Abstract
Pseudomonas aeruginosa strains are opportunistic pathogens associated with infections in immunocompromised hosts and patients with cystic fibrosis. Like many other mucosal pathogens, P. aeruginosa cells express flagella which provide motility and chemotaxis toward preferred substrates but also provide a ligand for clearance by phagocytic cells. We tested the role of flagella in the initial stages of respiratory tract infection by comparing the virulence of fliC mutants in a neonatal mouse model of pneumonia. In the absence of fliC, there was no mortality, compared with 30% mortality attributed to the parental strain PAK or 15% mortality associated with infection due to a pilA mutant PAK/NP (P < 0.0001). The fliC mutants caused pneumonia in only 25% of the mice inoculated, regardless of whether there was expression of the pilus, whereas the parental strain was associated with an 80% rate of pneumonia. Histopathological studies demonstrated that the fliC mutants caused very focal inflammation and that the organisms did not spread through the lungs as seen in infection due to either PAK or PAK/NP. Purified flagellin elicited an intense inflammatory response in the mouse lung. 125I-labeled flagellin bound to the glycolipids GM1 and GD1a and to asialoGM1 in an in vitro binding assay. However, flagellin-mediated binding to epithelial gangliosides was a relatively unusual event, as quantified by binding assays of wild-type or fliC mutant organisms to CHO Lec-2 cells with membrane-incorporated GM1. Fla+ organisms but not fliC mutants were efficiently taken up by murine macrophages. P. aeruginosa flagella are important in the establishment of respiratory tract infection and may act as a tether in initial interactions with epithelial membranes. This function is offset by the contribution of flagella to host clearance mechanisms facilitating phagocytic clearance and the role of flagellar genes in mucin binding and clearance.
Flagella are highly complex bacterial organelles which are unusually well conserved among diverse bacterial species. Over 50 genes are involved in the synthesis and function of flagella, suggesting that their preservation and role in chemotaxis and motility are important in the survival of many organisms (24). Flagella facilitate the acquisition of essential nutrients; thus, it seems likely that these organelles have a role in the virulence of pathogenic organisms. However, flagella are known to be highly immunogenic (16), and in certain settings Fla+ bacteria may be more readily cleared than Fla− organisms (1).
The contribution of flagella to the virulence of the opportunistic pathogen Pseudomonas aeruginosa has been examined in several animal models of infection (11, 19). Fla− mutants were less invasive than motile strains in a mouse burn infection, and the administration of antiflagellum antibody had a significant protective effect (8). In a model of mucosal colonization, globally defective RpoN mutants (Pil− Fla−) of P. aeruginosa were more deficient in their ability to colonize than to persist within the murine gastrointestinal tract (21). A rat model of Pseudomonas pneumonia was used to demonstrate the efficacy of human antiflagellum monoclonal antibody in attenuating pulmonary infection and reducing the spread of the organism within the rat lung (13). In these instrumented animals, large inocula of bacteria were instilled directly into the trachea. These studies do not directly address how flagella function in either the initial colonization of the upper respiratory tract or the subsequent infection of the lung by aspiration. Several flagellar genes, although not the flagellin structural gene fliC, have been shown to contribute to binding to respiratory mucin (2, 25). Attachment to mucin facilitates clearance from the respiratory tract via the normal mucociliary escalator. Flagella can function as ligands for macrophages and polymorphonuclear leukocytes (PMNs) which clear organisms from mucosal surfaces. In vivo selection of Fla− mutants of P. aeruginosa has been demonstrated in human pulmonary infection in cystic fibrosis (CF) (14, 15). Although the first of sequential Pseudomonas isolates from a patient were motile and piliated, such as environmental strains of P. aeruginosa, genotypically identical isolates over the course of a chronic infection had an RpoN-like phenotype and failed to express functional flagella (15). There may be significant selective pressure for the emergence of Fla− mutants which are less efficiently cleared by phagocytic cells (16, 29). Some respiratory pathogens such as Bordetella pertussis are nonmotile, and recent studies suggest that a bovine pathogen, Bordetella bronchoseptica, which can be motile, specifically turns off flagellar expression during the initial stages of infection (1).
Since P. aeruginosa flagella are antigenically relatively homogeneous, falling into two large groups based on structural analysis and reactivity to antisera (28), they may provide a useful target for vaccine development. The ability of antiflagellum antibody to ameliorate infection in the rat lung suggests that flagella may provide a useful target for immune intervention and may be most effective as a strategy to block the initial infection of the respiratory tract by motile strains. However, the importance of normal flagellar function in the earliest stages of P. aeruginosa respiratory infection has not been established for genetically characterized strains. The purpose of this study was to examine (i) the virulence of wild-type and fliC mutant strains in the development of murine pneumonia and (ii) participation of flagella in stimulation of the subsequent immune response to the organism.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains used for these studies were PAK, a wild-type piliated, motile strain (23), PAK/NP (pilA (23), and PAK/fliC and PAK/NP/fliC, both constructed as described below. PAO1, another prototypic strain of P. aeruginosa, has been previously well characterized (7). All strains were grown in M9 minimal medium supplemented with glutamine as necessary. Gentamicin (100 μg/ml) and piperacillin (100 μg/ml) were used for selection in P. aeruginosa. Fla− mutants of PAK and PAK/NP were isolated by gene replacement. Each strain was transformed by electroporation with pMS590, a pUC19 β-lactamase-producing (piperacillin-resistant) plasmid vector containing PAK fliC interrupted by a gentamicin resistance (Gmr) cassette (25). Gmr piperacillin-sensitive clones were screened on motility agar plates (0.3%) and compared with motile (PAK) and nonmotile PAK-N1 (rpoN) controls (34). These strains were also transformed by electroporation with pLS10, which consists of pUCP18+GFP (green fluorescent protein) cloned on a 700-bp HindIII-BamHI fragment obtained from S. Falkow (5) and inserted into the pUCP18 polylinker. Chemicals and media were purchased from Sigma Chemical Co., St. Louis, Mo., unless otherwise specified.
Epithelial cell culture.
1HAEo- cells, simian virus 40-immortalized human airway cells whose epithelial properties have been well characterized (10), were grown to confluence in Dulbecco modified Eagle medium–F-12 medium containing 10% fetal calf serum (FCS) in 24-well tissue culture plates and used for adherence studies and interleukin-8 (IL-8) assays. CHO Lec-2 cells (ATCC CRL-1736) (31) were obtained from the American Type Culture Collection and grown in alpha minimal essential medium (Gibco BRL, Grand Island, N.Y.)–10% FCS; the CHO Lec-2 cell line is a mutant clonal derivative of the CHO clone Pro-5 which exhibits a drastic reduction in the transport of CMP-sialic acid into the Golgi compartment and has minimal sialylation of glycosylated macromolecules.
Bacterial adherence assays using 35S-labeled organisms.
The number of P. aeruginosa adhering to the epithelial monolayers was quantified as previously described (23). The bacteria were grown to late log phase, metabolically labeled with [35S]methionine, and washed with 10 mM NaCl, and a 0.1-ml inoculum of 5 × 108 CFU/ml was added to each well of a confluent layer of 1HAEo- cells or serum-starved CHO cells for 60 min at 37°C. Nonadherent organisms were removed with three phosphate-buffered saline (PBS) rinses. The monolayers were solubilized in 200 μl of 0.2% sodium dodecyl sulfate (SDS), and scintillations were counted. The scintillations associated with a known number of organisms, quantified by serial dilution, were counted to provide the CFU/counts per minute. Each point was performed in triplicate, and a mean and standard deviation were determined.
Statistical analysis.
The differences in adherence between the wild-type and fliC mutant strains were analyzed by using a χ2 test to compare the populations of >10,000 events. The directly computed χ2 value was compared to appropriate critical values based on the large number of degrees of freedom in the samples, and the probability of exceeding that value were calculated. If the P was <0.05, the null hypothesis (that there was no difference between the groups) could properly be rejected.
Isolation of flagella.
PAK/NP or PAO/NP cells were used to inoculate large cookie sheets with M9 agar–Casamino Acids. After 72 h at 37°C, bacteria were scraped off the surface of the agar and suspended into M9 salts. Flagella were sheared by blending for 1 min at maximum speed in a Waring blender. Bacteria were removed by centrifugation at 8,000 rpm in a Sorvall SS34 rotor for 10 min, and the flagella were further centrifuged at 15,000 rpm for 90 min. This crude flagellar preparation was then purified by SDS-polyacrylamide gel electrophoresis, eluted from the acrylamide, and dialyzed extensively. Purity was checked by demonstration of a single 53-kDa protein band on an SDS-polyacrylamide gel stained with Coomassie blue which was also reactive with antiflagellum antisera by Western hybridization.
Flagellin binding to isolated glycolipids by thin-layer chromatography–overlay assay.
The affinity of purified flagella for isolated glycolipids was determined by monitoring the binding of 125I-labeled flagella to glycolipids separated by thin-layer chromatography (23). A mixture of GM1 derivatives with none, one, two, or three sialic acid residues (Calbiochem, La Jolla, Calif.) was separated by thin-layer chromatography on aluminum-backed plates (EM Separations, Gibbstown, N.J.), using a solvent system consisting of methanol-chloroform-CaCl2 (45:50:0.02). The plate was fixed by submersion in polyisobutyl methacrylate (1.25 g in 50 ml of chloroform and 450 ml of n-hexane) and incubated overnight with 125I-labeled PAO1 flagella. Flagellin binding to specific glycolipids was detected by autoradiography. Visualization of the glycolipids was performed by using an orcinol-ferric chloride spray.
Enzyme-linked immunosorbent assay (ELISA) to measure flagellin binding to glycolipids.
Each well of microtiter plates was coated with 200 μl of stock solution of either the monosialoganglioside GM1 (0.22 mg/ml) or asialoGM1 (0.22 mg/ml) in 10% chloroform in methanol. A solution of 10% chloroform in methanol served as a control. The plates were allowed to evaporate to dryness at 4°C and then blocked with 5% bovine serum albumin in PBS (pH 7.4) for 1 h at 37°C. The wells were washed, and a saturating amount of flagellin, 5 μl (768 μg/ml) in PBS, was added and allowed to adhere for 1 h at 37°C. N-Acetylneuraminic acid and N-acetylglucosamine were added to some wells at concentrations of 40 and 400 mg/ml as possible competitive inhibitors of binding. Following three washes with blocking solution, rabbit anti-PAO flagellin antiserum (1:1,000) was added for 1 h followed by washing. The bound antibody was detected with alkaline phosphatase-conjugated goat anti-rabbit antiserum (1:10,000). Visualization was performed with an alkaline phosphatase substrate kit (Bio-Rad Laboratories, Hercules, Calif.), reading optical density at 405 nm (OD405) after 30 min of incubation at 37°C in a kinetic microplate reader (Molecular Devices, Menlo Park, Calif.). As a positive control, purified PAO1 pilin in an equivalent concentration (previously shown to result in maximal binding) was used instead of flagella to bind to the coated plates and identified with rabbit antipilin antisera.
Fluorescence-activated cell sorting analysis of binding of P. aeruginosa.
Flow cytometry was used to measure the number of CHO Lec-2 cells which had adherent bacteria under control conditions or in the presence of exogenous GM1. Fifty percent confluent monolayers of the CHO Lec-2 cells were starved for FCS for 48 h, and then wells were incubated with either 10% FCS–100 μM GM1, 10% FCS, or alpha minimal essential medium alone. PAO1 cells (5 × 108 CFU/ml) were incubated with selected wells for 2 h at 37°C and washed with PBS. Bound bacteria were tagged with rabbit polyclonal antiserum raised against PAO outer membrane protein, detected with fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit immunoglobulin G (Caltag, Burlingame, Calif.). Additional wells were incubated with the B subunit of cholera toxin (CTB) labeled with FITC (List Biologicals, Campbell, Calif.) to quantify the uptake of GM1 into the cell membranes. The CHO Lec-2 cells were removed from the tissue culture wells by treatment with 0.02% EGTA in PBS–20 mM HEPES, fixed in 0.8% paraformaldehyde, and analyzed by FACStar flow cytometry (Becton Dickinson). Results were analyzed for statistical significance by using a χ2 analysis to compare the populations of >10,000 events as measured by flow cytometry (Excel software).
Mouse model of infection.
Individual litters of 7- to 10-day-old BALB/cByJ mice (Jackson Laboratory, Bar Harbor, Maine) housed in a room free of known murine pathogens were inoculated with a single strain of P. aeruginosa as previously described (33). Each animal was weighed and then inoculated with 2-μl aliquots of a 10-μl bacterial suspension (approximately 2 × 108 CFU/ml) placed directly in the nares. The mice were returned to the mother following inoculation and sacrificed 24 h after inoculation. The chest was opened with sterile technique, and the left lung was homogenized in 100 μl of sterile PBS and plated on MacConkey-lactose plates. The spleen was similarly prepared and cultured. The right lung and selected spleens were placed in 10% buffered formalin for histopathological studies. Animals found dead at 24 h were treated similarly. Growth of P. aeruginosa from splenic cultures was considered evidence of bacteremia. Organisms isolated from the lungs and spleen were checked for the phenotypes of the infecting strain, i.e., phage susceptibility and antimicrobial resistance. Pneumonia was defined as the recovery of >1,000 CFU/lung and confirmed histologically.
Statistical analysis.
Differences in the rates of pneumonia, bacteremia, and mortality attributed to each strain were compared by using a two-tailed Z test of proportions (Excel software). Following calculation of the mean and standard error of each population, a Z score was computed and compared to a table of critical values. If the calculated Z value exceed the critical value for a given probability (P < 0.05), the null hypothesis was rejected and the samples were deemed to be significantly different.
Histopathological studies.
Tissues fixed in formalin were embedded in paraffin and sections stained with hematoxylin and eosin in addition to Gram staining. A histological diagnosis of pneumonia by a veterinary pathologist was determined by the presence of gram-negative rods, leukocyte infiltration, consolidation with fibrin deposition, and hemorrhage into the bronchi and alveoli.
Macrophage studies.
RAW 264.7 cells (murine macrophage cell line ATCC TIB71) were grown at 37°C in 5% CO2 in RPMI 1640 containing glutamine and 10% FCS in 24-well tissue culture plates and used after 24 to 48 h of growth. Monolayers were incubated with an inoculum of PAK(pLS10 [expressing an enhanced GFP]), with PAK/NP(pLS10), or with PAK/NP/fliC(pLS10) at 37°C in 5% CO2 for 1 h to allow ingestion to take place and were then put on ice. Unattached bacteria were removed with three washes with PBS. The cells were taken up from the tissue culture wells by treatment with 0.02% EGTA and fixed with 0.8% paraformaldehyde. The macrophages were analyzed by flow cytometry using a dual laser FACStar (Becton Dickinson), and 10,000 cells counted and quantified for the fluorescence associated with GFP (maximum absorbance 488 nm; maximum emission 507 nm). Statistical analysis was done by using χ2 as described above.
RESULTS
Characterization of fliC mutants.
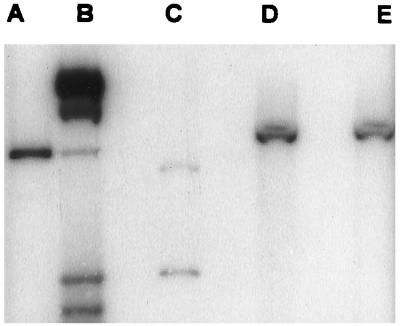
Nonmotile mutants of PAK were analyzed to confirm that the expected gene replacement events, in which the interrupted copy of fliC, the gene encoding flagellin protein, introduced into PAK and PAK/NP had replaced the wild-type coding sequence, had occurred. A 659-bp EcoRI-SalII DNA fragment from pMS590 was used to probe genomic DNA from nonmotile, Gmr piperacillin-sensitive PAK and PAK/NP mutants. A 2.35-kb EcoRI-NruI fragment was detected in the wild-type strain PAK (Fig. 1, lane C), whereas the nonmotile mutants had hybridizing DNA fragments of 5.0 kb (lanes D and E) consistent with the insertion of the 2.7-kb Gmr cassette within the fliC coding region. PAK/fliC was susceptible to the pilus-specific phage PO4, while PAK/NP/fliC was resistant. The fliC mutants exhibited no motility when viewed directly in the microscope, nor did they form the concentric rings of growth typical of motile organisms when inoculated on 0.3% agar plates.
FIG. 1.
Construction of fliC mutants demonstrated by Southern hybridization. A fliC probe consisting of a 659-bp EcoRI-SalI DNA fragment from pMS590 was used to detect homologous DNA cleaved with EcoRI and NruI in pMS590 (fliC interrupted by a Gm cassette) (lane A), 32P-λ HindIII-labeled molecular weight markers (lane B), PAK genomic digest (lane C), PAKfliC (lane D), and PAK/NPfliC (lane E).
Flagella bind to sialylated and asialylated epithelial glycolipids.
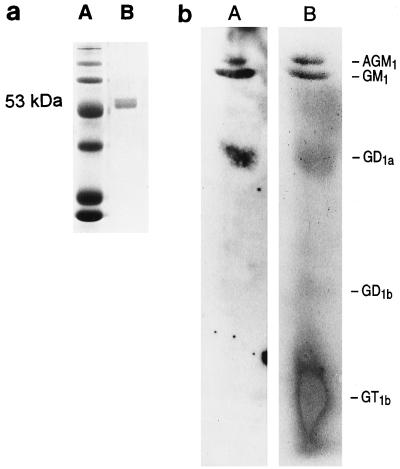
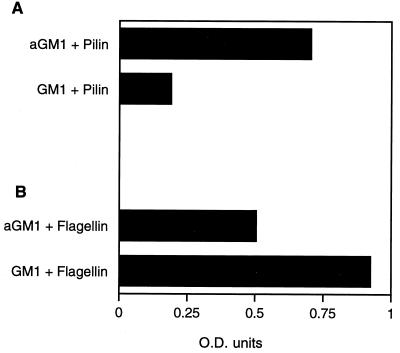
Flagella have been suggested to act as ligands for various eukaryotic receptors in addition to their role in motility. Flagellar genes, although not fliC, contribute to mucin binding (2, 25), and isolated flagella have been suggested to be capable of binding to glycolipid receptors such as asialoGM1 (9). Flagellin gel purified from PAO/NP ran as a single 53-kDa protein band identified by Coomassie blue staining of the preparation electrophoresed on an SDS–12% polyacrylamide gel (Fig. 2A). The same protein was identified with anti-PAO flagellin antiserum by Western hybridization (data not shown). The binding of gel-purified 125I-labeled PAO1 flagellin to purified glycolipids separated by thin-layer chromatography was performed by using an overlay assay (Fig. 2B). Flagellin bound to the monosialylated glycolipid GM1 as well as to both the disialylated ganglioside GD1a and asialoGM1. The relative affinity of flagellin for the glycolipids GM1 and asialoGM1 was directly compared by ELISA, using the known affinity of pilin for asialoGM1 as a positive control (Fig. 3). Flagellin binding to GM1 was 1.8 times greater than the binding to asialoGM1, whereas the ratio of pilin binding to GM1 over that to asialoGM1 was 0.28, suggesting that flagellin has greater affinity for the sialylated ganglioside GM1. However, the addition of sialic acid or N-acetylglucosamine at 40 or 400 mg/ml did not competitively inhibit flagellin recognition of GM1 (data not shown).
FIG. 2.
(a) Purified flagellin. Lanes: A, molecular weight markers; B, gel-purified flagellin isolated from PAO/NP electrophoresed on a 12% polyacrylamide gel and stained with Coomassie blue. (b) Binding of 125I-labeled flagellin to glycolipids. Lanes: A, autoradiograph of 125I-labeled flagellin from PAO/NP overlaid on the glycolipids separated by thin-layer chromatography; B, visualization of the glycolipids with orcinol-ferric chloride following autoradiography. AGM1, asialoGM1.
FIG. 3.
Binding of flagellin and pilin to GM1 and asialoGM1 quantified by ELISA. (A) Pilin binding to microtiter plates coated with either asialoGM1 (AGM1) or GM1, detected by using antipilin antisera and alkaline phosphatase-conjugated goat anti-rabbit antibody and plotted by the relative OD405 determined in an ELISA reader. (B) Flagellin binding to asialoGM1 and GM1, similarly compared by using antiflagellin antisera and alkaline phosphatase-conjugated goat anti-rabbit antibody as measured by ELISA.
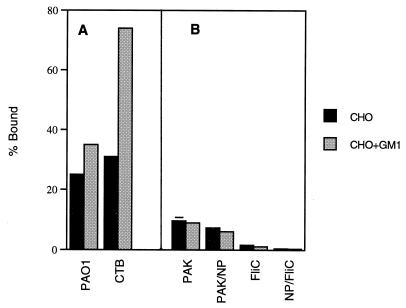
Having found that flagellin binds to GM1 in vitro, we wanted to determine if GM1 in the context of a cell membrane is capable of serving as a receptor for intact flagella. The adherence of PAK and the fliC mutants to CHO Lec-2 cells in the presence of exogenous GM1 was quantified (Fig. 4). These mutant CHO cells are relatively ganglioside deficient but can incorporate exogenous GM1 into their membranes (31). The CHO Lec-2 cells were incubated with GM1 and demonstrated to have increased GM1 content, as quantified by an increase in binding of FITC-labeled CTB from 31% of the cells with an available GM1 receptor to 74% (P < 0.0001, χ2), measured by flow cytometry (Fig. 4A). Accompanying the increase in membrane-associated GM1, there was a modest (but statistically significant [P < 0.001]) increase in the number of CHO cells with adherent PAO1, as identified by flow cytometry, from 25 to 35%, suggesting that a small fraction of membrane-incorporated GM1 can function as receptors for P. aeruginosa PAO1 binding. To determine if the expression of flagellin affects the binding to CHO Lec-2 cells with added GM1, the adherence of 35S-labeled PAK, PAK/NP, PAKfliC, and PAK/NP/fliC was quantified (Fig. 4B). There was no increase in the binding of either PAK or any of the fliC mutant strains to the CHO Lec-2 cells with added GM1, as quantified by measuring the adherence of 35S-labeled organisms, a method which is more sensitive to alterations in binding due to differences in the nature of the inoculating strain (Fig. 4B). There was little binding to the CHO cells under any circumstances, and the addition of GM1 did not increase the binding of Fla+ strain PAK. In assays using respiratory epithelial cells, binding of 35S-labeled PAO1 in the presence of CTB, a ligand for available GM1, was found to be decreased by approximately 10% (data not shown), confirming the results obtained with the CHO Lec-2 cells and similarly indicating a modest role for GM1 as a Pseudomonas receptor under these in vitro conditions.
FIG. 4.
Bacterial adherence to CHO cells in the presence or absence of exogenous GM1. (A) The percentage of CHO Lec-2 cells, preincubated with or without exogenous GM1 (n = 10,000), which had adherent PAO1 (labeled with GFP) was quantified by flow cytometry under control conditions. The amount of GM1 incorporation into the CHO cells was estimated by measuring the percentage of cells which could be labeled with FITC-conjugated CTB, a GM1-specific ligand. (B) Binding of 35S-labeled PAK, PAK/NP, PAK/fliC, and PAK/NP/fliC mutants to the CHO cells under control conditions or with added GM1 (as in panel A) was quantified. For these strains, the percent bound reflects the percentage of the bacterial inoculum added which bound to the CHO Lec-2 cells. The error bars are contained within the graph for many of the data points.
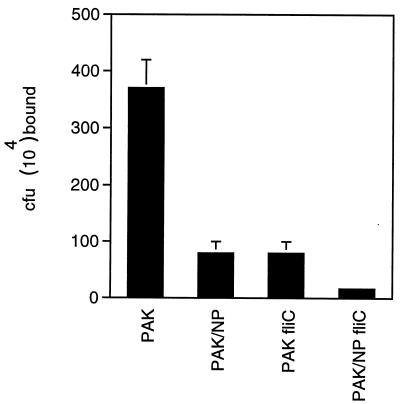
Epithelial binding of fliC and fliC pilA mutants is significantly impaired.
The contribution of fliC and motility to overall bacterial adherence was quantified by measuring the binding of 35S-labeled organisms to confluent monolayers of 1HAEo- cells (Fig. 5). The fliC mutant was significantly impaired in binding, with a 78% decrease in the number of adherent organisms compared with the parental strain (P < 0.001, χ2). Binding characteristics of the fliC mutant and a pilA mutant were very similar, whereas the fliC pilA double mutant (PAK/NP/fliC) was even more impaired than either single mutant alone, with a 95% reduction in the number of adherent organisms (P < 0.001, χ2).
FIG. 5.
P. aeruginosa adherence to 1HAEo- cells. The adherence of 35S-labeled PAK and mutant strains (from an inoculum of 5 × 108 CFU/ml) was quantified. (Some of the error bars fall within the data bar.)
Virulence of fliC mutants in a mouse model of pulmonary infection.
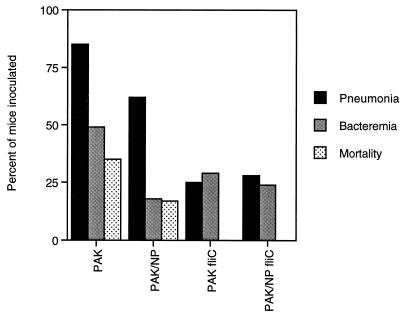
The fliC mutant strains were significantly hampered in their ability to cause disease in neonatal BALBc/ByJ mice. Mice inoculated intranasally with wild-type PAK had 35% mortality and an 85% rate of acquiring pneumonia (Fig. 6). Twenty-four hours after inoculation, these mice looked ill and most had failed to gain weight. In contrast, mice inoculated with either of the fliC mutants remained healthy: there was no mortality (P < 0.001) or obvious sign of infection. Approximately 25% of the mice inoculated with PAK/fliC or PAK/NP/fliC developed pneumonia, as defined by the recovery of >103 CFU from homogenized lung, a significantly lower rate of infection than that attributed to PAK (P < 0.0001) for either fliC mutant strain. Neither of the fliC mutants caused death (P < 0.0001), in contrast to infection with the Fla+Pil− mutant PAK/NP, which was less virulent than the parental strain but still associated with a 20% rate of mortality at 24 h and a 55% rate of pneumonia. In the absence of flagella, the expression of pili did not contribute significantly to virulence, and the types and severity of infection due to either PAK/fliC or PAK/NP/fliC were equivalent. Bacteremia occurred approximately half as often in animals infected with the fliC mutant or with the PAK/NP/fliC mutant, but the differences did not achieve statistical significance.
FIG. 6.
Virulence of fliC mutants in a neonatal BALBc/ByJ mice. The percentage of mice inoculated which developed pneumonia (recovery of >103 CFU/lung), bacteremia, or mortality is indicated. Numbers of mice inoculated with the various strains: PAK, 30; PAK/NP, 29; PAK/fliC, 28; and PAK/NP/fliC, 25.
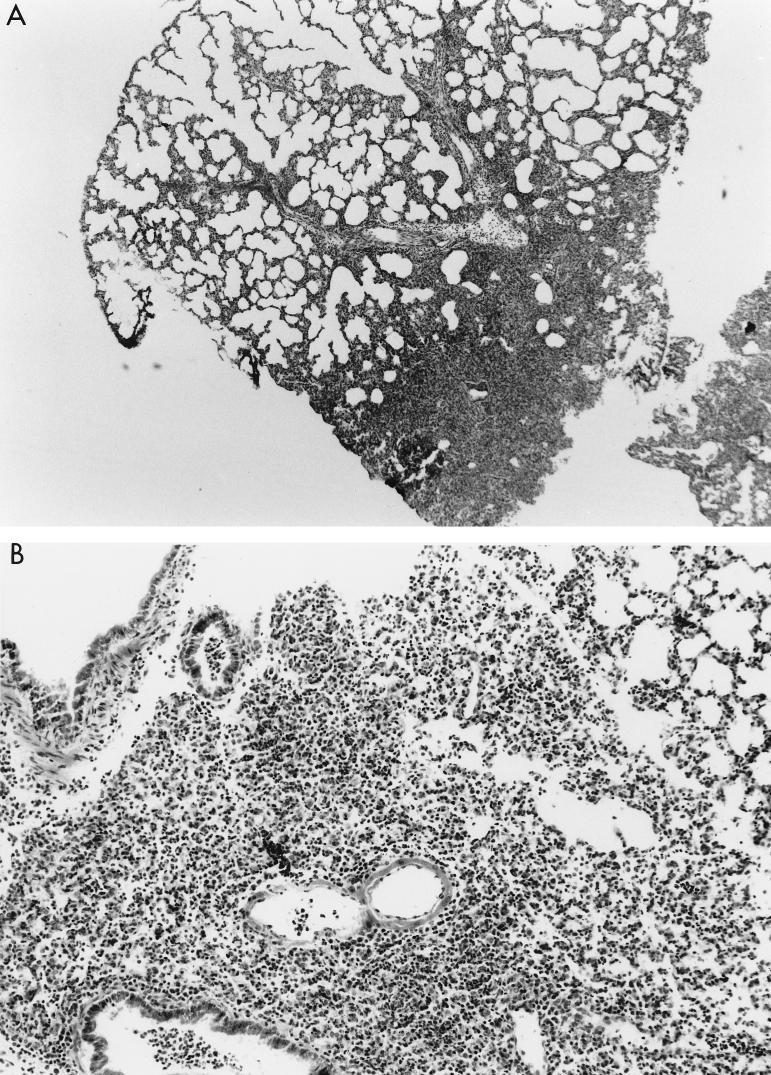
The pathology associated with the pulmonary infections caused by the fliC mutants consisted of focal consolidation with an intense inflammatory response. In all of the lungs examined, there were large areas of normal tissue (Fig. 7), suggesting that these organisms were limited in their ability to spread. Thus, the pulmonary damage, marked by PMN infiltration into the airways and into the stromal tissues, edema of the interstitium, and some areas of hemorrhage, is typical of that seen in acute P. aeruginosa infection but was limited to very discrete areas of the lung.
FIG. 7.
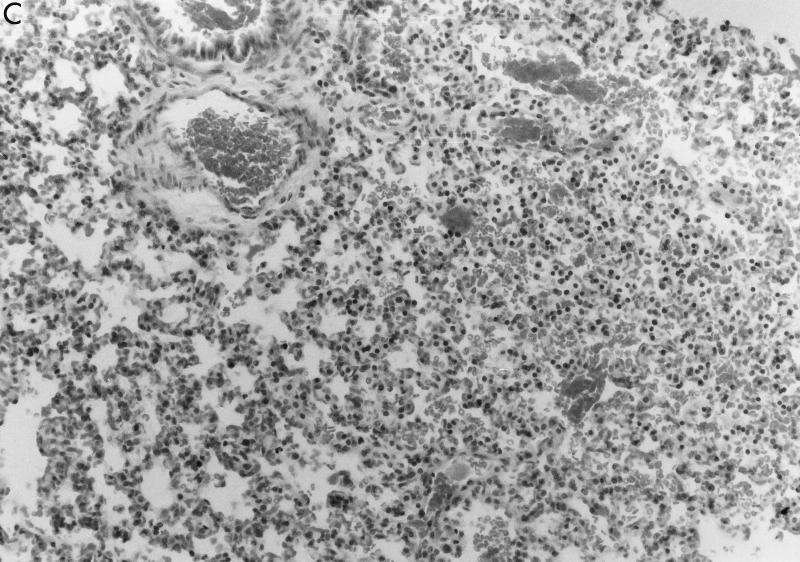
Pathology associated with infection due to fliC mutants. (A) Inoculation of the mice with PAK/fliC resulted in a focal infiltrate involving a very limited portion of the lung. (B) Infection with wild-type PAK shown at higher magnification (×100) demonstrates the typical response to P. aeruginosa with edema, infiltration of PMNs, and some hemorrhage. A small area of relatively normal appearing lung is visible at the upper right. (C) Pathology induced by the introduction of purified flagellin into the mouse lung.
The introduction of purified flagellin into the airways of the mouse similarly elicited an inflammatory response (Fig. 7C). Although the mice were seemingly healthy when sacrificed 24 h after the instillation of 10 μg of flagellin per ml, histological studies of the lung parenchyma revealed a PMN response, hemorrhage, and edema in a very limited and focal distribution. There was no apparent pathology associated with the instillation of an equivalent dose of bovine serum albumin (data not shown).
Macrophage interactions with fliC mutants are impaired.
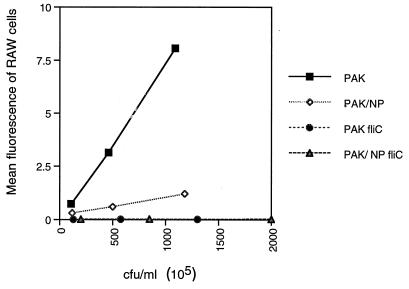
Flagella act as a major ligand for nonopsonic phagocytosis of P. aeruginosa (16). This process is likely to be important in the pathogenesis of pneumonia in nonimmune animals, such as neonatal mice, used in the virulence studies, since the mothers of the mice have no detectable antibody to P. aeruginosa (data not shown). We tested the efficiency of murine macrophage ingestion of P. aeruginosa PAK and fliC mutants, using flow cytometry to quantify the number of RAW cells with associated bacteria tagged with a plasmid expressing GFP (Fig. 8). The parental strain was efficiently ingested or associated with the macrophages (the technique for monitoring the number of RAW cells with associated bacteria did not distinguish between internalized and external organisms). The pil mutant was associated with the RAW cells approximately 50% less than the parental strain, and the RAW cells did not associate with the fliC mutants at all. Although we did not specifically quantify ingested organisms, in identical experiments performed in the presence of 1 mM cytochalasin D, the number of bacteria associated with the RAW cells was decreased by 60%, suggesting that under these experimental conditions, the majority of bacteria associated with the RAW cells are actually ingested.
FIG. 8.
Adherence and uptake of PAK strains by RAW 264.7 cells. PAK strains transformed with a plasmid encoding GFP were incubated with RAW cells for 15 min, and the mean fluorescence of RAW cells with associated bacteria was quantified by flow cytometry. Mean fluorescence was normalized by dividing the fluorescence of the RAW cells plus bacteria by the mean fluorescence of each bacterial strain.
DISCUSSION
In the model of acute P. aeruginosa pulmonary infection presented, Pseudomonas flagella are essential to the virulence of the organism just as they have been found to be important in the pathogenesis of other mucosal infections in which chemotaxis and motility are required for pathogenicity (18). Functional flagella provide a means for the organism to spread throughout the respiratory tract, and associated chemotaxis directs the organisms toward preferred substrates, which for P. aeruginosa include amino acids and Pi as well as other known components of human mucin (17, 20). The most striking feature of the infections ascribed to the fliC mutants was their extremely focal distribution. Nonpilus adhesins with affinity for mucins, which are expressed in the fliC mutant strains, were likely to be responsible for the clearance of the majority of the inoculated organisms from the upper respiratory tract. Areas of lower respiratory tract infection were limited to the segments of lung into which organisms could be directed aspirated from above. To establish that it is the motility function provided by flagella which is critical for virulence, it will be necessary to characterize a Fla+ Mot− mutant in this model of respiratory tract infection.
The control of flagella synthesis is complex, involving at least two ς factors, the products of rpoN (ς54) (34) and fliA (rpoF), a member of the ς28 class of alternative sigma factors (32). Unlike pili which are antigenically diverse among strains of P. aeruginosa, flagella are highly conserved, falling into two groups: type a, which is relatively heterogeneous, ranging in size from 45 to 52 kDa; and type b, with a molecular mass of 53 kDa, which are more homogeneous. Both types of flagella have phosphorylated tyrosines (27), a property unusual for prokaryotes, whose functional significance remains unclear. In addition, it has been speculated that glycosylation of flagellin, also an unusual property, may account for the heterogeneity in the sizes of the type a flagella (4). These sophisticated modifications of an already complex machinery suggest that these structures are of considerable importance to the survival or the organism and its success as a pathogen.
Flagella also can function as adhesins to epithelial membranes. As first suggested by Gehring and Baker (9), purified flagellin binds to glycolipids, particularly to the common membrane constituent GM1. The significance of this ligand function is difficult to judge in the absence of a mutant which retains motility but lacks the flagellin adhesin domain, since motility seems to be paramount in these host-bacterium interactions. Much of the membrane-embedded GM1 appears inaccessible to the ligand domain of flagellin, as the incorporation of GM1 in the ganglioside-deficient CHO cell membrane was not paralleled by an equivalent increase in bacterial binding, and despite saturating inocula of bacteria, there was only a modest increase in overall adherence of strain PAO and no increase in adherence of strain PAK. Recognition of a membrane form of GM1 by the organisms is probably an even less frequent event in vivo, since few organisms penetrate the glycocalyx barrier of mucin glycoproteins which protects the apical epithelial surface. Recognition of asialoGM1 residues by flagella as occasionally exposed following epithelial damage (6) or in CF (23) may account for some adhesin function. Flagella may “browse” the epithelial surface and tether the organism to an exposed site with an accessible GM1 or asialoGM1 moiety as a very early event in establishing a nidus of infection.
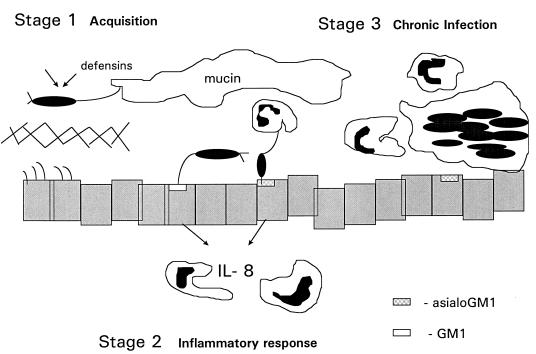
The ligand function of flagella may also act to signal the host immune response. Bacteria which persist in the respiratory tract provoke an immediate immune response, as the respiratory epithelium is an important component of the mucosal immune system, fully capable of cytokine synthesis and response to exogenous stimuli (22, 30). Flagellar recognition of epithelial glycolipids is a potent stimulus for the production of IL-8 (7), the polymorphonuclear chemokine which serves to target PMN migration as well as activating PMNs and macrophages. Flagella then function as the ligand for nonopsonic phagocytosis, facilitating phagocytic ingestion. Thus, very shortly after reaching the epithelial surface, flagella become a liability, eliciting and facilitating PMN-directed clearance. It was interesting that the fliC mutants caused bacteremia at rates not statistically different from that for the parental strain PAK, suggesting that there was some delay in clearing the bloodstream of these organisms. However, these rates of bacteremia were not accompanied by mortality, as was the case for the FliC+ strains. The potential roles of flagella in pathogenesis are clearly complex. It may be useful to separate the pathogenesis of respiratory tract infection into three phases (Fig. 9).
FIG. 9.
Model of respiratory tract infection. Stage 1, acquisition of organisms from the environment. Motile, piliated organisms are inhaled and usually killed by local defensins (arrows) and removed by mucociliary clearance before penetrating the glycocalyx. Stage 2, immunostimulatory phase. In CF, due to diminished defensin activity, organisms produce exoproducts which destroy the protective glycocalyx, allowing access to the epithelial surface. Flagella may function to tether the bacteria to the mucosal surface. The close apposition of the organism to glycolipid receptors found predominantly on cells with mutant CF transmembrane conductance regulator function allows pilin-mediated attachment to occur, followed by stimulation of epithelial IL-8 expression and migration of PMNs to the airway. Stage 3, adaptation and chronic infection. In response to the immune pressure of the host, mutants which do not express flagella as well as the alginate-producing strains are selected and predominate due to their ability to avoid phagocytic clearance.
Model of respiratory tract infection by P. aeruginosa. (i) Stage 1: acquisition of environmental organisms.
In the initial phase of infection, environmental strains of P. aeruginosa from any aqueous setting are inadvertently inhaled or otherwise delivered to the upper respiratory tract. It is at this stage where the expression of functional flagella is critical, providing chemotaxis toward desirable substrates, such as mucin, and the motility essential for widespread dissemination. In the normal host, the presence of defensins (26) and additional antimicrobial peptides, the affinity of flagella components for mucin glycopeptides (2), and the process of mucociliary clearance are likely to eradicate such transiently inhaled organisms from the respiratory tract before any epithelial immune response is elicited.
(ii) Stage 2: Immunostimulatory phase of infection.
In patients with CF and probably in those with nosocomial P. aeruginosa pneumonia, there is a second stage of P. aeruginosa respiratory tract infection characterized by the development of airway inflammation in response to the organism. As a consequence of diminished defensin activity in the milieu specific to the CF airway (or iatrogenic impairment of mucociliary clearance via intubation or mechanical damage to the epithelium), organisms are not efficiently cleared. In concert with the production of proteases, elastase, neuraminidase, and additional exoenzymes, bacteria broach the glycocalyx barrier overlying the ciliated apical surface of the epithelium. The long flagella may then function as a tether, recognizing accessible GM1 moieties on the apical surface of respiratory epithelial cells or asialoGM1 which may be exposed in areas of regenerating epithelium following damage (6, 7). Juxtaposition of the bacteria and epithelial membranes may then provide the opportunity for more intimate contact as mediated by the N-MetPhe pili of P. aeruginosa which bind to asialylated glycolipid receptors. Access to these asialoGM1 receptors is limited, but flagellar recognition of GM1 may also initiate the cascade of epithelial signaling events resulting in IL-8 expression (7). Since IL-8 is a potent chemokine, flagellated organisms, with their convenient ligand for the Glut1 receptor of macrophages and increased clearance by PMN (3), may be eradicated by the inflammatory response.
(iii) Stage 3: adaptation.
In CF virtually exclusively, there is a third stage of infection which occurs when there are enough bacteria within the airway to allow for the selection of mutants which can adapt specifically to this ecologic niche. To avoid clearance by the host phagocytic cells, Fla− mutants proliferate as was originally described by Luzar et al. (14). The molecular basis for loss of motility in this clinical setting was demonstrated by Mahenthirlingham and Speert (15), who found that organisms from CF patients chronically infected with P. aeruginosa may have substantial deletions of genomic DNA associated with an RpoN−-like phenotype. They are Fla−, and some can be complemented with rpoN introduced on a plasmid. Thus, the negative consequences of flagellar expression are well illustrated by the repeated isolation of Fla− mutants from this specific clinical setting. The selection of mucoid mutants of P. aeruginosa which overexpress alginate, an additional antiphagocytic virulence factor, also occurs at this stage.
Flagellated organisms are likely to be involved in the earliest stages of infection, with the eventual selection of Fla− mutants in response to host immune pressure. As flagellar structure is highly conserved in P. aeruginosa, this appendage may serve as a convenient target for preventative therapy, either as a vaccine antigen or for passive immunotherapy for uninfected patients prior to the acquisition of the organism from environmental exposure. With the increased recognition of the negative implications of any P. aeruginosa infection in CF (12), there has been renewed interest in therapeutic modalities to delay the development of chronic P. aeruginosa infection, instead of accepting it as an inevitable consequence of this disease. Strategies to improve the clearance of the motile environmental strains of P. aeruginosa which are inadvertently inhaled may contribute to the prevention of lung disease.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant DK39693 (A.P.) and by the Cystic Fibrosis Foundation (A.P. and S.R.).
REFERENCES
- 1.Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 2.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. Cloning and characterization of Pseudomonas aeruginosa fliF, necessary for flagellar assembly and bacterial adherence to mucin. Infect Immun. 1996;64:2130–2136. doi: 10.1128/iai.64.6.2130-2136.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bargouthi S, Everett K D E, Speert D P. Nonopsonic phagocytosis of Pseudomonas aeruginosa requires facilitated transport of d-glucose by macrophages. J Immunol. 1995;154:3420–3428. [PubMed] [Google Scholar]
- 4.Brimer CD, Kelly-Wintenberg K, Montie T C. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Cloning and characterization of P. aeruginosa a-type flagellin genes, abstr. D-43. [Google Scholar]
- 5.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 6.De Bentzmann S, Roger P, Dupuit F, Bajolet-Laudinat O, Fuchey C, Plotkowski M C, Puchelle E. AsialoGM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect Immun. 1996;64:1582–1588. doi: 10.1128/iai.64.5.1582-1588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMango E, Zar H J, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake D, Montie T C. Protection against Pseudomonas aeruginosa infection by passive transfer of anti-flagellar serum. Can J Microbiol. 1987;33:755–763. doi: 10.1139/m87-130. [DOI] [PubMed] [Google Scholar]
- 9.Gehring K, Baker N R. Abstracts of the 94th General Meeting of the American Society for Microbiology 1994. Washington, D.C: American Society for Microbiology; 1994. Evidence for a role of flagella in the binding of Pseudomonas aeruginosa to glycosphingolipid receptors, abstr. D-134. [Google Scholar]
- 10.Grunert D C, Basbaum C B, Welsh M J, Finkbeiner W E, Nadel J A. Characterization of human tracheal epithelial cells transformed by an origin defection simian virus 40. Proc Natl Acad Sci USA. 1988;85:5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holder I A, Wheeler R, Montie T C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect Immun. 1982;35:276–280. doi: 10.1128/iai.35.1.276-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubesch P, Dork T, Wulbrand U, Kalin N, Neumann T, Wulf B, Geerlings H, Weisbrodt H, von der Hardt H, Tummler B. Genetic determinants of airways’ colonization with Pseudomonas aeruginosa in cystic fibrosis. Lancet. 1993;341:189–193. doi: 10.1016/0140-6736(93)90062-l. [DOI] [PubMed] [Google Scholar]
- 13.Landsperger W J, Kelly-Wintenberg K D, Montie T C, Knight L S, Hansen M B, Huntenburg C C, Schneidkraut M J. Inhibition of bacterial motility with human antiflagellar monoclonal antibodies attenuates Pseudomonas aeruginosa-induced pneumonia in the immunocompetent rat. Infect Immun. 1994;62:4825–4830. doi: 10.1128/iai.62.11.4825-4830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luzar M A, Thomassen M J, Montie T C. Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical condition. Infect Immun. 1985;50:577–582. doi: 10.1128/iai.50.2.577-582.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahenthiralingam E, Speert D P. Nonopsonic phagocytosis of Pseudomonas aeruginosa by macrophages and polymorphonuclear leukocytes requires the presence of the bacterial flagellum. Infect Immun. 1995;63:4519–4523. doi: 10.1128/iai.63.11.4519-4523.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahenthiralingam E, Campbell M E, Speert D P. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun. 1994;62:596–605. doi: 10.1128/iai.62.2.596-605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masduki A, Nakamura J, Ohga T, Umezaki R, Kato J, Ohtake H. Isolation and characterization of chemotaxis mutants and genes of Pseudomonas aeruginosa. J Bacteriol. 1995;177:948–952. doi: 10.1128/jb.177.4.948-952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mobley H L T, Belas R, Lockatell V, Chippendale G, Trifillis A L, Johnson D E, Warren J W. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1996;64:5332–5340. doi: 10.1128/iai.64.12.5332-5340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montie T C, Doyle-Huntzinger D, Craven R C, Holder I A. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned mouse model. Infect Immun. 1982;38:1296–1298. doi: 10.1128/iai.38.3.1296-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson J W, Tredgett M W, Sheehan J K, Thornton D J, Notman D, Govan J R W. Mucinophilic and chemotactic properties of Pseudomonas aeruginosa in relation to pulmonary colonization in cystic fibrosis. Infect Immun. 1990;58:1489–1495. doi: 10.1128/iai.58.6.1489-1495.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pier G B, Meluleni G, Goldberg J B. Clearance of Pseudomonas aeruginosa from the murine gastrointestinal tract is effectively mediated by O-antigen-specific circulating antibodies. Infect Immun. 1995;63:2818–2825. doi: 10.1128/iai.63.8.2818-2825.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richman-Eisenstat J B, Jorens P G, Hebert C A, Ueki I, Nadel J A. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway disease. Am J Physiol. 1993;264(Lung Cell Mol. Physiol. 8):L413–L418. doi: 10.1152/ajplung.1993.264.4.L413. [DOI] [PubMed] [Google Scholar]
- 23.Saiman L, Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Invest. 1993;92:1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro L. The bacterial flagellum: from genetic network to complex architecture. Cell. 1995;80:525–527. doi: 10.1016/0092-8674(95)90505-7. [DOI] [PubMed] [Google Scholar]
- 25.Simpson D A, Ramphal R, Lory S. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect Immun. 1992;60:3771–3779. doi: 10.1128/iai.60.9.3771-3779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 27.South S L, Nichols R, Montie T C. Tyrosine kinase activity in Pseudomonas aeruginosa. Mol Microbiol. 1994;12:903–910. doi: 10.1111/j.1365-2958.1994.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 28.Spangenberg C, Heuer T, Burger C, Tummler B. Genetic diversity of flagellins of Pseudomonas aeruginosa. FEBS Lett. 1996;396:213–217. doi: 10.1016/0014-5793(96)01099-x. [DOI] [PubMed] [Google Scholar]
- 29.Speert D P, Loh B H, Cabral D A, Salit I E. Nonopsonic phagocytosis of nonmucoid Pseudomonas aeruginosa by human neutrophils and monocyte-derived macrophages is correlated with bacterial piliation and hydrophobicity. Infect Immun. 1986;53:207–212. doi: 10.1128/iai.53.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standiford T J, Kunkel S L, Basha M A, et al. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1991;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanley P, Siminovitch L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 1977;3:391–405. doi: 10.1007/BF01542968. [DOI] [PubMed] [Google Scholar]
- 32.Starnbauch M N, Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol Microbiol. 1992;6:459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- 33.Tang H, Kays M, Prince A. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect Immun. 1995;63:1278–1285. doi: 10.1128/iai.63.4.1278-1285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Totten P A, Lara J C, Lory S. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990;172:389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]