Abstract
Background
Allogeneic haematopoietic stem-cell transplant is an option, potentially curative, for high-risk acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS) patients. Post-transplant cyclophosphamide administration allows for the selection of haploidentical donors in patients who are eligible for the procedure but do not have a fully matched donor since it can overcome the HLA barrier. There is still an active debate on whether intensifying the conditioning regimen is necessary with haploidentical donors when peripheral blood stem cells are used as the graft source. Herein, we report our decennial experience of haploidentical stem-cell transplant using peripheral blood stem cells (haplo-PBSC) at King’s College Hospital.
Objectives
The primary objective was to evaluate overall survival (OS) following haplo-PBSC. Secondary objectives were total OS for patients with less than two previous lines of therapy, OS according to cytomegalovirus (CMV) reactivation, incidence of transplant-related mortality (TRM), graft-versus-host disease (GVHD) and GVHD-relapse-free survival (GRFS).
Results
One-year and three-year total OS were 62% and 43%, respectively, with a median OS of 22 months. One-year and three-year OS for patients with ≤2 and those with >2 previous lines of therapy were 72% and 55%, and 60% and 22%, respectively (p-value=0.04). The median OS in patients with >2 previous and ≤2 lines of therapy was 16 and 49 months, respectively. Cumulative incidence (CI) of relapse was 25% with a median time to relapse of 5 months (range 1 – 38 months).
Conclusions
Haploidentical haematopoietic stem-cell transplant is potentially curative in chemosensitive AML and MDS and offers a high rate of prolonged remission. Our cohort further confirms the role of consolidative haploidentical transplant in patients in complete remission and highlights that patients with heavily pre-treated disease may not benefit from this strategy.
Keywords: Haplo-identical stem cell transplantation, AML, MDS
Introduction
Curative strategy for high-risk acute myeloid leukaemia (AML) and myelodysplastic syndromes (MDS) still relies on the graft-versus-leukaemia (GVL) effect following allogeneic haematopoietic stem cell transplant (allo-HSCT).1,2
Despite the improvement in the HLA tissue typing techniques and the evolution of mismatched unrelated donor transplants, a non-negligible proportion of allo-HSCT eligible patients lack a fully matched donor, and the guidelines still need to be provided for optimal donor selection for these patients.3
The administration of post-transplant cyclophosphamide (PTCY) after the infusion of bone marrow cells from haploidentical sibling donors4 has become a viable option to remove the HLA barrier, and nowadays, mismatched related donors can be selected in the absence of a fully matched donor. As with other donor types, the main reasons for transplant failure remain disease relapse and transplant-related mortality (TRM).
Over the past two decades, several strategies have been implemented in an attempt to improve the anti-leukaemia effect of haplo-HSCT, including the use of peripheral blood stem cells (PBSC) instead of bone marrow (BM),5–7 intensification of the conditioning regimen8,9 or a combination of both.
A single-centre retrospective study reported a higher rate of both grade III/IV acute graft-versus-host disease (GVHD) and steroid-refractory GVHD (SR-GVHD) with haploidentical PBSC, without any difference in the relapse rate compared to those that received BM.10
A multicentric study suggested lower relapse rates with the use of PBSC compared to BM as a source of the graft,7 and despite initial concerns regarding an increased risk of GVHD with haplo-PBSC, different groups demonstrated similar outcomes compared to BM.6,11
Data from a large retrospective haplo-HSCT study suggests that the degree of HLA mismatch is the best predictor of success in this setting, regardless of the type of conditioning (myeloablative conditioning (MAC) or reduced intensity conditioning (RIC)).12,13
Recently, a large retrospective study showed that haplo-HSCT and matched sibling donors (MSD) have similar 1-year and 3-year overall survival (OS) rates.14 The administration of PTCY following haplo-HSCT significantly reduced the incidence of chronic GVHD compared to MSD (26% versus 56%). Despite the decreased incidence of chronic GVHD within the haplo-HSCT cohort, there was no survival advantage due to late-onset lethal infections and the occurrence of secondary malignancies.14
Also, an EBMT study performed in acute leukaemia patients undergoing transplant in first complete remission reported no differences in OS and leukaemia-free survival between haplo-BM and PBSC from matched unrelated donors. Interestingly, this study showed a significantly reduced incidence of chronic GVHD and extensive GVHD in patients who underwent haplo-BM.15 In 2017, a CIBMTR study enriched for RIC cases found that although OS was not different, peripheral blood compared to BM had aGVHD and cGVHD risk greater and relapse risk less.16
Herein, we report the outcome of AML and MDS patients transplanted with haploidentical PBSC with a uniform GVHD prophylaxis regimen with PTCY, tacrolimus and mycophenolate mofetil.
Patients and Methods
Between August 2010 and August 2021, 72 haploidentical hematopoietic stem-cell transplants were performed at King’s College Hospital in London, United Kingdom.
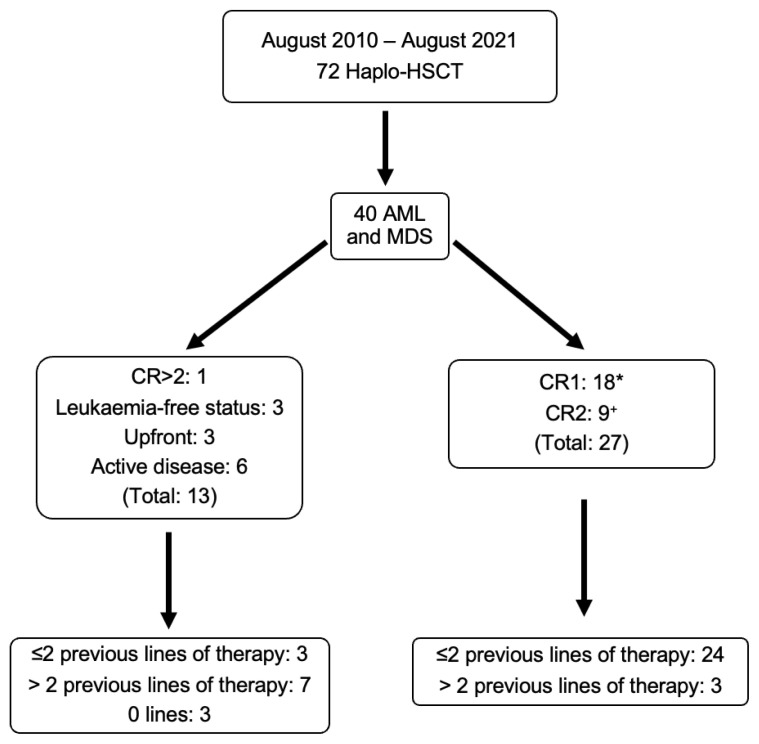
Forty cases of AML and MDS were analysed in this single-centre retrospective cohort study (Figure 1). AML and MDS were diagnosed according to WHO criteria and stratified according to the disease risk index as previously described.17,18
Figure 1.
Haplo-HSCT population at King’s College Hospital in 10 years. *Two cases of primary induction failure achieved first complete remission (CR1) after two lines of therapies; one patient achieved CR1 after three lines of therapy. +Two cases needed three lines of therapy to achieve second complete remission (CR2).
Neutrophil engraftment was defined as a neutrophil count of ≥1,000/μL for two consecutive days without GCSF support. Platelet engraftment was defined as platelets count ≥20,000/μL for two consecutive days without platelets transfusion in the two previous days.
Chimerism analysis of peripheral blood and bone marrow was performed on days +28, +56, +100, +180 and +365 with short tandem repeat (STR) testing by polymerase chain reaction (PCR) followed by fragment-length analysis as previously described.19
All patients signed consent forms approved by the institutional review board.
HLA tissue typing and matching
Donors and recipients were typed using Third Generation Sequencing (TGS) and Next Generation Sequencing (NGS) techniques for HLA-A, -B, -C, -DRB1, -DQ with high resolution. Donors were considered haploidentical if they shared a haplotype with the patient.
Transplant procedure
HSCT was performed with GCSF mobilised PBSC. Nonmyeloablative (NMA) regimen consisted of cyclophosphamide 14.5 mg/kg on days −6 and −5, fludarabine 30 mg/m2/day from day −6 to day −2, and low-dose total body irradiation (2 Gy) on day −1 (FCTBI). Myeloablative MAC regimen included Thiotepa 5 mg/Kg on days −6 and −5, Busulfan 3.2 mg/Kg on days −4, −3, −2 and fludarabine 50 mg/m2 from day −4 till day −2.
GVHD prophylaxis consisted of post-transplant cyclophosphamide (50 mg/kg) on days +3 and +4. On day +5, tacrolimus and mycophenolate mofetil (MMF) were started. Tacrolimus (at a total dose of 1 mg) was administered as a once-a-day infusion and switched to oral tablets at discharge. The doses were adjusted to obtain serum levels between 10 and 15 ng/ml. MMF was administered at 15 mg/kg p.o. three times per day until day +35. G-CSF was started on day +5 in all patients.
NMA conditioning was the standard of care for patients undergoing haplo-HSCT. MAC was offered exceptionally only to patients aged <55 years and with an HCT-CI score of <3 or with either active disease or leukaemia-free status.
Supportive Care
Anti-microbial prophylaxis was initiated at the time of conditioning. It consisted of Acyclovir 400 mg twice daily, Ciprofloxacin 500 mg once daily, and Posaconazole 300 mg once daily until neutrophil engraftment. At the resolution of neutropenia, patients started fluconazole prophylaxis until tacrolimus administration was discontinued. Penicillin V 500 mg BD was started at neutrophil engraftment and continued for life. Letermovir for cytomegalovirus (CMV) prophylaxis was offered in November 2019. Biweekly CMV, adenovirus and Epstein-Bar virus (EBV) monitoring by quantitative PCR was performed from the start of conditioning until day +100 and weekly until day +180. Patients received red blood cell and platelet transfusions according to standard operative procedures at our institution.
Diagnosis and Treatment of Graft-Versus-Host Disease
Clinical diagnosis of acute and chronic GVHD (aGVHD and cGVHD, respectively) was made based on standard criteria20. When possible, confirmation by histologic analysis of skin and/or gastrointestinal biopsy specimens was performed. First-line and second-line therapy for GVHD were provided according to institutional protocols.
Statistical Analysis
Continuous variables were described as median and range, and categorical variables as frequencies.
Overall survival (OS) was estimated using the Kaplan-Meier curves and defined as the time from starting HSCT to death from any cause or the last follow-up for living patients.
Progression-free survival (PFS) was estimated with Kaplan-Meier curves and was defined as the time from HSCT to relapse or death (whichever came first) or last follow-up.
GVHD-Relapse free survival (GRFS) was defined as the time from HSCT to either grade 3/4 acute GVHD or moderate to severe chronic GVHD or relapse or death from any cause.
Cumulative incidence (CI) analysis was performed for transplant-related mortality (TRM), relapse and GVHD incidence (either acute or chronic). TRM was defined as death due to any cause other than progression of the underlying malignancy, with death due to relapse as a competing event. Relapse was defined as recurrence of the underlying hematologic malignancy, and death due to any other cause (TRM) was a competing event for this analysis.
For cumulative incidence analysis of GVHD, death without aGVHD in the first 100 days was considered a competing event for the aGVHD, whereas relapse or death in the absence of cGVHD was considered a competing event for cGVHD.
Chi-square statistics was used to compare categorical variables, and the Mann-Whitney test was used for continuous variables.
The primary objective was to evaluate OS following transplantation with haploidentical PBSC.
Secondary objectives were OS for patients with less than two previous lines of therapy, OS according to CMV reactivation, and the incidence of TRM, GVHD and GRFS. All analyses were done with SPSS software Version 29.0.1.0, and two-tailed p-values ≤ 0.05 were considered significant.
Results
Patient characteristics
Twenty/eight AML patients (13 females and 15 males) and twelve MDS patients (12 males) were transplanted with haploidentical sibling donors. Patient baseline characteristics are summarised in Table 1. 90% of patients received NMA conditioning, and the remaining 10% received MAC.
Table 1.
Baseline characteristics.
| Characteristic | Number of patients (%) |
|---|---|
|
| |
| Total | 40 (100) |
| Male | 28 (70) |
| Female | 12 (30) |
|
| |
| Median age (range) | 51 (21–69) |
| Diagnosis | |
| AML | 28 (70) |
| MDS | 12 (30) |
|
| |
| Disease risk index | |
| Low | 1 (3) |
| Intermediate | 10 (25) |
| High | 25 (62) |
| Very high | 4 (10) |
|
| |
| Donor relationship | |
| Brother/Sister | 18 (45) |
| Son/Daughter | 19 (47) |
| Parents | 3 (8) |
|
| |
| Disease status | |
| CR1 | 18 (45) |
| CR2 | 9 (23) |
| CR>2 | 1 (3) |
| Active disease* | 6 (15) |
| Leukaemia free status* | 3 (7) |
| Upfront transplant+ | 3 (7) |
|
| |
| HCT-CI | |
| <3 | 21(53) |
| ≥3 | 19 (48) |
|
| |
| Patients undergoing transplant after 1 line of therapy | 14 (35) |
|
| |
| Median months from diagnosis to transplant (range) | 14 (2–66) |
|
| |
| Lines of previous chemotherapy | |
| 0 | 3 (7) |
| ≤2 | 27 (68) |
| >2 | 10 (25) |
|
| |
| Female donor male recipient | 12 (30) |
|
| |
| Procedure to rescue a graft failure from a previous transplant procedure | 2 (5) |
|
| |
| CMV serology (donor/recipient) | |
| +/+ | 21 (53) |
| +/− | 2 (5) |
| −/+ | 7 (18) |
| −/− | 8 (20) |
AML= acute myeloid leukaemia; MDS= myelodysplastic syndrome; CR=complete remission. +Upfront transplant was offered to few MDS patients as per institutional standard operative procedure in MDS with >5% of blasts but <10%.
It applies only to the AML patients.
The median age of the cohort was 51 (range 21 – 69); 19 patients were 55 or older.
Eighteen patients (45%) underwent allo-HSCT in first complete remission (CR1), nine (23%) when a second complete remission (CR2) was achieved, one patient (3%) was transplanted in CR>2, six AML patients (15%) had active disease and three AML patients were in morphologic leukaemia free state2 (7%). Three (7%) MDS patients underwent upfront allo-HSCT.
88% of the patients in CR1 and CR2 had ≤2 previous lines of therapy, and 12% had >2 lines of therapy. 53% of the remaining patients had >2 lines of therapy. All but one patient with active disease had >2 lines of therapy. Median follow-up time was 23 months (range 1 – 144 months).
Graft characteristics, engraftment and chimerism
Unmanipulated peripheral blood haematopoietic stem cells with a median of 5.9x106 CD34+cells/Kg (3.37 – 14.6) were infused. Median time to neutrophils ≥ 1000/μL was 18 days (13–42), and 25 days (14–44) to platelets ≥ 20.000/μL. Two cases of primary graft failure occurred. Median unfractionated, CD3+ and CD15+ chimerism at 365 days after transplant were 100%, 100% and 100%, respectively.
Overall survival, progression-free survival, and relapse rate
One-year and three-year total OS were 62% and 43%, respectively, with a median OS of 22 months (Figure 2; range 1–142).
Figure 2.
Overall survival for AML and MDS at haplo-HSCT at King’s College Hospital between August 2010 and August 2021.
One-year and three-year OS for patients with ≤2 and those with >2 previous lines of therapy were 72% and 55%, and 60% and 22%, respectively (p-value=0.04 – Log Rank, Figure 3). The median OS in patients with >2 previous and ≤2 lines of therapy were 16 and 49 months, respectively.
Figure 3.
OS for patients with ≤2 previous line of therapy (red line) versus OS for patients with >2 previous line of therapy (blue line).
Twenty-eight patients (70%) were CMV IgG positive. One-year and three-year OS for patients with and without CMV reactivation were 65% and, 58%, and 40% and 51%, respectively (p-value not significant).
One-year and three-year total PFS was 57% and 46%, respectively.
One-year and three-year PFS for patients with ≤2 and those with >2 previous lines of therapy were 62% and 40%, and 50% and 30%, respectively (p-value not significant).
Cumulative incidence (CI) of relapse was 25% with a median time to relapse of 5 months (range 1 – 38 months).
One-year and three-year CI of relapse for patients with ≤2 and in patients with >2 previous lines of therapy were 13% and 49%, and 17% and 49%, respectively (p-value 0.05)
Graft versus host disease
Acute GVHD (aGVHD) grade I–II occurred in 50% of the patients, with no grade III/IV observed. The median time from transplant to aGVHD onset was 55 days (range 28 – 210).
Mild chronic GVHD (cGVHD) occurred in 3% of the patients. The incidence of moderate and severe chronic GVHD was 5% and 10% of all patients, respectively (Figure 4). The median time to chronic GVHD was 280 days (range 111 – 552).
Figure 4.
Cumulative incidence of chronic GVHD.
Transplant related mortality
One-year and three-year TRM was 21% and 35%, respectively. The median time to TRM was 4 months (range 1 – 49). The most common cause of death was infection (50% of total TRM), followed by GVHD (30%), post-transplant lymphoproliferative disease (PTLD) (6%), unknown cause (6%), graft failure (8%).
Patients who underwent allograft with either active disease or >2 previous lines of therapy had a global TRM of 90% (the two remaining patients in this cohort succumbed to relapsed disease). In contrast, patients who had achieved CR or have had ≤2 previous lines of therapy had a global TRM of 40%.
GVHD-relapse free survival
The one-year GRFS rate was 51%, and the three-year GRFS was 41%, with a median total GRFS of 13 months (range 1 – 142). One-year and three-year GRFS in patients with ≤2 and >2 previous lines of therapy were 39% and 33%, and 22% and 27%, respectively.
Viral reactivation
CMV reactivation occurred in 50% of patients at a median time of 46 days post-transplant (range 13–415 days) without any evidence of organ damage. Notably, CMV prophylaxis with letermovir became available only in November 2019; therefore, only 7% of patients received the treatment. EBV reactivation was detected in 67% of the patients at a median time of 46 days post-transplant (range 9 – 1218). Only one patient developed monomorphic PTLD that required multiple lines of therapy and had a dismal outcome. Adenovirus reactivation was recorded in 10% of patients at a median time of 45 days post-transplant (range 21 – 126); no cases of adenovirus disease were diagnosed.
Discussion
Haplo-HSCT is nowadays considered an established transplant option for AML and MDS patients lacking a fully matched donor.21,22
To minimise the risk of relapse in AML and MDS patients, both intensification of the conditioning regimen and/or substitution of BM with PBSC as source of the graft have been implemented. While intensification of the conditioning regimen led to ambiguous results,4,8,9,23 the utilisation of PBSC has led to decreased relapse rate6,7 with a resulting increased risk of grade II/IV acute GVHD seen only after the administration of MAC.5 However, studies focused on the source of stem cells showed contrasting results. A retrospective EBMT study in patients with relapsed/refractory or active AML at the time of haplo-HSCT showed a better leukaemia-free survival with BM rather than with PBSC in patients ≥55 years of age.24 This was mainly a consequence of a higher TRM within the PBSC group. Our study showed a similar finding: 50% of patients were 55 or older at the time of transplant and had only PBSC as a source of stem cells. The use of haplo PBSC within an older population could have contributed to a higher TRM, especially within the group of patients undergoing transplantation with >2 lines of previous therapy.
Despite our cohort being heavily pre-treated, the current study showed a 25% 2-year relapse rate, which is quite similar to one reported by Mehta et al. for patients receiving haplo-PBSC.10 Notably, the cumulative incidence of relapse is inferior compared to a large retrospective EBMT study.24 The retrospective nature of the current report is an important bias; however, it is worth mentioning that it is difficult to compare with one or more studies due to many differences on one or more points (conditioning, stem cell source, haematological diseases, etc). A possible explanation of a relatively low relapse rate could be a higher CD3-positive T-cells and NK-cells in the graft, allowing a stronger anti-leukaemia effect of haplo-PBSC.
In our decennial experience of haploidentical transplantation, the 2-year OS for AML and MDS patients was 46%. In patients with ≤2 previous lines of therapy, the 2-year OS was 60%, similar to the estimated 2-year OS of other groups.9,25
Additionally, in our study, the incidence of grade I/II acute GVHD was 50%, with no cases of grade III/IV, further confirming the safety of haplo-PBSC after NMA conditioning.
In terms of chronic GVHD, the administration of PTCY in combination with MMF and tacrolimus was effective in minimising the risk of moderate to severe cases. This result highlights the protective effect of PTCY even in the context of haplo-PBSC.
Interestingly, all cases of severe GVHD within our cohort received a graft with a CD34+ dose >5x106/Kg, and this result suggests that defining a maximum haplo-CD34+ cell dose might be of benefit.
Our cohort also showed a grim outcome in patients undergoing haplo-HSCT with active disease or with >2 lines of therapy due to increased TRM and relapse rates. This suggests that haplo-HSCT should be offered at an early stage to patients for whom a fully matched donor cannot be identified to avoid increased chances of disease relapse. Our results are a further confirmation that advanced disease at the time of transplant is a strong negative prognostic factor for both TRM and early relapse.
This study suggests the feasibility and safety of haplo-PBSC in MDS and AML patients and highlights the importance of PTCY administration to ensure an acceptable risk of GVHD. Nevertheless, our results further contribute to the debate about the optimal graft source within the haplo-HSCT platform.
This is the first study examining the role of the number of previous lines of therapy on transplant outcomes. This single-centre experience in a limited number of heavily pre-treated patients suggests that the GVL effect may not suffice to guarantee durable remission, and this cohort of patients may require maintenance therapy to minimise the risk of relapse. Additionally, the elevated TRM in patients with >2 lines of therapy suggests they may benefit from more frequent surveillance to minimise the risk of lethal infectious events.
Conclusions
Haplo-PBSC after NMA conditioning is potentially curative in AML and MDS patients with chemo-sensitive disease and seems to offer a high rate of prolonged remission with relatively low rates of severe GVHD and relapse.
Footnotes
Competing interests: The authors declare no conflict of Interest.
Author contribution.: DA and FS collected the data. DA performed statistical analysis. DA, LBS, FS, and SB wrote the manuscript and were involved in patient care. VP supervised the project, was involved in patient care, and wrote the manuscript. The other authors were involved in patient care and reviewed the manuscript. All the authors approved the final version.
References
- 1.Sweeney C, Vyas P. The Graft-Versus- Leukemia Effect in AML Front Oncol. 2019;9:1217. doi: 10.3389/fonc.2019.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Döhner H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 140(2022):1345–1377. doi: 10.1182/blood.2022016867. [DOI] [PubMed] [Google Scholar]
- 3.Nagler A, Mohty M. In 2022, which is preferred: haploidentical or cord transplant? Hematology. 2022;64–73(2022) doi: 10.1182/hematology.2022000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luznik L, et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggeri A, et al. Bone marrow versus mobilised peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer. 2018;124:1428–1437. doi: 10.1002/cncr.31228. [DOI] [PubMed] [Google Scholar]
- 6.Castagna L, et al. Bone Marrow Compared with Peripheral Blood Stem Cells for Haploidentical Transplantation with a Nonmyeloablative Conditioning Regimen and Post-transplantation. Cyclophosphamide. 2014. [DOI] [PubMed]
- 7.O’Donnell PV, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2002;8:377–86. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 8.Raiola AM, et al. Unmanipulated Haploidentical Bone Marrow Transplantation and Posttransplantation Cyclophosphamide for Hematologic Malignancies after Myeloablative Conditioning. Biol Blood Marrow Transplant. 2013;19:117–122. doi: 10.1016/j.bbmt.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SR, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2012;18:1859–1866. doi: 10.1016/j.bbmt.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RS, et al. Bone Marrow versus Peripheral Blood Grafts for Haploidentical Hematopoietic Cell Transplantation with Post-Transplantation Cyclophosphamide. Transplant Cell Ther Off Publ Am Soc Transplant Cell Ther. 2021;27:1003e1–1003.e13. doi: 10.1016/j.jtct.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sammassimo S. A Cellular Therapy with Haploidentical Peripheral Hematopoietic STEM CELL Transplantation MAY be a Therapeutic Option in Patients with Relapsed Lymphoma with Chemorefractory Disease. 2018. [DOI]
- 12.Ciurea SO, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2012;18:1835–1844. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piemontese S, et al. A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1069–1075. doi: 10.1038/leu.2014.336. [DOI] [PubMed] [Google Scholar]
- 14.Rashidi A, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019;3:1826–1836. doi: 10.1182/bloodadvances.2019000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagler A, et al. Comparison of Haploidentical Bone Marrow versus Matched Unrelated Donor Peripheral Blood Stem Cell Transplantation with Posttransplant Cyclophosphamide in Patients with Acute Leukemia. Clin Cancer Res. 27(2021):843–851. doi: 10.1158/1078-0432.CCR-20-2809. [DOI] [PubMed] [Google Scholar]
- 16.Bashey A, et al. Mobilised Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol. 2017;35:3002–3009. doi: 10.1200/JCO.2017.72.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury JD, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703–1719. doi: 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armand P, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blouin AG, Ye F, Williams J, Askar MA, Practical Guide To. Chimerism Analysis: Review of The Literature and Testing Practices Worldwide. Hum Immunol. 82(2021):838–849. doi: 10.1016/j.humimm.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toubai T, Sun Y, Reddy P. GVHD pathophysiology: is acute different from chronic? Best Pract Res Clin Haematol. 2008;21:101–117. doi: 10.1016/j.beha.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Appelbaum FR. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia when a matched related donor is not available. Hematol Am Soc Hematol Educ Program. 2008:412–417. doi: 10.1182/asheducation-2008.1.412. doi: 10.1182/asheducation-2008.1.412. [DOI] [PubMed] [Google Scholar]
- 22.McCurdy SR, Fuchs EJ. Selecting the best haploidentical donor. Semin Hematol. 2016;53:246–251. doi: 10.1053/j.seminhematol.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Devillier R, et al. Reduced intensity versus non-myeloablative conditioning regimen for haploidentical transplantation and posttransplantation cyclophosphamide in complete remission acute myeloid leukemia: a study from the ALWP of the EBMT. Bone Marrow Transplant. 57(2022):1421–1427. doi: 10.1038/s41409-022-01674-x. [DOI] [PubMed] [Google Scholar]
- 24.Baron F, et al. Human leukocyte antigen-haploidentical transplantation for relapsed/refractory acute myeloid leukemia: Better leukemia-free survival with bone marrow than with peripheral blood stem cells in patients ≥55 years of age. Am J Hematol. 97(2022):1065–1074. doi: 10.1002/ajh.26627. [DOI] [PubMed] [Google Scholar]
- 25.Raiola AM, et al. Impact of HLA Disparity in Haploidentical Bone Marrow Transplantation Followed by High-Dose Cyclophosphamide. Biol Blood Marrow Transplant. 2017 doi: 10.1016/j.bbmt.2017.10.002. doi: 10.1016/j.bbmt.2017.10.002. [DOI] [PubMed] [Google Scholar]