Abstract
Helicobacter pylori NCTC 11637 lipopolysaccharide (LPS) expresses the human blood group antigen Lewis x (Lex) in a polymeric form. Lex is β-d-galactose-(1-4)-[α-l-fucose-(1-3)]-β-d-acetylglucosamine. Schematically the LPS structure is (Lex)n-core-lipid A. In this report, we show that Lex expression is not a stable trait but that LPS displays a high frequency (0.2 to 0.5%) of phase variation, resulting in the presence of several LPS variants in one bacterial cell population. One type of phase variation implied the loss of α1,3-linked fucose, resulting in variants that expressed nonsubstituted polylactosamines (also called the i antigen), i.e., Lex minus fucose; LPS: (lactosamine)n-core-lipid A. The switch of Lex to i antigen was reversible. A second group of variants arose by loss of polymeric main chain which resulted in expression of monomeric Ley; LPS: (Ley)-core-lipid A. A third group of variants arose by acquisition of α1,2-linked fucose which hence expressed Lex plus Ley; LPS: (Ley)(Lex)n-core-lipid A. The second and third group of variants switched back to the parental phenotype [(Lex)n-core-lipid A] in lower frequencies. Part of the variation can be ascribed to altered expression levels of glycosyltransferase levels as assessed by assaying the activities of galactosyl-, fucosyl-, and N-acetylglucosaminyltransferases. Clearly phase variation increases the heterogeneity of H. pylori, and this process may be involved in generating the very closely related yet genetically slightly different strains that have been isolated from one patient.
Helicobacter pylori is involved in the pathogenesis of gastritis, gastric glandular atrophy, duodenal and gastric ulcer, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma (12). How bacteria belonging to a single species can give rise to such a diversity of disease entities is currently under investigation. H. pylori is genetically very diverse (9): strains from different sources all are different from each other at the DNA level (15). This genetic heterogeneity is in part due to the natural competence of H. pylori that renders this bacterium naturally transformable (24). In addition, it has been reported that recombinational events involving insertion elements occur (7). A mechanism used by other mucosal pathogens like Haemophilus influenzae (19) and Neisseria spp. (30) to increase diversity is phase variation. Phase variation (also called antigenic variation) is the reversible on-and-off switching of surface epitopes, e.g., those present on adhesins or lipopolysaccharide (LPS). Switch frequencies of 0.1% in these species are common. Genetically, this is paralleled by on-and-off switching of specific genes coding for surface structures, such as the glycosyltransferase genes involved in LPS biosynthesis. Often, those glycosyltransferase genes contained oligonucleotide repeats in the 5′ end, and one mechanism for phase variation involved replication errors due to slipped-strand base pairing in the repeat region. Recently, oligonucleotide repeats have been identified in the fucosyltransferase (FucT) genes of H. pylori (8, 16, 23). The result of phase variation is a more versatile, more heterogeneous microorganism that can cope better with a variety of different environments: with one set of genes switched on, the microorganism may be able to adhere to mucosal cells of the nasopharynx but not to survive complement attack in serum; with that particular set switched off, the reverse may apply.
H. pylori LPS O antigen expresses Lewis x and/or y (Fig. 1), and only 15% of the strains tested lacked these blood group antigens (22). The surface localization of the bacterial Lewis antigens was demonstrated by immunoelectron microscopy (6, 21). The enzymatic activity of two enzymes involved in LPS O-antigen biosynthesis (α1,3-FucT and β1,4-galactosyltransferase [GalT]) has been demonstrated (1, 21), but it is not clear by what mechanisms serotype diversity is generated.
FIG. 1.
Structures of Lewis-related antigens and H. pylori LPS. Abbreviations: Gal, d-galactose; Fuc, l-fucose; GlcNAc, N-acetyl-d-glucosamine.
Thus, the H. pylori O antigen expresses structures identical to that of the corresponding human blood group antigens present in the gastric mucosa (molecular mimicry) (2–4, 6, 21, 22). The identity of these LPS epitopes with molecules of the host may play a role in the induction of H. pylori-associated autoantibodies (1, 17, 18) and facilitate persistence in the gastric niche of humans (31).
We now report that expression of H. pylori LPS in a given strain is not a stable trait and that H. pylori LPS displays phase variation. From a single strain, we isolated various serotypes, all expressing different LPS structures and having different glycosyltransferase levels, which demonstrates that also in H. pylori, phase variation contributes to increased heterogeneity.
MATERIALS AND METHODS
Bacterial strains and cultivation procedures.
We investigated three strains, all expressing Lewis x: the laboratory strain NCTC 11637; strain 92-1152, isolated from a patient with gastric carcinoma; and strain 3B3, isolated from the oral cavity of a patient with gastritis (1, 22). Bacteria were stored at −80°C in 20% glycerol and were subcultured on solid medium (blood agar base plus Dent supplement) before growth in the fluid phase (brucella broth with 3% fetal calf serum) under microaerobic conditions (1, 22). Apart from B1.3 (see below), none of the strains autoagglutinated.
MAbs.
The following murine immunoglobulin M monoclonal antibodies (MAbs) were used: MAb 54.1F6A, specific for Lewis x (G. van Dam, Leiden, The Netherlands [25]); MAb 1E52, specific for Lewis y (R. Negrini, Brescia, Italy [1, 17, 18]); MAb 19-O-Le, specific for H type 2 and Lewis y (Bioprobe, Amstelveen, The Netherlands [1]); MAb 4D2, specific for H type 1 (R. Negrini [1, 17, 18]); and MAb NAM61-1A2, specific for i antigen (D. Blanchard, Nantes, France [5]). MAbs specific for Lewis x did not cross-react with H type 2/Lewis y or vice versa; the anti-i MAb did not react with Lewis x, H type 2, or Lewis y.
Isolation of H. pylori LPS mutants after UV irradiation.
Overnight cultures of strain NCTC 11637 in brucella broth plus 3% fetal calf serum were centrifuged, suspended in 1 M MgSO4, serially diluted in phosphate-buffered saline, pH 7.5 (PBS), plated, and UV irradiated. Time, distance between plates UV light source, and dilution were chosen such that 10% of bacteria survived, resulting in 300 colonies per plate. Individual colonies were picked, transferred to each of two identical plates, and grown. The colonies were transferred to a nitrocellulose filter (0.45-μm pore size; Millipore Corporation, Bedford, Mass.) by pressing the filter to the surface of the plate. Mutants not expressing Lewis x were isolated as follows: colony blots were baked (1 h at 80°C), washed three times in PBS with 0.05% Tween 80 (PBST), and blocked with blocking buffer (Boehringer Mannheim, Almere, The Netherlands). Then blots were incubated overnight with anti-Lewis x MAb 54.1F6a, diluted in blocking buffer-PBST (1:1) at a concentration of 1 μg/ml. Blots were washed, incubated (1 h, 37°C) with goat anti-mouse immunoglobulin M-peroxidase (American Qualex, La Mirada, Calif.), diluted 1:1,000 in PBST plus 0.5% preimmune goat serum, and developed as described previously (29). Blots were inspected under a stereomicroscope at magnifications of up to ×64 and, when necessary, photographed. Nonreactive colonies were identified and subcultured at least twice, and purity was checked by colony blotting; finally, cells were grown in fluid medium, washed in PBS, and used in enzyme-linked immunosorbant assay (ELISA) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for further characterization. A modification of the procedure described above was also used: the nitrocellulose blotting paper was put on the irradiated plates, which resulted in almost complete transfer of the bacteria to the paper. Replicas of this first colony blot to a second and third nitrocellulose paper were made. One of the nitrocellulose replicas was put, colonies upward, on a fresh culture plate, a procedure that kept the bacteria alive. The remaining two blots were immunoprobed.
Isolation of variants.
Spontaneously arising LPS variants were isolated from bacteria not subjected to UV irradiation by following the above-described modified protocol; i.e., bacteria were first grown in the fluid phase and distributed over solid media, after which immunodetection took place.
ELISA.
The expression of various LPS epitopes on the parent strain, mutants, and variants was measured in ELISA. Bacteria grown in the fluid phase were washed in PBS, and coated at 7.5 × 106 CFU/ml on ELISA plates, and tested for reactivity with MAbs (1 μg/ml) as described elsewhere (1, 22).
SDS-PAGE, silver staining, and immunoblotting.
The size distribution of LPS molecules of parent, mutants, and variants was analyzed by SDS-PAGE and silver staining. Bacterial cells were first digested with proteinase K and subjected to SDS-PAGE in 12% gels, and part of the gel was silver stained for LPS (13, 29). To localize particular LPS epitopes to particular LPS components, part of the gel was electroblotted to nitrocellulose. LPS epitopes were visualized as described above for colony blotting.
Glycosyltransferase assays.
GlcNAcβ-O-(CH2)8-COOCH3 was generously donated by O. Hindsgaul (University of Alberta, Edmonton, Alberta, Canada), Galβ1→4GlcNAcβ-O-(CH2)8-COOCH3 was derived therefrom as described before (11). GDP-[3H]Fuc (7.0 Ci/mmol), UDP-[3H]Gal (50 Ci/mmol), and UDP-[3H]GlcNAc (30.4 Ci/mmol) were purchased from New England Nuclear (Boston, Mass.). The radioactive nucleotide sugars were diluted to the desired specific radioactivity with unlabeled GDP-Fuc (kind gift of H. Lönn and T. Nordberg, Biocarb, Lund, Sweden), UDP-Gal, and UDP-GlcNAc (Sigma), respectively.
Cells (1010) of each of the variants cultivated as described above were sonicated in 250 μl of PBS at 0°C, using a microprobe tip (five bursts of three s each, with intermittent cooling for 2 min). The resulting suspension was used as an enzyme preparation in the glycosyltransferase assays. Incubation mixtures contained, in 50 μl, the following: for GalT, 5 μmol of sodium cacodylate buffer (pH 7.0), 1 μmol of MnCl2, 0.2 μmol of ATP, 0.25 μl of Triton X-100, 25 nmol of UDP-[3H]Gal (specific radioactivity, 1 Ci/mol), 50 nmol of GlcNAcβ-O-(CH2)8-COOCH3, and 1 μl of enzyme; for N-acetylglucosaminyltransferase (GlcNAcT), 5 μmol of sodium cacodylate buffer (pH 7.0), 1 μmol of MnCl2, 0.2 μmol of ATP, 0.25 μl of Triton X-100, 25 nmol of UDP-[3H]GlcNAc (specific radioactivity, 0.75 Ci/mol), 50 nmol of Galβ1→4GlcNAcβ-O-(CH2)8-COOCH3, and 10 μl of enzyme; and for FucT, 5 μmol of sodium cacodylate buffer (pH 7.2), 1 μmol of MnCl2, 0.2 μmol of ATP, 0.25 μl of Triton X-100, 50 nmol of dithiothreitol, 25 nmol of UDP-[3H]Fuc (specific radioactivity, 6.9 Ci/mol), 50 nmol of Galβ1→4GlcNAcβ-O-(CH2)8-COOCH3, and 10 μl of enzyme. Mixtures were incubated for 1 h (GalT) or 5 h (GlcNAcT and FucT) at 37°C. Radioactivity incorporated into the acceptor substrate was determined by liquid scintillation counting after isolation of the products by using Sep-Pak C18 cartridges (Waters, Milford, Mass.) as described previously (6). Control assays lacking acceptor were carried out to correct for incorporation into endogenous substrates. One unit of enzyme activity is defined as the amount of enzyme catalyzing the transfer of 1 μmol of sugar per min.
RESULTS
Phase variation in an H. pylori LPS mutant.
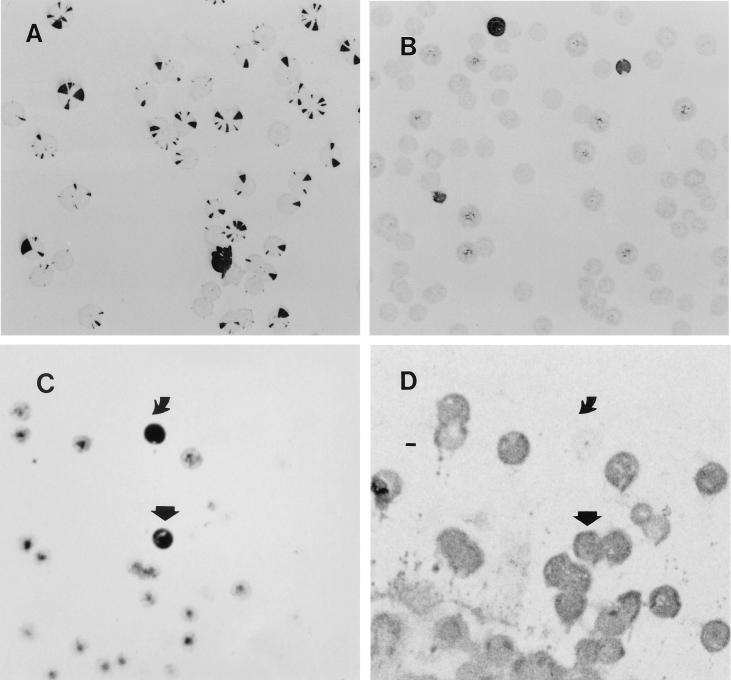
Following UV irradiation, an LPS mutant (named B1.5) that lacked Lewis x expression and was strongly positive for Lewis y was isolated (for further characterization, see below). A colony blot of this mutant, probed with MAb 4D2, specific for H type 1, is shown in Fig. 2A. There are completely nonreactive colonies, colonies with reactive sectors only, and completely reactive (dark) colonies; this pattern indicates a spontaneous high-frequency clonal on switching of the H type 1 epitope.
FIG. 2.
(A) Colony blot of LPS mutant B1.5 probed with MAb 4D2 Mab (anti-H type 1). (B) Colony blot of nonirradiated strain NCTC 11637 probed with MAb NAM61-1A2 (anti-i). (C) Colony blot of nonirradiated strain NCTC 11637 probed with MAb 19-O-Le (anti-Lewis y). (D) Colony blot of nonirradiated strain NCTC 11637 probed with MAb 54.1F6A (anti-Lewis x). Variant 1c ( ) expresses both Lewis x and y; variant 1b ( ) expresses Lewis y but lacks Lewis x.
Isolation of LPS variants from nonirradiated bacterial cells and demonstration of reversibility.
Nonirradiated cells of strain NCTC 11637 and the two clinical isolates investigated also displayed phase variation behavior (Fig. 2B) but at a lower frequency. Figures 1 and 2B shows that NCTC 11637, which expresses Lewis x, has the ability to switch spontaneously to the expression of i antigen; strains expressing the i antigen did not express Lewis x (Table 1). Several of the colonies reactive with the anti-i MAb but nonreactive with anti-Lewis x, including variant K4.1, were subcultured. K4.1 was tested again to detect whether it could switch back to phenotype of the parent strain NCTC 11637. Indeed, K4.1 spontaneously switched to variants that reacted with anti-Lewis x MAbs but not with the anti-i MAb. The frequency of switching from Lewis x to i was determined in multiple experiments and equaled 49/11,080 (0.44%); the switchback frequency was 17/3,095 (0.55%). One of the switched-back variants (strain K5.1 [Table 1]) was isolated. Switching from Lewis x to i-antigen expression also took place in the two clinical isolates tested (not shown). Forty percent of the 106 clinical isolates tested proved positive for the i antigen. Parallel colony blots of NCTC 11637 probed with 19-O-Le (anti-Lewis y) and 54.1F6A (anti-Lewis x) (Fig. 2C and D, respectively) showed the presence of two other variants, i.e., variants that strongly expressed both Lewis x and y (for example, strain 1c [see below]; frequency, 0.5%) and variants that were strongly positive for Lewis y but did not express Lewis x (for example, strain 1b [see below]; frequency, 1.5%). The majority of the colonies strongly expressed Lewis x and reacted weakly with the anti-Lewis y MAb. Three such colonies (2a, 2b, and 3c) were subcultured and characterized. Their reactivity pattern was identical to that of the parental strain (Table 1).
TABLE 1.
Characterization of H. pylori NCTC 11637 LPS mutants and variants
| Strain | ELISA
reactivity with MAbsa
|
|||
|---|---|---|---|---|
| Anti-Lewis y | Anti-Lewis x | Anti-H type 1 | Anti-i | |
| NCTC 11637 | + | +++ | +++ | − |
| Mutants | ||||
| B1.5 | ++ | − | + | − |
| B2.1 | ++ | − | + | − |
| K1.4 | − | − | + | + |
| K2.1 | − | − | ++ | ++ |
| K3.1 | − | − | ++ | ++ |
| D1.1b | − | − | − | +c |
| Variants | ||||
| K4.1 | − | − | + | ++ |
| K5.1 | − | ++ | ++ | − |
| B3.1 | +++ | − | ++ | − |
| 1b | +++ | − | + | − |
| 1c | +++ | +++ | ++ | − |
| 2a | + | +++ | +++ | − |
| 2b | + | +++ | ++ | − |
| 3c | + | +++ | ++ | − |
| 3a | − | +++ | +d | − |
−, optical density (OD) < 0.3; +, 0.3 < OD < 1.3; ++, 1.3 < OD < 2.3; +++, OD > 2.3.
D1.1 was isolated as an anti-H type 1-negative variant of the K3.1 mutant.
OD < 0.35.
OD < 0.8.
Immunochemical analysis of variants.
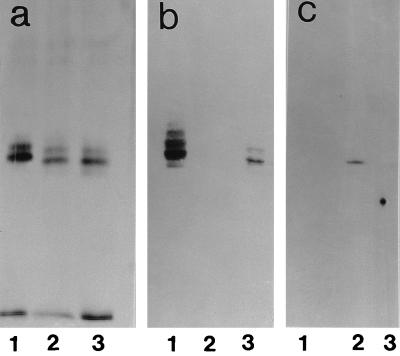
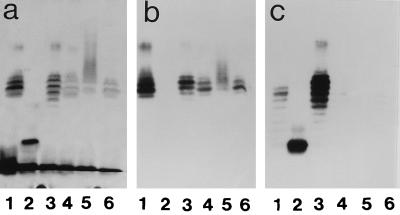
Immunochemical data for all mutants and variants isolated by us from strain NCTC 11637 are shown in Table 1 and Fig. 3 and 4. Variant K4.1 (Fig. 3a) expressed an LPS similar to that of the parent strain; both in ELISA and in blot analysis, K4.1 (Fig. 3b) reacts with the anti-i MAb but not with anti-Lewis x. Variant K5.1, a switchback variant isolated from K4.1, behaves serologically like the parent strain: the O antigen expressed Lewis x, not i antigen. We hypothesized that the switch from parent to K4.1 implied the loss of α1,3-fucose, which was regained upon switching from K4.1 to K5.1. Serologically, the mutants K1.4, K2.1, and K3.1 were identical to the K4.1 variant. Figure 4 shows that variant 1b had lost its polymeric main chain and now synthesized a low-molecular-mass LPS expressing Lewis y but not Lewis x. Serologically, mutants B1.5 and B2.1 are identical to variants B3.1 and 1b. Strain 1c expressed a polymeric Lewis x ladder, runs of the ladder being capped with Lewis y and with slightly more runs in the lower-molecular-weight zone compared to the parent. Variant 3a reacted less than the parent or not at all with 4D2 in ELISA or blot analysis, respectively. This variant expressed LPS that upon SDS-PAGE yielded a smear instead of a ladder (see silver stain and blot in Fig. 4).
FIG. 3.
(a) SDS-PAGE and silver stain. Lane 1, NCTC 11637; lane 2, K4.1; lane 3, K5.1. (b) Blot (after SDS-PAGE) probed with MAb 54.1F6A (anti-Lewis x). (c) Blot probed with MAb NAM61-1A2 (anti-i).
FIG. 4.
(a) SDS-PAGE and silver stain. Lane 1, NCTC 11637; lane 2, strain 1b; lane 3, strain 1c; lane 4, strain 2b; lane 5, strain 3a; lane 6, strain 3c. (b) Blot probed with MAb 54.1F6A (anti-Lewis x). (c) Blot probed with MAb 1E52 (anti-Lewis y).
Additional data on switch frequencies of H. pylori NCTC 11637, LPS mutants, and variants are shown in Table 2. The switching behavior of mutants was similar to that of the variants: mutant K1.4 (Lewis x−, i+) switched back to Lewis x+, i− at a frequency of 0.2%. One K1.4 derived switchback variant (called K6.1 [data not shown]) was isolated and found to be able to switch to Lewis x−, i+, which is further proof of the reversibility of the Lewis x-to-i-antigen switch. In contrast, mutants and variants that were Lewis x−, Lewis y+ did not switch back (mutant B1.5, variant B3.1) or did so in extremely low frequencies (variant 1b, switchback frequency, 1/1,400). We also did not find switchbacks of variant 1c (Lewis x+, Lewis y+). The serotype that most often is expressed by colonies of NCTC 11637 (strong Lewis x+, weak Lewis y+; variants 2b and 3c) switched to strong expression of Lewis y+ at frequencies of 0.28 to 1%.
TABLE 2.
Frequencies of LPS phase variation in H. pylori NCTC 11637, its mutants, and its variants
| Strain | MAb | Estimated no. of colonies on blots | No. (%) of positive colonies |
|---|---|---|---|
| K1.4 mutant | Anti-Lewis x | 3,000 | 5 (0.2) |
| K6.1 varianta | Anti-i | 5,000 | 39 (0.78) |
| B1.5 mutant | Anti-Lewis x | 900 | 0 (0) |
| B3.1 variant | Anti-Lewis x | 6,386 | 0 (0) |
| 1b variant | Anti-Lewis x | 1,400 | 1 (0.07) |
| 2b variant | Anti-Lewis y | 1,850 | 19 (1) |
| 3c variant | Anti-Lewis y | 6,400 | 18 (0.28) |
| 1c variant | Anti-Lewis x | 3,200 | 0b |
| Anti-Lewis y |
K6.1 is a phase variant (Lewis x+,i−) isolated from K1.4 mutant.
Number of negative colonies.
Glycosyltransferase assays.
To study the enzymatic basis for the phenotypic differences observed, the activities of three relevant glycosyltransferases were assayed in some of the variants. Various activities were found (Table 3), with highest GalT activity in the parental strain (NCTC 11637) and highest FucT activity in variant 1c, while the activity of GlcNAcT was below the detection limit in variant 1b. Except for the latter variant, the GlcNAcT, GalT, and FucT activities appeared to vary over ranges of 2-, 8-, and 60-fold, respectively, suggesting that the gene coding for the latter enzyme in particular might be a target of mechanisms that causes phase variations.
TABLE 3.
Glycosyltransferase activities in H. pylori 11637 LPS variants
| Strain or variant | Glycosyltransferase activity
|
||
|---|---|---|---|
| GalT (mU/1010 cells) | GlcNAcT (μU/1010 cells) | FucT (μU/1010 cells) | |
| NCTC 11637 | 20.0 | 33.8 | 46.0 |
| K4.1 | 9.3 | 59.4 | 15.3 |
| K5.1 | 6.7 | 28.2 | 3.6 |
| 1b | 2.8 | <0.1 | 19.6 |
| 1c | 7.3 | 29.9 | 216 |
DISCUSSION
In this report, we show that H. pylori LPS displays phase variation. Reversible Lewis x-to-i-antigen switching was observed, at frequencies of about 0.4%. We also observed switches implying loss of polymeric main chain (Lewis x−, Lewis y+; for example, variant 1b) as well as switches implying strong expression of Lewis y (Lewis x+, Lewis y+; for example, variant 1c). By chemical means, strain NCTC 11637 was shown to express Lewis x (2), but in the past we have described the presence of low amounts of Lewis y in this strain as measured by serology (1); this finding was confirmed in this study. We assume that serology is more sensitive than structural-chemical methods. The presence of nonfucosylated polylactosamine stretches (i.e., the i antigen) has already been demonstrated by chemical methods (2). Our report is the first one on phase variation in H. pylori LPS; however, Bukholm et al. (5a) have reported the occurrence of reversible variants in colony morphology.
In previous reports (1, 18), we have suggested a pathogenic role for H. pylori LPS-induced anti-Lewis antibodies that cross-react with gastric mucosal antigens of the host. To further study the role of Lewis x, we isolated UV-induced Lewis x− mutants (Table 1). By serendipity, we discovered that a mutant (B1.5 [Fig. 2A]) displayed clonal expression of the epitope recognized by MAb 4D2, specific for H type 1 antigen. However, no H type 1 antigen can be detected in LPS of strain NCTC 11637 by structural-chemical means, and possibly the reactivity with strain 11637 represents a cross-reaction. Due to these uncertainties, we did not study phase variation of this epitope any further.
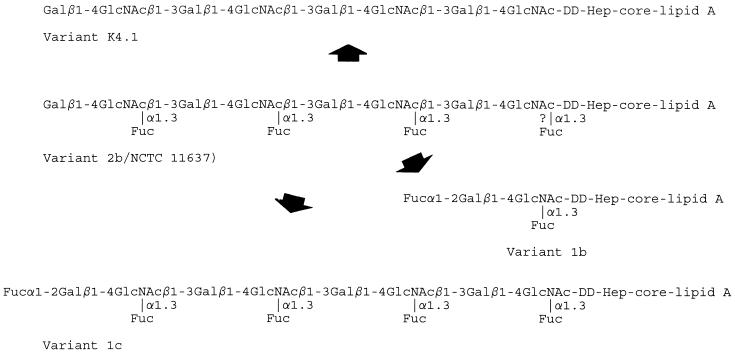
In contrast, the events taking place during phase variation of Lewis x to i antigen (and vice versa) can be understood at the molecular level, based on serological data (Table 1; Fig. 3) and on the primary structure of the LPS of H. pylori NCTC 11637 (Fig. 1). We postulate that the Lewis x antigen of strain 11637 switches to the i antigen by loss of the α1,3-linked fucose. A summary of the postulated LPS structures of the various variants and their possible interrelationships is shown in Fig. 5. The chemical structures of the LPS of the variants is under investigation. The presence of FucT enzymatic activity in H. pylori has been reported (1, 6), and hence it was conceivable that this Lewis x-to-i-antigen switch actually involved the on and off switching of the gene coding for H. pylori α3-FucT. Variant K4.1 shows FucT activity yet is Lewis x negative. Whether the measurement of FucT activities of a value in this respect is not clear, because in the past it has been shown that strains strongly positive for Lewis x may have lower FucT activities than strains expressing less Lewis x (6). It is also conceivable that genes coding for enzymes of the pathway leading to the donor substrate for FucT, GDP-Fuc, are involved in the Lewis x-to-i phase variation.
FIG. 5.
Proposed schematic LPS structures of variants and their interrelationships. The actual number of Lewis x repeats in NCTC 11637 is higher than indicated. The question mark in the NCTC 11637 LPS structure indicates that this LPS contains nonfucosylated lactosamines, adjacent to the core. dd-Hep, d-glycero-d-manno-heptose. For other abbreviations, see the legend to Fig. 1.
We observed the occurrence of i antigen to Lewis x switches first in strains that had been obtained from NCTC 11637 after UV irradiation (strains K1.4, K2.1, and K3.1). Later, switches were shown to occur also in nonirradiated cells. The reversibility of the phenomenon was investigated extensively, and we demonstrated that both spontaneous Lewis x-to-i-antigen switches and i antigen-to-Lewis x switches occurred at the same frequency, 0.44 and 0.55%, respectively. By using our colony blot procedures, we succeeded in isolating variants that had spontaneously switched from Lewis x to i antigen (e.g., K4.1) and a variant (K5.1) that had switched back again from i antigen to Lewis x. Mutants K1.4, K2.1, and K3.1 (all i+) also switched back spontaneously to Lewis x-positive cells, and one Lewis x-positive variant (K6.1 [data not shown]) that again switched to i+ at a frequency of 0.78% was isolated. In short, phase variation in the Lewis x to i antigen is reversible.
The presence in human sera of antibodies to the i antigen has been reported; these antibodies belong to the group of so-called cold agglutinins (CAs) (20). CAs recognize epitopes on erythrocytes and cause agglutination. As many H. pylori strains express the i antigen, it is possible that they induce CAs.
Second, we also studied mutants and variants that strongly expressed Lewis y and were negative for Lewis x (B1.5, B2.1, B3.1, and 1b [Table 1; Fig. 4]). Analysis of the lengths of these LPSs by SDS-PAGE showed that the polymeric main chain was lost. Apart from the core, only a single band could be seen. Strains that express Lewis y only, as determined both by chemical-structural analysis and by serology, have been described before (strain MO19 [4] and serotype O6 [1b]), and likely strains B1.5, B2.1, B3.1, and 1b are similar to MO19 and serotype O6 (Fig. 1). The single, Lewis y-expressing band (lanes 2 in Fig. 4a and 5b) likely represents a single Lewis y unit, covalently linked to the core. In contrast to the readily detected reversible phase variation Lewis x to i antigen, the switch from Lewis y to Lewis x was nondetectable in mutants B1.5 and variant B3.1 and with only one observed event of backswitching in strain 1b (Table 2). Again the similarity of the mutant and the variants is striking, and it is possible that those strains that we classified as mutants because they were isolated after UV irradiation simply are spontaneous phase variants.
In variant 1b, the switch from polylactosamine with multimeric Lewis x to a single, Lewis y-carrying lactosamine unit appears to be due to a complete turn off of a chain-elongating GlcNAcT, as no activity was found with a substrate specific for such an enzyme. In mammals, the GlcNAcTs that link GlcNAc residues to the core regions of protein- and lipid-linked glycans differ from the aglycon-nonspecific β3-GlcNAcT that is involved in the elongation of lactosamine chains (26, 27). Because in variant 1b only one lactosamine unit is present, it is likely that also in H. pylori the attachment of a GlcNAc to the d-glycero-α-d-manno-heptose unit of the LPS core is catalyzed by a GlcNAcT that differs from the elongating enzyme. Likely, the high expression of Lewis y by variant 1b is caused by the absence of the GlcNAcT, which may compete with the α2-FucT for linking a glycosyl group to galactose. In addition, a preferred action of the α2-FucT on short lactosamine chains rather than elongated ones is possible.
Third, a switch from Lewis x to Lewis x plus Lewis y was observed (strain 1c [Table 1; Fig. 4]). This variant expressed polymeric Lewis x, forming a ladder structure like its parent strain but with a much stronger expression of Lewis y; i.e., terminal α1,2-linked fucose is strongly increased. Strains with this phenotype, as determined by structural analysis and serology, have been described before (strain P466 [4] and serotype O3 [1b]). We did not observe backswitches of 1c to the parent phenotype. The mechanism involved may be a switching from low to high levels of expression of α2-FucT. It is known that in H. influenzae LPS, phase variation may involve both off-to-on switches and switches from low to high levels of expression (10).
The substrate used to assay FucT activity [Galβ1→4GlcNAcβ-O-(CH2)8-COOCH3] did not allow us to discriminate between α2- and α3-FucT. Previously it has been shown that using the same acceptor substrate the FucT in several strains of H. pylori only catalyzed the transfer of Fuc to GlcNAc in α1→3 linkage to yield the Lewis x structure (6). The high FucT activity in variant 1c might, however, in part be due to a strongly enhanced activity of an α2-FucT leading to the high expression of Lewis y. No such transfer could be demonstrated in assays using a substrate (Galβ-O-para-nitrophenol) that is known as a specific acceptor for mammalian α2-FucTs (data not shown). It has, however, been suggested that in the formation of Lewis y in H. pylori, α2-fucosylation may have to precede α3-fucosylation (16). Thus, it is conceivable that the high FucT activity in variant 1c reflects the consecutive transfers of Fuc residues in α1→3 and α1→2 linkage. Thus, α2-FucT may be involved in the switch from parental type to variant 1c. H. pylori FucT genes have been identified (8, 16, 23) and expressed (8, 16); these genes contain poly(C) tracts. Interestingly, in strain NCTC 11637 (expressing Lewis x), the reported FucT gene contains a C9 tract (gene off, truncated enzyme), while in strain 826695 (expressing Lewis xy [1a]), C13 is present (gene on, full-length enzyme). These data suggest the possibility that the expression of serotypes is regulated on the genetic level by means of variable-length oligonucleotide repeats. They also suggest that the mechanism of phase variation in H. pylori may mimic that of H. influenzae. When screened with anti-Lewis y MAbs, most of the colonies of strain NCTC 11637 stained only faintly, and we demonstrated that those faintly staining colonies (that also strongly express Lewis x) are the ones that switch to the variant with a high expression of Lewis y (i.e., variants 1b and 1c [Fig. 2]).
Finally, variant 3a that expressed LPS that was less reactive or not reactive with MAb 4D2 did not yield the characteristic LPS ladder pattern. As yet, we have no indication as to the structural change that has taken place in this variant.
Phase variation contributes to the heterogeneity of H. pylori and may explain the finding that from one patient, several highly related yet different isolates may be obtained (28, 32). Whether phase variation-induced heterogeneity is functional or merely an epiphenomenon has not yet been assessed. The phenomenon occurred not only in the laboratory strain NCTC 11637, which has undergone many in vitro passages, but also in clinical isolates that had been passaged only a few times. Phase variation in LPS of N. gonorrhoeae or H. influenzae serves a biological role, one variant being more adequate in one situation (e.g., adherence to mucosal cells) and the other one being more resistant to killing by complement. It would be interesting to investigate if variants of one strain can be isolated differentially from different gastric sites of one patient or experimental animal (14) and to investigate whether certain variants adhere better. Further studies are required to assess the molecular mechanism of phase variation in H. pylori LPS and its biological relevance.
ACKNOWLEDGMENTS
We thank S. L. Martin (GlaxoWellcome, Stevenage, England), D. E. Taylor (University of Alberta, Edmonton, Alberta, Canada), and G. O. Aspinall (York University, North York, Ontario, Canada) for providing unpublished data. We thank R. Negrini (Brescia, Italy) and G. Van Dam (Leiden, The Netherlands) for providing MAbs.
REFERENCES
- 1.Appelmelk B J, Simoons-Smit I M, Negrini R, Moran A P, Aspinall G O, Forte J G, De Vries T, Quan H, Verboom T, Maaskant J J, Ghiara P, Kuipers E J, Bloemena E, Tadema T M, Townsend R R, Tyagarajan K, Crothers J M, Jr, Monteiro M A, Savio A, de Graaff J. Potential role of molecular mimicry between Helicobacter pylorilipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64:2031–2040. doi: 10.1128/iai.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Appelmelk, B. J. Unpublished data.
- 1b.Aspinall G O, Monteiro M, Shaver R T, Kurjanczyk L A, Penner J L. Lipopolysaccharides of Helicobacter pylori serogroups O:3 and O:6. Structures of a class of lipopolysaccharides with reference to the location of oligomeric units of d-glycero-α-d-manno-heptose residues. Eur J Biochem. 1997;248:592–601. doi: 10.1111/j.1432-1033.1997.00592.x. [DOI] [PubMed] [Google Scholar]
- 2.Aspinall G O, Monteiro M A, Pang H, Walsh E J, Moran A P. O antigen chains in the lipopolysaccharide of Helicobacter pyloriNCTC 11637. Carbohydr Lett. 1994;1:151–156. [Google Scholar]
- 3.Aspinall G O, Monteiro M A, Pang H, Walsh E J, Moran A P. Lipopolysaccharide of the Helicobacter pyloritype strain NCTC 11637 (ATCC 43504): structure of the O antigen and core oligosaccharide regions. Biochemistry. 1996;35:2489–2497. doi: 10.1021/bi951852s. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall G O, Monteiro M A. Lipopolysaccharides of Helicobacter pyloristrains P466 and MO19: structures of the O antigen and core oligosaccharide strains. Biochemistry. 1996;35:2498–2504. doi: 10.1021/bi951853k. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard D, Bernard D, Loirat M J, Frioux Y, Guimbretiere J, Guimbretiere L. Characterization of murine monoclonal antibodies directed to fetal erythrocytes. Rev Fr Transfus Hemobiol. 1992;35:239–254. doi: 10.1016/s1140-4639(05)80102-9. [DOI] [PubMed] [Google Scholar]
- 5a.Bukholm G, Tannaes T, Nedenskov P, Esbensen Y, Gray H J, Hovig T, Ariansen S, Goldvog I. Colony variation of Helicobacter pylori: pathogenic potential is correlated to cell wall lipid composition. Scand J Gastroenterol. 1997;32:445–454. doi: 10.3109/00365529709025079. [DOI] [PubMed] [Google Scholar]
- 6.Chan N W C, Stangier K, Sherburne R, Taylor D E, Zhang Y, Dovichi N J, Palcic M M. The biosynthesis of Lewis x in Helicobacter pylori. Glycobiology. 1995;5:683–688. doi: 10.1093/glycob/5.7.683. [DOI] [PubMed] [Google Scholar]
- 7.Covacci A, Falkow S, Berg D E, Rappuoli R. Did the inheritance of a pathogenicity island motif modify the virulence of Helicobacter pylori? Trends Microbiol. 1997;5:205–208. doi: 10.1016/S0966-842X(97)01035-4. [DOI] [PubMed] [Google Scholar]
- 8.Ge Z, Chan N W C, Palcic M, Taylor D E. Cloning and heterologous expression of an α1,3-fucosyltransferase gene from the gastric pathogen Helicobacter pylori. J Biol Chem. 1997;272:21357–21363. doi: 10.1074/jbc.272.34.21357. [DOI] [PubMed] [Google Scholar]
- 9.Go M F, Kapur V, Graham D Y, Musser J M. Population genetic analysis of Helicobacter pyloriby multilocus enzyme electrophoresis: extensive allelic diversity and recombinational population structure. J Bacteriol. 1996;178:3934–3938. doi: 10.1128/jb.178.13.3934-3938.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.High N J, Deadman M E, Moxon E R. The role of a repetitive DNA motif (5′-CAAT-3′) in the variable expression of the Haemophilus influenzaelipopolysaccharide epitope Gal(1-4)betaGal. Mol Microbiol. 1993;9:1275–1282. doi: 10.1111/j.1365-2958.1993.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 11.Hokke C H, Zervosen A, Elling L, Joziasse D H, Van den Eijnden D H. One-pot enzymatic synthesis of the Galα1→3Galβ1→4GlcNAc sequence with in situUDP-Gal regeneration. Glycoconjugate J. 1996;13:687–692. doi: 10.1007/BF00731458. [DOI] [PubMed] [Google Scholar]
- 12.Kuipers, E. J. 1997. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment. Pharmacol. Therapeut. 11(Suppl. 1):71–88. [DOI] [PubMed]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lee A, Orourke J, Deungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pyloriinfection—introduction of the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 15.Logan P H, Berg D E. Genetic diversity of Helicobacter pylori. Lancet. 1996;348:1462–1463. doi: 10.1016/s0140-6736(05)65885-0. [DOI] [PubMed] [Google Scholar]
- 16.Martin S L, Edbroke M R, Hodgman T C, van den Eijnden D H, Bird M I. Lewis x biosynthesis in Helicobacter pylori: molecular cloning of an α-(1,3)-fucosyltransferase gene. J Biol Chem. 1997;272:21349–21356. doi: 10.1074/jbc.272.34.21349. [DOI] [PubMed] [Google Scholar]
- 17.Negrini R, Lisato L, Zanella I, Cavazzini S, Gullini S, Villanacci V, Poiesi C, Albertini A, Ghielmi S. Helicobacter pyloriinfection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991;101:437–445. doi: 10.1016/0016-5085(91)90023-e. [DOI] [PubMed] [Google Scholar]
- 18.Negrini R, Savio A, Poiesi C, Appelmelk B J, Buffoli F, Paterlini A, Cesari P, Graffeo M, Vaira D, Franzin G. Antigenic mimicry between Helicobacter pyloriand gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996;111:655–665. doi: 10.1053/gast.1996.v111.pm8780570. [DOI] [PubMed] [Google Scholar]
- 19.Roche R J, Moxon E R. Phenotypic variation of carbohydrate surface antigens and the pathogenesis of Haemophilus influenzaeinfections. Trends Microbiol. 1995;3:304–309. doi: 10.1016/s0966-842x(00)88959-3. [DOI] [PubMed] [Google Scholar]
- 20.Roelcke D. Serology, biochemistry, and pathology of antigens defined by cold agglutinins. In: Catron J P, Rouger P, editors. Blood cell biochemistry. 6. Molecular basis of major human blood group antigens. New York, N.Y: Plenum Press; 1995. pp. 117–152. [Google Scholar]
- 21.Sherburne R, Taylor D E. Helicobacter pyloriexpresses a complex surface carbohydrate, Lewis x. Infect Immun. 1995;63:4564–4568. doi: 10.1128/iai.63.12.4564-4568.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simoons-Smit I M, Appelmelk B J, Verboom T, Negrini R, Penner J L, Aspinall G O, Moran A P, Fei-fei S, Bi-shan S, Rudnica W, de Graaff J. Typing of Helicobacter pyloriwith monoclonal antibodies against Lewis antigens in lipopolysaccharide. J Clin Microbiol. 1996;34:2196–2200. doi: 10.1128/jcm.34.9.2196-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischman R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenney K, Fitzgerald L M, Lee N, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W S, Bordovsky M, Karp P D, Smith H O, Fraser C M, Venter C J. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda M, Karita M, Nakazawi T. Genetic transformation in Helicobacter pylori. Microbiol Immunol. 1993;37:85–89. doi: 10.1111/j.1348-0421.1993.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 25.Van Dam G J, Bergwerff A A, Thomas-Oates J E, Rotmans J P, Kamerling J P, Vliegenthart J F, Deelder A M. The immunologically reactive O-linked polysaccharide chains derived from circulating cathodic antigen isolated from human blood fluke Schistosoma mansonihave Lewis x as repeating unit. Eur J Biochem. 1994;225:467–82. doi: 10.1111/j.1432-1033.1994.00467.x. [DOI] [PubMed] [Google Scholar]
- 26.Van den Eijnden D H, Koenderman A H L, Schiphorst W E C M. Biosynthesis of blood group i-active polylactosaminoglycans. Partial purification and properties of an UDP-GlcNAc: N-acetyllactosaminide β1→3-N-acetylglucosaminyltransferase from Novikoff tumor cell ascites fluid. J Biol Chem. 1988;263:12461–12471. [PubMed] [Google Scholar]
- 27.Van den Eijnden D H, Bakker H, Neeleman A P, Van den Nieuwenhof I M, Van Die I. Novel pathways in complex-type oligosaccharide synthesis: new vistas opened by studies in invertebrates. Biochem Soc Trans. 1997;25:886–89328. doi: 10.1042/bst0250887. [DOI] [PubMed] [Google Scholar]
- 28.Van der Ende A, Rauws E A J, Feller M, Mulder C J J, Tytgat G N J, Dankert J. Heterogenous Helicobacter pyloriisolates from members of a family with a history of peptic ulcer disease. Gastroenterology. 1996;111:638–647. doi: 10.1053/gast.1996.v111.pm8780568. [DOI] [PubMed] [Google Scholar]
- 29.Van der Meer N M, Appelmelk B J, Verweij-van Vught A M J J, Nimmich W, Kosma P, Thijs L G, MacLaren D M. Binding studies of a monoclonal antibody specific for 3-deoxy-d-manno-octulosonic acid (Kdo) with a panel of Klebsiella pneumoniaelipopolysaccharides representing all the O serotypes. Infect Immun. 1994;62:1052–1057. doi: 10.1128/iai.62.3.1052-1057.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Putten J P M, Robertson B D. Molecular mechanisms and implications for infection of lipopolysaccharide variation in Neisseria. Mol Microbiol. 1995;16:847–853. doi: 10.1111/j.1365-2958.1995.tb02312.x. [DOI] [PubMed] [Google Scholar]
- 31.Wirth H P, Yang M, Peek R M, Tham K T, Blaser M J. Helicobacter pyloriLewis expression is related to the host Lewis phenotype. Gastroenterology. 1997;113:1091–1098. doi: 10.1053/gast.1997.v113.pm9322503. [DOI] [PubMed] [Google Scholar]
- 32.Wirth H P, Yang M, Peek R M, Hook-Nikanne J, Blaser M J. Phenotypic diversity in Lewis expression of H. pylori colonies derived from the same biopsy. Gastroenterology. 1997;112:A331. [Google Scholar]