Abstract
Background
While exercise training (ET) programs show positive outcomes in cognition, motor function, and physical fitness in pediatric brain tumor (PBT) survivors, little is known about the optimal timing of intervention. The aim of this work was to explore the feasibility and benefits of ET based on its timing after radiotherapy.
Methods
This retrospective analysis (ClinicalTrials.gov, NCT01944761) analyzed data based on the timing of PBT survivors' participation in an ET program relative to their completion of radiotherapy: <2 years (n = 9), 2–5 years (n = 10), and > 5 years (n = 13). We used repeated measures analysis of variance to compare feasibility and efficacy indicators among groups, as well as correlation analysis between ET program timing postradiotherapy and preliminary treatment effects on cognition, motor function and physical fitness outcomes.
Results
Two to five years postradiotherapy was the optimal time period in terms of adherence (88.5%), retention (100%), and satisfaction (more fun, more enjoyable and recommend it more to other children). However, the benefits of ET program on memory recognition (r = −0.379, P = .047) and accuracy (r = −0.430, P = .032) decreased with increased time postradiotherapy. Motor function improved in all groups, with greater improvements in bilateral coordination (P = .043) earlier postradiotherapy, and in running (P = .043) later postradiotherapy. The greatest improvement in pro-rated work rate occurred in the < 2-year group (P = .008).
Conclusion
Participation in an ET program should be offered as part of routine postradiotherapy care in the first 1–2 years and strongly encouraged in the first 5 years.
Keywords: brain recovery, cranial radiation, exercise, neuroplasticity, pediatric brain tumor
While advances in pediatric neuro-oncology improved survival rates in a large proportion of children with pediatric brain tumor (PBT),1 concerns about long-term physical, neurological, cognitive, and psychosocial sequelae have becoming increasingly prominent over the past 20 years.2 To limit these side-effects, two main research areas have been explored: 1) restrict the damage caused by the treatments by limiting their toxicity to the healthy brain structures, and 2) develop therapies to enhance brain self-repair and recovery. Over the past 10 years, clinical trials in animals and humans have confirmed the feasibility and efficacy of such interventions after PBT.3 Among them, exercise training (ET) programs have demonstrated positive effects after pediatric cancer4 and PBT.5–8 ET is as an efficient neuro-rehabilitation intervention as it allows for improvements in both cognitive and motor function, and these improvements are linked with structural and functional changes within the brain.9 However, the timing of the ET program relative to treatment completion has been highly variable, which could affect program feasibility and its impact on brain neuroplasticity.
On one hand, early intervention may be required to minimize the toxicity of radiotherapy on brain structures and its evolving impact on cognition. Early brain changes induced by radiotherapy include a decline in oligodendrocytes, microvascular damage, subtle white matter compromise, neuroinflammation, and disturbances of neuronal micro-morphophysiology. These changes can interact and progressively alter neuronal stem cell niches to impede neuronal function, viability, and progenitor cell differentiation leading to progressive long-term brain changes.10 In children treated with focal and/or craniospinal irradiation (CSI), these changes lead to a progressive decline in cognitive function, and corresponds with decreased learning of new skills.11–13 Some studies suggest that the trajectory of this decline is not linear but more pronounced in the three years following diagnosis, followed by a slower rate of decline.14,15 In their literature review, Bernal et al. (2022) found that studies involving participants with longer time from cancer treatment had significantly smaller improvements in cognitive function.4
However, commencing an ET program too soon after completion of cancer treatment may not necessarily be better. Radiotherapy-induced transient neuroinflammation10 in the first four to six months following cancer treatment can be accompanied by a “pseudo-progression” in MRI images with clinical symptoms of headache, fatigue, nausea/vomiting, and sensitivity to corticosteroid treatment.16 Recent research in adults with stroke suggests that neuroinflammation could be deleterious to brain plasticity and challenges the idea that rehabilitation is all the more effective if it is done early.17 Moreover, adherence to an ET program will be particularly challenging in this early period due to the postoperative recovery period and side effects associated with adjuvant chemotherapy (eg, pancytopenia, nausea, vomiting, asthenia), and the need for intensive medical follow-up.18,19
Given these different factors, it is crucial to understand the optimal timing of an ET program following diagnosis and treatment for PBT. To date, limited work has been conducted in this area. As such, we performed a retrospective analysis of published clinical trial data from a 12-week ET program for PBT survivors,5–8 to examine the optimal timing of ET program participation postradiotherapy as measured by feasibility and impact on neurocognition, motor function, and physical fitness. We divided participant data into three groups depending on the timing of their participation in the ET program relative to the completion of irradiation (less than 2 years (<2y), two to five years (2–5 y), and greater than 5 years (>5 y)) to examine the impact of time dependent factors on neural function and cognitive recovery. The chosen timeframe for each subgroup was based on: 1) neuropsychological data suggesting an early cognitive decline after radiotherapy, 2) the decreasing intensity and frequency of medical follow-up with time (ie, MRI and medical exam quarterly in the first 2 years post-treatment, 2-3 times a year in the third to fifth year, and then annual MRIs and medical exams thereafter until 10 years post-treatment), and 3) the decreasing risk of relapse with increased time since the end of treatment. We examined ET program participation, adherence, retention, and satisfaction by subgroup to determine if timing of the ET program influenced its feasibility. Then, using both a group comparison approach and correlation analysis, we explored how the benefits of an ET program varied with time postradiotherapy and age at ET program completion. We hypothesized that the 2–5 y period postradiotherapy would be optimal in terms of feasibility, and motor and cognitive improvements induced by ET would decrease with increased time postradiotherapy. No study has previously explored neuroplasticity in the early postradiotherapy period, during which (effects such as) neuro-inflammation may occur. Based on interventional trials in adults with stroke, we hypothesize that the neuroplasticity in the first year postradiotherapy could be altered by radiotherapy-induced neuroinflammation.
Materials and Methods
Participants
This retrospective analysis involved children 7–18 years old, one to ten years after PBT diagnosis, in remission, treated with focal radiotherapy or CSI who participated in the ET program interventional trial (ClinicalTrials.gov, NCT01944761). Participants were recruited from the Hospital for Sick Children (Toronto, Canada) and McMaster Children’s Hospital (Hamilton, Canada). The institutional review boards at each site approved the study protocol. Either written informed consent or assent and parental consent (where applicable) was obtained.
To examine the effects of ET program timing, we categorized participants into three groups based on the time between completion of radiotherapy and commencement of the ET program: <2 y (n = 9), 2–5 y (n = 10), and > 5 y (n = 13). The timing postradiotherapy was used instead of the time post-treatment due to the well-known radiotherapy associated preponderant impact on the brain.
Procedure
The study used a crossover design in which participants were randomized to either: (1) start the ET program without delay (ie, the intervention group) or (2) wait 12 weeks before starting the ET program (ie, the control group). Participants were recruited through the neuro-oncology clinic at any stage of recovery as long as they were in remission. Assessments were performed at baseline (pretraining), post-training and after 12 weeks in the intervention group, and at baseline (ie, before the delay period), pre- and post-training in the control group. Each assessment included an evaluation of cognitive, motor, and fitness function. The methods and procedures have been previously described.5–8 The goal of the ET program was to increase and maintain each child’s heart rate at 80% of their maximum heart rate achieved during baseline fitness testing, for at least 30 min per session. In this work, we explored the pre- and post-training assessments regardless of group allocation.
Assessment of feasibility
The ET program study was evaluated using several feasibility indicators: recruitment, retention, adherence, and satisfaction.20 While recruitment, retention and adherence rate was previously published for the entire sample,5 we re-analyzed these data. For eligible children who did not enroll in the study, we recorded the time between completion of radiotherapy and when they were approached to participate in the study. Then, we allocated them to time postradiotherapy groups (<2 y, 2–5 y or > 5 y). For each group, the recruitment rate was calculated as the percentage of children who enrolled in the study out of all eligible children within the same period postradiotherapy. Reasons that eligible children declined to participate were descriptively analyzed by group. For each group, the retention rate was calculated as the percentage of participants who completed the ET program (including postintervention assessment) and the adherence rate was the percentage of exercise sessions completed by each participant out of the total number of sessions.
Unpublished data, from a study-specific questionnaire completed by parents and children at the end of the ET program were used to evaluate satisfaction. Each item on this 47-item questionnaire was rated on a seven-point Likert scale and explored several aspects of satisfaction: the burden of completing the fitness tests, the MRI scans, the psychological testing, and the questionnaires; feelings about participating in the study; the effect of the ET program on quality of life, physical fitness, fatigue, happiness, sleep quality, depressed feelings, anxious feelings, stress, body weight or shape, and illness or injury; the potential barriers; and the support received. Internal consistency, a measure of questionnaire reliability, was assessed using Cronbach’s α. We analyzed these questionnaires in two ways, first comparing item responses within each postradiotherapy time group, then performing profile analyses based on each responder’s answers for all questionnaire items and comparing the distribution of profile between groups.
Assessment of cognition, motor function and fitness
We selected the assessments for which the first analyses had shown a benefit of ET program at the level of all participants.5–8 We re-analyzed these data for each postradiotherapy time group to determine whether these benefits were greater for a specific time postradiotherapy. Because radiotherapy has affects the hippocampus and episodic memory outcomes, we also analyzed previously unpublished data for an episodic memory assessment.
The Cambridge Neuropsychological Test Automated Battery (Third Edition) (CANTAB) was used to assess verbal anterograde memory, and obtain a composite score for mean reaction times and accuracy.21 Mean reaction time and accuracy were averaged across the subtests measuring attention (Rapid Visual Information Processing, Match to Sample Visual Search), processing speed (Simple Reaction Time, Choice Reaction Time), and short-term memory (Delayed Matching to Sample) as reported in a previous study.5 We analyzed previously unpublished data from the Verbal Recognition Memory (VRM) test that assesses immediate and delayed memory of verbal information under free recall and forced choice recognition conditions. The gross motor subtests of the Bruininks Oseretsky Test of Motor Performance (2nd Edition) (BOT-2) were used to assess motor function: bilateral coordination, balance, strength, and running speed and agility as reported in a previous study.6,22 Scaled scores were calculated for each subtest (mean Z 15, SD Z 5).
Physical fitness was assessed using the Six-Minute Walk Test (6MWT) and pro-rated work rate as previouslyreported.6,23 The 6MWT is the distance walked (in meters) in 6 min. The pro-rated work rate (the power output data from an electrically braked cycle ergometer) was used as an estimation of physical fitness and was calculated in two-minute increments using the McMaster All-out Protocol [2nd to last work rate þ ((time at last work rate in seconds/120 s) * increment in work rate), in watts].24
Statistical analyses
Data were analyzed using JAMOVI (1.6.15.0) software.25 Feasibility and pre- and post-training outcome variables are compared as a function of group (ie, time postradiotherapy). To address variation with time, we performed correlation analyses between the difference in pre- and post-training performance, reflecting the impact of ET program, and time postradiotherapy. As participant age differed within groups, we explored the potential impact of age in outcomes by the mean of correlation analysis.
For population characteristics, adherence rates, and satisfaction questionnaire scores, we performed comparisons between groups using non-parametric Kruskal Wallis analysis of variance (ANOVA). A P-value of < .05 indicated statistical significance. If the ANOVA was significant, post hoc analysis was conducted using Dwass–Steel–Critchlow–Fligner pairwise comparisons.
For the satisfaction analysis, we also used a cluster analysis to identify profiles of responders’ satisfaction using K-means analysis. The optimal number of clusters was determined on an elbow diagram. We then identified the number of satisfaction profiles in each group and compared them by the mean of nonparametric ANOVA.
To investigate the impact of time postradiotherapy on the benefit of ET program, we performed 1) repeated measures (RM) ANOVA using groups as the between participants factor, and pre- and post-training behavioral scores as the repeated measure factor, and 2) Pearson or Spearman correlations (depending on the normality of data distribution) for the difference in pre- and post-training scores between a) the number of years postradiotherapy and b) participant age at commencement of the ET program. Supplementary analyses were conducted in medulloblastoma patients to explore the specific impact of ET in a more homogeneous group. To explore the specific impact of ET in a homogeneous subgroup, a Mann–Whitney U (MW) test compared no-training (ie, control) and training treatment arms in the first period of the crossover study among medulloblastoma patients.
Results
Population
All children involved in the trial received surgery and radiotherapy as treatment, sometimes followed by chemotherapy (eg, medulloblastoma). Nine children enrolled in the ET program were < 2 y (from 11 months to 23 months), 10 were 2–5 y, and 13 were > 5 y (from 5 to 10 years and 2 months) postradiotherapy. Children in the < 2 y and 2–5-y postradiotherapy groups were significantly younger than children who participated after 5 years postradiotherapy (P = .007) and tended to have more infratentorial tumors (P = .058). The body mass index (BMI) tended to be lower in the < 2 y postradiotherapy group (P = .095). Subgroup characteristics are reported in Table 1.
Table 1.
Characteristics of Participants Depending on the Delay from the End of Radiotherapy.
| Delay from end of radiotherapy (Number of participants) |
<2y (n = 9) |
2–5y (n = 10) | >5 y (n = 13) | P-value | |
|---|---|---|---|---|---|
| Participant characteristics | |||||
| Age at baseline assessment (years) .007 | |||||
| Mean | 9.72 | 10.65 | 13.65 | ||
| Standard deviation | 1.38 | 2.87 | 2.82 | ||
| Range | 7.5–11.8 | 7.49–17.0 | 9.0–16.9 | ||
| Sex (male) | 5 (55.6%) | 4 (40.0%) | 9 (69.2%) | .386 | |
| Handedness (right) | 7 (100%) | 9 (90%) | 12 (92%) | .713 | |
| Number of years of education—Mother .241 | |||||
| Mean | 13.8 | 16.2 | 15.0 | ||
| Standard deviation | 1.92 | 2.49 | 2.16 | ||
| Range | 12–17 | 14–20 | 12–18 | ||
| Number of years of education—Father .238 | |||||
| Mean | 15.6 | 16.6 | 13.57 | ||
| Standard deviation | 2.70 | 2.07 | 2.64 | ||
| Range | 11–18 | 15–20 | 10–17 | ||
| Tumor and treatment | |||||
| Age at diagnosis .180 | |||||
| Mean | 7.18 | 6.30 | 5.44 | ||
| Standard deviation | 2.72 | 1.99 | 2.22 | ||
| Range | 2.25–10.67 | 3.0–9.17 | 1.92–9.33 | ||
| Tumor type .237 | |||||
| Medulloblastoma | 7 | 7 | 6 | ||
| Ependymoma | 2 | 1 | 3 | ||
| High Grade Glioma | 0 | 0 | 2 | ||
| Germ cell tumor | 0 | 1 | 1 | ||
| Pineoblastoma | 0 | 1 | 0 | ||
| Sarcoma | 0 | 0 | 1 | ||
| Tumor location .058 | |||||
| Infratentorial | 9 | 8 | 8 | ||
| Supratentorial | 0 | 1 | 5 | ||
| Chemotherapy | 8 | 9 | 11 | .920 | |
| Hydrocephalus .631 | |||||
| With no treatment | 3 | 1 | 6 | ||
| Requiring CSF diversion | 3 | 6 | 3 | ||
| Radiation type .490 | |||||
| Focal | 1 | 2 | 6 | ||
| Cranio-spinal 23.4 Gy Cranio-spinal 36.0 Gy |
2 5 |
2 5 |
2 5 |
||
| Number of surgeries .853 | |||||
| 1 surgery | 6 | 6 | 8 | ||
| 2 surgeries | 1 | 2 | 5 | ||
| 3 surgeries | 1 | 1 | 0 | ||
| Relapse | 2 | 1 | 1 | ||
| Exercise level before training | |||||
| Exercise activity before trial .509 | |||||
| Everyday | 1 | 1 | 0 | ||
| More than 3 times a week | 2 | 2 | 5 | ||
| Less than 3 times a week | 2 | 1 | 4 | ||
| Sedentary | 0 | 3 | 0 | ||
| BMI (z-score) .095 | |||||
| Mean | −0.164 | 0.885 | 0.929 | ||
| Standard deviation | 1.23 | 1.01 | 0.868 | ||
| Range | −2.07 to 1.69 | −1.09 to 2.24 | −0.290 to .56 | ||
| Intervention | |||||
| Intervention or control group .273 | |||||
| Intervention | 3 | 7 | 6 | ||
| Control | 6 | 3 | 7 | ||
| Group training or combined training .810 | |||||
| Group training | 2 | 5 | 5 | ||
| Combined training | 4 | 5 | 7 | ||
Feasibility
Recruitment
Medical data were available for 47 (67%) of children who did not enroll in the study (ie, nonparticipants) and were assigned to the appropriate time postradiotherapy group: 11 were in the < 2 y group, 19 were in the 2–5 y group, and 17 were in the > 5 y group. Recruitment rate by group (45, 39, and 43%, respectively) did not differ (P = .698).
Then we analyzed the reason provided for nonparticipation by group. Twenty-two children and their families (n<2y = 5, n2–5y = 11, and n>5y = 6) provided reasons for nonparticipation (several reasons could be reported for one family). Reasons reported in the < 2 y group were: fear of missing school (n = 2, 40%), lack of time (n = 2, 40%), did not wish to participate in research (n = 2, 40%), and too many study visits (n = 1, 20%). In the 2–5 y group, travel distance was reported as primary reason for nonparticipation (n = 9, 82%), followed by fear of missing school (n = 1, 9%), lack of time (n = 1, 9%) and too many study visits (n = 1, 9%)). In the > 5 y group, the reasons for nonparticipation were: travel distance (n = 5, 83%), lack of time (n = 3, 50%), and fear of missing school (n = 1, 17%).
Retention
Retention (ie, the percentage of children who enrolled and completed the study) differed between groups. Only six (66%) children in the < 2 y group completed the study. One child with anaplastic medulloblastoma stopped the ET program because of a relapse in the first year postradiotherapy, and two more children with medulloblastoma withdrew before the ET program started (one 7-year old girl who was 1.6 years postradiotherapy and one 10-year-old boy who was 1.4 years postradiotherapy). All children (n = 10, 100%) in the 2–5 y group completed the study. Twelve of the 13 (92%) children in the > 5 y group completed the study. One boy (16-year-old, treated for medulloblastoma, 7.6 years postradiotherapy) withdrew just after the pretraining assessment and had expressed decreased motivation to participate in the study in a survey.
Adherence
The mean adherence rate did not statistically differ between groups (P = 0.859). The < 2 y group had a mean adherence rate of 77% (SD: 29.1; R: 19.1–93.8), that increased to 88.5% after exclusion of one participant who had poor adherence due to various problems including car breakdown and viral infections. Adherence rates were 88.5% (SD: 7.1; R: 76.6–97.0) in the 2–5 y group and 85.6% (SD: 13.3; R: 59.6–100) in the > 5 y group.
Satisfaction
Questionnaire reliability
Internal reliability of the whole questionnaire was close to 0.90, an indication of excellence, with a Cronbach’s α = 0.896.
Items answer by group
Nonparametric ANOVA indicated that between groups, responses differed significantly for how useful the child felt the ET program was (P = .048) and whether they would recommend it to other childhood brain tumor survivors (P = .033). The 2–5 y group recommended it more than the > 5y group (post hoc P = .031). The 2–5 y group found the ET program more enjoyable (P = .030) than the < 2 y group (post hoc P = .043) and the > 5 y group (post hoc P = .030), and more fun than the > 5 y group (post hoc P = .045). Participants in the < 2y group reported significantly more improvement on physical fitness (P = .002) than participants in the > 5 y group. Finally, there was a trend towards a significant group difference in the support provided by family members (P = .062), with a greater support for children in 2-5 y group. Satisfaction responses in the questionnaires were reported in Figure 1.
Figure 1.

Satisfaction questionnaire: Details of responses by item and by groups. This bar chart shows the frequency of responses to the Likert items on the satisfaction questionnaire, which measures respondents’ levels of agreement (right part, green shading) or disagreement (left part, red shading) with each item. ET program: exercise training program. * significant difference between group (P < .05).
Profile of questionnaire answer by group
Using K-means analysis, we individualized three profiles of responders. Two children of the 2–5 y group were excluded from this analysis because they did not answer all of the questions. Profiles 1 and 2 had a high level of satisfaction while Profile 3 was slightly satisfied. Compared to Profile 1, Profile 2 had more improvement in happiness, sleep quality, depressed and anxious feelings, and stress.
Participants from all three groups (ie, time postradiation) were classified as Profile 1 or Profile 2. Only participants from > 5 y (4/12) were classified as Profile 3. Profile 1 was predominantly represented by the < 2 y and 2–5 y groups (5/6 and 5/8, respectively), followed by > 5y (3/12). Profile 2 was predominantly > 5y (5/12), followed by 2–5y (3/8), and < 2y (1/6). ANOVA analysis showed a significant difference in profile of satisfaction between groups (P = .025) (see Supplementary Data).
Time postradiotherapy impacts ET program effectiveness
Results before and after ET program in each group in cognition, motor function, and physical fitness are reported in Table 2. Although there was no significant difference between the groups on pretraining or post-training performances, children in the < 2 y group performed worse in pretraining than the others on all measures except memory but performed better than the others in post-training on recognition score of memory, strength, and physical fitness as estimated by pro-rated work rate score.
Table 2.
Results Before and After the ET Program in Each Group for Neurocognition, Motor Function, and Physical Fitness.
| <2 y | 2–5 y | >5 y | |||||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | P-value | |
| Neurocognition (CANTAB) | |||||||
| Free recall | 4.8 [1.2–8.5] |
5.3 [3.5–7.2] |
4.2 [3.1–5.3] |
5.4 [4.0–6.8] |
6.2 [4.2–8.1] |
6.1 [4.6–7.6] |
.212 |
| Recognition | 22.3 [21.8–22.9] |
23.3 [22.5–24.2] |
21.9 [20.2–23.6] |
22.8 [21.5–24.1] |
22.2 [19.9–24.5] |
22.1 [20.0–24.2] |
.102 |
| Mean RT (ms) | 1961 [913–3009] |
1873 [1162–2583] |
1553 [980–2128] |
1643 [1049–2237] |
1607 [1293–1922] |
1461 [1184–1738] |
.375 |
| Mean accuracy | 76 [64–82] |
78 [71–84] |
81 [78–84] |
83 [79–88] |
82 [76–88] |
82 [76–87] |
.147 |
| Motor function (BOT-2) | |||||||
| Bilateral coordination | 9.0 [4.7–13.3] |
11.0 [7.4–14.6] |
9.8 [5.3–14.3] |
11.8 # [8.1–15.5] |
10.4 [5.9–14.9] |
11.2 [6.8–15.5] |
.012* |
| Balance | 3.7 [2.2–5.1] |
4.7 [1.3–8.0] |
4.3 [2.9–5.7] |
4.7 [2.5–6.9] |
6.2 [3.4–8.9] |
6.9 [3.8–10.0] |
.119 |
| Running speed and agility | 5.4 [1.8–9.0] |
6.5 [1.5–11.5] |
7.3 [4.6–10.0] |
7.8 [4.7–10.9] |
7.7 [4.7–10.6] |
9.1 # [5.8–12.4] |
.028* |
| Strength | 7.0 [3.1–10.9] |
8.7 [4.2–13.2] |
7.3 [4.6–10.0] |
6.9 [5.0–8.8] |
7.5 [4.5–10.5] |
8.3 [5.0–11.5] |
.084 |
| Physical Fitness | |||||||
| 6MWT (m) | 432 [346–519] |
450 [368–531] |
453 [398–508] |
468 [399–537] |
481 [437–525] |
526 [478–573] |
.111 |
| Prorated work rate | 51.1 [28.4–73.9] |
73.8 # [45–102.5] |
69.9 [47.3–92.5] |
80.5 [50.4–110.6] |
67.6 [44.5–90.6] |
71.8 [40.9–102.8] |
.006* |
Mean [IC95%]. * repeated-measure ANOVA significant results (P < .05). # post hoc significative difference after and before exercise training program within the group.
Cognition: increased improvements in memory and accuracy when the ET program is performed earlier
Episodic memory
Free recall and recognition scores improved after ET program for < 2y and 2-5y groups but were unchanged in group > 5y (Table 2). The benefits of ET program on recognition significantly decreased with time postradiotherapy (Spearman’s rho = −0.379, P = .047) (Figure 2). There was a trend toward a negative correlation between age at ET program for episodic memory (Spearman’s rho = −0.351, P = .087) with no child over 13 years of age demonstrating improvements (Figure 3). However, the test had a ceiling effect with 9 children having a maximum pre-training score of 24, including 5 of 7 participants who were older than 13 years.
Figure 2.
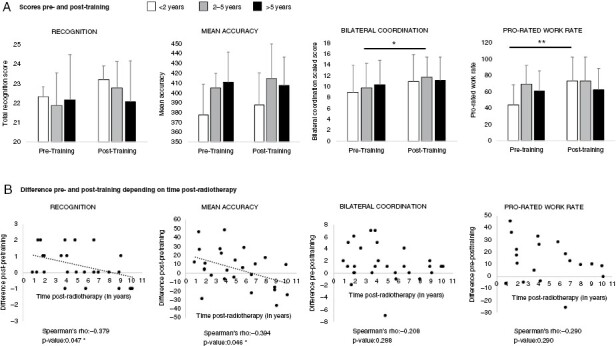
Mean impacts of a 12-week exercise training program (ET program) in irradiated brain tumor survivors depending on timing of exercise postradiotherapy. (A) Scores before and after training depending on the time category postradiotherapy. (B) Correlation between time postradiotherapy and benefit in recognition, mean accuracy, bilateral coordination, and prorated work rate. A positive difference means a benefit of training while a null or a negative difference means an absence or unexpected negative impact of training. The benefit of ET program on recognition and accuracy scores decreased significantly with the delay postradiotherapy. At group level, performances remained stable in > 5 y group and the increase between pre and post scores in < 2 y and 2–5 y groups was not statistically significant. For motor coordination and prorated work rate, improvement in performance was higher in the < 5 y and < 2 y groups, respectively, but no statistically significant correlations were found with delay postradiotherapy.
Figure 3.
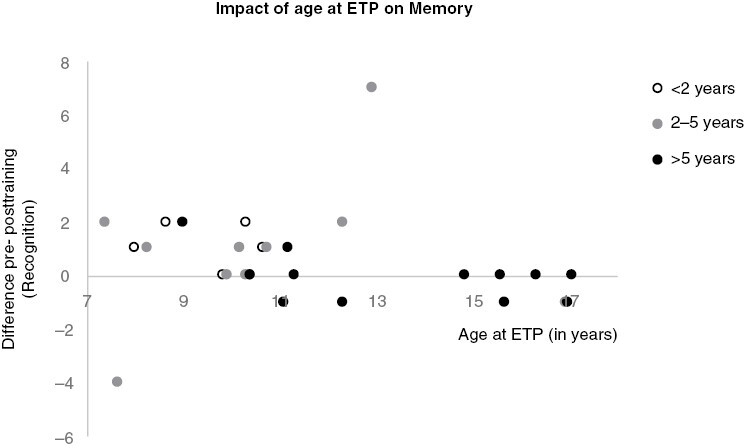
Impact of age at exercise training program on episodic memory. Grey zone showed period of benefits on cognition between age 7 and 13 years old.
Mean reaction time and accuracy on CANTAB
After the ET program, children in groups < 2 y and > 5 y had improved reaction time while no improvement was found in no-training participants in > 5 y group (training median = −253; no-training median = 14; P = .032; training n = 6, no-training n = 6) compared to the 2–5 y group. However, this difference was not significant and there was no significant impact of the timing postradiotherapy or age at ET program on mean reaction time. The children in groups < 2 y and 2–5 y saw slight improvement in accuracy scores after the ET program, while children in the > 5 y group did not. The benefits of the ET program on accuracy significantly decreased with increased time postradiotherapy (Spearman’s rho = −0.430, P = .032) (Figure 2) with trend towards decreased efficacy of ET over time (Spearman’s rho = −0.378, P = .057) without a clear cut-off.
When restricting the analysis to the 16 survivors of medulloblastoma involved in this study (four in < 2 y group, seven in 2–5 y group and five in > 5 y group), the relationship between lower benefits of ET and greater delay postradiotherapy remained weak (Spearman’s rho between 0.20 and 0.39) for mean accuracy (Spearman’s rho: −0.271, P = .327) and moderate (Spearman’s rho between 0.40 and 0.59) for recognition (Spearman’s rho: −0.423, P = .103) (Supplementary Data).
Motor function: ET program benefits at all times postradiotherapy
The ET program had a significant effect on bilateral coordination scores (F(1,25) = 7.317, P = .012), with more improvement in the 2–5 y group (mean difference −2, P = .043) and the < 2 y group (mean difference −2, P = .111) compared to the > 5 y group (mean difference −0.75, P = .390). Compared to no-training participants’ performances, the impact of ET on bilateral coordination was significant in the < 2 y postradiotherapy group (training median = 5; no-training median = 0; P = .05; training n = 3, no-training n = 3) and in the 2–5 y postradiotherapy group (training median = 4; no-training median = 0; P = 0.033; training n = 7, no-training n = 3).There was also a significant effect on the running speed and agility score (F(1,24) = 5.439, P = .028), with more improvement in the > 5 y (mean difference −1.4, P = .043) and < 2 y groups (mean difference −1.4, P = .186) compared to the 2–5 y group (mean difference −0.5, ns). There was also a trend toward increased strength (F(1,25) = 3.23, P = .084) with more improvement in the < 2 y group (mean difference −1.67, P = .041) compared to the 2–5 y (mean difference 0.4, P = .511) and the > 5 y (mean difference −0.75, P = .183) groups.
Greater improvements in strength were correlated with younger age at ET program (Spearman’s rho = −0.413, P-value = .040). There were no significant correlations between the time postradiotherapy and each of the four BOT-2 difference scores. However, there were greater differences in bilateral coordination with less time postradiotherapy (Figure 2).
Physical Fitness: children earlier postradiotherapy had lower initial prorated work rate scores and greater improvements after ET program
The 6MWT improved with training in all groups with a trend toward significance (F(1,25) = 2.72, P = .111) but without impact of time postradiotherapy.
Prorated work rate improved significantly after the ET program (F(1,15) = 10.14, P = .006) in all groups, with particular benefits in the < 2 y group (mean difference −22.6, P = .008, vs −10.6 and –4.27, respectively, in groups 2–5 y and > 5 y). No significant correlations were found between time postradiotherapy or age at ET program and physical fitness benefits of ET program. However, greater differences in scores pre- and post-training were seen in prorated work rate with less time postradiotherapy (Figure 2).
Discussion
As predicted, there were differences in feasibility and effectiveness of an ET program in PBT survivors depending on its timing postradiotherapy, with better adherence, feasibility, and satisfaction seen 2–5 y period postradiotherapy. Moreover, involvement up to five years postradiotherapy in the ET program was associated with greater improvements in bilateral coordination, physical fitness, verbal memory, and accuracy.
Greater feasibility: not too early, not too late...
Our results suggest that, while recruitment was similar across all time periods postradiotherapy, children in the first two years postradiotherapy were less likely to complete the ET program (ie, only 66% in our study). Interestingly, the main reasons of nonparticipation in the < 2 y group were not linked with travel distance but more with worries about missing school, wish to return to “back to normal”, parents missing work, or need to spend more time with siblings. Notably, the exercise sessions were scheduled in the evenings while the assessments occurred during the day. These concerns are understandable due to the need for frequent medical follow-up (ie, every 3–4 months the first 2 years) because of a higher risk of tumor relapse,18 and the physical, neurocognitive, and psychological adjustments associated with return to school after cancer treatment. The appropriateness of an ET program during this early time frame should be determined on an individual basis, taking the following factors into consideration: 1) termination of after-effects of treatment (ie, fatigue), 2) high motivation of the child and family, 3) good individual prognosis, 4) low interference between ET program and other important daily activities and interactions. Home-based exercise training using virtual reality and exergaming may be an engaging approach that addresses some of these barriers.
Beyond five years postradiotherapy, the desire to participate in an ET program study appeared to decrease with one teenager withdrawing due to decreased motivation, and reasons for nonparticipation predominantly related to a lack of time. Participants in the > 5 y group also reported lower satisfaction than the other two groups. Children in the > 5 y group were generally older suggesting that it may have been more difficult to motivate teenagers to participate in the ET program than school-age children. Finally, the 2–5 y time postradiotherapy seemed to be the most favorable for an ET program, with higher retention and satisfaction ratings. This timing postradiotherapy is associated with a lower risk of relapse and frequency of medical follow-up which may decrease psychological stressors and ultimately increase the feasibility of an ET program.
In practice, pediatric centers should offer ET programs as part of their postradiotherapy care. A pilot clinical trial (NCT05367076) is currently evaluating the use of a web-based platform to deliver exercise training by community-based instructors, to make such programs more widely available.
Positive impact of ET program on motor function and physical fitness: specific benefits for each period
The ET program had a positive impact on motor function and physical fitness on all participants regardless of its timing postradiotherapy. However, the domains of improvement differed depending on timing. In the period < 2 y postradiotherapy, patients had lower BMI and fitness scores at baseline, and experienced greater improvements in strength and pro-rated work rate with the ET program, which is likely attributed more to exercise conditioning than neuroplasticity. However, the large improvements in bilateral coordination in this group and the 2–5 y group support the prolonged opportunity for ET program to boost neuroplasticity in the motor areas of the brain and improve interhemispheric communication.5,8
Finally, participants > 5 y postradiotherapy experienced the greatest improvement in endurance and cardio-respiratory function (ie, 6MWT, and the running speed and agility subtest of BOT-2). However, this group had the least improvement in other aspects of motor function (ie, bilateral coordination, strength, balance), suggesting that the aerobic element of the ET program played a critical role in their improvements in motor function.
Cognitive impact of earlier ET program in episodic memory and accuracy
Our results indicate that ET program had a greater impact on cognition, especially long-term memory, in the earlier periods postradiotherapy. Higher scores and improvement suggest a specific impact on episodic memory. Episodic memory is mainly supported by the medial temporal lobe and this structure is particularly sensitive to irradiation with progressive damage that can decrease neuroplasticity.26–28 The ET program predicted improved cortical thickness within bilateral parahippocampal gyrus to a level similar to healthy controls8 and increased hippocampal volume.5 Early participation in an ET program may have a neuroprotective effect on memory, as memory outcomes were higher in children who completed the ET program earlier after radiation and there were no improvements observed when the ET program took place beyond 8 years postradiotherapy. However, without long-term follow-up data, we cannot draw conclusions about the long-term neuroprotective effect of ET on memory. A longitudinal follow-up is necessary to determine if an ET program can change the natural history of memory decline or if it provides an initial increase in performance followed by a gradual decline or stabilization. Future studies will also determine if repetition of exercise programs is necessary over time and at what rate. Another explanation of the higher improvement of episodic memory with training in patients with delay from radiotherapy under five years could be linked with their younger age at training. Indeed, episodic memory performance improved during childhood with a peak between 8 and 10 years old (the mean ages of the < 2 y and 2–5 y groups were 9.72 and 10.65 years old, respectively). Children in the > 5 years postradiotherapy group were on average 13.65 years old at the time of ET program, typically an age where memory performance stabilizes.29 Thus, we can hypothesize that a window of opportunity might exist between the ages of 8 and 10 years for interventions that target episodic memory.
Accuracy scores were lower in the < 2 y group compared to the other groups with greater improvements after the ET program, suggesting an enhanced effect of ET program on spontaneous recovery in this group.
Potential mechanisms of recovery involved over time
Depending on the type of brain lesions in PBT survivors (fixed or progressive), the evolution of sequelae may differ. “Fixed” lesions do not worsen with time and are the result of: 1) direct damage from the tumor itself, 2) indirect damage caused by compression of surrounding structures (eg, triventricular hydrocephalus secondary to compression of the fourth ventricle from a posterior fossa tumor), or 3) localized surgical damage. In these instances, recovery can be mediated over time through a combination of restitution (resolution of the diaschisis, removal of the initial lesion) and compensatory mechanisms (anatomical reorganization, creation of new circuits, creation of new synapses, rupture of inhibitory circuits).30 This type of recovery follows a logarithmic trajectory like recovery poststroke where most of the natural recovery occurs in the first few weeks following surgery.31 In contrast, the rate and extent of recovery is disrupted when the tumor requires radiotherapy. Radiotherapy induces repeated damage to healthy brain tissue and has additional acute and long-term effects.10 The progressive lesions associated with cranial irradiation (loss of neuronal precursors in hippocampus, altered signaling in hippocampal microenvironment, failure of differentiation and long-term neuronal loss) accumulate over time, leading to structural changes detectable using MRI and cognitive decline like a neurodegenerative condition. However, in the developing brain, the impact of radiotherapy could also manifest as reduced ability to learn new skills and lower recovery rate. ET program has been shown to enhance neuroplasticity of fixed lesions in stroke and traumatic brain injuries32 and has demonstrated neuroprotection against progressive lesions in elderly individuals.33 At present, a detailed understanding of the mechanisms of recovery for an ET program in irradiated PBT is not well defined. However, a better understanding of the role of intervention timing in PBT survivors is essential to elucidate the potential mechanisms of recovery involved. Figure 4 provides a theoretical illustration of our main results with respect to the healthcare pathway and possible evolution of a brain lesion over time.
Figure 4.
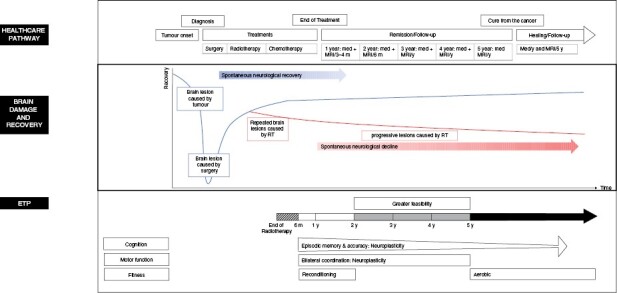
The time course of a pediatric brain tumor and its treatments, brain damage and mechanisms of recovery and main benefits of Exercise training program depending on time postradiotherapy.
Limitations
This study had several limitations. First, this retrospective subgroup analysis limits the number of participants per group and, thus, reduces the power of our statistical analysis and the conclusions that can be drawn from them, especially in the < 2 y group. Furthermore, the small subgroup size prevented us from controlling for additional factors that influence the benefits of ET program on cognition (eg, type of tumor, age at treatment or assessment, and sex).4 In addition, while we wanted to explore the first six months postradiotherapy, a period during which neuroinflammation could negatively impact intervention efficacy, none of the participants in the study met this criterion suggesting that participation in an ET program is already difficult from a feasibility standpoint. Finally, we chose the time postradiotherapy for our groups because radiotherapy appeared to have the greatest impact on the brain and cognition. However, this approach does not take the length of treatment protocols that include chemotherapy after radiotherapy into consideration. While the completion of radiotherapy in children treated for ependymoma (ie, surgery and radiotherapy) is the end of cancer treatment, children treated for medulloblastoma (ie, surgery, radiotherapy, and chemotherapy) have cancer treatment for up to one year after radiation, which has an additional impact on recovery. Undoubtedly, every child and situation are different and, for this reason, many factors need to be considered to determine the timing of an ET program: importance of sequelae, recovery of the general state after treatment, risk of relapse, motivation, environmental support, previous physical activity level.
Conclusion
Based on the feasibility and preliminary cognitive, motor, and physical benefits of an ET program in PBT survivors, participation in an ET program should be offered as part of routine post-treatment care in the first 1–2 years postradiotherapy and is strongly encouraged in the first 5 years. While the side effects of treatment likely limit the feasibility of a very early ET program, the impact of neuroinflammation on neuroplasticity in the first 6 months postradiotherapy has yet to be studied. Starting an ET program after five years postradiotherapy continues to have a positive impact on physical fitness, but may have less impact on certain aspects of cognitive and motor function. Future research should consider time since treatment, or specifically postradiotherapy, when assessing the feasibility and efficacity of intervention to enhance neural and cognitive recovery, especially memory. The possibility of a window of opportunity for episodic memory rehabilitation in the neurodevelopmental period between 7 and 13 years of age requires further work.
Supplementary Material
Acknowledgments
We would like to thank the participants in this study and their parents, and the research personnel and therapists who made this trial possible.
Contributor Information
Éloïse Baudou, Neurosciences and Mental Health, The Hospital for Sick Children, Toronto, Ontario, Canada.
Jennifer L Ryan, Neurosciences and Mental Health, The Hospital for Sick Children, Toronto, Ontario, Canada.
Elizabeth Cox, Neurosciences and Mental Health, The Hospital for Sick Children, Toronto, Ontario, Canada.
Lisa Nham, Neurosciences and Mental Health, The Hospital for Sick Children, Toronto, Ontario, Canada.
Krista Johnston, Divisions of Hematology/Oncology, The Hospital for Sick Children, Toronto, Ontario, Canada.
Éric Bouffet, Divisions of Hematology/Oncology, The Hospital for Sick Children, Toronto, Ontario, Canada.
Ute Bartels, Divisions of Hematology/Oncology, The Hospital for Sick Children, Toronto, Ontario, Canada.
Brian Timmons, Department of Pediatrics, Child Health and Exercise Medicine Program, McMaster University, Hamilton, ON, Canada.
Cynthia de Medeiros, Neurosciences and Mental Health, The Hospital for Sick Children, Toronto, Ontario, Canada.
Donald J Mabbott, Neurosciences and Mental Health, The Hospital for Sick Children, Toronto, Ontario, Canada; Department of Psychology, University of Toronto, Toronto, Ontario, Canada.
Funding
This work was supported by the Canadian Institute of Health Research (2009-03-23), Canadian Cancer Society (#701423), Sunshine Kids Foundation, and Brain Canada.
We also wish to thank the Toulouse Recherche Enfant Cancer (TREC), AT3C and “Timeo notre heros” associations that supported E.B.’s research fellowship and the Clinician Scientist Training Program at The Hospital for Sick Children that supported J.R.’s research fellowship.
Conflict of interest:
None
References
- 1. Girardi F, Allemani C, Coleman MP.. Worldwide trends in survival from common childhood brain tumors: a systematic review. J Glob Oncol. 2019;5:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oyefiade A, Paltin I, De Luca CR, et al. Cognitive risk in survivors of pediatric brain tumors. J Clin Oncol. 2021;39(16):1718–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al Dahhan NZ, Cox E, Nieman BJ, Mabbott DJ.. Cross-translational models of late-onset cognitive sequelae and their treatment in pediatric brain tumor survivors. Neuron. 2022;110(14):2215–2241. [DOI] [PubMed] [Google Scholar]
- 4. Bernal J, Recchia F, Chin E, et al. Physical activity for cancer-related cognitive impairment in childhood cancer survivors: a systematic review and meta-analysis. SSRN Electron J. 2022;4642(22):1–12. [Google Scholar]
- 5. Riggs L, Piscione J, Laughlin S, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro Oncol. 2017;19(3):440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piscione PJ, Bouffet E, Timmons B, et al. Exercise training improves physical function and fitness in long-term paediatric brain tumour survivors treated with cranial irradiation. Eur J Cancer. 2017;80:63–72. [DOI] [PubMed] [Google Scholar]
- 7. Cox E, Bells S, Timmons BW, et al. A controlled clinical crossover trial of exercise training to improve cognition and neural communication in pediatric brain tumor survivors. Clin Neurophysiol. 2020;131(7):1533–1547. [DOI] [PubMed] [Google Scholar]
- 8. Szulc-Lerch KU, Timmons BW, Bouffet E, et al. Repairing the brain with physical exercise: cortical thickness and brain volume increases in long-term pediatric brain tumor survivors in response to a structured exercise intervention. NeuroImage Clin. 2018;18:972–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dobkin BH. Rehabilitation and functional neuroimaging dose-response trajectories for clinical trials. Neurorehabil Neural Repair. 2005;19(4):276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S.. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol. 2017;13(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19(8):2302–2308. [DOI] [PubMed] [Google Scholar]
- 12. Sekeres MJ, Riggs L, Decker A, et al. Impaired recent, but preserved remote, autobiographical memory in pediatric brain tumor patients. J Neurosci. 2018;38(38):8251–8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Partanen M, Bouffet E, Laughlin S, et al. Early changes in white matter predict intellectual outcome in children treated for posterior fossa tumors. NeuroImage Clin. 2018;20:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ.. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22(4):706–713. [DOI] [PubMed] [Google Scholar]
- 15. Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32(17):1760–1768. [DOI] [PubMed] [Google Scholar]
- 16. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ.. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 17. Zhao LR, Willing A.. Enhancing endogenous capacity to repair a stroke-damaged brain: an evolving field for stroke research. Prog Neurobiol. 2018;163-164:5–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hill RM, Richardson S, Schwalbe EC, et al. Time, pattern, and outcome of medulloblastoma relapse and their association with tumour biology at diagnosis and therapy: a multicentre cohort study. Lancet Child Adolesc Heal. 2020;4(12):865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ritzmann TA, Rogers HA, Paine SML, et al. A retrospective analysis of recurrent pediatric ependymoma reveals extremely poor survival and ineffectiveness of current treatments across central nervous system locations and molecular subgroups. Pediatr Blood Cancer. 2020;67(9):1–12. [DOI] [PubMed] [Google Scholar]
- 20. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luciana M. Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB). J Child Psychol Psychiatry. 2003;44(5):649–663. [DOI] [PubMed] [Google Scholar]
- 22. Bruininks RH, Bruininks DB.. The Bruininks-Oseretsky Test of Motor Proficiency, 2nd edn. Minneapolis, MN: Pearson Assessment; 2005. [Google Scholar]
- 23. Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150(4):395–9, 399.e1. [DOI] [PubMed] [Google Scholar]
- 24. Bar-or O, Rowland TW.. Pediatric Exercise Medicine: From Physiologic Principles to Health Care Application. Champaign: Human Kinetics; 2004. [Google Scholar]
- 25. The Jamovi Project. Published online2021.
- 26. Riggs L, Bouffet E, Laughlin S, et al. Changes to memory structures in children treated for posterior fossa tumors. J Int Neuropsychol Soc. 2014;20(2):168–180. [DOI] [PubMed] [Google Scholar]
- 27. Decker AL, Szulc KU, Bouffet E, et al. Smaller hippocampal subfield volumes predict verbal associative memory in pediatric brain tumor survivors. Hippocampus. 2017;27(11):1140–1154. [DOI] [PubMed] [Google Scholar]
- 28. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guillery-Girard B, Martins S, Deshayes S, et al. Developmental trajectories of associative memory from childhood to adulthood: a behavioral and neuroimaging study. Front Behav Neurosci. 2013;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hylin MJ, Kerr AL, Holden R.. Understanding the mechanisms of recovery and/or compensation following injury. Neural Plast. 2017;2017:7125057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Langhorne P, Bernhardt J, Kwakkel G.. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702. [DOI] [PubMed] [Google Scholar]
- 32. Vanderbeken I, Kerckhofs E.. A systematic review of the effect of physical exercise on cognition in stroke and traumatic brain injury patients. NeuroRehabilitation. 2017;40(1):33–48. [DOI] [PubMed] [Google Scholar]
- 33. Mahalakshmi B, Maurya N, Lee SD, Kumar VB.. Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int J Mol Sci. 2020;21(16):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


