± SD) | 1.3 ± 1.7 | 2.9 ± 3.6 | 0.04 | |
| Median OS from cancer diagnosis (mo ± SD) | 12.7 ± 4.2 | 42.2 ± 6.7 | 69.7 ± 14.7 | 0.08 |
| Median OS from LMD diagnosis (mo ± SD) | 12.7 ± 4.2 | 3.8 ± 0.7 | 17.9 ± 8.9 | 0.01 |
| Median OS from shunt procedure (mo ± SD) | 8.9 ± 3.7 | 2.8 ± 0.7 | 11.7 ± 3.6 | 0.02 |
IT, intrathecal therapy; SD, standard deviation; n.s., not significant (p > 0.05); CSF, cerebrospinal fluid; OS, overall survival; LMD, leptomeningeal disease.
The most common primary cancers were breast carcinoma (n = 20, 34%) and melanoma (n = 19, 33%). Carcinomas of the lung and gastrointestinal tract together accounted for 16 (28%), and the other 3 patients (5%) were two patients with sarcoma and one with transitional cell carcinoma of the bladder.
In terms of presenting signs and symptoms (Table 2), just over half of patients (52%) presented with headache, 31% with nausea and vomiting, 28% with altered mental status, and 22% with gait abnormalities. Urinary incontinence (7%) and papilledema (3%) were less common. Most of these signs and symptoms improved after placement of the shunt system as assessed at their first postoperative visit; only gait abnormality (improved in 4 patients, 31%) and urinary incontinence (improved in 1 patient, 25%) showed improvement in <80% of affected patients.
Table 2.
Signs and Symptoms Before and After Shunt Placement
| Before Shunt Placement | Resolved After Shunt Placement | Median time after surgery (wks) | |
|---|---|---|---|
| Signs or Symptoms | |||
| Headache | 30 (52%) | 24 (80%) | 1.57 |
| Nausea and vomiting | 18 (31%) | 17 (94%) | 1.21 |
| Altered mental status | 16 (28%) | 14 (88%) | 2.01 |
| Gait abnormality | 13 (22%) | 4 (31%) | 1.64 |
| Urinary incontinence | 4 (7%) | 1 (25%) | 2.24 |
| Papilledema | 2 (3%) | 2 (100%) | 1.42 |
Resolution based on 1st post-operative visit.
With regard to postplacement use of the on-off valve VPS/Ommaya system, 24 patients (41%) received no IT treatments; 8 patients (14%) received one or two IT treatments; and 26 patients (45%) received three or more IT treatments (45%). The IT regimens were patient specific based on pathology and provider preferences and/or protocols. In general, patients with melanoma received IT Interleukin-2 (IL-2), those with breast and other etiologies had IT regimens with various combinations or cycles/sequences of topotecan, cytarabine, and methotrexate. No immunotherapy checkpoint inhibitors were given by IT. Radiotherapy was utilized primarily postoperatively, but at a similar frequency in those patients that received no IT treatments and those that received three or more IT treatments (Table 1). Most patients in both groups did not have progressive systemic disease at the time of the procedure (Table 1). The median age at cancer diagnosis was similar between the two groups of no treatment vs. three or more treatments, as was the presence of ventriculomegaly on preoperative MRI, median opening pressure, and the presence of malignant cells in the CSF. Although median survival time from the original cancer diagnosis was not prolonged in patients who received ≥ 3 treatments vs. those who received none, receipt of ≥ 3 treatments was significantly associated with median survival after LMD diagnosis and after VPS/Ommaya insertion, with treated patients experiencing a survival advantage over untreated patients (Table 1).
Surgical complications occurred in 12 patients (14 events) (Table 3), with the most common complications being infection (6.9%), subdural or intraventricular hematoma (5.2%), and shunt failure (5.2%). Of the two patients who experienced peritoneal seeding, one had melanoma, did not receive IT chemotherapy, and lived for 15.7 months after the shunt placement; the other had breast carcinoma, received 25 IT chemotherapy sessions, and survived for 10.1 months after shunt placement. Neither patient with periotoneal seeding was symptomatic nor did either require additional interventions. One patient died from intracranial hypertension exacerbated by prolonged occlusion of the shunt valve during administration of IT therapy at 2 months after shunt insertion.
Table 3.
Complications after Placement of a Ventriculoperitoneal Shunt with an Ommaya Reservoir and an On-Off Valve
| Complications | No. (%) |
|---|---|
| Subdural/intraventricular hematoma | 3 (5.2%) |
| Infection (meningitis) | 4 (6.9%) |
| Shunt failure (requiring reoperation) | 3 (5.2%) |
| Peritoneal seeding | 2 (3.4%) |
| CSF leak (incision) | 1 (1.7%) |
| Death (unopened valve) | 1 (1.7%) |
CSF, cerebrospinal fluid.
Discussion
This study reports the largest series of LMD patients with shunt systems consisting of a combined VPS plus Ommaya reservoir system with a mechanical on–off valve. We showed that this valve system allowed ongoing IT treatments while relieving symptoms of elevated ICP, thereby improving patients’ quality of life by sparing them multiple lumbar punctures, and perhaps improving survival by allowing ongoing IT therapy. We found significantly longer survival from the date of LMD diagnosis and from the time at which the procedure was performed for patients who underwent VPS placement and had more than three subsequent IT therapy sessions.
A previous report of 37 patients showed that use of VPS for LMD-related hydrocephalus resulted in improvement of symptoms in 77% of patients despite a relatively short median survival time of two months after shunt placement.17 In a study of hydrocephalus in patients with brain metastasis (80% of whom had LMD), Lee et al. correlated ventricular opening pressure with cumulative survival, and found that an opening pressure of > 30 cm of water was an independent risk factor for shorter survival.12 Another study by Jung et al showed that patients with surgically untreated LMD-related hydrocephalus had poorer overall survival than did those with surgically treated hydrocephalus.21 The overall survival time from LMD diagnosis for the 24 patients in our study who did not receive IT treatment (3.8 ± 0.7 months) was consistent with the 5.7-month survival time reported by Jung et al.21 Another smaller study of patients showed that survival after LMD-related hydrocephalus from lung adenocarcinoma was 4.5 months after placement of a VPS.22 Our experience echoes these earlier results indicating that CSF diversion for hydrocephalus is effective for relieving symptoms and improving quality of life while also possibly contributing meaningfully to prolonged survival for patients with LMD.23
A major concern for using a VP shunt in patients with LMD is dissemination of disease to the peritoneum. Reported rates of intra-abdominal metastasis secondary to diversion of CSF into the abdominal cavity have ranged from 1% to 27.3%.24–28 However, these reports involved patients with solid tumors in brain parenchyma (or intracranial germinoma, which can also cause LMD) rather than specifically LMD from brain metastasis.24–28 The incidence of peritoneal carcinomatosis in LMD has been difficult to ascertain and has been described largely in anecdotal case reports.17,24,29 In our study, surgical site complications were modest overall, and peritoneal seeding was seen in only 2 patients (3.4%).
That ongoing IT treatments can confer survival benefit has been documented by others, and when such therapy is feasible it should be initiated as soon as possible.1,4,15 The median survival time of patients after diagnosis of LMD is 4–6 months.4,15 However, in our study, patients who received three or more IT treatments had a median survival time of 17.9 ± 8.9 months from the time of LMD diagnosis. This difference in survival cannot be explained by a preponderance of a particular type of primary tumor, because our study had similar proportions of patients with the most common culprits provoking LMD, that is, melanoma and breast carcinoma. Other possible confounding factors, such as KPS and time to diagnosis or to treatment, were also similar in these two treatment groups. Although patients with type 1 LMD live longer than those with type 2, most of the patients in both groups had type 1 LMD, so the survival advantage cannot be ascribed to differences in LMD type. Even so, our patients who underwent three or more IT treatments may have had other unrecognized features that predisposed them to longer survival times: they may have had a smaller burden of systemic disease or a lower overall burden of LMD, and may have been more physically robust, than those who received no IT treatments. For that reason, a true cause-and-effect link between IT therapy and increased survival cannot be rigorously established from our data. Among patients in our cohort who received the on–off valve VPS/Ommaya system but did not undergo IT therapy, the survival time of ~4 months was similar to historical reports of “treated” LMD.30
We acknowledge that comparisons among studies such as these are problematic, for several reasons. First, the options for IT treatment have changed over time. Also, we were unable to reliably determine why some patients in this retrospective review received the Ommaya reservoir but did not undergo IT therapy; possibilities include non-random distribution of factors affecting survival, including but not limited to tumor subtypes that were more amenable to IT therapy; effective vs. ineffective therapies; selection of patients that were in better condition (KPS was similar between groups but may not be true reflection of condition) or differences in systemic disease burden between the two groups being compared. Notably, the apparent survival benefit suggested by our findings cannot be statistically ascribed to a specific underlying cause, given the small number of patients with varied clinical histories (eg, different tumor types, modes of IT therapy, and interventions [shunt vs. IT therapy]); we did not attempt such an analysis. Our finding of survival benefit for patients who got a shunt and more than two IT therapy sessions (vs. those who only received a shunt without subsequent IT therapy) should provide a starting point for future prospective studies that include multivariable analysis to dissect factors that influence the outcomes after IT therapy for LMD from specific types of tumors. Nevertheless, our findings do suggest that the value of managing hydrocephalus to reduce morbidity in patients with LMD should not be underestimated, and they further suggest that using a reversible occlusion shunt system such the one described here can facilitate ongoing IT treatments that have positive therapeutic benefit while also treating the underlying hydrocephalus, control of which improves the patients’ quality of life.
Despite the clear benefits of this system, management of the VPS/reservoir/on–off valve system requires clear communication among the patient, the patient’s caregivers, and medical staff. First, because the valve can be inadvertently turned off, patients and caregivers should be educated as to how to turn the valve on and off. Second, the positioning of the valve is critical, in that it must not be occluded when the patient lies on his or her back or side. Third, our experience with the one patient who died from intracranial hypertension after VPS valve occlusion leads us to recommend that the maximum time that a valve should be left in the “off” position, for IT treatments or other related procedures, is six hours. This patient was considered highly “shunt-dependent,” and became comatose with tonsillar herniation nine hours after the shunt had been closed. Because none of the patients in our cohort who received IT treatment showed neurological decline when the shunt was occluded for less than six hours, our current policy is that all on–off valves must be reopened within six hours of shunt occlusion. Fourth, medical practitioners and caregivers also require education to avoid confusion on valve management, and specific training about the procedures for closing and opening the valve. Finally, oncologists must be educated in best practices for the use of these shunts; many are not familiar with shunt systems such as these that can prolong survival by enabling IT therapy and control symptoms of elevated ICP. Education on this topic will aid in patient selection and may reduce the proportion of patients for whom Ommaya/shunt placement is requested but IT chemotherapy is not used. In some such patients, rapid progression of either LMD or systemic disease can prompt a shift from active to palliative care, but in others the lack of use of the Ommaya system after its placement may reflect a lack of clarity in plans for patient care.
We recognize that this study had several limitations, chief among them its retrospective nature and its inclusion of patients treated over the course of 25 years. Treatments for LMD and systemic solid tumors have changed during that time, as has our ability to detect LMD at earlier stages. IT treatment agents and regimens can vary by the prescribing oncologist or be based on previously attempted regimens, factors that may have different effects on survival benefits across treatment centers. Also, although in this study most patients were treated by the same neurosurgeon [I.M.], any surgical procedure requires a learning curve for optimal placement and avoidance of complications, and patient selection will vary among neurosurgeons performing this procedure.
Conclusions
In this retrospective analysis, administration of IT chemotherapy via a VPS/Ommaya reservoir system with an on–off valve was associated with significant survival benefit among patients given three or more IT treatments for LMD associated with hydrocephalus. These valve systems can relieve symptoms of elevated ICP and allow meaningful administration of IT therapy without compromising ICP control. In our hands, the incidence of complications associated with placement of this system was modest. We believe that such systems are underused in clinical oncological practice and should be studied prospectively in a large patient population to validate our findings ideally in concert with clinical trials (eg, NCT04588545, NCT03025256). In addition, the impact of this system in the advent of immunotherapy and more targeted therapy should be evaluated. However, any prospective trial should evaluate quality of life metrics as prolongation of life in the setting of LMD can be accompanied by severe neurological symptoms in some patients. However, collectively these results hold promise for treating LMD in patients with associated hydrocephalus.
Contributor Information
Kristin M Huntoon, Department of Neurosurgery, The University of Texas M D Anderson Cancer Center, Houston, Texas, USA.
Jaime Gasco, Department of Neurosurgery, University Medical Center of El Paso, El Paso, Texas, USA.
Isabella C Glitza Oliva, Department of Melanoma Medical Oncology, The University of Texas M D Anderson Cancer Center, Houston, Texas, USA.
Sherise D Ferguson, Department of Neurosurgery, The University of Texas M D Anderson Cancer Center, Houston, Texas, USA.
Nazarin K Majd, Department of Neuro-Oncology, The University of Texas M D Anderson Cancer Center, Houston, Texas, USA.
Ian E McCutcheon, Department of Neurosurgery, The University of Texas M D Anderson Cancer Center, Houston, Texas, USA.
Funding
Supported in part by Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center (PI: PW Pisters).
Conflict of Interest:
The authors have no relevant financial or non-financial interests to disclose.
Parts of this work were presented at the Society of University Neurosurgeons Annual Meeting, Prague, Czech Republic, June 29th-July 3, 2022.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Kristin Huntoon, and Jaime Gasco. The first draft of this manuscript was written by Kristin Huntoon and Ian McCutcheon, and all authors provided comments on the manuscript and read and approved the final version.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
Institutional IRB approval was granted for this retrospective study.
References
- 1. Grossman SA, Krabak MJ.. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25(2):103–119. [DOI] [PubMed] [Google Scholar]
- 2. Balm M, Hammack J.. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996;53(7):626–632. [DOI] [PubMed] [Google Scholar]
- 3. Sener U, Kumthekar P, Boire A.. Advances in the diagnosis, evaluation, and management of leptomeningeal disease. Neuro-Oncology Adv. 2021;3(Supplement_5):v86–v95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clarke J, Perez H, Jacks L, Panageas K, Deangelis L.. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suto C, Oohira A, Funaki C, Kanno S, Mori Y.. Pathological findings of optic neuropathy from metastatic leptomeningeal carcinomatosis. Jpn J Ophthalmol. 2007;51(5):396–398. [DOI] [PubMed] [Google Scholar]
- 6. Jeffs GJ, Lee GY, Wong GT.. Leptomeningeal carcinomatosis: an unusual cause of sudden onset bilateral sensorineural hearing loss. J Clin Neurosci. 2006;13(1):116–118. [DOI] [PubMed] [Google Scholar]
- 7. Habek M, Petravic D, Gjadrov-Kuvezdic K, Mahovic Lakusic D.. Leptomeningeal carcinomatosis: cerebrospinal fluid findings. Arch Neurol. 2006;63(6):910. [DOI] [PubMed] [Google Scholar]
- 8. Park JS, van den Noort S, Kim RC, Walot I, Licht H.. Primary diffuse leptomeningeal gliomatosis with signs of increased intracranial pressure and progressive meningeal enhancement on MRI. J Neuroimaging. 1996;6(4):250–254. [DOI] [PubMed] [Google Scholar]
- 9. Hegde U, Filie A, Little RF, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105(2):496–502. [DOI] [PubMed] [Google Scholar]
- 10. Wilcox JA, Li MJ, Boire AA.. Leptomeningeal metastases: new opportunities in the modern era. Neurotherapeutics. 2022;19(6):1782–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le Rhun E, Devos P, Weller J, et al. Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. Neuro-oncology. 2021;23(7):1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee SH, Kong DS, Seol HJ, Nam D-H, Lee J-I.. Ventriculoperitoneal shunt for hydrocephalus caused by central nervous system metastasis. J Neurooncol. 2011;104(2):545–551. [DOI] [PubMed] [Google Scholar]
- 13. Chamberlain MC. Radioisotope CSF flow studies in leptomeningeal metastases. J Neurooncol. 1998;38(2-3):135–140. [DOI] [PubMed] [Google Scholar]
- 14. Chamberlain MC, Kormanik PA.. Prognostic significance of 111indium-DTPA CSF flow studies in leptomeningeal metastases. Neurology. 1996;46(6):1674–1677. [DOI] [PubMed] [Google Scholar]
- 15. Eltobgy M, Huntoon K, Musgrave N, et al. Impact of cerebrospinal fluid flow study in patients undergoing intrathecal chemotherapy via ventricular catheter reservoir. J Neurooncol. 2021;153(1):161–167. [DOI] [PubMed] [Google Scholar]
- 16. Chamberlain MC, Glantz M.. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2006;66(5):783; author reply 783. [DOI] [PubMed] [Google Scholar]
- 17. Omuro AM, Lallana EC, Bilsky MH, DeAngelis LM.. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2005;64(9):1625–1627. [DOI] [PubMed] [Google Scholar]
- 18. Czech T, Reinprecht A, Dietrich W, Hainfellner JA, Slavc I.. Reversible occlusion shunt for intraventricular chemotherapy in shunt-dependent brain tumor patients. Pediatr Hematol Oncol. 1997;14(4):375–380. [DOI] [PubMed] [Google Scholar]
- 19. Portnoy HD, Schulte RR, Fox JL, Croissant PD, Tripp L.. Anti-siphon and reversible occlusion valves for shunting in hydrocephalus and preventing post-shunt subdural hematomas. J Neurosurg. 1973;38(6):729–738. [DOI] [PubMed] [Google Scholar]
- 20. Sandberg DI, Bilsky MH, Souweidane MM, Bzdil J, Gutin PH.. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery. 2000;47(1):49–54; discussion 54–54. [DOI] [PubMed] [Google Scholar]
- 21. Jung T-Y, Chung W-K, Oh I-J.. The prognostic significance of surgically treated hydrocephalus in leptomeningeal metastases. Clin Neurol Neurosurg. 2014;119:80–83. [DOI] [PubMed] [Google Scholar]
- 22. Mitsuya K, Nakasu Y, Hayashi N, et al. Palliative cerebrospinal fluid shunting for leptomeningeal metastasis-related hydrocephalus in patients with lung adenocarcinoma: a single-center retrospective study. PLoS One. 2019;14(1):e0210074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murakami Y, Ichikawa M, Bakhit M, et al. Palliative shunt surgery for patients with leptomeningeal metastasis. Clin Neurol Neurosurg. 2018;168:175–178. [DOI] [PubMed] [Google Scholar]
- 24. Magtibay PM, Friedman JA, Rao RD, Buckner JC, Cliby WA.. Unusual presentation of adult metastatic peritoneal medulloblastoma associated with a ventriculoperitoneal shunt: a case study and review of the literature. Neuro-oncology. 2003;5(3):217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barajas RF Jr, Phelps A, Foster HC, et al. Metastatic diffuse intrinsic pontine glioma to the peritoneal cavity via ventriculoperitoneal shunt: case report and literature review. J Neurol Surg Rep. 2015;76(01):e91–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Back M, Hu B, Rutgers J, French S, Moore T.. Metastasis of an intracranial germinoma through a ventriculoperitoneal shunt: recurrence as a yolk-sac tumor. Pediatr Surg Int. 1997;12(1):24–27. [DOI] [PubMed] [Google Scholar]
- 27. Haimovic IC, Sharer L, Hyman RA, Beresford HR.. Metastasis of intracranial germinoma through a ventriculoperitoneal shunt. Cancer. 1981;48(4):1033–1036. [DOI] [PubMed] [Google Scholar]
- 28. Murray MJ, Metayer LE, Mallucci CL, et al. Intra-abdominal metastasis of an intracranial germinoma via ventriculo-peritoneal shunt in a 13-year-old female. Br J Neurosurg. 2011;25(6):747–749. [DOI] [PubMed] [Google Scholar]
- 29. Lee MH, Lee J-I.. Malignant ascites after subduroperitoneal shunt in a patient with leptomeningeal metastasis. J Korean Neurosurg Soc. 2011;50(4):385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buszek SM, Chung C.. Radiotherapy in leptomeningeal disease: a systematic review of randomized and non-randomized trials. Front Oncol. 2019;9:1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


