Abstract
Background:
Stress from preterm infant admission to the neonatal intensive care unit (NICU) is associated with infant and maternal physiologic changes, including endocrine and epigenetic alterations. Little is known about the mechanisms connecting NICU stress to biologic changes, and whether preterm infant and maternal stress are reciprocal. As a preliminary step, feasibility, and acceptability of measuring indicators of stress is required.
Purpose:
This study evaluated the feasibility and acceptability of research examining perceptions and biologic markers of stress in premature infant-maternal dyads during and after NICU hospitalization.
Methods:
We evaluated study feasibility using a longitudinal descriptive design. Acceptability was measured via a maternal questionnaire. Exploratory data regarding hospitalization, perceptions of stress, social support and social determinants of health, and biologic markers of stress were collected during the first week of life and again three months after NICU.
Results:
Forty-eight mothers were eligible for the study, 36 mothers were approached, 20 mothers consented to participate, and 14 mothers completed data collection. Mothers reported high levels of study acceptability despite also voicing concern about the sharing of genetic data. Exploration of DNA methylation of SLC6A4 in preterm infants was significant for a strong correlation with perception of total chronic stress.
Implications for Practice and Research:
Clinical practice at the bedside in the NICU should include standardized screening for and early interventions to minimize stress. Complex research of stress is feasible and acceptable. Future research should focus on linking early life stress with epigenetic alterations and evaluation of the dyad for reciprocity.
Keywords: NICU, preterm infants, mothers, stress, feasibility study, DNA methylation
Background and Significance
Many premature infants and their biologic mothers experience significant stress during hospitalization in the neonatal intensive care unit (NICU).1,2 This stress arises from several sources and may include compounding, multisensory and painful stimuli,2 separation from support systems,3 the immediate- and threatened loss of life, and parental role alterations.4 Some amount of mild-moderate stress is to be expected and can be positive for infants, contributing to healthy development.5 When stress becomes severe and frequent, though, it is toxic and can overwhelm an infant’s physiologic capacity and lead to negative short- and long-term consequences.6 For example, adverse neurobehavioral outcomes7 alterations in gut microbiome,8 and epigenetic alterations have all been associated with excessive stress in premature infants.9 Similarly, mothers of premature infants’ have also reported adverse outcomes, including increased perinatal posttraumatic stress,10,11 and altered early infant-maternal relationships.12 Further, maternal stress has been found to pass generationally, as mothers with a history of elevated stress are more likely to have children with mental and physical adverse outcomes.13,14 The implications of stress for premature infants and mothers are similarly the results of complex interactions among beneficial and adverse experiences.15 Correspondingly, the risks for adverse mental and physical health extend to both premature infants and their mothers.
Assessing for and addressing stressful experiences resulting from NICU hospitalization has been a priority for NICU clinicians as research focused on NICU stress has expanded over the last 20 years.2 However, the mechanism connecting the stress experienced in the NICU to changes in both premature infants and their biologic mothers (the individual whose genetic material was transmitted to the infant) remains a gap in our understanding of this clinical issue. Furthermore, these premature infant-maternal dyads are frequently considered to be two independent people and not a true dyad. This misconstruction leads to a failure to consider the dyadic interplay of their stress responses. The possibility that the stress experience, both while in the NICU and after discharge, is reciprocal within the dyad has not been investigated. This has led to a missed opportunity in exploring and further understanding these meaningful relationships.
Literature Review
Perceptions of NICU Stress.
Perceptions of the NICU stress experience have been reported for both premature infants and mothers.16,17 Since premature infants are unable to self-report perceptions of their stress, researchers and clinicians must rely on observation to evaluate the level of the neonate’s stress experience.17 Stress is often conceptualized as pain and measured using physiologic indicators such as heart rate in premature infants.18 While pain is a component of the stress experience, premature infants in the NICU often experience stress from other sources, including excessive light, sound, and separation from parents.19 Consideration of the range of sources of stress for premature infants requires thoughtful and comprehensive assessments of the NICU environment.19 Mothers of premature infants also report excessive acute stress while their premature infants are in the NICU1. Furthermore, the risk for the continuation of this stress is high for mothers of premature infants, with up to 79% of mothers reporting at least one symptom of perinatal post-traumatic stress disorder (PPTSD).20
Objective Measures of NICU Stress:
Biologic markers of stress provide an objective measure of maternal stress.21 For example, maternal measurements of cortisol are associated with increased self-reported stress,22 while maternal oxytocin has been found to be associated with lower levels of self-reported stress in mothers of infants in the NICU,23 increased maternal engagement,24 and alterations in maternal interactive behavior during the perinatal period.24 Measurements of biologic markers of infant stress are a newer source of objective stress data and have focused on hormonal and epigenetic alterations. Premature infant oxytocin is associated with less infant stress 25 and is an important neuromodulator in infant-parent synchrony.26 Preterm Behavioral Epigenetics is a contemporary field of study offering a promising framework that links early life stress, gene modifications, and risk for poor health.27 The FKBP5 genotype and early NICU related stress are associated with neurobehavioral outcomes, specifically that the minor allele genotype in conjunction with a stressful environment is associated with poorer infant development.28 DNA methylation (DNAm) of the serotonin promoter gene SLC6A4 is the one of the most frequently measured genes in research associated with stress in premature infants.9 DNAm of SLC6A4 is a known marker of life adversity and is susceptible to stress and trauma.29 In premature infants, increased DNAm of SLC6A4 is associated with higher levels of early life stress.30
Studying the intersection of premature infant and maternal stress responses will require complex research methods measuring the multiple factors impacting the stress experienced during and after NICU hospitalization. As a preliminary phase for this program of research, feasibility issues must be examined. Our study is, to our knowledge, the first to explore the feasibility of research examining infant and maternal stress using both perceptions and hormonal and epigenetic markers of stress. Specifically, our aims were to: (1) examine the feasibility of timely recruitment of premature infant-maternal dyads during NICU hospitalization, (2) evaluate maternal acceptability of study participation and study measures, and (3) preliminarily explore the relationships between perceptions and biologic indicators of stress in infants and mothers both during the first week of NICU hospitalization and three months after NICU discharge.
Methods
Theoretical Framework:
Hill’s Family Stress Theory (FST)31 provided a framework that accounts for the complex stress processes that occur in premature infant-maternal dyads in the NICU. The FST asserts that the degree of stress experienced is influenced by the complex relationships between the a) stressful event, b) perceptions of the event, and c) resources.31 In our study, the focus of the premature infant-maternal dyad is a starting point to begin to describe the complex stress experiences that occur in the NICU. We operationalized the theory concepts as follows: a) the event or situation = NICU hospitalization, including NICU nurse documentation of stressful stimuli in the NICU, b) resources = social determinants of health and social support, c) perceptions of stress = maternal self-report perceptions. Table 1 displays the relationships among the theory concepts, study concepts, and study measures.
Table 1:
Relationships among the theory concepts, study concepts, and study measures
| Theory Concepts | Study Concepts | Study Measures |
|---|---|---|
| Event or Situation | NICU Hospitalization | Demographic Data Neonatal Infant Stressor Scale |
| Resources | Social Determinants of Health Social Support |
Protocol for Responding to and Assessing Parents’ Assets, Risks, and Experiences Multidimensional Scale of Perceived Social Support |
| Perceptions of Stress | Maternal Perception of Stress | Stanford Acute Stress Reaction Perinatal Posttraumatic Stress Disorder Questionnaire |
| Degree of Stress | Infant Stress Maternal Stress |
DNA Methylation of SLC6A4 Salivary Cortisol and Oxytocin |
Design:
A longitudinal descriptive design was approved by the Institutional Review Board of the study institution.
Setting:
The study setting was a Midwestern, 70-bed, level IV NICU in the United States. The NICU reports an average yearly admission rate of 800 infants.
Sample:
Convenience sampling, using a previously successful team-based approach for eligibility screening32 was used to identify eligible infant-maternal dyads. Infant eligibility criteria included: less than 37 weeks gestation at birth, admitted to the NICU, less than seven days old at enrollment, and planned discharged to the biologic mother. Infants were excluded if they had been discharged home prior to NICU admission, were transferred to the cardiac intensive care unit during hospitalization, had a known genetic abnormality, or had a lethal diagnosis as determined by the NICU provider. Maternal eligibility criteria included: biologic mother, primary language was English, and over the age of majority. Mothers who would not have legal custody of their infant were excluded. If the infant died after initial enrollment, the family was excluded from any further assessments and the research team mailed a condolence card. A sample of 20 infant-maternal dyads was targeted to determine feasibility and acceptability of the study design. Because the aim related to the relationship of perceptions and biologic indicators of stress in the premature infant-maternal dyad were preliminary with no basis for sample size estimation, a power analysis was not completed.
Measures
Aims 1 & 2:
Feasibility was measured by assessment of NICU census data, number of eligible dyads, number of mothers approached in the NICU, timely recruitment of study participants, and percent of dyads who returned for the three-month follow-up visit. Acceptability of the study was measured using a five-question questionnaire developed by the investigator. One open-ended question which allowed mothers to offer any other thoughts about the study was included. Completeness of data collection was measured using an assessment of all study measurements for percentage complete.
Aim 3:
The degree of stress was assessed using biologic measures of stress in both premature infants and mothers. Premature infant stress was measured using DNAm of the SLC6A4 gene from buccal cells. Genomic DNA was extracted from buccal cells using the Buccal Swab DNA kit (Promega, Madison, WI) implemented on the Maxwell RSC48 extraction device. Genomic DNA was bisulfite treated with the EZ DNA Methylation-Lightning Kit (D5030; Zymo Research) according to the manufacturer’s instructions. The target region was amplified using primers previously designed 33 using a two-stage PCR protocol, as described previously.34 Briefly, primers were synthesized with linker sequences (CS1 and CS2 for forward and reverse primers, respectively). Primers were CS1_SLC6A4F(ACACTGACGACATGGTTCTACAGYGGGTTTTTATATGGTTTGATTTTTAG) and CS2_SLC6A4R (TACGGTAGCAGAGACTTGGTCTCRAAAATCCCTCCCCTCCTAACTCTAAAATC), with linker sequences underlined. PCR amplifications were performed using ZymoTaq PreMix (E2003) in 25 microliter reactions, according to manufacturer’s instructions. First stage PCR amplification was performed with a 10-minute initial denaturation at 95°C, followed by 40 cycles of amplification of 30” at 94°C, 40” at 58°C and 60” at 72°C. Subsequently, a second PCR amplification was performed in 10 microliter reactions in 96-well plates using repliQa HiFi ToughMix (Quantabio). Each well received a separate primer pair with a unique 10-base barcode, obtained from the Access Array Barcode Library for Illumina (Fluidigm, South San Francisco, CA; Item# 100–4876). One microliter of PCR product from the first stage amplification was used as template for the 2nd stage, without cleanup. Cycling conditions were 98°C for 2 minutes, followed by 8 cycles of 98°C for 10”, 60°C for 1” and 68°C for 1”. Libraries were then pooled and sequenced with a 20% phiX spike-in on an Illumina MiniSeq sequencer employing a mid-output flow cell (2×150 paired-end reads). Raw sequence data were processed through a pipeline within the software package CLC Genomics Workbench (v22; Qiagen). Before mapping to a bisulfite converted reference with “N” substitutions for potential methylation sites, data were merged, and primers trimmed. Mapping was subsequently performed, and mapping coverages were exported to calculate percent methylation at CpG sites. DNA extraction, bisulfite conversion, library preparation, pooling, sequencing, and sequence data processing were performed at the Genomics and Microbiome Core Facility (GMCF) at Rush University. Two samples were collected at each point in time to evaluate reproducibility and data robustness.
Maternal stress was measured via salivary cortisol and oxytocin. Mothers donated 3 ml of saliva using a passive drool technique. Mothers were instructed to not eat, drink, smoke, or vape for at least 30 minutes prior to saliva donation. Samples were stored in a freezer at −80° C in the study site’s Translational Research Unit (TRU) until shipped on dry ice for analysis at Dr. Sue Carter’s lab at Indiana University. Highly sensitive Enzyme Immunoassay (EIA; Arbor Assays LLC., Ann Arbor, Michigan, USA) was used to measure oxytocin and cortisol concentrations, as it has a minimal detection rate of 16.36 pg/mL for oxytocin and 50 pg/mL for cortisol. In addition, the EIA has minimal cross-reactivity for other neuropeptides. To increase reliability within the assays, all samples were run at the same time. Coefficients of variance were less than 3.96 for oxytocin and 5.55 for cortisol in the intra-assays. Assays were conducted in duplicate.
Measurements of DNAm of SLC6A4 as the biologic measure of stress in the infant and not in the mothers was secondary to the lack of evidence of alterations in DNAm of SLC6A4 associated with stress in mothers. There is, however, evidence that both maternal cortisol35 and oxytocin23 are reliable biologic measures of maternal stress.
The stressful event was operationalized as NICU hospitalization. Data regarding the NICU hospitalization that are associated with both infant and maternal stress were collected from the electronic health record at the time of NICU discharge. These variables included: gestational age32, birth weight32, Apgar scores10, length of NICU hospitalization16,32, presence of sepsis2, days of mechanical ventilation2, hypoxic ischemic encephalopathy2, and periventricular leukomalacia.2 To measure infant stress further, the Neonatal Stressor Scale (NISS)17 was calculated using the infant’s electronic health record to score stressful events occurring within the 24 hours prior to collection of the buccal swab. Either the primary investigator or the research assistant (both NICU clinicians who were trained to criterion) calculated the total NISS. The NISS is a validated measure of 44 acute and 24 chronic stressful NICU events. Numerical values for each stressful event are assigned and then added together to calculate a final total score, with higher scores indicating higher acute and chronic neonatal stress.17 Reliability and construct validity36 have been reported in other samples of neonates requiring NICU hospitalization, with Cronbach alphas of 0.88.17 For this sample, the Cronbach alpha was 0.87.
Perceptions of maternal stress was measured twice during the study with relevant measures for acute and perinatal post-traumatic stress. First, while in the NICU, mothers completed the Stanford Acute Stress Reaction (SASRQ) to measure acute stress.37This measurement occurred at the first time of data collection, occurring within the first week of the infant’s life. The SASRQ is a 30-item instrument that measures frequency and symptoms of acute stress disorder. Using a Likert scale with 0 to 5 scaling format, with 0 = “not experienced” and 5 = “very often experienced”, and a possible total scores range from 0 and 150. Higher scores indicate higher levels of self-reported acute stress. Reliability and validity of the SASRQ in parents with infants in the NICU have been reported, with Chronbach alphas of 0.92.38The Cronbach alpha in this sample was 0.97.
At three months after infant’s discharge from the NICU, perinatal posttraumatic stress disorder was screened for using the Perinatal Post-Traumatic Stress Disorder Questionnaire (PPQ). The timing of this data collection occurred at different post menstrual ages, depending on the length of NICU hospitalization. The PPQ uses a 14-item questionnaire to assess symptoms of PPTSD associated with birth of an infant.39 Using a 5-point Likert scaling format, with 0 = “not at all experienced” and 4 = “often, for more than one month”, a possible total scores range of 0 to 56. Scores ≥ 19 are considered a positive screening for PPTSD and require clinical treatment.39 Reliability and construct validity have been reported in samples of mothers and fathers, with Cronbach alphas between 0.84 and 0.9.10,35 For this sample, the Cronbach alpha was 0.78.
Maternal Resources were measured using the Protocol for Responding to and Assessing Parents’ Assets, Risks, and Experiences instrument (PRAPARE) to assess social determinants of health36 and the Multidimensional Scale of Perceived Social Support (MSPSS).37 The PRAPARE instrument is used to screen, assess, and act on individuals’ social determinants of health in clinical and community settings.36 Screening questions regarding housing, employment, income, transportation, insurance, social support, material security, social support and stress were answered by the mothers on a study tablet. The 12-item MSPSS measures perception of social support in the domains of family, friends, and social support using a 7-point Likert scale. Scores range between 18 and 84 with lower scores indicating lower perceived social support. Previous Cronbach’s alphas in parents of infants in the NICU range between 0.85 and 0.95 in samples of mothers of premature infants.37,38
Data Analysis:
Descriptive statistics were used to summarize sample characteristics. Completeness of all the study measures were analyzed and reported as frequencies and percentages. No mothers provided any written answers to the open-ended question asking for any other feedback about the study. Mothers’ comments and questions to the PI and RA regarding consenting for the study were recorded. For Aim 1, timeliness of enrollment was calculated. For Aim 2, each acceptability question was summarized with descriptive statistics, including means and standard deviations and/or median, and interquartile range. For Aim 3, data were examined for normal distribution and measures of association, including t-test, chi-square, spearman’s rho, and Wilcoxon Signed Ranks test. For all statistical analysis, an alpha of 0.05 was used. Data analysis was conducted using STATA version 15.0.39
Procedures:
Mothers were approached in the NICU by the PI or RA and informed consent was obtained in person. Collection of instrument data and biologic markers occurred at the bedside at the time of enrollment (T1) and at the study site’s Translational Research Unit (TRU) at the three-month after NICU discharge follow-up appointment (T2). Data from the electronic health record were collected at the time of NICU discharge. Mothers who screened positive for PPTSD were provided a list of local mental health resources. Mothers received a $20 gift card at the completion of data collection both while in the NICU and at three months after NICU discharge.
Results
Recruitment occurred between July 2021 and September 2021, with 20 mothers consenting to participate in the study. Sample characteristics are displayed in Table 2. The average daily NICU census was 63 during recruitment. Forty-eight dyads met eligibility criteria. Thirty-eight (79%) of eligible mothers were approached at the bedside by either the RA or the PI in the first week of life. Fifty-three percent (N=20) of mothers approached consented for enrollment. Figure 2 displays the Consort Diagram.
Table 2:
Sample Characteristics
| Variable | Total (N) | % | Mean (SD) | Minimum – Maximum |
|---|---|---|---|---|
| Infant Characteristics | 20 | |||
| Gestational Age at Birth (weeks) | ||||
| 22–25 | 1 | 5% | ||
| 26–28 | 1 | 5% | ||
| 29–31 | 6 | 30% | ||
| 32–36 | 12 | 60% | ||
| Birth Weight (grams) | 20 | 2011 (781) | 670 – 3714 | |
| Length of NICU Hospitalization in Days | 20 | 38.6 (27.4) | 5 – 122 | |
| Apgar Score at 1 min | 20 | 4.5 (2.3) | 1 – 8 | |
| Apgar score at 5 min | 20 | 6.9 (2.01) | 1 – 9 | |
| Days of Ventilation | ||||
| 0 | 12 | 52% | ||
| 1–7 | 8 | 35% | ||
| 8–30 | 3 | 13% | ||
| >30 | 0 | |||
| Presence of sepsis during NICU hospitalization | ||||
| Yes | 0 | 0% | ||
| No | 20 | 100% | ||
| Neonatal Infant Stressor Score: Acute Score | 20 | 90.34 (23.2) | 51–142 | |
| Neonatal Infant Stressor Score: Chronic Score | 20 | 9.2 (5.6) | 0–20 | |
| Presence of Periventricular leukomalacia | ||||
| Yes | 0 | 0% | ||
| No | 20 | 100% | ||
| Hypoxic Ischemic Encephalopathy Requiring Cooling | ||||
| Yes | 0 | 0% | ||
| No | 20 | 100% | ||
| % methylation of SLC6A4 in NICU (T1) | 20 | 1.19 (.21) | 0.68 – 1.67 | |
| % methylation of SLC6A4 3 months after NICU (T2) | 14 | 1.15 (.11) | 1.01 – 1.43 | |
| Race | ||||
| White | 14 | 70% | ||
| Asian | 1 | 5% | ||
| Black | 1 | 5% | ||
| Unanswered | 3 | 20% | ||
| Multidimensional Scale of Perceived Social Support at enrollment | 20 | 72.5 (13) | 30 – 84 | |
| Multidimensional Scale of Perceived Social Support 3 Months after NICU Discharge | 14 | 75.1 (9.5) | 50 – 84 | |
| Sandford Acute Stress Reaction Questionnaire | 20 | 48.7 (33) | 0 – 108 | |
| Perinatal Posttraumatic Stress Disorder | ||||
| Screened positive | 2 | 18% | ||
| Screen negative | 9 | 82% | ||
| Salivary Oxytocin in NICU (pg/ml) | 19 | 32.7 (9.5) | 15.3 – 47.4 | |
| Salivary Cortisol in NICU (pg/ml) | 20 | 1170.93 (759.5) | 335.4 – 2864 | |
| Salivary Oxytocin 3 months after NICU discharge (pg/ml) | 13 | 25.5 (9.45) | 18.2 – 50.92 | |
| Salivary Cortisol 3 months after NICU (pg/ml) | 13 | 762.5 (462.55) | ||
| PRAPARE TOOL | ||||
| Housing | ||||
| Has housing | 15 | 94% | ||
| Does not have housing | 1 | 6% | ||
| Current work situation | ||||
| Unemployed | 5 | 31% | ||
| Part-time or temporary work | 3 | 19% | ||
| Full-time work | 8 | 50% | ||
| Income | ||||
| < $20,000/year | 3 | 18.75% | ||
| $20,001 - $40,000/year | 3 | 18.75% | ||
| $40,001 - $60,000/year | 3 | 18.75% | ||
| $60,001 - $80,000/year | 3 | 18.75% | ||
| >$80,000/year | 4 | 25% | ||
| Lack of transportation | ||||
| Yes | 1 | 94% | ||
| No | 15 | 6% | ||
| Insurance | ||||
| Public insurance | 9 | 56% | ||
| Private insurance | 7 | 44% | ||
| Number of family members currently live with? | ||||
| 1 | 3 | 18.75% | ||
| 2 | 5 | 31.25% | ||
| 4 | 6 | 37.5% | ||
| 7 | 2 | 12.5% | ||
| Unable to get the following when it was really needed? | ||||
| Food | 2 | 9% | ||
| Utilities | 2 | 9% | ||
| Clothing | 2 | 9% | ||
| Childcare | 2 | 9% | ||
| Medicine or any health care | 2 | 9% | ||
| Phone | 2 | 9% | ||
| None | 12 | 52% | ||
| How often do you see or talk to people that you care about/feel close to? | ||||
| 1–2 times/week | 4 | 25% | ||
| 3–5 times/week | 2 | 12.5% | ||
| 5 or more times/week | 10 | 62.5% | ||
| How stressed are you? | ||||
| Not at all | 2 | 12.5% | ||
| A little | 6 | 37.5% | ||
| Moderately | 1 | 6.25% | ||
| Quite a bit | 6 | 37.5% | ||
| Very much | 1 | 6.25% |
Total possible Multidimensional Scale of Perceived Social Support range from 12 to 84.
Total possible Stanford Acute Stress Reaction Questionnaire range from 0 to 150.
Total possible scores on the Perinatal Posttraumatic Stress Disorder Questionnaire range from zero to 56 with a score of ≥ 19 considered a positive screen for perinatal PPTSD.
Figure 2:
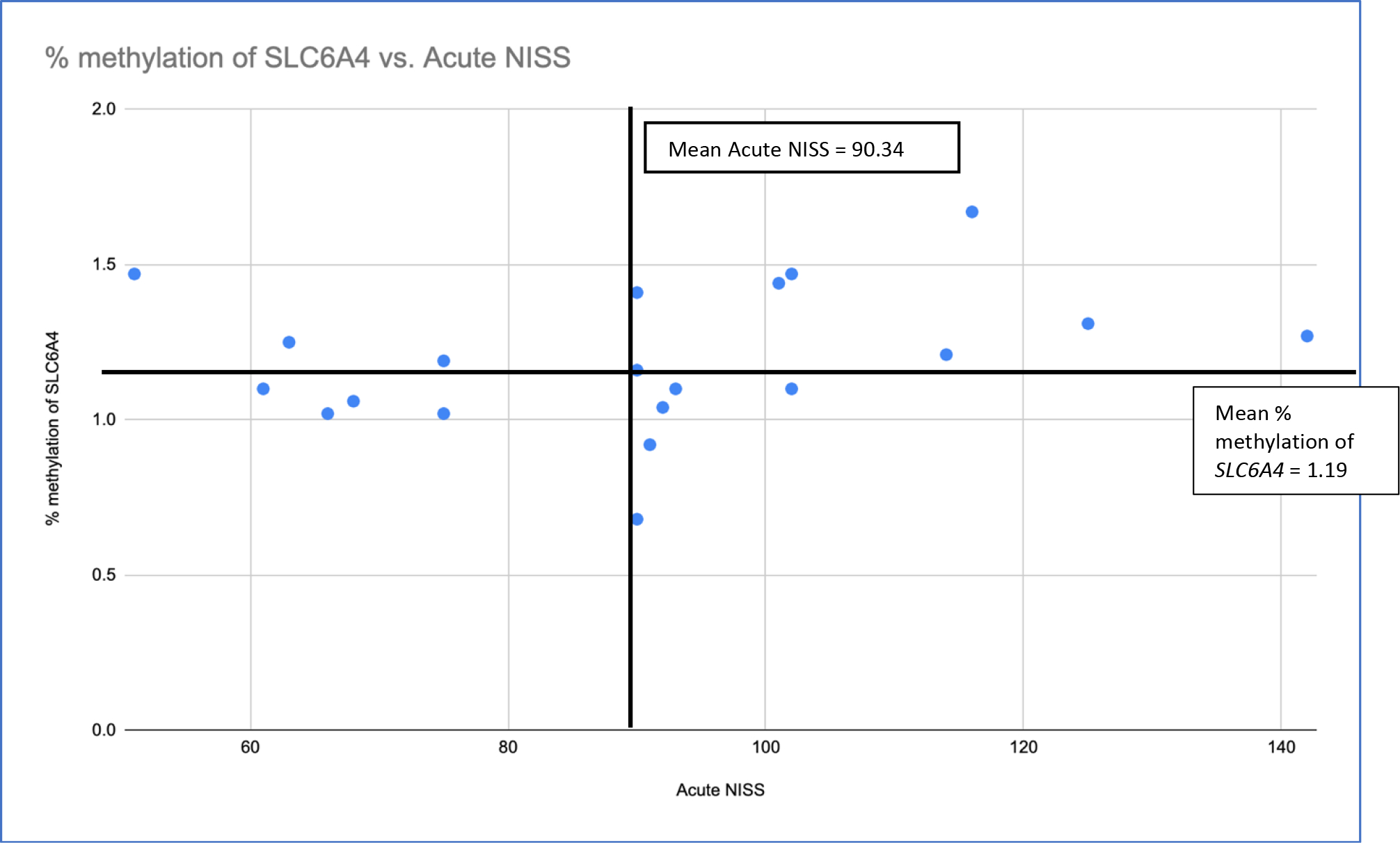
Scatter plot of infant percentage of DNAm of SLC6A4 in relationship to acute NISS score at the time of enrollment in the study.
Acceptability questions were completed by 14 mothers at the three-month post NICU discharge follow-up visit. Reasons for not attending the three-moth follow-up visit are listed in Figure 1. Mothers reported high levels of acceptance of the study, with all mothers reporting either agreement or strong agreement to the statement, “I would participate in another research study similar to this”. Only one mother reported difficulty with donating saliva. Results of acceptance of study questions are displayed in Table 3. Analysis of completeness of study questions revealed 65% of participants completed all questions included in the PRAPARE instrument and 71% of participants completed all questions included in the PPQ. The remaining questionnaires were completed by 100% of participants at both the time of enrolment and at three months after discharge from the NICU. No mothers answered the one open-ended question asking for any other feedback about the study. Notably, though, more than one mother did ask questions about banking of genetic data when consenting for enrolment. Specifically, mothers asked that genetic data not to be shared with other researchers or outside organizations. These same mothers did consent to participate in the study.
Figure 1:
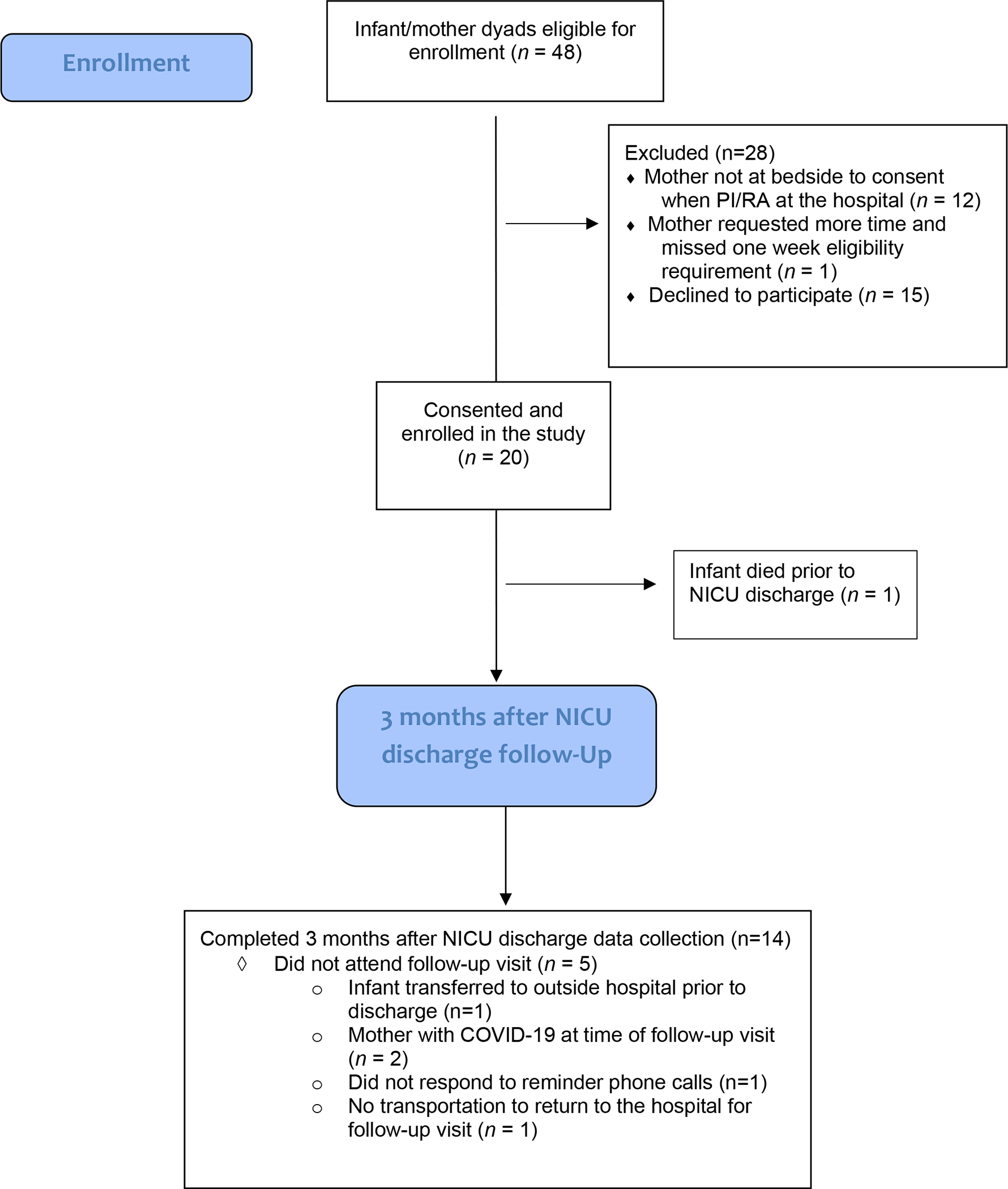
Diagram of study enrollment and follow-up.
Table 3:
Acceptability of Study
| Questions | Total (N) | % |
|---|---|---|
| It was difficult for me to answer all the questions asked during the study | ||
| Strongly disagree | 8 | 57% |
| Disagree | 5 | 36% |
| Neither agree or disagree | 1 | 7% |
| Agree | 0 | |
| Strongly agree | 0 | |
| The amount of time it took to answer all the question for the study was reasonable. | ||
| Strongly disagree | 0 | |
| Disagree | 0 | |
| Neither agree or disagree | 0 | |
| Agree | 2 | 13% |
| Strongly agree | 12 | 87% |
| It was easy to donate my saliva for the purpose of this study | ||
| Strongly disagree | 1 | 7% |
| Disagree | 0 | |
| Neither agree or disagree | 0 | |
| Agree | 1 | 7% |
| Strongly agree | 13 | 86% |
| I felt comfortable allowing the researchers to swab my child’s cheek | ||
| Strongly disagree | 0 | |
| Disagree | 0 | |
| Neither agree or disagree | 0 | |
| Agree | 0 | |
| Strongly agree | 14 | 100% |
| I would participate in another research study similar to this | ||
| Strongly disagree | 0 | |
| Disagree | 0 | |
| Neither agree or disagree | 0 | |
| Agree | 3 | 22% |
| Strongly agree | 11 | 78% |
Relationships of perceptions and biologic indicators of stress in premature infants and their mothers were exploratory in nature considering the feasibility study design. Of the repeated measures (MSPSS, oxytocin, cortisol, DNAm of SLC6A4), only maternal oxytocin changed significantly between the time of study enrollment in the NICU (T1) and three months after NICU discharge (T2) (z=2.55, p < 0.01). Relationships among measurements of resources (MSPSS and PRAPARE instrument), perceptions of stress, and biologic markers of stress were not statistically significant at the time of enrollment nor at the three months after NICU discharge follow-up appointment.
In exploration of the stressful event and biologic markers of stress, infant DNAm of SLC6A4 was strongly correlated with the total chronic stress score on the NISS (nurse perception of stress), (r (18) = 0.54, p = 0.014). No statistically significant relationships between DNAm of SLC6A4 and perceptions of stress were identified. To further evaluate the relationship of infant percentage of DNAm of SLC6A4 with NISS scores while in the NICU and three months after NICU discharge, percent methylation of SLC6A4 was plotted against NISS scores (Figure 2). Notably, 60% of infants with percentage of DNAm of SLC6A4 at or above the mean (M=1.19) while in the NICU, also demonstrated a total NISS score above the mean (M=90.34) for the sample at the same point in time. Similarly, 67% of infants with percentage of DNAm of SLC6A4 above the sample mean (M=1.15) three months after NICU discharge, demonstrated a NISS score above the mean for the entire sample at while in the NICU.
While in the NICU and three months after NICU discharge, there were no statistically significant relationships between maternal salivary oxytocin or cortisol and total score on the SASRQ and the PPQ. There was, however, a strong positive correlation between maternal acute stress while in the NICU (total SASRQ) and maternal PPTSD (total PPQ) three months after NICU discharge, (r (8) = 0.65, p = 0.039). Relationships between variables and effect sizes of biologic measures of stress are displayed in Table 4.
Table 4.
Relationships between variables and effect size of biologic measures of stress
| Spearman’s rho | Significance (2-tailed) | Intervals (2-tailed) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Cortisol T1 and Cortisol T2 | −0.41 | 0.16 | −0.79 | 0.19 |
| Cortisol T1 and Oxytocin T1 | −0.06 | 0.80 | −0.51 | 0.42 |
| Cortisol T1 and Oxytocin T2 | −0.28 | 0.35 | −0.73 | 0.33 |
| Cortisol T1 and % DNAm T1 | 0.06 | 0.79 | −0.40 | 0.50 |
| Cortisol T1 and %DNAm T2 | 0.42 | 0.13 | −0.16 | 0.78 |
| Cortisol T2 and Oxytocin T1 | 0.13 | 0.69 | −0.49 | 0.67 |
| Cortisol T2 and %DNAm T1 | 0.50 | 0.08 | −0.83 | 0.08 |
| Cortisol T2 and %DNAm T2 | 0.40 | 0.20 | −0.80 | 0.24 |
| %DNAm T1 and %DNAm T2 | 0.33 | 0.24 | −0.26 | 0.74 |
| %DNAm T1 and Oxytocin T1 | −0.38 | 0.11 | −0.72 | 0.10 |
| %DNAm T1 and Oxytocin T2 | 0.61 | 0.02* | −0.87 | −0.08 |
| %DNAmT2 and Oxytocin T1 | −0.43 | 0.14 | −0.80 | 0.17 |
| %DNAm T2 and Oxytocin T2 | −0.28 | 0.38 | −0.75 | 0.37 |
Oxytocin and cortisol are reported in pg/ml
=p < 0.05
Discussion
Our team documented the feasibility and acceptability of a complex research protocol. Additionally, we examined the relationships among NICU stress, perceptions of stress, maternal resources, and biologic changes in both premature infants and their mothers both while in the NICU and three months after NICU discharge. The results provide essential information required for planning of larger future studies.
The results indicate high feasibility of enrolling premature infant-maternal dyads in a reasonable timeframe for longitudinal research focused on NICU stress. Consideration for enrollment of a diverse sample of premature infants is imperative. The enrolled sample was majority white, non-Latinx. Additionally, 60% of the premature infants enrolled in the study were born between 32- and 36- weeks gestation. The NICU data showed that 44% of infants admitted annually are born between 32- and 36- weeks gestation. The study site NICU data revealed a racial/ethnic mix of the study site is 65% White, 14% Black, 7% Latinx, and 4% Asian. Our study did not fully replicate this diversity, underscoring the importance of customizing research protocols to include elements such as diverse research staff, previous NICU families, longer and targeted enrollment, and collaboration with clinical staff in future studies.40,41 Feasibility of follow-up data collection after NICU discharge was also affirmed. Forty percent of the premature infant-maternal dyads who did not attend the three months after NICU discharge appointment listed current COVID-19 infection as the reason for failure to attend. Our study was conducted after the start of the COVID-19 pandemic and the majority of the 3-month follow-up study visits were scheduled during the Omicron wave of COVID-19, in December 2021 and January 2022. One mother reported she was unable to attend the follow-up visit because of a lack of transportation. Amendments to future research protocols including budget for transportation vouchers and adequate reimbursement need to be considered a priority. Completing a telehealth follow-up visit was not possible for the study because of the need to collect biologic materials. Future research requiring biologic materials should test the feasibility of clinician-observed, patient-collected biologic samples using telehealth technologies. Study designs leveraging patient-collected biologic sample designs have previously been successful and are more frequently needed with the introduction of COVID-19.42
Overwhelmingly, the mothers in our study reported high acceptance of the research protocol. All but one participating mother reported high degrees of acceptability of collecting samples of biologic materials. Importantly, non-invasive collection of DNA materials from premature infants was not an inhibiting factor in decision making about enrollment. These findings are comparable to previous research using buccal swabs for DNA collection in infants.43 Maternal questions at the time of consent regarding banking of DNA data supports the possibility that parents have heard of DNA research and popular culture stories about the use of publicly available DNA banking companies and privacy may influence decisions regarding acceptability of genetic research.44 Study protocols and parent information that are explicit and understandable regarding the use of genetic data in research are needed. Reaffirmation of privacy, especially with genetic data, should be included when discussing epigenetic research with parents.
The exploratory aim of our feasibility study provided encouraging data for future research of premature infant-maternal stress. Connecting the relationships among early stress, epigenetic changes, neurodevelopmental outcomes, and the role of maternal stress is a research priority. Understanding the impact of early life stress on gene expression and which the timing of these changes in premature infants would provide the foundation of targeted nursing interventions to decrease stress and improve long-term outcomes. Larger studies are needed to build on our results and validate current measurements of stress experienced by the small sample of premature infants and their mothers in our study. These future studies should also consider the impact of significant prenatal maternal mental health problems and gestational age on the extent of biological alterations and timing of interventions. Enrollment of younger, more premature infants in research may be more challenging. 40 Further, the differences in the degree of maternal stress experienced in mothers of more premature infants as compared to older infants need to be described. Finally, future research including fathers and other parents examining the stress interactions among family members are needed.
Maternal oxytocin is an important biologic factor influencing infant and maternal attachment.45 Our data supports the role of oxytocin as a buffer in premature infant-maternal stress. Additional data (both perception and biologic) linking the stress experienced by the premature infant and their mother is needed to provide foundational methods in improving the health of the dyad. Leveraging resources to both the premature infant and the mother will be an essential aspect of successful intervention development.2,46,47
Our study had several limitations. The small sample size, secondary to the nature of the study design, prevents us from drawing conclusions about relationships among the variables. Based on our results, future research can be powered appropriately. The lack of diversity in our sample limits the generalizability of our results. The feasibility and acceptability of stress research in the NICU may not extend to more diverse samples of families. The variations in length of hospitalization resulted in differences in infant age at the second data collection time. As a result, we are unable to make conclusions regarding the impact of age on both the acceptability of longitudinal research and the stress experienced after NICU hospitalization. We did not measure maternal presence in the NICU, limiting our ability to assess the protective factors of family integrated care.48 We did not account for the timing of saliva collection, which is known to vary in women based on timing of breastfeeding or pumping.49 The need to control the timing of saliva collection may impact the feasibility of future studies. Finally, our use of an older theoretical framework and the exclusion fathers when conceptualizing family stress simplistic. Future research needs to include fathers/other parents and should account for the known complexities of family stress.
Despite these limitations, our feasibility study provides much needed information needed for the development of future research protocols focused on the stress experience of premature infants and their mothers. Through sharing the results of feasibility research, we have answered the call for foundational information required for future research.50 Addressing issues of sampling and loss to follow-up in the era of COVID-19 will continue impact methodological decisions. Defining the mechanisms of premature infants and their mothers’ stress resulting from NICU hospitalization remains a priority. Our results provide insight into the important phenomenon of infant and maternal stress, which is highly complex and requires a fuller panel of infant and maternal biologic, psychosocial, and behavioral measures to fully elucidate.
Financial Disclosure Statement:
Kathryn J. Malin is supported by a grant from The National Association of Neonatal Nurses: Small Grants, The Marquette University Regular Research Grant, The Marquette University Regnar Research Grant, and the National Institutes of Health (NIH) CTSA award (UL TR001436).
Acronyms and Abbreviations:
- NICU
Neonatal Intensive Care Unit
- DNAm
DNA methylation
- Dyads
premature infants and their mothers
- PPTSD
perinatal post-traumatic stress disorder
- FST
Family Stress Theory
- NISS
Neonatal Infant Stressor Scale
- SASRQ
Stanford Acute Stress Reaction
- PPQ
Perinatal Post-Traumatic Stress Disorder Questionnaire
- PRAPARE
Parents’ Assets, Risks, and Experiences Instrument
- MSPSS
Multidimensional Scale of Perceived Social Support
- TRU
Translational Research Unit
Footnotes
Competing Interests Statement: All authors report no competing interests.
Ethical Approval: Ethical approval was provided by the Institutional Review Board of Children’s Wisconsin in advance of implementation. Written informed consent was obtained from the patients/guardians.
Clinical Trial Registration: Not Applicable
Preprint Disclosure: Not Applicable
References
- 1.Roque ATF, Lasiuk GC, Radünz V, Hegadoren K. Scoping review of the mental health of parents of infants in the NICU. Journal of obstetric, gynecologic, and neonatal nursing. 2017;46(4):576–587. doi: 10.1016/j.jogn.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Weber A, Harrison TM. Reducing toxic stress in the NICU to improve infant outcomes. Nursing outlook. 2019;67(2):169–189. doi: 10.1016/j.outlook.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mäkelä H, Axelin A, Feeley N, Niela-Vilén H. Clinging to closeness: The parental view on developing a close bond with their infants in a NICU. Midwifery. 2018;62:183–188. doi: 10.1016/j.midw.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Caporali C, Pisoni C, Gasparini LL, et al. A global perspective on parental stress in the neonatal intensive care unit: A meta-analytic study. J Perinatol. 2020;40(12):1739. doi: 10.1038/s41372-020-00798-6. [DOI] [PubMed] [Google Scholar]
- 5.Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics (Evanston). 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 6.Zhao T, Griffith T, Zhang Y, et al. Early-life factors associated with neurobehavioral outcomes in preterm infants during NICU hospitalization. Pediatr Res. 2022. doi: 10.1038/s41390-022-02021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cong X, Wu J, Vittner D, et al. The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early human development. 2017;108:9–16. doi: 10.1016/j.earlhumdev.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’agata AL, Wu J, Welandawe MKV, Dutra SVO, Kane B, Groer MW. Effects of early life NICU stress on the developing gut microbiome. Dev Psychobiol. 2019;61(5):650. doi: 10.1002/dev.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malin KJ, Gondwe KW, Fial A, et al. Early toxic stress and epigenetic alterations in the neonatal intensive care unit: A scoping review. Nursing Research. 2023; 72(3): 218–228. doi: 10.1097/NNR.0000000000000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aftyka A, Rybojad B, Rosa W, Wróbel A, Karakuła-Juchnowicz H. Risk factors for the development of post-traumatic stress disorder and coping strategies in mothers and fathers following infant hospitalisation in the neonatal intensive care unit. J Clin Nurs. 2017;26(23–24):4436. doi: 10.1111/jocn.13773. [DOI] [PubMed] [Google Scholar]
- 11.Malin KJ, Johnson TS, Brown RL, et al. Uncertainty and perinatal post-traumatic stress disorder in the neonatal intensive care unit. Research in nursing & health. 2022;45(6):717. doi: 10.1002/nur.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Givrad S, Hartzell G, Scala M. Promoting infant mental health in the neonatal intensive care unit (NICU): A review of nurturing factors and interventions for NICU infant-parent relationships. Early human development. 2021;154:105281. doi: 10.1016/j.earlhumdev.2020.105281. [DOI] [PubMed] [Google Scholar]
- 13.Bowers ME, Yehuda R. Intergenerational transmission of stress in humans. Neuropsychopharmacol. 2016;41(1):232. doi: 10.1038/npp.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGowan PO, Matthews SG. Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology. 2018;159(1):69–82. doi: 10.1210/en.2017-00896. [DOI] [PubMed] [Google Scholar]
- 15.Carrero-Castillero A, Morton SU., Nelson C A, Smith V C. Psychosocial stress and adversity: Effects from the perinatal period to adulthood. NeoReviews. 2019;20(12): e686–e696. 10.1542/neo.20–12-e686. doi: 10.1542/neo.20-12-e686. [DOI] [PubMed] [Google Scholar]
- 16.Caporali C, Pisoni C, Gasparini L, et al. A global perspective on parental stress in the neonatal intensive care unit: A meta-analytic study. J Perinatol. 2020;40(12):1739. doi: 10.1038/s41372-020-00798-6. [DOI] [PubMed] [Google Scholar]
- 17.Newnham CA, Inder TE, Milgrom J. Measuring preterm cumulative stressors within the NICU: The neonatal infant stressor scale. Early human development. 2009;85(9):549–555. doi: 10.1016/j.earlhumdev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Zeiner V, Storm H, Doheny KK. Preterm infants’ behaviors and skin conductance responses to nurse handling in the NICU. The Journal of Maternal-Fetal and Neonatal Medicine. 2015:1. doi: 10.3109/14767058.2015.1092959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Agata AL, Sanders MR, Grasso DJ, Young EE, Mcgrath JM. Unpacking the burden of care for infants in the NICU. Infant Mental Health Journal. 2017;38(2):306. doi: 10.1002/imhj.21636. [DOI] [PubMed] [Google Scholar]
- 20.Beck CT, Harrison L. Posttraumatic stress in mothers related to giving birth prematurely: A mixed research synthesis. Journal of the American Psychiatric Nurses Association. 2017;23(4):241–257. doi: 10.1177/1078390317700979. [DOI] [PubMed] [Google Scholar]
- 21.Caparros-Gonzalez RA, Lynn F, Alderdice F, Peralta-Ramirez MI. Cortisol levels versus self-report stress measures during pregnancy as predictors of adverse infant outcomes: A systematic review. Stress. 2022;25(1):189. doi: 10.1080/10253890.2022.2059348. [DOI] [PubMed] [Google Scholar]
- 22.Garfield CF, Simon CD, Rutsohn J, Lee YS. Stress from the neonatal intensive care unit to home. Journal of Perinatal and Neonatal Nursing. 2018;32(3):257. doi: 10.1097/jpn.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garfield L, Holditch-Davis D, Carter C, et al. A pilot study of oxytocin in low-income women with a low birth-weight infant: Is oxytocin related to posttraumatic stress? Advances in Neonatal Care. 2019;19(4):E12–E21. doi: 10.1097/ANC.0000000000000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White-Traut R, Gillette P, Simpson P, Zhang L, Nazarloo HP, Carter CS. Early postpartum maternal and newborn responses to auditory, tactile, visual, vestibular, and olfactory stimuli. Journal of obstetric, gynecologic, and neonatal nursing. 2022;51(4):402–417. doi: 10.1016/j.jogn.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Vittner D, D’Agata A, Choi BY, McGrath J. Release of oxytocin and cortisol is associated with neurobehavioral patterns in premature infants. Journal of obstetric, gynecologic, and neonatal nursing. 2023;52(3):248–256. doi: 10.1016/j.jogn.2023.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Vittner D, McGrath J, Robinson J, et al. Increase in oxytocin from skin-to-skin contact enhances development of parent–infant relationship. Biological Research for Nursing. 2018;20(1):54–62. doi: 10.1177/1099800417735633. [DOI] [PubMed] [Google Scholar]
- 27.Montirosso R & Provenzi L. Implications of epigenetics and stress regulation on research and developmental care of preterm infants. Journal of obstetric, gynecologic, and neonatal nursing. 2015;44(2):174–182. doi: 10.1111/1552-6909.12559. [DOI] [PubMed] [Google Scholar]
- 28.D’Agata A, Walsh S, Vittner D, Cong X, McGrath JM, & Young EE FKBP5 genotype and early life stress exposure predict neurobehavioral outcomes for preterm infants. Developmental Psychobiology. 2017;59:410–418. doi: 10.1002/dev.21507. [DOI] [PubMed] [Google Scholar]
- 29.Provenzi L, Giorda R, Beri S, Montirosso R. SLC6A4 methylation as an epigenetic marker of life adversity exposures in humans: A systematic review of literature. Neuroscience and biobehavioral reviews. 2016;71:7–20. doi: 10.1016/j.neubiorev.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Fumagalli M, Provenzi L, De Carli P, et al. From early stress to 12-month development in very preterm infants: Preliminary findings on epigenetic mechanisms and brain growth. PLos One. 2018; 13(1): e01906–2. doi: 10.1371/journal.pone.0190602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill Reuben. Families under stress. Westport, CT: Greenwood; 1949. [Google Scholar]
- 32.Malin KJ, Johnson TS, McAndrew S, Westerdahl J, Leuthner J, Lagatta J. Infant illness severity and perinatal post-traumatic stress disorder after discharge from the neonatal intensive care unit. Early human development. 2020;140:104930. doi: 10.1016/j.earlhumdev.2019.104930.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provenzi L, Fumagalli M, Scotto di Minico G, et al. Pain-related increase in serotonin transporter gene methylation associates with emotional regulation in 4.5-year-old preterm-born children. Acta Paediatrica. 2020;109(6):1166–1174. doi: 10.1111/apa.15077. [DOI] [PubMed] [Google Scholar]
- 34.Naqib A, Poggi S, Wang W, Hyde M, Kunstman K, & Green SJ Gene expression analysis. New York NY: Humana Press; 2018:149–169. [Google Scholar]
- 35.Janevski MR, Vujičić AĐ, Đukić SM. Salivary cortisol as a biomarker of stress in mothers and their low birth weight infants and sample collecting challenges. Journal of Medical Biochemistry. 2016;35(2):118–122. doi: 10.1515/jomb-2015-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pourkaviani S, Zhang X, Spear EA, et al. Clinical validation of the neonatal infant stressor scale with preterm infant salivary cortisol. Pediatr Res. 2019;87(7):1237. doi: 10.1038/s41390-019-0713-0.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw RJ, Bernard RS, DeBlois T, Ikuta LM, Ginzburg K, Koopman C. The relationship between acute stress disorder and posttraumatic stress disorder in the neonatal intensive care unit. Psychosomatics (Washington, D.C.). 2009;50(2):131–137. doi: 10.1176/appi.psy.50.2.131. [DOI] [PubMed] [Google Scholar]
- 38.Shaw RJ, Bernard RS, DeBlois T, Ikuta LM, Ginzburg K, Koopman C. The relationship between acute stress disorder and posttraumatic stress disorder in the neonatal intensive care unit. Psychosomatics (Washington, D.C.). 2009;50(2):131–137. doi: 10.1176/appi.psy.50.2.131. [DOI] [PubMed] [Google Scholar]
- 39.Callahan JL, Borja SE, Hynan MT. Modification of the perinatal PTSD questionnaire to enhance clinical utility. Journal of Perinatology. 2006;26(9):533–539. doi: 10.1038/sj.jp.7211562.. [DOI] [PubMed] [Google Scholar]
- 40.Weiss EM, Olszewski AE, Guttmann KF, et al. Parental factors associated with the decision to participate in a neonatal clinical trial. JAMA Netw Open. 2021;4(1). doi: 10.1001/jamanetworkopen.2020.32106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burris HH, Duncan AF. Rethinking how to persuade more parents from diverse or disadvantaged backgrounds to enroll infants in neonatal clinical trials. JAMA Network Open. 2021;4(1). doi: 10.1001/jamanetworkopen.2020.32137. [DOI] [PubMed] [Google Scholar]
- 42.Kissler K, Breman RB, Carlson N, Tilden E, Erickson E, Phillippi J. Innovations in prospective perinatal research as a result of the COVID-19 pandemic. J Midwife Womens Health. 2022;67(2):264. doi: 10.1111/jmwh.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatfield LA, Hoffman RK, Polomano RC, Conley Y. Epigenetic modifications following noxious stimuli in infants. Biological research for nursing. 2018;20(2):137–144. doi: 10.1177/1099800417754141. [DOI] [PubMed] [Google Scholar]
- 44.Brewer PR, Ley BL. Media use and public perceptions of DNA evidence. Science communication. 2010;32(1):93–117. doi: 10.1177/1075547009340343. [DOI] [Google Scholar]
- 45.Scatliffe N, Casavant S, Vittner D, Cong X. Oxytocin and early parent-infant interactions: A systematic review. International Journal of Nursing Sciences. 2019;6(4):445. doi: 10.1016/j.ijnss.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabnis A, Fojo S, Nayak SS, Lopez E, Tarn DM, Zeltzer L. Reducing parental trauma and stress in neonatal intensive care: Systematic review and meta-analysis of hospital interventions. J Perinatol. 2019;39(3):375. doi: 10.1038/s41372-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lean RE, Rogers CE, Paul RA, Gerstein ED. NICU hospitalization: Long-term implications on parenting and child behaviors. Curr Treat Options Peds. 2018;4(1):49–69. doi: 10.1007/s40746-018-0112-5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Brien D, Robson K, Bracht M, et al. Effectiveness of family integrated care in the neonatal intensive care units on infants and parent outcomes: a multicenter, multinational, cluster-randomised controlled trial. Lancet Child Adolesc Health.2018; 2(4): 245–254. doi: 10.1016/S2352-4642(18)30039-7.. [DOI] [PubMed] [Google Scholar]
- 49.White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A., Carer CS. Detection of salivary oxytocin levels in lactating women. Dev Psychobiol. 2009; 51(4):367–373. doi: 10.1002/dev.20376.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Story DA. Feasibility and pilot studies: Dropping the fig leaf. Anesthesia. 2020;75(2):152–154. doi: 10.1111/anae.14865. [DOI] [PubMed] [Google Scholar]


