Abstract
In recent years, the severity of plant diseases caused by plant pathogenic fungi and viruses has been on the rise. However, there is a limited availability of pesticide chemicals in the market for effectively controlling both fungal and viral infections. To solve this problem, a series of novel pyrimidine derivatives containing a 1,3,4-oxadiazole thioether fragment were synthesized. Among them, compound 6s exhibited remarkable in vivo protection activity against tobacco mosaic virus, demonstrating the superior 50% effective concentration (EC50) value of 0.42 μM, outperforming ningnanmycin (0.60 μM). Meanwhile, compound 6s exhibited remarkable antifungal activity against Botrytis cinerea Pers. in postharvest blueberry in vitro, with an EC50 value of 0.011 μM, surpassing the inhibition rate of Pyrimethanil (0.262 μM). Additionally, compound 6s also demonstrated remarkable curative and protection activities against blueberry fruit gray mold in vivo, with control efficiencies of 54.2 and 60.4% at 200 μg/mL concentration, respectively, which were comparable to those of Pyrimethanil (49.3 and 63.9%, respectively). Scanning electron microscopy showed that the compound 6s-treated hyphae of B. cinerea Pers. in postharvest blueberry became abnormally collapsed and shriveled. Furthermore, the molecular docking simulation demonstrated that compound 6s formed hydrogen bonds with SER-17, ARG-43, and SER-39 of succinate dehydrogenase (SDH), providing a possible explanation for the mechanism of action between the target compounds and SDH. This study represents the first report on the antiviral and antifungal activities of novel pyrimidine derivatives containing a 1,3,4-oxadiazole thioether fragment.
1. Introduction
The future direction of pesticide development will be oriented toward highly potent, low residual, and eco-friendly pesticides in order to meet the demands of sustainable development in modern agriculture. Compounds featuring diverse heterocyclic groups hold significant importance in the field of drug development.1 The majority of current pesticide patents revolve around heterocyclic compounds, particularly those containing nitrogen (N) atoms that are widely present in natural products and frequently utilized in pesticides and pharmaceuticals due to their unique biological and pharmaceutical activities.2 Thus, the incorporation of nitrogen heterocyclic structures into organic compounds often introduces novel functional properties and garners considerable attention.3−5
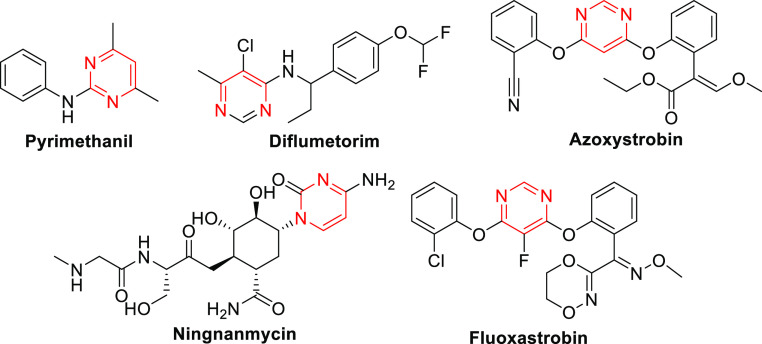
Pyrimidine and its derivatives, a class of heterocyclic compounds containing two N atoms, which are widely distributed throughout various organisms and play a crucial role in nucleic acid structures.6 Pyrimidine has found extensive applications in the fields of medicine and pesticides, with many commercial pesticides incorporating a pyrimidine moiety (Figure 1).7−11 Numerous studies have reported that a plethora of pyrimidine derivatives exhibit diverse biological activities, including antitubercular, antibacterial, antiviral, antitumor, anti-inflammatory, insecticidal, antifungal, and herbicidal properties.12−24 Therefore, it has been extensively utilized in the fields of medicine and pesticides. Particularly in recent years, a large number of literature reports have documented the antifungal and antiviral activities of pyrimidine and its derivatives, garnering significant attention from researchers.25 Meanwhile, pyrimidine derivatives have shown promise as a potential starting point for the discovery of novel inhibitors targeting succinate dehydrogenase (SDH).26−28
Figure 1.
Structures of some commercial pesticides containing a pyrimidine moiety.
The 1,3,4-oxadiazole skeleton, a class of heterocyclic compounds containing two N atoms, is widely utilized as a fundamental building block in natural products and pharmaceutical molecules due to its remarkable biological and pharmacological activities.29−34 Therefore, the development of synthetic methodologies for 1,3,4-oxadiazole frameworks has consistently been a focal point of research in the chemical community. Notable commercially available pesticides containing a 1,3,4-oxadiazole moiety include oxadiazon and metoxadiazone. Furthermore, several studies from the literature have also documented the remarkable antiviral and fungicidal activities exhibited by derivatives of 1,3,4-oxadiazoles.35 Meanwhile, thioether compounds are abundantly found in natural products and possess significant research value in the fields of pharmaceutical chemistry and pesticides. These compounds exhibit diverse pesticidal activities including antiviral, antibacterial, antifungal, and herbicidal properties.36−39 Additionally, research has demonstrated that the incorporation of an electron-donating S atom to simultaneously connect multiple heterocyclic active centers in the molecule, specifically through a thioether structure, is conducive to enhancing the receptor–ligand affinity and effectively improving the compound’s biological activity.40−42 Recently, there have been many researchers focusing on the 1,3,4-oxadiazole thioether derivatives with diverse biological activities.43−51
Motivated by the above findings and to find novel lead compounds with good biological activity, the aims of this study are to (1) introduce the active structure of 1,3,4-oxadiazole thioether group into the pyrimidine ring to build a series of novel pyrimidine derivatives containing a 1,3,4-oxadiazole thioether fragment; (2) investigate the in vivo antiviral activity against tobacco mosaic virus (TMV) using the half-leaf method; (3) investigate the in vitro antifungal activities against Botryosphaeria dothidea (Moug.) Ces. De Not. in postharvest kiwifruit, Phomopsis sp. in postharvest kiwifruit, Botrytis cinerea Pers. in postharvest blueberry, B. cinerea Pers. in postharvest cucumber, Sclerotinia sclerotiorum (Lib.) de Bary in oilseed rape, B. cinerea Pers. in postharvest tobacco, B. cinerea Pers. in postharvest strawberry, Colletotrichum sp. in postharvest blueberry, and Magnaporthe grisea (Hebert) Barr in rice; (4) investigate the in vivo activity against blueberry fruit gray mold; (5) molecular docking study of the target compounds to SDH.
2. Materials and Methods
2.1. Instruments and Chemicals
All chemical reagents were purchased from Aladdin Reagent (Shanghai, China) and Energy Chemical (Shanghai, China), respectively. The melting points (m.p.) of all the target compounds were determined on an X-4B microscope m.p. apparatus and were uncorrected (Shanghai electrophysics optical Instrument Co., LTD, China). All 1H NMR and 13C NMR spectra data were recorded on a Bruker NEO-600 spectrometer. High-resolution mass spectrometry (HRMS) data of the target compounds were obtained using a Thermo Scientific Q-Exactive (Thermo Fisher Scientific, MA, USA).
2.2. Chemical Synthesis
2.2.1. General Procedure for the Synthesis of Intermediates 1–5
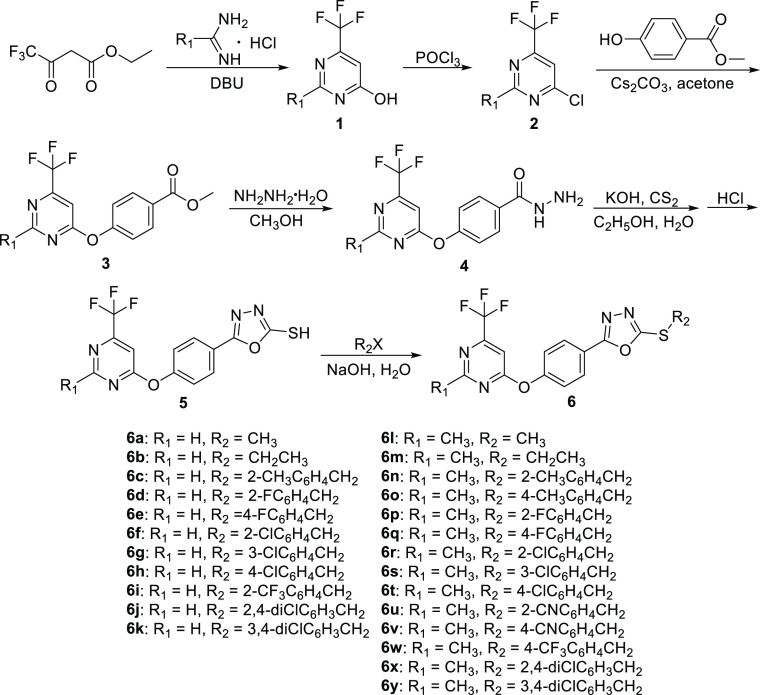
As shown in Scheme 1, to a 100 mL three-necked round-bottomed flask, ethyl trifluoroacetoacetate (50 mmol), formimidamide hydrochloride or acetamidine hydrochloride (80 mmol), and diazabicycloundecene (50 mmol) were added. After refluxing for 10 h, the solvent was evaporated under vacuum. Subsequently, the resulting mixture was treated with 50 mL of distilled water and extracted with ethyl acetate followed by evaporating excess solvent to obtain intermediate 1.
Scheme 1. Synthetic Route of the Target Compounds 6a–6y.
Intermediate 1 (40 mmol) was introduced into a 100 mL three-necked round-bottomed flask, followed by the addition of POCl3 (60 mmol), acetonitrile (40 mL), and N,N-diisopropylethylamine (30 mmol). After refluxing for 5 h, the excess solvent was removed under vacuum. Subsequently, the resulting mixture was treated with 50 mL of distilled water and adjusted to pH = 9 using 10% NaHCO3 solution. The aqueous phase was extracted twice with dichloromethane before evaporating the excess solvent to obtain intermediate 2.
Intermediate 2 (10 mmol), ethyl 4-hydroxybenzoate (12 mmol), Cs2CO3 (20 mmol), and acetone (50 mL) were added to a 100 mL three-necked round-bottomed flask. The reaction was carried out at room temperature for 2–4 h. After the reaction was completed, the excess solvent was vacuum evaporated and then recrystallization with ethanol to obtain intermediate 3.
To a 100 mL three-necked round-bottomed flask, intermediate 3 (20 mmol), 80% hydrazine hydrate (60 mmol), and methanol (40 mL) were added and reacted under reflux conditions for 5–7 h. After cooling to room temperature, the white solid precipitated from the reaction solution was filtered and recrystallized from absolute ethanol to obtain intermediate 4.
Carbon disulfide (36 mmol) was added dropwise to the mixture of intermediate 4 (30 mmol), KOH (45 mmol), and ethanol (500 mL) in a 1000 mL three-necked round-bottomed flask. After refluxing at 85 °C for 8 h, the white precipitate was filtered and neutralized with saturated NaHCO3 solution. The filtrate was acidified by 5% HCl solution, and the obtained solid was filtered. The solid was washed with water for 3 times. The key intermediate 5 was obtained by recrystallization with ethanol.
2.2.2. General Procedure for the Synthesis of the Target Compounds 6a–6y
As shown in Scheme 1, intermediate 5 (2 mmol), different substituted halogenated hydrocarbons or benzyl chlorides (2 mmol), and NaOH (2.2 mmol) dissolved in water (15 mL) were added to a 50 mL three-necked round-bottomed flask and stirred at room temperature for 8–10 h. After the reaction was completed, the residue was filtered and recrystallized from ethanol to obtain target compounds 6a–6y.
2.3. Anti-TMV Activity Test In Vitro
The anti-TMV activity of the target compounds was determined according to the half-leaf method.52,53 The inhibition rate I (%) was calculated according to the following formula, where C was the average local lesion number of the CK group and T was the average local lesion number of the treatment group.
2.3.1. Curative Activity Test of the Target Compounds against TMV In Vivo
The leaves of Nicotiana tabacum L. of the same age were inoculated with TMV (at a concentration of 6 × 10–3 mg/mL) through immersion and gentle brushing, after being pretreated with silicon carbide abrasion. Following inoculation, the leaves were rinsed with water for 0.5 h. A compound solution was applied to the left side of the leaves, while a solvent was applied to the right side as a control. The number of localized lesions was counted and recorded 3–4 days postinoculation. Three replications were conducted for each compound.
2.3.2. Protection Activity Test of the Target Compounds against TMV In Vivo
Each target compound solution was applied to the left side of growing N. tabacum L. leaves of the same age, while the solvent was applied to the right side as a control. The leaves, previously dusted with silicon carbide, were inoculated with TMV using a brush dipped in a concentration of 6 × 10–3 mg/mL after 12 h and subsequently washed with water and gently rubbed along the veins once or twice. The number of local lesions that appeared 3–4 days after inoculation was recorded. Three replications were conducted for each compound.
2.3.3. Inactivation Activity Test of the Target Compounds against TMV In Vivo
The virus was effectively inhibited when it was mixed with an equimolar target compound solution for 30 min. As a control, the solvent and virus mixture were inoculated on the right side of N. tabacum L. leaves, which had been previously treated with silicon carbide. The number of local lesions was recorded 3–4 days after inoculation. Three replications were conducted for each compound.
2.4. Antifungal Activity Test In Vitro
The in vitro antifungal activities of the target compound 6a–6y against nine pathogenic fungi (B. dothidea (Moug.) Ces. De Not. in postharvest kiwifruit, Phomopsis sp. in postharvest kiwifruit, B. cinerea Pers. in postharvest blueberry, B. cinerea Pers. in postharvest cucumber, S. sclerotiorum (Lib.) de Bary in oilseed rape, B. cinerea Pers. in postharvest tobacco, B. cinerea Pers. in postharvest strawberry, Colletotrichum sp. in postharvest blueberry, and M. grisea (Hebert) Barr in rice) were determined by the mycelial growth rate method.46 The target compound (5 mg) was dissolved in 1 mL of dimethyl sulfoxide (DMSO), followed by the addition of 9 mL of sterile water. The mixture was then combined with 90 mL of potato dextrose agar (PDA) medium and thoroughly shaken to form a solution with a concentration of 50 μg/mL. Subsequently, the mixed PDA medium was poured into 9 Petri dishes, with each treatment being repeated three times. After that, mycelia dishes measuring approximately 0.5 cm in diameter were taken from the middle of the PDA plates using a sterile inoculation needle. The inoculated PDA plates were incubated at 28 °C for a period of 3–4 days until the mycelium growth on the CK group reached a diameter of 5–6 cm. The inhibition rate I (%) was calculated using formula (2), where C (cm) and T (cm) represented the fungal diameters on untreated and treated PDA plates, respectively.
2.5. Antifungal Activity Test In Vivo
The in vivo curative and protection activities of compound 6s against blueberry fruit gray mold were determined according to the reported method.54,55 The control efficacy of compound 6s against blueberry fruit gray mold was calculated by the following formula (3), where A0 (cm) and A1 (cm) were the lengths of untreated and treated lesions, respectively.
2.5.1. In Vivo Curative Effect of Compound 6s against Blueberry Fruit Gray Mold
The fresh blueberry fruits were washed and then sterilized by evenly applying 75% ethanol. Subsequently, the surface of the fruits was punctured with a sterile toothpick, and mycelia dishes of B. cinerea Pers. in postharvest blueberry measuring approximately 0.5 cm in diameter were inoculated at the puncture site. After 24 h, prepared solutions of compound 6s with concentrations of 100, 50, and 25 μg/mL were uniformly applied to the fresh blueberry fruits. The treated fruits were cultured in a light incubator (temperature: 25 °C; relative humidity: 100%) for a period of 3–4 days, during which the length of lesions on the blueberry fruits was measured. Each treatment group consisted of three replicates with nine fresh blueberry fruits per replicate, while Pyrimethanil served as the control agent.
2.5.2. In Vivo Protection Activity of Compound 6s against Blueberry Fruit Gray Mold
The blueberry fruits were washed and then treated with 75% ethanol applied evenly. Subsequently, the blueberry fruits were disinfected by applying a solution containing compound 6s at concentrations of 100, 50, and 25 μg/mL. Afterward, the solution was uniformly distributed onto the surface of the blueberry fruits. Following a period of 24 h, sterile toothpicks were used to puncture the fruit’s surface, and mycelia dishes (approximately 0.5 cm in diameter) of B. cinerea Pers. in postharvest blueberry were inoculated at each puncture site. The blueberries were then cultured in a light incubator under conditions of temperature (25 °C) and relative humidity (100%) for a duration of 3–4 days, during which the length of lesions on the blueberry fruits was measured. Each treatment group consisted of three replicates with nine blueberry fruits per replicate; Pyrimethanil served as the control agent.
2.6. SEM Observations
The morphological changes of B. cinerea Pers. in postharvest blueberries treated by compound 6s were studied by scanning electron microscopy (SEM) according to the reported method.56 A mycelia dish (approximately 0.5 cm in diameter) of B. cinerea Pers. in postharvest blueberry was inoculated on the PDA medium containing compound 6s at 25 μg/mL concentration. When the diameter of the fungus cake is 1–2 cm, the in situ fixation method is used to cut the culture with the mycelium site into a square with a size of less than 1 cm and a thickness of 0.2 cm, and directly put it into 2.5% glutaraldehyde to fix Bbo cells at 4 °C. First, the mycelium is picked out, dehydrated with different concentrations of ethanol gradient, and transited with isoamyl acetate. Finally, the mycelium sample is placed in the critical point dryer for drying, and then the dried mycelium sample adheres to the platform, and the gold coating is sprayed by an ion sputtering instrument. The morphological changes of B. cinerea Pers. in postharvest blueberries treated by compound 6s were observed under the scanning electron microscope.
2.7. Molecular Docking
The enzyme SDH, which plays a crucial role in connecting the respiratory electron transport chain and tricarboxylic acid cycle, has been identified as an optimal target for the development of potent fungicides.26−28 The three-dimensional structure of compound 6s was generated using ChemDraw Ultra 20.0 software (PerkinElmer, Waltham, MA, USA), while the protein SDH receptor structure (PDB: 2FBW) was obtained from the RCBs PDB database (https://www.rcsb.org/structure/2FBW). A molecular docking study was conducted to investigate the binding mode of compound 6s with SDH using Discovery Studio 2.5 software (Accelrys Inc., San Diego, USA) following a previously reported method.57,58
3. Results and Discussion
3.1. Chemistry
Using ethyl trifluoroacetate as the starting material, the target compounds 6a–6y were synthesized with yields of 42.5–84.6%. All the structures of the target compound were confirmed by 1H NMR, 13C NMR, and HRMS. The physical characteristics, 1H NMR, 13C NMR, and HRMS data of the target compounds 6a–6y are shown below, while the spectra of 1H NMR, 13C NMR, and HRMS for the target compounds 6a–6y are shown in Supporting Information. In the 1H NMR spectra of compound 6s, the presence of a hydrogen (H) atom in the pyrimidine structure was confirmed by a singlet at 7.60 ppm. Meanwhile, in the 13C NMR spectra of compound 6s, the presence of a carbon (C) atom in the CF3 group was revealed by a quartet at 121.80 ppm. Furthermore, accurate assignment of the molecular weight for compound 6s was achieved through HRMS data, which showed [M + Na]+ ions with an m/z value of 501.03616.
3.1.1. 2-(Methylthio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6a)
Yellow solid; yield 50.8%; mp 123.2–124.7 °C; 1H NMR (DMSO-d6, 600 MHz, ppm): δ 8.99 (s, 1H, pyrimidine-H), 8.09 (d, 2H, J = 8.40 Hz, Ph-H), 7.88 (s, 1H, pyrimidine-H), 7.86 (s, 1H, Ph-H), 7.53 (d, 2H, J = 9.00 Hz, Ph-H), 2.79 (s, 3H, CH3); 13C NMR (DMSO-d6, 150 MHz, ppm): δ 170.25, 165.29, 164.95, 159.72, 156.24 (q, J = 36.60 Hz), 154.70, 128.75, 123.34, 121.78 (q, J = 273.45 Hz), 121.49, 107.09, 14.79; HRMS(ESI) calcd for C14H9F3N4O2S: [M + Na]+, 377.02832; found, 377.02905.
3.1.2. 2-(Ethylthio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6b)
Yellow solid; yield 66.8%; mp 58.8–60.1 °C; 1H NMR (DMSO-d6, 600 MHz, ppm): δ 8.99 (s, 1H, pyrimidine-H), 8.09 (d, 2H, J = 9.00 Hz, Ph-H), 7.85 (s, 1H, pyrimidine-H), 7.53 (d, 2H, J = 9.00 Hz, Ph-H), 3.35 (t, 2H, J = 7.20 Hz, CH2), 1.45 (q, 3H, J = 7.80 Hz, CH3); 13C NMR (DMSO-d6, 150 MHz, ppm): δ 170.24, 165.94, 164.40, 159.72, 156.25 (q, J = 35.25 Hz), 154.70, 128.76, 123.33, 121.77 (q, J = 273.30 Hz), 121.49, 115.66, 107.07, 27.16, 15.32; HRMS (ESI) calcd for C15H11F3N4O2S: [M + Na]+, 391.04382; found, 391.04470.
3.1.3. 2-((2-Methylbenzyl)thio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6c)
Yellow solid; yield 75.8%; mp 76.8–77.6 °C; 1H NMR (DMSO-d6, 600 MHz, ppm): δ 9.00 (s, 1H, pyrimidine-H), 8.10 (d, 2H, J = 8.40 Hz, Ph-H), 7.88 (s, 1H, pyrimidine-H), 7.61 (s, 1H, Ph-H), 7.55 (d, 2H, J = 8.40 Hz, Ph-H), 7.42 (d, 1H, J = 7.80 Hz, Ph-H), 7.25–7.21 (m, 2H, Ph-H), 7.18–7.15 (m, 1H, Ph-H), 4.62 (s, 2H, CH2), 2.42 (s, 3H, CH3); 13C NMR (DMSO-d6, 150 MHz, ppm): δ 170.25, 165.20, 163.72, 159.73, 156.25 (q, J = 35.10 Hz), 154.81, 137.34, 134.24, 130.98, 130.52, 128.84, 128.73, 126.60, 123.38, 121.80 (q, J = 273.45 Hz), 121.40, 116.1, 107.12, 35.03, 19.22; HRMS (ESI) calcd for C21H15F3N4O2S: [M + Na]+, 467.07529; found, 467.07600.
3.1.4. (2-((2-Fluorobenzyl)thio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6d)
Brown solid; yield 49.8%; mp 91.7–92.9 °C; 1H NMR (DMSO-d6, 600 MHz, ppm): δ 8.98 (s, 1H, pyrimidine-H), 8.03–8.01 (m, 2H, Ph-H), 7.86 (s, 1H, pyrimidine-H), 7.58–7.56 (m, 1H, phenyl-H), 7.50–7.47 (m, 2H, Ph-H), 7.39–7.35 (m, 1H, Ph-H), 7.25–7.22 (m, 1H, Ph-H), 7.20–7.18 (m, 1H, Ph-H), 4.65 (s, 2H, –CH2−); 13C NMR (DMSO-d6, 150 MHz, ppm): δ 170.34, 167.98, 164.83, 161.79 (q, J = 245.24 Hz), 159.75, 156.24 (q, J = 34.83 Hz), 154.34, 132.03 (d, J = 3.50 Hz), 130.69 (d, J = 8.43 Hz), 129.78, 127.62, 125.11 (d, J = 3.45 Hz), 123.90 (d, J = 14.66 Hz), 121.82 (q, J = 273.60 Hz), 116.09 (d, J = 20.88 Hz), 107.07, 31.85 (d, J = 2.30 Hz); HRMS (ESI) calcd for C20H12F4N4O2S: [M + Na]+, 471.05014; found, 471.05093.
3.1.5. 2-((4-Fluorobenzyl)thio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6e)
Yellow solid; yield 80.5%; mp 105.2–107.0 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 9.00 (s, 1H, pyrimidine-H), 8.09 (d, 2H, J = 9.00 Hz, Ph-H), 7.86 (s, 1H, pyrimidine-H), 7.56–7.53 (m, 4H, Ph-H), 7.20 (t, 1H, J = 9.00 Hz, Ph-H), 4.60 (s, 2H, CH2); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.24, 165.20, 163.82, 162.93, 161.31, 159.72, 156.31 (q, J = 35.40 Hz), 154.82, 1133.42, 133.41, 131.64, 131.59, 128.82, 123.34, 121.79 (q, J = 273.30 Hz), 121.40, 115.93, 107.09, 35.63; HRMS (ESI) calcd for C20H12F4N4O2S: [M + Na]+, 471.05048; found, 471.05093.
3.1.6. 2-((2-Chlorobenzyl)thio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6f)
Yellow solid; yield 82.2%; mp 93.1–94.9 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 9.00 (s, 1H, pyrimidine-H), 8.09 (d, 2H, J = 8.40 Hz, Ph-H), 7.88 (s, 1H, pyrimidine-H), 7.65 (dd, 1H, J1 = 2.40 Hz, J2 = 4.80 Hz, Ph-H), 7.55 (d, 2H, J = 9.00 Hz, Ph-H), 7.53 (dd, 1H, J1 = 1.80 Hz, J2 = 6.00 Hz, Ph-H), 7.48 (m, 2H, Ph-H), 4.67 (s, 2H, CH2); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.25, 165.40, 163.38, 159.73, 156.25 (q, J = 34.95 Hz), 154.85, 134.37, 133.80, 132.10, 130.49, 130.12, 128.87, 127.98, 123.40, 121.80 (q, J = 273.45 Hz), 121.37, 107.14, 34.77; HRMS (ESI) calcd for C20H12ClF3N4O2S: [M + Na]+, 487.02057; found, 487.02138.
3.1.7. 2-((3-Chlorobenzyl)thio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6g)
White solid; yield 65.5%; mp 105.6–106.5 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 9.00 (s, 1H, pyrimidine-H), 8.09 (d, 2H, J = 8.40 Hz, Ph-H), 7.88 (s, 1H, pyrimidine-H), 7.61 (s, 1H, Ph-H), 7.54 (d, 2H, J = 9.00 Hz, Ph-H), 7.20 (d, 1H, J = 7.20 Hz, Ph-H), 7.48 (m, 2H, Ph-H), 4.60 (s, 2H, CH2); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.25, 165.25, 163.72, 159.73, 156.45 (q, J = 35.40 Hz), 154.81, 139.93, 133.46, 130.90, 129.42, 128.83, 128.25, 128.18, 123.37, 121.79 (q, J = 272.85 Hz), 121.36, 107.12, 35.57; HRMS (ESI) calcd for C20H12ClF3N4O2S: [M + Na]+, 487.02130; found, 487.02138.
3.1.8. 2-((4-Chlorobenzyl)thio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6h)
White solid; yield 62.4%; mp 83.0–84.5 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 9.00 (s, 1H, pyrimidine-H), 8.09 (d, 2H, J = 8.40 Hz, Ph-H), 7.88 (s, 1H, pyrimidine-H), 7.54–7.52 (m, 4H, Ph-H), 7.43 (d, 2H, J = 8.40 Hz, Ph-H), 4.60 (s, 2H, CH2); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.25, 165.20, 163.78, 159.73, 156.45 (q, J = 35.40 Hz), 154.82, 136.40, 131.43, 129.03, 128.84, 123.36, 121.80 (q, J = 273.15 Hz), 121.36, 107.14, 35.55; HRMS (ESI) calcd for C20H12ClF3N4O2S: [M + Na]+, 487.02029; found, 487.02138.
3.1.9. 2-((2-(Trifluoromethyl)benzyl)thio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6i)
White solid; yield 43.5%; mp 112.2–113.4 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 9.00 (s, 1H, pyrimidine-H), 8.08 (d, 2H, J = 8.40 Hz, Ph-H), 7.78 (s, 1H, pyrimidine-H), 7.81 (d, 1H, J = 7.80 Hz, Ph-H), 7.79 (d, 1H, J = 7.80 Hz, Ph-H), 7.79 (t, 1H, J = 7.20 Hz, Ph-H), 7.58 (d, 1H, J = 7.80 Hz, Ph-H), 7.54 (d, 2H, J = 9.00 Hz, Ph-H), 4.75 (s, 2H, CH2); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.24, 165.45, 163.23, 159.73, 156.45 (q, J = 34.80 Hz), 154.87, 134.79, 132.58, 129.25, 128.86, 127.74 (q, J = 29.70 Hz), 126.83, 125.83 (q, J = 272.85 Hz), 121.78 (q, J = 273.00 Hz), 121.33, 107.13, 33.62; HRMS (ESI) calcd for C21H12F6N4O2S: [M + Na]+, 521.04742; found, 521.04774.
3.1.10. 2-((3,4-Dichlorobenzyl)thio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6j)
Yellow solid; yield 66.4%; mp 102.8–104.6 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.99 (s, 1H, pyrimidine-H), 8.07 (d, 2H, J = 9.00 Hz, Ph-H), 7.85 (s, 1H, pyrimidine-H), 7.80 (d, 1H, J = 1.80 Hz, Ph-H), 7.61 (d, 1H, J = 6.00 Hz, Ph-H), 7.53–7.50 (m, 3H, Ph-H), 4.59 (s, 2H, CH2); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.23, 165.27, 163.60, 159.72, 156.03 (q, J = 34.95 Hz), 154.80, 138.74, 131.57, 131.42, 131.15, 130.85, 129.90, 128.81, 123.34, 121.77 (q, J = 273.15 Hz), 121.34, 107.09, 35.96; HRMS (ESI) calcd for C20H11Cl2F3N4O2S: [M + Na]+, 520.98193; found, 520.98241.
3.1.11. 2-((2,4-Dichlorobenzyl)thio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6k)
White solid; yield 62.4%; mp 89.2–91.0 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.99 (s, 1H, pyrimidine-H), 8.07 (d, 2H, J = 8.40 Hz, Ph-H), 7.88 (s, 1H, pyrimidine-H), 7.69 (d, 1H, J = 1.80 Hz, Ph-H), 7.68 (d, 1H, J = 8.40 Hz, Ph-H), 7.55 (d, 2H, J = 9.00 Hz, Ph-H), 7.48 (dd, 1H, J1 = 1.80 Hz, J2 = 6.60 Hz, Ph-H), 4.65 (s, 2H, CH2); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.24, 165.47, 163.18, 159.73, 156.45 (q, J = 35.10 Hz), 134.79, 134.08, 133.70, 133.32, 129.60, 128.88, 128.11, 123.39, 121.79 (q, J = 272.70 Hz), 121.34, 116.13, 107.13, 34.16; HRMS (ESI) calcd for C20H11Cl2F3N4O2S: [M + Na]+, 520.98169; found, 520.98241.
3.1.12. 2-(4-((2-Methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-5-(methylthio)-1,3,4-oxadiazole (6L)
Pink solid; yield 42.5%; mp 127.4–129.4 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.07 (d, 2H, J = 8.40 Hz, Ph-H), 7.60 (s, 1H, pyrimidine-H), 7.52 (d, 2H, J = 9.00 Hz, Ph-H), 2.78 (s, 3H, CH3), 2.52 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.12, 169.67, 165.24, 164.94, 156.44 (q, J = 34.80 Hz), 154.76, 128.72, 123.20, 121.80 (q, J = 273.15 Hz), 121.32, 103.85, 25.87, 14.78; HRMS (ESI) calcd for C15H11F3N4O2S: [M + Na]+, 391.04370; found, 391.04470.
3.1.13. 2-(Ethylthio)-5-(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6m)
Pink solid; yield 65.5%; mp 104.9–106.6 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.08 (d, 2H, J = 9.00 Hz, Ph-H), 7.60 (s, 1H, pyrimidine-H), 7.52 (d, 2H, J = 9.00 Hz, Ph-H), 3.32 (q, 2H, J = 7.80 Hz, CH2), 2.52 (s, 3H, CH3), 1.45 (t, 3H, J = 7.20 Hz, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.12, 169.67, 164.97, 164.34, 156.44 (q, J = 34.80 Hz), 154.77, 128.74, 121.79 (q, J = 276.60 Hz), 121.33, 103.84, 27.17, 25.86, 15.34; HRMS (ESI) calcd for C16H13F3N4O2S: [M + Na]+, 405.05978; found, 405.06035.
3.1.14. 2-(4-((2-Methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-5-((2-methylbenzyl)thio)-1,3,4-oxadiazole (6n)
White solid; yield 45.8%; mp 93.8–94.2 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.09 (d, 2H, J = 8.40 Hz, Ph-H), 7.62 (s, 1H, pyrimidine-H), 7.53 (d, 2H, J = 9.00 Hz, Ph-H), 7.43 (d, 1H, J = 7.20 Hz, Ph-H), 7.24–7.21 (m, 2H, Ph-H), 7.18–7.15 (m, 2H, Ph-H), 4.62 (s, 2H, CH2), 2.53 (s, 3H, CH3), 2.42 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.12, 169.67, 163.68, 156.43 (q, J = 35.10 Hz), 154.87, 137.33, 134.22, 130.98, 130.51, 128.81, 128.73, 126.60, 123.23, 121.81 (q, J = 273.30 Hz), 103.89, 35.03, 25.88, 19.22; HRMS (ESI) calcd for C22H17F3N4O2S: [M + Na]+, 481.09140; found, 481.09165.
3.1.15. 2-(4-((2-Methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-5-((4-methylbenzyl)thio)-1,3,4-oxadiazole (6o)
Brown solid; yield 52.3%; mp 111.2–112.8 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.08 (d, 2H, J = 8.40 Hz, Ph-H), 7.62 (s, 1H, pyrimidine-H), 7.53 (d, 2H, J = 8.40 Hz, Ph-H), 7.38 (d, 2H, J = 8.40 Hz, Ph-H), 7.17 (d, 2H, J = 7.80 Hz, Ph-H), 4.56 (s, 2H, CH2), 2.53 (s, 3H, CH3), 2.28 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.13, 169.67, 165.14, 163.89, 156.45 (q, J = 34.80 Hz), 154.86, 137.59, 133.88, 129.63, 129.45, 128.79, 123.25, 121.81 (q, J = 273.30 Hz), 121.23, 103.91, 36.27, 25.89, 21.16; HRMS (ESI) calcd for C22H17F3N4O2S: [M + Na]+, 481.09097; found, 481.09072.
3.1.16. 2-((4-Fluorobenzyl)thio)-5-(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadiazole (6p)
Yellow solid; yield 56.5%; mp 74.2–75.1 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.07 (d, 2H, J = 9.00 Hz, Ph-H), 7.59 (t, 2H, J = 6.60 Hz, Ph-H and pyrimidine-H), 7.52 (d, 2H, J = 9.00 Hz, Ph-H), 7.38–7.34 (m, 1H, Ph-H), 7.21–7.17 (m, 2H, Ph-H), 4.61 (s, 2H, CH2), 2.52 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.08, 169.68, 165.35, 163.36, 161.69, 160.06, 156.48 (q, J = 34.80 Hz), 154.88, 131.91, 130.73, 128.75, 125.04, 124.04, 123.19, 121.78 (q, J = 273.15 Hz), 121.17, 116.03, 103.79, 30.31, 25.83; HRMS (ESI) calcd for C21H14F4N4O2S: [M + Na]+, 485.06638; found, 485.06658.
3.1.17. 2-((4-Fluorobenzyl)thio)-5-(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy) phenyl)-1,3,4-oxadiazole (6q)
White solid; yield 54.6%; mp 87.7–89.6 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.08 (d, 2H, J = 9.00 Hz, Ph-H), 7.62 (s, 1H, pyrimidine-H), 7.53 (q, 2H, J = 5.40 Hz, Ph-H), 7.53 (d, 1H, J = 8.40 Hz, Ph-H), 7.21 (t, 2H, J = 9.00 Hz, Ph-H), 4.61 (s, 2H, CH2), 2.52 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.11, 169.67, 165.20, 163.78, 162.91, 161.29, 156.46 (q, J = 35.25 Hz), 154.86, 133.42, 131.66, 128.78, 123.22, 121.80 (q, J = 273.45 Hz), 121.21, 115.93, 115.79, 103.87, 35.56, 25.87; HRMS (ESI) calcd for C21H14F4N4O2S: [M + Na]+, 485.06616; found, 485.06658.
3.1.18. 2-((2-Chlorobenzyl)thio)-5-(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy) phenyl)-1,3,4-oxadiazole (6r)
Yellow solid; yield 62.7%; mp 89.8–91.6 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.08 (d, 2H, J = 9.0 Hz, Ph-H), 7.80 (dd, 1H, J1 = 1.80 Hz, J2 = 6.60 Hz, pyrimidine-H), 7.61 (s, 2H, Ph-H), 7.52–7.50 (m, 3H, Ph-H), 7.38–7.33 (m, 2H, Ph-H), 4.67 (s, 2H, CH2), 2.52 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.10, 169.67, 163.34, 156.46 (q, J = 35.10 Hz), 154.90, 134.34, 133.81, 132.07, 130.46, 130.10, 128.82, 127.96, 123.22, 121.80 (q, J = 273.45 Hz), 121.19, 103.86, 34.76, 25.87; HRMS (ESI) calcd for C21H14ClF3N4O2S: [M + Na]+, 501.03671; found, 501.03703.
3.1.19. 2-((3-Chlorobenzyl)thio)-5-(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy) phenyl)-1,3,4-oxadiazole (6s)
Gray solid; yield 67.5%; mp 89.2–90.2 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.07 (d, 2H, J = 9.00 Hz, Ph-H), 7.60 (s, 2H, Ph-H and pyrimidine-H), 7.52 (d, 2H, J = 9.00 Hz, Ph-H), 7.48 (d, 1H, J = 7.80 Hz, Ph-H), 7.40–7.35 (m, 2H, Ph-H), 4.60 (s, 2H, CH2), 2.52 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.11, 169.66, 165.25, 163.67, 156.45 (q, J = 34.80 Hz), 155.99, 139.92, 133.46 130.89, 129.42, 128.78, 128.24, 123.62, 121.80 (q, J = 273.45 Hz), 121.19, 103.87, 35.57, 25.87; HRMS (ESI) calcd for C21H14ClF3N4O2S: [M + Na]+, 501.03616; found, 501.03703.
3.1.20. 2-((4-Chlorobenzyl)thio)-5-(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy) phenyl)-1,3,4-oxadiazole (6t)
Yellow solid; yield 84.6%; mp 90.1–91.9 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.07 (d, 2H, J = 8.40 Hz, Ph-H), 7.61 (s, 1H, pyrimidine-H), 7.53 (dd, 4H, J1 = 6.60 Hz, J2 = 1.80 Hz, Ph-H), 7.38 (d, 2H, J = 8.40 Hz, Ph-H), 7.42 (d, 2H, J = 8.40 Hz, Ph-H), 4.59 (s, 2H, CH2), 2.52 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.12, 169.66, 165.24, 163.89, 156.45 (q, J = 34.65 Hz), 154.87, 136.37, 132.88, 131.41, 129.02, 128.80, 123.62, 121.80 (q, J = 273.45 Hz), 121.20, 103.89, 35.57, 25.88; HRMS (ESI) calcd for C21H14ClF3N4O2S: [M + Na]+, 501.03635; found, 501.03703.
3.1.21. 2-(((5-(4-((2-Methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadia zol-2-yl)thio)methyl)benzonitrile (6u)
Gray solid; yield 56.3%; mp 125.2–127.0 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.08 (d, 2H, J = 8.40 Hz, Ph-H), 7.89 (d, 1H, J = 7.80 Hz, Ph-H), 7.77 (d, 1H, J = 7.20 Hz, Ph-H), 7.73–7.70 (m, 1H, Ph-H), 7.63 (s, 1H, pyrimidine-H), 7.54–7.51 (m, 3H, Ph-H), 4.75(s, 2H, CH2), 2.53 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.11, 169.67, 165.60, 162.78, 156.45 (q, J = 34.65 Hz), 154.95, 140.62, 134.01, 133.75, 131.06, 129.03, 128.89, 123.23, 121.81 (q, J = 272.85 Hz), 121.16, 117.62, 112.29, 103.91, 34.98, 25.89; HRMS (ESI) calcd for C22H14F3N5O2S: [M + Na]+, 492.07050; found, 492.07125.
3.1.22. 4-(((5-(4-((2-Methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-oxadia zol-2-yl)thio)methyl)benzonitrile (6v)
Yellow solid; yield 67.3%; mp 110.1–111.4 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.05 (d, 2H, J = 9.00 Hz, Ph-H), 7.84 (d, 2H, J = 8.40 Hz, Ph-H), 7.72 (d, 2H, J = 8.40 Hz, Ph-H), 7.60 (s, 1H, pyrimidine-H), 7.52 (d, 2H, J = 9.00 Hz, Ph-H), 4.67 (s, 2H, CH2), 2.52 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.10, 169.66, 165.31, 163.50, 156.46 (q, J = 34.80 Hz), 154.88, 143.29, 132.92, 130.50, 128.79, 123.21, 121.79 (q, J = 273.00 Hz), 121.16, 119.07, 110.93, 103.86, 35.76, 25.87; HRMS (ESI) calcd for C22H14F3N5O2S: [M + Na]+, 492.07019; found, 492.07125.
3.1.23. 2-(4-((2-Methyl-6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-5-((4-(trifluoromethyl)benzyl)thio)-1,3,4-oxadiazole (6w)
White solid; yield 45.9%; mp 97.7–99.2 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.06 (d, 2H, J = 9.00 Hz, Ph-H), 7.73 (br, 4H, Ph-H), 7.61 (s, 1H, pyrimidine-H), 7.52 (d, 2H, J = 9.00 Hz, Ph-H), 4.69 (s, 2H, CH2), 2.52 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.12, 169.66, 165.30, 163.58, 156.45 (q, J = 34.80 Hz), 154.88, 142.33, 130.34, 128.80, 128.59 (q, J = 32.10 Hz), 125.89, 125.52 (q, J = 270.15 Hz), 123.23, 121.80 (q, J = 273.00 Hz), 121.18, 103.89, 35.63, 25.87; HRMS (ESI) calcd for C22H14F6N4O2S: [M + Na]+, 535.06274; found, 535.06339.
3.1.24. 2-((2,4-Dichlorobenzyl)thio)-5-(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl) oxy)phenyl)-1,3,4-oxadiazole (6x)
White solid; yield 56.8%; mp 103.3–104.1 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.08 (d, 2H, J = 8.40 Hz, Ph-H), 7.69–7.62 (m, 3H, Ph-H and pyrimidine-H), 7.53 (d, 1H, J = 9.00 Hz, Ph-H), 7.46–7.37 (m, 1H, Ph-H), 4.72 (s, 2H, CH2), 2.53 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.11, 169.66, 165.49, 163.14, 156.45 (q, J = 34.50 Hz), 154.92, 137.16, 134.79, 134.08, 133.70, 133.31, 132.63, 131.89, 130.78, 129.59, 128.85, 128.79, 128.10, 123.24, 121.80 (q, J = 272.85 Hz), 121.17, 103.89, 35.46, 25.88; HRMS (ESI) calcd for C21H13Cl2F3N4O2S: [M + Na]+, 534.99756; found, 534.99806.
3.1.25. 2-((3,4-Dichlorobenzyl)thio)-5-(4-((2-methyl-6-(trifluoromethyl)pyrimidin-4-yl) oxy)phenyl)-1,3,4-oxadiazole (6y)
Yellow solid; yield 74.3%; mp 86.2–87.7 °C; 1H NMR (600 MHz, DMSO-d6, ppm): δ 8.04 (d, 2H, J = 9.00 Hz, Ph-H), 7.80 (d, 1H, J = 1.80 Hz, Ph-H), 7.61 (d, 2H, J = 8.40 Hz, Ph-H), 7.59 (s, 2H, Ph-H and pyrimidine-H), 7.51 (d, 3H, J = 8.40 Hz, Ph-H), 4.59 (s, 2H, CH2), 2.52 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6, ppm): δ 170.09, 169.66, 165.29, 163.55, 156.46 (q, J = 35.10 Hz), 154.86, 138.74, 131.57, 131.42, 131.14, 130.85, 128.76, 123.19, 121.78 (q, J = 273.00 Hz), 121.17, 103.81, 34.97, 25.85; HRMS (ESI) calcd for C21H13Cl2F3N4O2S: [M + Na]+, 534.99689; found, 534.99806.
3.2. In Vivo Anti-TMV Activity Test
The anti-TMV activity of the target compounds 6a–6y are illustrated in Table 1. Table 1 shows that the target compounds had moderate to good inhibitory activity against TMV at a concentration of 500 μg/mL. In terms of curative activity against TMV, compound 6r exhibited a slightly superior inhibition rate of 61.3% compared to ningnanmycin (56.4%). Meanwhile, compound 6s demonstrated the highest protection activity against TMV with a rate of 68.7%, which was comparable to that of ningnanmycin (66.4%). However, the inactivation activity of all target compounds against TMV was found to be lower than that of ningnanmycin.
Table 1. In Vivo Antiviral Activity of the Target Compounds against TMV at 500 μg/mL.
| inhibition
rate (%)a |
|||
|---|---|---|---|
| compounds | curative activity | protection activity | inactivation activity |
| 6a | 38.6 ± 1.9 | 35.4 ± 2.1 | 34.7 ± 2.6 |
| 6b | 43.4 ± 3.0 | 38.9 ± 1.7 | 42.3 ± 3.2 |
| 6c | 27.3 ± 2.2 | 51.4 ± 0.8 | 58.6 ± 2.1 |
| 6d | 58.2 ± 1.5 | 47.5 ± 1.5 | 50.2 ± 2.0 |
| 6e | 34.9 ± 3.1 | 56.5 ± 1.3 | 62.9 ± 3.2 |
| 6f | 58.8 ± 2.8 | 47.5 ± 3.3 | 69.8 ± 2.8 |
| 6g | 45.3 ± 2.3 | 54.4 ± 2.6 | 70.2 ± 3.3 |
| 6h | 40.5 ± 3.1 | 58.8 ± 1.3 | 78.0 ± 1.9 |
| 6i | 50.6 ± 1.5 | 43.5 ± 2.0 | 52.5 ± 2.5 |
| 6j | 49.5 ± 3.2 | 46.8 ± 2.4 | 64.4 ± 1.5 |
| 6k | 48.3 ± 2.5 | 50.0 ± 2.2 | 60.3 ± 2.1 |
| 6L | 46.8 ± 2.2 | 39.3 ± 3.1 | 38.6 ± 1.8 |
| 6m | 50.6 ± 1.0 | 46.2 ± 1.4 | 47.2 ± 3.3 |
| 6n | 36.3 ± 2.2 | 55.5 ± 1.2 | 62.3 ± 2.7 |
| 6o | 29.3 ± 1.7 | 49.4 ± 1.3 | 54.9 ± 1.6 |
| 6p | 47.3 ± 1.5 | 64.2 ± 1.2 | 72.1 ± 2.0 |
| 6q | 43.1 ± 3.6 | 59.5 ± 1.3 | 70.2 ± 1.2 |
| 6r | 61.3 ± 2.6 | 55.7 ± 1.3 | 73.2 ± 1.5 |
| 6s | 55.3 ± 1.0 | 68.7 ± 2.6 | 76.9 ± 1.2 |
| 6t | 43.2 ± 0.6 | 61.3 ± 1.3 | 80.0 ± 1.1 |
| 6u | 45.8 ± 1.9 | 39.5 ± 1.8 | 47.3 ± 2.9 |
| 6v | 39.4 ± 1.4 | 22.0 ± 2.7 | 43.2 ± 2.8 |
| 6w | 49.3 ± 1.1 | 43.8 ± 0.9 | 49.6 ± 1.2 |
| 6x | 54.5 ± 2.2 | 49.9 ± 1.4 | 68.7 ± 1.2 |
| 6y | 51.9 ± 3.1 | 54.2 ± 1.2 | 64.3 ± 2.5 |
| Ningnanmycin | 56.4 ± 1.5 | 66.4 ± 2.0 | 94.0 ± 1.5 |
The mean value derived from three repeated measurements.
To further demonstrate the antiviral potential of the promising target compounds, several candidates exhibiting relatively high primary inhibitory activity against TMV were subjected to effective concentration (EC50) value testing, and the results were listed in Table 2. It can be observed from Table 2 that compounds 6f and 6r demonstrated remarkable curative activity against TMV, exhibiting EC50 values of 0.59 and 0.48 μM respectively, surpassing even the efficacy of ningnanmycin (0.69 μM). Additionally, compound 6s displayed exceptional protection activity against TMV with an EC50 value of 0.42 μM, outperforming ningnanmycin (0.60 μM).
Table 2. EC50 Values of Some of the Target Compounds against TMV in vivo.
| EC50 (μM)a |
||
|---|---|---|
| compounds | curative activity | protection activity |
| 6d | 0.66 ± 0.005 | |
| 6f | 0.59 ± 0.004 | |
| 6p | 0.62 ± 0.006 | |
| 6r | 0.48 ± 0.004 | |
| 6s | 0.70 ± 0.006 | 0.42 ± 0.006 |
| 6t | 0.66 ± 0.007 | |
| 6x | 0.72 ± 0.005 | |
| ningnanmycin | 0.69 ± 0.007 | 0.60 ± 0.006 |
The mean value derived from three repeated measurements.
3.3. In Vitro Antifungal Activity Test
As shown in Table 3, the target compounds 6a–6y exhibited certain in vitro antifungal activities against B. dothidea (Moug.) Ces. De Not. in postharvest kiwifruit (40.13–77.85%), Phomopsis sp. in postharvest kiwifruit (47.99–94.43%), B. cinerea Pers. in postharvest blueberry (44.71–100%), B. cinerea Pers. in postharvest cucumber (35.35–58.01%), S. sclerotiorum (Lib.) de Bary in oilseed rape (23.38–75.19%), B. cinerea Pers. in postharvest tobacco (34.77–66.17%), B. cinerea Pers. in postharvest strawberry (31.96–100%), Colletotrichum sp. in postharvest blueberry (13.20–75.05%), and M. grisea (Hebert) Barr in rice (8.20–52.46%). Among them, the inhibitory activities of compounds 6s and 6x against Phomopsis sp. in postharvest kiwifruit were found to be 94.12 and 94.43% respectively, surpassing that of Pyrimethanil (85.13%). Meanwhile, compounds 6c, 6g, and 6s exhibited complete inhibition (100.00%) against B. cinerea Pers. in postharvest blueberry, outperforming Pyrimethanil (82.83%). Additionally, compounds 6f and 6j demonstrated inhibition rates of 100.00 and 91.30%, respectively toward B. cinerea Pers. in postharvest strawberries which were superior to Pyrimethanil (84.88%).
Table 3. In Vitro Antifungal Activity of the Target Compounds 6a–6y at 50 μg/mL.
| inhibition
rate (%)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| compounds | Botryosphaeria dothidea(Moug.) Ces. De Not. in postharvest kiwifruit | Phomopsis sp. in postharvest kiwifruit | Botrytis cinerea Pers. in postharvest blueberry | Botrytis cinerea Pers. in postharvest cucumber | Sclerotinia sclerotiorum (Lib.) de Bary in oilseed rape | Botrytis cinerea Pers. in postharvest tobacco | Botrytis cinerea Pers. in postharvest strawberry | Colletotrichum sp. in postharvest blueberry | Magnaporthe grisea (Hebert) Barr in rice |
| 6a | 43.78 ± 3.16 | 54.34 ± 1.88 | 56.14 ± 2.26 | 40.94 ± 1.26 | 68.31 ± 3.88 | 39.81 ± 1.55 | 68.67 ± 2.97 | 41.77 ± 3.25 | 14.75 ± 1.32 |
| 6b | 54.35 ± 1.56 | 59.61 ± 2.67 | 50.04 ± 2.32 | 58.01 ± 1.32 | 53.25 ± 0.97 | 66.17 ± 1.99 | 82.12 ± 2.88 | 46.84 ± 1.38 | 52.46 ± 2.31 |
| 6c | 67.41 ± 1.63 | 73.53 ± 2.10 | 100.00 | 40.03 ± 1.64 | 63.12 ± 1.75 | 41.68 ± 1.51 | 55.38 ± 1.34 | 40.33 ± 1.20 | 46.99 ± 2.00 |
| 6d | 65.98 ± 3.12 | 79.41 ± 3.08 | 49.48 ± 2.18 | 46.53 ± 1.14 | 59.22 ± 1.56 | 43.93 ± 1.71 | 50.95 ± 4.20 | 34.36 ± 1.85 | 25.32 ± 1.37 |
| 6e | 45.63 ± 2.87 | 50.46 ± 2.31 | 56.23 ± 2.11 | 46.07 ± 1.28 | 31.04 ± 0.93 | 51.78 ± 3.79 | 52.69 ± 1.43 | 13.20 ± 1.05 | 8.20 ± 1.06 |
| 6f | 59.97 ± 1.65 | 69.97 ± 2.62 | 79.35 ± 2.12 | 35.35 ± 3.30 | 74.16 ± 1.85 | 38.88 ± 2.02 | 100.00 | 75.05 ± 2.53 | 18.76 ± 1.15 |
| 6g | 70.41 ± 2.33 | 80.03 ± 2.99 | 100.00 | 36.56 ± 2.28 | 23.38 ± 1.35 | 34.77 ± 2.90 | 31.96 ± 1.47 | 20.25 ± 1.35 | 26.96 ± 2.37 |
| 6h | 71.84 ± 2.37 | 50.00 ± 3.04 | 81.18 ± 2.06 | 36.40 ± 1.21 | 74.68 ± 2.94 | 41.31 ± 1.81 | 65.98 ± 1.57 | 72.15 ± 3.46 | 20.58 ± 1.23 |
| 6i | 71.68 ± 2.44 | 74.92 ± 2.37 | 44.71 ± 2.99 | 41.84 ± 2.41 | 65.06 ± 2.25 | 42.99 ± 4.82 | 70.25 ± 2.13 | 37.43 ± 1.85 | 30.42 ± 1.21 |
| 6j | 64.56 ± 1.99 | 57.74 ± 3.83 | 60.61 ± 1.42 | 44.56 ± 2.55 | 75.19 ± 1.90 | 41.12 ± 1.79 | 91.30 ± 2.87 | 19.89 ± 1.24 | 33.33 ± 2.22 |
| 6k | 74.37 ± 2.01 | 70.28 ± 2.26 | 76.19 ± 2.20 | 44.71 ± 2.97 | 64.16 ± 3.79 | 42.43 ± 1.87 | 43.35 ± 1.75 | 32.55 ± 1.62 | 17.85 ± 3.13 |
| 6L | 40.13 ± 2.62 | 61.01 ± 1.33 | 66.33 ± 2.27 | 47.13 ± 1.62 | 60.91 ± 2.33 | 39.44 ± 1.27 | 56.49 ± 1.56 | 43.58 ± 3.41 | 31.88 ± 2.00 |
| 6m | 43.74 ± 2.17 | 67.29 ± 182 | 67.67 ± 3.26 | 48.34 ± 1.17 | 65.19 ± 2.82 | 40.37 ± 1.26 | 77.53 ± 2.24 | 50.81 ± 2.42 | 29.51 ± 1.96 |
| 6n | 72.63 ± 2.30 | 47.99 ± 3.88 | 59.48 ± 3.41 | 36.25 ± 1.74 | 62.34 ± 1.39 | 37.57 ± 1.69 | 62.34 ± 1.58 | 28.57 ± 1.32 | 10.02 ± 1.46 |
| 6o | 64.87 ± 2.30 | 70.90 ± 2.13 | 64.35 ± 1.12 | 37.46 ± 1.30 | 67.53 ± 1.87 | 37.20 ± 1.73 | 43.20 ± 1.66 | 34.54 ± 2.01 | 15.85 ± 1.51 |
| 6p | 68.04 ± 3.18 | 65.48 ± 3.56 | 66.94 ± 3.30 | 48.04 ± 1.88 | 60.78 ± 3.69 | 37.57 ± 1.91 | 72.47 ± 2.32 | 51.72 ± 2.73 | 44.99 ± 1.71 |
| 6q | 68.83 ± 3.10 | 53.41 ± 1.78 | 68.71 ± 2.98 | 39.43 ± 1.90 | 74.42 ± 4.08 | 37.20 ± 2.72 | 61.87 ± 1.56 | 48.28 ± 1.69 | 29.87 ± 1.27 |
| 6r | 71.99 ± 2.80 | 54.95 ± 3.43 | 76.25 ± 3.93 | 45.17 ± 1.23 | 44.29 ± 1.06 | 45.61 ± 1.48 | 62.34 ± 1.42 | 45.03 ± 1.77 | 32.06 ± 2.09 |
| 6s | 65.98 ± 2.03 | 94.12 ± 3.02 | 100.00 | 41.54 ± 1.67 | 54.68 ± 3.07 | 45.23 ± 1.78 | 57.91 ± 3.47 | 28.03 ± 2.60 | 30.05 ± 1.56 |
| 6t | 64.08 ± 2.39 | 77.09 ± 2.73 | 47.90 ± 1.10 | 43.20 ± 1.37 | 62.86 ± 1.40 | 44.30 ± 1.88 | 56.17 ± 1.67 | 41.23 ± 2.11 | 41.89 ± 1.42 |
| 6u | 68.35 ± 2.45 | 81.58 ± 3.57 | 55.35 ± 3.12 | 39.27 ± 1.44 | 59.48 ± 1.55 | 42.43 ± 1.77 | 71.99 ± 3.34 | 44.67 ± 1.64 | 31.15 ± 1.19 |
| 6v | 74.34 ± 2.52 | 77.71 ± 3.40 | 51.30 ± 3.96 | 48.04 ± 1.41 | 38.96 ± 5.26 | 45.05 ± 1.35 | 63.77 ± 2.77 | 45.39 ± 1.39 | 31.33 ± 2.28 |
| 6w | 58.23 ± 2.06 | 69.97 ± 1.78 | 68.84 ± 3.60 | 46.53 ± 1.45 | 64.94 ± 1.68 | 41.68 ± 1.42 | 53.48 ± 1.42 | 34.54 ± 1.43 | 17.67 ± 1.72 |
| 6x | 65.03 ± 2.52 | 94.43 ± 3.40 | 76.87 ± 2.96 | 43.81 ± 1.71 | 50.78 ± 2.31 | 42.80 ± 3.02 | 56.01 ± 2.52 | 37.43 ± 1.60 | 18.40 ± 2.00 |
| 6y | 77.85 ± 3.30 | 68.89 ± 3.25 | 67.62 ± 2.46 | 44.71 ± 1.22 | 60.39 ± 2.05 | 43.74 ± 2.04 | 58.54 ± 2.09 | 41.23 ± 2.58 | 44.26 ± 3.10 |
| pyrimethanil | 87.16 ± 1.54 | 86.56 ± 1.76 | 81.99 ± 2.34 | 81.49 ± 1.34 | 68.39 ± 2.13 | 70.11 ± 3.76 | 84.88 ± 1.63 | 65.12 ± 1.54 | 55.41 ± 1.65 |
The mean value derived from three repeated measurements.
The EC50 values of some target compounds against B. cinerea Pers. in postharvest blueberry, Phomopsis sp. in postharvest kiwifruit, and B. cinerea Pers. in postharvest strawberry were determined and the results are shown in Table 4. The results presented in Table 4 demonstrate that compounds 6c, 6g, and 6s exhibited superior antifungal activity against B. cinerea Pers. in postharvest blueberry, with EC50 values of 0.018, 0.031, and 0.011 μM respectively, outperforming Pyrimethanil (0.262 μM). Meanwhile, compounds 6s and 6x displayed excellent antifungal activity against Phomopsis sp. in postharvest kiwifruit, with EC50 values of 0.030 and 0.038 μM, respectively, which were better than Pyrimethanil (0.176 μM). Additionally, compound 6j demonstrated good antifungal efficacy against B. cinerea Pers. in postharvest strawberries with an EC50 value of 0.032 μM, surpassing that of Pyrimethanil (0.193 μmol/mL).
Table 4. EC50 Values of Some of the Target Compounds against the Test Fungi.
| EC50 (μM)a |
|||
|---|---|---|---|
| compounds | Botrytis cinerea Pers. in postharvest blueberry | Phomopsis sp. in postharvest kiwifruit | Botrytis cinerea Pers. in postharvest strawberry |
| 6c | 0.018 ± 0.003 | ||
| 6f | 0.183 ± 0.003 | ||
| 6j | 0.032 ± 0.006 | ||
| 6g | 0.031 ± 0.004 | ||
| 6s | 0.011 ± 0.003 | 0.030 ± 0.003 | |
| 6x | 0.038 ± 0.007 | ||
| Pyrimethanil | 0.262 ± 0.012 | 0.176 ± 0.010 | 0.193 ± 0.011 |
The mean value derived from three repeated measurements.
3.4. In Vivo Control Efficacy Test against Blueberry Fruit Gray Mold
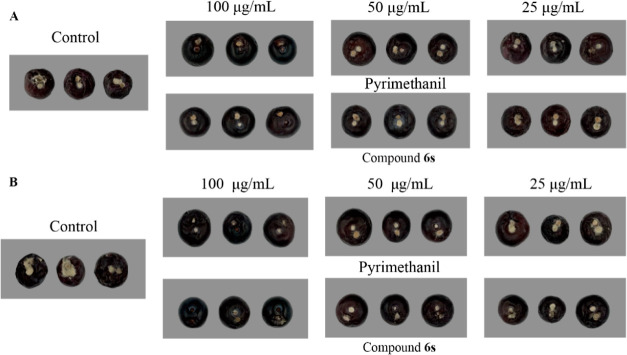
The in vivo evaluation was conducted to assess the curative and protection control efficacy of compound 6s against blueberry fruit gray mold, and the corresponding results are presented in Table 5. According to the data in Table 5, compound 6s exhibited remarkable curative effects against blueberry fruit gray mold at concentrations of 100, 50, and 25 μg/mL with values of 54.2, 23.9, and 8.9% respectively; however, these values were comparable to those observed for Pyrimethanil (49.3, 24.4, and 7.5%). Meanwhile, the protection effect of compound 6s at a concentration of 100 μg/mL was found to be equivalent to Pyrimethanil (63.9%), with a value of 60.4%; however, at concentrations of 50 and 25 μg/mL, the protection effect of compound 6s was slightly higher than Pyrimethanil (16.1 and 12.6%) at values of 29.1 and 22.6%, respectively. Moreover, it is noteworthy that the in vivo curative and protection effects of compound 6s exhibited concentration-dependent properties, and compound 6s was proven to be safe for blueberry fruit even at high concentrations (Figure 2).
Table 5. In Vivo Curative and Protection Effects of Compound 6s against Blueberry Fruit Grey Mold.
| curative
effecta |
protection
effecta |
||||
|---|---|---|---|---|---|
| treatment | concentration (μg/mL) | lesion length (mm) | control efficacy (%) | lesion length (mm) | control efficacy (%) |
| 6s | 200 | 0.9 ± 0.6 | 54.2 ± 1.6 | 0.9 ± 0.2 | 60.4 ± 1.3 |
| 100 | 1.5 ± 1.1 | 23.9 ± 1.8 | 1.6 ± 0.1 | 29.1 ± 1.0 | |
| 50 | 1.8 ± 2.3 | 8.9 ± 2.4 | 1.8 ± 2.1 | 22.6 ± 1.9 | |
| Pyrimethanil | 200 | 1.0 ± 0.9 | 49.3 ± 1.1 | 0.8 ± 0.2 | 63.9 ± 1.2 |
| 100 | 1.5 ± 0.3 | 24.4 ± 1.0 | 1.9 ± 0.3 | 16.1 ± 1.4 | |
| 50 | 1.9 ± 1.5 | 7.5 ± 2.3 | 0.8 ± 0.7 | 12.6 ± 1.6 | |
| CK | 2.0 ± 0.8 | 2.3 ± 1.6 | |||
The mean value derived from three repeated measurements.
Figure 2.
In vivo curative effect (A) and protection effect (B) of compound 6s against blueberry fruit gray mold.
3.5. Effect on the Hyphae Morphology
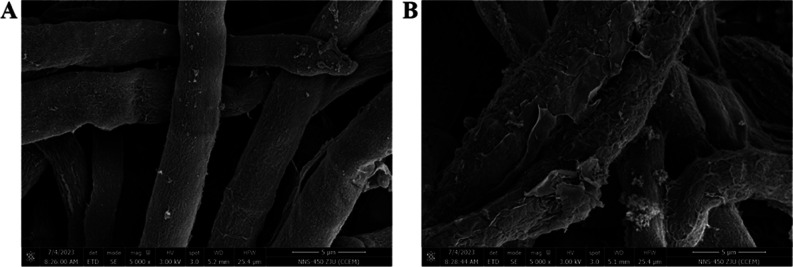
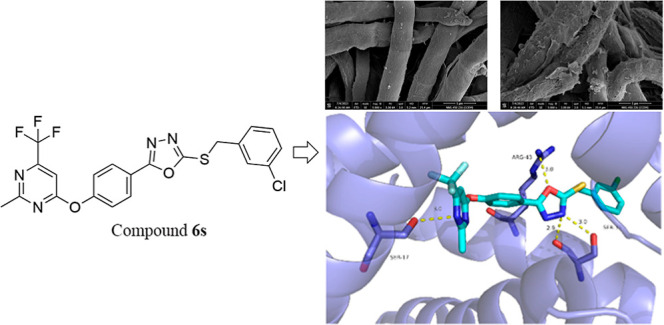
The impact of compound 6s on the hyphal morphology of B. cinerea Pers. in postharvest blueberry was assessed using SEM. Figure 3 illustrates that the negative control (CK) group exhibited a typical and characteristic morphology, with uniformly distributed and linear hyphae compared to evident collapse; however, treatment with compound 6s at a concentration of 12.5 μg/mL resulted in abnormal collapse, shriveling, and damage to the hyphae of B. cinerea Pers. in postharvest blueberry. This suggests that compound 6s may disrupt the cell membrane or cell wall, thereby influencing the subsequent growth and reproduction of B. cinerea Pers. in postharvest blueberry.
Figure 3.
Impact of compound 6s on the hyphal morphology of Botrytis cinerea Pers. in postharvest blueberry. (A) CK group and (B) treatment group.
3.6. Molecular Docking Study
To elucidate the binding mode of the target compounds to the SDH receptor, compound 6s with the highest activity was subjected to molecular docking within the active site of the SDH receptor. The resulting docking conformation, depicted in Figure 4, revealed that a nitrogen atom in the pyrimidine ring of compound 6s engaged in a hydrogen bond interaction with amino acid residue SER-17 at a distance of 3.0 Å. Simultaneously, an oxygen atom within the 1,3,4-oxadiazole moiety formed a hydrogen bond interaction with amino acid residue ARG-43 at a distance of 3.0 Å. Furthermore, another nitrogen atom within the 1,3,4-oxadiazole group established two hydrogen bonds with amino acid residue SER-39 at distances of 3.0 and 2.9 Å respectively.
Figure 4.
Docking binding mode of compound 6s to SDH.
4. Conclusions
In this study, a total of 25 novel pyrimidine derivatives containing a 1,3,4-oxadiazole thioether fragment were designed and synthesized. Among them, several target compounds exhibited significant anti-TMV and antifungal activities both in vitro and in vivo. Meanwhile, the SEM results demonstrated that the hyphae of B. cinerea Pers. in postharvest blueberry treated with compound 6s caused abnormal collapse and shrinkage. Additionally, molecular docking simulations revealed that compound 6s formed hydrogen bonds with SER-17, ARG-43, and SER-39 residues of the SDH enzyme, providing a potential explanation for the mechanism of action between the target compounds and SDH. Therefore, pyrimidine derivatives incorporating a 1,3,4-oxadiazole thioether moiety can be regarded as a suitable model for identifying potential candidates against viral infections and fungal-induced plant diseases.
Acknowledgments
This work was supported by the Science and Technology Fund Project of Guizhou (no. [2020]1Z023), Science and Technology Fund Project of Guizhou (Qian Ke He Pingtai Rencai-CXTD [2022]002), Education Department of Guizhou Province-Natural Science Research Project (QJJ[2023]042), Young Sci-Tech Talents Growth Program from the Department of Education of Guizhou Province (QJHKYZ[2020]086), and Discipline and Master’s Site Construction Project of Guiyang University by Guiyang City Financial Support Guiyang University (KJY-2020).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c07820.
1H NMR, 13C NMR, and HRMS spectra for all compounds (PDF)
Author Contributions
§ N.P. and H.W. are contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Thanikachalam P. V.; Maurya R. K.; Garg V.; Monga V. An insight into the medicinal perspective of synthetic analogs of indole: A review. Eur. J. Med. Chem. 2019, 180, 562–612. 10.1016/j.ejmech.2019.07.019. [DOI] [PubMed] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: miniperspective. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Kakkar S.; Narasimhan B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem. 2019, 13, 16. 10.1186/s13065-019-0531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandewale M. C.; Patil U. C.; Shedge S. V.; Dappadwad U. R.; Yamgar R. S. A review on quinoline hydrazone derivatives as a new class of potent antitubercular and anticancer agents. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 354–361. 10.1016/j.bjbas.2017.07.005. [DOI] [Google Scholar]
- Xu Z.; Zhao S. J.; Liu Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. 10.1016/j.ejmech.2019.111700. [DOI] [PubMed] [Google Scholar]
- Wu Z. B.; Yang W. Q.; Hou S. T.; Xie D.; Yang J. X.; Liu L. W.; Yang S. In vivo antiviral activity and disassembly mechanism of novel 1-phenyl-5-amine-4-pyrazole thioether derivatives against Tobacco mosaic virus. Pestic. Biochem. Physiol. 2021, 173, 104771. 10.1016/j.pestbp.2021.104771. [DOI] [PubMed] [Google Scholar]
- Lloyd A. W.; Percival D.; Langille M. G. I.; Yurgel S. N. Changes to Soil Microbiome Resulting from Synergetic Effects of Fungistatic Compounds Pyrimethanil and Fluopyram in Lowbush Blueberry Agriculture, with Nine Fungicide Products Tested. J. Microorganisms 2023, 11, 410. 10.3390/microorganisms.11020410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.; Wang L. Z.; Zhang J. B.; Li Z. N. Synthesis and Bioactivity of Diflumetorim. Agrochemicals 2013, 52, 868–870. 10.16820/j.cnki.1006-0413.2013.12.003. [DOI] [Google Scholar]
- Wang J. B.; Yue T.; He C.; Zhou Y. F.; Bai Y. S.; Li Q. W.; Jiang W.; Huang Y. N.; Liu X. F. Biocontrol of tomato bacterial wilt by a combination of Bacillus subtilis GSJB-1210 and ningnanmycin. Sci. Hortic. 2023, 321, 112296. 10.1016/j.scienta2023.112296. [DOI] [Google Scholar]
- Liu M. T.; Tian X. L. Toxicity test and field control effect of fluoxastrobin on tomato grey mildew. North. Hortic. 2015, 10, 116–118. 10.11937/bfyy.201510028. [DOI] [Google Scholar]
- Rossi A. S.; Michlig M. P.; Repetti M. R.; Cazenave J. Single and joint toxicity of azoxystrobin and cyproconazole to Prochilodus lineatus: Bioconcentration and biochemical responses. Sci. Total Environ. 2024, 907, 167992. 10.1016/j.scitotenv.2023.167992. [DOI] [PubMed] [Google Scholar]
- Xu W. M.; Li S. Z.; He M.; Yang S.; Li X. Y.; Li P. Synthesis and bioactivities of novel thioether/sulfone derivatives containing 1,2,3-thiadiazole and 1,3,4-oxadiazole/thiadiazole moiety. Bioorg. Med. Chem. Lett. 2013, 23, 5821–5824. 10.1016/j.bmcl.2013.08.107. [DOI] [PubMed] [Google Scholar]
- Naikoo R. A.; Kumar R.; Kumar V.; Bhargava G. Recent developments in the synthesis of tricyclic condensed pyrimidinones. Synth. Commun. 2021, 51, 1451–1527. 10.1080/00397911.2021.1885718. [DOI] [Google Scholar]
- Finger V.; Kufa M.; Soukup O.; Castagnolo D.; Roh J.; Korabecny J. Pyrimidine derivatives with antitubercular activity. Eur. J. Med. Chem. 2023, 246, 114946. 10.1016/j.ejmech.2022.114946. [DOI] [PubMed] [Google Scholar]
- Wu R.; Liu T.; Wu S. K.; Li H. D.; Song R. J.; Song B. A. Synthesis, antibacterial activity, and action mechanism of novel sulfonamides containing oxyacetal and pyrimidine. J. Agric. Food Chem. 2022, 70, 9305–9318. 10.1021/acs.jafc.2c02099. [DOI] [PubMed] [Google Scholar]
- Ding R. C.; Wang X. X.; Fu J. F.; Chang Y. Y.; Li Y. X.; Liu Y. J.; Liu Y.; Ma J. L.; Hu J. X. Design, synthesis and antibacterial activity of novel pleuromutilin derivatives with thieno[2,3-d]pyrimidine substitution. Eur. J. Med. Chem. 2022, 237, 114398. 10.1016/j.ejmech.2022.114398. [DOI] [PubMed] [Google Scholar]
- Zhan W. L.; Mao P.; Yuan C. M.; Zhang Y. Q.; Zhang T.; Liu Y.; Tian J.; Xue W. Design, synthesis and antiviral activities of chalcone derivatives containing pyrimidine. J. Saudi Chem. Soc. 2023, 27, 101590. 10.1016/j.jscs.2022.101590. [DOI] [Google Scholar]
- Tang X. M.; Zhan W. L.; Chen S.; Zhou R.; Hu D.; Sun N.; Fei Q.; Wu W. N.; Xue W. Synthesis, bioactivity and preliminary mechanism of action of novel trifluoromethyl pyrimidine derivatives. Arab. J. Chem. 2022, 15, 104110. 10.1016/j.arabjc.2022.104110. [DOI] [Google Scholar]
- Lamie P. F.; El-Kalaawy A. M.; Abdel Latif N. S.; Rashed L. A.; Philoppes J. N. Pyrazolo[3,4-d]pyrimidine-based dual EGFR T790M/HER2 inhibitors: Design, synthesis, structure-activity relationship and biological activity as potential antitumor and anticonvulsant agents. Eur. J. Med. Chem. 2021, 214, 113222. 10.1016/j.ejmech.2021.113222. [DOI] [PubMed] [Google Scholar]
- Sondhi S. M.; Singh N.; Johar M.; Kumar A. Synthesis, anti-inflammatory and analgesic activities evaluation of some mono, bi and tricyclic pyrimidine derivatives. Bioorg. Med. Chem. 2005, 13, 6158–6166. 10.1016/j.bmc.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Wang H. W.; Cui E. J.; Li J. M.; Ma X. D.; Jiang X. Y.; Du S. S.; Qian S. H.; Du L. Design and synthesis of novel indole and indazole-piperazine pyrimidine derivatives with anti-inflammatory and neuroprotective activities for ischemic stroke treatment. Eur. J. Med. Chem. 2022, 241, 114597. 10.1016/j.ejmech.2022.114597. [DOI] [PubMed] [Google Scholar]
- Wen Y. H.; Cheng L.; Xu T. M.; Liu X. H.; Wu N. J. Synthesis, insecticidal activities and DFT study of pyrimidin-4-amine derivatives containing the 1,2,4-oxadiazole motif. Front. Chem. Sci. Eng. 2022, 16, 1090–1100. 10.1007/s11705-021-2091-5. [DOI] [Google Scholar]
- Zhang D. S.; Zhang J.; Liu T.; Wu S.; Wu Z. X.; Wu S. K.; Song R. J.; Song B. A. Discovery of pyrido[1,2-a]pyrimidine mesoionic compounds containing benzo[b]thiophene moiety as potential pesticide candidates. J. Agric. Food Chem. 2022, 70, 8598–8608. 10.1021/acs.jafc.2c01899. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Yang Z. H.; Liu Q. S.; Sun X. B.; Chen L. L.; Sun L.; Gu W. Design, synthesis, and fungicidal evaluation of novel 1,3-benzodioxole-pyrimidine derivatives as potential succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2022, 70, 7360–7374. 10.1021/acs.jafc.2c00734. [DOI] [PubMed] [Google Scholar]
- Zhang X. M.; Yang Z. K.; Xu H.; Liu Y. S.; Yang X. L.; Sun T. D.; Lu X.; Shi F. S.; Yang Q.; Chen W.; Duan H. X.; Ling Y. Synthesis, antifungal activity, and 3D-QASR of novel 1,2,3,4-tetrahydroquinoline derivatives containing a pyrimidine ether scaffold as chitin synthase inhibitors. J. Agric. Food Chem. 2022, 70, 9262–9275. 10.1021/acs.jafc.2c01348. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Yang Z. H.; Liu Q. S.; Sun X. B.; Chen L. L.; Sun L.; Gu W. Design, Synthesis, and Fungicidal Evaluation of Novel 1,3-Benzodioxole-Pyrimidine Derivatives as Potential Succinate Dehydrogenase Inhibitors. J. Agric. Food Chem. 2022, 70, 7360–7374. 10.1021/acs.jafc.2c00734. [DOI] [PubMed] [Google Scholar]
- Yan Y. K.; Cheng W.; Xiao T. T.; Zhang G. L.; Zhang T. T.; Lu T.; Tang X. R. Discovery of Novel 2,4,6-Trisubstituted Pyrimidine Derivatives as Succinate Dehydrogenase Inhibitors. Chin. J. Org. Chem. 2020, 40, 4237–4248. 10.6023/cjoc202005057. [DOI] [Google Scholar]
- Shi Y. H.; Zhang S.; Wan F. X.; Sun C. X.; Jiang L. Synthesis, Fungicidal Activity and Molecular Docking Study of Novel N-[2-((Substitutedphenyl)amino)pyridin-3-yl]-pyrimidine-4-carboxamides. Chin. J. Org. Chem. 2020, 40, 1948–1954. 10.6023/cjoc202002019. [DOI] [Google Scholar]
- Yang Z. H.; Sun Y.; Liu Q. S.; Li A. L.; Wang W. Y.; Gu W. Design, synthesis, and antifungal activity of novel thiophene/furan-1,3,4-oxadiazole carboxamides as potent succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2021, 69, 13373–13385. 10.1021/acs.jafc.1c03857. [DOI] [PubMed] [Google Scholar]
- Zhu J. J.; Wang P. Y.; Long Z. Q.; Xiang S. Z.; Zhang J. R.; Li Z. X.; Wu Y. Y.; Shao W. B.; Zhou X.; Liu L. W.; Yang S. Design, Synthesis, and Biological Profiles of Novel 1,3,4-Oxadiazole-2-carbohydrazides with Molecular Diversity. J. Agric. Food Chem. 2022, 70, 2825–2838. 10.1021/acs.jafc.1c07190. [DOI] [PubMed] [Google Scholar]
- Wu Y. Y.; Shao W. B.; Zhu J. J.; Long Z. Q.; Liu L. W.; Wang P. Y.; Li Z.; Yang S. Novel 1,3,4-Oxadiazole-2-carbohydrazides as Prospective Agricultural Antifungal Agents Potentially Targeting Succinate Dehydrogenase. J. Agric. Food Chem. 2019, 67, 13892–13903. 10.1021/acs.jafc.9b05942. [DOI] [PubMed] [Google Scholar]
- Zeng D.; Liu S. S.; Shao W. B.; Zhang T. H.; Qi P. Y.; Liu H. W.; Zhou X.; Liu L. W.; Zhang H.; Yang S. New Inspiration of 1,3,4-Oxadiazole Agrochemical Candidates: Manipulation of a Type III Secretion System-Induced Bacterial Starvation Mechanism to Prevent Plant Bacterial Diseases. J. Agric. Food Chem. 2023, 71, 2804–2816. 10.1021/acs.jafc.2c07486. [DOI] [PubMed] [Google Scholar]
- Peng F.; Liu T. T.; Wang Q. F.; Liu F.; Cao X.; Yang J. S.; Liu L. W.; Xie C. W.; Xue W. Antibacterial and Antiviral Activities of 1,3,4-Oxadiazole Thioether 4H-Chromen-4-one Derivatives. J. Agric. Food Chem. 2021, 69, 11085–11094. 10.1021/acs.jafc.1c03755. [DOI] [PubMed] [Google Scholar]
- Hou S. T.; Shi H. B.; Zhang H.; Wu Z. B.; Hu D. Y. Synthesis, Antifungal Evaluation, 3D-QSAR, and Preliminarily Mechanism Study of Novel Chiral Mandelic Acid Derivatives. J. Agric. Food Chem. 2023, 71, 7631–7641. 10.1021/acs.jafc.2c09006. [DOI] [PubMed] [Google Scholar]
- Yang G. Q.; Zheng H. L.; Shao W. B.; Liu L. W.; Wu Z. B. Study of the in vivo antiviral activity against TMV treated with novel 1-(t-butyl)-5-amino-4-pyrazole derivatives containing a 1,3,4-oxadiazole sulfide moiety. Pestic. Biochem. Physiol. 2021, 171, 104740. 10.1016/j.pestbp.2020.104740. [DOI] [PubMed] [Google Scholar]
- Li J. H.; Wang Y.; Wu Y. P.; Li R. H.; Liang S.; Zhang J.; Zhu Y. G.; Xie B. J. Synthesis, herbicidal activity study and molecular docking of novel pyrimidine thiourea. Pestic. Biochem. Physiol. 2021, 172, 104766. 10.1016/j.pestbp.2020.104766. [DOI] [PubMed] [Google Scholar]
- Kumer A.; Kobir M. E.; Alam M.; Chakma U.; Akter P.; Bhuiyan M. M. H. Antibacterial, antifungal and antiviral activities of pyrimido [4,5-d]pyrimidine derivatives through computational approaches. Org. Commun. 2022, 15, 239–260. 10.25135/acg.oc.133.2204.2439. [DOI] [Google Scholar]
- Fan Z. J.; Shi J.; Luo N.; Ding M. H.; Bao X. P. Synthesis, Crystal Structure, and Agricultural Antimicrobial Evaluation of Novel Quinazoline Thioether Derivatives Incorporating the 1,2,4-Triazolo[4,3-a]pyridine Moiety. J. Agric. Food Chem. 2019, 67, 11598–11606. 10.1021/acs.jafc.9b04733. [DOI] [PubMed] [Google Scholar]
- Li C. K.; Liu Y. H.; Ren X. L.; Tan Y. N.; Jin L. H.; Zhou X. Design, Synthesis and Bioactivity of Novel Pyrimidine Sulfonate Esters Containing Thioether Moiety. Int. J. Mol. Sci. 2023, 24, 4691. 10.3390/ijms24054691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. E.; Yang D. C.; Huo J. Q.; Chen L.; Kang Z. H.; Mao J. Y.; Zhang J. L. Design, Synthesis, and Herbicidal Activity of Thioether Containing 1,2,4-Triazole Schiff Bases as Transketolase Inhibitors. J. Agric. Food Chem. 2021, 69, 11773–11780. 10.1021/acs.jafc.1c01804. [DOI] [PubMed] [Google Scholar]
- Sun C. X.; Zhang S.; Qian P.; Li Y.; Ren W. S.; Deng H.; Jiang L. Synthesis and fungicidal activity of novel benzimidazole derivatives bearing pyrimidine-thioether moiety against Botrytis cinerea. Pest Manag. Sci. 2021, 77, 5529–5536. 10.1002/ps.6593. [DOI] [PubMed] [Google Scholar]
- Bogolubsky A. V.; Moroz Y. S.; Mykhailiuk P. K.; Ostapchuk E. N.; Rudnichenko A. V.; Dmytriv Y. V.; Bondar A. N.; Zaporozhets O. A.; Pipko S. E.; Doroschuk R. A.; Babichenko L. N.; Konovets A. I.; Tolmachev A. One-Pot Parallel Synthesis of Alkyl Sulfides, Sulfoxides, and Sulfones. ACS Comb. Sci. 2015, 17, 348–354. 10.1021/acscombsci.5b00024. [DOI] [PubMed] [Google Scholar]
- Li P.; Hu D. Y.; Xie D. D.; Chen J. X.; Jin L. H.; Song B. A. Design, Synthesis, and Evaluation of New Sulfone Derivatives Containing a 1,3,4-Oxadiazole Moiety as Active Antibacterial Agents. J. Agric. Food Chem. 2018, 66, 3093–3100. 10.1021/acs.jafc.7b06061. [DOI] [PubMed] [Google Scholar]
- Li P.; Tian P. Y.; Chen Y. Z.; Song X. P.; Xue W.; Jin L. H.; Hu D. Y.; Yang S.; Song B. A. Novel bisthioether derivatives containing a 1,3,4-oxadiazole moiety: design, synthesis, antibacterial and nematocidal activities. Pest Manag. Sci. 2018, 74, 844–852. 10.1002/ps.4762. [DOI] [PubMed] [Google Scholar]
- Shi J.; Luo N.; Ding M. H.; Bao X. P. Synthesis, in vitro antibacterial and antifungal evaluation of novel 1,3,4-oxadiazole thioether derivatives bearing the 6-fluoroquinazolinylpiperidinyl moiety. Chin. Chem. Lett. 2020, 31, 434–438. 10.1016/j.cclet.2019.06.037. [DOI] [Google Scholar]
- Li P.; Chi J. Y.; Xiao L. L.; Yu L. Synthesis and antibacterial and antifungal activities of novel thiochroman-4-one derivatives incorporating oxime ether and 1,3,4-oxadiazole thioether moieties. Phosphorus Sulfur 2022, 197, 1063–1068. 10.1080/10426507.2022.2056736. [DOI] [Google Scholar]
- Yang Z. B.; Li P.; He Y. J.; Luo J.; Zhou J.; Wu Y. H.; Chen L. T. Novel pyrethrin derivatives containing an 1,3,4-oxadiazole thioether moiety: Design, synthesis, and insecticidal activity. J. Heterocyclic Chem. 2020, 57, 81–88. 10.1002/jhet.3750. [DOI] [Google Scholar]
- Pan N. J.; Wu R. R.; Yan C.; Zhou M.; Fei Q.; Li P.; Wu W. N. Design, synthesis, antifungal activity, and molecular docking of novel trifluoromethyl pyrimidine derivatives containing 1,3,4-oxadiazole and thioether moieties as potential succinate dehydrogenase inhibitors. J. Heterocyclic. Chem. 2023, 60, 1768–1777. 10.1002/jhet.4719. [DOI] [Google Scholar]
- Zhou W. J.; Zhang L.; Xiao W.; Chen H. J.; Wu W. N.; Ouyang G. P. Synthesis and Biological Activity of Anthranilic Diamide Derivatives Incorporating 1,3,4-oxadiazole or Nitrogen-containing Saturated Heterocyclic Moieties. J. Heterocyclic Chem. 2017, 54, 1423–1429. 10.1002/jhet.2724. [DOI] [Google Scholar]
- Wu W. N.; Gao M. N.; Tu H.; Ouyang G. P. Synthesis and Antibacterial Activity of Novel Substituted Purine Derivatives. J. Heterocyclic Chem. 2016, 53, 2042–2048. 10.1002/jhet.2527. [DOI] [Google Scholar]
- Wu W. N.; Chen Q.; Tai A. Q.; Jiang G. Q.; Ouyang G. P. Synthesis and antiviral activity of 2-substituted methylthio-5-(4-amino-2-methylpyrimidin-5-yl)-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 2243–2246. 10.1016/j.bmcl.2015.02.069. [DOI] [PubMed] [Google Scholar]
- Gooding G. V.; Hebert T. T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1287. [PubMed] [Google Scholar]
- Song B. A.; Zhang H. P.; Wang H.; Yang S.; Jin L. H.; Hu D. Y.; Pang L.; Xue W. Synthesis and Antiviral Activity of Novel Chiral Cyanoacrylate Derivatives. J. Agric. Food Chem. 2005, 53, 7886–7891. 10.1021/jf051050w. [DOI] [PubMed] [Google Scholar]
- Lachhab N.; Sanzani S. M.; Bahouaoui M. A.; Boselli M.; Ippolito A. Effect of some protein hydrolysates against gray mold of table and wine grapes. Eur. J. Plant Pathol. 2016, 144, 821–830. 10.1007/s10658-015-0749-x. [DOI] [Google Scholar]
- Wu W. N.; Cao S.; Chen H. J.; Ruan L. X.; Lei Q. Q.; Xu S.; Li J. K. Effects of Ozone Fumigation on the Main Postharvest Pathogenic Fungi Penicillium sp. And the Storage Quality of Blueberry in Majiang County, China. Front. Plant Sci. 2022, 13, 898994. 10.3389/fpls.2022.898994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Yan L. T.; Yuan E. L.; Ding H. X.; Ye H. C.; Zhang Z. K.; Yan C.; Liu Y. Q.; Feng G. Antifungal Activity of Compounds Extracted from Cortex Pseudolaricis against Colletotrichum gloeosporioides. J. Agric. Food Chem. 2014, 62, 4905–4910. 10.1021/jf500968b. [DOI] [PubMed] [Google Scholar]
- Tao Q. Q.; Liu L. W.; Wang P. Y.; Long Q. S.; Zhao Y. L.; Jin L. H.; Xu W. M.; Chen Y.; Li Z.; Yang S. Synthesis and In Vitro and In Vivo Biological Activity Evaluation and Quantitative Proteome Profiling of Oxadiazoles Bearing Flexible Heterocyclic Patterns. J. Agric. Food Chem. 2019, 67, 7626–7639. 10.1021/acs.jafc.9b02734. [DOI] [PubMed] [Google Scholar]
- Wang J. M.; Wang W.; Kollman P. A.; Case D. A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. 10.1016/j.jmgm.2005.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.