Abstract
Background:
Oral nirmatrelvir/ritonavir is approved as treatment for acute COVID-19, but the effect of treatment during acute infection on risk of Long COVID is unknown. We hypothesized that nirmatrelvir treatment during acute SARS-CoV-2 infection reduces risk of developing Long COVID and rebound after treatment is associated with Long COVID.
Methods:
Observational cohort study within the Covid Citizen Science (CCS) study, an online cohort study with over 100,000 participants. We included vaccinated, non-hospitalized, non-pregnant individuals who reported their first SARS-CoV-2 positive test March-August 2022. Oral nirmatrelvir/ritonavir treatment was ascertained during acute SARS-CoV-2 infection. Patient-reported Long COVID symptoms, symptom rebound and test-positivity rebound were asked on subsequent surveys at least 3 months after SARS-CoV-2 infection.
Results:
4684 individuals met the eligibility criteria, of whom 988 (21.1%) were treated and 3696 (78.9%) were untreated; 353/988 (35.7%) treated and 1258/3696 (34.0%) untreated responded to the Long COVID survey (n=1611). Among 1,611 participants, median age was 55 years and 66% were female. At 5.4±1.3 months after infection, nirmatrelvir treatment was not associated with subsequent Long COVID symptoms (OR 1.15; 95%CI 0.80–1.64; p=.45). Among 666 treated who answered rebound questions, rebound symptoms or test positivity were not associated with Long COVID symptoms (OR 1.34; 95%CI 0.74–2.41; p=.33).
Conclusions:
Within this cohort of vaccinated, non-hospitalized individuals, oral nirmatrelvir treatment during acute SARS-CoV-2 infection and rebound after nirmatrelvir treatment were not associated with Long COVID symptoms more than 90 days after infection.
Keywords: nirmatrelvir, post-acute sequelae of COVID-19, Long COVID, rebound, Paxlovid
Introduction
Symptoms after SARS-CoV-2 may persist as Long COVID, a type of post-acute sequalae of SARS-CoV-2 infection (PASC) defined as unexplained symptoms attributed to COVID-19 1. Vaccination reduces but does not eliminate risk of Long COVID 2. Higher acute viral load or prolonged shedding may be associated with increased risk 3,4. Recent studies suggest viral persistence in a subset of individuals with PASC, including prolonged gastrointestinal shedding, ongoing Spike and Nucleocapsid antigen in neuronal and astrocytic exosomes, and evidence of persistent viral RNA or proteins in deep tissues and plasma 5–10.
Among high-risk, unvaccinated, non-hospitalized individuals with symptomatic COVID-19, nirmatrelvir, a novel orally-administered SARS-CoV-2 main protease (Mpro) inhibitor in combination with ritonavir reduces viral load and progression to severe disease 11. Anecdotal reports suggest that nirmatrelvir may improve Long COVID symptoms 12–14. However, whether treatment with nirmatrelvir during acute infection reduces post-COVID conditions is uncertain, with two studies finding conflicting results in the same population15,16. Use of EHR-based diagnosis of post-COVID conditions, as in those studies, relies on patient reporting and clinician documentation of medical diagnoses so may not capture the possible treatment effect on Long COVID symptoms. A third study, which surveyed individuals 4 months after SARS-CoV-2 infection, found no association between nirmatrelvir use and prevalent Long COVID symptoms, but reported a prevalence of symptoms of nearly 50% in both groups 17.
Therefore, the objectives were to test the hypothesis that nirmatrelvir treatment during acute SARS-CoV-2 infection is associated with a lower prevalence of patient-reported Long COVID symptoms >90 days after infection, and that rebound of acute symptoms or rebound test-positivity after treatment is associated with higher prevalence of Long COVID symptoms.
Methods
Design, Setting, and Participants
The COVID-19 Citizen Science (CCS) Study is an online cohort study hosted on the Eureka Research Platform 18. Recruitment occurred through email invitations to existing Eureka participants, referrals from partner organizations, and word of mouth. To participate, individuals must register for an account, agree to participate in English and provide electronic consent. After consenting, participants complete baseline and follow-up surveys. We used data collected from March 26, 2020, to December 22, 2022. We included individuals who enrolled prior to first known SARS-CoV-2 infection first infected when nirmatrelvir was available in March 2022 through August 2022. Outcomes were ascertained only for those who responded to surveys about Long COVID symptoms in November and December 2022. The primary outcome was defined as at least one patient-reported Long COVID symptom >90 days after acute SARS-CoV-2 infection 1.
Inclusion and Exclusion Criteria
We included CCS participants enrolled March 2020-August 2022 (median March 2021, IQR August 2020-September 2021) with first-reported positive SARS-CoV-2 antigen or PCR test starting in March 2022. We excluded those treated with molnupiravir, remdesivir, and monoclonal antibodies. Following the Emergency Use Authorization, we excluded hospitalized and pregnant individuals. Because >98% were vaccinated prior to infection we limited analysis to vaccinated individuals. We chose not to limit inclusion to “eligible” patients as in our clinical experience the “high risk” criteria are loosely interpreted but conducted sensitivity analyses limiting inclusion criteria to those defined as at high-risk by the Centers for Disease Control (CDC).
Exposures
The primary exposure was self-reported nirmatrelvir treatment within 30 days of first reported positive SARS-CoV-2 antigen or PCR test on a weekly survey from March 2022-August 2022. Participants who reported taking nirmatrelvir were subsequently invited to respond to a survey in December 2022 to assess for rebound symptoms or test positivity. Rebound symptoms were defined as symptom worsening after initial improvement. Rebound test positivity was defined as testing negative and then positive on antigen test after completing treatment.
Outcomes
The primary outcome was ≥1 self-reported prevalent Long COVID symptom >90 days after first SARS-CoV-2 positive test on a cross-sectional survey distributed in November and December 2022 that asked about presence, duration, and severity of Long COVID symptoms using a published non-validated instrument.19 We decided a priori to use the 90-day World Health Organization definition as a significant proportion experience symptom resolution in the 4 week to 90 day period 19. Symptoms queried included fatigue, shortness of breath, confusion, headache, altered taste and smell, joint pain, muscle aches, cough, chest pain, scratchy throat, nausea, vomiting, diarrhea, fever, chills, red or painful eyes, sore throat, and other. Because the survey was developed initially based on symptoms during acute COVID-19 prior to thorough understanding of common Long COVID symptoms, the queried symptoms do not include every symptom reported in Long COVID (brain fog and post-exertional malaise are excluded, for example), but the inclusion of an “other” symptom question helps overcome this limitation. Severity was assessed using a Likert-scale asked for each symptom (1–5, very mild to very severe).
Covariates
Other variables were self-reported including demographics, medical history, vaccine history, and lifestyle factors 19. Only body mass index (BMI) had >1% of data missing (650 missing, 40%). For the propensity adjusted model, we imputed missing BMI from age, sex, race/ethnicity, and past medical history.
Statistical Analysis
The analysis plan was developed prior to outcome data collection and was designed to emulate a randomized clinical trial using a “target trial” approach 20. First, we compared treated and untreated individuals using Chi-squared tests for categorical variables and T-tests for continuous variables. We modeled nirmatrelvir use propensity with logistic regression models including all individuals who met the inclusion/exclusion criteria including age, sex, race, ethnicity, socioeconomic status, education, employment, past medical history, substance use, number of vaccines. After checking the Hosmer-Lemshow goodness of fit and balance of key covariates by propensity score quintile, we used logistic regression models to model the association between nirmatrelvir and Long COVID among survey respondents adjusted for age, sex, time since SARS-CoV-2 test positivity, and the cubic spline of the propensity score. As a sensitivity analysis, we repeated the analysis using inverse probability of treatment weighting, truncating extreme weights >95th percentile. As a post-hoc sensitivity analysis we restricted the inclusion criteria to United States residents at risk for severe COVID-19 defined according to the CDC using two definitions (age ≥50, one or more comorbid condition, or BMI≥25 kg/m2; age ≥65, one or more comorbid condition, or BMI≥30 kg/m2). The next post-hoc sensitivity analyses used propensity matching to estimate the average treatment effect using 1:1 nearest neighbor matching by propensity score or by Mahalanobis distance (inverse sample covariate covariance). For analyses of rebound, we used Fisher’s exact test and logistic regression for unadjusted analyses given the lack of evident confounders. Analyses were conducted in SAS version 9.4 and STATA 17.0.
Results Reporting and Informed Consent
Results are reported in accordance with STROBE guidelines 21. All participants provided digitally-signed informed consent. The study was reviewed and approved by the UCSF Institutional Review Board (#17–21879).
Results
Within the CCS, 4684 eligible vaccinated individuals reported their first positive SARS-CoV-2 test during the study period (Omicron era), of whom 988 (21.1%) were treated with nirmatrelvir and 3696 (78.9%) were untreated (Figure 1). Among those eligible, 353/988 (35.7%) treated and 1258/3696 (34.0%) untreated individuals responded to the Long COVID survey at 5.4±1.3 months after acute infection. Baseline characteristics and time since SARS-CoV-2 infection were similar among respondents and nonrespondents (Supplemental Table 1). As expected based on prescribing guidelines 22, individuals treated with nirmatrelvir were older, more likely to be male, and had more comorbidities (Table 1).
Figure 1.
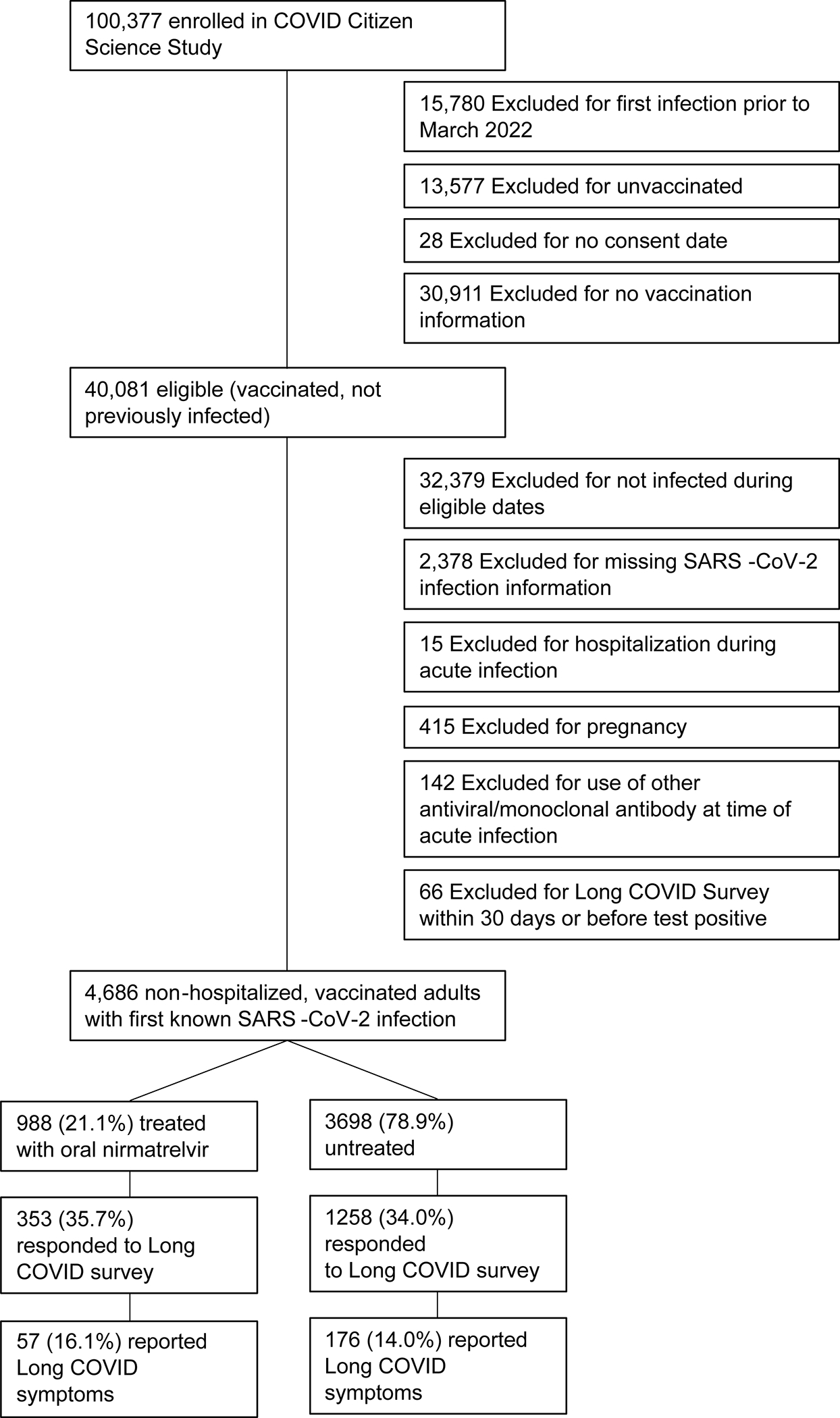
Flow diagram for participants included in the primary analysis.
Table 1.
Baseline characteristics among Long COVID survey respondents. For race and ethnicity, participants could select all that apply so these are not mutually exclusive categories.
| Treated with nirmatrelvir/ritonavir, N = 353 | SARS-CoV-2 infected, untreated, N = 1258 |
||||
|---|---|---|---|---|---|
| Characteristic | N | Mean ± std or n (%) | N | Mean ± std or n (%) | P value treated vs not |
| Age | 352 | 62.1 ± 12.7 | 1258 | 55.1 ± 13.6 | 0.0000 |
| Female sex assigned at birth | 349 | 186 (53.3%) | 1250 | 811 (64.9%) | 0.0001 |
| Race/Ethnicity* | |||||
| White | 347 | 327 (94.2%) | 1245 | 1156 (92.9%) | 0.37 |
| Black or African American | 347 | 4 (1.2%) | 1245 | 28 (2.2%) | 0.20 |
| Asian | 347 | 14 (4.0%) | 1245 | 61 (4.9%) | 0.50 |
| Native Hawaiian or Pacific Islander | 1245 | 1 (0.1%) | 0.60 | ||
| American Indian or Alaska Native | 347 | 5 (1.4%) | 1245 | 17 (1.4%) | 0.92 |
| Other/Donť know | 347 | 7 (2.0%) | 1245 | 29 (2.3%) | 0.73 |
| Hispanic Ethnicity | 349 | 11 (3.2%) | 1250 | 73 (5.8%) | 0.05 |
| Highest Educational Level | 0.28 | ||||
| No high school degree | 349 | 2 (0.6%) | 1250 | 1 (0.1%) | |
| High school graduate | 349 | 7 (2.0%) | 1250 | 25 (2.0%) | |
| College degree | 349 | 151 (43.3%) | 1250 | 587 (47.0%) | |
| Graduate degree | 349 | 187 (53.6%) | 1250 | 628 (50.2%) | |
| Other | 349 | 2 (0.6%) | 1250 | 9 (0.7%) | |
| MacArthur Socioeconomic Status | 349 | 7.23 ± 1.55 | 1249 | 7.17 ± 1.47 | 0.46 |
| US resident | 353 | 353 (100.0%) | 1258 | 1220 (97.0%) | 0.001 |
| Body Mass Index | 216 | 27.9 ± 6.5 | 745 | 27.6 ± 6.0 | 0.50 |
| Tobacco use | 353 | 10 (2.8%) | 1258 | 55 (4.4%) | 0.19 |
| Marijuana use | 353 | 23 (6.5%) | 1258 | 87 (6.9%) | 0.79 |
| Alcoholic drinks/week pre-COVID | 353 | 4.52 ± 5.48 | 1254 | 4.59 ± 5.77 | 0.85 |
| Financial insecurity pre-COVID | 352 | 61 (17.3%) | 1250 | 200 (16.0%) | 0.55 |
| Average Anxiety (GAD-7) pre-COVID | 352 | 3.27 ± 3.70 | 1250 | 3.55 ± 3.55 | 0.20 |
| Average Depression (PHQ-8) pre-COVID | 352 | 3.32 ± 3.71 | 1250 | 3.58 ± 3.59 | 0.24 |
| Hypertension | 353 | 128 (36.3%) | 1257 | 364 (29.0%) | 0.01 |
| Diabetes | 353 | 30 (8.5%) | 1257 | 69 (5.5%) | 0.10 |
| Coronary Artery Disease | 353 | 23 (6.5%) | 1257 | 62 (4.9%) | 0.24 |
| Heart Failure | 353 | 3 (0.8%) | 1257 | 15 (1.2%) | 0.10 |
| Stroke or Transient Ischemic Attack | 353 | 12 (3.4%) | 1257 | 23 (1.8%) | 0.12 |
| Atrial Fibrillation | 353 | 27 (7.6%) | 1257 | 54 (4.3%) | 0.04 |
| Sleep Apnea | 352 | 69 (19.6%) | 1257 | 154 (12.3%) | 0.001 |
| Chronic Obstructive Pulmonary Disease | 353 | 7 (2.0%) | 1257 | 18 (1.4%) | 0.76 |
| Asthma | 353 | 37 ( 10.5%) | 1257 | 79 ( 6.3%) | 0.02 |
| Cancer | 353 | 41 ( 11.6%) | 1257 | 78 ( 6.2%) | 0.0006 |
| Immunodeficiency | 353 | 17 ( 4.8%) | 1257 | 12 ( 1.0%) | 0.0000 |
| HIV | 353 | 3 ( 0.8%) | 1257 | 8 ( 0.6%) | 0.79 |
| Anemia | 353 | 26 ( 7.4%) | 1257 | 121 ( 9.6%) | 0.33 |
| Days since COVID-19 | 353 | 162 ± 40 | 1258 | 165 ± 45 | 0.16 |
| Number of acute symptoms | 353 | 5.05 ± 2.20 | 1250 | 4.91 ± 2.27 | 0.31 |
| Number of Long COVID Symptoms among those with any, median (IQR) | 57 | 1 (IQR 1–4) | 176 | 2 (IQR 1–4) | 0.35 |
| Number of SARS-CoV-2 vaccines received prior to COVID-19 | 353 | 2.41 ± 0.62 | 1258 | 2.46 ± 0.61 | 0.25 |
| Number of Vaccines received after COVID-19 | 353 | 0.01 ± 0.09 | 1258 | 0.00 ± 0.07 | 0.41 |
Association of nirmatrelvir/ritonavir treatment with Long COVID symptoms
Among those treated with nirmatrelvir, 57/353 (16.1%) reported Long COVID symptoms compared to 176/1258 (14.0%) untreated (OR 1.18; 95%CI 0.84–1.65; p=.31; Table 2). Commonly reported symptoms included fatigue, shortness of breath, confusion, headache, and altered taste and smell. The propensity score successfully modeled probability of treatment, and overall characteristics were similar by propensity score quintile (Supplemental Tables 2 & 3, Supplemental Figure). In the propensity-adjusted model, nirmatrelvir was not associated with self-reported Long COVID symptoms (OR 1.15; 95%CI 0.80–1.64; p=.45; Figure 2). Results were similar when incorporating demographics, past medical history, and substance use (OR 1.17; 95%CI 0.81–1.69). Similarly, using inverse probability of treatment weighting as an alternative analytic strategy yielded the same estimate (OR 1.17; 95%CI 0.80–1.71). Restricting to those at high risk yielded similar results for sensitive (n=968 treated & n=3,326; OR 1.11; 95%CI 0.77–1.59) and specific definitions (n=890 treated & n=2,840 untreated); OR 1.12, 95%CI 0.78–1.60). Finally, among those at high risk for severe COVID-19 we estimated average treatments effects of 0.032 (95%CI −0.017–0.081; p=.20) using propensity matching and 0.012 (95%CI −0.04–0.07; p=.67) using nearest neighbor matching, both consistent with no benefit of treatment.
Table 2.
This table reports the number and percentage among the treated and untreated who did and did not report Long COVID symptoms.
| Not treated | Nirmatrelvir treated | Total | |
|---|---|---|---|
| No Long COVID symptoms | 1082 (86.0%) | 176 (83.9%) | 1258 (85.5%) |
| ≥1 Long COVID symptom | 296 (14.0%) | 57 (16.1%) | 353 (14.5%) |
| Total | 1378 (100%) | 233 (100%) | 1611 (100%) |
Figure 2.
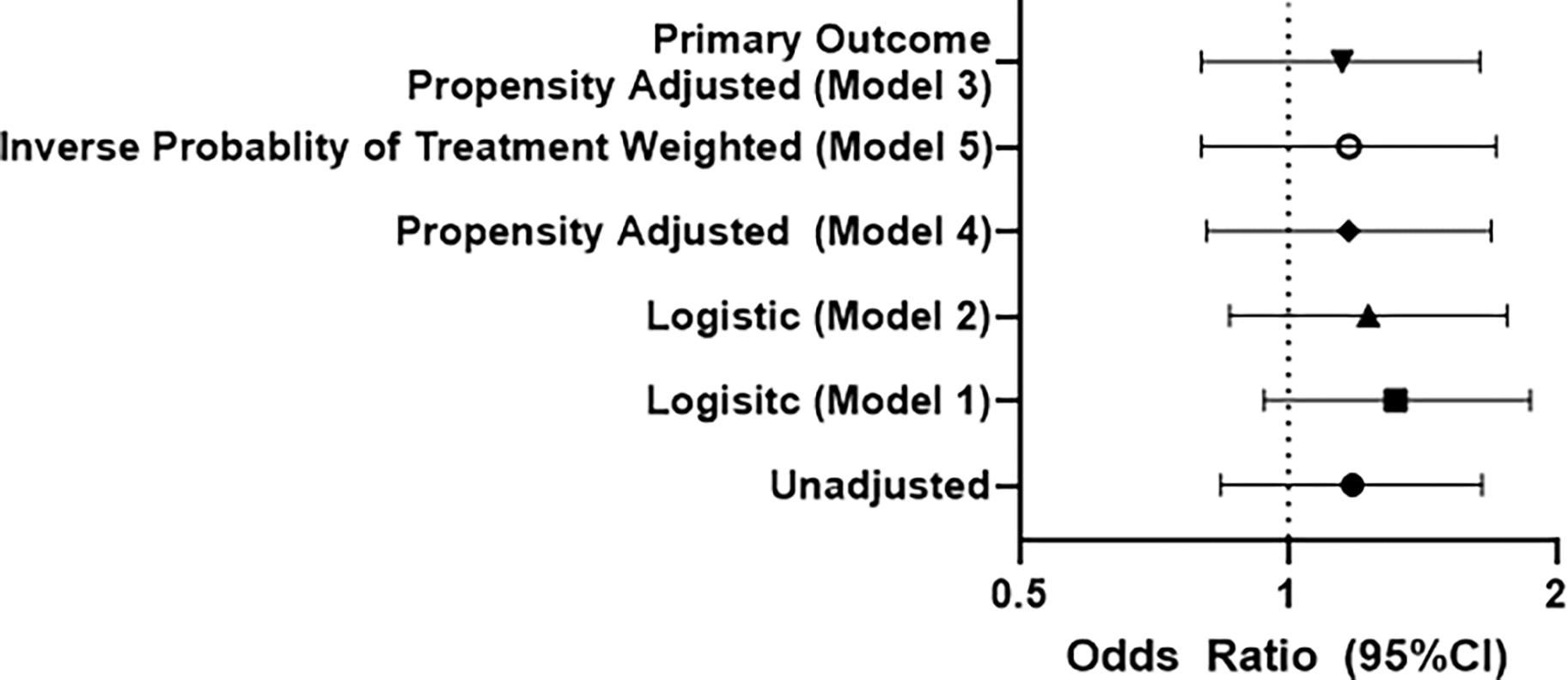
In all models, treatment with oral nirmatrelvir was not associated with lower odds of patient reported Long COVID symptoms with confidence intervals that cross 1, which suggests that treatment is not beneficial in reducing the risk of Long COVID. Model 3, the pre-specified primary result, includes age, sex, and time since SARS-CoV-2 infection, and the restricted cubic spline of the propensity score. Model 1 includes age, sex, and time since SARS-CoV-2 infection. Model 2 includes age, sex, time since SARS-CoV-2 infection, race/ethnicity, past medical history and substance use. Model 4 is the propensity-adjusted version of Model 2. Finally, Model 5 incorporates inverse probability of treatment weighting and age, sex, and time since SARS-CoV-2 infection. Results were similar in additional sensitivity analyses.
Among those treated with nirmatrelvir reporting at least one Long COVID symptom, the median number of Long COVID symptoms was 1 (IQR 1–4) compared to 2 (IQR 1–4) among those untreated (p=.35); the mean number of symptoms was 2.86±2.42 and 3.06±2.54, respectively (p=.70). Few reported at least one severe or very severe Long COVID symptom in either group: 6 (1.7% overall, 10.5% with Long COVID) among the treated and 18 (1.4% overall, 10.2% with Long COVID) among the untreated (p=.18); therefore, we could not exclude an effect on symptom severity.
Rebound
Among 666 individuals treated with nirmatrelvir who responded to the rebound survey, 139 of the 650 who experienced symptomatic improvement during nirmatrelvir treatment (21%) reported rebound symptoms. Among those who repeated antigen testing after testing negative and completing treatment, 97/377 (25.7%) reported rebound test positivity. In total, 174/666 (26.1%) reported rebound symptoms or test positivity. In total 634 of the respondents to the rebound survey also responded to the Long COVID questions: 166 with rebound and 468 without rebound. Among those with rebound, 18/166 (10.8%) reported 1 or more Long COVID symptom compared to 39/468 (8.3%) without rebound (OR 1.34; 95%CI 0.74–2.41; p=.33; Table 3). Results were similar for symptoms (9.9% vs 8.4%; OR 1.20; 95%CI 0.62–2.31) and test positivity (10.8% vs 7.7%; OR 1.45; 95%CI 0.66–3.21). Results were similar using a more sensitive outcome of those who had not experienced full recovery (14.9% with rebound vs 12.8% without; OR 1.20; 95%CI 0.73–1.96). Results were similar restricting analyses only to those who reported completing the full ten-dose course of nirmatrelvir (n=621): OR 1.20 (95% CI 0.62–2.30; p=.59).
Table 3.
Among 634 who responded to questions about rebound and Long COVID, although 26% experienced rebound symptoms, rebound test positivity, or both, there was not a statistically significant difference in the presence of Long COVID among those who experienced rebound compared to those who did not (OR 1.34; 95%CI 0.74–2.41; p=.33).
| No Rebound | Rebound Symptoms or Test Positivity | Total | |
|---|---|---|---|
| No Long COVID symptoms | 429 (91.7%) | 148 (90.2%) | 577 (91.0%) |
| ≥1 Long COVID symptom | 39 (8.3%) | 18 (10.8%) | 57 (9.0%) |
| Total | 468 (73.8%) | 166 (26.2%) | 634 (100%) |
Results were similar limiting analysis to respondents who completed treatment more than 90 days prior (n=157). Of those, 31/157 (20.2%) experienced rebound symptoms and 17/84 (19.7%) had a rebound positivity with 38/157 (24.2%) classified as having any rebound. Only 2/38 (5.2%) individuals who experienced rebound reported Long COVID symptoms compared to 19/119 (16.0%) who did not experience rebound (p=.11). Among those who experienced rebound test positivity, none reported Long COVID symptoms compared to 10/67 (14.9%) without rebound test positivity (p=.20).
Discussion
Within an online observational cohort, treatment with nirmatrelvir among vaccinated, non-hospitalized individuals during first known SARS-CoV-2 infection was not associated with a lower prevalence of patient-reported Long COVID symptoms >90 days after infection. Treatment was not associated with fewer Long COVID symptoms or severe symptoms, although these endpoints were limited by rarity of these outcomes. Rebound symptoms or test positivity after nirmatrelvir treatment were not associated with Long COVID symptoms.
Prior studies of nirmatrelvir, including two randomized clinical trials, EPIC-HR and EPIC-SR, focused on acute outcomes of SARS-CoV-2 infection among unvaccinated individuals. EPIC-HR demonstrated a reduction in hospitalization and mortality by day 28 among those at high risk of disease progression treated with nirmatrelvir compared to placebo 11, but the EPIC-SR study of standard risk individuals was stopped early for lack of benefit with negative results now posted on clinicaltrials.gov but not published 23. “Real-world” observational studies have found similar results, with reductions in hospitalization and mortality among higher risk and vaccinated individuals.24–29 Higher viral loads and prolonged viral shedding have been associated with risk of Long COVID 3,4, and nirmatrelvir results in faster viral clearance 30,31, supporting the hypothesis that nirmatrelvir may prevent Long COVID. Secondly, in this cohort, the number of symptoms during acute infection is associated with Long COVID symptoms independent of vaccination and variant wave 19, but whether reducing acute symptoms with antiviral therapy prevents Long COVID has not been demonstrated.
Three prior studies have considered the effect of nirmatrelvir during acute infection on EHR-ascertained post-COVID outcomes. Two found contradictory results despite both including Veterans and examining EHR-diagnosed post-COVID conditions by ICD-10 diagnostic codes as the outcome of interest 15,16. Bajema et al found that nirmatrelvir improved 30-day outcomes but not EHR-diagnosed post-COVID conditions at 6 months, whereas Xie et al found decreased post-COVID conditions, hospitalizations, and mortality among treated individuals from 30–90 days. Similar to our design, Bajema et al used a target-trial emulation study design, which is more robust to common pitfalls of observational studies of treatment effects. Both included an older, mostly male patient population with significant comorbidities and are limited by differential outcome ascertainment, differential misclassification, and lack of ascertainment that ICD-codes correspond to symptoms. A third study, by Patel et al, used EHR records from the TriNetX research network, but included individuals with pre-existing Long COVID in the control group which may explain their finding of a benefit of nirmatrelvir 32.
In contrast to EHR-based studies focused on ascertainment of post-COVID-conditions using ICD-10 codes, our study evaluated whether nirmatrelvir is associated with patient-reported symptoms. Patient-reported symptoms is a closer approximation of the condition of interest (Long COVID, defined as unexplained symptoms persisting for >90 days following initial infection) 1. One other published study surveyed participants from a single medical center four months after SARS-CoV-2 infection and compared the prevalence of self-reported prevalent Long COVID symptoms among people who reported nirmatrelvir use to those who were not treated with nirmatrelvir 17. Our findings are consistent with their finding of no association between nirmatrelvir use and subsequent Long COVID symptoms. Our inclusion of participants already enrolled in the COVID Citizen Science study without prior infection, use of a target-trial framework, and propensity-adjustment further strengthens the validity of our findings. In sum, our finding that nirmatrelvir treatment during acute infection is not associated with lower odds of Long COVID is consistent with the Bajema report of no difference in post-COVID conditions at 6 months 16 and with the Congdon survey study at 4 months after infection 17.
We found a higher proportion with clinical rebound than previously reported 33,34,35, but did not identify an effect of post-treatment rebound on Long COVID symptoms. Since antivirals entered widespread use, there has been intense interest in this phenomenon, and a case report suggested the development of Long COVID among those with post-treatment rebound 12. The observation that antiviral-associated rebound is not associated with Long COVID suggests that the potential for treatment-related rebound should not discourage antiviral use, and that individuals experiencing this phenomenon are not at elevated risk for Long COVID. Rebound test positivity was common in the early days of the pandemic 36; we were not able to determine whether rebound unrelated to treatment increases risk of Long COVID.
Our study does not consider whether nirmatrelvir may be effective at treating (rather than preventing) Long COVID, which is currently under investigation in one ongoing and two planned clinical trials (NCT05576662, NCT05595369, NCT05668091).
Limitations
The primary limitations arise from its observational nature which is at risk for selection bias and confounding. The cohort was relatively homogeneous; most individuals identified as White with advanced education, in large part because we limited this study to those privileged enough to avoid infection until March 2022. We relied on self-report of treatment and Long COVID symptoms. Baseline data was collected prior to infection, and SARS-CoV-2 testing and nirmatrelvir use were collected at the time of testing and treatment, respectively, limiting the impact of recall bias. Nonetheless differential reporting of test positivity or survey response (which was non-differential by treatment) may induce selection bias. The survey did not include all Long COVID symptoms, such as post-exertional malaise, insomnia, decreased exercise tolerance, and menstrual cycle changes, for example. Our study differs from EHR-based reports in that we assessed Long COVID symptoms rather than ICD-10 codes. We therefore could not determine whether treatment had an impact on post-acute diagnoses. We also did not include objectively measured post-COVID outcomes (e.g., exercise capacity 37, neurocognitive performance 38, or other measurable physiologic perturbations). We used propensity scores and inverse probability of treatment weighting to adjust for baseline differences between propensity of treatment between the treated and untreated, but residual confounding may still bias the results. Rebound testing was not performed systematically, and results were based on participant test self-report.
Conclusions
Among vaccinated, non-hospitalized adults in the COVID Citizen Science online cohort, nirmatrelvir treatment during acute SARS-CoV-2 infection was not associated with Long COVID symptoms >90 days after infection. Among those treated, rebound was not associated with Long COVID symptoms.
Supplementary Material
Acknowledgements:
We would like to thank all of the research participants and all of the Patient-Centered Outcomes Research Institute sites and investigators who recruited participants.
Funding:
Eureka Research Platform is supported by NIH/NIBIB 3U2CEB021881-05S1. The COVID-19 Citizen Science Study is supported by Patient-Centered Outcomes Research Institute (PCORI) contract COVID-2020C2-10761 and Bill and Melinda Gates Foundation contract INV-017206. Dr. Durstenfeld is supported by NIH/NHLBI grant K12HL143961.
Footnotes
Disclosures/Conflicts of Interest: Dr. Peluso has served as a consultant for AstraZeneca and Gilead Sciences, outside the submitted work. Dr. Beatty was formerly employed by (2018–2019) and held stock in (2019–2021) Apple Inc. All other authors report no conflicts.
Work performed at University of California, San Francisco
Contributor Information
Matthew S. Durstenfeld, Division of Cardiology at ZSFG and Department of Medicine, University of California, San Francisco (UCSF), USA.
Michael J. Peluso, Division of HIV, Infectious Disease, & Global Medicine, UCSF, USA.
Feng Lin, Department of Epidemiology and Biostatistics, UCSF, USA.
Noah D. Peyser, Department of Epidemiology and Biostatistics, UCSF, USA
Carmen Isasi, Department of Epidemiology & Population Health, Albert Einstein College of Medicine.
Thomas W. Carton, Louisiana Public Health Institute, USA
Timothy J. Henrich, Department of Experimental Medicine, UCSF, USA
Steven G. Deeks, Division of HIV, Infectious Disease, & Global Medicine, UCSF, USA
Jeffrey E. Olgin, Division of Cardiology, Department of Medicine, UCSF, USA
Mark J. Pletcher, Department of Epidemiology and Biostatistics, UCSF, USA
Alexis L. Beatty, Department of Epidemiology and Biostatistics and Division of Cardiology, Department of Medicine, UCSF, USA
Gregory M. Marcus, Division of Cardiology, Department of Medicine, UCSF, USA
Priscilla Y. Hsue, Division of Cardiology at ZSFG and Department of Medicine, University of California, San Francisco (UCSF), USA
Data Availability:
Data are available by application to the COVID Citizen Science leadership committee. Data may be requested by emailing covid19@eurekaplatform.org.
References
- 1.Soriano JB, Murthy S, Marshall JC, Relan P, & Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. The Lancet Infectious Diseases, 2022; 22(4): e102–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe A, Iwagami M, Yasuhara J, Takagi H, Kuno T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881–895 e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antar AAR, Yu T, Demko ZO, et al. Long COVID brain fog and muscle pain are associated with longer time to clearance of SARS-CoV-2 RNA from the upper respiratory tract during acute infection. Front Immunol. 2023; 14:1147549. doi: 10.3389/fimmu.2023.1147549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein SR, Ramelli SC, Grazioli A, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612(7941):758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swank Z, Senussi Y, Manickas-Hill Z, et al. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Clin Infect Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natarajan A, Zlitni S, Brooks EF, et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med (N Y). 2022;3(6):371–387 e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peluso MJ, Deeks SG, Mustapic M, et al. SARS-CoV-2 and Mitochondrial Proteins in Neural-Derived Exosomes of COVID-19. Ann Neurol. 2022;91(6):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peluso MJ, Swank ZN, Goldberg SA, et al. Plasma-based antigen persistence in the post-acute phase of SARS-CoV-2 infection. medRxiv. 2023:2023.2010.2024.23297114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peluso MJ, Ryder D, Flavell R, et al. Multimodal Molecular Imaging Reveals Tissue-Based T Cell Activation and Viral RNA Persistence for Up to 2 Years Following COVID-19. medRxiv. 2023:2023.2007.2027.23293177. [Google Scholar]
- 11.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peluso MJ, Anglin K, Durstenfeld MS, et al. Effect of Oral Nirmatrelvir on Long COVID Symptoms: 4 Cases and Rationale for Systematic Studies. Pathog Immun. 2022;7(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visvabharathy L, Orban ZS, Koralnik IJ. Case report: Treatment of long COVID with a SARS-CoV-2 antiviral and IL-6 blockade in a patient with rheumatoid arthritis and SARS-CoV-2 antigen persistence. Front Med (Lausanne). 2022;9:1003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng LN, Bonilla HF, Shafer RW, Miglis MG, Yang PC. The Use of Nirmatrelvir-ritonavir in a Case of Breakthrough Long COVID. Exploratory Research and Hypothesis in Medicine. 2023;000(000):000–000. [Google Scholar]
- 15.Xie Y, Choi T, Al-Aly Z. Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition. JAMA Intern Med. 2023;183(6):554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajema KL, Berry K, Streja E, et al. Effectiveness of COVID-19 Treatment With Nirmatrelvir-Ritonavir or Molnupiravir Among U.S. Veterans: Target Trial Emulation Studies With One-Month and Six-Month Outcomes. Ann Intern Med. 2023;176(6):807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Congdon S, Narrowe Z, Yone N, et al. Nirmatrelvir/ritonavir and risk of long COVID symptoms: a retrospective cohort study. Scientific Reports. 2023;13(1):19688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beatty AL, Peyser ND, Butcher XE, et al. The COVID-19 Citizen Science Study: Protocol for a Longitudinal Digital Health Cohort Study. JMIR Res Protoc. 2021;10(8):e28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durstenfeld MS, Peluso MJ, Peyser ND, et al. Factors Associated With Long COVID Symptoms in an Online Cohort Study. Open Forum Infect Dis. 2023;10(2):ofad047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. American Journal of Epidemiology. 2016;183(8):758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. [DOI] [PubMed] [Google Scholar]
- 22.Administration FaD. Fact Sheet for Healthcare Providers. In:2022. [Google Scholar]
- 23.Pfizer Reports Additional Data on PAXLOVID™ Supporting Upcoming New Drug Application Submission to U.S FDA; [press release]. 2022. [Google Scholar]
- 24.Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients. Clin Infect Dis. 2022;76(3):e342–e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System : A Population-Based Cohort Study. Ann Intern Med. 2023;176(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wai AK, Chan CY, Cheung AW, et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health West Pac. 2023;30:100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah MM, Joyce B, Plumb ID, et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19 - United States, April-September 2022. Am J Transplant. 2023;23(1):150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22(12):1681–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen K, Makkar SR, Sahner D, et al. Paxlovid (nirmatrelvir/ritonavir) effectiveness against hospitalization and death in N3C: A target trial emulation study. medRxiv. 2023:2023.2005.2026.23290602. [Google Scholar]
- 30.Qi T, Jin Y, Wang H, et al. Nirmatrelvir-ritonavir Therapy and COVID-19 Vaccination Improve Clinical Outcomes of SARS-CoV-2 Omicron Variant Infection. J Med Virol. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Zhao D, Liu X, Chen X, Xiao W, Feng L. Early administration of Paxlovid reduces the viral elimination time in patients infected with SARS-CoV-2 Omicron variants. J Med Virol. 2023;95(1):e28443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel R, Dani S, Khadke S, et al. Incidence of Symptoms Associated with Post-Acute Sequelae of SARS-CoV-2 infection in Non-Hospitalized Vaccinated Patients Receiving Nirmatrelvir-Ritonavir. medRxiv. 2023:2023.2004.2005.23288196. [Google Scholar]
- 33.Wong GL, Yip TC, Lai MS, Wong VW, Hui DS, Lui GC. Incidence of Viral Rebound After Treatment With Nirmatrelvir-Ritonavir and Molnupiravir. JAMA Netw Open. 2022;5(12):e2245086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranganath N, O’Horo JC, Challener DW, et al. Rebound Phenomenon After Nirmatrelvir/Ritonavir Treatment of Coronavirus Disease 2019 (COVID-19) in High-Risk Persons. Clinical Infectious Diseases. 2022;76(3):e537–e539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edelstein GE, Boucau J, Uddin R, et al. SARS-CoV-2 Virologic Rebound With Nirmatrelvir-Ritonavir Therapy : An Observational Study. Ann Intern Med. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhm JS, Ahn JY, Hyun J, et al. Patterns of viral clearance in the natural course of asymptomatic COVID-19: Comparison with symptomatic non-severe COVID-19. Int J Infect Dis. 2020;99:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durstenfeld MS, Peluso MJ, Kaveti P, et al. Reduced Exercise Capacity, Chronotropic incompetence, and early systemic inflammation in cardiopulmonary phenotype long coronavirus disease 2019. J Infect Dis. 2023; 228(5): 542–554. doi: 10.1093/infdis/jiad131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apple AC, Oddi A, Peluso MJ, et al. Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. Ann Clin Transl Neurol. 2022;9(2):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available by application to the COVID Citizen Science leadership committee. Data may be requested by emailing covid19@eurekaplatform.org.


