Abstract
The in vivo role of endogenous interleukin 12 (IL-12) in modulating intrapulmonary growth of Legionella pneumophila was assessed by using a murine model of replicative L. pneumophila lung infection. Intratracheal inoculation of A/J mice with virulent bacteria (106 L. pneumophila cells per mouse) resulted in induction of IL-12, which preceded clearance of the bacteria from the lung. Inhibition of endogenous IL-12 activity, via administration of IL-12 neutralizing antiserum, resulted in enhanced intrapulmonary growth of the bacteria within 5 days postinfection (compared to untreated L. pneumophila-infected mice). Because IL-12 has previously been shown to modulate the expression of cytokines, including gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-10, which regulate L. pneumophila growth, immunomodulatory effects of endogenous IL-12 on intrapulmonary levels of these cytokines during replicative L. pneumophila lung infection were subsequently assessed. Results of these experiments demonstrated that TNF-α activity was significantly lower, while protein levels of IFN-γ and IL-10 in the lung were similar, in L. pneumophila-infected mice administered IL-12 antiserum, compared to similarly infected untreated mice. Together, these results demonstrate that IL-12 is critical for resolution of replicative L. pneumophila lung infection and suggest that regulation of intrapulmonary growth of L. pneumophila by endogenous IL-12 is mediated, at least in part, by TNF-α.
Legionella pneumophila, the causative agent of Legionnaires’ disease, is an intracellular pathogen of mononuclear phagocytic cells (MPCs) (37, 43, 45). Pulmonary infection usually develops following inhalation of L. pneumophila-contaminated water aerosols or microaspiration of contaminated water sources (9). Following inhalation, the bacteria invade and replicate in host MPCs, primarily in alveolar MPCs (34, 36, 37, 43, 45). Intracellular growth of L. pneumophila results in eventual lysis of infected MPCs, the release of bacterial progeny, and reinfection of additional pulmonary cells (34, 36). Severe lung damage, mediated by tissue-destructive substances likely derived from both damaged host cells and the bacteria, ensues (20, 21).
Previous studies have demonstrated that resistance to primary replicative L. pneumophila lung infection is dependent on the induction of cellular immunity and is mediated in part by cytokines including gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (8, 12, 14, 15, 23, 27, 28, 35, 57). Growth of L. pneumophila within permissive MPCs requires iron. IFN-γ limits MPC iron, thereby converting the MPC intracellular environment from one that is permissive to one that is nonpermissive for L. pneumophila replication (14, 15). IFN-γ in combination with other cytokines including TNF-α facilitates elimination of L. pneumophila from infected MPCs, likely through the induction of effector molecules including nitric oxide (12). In contrast, other cytokines including interleukin 10 (IL-10) facilitate growth of L. pneumophila in permissive MPCs, due in part to IL-10-mediated inhibition of TNF-α secretion and IFN-γ-mediated MPC activation (46).
IL-12 is a recently described cytokine with pleiotropic effects on T cells and natural killer (NK) cells which include (i) regulation of expression of cytokines including IFN-γ, TNF-α, and IL-10 by T cells and/or NK cells, (ii) induction of T-cell and/or NK cell proliferation and/or differentiation, and (iii) enhancement of NK cell and T-cell cytotoxic activity (4, 5, 19, 32, 33, 39, 44, 47, 48, 50, 56). While systemic administration of exogenous IL-12 has been demonstrated to increase host resistance to several intracellular pathogens, including Leishmania major, Toxoplasma gondii, Listeria monocytogenes, Mycobacterium tuberculosis, Mycobacterium avium, and Plasmodium chabaudi, in mice (26, 29, 33, 40, 51, 52, 55), the role of endogenous IL-12 in innate immunity to intracellular pathogens including L. pneumophila has not been thoroughly investigated. We have recently developed a model of replicative L. pneumophila lung infection in A/J mice inoculated intratracheally with virulent bacteria and have used this model system to identify immune responses which mediate host resistance to legionellosis (10–12). Using this murine model of Legionnaires’ disease, we assessed the biologic relevance and immunomodulatory role of endogenous IL-12 in innate immunity to replicative L. pneumophila lung infection.
MATERIALS AND METHODS
Mice.
Female pathogen-free 6- to 8-week-old A/J mice (Jackson Laboratory, Bar Harbor, Maine) were used for all experiments. The animals were house in microisolator cages in Horsefall units and were cared for in accordance with standard guidelines.
Preparation of bacteria.
L. pneumophila serogroup 1, strain AA100, a redesignation of a primary clinical isolate from the Wadsworth Veterans Administration Hospital (Wadsworth, Calif.), was provided by Paul Edelstein. For preparation of the intratracheal inoculum, L. pneumophila was quantified on buffered charcoal-yeast extract agar (Becton Dickinson, Cockeysville, Md.) that had been incubated for 48 h and resuspended in phosphate-buffered saline (PBS) at 4 × 107 organisms/ml (10, 25).
Inoculation of A/J mice with L. pneumophila.
A/J mice were inoculated intratracheally with L. pneumophila as previously described (10, 49). Briefly, the mice were anesthetized with ketamine (2.5 mg/mouse intraperitoneally) and tethered, and an incision was made through the skin of the ventral neck. The trachea was isolated, and 25 μl of the bacterial suspension (i.e., containing 106 L. pneumophila cells), followed by 10 μl of air, was injected directly into the trachea with a 26-gauge needle. The skin incision was closed with a sterile wound clip.
Recovery of L. pneumophila from infected lung tissue.
At specific time points postinoculation (p.i.), the mice were humanely euthanized and the lungs were removed. Lung tissue was finely minced in sterile water (10 ml/lung) and subsequently homogenized (2 min/sample) with a Stomacher (Tekmar, Cincinnati, Ohio) (6, 10). Lung homogenates were subsequently serially diluted in sterile water and cultured on buffered charcoal-yeast extract agar containing polymyxin B, cefamandole, and anisomycin (Becton Dickinson) for 72 h (10, 22). The lower limit of detection of L. pneumophila with this system is 103 CFU per lung.
Collection of lung homogenate supernatant and BALF for cytokine analysis.
Lung homogenate supernatant was procured by filtering lung homogenates, prepared as described above, through a 0.45-μm-pore-size filter (Gelman Sciences, Ann Arbor, Mich.) to remove the bacteria. Alternatively, for collection of bronchoalveolar lavage fluid (BALF), the mice were humanely sacrificed and their lungs were lavaged with 1.6 ml of PBS (6). The resultant lavage fluid was subsequently filtered as described above. Filtered lung homogenates and BALF were stored at −20°C until use for cytokine analysis.
Cytokine analysis.
IFN-γ, IL-10, and IL-12 protein levels in lung homogenates and/or BALF were measured by commercially available cytokine-specific murine enzyme-linked immunosorbent assay (ELISA) kits (Intertest-γ, Intertest-IL-10, and Intertest-IL-12X total mouse IL-12, respectively; Genzyme Corp., Cambridge, Mass.) according to the manufacturer’s directions. This IL-12 ELISA detects all three forms of IL-12 (i.e., p70 heterodimer, p402 homodimer, and p40 monomer). TNF-α activity in lung homogenate was measured by a cytotoxicity assay using the WEHI 164 subclone 13 cell line as previously described (24). Briefly, lung homogenate samples were serially diluted directly into 96-well microtiter plates (Costar, Cambridge, Mass.). The WEHI cells were suspended at 5 × 105 cells per ml in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 0.5 μg of actinomycin D (Calbiochem, La Jolla, Calif.) per ml and added to the samples. A standard of human recombinant TNF-α was run in each assay. Samples were incubated overnight at 37°C, after which 20 μl of dimethyl-azole tetrazolium bromide (MTT) (5 mg/ml; Sigma, St. Louis, Mo.) was added to the wells and allowed to incubate at 37°C for an additional 4 h. Viable cells (i.e., cells not lysed by TNF-α) metabolize the MTT-tetrazolium to produce dark formazan crystals. The crystals were dissolved in isopropanol-HCl, and the plates were read in a microELISA reader (Bio-Tek Instruments, Inc., Winooski, Vt.) at 550 nm. TNF-α activity was calculated based on the human recombinant TNF-α standard that was run in the same assay.
Interventional studies.
Mice were depleted of endogenous IL-12 by intraperitoneal inoculation with rabbit serum containing neutralizing antibody to IL-12 (0.5 ml/mouse) 2 h prior to intratracheal inoculation with L. pneumophila. This antiserum, a generous gift from Steven Kunkel, Department of Pathology, University of Michigan Medical School, Ann Arbor, has previously been shown to be efficacious in neutralizing endogenous intrapulmonary IL-12 in other murine models of lung injury (31). Results of preliminary experiments demonstrated that this concentration of antiserum neutralized ≥95% of endogenous IL-12 activity in lung homogenates of L. pneumophila-infected mice for up to 5 days (data not shown). Alternatively, in selected experiments, mice were inoculated with preimmune rabbit antisera (0.5 ml/mouse) prior to intratracheal inoculation with L. pneumophila. Results of these preliminary experiments demonstrated that neither recovery of L. pneumophila nor cytokine levels in BALF were significantly altered in mice treated with preimmune rabbit serum, compared to similarly infected mice not administered antiserum (data not shown). Consequently, in all subsequent experiments, L. pneumophila-infected mice administered anti-IL-12 serum were compared to similarly infected untreated mice.
Statistical analysis.
Student’s t test was used to compare differences between treatment groups. For comparison of multiple groups to a single control, analysis of variance with post-hoc Tukey analysis was performed. P < 0.05 was considered significant.
RESULTS
Endogenous IL-12 facilitates resolution of primary replicative L. pneumophila lung infection.
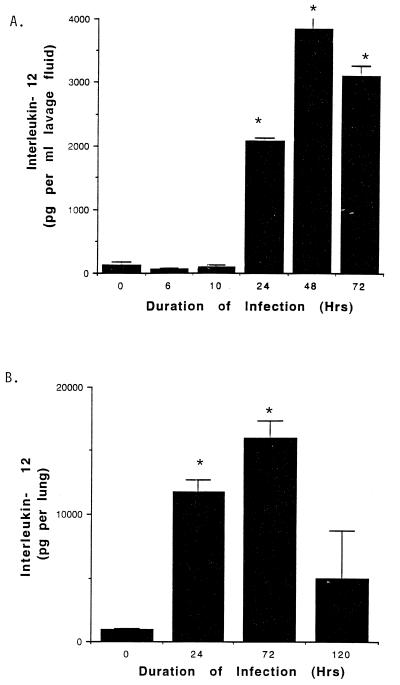
In initial experiments, induction of intrapulmonary IL-12 during replicative L. pneumophila lung infection was assessed. A/J mice were inoculated intratracheally with L. pneumophila (106 organisms per mouse). At 0 to 120 h p.i., the mice were humanely euthanized and the lungs were either lavaged or homogenized. IL-12 activity in BALF and in whole lung homogenates was subsequently quantified by a murine-specific IL-12 ELISA. As shown in Fig. 1, IL-12 was significantly enhanced in BALF and in whole lung homogenates within 24 h p.i. Furthermore, whole lung homogenates from infected mice contained greater than fivefold more IL-12 than did BALF from similarly infected mice.
FIG. 1.
IL-12 activity in BALF and lung homogenates of A/J mice inoculated intratracheally with L. pneumophila. A/J mice were inoculated intratracheally with L. pneumophila as described in Materials and Methods. At specific time points thereafter, the mice were sacrificed and IL-12 was assessed in BALF (A) and whole lung homogenates (B) by ELISA. Results represent the means ± standard errors of the means for three to five animals per time point, ∗, significantly greater than value for uninfected mice (i.e., 125 pg of IL-12 per ml of BALF; 935 pg of IL-12 per lung) (analysis of variance, P < 0.05).
We have previously demonstrated that A/J mice inoculated intratracheally with virulent L. pneumophila (106 bacteria per mouse) develop replicative L. pneumophila lung infections, with logarithmic growth of the bacteria within the first 48 h p.i. followed by gradual clearance of the bacteria from the lung at ≥72 h P.I. (10). Because induction of IL-12 activity in the lung of L. pneumophila-infected A/J mice (i.e., at ≥24 h p.i. [Fig. 1]) precedes clearance of the bacteria from the lung (i.e., at ≥72 h p.i.), the potential role of endogenous IL-12 in resistance to primary replicative L. pneumophila lung infection was evaluated. Mice were depleted of endogenous IL-12 by administration of rabbit IL-12 antiserum as described in Materials and Methods. At 24, 72, and 120 h p.i., the mice were humanely euthanized, the lungs were excised and homogenized, and L. pneumophila CFU were quantified in lung homogenates. As shown in Table 1, while there was no significant difference in recovery of L. pneumophila from the lung of mice treated with anti-IL-12 antiserum and similarly infected immunocompetent mice within the first 72 h p.i., significantly more bacteria were recovered in lung homogenates from animals depleted of endogenous IL-12 and sacrificed at 5 days p.i. (compared to similarly infected immunocompetent mice [Student’s t test, P <0.05]). Furthermore, 10-fold more bacteria were recovered in lung homogenates of L. pneumophila-infected mice treated with IL-12 antiserum at 5 days p.i. compared to the inoculating dose of bacteria (i.e., 106 L. pneumophila per mouse), suggesting that A/J mice depleted of endogenous IL-12 activity develop persistent replicative intrapulmonary L. pneumophila infection. Taken together, these results demonstrate that IL-12 is induced in the lung in response to L. pneumophila and plays a key role in innate immunity to the bacteria.
TABLE 1.
Effect of IL-12 neutralizing antibody on intrapulmonary replication of L. pneumophilaa
| Duration of infection (h) | Antibody titerb
|
|
|---|---|---|
| Untreated | Anti-IL-12 | |
| 24 | (6.00 ± 2.67) × 107 | (1.27 ± 4.68) × 108 |
| 72 | (1.17 ± 0.43) × 107 | (2.23 ± 0.63) × 107 |
| 120 | (4.53 ± 3.21) × 105 | (1.37 ± 0.38) × 107* |
A/J mice were administered IL-12 neutralizing antibody as described in Materials and Methods prior to intratracheal inoculation with L. pneumophila (106 bacteria per mouse). At selected time points thereafter, the mice were euthanized and growth of L. pneumophila in the lung was determined and compared to that of untreated mice (i.e., immunocompetent mice inoculated intratracheally with L. pneumophila).
Mean ± standard error of the mean for 8 to 14 animals per treatment group. *, significantly greater than value for control mice (Student’s t test, P < 0.05).
Immunomodulatory activity of endogenous IL-12.
Previous in vitro and in vivo studies have demonstrated that growth of L. pneumophila in permissive MPCs is modulated by cytokines including IFN-γ, TNF-α, and IL-10 (10, 12, 14, 15, 46). Because IL-12 has previous been shown to modulate the production of these cytokines (44), in subsequent experiments the effect of endogenous IL-12 on intrapulmonary levels of IFN-γ, TNF-α, and IL-10 during replicative L. pneumophila lung infection was assessed. A/J mice were administered anti-IL-12 serum and inoculated intratracheally with L. pneumophila (106 bacteria per mouse) as described in Materials and Methods. At 24, 72, and 120 h p.i., the mice were humanely euthanized, the lungs were excised, and filtered lung homogenates were obtained. TNF-α, IFN-γ, and IL-10 were subsequently quantified in filtered lung homogenates obtained from anti-IL-12-treated and untreated L. pneumophila-infected mice by cytokine-specific bioassay or ELISA.
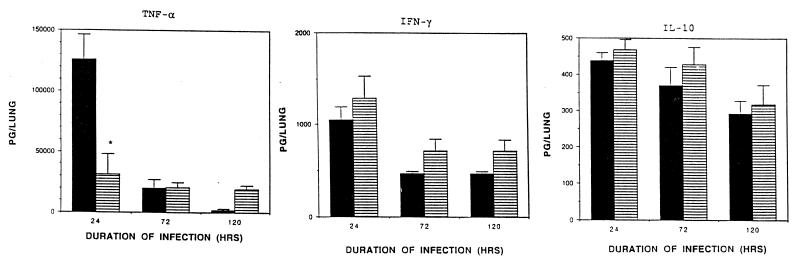
In agreement with our previous studies (10, 12), both TNF-α and IFN-γ were significantly induced in the lung of immunocompetent L. pneumophila-infected mice within 24 h p.i. compared to uninfected mice (<10 pg of TNF and 170 pg of IFN-γ per lung of uninfected mice) (Fig. 2). In contrast, IL-10 was not significantly induced in the lung of L. pneumophila-infected immunocompetent mice at any time point studied compared to uninfected mice (330 pg of IL-10 per lung of uninfected mice). Administration of anti-IL-12 serum to A/J mice prior to intratracheal inoculation with L. pneumophila resulted in a significant decrease in intrapulmonary TNF-α activity within 24 h p.i. compared to similarly infected immunocompetent mice (Student’s t test, P <0.05). In contrast, there was no significant difference between intrapulmonary levels of IFN-γ or IL-10 in L. pneumophila-infected mice depleted of endogenous IL-12 compared to similarly infected immunocompetent mice at any time point studied. We have previously demonstrated that TNF-α plays a key role in elimination of L. pneumophila from the lung in this murine model system (12). Together, these results suggest that regulation of intrapulmonary growth of L. pneumophila by endogenous IL-12 is likely mediated at least in part by TNF-α.
FIG. 2.
Effect of IL-12 on endogenous cytokine activity in the lung during replicative L. pneumophila lung infection. A/J mice were administered IL-12 antiserum as described in Materials and Methods prior to intratracheal inoculation of L. pneumophila. At specific time points p.i., the mice were sacrificed. The lungs were excised, homogenized, and filtered. Levels of TNF-α, IFN-γ, and IL-10 were quantified in filtered lung homogenates of L. pneumophila-infected mice depleted of endogenous IL-12 by ELISA or by bioassay and compared to those of similarly infected immunocompetent (i.e., untreated) mice. Results represent the means ± standard errors of the means for five mice per treatment group. ▪, untreated mice; ▤, anti-IL-12-treated mice. ∗, significantly less than value for untreated mice (Student’s t test, P < 0.05).
DISCUSSION
In this study, the role of endogenous IL-12 in innate immunity to replicative L. pneumophila lung infection was assessed in vivo, using a murine model of Legionnaires’ disease in A/J mice inoculated intratracheally with virulent bacteria. We demonstrate that IL-12 is induced in the lung during replicative L. pneumophila lung infection and that neutralization of endogenous IL-12 by administration of IL-12 antiserum resulted in impaired ability of A/J mice to resolve a primary replicative L. pneumophila lung infection. These results identify a key role of endogenous IL-12 in innate immunity to L. pneumophila pulmonary infection.
In subsequent experiments, immunomodulatory effects of endogenous IL-12 on intrapulmonary IFN-γ, TNF-α, and IL-10 during replicative L. pneumophila lung infection were assessed. This is of particular interest, as IFN-γ, TNF-α, and IL-10 have previously been shown to regulate growth of the bacteria in permissive MPCs in vivo and/or in vitro (10, 12, 46). Results of these studies demonstrated that enhanced growth of L. pneumophila in mice depleted of endogenous IL-12 was positively correlated with a significant reduction in TNF-α activity in the lung within 24 h p.i.; however, intrapulmonary levels of neither IFN-γ nor IL-10 were significantly altered by this therapy (Fig. 2). TNF-α has previously been shown to play a key role in resolution of replicative L. pneumophila infection, as it enhances polymorphonuclear leukocyte bacteriocidal activity (7), is directly toxic for L. pneumophila (42), and in combination with other cytokines, including IFN-γ, induces MPC production of reactive nitrogen intermediates, including nitric oxide, which limit L. pneumophila growth and viability (12). Together, these results suggest that regulation of intrapulmonary L. pneumophila replication by endogenous IL-12 is likely mediated, at least in part, by TNF-α.
Previous studies have demonstrated that IL-12 is a potent inducer of IFN-γ (41). Furthermore, IL-12-mediated inhibition of other pathogenic microbes, including T. gondii (30), Histoplasma capsulatum (1), and Listeria monocytogenes (54, 55), has been shown to occur through an IFN-γ-mediated mechanism. Therefore, we were somewhat surprised that neutralization of endogenous IL-12 in L. pneumophila-infected A/J mice did not significantly alter intrapulmonary levels in IFN-γ. However, our results demonstrating IFN-γ-independent effects of IL-12 on host immune responses to L. pneumophila concur with those of a recent in vitro study by Bermundez et al., who showed that IL-12-induced NK cell-mediated mycobactericidal activity in human MPCs is mediated by a TNF-α-dependent, IFN-γ-independent mechanism (3). Together, these studies suggest that the role of endogenous IL-12 in cytokine networking and host resistance to pathogenic microbes differs with respect to different intracellular pathogens.
While our studies have focused on elucidating immunomodulatory effects of IL-12 on the expression of cytokines which mediate resistance to replicative L. pneumophila lung infection, it is likely that endogenous IL-12 also contributes to innate immunity to L. pneumophila by cytokine-independent mechanisms. Specifically, costimulation of NK cells and/or T cells with IL-12 and other cytokines, including IL-15 or TNF-α, has been shown to enhance NK cell and T-cell cytotoxicity (2, 13, 16–18, 38, 53). The potential role of cytotoxic T cells and/or NK cells in resistance to primary replicative L. pneumophila lung infection remains to be thoroughly explored.
In summary, using a murine model of Legionnaires’ disease in A/J mice, we have demonstrated that endogenous IL-12 plays a key role in innate immunity to legionellosis, likely in part by its ability to modulate intrapulmonary activity of other cytokines, including TNF-α. Further studies, which will identify the potential synergy between intrapulmonary IL-12 and other cytokines such as IL-15 in innate immunity to replicative L. pneumophila lung infection are warranted and will likely provide a rational approach to immunotherapy for treatment of Legionnaires’ disease.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants RR00200 and R-29-HL-49136.
REFERENCES
- 1.Allendoerfer R, Boivin G P, Deepe G S., Jr Modulation of immune responses in murine pulmonary histoplasmosis. J Infect Dis. 1997;175:905–914. doi: 10.1086/513989. [DOI] [PubMed] [Google Scholar]
- 2.Bamford R N, Grant A J, Burton J D, Peters C, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. The interleukin (IL) 2 receptor β-chain is shared by IL-2 and a cytokine provisionally designated IL-T that stimulates T cell proliferation and the induction of lymphokine activated killer cells. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez L E, Wu M, Young L S. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect Immun. 1995;63:4099–4104. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertagnolli M M, Lin B Y, Young D, Herrmann S H. IL-12 augments antigen-dependent proliferation of activated T lymphocytes. J Immunol. 1992;149:3778–3783. [PubMed] [Google Scholar]
- 5.Biron C A, Gazzinelli R T. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard D K, Djeu J Y, Klein T W, Friedman H, Stewart W E, 2nd, Stewart W E. Protective effects of tumor necrosis factor in experimental Legionella pneumophila infections of mice via activation of PMN function. J Leukocyte Biol. 1988;43:429–435. doi: 10.1002/jlb.43.5.429. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard D K, Friedman H, Klein T W, Djeu J Y. Induction of interferon gamma and tumor necrosis factor by Legionella pneumophila. Augmentation of human neutrophil bactericidal activity. J Leukocyte Biol. 1989;45:538–545. doi: 10.1002/jlb.45.6.538. [DOI] [PubMed] [Google Scholar]
- 8.Blander S J, Horwitz M A. Vaccination with Legionella pneumophila membranes induces cell mediated and protective immunity in a guinea pig model of Legionnaires’ disease. Protective immunity independent of the major secretory protein of Legionella pneumophila. J Clin Invest. 1991;87:1054–1059. doi: 10.1172/JCI115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breiman R F. Modes of transmission in epidemic and nonepidemic Legionella infection: directions for further study. In: Barbaree J M, Breiman R F, Duflour A P, editors. Legionella: current status and emerging perspectives. Washington, D.C: American Society for Microbiology; 1993. pp. 30–35. [Google Scholar]
- 10.Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, Engleberg C. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires’ disease. Am J Pathol. 1994;145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 11.Brieland J K, Heath L A, Huffnagle G B, Remick D G, McClain M S, Hurley M C, Kunkel R K, Fantone J C, Engleberg N C. Humoral immunity and regulation of intrapulmonary growth of Legionella pneumophila in the immunocompetent host. J Immunol. 1996;157:5002–5008. [PubMed] [Google Scholar]
- 12.Brieland J K, Remick D G, Freeman P T, Hurley M C, Fantone J C, Engleberg N C. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous tumor necrosis factor alpha and nitric oxide. Infect Immun. 1995;63:3253–3258. doi: 10.1128/iai.63.9.3253-3258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton J D, Bamford R N, Peters C, Grant A J, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. A lymphokine, provisionally designated interleukin T and produced by a human adult T cell leukemia line, stimulates T cell proliferation and induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd T F, Horwitz M A. Interferon gamma- activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrd T F, Horwitz M A. Interferon gamma-activated human monocytes downregulate the intracellular concentration of ferritin: a potential new mechanism for limiting iron availability to Legionella pneumophila and subsequently inhibiting intracellular multiplication. Clin Res. 1990;38:481. . (Abstract.) [Google Scholar]
- 16.Carson W E, Caliguiri M A. Interleukin 15: a potential player during the innate immune responses to infection. Exp Parasitol. 1996;84:291–294. doi: 10.1006/expr.1996.0115. [DOI] [PubMed] [Google Scholar]
- 17.Carson W E, Giri J G, Lindemann M, Linett M L, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri M A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carson W E, Ross M E, Baiocchi R A, Marien M J, Boiani N, Grabstein K, Caligiuri M A. Endogenous production of IL-15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J Clin Invest. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan S H, Perussia B, Gupta J W, Kobayashi M, Pospisil M, Young H A, Wolf S F, Young D, Clark S C, Trinchieri G. Induction of IFN-gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conlan J W, Baskerville A, Ashworth L A. Separation of Legionella pneumophila proteases and purification of a protease which produces lesions like those of Legionnaires’ disease in guinea-pig lung. J Gen Microbiol. 1986;132:1565–1574. doi: 10.1099/00221287-132-6-1565. [DOI] [PubMed] [Google Scholar]
- 21.Conlan J W, Williams A, Ashworth L A. In vivo production of a tissue-destructive protease by Legionella pneumophila in the lungs of experimentally infected guinea pigs. J Gen Microbiol. 1988;134:143–149. doi: 10.1099/00221287-134-1-143. [DOI] [PubMed] [Google Scholar]
- 22.Edelstein P H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981;14:298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenstein T, Friedman H. Immunity to Legionella. In: Katz S M, editor. Legionellosis. Vol. 2. Boca Raton, Fla: CRC Press; 1985. pp. 159–169. [Google Scholar]
- 24.Eskandari M K, Nguyen D T, Kunkel S L, Remick D G. Wehi 164 subclone 13 assay for TNF: sensitivity, specificity and reliability. Immunol Invest. 1990;19:69–79. doi: 10.3109/08820139009042026. [DOI] [PubMed] [Google Scholar]
- 25.Feeley J C, Gibson R J, Gorman G W, Langford N C, Raheed J K, Mackel D C, Baine W B. Charcoal-yeast extract agar: primary medium for Legionella pneumophila. J Clin Microbiol. 1979;10:437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn J L, Goldstein M M, Triebold K J, Sypek J, Wolf S, Bloom B R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 27.Friedman H, Widen R, Klein T, Searls L, Cabrian K. Legionella pneumophila induced blastogenesis of murine lymphoid cells in vitro. Infect Immun. 1984;43:314–319. doi: 10.1128/iai.43.1.314-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman H, Widen R, Lee I, Klein T. Cellular immunity to Legionella pneumophila in guinea pigs assessed by direct and indirect migration inhibition reactions in vitro. Infect Immun. 1983;41:1132–1137. doi: 10.1128/iai.41.3.1132-1137.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gazzinelli R T, Hieny S, Wynn T A, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon-γ by an intracellular parasite and induces resistance in T-cell deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 31.Greenberger M J, Kunkel S L, Strieter R M, Lukacs N W, Bramson J, Gauldie J, Graham F L, Hitt M, Danforth J M, Standiford T J. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol. 1996;157:3006–3012. [PubMed] [Google Scholar]
- 32.Gubler U, Chua A O, Schoenhaut D S, Dwyer C M, McComas W, Motyka R, Nabavi N, Wolitzy A G, Quinn P M, Familletti P C, Gately M K. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinzel F P, Schoenhaut D S, Rerko R M, Rosser L E, Gately M K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horwitz M A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horwitz M A. Cell-mediated immunity in Legionnaires’ disease. J Clin Invest. 1983;71:1686–1697. doi: 10.1172/JCI110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horwitz M A. Phagocytosis of Legionnaires’ disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 37.Horwitz M A, Silverstein S C. Legionnaires’ disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Invest. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jullien D, Sieling P A, Uyemura K, Mar N D, Rea T H, Modlin R L. IL-15, an immunomodulator of T cell responses in intracellular infection. J Immunol. 1997;158:800–806. [PubMed] [Google Scholar]
- 39.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi K, Kasama T, Yamazaki J, Hosaka M, Katsura T, Mochizuki T, Soejima K, Nakamura R M. Protection of mice from Mycobacterium avium infection by recombinant interleukin-12. Antimicrob Agents Chemother. 1995;39:1369–1371. doi: 10.1128/aac.39.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma X, Aste-Amezaga A, Trichieri G. Regulation of interleukin-12 production. NY Acad Sci. 1996;795:13–25. doi: 10.1111/j.1749-6632.1996.tb52651.x. [DOI] [PubMed] [Google Scholar]
- 42.Matsiota-Bernard P, Lefebre C, Sedqui M, Cornillet P, Guenounou M. Involvement of tumor necrosis factor alpha in intracellular multiplication of Legionella pneumophila in human monocytes. Infect Immun. 1993;61:4980–4983. doi: 10.1128/iai.61.12.4980-4983.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDade J E, Shepard C C, Fraser D W, Tsai T R, Redus M A, Dowdle W R. Legionnaires’ disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 44.Morris S C, Madden K B, Adamovicz J J, Gause W C, Hubbard B R, Gately M K, Finkelman F D. Effects of IL-12 in in vivo cytokine gene expression and Ig isotype selection. J Immunol. 1994;152:1047–1056. [PubMed] [Google Scholar]
- 45.Nash T W, Libby D M, Horwitz M A. Interaction between the Legionnaires’ disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines and hydrocortisone. J Clin Invest. 1984;74:771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park D R, Skerrett S J. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-γ. Differential responses of blood monocytes and alveolar macrophages. J Immunol. 1996;157:2528–2538. [PubMed] [Google Scholar]
- 47.Perussia B, Chan S H, D’Andrea A, Tsuji K, Santoli D, Pospisil M, Young D, Wolf S F, Trinchieri G. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-αβ+, TCR-γδ+ T lymphocytes and NK cells. J Immunol. 1992;149:3495–3502. [PubMed] [Google Scholar]
- 48.Robertson M J, Soiffer R J, Wolf S F, Manley T J, Donahue C, Young D, Herrmann S H, Ritz J. Response of human natural killer cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992;175:779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986;136:1435–1441. [PubMed] [Google Scholar]
- 50.Stern A S, Podlaski F J, Hulmes J D, Pan Y C, Quinn P M, Wolitzky A G, Familletti P C, Stremlo D L, Truitt T, Chizzonite R. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87:6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 52.Sypek J P, Chung C L, Mayor S E H, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeuchi E, Yanagawa H, Yano S, Haku T, Sone S. Induction of interleukin 15 of human killer cell activity against lung cancer cell lines and its regulatory mechanisms. Jpn J Cancer Res. 1996;87:1251–1258. doi: 10.1111/j.1349-7006.1996.tb03140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tripp C S, Gately M K, Hakimi J, Ling P, Unanue E. Neutralization of IL-12 decreases resistance to Listeria in SCID and CB-17 mice: reversal by IFN-γ. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 55.Wagner R D, Steinberg H, Brown J F, Czuprynski C J. Recombinant interleukin-12 enhances resistance of mice to Listeria monocytogenes infection. Microb Pathog. 1994;17:175–186. doi: 10.1006/mpat.1994.1064. [DOI] [PubMed] [Google Scholar]
- 56.Wolf S F, Temple P A, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Azzoni L, Hewick R M, Chan S H, Trinchieri G, Perussia B. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and NK cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 57.Yamamoto Y, Klein T W, Newton C, Friedman H. Differing macrophage and lymphocyte roles in resistance to Legionella pneumophila infection. J Immunol. 1992;148:584–589. [PubMed] [Google Scholar]