Abstract
Understanding medication regimen complexity is important to understand what patients may benefit from pharmacist interventions. Medication Regimen Complexity Index (MRCI), a 65-item tool to quantify the complexity by incorporating the count, dosage form, frequency, and additional administration instructions of prescription medicines, provides a more nuanced way of assessing complexity. The goal of this study was to construct and validate a computational strategy to automate the calculation of MRCI. The performance of our strategy was evaluated by comparing our calculated MRCI values with gold-standard values, using correlation coefficients and population distributions. The results revealed satisfactory performance to calculate the sub-score of MRCI that includes dosage form and frequency (76 to 80% match with gold standard), and fair performance for sub-score related to additional direction (52% match with gold standard). Our automated strategy shows potential to help reduce the effort for manually calculating MRCI and highlights areas for future development efforts.
Introduction
High complexity of medication regimen can potentially lead to several negative patient outcomes, including poor medication adherence (the degree to which the person’s behavior corresponds with the agreed recommendations from a health care provider)1. Furthermore, medication adherence has been found to be an important determinant for treatment success of patients with failure to adhere to prescribed medicines potentially leading to many severe results such as worsening of diseases, increased health care costs and even death2.
Clinical research to better understand the relationship between medication regimen complexity and medication adherence in patients is growing, with 35 studies to date showing that patients with more complex regimen were less likely to adherence to the pharmacotherapy3. Thus, understanding medication regimen complexity is important to understand what patients may benefit from pharmacist interventions. The medication regimen complexity is often defined by number of medications, the number of times those medications are taken per day4, and the doses at which a patient takes a medication, among other factors. Tools to quantify medication regimen complexity hold promise to allow clinical researchers and health care providers to evaluate the regimen complexity in a standardized and objective way. One commonly used method in clinical research is the medication regimen complexity index (MRCI) developed by George and colleagues in 20045.
MRCI is a 65-item tool to quantify the complexity of medication regimens by incorporating the medication counts, dosage forms, dosing frequencies, and special administration instructions of prescription medicines. The MRCI score is the sum of 3 different sub-scores: sub-score A - Dosage Forms (e.g., tablet, liquid), sub-score B - Dosing Frequency (e.g., once a day, as needed), and sub-score C - Additional Directions (e.g., crush, dissolve). Weights are assigned to each type of dosage form, frequency and special administration and the sum of all weights is the final MRCI score. Patient-level MRCI has been used widely in clinical studies of multiple patient cohorts including patients with chronic diseases6, critically ill patients7, and patients after surgery8. Many applications of MRCI have been shown to be effective in applications such as identifying patients for medication therapy management interventions9 and studying the substantial medication burden for patients after heart transplantation8. There are currently no widely used tools, however, to automate the calculation of MRCI from raw prescription data. Currently, calculating MRCI score requires researchers to manually go through the medication list and assign weights according to the MRCI table from Geroge, 20045. That manual approach can be time- and labor-intensive when dealing with a large dataset, thus motivating our goal and the goal of others to implement a strategy to automate this calculation.
There are several previous studies aiming to automate the MRCI calculation10, 11. The previous strategies, however, are limited by requiring preliminary manual processing of data or automating only part of the calculation. In addition, the existing strategies are not applicable for a range of data input formats. For example, in 2013, McDonald and colleagues developed a tool to automate the calculation of MRCI in the electronic health record (EHR) system at their institution10. While successfully automating the MRCI calculation, their “data translating” process did not have enough tolerance for incomplete data or for data of different formats. Furthermore, it was not usable by other institutions that were missing certain required data fields (e.g., “route” of dosage or “additional directions”)9. Acknowledging the limitations of previous strategies to automate the MRCI calculation, the goal of this study was to construct and validate a computational strategy that takes the prescription data of one or more patients as input, and then automatically calculates MRCI scores for each patient.
Methods
Data source
This study used data that were previously collected as part of the pilot study “ALIGN: Aligning Medications with What Matters Most,” a pharmacist-led deprescribing intervention12. Patients enrolled in the study were ≥65 years old with a diagnosis of Alzheimer’s disease or related dementia and taking at least five medications. Patients with fewer than 1 visit to a participating primary care clinic within the past year were excluded, as were individuals residing in long term care facilities or enrolled in hospice, and those who could not converse comfortably in English. The study was approved by the Advarra and Johns Hopkins Medicine Institutional Review Boards.
Data from 25 patients enrolled in ALIGN were included in this study. The raw data for those patients were extracted from the Johns Hopkins Medicine (JHM) EHR at two time points (baseline/enrollment date and 3-months after enrollment). Each medication record contained the patient’s identifier such as medical record number (MRN) and electronic medical record number (EMRN), the medication name, and the doctors’ signature to pharmacists (SIG). The National Drug Code (NDC) number was also included for a part of medications on the list.
Supplementary Database
The present study incorporated the NDC directory sourced from the U.S. Food and Drug Administration (FDA) as an additional data source to aid the dosage form determination. The NDC directory contains information on dosage form and route of administration for each drug product. The NDC number is a distinctive three-segment identifier for drugs developed by the FDA. Notably, the current downloadable released version of the NDC database contained information only about finished drug products available in the U.S. market, excluding those still under development and those are legal in other places; medications not covered in this database will go through alternative pipelines for dosage form determination.
Data selection
Using the medication records available through the JHM EHR, we identified a small subset of medication records of enrolled patients that should not be considered for MRCI calculation. These included medication records that lacked both medication names and SIGs (often indicated as “null” in the data set). Those records were unable to provide any useful information about the complexity of the regimen and were thus excluded. Additionally, one-time medications that did not require any adherence over a specific period, such as vaccines, were also omitted from the calculation. Similarly, medical devices, including miscellaneous medical and diagnostic supplies, were excluded since the MRCI did not apply to them.
Although the over-the-counter (OTC) medications such as vitamin supplements are often not considered with prescriptions, the regimen data we obtained contained a large number of records of these medications. To ensure that our strategy can work on real-world medication regimens that also frequently have vitamins and other supplements, we chose to include supplements such as vitamin B, C, E, Calcium, and Magnesium in the calculation of medication count (MC) and MRCI.
Data Processing Pipeline
We developed a data processing pipeline that accepts medication record data as input and then generates a numeric MRCI score as output. The pipeline consists of two stages: keyword screening and score calculation.
A patient has multiple medication records. For each medication record, the pipeline first screens for keywords from medication names (for sub-score A) and SIG (for sub-score A, B and C). If a keyword is detected from the medication record, the pipeline assigns the corresponding weight to that category and moves on to the next record for that patient. If no keyword is found, the program will proceed to the next keyword on the list to repeat the screening until a keyword is detected or until the end of the keyword list is reached. After all medication records for a patient have been screened for keywords, this pipeline calculates the score by adding up all the weights assigned to each category for the final MRCI score. The calculation of frequency (sub-score B) and additional instructions (sub-score C) follows the exact data processing pipeline.
The determination of dosage forms (sub-score A) is slightly different. To determine the dosage form, our strategy first checks the medication’s NDC number. If a valid NDC is found, the corresponding dosage form is determined from the combination of “dosage form” and “route” column embedded in the NDC directory. If the medication has no NDC code or the NDC directory cannot be searched in the current data directory, keyword screening is executed on the medication name and SIG to identify potential matching keywords for the dosage form. Figure 1 below shows a sample workflow of the pipeline.
Figure 1:
Sample Data Pipeline for Dosage Form Identification
Gold standard dataset
The gold standard dataset was created by the ALIGN study team. A previously developed Microsoft Access database was used to code components of the MRCI scores for each of the 25 enrolled patients represented in the dataset13. Medication lists at baseline and 3 months were coded by one research team member (RQ) and reviewed by another team member (AG) when there was uncertainty about the coding. The study team discussed specific cases and rationales and made decision rules for cases not clearly addressed in the MRCI tool instructions.
The creation of a gold standard dataset is an important step to facilitate evaluating the accuracy and effectiveness of our strategy to automatically calculate MRCI scores. The scores in the gold standard dataset are the “ground truth” for the performance evaluation. By comparing the output MRCI scores of our strategy to those in the gold standard dataset, we both evaluated the performance of the strategy and identified potential limitations. We considered performance measures sufficient for use in clinical research settings if correlation coefficient was above 0.8 and the p-value from rank sum test was above 0.05 (described in more detail in the following section).
Evaluating performance of MRCI calculation
To evaluate the performance of the MRCI calculation from our computational strategy, we utilized three different methods of comparison to the gold standard results. In addition, we generated a simple measure of medication count for comparison.
MRCI Score and MC Match: To assess MRCI scores and MC we directly compared whether the scores and count of medications produced by our strategy matched the gold standard values. Comparison findings are grouped into three levels of matching criteria: exact match (exactly equal to the gold standard), good match (gold standard ±15%) and fair match (gold standard ±30%). The three levels were not mutually exclusive—the subjects grouped as exact match can also be grouped as good match and so forth. Each level uses different criteria to define “matched”. For instance, if the automated result is 11 and the gold standard result is 10, this pair of score will be identified as “not matched” using exact match criteria but “matched” using the good match and fair match criteria. In addition to direct comparison, we used Pearson’s correlation coefficient to evaluate the alignment between the results from our strategy and the gold standard.
MRCI Value and MC Change: To assess MRCI value and MC change, we compared whether the calculated changes in MRCI value and MC from baseline to three months after enrollment matched the gold standard values. The change in MRCI value and MC is defined as the MRCI value/MC count three months after enrollment minus MRCI value/MC count at baseline. The changes can be either negative or positive. Similar to the first approach, this approach evaluated the performance using direct comparison (exact match, good match, fair match) and assessed correlation using Pearson’s correlation coefficient.
MRCI Patient Cohort: The third evaluation method assessed whether a patient falls into the same cohort or tier of MRCI as that of the gold standard when using our strategy. A cohort or tier is defined as the ranking of MRCI values within the whole population as high, medium, or low. The Wilcoxon rank-sum test, a nonparametric statistical test used to compare two independent samples and determine if there is a significant difference in their underlying distributions, was used for this evaluation. The null hypothesis for the rank-sum test was that the two groups of MRCI scores are equal in distribution.
Requirements for a medication regimen complexity calculation tool
We created an initial list of requirements for the potential use of our strategy to calculate medication regimen complexity as a Python module. Through discussions with the co-authors, we compiled this list to include in the initial release of the module.
Results
Decision-Making Keywords
Lists of keywords used for decision making in terms of dosage form, frequency, and additional instruction (Appendix I) were first determined by our clinical experts experienced in deprescribing. The each MRCI items in lists have been corresponds with the keywords concluded from ALIGN study and our data and they are not case sensitive. However, we chose to keep the keyword lists customizable so that keywords can be added, taken off, or modified slightly in case there are needs to accommodate special data.
The keyword list for sub-score A, dosage form, contained two columns of keywords: form keywords as primary determinant and route keywords as an optional determinant. When the search results from the NDC directory, which also contains fields of dosage form and route, are available, our strategy uses both sets of keywords to determine the dosage form. Otherwise, the combination of dosage form and route keywords will be used to align with the medication names for dosage form determination.
The list used for sub-score B and C contained keywords for inclusion and for exclusion. If any of the exclusion keywords was found in the SIG of the medication records, the frequency or additional direction was not assigned to a corresponding type. For example, in the SIG, “once daily as needed,” “once daily” was read as a keyword for both “Once daily prn” and “Once daily.” The exclusion keyword was first screened to distinguish these two frequencies. When the exclusion keyword “as needed” was identified in the text, our strategy assigns the frequency “Once daily” rather than “Once daily prn.”
Performance Evaluation
Performance was assessed with 556 medication records from 25 patients at both baseline and 3 months after. The average MC among the cohort from our strategy was 11.1 (± 0.09), compared to the MC from the gold standard dataset of 11.2 (± 0.09). Our strategy produced an average MRCI score of 18.71 (± 11.74), while the gold standard dataset yielded a score of 25.1 (± 20.46).
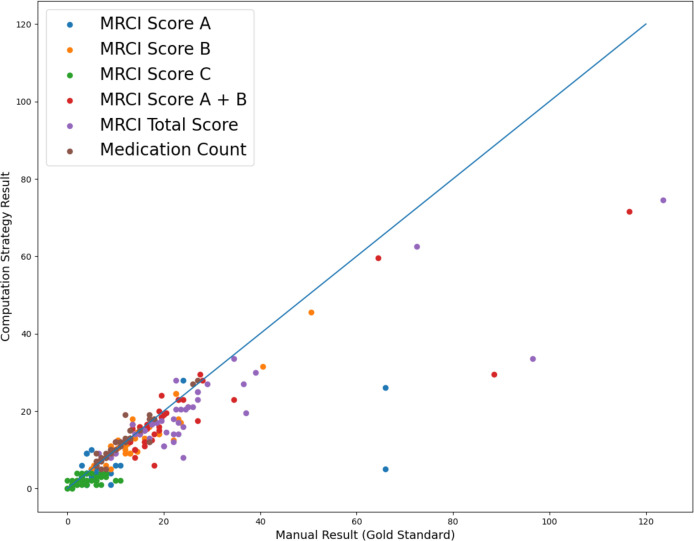
A summary of the match between calculated MRCI values and MC using our strategy and the gold standard values are shown in Figure 2. A total of 50 data points was plotted (one at each of 2 timepoints for each of 25 patients). Outliers were observed for patients with large MRCI scores (>60) in the comparison results shown in Figure 2. The overall distribution of MRCI sub-scores A, B, A+B, and total scores mostly follow the same trend as the gold standard results. The MC from the gold standard and our strategy are mostly consistent. More detailed statistics are presented in Table 1 and 2.
Figure 2.
MRCI Matching Comparison of Manual Result with Gold Standard
Table 1:
MC Matching Result Analysis Table
Table 2:
MRCI Matching Result Analysis Table
Matching rates for MC (Table 1) were 78% (exact match), 86% (good match) and 94% (fair match). Within MRCI scores (Table 2), sub-score A showed the best performance with matching rates of 60% (exact and good match) and 66% (fair match). For sub-score B, the matching rates were 24% (exact match), 58% (good match), and 86% (fair match). The least accurate sub-score was C, with matching rates of 28% (exact and good match) and 32% (fair match). Despite the relatively low exact match rate, the total MRCI score had an 80% matching rate using the fair match criteria. To investigate the influence of sub-score C on the overall performance, the sum of sub-scores A and B was evaluated further. We found that Score A + B had matching rates of 68% (good match) and 90% (fair match).
The Pearson’s correlation coefficient for MC, MRCI sub-score B, MRCI total score and MRCI sub-score A + B showed values that were larger than 0.8, indicating significance in correlation between our results and the gold standard results. Furthermore, the calculated and gold standard MRCI values showed a positive linear relationship. We selected the significance threshold of 0.8 based on standard criteria from previous medical research with a similar expectation of linear relationship and using the same statistical methods14.
The evaluation of changes in the MRCI and MC over the three-month period is shown in Table 1 and 3. The overall matching rate for change in MRCI is lower than the matching rate of absolute MRCI values. Using the same three matching criteria levels, the performance of changes in sub-score A showed the best matching rate, 76% (exact and good match) and 80% (fair match). Meanwhile, those of changes in score B and C are all around 52%. No correlation coefficients exceeded the significance threshold of 0.8, indicating only weak correlations between the changes in calculated MRCI and those in gold standard MRCI values. There was a negative correlation coefficient for sub-score A. Changes in MC over the 3-month period showed matching rates of 76% for all three matching criteria and a correlation coefficient of 0.638, indicating weak correlation.
Table 3:
Matching Result Table for Change in MRCI Scores over 3 Months
The results from Wilcoxon rank-sum tests for significant differences in calculated and gold standard scores are shown in Table 4. The significance level was set to be 0.05, the conventional statistical threshold15. From Table 4, the p-value of sub-score C was less than 0.05, indicating that the score C rejected the null hypothesis (p=0.00039). The p-values of sub-score A, B, and A+B, however, were all greater than 0.05, failing to reject the null hypothesis and showing that the MRCI scores from our strategy and the gold standard do not show statistically significant different distributions.
Table 4:
Rank-Sum Analysis Table
List of features for inclusion in a medication regimen complexity calculation tool
An initial list of features was proposed by our research team and is summarized in Table 5. This list includes functionalities to load input files, write output files, and the ability to customize decision-making keyword lists. Based on our finding that our strategy performs well to calculate MC, we included this option.
Table 5:
Proposed Features Table
| Feature Name | Description | Input(s) | Output(s) |
|---|---|---|---|
| Load File | Read in data file with modifiable column name | A file name for the input medication records list | An indicator showing whether the file loads successfully |
| Write file | Write MRCI output to an output file with modifiable column name | A file name for the output file, a column name for patient identifier (optional) | An indicator showing whether the file writes successfully |
| Customize Decision Keywords | Make modification of decision keyword list | A file name for the data file containing the updated keywords, identifier of list needing to be modified (A/B/C) | None |
| Calculate MRCI and MC (Single time point) | Calculate MRCI score and MC from the data read in | A file name for the input medication records list, a file name for the output result list, a column name for patient identifier (optional), a column name for medication name (optional), a column name for SIG (optional), a column name for dose information (optional), a column name for NDC code (optional), a true/false option for whether to include MC (optional) | An indicator showing whether the calculation successfully, a file contains MRCI scores and MC at one time point |
| Compare MRCI and MC (Two time points) | Calculate MRCI score and MC from the data read in when there are time identifiers | A file name for the input medication records list, a file name for the output result list, a column name for patient identifier (optional), a column name for medication name (optional), a column name for SIG (optional), a column name for dose information (optional), a column name for NDC code (optional), a column name for time idenfier (optional), the name for one of the time points (optional), a true/false option for whether to include MC (optional) | An indicator showing whether the calculation successfully, a file contains MRCI scores and MC at two time points |
Discussion and Conclusions
Performance Evaluation
We implemented a data processing pipeline to automate the calculation of MRCI scores for a cohort of 25 patients. We then conducted a series of performance evaluations. Our findings showed that among MRCI sub-scores, sub-score A exhibited the best performance in exact match, while sub-score B performed the best using the fair match criteria. The calculation of sub-score C had the lowest match with the gold standard, which may be attributed to data quality. The MC exhibited an overall high matching rates, with only a few exceptions. Meanwhile, the computed total MRCI score matched the gold standard 80% of the time using fair match criteria (gold standard ±30%) and a correlation coefficient of 0.88, demonstrating a strong correlation. Sub-score B and sub-score A+B had even higher matching rates and a significant p-value in the rank sum test. These results indicate that our automated strategy successfully extracted and quantified medication form, and frequency.
Although the sum of sub-score A and B performed well, sub-score C showed poor performance. Processing additional directions presents obstacles due to the subjectivity of categorization and dependence on free text data. For instance, the direction “break or crush tablet” might be expressed in various ways, including “cut”, “break”, “crush” or “divide” into “half”, “0.5”, or “1/2”. The decision-making keywords are designed to fit general data rather than institution-specific data, which can result in a low matching rate when the SIG is written in uncommon or inconsistent wording.
Our results also indicated little difference between the exact match, good match, and fair match of sub-score A for MRCI values and MRCI changes. This may be due to the fact that most of the dosage forms in our medication data list are tablets and capsules, which were successfully extracted, resulting in a high exact match rate. Our strategy had more difficulty distinguishing less clearly documented dosage forms such as prefilled injection and ampoules/vials injection.
In our comparison of computed MC with those from the gold-standard dataset, we observed that OTC medications, particularly vitamins, were the primary discrepancies that hindered exact matches of the MC. Notably, we opted to incorporate all OTC medications in our computation of the MC and MRCI, a methodology that is not consistently adopted in the manual calculation in gold-standard data, which may exclude some OTC medications, but not all. Moreover, there are no established guidelines for inclusion or exclusion of OTC medications in the original medication regimen complexity calculation design5.
Limitations
Although our results demonstrate the feasibility of our computational approach, we observed certain limitations. The performance evaluation is constrained by the small dataset available to us, with only 25 patients enrolled and a total of 50 calculated MRCI values, which may not be substantial enough to draw significant conclusions. Another potential limitation for the data in this study is that they are all from one institution. Given that physicians often write additional directions in various styles, our current keywords may not encompass written expressions that are commonly used at other institutions. This “overfitting” issue may limit the generalizability of our MRCI calculations. Furthermore, the gold standard dataset was generated by a single researcher without cross-validation by other researchers to ensure accuracy. Potential transcriptional errors and estimation errors due to limited dataset and human operations could also negatively affect the quality of our gold standard dataset.
In comparing the two sets of MRCI data, we employed three-level matching criteria during the performance evaluation. However, we could not find any established standard for matching thresholds from previous clinical studies. As a result, we designed these matching criteria from a statistical perspective. The clinical significance of these matching criteria remains uncertain, preventing us from reaching a quantitative conclusion to confirm the universal applicability in real-world clinical studies. Additionally, while previous study5 demonstrated the strong correlation in the number of unduplicated medications and MRCI score, we now base our matching criteria on the MRCI score percentage instead of medication counts. Our performance study would benefit from matching thresholds proportional or related to medication counts instead of the current fixed-value ones.
Related and Future Work
Medication regimen complexity is increasingly used to predict risk of poor clinical outcomes such as hospital readmission, patient acuity, and inpatient mortality16. Which characteristics of medication regimens are important to encode in calculations of medication regimen complexity, however, may depend on the problem being investigated. One study producing a model to predict health care costs, for example, showed better performance with use of medication count than MRCI score16. Here we focus our investigation on MRCI that encodes a range of medication data such as dosage form, frequency, and additional instructions. Producing a tool to automate the calculation of both MRCI and simple measures of medication complexity (e.g., medication count) for use by diverse research communities would simplify investigations using those measures as covariates. It would also enable future discovering on whether encoding more or less information on medication complexity is more beneficial for prediction tasks. This work lays the foundation for further testing on larger datasets.
The tool developed from our computational strategy is published as a Python package called MedX (Appendix II). We will provide mechanisms for ongoing user feedback from those choosing to use our tool. Collecting these data will enable us to assess performance and prioritize areas to improve our computational strategy.
Conclusion
While patient-level MRCI is a useful tool for characterizing drug complexity in clinical studies, it has not yet been widely used. Low use may in part be due to the difficulty of performing the calculation. Our strategy provides a framework to transform raw EHR data for input into our automated pipeline that calculates MRCI. This framework holds promise to reduce time and labor required to perform MRCI calculations. It also lays the foundation for broader dissemination as a Python module for use by diverse research communities.
Acknowledgements
We thank Anne Libby, PhD, Department of Emergency Medicine, University of Colorado School of Medicine, for developing the Microsoft Access database used to code components of the MRCI score in the manual calculation and for graciously allowing us to use it for the ALIGN pilot study.
Appendixes
Appendix I: MRCI Calculation Decision Keyword Lists
Sub-score A: Dosage Form
| MRCI Dosage Form | Form Keywords | Route Keywords (Optionally) | Weighting | |
|---|---|---|---|---|
| Oral | Capsules/Tablets | TABLET, CAPSULE, VITAMIN | MOUTH, ORAL | 1 |
| Gargles/Mouthwashes | MOUTHWASH | MOUTH, ORAL | 2 | |
| Gums/Lozenges | LOZENGE, GUM | MOUTH, ORAL | 2 | |
| Liquids | LIQUID, SOLUTION | MOUTH, ORAL | 2 | |
| Powders/Granules | POWDER, GRANULE | MOUTH, ORAL | 2 | |
| Sublingual sprays/tabs | SPRAY, AEROSOL, TABLET | SUBLINGUAL | 2 | |
| Topical | Creams/Gels/Ointments | CREAM, GEL, OINTMEN | TOPICAL, CUTANEOUS, TRANSDERMAL | 2 |
| Dressings | DRESSING | TOPICAL, CUTANEOUS, TRANSDERMAL | 3 | |
| Paints/Solutions | SOLUTION | TOPICAL, CUTANEOUS, TRANSDERMAL | 2 | |
| Pastes | PASTE | TOPICAL, CUTANEOUS, TRANSDERMAL | 3 | |
| Patches | PATCH | TOPICAL, CUTANEOUS, TRANSDERMAL | 2 | |
| Spray | SPRAY, AEROSOL | TOPICAL, CUTANEOUS, TRANSDERMAL | 1 | |
| Ear, Eye & Nose | Ear drops/creams/ointments | GEL, OINTMENT | OPHTHALMIC, INTRAOCULAR, AURICULAR | 3 |
| Eye drops | SOLUTION | OPHTHALMIC, INTRAOCULAR, AURICULAR | 3 | |
| Eye gels/ointments | GEL, OINTMENT | OPHTHALMIC, INTRAOCULAR, AURICULAR | 3 | |
| Nasal drops/cream/ointment | DROP, CREAM, OINTMENT | NASAL, NOSTRIL, NARE | 3 | |
| Nasal spray | SPRAY, AEROSOL | NASAL, NOSTRIL, NARE | 2 | |
| Inhalation | Accuhalers | ACCUHALER | INHALE, INHALATION, INTRABRONCHIAL, RESPIRATORY | 3 |
| Aerolizers | AEROLIZER | INHALE, INHALATION, INTRABRONCHIAL, RESPIRATORY | 3 | |
| Metered dose inhalers | METERED | INHALE, INHALATION, INTRABRONCHIAL, RESPIRATORY | 4 | |
| Nebuliser | SOLUTION | INHALE, INHALATION, INTRABRONCHIAL, RESPIRATORY | 5 | |
| Oxygen/Concentrator | GAS | INHALE, INHALATION, INTRABRONCHIAL, RESPIRATORY | 3 | |
| Turbuhalers | TURBUHALER | INHALE, INHALATION, INTRABRONCHIAL, RESPIRATORY | 3 | |
| Other DPIS | DPI | INHALE, INHALATION, INTRABRONCHIAL, RESPIRATORY | 3 | |
| Others | Dialysate | DIALYSATE | 5 | |
| Enemas | ENEMA, LIQUID | RECTAL | 2 | |
| Injections: Prefilled | INJECTION, INJECTABLE, PREFILLED SOLUTION | SUBCUTANEOUS, INTRAVENOUS, INTRAMUSULAR, PERINEURAL, INTRAVASCULAR, INTRA-ARTERIAL, INJECT | 3 | |
| Injections: Ampoules/Vials | INJECTION, INJECTABLE | SUBCUTANEOUS, INTRAVENOUS, INTRAMUSULAR, PERINEURAL, INTRAVASCULAR, INTRA-ARTERIAL, INJECT | 4 | |
| Pessaries | PESSARIES, PESSARY | VAGINAL | 3 | |
| Patient controlled analgesia | PCA, ANALGESIA | 2 | ||
| Suppositories | SUPPOSITORY | 2 | ||
| Vaginal creams | CREAM | VAGINAL | 2 |
Sub-score B: Frequency
| Frequency | Keywords | Exclusion Keywords | Weighting |
|---|---|---|---|
| Once daily prn | “once daily”, “nightly”, “every day”, “every night”, “every evening”, “every afternoon” | “times”, “as needed”, “prn” | 1 |
| Once daily | “once daily”, “nightly”, “every day”, “every night”, “every evening”, “every afternoon” | “times” | 0.5 |
| Twice daily | “twice daily”, “2 times daily”, “two times daily”, “twice a day”, “2 times a day”, “two times a day” | “as needed”, “prn” | 2 |
| Twice daily prn | “twice daily”, “2 times daily”, “two times daily”, “twice a day”, “2 times a day”, “two times a day” | “” | 1 |
| Three times daily | “three times a day”, “three times daily”, “3 times a day”, “3 times daily” | “as needed”, “prn” | 3 |
| Three times daily prn | “three times a day”, “three times daily”, “3 times a day”, “3 times daily” | “” | 1.5 |
| Four times daily | “four times a day”, “four times daily”, “4 times a day”, “4 times daily” | “as needed”, “prn” | 4 |
| Four times daily prn | “four times a day”, “four times daily”, “4 times a day”, “4 times daily” | “” | 2 |
| q 12h | “every 12h”, “every 12 hours”, “every twelve hours” | “as needed”, “prn” | 2.5 |
| q 12h prn | “every 12h”, “every 12 hours”, “every twelve hours” | “” | 1.5 |
| q 8h | “every 8h”, “every 8 hours”, “every eight hours” | “as needed”, “prn” | 3.5 |
| q 8h prn | “every 8h”, “every 8 hours”, “every eight hours” | “” | 2 |
| q 6h | “every 6h”, “every 6 hours”, “every six hours” | “as needed”, “prn” | 4.5 |
| q 6h prn | “every 6h”, “every 6 hours”, “every six hours” | “” | 2.5 |
| q 4h | “every 4h”, “every 4 hours”, “every four hours” | “as needed”, “prn” | 6.5 |
| q 4h prn; | “every 4h”, “every 4 hours”, “every four hours” | “” | 3.5 |
| q 2h | “every 2h”, “every 2 hours”, “every two hours” | “as needed”, “prn” | 12.5 |
| q 2h prn | “every 2h”, “every 2 hours”, “every two hours” | “” | 6.5 |
| prn/sos | “as needed”, “prn” | “[number]” | 0.5 |
| On alternating days or less frequently | ““MWF,” “MONDAY,” “WEDNESDAY,” “FRIDAY,” “once weekly,” “once a week,” “TWICE A WEEK,” “TIMES PER WEEK,” “ONCE A MONTH,” “every other day,” “on dialysis days,” “after dialysis,” “every 6 months”” | “” | 2 |
| Oxygen prn | “oxygen” | 1 | |
| Oxygen <15hrs | “oxygen” | “as needed”, “prn” | 2 |
| Oxygen >15hrs | “oxygen continous”, “continous oxygen”, “coontinuously” | “as needed”, “prn” | 3 |
Sub-score C: Additional Directions
| Additional Directions | Keywords | Exclusion Keywords | Weighting |
|---|---|---|---|
| Break or crush tablet | “G-tube tablet”, “PEG tab”, “PEG EC tab”, “crush”, “[number].[number]”, “1/[number]”, “half”, “quarter”, “cut”, “break” | “do not crush” | 1 |
| Dissolve tablet/powder | “dissolve” | 1 | |
| Multiple units at one time (e.g. 2 tabs, 2 puffs) | “tabs”, “tablets”, “capsules”, “caps”, “puffs”, “inhalations”, “drops”, “patches”, “combine with”, “together with”, “along with”, “give with” | 1 | |
| Take/use at specified times (e.g. 8 am) | “AM”, “PM”, “morning”, “afternoon”, “noon”, “evening”, “bedtime”, “night”, “before bed”, “pm”, “HS,before” | 1 | |
| Relation to food (e.g. pc, ac, with food) | “breakfast”, “lunch”, “food”, “meal”, “with meals”, “supper”, “dinner”, “carb”, “eating”, “EMPTY STOMACH”, “AC” | 1 | |
| Take with specific fluid | “water”, “juice”, “soda”, “clear liquid”, “clearliquid”, “liquid of choice”, “non-carbonated”, “beverage” | “liquid tears” | 1 |
| Take/use as directed | “as directed”, “adjust dose”, “hold if”, “bridge”, “INR”, “weight gain”, “weight increase”” | 2 | |
| Tapering/increasing dose | “taper”, “decrease”, “increase”, “increase weekly”, “increasing”, “wean”, “then” | 2 | |
| Variable dose | “may repeat”, “may take additional”, “[number]-[number]” | 1 | |
| Alternating dose (e.g. one mane and 2 nocte) | “alternat”, “other days”, “even”, “odd” | 2 |
Appendix II: GitHub repository for MedX
Author Contributions
YL contributed to manuscript drafts and code development. YL, CT, AG and RQ contributed to the study design, theory development, data collection and data analysis. CT and AG provided project supervision.
Figures & Tables
References
- 1.Dobbels F, Damme-Lombaert RV, Vanhaecke J, Geest SD. Growing pains: Non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatric Transplantation. 2005 Jun;9(3):381–90. doi: 10.1111/j.1399-3046.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 2.Jimmy B, Jose J. Patient medication adherence: Measures in daily practice. Oman Medical Journal. 2011 May;26(3):155–9. doi: 10.5001/omj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantuzza LL, Ceccato Mdas, Silveira MR, Junqueira LM, Reis AM. Association between medication regimen complexity and pharmacotherapy adherence: A systematic review. European Journal of Clinical Pharmacology. 2017 Aug 4;73(11):1475–89. doi: 10.1007/s00228-017-2315-2. [DOI] [PubMed] [Google Scholar]
- 4.Muir AJ, Sanders LL, Wilkinson WE, Schmader K. Reducing medication regimen complexity. Journal of General Internal Medicine. 2001;16(2):77–82. doi: 10.1046/j.1525-1497.2001.016002077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George J, Phun Y-T, Bailey MJ, Kong DC, Stewart K. Development and validation of the medication regimen complexity index. Annals of Pharmacotherapy. 2004;38(9):1369–76. doi: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- 6.Tesfaye WH, Peterson GM, Castelino RL, McKercher C, Jose MD, Wimmer BC, et al. Medication regimen complexity and hospital readmission in older adults with chronic kidney disease. Annals of Pharmacotherapy. 2018 Aug 2;53(1):28–34. doi: 10.1177/1060028018793419. [DOI] [PubMed] [Google Scholar]
- 7.Gwynn ME, Poisson MO, Waller JL, Newsome AS. Development and validation of a medication regimen complexity scoring tool for critically ill patients. American Journal of Health-System Pharmacy. 2019 Jun 17;76(Supplement_2) doi: 10.1093/ajhp/zxy054. [DOI] [PubMed] [Google Scholar]
- 8.Bryant BM, Libby AM, Metz KR, Page RL, Ambardekar AV, Lindenfeld JA, et al. Evaluating patient-level medication regimen complexity over time in heart transplant recipients. Annals of Pharmacotherapy. 2016 Jul 19;50(11):926–34. doi: 10.1177/1060028016657552. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch JD, Metz KR, Hosokawa PW, Libby AM. Validation of a patient‐level medication regimen complexity index as a possible tool to identify patients for medication therapy management intervention. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2014 Jun 20;34(8):826–35. doi: 10.1002/phar.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald MV, Peng TR, Sridharan S, Foust JB, Kogan P, Pezzin LE, et al. Automating the medication regimen complexity index. Journal of the American Medical Informatics Association. 2013 May;20(3):499–505. doi: 10.1136/amiajnl-2012-001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dierich M, Adam T, Westra BL, Olson CH. Optimization of Decision Support Tool using medication regimens to assess rehospitalization risks. Applied Clinical Informatics. 2014 Aug 27;05(03):773–88. doi: 10.4338/ACI-2014-04-RA-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green A. Align: Aligning Medications with What Matters Most, a pharmacist-led deprescribing intervention. 2022Jun21 [cited 2023Mar21]. Available from: https://beta.clinicaltrials.gov/study/NCT04938648.
- 13.Metz KR, Fish DN, Hosokawa PW, Hirsch JD, Libby AM. Patient-level medication regimen complexity in patients with HIV. Annals of Pharmacotherapy. 2014;48(9):1129–37. doi: 10.1177/1060028014539642. [DOI] [PubMed] [Google Scholar]
- 14.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Medical Journal. 2012 Sep;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 15.Grabowski B. “p< 0.05” might not mean what you think: American statistical association clarifies p. values. Journal of the National Cancer Institute. 2016Aug10;108(8) doi: 10.1093/jnci/djw194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kharrazi H, Ma X, Chang H-Y, Richards TM, Jung C. Comparing the predictive effects of patient medication adherence indices in electronic health record and claims-based risk stratification models. Population Health Management. 2021;24(5):601–9. doi: 10.1089/pop.2020.0306. [DOI] [PubMed] [Google Scholar]