Abstract
Rhizosphere microbes play critical roles for plant’s growth and health. Among them, the beneficial rhizobacteria have the potential to be developed as the biofertilizer or bioinoculants for sustaining the agricultural development. The efficient rhizosphere colonization of these rhizobacteria is a prerequisite for exerting their plant beneficial functions, but the colonizing process and underlying mechanisms have not been thoroughly reviewed, especially for the nonsymbiotic beneficial rhizobacteria. This review systematically analyzed the root colonizing process of the nonsymbiotic rhizobacteria and compared it with that of the symbiotic and pathogenic bacteria. This review also highlighted the approaches to improve the root colonization efficiency and proposed to study the rhizobacterial colonization from a holistic perspective of the rhizosphere microbiome under more natural conditions.
Keywords: rhizosphere, bacteria, root colonization, plant-microbe interactions, root exudates
We summarized the root colonizing process of the nonsymbiotic beneficial rhizobacteria, compared that with pathogenic and symbiotic rhizobacteria, and reviewed the approaches to improve the colonization efficiency.
Introduction
The significance of plant- and animal-associated microbiomes to their hosts has been well recognized for decades (Mendes et al. 2013). Microbes inhabiting the rhizosphere are critical determinants of plant growth and health. Beneficial rhizobacteria show great potential in agricultural production since they offer a variety of beneficial functions for plants, such as promoting plant growth and enhancing plant abiotic stress tolerance by secreting phytohormones and some specific signaling molecules and protecting host plants by inducing systemic resistance and direct antagonism with soil-borne pathogens (Pieterse et al. 2014). These beneficial bacteria can generally be used in agriculture as biofertilizers or microbial agents and are essential in green agricultural production. Rhizosphere colonization is one of the most important features of rhizobacteria that determines their survival and propagation, which are prerequisites for versatile bacteria to exert their beneficial functions on host plants (Mendes et al. 2013).
The rhizosphere includes plant roots and the surrounding soil influenced by root exudates (Dessaux et al. 2016), therefore, bacteria surviving and forming firmly community in rhizosphere soil, on rhizoplane and in root endosphere were all defined as the term “rhizosphere colonization” (Fig. 1). They can selectively colonize distinctively on primary root or lateral root, on spatial axis of the root, inside root, or root surface. Rhizobacteria colonize the plant root in a highly heterogeneous manner, covering 10%–40% of the root surface (Danhorn and Fuqua 2007), and some endophytic bacteria can also live inside root tissue. Since the colonization process of symbiotic bacteria, which reside in living plant cells or is surrounded by a membrane compartment (Reinhold-Hurek and Hurek 2011), has been thoroughly reviewed (Roy et al. 2020, Soyano et al. 2021, Yang et al. 2022, González-Guerrero et al. 2023, Jain et al. 2023, Rahmat et al. 2023, Xu and Wang 2023), this review only focuses on the root colonization of nonsymbiotic beneficial rhizobacteria.
Figure 1.
Rhizosphere colonization process of nonsymbiotic bacteria. Rhizosphere consists of the roots and the surrounding soil, and the rhizosphere colonization includes rhizosphere soil-, rhizoplane- and endophytic colonization. According to different bacterial species, the colonization process can be divided into several steps, including chemotaxis and motility, root surface attachment, growth and rhizoplane biofilm formation, and endophytic penetration. Chemotaxis and motility determine the moving toward rhizosphere, the initial site selection, and migration of colonization site. Attachment to the root surface is followed, during which the bacteria must overcome plant immunity. Bacterial growth using root exudates as the carbon resources and competing scarce elements in rhizosphere is necessary for biofilm formation, which is required by most rhizosphere soil and rhizoplane colonizing bacteria. Endophytic bacterial species penetrate intercellular spaces within root tissue through unique mechanisms after root attachment or biofilm formation.
Plants are the major players in the rhizosphere and they affect bacterial colonization. Plants secrete 11%–40% of photosynthesis products into the rhizosphere as root exudates (Zhalnina et al. 2018, Du et al. 2021), which cause the rhizosphere to be a highly active site for microbial colonization than bulk soil. Undoubtedly, the colonization of beneficial rhizobacteria is largely impacted by the abundance and composition of root exudates. Root exudates can be divided into the low molecular weight and high molecular weight compounds. Low molecular weight compounds include sugars, organic acids, amino acids, alcohols, volatile compounds, and some secondary metabolites. The high molecular weight compounds are less diverse but yield a higher mass % of root exudates, and those compounds are mostly polysaccharides and proteins (Chagas et al. 2018). Although the rhizosphere is rich in carbon resources for bacterial growth, it is generally accepted that plants are able to expel unfavorable bacteria through the plant immune system, which is also a crucial factor that determines bacterial colonization in the rhizosphere (Shu et al. 2023). The concept of plant immunity has been well-established in interactions with pathogens and symbiotic microbes. Recently, the importance of plant immunity in modulating nonsymbiotic rhizobacteria colonization has been fully recognized (Shu et al. 2023). Additionally, a “cry-for-help” theory proposed that a stressed plant can recruit beneficial bacteria to colonize the rhizosphere (Lebeis et al. 2015, Rolfe et al. 2019). All these factors influence the rhizosphere colonization of the nonsymbiotic beneficial bacteria.
The biology of root colonization by rhizobacteria has advanced in recent years. Rhizosphere colonization is a complex process involving several steps that depend on bacterial lifestyles. They can colonize in rhizosphere soil, on rhizoplane, or endophytically based on some of these steps (Fig. 1). In general, rhizobacteria colonize the root in a sequential process that begins with rhizosphere chemotaxis, root attachment, sometimes followed by rhizoplane biofilm formation or endophytic colonization for some strains. Bacterial chemotactic motility involves a conserved intracellular signal transduction pathway and varied signal sensors and drives the selection of initial sites for attachment and colonization site migration, which vary depending on the strain and plant species (Sampedro et al. 2015, Li et al. 2022). After moving to the rhizosphere, some bacterial strains need to stop moving and adhere to the root surface, which is defined as root attachment (Knights et al. 2021). During this period, bacteria must exert their role to overcome the plant immune response for further colonization. Rapid proliferation using root exudates as the main carbon resources is one of most important process for colonization. Some of the rhizobacteria formed biofilm on the rhizoplane in a multispecies manner (Beauregard et al. 2013). During this period, bacteria have to compete for some scarce elements in the rhizosphere to support proliferation and biofilm formation (Liu et al. 2023). Additionally, some endophytes begin penetrating into plant tissue during life on the root surface (Dudeja et al. 2021, Mushtaq et al. 2023). In general, these processes involve complicated lifestyle transformation and intracellular signal transduction that are influenced by plants and the environment. However, the current understanding of bacterial colonization in the rhizosphere is scattered, especially for beneficial nonsymbiotic rhizobacteria.
In this review, we will summarize the knowledge on the rhizosphere colonization of nonsymbiotic beneficial bacteria along with the sequential process and conclude the underlying regulatory molecular mechanism, the important bacterial genes involved in the processes, and the influencing factors. We will also review the advances in “cry-for-help” theory. The difference in colonization processes and the plant‒microbe interactions that determine colonization between nonsymbiotic bacteria will be compared with that of symbiotic/pathogenic bacteria. Finally, we propose several artificial strategies to enhance the colonization of beneficial rhizobacteria, which would benefit the application of beneficial rhizobacteria in agriculture. The scope of this review is comprehensively summarizing the rhizosphere colonization processes of the nonsymbiotic bacteria to promote the application of beneficial rhizobacteria in agriculture.
Chemotaxis and motility
Chemotaxis is a motility-based ability of microbes to sense chemical gradients and direct their movement either up the gradient toward the source (attraction) or down the gradient away from the source (repulsion). Motility and chemotaxis of vegetative bacterial cells are essential for rhizosphere colonization, as well as for establishing primary bacteria–root interactions (Feng et al. 2021a). Root exudates activate chemosensory pathways and cause motile bacteria to move toward the root. Rhizobacterial motility can be achieved by various mechanisms, including flagellar swimming, swarming, twitching, and gliding motility (Kearns 2010). Bacterial swimming is achieved by rotating flagella to generate a force that moves the cell forward (Sampedro et al. 2015). Swarming is a multicellular movement over a solid surface that is driven by a raft-like flagellar complex from the community (Kearns 2010). Twitching is a motility based on the extension–tethering–retraction–extension of type IV pili (Sampedro et al. 2015). Gliding motility is a definition of cells moving smoothly along their long axis in the absence of any visible organelle (Mignot 2007).
Chemotaxis and motility then drive the selection of the initial contact site on the root. The success of these processes determines the root colonization efficiency. It is evident that either inactivation of chemosensory activity by knocking out all the chemotaxis receptors or blocking motility by deleting the genes responsible for synthesizing flagellin in a rhizobacterium led to a 100-fold decrease in root colonization efficiency (Feng et al. 2018, Tzipilevich et al. 2021).
Chemotaxis process and signaling
Chemotaxis intracellular signaling is conserved in many bacterial species. Bacterial chemotaxis toward root exudates is initiated by the perception of chemoeffectors in root exudates by bacterial transmembrane chemotaxis receptors, which are specifically termed methyl-accepting chemotaxis proteins (MCPs) (Feng et al. 2021a). Generally, chemotaxis receptor proteins always exist in a ternary complex with the CheA histidine kinase and the coupling protein CheW. Chemotaxis receptors are transmembrane proteins that constitute a highly varied ligand-binding domain (LBD) in the extracellular space for signal sensing, an intracellular highly conserved methyl-accepting (MA) domain for adaptation, which is the standard criterion for the annotation of proteins as MCPs (Sampedro et al. 2015). The MCPs selectively recognize and bind to specific ligands, such as root exudates, resulting in molecular signals that transduce across the cellular membrane. This transduction subsequently modulates the autophosphorylation rate of the histidine kinase CheA in a CheW-dependent manner (Lacal et al. 2010). CheA and CheY constitute a two-component system. The phosphorylation of CheA affects the transphosphorylation of the CheY response regulator. Phosphorylated CheY binds to motor proteins that are responsible for driving various kinds of motility in different bacteria. In addition, the turnover of methylation and demethylation of the MA domain of the MCPs was deployed as an adaptation system, and methylation increased while demethylation decreased the autophosphorylation activity of CheA (Sampedro et al. 2015). This whole signaling pathway is extremely well-conserved in many bacteria, including Escherichia coli, Bacillus spp. and Pseudomonas spp.
The variety of MCPs with different LBDs determines the molecules to be sensed by the bacteria (Sanchis-López et al. 2021). In rhizobacteria, an expansive array of MCPs and their corresponding ligands have been identified, with notable examples found in species such as Pseudomonas putida, Bacillus velezensis, and Sinorhizobium meliloti. Allard-Massicotte et al. (2016) demonstrated that root colonization of Bacillus subtilis involves multiple chemotaxis receptors. An efficient colonizer in the rhizosphere should respond to a broad range of compounds in root exudates. For example, the colonization of P. putida KT2440 and B. velezensis SQR9 was regulated by various compounds in root exudates (Ortega et al. 2017, Feng et al. 2019). Notably, Pseudomonas spp. exhibit chemotactic responses to an impressive repertoire of over 140 compounds, thereby setting them as exemplary models for elucidating the structure‒function relationships between MCPs (Sampedro et al. 2015). A comprehensive analysis revealed that P. putida KT2440 harbors as many as 27 distinct MCPs (Corral-Lugo et al. 2016), each specific to detect a myriad of signaling molecules, including polyamines, amino acids, fatty acids, sugars, and many secondary metabolites. Bacillus velezensis SQR9 is endowed with eight unique MCPs, explicitly enumerated as McpA, McpB, McpC, McpR, TlpA, TlpB, YfmS, and HemAT (Liu et al. 2020b). However, the functions of homologous MCPs can be different between strains. For example, McpA in B. velezensis SQR9 orchestrates chemoattraction to a wide range of 20 ligands, including organic acids, sugars, and amino acids (Feng et al. 2019). Its homologs in B. subtilis NCIB 3610 are predominantly predisposed to sugar ligands, specifically glucose and α-methylglucoside (Allard-Massicotte et al. 2016). Through rigorous molecular investigations coupled with site-directed mutagenesis experiments, it has been elucidated that McpA in strain SQR9 boasts a broad ligand-sensing capacity arising from its capability to harness both the distal and proximal membrane regions of its LBD. (Feng et al. 2022). Root-secreted glucose can act as a chemoattractant to many beneficial rhizobacteria (Feng et al. 2019, Sánchez-Gil et al. 2023). Cucumber root-secreted d-galactose serves as a ligand of McpA in strain SQR9 to enhance chemotaxis (Liu et al. 2020b). Compounds that act as chemoeffectors in root exudates are mainly low molecular weight compounds, such as organic acids, amino acids, sugars, sugar alcohols, and flavonoids. Some of these compounds also act as repellents. Detailed MCPs and their sensed root exudate compounds have been summarized by Feng et al. (2021a).
In addition to acting as a chemoeffector attracting bacteria, a range of compounds in root exudates enhance the motility of rhizobacteria. Root-secreted sucrose activates the bacterial production of extracellular polymeric levan, which in turn regulates the flagellar synthesis of B. subtilis, and B. subtilis cannot effectively colonize roots of Arabidopsis mutants that are deficient in root sucrose secretion (Tian et al. 2021b). Interestingly, Bacillus-produced surfactin, an antibiotic essential for bacterial motility and thus rhizosphere colonization, is also promoted by other root exudates, such as polysaccharides (Debois et al. 2015, Hoff et al. 2021). Recent studies revealed that root-secreted inositol can act as a signaling molecule to stimulate swimming motility in Pseudomonas via inositol-induced repression of DksA, a transcriptional regulator involved in inhibiting swimming motility and thus chemotaxis to the rhizosphere (Vílchez et al. 2020, O’Banion et al. 2023, Sánchez-Gil et al. 2023). The Arabidopsis root-secreted flavonoids attract Aeromonas sp. H1 by upregulating transcripts of flagellum biogenesis and inhibiting fumarate reduction for smooth swims (He et al. 2022).
Notably, the diffusion range of root exudates is inherently limited, leading to reduced concentrations at greater distances from the root. In light of emerging theories on bacterial chemotaxis, there appears to be a sophisticated relay of chemotactic signals between distinct bacterial cells (Cremer et al. 2019, Insall et al. 2022). Although they have not identified the signaling molecules secreted by the bacteria yet (Cremer et al. 2019), it supports that bacterial self-generated chemotactic signals might be essential in facilitating movement to the rhizosphere. Besides by sensing self-produced signal, bacterial chemotaxis may also be achieved through microbe–microbe interactions (Tian et al. 2021a), sometimes even by attraction to the exudates of root-associated fungi (Jiang et al. 2021, Mesny et al. 2023). To encapsulate this dynamic, microbes near the roots will sense root-secreted chemotactic signals and secrete chemotactic cues from their locus. This results in the establishment of a secondary chemotactic signal gradient, effectively drawing in more bacterial cells and mediating bacterial advancement toward the roots.
Colonization site selection and migration
Bacterial chemotaxis and motility determine colonization site selection and migration. The colonization sites can differ between bacteria, even between phylogenetically close strains (Fan et al. 2012, Gao et al. 2013, Tovi et al. 2019, O’Neal et al. 2020). It can be expected that sites with high exudation are possible colonization hotspots for the whole community because the high concentration of root exudates would attract bacteria (Darrah 1991, Marschner et al. 2011). Root hairs promote plants to allocate more carbon to root exudates (Holz et al. 2018), but it is generally agreed that the exudation rates are high in the elongation zone just behind the root tips rather than in the mature root zones. The colonization site is temporally changed along the root axis or between different root branches during the colonizing life cycle (Trivedi et al. 2020). The long-term colonization site may be different from the initial contact site. For instance, Bacillus megaterium NCT-2 cells were mostly distributed in the epidermis of the root elongation zone of maize at 3 days postinoculation (dpi), while colonization was observed along the meristematic zone, elongation zone, and root hair region at 11 dpi (Chu et al. 2018).
First, bacterial chemotaxis and motility contribute decisively to the selection of the initial site for colonization. O’Neal et al. (2020) found that the Azospirillum brasilense mutant lacking the major chemoreceptors that are responsible for root exudate chemotaxis is impaired in preferentially accumulating on rhizoplane and inside tissue of maturation and elongation zones. The factors influencing bacterial chemotaxis and motility for selecting root colonization sites are complex, including the diversity and concentration of each component in root exudates at different sites and the immune response of different cell types and some locally secreted antimicrobial compounds (Fröschel et al. 2021, Verbon et al. 2023). For example, reactive oxygen species (ROS) produced by roots also act as repellents to drive bacterial repulsion from the wheat root tip for initial colonization (O’Neal et al. 2020). Second, in addition to having a decisive role in the initial contact site, bacterial chemotaxis and motility also drive colonization site migration after root surface attachment. Root cell development changes the root exudation site, and bacterial migration could follow the changed root exudation sites, which are dynamically moving, following the expansion of the root system (Zboralski and Filion 2020). The migration of bacterial colonization site on roots after initial colonization can also result from evasion of immune-activating sites. Spatiotemporal root immune responses during microbial colonization are an important factor that determines the bacterial colonization site (Tsai et al. 2023). Liu et al. (2018) suggested that the ΔmorA mutant of Pseudomonas is a poor rhizosphere colonizer due to its inability to move from the initial site of colonization after triggering plant immune responses, indicating that migration along the root may occur to evade plant defense after initial colonization.
Overall, there is no doubt that bacterial chemotaxis and motility determine the site preferences for colonization in different root zones. However, most of the current research measuring rhizosphere colonization is mainly based on overall quantitative measurements, while measurements of colonization in different root zones are lacking, which will lead to many objectively existing differences in colonization being ignored or some differences in colonization being misinterpreted. The key problem for this status is the difficulty in measuring bacterial colonization within distinct root zones. Most current studies regarding colonization sites are based on microscopic observations, including fluorescence-, GUS- and FISH-based methods (Cao et al. 2023b). These strategies can well-reflect in situ bacterial colonization, but they are not as accurate as traditional plate counting methods in quantification. Moreover, due to the limitation of displaying only partial root zones under the microscope, it can sometimes be influenced by subjective bias.
Root surface attachment and interaction with plant immunity
Root surface colonization begins immediately after chemotaxis toward root, with successful adhesion to the root being the critical step for rhizoplane and endophytic colonization. In brief, bacteria need to stop moving and bind to the root surface, in which a transformation of lifestyle processes controlled by complex signal transduction is involved. Comprehensive studies on representative rhizobacteria, including Pseudomonas, Bacillus, Bradyrhizobium, Azospirillum, Agrobacterium, and Salmonella, have unveiled the molecular intricacies of root attachment. It has been established that agriculturally important microbial species share a biphasic model for root attachment (Wheatley and Poole 2018, Knights et al. 2021). In most cases, this biphasic model involves two steps: initial attachment occurs when rhizobacteria are reversibly bound to a root surface, followed by secondary attachment that results in their irreversible attachment (Knights et al. 2021). The current knowledge on root attachment based on these two steps will be reviewed here. In addition, upon attachment to the root surface, plant immunity functions as an important factor influencing bacterial colonization, which will also be discussed for the strategies bacteria deployed to address plant immunity.
Root surface attachment process
Reversible initial attachment
In general, initial attachment is weak, reversible, and nonspecific, allowing single cells to attach to the root surface. Compared to later-stage secondary attachment, the initial attachment is relatively poorly characterized. Numerous physiochemical and electrostatic forces influence the initial interactions between the surface molecules of the root and bacterial cell envelope, including van der Waals forces, electrostatic forces, and hydrophobic interactions. To overcome these repulsive forces, rhizobacteria use adhesive pili (T-pili), flagella, the polar flagellum, and fimbriae to overcome the electrostatic repulsion that occurs between negatively charged cell envelopes and root surfaces (Berne et al. 2015, Knights et al. 2021). For instance, the flagella-deficient mutant of A. brasilense is unable to adhere to wheat or maize roots. Moreover, the polar flagella purified from A. brasilense bind to wheat roots directly (Rossi et al. 2016). In addition to this universal force of attachment, rhizobacteria can exhibit numerous species-specific mechanisms for attachment and colonization. The major membrane porin, outer membrane proteins, and polysaccharides are considered to play a role in root adhesion during the early stages of root establishment (Berne et al. 2015). An outer membrane porin F (OprF) from Pseudomonas shows adhesive properties toward the roots of cucumbers and tomatoes. It was found that OprF-deficient mutants of P. fluorescens are significantly less capable of loosely adhering to roots than wild-type plants, which indicates that OprF plays an important role in primary attachment (Alvarez Crespo and Valverde 2009). Although OprF in Pseudomonas appears to play a role in initial attachment, its molecular mechanism remains unclear.
Irreversible secondary attachment
In the following stages of initial bacterial attachment, only a small percentage of rhizobacteria switch to a stronger, more specific binding mode and generate extracellular fibrils that facilitate bacterial accumulation and aggregation, called secondary attachment (Wheatley and Poole 2018). A range of species-specific strategies are employed by rhizobacteria for secondary attachment. Pseudomonas spp. secrete a Ca2+-binding protein, LapA, via ATP-binding cassette transporters. This protein loosely associates with bacterial surfaces, facilitating interactions with the root surface (Hinsa et al. 2003). LapA of P. putida is also necessary for attachment to abiotic surfaces and to plant seeds (Espinosa-Urgel et al. 2000). Notably, P. fluorescens mutants lacking LapA exhibit diminished initial attachment to abiotic surfaces and compromised biofilm formation abilities. The O-antigenic chains of Pseudomonas spp. lipopolysaccharides have also been linked to root attachment in crops such as tomatoes and potatoes (Spiers and Rainey 2005). Zhao et al. (2016) demonstrated that collagen-like proteins of B. velezensis FZB42 are critical for root attachment. Recently, Huang et al. (2022) demonstrated that the wall teichoic acid, flagellar protein FliD, and YhaN (a putative ABC transporter) of B. velezensis SQR9 function as adhesins on both cucumber root surfaces and abiotic surfaces and are involved in rhizosphere colonization (Huang et al. 2022). Cyclic di-AMP, a common bacterial second messenger, influences the formation of biofilms and plant root attachments in B. subtilis (Townsley et al. 2018). These investigations underscore that root attachment mechanisms are pivotal for successful rhizosphere colonization by bacteria.
Interaction with plant immunity
Plant immunity is one of the barriers that rhizobacteria must overcome during attachment to the root surface. The first process depends on recognizing highly conserved microbe-associated molecular patterns (MAMPs), including flg22, chitin, peptidoglycan, and lipopolysaccharide, by pattern recognition receptors (PRRs) and activating pattern-triggered immunity (PTI), which forms a primary defense against microbial colonization. The second layer of plant immunity is referred to as effector-triggered immunity. Plants have evolved nucleotide binding and oligomerization domain-like receptors, which sense microbial effectors either directly or through effector-induced modifications of host structures (Wang et al. 2022b). H+/Ca2+ ion fluxes and bursts of ROS are two typical cellular responses occurring within minutes after immune signaling responses. Other responses include triggering downstream defense-related gene activation, defense hormone regulation, callose deposition, camalexin biosynthesis, and antimicrobial compound accumulation. This local immune response is always accompanied by growth inhibition as a result of the growth-defense trade-off (Liu et al. 2013). In addition to triggering the local immune response, beneficial rhizobacteria can also elicit the induction of systemic resistance (ISR) (Pieterse et al. 2014).
Evidence show that at least the PTI is engaged and influences root colonization by beneficial rhizobacteria (Yu et al. 2019b). A recent study demonstrated that the Arabidopsis root bacterial community is involved in PTI regulation, and a group of robust, taxonomically diverse PTI-inhibiting strains that are efficient root colonizers were identified (Teixeira et al. 2021). In addition to facilitating the colonization of PTI-regulating bacteria themselves, both individual strains and synthetic consortia that regulate PTI can increase the ability of other beneficial bacteria to colonize roots (Ma et al. 2021, Teixeira et al. 2021). This suggests that the interaction with plant immunity highly influences the root colonization of beneficial rhizobacteria.
Suppressing the root immune response
Increasing evidence demonstrates that beneficial rhizobacteria can avoid being detected by root receptors that elicit immune responses, which are negative for bacterial colonization and plant growth. One aspect is the variation in the MAMPs, which is evidenced by the variation in flg22, one of the well-studied MAMPs. Colaianni et al. (2021) showed that most of the flg22 peptide variants from beneficial bacteria failed to activate PRR FLS2 (64%) and did not significantly inhibit plant host growth (80%), suggesting no activation of an energy-costly immune response. This kind of flg22 peptide variant altered PTI signaling output by interfering with coreceptor enlistment and by another, unidentified mechanism that triggered the typical ROS response, resulting in modulation of plant immunity (Colaianni et al. 2021). This finding suggests that beneficial rhizobacteria may avoid eliciting the root immune response by deploying flagella with low immunogenic sequences to facilitate rhizosphere colonization. The advantages of a low-immune-response-eliciting flagellin also drive the evolution of bacterial flagellar sequences with a trade-off of motility (Parys et al. 2021). In addition, there are beneficial rhizobacteria that possess immunogenic MAMPs that are very similar to those of pathogens. They have, therefore, evolved the ability to evade PRR recognition by inhibiting the interaction of their MAMP with PRRs, including through modification of the MAMP epitope, inhibition of the biosynthesis of MAMP-containing molecules, or alteration of microbial cell wall compositions (Yu et al. 2019b). In contrast to the phytopathogen Pseudomonas syringae, which suppresses the root immune response by producing the low molecular weight phytotoxin COR, the beneficial rhizobacterium Pseudomonas suppresses the flg22-triggered immune response without producing COR (Millet et al. 2010). Instead, Yu et al. (2019a) demonstrated that Pseudomonas capeferrum WCS358 reduces the rhizosphere pH by producing gluconic acid and its derivative 2-keto gluconic acid, therefore inhibiting the flg22-binding activity of FLS2, which requires a neutral pH environment. The inhibition of FLS2 activity further suppresses the flg22-mediated oxidative burst and root immunity, thereby facilitating colonization (Yu et al. 2019a). Similarly, the beneficial B. subtilis FB17 can suppress flg22-induced early root immune responses in Arabidopsis by releasing an unidentified low molecular weight compound, which controls the JA signaling components JAR1, JIN1, and MYC2 (Lakshmanan et al. 2012). This suggests that beneficial rhizobacteria actively interfere with plant immune signaling by delivering immune-suppressive compounds. However, current knowledge on suppressing PTI is mainly aimed at flg22, and more efforts aimed at other MAMPs on a large scale should be made to reveal immune suppression by beneficial rhizobacteria during colonization.
Tolerance of root immune response
Once plant immunity is activated, some beneficial rhizobacteria can also utilize strategies to address the activated immune response. The root cell-type-specific transcriptome in response to a beneficial rhizobacterium Pseudomonas simiae WCS417 revealed a spatial difference in immune activation of root hairs, cortex and endodermal barrier during colonization of this strain, suggesting that a spatial selection of the colonization site would benefit immune response evasion (Verbon et al. 2023). A genome-wide screen in rhizosphere Pseudomonas identified two genes, morA and spuC, that are essential in rhizosphere colonization, and the authors speculated that these two genes may confer the bacterium an ability to disperse from the initial site of colonization after triggering plant immune responses (Liu et al. 2018). This case proposed a potential bacterial strategy that evades root immunity through spatial mitigation of the colonization site. In addition to spatial evasion, higher tolerance is another strategy to address the activated root immune response, such as the ROS burst. Recently, Song et al. (2021) demonstrated that ROS in roots regulate the levels of rhizosphere beneficial Pseudomonas. The auxin produced by the beneficial bacterium B. velezensis FZB42 is essential for root colonization by antagonizing ROS produced as part of the receptor EFR-triggered immune response (Tzipilevich et al. 2021). Moreover, ROS induce auxin synthesis in B. velezensis FZB42 (Tzipilevich et al. 2021). The beneficial rhizobacterium B. velezensis SQR9 possesses a specific two-component regulatory system (TCS), ResDE, to tolerate the ROS produced during the flg22-triggered root immune response, which promotes rhizosphere colonization of this strain (Zhang et al. 2021).
However, it is still unclear whether the suppression of PTI in roots by beneficial rhizobacteria increases the risk of root infection by soil-borne pathogens. From the results reported by Ma et al. (2021), it seems that suppression of root PTI by beneficial rhizobacteria renders plants more susceptible to opportunistic Pseudomonas pathogens. Moreover, beneficial rhizobacteria can stimulate ISR, but the plant immune system actively or passively overlooks colonization by beneficial rhizobacteria during interactions. Whether this resistance impacts the colonization of nonsymbiotic beneficial rhizobacteria and its relationship with local plant immunity is unclear.
Bacterial growth and biofilm formation
In the rhizosphere, bacterial growth using root exudates as carbon resources is an important factor influencing root colonization. In addition to carbon resources, some scarce elements, such as phosphorus and iron, are also factors limiting the colonization of bacteria. Many bacterial species have evolved fascinating strategies to compete for scarce elements. Moreover, biofilm formation is an important process for many rhizoplane-colonizing bacterial species, motile flagellated bacterial cells differentiate into matrix-producing cells, which stop agglutinating, begin and form extracellular matrix surrounding chains (Karygianni et al. 2020). The biofilm matrix binds cells and imparts many key features to the biofilm, and therefore rhizosphere colonization (Flemming et al. 2023). The biofilms in rhizosphere are generally formed by bacteria from multispecies, and the matrix provides a spatial structure and multiple levels of protection for the community within biofilm.
Bacterial growth using root exudates
Bacterial growth and nutrition are the most important factors influencing bacterial colonization in the rhizosphere (López et al. 2023), and root exudate compounds can serve as nutrients that support bacterial colonization. The ability to utilize nutrients in root exudates is critical for rhizobacteria to occupy rhizosphere niches. Sugars and organic acids constitute a large fraction of exudates and are the major carbon sources for rhizobacteria (Sasse et al. 2018, Korenblum et al. 2022); some root-sourced VOCs, such as terpenes, can also act as nutrient sources (Schulz-Bohm et al. 2018). Plant root exudate nutrients can selectively promote the colonization of specific bacteria (Wang et al. 2022a). For instance, Huang et al. (2019) discovered that the specialized triterpenes thalianin, thalianyl fatty acid esters, and arabidin in root exudates of Arabidopsis modulate the root microbiota by enhancing or inhibiting specific bacterial growth. Rhizobacteria that can selectively metabolize certain triterpenes as carbon sources for growth have more efficient rhizosphere colonization. The root-secreted compound 1-aminocyclopropane-1-carboxylic acid (ACC), which is the precursor of ethylene, can be used only by bacteria with ACC deaminase. These bacteria can degrade ACC as a nitrogen source, giving them a significant advantage in rhizosphere colonization (Li et al. 2019). Recently, several publications demonstrated that plant secreted inositol as a nutrient is important for regulating rhizobacteria colonization (O’Banion et al. 2023), and a conserved inositol metabolism cluster in root Pseudomonas contributes to the competition for nutrients in the rhizosphere (Sánchez-Gil et al. 2023). In addition to the direct effect, compounds in root exudates can be degraded by specific bacteria, and the resulting metabolites will promote colonization by other bacteria. This kind of effect is expected to greatly participate in modulating root colonization by beneficial rhizobacteria (Sasse et al. 2018).
Some broad-spectrum antimicrobial substances in root exudates also impact the colonization of beneficial rhizobacteria by serving as carbon resources. Many plant secondary metabolites and small peptides exert variable antimicrobial activity (Chagas et al. 2018) and function as bioprotectants against pathogens. However, some of these compounds have selective antimicrobial activity and can act as carbon resources for certain beneficial rhizobacteria. Rhizobacteria that can metabolize root-secreted antimicrobial substances will have higher rhizosphere colonization efficiency and succeed in root colonization. The root-secreted toxic compounds camalexin and benzoxazinoids, which are signatures of the root immune response, also promoted colonization by beneficial Pseudomonas (Hu et al. 2018, Koprivova et al. 2019). Many VOCs produced by roots can serve as antimicrobial compounds, such as terpenes and terpenoids, to inhibit pathogen growth, and interestingly, they can also promote specific beneficial rhizobacterial growth (Chagas et al. 2018, Schulz-Bohm et al. 2018). In addition, aromatic compounds released by roots also mediate defense mechanisms against pathogens and attract some microbes by serving as carbon sources (Lattanzio et al. 2006). Indeed, Lebeis et al. (2015) demonstrated that salicylic acid, an aromatic signaling molecule responsible for many kind of plant defense response, can be used by some beneficial bacterial strains as a growth signal or as a carbon source.
Some specific transporters from either plants or bacteria have been suggested to be involved in the process of bacterial acquisition of root secreted carbon resource and contribute to the bacterial colonization in rhizosphere. Plants have developed active mechanisms for root exudation. Numerous studies have established that specific transporters located on the plasma membrane of root may be responsible for recruiting beneficial bacteria (Hennion et al. 2019, Vives-Peris et al. 2020). The plant transporter ALMT1 plays a role in exudation of the malate and the gamma-aminobutyric acid (GABA), which is one of the major carbon resources for rhizobacteria (Lakshmanan et al. 2012, 2013, Kamran et al. 2020). Arabidopsis amino acid transporter, LHT1, modulates P. simiae metabolism in the rhizosphere, which influence its colonization efficiency (Agorsor et al. 2023). Bacterial also deploy a range of transporters to acquire the root exudates. Using a combination of comparative genomics and exometabolomics, Zhalnina et al. (2018) revealed that the uptake of root-secreted carbon resources by specific transporters of rhizobacteria determines their colonization, and a bacterium with an uptake transporter of the highly abundant nutritional compounds of root exudates will be highly advantageous in rhizosphere colonization. They also found that the uptake of certain substances is highly variable among rhizobacteria (Zhalnina et al. 2018). Under controlled conditions, Lin et al. (2020) demonstrated that knockout of the ptsG gene encoding the main glucose transporter in Bacillus cereus C1 L led to a sharp decrease in root colonization, suggesting the importance of bacterial transporter of root secreted carbon resources in bacterial colonization.
Biofilm formation
The formation of a biofilm is a way to maintain a critical cell mass in a specific location that is sufficient to initiate beneficial interactions with host plants (Flemming and Wuertz 2019). Biofilms increase resistance to certain environmental stresses as well as antimicrobial tolerance, protection from protozoan predation, consortia metabolism, or the opportunity for horizontal gene transfer (Arnaouteli et al. 2021). The biofilm matrix consists of extracellular polymeric substances, including polysaccharides, proteins, amyloids, lipids, and extracellular DNA, as well as membrane vesicles and humic-like refractories (Flemming et al. 2023).
Global transcription factors in biofilm formation
Mature biofilm formation generally indicates successful rhizosphere colonization. Rhizobacterial biofilm formation on the root surface is a highly regulated process, as each species has its own molecular mechanism for responding to environmental cues (Trivedi et al. 2020). The cessation of movement and initiation of biofilm formation by beneficial rhizobacteria are typically governed by one or several global transcriptional regulators within the bacterium. Consequently, these two cellular decisions are always coupled. When cells opt to transition into a biofilm state, the gene transcription associated with motility and chemotaxis is simultaneously downregulated. For example, biofilm formation by beneficial Bacillus in rhizosphere is governed by two global transcription factors, Spo0A and DegU (Arnaouteli et al. 2016, Kobayashi and Ikemoto 2019). DegU controls both motility and biofilm formation by different phosphorylation levels (Kobayashi and Ikemoto 2019). Spo0A also controls sporulation and biofilm formation by different phosphorylation levels (Xu et al. 2019a). Pseudomonas deploys different oligomerization of the global transcriptional regulator FleQ to adjudge the decision of motility and biofilm formation (Nie et al. 2022). Deficiency of these global transcriptional regulators in bacteria always leads to sharply reduced rhizosphere colonization (Xu et al. 2014, 2017, Emonet et al. 2021), suggesting the critical role of lifestyle transitions in rhizosphere colonization. Such a mechanism will prevent the contradictory coactivation of biofilm formation and motility during rhizosphere colonization.
The global transcriptional regulators that direct the shift from bacterial motility to biofilm formation respond to environmental cues, such as root exudates (Ivanova et al. 2023). This sensory mechanism is generally mediated by cell surface receptors such as histidine kinases, notably KinD in Bacillus (Liu et al. 2020a). Upon perceiving specific rhizosphere signals, these receptors communicate with global regulatory factors in various ways depending on bacterial variations (Arnaouteli et al. 2021, Nie et al. 2022, Wang et al. 2022), prompting cells to initiate biofilm formation on root surfaces. Certain plant polysaccharides, the major components of the plant cell wall, were also shown to enhance the biofilm of B. subtilis by acting as signals for controlling the phosphorylation level of the master regulator Spo0A and as carbon resources for producing the matrix exopolysaccharide (Beauregard et al. 2013). Interestingly, some signaling molecules induce both biofilm formation and trigger chemotaxis in beneficial rhizobacteria, such as cucumber root-secreted d-galactose, which could be induced by B. velezensis SQR9, serving as a signal for enhancing chemotaxis and biofilm formation of strain SQR9 in a McpA-dependent manner (Liu et al. 2020b). The organic acids in the root exudates of peanut, including citric, malic, and oxalic acids, promoted bacterial biofilm formation of the beneficial rhizobacterium Burkholderia pyrrocinia strain P10 in rhizosphere (Han et al. 2023). In addition, the flavones in rice root exudates enhance biofilm formation of the nitrogen-fixing bacterium Gluconacetobacter diazotrophicus, and biofilm formation in turn recruits diazotrophic bacteria in the rhizosphere (Yan et al. 2022). While these are distinct processes in rhizosphere colonization, it can be expected that bacteria might exhibit differential responses to different concentrations of the same signaling molecule. Thus, a molecule could stimulate chemotaxis at greater distances from roots but favor biofilm formation on the root surface. Such dose-dependent signaling is very common in biofilm and chemotaxis regulation among rhizobacteria.
Effect of self-produced secondary metabolites on biofilm formation
Rhizosphere microorganisms can produce many secondary metabolites, which also impact biofilm formation. Root-secreted sucrose activates the bacterial production of extracellular polymeric levan, which in turn regulates the synthesis of surfactin and hyperflagellation of the bacterium (Tian et al. 2021b). Interestingly, by causing potassium leakage, surfactin was demonstrated to be an essential signaling molecule in the establishment of biofilms and root colonization in B. subtilis NCIB3610 (Lopez et al. 2009). It has also been shown that another lipopeptide antibiotic, bacillomycin D, contributes to biofilm formation by facilitating iron acquisition. In B. velezensis SQR9, bacillomycin D specifically promotes transcription of the iron ABC transporter FeuABC by binding to its transcription factor, called Btr (Xu et al. 2019a). Additionally, using a novel branched-chain fatty acid, bacillunoic acid, allows B. velezensis SQR9 to utilize a novel branched-chain fatty acid called bacillunoic acid to establish a policing system for punishing cheaters within the biofilm community and to improve the community’s fitness in a variety of conditions, including the root colonization process (Huang et al. 2023). Importantly, numerous studies have observed that siderophores play an important role in rhizobacterial biofilm formation of Bacillus spp. and Pseudomonas spp. siderophore-defective mutants in different PGPR strains fail to form biofilms and are unable to competitively colonize plant roots (Pizarro-Tobías et al. 2015, Qin et al. 2019, Singh et al. 2022a). Owing to the complexity of secondary metabolites in the rhizosphere, there are numerous secondary metabolites that affect the interaction between plants and rhizobacteria, which needs to be investigated further.
Multispecies biofilm in the rhizosphere
It has been recognized that multispecies biofilms, rather than single-species biofilms, are the most dominant bacterial lifestyle naturally found in the rhizosphere, a consortium of bacterial isolates may form stronger biofilm on rhizoplane thus an enhanced colonization can be expected (Burmølle et al. 2014, Sadiq et al. 2021). There have been numerous recent studies that provide insight into the synergistic effects of multispecies biofilms in rhizosphere soil, resulting in beneficial properties for plants. For example, a four-species biofilm consortium exhibited higher biomass than single species, as well as increased tolerance to environmental stress (Ren et al. 2015, Yang et al. 2021). In one particular instance, a consortium of five rhizosphere native bacterial isolates forms synergistic biofilms in vitro and colonizes a larger area on the root than the individual strains (Santhanam et al. 2015, 2019). Inoculation of cucumber rhizosphere with B. velezensis could increase the colonization of resident plant-beneficial Pseudomonas stutzeri through synergic biofilm formation (Sun et al. 2022). Furthermore, a study demonstrated that a three-species combination composed of Xanthomonas, Stenotrophomonas, and Microbacterium spp. showed increasing biofilm production compared to their individual members and thus increasing beneficial function on Arabidopsis (Berendsen et al. 2018).
Competition for scarce elements for growth and biofilm formation
Because of the large number of organisms in the rhizosphere, there are inevitable wars for limited elements, especially for the relatively scarce nutrient elements that are essential for rhizobacterial colonization, such as phosphorus, iron, zinc, and manganese (Dennis et al. 2010, Tsai and Schmidt 2017). Here, the scarce element nutrient is defined as the limited amount of this element in the rhizosphere becomes a limiting factor for bacterial growth and biofilm formation. In addition, plants also need these elements for growth, leading to fierce competition for phosphorus and iron in the rhizosphere.
Phosphorus generally reacts with calcium and magnesium in alkaline soils or with aluminum and iron in acidic soils to be fixed, which is difficult to absorb and utilize, resulting in a low level of phosphorus availability for bacteria (Earth System Science Data Discussions 2017). Rapid root absorption and poor mobility often lead to phosphorus depletion in the rhizosphere (Ceulemans et al. 2017, Sakuraba et al. 2018). Soil phosphorus is divided into inorganic P (Pi) and organic P (Po); inorganic phosphorus mainly exists in the form of phosphate, and organic P is an insoluble complex formed with organic monoesters, diesters, and inositol phosphates (Turner 2008, Liu et al. 2022). To cope with such situations, a range of beneficial rhizobacteria secrete different phosphatases to dissolve organic phosphorus in soil and utilize the unique phosphorus transport system for uptake and utilization (Fitriatin et al. 2011). The general phosphorus solubilization and uptake system in rhizobacteria consists of four categories of genes, including the phosphorus regulatory transcription factor pho and the TCS phoB/phoR, transport system genes such as pit, pstA, pstB, and ugpQ, the inorganic phosphorus solubilization genes gcd, ppa, and ppx, and organic phosphate mineralization genes such as phoA and phoD (Wu et al. 2022). The phosphorus regulatory transcription regulator pho and the downstream TCS, which are conserved in most bacterial species, are essential in activating phosphorus solubilization and uptake genes in response to a low phosphorus environment. Activation of pho generally induces the expression of a series of downstream reactions to secrete phosphatases and organic acids, therefore mineralizing insoluble organic phosphates (Hulett 1996). In recent years, it has been reported that the constitutive phosphatase (PafA) activity expressed by Flavobacteria in the rhizosphere is stronger than that of Pseudomonas, which enables Flavobacteria to occupy unique phosphorus clearance sites in the rhizosphere and enhance the ability of phosphorus acquisition (Lidbury et al. 2021), making the Flavobacteria successful colonizers of the phosphorus solubilizing niche in the rhizosphere.
Iron is an indispensable element that participates in many important biological metabolic processes; in particular, bacterial biofilm formation requires sufficient iron (Qin et al. 2019, Xu et al. 2019a). The total iron in soil is abundant, estimated to be 20–40 g/kg (Bowles 1997); however, most iron is present in insoluble iron oxide precipitates or insoluble high-valence forms. Iron availability is extremely low in neutral and alkaline soils (Moreno-Jiménez et al. 2019). Moreover, plant roots also deploy a strategy that takes up iron and withholds excess iron in vacuoles to restrict pathogen virulence. Therefore, soluble iron is extremely scarce for bacteria in the rhizosphere (Trapet et al. 2021). To increase their competitiveness for iron nutrition in the rhizosphere, most rhizobacteria produce siderophores to chelate ferric iron for colonization in rhizosphere (Stringlis et al. 2018b). Bacterial siderophores can be hijacked by other bacteria to compete for iron (Gu et al. 2020). In addition to competition for soil iron by siderophores, iron competition between rhizobacteria and plants is also a canonical battle field (Xing et al. 2021). It has been recently found that beneficial rhizobacteria also trade with iron resources during bacterial colonization. Bacillus velezensis SQR9 deploys the type VII secretion system to export YukE, which inserts into the plant root cell membrane to cause iron leakage to facilitate the iron nutrition and rhizosphere colonization of this strain (Liu et al. 2023).
Endophyte penetration
Endophytic bacteria colonize the host tissue. Some endophytes can colonize roots from vertical transmission and have been reviewed on vertical transmission (Frank et al. 2017, Guo et al. 2021, Soluch et al. 2021). Here, we focus on the endophytic process after root attachment of the bacteria. The intercellular colonization process has been demonstrated with several model endophytes, such as Azoarcus spp., Paraburkholderia phytofirman, and Klebsiella spp. (Reinhold-Hurek et al. 2007, Turner et al. 2013). The key process is penetration into plant tissue (Hallmann 2001). The infection site selection and the bacterial features involved in lifestyle of root colonization are the key points here.
Infection site
The infection sites of rhizosphere endophytes are selective. It has been reported that many microorganisms enter plant root tissue by the following three putative pathways: the root tip in the elongation and differentiation zone, the points where lateral roots emerge, and the axils of emerging or developed lateral roots (Reinhold-Hurek and Hurek 1998, James 2000, Mushtaq et al. 2023). James et al. (2002) deployed a GUS-marked strain of the endophyte Herbaspirillum seropedicae, a nitrogen-fixing bacterium, to study the rhizosphere colonization site in rice. This bacterium is most abundant on coleoptiles, lateral roots, and at the junctions of the major and lateral roots in the initial step (James et al. 2002, Balsanelli et al. 2010). It enters roots via cracks at the points of lateral root emergence and subsequently colonizes the intercellular spaces of roots (James et al. 2002). Histochemical analysis of seedlings of maize, sorghum, wheat, and rice grown in vermiculite showed that strain H. seropedicae LR15 colonized inner tissues. In the early steps of the endophytic association, H. seropedicae colonized intercellular spaces of the root cortex; it then occupied the vascular tissue. Colonization was also observed in the external mucilaginous root material at 8 dpi (Roncato-Maccari et al. 2003). Bacillus megaterium NCT-2 could penetrate into maize roots through the root tip in the elongation and differentiation zone (Chu et al. 2018). Compant et al. (2005) labeled Burkholderia sp. PsJN with GFP and observed the bacterial cells enriched in high numbers at the sites of lateral root emergence. Growing evidence support the idea that the endophytic colonization site is highly restricted by plant, such as by the plant immunity, the suberin, the casparian strip, and some antimicrobial metabolites in root tissues (Philippe et al. 2020, Durr et al. 2021, Fröschel et al. 2021, Kashyap et al. 2022, Verbon et al. 2023).
Specific features of bacterial endophytes
It seems that the decision of endophytic colonization can be distinct even between bacterial strains with close phylogenetic relationships. For instance, two efficient avocado root tip colonizers, P. alcaligenes AVO73 and P. pseudoalcaligenes AVO110, display distinct colonization sites; the latter colonizes root wounds and intercellular spaces between root epidermal cells, while the former colonizes only the root surface (Pliego et al. 2008). It is generally agreed that the factors influencing bacterial endophytism are complex and varied. Chen et al. (2020) explored the transcriptome profile of rice upon infection by two endophyte isolates, Azoarcus olearius BH72 and Azospirillum sp. B510 and found that plants respond quite differently to these two endophytes, suggesting a large variation in molecular interactions during endophytic colonization. But knowledge on the bacterial genetic features that responsible for penetration into root tissue and intercellular lifestyle is still very limited.
Cell wall degradation is expected to be a fundamental skill of endophytic bacteria, even if they do not need to enter the intracellular space. The secretion of cell wall-degrading enzymes, mainly pectinases and cellulases, is known to be involved in bacterial penetration into plant tissue (Compant et al. 2005). A mutant of A. olearius BH72 devoid of endoglucanase activity had a decreased ability to colonize rice (Reinhold-Hurek et al. 2006). Rat et al. (2021) tested 197 endophytic bacteria of medicinal plant Alkanna tinctoria and found strains expressing cell-wall degrading enzymatic activities might have strong plant growth-promoting activity due to their ability to colonize plant.
A unique respiratory type of metabolism may be essential for an endophyte because the carbon resources and the oxygen in plant tissue are quite different from those in the rhizoplane and soil. For example, the well-studied endophyte A. olearius BH72 has a strictly respiratory type of metabolism and cannot utilize common carbohydrates (Krause et al. 2006). A highly adaptive respiratory type can be expected to be essential for root endophytic life of bacteria.
Unique motility may function in evading plant tissue. Böhm et al. (2007) demonstrated that a type IV pili-dependent twitching motility, but not the type-pili itself, mediated the endophyte A. olearius BH72 invasion of and establishment inside the plant.
The interaction with plant immunity is expected to be a major trait for the adaptive lifestyle of endophytes. It has been shown that a plant-beneficial endophyte generally elicits a weaker immune response than pathogens. Moreover, Deng et al. (2019) demonstrated that an endophyte B. subtilis strain could evade plant defense by producing subtilomycin to mask self-produced flg22. Activation of the immune response or other stress responses is always accompanied by oxidative bursts, which lead to osmotic stress in endophytes, so it can be expected that a successful endophyte also harbors ROS tolerance to address the plant immune response and the ROS produced by plants under stressful conditions. Alquéres et al. (2013) found that the endophyte G. diazotrophicus PAL5 showed increased expression of genes encoding ROS-detoxifying enzymes during colonization in rice roots.
In conclusion, knowledge on the molecular mechanism underlying the endophytic lifestyle is still lacking. First, although the feasible and independent solutions for endophyte isolation have been demonstrated, a standardized and unbiased method is urgently needed. A comprehensive genomic comparison will help to determine whether there is a common trait in the genome of bacterial endophytes. To identify genes involved in the endophytic lifestyle rather than contributing to the colonizing process before entering plant tissue using mutational experiments, comparing colonization both on the root surface and in root tissue is necessary. In addition, it could also be that endophytism is transient and opportunistic rather than a strict lifestyle.
“Cry-for-help” theory for root colonization of rhizobacteria
Several papers demonstrated that stressed plants recruit beneficial bacteria to colonize the root, thereby facilitating the stress-induced opposite effect on plant growth and health (Berendsen et al. 2018, Yuan et al. 2018, Santoyo 2022, Xie et al. 2022, Wen et al. 2023). It is a noteworthy factor that influences bacterial colonization. One of the well-known strategies is the “cry for help” hypothesis, which explains the long-term disease suppressive soil feedback to foliar pathogen attack. The underlying mechanism still remain to be demonstrated (Wang and Song 2022). Although the current understanding of the cross-talk between root exudation, the root immune system, and the “cry for help” response is limited, it can be expected or confirmed that they may be linked internally. Rolfe et al. (2019) proposed three stages for this plant disease-induced long-term response: root immune responses to attackers, stress-induced changes in root exudation of antimicrobials and signaling chemicals, and impacts of root exudates on the rhizosphere microbiome. In addition, evidence has shown that root exudation from abiotic stressed plants also promotes colonization of beneficial rhizobacteria, which function to relieve the stress response of the plant. This stress-induced host selection would highly influence the colonization of beneficial rhizobacteria by changing the immune response and root exudation.
Biotic stress triggered “cry for help” response
Rudrappa et al. (2008) were the first to provide experimental evidence that aboveground disease alters root exudation of a primary root metabolite, l-malic acid, resulting in increased root colonization by a beneficial rhizobacterial strain. The authors propose that P. syringae pathovar tomato DC3000 (Pst DC3000) infection of Arabidopsis leaves induces root secretion of l-malic acid, which acts as a specific signal for chemotaxis and colonization of the biocontrol bacterium B. subtilis FB17 in the rhizosphere (Rudrappa et al. 2008). A follow-up study demonstrated that either MAMPs, such as flg22, or the pathogen-derived phytotoxin COR are necessary to induce plants to secrete l-malic acid to promote colonization by B. subtilis FB17 (Lakshmanan et al. 2012).
However, the mechanism that triggers the colonization promotion response is unclear. Regulation of the immune system upon perception of foliar pathogens was thought to contribute to influencing root microbiome composition (Lebeis et al. 2015). Foliar attack by pathogens or insects can influence belowground direct and indirect plant defense responses (Bezemer and Van Dam 2005), but the root immune system needs to differentiate between beneficial and pathogenic microbes and mount appropriate, yet diametrically opposed, colonization-enabling or defense responses. However, COR, as a mimic of JA-Ile, was proposed to suppress SA signaling and the flg22-triggered immune response (Li et al. 2005, Melotto et al. 2006), since both flg22 and COR could trigger the colonization promotion response. It is ambiguous how the immune response in aboveground tissue is involved in promoting root colonization by Bacillus. It is hypothesized that some defense signaling activated upon infection by pathogen may be positive for beneficial rhizobacterial colonization. Indeed, Yang et al. (2023) found that the SA signaling pathway is essential for eliciting plants to promote root colonization of some biocontrol bacteria for bacterial wilt disease.
Another important case comes from the interaction between Fusarium and plants. Liu et al. (2017) used a split-root system to show that inoculation of part of the cucumber root system with Fusarium changes numerous root exudates and promotes colonization of the beneficial rhizobacterium B. velezensis SQR9 in distal roots, which was linked to increased exudation of tryptophan, a biofilm formation stimulator of strain SQR9. This finding was also corroborated by a comics study by Wen et al. (2023), who found that Fusarium-infected cucumber also attracted Sphingomonas in addition to Bacillus for root colonization by stimulating the genes involved in motility and chemotaxis (Wen et al. 2023). Similarly, Schulz-Bohm et al. (2018) found that upon infection with the fungal pathogen Fusarium culmorum, Carex arenaria changed the blend of root-secreted VOCs that promote the colonization of specific bacteria with antifungal properties. Root exudates from Fusarium-infected maize also stimulate root colonization of B. amyloliquefaciens OR2-30 by stimulating chemotaxis and motility (Xie et al. 2022). In wheat, Fusarium infection leads to higher root colonization of Stenotrophomonas rhizophila SR80, a dominant beneficial bacterium that induces strong disease resistance by boosting plant defense in aboveground plant parts (Liu et al. 2021).
Upon infection by phytopathogens, plant roots release several antimicrobial compounds, but little is known about their effects on root colonization by beneficial rhizobacteria. One interesting field of how these antimicrobial compounds contribute to the “cry for help” response and affect beneficial bacterial colonization is studies on the rhizosphere function of coumarin. Coumarin is a class of phenolic secondary metabolites synthesized by Arabidopsis that can stimulate biofilm formation of B. subtilis (Korenblum et al. 2022). Stringlis et al. (2018a) revealed that coumarin scopoletin selectively inhibits the soil-borne fungal pathogens Fusarium oxysporum and Verticillium dahliae, while growth-promoting and resistance-inducing Pseudomonas are highly tolerant to scopoletin. Vismans et al. (2022) found that foliar infection of Arabidopsis thaliana by the biotrophic downy mildew pathogen Hyaloperonospora arabidopsidis recruits beneficial bacteria that can enhance plant resistance, while it is evident that the coumarin biosynthesis genes MYB72 and F6’H1 in Arabidopsis are essential for recruiting beneficial bacterial colonization upon infection. These findings draw the outline of a fascinating “cry for help” response.
Abiotic stress triggered “cry for help” response
The colonization of beneficial rhizobacteria on roots can also be activated by plants under abiotic stress. For instance, rice during and after drought recruits beneficial Streptomyces to colonize the root endosphere (Santos-Medellín et al. 2021). Drought typically decreases the root exudation of plants, but drought-stressed trees have increased root exudation of phenolic acid compounds and quinate to recruit beneficial Bacillus and Pseudomonas for colonization (Oppenheimer-Shaanan et al. 2022). Root secretion of flavonoids, which is often elevated in plants under abiotic stress, may also be involved in promoting colonization upon stress production. Arabidopsis roots under dehydration stress increased flavonoid accumulation within 15 min. The flavonoid naringenin enhances root colonization of Aeromonas sp. H1, which is identified as a plant beneficial bacterium capable of enhancing plant resistance to dehydration through transcriptional enhancement of bacterial motility and colonization (He et al. 2022). Hou et al. (2021) demonstrated that Arabidopsis under low photosynthesis drives the recruitment of specific rhizobacteria with beneficial effects. Plants under salt stress employ a species-specific strategy to promote colonization by beneficial bacteria in the rhizosphere. Root exudates from the salt-stressed coastal halophyte Limonium sinense promote the growth, chemotaxis and finally root colonization of the bacterium B. flexus KLBMP 4941 (Li et al. 2021d). An interesting example is coumarins, besides mediating the pathogen-infection-triggered “cry for help” response, coumarins have also demonstrated to be secreted by A. thaliana upon iron-deficiency stress to recruit beneficial bacteria (Harbort et al. 2020). Besides the specific molecules, stress-induced plant hormones are essential for plants to recruit beneficial bacteria. Indeed, Chen et al. (2020) found that peanut root could sense the cyanide stress produced by neighboring cassava plants and produce ethylene to recruit beneficial bacteria to adjust to the stressful environment.
Comparison with pathogenic/symbiotic bacteria for rhizosphere colonization mechanisms
Pathogenic, symbiotic, and nonsymbiotic rhizobacteria represent three groups of root colonizers that are tightly associated with plant. But the comparison of the difference of their colonization mechanisms in rhizosphere is lack. The rhizosphere chemotaxis and root attachment of these bacterial groups are similar, which are mainly by sensing root secreted signals, moving toward rhizosphere, and adhering to root surface, although the signals or cellular molecular pathway involved may different. The colonization process for pathogenic/symbiotic bacteria and the nonsymbiotic beneficial bacteria differed mainly in their specific lifestyles. Most nonsymbiotic rhizobacteria colonize the rhizoplane as a community, some endophytes colonize the intercellular spaces of the root at a controlled low density (Lugtenberg and Kamilova 2009). However, symbiotic bacteria colonize roots intracellularly and sometimes they induce root to develop specific organs, which allow their high populations in root (Tang et al. 2020). Pathogenic bacteria infect root tissues and always grow to a very high density, which is needed for expression of virulence factors (von Bodman et al. 2003). The different lifestyles lead to difference of host specificity, nutrition and metabolism and strategies against plant immunity during colonization in the rhizosphere (Fig. 2).
Figure 2.
Comparison of the colonization of nonsymbiotic rhizobacteria with symbiotic and pathogenic bacteria. The relative bacterial density of a nonsymbiotic rhizobacterium in its colonization site is lower than that of symbiotic and pathogenic bacteria. Nonsymbiotic rhizobacteria generally have broad host range, while symbiotic and pathogenic bacteria have very specific host. Symbiotic bacteria acquire carbon resources directly from the root cells and feed root cells with nitrogen, pathogenic bacteria hijack plant metabolism and nutrition, while nonsymbiotic rhizobacteria mainly use root exudates and the secretions in intercellular spaces. Symbiotic bacteria have specific interaction with plant immunity to establish infection and symbiosis, pathogenic bacteria block plant immune response by injecting effectors into root cells.
Host specificity
Generally, a nonsymbiotic beneficial rhizobacterium can colonize a broad range of host plants. For example, B. velezensis SQR9 was isolated from the rhizosphere of cucumber and can colonize Arabidopsis, maize and rice efficiently (Liu et al. 2014, Cao et al. 2023a). Pseudomonas simiae WCS417 was isolated from the rhizosphere of wheat and induced systemic resistance in Arabidopsis, tomato, and many other plant species, suggesting efficient colonization of these plant species (Berendsen et al. 2015). The endophytes A. olearius BH72 was isolated from Kallar grass (Leptochloa fusca L. Kunth), while it also endophytically colonized rice (Hurek and Reinhold-Hurek 2003). However, relatively strict host selection is observed for symbiotic and pathogenic bacteria. Isolates belonging to Rhizobiaceae only infect legumes as a very specific host. One rhizobium strain can not colonize different cultivars from the same host plant species. This opinion is highly supported by the results from Dong et al. (2021), who found that the legume Medicago truncatula possesses an SHR–SCR stem cell program in cortical cells to specifically interact with rhizobia for nodulation. Pathogenic bacteria also have strict host selection. For example, one strain from P. syringae generally has a very limited host plant species and even a few cultivars from a single plant species, based on which the basis of the pathogenic P. syringae can be grouped into pathovars (Xin and He 2013).
The narrow host spectrum for symbiotic and pathogenic bacteria is generally due to their host selection genes, and the presence or absence of these genes determines the infection of a specific host. For example, a common concept of the presence of pathogenic bacteria and symbiotic strains is called avirulent genes, which enable specific nonhost plants to specifically prevent the infection of that strain. These avirulent genes typically mediate immune recognition by nonhost plants (Yang et al. 2010). In contrast, there are currently no reported host selection genes in nonsymbiotic beneficial rhizobacteria. But nonsymbiotic rhizobacteria do have a host preference, which suggest the existence of specific genes determines the colonization of these bacteria (Wippel et al. 2021). Even though, here is currently a tendency to believe that such bacteria use lower amplification rates in association with host plant in exchange for a wider host range.
Nutrition and metabolism
Lifestyle determines the metabolism of the bacteria. Due to the intracellular life of symbiotic bacteria, their metabolism and carbon resources are largely dependent on their host cells, and therefore, they generally have a more specific metabolites exchange with the host. Intracellular colonization is established and partially controlled by plant genes. For example, rhizobia mainly use the carbohydrates of host plants as carbon resources and feed plants with ammonia during root nodule symbiosis (Yang et al. 2022). Moreover, the respiratory type and redox potential of symbiotic bacteria are highly influenced by the host plant (Yu et al. 2018). Specific metabolism was also observed in the well-studied Agrobacteria strategy, during which pathogenic Agrobacterium hijacks plant cells by injecting a part of the DNA sequence from the Ti plasmid to produce opines as dedicated carbon resources for Agrobacterium itself (Lang et al. 2013, González-Mula et al. 2018, Matveeva and Otten 2021). The plant pathogen Ralstonia solanacearum is also able to manipulate plant metabolism to produce GABA to support bacterial nutrition during colonization (Xian et al. 2020).
The nutrition and metabolism of most nonsymbiotic rhizobacteria are not strictly dependent on the host. They mainly use a broad range of organic compounds in root exudates for colonization (Badri and Vivanco 2009). In contrast to the specific carbon resources for bacteria during nodulation or infection, due to the much higher diversity of bacteria than intercellular and intracellular spaces, the bacteria colonizing the root surface should have a broader carbon source utilization spectrum to compete for nutrients in root exudates (Mataigne et al. 2022). The diversity of the bacteria in the rhizosphere led them to share the various compounds of the root exudates (Yang et al. 2017, 2019). Moreover, most nonsymbiotic rhizobacteria can degrade and use the soil-derived carbon resources.
Plant immunity evading strategy
The lifestyle of pathogenic, symbiotic, and nonsymbiotic bacteria is largely distinctive, leading a quite different strategy to interact with plant immunity. Due to the intracellular lifestyle of symbiotic bacteria, activation of the plant immune response is believed to be harmful to the interaction (Feng et al. 2021b). Most pathogenic bacteria infect root tissue in a high density, eliciting a stressful and PAMP-rich environment; when pathogens do not have immune-blocking strategies, strong PTI and sharply reduced colonization can be expected (Wei et al. 2018). Nonsymbiotic bacteria generally colonize the rhizosphere at a relatively lower density, but ROS accumulation or establishment of immune response within roots has a weaker influence to the colonization of nonsymbiotic bacteria than to the pathogenic and symbiotic bacteria (Buschart et al. 2012, Zhang et al. 2021). This may rely on the different concentrations of antibacterial compounds, such as ROS, in root cells, intercellular spaces, and rhizoplane. The difference has been evident by several studies that blocking the plant immune response evading mechanism in bacteria has a much stronger impact on colonization of rhizobia and pathogenic bacteria than that of nonsymbiotic beneficial bacteria (Liang et al. 2013, Wei et al. 2015, Deng et al. 2019, Pfeilmeier et al. 2019, Yu et al. 2019a, Zhang et al. 2021). To fit their unique lifestyles, pathogenic, symbiotic, and nonsymbiotic bacteria deployed different strategies to evade plant immunity.
Pathogenic and symbiotic bacteria possess highly immunogenic MAMPs. Although many MAMPs from nonsymbiotic rhizobacteria have been identified, current researches suggest those MAMPs elicit a weaker response than that derived from pathogens, which is shown by a lower elicitation of defense gene transcription, a lower oxidative burst, and a higher concentration needed for seedling growth inhibition (Colaianni et al. 2021, Zhang et al. 2021). For example, Colaianni et al. (2021) demonstrated that the flg22 variant from beneficial Bacillus can not trigger seedling growth inhibition when applied to a final concentration of 10 nM, a concentration the flg22 variant from Pst DC3000 did. However, pathogens use unique secretion system to interfere the PTI therefore establishing disease (Shu et al. 2023). For example, both pathogenic P. syringae and R. solanacearum deliver effectors into plant cells through the type III secretion system to interfere with the plant immune response for efficient colonization (Yuan et al. 2021, Yu et al. 2022). The nodulation out proteins secreted by symbiotic bacteria have been reported to suppress PTI (Xin et al. 2012). Both symbiotic and pathogenic bacteria show specific interactions with the plant immune system, such as R genes. For rhizobia, it has also been demonstrated that R genes in legumes control the host specificity of rhizobium symbiosis. But different with pathogen, balanced regulation of innate immunity is required for rhizobial infection and symbiosis (Cao et al. 2017, Yang et al. 2022). In contrast, nonsymbiotic rhizobacteria regulate the plant immune response in general as reviewed in the section “Interaction with plant immunity”, rather than through specific interactions as that of pathogenic bacteria and have never been shown to interact with R genes in plants.
Artificial enhancement of root colonization by beneficial rhizobacteria
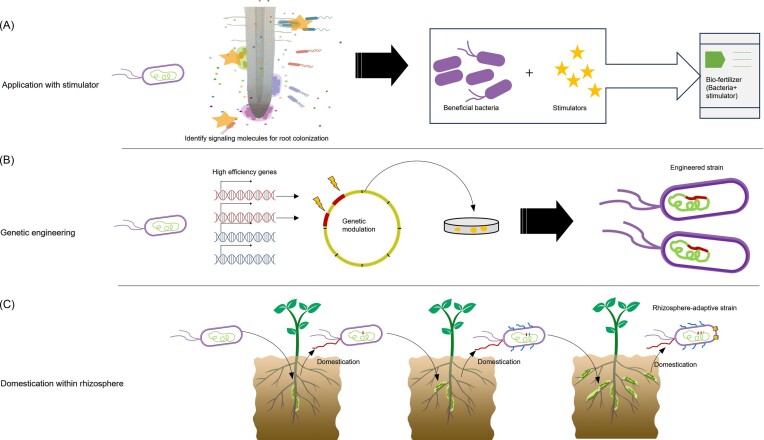
The field application of beneficial rhizobacteria is an effective practice for sustainable agriculture, the efficient root colonization of these bacteria is critical for the performance of their beneficial functions. Hence, it is important to develop strategies to enhance the root colonization of beneficial rhizobacteria. This review proposes three strategies, which include the addition of colonization-enhancing substrates, bacterial genetic modulation, and evolution of beneficial rhizobacteria (Fig. 3).
Figure 3.
Strategies to promote rhizosphere colonization of nonsymbiotic bacteria. (A) Many compounds in rhizosphere, mainly from the root exudates, have been identified to be positive signaling molecules for beneficial bacterial colonization in rhizosphere. It is a practicable way to develop such molecules as colonization stimulator and applied with the beneficial bacteria together in agriculture. (B) Many bacterial genes have been identified to be positive for rhizosphere colonization with clear mechanisms. Genetic modulation of the beneficial bacteria by introducing “colonization positive” genes would generate engineered strains as better colonizers. (C) Efficient rhizosphere colonization is a beneficial trait for bacteria itself, because rhizosphere supplied more nutrient for bacterial proliferation, therefore a continuous life in rhizosphere is expected to drive the accumulation of “colonization positive” mutations in bacterial genome. So, round-by-round inoculation and reisolation of bacteria in rhizosphere will domesticate an evolved strain as a better colonizer.
It can be expected that the application of some compounds in root exudates or microbial metabolites may serve as root colonization stimulators given that many studies have demonstrated the role of these compounds in modulating the root colonization of beneficial rhizobacteria. For example, the application of organic acids, such as malic acids, citric acid, and fumaric acid, can enhance root colonization of the beneficial strains Hansschlegelia zhihuaiae, B. velezensis SQR9, and B. pyrrocini (Zhang et al. 2014, 2015, 2018, 2022, Feng et al. 2018, Han et al. 2023). Therefore, soil amendments can be used to promote beneficial bacterial colonization.
Genetic engineering of beneficial rhizobacteria to respond to specific root exudate compounds is another strategy to enhance colonization. Xu et al. (2019b) developed a xylose-inducible degQ genetically engineered strain of B. velezensis SQR9, which can use root secreted xylose as a signal to regulate the phosphorylation level of DegU and then promoted its ability to form biofilm on the root surface. Compared to the wild-type strain, the genetically engineered strain showed greater root colonization ability and biocontrol efficacy in cucumber and tomato (Xu et al. 2019b). Singh et al. (2022b) engineered the beneficial bacterium A. brasilense Sp7 with enhanced d-glucose utilization ability and showed significantly increased root colonization in rice compared with the wild-type strain.
One imaginative strategy for improving root colonization ability of beneficial rhizobacteria is coevolution of the strain with the target plant to get the evolved strain, which is termed as targeted domestication. It is known that natural genetic mutations, such as random point mutation and horizontal gene transfer, drive the evolution of bacteria, for example, phage infection drive the evolution of bacterial resistance to phage (Hussain et al. 2021). Therefore, if a bacterial strain was inoculated to the rhizosphere, isolated and reinoculated again, then, after rounds of repeating, the genetic mutations in the evolved bacterial genome that benefit its life in rhizosphere will accumulate due to the survival of the fittest theory. It can be inferred that artificial domestication of bacterial strains within the rhizosphere under monoassociation conditions may accelerate evolution and drive the direction to a better rhizosphere colonizer. Indeed, Blake et al. (2021) found that B. subtilis NCIB 3610 differentiated into three different colony morphologies after experimental evolution within the Arabidopsis rhizosphere and that a mixture of the three morphotypes colonized the rhizosphere better than each colony alone. Li et al. (2021c) repeatedly inoculated Pseudomonas protegens CHA0 in the rhizosphere of A. thaliana cultivated in sandy soil for six growth cycles, and they detected 35 mutations within 28 genes in the genome of the evolved isolates. Among them, mutations affecting global regulators, bacterial cell surface structure, and motility accumulated in parallel across multiple evolved strains (Li et al. 2021c). Moreover, the relationship between bacteria and plants has evolved from antagonism to mutualistic cooperation, which is manifested in a stronger ability to utilize rhizosphere exudates and a stronger tolerance to antibacterial substances secreted by plants (Li et al. 2021a). However, the entire trait correlation networks of P. protegens CHA0 are recombined after adaptive evolution, showing the loss of stress resistance modules and the linking of those modules that enhance ability after evolution (Li et al. 2021b). Compared with the solid substrate environment, domestication within the Arabidopsis rhizosphere under a hydroponic environment places more emphasis on the mobility and recolonization ability of strains (Nordgaard et al. 2022). Rotating croplands provide a more complex ecological environment for bacteria. In an evolutionary experimental study, the evolutionary strains in alternate host environments had a higher degree of parallel evolution at the gene level (Hu et al. 2023). Adaptive mutations in B. subtilis NCIB 3610 occurred earlier in the presence of Pseudomonas in the rhizosphere, suggesting that a competitive environment accelerates this capacity change (Pomerleau et al. 2023). In conclusion, evolution experiments can be used as an important means to breed beneficial rhizobacteria with improved root colonization and agricultural application. However, this evolution-based domestication is also risky because a slight environmental difference may lead to a butterfly effect on the resultant strains’ features. Moreover, domestication of the bacteria in a simplified environment would weaken the bacterial ability in other environments, such as stress tolerance (Li et al. 2021a, b, c).
Conclusion and outlook
The importance of rhizobacteria in plant growth, development, and health has been well recognized. Recent studies have revealed many fascinating models that describe complex interactions between rhizobacteria and plant and soil environments. However, compared with the soil-borne pathogenic and symbiotic bacteria of rhizobia, the root colonization of beneficial rhizobacteria has not been comprehensively concluded. Here, we summarized the root colonization of rhizobacteria into several steps. We also compared the difference in the colonization process of those nonsymbiotic beneficial rhizobacteria with symbiotic and pathogenic bacteria. Finally, we discussed the efforts made to improve the root colonization of beneficial rhizobacteria, which will facilitate their agricultural application.
The nonsymbiotic rhizobacteria represent the plant-associated bacteria with the largest abundance and diversity in the rhizosphere. The mechanism of root colonization of nonsymbiotic bacteria is significantly different from that of symbiotic and pathogenic bacteria. The colonization of any nonsymbiotic strain can not reach the abundance level as that of symbiotic or pathogenic bacteria. The symbiotic and pathogenic bacteria colonize the inside root tissues with very high abundance, while most nonsymbiotic beneficial rhizobacteria colonize the root surface or inside root tissue with a low abundance. The differences in colonization site and abundance suggest that the nonsymbiotic rhizobacteria have different root–bacteria interaction mechanisms. In particular, how do plants recognize nonsymbiotic beneficial rhizobacteria and allow colonization?
The rhizosphere environment is rich in other organisms, including fungi, protozoans, viruses, and other bacteria. Moreover, the microbiome in the rhizosphere is dominated by nonplant factors and varies largely depending on environmental factors, such as soil type, temperature, and humidity. Based on these concerns, the root colonization study of beneficial rhizobacteria in more natural conditions and under the holistic view of the rhizosphere microbiome and even the multitrophic interaction level will provide an in-depth understanding of the process and mechanisms in the future. Benefiting from the development of sequencing technology, many studies have made great efforts to use bioinformatic methods to analyze the rhizosphere microbiome.
Finally, the study of rhizobacterial root colonization aims to improve the agricultural application efficiency of biofertilizers, which are mostly isolated from beneficial rhizobacteria. Therefore, our future study of rhizobacterial root colonization should pay more attention to the development of products or biotechnologies based on the process and mechanism understanding to improve the field application effect of beneficial rhizobacteria. More efforts to develop a new generation of biofertilizers that enhance beneficial rhizobacterial colonization should be made to promote the sustainable development of agriculture.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (32370135), the National Key Research and Development Program (2022YFF1001804 and 2021YFF1000400), the Innovation Program of Chinese Academy of Agricultural Sciences (CAAS-CSAL-202302), the Central Public-interest Scientific Institution Basal Research Fund (Y2022QC15), and the Agricultural Science and Technology Innovation Program (CAAS-ZDRW202308).
Contributor Information
Yunpeng Liu, State Key Laboratory of Efficient Utilization of Arid and Semi-Arid Arable Land in Northern China, The Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, 12 Zhongguancun South Street, Beijing 100081, P.R. China.
Zhihui Xu, Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, National Engineering Research Center for Organic-Based Fertilizers, Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing Agricultural University, 6 Tongwei Road, Nanjing 210095, P.R. China.
Lin Chen, Experimental Center of Forestry in North China, Chinese Academy of Forestry, 1 Shuizha West Road, Beijing 102300, P.R. China.
Weibing Xun, Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, National Engineering Research Center for Organic-Based Fertilizers, Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing Agricultural University, 6 Tongwei Road, Nanjing 210095, P.R. China.
Xia Shu, State Key Laboratory of Efficient Utilization of Arid and Semi-Arid Arable Land in Northern China, The Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, 12 Zhongguancun South Street, Beijing 100081, P.R. China; State Key Laboratory of Agricultural Microbiology, College of Life Science and Technology, Huazhong Agricultural University, 1 Shizishan Street, Wuhan, P.R. China.
Yu Chen, Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, National Engineering Research Center for Organic-Based Fertilizers, Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing Agricultural University, 6 Tongwei Road, Nanjing 210095, P.R. China.
Xinli Sun, Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, National Engineering Research Center for Organic-Based Fertilizers, Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing Agricultural University, 6 Tongwei Road, Nanjing 210095, P.R. China.
Zhengqi Wang, Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, National Engineering Research Center for Organic-Based Fertilizers, Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing Agricultural University, 6 Tongwei Road, Nanjing 210095, P.R. China.
Yi Ren, Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, National Engineering Research Center for Organic-Based Fertilizers, Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing Agricultural University, 6 Tongwei Road, Nanjing 210095, P.R. China.
Qirong Shen, Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, National Engineering Research Center for Organic-Based Fertilizers, Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing Agricultural University, 6 Tongwei Road, Nanjing 210095, P.R. China.
Ruifu Zhang, State Key Laboratory of Efficient Utilization of Arid and Semi-Arid Arable Land in Northern China, The Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, 12 Zhongguancun South Street, Beijing 100081, P.R. China; Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, National Engineering Research Center for Organic-Based Fertilizers, Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing Agricultural University, 6 Tongwei Road, Nanjing 210095, P.R. China.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Agorsor IDK, Kagel BT, Danna CH. The Arabidopsis LHT1 amino acid transporter contributes to Pseudomonas simiae-mediated plant growth promotion by modulating bacterial metabolism in the rhizosphere. Plants. 2023;12:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard-Massicotte R, Tessier L, Lécuyer Fet al. Bacillus subtilis early colonization of Arabidopsis thaliana roots involves multiple chemotaxis receptors. mBio. 2016;7:e01664–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alquéres S, Meneses C, Rouws Let al. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. MPMI. 2013;26:937–45. [DOI] [PubMed] [Google Scholar]
- Alvarez Crespo MC, Valverde C. A single mutation in the oprF mRNA leader confers strict translational control by the Gac/Rsm system in Pseudomonas fluorescens CHA0. Curr Microbiol. 2009;58:182–8. [DOI] [PubMed] [Google Scholar]
- Arnaouteli S, Bamford NC, Stanley-Wall NRet al. Bacillus subtilis biofilm formation and social interactions. Nat Rev Micro. 2021;19:600–14. [DOI] [PubMed] [Google Scholar]
- Arnaouteli S, MacPhee CE, Stanley-Wall NR. Just in case it rains: building a hydrophobic biofilm the Bacillus subtilis way. Curr Opin Microbiol. 2016;34:7–12. [DOI] [PubMed] [Google Scholar]
- Badri DV, Vivanco JM. Regulation and function of root exudates. Plant Cell Environ. 2009;32:666–81. [DOI] [PubMed] [Google Scholar]
- Balsanelli E, Serrato RV, de Baura VAet al. Herbaspirillum seropedicae rfbB and rfbC genes are required for maize colonization. Environ Microbiol. 2010;12:2233–44. [DOI] [PubMed] [Google Scholar]
- Beauregard PB, Chai Y, Vlamakis Het al. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci USA. 2013;110:E1621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen RL, van Verk MC, Stringlis IAet al. Unearthing the genomes of plant-beneficial Pseudomonas model strains WCS358, WCS374 and WCS417. BMC Genomics. 2015;16:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen RL, Vismans G, Yu Ket al. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018;12:1496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne C, Ducret A, Hardy GGet al. Adhesins involved in attachment to abiotic surfaces by Gram-negative bacteria. Microbiol Spectr. 2015;3:163–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezemer TM, Van Dam NM. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol. 2005;20:617–24. [DOI] [PubMed] [Google Scholar]
- Blake C, Nordgaard M, Maróti Get al. Diversification of Bacillus subtilis during experimental evolution on Arabidopsis thaliana and the complementarity in root colonization of evolved subpopulations. Environ Microbiol. 2021;23:6122–36. [DOI] [PubMed] [Google Scholar]
- Böhm M, Hurek T, Reinhold-Hurek B. Twitching motility is essential for endophytic rice colonization by the N2-fixing endophyte Azoarcus sp. strain BH72. MPMI. 2007;20:526–33. [DOI] [PubMed] [Google Scholar]
- Bowles JFW The iron oxides: structure, properties reactions occurrence and uses. Mineral Mag. 1997;61:740–1. [Google Scholar]
- Burmølle M, Ren D, Bjarnsholt Tet al. Interactions in multispecies biofilms: do they actually matter?. Trends Microbiol. 2014;22:84–91. [DOI] [PubMed] [Google Scholar]
- Buschart A, Sachs S, Chen Xet al. Flagella mediate endophytic competence rather than act as MAMPs in rice-Azoarcus sp. strain BH72 interactions. MPMI. 2012;25:191–9. [DOI] [PubMed] [Google Scholar]
- Cao Y, Halane MK, Gassmann Wet al. The role of plant innate immunity in the legume-rhizobium symbiosis. Annu Rev Plant Biol. 2017;68:535–61. [DOI] [PubMed] [Google Scholar]
- Cao Y, Wang Y, Gui Cet al. Beneficial rhizobacterium triggers induced systemic resistance of maize to Gibberella stalk rot via calcium signaling. MPMI. 2023a;36:516–28. [DOI] [PubMed] [Google Scholar]
- Cao Z, Zuo W, Wang Let al. Spatial profiling of microbial communities by sequential FISH with error-robust encoding. Nat Commun. 2023b;14:1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans T, Bodé S, Bollyn Jet al. Phosphorus resource partitioning shapes phosphorus acquisition and plant species abundance in grasslands. Nat Plants. 2017;3:16224. [DOI] [PubMed] [Google Scholar]
- Chagas FO, Pessotti RDC, Caraballo-Rodríguez AMet al. Chemical signaling involved in plant-microbe interactions. Chem Soc Rev. 2018;47:1652–704. [DOI] [PubMed] [Google Scholar]
- Chen X, Marszałkowska M, Reinhold-Hurek B. Jasmonic acid, not salicyclic acid restricts endophytic root colonization of rice. Front Plant Sci. 2020;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bonkowski M, Shen Yet al. Root ethylene mediates rhizosphere microbial community reconstruction when chemically detecting cyanide produced by neighbouring plants. Microbiome. 2020;8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Zhang D, Zhi Yet al. Enhanced removal of nitrate in the maize rhizosphere by plant growth-promoting Bacillus megaterium NCT-2, and its colonization pattern in response to nitrate. Chemosphere. 2018;208:316–24. [DOI] [PubMed] [Google Scholar]
- Colaianni NR, Parys K, Lee HSet al. A complex immune response to flagellin epitope variation in commensal communities. Cell Host Microbe. 2021;29:635–649.e9. [DOI] [PubMed] [Google Scholar]
- Compant S, Reiter B, Sessitsch Aet al. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl Environ Microb. 2005;71:1685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Lugo A, De la Torre J, Matilla MAet al. Assessment of the contribution of chemoreceptor-based signalling to biofilm formation. Environ Microbiol. 2016;18:3355–72. [DOI] [PubMed] [Google Scholar]
- Cremer J, Honda T, Tang Yet al. Chemotaxis as a navigation strategy to boost range expansion. Nature. 2019;575:658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhorn T, Fuqua C. Biofilm formation by plant-associated bacteria. Annu Rev Microbiol. 2007;61:401–22. [DOI] [PubMed] [Google Scholar]
- Darrah PR. Models of the rhizosphere—I. Microbial population dynamics around a root releasing soluble and insoluble carbon. Plant Soil. 1991;133:187–99. [Google Scholar]
- Debois D, Fernandez O, Franzil Let al. Plant polysaccharides initiate underground crosstalk with Bacilli by inducing synthesis of the immunogenic lipopeptide surfactin. Environ Microbiol Rep. 2015;7:570–82. [DOI] [PubMed] [Google Scholar]
- Deng Y, Chen H, Li Cet al. Endophyte Bacillus subtilis evade plant defense by producing lantibiotic subtilomycin to mask self-produced flagellin. Commun Biol. 2019;2:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PG, Miller AJ, Hirsch PR. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities?. FEMS Microbiol Ecol. 2010;72:313–27. [DOI] [PubMed] [Google Scholar]
- Dessaux Y, Grandclément C, Faure D. Engineering the rhizosphere. Trends Plant Sci. 2016;21:266–78. [DOI] [PubMed] [Google Scholar]
- Dong W, Zhu Y, Chang Het al. An SHR–SCR module specifies legume cortical cell fate to enable nodulation. Nature. 2021;589:586–90. [DOI] [PubMed] [Google Scholar]
- Du JX, Li Y, Ur-Rehman Set al. Synergistically promoting plant health by harnessing synthetic microbial communities and prebiotics. iScience. 2021;24:102918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeja SS, Suneja-Madan P, Paul Met al. Bacterial endophytes: molecular interactions with their hosts. J Basic Microbiol. 2021;61:475–505. [DOI] [PubMed] [Google Scholar]
- Durr J, Reyt G, Spaepen Set al. A novel signaling pathway required for Arabidopsis endodermal root organization shapes the rhizosphere microbiome. Plant Cell Physiol. 2021;62:248–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earth System Science Data Discussions . Global and regional phosphorus budgets in agricultural systems and their implications for phosphorus-use efficiency, Vol. 41. Göttingen: Copernicus GmbH, 2017, 1–45. [Google Scholar]
- Emonet A, Zhou F, Vacheron Jet al. Spatially restricted immune responses are required for maintaining root meristematic activity upon detection of bacteria. Curr Biol. 2021;31:1012–1028.e7. [DOI] [PubMed] [Google Scholar]
- Espinosa-Urgel M, Salido A, Ramos JL. Genetic analysis of functions involved in adhesion of Pseudomonas putida to seeds. J Bacteriol. 2000;182:2363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B, Borriss R, Bleiss Wet al. Gram-positive rhizobacterium Bacillus amyloliquefaciens FZB42 colonizes three types of plants in different patterns. J Microbiol. 2012;50:38–44. [DOI] [PubMed] [Google Scholar]
- Feng H, Fu R, Hou Xet al. Chemotaxis of beneficial rhizobacteria to root exudates: the first step towards root–microbe rhizosphere interactions. Int J Mol Sci. 2021a;22:6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Wu P, Liu Cet al. Suppression of LjBAK1-mediated immunity by SymRK promotes rhizobial infection in Lotus japonicus. Mol Plant. 2021b;14:1935–50. [DOI] [PubMed] [Google Scholar]
- Feng H, Lv Y, Krell Tet al. Signal binding at both modules of its dCache domain enables the McpA chemoreceptor of Bacillus velezensis to sense different ligands. Proc Natl Acad Sci USA. 2022;119:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Zhang N, Du Wet al. Identification of chemotaxis compounds in root exudates and their sensing chemoreceptors in plant-growth-promoting rhizobacteria Bacillus amyloliquefaciens SQR9. MPMI. 2018;31:995–1005. [DOI] [PubMed] [Google Scholar]
- Feng H, Zhang N, Fu Ret al. Recognition of dominant attractants by key chemoreceptors mediates recruitment of plant growth-promoting rhizobacteria. Environ Microbiol. 2019;21:402–15. [DOI] [PubMed] [Google Scholar]
- Fitriatin BN, Arief DH, Simarmata Tet al. Phosphatase-producing bacteria isolated from Sanggabuana forest and their capability to hydrolyze organic phosphate. J Soil Sci Environ Manag. 2011;2:299–303. [Google Scholar]
- Flemming HC, van Hullebusch ED, Neu TRet al. The biofilm matrix: multitasking in a shared space. Nat Rev Micro. 2023;21:70–86. [DOI] [PubMed] [Google Scholar]
- Flemming HC, Wuertz S. Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Micro. 2019;17:247–60. [DOI] [PubMed] [Google Scholar]
- Frank AC, Guzmán JPS, Shay JE. Transmission of bacterial endophytes. Microorganisms. 2017;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröschel C, Komorek J, Attard Aet al. Plant roots employ cell-layer-specific programs to respond to pathogenic and beneficial microbes. Cell Host Microbe. 2021;29:299–310.e7. [DOI] [PubMed] [Google Scholar]
- Gao S, Wu H, Wang Wet al. Efficient colonization and harpins mediated enhancement in growth and biocontrol of wilt disease in tomato by Bacillus subtilis. Lett Appl Microbiol. 2013;57:526–33. [DOI] [PubMed] [Google Scholar]
- González-Guerrero M, Navarro-Gómez C, Rosa-Núñez Eet al. Forging a symbiosis: transition metal delivery in symbiotic nitrogen fixation. New Phytol. 2023;239:2113–25. [DOI] [PubMed] [Google Scholar]
- González-Mula A, Lang J, Grandclément Cet al. Lifestyle of the biotroph Agrobacterium tumefaciens in the ecological niche constructed on its host plant. New Phytol. 2018;219:350–62. [DOI] [PubMed] [Google Scholar]
- Gu S, Yang T, Shao Zet al. Siderophore-mediated interactions determine the disease suppressiveness of microbial consortia. mSystems. 2020;5:e00811–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Ling N, Li Yet al. Seed-borne, endospheric and rhizospheric core microbiota as predictors of plant functional traits across rice cultivars are dominated by deterministic processes. New Phytol. 2021;230:2047–60. [DOI] [PubMed] [Google Scholar]
- Hallmann J. Plant interactions with endophytic bacteria. In: Biotic Interactions in Plant-Pathogen Associations. Wallingford: CABI Publishing, 2001, 87–119. [Google Scholar]
- Han L, Zhang H, Bai Xet al. The peanut root exudate increases the transport and metabolism of nutrients and enhances the plant growth-promoting effects of Burkholderia pyrrocinia strain P10. BMC Microbiol. 2023;23:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbort CJ, Hashimoto M, Inoue Het al. Root-secreted coumarins and the microbiota interact to improve iron nutrition in Arabidopsis. Cell Host Microbe. 2020;28:825–837.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Singh SK, Peng Let al. Flavonoid-attracted Aeromonas sp. from the Arabidopsis root microbiome enhances plant dehydration resistance. ISME J. 2022;16:2622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennion N, Durand M, Vriet Cet al. Sugars en route to the roots. Transport, metabolism and storage within plant roots and towards microorganisms of the rhizosphere. Physiol Plant. 2019;165:44–57. [DOI] [PubMed] [Google Scholar]
- Hinsa SM, Espinosa-Urgel M, Ramos JLet al. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol Microbiol. 2003;49:905–18. [DOI] [PubMed] [Google Scholar]
- Hoff G, Arias AA, Boubsi Fet al. Surfactin stimulated by pectin molecular patterns and root exudates acts as a key driver of the Bacillus-plant mutualistic interaction. mBio. 2021;12:e0177421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz M, Zarebanadkouki M, Kuzyakov Yet al. Root hairs increase rhizosphere extension and carbon input to soil. Ann Bot. 2018;121:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Thiergart T, Vannier Net al. A microbiota–root–shoot circuit favours Arabidopsis growth over defence under suboptimal light. Nat Plants. 2021;7:1078–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Wang Y, Blake Cet al. Parallel genetic adaptation of Bacillus subtilis to different plant species. Microb Genomics. 2023;9:001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Robert CAM, Cadot Set al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat Commun. 2018;9:2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AC, Jiang T, Liu YXet al. A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science. 2019;364:eaau6389. [DOI] [PubMed] [Google Scholar]
- Huang R, Feng H, Xu Zet al. Identification of adhesins in plant beneficial rhizobacteria Bacillus velezensis SQR9 and their effect on root colonization. MPMI. 2022;35:64–72. [DOI] [PubMed] [Google Scholar]
- Huang R, Shao J, Xu Zet al. A toxin-mediated policing system in Bacillus optimizes division of labor via penalizing cheater-like nonproducers. eLife. 2023;12:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett FM. The signal-transduction network for Pho regulation in Bacillus subtilis. Mol Microbiol. 1996;19:933–9. [DOI] [PubMed] [Google Scholar]
- Hurek T, Reinhold-Hurek B. Azoarcus sp. strain BH72 as a model for nitrogen-fixing grass endophytes. J Biotechnol. 2003;106:169–78. [DOI] [PubMed] [Google Scholar]
- Hussain FA, Dubert J, Elsherbini Jet al. Rapid evolutionary turnover of mobile genetic elements drives bacterial resistance to phages. Science. 2021;374:488–92. [DOI] [PubMed] [Google Scholar]
- Insall RH, Paschke P, Tweedy L. Steering yourself by the bootstraps: how cells create their own gradients for chemotaxis. Trends Cell Biol. 2022;32:585–96. [DOI] [PubMed] [Google Scholar]
- Ivanova LA, Egorov VV, Zabrodskaya YAet al. Matrix is everywhere: extracellular DNA is a link between biofilm and mineralization in Bacillus cereus planktonic lifestyle. npj Biofilms Microbiomes. 2023;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain D, Jones L, Roy S. Gene editing to improve legume-rhizobia symbiosis in a changing climate. Curr Opin Plant Biol. 2023;71:102324. [DOI] [PubMed] [Google Scholar]
- James EK. Nitrogen fixation in endophytic and associative symbiosis. Field Crops Res. 2000;65:197–209. [Google Scholar]
- James EK, Gyaneshwar P, Mathan Net al. Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. MPMI. 2002;15:894–906. [DOI] [PubMed] [Google Scholar]
- Jiang F, Zhang L, Zhou Jet al. Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol. 2021;230:304–15. [DOI] [PubMed] [Google Scholar]
- Kamran M, Ramesh SA, Gilliham Met al. Role of TaALMT1 malate-GABA transporter in alkaline pH tolerance of wheat. Plant Cell Environ. 2020;43:2443–59. [DOI] [PubMed] [Google Scholar]
- Karygianni L, Ren Z, Koo Het al. Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol. 2020;28:668–81. [DOI] [PubMed] [Google Scholar]
- Kashyap A, Jiménez-Jiménez ÁL, Zhang Wet al. Induced ligno-suberin vascular coating and tyramine-derived hydroxycinnamic acid amides restrict Ralstonia solanacearum colonization in resistant tomato. New Phytol. 2022;234:1411–29. [DOI] [PubMed] [Google Scholar]
- Kearns DB. A field guide to bacterial swarming motility. Nat Rev Micro. 2010;8:634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knights HE, Jorrin B, Haskett TLet al. Deciphering bacterial mechanisms of root colonization. Environ Microbiol Rep. 2021;13:428–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Ikemoto Y. Biofilm-associated toxin and extracellular protease cooperatively suppress competitors in Bacillus subtilis biofilms. PLoS Genet. 2019;15:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivova A, Schuck S, Jacoby RPet al. Root-specific camalexin biosynthesis controls the plant growth-promoting effects of multiple bacterial strains. Proc Natl Acad Sci USA. 2019;116:15735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenblum E, Massalha H, Aharoni A. Plant–microbe interactions in the rhizosphere via a circular metabolic economy. Plant Cell. 2022;34:3168–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A, Ramakumar A, Bartels Det al. Complete genome of the mutualistic, N2-fixing grass endophyte Azoarcus sp. strain BH72. Nat Biotechnol. 2006;24:1384–90. [DOI] [PubMed] [Google Scholar]
- Lacal J, García-Fontana C, Muñoz-Martínez Fet al. Sensing of environmental signals: classification of chemoreceptors according to the size of their ligand binding regions. Environ Microbiol. 2010;12:2873–84. [DOI] [PubMed] [Google Scholar]
- Lakshmanan V, Castaneda R, Rudrappa Tet al. Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta. 2013;238:657–68. [DOI] [PubMed] [Google Scholar]
- Lakshmanan V, Kitto SL, Caplan JLet al. Microbe-associated molecular patterns-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol. 2012;160:1642–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J, Planamente S, Mondy Set al. Concerted transfer of the virulence Ti plasmid and companion At plasmid in the Agrobacterium tumefaciens-induced plant tumour. Mol Microbiol. 2013;90:1178–89. [DOI] [PubMed] [Google Scholar]
- Lattanzio V, Lattanzio VMT, Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochemistry. 2006;661:23–67. [Google Scholar]
- Lebeis SL, Paredes SH, Lundberg DSet al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science. 2015;349:860–4. [DOI] [PubMed] [Google Scholar]
- Li E, de Jonge R, Liu Cet al. Rapid evolution of bacterial mutualism in the plant rhizosphere. Nat Commun. 2021a;12:3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Ryo M, Kowalchuk GAet al. Rapid evolution of trait correlation networks during bacterial adaptation to the rhizosphere. Evolution. 2021b;75:1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Zhang H, Jiang Het al. Experimental-evolution-driven identification of Arabidopsis rhizosphere competence genes in Pseudomonas protegens. mBio. 2021c;12:e0092721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, La S, Zhang Xet al. Salt-induced recruitment of specific root-associated bacterial consortium capable of enhancing plant adaptability to salt stress. ISME J. 2021d;15:2865–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang N, Ding Jet al. Spatial distribution of the Pepper Blight (Phytophthora capsici) suppressive microbiome in the rhizosphere. Front Plant Sci. 2022;12:748542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhang J, Shen Cet al. 1-aminocyclopropane-1-carboxylate: a novel and strong chemoattractant for the plant beneficial rhizobacterium Pseudomonas putida UW4. MPMI. 2019;32:750–9. [DOI] [PubMed] [Google Scholar]
- Li X, Lin H, Zhang Wet al. Flagellin induces innate immunity in nonhost interactions that is suppressed by Pseudomonas syringae effectors. Proc Natl Acad Sci USA. 2005;102:12990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Cao Y, Tanaka Ket al. Nonlegumes respond to rhizobial nod factors by suppressing the innate immune response. Science. 2013;341:1384–7. [DOI] [PubMed] [Google Scholar]
- Lidbury IDEA, Borsetto C, Murphy ARJet al. Niche-adaptation in plant-associated bacteroidetes favours specialisation in organic phosphorus mineralisation. ISME J. 2021;15:1040–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lu CY, Tseng ATet al. The ptsG gene encoding the major glucose transporter of Bacillus cereus C1L participates in root colonization and beneficial metabolite production to induce plant systemic disease resistance. MPMI. 2020;33:256–71. [DOI] [PubMed] [Google Scholar]
- Liu H, Li J, Carvalhais LCet al. Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 2021;229:2873–85. [DOI] [PubMed] [Google Scholar]
- Liu S, He F, Kuzyakov Yet al. Nutrients in the rhizosphere: a meta-analysis of content, availability, and influencing factors. Sci Total Environ. 2022;826:153908. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen L, Wu Get al. Identification of root-secreted compounds involved in the communication between cucumber, the beneficial Bacillus amyloliquefaciens, and the soil-borne pathogen Fusarium oxysporum. MPMI. 2017;30:53–62. [DOI] [PubMed] [Google Scholar]
- Liu Y, Feng H, Chen Let al. Root-secreted spermine binds to Bacillus amyloliquefaciens SQR9 histidine kinase KinD and modulates biofilm formation. MPMI. 2020;33:423–32. [DOI] [PubMed] [Google Scholar]
- Liu Y, Feng H, Fu Ret al. Induced root-secreted d-galactose functions as a chemoattractant and enhances the biofilm formation of Bacillus velezensis SQR9 in an McpA-dependent manner. Appl Microbiol Biotechnol. 2020;104:785–97. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shu X, Chen Let al. Plant commensal type VII secretion system causes iron leakage from roots to promote colonization. Nat Microbiol. 2023;8:1434–49. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang N, Qiu Met al. Enhanced rhizosphere colonization of beneficial Bacillus amyloliquefaciens SQR9 by pathogen infection. FEMS Microbiol Lett. 2014;353:49–56. [DOI] [PubMed] [Google Scholar]
- Liu Z, Beskrovnaya P, Melnyk RAet al. A genome-wide screen identifies genes in rhizosphere-associated Pseudomonas required to evade plant defenses. Lindow SE (ed.), mBio. 2018;9:e00433–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wu Y, Yang Fet al. BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc Natl Acad Sci USA. 2013;110:6205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D, Fischbach MA, Chu Fet al. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci USA. 2009;106:280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López JL, Fourie A, Poppeliers SWMet al. Growth rate is a dominant factor predicting the rhizosphere effect. ISME J. 2023;17:1396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 2009;63:541–56. [DOI] [PubMed] [Google Scholar]
- Ma KW, Niu Y, Jia Yet al. Coordination of microbe–host homeostasis by crosstalk with plant innate immunity. Nat Plants. 2021;7:814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner P, Crowley D, Rengel Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis—model and research methods. Soil Biol Biochem. 2011;43:883–94. [Google Scholar]
- Mataigne V, Vannier N, Vandenkoornhuyse Pet al. Multi-genome metabolic modeling predicts functional inter-dependencies in the Arabidopsis root microbiome. Microbiome. 2022;10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveeva T, Otten L. Opine biosynthesis in naturally transgenic plants: genes and products. Phytochemistry. 2021;189:112813. [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan Jet al. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–80. [DOI] [PubMed] [Google Scholar]
- Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev. 2013;37:634–63. [DOI] [PubMed] [Google Scholar]
- Mesny F, Hacquard S, Thomma BP. Co-evolution within the plant holobiont drives host performance. EMBO Rep. 2023;24:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot T. The elusive engine in Myxococcus xanthus gliding motility. Cell Mol Life Sci. 2007;64:2733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet YA, Danna CH, Clay NKet al. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell. 2010;22:973–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Jiménez E, Plaza C, Saiz Het al. Aridity and reduced soil micronutrient availability in global drylands. Nat Sustain. 2019;2:371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq S, Shafiq M, Tariq MRet al. Interaction between bacterial endophytes and host plants. Front Plant Sci. 2023;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Xiao Y, Song Met al. Wsp system oppositely modulates antibacterial activity and biofilm formation via FleQ-FleN complex in Pseudomonas putida. Environ Microbiol. 2022;24:1543–59. [DOI] [PubMed] [Google Scholar]
- Nordgaard M, Blake C, Maróti Get al. Experimental evolution of Bacillus subtilis on Arabidopsis thaliana roots reveals fast adaptation and improved root colonization. iScience. 2022;25:104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Banion BS, Jones P, Demetros AAet al. Plant myo-inositol transport influences bacterial colonization phenotypes. Curr Biol. 2023;33:3111–3124.e5. [DOI] [PubMed] [Google Scholar]
- O'Neal L, Vo L, Alexandre G. Specific root exudate compounds sensed by dedicated chemoreceptors shape Azospirillum brasilense chemotaxis in the rhizosphere. Appl Environ Microb. 2020;86:e01026–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer-Shaanan Y, Jakoby G, Starr MLet al. A dynamic rhizosphere interplay between tree roots and soil bacteria under drought stress. eLife. 2022;11:e79679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega Á, Zhulin IB, Krell T. Sensory repertoire of bacterial chemoreceptors. Microbiol Mol Biol Rev. 2017;81:e00033–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys K, Colaianni NR, Lee HSet al. Signatures of antagonistic pleiotropy in a bacterial flagellin epitope. Cell Host Microbe. 2021;29:620–634.e9. [DOI] [PubMed] [Google Scholar]
- Pfeilmeier S, George J, Morel Aet al. Expression of the Arabidopsis thaliana immune receptor EFR in Medicago truncatula reduces infection by a root pathogenic bacterium, but not nitrogen-fixing rhizobial symbiosis. Plant Biotechnol J. 2019;17:569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe G, Sørensen I, Jiao Cet al. Cutin and suberin: assembly and origins of specialized lipidic cell wall scaffolds. Curr Opin Plant Biol. 2020;55:11–20. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Zamioudis C, Berendsen RLet al. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 2014;52:347–75. [DOI] [PubMed] [Google Scholar]
- Pizarro-Tobías P, Udaondo Z, Roca Aet al. Events in root colonization by Pseudomonas putida. In: Ramos J-L, Goldberg JB, Filloux A (eds.), Pseudomonas. Dordrecht: Springer, 2015, 251–86. [Google Scholar]
- Pliego C, De Weert S, Lamers Get al. Two similar enhanced root-colonizing Pseudomonas strains differ largely in their colonization strategies of avocado roots and Rosellinia necatrix hyphae. Environ Microbiol. 2008;10:3295–304. [DOI] [PubMed] [Google Scholar]
- Pomerleau M, Charron-Lamoureux V, Léonard Let al. Adaptative laboratory evolution reveals biofilm regulating genes as key players in B. subtilis root colonization. Biorxiv. 2023. 10.1101/2023.07.04.547689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, He Y, She Qet al. Heterogeneity in respiratory electron transfer and adaptive iron utilization in a bacterial biofilm. Nat Commun. 2019;10:3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmat Z, Sohail MN, Perrine-Walker Fet al. Balancing nitrate acquisition strategies in symbiotic legumes. Planta. 2023;258:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rat A, Naranjo HD, Krigas Net al. Endophytic bacteria from the roots of the medicinal plant Alkanna tinctoria Tausch (Boraginaceae): exploration of plant growth promoting properties and potential role in the production of plant secondary metabolites. Front Microbiol. 2021;12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold-Hurek B, Hurek T. Life in grasses: diazotrophic endophytes. Trends Microbiol. 1998;6:139–44. [DOI] [PubMed] [Google Scholar]
- Reinhold-Hurek B, Hurek T. Living inside plants: bacterial endophytes. Curr Opin Plant Biol. 2011;14:435–43. [DOI] [PubMed] [Google Scholar]
- Reinhold-Hurek B, Krause A, Leyser Bet al. The rice apoplast as a habitat for endophytic n2-fixing bacteria. In: The Apoplast of Higher Plants: Compartment of Storage, Transport and Reactions. Dordrecht: Springer, 2007, 427–43. [Google Scholar]
- Reinhold-Hurek B, Maes T, Gemmer Set al. An endoglucanase is involved in infection of rice roots by the not-cellulose-metabolizing endophyte Azoarcus Sp. strain BH72. MPMI. 2006;19:181–8. [DOI] [PubMed] [Google Scholar]
- Ren D, Madsen JS, Sørensen SJet al. High prevalence of biofilm synergy among bacterial soil isolates in cocultures indicates bacterial interspecific cooperation. ISME J. 2015;9:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe SA, Griffiths J, Ton J. Crying out for help with root exudates: adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr Opin Microbiol. 2019;49:73–82. [DOI] [PubMed] [Google Scholar]
- Roncato-Maccari LDB, Ramos HJO, Pedrosa FOet al. Endophytic Herbaspirillum seropedicae expresses nif genes in gramineous plants. FEMS Microbiol Ecol. 2003;45:39–47. [DOI] [PubMed] [Google Scholar]
- Rossi FA, Medeot DB, Liaudat JP. et al. Azospirillum brasilense, mutations in flmA or flmB genes affect polar flagellum assembly, surface polysaccharides, and attachment to maize roots. Microbiol Res. 2016;190:55–62. [DOI] [PubMed] [Google Scholar]
- Roy S, Liu W, Nandety RSet al. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell. 2020;32:15–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrappa T, Czymmek KJ, Paré PWet al. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008;148:1547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadiq FA, Burmølle M, Heyndrickx Met al. Community-wide changes reflecting bacterial interspecific interactions in multispecies biofilms. Crit Rev Microbiol. 2021;47:338–58. [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Kanno S, Mabuchi Aet al. A phytochrome-B-mediated regulatory mechanism of phosphorus acquisition. Nat Plants. 2018;4:1089–101. [DOI] [PubMed] [Google Scholar]
- Sampedro I, Parales RE, Krell Tet al. Pseudomonas chemotaxis. FEMS Microbiol Rev. 2015;39:17–46. [DOI] [PubMed] [Google Scholar]
- Sánchez-Gil JJ, Poppeliers SWM, Vacheron Jet al. The conserved iol gene cluster in Pseudomonas is involved in rhizosphere competence. Curr Biol. 2023;33:3097–3110.e6. [DOI] [PubMed] [Google Scholar]
- Sanchis-López C, Cerna-Vargas JP, Santamaría-Hernando Set al. Prevalence and specificity of chemoreceptor profiles in plant-associated bacteria. mSystems. 2021;6:e00951–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam R, Luu VT, Weinhold Aet al. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc Natl Acad Sci USA. 2015;112:E5013–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam R, Menezes RC, Grabe Vet al. A suite of complementary biocontrol traits allows a native consortium of root-associated bacteria to protect their host plant from a fungal sudden-wilt disease. Mol Ecol. 2019;28:1154–69. [DOI] [PubMed] [Google Scholar]
- Santos-Medellín C, Liechty Z, Edwards Jet al. Prolonged drought imparts lasting compositional changes to the rice root microbiome. Nat Plants. 2021;7:1065–77. [DOI] [PubMed] [Google Scholar]
- Santoyo G. How plants recruit their microbiome? New insights into beneficial interactions. J Adv Res. 2022;40:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse J, Martinoia E, Northen T. Feed Your Friends: do plant exudates shape the root microbiome?. Trends Plant Sci. 2018;23:25–41. [DOI] [PubMed] [Google Scholar]
- Schulz-Bohm K, Gerards S, Hundscheid Met al. Calling from distance: attraction of soil bacteria by plant root volatiles. ISME J. 2018;12:1252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu LJ, Kahlon PS, Ranf S. The power of patterns: new insights into pattern-triggered immunity. New Phytol. 2023;240:960–7. [DOI] [PubMed] [Google Scholar]
- Singh P, Singh RK, Zhou Yet al. Unlocking the strength of plant growth promoting Pseudomonas in improving crop productivity in normal and challenging environments: a review. J Plant Interact. 2022;17:220–38. [Google Scholar]
- Singh VS, Dubey BK, Rai Set al. Engineering D-glucose utilization in Azospirillum brasilense Sp7 promotes rice root colonization. Appl Microbiol Biotechnol. 2022;106:7891–903. [DOI] [PubMed] [Google Scholar]
- Soluch R, Hülter NF, Romero Picazo Det al. Colonization dynamics of Pantoea agglomerans in the wheat root habitat. Environ Microbiol. 2021;23:2260–73. [DOI] [PubMed] [Google Scholar]
- Song Y, Wilson AJ, Zhang XCet al. FERONIA restricts Pseudomonas in the rhizosphere microbiome via regulation of reactive oxygen species. Nat Plants. 2021;7:644–54. [DOI] [PubMed] [Google Scholar]
- Soyano T, Liu M, Kawaguchi Met al. Leguminous nodule symbiosis involves recruitment of factors contributing to lateral root development. Curr Opin Plant Biol. 2021;59:102000. [DOI] [PubMed] [Google Scholar]
- Spiers AJ, Rainey PB. The Pseudomonas fluorescens SBW25 wrinkly spreader biofilm requires attachment factor, cellulose fibre and LPS interactions to maintain strength and integrity. Microbiology. 2005;151:2829–39. [DOI] [PubMed] [Google Scholar]
- Stringlis IA, Yu K, Feussner Ket al. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc Natl Acad Sci USA. 2018a;115:E5213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringlis IA, Zhang H, Pieterse CMJet al. Microbial small molecules-weapons of plant subversion. Nat Prod Rep. 2018b;35:410–33. [DOI] [PubMed] [Google Scholar]
- Sun X, Xu Z, Xie Jet al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022;16:774–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Bouchez O, Cruveiller Set al. Modulation of quorum sensing as an adaptation to nodule cell infection during experimental evolution of legume symbionts. mBio. 2020;11:e03129–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira PJPL, Colaianni NR, Law TFet al. Specific modulation of the root immune system by a community of commensal bacteria. Proc Natl Acad Sci USA. 2021;118:e2100678118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Zhang C, Zhang Ret al. Collective motion enhances chemotaxis in a two- dimensional bacterial swarm. Biophys J. 2021a;120:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Sun B, Shi Het al. Sucrose triggers a novel signaling cascade promoting Bacillus subtilis rhizosphere colonization. ISME J. 2021b;15:2723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovi N, Frenk S, Hadar Yet al. Host specificity and spatial distribution preference of three Pseudomonas isolates. Front Microbiol. 2019;9:3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley L, Yannarell SM, Huynh TNet al. Cyclic di-AMP acts as an extracellular signal that impacts Bacillus subtilis biofilm formation and plant attachment. mBio. 2018;9:e00341–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapet PL, Verbon EH, Bosma RRet al. Mechanisms underlying iron deficiency-induced resistance against pathogens with different lifestyles. J Exp Bot. 2021;72:2231–41. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Leach JE, Tringe SGet al. Plant–microbiome interactions: from community assembly to plant health. Nat Rev Micro. 2020;18:607–21. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Schmidt W. Mobilization of iron by plant-borne coumarins. Trends Plant Sci. 2017;22:538–48. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Wang J, Geldner Net al. Spatiotemporal control of root immune responses during microbial colonization. Curr Opin Plant Biol. 2023;74:102369. [DOI] [PubMed] [Google Scholar]
- Turner BL. Resource partitioning for soil phosphorus: a hypothesis. J Ecol. 2008;96:698–702. [Google Scholar]
- Turner TR, James EK, Poole PS. The plant microbiome. Genome Biol. 2013;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipilevich E, Russ D, Dangl JLet al. Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria. Cell Host Microbe. 2021;29:1507–1520.e4. [DOI] [PubMed] [Google Scholar]
- Verbon EH, Liberman LM, Zhou Jet al. Cell-type-specific transcriptomics reveals that root hairs and endodermal barriers play important roles in beneficial plant-rhizobacterium interactions. Mol Plant. 2023;16:1160–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vílchez JI, Yang Y, He Det al. DNA demethylases are required for myo-inositol-mediated mutualism between plants and beneficial rhizobacteria. Nat Plants. 2020;6:983–95. [DOI] [PubMed] [Google Scholar]
- Vismans G, van Bentum S, Spooren Jet al. Coumarin biosynthesis genes are required after foliar pathogen infection for the creation of a microbial soil-borne legacy that primes plants for SA-dependent defenses. Sci Rep. 2022;12:22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Peris V, de Ollas C, Gómez-Cadenas Aet al. Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep. 2020;39:3–17. [DOI] [PubMed] [Google Scholar]
- von Bodman SB, Bauer WD, Coplin DL. Quorum sensing in plant-pathogenic bacteria. Annu Rev Phytopathol. 2003;41:455–82. [DOI] [PubMed] [Google Scholar]
- Wang L, Chen S, Yu B. Poly-γ-glutamic acid: recent achievements, diverse applications and future perspectives. Trends Food Sci Technol. 2022;119:1–12. [Google Scholar]
- Wang W, Jia T, Qi Tet al. Root exudates enhanced rhizobacteria complexity and microbial carbon metabolism of toxic plants. iScience. 2022;25:105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Pruitt RN, Nürnberger Tet al. Evasion of plant immunity by microbial pathogens. Nat Rev Micro. 2022;20:449–64. [DOI] [PubMed] [Google Scholar]
- Wang Z, Song Y. Toward understanding the genetic bases underlying plant-mediated “cry for help” to the microbiota. Imeta. 2022;1:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei HL, Chakravarthy S, Mathieu Jet al. Pseudomonas syringae pv. tomato DC3000 type III secretion effector polymutants reveal an interplay between HopAD1 and AvrPtoB. Cell Host Microbe. 2015;17:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei HL, Zhang W, Collmer A. Modular study of the type III effector repertoire in Pseudomonas syringae pv. tomato DC3000 reveals a matrix of effector interplay in pathogenesis. Cell Rep. 2018;23:1630–8. [DOI] [PubMed] [Google Scholar]
- Wen T, Ding Z, Thomashow LSet al. Deciphering the mechanism of fungal pathogen-induced disease-suppressive soil. New Phytol. 2023;238:2634–50. [DOI] [PubMed] [Google Scholar]
- Wheatley RM, Poole PS. Mechanisms of bacterial attachment to roots. FEMS Microbiol Rev. 2018;42:448–61. [DOI] [PubMed] [Google Scholar]
- Wippel K, Tao K, Niu Yet al. Host preference and invasiveness of commensal bacteria in the Lotus and Arabidopsis root microbiota. Nat Microbiol. 2021;6:1150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Rensing C, Han Det al. Genome-resolved metagenomics reveals distinct phosphorus acquisition strategies between soil microbiomes. mSystems. 2022;7:e01107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian L, Yu G, Wei Yet al. A bacterial effector protein hijacks plant metabolism to support pathogen nutrition. Cell Host Microbe. 2020;28:548–557.e7. [DOI] [PubMed] [Google Scholar]
- Xie S, Jiang L, Wu Qet al. Maize root exudates recruit Bacillus amyloliquefaciens OR2-30 to inhibit Fusarium graminearum infection. Phytopathology®. 2022;112:1886–93. [DOI] [PubMed] [Google Scholar]
- Xin DW, Liao S, Xie ZPet al. Functional analysis of NopM, a novel E3 ubiquitin ligase (NEL) domain effector of Rhizobium sp. strain NGR234. PLoS Pathog. 2012;8:e1002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin XF, He SY. Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu Rev Phytopathol. 2013;51:473–98. [DOI] [PubMed] [Google Scholar]
- Xing Y, Xu N, Bhandari DDet al. Bacterial effector targeting of a plant iron sensor facilitates iron acquisition and pathogen colonization. Plant Cell. 2021;33:2015–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Wang E. Diversity and regulation of symbiotic nitrogen fixation in plants. Curr Biol. 2023;33:R543–59. [DOI] [PubMed] [Google Scholar]
- Xu S, Yang N, Zheng Set al. The spo0A-sinI-sinR regulatory circuit plays an essential role in biofilm formation, nematicidal activities, and plant protection in Bacillus cereus AR156. MPMI. 2017;30:603–19. [DOI] [PubMed] [Google Scholar]
- Xu Z, Mandic-Mulec I, Zhang Het al. Antibiotic bacillomycin D affects iron acquisition and biofilm formation in Bacillus velezensis through a Btr-mediated FeuABC-dependent pathway. Cell Rep. 2019;29:1192–1202.e5. [DOI] [PubMed] [Google Scholar]
- Xu Z, Xie J, Zhang Het al. Enhanced control of plant wilt disease by a xylose-inducible degQ gene engineered into Bacillus velezensis strain SQR9XYQ. Phytopathology®. 2019;109:36–43. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhang R, Wang Det al. Enhanced control of cucumber wilt disease by Bacillus amyloliquefaciens SQR9 by altering the regulation of its DegU phosphorylation. Appl Environ Microb. 2014;80:2941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Tajima H, Cline LCet al. Genetic modification of flavone biosynthesis in rice enhances biofilm formation of soil diazotrophic bacteria and biological nitrogen fixation. Plant Biotechnol J. 2022;20:2135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Zheng M, Dong Wet al. Plant disease resistance-related pathways recruit beneficial bacteria by remodeling root exudates upon Bacillus cereus AR156 treatment. Microbiol Spectr. 2023;11:e0361122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Dong Y, Friman VPet al. Carbon resource richness shapes bacterial competitive interactions by alleviating growth-antibiosis trade-off. Funct Ecol. 2019;33:868–75. [Google Scholar]
- Yang J, Lan L, Jin Yet al. Mechanisms underlying legume–rhizobium symbioses. JIPB. 2022;64:244–67. [DOI] [PubMed] [Google Scholar]
- Yang N, Nesme J, Røder HLet al. Emergent bacterial community properties induce enhanced drought tolerance in Arabidopsis. npj Biofilms Microbiomes. 2021;7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Tang F, Gao Met al. R gene-controlled host specificity in the legume-rhizobia symbiosis. Proc Natl Acad Sci USA. 2010;107:18735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Wei Z, Friman VPet al. Resource availability modulates biodiversity-invasion relationships by altering competitive interactions. Environ Microbiol. 2017;19:2984–91. [DOI] [PubMed] [Google Scholar]
- Yu G, Derkacheva M, Rufian JSet al. The Arabidopsis E3 ubiquitin ligase PUB4 regulates BIK1 and is targeted by a bacterial type-III effector. EMBO J. 2022;41:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Xiao A, Dong Ret al. Suppression of innate immunity mediated by the CDPK-Rboh complex is required for rhizobial colonization in Medicago truncatula nodules. New Phytol. 2018;220:425–34. [DOI] [PubMed] [Google Scholar]
- Yu K, Liu Y, Tichelaar Ret al. Rhizosphere-associated Pseudomonas suppress local root immune responses by gluconic acid-mediated lowering of environmental pH. Curr Biol. 2019;29:3913–3920.e4. [DOI] [PubMed] [Google Scholar]
- Yu K, Pieterse CMJ, Bakker PAHMet al. Beneficial microbes going underground of root immunity. Plant Cell Environ. 2019;42:2860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Zhao J, Wen Tet al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome. 2018;6:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Jiang Z, Bi Get al. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature. 2021;592:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zboralski A, Filion M. Genetic factors involved in rhizosphere colonization by phytobeneficial Pseudomonas spp. Comput Struct Biotechnol J. 2020;18:3539–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhalnina K, Louie KB, Hao Zet al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol. 2018;3:470–80. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chen F, Zhao Het al. Colonization on cucumber root and enhancement of chlorimuron-ethyl degradation in the rhizosphere by Hansschlegelia zhihuaiae S113 and root exudates. J Agric Food Chem. 2018;66:4584–91. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu Y, Wu Get al. Bacillus velezensis tolerance to the induced oxidative stress in root colonization contributed by the two-component regulatory system sensor ResE. Plant Cell Environ. 2021;44:3094–102. [DOI] [PubMed] [Google Scholar]
- Zhang H, Qian Y, Fan Det al. Biofilm formed by Hansschlegelia zhihuaiae S113 on root surface mitigates the toxicity of bensulfuron-methyl residues to maize. Environ Pollut. 2022;292:118366. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wang D, Liu Yet al. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil. 2014;374:689–700. [Google Scholar]
- Zhang N, Yang D, Wang Det al. Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genomics. 2015;16:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang R, Shang Qet al. The new flagella-associated collagen-like proteins ClpB and ClpC of Bacillus amyloliquefaciens FZB42 are involved in bacterial motility. Microbiol Res. 2016;184:25–31. [DOI] [PubMed] [Google Scholar]