Abstract
Chemical methods for the extraction and refinement of technologically critical rare earth elements (REEs) are energy-intensive, hazardous, and environmentally destructive. Current biobased extraction systems rely on extremophilic organisms and generate many of the same detrimental effects as chemical methodologies. The mesophilic methylotrophic bacterium Methylobacterium extorquens AM1 was previously shown to grow using electronic waste by naturally acquiring REEs to power methanol metabolism. Here we show that growth using electronic waste as a sole REE source is scalable up to 10 L with consistent metal yields without the use of harsh acids or high temperatures. The addition of organic acids increases REE leaching in a nonspecific manner. REE-specific bioleaching can be engineered through the overproduction of REE-binding ligands (called lanthanophores) and pyrroloquinoline quinone. REE bioaccumulation increases with the leachate concentration and is highly specific. REEs are stored intracellularly in polyphosphate granules, and genetic engineering to eliminate exopolyphosphatase activity increases metal accumulation, confirming the link between phosphate metabolism and biological REE use. Finally, we report the innate ability of M. extorquens to grow using other complex REE sources, including pulverized smartphones, demonstrating the flexibility and potential for use as a recovery platform for these critical metals.
Keywords: bioaccumulation, bioconcentration, electronic waste, lanthanide, metal-binding protein, neodymium, bioleaching, acid-free leaching
Short abstract
REE production requires harsh acids, high temperatures and pressures, is costly, and is environmentally taxing. This study shows a scalable and sustainable bacterial platform for nonacidic, targeted recovery of REE from E-waste.
Introduction
Global demand for rare earth elements (REEs) is at an all-time high and is steadily increasing, but their supply is susceptible to market and national interest perturbations.1−3 REEs, composed of the lanthanides (Lns), scandium, and yttrium, are critical metals for modern clean energy, communication, advanced transportation, consumer electronics, and defense technologies, underpinning a broader economy.4−6 China maintains its status as the world’s foremost producer of REEs, generating heavy reliance on a single market that could jeopardize supply and compromise national security.7,8 Though domestic REE production is on the rise, environmental and health concerns loom over traditional mining operations.9−11 The development of technologies for REE reuse and recycling has garnered interest as a means of moving toward independence from foreign importation while simultaneously generating a robust, resilient supply of these critical metals.12
Several challenges continue to stymie the development of a safer, more environmentally sustainable REE supply chain. These include (1) the requirement of high temperatures and pressures and the inclusion of harsh acids for the extraction of poorly soluble REE, which can produce radioactive waste products; (2) the preference for high-grade sources (>0.2% REE m/m) for cost-efficient recovery, leaving low-grade and waste sources as untapped reservoirs of valuable REE; and (3) the necessity for hundreds of costly, hazardous processing steps for successful separation of co-occurring REE in minerals.2,13
Microbiological REE leaching and extraction methods offer promising alternatives to current state-of-the-art methods (hydrometallurgical, pyrometallurgical, and electrometallurgical approaches) that produce large quantities of sludge, acidic wastewater, atmospheric pollution, and radioactive tailings.9,14−16 Given that conventional rare earth ores (e.g., bastnaesite, monazite, and xenotime) are typically oxidized and nonsulfidic,17 conventional bioleaching using acidophilic sulfur and iron oxidizers, which has been widely practiced commercially for sulfidic copper and gold ores, is not directly applicable. Rather, REE bioleaching approaches typically involve the production of organic acids (e.g., citric, gluconic, and oxalic acids)18,19 from sugar-based carbon sources by heterotrophic bacteria or fungi to leach REEs into solution. While these approaches can be effective for overall leaching and even provide an economical benefit,19−21 they indiscriminately dissolve metal feedstocks and require additional process(es) to separate desired metals from the remaining leachate. Microbe-mediated REE adsorption using native and engineered cell surface functional groups (e.g., carboxylates, phosphates, and metal-binding outer membrane proteins)22−25 can be an effective and potentially economic strategy26,27 for selective sequestration of REE from bulk samples, but this method requires solution-based feedstocks derived from costly chemical leaching and pH-adjustment processes.
Microbial metal accumulation and biomineralization have been shown to be effective strategies for the recovery of Hg,28 Au,29−31 and U,32,33 but has been underexplored as an REE recovery approach. Bioaccumulation and biomineralization of REEs in mesophilic bacteria were first shown in the model methylotroph Methylobacterium (also known as Methylorubrum) extorquens AM1.34 Methylotrophic bacteria, organisms that thrive on inexpensive, readily available one-carbon compounds such as methane and methanol, therefore provide an attractive new approach for REE bioleaching and extraction due to their natural ability to acquire Lns from the surrounding environment.35,36 This includes soluble and insoluble REE sources, such as electronic waste (E-waste).37−39M. extorquens AM1 was the first organism reported to grow using REE from electronic waste.36 Mesophilic methylotrophs like M. extorquens AM1 have dedicated systems for acquisition, uptake, and intracellular storage of Lns as polyphosphate granules, making them effective agents of bioleaching and bioaccumulation without the need for high acidity or temperature.34 REE use by mesophilic methylotrophs was thought to be restricted to the light Ln, but recently, a genetic variant of M. extorquens AM1 was isolated and characterized that can transport, store, and grow using the heavy Ln, gadolinium.40 Detailed genetic and Ln uptake studies indicate the likely possibility of an additional system dedicated to the acquisition and transport of heavy Lns. Thus, methylotrophs, such as M. extorquens AM1, may already possess the biological means to separate light and heavy Lns, and have the potential to be engineered for uptake of specific Ln species from complex feedstocks. To date, the potential of methylotrophs as a platform for REE recovery has not been rigorously investigated, including the selectivity of the REE uptake.
Here, we show the scalability of this growth up to 10 L with Nd magnet swarf, waste powders, and filings generated during magnet production and an E-waste analogue without the requirements of high temperatures or harsh acids. We also show the potential for process improvement by leveraging the genetic tractability and suite of genetic tools available for M. extorquens AM1 to engineer enhanced, nonacidic REE bioleaching (∼20-fold) and bioaccumulation (∼50-fold) attributes. This study provides a proof-of-principle demonstration of M. extorquens AM1 as a scalable platform for biomining and bioaccumulation of REEs from complex feedstocks, such as magnet swarf, without the need of harsh acids and high temperatures, greatly reducing potential hazards and obstacles to generating a circular economy (Figure 1).
Figure 1.
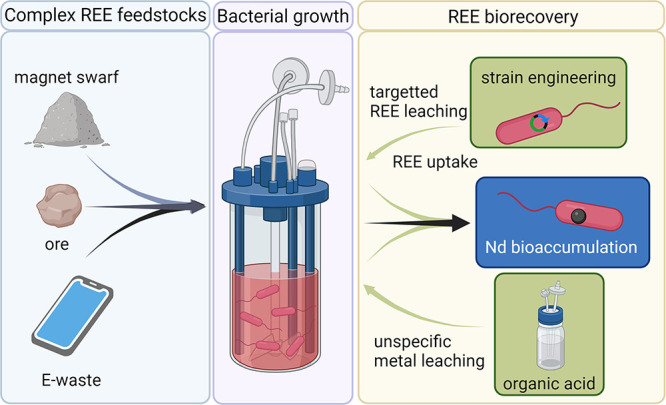
M. extorquens AM1 is a platform for Nd recovery through efficient, high-yield bioaccumulation from complex sources. REEs in complex feedstocks are accessible to M. extorquens AM1 due to its innate ability to selectively acquire, transport, and accumulate metals in polyphosphate granules. Metal leaching and REE uptake can be enhanced by the addition of organic acid to the culture medium and strain engineering, resulting in greater Nd recovery yields.
Materials and Methods
Chemicals
All chemicals were purchased from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Mineral ores containing lanthanides were purchased from vendors via Ebay.com. Magnets were removed from their protective stainless-steel cases prior to processing. Lanthanide-containing ores and magnets were powdered for subsequent experiments. NdFeB magnet swarf was provided by Urban Mining Company (Austin, TX).
Bacterial Strains and Growth Conditions
All growth studies were performed using the ΔmxaF strain of M. extorquens AM1. The mxaF gene encodes the large subunit of the calcium-dependent methanol dehydrogenase, MxaFI. Deletion of this gene ensures REE dependency for growth with methanol via primary oxidation catalyzed by alternative REE-dependent alcohol dehydrogenases.41 For routine culturing of M. extorquens AM1, strains were grown in 16 × 125 mM borosilicate culture tubes shaking at 200–220 rpm, 29–30 °C in modified Methylobacterium PIPES (MP) or Hypho minimal medium containing 2–20 μM calcium chloride,41,42 with 1.75 g/L succinate as a sole carbon source. Except when indicated otherwise, a modified formulation of Hypho (HyphoMOD) containing 1.27 g/L of K2HPO4 and 1.30 g/L of NaH2PO4.H2O was used. For growth measurements with solid ores and minerals, cells were grown in 16 × 125 or 25 × 150 mm borosilicate culture tubes using an Excella E25 shaking incubator (New Brunswick Scientific, Edison, NJ) at 180 rpm.
Growth Analysis with Organic Acids
M. extorquens AM1 ΔmxaF was grown overnight in 3 mL of MP medium with 1.77 g/L succinate as a sole carbon source at 30 °C and 220 rpm in an I 24 shaking incubator (New Brunswick Scientific, Edison, NJ). Cells were harvested via centrifugation at 6,900g for 10 min (Centrifuge 5420; Eppendorf, Hamburg, Germany), then washed with 1 volume of fresh, HyphoMOD medium. The resulting cell pellet was resuspended in 0.1 volume of fresh medium, then used to inoculate 1 mL cultures in 24-well suspension culture plates (Greiner Bio-One, Monroe, NC) at a final dilution rate of 1:10. Control cultures were incubated at 30 °C and 567 rpm in a microplate reader (BioTek Synergy H1; Agilent, Santa Clara, CA), and growth was monitored continuously by measuring optical density at 600 nm (OD600). Cultures with magnet swarf were incubated at 30 °C and 500 rpm in a Thermoshaker PHMP-4 (Grant Instruments, Royston, U.K.), and periodically removed to measure OD600 in the microplate reader. These cultures were passed over a permanent magnet to clear the well center of magnet swarf particles prior to measurements. To investigate the effect of organic acids on methylotrophic bioleaching and bioaccumulation with 1% (w/v) Nd magnet swarf and 1.6 g/L methanol as sole sources of REE and carbon, respectively, cultures were grown with and without 5 mM or 15 mM citrate, 55 mM gluconate, or 5 mM oxalate. These organic acids were provided as trisodium citrate dihydrate, sodium gluconate, and oxalic acid. The cells were harvested at the early stationary phase by transferring the culture into a 1.5 mL microcentrifuge tube to separate from the remaining magnet swarf. The cultures were then centrifuged, and cell pellet and spent medium fractions were separately stored at −20 °C. Biological triplicates were analyzed for every condition.
Bioreactor Cultivation
Precultures grown in HyphoMOD medium with succinate were prepared as mentioned and then subcultured into 50 mL of HyphoMOD medium with 1.75 g/L succinate and 1.6 g/L methanol in 250 mL shake flasks. Cultures were incubated with shaking at 200 rpm, 30 °C in an Innova S44i shaker (Eppendorf, Hamburg, Germany) for 16 h. 30 mL of culture was then transferred to a 2 L BIOne 1250 bioreactor (Distek, North Brunswick, New Jersey) with 0.75 L of HyphoMOD medium and 1% (w/v) NdFeB magnet swarf. For 10 L bioreactor cultivations, 500 mL of overnight cultures was transferred to 9.5 L of HyphoMOD medium. Bioreactor parameters were as follows: agitation, 500 rpm; air flow, 200–2000 sccm; temperature, 29.5 °C; and pH, 6.9. The pH of the bioreactor was maintained by using 1 M NaOH.
Metal Quantification
Samples for the determination of Nd content were prepared as follows: 10 mL of culture was removed from the bioreactor, and cells were pelleted by centrifugation at 4121 × g in a Multifuge X Pro Series centrifuge (Thermo Fisher Scientific, Waltham, MA) for 12 min. The culture supernatant was decanted, and cell pellets were washed four times with double-distilled water before drying at 65 °C. After achieving complete dryness, dry weight was measured, and then cells were deconstructed in 20% metal-grade nitric acid at 90 °C and diluted to 2.3% acid before analysis by inductively coupled plasma mass spectrometry (ICP-MS) at the Laboratory for Environmental Analysis (Center of Applied Isotope Studies, University of Georgia). Values were determined by ICP-MS and normalized per unit dry weight as recommended for comparison of bioaccumulation across samples and methods.43
Cell pellets obtained from growth in 24-well plates were acidified in 35% (v/v) HNO3 for 2 h at 95 °C with mixing by inversion every 30 min. Prior to dilution, the REE concentrations in spent medium and acidified cell samples were quantified by the Arsenazo III assay, as described previously.44 Briefly, 40 μL of sample was combined with 40 μL of 12.5% (w/v) trichloroacetic acid (TCA), then added with 120 μL of filtered 0.1% (w/v) Arsenazo in 6.25% (w/v) TCA. Absorbance at 652 nm was measured, and REE concentration was calculated based on a calibration curve with Nd. For metal content analysis using ICP-MS, digested cell suspensions and spent medium samples were diluted with Milli-Q water or 4% (v/v) HNO3, respectively, to achieve a final HNO3 concentration of 2%. The amount of metal associated with cells was normalized to biomass using a predetermined factor of 0.422 ± 0.018 mg dry weight mL–1 OD600–1 (n = 5).
Statistical Analysis
Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test to determine significant differences between conditions. Pairwise comparisons were performed by using the Student’s t test with Bonferroni correction. Maximum growth rates in 24-well cultures were determined via linear fitting of natural log transformed OD600 using the R package growth rates.45
DNA Manipulation, Molecular Cloning, and Mutagenesis
To increase REE bioaccumulation in polyphosphate granules, the gene ppx, encoding the exopolyphosphatase enzyme that catalyzes polyphosphate degradation, was deleted. A genetic system for overproducing the lanthanophore was constructed and transformed into M. extorquens AM1 with the purpose of increasing nonacidic bioleaching of REEs. Detailed methods for the construction of the ppx deletion mutant and for pAZ1, for the overproduction of the lanthanophore, can be found in the Supporting Information (Supplemental Methods and Figure S9).
Results and Discussion
Scalable REE Bioleaching and Bioaccumulation from Electronic Waste
M. extorquens growth performance with 1% (m/v) Nd magnet swarf was benchmarked in a 0.75 L benchtop bioreactor with 1.6 g/L methanol (Figure 2A). Next, we investigated the impact of inorganic phosphate on growth performance because REEs are poorly soluble as phosphate compounds,46 the formation of which could hinder REE-dependent methanol growth in media with high phosphate concentrations. Comparative growth analysis showed that lowering inorganic phosphate concentrations in the growth medium resulted in higher growth rates (Figures 2B and S1) but limited growth yields if reduced too much (Figure S1). Therefore, we used HyphoMOD medium containing half the standard phosphate concentration (see Methods) and 1% (m/v) Nd magnet swarf for all growth experiments going forward. M. extorquens AM1 cultures grown in HyphoMOD in a 0.75 L benchtop bioreactor generated growth rates that were significantly increased compared to microplate (+2.3-fold) (Figure 2B,D) and shake flask cultures (+1.5-fold) (Figure 2C,D). In the bioreactor, cultures reached maximum densities in 25 h, representing a 2.2-fold decrease in growth period relative to culturing in microplates and a 0.5-fold reduction compared to growth in shake flasks (Table 1). At higher pulp densities, the leaching of REEs and/or other metals, such as iron, could negatively impact growth performance and bioaccumulation. However, when the magnet swarf was increased to 10% (m/v) in the same bioreactor setup, growth rates (Figure 2E) and cycle times (Table 1) were similar to what was observed at the lower pulp density (Figure 2D), indicating that growth was not inhibited.
Figure 2.
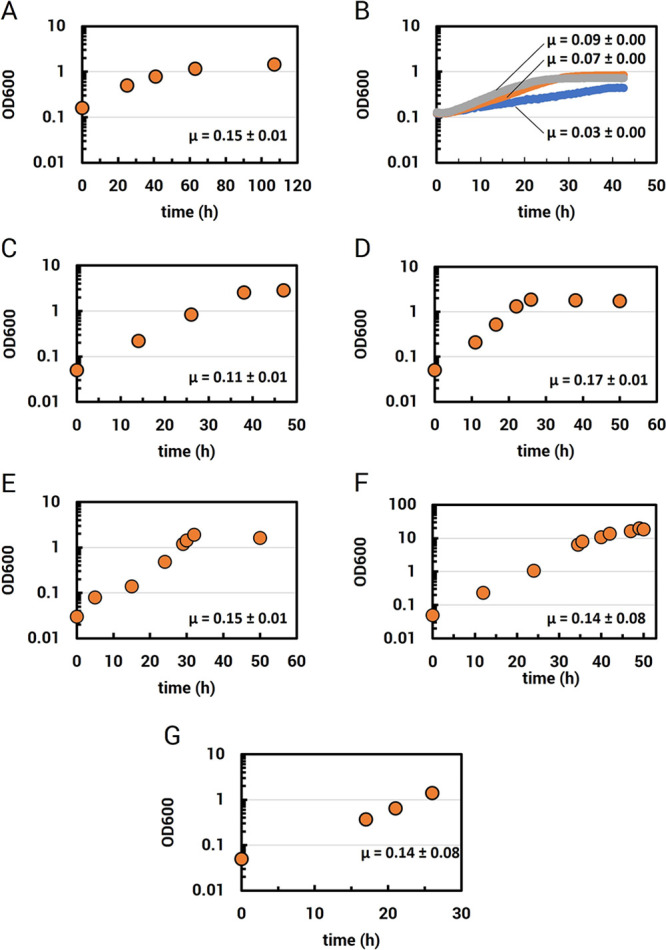
M. extorquens growth with Nd magnet swarf and methanol. (A) 0.75 L bioreactor growth in full inorganic phosphate Hypho minimal medium (see Methods) and 1% (w/v) Nd magnet swarf. (B) Microplate growth with full phosphate (blue), half phosphate (HyphoMOD; orange), and quarter phosphate (gray) medium and 1% (w/v) magnet swarf. (C) 50 mL shake flask culture growth with HyphoMOD and 1% (w/v) magnet swarf. (D) 0.75 L bioreactor growth with HyphoMOD medium and 1% (w/v) magnet swarf. (E) 0.75 L bioreactor growth with HyphoMOD medium and 10% magnet swarf. (F) 0.75 L bioreactor growth with 1% (w/v) magnet swarf. (G) 10 L bioreactor growth with HyphoMOD medium and 1% magnet swarf. Growth curves are representative of each condition (bioreactors n = 2, microplates n = 6, flasks n = 3). Growth rates are the mean rates for each condition, with standard deviations.
Table 1. Growth Performance and Bioaccumulation of Nd by M. extorquens AM1 during Bioreactor Cultivation with a Nd Magnet Swarfa.
| growth setupc | growth cycle (h) | bioaccumulation (mg Nd/g DWb) | leachate (mg/L) |
|---|---|---|---|
| bioreactor (baseline) | 63 | 3.2 ± 0.1 | n.m. |
| microplate | 28–54 | n.m. | n.m. |
| flask | 38 | n.m. | n.m. |
| bioreactor | 25 | 11.5 ± 0.3 | <0.001 |
| bioreactor (10% pulp) | 30 | 10.8 ± 0.2 | <0.001 |
| bioreactor (fed batch) | 50 | 11.0 ± 0.4 | <0.001 |
| bioreactor (10 L) | 26 | 13.2 ± 2.9 | <0.001 |
n.m. is not measured.
Nd content of cell samples were measured by ICP-MS and normalized to dry weight. Means and standard deviations of 3 independent measurements.
Bioreactor measurements are from two independent replicates with 1% Nd magnet swarf pulp density and 0.75 L volume, unless indicated otherwise. All bioreactors were run with HyphoMOD medium except baseline. 10% pulp indicates increase of swarf pulp density to 10%. Fed batch indicates methanol growth substrate was refed until maximum optical density of the culture was reached. 10 L indicates a scale increase to a 10 L bioreactor system.
M. extorquens AM1 stores REE as intracellular polyphosphate granules.34 Baseline Nd bioaccumulation levels were measured from bioreactor cultures grown in standard Hypho minimal medium (Table 1). Relative to the baseline bioaccumulation level, M. extorquens AM1 grown in HyphoMOD accumulated 3.6-fold more Nd when grown with 1% pulp density (Table 1). Reducing inorganic phosphate, therefore, not only boosted growth performance in the bioreactor (Figure 2A,D), but also positively affected REE bioaccumulation, underscoring the link between phosphate metabolism and REE use in microbial systems. Removal of calcium from the growth medium did not significantly impact REE bioaccumulation (p > 0.05 by ANOVA and Tukey’s HSD) (Figure S2). Even at 10% pulp density, growth rate REE bioaccumulation by M. extorquens AM1 was still high (Figure 2E and Table 1) showing that the process is not inhibited by a 10-fold increase in REE feedstock.
Next, we tested the performance of M. extorquens AM1 under methanol fed-batch conditions in a 0.75 L bioreactor. By repeated feeding of methanol over the run cycle, culture densities of 20 OD were achieved without a significant reduction in growth rate (Figure 2F). In total, ∼17.3 g/L of methanol was fed over a cycle time of ∼50 h. Again, there was no detectable residual Nd found in the leachate, and the REE to biomass ratio was the same as that in batch cultures (Table 1). Finally, we assessed process performance at a 10 L scale using optimized media and 1% Nd magnet swarf pulp density. The growth cycle and growth rate were like the 0.75 L scale, and Nd yields increased by nearly 15 ± 17%, but the differences in each of these parameters between the 0.75 and 10.0 L scales were not statistically significant (Student’s t test with Bonferroni correction, pBonf > 0.05). Together, these results show the scalability of this process without loss of efficiency in REE recovery from E-waste.
Selective Bioaccumulation of REE from Nd Magnet Swarf
Even high-grade feedstocks like Nd magnet swarf are of mixed composition and therefore vary in the content of highly valuable REE and associated metals,47 making selectivity crucial for the development of a successful recovery platform. We determined the precise metal content of the Nd magnet swarf used in our growth and recovery experiments using ICP-MS. Iron was the primary metal component of Nd magnet swarf (68.0% Fe), rendering nonselective leaching and uptake mechanisms insufficient for effective REE bioaccumulation. Nd was the second most abundant metal measured (26.7% Nd). In addition to Nd, the magnet swarf contained significant amounts of the light Ln praseodymium (Pr, 4.35%) and the heavy Ln dysprosium (Dy, 3.34%), both of which have high technological value.48,49
To assess the selectivity of M. extorquens AM1 uptake for REE, we determined the metal content of the abiotic leachate and cells and supernatants of cultures grown with 1% (w/v) magnet swarf. Approximately 60% of the metal leached by uninoculated HyphoMOD medium consisted of REE (Figure 3A, abiotic control). In cultures grown under the same conditions, most of the available REE was taken up by the cell, leaving the supernatant with 98% Fe (Figure 3A). Next, we assessed the specificity of REE bioaccumulation in batch bioreactor cultures with 1% magnet swarf (w/v). Cultures were grown to maximum culture density of 1.8 OD (600 nm) with 1.6 g/L methanol, after which Fe, Nd, Pr, and Dy content in cells and supernatants were measured. Supernatant metal content consisted of mostly Fe (95.9%) with low amounts of REE (Nd, 3.6%; Pr, < 1%; Dy, < 1%) (Figure 4). In cell samples, 98.0% of the accumulated metal was REE, 96.8% of which was Nd, while Pr and Dy made up a combined 1.2% (Figure 3B). This was ∼1.6-fold higher than what was observed in the 1 mL microplate cultures as mentioned above and could possibly be accounted for by more efficient mixing of swarf with M. extorquens AM1 in a stirred-tank system. Fe accounted for only 2.0% of the measured intracellular metal content (Figure 3B). Given that cells acquire Fe as a micronutrient, it is difficult to differentiate Fe from the growth medium from that obtained from the magnet swarf, but it can be concluded that the latter is less than 2.0%. Together, these data show preferential REE uptake and bioaccumulation in the bioreactor.
Figure 3.
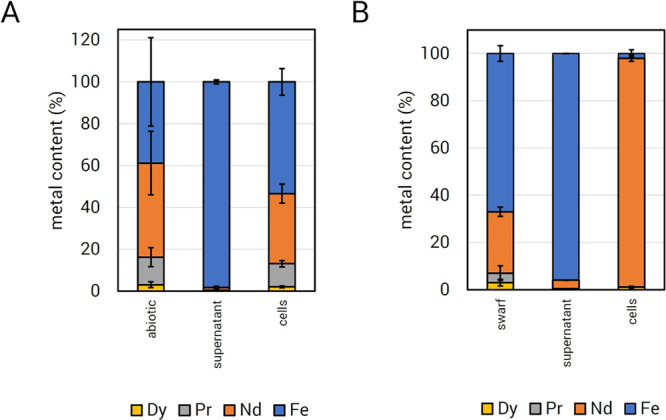
Selective bioconcentration of REE. M. extorquens AM1 was grown in HyphoMOD methanol medium with a 1% Nd magnet swarf. Cell and supernatant samples were taken once the culture reached the early stationary growth phase. (A) Metal content from 1 mL cultures. Abiotic data represents leachates of inoculated growth medium incubated simultaneously as cultures. (B) Metal content from 0.75 L benchtop bioreactor cultures. Swarf data shows the composition of the REE source material used. Cell and supernatant samples were taken once the culture reached the early stationary growth phase. Plots reflect the mean metal contents of three independent growth experiments, with error bars showing standard deviations. Dy, dysprosium; Pr, praseodymium; Nd, neodymium; Fe, iron.
Figure 4.
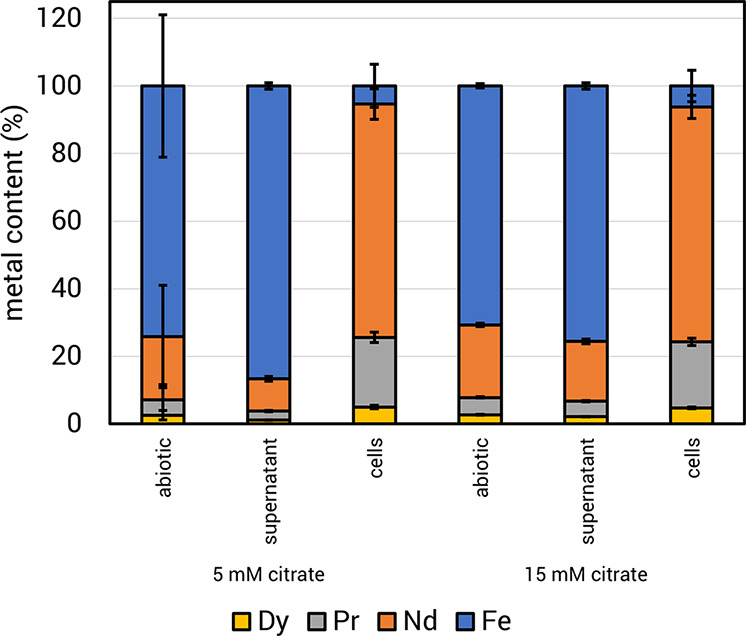
Selective bioconcentration of REE. M. extorquens AM1 was grown in 1 mL of HyphoMOD methanol medium with 1% Nd magnet swarf and 0, 5, or 15 mM citrate. Cell and supernatant samples were taken once the culture reached the early stationary growth phase. Cell and supernatant samples were taken once the culture reached the early stationary growth phase. Plots reflect the mean metal contents of three independent growth experiments, with error bars showing standard deviations. Dy, dysprosium; Pr, praseodymium; Nd, neodymium; and Fe, iron.
Chemical Strategies for Increased REE Leaching from Nd Magnet Swarf
Only trace amounts of REE were detected after process runs in bioreactor culture supernatants, indicating that nearly all leached REE was scavenged (Table 1). REE leaching, therefore, could be a limiting factor for bioaccumulation. Microbially produced organic acids are an environmentally and financially attractive alternative to traditional hydrometallurgical methods.50−52 Organic acids derived from heterotrophic microbial metabolism have been successfully employed as biolixiviants for metal dissolution, including REEs.18,19,53−62
Adding citric acid to HyphoMOD medium with a magnet swarf increased the level of chemical leaching of all metals (Table S1 and Figure S3). After 28 h of incubation, 8.2 ± 0.1 and 58.7 ± 0.6 ppm of REE were leached into the supernatant supplemented with 5 mM and 15 mM citrate, respectively, which were 15- and 108-fold greater than that found in the supernatant with no chelator (Figure S3). However, Fe was preferentially leached to concentrations of 23.7 ± 2.5 and 142.0 ± 3.2 ppm in the presence of citrate, i.e., there was a 67- and 404-fold increase in Fe concentrations under these conditions (Table S1). The increase in leached Dy with the addition of citrate was twice that of Nd or Pr (Table S1), which may be attributable to its higher complex formation constant with citrate (logK = 10.69 vs 9.76–9.94).63 Addition of 55 mM gluconate or 5 mM oxalate, the highest permissible concentration of each for comparable growth of M. extorquens, did not increase REE leaching as compared to the negative control condition, though Fe concentrations did increase by 12- and 4.2-fold, respectively (Table S1). Gluconic acid was previously shown to promote leaching of REEs from solid feedstocks, though the pH conditions were more acidic compared to the buffered pH of HyphoMOD medium (<pH 3.3 vs pH 6.9).19
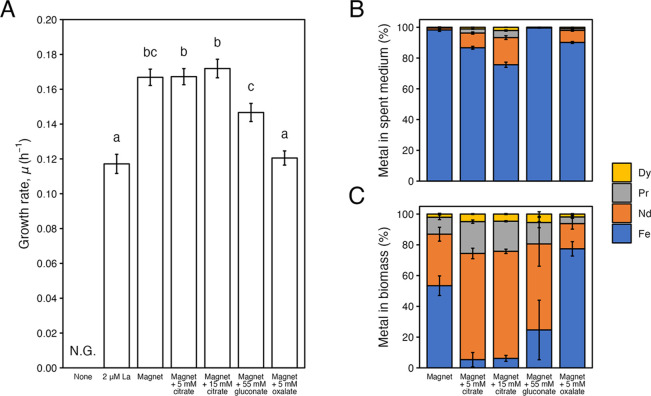
We investigated whether nonspecific leaching by the biolixiviant citrate would alter the specificity of REE uptake by M. extorquens AM1. When 5 or 15 mM citrate was added to the medium, there was a ∼2-fold increase in cellular REE content, from 47 to >93% (Student’s t test with Bonferroni correction; pBonf = 0.001 and 0.007, respectively), consisting of 69% Nd, 20% Pr, and 5% Dy. The REE content in the culture supernatant also increased from 2% to 13% and 24%, respectively (Figure 4). M. extorquens AM1 ΔmxaF grew well with 1% (w/v) magnet swarf as the sole source of REE, with a significantly higher growth rate as compared to that grown with soluble La (Figures 5A and S4; ANOVA with Tukey HSD, pTukey = 5.4 × 10–9).
Figure 5.
Growth of M. extorquens AM1 ΔmxaF in HyphoMOD medium with 1.6 g/L MeOH and 2 μM La, 1% (w/v) magnet swarf, or 1% (w/v) magnet swarf supplemented with citrate (5 or 15 mM), gluconate (55 mM), or oxalate (5 mM). (A) Growth rate of M. extorquens AM1 ΔmxaF, (B) metals leached into the spent medium, and (C) metals associated with biomass were determined based on biological triplicates. Bars and error bars represent model estimates and the standard error of growth rates, or the mean and standard deviation of metal content. The letters above bars represent statistical significance as determined by ANOVA followed by the Tukey HSD test (p < 0.05). N.G. represents no growth.
Addition of citrate to the growth medium with magnet swarf did not significantly affect growth (pTukey ≥ 0.98). However, addition of gluconate reduced the growth rate as compared to that of citrate (pTukey ≤ 0.042), and oxalate further negatively impacted growth (pTukey ≤ 1.6 × 10–10). As expected from the abiotic leaching results (Figure S3), addition of citrate greatly improved REE leaching (Figures 5B and S5A; pBonf ≤ 0.001), and led to greater REE uptake by M. extorquens AM1 ΔmxaF (Figures 5C and S5B; pBonf ≤ 0.001). Interestingly, more REE was associated with biomass when 5 mM, rather than 15 mM, citrate was supplemented, suggesting that there exists a balance between abiotic leaching and biological uptake at the scale tested (Figure S5B; pTukey ≤ 0.04). In fact, upon scale-up of the culture volume to 100 mL, 15 mM citrate inhibited growth of M. extorquens AM1 ΔmxaF (Figure S6). Importantly, unlike the increased uptake of REE in the presence of citrate, the level of Fe associated with the cell remained constant across all conditions (Figure S5B; pTukey ≥ 0.34).
Next, we assessed how adding 5 mM citrate impacts batch bioreactor cultures and found that total REE bioaccumulation increased to 98.8% of metal uptake with Nd accounting for 88.9%, Pr 9.2%, and Dy 0.7% (Figure S7). Supernatant REE concentrations were notably higher with the addition of citrate to the growth medium. Nd accounted for 24.5%, Pr 2.3%, and Dy 7.9%, with Fe accounting for only 65.2% (Figure S7). Nd and Fe concentrations in citrate-supplemented supernatants were similar to those of the original swarf composition, but Pr and Dy concentrations were notably different. Pr in the supernatant was ∼2-fold lower and Dy ∼2-fold higher in supernatant, indicating preferential uptake of the light REE and/or preferential leaching of the heavy REE. Overall, these data show highly selective, ∼3.6-fold bioconcentration of REE from magnet swarf by M. extorquens AM1. Based on these highly promising results, M. extorquens AM1 appears naturally poised as a competitive REE bioaccumulation microbe with scalable growth.
Current REE bioaccumulation yields do not account for 100% of the REE in the magnet swarf. We tested whether a single magnet swarf batch could be processed for further REE extraction in both small- and large-scale cultivations. In 1 mL cultures, the same batch of magnet swarf was able to sustain growth of M. extorquens AM1 ΔmxaF for five cycles, though the final OD and total leached REE concentrations decreased with continuing growth cycles (Figure S7). Based on the bioaccumulation yields from the first bioreactor run (Figure 6), we estimated 8.25 ± 0.04% of the swarf Nd could be recovered in a single fed-batch process, reaching a culture OD of 20 when 5 mM citrate is added to increase leaching. With a second consecutive process run, using the same swarf batch as that of the first run, bioaccumulation of Nd accounted for another 7.75 ± 0.12% of the original amount (Figure 6). These promising results suggest that a continuous process run could feasibly be used to recover an even higher proportion of REE in a swarf batch.
Figure 6.
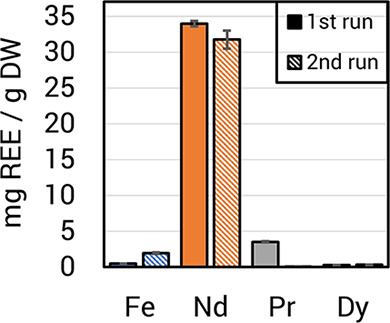
Total REE bioaccumulation is increased by reusing magnet swarf feedstock. Each experiment consisted of two complete process runs in which the ΔmxaF strain was grown with 1% magnet swarf (w/v) in a 0.75 L bioreactor with 1.6 g/L methanol and 5 mM citrate. After growth, cell samples were taken for ICP-MS metal content measurements, and the magnet swarf was removed from the bioreactor, rinsed 4 times with ddH2O, and dried at 65 °C for 72 h before reuse. Bars show the means of cellular REE content normalized to dry weight (DW) for two replicate experiments. A distinct swarf batch was used for each individual experiment. Error bars indicate standard deviations.
A recent techno-economic analysis reported the potential economic feasibility of using biolixiviants to increase REE leaching from waste materials and found that the carbon substrate (glucose) is a major contributor to cost.20 Our results showed that addition of low molarity citrate significantly increased REE leaching and bioaccumulation from the Nd magnet swarf. Citrate can be added directly to the bioreactor, or in the future, process development could be linked via coculture with a heterotrophic acid-producing microbe, such as Gluconobacter oxidans. However, to truly limit the costs of an REE recovery process, an acid-independent approach would be preferred, especially in areas in which secondary hazardous waste streams are of concern.
Approaches for Enhanced REE-Specific Bioleaching and Bioaccumulation
The genetic tractability and availability of genetic tools for strain engineering make M. extorquens AM1 a uniquely suitable microbial platform for REE recovery. A biosynthetic pathway for an REE-chelator (lanthanophore) has been previously reported and was named mll after its product methylolanthanin.64 Expression of the mll has already been shown to increase Nd uptake in M. extorquens AM1 from both soluble chloride and poorly soluble oxide forms in a highly specific manner.64 We tested the impact of expressing the mll in trans during growth with magnet swarfs and saw increases in REE bioaccumulation. Nd, Pr, and Dy accumulation increased by more than 3-fold with mll expression, reaching 80 mg of Nd/g of DW, 15 mg of Pr/g of DW, and 8 mg of Dy/g of DW (Figure 7A), while Fe accumulation increased only marginally. Notably, expression of the mll was under control of the lac promoter, a comparatively weak promoter in M. extorquens AM1.65 Generation of a stronger expression system is underway and in theory should boost REE bioaccumulation even further.
Figure 7.
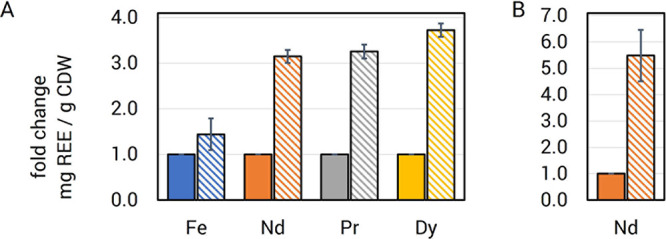
Strain engineering for increased REE bioaccumulation. (A), in trans expression of the mll enhances REE (Nd, neodymium; Pr, praseodymium; Dy, dysprosium) bioaccumulation from magnet swarf in M. extorquens AM1, with minimal Fe uptake. ΔmxaF, solid bars; ΔmxaF/pAZ1, hatched bars. A detailed description of pAZ1 can be found in the Supporting Information (Supplemental Methods and Figure S9). (B), deletion of the exopolyphosphatase-encoding ppx gene results in higher Nd bioaccumulation. ΔmxaF, solid bars; Δppx, hatched bars. For A and B, the metal content was determined by ICP-MS and normalized to cell dry weight. Values represent the mean of samples from 3 independent cultures grown in shake flasks. Error bars reflect standard deviations.
Pyrroloquinoline quinone (PQQ) has been shown to directly bind REEs66 and has been implicated in microbial REE solubilization.62 We recently reported M. extorquens AM1 strain evo-HLn40 that exhibited enhanced PQQ production in the presence of the heavy REE, gadolinium. We measured Nd bioaccumulation in evo-HLn and saw a 53% increase compared to ΔmxaF.
REEs are stored in intracellular polyphosphate granules in M. extorquens AM1. Depolymerization of polyphosphate is catalyzed by exopolyphosphatase activity. We hypothesized that limiting the cellular capacity for polyphosphate depolymerization could generate higher levels of REE bioaccumulation. A ppx (encoding exopolyphosphatase) deletion strain was generated and assessed for REE bioaccumulation, showing a ∼5.5-fold increase in Nd levels reaching 202 mg Nd/g DW (Figure 7B).
The results shown herein demonstrate that selective targeting of specific processes for REE metabolism, generated by a detailed understanding of the physiology of M. extorquens AM1, is an effective strategy for engineering enhanced REE storage. Reduction of inorganic phosphate in the growth medium, likely allowing for increased uptake and granulation with REE, resulted in a nearly 4-fold enhancement of Nd yields over baseline levels (Figure 8). Identification of REE-binding ligands like methylolanthanin allows for nonacidic, highly specific leaching from feedstocks. Genetic engineering to increase methylolanthanin levels produced Nd yields more than 20-fold higher than baseline values (Figure 7). Likewise, detailed knowledge of REE storage granulation and storage processes has allowed for genetic manipulation and engineering to further boost intracellular REE content. By engineering reduced capacity for granule deconstruction, we were able to increase the Nd storage capacity over 50-fold (Figure 7) relative to the baseline condition with ΔmxaF. These improved yields, combined with a culture OD of 20 in a 0.75 L bioreactor, would allow Nd recovery of 1.3–2.1 g of Nd/L, representing a recovery between 65 and 100% of Nd in a single process run when using 1% Nd magnet swarf pulp density.
Figure 8.
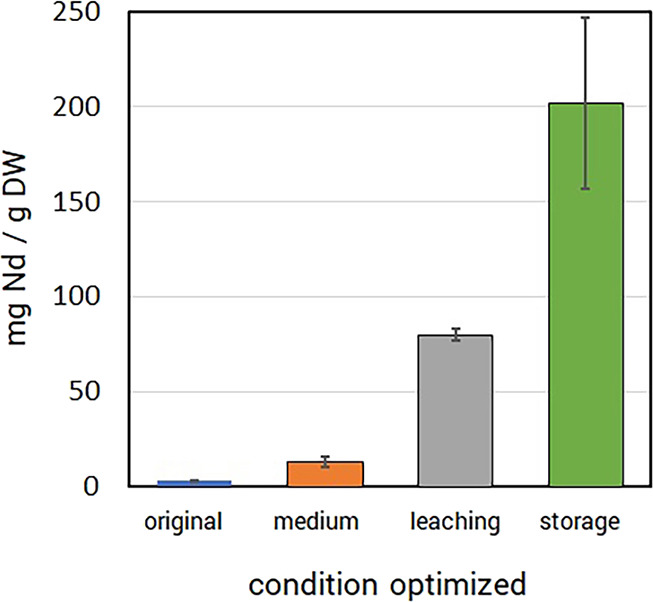
Process performance enhancement. Chemical and genetic targeting of specific metabolic processes generates improved REE bioaccumulation relative to the original (baseline) measurements. Manipulations to growth medium inorganic phosphate (medium), lanthanophore production (leaching), and REE/phosphate granulation (storage) each generated elevated REE bioaccumulation yields. Bioaccumulation was measured by ICP-MS and is normalized to dry weight. Error bars represent standard deviations of biological triplicates.
Environmental Implications
Current REE mining and refinement practices are costly, environmentally destructive, and unsustainable.67 Safer, cleaner alternatives are needed. Microbes have emerged as a promising biological alternative to harsh chemical extraction methods for leaching REE from complex sources and waste streams, opening new avenues for processing feedstocks and recycling these critical metals. M. extorquens AM1, a bacterium with the natural ability to solubilize and accumulate REE, provides a unique pathway toward selective REE bioleaching, bioaccumulation, and recovery that is scalable and does not rely on extremophile organisms and their inherent limitations. Future work will investigate this further with a focus on end-to-end conversion of feedstock to scalable rare earth oxide products.
We have reported optimization of several parameters to increase REE recovery yields in a 10 L bioreactor format, reaching yields of over 1.3% dry weight with the potential for up to 20% dry weight with further strain engineering. Our yields rival current state-of-the-art technologies when benchmarked against conditions when REE leaching is not limited (with citrate or with mll overexpression),43 and our process adds the major advantage of comparatively high REE uptake and storage from solid feedstocks. The potential of M. extorquens AM1 to recover REE is not limited to E-waste, as our process is compatible with even the most unrefined sources, such as monazite and bastnäsite ores, and pulverized smartphones (Table S2). M. extorquens AM1 is uniquely suited biologically for REE recovery through its production of lanthanophores, dedicated REE transport machinery, and its capability for producing REE-phosphate granules that will facilitate purification. The potential reduction in REE mining and refinement pollution and hazardous exposure make M. extorquens AM1 an attractive, green alternative to current chemical methodologies.
Acknowledgments
We would like to thank Yi Yao and Samir Budhathoki from the University of Wyoming for their assistance with some ICP-MS sample analysis, and Bang Luong, Caitlin Hoeber, Clarisse Hufana, Deval Patel, James Cai, Kim Luu, Krisha Gupta, Mariam Jacob, Nicholas Lien, Alice Huang, and Jennifer Lepe-Rodriguez for assistance with CFU measurements for smartphone growth curves. Part of this work was carried out under the auspices of the DOE by Lawrence Livermore National Laboratory under contract DEAC52-07NA27344 (LLNL-JRNL-851205).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c06775.
Supplemental methods; impact of inorganic phosphate on strain performance; effect of calcium in growth medium on Nd uptake; abiotic leaching of REE; growth rates of M. extorquens AM1 with organic acid addition; bioleaching and bioaccumulation measurements after growth with organic acids; effect of citrate on bioleaching and bioaccumulation; selective bioconcentration of REE; iterative bioaccumulation from a single swarf batch; plasmid map of pAZ1 for mll expression in trans; ratio of metals chemically leached from magnet swarf by organic acids; growth of M. extorquens AM1 with pure and complex REE sources; and flexibility of M. extorquens for REE bioleaching and bioaccumulation from complex sources (PDF)
Author Contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.
The information, data, or work presented herein was funded in part by the Advanced Research Projects Agency-Energy, United States Department of Energy, under Award Number DE-AR0001337.
The authors declare no competing financial interest.
Supplementary Material
References
- Summaries. United States Geological Survey: 2016; p 202. https://d9-wret.s3.us-west-2.amazonaws.com/assets/palladium/production/mineral-pubs/mcs/mcs2016.pdf.
- Alonso E.; Sherman A. M.; Wallington T. J.; Everson M. P.; Field F. R.; Roth R.; Kirchain R. E. Evaluating Rare Earth Element Availability: A Case with Revolutionary Demand from Clean Technologies. Environ. Sci. Technol. 2012, 46 (6), 3406–3414. 10.1021/es203518d. [DOI] [PubMed] [Google Scholar]
- Kingsnorth . Meeting the Challenges of Rare Earths Supply in the next Decade; Industrial Minerals Company of Australia Pty Ltd.: 2010.
- Binnemans K.; Jones P. T.; Blanpain B.; Van Gerven T.; Yang Y.; Walton A.; Buchert M. Recycling of Rare Earths: A Critical Review. J. Clean. Prod. 2013, 51, 1–22. 10.1016/j.jclepro.2012.12.037. [DOI] [Google Scholar]
- Graedel T. E.; Harper E. M.; Nassar N. T.; Reck B. K. On the Materials Basis of Modern Society. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (20), 6295–6300. 10.1073/pnas.1312752110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe, U. S. Department of Energy . Critical Materials Strategy (2010); Department of Energy (DOE): Washington, United States of America: 2011.
- Mancheri N. A.; Sprecher B.; Bailey G.; Ge J.; Tukker A. Effect of Chinese Policies on Rare Earth Supply Chain Resilience. Resour. Conserv. Recycl. 2019, 142, 101–112. 10.1016/j.resconrec.2018.11.017. [DOI] [Google Scholar]
- Zhang S. Study on Economic Significance of Rare Earth Mineral Resources Development Based on Goal Programming and Few-Shot Learning. Comput. Intell. Neurosci. 2022, 2022, 7002249 10.1155/2022/7002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Research ; Development. Rare Earth Elements: A Review of Production, Processing, Recycling, and Associated Environmental Issues. 2013.
- Balaram V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geoscience Frontiers 2019, 10 (4), 1285–1303. 10.1016/j.gsf.2018.12.005. [DOI] [Google Scholar]
- Talan D.; Huang Q. A Review of Environmental Aspect of Rare Earth Element Extraction Processes and Solution Purification Techniques. Miner. Eng. 2022, 179, 107430 10.1016/j.mineng.2022.107430. [DOI] [Google Scholar]
- Sahoo P. K.; Kim K.; Powell M. A.; Equeenuddin S. Recovery of Metals and Other Beneficial Products from Coal Fly Ash: A Sustainable Approach for Fly Ash Management. International Journal of Coal Science & Technology 2016, 3 (3), 267–283. 10.1007/s40789-016-0141-2. [DOI] [Google Scholar]
- Kumari A.; Panda R.; Jha M. K.; Kumar J. R.; Lee J. Y. Process Development to Recover Rare Earth Metals from Monazite Mineral: A Review. Miner. Eng. 2015, 79, 102–115. 10.1016/j.mineng.2015.05.003. [DOI] [Google Scholar]
- Liang T.; Li K.; Wang L. State of Rare Earth Elements in Different Environmental Components in Mining Areas of China. Environ. Monit. Assess. 2014, 186 (3), 1499–1513. 10.1007/s10661-013-3469-8. [DOI] [PubMed] [Google Scholar]
- Zhou L.; Li Z.; Liu W.; Liu S.; Zhang L.; Zhong L.; Luo X.; Liang H. Restoration of Rare Earth Mine Areas: Organic Amendments and Phytoremediation. Environ. Sci. Pollut. Res. Int. 2015, 22 (21), 17151–17160. 10.1007/s11356-015-4875-y. [DOI] [PubMed] [Google Scholar]
- Khawassek Y. M.; Eliwa A. A.; Gawad E. A.; Abdo S. M. Recovery of Rare Earth Elements from El-Sela Effluent Solutions. Journal of Radiation Research and Applied Sciences 2015, 8 (4), 583–589. 10.1016/j.jrras.2015.07.002. [DOI] [Google Scholar]
- Van Gosen B. S.; Verplanck P. L.; Seal R. R. II; Long K. R.; Gambogi J.. Rare-Earth Elements; US Geological Survey: 2017. 10.3133/pp1802O. [DOI] [Google Scholar]
- Brisson V. L.; Zhuang W.-Q.; Alvarez-Cohen L. Bioleaching of Rare Earth Elements from Monazite Sand. Biotechnol. Bioeng. 2016, 113 (2), 339–348. 10.1002/bit.25823. [DOI] [PubMed] [Google Scholar]
- Reed D. W.; Fujita Y.; Daubaras D. L.; Jiao Y.; Thompson V. S. Bioleaching of Rare Earth Elements from Waste Phosphors and Cracking Catalysts. Hydrometallurgy 2016, 166, 34–40. 10.1016/j.hydromet.2016.08.006. [DOI] [Google Scholar]
- Thompson V. S.; Gupta M.; Jin H.; Vahidi E.; Yim M.; Jindra M. A.; Nguyen V.; Fujita Y.; Sutherland J. W.; Jiao Y.; Reed D. W. Techno-Economic and Life Cycle Analysis for Bioleaching Rare-Earth Elements from Waste Materials. ACS Sustainable Chem. Eng. 2018, 6 (2), 1602–1609. 10.1021/acssuschemeng.7b02771. [DOI] [Google Scholar]
- Jin H.; Reed D. W.; Thompson V. S.; Fujita Y.; Jiao Y.; Crain-Zamora M.; Fisher J.; Scalzone K.; Griffel M.; Hartley D.; Sutherland J. W. Sustainable Bioleaching of Rare Earth Elements from Industrial Waste Materials Using Agricultural Wastes. ACS Sustainable Chem. Eng. 2019, 7 (18), 15311–15319. 10.1021/acssuschemeng.9b02584. [DOI] [Google Scholar]
- Park D. M.; Brewer A.; Reed D. W.; Lammers L. N.; Jiao Y. Recovery of Rare Earth Elements from Low-Grade Feedstock Leachates Using Engineered Bacteria. Environ. Sci. Technol. 2017, 51 (22), 13471–13480. 10.1021/acs.est.7b02414. [DOI] [PubMed] [Google Scholar]
- Čížková M.; Mezricky D.; Rucki M.; Tóth T. M.; Náhlík V.; Lanta V.; Bišová K.; Zachleder V.; Vítová M. Bio-Mining of Lanthanides from Red Mud by Green Microalgae. Molecules 2019, 24 (7), 071356 10.3390/molecules24071356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N.; Das D. Recovery of Rare Earth Metals through Biosorption: An Overview. J. Rare Earths 2013, 31 (10), 933–943. 10.1016/S1002-0721(13)60009-5. [DOI] [Google Scholar]
- Moriwaki H.; Masuda R.; Yamazaki Y.; Horiuchi K.; Miyashita M.; Kasahara J.; Tanaka T.; Yamamoto H. Application of Freeze-Dried Powders of Genetically Engineered Microbial Strains as Adsorbents for Rare Earth Metal Ions. ACS Appl. Mater. Interfaces 2016, 8 (40), 26524–26531. 10.1021/acsami.6b08369. [DOI] [PubMed] [Google Scholar]
- Alipanah M.; Park D. M.; Middleton A.; Dong Z.; Hsu-Kim H.; Jiao Y.; Jin H. Techno-Economic and Life Cycle Assessments for Sustainable Rare Earth Recovery from Coal Byproducts Using Biosorption. ACS Sustainable Chem. Eng. 2020, 8 (49), 17914–17922. 10.1021/acssuschemeng.0c04415. [DOI] [Google Scholar]
- Jin H.; Park D. M.; Gupta M.; Brewer A. W.; Ho L.; Singer S. L.; Bourcier W. L.; Woods S.; Reed D. W.; Lammers L. N.; Sutherland J. W.; Jiao Y. Techno-Economic Assessment for Integrating Biosorption into Rare Earth Recovery Process. ACS Sustainable Chem. Eng. 2017, 5 (11), 10148–10155. 10.1021/acssuschemeng.7b02147. [DOI] [Google Scholar]
- Kiyono M.; Omura H.; Omura T.; Murata S.; Pan-Hou H. Removal of Inorganic and Organic Mercurials by Immobilized Bacteria Having mer-ppk Fusion Plasmids. Appl. Microbiol. Biotechnol. 2003, 62 (2–3), 274–278. 10.1007/s00253-003-1282-y. [DOI] [PubMed] [Google Scholar]
- Johnston C. W.; Wyatt M. A.; Li X.; Ibrahim A.; Shuster J.; Southam G.; Magarvey N. A. Gold Biomineralization by a Metallophore from a Gold-Associated Microbe. Nat. Chem. Biol. 2013, 9 (4), 241–243. 10.1038/nchembio.1179. [DOI] [PubMed] [Google Scholar]
- Reith F.; Etschmann B.; Grosse C.; Moors H.; Benotmane M. A.; Monsieurs P.; Grass G.; Doonan C.; Vogt S.; Lai B.; Martinez-Criado G.; George G. N.; Nies D. H.; Mergeay M.; Pring A.; Southam G.; Brugger J. Mechanisms of Gold Biomineralization in the Bacterium Cupriavidus metallidurans. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (42), 17757–17762. 10.1073/pnas.0904583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok R. Inner Workings: How Bacteria Could Help Recycle Electronic Waste. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (3), 711–713. 10.1073/pnas.1820329116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S.; Misra C. S.; Gupta A.; Ballal A.; Apte S. K. Interaction of Uranium with Bacterial Cell Surfaces: Inferences from Phosphatase-Mediated Uranium Precipitation. Appl. Environ. Microbiol. 2016, 82 (16), 4965–4974. 10.1128/AEM.00728-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basnakova G.; Stephens E. R.; Thaller M. C.; Rossolini G. M.; Macaskie L. E. The Use of Escherichia coli Bearing a phoN Gene for the Removal of Uranium and Nickel from Aqueous Flows. Appl. Microbiol. Biotechnol. 1998, 50 (2), 266–272. 10.1007/s002530051288. [DOI] [PubMed] [Google Scholar]
- Roszczenko-Jasińska P.; Vu H. N.; Subuyuj G. A.; Crisostomo R. V.; Cai J.; Lien N. F.; Clippard E. J.; Ayala E. M.; Ngo R. T.; Yarza F.; Wingett J. P.; Raghuraman C.; Hoeber C. A.; Martinez-Gomez N. C.; Skovran E. Gene Products and Processes Contributing to Lanthanide Homeostasis and Methanol Metabolism in Methylorubrum extorquens AM1. Sci. Rep. 2020, 10 (1), 12663. 10.1038/s41598-020-69401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovran E.; Martinez-Gomez N. C. Microbiology. Just Add Lanthanides. Science 2015, 348 (6237), 862–863. 10.1126/science.aaa9091. [DOI] [PubMed] [Google Scholar]
- Martinez-Gomez N. C.; Vu H. N.; Skovran E. Lanthanide Chemistry: From Coordination in Chemical Complexes Shaping Our Technology to Coordination in Enzymes Shaping Bacterial Metabolism. Inorg. Chem. 2016, 55 (20), 10083–10089. 10.1021/acs.inorgchem.6b00919. [DOI] [PubMed] [Google Scholar]
- Maroufi S.; Nekouei R. K.; Hossain R.; Assefi M.; Sahajwalla V. Recovery of Rare Earth (i.e., La, Ce, Nd, and Pr) Oxides from End-of-Life Ni-MH Battery via Thermal Isolation. ACS Sustainable. Chem. Eng. 2018, 6 (9), 11811–11818. 10.1021/acssuschemeng.8b02097. [DOI] [Google Scholar]
- Deshmane V. G.; Islam S. Z.; Bhave R. R. Selective Recovery of Rare Earth Elements from a Wide Range of E-Waste and Process Scalability of Membrane Solvent Extraction. Environ. Sci. Technol. 2020, 54 (1), 550–558. 10.1021/acs.est.9b05695. [DOI] [PubMed] [Google Scholar]
- Peelman S.; Kooijman D.; Sietsma J.; Yang Y. Hydrometallurgical Recovery of Rare Earth Elements from Mine Tailings and WEEE. Journal of Sustainable Metallurgy 2018, 4 (3), 367–377. 10.1007/s40831-018-0178-0. [DOI] [Google Scholar]
- Good N. M.; Lee H. D.; Hawker E. R.; Su M. Z.; Gilad A. A.; Martinez-Gomez N. C. Hyperaccumulation of Gadolinium by Methylorubrum extorquens AM1 Reveals Impacts of Lanthanides on Cellular Processes Beyond Methylotrophy. Front. Microbiol. 2022, 13, 820327 10.3389/fmicb.2022.820327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu H. N.; Subuyuj G. A.; Vijayakumar S.; Good N. M.; Martinez-Gomez N. C.; Skovran E. Lanthanide-Dependent Regulation of Methanol Oxidation Systems in Methylobacterium extorquens AM1 and Their Contribution to Methanol Growth. J. Bacteriol. 2016, 198 (8), 1250–1259. 10.1128/JB.00937-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney N. F.; Kaczmarek M. E.; Ward L. M.; Swanson P. K.; Lee M.-C.; Marx C. J. Development of an Optimized Medium, Strain and High-Throughput Culturing Methods for Methylobacterium extorquens. PLoS One 2013, 8 (4), e62957 10.1371/journal.pone.0062957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep P.; Mahadevan R.; Yakunin A. F. Heavy Metal Removal by Bioaccumulation Using Genetically Engineered Microorganisms. Front Bioeng Biotechnol 2018, 6, 157. 10.3389/fbioe.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogendoorn C.; Roszczenko-Jasińska P.; Martinez-Gomez N. C.; de Graaff J.; Grassl P.; Pol A.; Op den Camp H. J. M.; Daumann L. J. Facile Arsenazo III-Based Assay for Monitoring Rare Earth Element Depletion from Cultivation Media for Methanotrophic and Methylotrophic Bacteria. Appl. Environ. Microbiol. 2018, 84 (8), e02887 10.1128/AEM.02887-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzoldt T.Growthrates: R Package Growthrates; Github.
- Firsching F. H.; Brune S. N. Solubility Products of the Trivalent Rare-Earth Phosphates. J. Chem. Eng. Data 1991, 36 (1), 93–95. 10.1021/je00001a028. [DOI] [Google Scholar]
- Chowdhury N. A.; Deng S.; Jin H.; Prodius D.; Sutherland J. W.; Nlebedim I. C. Sustainable Recycling of Rare-Earth Elements from NdFeB Magnet Swarf: Techno-Economic and Environmental Perspectives. ACS Sustainable Chem. Eng. 2021, 9 (47), 15915–15924. 10.1021/acssuschemeng.1c05965. [DOI] [Google Scholar]
- Binnemans K.; Jones P. T. Rare Earths and the Balance Problem. Journal of Sustainable Metallurgy 2015, 1 (1), 29–38. 10.1007/s40831-014-0005-1. [DOI] [Google Scholar]
- Yang Y.; Walton A.; Sheridan R.; Güth K.; Gauß R.; Gutfleisch O.; Buchert M.; Steenari B.-M.; Van Gerven T.; Jones P. T.; Binnemans K. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. Journal of Sustainable Metallurgy 2017, 3 (1), 122–149. 10.1007/s40831-016-0090-4. [DOI] [Google Scholar]
- Priya A.; Hait S. Comparative Assessment of Metallurgical Recovery of Metals from Electronic Waste with Special Emphasis on Bioleaching. Environ. Sci. Pollut. Res. Int. 2017, 24 (8), 6989–7008. 10.1007/s11356-016-8313-6. [DOI] [PubMed] [Google Scholar]
- Brierley C. L.; Brierley J. A. Progress in Bioleaching: Part B: Applications of Microbial Processes by the Minerals Industries. Appl. Microbiol. Biotechnol. 2013, 97 (17), 7543–7552. 10.1007/s00253-013-5095-3. [DOI] [PubMed] [Google Scholar]
- Zhuang W.-Q.; Fitts J. P.; Ajo-Franklin C. M.; Maes S.; Alvarez-Cohen L.; Hennebel T. Recovery of Critical Metals Using Biometallurgy. Curr. Opin. Biotechnol. 2015, 33, 327–335. 10.1016/j.copbio.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N.; Sharma D. K. Biohydrometallurgy for Nonsulfidic Minerals—A Review. Geomicrobiol. J. 2004, 21 (3), 135–144. 10.1080/01490450490275271. [DOI] [Google Scholar]
- Kermer R.; Hedrich S.; Bellenberg S.; Brett B.; Schrader D.; Schönherr P.; Köpcke M.; Siewert K.; Günther N.; Gehrke T.; Sand W.; Räuchle K.; Bertau M.; Heide G.; Weitkämper L.; Wotruba H.; Ludwig H.-M.; Partusch R.; Schippers A.; Reichel S.; Glombitza F.; Janneck E. Lignite Ash: Waste Material or Potential Resource - Investigation of Metal Recovery and Utilization Options. Hydrometallurgy 2017, C (168), 141–152. 10.1016/j.hydromet.2016.07.002. [DOI] [Google Scholar]
- Glombitza F.; Reichel S. Metal-Containing Residues from Industry and in the Environment: Geobiotechnological Urban Mining. Adv. Biochem. Eng. Biotechnol. 2013, 141, 49–107. 10.1007/10_2013_254. [DOI] [PubMed] [Google Scholar]
- Shin D.; Kim J.; Kim B.-S.; Jeong J.; Lee J.-C. Use of Phosphate Solubilizing Bacteria to Leach Rare Earth Elements from Monazite-Bearing Ore. Minerals 2015, 5 (2), 189–202. 10.3390/min5020189. [DOI] [Google Scholar]
- Hassanien W. A. G.; Desouky O. A. N.; Hussien S. S. E. Bioleaching of Some Rare Earth Elements from Egyptian Monazite Using Aspergillus ficuum and Pseudomonas aeruginosa. Walailak J. Sci. Technol. 2014, 11 (9), 809–823. 10.2004/wjst.v11i6.481. [DOI] [Google Scholar]
- Amiri F.; Yaghmaei S.; Mousavi S. M. Bioleaching of Tungsten-Rich Spent Hydrocracking Catalyst Using Penicillium simplicissimum. Bioresour. Technol. 2011, 102 (2), 1567–1573. 10.1016/j.biortech.2010.08.087. [DOI] [PubMed] [Google Scholar]
- Qu Y.; Lian B. Bioleaching of Rare Earth and Radioactive Elements from Red Mud Using Penicillium Tricolor RM-10. Bioresour. Technol. 2013, 136, 16–23. 10.1016/j.biortech.2013.03.070. [DOI] [PubMed] [Google Scholar]
- Maes S.; Zhuang W.-Q.; Rabaey K.; Alvarez-Cohen L.; Hennebel T. Concomitant Leaching and Electrochemical Extraction of Rare Earth Elements from Monazite. Environ. Sci. Technol. 2017, 51 (3), 1654–1661. 10.1021/acs.est.6b03675. [DOI] [PubMed] [Google Scholar]
- Corbett M. K.; Eksteen J. J.; Niu X.-Z.; Croue J.-P.; Watkin E. L. J. Interactions of Phosphate Solubilising Microorganisms with Natural Rare-Earth Phosphate Minerals: A Study Utilizing Western Australian Monazite. Bioprocess Biosyst. Eng. 2017, 40 (6), 929–942. 10.1007/s00449-017-1757-3. [DOI] [PubMed] [Google Scholar]
- Schmitz A. M.; Pian B.; Medin S.; Reid M. C.; Wu M.; Gazel E.; Barstow B. Generation of a Gluconobacter oxydans Knockout Collection for Improved Extraction of Rare Earth Elements. Nat. Commun. 2021, 12 (1), 6693. 10.1038/s41467-021-27047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y. Organic Species of Lanthanum in Natural Environments: Implications to Mobility of Rare Earth Elements in Low Temperature Environments. Appl. Geochem. 2011, 26 (7), 1130–1137. 10.1016/j.apgeochem.2011.04.003. [DOI] [Google Scholar]
- Zytnick A. M.; Gutenthaler S. M.; Aron A. T.; Reitz Z. L.; Phi M. T.; Good N. M.; Petras D.; Daumann L. J.; Martinez-Gomez N. C. Discovery and Characterization of the First Known Biological Lanthanide Chelator. bioRxiv 2023, 476857 10.1101/2022.01.19.476857. [DOI] [Google Scholar]
- Marx C. J.; Lidstrom M. E. Development of Improved Versatile Broad-Host-Range Vectors for Use in Methylotrophs and Other Gram-Negative Bacteria. Microbiology 2001, 147 (Pt 8), 2065–2075. 10.1099/00221287-147-8-2065. [DOI] [PubMed] [Google Scholar]
- Lumpe H.; Daumann L. J. Studies of Redox Cofactor Pyrroloquinoline Quinone and Its Interaction with Lanthanides(III) and Calcium(II). Inorg. Chem. 2019, 58 (13), 8432–8441. 10.1021/acs.inorgchem.9b00568. [DOI] [PubMed] [Google Scholar]
- Arshi P. S.; Vahidi E.; Zhao F. Behind the Scenes of Clean Energy: The Environmental Footprint of Rare Earth Products. ACS Sustainable Chem. Eng. 2018, 6 (3), 3311–3320. 10.1021/acssuschemeng.7b03484. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.