Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease, is characterized by hepatic steatosis and metabolic dysfunction and is often associated with obesity and insulin resistance. Recent research indicates a rapid escalation in MASLD cases, with projections suggesting a doubling in the United States by 2030. This review focuses on the central role of mitochondria in the pathogenesis of MASLD and explores potential therapeutic interventions. Mitochondria are dynamic organelles that orchestrate hepatic energy production and metabolism and are critically involved in MASLD. Dysfunctional mitochondria contribute to lipid accumulation, inflammation, and liver fibrosis. Genetic associations further underscore the relationship between mitochondrial dynamics and MASLD susceptibility. Although U.S. Food and Drug Administration-approved treatments for MASLD remain elusive, ongoing clinical trials have highlighted promising strategies that target mitochondrial dysfunction, including vitamin E, metformin, and glucagon-like peptide-1 receptor agonists. In preclinical studies, novel therapeutics, including nicotinamide adenine dinucleotide+ precursors, urolithin A, spermidine, and mitoquinone, have shown beneficial effects, such as improving mitochondrial quality control, reducing oxidative stress, and ameliorating hepatic steatosis and inflammation. In conclusion, mitochondrial dysfunction is central to MASLD pathogenesis. The innovative mitochondria-targeted approaches discussed in this review offer a promising avenue for reducing the burden of MASLD and improving global quality of life.
Keywords: Metabolic dysfunction-associated steatotic liver disease, Mitochondria, Mitochondrial quality control
INTRODUCTION
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a new name for non-alcoholic fatty liver disease (NAFLD) to focus on steatotic liver disease associated with metabolic dysfunction and includes the presence of at least one of five cardiometabolic risk factors of obesity, glucose intolerance, dyslipidemia, and hypertension.1 With a global prevalence of approximately 30%, MASLD is the most common chronic liver disease worldwide.2 A recent study has highlighted a significantly rapid increase in the number of patients with MASLD.3 Projections based on previous estimates have indicated a doubling of MASLD cases in the United States by 2030.4,5 Approximately 60% to 80% of individuals with type 2 diabetes mellitus and 80% of individuals with obesity are affected by MASLD.6,7 Although obesity is closely related to MASLD,8 lean individuals can experience the disease, which is a significant health concern. The development of lean MASLD can be influenced by loss of muscle mass and visceral obesity among other factors.9
The hallmark of MASLD is greater than 5% fat accumulation in the liver upon histological examination. This steatosis can progress to inflammatory metabolic dysfunction-associated steatohepatitis (MASH), followed by fibrosis, cirrhosis, and hepatocellular carcinoma (HCC), culminating in liver failure.10 In patients with MASLD, 41.9% experience steatohepatitis; approximately 32% with MASH experience liver fibrosis progression or regression.11 This complex situation demonstrates how MASLD contributes to the increasing incidence of hepatic cirrhosis and HCC worldwide. In addition, MASLD was associated with risk of all-cause and cardiovascular death in a large population-based cohort of Korean adults.12 Owing to the serious effects associated with these illnesses, it is necessary to explore the causes of MASLD and to determine ways to develop effective therapeutic interventions.
Several studies have shown the existence of molecular, biochemical, and biophysical perturbations in mitochondrial dynamics relevant to MASLD.13-15 Despite the insights gained from these studies, the mechanistic orchestration leading to mitochondrial dysfunction and the triggering of NAFLD pathogenesis and progression remain to be elucidated. In this review, we discussed the recent findings on the role of mitochondria in metabolism and their clinical implications in MASLD treatment.
ROLE OF MITOCHONDRIA IN THE LIVER
Mitochondria as a metabolic hub in the liver
The liver is a central organ of metabolic adaptability in humans and is characterized by dynamic responsiveness to shifts in energy demand and availability. Within hepatocytes, mitochondria are critical organelles that are involved in energy production and act as metabolic hubs.16,17 Mitochondria play a role in hepatic-specific anabolic pathways, including de novo lipogenesis, gluconeogenesis, and nonspecific one-carbon metabolism, and in catabolic pathways such as tricarboxylic acid and urea cycles, β-oxidation, ketogenesis, and the electron transport chain linked to the production of reactive oxygen species (ROS).16
Amid this intricate adaptation, hepatocyte mitochondria have emerged as key collaborators that finely tune metabolic flexibility. When these delicate processes are disrupted, mitochondrial problems cause several cellular stresses, such as increased ROS production, impaired oxidative phosphorylation (OxPhos), and a deranged immune response.18 These disturbances can lead to the development of metabolic diseases in the liver.
Mitochondrial oxidative stress
Recent studies have highlighted the importance of mitochondrial processes, specifically oxidative stress, mitophagy, and quality control, in the balance between liver function and disease progression.19,20 Mitochondria are essential for energy production. OxPhos, which occurs within mitochondria, is a significant source of oxidative stress as it generates adenosine triphosphate (ATP), superoxide anions, and hydrogen peroxide (H2O2) as byproducts.19,21 Increased β-oxidation within mitochondria and peroxisomes is also a source of ROS production. In addition to the mitochondria, the endoplasmic reticulum (ER) can also produce ROS.19
Antioxidant defense systems consist of enzymatic and nonenzymatic components.22 The enzymatic components comprise a collection of enzymes that effectively counteract ROS production. Prominent examples include alpha-dioxygenase, ascorbate peroxidase, superoxide dismutase, catalase, glutathione peroxidase (GPX), and glutathione (GSH) reductase.22 In contrast, nonenzymatic elements include small molecules such as GSH, ascorbic acid (vitamin C), retinol (vitamin A), melatonin, and tocopherol (vitamin E).22 These molecules act as electron acceptors and protect biomolecules and cellular structures from ROS-induced damage.
Excessive ROS production overwhelms antioxidant defenses, leading to oxidative stress and subsequent cellular damage. Additionally, the inflammatory response significantly affects oxidative stress.23 Disruption of the redox balance impairs insulin signaling and lipid metabolism, leading to lipid accumulation and liver inflammation.19 These are important processes in the progression of liver injury. When the liver is injured, oxidative stress triggers the activation of redox-sensitive transcription factors, such as nuclear factor kappa B (NF-κB), early growth factor 1, and activator protein 1. As a result, an inflammatory response follows, coupled with the initiation of cell death pathways within hepatocytes.19,24,25 Taken together, these findings highlight the importance of proper regulation of ROS levels and antioxidant defenses in cellular health and prevention of harmful outcomes.26
In response to oxidative stress, mitochondria possess an efficient system to repair oxidatively damaged macromolecules.27 The lipid composition of mitochondrial membranes makes them highly prone to ROS-induced oxidative damage. In particular, cardiolipin (CL), a glycerophospholipid dimer within the inner mitochondrial membrane, serves as an anchoring point for respiratory supercomplexes and mitochondrial DNA (mtDNA) during replication and mitochondrial protein transport.28 Maintaining the integrity of CL is critical for mitochondrial health because its oxidation is associated with cytochrome c release and increased mitochondrial membrane permeability to apoptotic factors.
Oxidative modification of CL and its degradation products attenuates respiratory chain complex activities while promoting mitochondrial pore opening and permeability transitions. As damaged CL is detrimental to mitochondria, oxidized CL is rapidly degraded.28 CL phospholipase hydroxysteroid 17-β dehydrogenase 10 was recently shown to mediate the degradation of oxidized CL.28,29 Key enzymes such as GPX 4 counteract mitochondrial lipid peroxidation through direct detoxification of membrane lipid hydroperoxides.30 In addition, ubiquinol is involved in the repair of peroxidized mitochondrial lipids, and dihydroorotate dehydrogenase plays a role in generating ubiquinol to counteract the effects of lipid peroxidation and ferroptosis.31,32
Mitochondrial quality control
In MASLD, several factors contribute to excessive ROS production, including decreased expression of intracellular antioxidant enzymes, GSH depletion, imbalances in ROS production and detoxification due to inflammatory reactions, and leukocyte accumulation in the liver. The abundance of ROS generated by these processes disrupts the balance of antioxidant defense systems in the liver, exacerbating oxidative damage.33 Therefore, an appropriate ROS balance is essential for mitochondrial integrity and function through mitochondrial quality control (MQC) processes.34
Mitochondria have a diverse array of mechanisms for maintenance of their intricate homeostasis.35 First, they possess an intrinsic proteolytic system that degrades misfolded proteins that can potentially compromise mitochondrial function.36 Mitochondrial proteases and the cytosolic ubiquitin–proteasome system (UPS) act as the first-line of cellular defense by eliminating damaged, oxidized, or misfolded mitochondrial protein.37 There are two membrane-bound ATPases associated with diverse cellular activities (AAA) that are responsible for quality control along the inner mitochondrial membrane and are part of the AAA+ superfamily.38 Matrix AAA protease targets the matrix, whereas intermembrane AAA protease targets the intermembrane spac.38 The OMA1 zinc metallopeptidase (OMA1) and Lon protease also contribute to this process. The ClpXP protease, a well-characterized and established AAA+ protease, comprises hexamers of AAA+ ATPase (ClpX) and tetradecameric peptidase (ClpP)39 and regulates the mitochondrial unfolded protein response (mtUPR).40-43 Notably, the cytosolic UPS also aids in MQC by detecting and eliminating misdirected or misfolded proteins. To restore cellular balance, cytosolic UPS also degrades mitochondrial outer membrane proteins in a process termed outer mitochondrial membrane-associated degradation, which is similar to ER-associated protein degradation.44,45
Second, the continuous process of mitochondrial dynamics involving fission and fusion provides a dynamic repair mechanism that eliminates dysfunctional components through fission-driven segregation and promotes material exchange between intact mitochondria through fusion-mediated interaction.46 Fission and fusion are essential for maintaining mitochondrial integrity. During fission, damaged portions are selectively removed, leaving healthy segments. Conversely, fusion structurally complements impaired mitochondria and facilitates the exchange of mtDNA. Proteins regulating mitochondrial dynamics are significantly associated with MASLD. The levels of proteins that are associated with mitochondrial dynamics, such as dynamin-related protein 1 (Drp1), mitochondrial fission 1 protein (Fis1), and mitofusion-2, decrease in mice after 6 months of a Western diet,47,48 whereas overexpression of Drp1 and Fis1 can alleviate hepatic injury.13
Third, oxidative stress causes a group of mitochondria to bud and create mitochondria-derived vesicles (MDVs). MDVs show remarkable size uniformity, ranging from 70 to 150 nm, and undergo cleavage independent of Drp1. MDVs bifurcate into two pathways: they are either directed to the late endosome/multivesicular body and then combine with lysosomes to coordinate the breakdown of oxidized mitochondrial proteins within the MDVs,49-51 or they embark on a distinct trajectory, targeting a specialized subset of peroxisomes.52 The PTEN induced kinase 1 (PINK1) and cytosolic ubiquitin E3 ligase parkin are required for the binding of MDVs to lysosomes.53 Both PINK1 and parkin are mutated in familial cases of Parkinson’s disease and function in a shared pathway in MQC.53
Disrupted mitochondria undergo a transformative pathway, coalescing into mitochondrial spheroids and acquiring lysosomal markers, potentially serving as an alternative pathway for eliminating compromised mitochondria. Mitophagy is the process of autophagy in which damaged mitochondria are engulfed and degraded within lysosomes as they become irreparable through fission and fusion.26,54 Dysregulated mitophagy compromises mitochondrial turnover and quality control, leading to impaired OxPhos and energy production, culminating in abnormal lipid metabolism and hepatic steatosis and contributing to the pathogenesis of metabolic disorders.
MITOCHONDRIAL DYSFUNCTION-MEDIATED METABOLIC DYSFUNCTION-ASSOCIATED STEATOTIC LIVER DISEASE
Role of mitochondrial dysfunction in MASLD
In MASLD, hepatic mitochondria are structurally and molecularly altered.55 Mitochondrial abnormalities, including altered cristae and reduced ATP production, have been observed in both mice and human studies.55 In patients with MASH, mitochondria have low maximal respiration, increased H2O2 production, lipid peroxidation, and decreased antioxidant capacity.56 Therefore, mitochondria have been implicated in the pathogenesis and progression of MASLD.57
Mitochondrial dysfunction exacerbates hepatic lipid accumulation, initiates inflammatory and fibrogenic responses, and induces cell death. Obesity resulting from overnutrition causes an excess of free fatty acids (FFAs)58 in the circulation, and elevated levels of FFAs in hepatocytes are characteristic of MASLD. Although liver mitochondria initially attempt to increase the oxidation of fatty acids to counteract the augmented fat accumulation, this compensatory response is insufficient to manage the increasing hepatic burden of FFAs. Excess FFAs are subsequently directed to triglyceride synthesis, resulting in the development of steatosis and hypertriglyceridemia.57,59
As steatosis progresses, there is a significant reduction in the mitochondrial redox capacity, which is primarily responsible for the impairment of respiratory chain function. Increased mitochondrial fatty acid delivery can lead to elevated level of uncoupled protein 2.60 This elevation results in increased mitochondrial respiration and decreased ATP synthesis, which result in increased ROS generation. The resulting ROS can impair the respiratory chain activity and induce mutations in mtDNA, which contribute to mitochondrial dysfunction.61
When the efficiency of the respiratory chain declines, mitochondria are unable to completely oxidize excess FFAs. Therefore, extramitochondrial oxidation is an important pathway of FFA degradation, leading to the production of lipid peroxidation products and additional ROS.62 These lipid peroxidation products damage mtDNA and crucial mitochondrial proteins, such as cytochrome C oxidase and adenine nucleotide translocator.63 This damage culminates in a cascading cycle of mitochondrial impairment, increased lipid peroxidation, and heightened ROS production. The augmented production of ROS can stimulate inflammation directly by activating diverse inflammatory signaling pathways, including NF-κB and c-Jun N-terminal kinase (JNK), and indirectly by upregulating the expression of inflammatory cytokines, such as tumor necrosis factor-α and transforming growth factor-β.64,65 These inflammatory mediators subsequently potentiate various pathological outcomes.
Recent studies have reported increased extracellular mtDNA levels in mice and humans with metabolic dysfunction-associated fatty liver disease (MAFLD).66 The release of oxidized mtDNA contributes to inflammasome activation, establishing an association between mitochondrial dysfunction and perpetuation of the inflammatory response.67 Within cytosolic and extracellular environments, mtDNA serves as a damage-associated molecular pattern, initiating and propagating an inflammatory response through several signaling pathways. The Toll-like receptor 9, inflammasome, and stimulator of interferon gene pathways play a significant role in this complex cascade.66 Activation of these pathways enhances damage to hepatocytes and potentially spreads injurious effects to other organs.67
Mitochondrial dysfunction is also associated with ER stress response in the liver. ER stress is induced by reduced ATP level and elevated ROS levels, resulting in activation of the unfolded protein response. This activation upregulates hepatic enzymes involved in lipid synthesis, leading to an increase in hepatic fat accumulation.68 In addition, it amplifies the JNK signaling pathway, creating a pro-inflammatory environment.
Genetic association between mitochondria and MASLD
Through a comprehensive investigation of genetic variations across large cohorts, genome-wide association studies (GWAS) have consistently revealed genetic loci and variants intricately associated with mitochondrial function and dynamics in the context of MAFLD pathogenesis.69 Of the single-nucleotide polymorphisms related to MAFLD in GWAS, those located in mitochondrial genes were also associated with the risk of MASLD. Aldehyde dehydrogenase 2 (ALDH2) is a mitochondrial enzyme in liver physiology responsible for converting acetaldehyde into nontoxic acetic acid, which is essential for detoxification processes.70 In addition to its canonical function, ALDH2 increases the antioxidant capacity of the liver.71 ALDH2 activity can be impeded by oxidative stress, which may compromise its protective function, and ALDH2 inhibition has deleterious effects in a murine model of liver disease.71-73 ALDH2 rs671 polymorphism is positively associated with a high risk of MASLD in Chinese74 and Japanese75 subjects. Additionally, several cohort studies have revealed a significant association between ALDH2 polymorphism and other liver diseases, such as alcoholic liver disease and hepatic cellular carcinoma.76
The sorting and assembly machinery component 50 (SAMM50) gene encodes a β-barrel protein that comprises a component of the sorting and assembly machinery (SAM) complex located in the outer membrane of mitochondria.77 The SAM complex is responsible for β-barrel protein sorting and assembly, ensuring proper mitochondrial structure and functionality.77 In human hepatoma cell lines, SAMM50 knockdown leads to increased lipid accumulation due to reduced fatty acid oxidation. In contrast, SAMM50 overexpression boosts fatty acid oxidation and reduces intracellular lipid accumulation.78 In a large multiethnic cohort, SAMM50 polymorphisms, including rs2143571, rs3761472, rs2073080, rs738491, rs2073082, rs738409, rs738408, rs3747207, and rs44391686, were positively associated with an increased risk of MASLD.78-81 In addition, polymorphisms in mitochondria-related genes, including fatty acid-binding protein 1, glycerol-3-phosphate acyltransferase, lysophospholipase-like 1, and mitochondrial amidoxime-reducing component 1, are significantly associated with MASLD (Table 1).74,78-84 Taken together, these comprehensive data obtained from the GWAS emphasize the significant influence of mitochondrial involvement on MASLD formation. Ongoing and diligent efforts in GWAS will enable a deeper understanding of the complex interactions between mitochondrial mechanisms and MASLD development, triggering innovative therapeutic approaches.
Table 1.
List of mitochondrial single-nucleotide polymorphism sites associated with MASLD
| Gene | Name | Polymorphism | Association with MASLD | Cohort | Reference |
|---|---|---|---|---|---|
| ALDH2 | Aldehyde dehydrogenase 2 | rs671 | Associated with increased probability of MASLD | Chinese patients with MASLD | 74 |
| FABP1 | Fatty acid-binding protein 1 | rs72943235 | Associated with increased risk of fibrosis | Participants from the Electronic Medical Records and Genomics (eMERGE) Network | 79 |
| GPAM | Glycerol-3-phosphate acyltransferase | rs2792751 | Associated with steatosis and liver damage | UK Biobank samples | 82 |
| LYPLAL1 | Lysophospholipas-like 1 | rs12137855 | Associated with increased risk of steatosis and fibrosis | Young and middle-aged Finns | 83 |
| MTARC1 | Mitochondrial amidoxime-reducing component 1 | rs2642438 | Independent protective factor against fibrosis | Caucasian Polish patients who underwent liver biopsy during weight loss surgery | 84 |
| SAMM50 | Sorting and assembly machinery component 50 homolog | rs2143571 rs3761472 rs2073080 |
Associated with the presence and severity of MASLD | Korean patients with MASLD | 80 |
| rs738491 rs2073082 |
Risk and severity of MASLD | Chinese Han patients with MASLD | 78 | ||
| rs738409 rs738408 rs3747207 |
Associated with MASLD risk | Participants from the eMERGE Network | 79 | ||
| rs44391686 | Associated with MASLD risk | Patients with MASLD from five ethnic groups | 81 |
MASLD, metabolic steatosis-associated steatotic liver disease; PNPLA3, patatin like phospholipase domain containing 3.
NEW THERAPEUTICS FOR MASLD
Current clinical trials for MASLD
Currently, there are no U.S. Food and Drug Administration (FDA)-approved treatments for MASLD. However, potential treatment options are being actively researched.85 Because mitochondrial dysfunction plays a central role in the pathological mechanisms underlying NAFLD, therapeutic approaches targeting the mitochondria have been developed in recent years. Table 2 shows the clinical trials of mitochondria-targeting drugs for MASLD treatment.86-93 Vitamin E is a potent antioxidant94 that has been studied in human clinical trials for MASLD treatment.86,94,95 It improves serum enzyme levels, liver steatosis, inflammation, and fibrosis in patients without type 2 diabetes mellitus at a high dose of 800 IU/day for 96 weeks.86 Recent meta-analyses have suggested that vitamin E reduces transaminase activity and potentially improves non-alcoholic steatohepatitis (NASH) histopathology. However, there is no significant improvement in liver fibrosis. Vitamin E is not recommended for the treatment of MASH associated with diabetes, MASLD without liver biopsy, NASH cirrhosis, or cryptogenic cirrhosis.96
Table 2.
Selected clinical trials of mitochondria-centric drugs
| Drug | Author (year) | National Clinical Trial numbers | Mechanism of action | Study outcomes | |
|---|---|---|---|---|---|
| Liver enzymes | Histology | ||||
| Vitamin E | Sanyal et al. (2010)86 |
NCT00063622 NCT02690792 NCT01792115 NCT02962297 NCT04198805 NCT01147523 NCT01934777 NCT00655018 |
Restoration the hepatic glutathione level | Improved | Improved steatosis and inflammation |
| Metformin | Loomba et al. (2009)87 |
NCT00063232 NCT00736385 NCT02696941 NCT05521633 |
Activation of AMPK signaling | Improved | Improved parenchymal inflammation and cellular injury |
| Resveratrol | Faghihzadeh et al. (2014)93 |
NCT01446276 NCT02030977 NCT01464801 NCT02216552 |
Activation of mitochondrial biogenesis and mitochondria-located antioxidant enzymes | Improved | Improved hepatic steatosis |
| Betaine | Abdelmalek et al. (2009)88 |
NCT00586911 NCT03073343 |
Restoration of hepatic mitochondrial glutathione and S-adenosyl methionine | No improvement | Improved hepatic steatosis |
| Pentoxifylline | Zein et al. (2011)89 |
NCT00267670 NCT00590161 NCT05284448 |
Increasing Nrf2 and PGC-1α through the cAMP–CREB pathway | No improvement | Improved steatosis, inflammation, and fibrosis |
| Liraglutide | Armstrong et al. (2016)90 |
NCT02147925 NCT03068065 NCT01399645 NCT03233178 NCT01237119 NCT05041673 NCT05779644 NCT02654665 |
Enhancing mitochondrial architecture through the SIRT1/SIRT3 signaling | Improved | Improved hepatic steatosis |
| Exenatide | Liu et al. (2021)91 |
NCT02303730 NCT01006889 NCT01208649 NCT00650546 NCT00529204 |
Enhancing mitochondrial architecture through the SIRT1/SIRT3 signaling | Improved | Improved hepatic steatosis |
| Semaglutide | Newsome et al. (2021)92 | NCT02970942 | Enhancing mitochondrial architecture through the SIRT1/SIRT3 signaling | Improved | Improved hepatic fibrosis and reduced liver-enzyme levels |
AMPK, AMP-activated protein kinase; Nrf2, nuclear factor erythroid 2-related factor 2; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; cAMP, cyclic adenosine monophosphate; CREB, cAMP-response element binding protein; SIRT1, sirtuin 1; SIRT3, sirtuin 3.
Metformin is the oldest and most widely used first-line antidiabetic drug.97 Metformin has several pharmacological effects that have inspired researchers to develop and reuse this drug.98 Although the details of its metabolism are not fully understood, mitochondria play a central role in the activity of metformin.98 Metformin inhibits complex I-dependent respiration and mitochondrial glycerophosphate dehydrogenase and activates sirtuin 1 (SIRT1) and SIRT3.98 It controls blood glucose levels by suppressing hepatic glucose production via AMP-activated protein kinase (AMPK) activation.99 In several clinical trials,87,100 metformin has been shown to have beneficial effects on fatty liver disease, despite the controversy in the effect of metformin on MASLD found in the meta-analysis of randomized controlled trials.101 Another meta-analysis revealed that metformin decreased transaminase activities and total cholesterol level and improved insulin sensitivity.102 However, metformin is not recommended for treatment of MAFLD or MASH due to unsuccessful outcomes in clinical trials. In contrast, clinical trials have highlighted the promising effects of various compounds, including betaine,88 pentoxifylline,89 liraglutide,90 exenatide,91 and semaglutide92 in ameliorating MASLD.
Potential new mitochondrial targets for MASLD treatment
Recently, several new therapeutics targeting MQC have emerged with potential benefits established through integrative analyses in mice and humans. Nicotinamide adenine dinucleotide (NAD+) serves as a central redox factor in energy metabolism and is a substrate for several enzymes, including SIRT.103 NAD+ plays an essential role as a precursor of reduced NAD phosphate, a critical component of the antioxidant defense mechanism in humans.103 Cellular NAD+ is a critical component of mitochondrial quality through processes such as the mtUPR.104 Furthermore, low NAD+ level is significantly associated with an increased risk of MASLD development.105 NAD+-boosting strategies are potential therapeutic targets for MASLD.104 NAD+ precursors, such as nicotinamide riboside and nicotinamide mononucleotide, are commercially available supplements.104 In preclinical models of MASLD, oral administration of these precursors increases hepatic NAD+ concentrations and consequently inhibits hepatic lipid accumulation.103 This intervention also improves hepatic mitochondrial respiration, steatosis, and oxidative stress in preclinical NAFLD models.103,106,107
Urolithin A (UA) is an endogenous substance synthesized by intestinal microorganisms through metabolic conversion of ingested ellagitannins and ellagic acid, which are complex polyphenolic compounds found in several dietary sources, such as pomegranates, berries, and nuts.108 UA promotes cellular health by enhancing mitophagy and mitochondrial competence and attenuating harmful inflammatory responses.108,109 Recent studies have demonstrated the beneficial effects of UA supplementation on MASLD through regulation of the AMPK signaling pathway110,111 and induction of lipophagy.112
Spermidine is a natural polyamine abundant in certain food sources, such as rice bran, soybeans, aged cheese, mushrooms, and broccoli.113 This polyamine has demonstrated notable beneficial effects under various pathological conditions owing to its ability to enhance mitochondrial functionality. Spermidine supplementation improves mitochondrial respiration, preserves mitochondrial membrane potential, and facilitates ATP synthesis.113,114 In a mouse model of diet-induced obesity (DIO), spermidine ameliorated DIO-induced hepatic steatosis by regulating AMPK signaling.115 In mice with Western diet-induced MASH, spermidine supplementation significantly attenuated hepatic lipid accumulation, insulin resistance, hepatic inflammation, and fibrosis.116
Mitoquinone (MitoQ) is an antioxidant that targets the matrix surface of the inner mitochondrial membrane and is particularly effective against lipid peroxidation.13 MitoQ supplementation increases the mitochondrial CL content, improves mitochondrial function, reduces oxidative damage, and prevents hepatic fat accumulation in animal models.117-119 These studies have highlighted novel therapeutic strategies targeting MQC with promising results in preclinical models. However, strict clinical trials are required to confirm their efficacy and safety.
CONCLUSION
The increasing global prevalence of MASLD, formerly known as NAFLD, is a pressing public health concern. A growing body of studies has highlighted the pivotal role of mitochondria in the etiology and progression of MASLD. These dynamic organelles serve as metabolic hubs regulating hepatic energy production, lipid metabolism, and redox homeostasis. Dysfunctional mitochondria, characterized by oxidative stress, impaired OxPhos, and defective quality control mechanisms, significantly contribute to lipid accumulation, inflammation, and fibrogenesis in the liver. GWAS have identified genetic loci related to mitochondrial function and dynamics that influence the risk of MASLD. These genetic associations highlight the complex interplay between mitochondrial genetics and MASLD susceptibility.
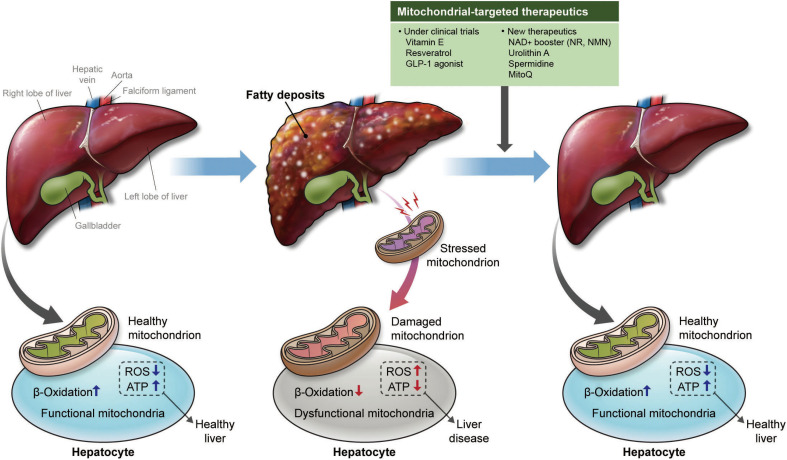
Although there are currently no FDA-approved treatments for MASLD, ongoing clinical trials exploring therapeutic options have identified promising strategies, including vitamin E and metformin, which target mitochondrial dysfunction to attenuate hepatic lipid accumulation and inflammation. Recent advancements have revealed novel mitochondria-targeted therapeutics, such as NAD+ precursors, UA, spermidine, and MitoQ. These compounds potentially improve MQC, mitigate oxidative stress, and restore metabolic balance (Fig. 1).
Figure 1.
Mitochondrial dysfunction in fatty liver. In fatty liver, stressed and damaged mitochondria cause greater oxidative stress and less energy production. New therapeutics that restore mitochondrial function could be a promising way to treat metabolic dysfunction-associated steatotic liver disease. GLP-1, glucagon-like peptide 1; NAD+, nicotinamide adenine dinucleotide; NR, nicotinamide riboside; NMN, nicotinamide mononucleotide; MitoQ, mitoquinone; ROS, reactive oxygen species; ATP, adenosine triphosphate.
In conclusion, mitochondrial dysfunction plays a key role in the pathogenesis of MASLD. The concerted efforts of researchers, clinicians, and drug developers are of critical importance for the prevention and treatment of this disease. Translating novel mitochondria-targeted approaches into effective and safe therapies will reduce disease burden and improve the quality of life of patients with MASLD worldwide.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2020R1C1C1004999), “GIST Research Institute IIBR” grants funded by the GIST in 2022, KHIDI-AZ Diabetes Research Program, Young Medical Scientist Research Grant through the Daewoong Foundation (DFY2107P), and by a National Research Council of Science & Technology (NST) grant by the Korean government (MSIT) (No. CAP23021-110).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: CMO; acquisition of data: SS, JK, and JYL; drafting of the manuscript: SS, JK, and CMO; critical revision of the manuscript: CMO; obtained funding: CMO; administrative, technical, or material support: CMO; and study supervision: CMO.
REFERENCES
- 1.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966–86. doi: 10.1097/HEP.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335–47. doi: 10.1097/HEP.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–33. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bril F, Cusi K. Nonalcoholic fatty liver disease: the new complication of type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2016;45:765–81. doi: 10.1016/j.ecl.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330–7. doi: 10.3748/wjg.v20.i28.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huh Y, Cho YJ, Nam GE. Recent epidemiology and risk factors of nonalcoholic fatty liver disease. J Obes Metab Syndr. 2022;31:17–27. doi: 10.7570/jomes22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu R, Pan J, Zhou W, Ji G, Dang Y. Recent advances in lean NAFLD. Biomed Pharmacother. 2022;153:113331. doi: 10.1016/j.biopha.2022.113331. [DOI] [PubMed] [Google Scholar]
- 10.Simões IC, Fontes A, Pinton P, Zischka H, Wieckowski MR. Mitochondria in non-alcoholic fatty liver disease. Int J Biochem Cell Biol. 2018;95:93–9. doi: 10.1016/j.biocel.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open. 2019;2:e1912565. doi: 10.1001/jamanetworkopen.2019.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KS, Hong S, Ahn HY, Park CY. Metabolic dysfunction-associated fatty liver disease and mortality: a population-based cohort study. Diabetes Metab J. 2023;47:220–31. doi: 10.4093/dmj.2021.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramanathan R, Ali AH, Ibdah JA. Mitochondrial dysfunction plays central role in nonalcoholic fatty liver disease. Int J Mol Sci. 2022;23:7280. doi: 10.3390/ijms23137280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dornas W, Schuppan D. Mitochondrial oxidative injury: a key player in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2020;319:G400–11. doi: 10.1152/ajpgi.00121.2020. [DOI] [PubMed] [Google Scholar]
- 15.Legaki AI, Moustakas II, Sikorska M, Papadopoulos G, Velliou RI, Chatzigeorgiou A. Hepatocyte mitochondrial dynamics and bioenergetics in obesity-related non-alcoholic fatty liver disease. Curr Obes Rep. 2022;11:126–43. doi: 10.1007/s13679-022-00473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morio B, Panthu B, Bassot A, Rieusset J. Role of mitochondria in liver metabolic health and diseases. Cell Calcium. 2021;94:102336. doi: 10.1016/j.ceca.2020.102336. [DOI] [PubMed] [Google Scholar]
- 17.Middleton P, Vergis N. Mitochondrial dysfunction and liver disease: role, relevance, and potential for therapeutic modulation. Therap Adv Gastroenterol. 2021;14:17562848211031394. doi: 10.1177/17562848211031394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang IW, López-Vicario C, Duran-Güell M, Clària J. Mitochondrial dysfunction in advanced liver disease: emerging concepts. Front Mol Biosci. 2021;8:772174. doi: 10.3389/fmolb.2021.772174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arroyave-Ospina JC, Wu Z, Geng Y, Moshage H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: implications for prevention and therapy. Antioxidants (Basel) 2021;10:174. doi: 10.3390/antiox10020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karkucinska-Wieckowska A, Simoes IC, Kalinowski P, Lebiedzinska-Arciszewska M, Zieniewicz K, Milkiewicz P, et al. Mitochondria, oxidative stress and nonalcoholic fatty liver disease: a complex relationship. Eur J Clin Invest. 2022;52:e13622. doi: 10.1111/eci.13622. [DOI] [PubMed] [Google Scholar]
- 21.Wong HS, Dighe PA, Mezera V, Monternier PA, Brand MD. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem. 2017;292:16804–9. doi: 10.1074/jbc.R117.789271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irato P, Santovito G. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants (Basel) 2021;10:579. doi: 10.3390/antiox10040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082–91. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe Y, Hines IN, Zibari G, Pavlick K, Gray L, Kitagawa Y, et al. Mouse model of liver ischemia and reperfusion injury: method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic Biol Med. 2009;46:1–7. doi: 10.1016/j.freeradbiomed.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Asmari AK, Khan AQ, Al-Masri N. Mitigation of 5-fluorouracil-induced liver damage in rats by vitamin C via targeting redox-sensitive transcription factors. Hum Exp Toxicol. 2016;35:1203–13. doi: 10.1177/0960327115626583. [DOI] [PubMed] [Google Scholar]
- 26.Fischer F, Hamann A, Osiewacz HD. Mitochondrial quality control: an integrated network of pathways. Trends Biochem Sci. 2012;37:284–92. doi: 10.1016/j.tibs.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Napolitano G, Fasciolo G, Venditti P. Mitochondrial management of reactive oxygen species. Antioxidants (Basel) 2021;10:1824. doi: 10.3390/antiox10111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudek J. Role of cardiolipin in mitochondrial signaling pathways. Front Cell Dev Biol. 2017;5:90. doi: 10.3389/fcell.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boynton TO, Shimkets LJ. Myxococcus CsgA, Drosophila sniffer, and human HSD10 are cardiolipin phospholipases. Genes Dev. 2015;29:1903–14. doi: 10.1101/gad.268482.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang H, Van Remmen H, Frohlich V, Lechleiter J, Richardson A, Ran Q. Gpx4 protects mitochondrial ATP generation against oxidative damage. Biochem Biophys Res Commun. 2007;356:893–8. doi: 10.1016/j.bbrc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 31.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–90. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J, Wang Y, Jiang R, Xue R, Yin X, Wu M, et al. Ferroptosis in liver disease: new insights into disease mechanisms. Cell Death Discov. 2021;7:276. doi: 10.1038/s41420-021-00660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M, Azarbayjani MA. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur J Sport Sci. 2019;19:994–1003. doi: 10.1080/17461391.2019.1571114. [DOI] [PubMed] [Google Scholar]
- 34.Rugarli EI, Langer T. Mitochondrial quality control: a matter of life and death for neurons. EMBO J. 2012;31:1336–49. doi: 10.1038/emboj.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker BM, Haynes CM. Mitochondrial protein quality control during biogenesis and aging. Trends Biochem Sci. 2011;36:254–61. doi: 10.1016/j.tibs.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Anand R, Langer T, Baker MJ. Proteolytic control of mitochondrial function and morphogenesis. Biochim Biophys Acta. 2013;1833:195–204. doi: 10.1016/j.bbamcr.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Opalińska M, Jańska H. AAA proteases: guardians of mitochondrial function and homeostasis. Cells. 2018;7:163. doi: 10.3390/cells7100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker TA, Sauer RT. ClpXP, an ATP-powered unfolding and protein-degradation machine. Biochim Biophys Acta. 2012;1823:15–28. doi: 10.1016/j.bbamcr.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaser M, Kambacheld M, Kisters-Woike B, Langer T. Oma1, a novel membrane-bound metallopeptidase in mitochondria with activities overlapping with the m-AAA protease. J Biol Chem. 2003;278:46414–23. doi: 10.1074/jbc.M305584200. [DOI] [PubMed] [Google Scholar]
- 41.Bender T, Lewrenz I, Franken S, Baitzel C, Voos W. Mitochondrial enzymes are protected from stress-induced aggregation by mitochondrial chaperones and the Pim1/LON protease. Mol Biol Cell. 2011;22:541–54. doi: 10.1091/mbc.e10-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell. 2007;13:467–80. doi: 10.1016/j.devcel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Haynes CM, Ron D. The mitochondrial UPR: protecting organelle protein homeostasis. J Cell Sci. 2010;123(Pt 22):3849–55. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- 44.Karbowski M, Youle RJ. Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr Opin Cell Biol. 2011;23:476–82. doi: 10.1016/j.ceb.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metzger MB, Scales JL, Dunklebarger MF, Loncarek J, Weissman AM. A protein quality control pathway at the mitochondrial outer membrane. Elife. 2020;9:e51065. doi: 10.7554/eLife.51065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Bliek AM, Shen Q, Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol. 2013;5:a011072. doi: 10.1101/cshperspect.a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnasamy Y, Gooz M, Li L, Lemasters JJ, Zhong Z. Role of mitochondrial depolarization and disrupted mitochondrial homeostasis in non-alcoholic steatohepatitis and fibrosis in mice. Int J Physiol Pathophysiol Pharmacol. 2019;11:190–204. [PMC free article] [PubMed] [Google Scholar]
- 48.Elgass K, Pakay J, Ryan MT, Palmer CS. Recent advances into the understanding of mitochondrial fission. Biochim Biophys Acta. 2013;1833:150–61. doi: 10.1016/j.bbamcr.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Soubannier V, Rippstein P, Kaufman BA, Shoubridge EA, McBride HM. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS One. 2012;7:e52830. doi: 10.1371/journal.pone.0052830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugiura A, McLelland GL, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 2014;33:2142–56. doi: 10.15252/embj.201488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol. 2012;22:135–41. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 52.Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, et al. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol. 2008;18:102–8. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 53.McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014;33:282–95. doi: 10.1002/embj.201385902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doblado L, Lueck C, Rey C, Samhan-Arias AK, Prieto I, Stacchiotti A, et al. Mitophagy in human diseases. Int J Mol Sci. 2021;22:3903. doi: 10.3390/ijms22083903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Einer C, Hohenester S, Wimmer R, Wottke L, Artmann R, Schulz S, et al. Mitochondrial adaptation in steatotic mice. Mitochondrion. 2018;40:1–12. doi: 10.1016/j.mito.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 56.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–46. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Li Z, Li Y, Zhang HX, Guo JR, Lam CW, Wang CY, et al. Mitochondria-mediated pathogenesis and therapeutics for non-alcoholic fatty liver disease. Mol Nutr Food Res. 2019;63:e1900043. doi: 10.1002/mnfr.201900043. [DOI] [PubMed] [Google Scholar]
- 58.Tumova J, Andel M, Trnka J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol Res. 2016;65:193–207. doi: 10.33549/physiolres.932993. [DOI] [PubMed] [Google Scholar]
- 59.Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria in steatohepatitis. Semin Liver Dis. 2001;21:57–69. doi: 10.1055/s-2001-12929. [DOI] [PubMed] [Google Scholar]
- 60.Geisler CE, Renquist BJ. Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones. J Endocrinol. 2017;234:R1–21. doi: 10.1530/JOE-16-0513. [DOI] [PubMed] [Google Scholar]
- 61.Dabravolski SA, Bezsonov EE, Baig MS, Popkova TV, Nedosugova LV, Starodubova AV, et al. Mitochondrial mutations and genetic factors determining NAFLD risk. Int J Mol Sci. 2021;22:4459. doi: 10.3390/ijms22094459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843. doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Surugihalli C, Porter TE, Chan A, Farley LS, Maguire M, Zhang C, et al. Hepatic mitochondrial oxidative metabolism and lipogenesis synergistically adapt to mediate healthy embryonic-to-neonatal transition in chicken. Sci Rep. 2019;9:20167. doi: 10.1038/s41598-019-56715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krstić J, Trivanović D, Mojsilović S, Santibanez JF. Transforming growth factor-beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longev. 2015;2015:654594. doi: 10.1155/2015/654594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014;5:e996. doi: 10.1038/cddis.2013.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inzaugarat ME, Wree A, Feldstein AE. Hepatocyte mitochondrial DNA released in microparticles and toll-like receptor 9 activation: a link between lipotoxicity and inflammation during nonalcoholic steatohepatitis. Hepatology. 2016;64:669–71. doi: 10.1002/hep.28666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, Wu X, Hu Q, Wu J, Wang G, Hong Z, et al. Mitochondrial DNA in liver inflammation and oxidative stress. Life Sci. 2019;236:116464. doi: 10.1016/j.lfs.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 68.Zheng Z, Zhang C, Zhang K. Role of unfolded protein response in lipogenesis. World J Hepatol. 2010;2:203–7. doi: 10.4254/wjh.v2.i6.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sookoian S, Flichman D, Scian R, Rohr C, Dopazo H, Gianotti TF, et al. Mitochondrial genome architecture in non-alcoholic fatty liver disease. J Pathol. 2016;240:437–49. doi: 10.1002/path.4803. [DOI] [PubMed] [Google Scholar]
- 70.Jackson B, Brocker C, Thompson DC, Black W, Vasiliou K, Nebert DW, et al. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Hum Genomics. 2011;5:283–303. doi: 10.1186/1479-7364-5-4-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, He W. Endogenous mitochondrial aldehyde dehydrogenase-2 as an antioxidant in liver. In: Patel VB, Rajendram R, Preedy VR, editors. The liver. Elsevier; 2018. pp. 247–59. [DOI] [Google Scholar]
- 72.Moon KH, Lee YM, Song BJ. Inhibition of hepatic mitochondrial aldehyde dehydrogenase by carbon tetrachloride through JNK-mediated phosphorylation. Free Radic Biol Med. 2010;48:391–8. doi: 10.1016/j.freeradbiomed.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang SS, Chen YH, Hu JT, Chiu CF, Hung SW, Chang YC, et al. Aldehyde dehydrogenase mutation exacerbated high-fat-diet-induced nonalcoholic fatty liver disease with gut microbiota remodeling in male mice. Biology (Basel) 2021;10:737. doi: 10.3390/genes10100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hao X, Zeng Q. The association and interaction of aldehyde dehydrogenase 2 polymorphisms with food group intake and probability of having non-alcoholic fatty liver disease. Diabetes Metab Syndr Obes. 2020;13:5049–57. doi: 10.2147/DMSO.S290491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oniki K, Morita K, Watanabe T, Kajiwara A, Otake K, Nakagawa K, et al. The longitudinal effect of the aldehyde dehydrogenase 2*2 allele on the risk for nonalcoholic fatty liver disease. Nutr Diabetes. 2016;6:e210. doi: 10.1038/nutd.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Q, Chang B, Li X, Zou Z. Role of ALDH2 in hepatic disorders: gene polymorphism and disease pathogenesis. J Clin Transl Hepatol. 2021;9:90–8. doi: 10.14218/JCTH.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu S, Gao Y, Zhang C, Li H, Pan S, Wang X, et al. SAMM50 affects mitochondrial morphology through the association of Drp1 in mammalian cells. FEBS Lett. 2016;590:1313–23. doi: 10.1002/1873-3468.12170. [DOI] [PubMed] [Google Scholar]
- 78.Li Z, Shen W, Wu G, Qin C, Zhang Y, Wang Y, et al. The role of SAMM50 in non-alcoholic fatty liver disease: from genetics to mechanisms. FEBS Open Bio. 2021;11:1893–906. doi: 10.1002/2211-5463.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Namjou B, Lingren T, Huang Y, Parameswaran S, Cobb BL, Stanaway IB, et al. GWAS and enrichment analyses of non-alcoholic fatty liver disease identify new trait-associated genes and pathways across eMERGE Network. BMC Med. 2019;17:135. doi: 10.1186/s12916-019-1364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung GE, Lee Y, Yim JY, Choe EK, Kwak MS, Yang JI, et al. Genetic polymorphisms of PNPLA3 and SAMM50 are associated with nonalcoholic fatty liver disease in a Korean population. Gut Liver. 2018;12:316–23. doi: 10.5009/gnl17306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Conti DV, Bogumil D, Sheng X, Noureddin M, Wilkens LR, et al. Association of genetic risk score with NAFLD in an ethnically diverse cohort. Hepatol Commun. 2021;5:1689–703. doi: 10.1002/hep4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jamialahmadi O, Mancina RM, Ciociola E, Tavaglione F, Luukkonen PK, Baselli G, et al. Exome-wide association study on alanine aminotransferase identifies sequence variants in the GPAM and APOE associated with fatty liver disease. Gastroenterology. 2021;160:1634–46. doi: 10.1053/j.gastro.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 83.Sliz E, Sebert S, Würtz P, Kangas AJ, Soininen P, Lehtimäki T, et al. NAFLD risk alleles in PNPLA3, TM6SF2, GCKR and LYPLAL1 show divergent metabolic effects. Hum Mol Genet. 2018;27:2214–23. doi: 10.1093/hmg/ddy124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalinowski P, Smyk W, Nowosad M, Paluszkiewicz R, Michałowski Ł, Ziarkiewicz-Wróblewska B, et al. MTARC1 and HSD17B13 variants have protective effects on non-alcoholic fatty liver disease in patients undergoing bariatric surgery. Int J Mol Sci. 2022;23:15825. doi: 10.3390/ijms232415825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cho Y, Lee YH. State-of-the-art overview of the pharmacological treatment of non-alcoholic steatohepatitis. Endocrinol Metab (Seoul) 2022;37:38–52. doi: 10.3803/EnM.2022.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–82. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abdelmalek MF, Sanderson SO, Angulo P, Soldevila-Pico C, Liu C, Peter J, et al. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50:1818–26. doi: 10.1002/hep.23239. [DOI] [PubMed] [Google Scholar]
- 89.Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–9. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y, Wang DW, Wang D, Duan BH, Kuang HY. Exenatide attenuates non-alcoholic steatohepatitis by inhibiting the pyroptosis signaling pathway. Front Endocrinol (Lausanne) 2021;12:663039. doi: 10.3389/fendo.2021.663039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 93.Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837–43. doi: 10.1016/j.nutres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 94.Perumpail BJ, Li AA, John N, Sallam S, Shah ND, Kwong W, et al. The role of vitamin E in the treatment of NAFLD. Diseases. 2018;6:86. doi: 10.3390/diseases6040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Larion S, Khurana S. Clinical studies investigating the effect of vitamin E therapy in patients with NASH. Clin Liver Dis (Hoboken) 2018;11:16–21. doi: 10.1002/cld.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sumida Y, Yoneda M, Seko Y, Takahashi H, Hara N, Fujii H, et al. Role of vitamin E in the treatment of non-alcoholic steatohepatitis. Free Radic Biol Med. 2021;177:391–403. doi: 10.1016/j.freeradbiomed.2021.10.017. [DOI] [PubMed] [Google Scholar]
- 97.Huang KH, Lee CH, Cheng YD, Gau SY, Tsai TH, Chung NJ, et al. Correlation between long-term use of metformin and incidence of NAFLD among patients with type 2 diabetes mellitus: a real-world cohort study. Front Endocrinol (Lausanne) 2022;13:1027484. doi: 10.3389/fendo.2022.1027484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng J, Wang X, Ye X, Ares I, Lopez-Torres B, Martínez M, et al. Mitochondria as an important target of metformin: the mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res. 2022;177:106114. doi: 10.1016/j.phrs.2022.106114. [DOI] [PubMed] [Google Scholar]
- 99.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–85. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–90. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 101.Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang Y, Wang X, Yan C, Li C, Zhang L, Zhang L, et al. Effect of metformin on nonalcoholic fatty liver based on meta-analysis and network pharmacology. Medicine (Baltimore) 2022;101:e31437. doi: 10.1097/MD.0000000000031437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dall M, Hassing AS, Treebak JT. NAD+ and NAFLD: caution, causality and careful optimism. J Physiol. 2022;600:1135–54. doi: 10.1113/JP280908. [DOI] [PubMed] [Google Scholar]
- 104.Cantó C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Komatsu M, Kanda T, Urai H, Kurokochi A, Kitahama R, Shigaki S, et al. NNMT activation can contribute to the development of fatty liver disease by modulating the NAD+ metabolism. Sci Rep. 2018;8:8637. doi: 10.1038/s41598-018-26882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sambeat A, Ratajczak J, Joffraud M, Sanchez-Garcia JL, Giner MP, Valsesia A, et al. Endogenous nicotinamide riboside metabolism protects against diet-induced liver damage. Nat Commun. 2019;10:4291. doi: 10.1038/s41467-019-12262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoshino J, Baur JA, Imai SI. NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27:513–28. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.D'Amico D, Andreux PA, Valdés P, Singh A, Rinsch C, Auwerx J. Impact of the natural compound urolithin A on health, disease, and aging. Trends Mol Med. 2021;27:687–99. doi: 10.1016/j.molmed.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 109.Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Félix AA, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22:879–88. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 110.Xu J, Tian H, Ji Y, Dong L, Liu Y, Wang Y, et al. Urolithin C reveals anti-NAFLD potential via AMPK-ferroptosis axis and modulating gut microbiota. Naunyn Schmiedebergs Arch Pharmacol. 2023;396:2687–99. doi: 10.1007/s00210-023-02492-8. [DOI] [PubMed] [Google Scholar]
- 111.ALTamimi JZ, Alshammari GM, AlFaris NA, Alagal RI, Aljabryn DH, Albekairi NA, et al. Ellagic acid protects against non-alcoholic fatty liver disease in streptozotocin-diabetic rats by activating AMPK. Pharm Biol. 2022;60:25–37. doi: 10.1080/13880209.2021.1990969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang C, Song Y, Yuan M, Chen L, Zhang Q, Hu J, et al. Ellagitannins-derived intestinal microbial metabolite urolithin A ameliorates fructose-driven hepatosteatosis by suppressing hepatic lipid metabolic reprogramming and inducing lipophagy. J Agric Food Chem. 2023;71:3967–80. doi: 10.1021/acs.jafc.2c05776. [DOI] [PubMed] [Google Scholar]
- 113.Oh CM, Ryu D, Cho S, Jang Y. Mitochondrial quality control in the heart: new drug targets for cardiovascular disease. Korean Circ J. 2020;50:395–405. doi: 10.4070/kcj.2019.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fairley LH, Lejri I, Grimm A, Eckert A. Spermidine rescues bioenergetic and mitophagy deficits induced by disease-associated tau protein. Int J Mol Sci. 2023;24:5297. doi: 10.3390/ijms24065297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gao M, Zhao W, Li C, Xie X, Li M, Bi Y, et al. Spermidine ameliorates non-alcoholic fatty liver disease through regulating lipid metabolism via AMPK. Biochem Biophys Res Commun. 2018;505:93–8. doi: 10.1016/j.bbrc.2018.09.078. [DOI] [PubMed] [Google Scholar]
- 116.Ni Y, Hu Y, Lou X, Rong N, Liu F, Yang C, et al. Spermidine ameliorates nonalcoholic steatohepatitis through thyroid hormone-responsive protein signaling and the gut microbiota-mediated metabolism of bile acids. J Agric Food Chem. 2022;70:6478–92. doi: 10.1021/acs.jafc.2c02729. [DOI] [PubMed] [Google Scholar]
- 117.Fink BD, Yu L, Coppey L, Obrosov A, Shevalye H, Kerns RJ, et al. Effect of mitoquinone on liver metabolism and steatosis in obese and diabetic rats. Pharmacol Res Perspect. 2021;9:e00701. doi: 10.1002/prp2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu J, Shen J, Yuan R, Jia B, Zhang Y, Wang S, et al. Mitochondrial targeting therapeutics: promising role of natural products in non-alcoholic fatty liver disease. Front Pharmacol. 2021;12:796207. doi: 10.3389/fphar.2021.796207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fouret G, Tolika E, Lecomte J, Bonafos B, Aoun M, Murphy MP, et al. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Biochim Biophys Acta. 2015;1847:1025–35. doi: 10.1016/j.bbabio.2015.05.019. [DOI] [PubMed] [Google Scholar]