Significance
Inorganic polyphosphate (polyP), a linear polymer of hundreds of phosphate residues, is synthesized by Polyphosphate kinase-1 (PPK-1). Studies have shown that polyP dysregulation impairs Mycobacterium tuberculosis growth in host tissues. Overall, our work provides a detailed mechanistic perspective on regulation of mycobacterial pathogenesis by deletion of ppk-1. We show that deletion of ppk-1 affects glucose utilization and lipid biosynthesis in M. tuberculosis. In addition, the lungs of Δppk-1 mutant strain–infected mice had reduced transcript levels of genes encoding for proteins involved in antimicrobial pathways. We have also identified a broad-spectrum inhibitor against the PPK-1 enzyme. We anticipate that PPK-1 targeting small molecules can reduce the duration of chemotherapy and be effective against bacteria that require polyP for virulence.
Keywords: Mycobacterium tuberculosis, inorganic polyphosphate, virulence-associated factors, target-based screening, pathogenesis
Abstract
Inorganic polyphosphate (polyP) is primarily synthesized by Polyphosphate Kinase-1 (PPK-1) and regulates numerous cellular processes, including energy metabolism, stress adaptation, drug tolerance, and microbial pathogenesis. Here, we report that polyP interacts with acyl CoA carboxylases, enzymes involved in lipid biosynthesis in Mycobacterium tuberculosis. We show that deletion of ppk-1 in M. tuberculosis results in transcriptional and metabolic reprogramming. In comparison to the parental strain, the Δppk-1 mutant strain had reduced levels of virulence-associated lipids such as PDIMs and TDM. We also observed that polyP deficiency in M. tuberculosis is associated with enhanced phagosome–lysosome fusion in infected macrophages and attenuated growth in mice. Host RNA-seq analysis revealed decreased levels of transcripts encoding for proteins involved in either type I interferon signaling or formation of foamy macrophages in the lungs of Δppk-1 mutant–infected mice relative to parental strain–infected animals. Using target-based screening and molecular docking, we have identified raloxifene hydrochloride as a broad-spectrum PPK-1 inhibitor. We show that raloxifene hydrochloride significantly enhanced the activity of isoniazid, bedaquiline, and pretomanid against M. tuberculosis in macrophages. Additionally, raloxifene inhibited the growth of M. tuberculosis in mice. This is an in-depth study that provides mechanistic insights into the regulation of mycobacterial pathogenesis by polyP deficiency.
Inorganic polyphosphate (polyP) is present in species from all three kingdoms of life, and enzymes involved in polyP metabolism have been extensively studied in prokaryotes. Polyphosphate kinase-1 (PPK-1) primarily synthesizes polyP using ATP as substrate (1). Polyphosphate kinase-2 (PPK-2) generates polyP from GTP or ATP and uses polyP as a phosphate donor to synthesize GTP or ATP (2–5). Exopolyphosphatases degrade polyP and sequentially remove the terminal phosphate (6, 7). Studies have shown that mycobacteria accumulate polyP during the stationary phase of growth and following exposure to various stress conditions and drugs (8–10). It has been demonstrated that Mycobacterium smegmatis and Mycobacterium tuberculosis secrete polyP upon infecting Dictyostelium discoideum or macrophages and inhibit phagosome acidification and phagosome–lysosome fusion (11). The genome of M. tuberculosis encodes homologs of polyphosphate kinases (PPK-1/Rv2984 and PPK-2/Rv3232c) and exopolyphosphatases (PPX-1/Rv0496 and PPX-2/Rv1026) (12). The PPK-2 homolog is required for the survival of M. smegmatis in acidic and low-oxygen conditions and is essential for M. tuberculosis growth in mice and guinea pigs (5, 13, 14). PPX enzymes from M. tuberculosis can utilize polyP, ATP, or GTP as substrates, and their activity is inhibited by (p)ppGpp (15). We have recently shown that simultaneous deletion of ppx-1 and ppx-2 from M. tuberculosis results in reduced expression of the dormancy-associated genes and attenuated growth in mice and guinea pigs (16).
Previously, we had shown that deletion of ppk-1 in M. tuberculosis is associated with polyP deficiency, increased susceptibility to isoniazid (INH), and attenuated growth in guinea pigs (8). Another study showed that the RNA polymerase binding protein RbpA and sigma factor B (sigB) regulate ppk-1 expression and contribute to INH tolerance (17). In agreement, relative to the wild-type strain, polyP accumulating strains (Δppk-2 strain) were resistant to INH-mediated killing (13, 14). Here, we have performed experiments to delineate the mechanisms by which polyP levels regulate M. tuberculosis pathogenesis. We show that polyP interacts with acyl CoA carboxylases (ACC) from M. tuberculosis, the enzymes that synthesize precursors for various lipids (18, 19). Further, the Δppk-1 mutant strain has reduced levels of lipids that contribute to mycobacterial pathogenesis relative to the wild-type and complemented strains. We also show enhanced phagosome–lysosome fusion in THP-1 macrophages infected with Δppk-1 mutant strain compared to wild-type and complemented strain–infected macrophages. In comparison to the parental strain, lung transcriptional profiling revealed reduced expression of proteins involved in inflammatory pathways in lung tissues from Δppk-1 mutant–infected mice. In the present study, we have identified raloxifene hydrochloride as a broad-spectrum PPK-1 inhibitor. The identified small molecule enhanced the activity of known anti-TB drugs in an additive manner and inhibited the growth of intracellular M. tuberculosis in macrophages and mice. Taken together, targeting the PPK-1 enzyme represents an approach for shortening the duration of therapy for drug-susceptible and drug-resistant M. tuberculosis.
Results
Acyl CoA Carboxylases from M. tuberculosis Interact with polyP.
In order to identify polyP-interacting proteins from M. tuberculosis, pull-down experiments were performed using biotinylated polyP700 (Fig. 1A). Using clarified lysates prepared from either mid-log or late-log phase, 11- and 20- polyP700 interacting proteins were identified (Fig. 1B). Among these, eight interacting proteins were common in lysates prepared from mid-log and late-log phase M. tuberculosis cultures (SI Appendix, Table S1 and Dataset S1). Interestingly, the majority of the commonly identified polyP700 interacting proteins belonged to the ACC family of enzymes. These included AccA1 (Rv2501c), AccD1 (Rv2502c), AccA2 (Rv0973c), AccD2 (Rv0974c), AccD4 (Rv3799c), AccD5 (Rv3280), and AccE5 (Rv3281) (SI Appendix, Table S1 and Dataset S1). Since polyP has been demonstrated to replace ATP as a phosphate donor in various metabolic enzymatic reactions, we hypothesized that ACC enzymes can bind both ATP and polyP (20, 21). In our bio-layer interferometry (BLI) experiments, (His)6-AccA1, (His)6-AccD1, and (His)6-PPK-1 showed a simple 1:1 binding with biotinylated polyP700 (Fig. 1C). We did not observe binding of biotinylated polyP700 with the remaining purified proteins. These observations suggest that AccA2, AccD2, AccD4, AccD5, and AccE5 might not interact directly and require an accessory M. tuberculosis protein or factor to interact with polyP. The dissociation constant (Kd) for (His)6-AccA1 binding with polyP700 and polyP45 was 1.43 µM and 1.1 µM, respectively (Fig. 1D). In comparison, (His)6-AccD1 displayed Kd values of 1.1 µM for both polyP700 and polyP45 (Fig. 1D). As shown in SI Appendix, Fig. S1 A and B, no interaction was observed between (His)6-AccA1 and (His)6-AccD1 with polyP3. Further, we performed molecular modeling and docking studies to identify polyP binding pockets in AccA1 and AccD1. We identified three and five potential clusters for polyP binding with AccA1 and AccD1, respectively (Fig. 1 E and F). Further, to establish the most likely orientation of polyP binding, focused docking was performed utilizing cluster representatives (as grid). For AccA1, the lowest docking energy and the highest population of polyP poses (number of conformations) were −4.0 kcal/mol and 32, respectively (Fig. 1E). For AccD1, the lowest docking energy and the highest population of polyP poses were −6.0 kcal/mol and 120, respectively (Fig. 1F). Interaction mapping analysis revealed that Lys576 and His578 of AccA1 and His352, Leu356, Lys359, and Arg360 of AccD1 might interact with polyP (Fig. 1 G and H). In agreement with molecular docking studies, in comparison to wild-type protein, we observed significantly reduced binding of (His)6-AccD1L356A with biotinylated polyP700 (SI Appendix, Fig. S1C). Taken together, these observations suggest that polyP interacts with AccA1 and AccD1 from M. tuberculosis.
Fig. 1.
Inorganic polyphosphate interacts with AccA1 and AccD1 enzymes from M. tuberculosis. (A) Schematic diagram showing the experimental design for identification of polyP700 interacting proteins from M. tuberculosis. (B) Venn diagrams depicting the number of polyP700 interacting proteins identified in three replicative samples from either mid-log or late-log phase cultures. (C) BLI sensogram showing the interaction between biotinylated polyP700 with either (His)6-AccA1 or (His)6-AccD1 or (His)6-PPK-1. (D) The binding affinity of (His)6-AccA1 and (His)6-AccD1 with either polyP700 or polyP45 was determined using MST. The data shown in panels (C) and (D) are representative of two independent experiments. (E and F) These panels represents the top clusters obtained from blind docking of polyP with AccA1 and AccD1. (G and H) The most likely pose of polyP binding on the modeled structure of AccA1 and AccD1 is shown. The zoom-in Inset highlights the critical residues of proteins that might be involved in polyP binding. The polyP and interacting residues are shown in licorice and colored atom-wise: C: magenta/white, N: blue, O: red, respectively.
Deletion of PPK-1 in M. tuberculosis Affects the Expression of Virulence-Associated Factors and Enhances Phagosome–Lysosome Fusion.
In order to understand the mechanisms by which PPK-1 contributes to mycobacterial pathogenesis, we compared the transcription profiles of mid-log phase cultures of wild-type and Δppk-1 mutant strains. We observed that, in comparison to the parental strain, the expression of 247 and 183 transcripts were decreased and increased, respectively, in Δppk-1 mutant strain (Fig. 2A and Dataset S2). Functional categorization of these differentially expressed genes (DEGs) revealed that the majority of these encodes for conserved hypothetical proteins (CHP) or proteins involved in either intermediary metabolism and respiration (IMR) or cell wall (CW) processes (SI Appendix, Table S2). This transcriptional reprogramming observed in the Δppk-1 mutant strain might be associated with the differential expression of various regulatory proteins such as Rv2034, Rv1845c, Rv1773c, Rv3082c, Rv0485, Rv3173c, Rv2745c, Rv1152, Rv0377, Rv2642, Rv0353, Rv3058c, Rv3132c, Rv1129c, Rv0894, Rv0490, Rv3416, and Rv2011c (Fig. 2B). There is a possibility that polyP regulates the activity of these transcription factors, as reported for RpoS from Escherichia coli (22). Few of the transcripts with reduced expression encode for metabolic enzymes involved in either the production of cobalamin or porphyrin, storage or transport of metal ions, or peptidoglycan synthesis (Fig. 2C). The transcript levels of genes encoding for various ribosomal and ribosome-associated proteins were also increased in the ∆ppk-1 mutant strain relative to the parental strain (Fig. 2D). These findings indicate a lower rate of translation in the ∆ppk-1 mutant strain relative to the wild type, a phenomenon observed in M. tuberculosis upon nutrient deprivation (23). We also observed that transcripts of genes encoding for various toxin-antitoxin (TA) systems were differentially expressed between these two strains (Fig. 2E). Among these, transcript levels of the vapBC15 TA system were increased by ~16.0 folds in the ∆ppk-1 mutant strain relative to the parental strain (Fig. 2E and Dataset S2).
Fig. 2.
(A–F) Effect of ppk-1 deletion on the transcriptional profiles of M. tuberculosis (A) This panel shows the volcano plot comparing the expression profiles obtained from mid-log phase cultures of wild-type and Δppk-1 mutant strain. (B–F) Heatmaps depicting differentially expressed transcripts involved in gene regulation (B), metal ion transport/cell wall biosynthesis (C), ribosomal proteins (D), toxin-antitoxin systems (E), and PDIMs biosynthesis/Type VII secretion system (F) in mid-log phase cultures of Δppk-1 mutant strain relative to the parental strain. The color intensity in heatmaps represents the log2 value of raw read counts. (G) The transcript levels of various differentially expressed transcripts between Δppk-1 mutant and parental strain were quantified using qPCR. The data obtained were normalized to sigA levels and are shown as mean ± SE of relative fold change with respect to the parental strain. (H) Effect of ppk-1 deletion on lipid biosynthesis in M. tuberculosis. This panel shows the profiles of apolar lipids isolated from mid-log phase cultures of various strains. WT, MT, and CT represent wild-type, Δppk-1 mutant, and complemented strains. TAG, triacylglycerol; PDIM, phthiocerol dimycocerosate; FAME, fatty acid methyl esters; MAME, mycolic acid methyl esters. The data shown in panels (A–F and H) are obtained from two biological replicates. The data shown in (G) are obtained from two independent experiments performed in duplicates. (I and J) Deletion of ppk-1 enhances phagosome–lysosome fusion in THP-1 macrophages infected with M. tuberculosis. (I) THP-1 macrophages were infected with FITC-labeled strains. The images were acquired using an Olympus FV3000 confocal microscope at 60× magnification. (Scale bars, 20 µm.) (J) The overlap coefficient for co-localization between red and green fluorescent signals for all three strains was calculated using Olympus cellSens software. The data shown in this panel are mean ± SE for the overlap coefficient calculated using 30 z-stacked images obtained from two independent experiments. The data were statistically analyzed by one-way ANOVA (***P < 0.001, ****P < 0.0001). Photo credit: Mr. Saurabh Chugh, Translational Health Science and Technology Institute.
Intriguingly, the mutant strain also had reduced transcript levels of genes encoding for enzymes involved in either phthiocerol dimycocerosates (PDIM) biosynthesis (pps gene cluster, ppsABCDE) or components of type VII secretion systems (eccD3, eccD5, esxG, esxN and associated protease mycP5, Fig. 2F). The differential expression of a subset of these DEGs in ∆ppk-1 mutant strain relative to the parental strain was confirmed by qPCR (Fig. 2G). In agreement with RNA-seq data, relative to the wild-type and complemented strain, levels of PDIMs, fatty acid methyl esters (FAMEs), and mycolic acid methyl esters (MAME-II) were reduced in mid-log phase cultures of ∆ppk-1 mutant strain (Fig. 2H). However, the levels of triacylglycerols and MAME-I were comparable in these strains (Fig. 2H). It has been previously shown that proteins belonging to ESX-1 and PDIMs are required for phagosome rupture and escape of M. tuberculosis to the cytosol (24, 25). In agreement, we found that THP-1 macrophages infected with the Δppk-1 mutant strain showed increased phagosome–lysosome fusion compared to wild-type and complemented strain–infected macrophages (Fig. 2 I and J). Taken together, these findings suggest that deletion of ppk-1 leads to reduced levels of PDIMs, FAMEs, and MAMEs and enhanced phagosome–lysosome fusion–mediated killing of M. tuberculosis.
Deletion of ppk-1 Perturbs the Central Carbon and Trehalose Metabolism in M. tuberculosis.
Previous studies have shown that M. tuberculosis glucokinase mainly utilizes polyP as the phosphate donor to convert glucose into glucose 6-phosphate (26, 27). Therefore, we next compared the growth patterns of various strains in a medium containing glucose, glycerol, or cholesterol as the sole carbon source. The Δppk-1 mutant strain displayed slower growth in a medium containing glucose in comparison to parental and complemented strains (Fig. 3A). However, the growth patterns of these strains were comparable in a medium containing either glycerol or cholesterol as the sole carbon source (SI Appendix, Fig. S2 A and B). We next performed untargeted metabolomics of samples prepared from mid-log phase cultures of wild-type and Δppk-1 mutant strains grown in either complete 7H9 or glucose-containing medium. Principal component analysis revealed that the metabolite profiles obtained for both strains in a glucose-containing medium were more distinct than those obtained in the 7H9 medium (SI Appendix, Fig. S2 C and D). The relative levels of 91 and 24 metabolites were significantly altered between these two strains upon culturing in glucose-containing or complete 7H9 medium, respectively (Fig. 3 B and C and Dataset S3). As expected, glucose 6-phosphate levels were significantly reduced in the Δppk-1 mutant strain relative to the wild-type strain in both growth conditions (Fig. 3E). Also, the levels of intermediates from the pentose phosphate pathway (ribose-5-phosphate) and tricarboxylic acid (TCA) cycle (citric acid, succinate, fumarate, and malate levels) were reduced in the Δppk-1 mutant strain upon culturing in glucose-containing medium (Fig. 3E). The levels of dihydroxyacetone phosphate and aconitate were non-significantly reduced in the Δppk-1 mutant strain in the glucose-containing medium in comparison to the parental strain. The peaks corresponding to other intermediates of glycolysis and the pentose phosphate pathway were not detected in our metabolite experiments. The intermediates of central carbon metabolism serve as precursors for amino acid synthesis in prokaryotes (Fig. 3D) (28). We observed that the levels of several amino acids were significantly reduced in Δppk-1 mutant strain in a glucose-containing medium compared to the parental strain (SI Appendix, Fig. S2E). We also compared the metabolomic profiles of polyP deficient (Δppk-1) and polyP accumulating (dkppx) strain in 7H9 medium. In comparison to dkppx strain, the levels of glucose-6-phosphate were reduced in the Δppk-1 mutant strain (SI Appendix, Fig. S3A). Previously, it has been shown that the levels of pyrophosphate, NAD+, NADH, nicotinamide, malate, succinate, 2-methyl citrate, acetyl-CoA, and metabolites belonging to arginine metabolism such as arginine, citrulline, and ornithine were increased in Δppx-1 mutant strain relative to the parental strain (29). In agreement, we noticed increased levels of NAD and ornithine in the dkppx strain relative to the Δppk-1 mutant strain (SI Appendix, Fig. S3A). Interestingly, the levels of metabolites belonging to purine metabolism were altered between Δppk-1 and dkppx strain (SI Appendix, Fig. S3B). We observed that the levels of ribose-5-phosphate, guanosine monophosphate, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranosyl 5′-monophosphate and inosine monophosphate were increased in dkppx strain in comparison to the Δppk-1 mutant strain (SI Appendix, Fig. S3C). Also, we observed decreased levels of guanosine, inosine, and hypoxanthine in the dkppx strain relative to the Δppk-1 mutant strain (SI Appendix, Fig. S3C). These observations suggest that polyP accumulation might inhibit the enzymes involved in the synthesis of guanosine, inosine, and hypoxanthine in M. tuberculosis.
Fig. 3.
Deletion of ppk-1 perturbs central carbon and trehalose metabolism in M. tuberculosis. (A) The growth pattern of wild-type, Δppk-1 mutant, and complemented strain was compared in the glucose-containing medium by measuring OD600nm at regular intervals. The data shown in this panel are representative of two independent experiments. (B and C) This panel shows the volcano plot highlighting the relative metabolite levels in Δppk-1 mutant and wild-type strains upon culturing in glucose-containing medium (B) or 7H9 (C) for 7 d. The data shown in panels (B) and (C) are obtained from three biological replicates. (D) A schematic showing the central carbon metabolism and TMM/TDM biosynthesis pathways in M. tuberculosis. The levels of metabolites highlighted in blue were reduced in the Δppk-1 mutant strain relative to wild-type strain in glucose-containing medium. (E) This panel shows the relative levels of various metabolites in wild-type (blue bar) and Δppk-1 mutant strain (red bar) upon culturing in either a complete 7H9 medium or in glucose-containing medium for 7 d. The data shown in this panel are mean ± SE obtained from three replicates. RPI represents relative peak intensity. The data were statistically analyzed using the paired-two-tailed t test (*P < 0.05, **P < 0.01, and ***P < 0.001). (F) Equal amount of TMM and TDM isolated from various strains was spotted on silica TLC plates, resolved, and analyzed. The data shown in this panel are representative of two independent experiments. TMM and TDM represent trehalose monomycolate and trehalose 6′6′-dimycolate, respectively. WT, MT, and CT represent wild-type, Δppk-1 mutant, and complemented strains. Photo credit: Mr. Saurabh Chugh, Translational Health Science and Technology Institute.
It has been previously shown that glucose-6-phosphate is a precursor for synthesizing trehalose-6-phosphate and ADP-glucose, key intermediates in trehalose biosynthesis (Fig. 3D) (12, 30–33). We observed that relative to the wild-type strain, levels of trehalose-6-phosphate, the glycosyl donor, ADP-glucose, maltose, and trehalose were reduced in Δppk-1 mutant strain in glucose-containing medium (Fig. 3E). We did not observe any significant differences in the levels of these intermediates between the two strains upon culturing in 7H9 medium (Fig. 3E). The levels trehalose-6-phosphate, trehalose, and ADP-glucose were also reduced in Δppk-1 mutant strain relative to the dkppx strain in 7H9 medium (SI Appendix, Fig. S3A). Trehalose acts as a precursor for the biosynthesis of trehalose monomycolate (TMM), trehalose 6-6′-dimycolate (TDM), and mycolyl-arabinogalactan (mAG) in M. tuberculosis (34, 35). In agreement with our metabolomics data, the levels of TMM and TDM were also reduced in the Δppk-1 mutant strain relative to the wild-type and complemented strains (Fig. 3F). In conclusion, we observed perturbations in central carbon metabolism and TMM/TDM biosynthesis upon deletion of ppk-1 in M. tuberculosis.
Characterization of the Lung Transcriptional Signatures of Mice Infected with Parental and Δppk-1 Mutant Strain.
Previously, we reported that, compared to the wild-type strain, the Δppk-1 mutant strain was attenuated for growth in guinea pigs (8). In the present study, we compared the growth of parental and Δppk-1 mutant strains in aerosol-infected mice. The mice infected with the Δppk-1 mutant strain displayed ~25.5-fold and ~95.0-fold reduced lung bacillary load in comparison to mice infected with the parental strain at 2 and 4 wk post-infection, respectively (Fig. 4A). In agreement, the splenic bacillary loads were also significantly reduced in Δppk-1 mutant strain–infected mice at 4-wk post-infection (Fig. 4A). It has been shown that lipids are key components of the immune evasion strategy and are essential for intracellular growth of M. tuberculosis (36). Since the virulence-associated lipids levels were reduced in the mutant strain, we next performed whole lung transcriptional profiling of wild-type and Δppk-1 mutant strain–infected mice at 4 wk post-infection to investigate the effect of polyP deficiency on modulation of host antimicrobial pathways. We observed that the levels of 1100 (334 decreased and 766 increased) and 618 (242 decreased and 376 increased) transcripts were significantly altered in the lung tissues of naive versus wild type–infected and naive versus Δppk-1-infected mice, respectively (Fig. 4B and SI Appendix, Fig. S4 A and B and Dataset S4). We observed that the expression of 453 and 289 transcripts was decreased and increased, respectively, in the lung tissues of animals infected with the Δppk-1 mutant strain relative to wild type–infected animals (Fig. 4C and Dataset S4). It has been shown that PDIMs are responsible for the coordinated secretion of ESAT-6 and CFP-10, which leads to phagosome rupture and the induction of Type I IFN signaling (25). In agreement, a subset of transcripts involved in Type I IFN signaling such as guanylate binding proteins (gbp2a, gbp5), socs1, il-27, stat1, and chemokines such as cxcl10, cxcl9, and ccl5 were reduced in lung tissues of Δppk-1 mutant–infected mice relative to mice infected with the parental strain (Fig. 4D and Dataset S4) (37). The death of M. tuberculosis–infected cells in a necrotic manner and subsequent inflammation has also been associated with phagosome rupture, an increase in cytosolic Ca2+, the generation of reactive oxygen species and ATP depletion (38, 39). We observed that the transcripts of genes associated with cell death (pdcd1lg2, skap1, pdcd1, bcl2I15, sirt3, fasl, and aif1), calcium transport channels (cacna1c, cant1, c2cd4a, cabp4, and s100a14), and potassium ions transporters (dpp6, kcnt1, kctd18, and kcnj10) were significantly decreased in the lung tissues of mice infected with Δppk-1 mutant (Fig. 4E and Dataset S4). Several studies have shown that zinc is essential for virulence of various bacterial pathogens, including M. tuberculosis (40, 41). In accordance, in mice infected with the mutant strain, the expression of zinc finger proteins and gene clusters involved in zinc ion binding (such as zfp661, zkscan5, zfx, zfp639, zfp60, zfp740, zfp219, zfp945, mefv, zfp37, zfp277, zfp386, and zfp82) was also decreased (SI Appendix, Fig. S4C and Dataset S4).
Fig. 4.
(A) Deletion of ppk-1 attenuates M. tuberculosis growth in mice. The data shown are mean ± SE of log10 of lung and splenic bacillary loads in mice infected with wild-type or Δppk-1 mutant strain at days 1, 14, and 28 post-infection. The data were statistically analyzed using the paired-two-tailed t test (***P < 0.001, ****P < 0.0001). (B and C) Comparison of transcriptional profiles obtained from lung tissues of naive or wild type–infected or Δppk-1 mutant strain–infected mice. (B) Venn diagram showing correlation of expression profiles obtained from lung tissues of uninfected or mice infected with either wild-type or Δppk-1 mutant strain at days 28 post-infection. (C) The volcano plot shows comparative gene expression profiles from mice infected with either wild-type or Δppk-1 mutant strain of M. tuberculosis. (D–F) Heatmaps showing differentially expressed transcripts in lungs of mice infected with either wild or Δppk-1 mutant strain. These transcripts showing differential expression are involved in either Type I IFN signaling (D) or cell death, calcium and potassium transport channel (E) or foamy macrophages and lipids metabolism (F). The data shown are obtained from three biological replicates. #: The expression of stat1 was reduced by 3.91-fold in Δppk-1 mutant–infected mice relative to wild type–infected mice. The color intensity in heatmaps represent the log2 value of raw read counts. (G and H) Nile Red staining in macrophages infected with either wild type or Δppk-1 mutant or complemented strain of M. tuberculosis. (G) THP-1 macrophages were infected with various strains and stained with Nile Red. The images were acquired using an Olympus FV3000 confocal microscope at 60× magnification. (Scale bars, 20 µm.) (H) The raw intensity of Nile red-stained macrophages was quantified using Image J software. The data shown in this panel are mean ± SE values of raw intensity obtained from 10 images from two independent experiments. The data obtained were statistically analyzed by one-way ANOVA (*P < 0.05; ****P < 0.0001). Photo credit: Mr. Saurabh Chugh, Translational Health Science and Technology Institute. (I) Oil red O and hematoxylin staining of lung sections of guinea pigs infected with various strains of M. tuberculosis. The representative Oil red O and hematoxylin stained lung sections from guinea pigs infected with various strains at days 28 post-infection is shown in this panel. The red color indicates the ORO-positive region. Images were acquired using an Olympus CX 43 microscope at 20× objective. (Scale bars, 20 µm.) Photo credit: Dr. Bhisma Panda, Translational Health Science and Technology Institute.
It has been reported that M. tuberculosis utilizes PDIM-related surface lipids to recruit neutrophils and permissive monocytes, which subsequently leads to inflammation and disease severity in host tissues (42). In addition, PDIMs also obscure mycobacterial pathogen-associated molecular patterns (PAMPs) to inhibit toll-like receptor-mediated recruitment of microbicidal macrophages (43). In agreement, levels of transcripts encoding for various chemokines involved in monocytes and PMN-recruitment, such as cxcl5, cxcl13, ccl2, ccl4, and ccl19, were decreased in lung tissues of Δppk-1 infected mice relative to animals infected with the wild-type strain (SI Appendix, Fig. S4D and Dataset S4). It has also been shown that M. tuberculosis cell wall lipids are crucial for mounting maximal T cell responses that contribute to TB pathogenesis (44). In agreement with reduced levels of virulence-associated lipids, we observed that the transcript levels of various pro-inflammatory and T cell cytokines, including ifn-γ, tnf-α, il-12, il-17, and il-21 as well as T cell activated related markers such as cd44 and il-2 were reduced in lung tissue of Δppk-1 infected mice when compared to wild type–infected mice (SI Appendix, Fig. S4E and Dataset S4). In concordance, the levels of IL-17A, IFN-γ, and TNF-α were reduced by 2.4-fold, 4.5-fold, and 3.0-fold, respectively, in lung homogenates of Δppk-1 mutant strain–infected animals in comparison to wild type–infected animals (SI Appendix, Fig. S4F). However, no differences were observed in IL-10 levels in lung homogenates of mice infected with either the parental or Δppk-1 mutant strain at days 28 post-infection (SI Appendix, Fig. S4F).
Upon infection with M. tuberculosis, macrophages differentiate into lipid-overloaded foamy macrophages that accumulate cholesteryl esters and triglycerides, which serve as primary carbon sources for M. tuberculosis growth in vivo (45–47). M. tuberculosis infection induces the expression of proinflammatory cytokines such as IFN-γ and TNF-α, which contribute to the formation of foamy cells (48, 49). In addition, M. tuberculosis–infected macrophages release mycobacterial cell wall lipid-containing vesicles (TDM and oxygenated forms of mycolic acids), transforming surrounding infected and uninfected macrophages into foamy cells (50). In our RNA-seq data, transcript levels of genes encoding for foamy cell markers such as scavenger receptors (msr1, cd163) and the checkpoint inhibitor pdl1 (cd274) were reduced in mice infected with the Δppk-1 mutant strain relative to the parental strain–infected mice. In addition, the transcript levels of genes involved in lipid catabolism (such as lmf1, apol10b, apoo, apol7e, pla2g7, pla2g6, apoc2, lcn2, apol11b, apol7b, ch25h, ccsl1, ccox1, pla2g4a, far1, gpcpd1, lpin3, acot3, and osbpl6) were also differentially expressed in Δppk-1 mutant–infected mice (Fig. 4F and Dataset S4). These observations suggest that infection of mice with the Δppk-1 mutant strain might reduce foamy macrophage formation in lung tissues. In agreement with the RNA-seq data, we observed that THP-1 macrophages infected with the Δppk-1 mutant strain had reduced lipid content compared to wild-type and complemented strain-infected macrophages (Fig. 4 G and H). The lung sections of wild-type infected guinea pigs showed well-formed caseous lesions with a central necrotic core where oil red O positive cells organized as a cuff surrounding the central necrotic core (Fig. 4I). The lung sections of guinea pigs infected with the Δppk-1 mutant strain showed significantly reduced oil red O staining in comparison to wild-type infected guinea pigs (Fig. 4I). We also observed that this defect was partially restored in guinea pigs infected with the complemented strain (Fig. 4I). Taken together, our data suggest that PPK-1 regulates M. tuberculosis virulence, the induction of type I interferon responses, inflammatory responses to exacerbate pathogenesis, and the formation of foamy macrophages in lung tissues.
Target-Based Screening Identifies Broad-Spectrum PPK-1 Inhibitor.
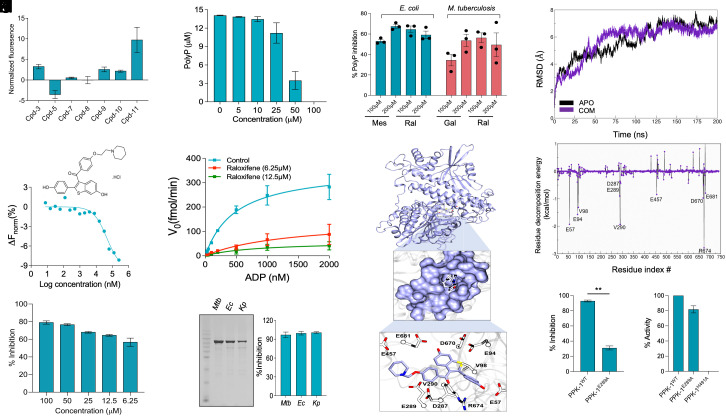
Previous studies have shown that various microorganisms require PPK-1 for stress adaptation, virulence, and persistence (51, 52). These observations suggest that PPK-1 is an attractive target to identify small molecule inhibitors with a specific mechanism of action. In the present study, a collection of 1,280 pharmacologically active compounds belonging to the Sigma Lopac 1280 library was screened to identify small molecule inhibitors of PPK-1 (SI Appendix, Fig. S5A). The preliminary screening performed at 100 µM concentration resulted in the identification of 60 compounds that inhibited PPK-1 enzymatic activity by at least 50% (SI Appendix, Fig. S5B). Among these, we shortlisted 11 molecules that were either FDA-approved or are in trials for clinical use against other diseases. These molecules did not inhibit luciferase activity but reduced PPK-1 enzymatic activity in vitro in a dose-dependent manner (SI Appendix, Fig. S5 C and D). The structure, names, and TC50 values of the identified PPK-1 inhibitors are shown in SI Appendix, Fig. S5E. Among the identified small molecules, antimycobacterial activity has already been reported for trifluperazine (Cpd-1), perphenazine (Cpd-2), prochlorperazine (Cpd-4), and fluphenazine (Cpd-6) (53–55). We next determined the interaction of the remaining small molecules with PPK-1 using microscale thermophoresis (MST). As shown in Fig. 5A, Cpd-11, raloxifene hydrochloride (raloxifene) showed the highest binding with PPK-1. We further show that raloxifene binds to PPK-1 in a dose-dependent manner and displays Kd value of 40 µM (Fig. 5B). As shown in Fig. 5 C and D, raloxifene inhibited both ATP and polyP synthesis activity associated with PPK-1 in a dose-dependent manner. We also observed that the Michaelis–Menten constant of PPK-1 for ADP increased by 2.73-fold and 1.70-fold in the presence of 6.25 µM and 12.5 µM of raloxifene, respectively (Fig. 5E). Further, in comparison to no drug control, Vmax of the enzymatic reaction decreased by 2.4-fold and 6.1-fold in the presence of 6.25 µM and 12.5 µM of raloxifene, respectively (Fig. 5E). These data suggest that raloxifene inhibits PPK-1 protein by binding to a pocket other than the substrate binding site.
Fig. 5.
Target-based screening identifies raloxifene hydrochloride as an inhibitor of PPK-1 from M. tuberculosis. (A) Microscale thermophoresis (MST) binding data of (His)6-PPK-1 with primary hits obtained from preliminary screening assays. The y-axis depicts the normalized fluorescence values, and the x-axis represents compound IDs. The data shown in this panel are mean ± SE of normalized fluorescence obtained from two independent experiments performed in duplicates. (B) Dose–response curve showing the interaction between M. tuberculosis (His)6-PPK-1 with various concentrations of raloxifene hydrochloride. The data obtained are plotted as response versus concentration and are representative of two independent experiments. The structure of raloxifene hydrochloride is also shown in this panel. (C and D) Dose-dependent inhibition of M. tuberculosis PPK-1 enzyme-dependent ATP (C) and PolyP (D) synthesis in the presence of varying raloxifene concentrations was calculated. (E) Michaelis–Menten (MM) plot for M. tuberculosis PPK-1 enzyme activity in the absence or presence of raloxifene. (F) SDS-PAGE showing the purified PPK-1 homologs from M. tuberculosis (Mtb), E. coli (Ec), and K. pneumoniae (Kp). (G) Inhibition of ATP synthesis activity of PPK-1 homologs from Mtb, Ec, and Kp in the presence of 100 µM raloxifene was calculated. The data shown in panels (C), (D), (E), and (G) are mean ± SE obtained from three independent experiments performed in duplicates. (H) Effect of mesalamine, gallein, and raloxifene on polyP accumulation in E. coli or M. tuberculosis. The data shown in this panel are mean ± SE of % polyP inhibition obtained from three independent experiments. (I) This panel depicts the docking of raloxifene on the modeled structure of M. tuberculosis PPK-1. In the upper panel, the protein (colored in ice blue and render in the new cartoon) and best conformer of raloxifene (colored atom-wise C: ice blue, O: red, and N: blue, rendered in licorice) is shown. In the middle panel, the residues lining the binding site of raloxifene are shown. In the lower panel, key residues of M. tuberculosis PPK-1 that are involved in interaction with raloxifene are shown in licorice: White and their respective Cα atoms are shown in Corey Pauling Koltun (CPK) representation. For clarity, only the sidechain and Cα of residues are shown. (J and K) Time-wise dynamic stability measurement of raloxifene binding in M. tuberculosis PPK-1 protein using molecular dynamics simulations. (J) Rms stability analysis in APO (black) and with complex (purple). (K) Per-residue decomposition of raloxifene interacting residues with PPK-1 homolog from M. tuberculosis. (L) Inhibition assay of M. tuberculosis PPK-1WT and PPK-1E289A enzyme-dependent ATP synthesis in the presence of 100 µM raloxifene. (M) Comparison of ATP synthesis activity of PPK-1WT, PPK-1E289A, and PPK-1H491A. The data shown in panels (L) and (M) are mean ± SE obtained from three independent experiments performed in duplicates. The data obtained were statistically analyzed using the paired-two-tailed t test (**P < 0.01).
PPK-1 is a highly conserved enzyme in various bacterial pathogens, including E. coli and Klebsiella pneumoniae (56). We observed raloxifene inhibited the enzymatic activity associated with PPK-1 homologs from E. coli and Klebsiella pneumoniae by >95% (Fig. 5 F and G). Recently, mesalamine and gallein have been identified as inhibitors of PPK-1 enzymes from E. coli and Pseudomonas aeruginosa, respectively (57, 58). We observed that gallein inhibited both ATP and polyP synthesis activity associated with the PPK-1 homolog from M. tuberculosis (SI Appendix, Fig. S6 A–C). However, mesalamine did not inhibit M. tuberculosis PPK1 enzymatic activity in vitro (SI Appendix, Fig. S6D). We next determined the levels of intracellular polyP in E. coli and M. tuberculosis in the presence of mesalamine, gallein, or raloxifene. As shown in Fig. 5H, exposure of E. coli to mesalamine and raloxifene significantly reduced the intracellular polyP levels by ~50 to 65%. Also, treatment with raloxifene and gallein reduced the levels of intracellular polyP by ~35 to 55% in M. tuberculosis (Fig. 5H). These results imply that raloxifene and gallein are broad-spectrum PPK-1 inhibitors.
Identification of Critical Residues Involved in Raloxifene Binding with PPK-1 Homologs from Various Microorganisms.
Using the SiteMap module, we identified Site1, Site2, and Site3 as probable raloxifene binding sites in the M. tuberculosis PPK-1 enzyme. The docking on these sites revealed that raloxifene binds at Site 1, Site 2, and Site 3 with GlideXp scores of −7.26 kcal/mol, −6.42 kcal/mol, and −4.14 kcal/mol, respectively (SI Appendix, Table S3). To further define the binding pose of raloxifene, Site 1 and Site 2 were re-scored using focused docking and MM-GBSA methods. We observed that raloxifene strongly binds to Site 2, with docking and MM-PBSA scores of −10.25 kcal/mol and −85.89 kcal/mol, respectively (SI Appendix, Table S3). The docking and MM-PBSA scores for raloxifene binding to Site 1 were −8.09 kcal/mol and −61.54 kcal/mol, respectively (SI Appendix, Table S3). We next identified hot-spot residues involved in establishing diverse interactions with raloxifene, such as electrostatic, non-polar, hydrogen bonds, and salt bridges. We noticed that raloxifene forms durable hydrogen bonds with Val98, Glu289, Arg674, and Asn675 of the PPK-1 protein from M. tuberculosis (Fig. 5I and SI Appendix, Table S4). Additionally, we observed the formation of a salt bridge between the Glu289 residue of PPK-1 protein and raloxifene (Fig. 5I and SI Appendix, Table S4). These observations suggest that Glu289 is critical for the stability of raloxifene inside the binding pocket as it forms intra hydrogen bonds with residues Arg310 and Phe462 in the APO protein (Fig. 5I). In addition, Glu57, Glu94, Val98, Asp287, Val290, Glu457, Asp670, and Glu681 of PPK-1 protein are also involved in raloxifene binding (Fig. 5I).
We also performed MD simulations to assess further the docking results for raloxifene binding with M. tuberculosis PPK-1. The rmsd analysis revealed that the complex was stable at around 6.7 Å till the completion of the trajectory (Fig. 5J). The converged trajectory was used for calculating the binding free energy using the MM-PBSA method, and it was observed to be −32.65 kcal/mol (SI Appendix, Table S5). Furthermore, per-residue energy decomposition analysis revealed that Glu57, Glu94, Val98, Glu289, Val290, and Arg674 contributed substantially with energy contributions greater than −1.0 kcal/mol (Fig. 5K). Molecular docking studies also revealed that the raloxifene binding site in PPK-1 homologs from E. coli, K. pneumoniae, and M. tuberculosis is similar (SI Appendix, Fig. S6 E–H and Table S4). We observed that raloxifene binds E. coli and K. pneumoniae PPK-1 with MM-GBSA scores of −96.84 and −61.69 kcal/mol, respectively. Molecular docking of gallein with M. tuberculosis PPK-1 revealed that it also binds close to the ATP binding site (SI Appendix, Fig. S6I). The residues involved in the binding of gallein with M. tuberculosis PPK-1 are shown in (SI Appendix, Table S4). In agreement with molecular docking experiments, we observed that mutation of Glu289 to alanine abrogated the ability of raloxifene to inhibit PPK-1 enzymatic activity (Fig. 5L). We also observed that the enzymatic activity associated with (His)6-PPK-1 and (His)6-PPK-1E289A proteins was comparable to each other (Fig. 5M). As reported earlier, (His)6-PPK-1H491A was inactive in our enzymatic assays (Fig. 5M) (59). Taken together, these results suggest that the binding pocket of raloxifene in PPK-1 homologs from various microorganisms is similar and Glu289 residue is important for the interaction of M. tuberculosis homolog with raloxifene.
Raloxifene Inhibits M. tuberculosis Growth in THP-1 Macrophages and Mice.
Since PPK-1 is required for the intracellular survival of M. tuberculosis in macrophages, we next investigated the activity of raloxifene alone or in combination with existing TB drugs on M. tuberculosis growth inside THP-1 macrophages (8). We observed ~19.4-fold and ~12.0-fold inhibition of M. tuberculosis growth in macrophages after exposure to INH and raloxifene, respectively (Fig. 6A). The addition of raloxifene increased the intracellular activity of INH by ~7.0-fold (Fig. 6A). We next determined the effect of raloxifene on the growth of an INH-resistant strain inside macrophages. We observed that exposure to raloxifene inhibited the growth of INH-resistant M. tuberculosis strain by ~25.0-fold in macrophages (Fig. 6A). We also observed ~14.0-fold and ~13.5-fold inhibition of M. tuberculosis growth inside macrophages after exposure to BDQ or PTM, respectively (Fig. 6B). The addition of raloxifene increased the intracellular activity of BDQ and PTM by ~32.0-fold and ~42.0-fold, respectively (Fig. 6B). In our checkerboard assays, we observed that raloxifene showed additive interactions with either INH or BDQ, or PTM in intracellular killing assays (SI Appendix, Fig. S7). We observed that the HSA synergy score for the combination of raloxifene with INH, BDQ, and PTM was 6.179, 6.723, and 3.599, respectively (SI Appendix, Fig. S7). We next determined whether oral administration of raloxifene inhibits M. tuberculosis growth in vivo. We observed that in comparison to the vehicle-treated group, administration of INH or raloxifene reduced the lung bacillary loads by ~16.0-fold and 3.0-fold, respectively, after 4 wk of treatment (Fig. 6C). In spleens, in comparison to the vehicle-treated group, we observed 197.0-fold and 7.0-fold reduction in bacterial burdens in INH or raloxifene-treated mice, respectively (Fig. 6D). We next performed experiments to determine the effect of oral administration of raloxifene on the intracellular growth of wild-type, Δppk-1 mutant, and complemented strain in chronic mice model of infection. As expected, Δppk-1 mutant displayed attenuated phenotype in the lung and spleens of mice in comparison to the parental strain at 4 wk post-infection. As reported in guinea pigs, we observed that this growth defect was partially restored in the complemented strain (8) (Fig. 6E). In comparison to the vehicle-treated group, the lung bacillary loads were reduced by ~4.5-folds and ~3.5-folds, respectively, in raloxifene-treated wild-type and complemented strain–infected mice at 4 wk post-treatment (Fig. 6F). Similarly, the splenic bacillary load was decreased by ~3.5-folds and ~6.5-folds, respectively, in raloxifene-treated wild-type and complemented infected mice, in comparison to vehicle-treated group (Fig. 6G). However, no differences were observed in lungs and splenic bacterial loads in vehicle-treated and raloxifene-treated Δppk-1 mutant–infected mice at 4 wk post-treatment (Fig. 6 F and G). Overall, these findings show that raloxifene enhanced the activity of known TB drugs and also inhibited the growth of M. tuberculosis in macrophages and mice by targeting PPK-1.
Fig. 6.
(A and B) Antimycobacterial activity of raloxifene alone or in combination with known TB drugs in macrophages. The intracellular killing experiment against M. tuberculosis was performed. The concentration of drugs used were isoniazid (INH, 10 µM), raloxifene (Ral, 50 µM), bedaquiline (BDQ, 10 µM), and pretomanid (PTM, 10 µM). The data shown in these panels are mean ± SE of log10 CFU obtained from two or three independent experiments performed in duplicates. (C and D) Oral administration of raloxifene inhibits the growth of M. tuberculosis in mice. Four-week aerosol infected female mice were treated with either 120 mg/kg raloxifene or 10 mg/kg INH for 4 wk. The data shown in these panels are mean ± SE of log10 CFU in lung (C) or spleens (D) obtained from various groups. (E–G) PPK-1 specific in vivo activity of raloxifene. Six- to eight-week-old female mice were infected with either wild type or Δppk-1 mutant or complemented strain via aerosol route for 4 wk. (E) This panel shows lung and splenic bacillary load in mice infected with wild type or Δppk-1 mutant or complemented strain. (F and G) The bacterial burdens were determined in the lungs and spleens of vehicle and raloxifene-treated mice infected with indicated groups at 4 wk post-treatment. The data obtained were statistically analyzed by one-way ANOVA [panels (A–E), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001] or paired two-tailed t test [panels (F) and (G), *P < 0.05, **P < 0.01]. (H) Deletion of ppk-1 from M. tuberculosis affects the expression of bacterial and host metabolic pathways implicated in disease pathogenesis. M. tuberculosis virulence factors such as PDIMs and ESX-1 induce phagosome rupture necrosis, and promote bacterial pathogenesis in host tissues. These virulence factors and TMM and TDM trigger lipid-laden foamy macrophage formation. These foamy macrophages provide a favorable environment and support bacterial nutrition and persistence. M. tuberculosis infection induces the secretion of various pro-inflammatory cytokines, which contribute to tissue damage, inflammation, and disease progression. In the present study, we show reduced levels of various virulence-associated factors such as PDIMs, TMM, and TDM in mid-log phase cultures of the Δppk-1 mutant strain. The reduced levels of these virulence factors are most likely to be associated with i) increased phagosome–lysosome fusion in macrophages, ii) reduced Type-I IFN signaling, inflammation, and formation of foamy macrophages and iii) faster clearance of Δppk-1 mutant strain from host tissues. Panel (H) was generated using BioRender.
Discussion
Previous studies have reported that polyP homeostasis influences M. tuberculosis pathogenesis and susceptibility to current TB drugs (8, 10, 13, 14, 16, 29). However, the regulation of M. tuberculosis pathogenesis by PolyP deficiency is still unclear. In the present study, we have identified several polyP interacting proteins. Many of these interacting proteins require ATP for cellular functions, which raises the possibility that polyP can substitute ATP for their enzymatic activity. We show that polyP interacts with AccA1 and AccD1 proteins from M. tuberculosis. This interaction was specific for long-chain polyP as no interaction was observed with short-chain polyP. Studies have shown that AccA1-AccD1 forms a multisubunit complex and is involved in the leucine degradation pathway (60). These enzymes catalyze the carboxylation of acetyl-CoA to malonyl-CoA, a precursor for fatty-acid biosynthesis (61). We speculate that polyP levels might regulate the activity of the AccA1-AccD1 enzyme complex and contribute to lipid biosynthesis in M. tuberculosis. Previously, it has been shown that polyP forms a complex with Lon protease and promotes degradation of ribosomal proteins during amino acid starvation (62). Interestingly, ClpP2 was also identified as a polyP interacting protein from late-log phase cultures of M. tuberculosis. It has been shown that various M. tuberculosis antitoxins belonging to TA systems are Clp protease substrates. The degradation of these antitoxins by cellular protease might result in derepression of the TA operon and toxin activation (63). Therefore, we hypothesize that polyP might regulate ClpP2-mediated degradation of antitoxins, eventually leading to differential expression of TA systems, including vapBC15, in the Δppk-1 mutant strain. DnaK, a chaperone protein involved in protein folding, the RNA polymerase subunits RpoB and RpoC and transcription terminator Rho were also identified as polyP interacting proteins from late-log phase cultures of M. tuberculosis. These observations suggest that polyP might regulate the rates of translation and transcription in M. tuberculosis, as reported for E. coli (64).
Previously, it has been shown that phosphoglucokinase from M. tuberculosis prefers polyP as a phosphoryl donor for conversion of glucose to glucose-6-phosphate (26, 27). As expected, the Δppk-1 mutant strain displayed a growth defect in the 7H9 medium containing glucose as the carbon source. Glucose-6-phosphate is a key intermediate in central carbon metabolism and acts as a precursor for trehalose biosynthesis. In agreement, the levels of glucose-6-phosphate, intermediates belonging to the TCA cycle, trehalose metabolism, and amino acids were significantly reduced in the Δppk-1 mutant strain relative to the parental strain in the glucose-containing medium. Also, the mutant strain had lower levels of virulence-associated lipids such as PDIMs, TDM, and TMM relative to the parental and complemented strains. In agreement with the reduced levels of PDIMs, we observed increased phagosome–lysosome fusion in macrophages and decreased Type I IFN signaling in lung tissues of mice upon infection with the Δppk-1 mutant strain relative to the wild-type strain. We also observed reduced expression of transcripts encoding for proteins involved in calcium signaling and cell death in the lungs of animals infected with the Δppk-1 mutant strain. It has been reported that the host limits the access of trace metal ions such as Fe2+, Zn2+, and Cu2+ to intracellular pathogens, which thwarts microbial growth (65). Recently, it has been shown that M. tuberculosis produces zinc-scavenging molecules kupyaphores, and these are essential to establish infection in mice (41). We observed reduced transcript levels of various zinc finger proteins in lung tissues of mice infected with the Δppk-1 mutant strain, suggesting a zinc-limiting environment. It has also been shown that M. tuberculosis deploys the ESX-3 system to maintain zinc and iron homeostasis (66, 67). In agreement, we observed the reduced transcript levels of eccD3, a component of the ESX-3 secretion system, in mid-log phase cultures of the Δppk-1 mutant strain.
Several studies have shown that M. tuberculosis infected macrophages in granulomas accumulate lipid droplets and differentiate into foamy macrophages. Foamy macrophages represent a favorable niche for the intracellular multiplication and dissemination of M. tuberculosis (50, 68). In agreement with the reduced levels of PDIMs, TMM, and TDM, we observed that transcript levels of markers associated with the formation of foamy macrophages were reduced in animals infected with the Δppk-1 mutant strain. Reduced neutral lipid content in THP-1 macrophages and lung tissues of Δppk-1 infected guinea pigs substantiates our RNA-seq data. It has also been shown that strains harboring deletions in proteins involved in mycolic acid biosynthesis or the ESX-1 secretion system generate a weaker TH1 immune response upon infection (69, 70). As expected, the levels of various inflammatory cytokines and chemokines were significantly reduced in mutant strain–infected mice relative to wild type strain–infected mice. This reduced expression of host inflammatory markers in the lungs of Δppk-1 mutant infected animals could be attributed to decreased bacterial burdens and alteration of host-pathogen interactions resulting from polyP deficiency. In support of the latter, it has been reported that M. smegmatis and M. tuberculosis secrete polyP and inhibit phagosome acidification and phagosome–lysosome fusion (11). In addition, it has also been demonstrated that polyP exacerbates LPS-mediated inflammatory response in macrophages and mice (71).
Using target-based screening, we have identified raloxifene as an inhibitor of PPK-1 homolog from M. tuberculosis. Raloxifene is a selective estrogen receptor modulator and is being used in the treatment of osteoporosis and invasive breast cancer (72). Several studies have identified small molecules such as gallein and mesalamine that inhibit PPK-1 homologs from E. coli P. aeruginosa or V. cholerae (51, 57). We show that gallein also inhibited the activity of PPK-1 homolog from M. tuberculosis, however, no inhibition was observed in the presence of mesalamine. In agreement, the levels of intracellular polyP were decreased upon exposure of M. tuberculosis to raloxifene or gallein in vitro. We also observed that raloxifene inhibited the activity of PPK-1 homologs from E. coli and K. pneumoniae. Also, the binding patterns of raloxifene with PPK-1 homologs of E. coli, K. pneumoniae, and M. tuberculosis were similar. Computational studies revealed that in APO protein, Glu289 is involved in the formation of an intra-hydrogen bond network with Arg310 and Phe462, and this network is destabilized by the binding of raloxifene. In agreement, the mutation of Glu289 to alanine reduced the ability of raloxifene to inhibit the M. tuberculosis PPK-1 enzyme. Previously, we have shown that polyP deficiency results in attenuation and enhanced susceptibility of M. tuberculosis against INH (8). In agreement, the M. tuberculosis strain deficient in the stringent response (ΔrelA, with reduced polyP levels) is also more susceptible to INH during the chronic phase of infection in mice lung tissues (73). Also, several studies have shown that polyP accumulating M. tuberculosis strains are tolerant to INH- mediated killing (13, 14). In agreement with our earlier observations, we observed that raloxifene was able to inhibit M. tuberculosis growth in macrophages, mice and also enhance INH, BDQ, and PTM intracellular activity. We observed that raloxifene also inhibited the growth of INH resistant M. tuberculosis strain in THP-1 macrophages. These observations suggest that inhibitors of stringent response pathway (PPK-1/RelA) in combination with existing drugs may shorten the duration of chemotherapy. Identification of PPK1-specific inhibitors, in addition to small molecules such as BDQ and Q-203, would further validate the processes of ATP synthesis as targets for TB drug discovery (74, 75).
This detailed study delineates the mechanisms by which polyP contributes to pathogenesis and identifies small molecule inhibitors against the PPK-1 enzyme from M. tuberculosis. We demonstrate that deletion of ppk-1 affects the expression of virulence-associated genes, lipid biosynthesis, and utilization of glucose as the carbon source in M. tuberculosis. The decreased levels of these lipid molecules might result in increased phagolysosome fusion with reduced necrosis and dissemination to the cytosol, reduced formation of foamy macrophages, metal ion deficient environment in granulomas, minimal inflammatory, and TH1 response in mice (Fig. 6H). The identified small molecules may be combined with current TB drugs to eradicate both drug-susceptible and drug-resistant populations in different metabolic states. Future experiments would be performed to delineate the exact mechanisms for the observed reduced expression of inflammatory markers in the lungs of Δppk-1 infected mice. Since polyP accumulation has been reported in M. tuberculosis at later stages of growth, we would also perform experiments to compare the transcriptional profiles of parental, polyP deficient and accumulating strains in these conditions (10). Since PPK-1 is a highly conserved enzyme, the identified PPK-1-specific inhibitors may possess broad-spectrum in vivo activity.
Materials and Methods
The detailed description of all protocols used in the study is provided in SI Appendix, Materials and Methods.
Bacterial Strains, Culture Conditions, and Plasmids.
Details are in SI Appendix The bacterial strains and plasmids used in the study are listed in SI Appendix, Table S6.
Pull-Down Assays.
In order to identify polyP700 interacting proteins, pull-down assays were performed using clarified lysates prepared from mid-log or late-log phase cultures of M. tuberculosis.
Cloning, Expression, and Protein Purification.
In order to purify recombinant proteins, various wild-type genes or their mutant derivatives were PCR amplified and cloned into pET28b.
Bacterial RNA-Seq Experiments.
Total RNA was isolated from mid-log phase cultures of various strains and shipped to AgriGenome Labs Pvt. Ltd. for sequencing on an Illumina HiSeq platform.
Metabolomics Experiments.
Total metabolites were extracted from various strains grown in 7H9 (wild-type, Δppk-1, and dkppx) and glucose medium (wild-type and Δppk-1) for 7 d. The mass-spectrometric data were collected in both positive and negative mode at 1,20,000 resolutions in MS1 mode and 30,000 resolution in data-dependent MS2 scan mode using an Orbitrap fusion tribrid mass spectrometer equipped with a heated electrospray ionization (HESI) source.
Lipid Extraction and Thin Layer Chromatography.
Apolar lipid fraction, TMM, and TDM were isolated from mid-log phase cultures of various M. tuberculosis strains.
Target-Based Screening Assays.
For target-based screening experiments, recombinant M. tuberculosis PPK-1 protein was purified, and activity assays were performed. PPK-1 reverse enzymatic reaction was performed in 50 mM Tris-Cl–pH-7.4, 40 mM (NH4)2SO4, 4 µM MgCl2, 100 nM ADP, and 300 nM (His)6-PPK-1 enzyme. All reaction plates included buffer-only, enzyme-only, and substrate-only control.
PPK-1-Dependent polyP Synthesis Assays.
The PPK-1-dependent forward enzymatic reaction was performed as previously described (57).
Quantification of Intracellular polyP Levels in E. coli and M. tuberculosis.
Intracellular polyP was extracted from E.coli, and M. tuberculosis after exposure to various drugs was extracted as per standard protocols. The quantification of polyP was performed using the toluidine blue method.
Bio-Layer Interferometry and Microscale Thermophoresis Experiments.
The protein-small molecule interactions were performed using BLI and MST.
Macrophage Experiments.
THP-1 macrophages were infected with M. tuberculosis at a multiplicity of infection of 1:10. The infected macrophages were subsequently treated with various drugs, and CFU enumeration or luciferase measurements were performed after 4 d.
Molecular Docking and Dynamics Studies.
Schrodinger-2017 was used for all docking-related experiments. Molecular dynamics (MD) simulations were performed using the Antechamber module of AmberTools, and protein parameters were assigned using the AMBER ff14SB force field.
Mice Virulence and Efficacy Experiments.
For virulence studies, 6- to 8-wk-old female Balb/c mice were infected with various strains via aerosol route. For efficacy experiments, M. tuberculosis–infected mice were orally administered with INH or raloxifene for 6 d a week for 28 d. The bacterial loads were determined in the lungs and spleens at designated time points as per standard protocols.
Host RNA-Seq and Cytokine Measurement Experiments.
For host transcriptional analysis, total RNA was isolated from lung tissues of uninfected or mice infected with wild-type or Δppk-1 mutant strain at 4 wk post-infection. The samples were shipped to Bionivid Technology Private Ltd. for RNA sequencing. The levels of cytokines in lung homogenates were determined by ELISA.
Nile Red Staining.
For Nile red staining, infected macrophages were fixed with 4% paraformaldehyde and stained with Nile red.
Hematoxylin and Oil Red O Staining of Lung Sections of Infected Guinea Pigs.
The lungs from 4-wk infected guinea pigs were fixed in 4% formalin and stained with hematoxylin and Oil Red O solution.
Statistical Analysis.
GraphPad Prism version 8 (GraphPad Software Inc.) was used for statistical analysis and the generation of graphs. The data were considered significant at P < 0.05. The statistical test used is mentioned in the respective figure legends.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Acknowledgments
We are thankful to the technical staff of IDRF and, SAF, THSTI, for their assistance during BSL-3 and animal experiments. S.C. and M.S. acknowledge the Council of Scientific and Industrial Research and the Department of Biotechnology, respectively, for their research fellowship. P.S. acknowledges the Department of Science and Technology for his fellowship (PDF/2018/002454). We sincerely thank Dr. Bill Jacobs (Albert Einstein College of Medicine, United States) for providing the INH-resistant strain of M. tuberculosis. The plasmid pMV306hsp+LuxG13 was a gift from Brian Robertson & Siouxsie Wiles (Addgene plasmid # 26161; http://n2t.net/addgene:26161; RRID:Addgene_26161). Mr. Amit (Institute of Liver and Biliary Science, India) is greatly acknowledged for oil red O staining of guinea lung sections. The authors sincerely thank Dr. Bhisma Panda (THSTI) for acquiring images of oil-red O-stained guinea lung sections. R.S. acknowledges the financial support from the Indian Council of Medical Research (5/8/5/21/2019/ECD-1) and Wellcome Trust-DBT India Alliance (IA/S/19/2/504646). S.A. acknowledge the funding received from Department of Biotechnology, India through Translational Research Program (BT/PR30159/MED/15/188/2018). The funders had no role in study design, results analysis and manuscript preparation.
Author contributions
S.A. and R.S. designed research; S.C., P.T., C.S., S.K.G., P.S., R.B., S.K., M.S., N.R.R., and S.A. performed research; S.C., P.T., C.S., S.K.G., P.S., R.B., S.K., M.S., N.R.R., Y.K., S.A., and R.S. analyzed data; and S.C., C.S., Y.K., S.A., and R.S. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. J.V. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
The RNA-seq data reported in this article has been obtained from mid-log phase cultures of Mycobacterium tuberculosis strains or lung tissues of uninfected and infected Mus musculus. The raw data sequence files have been deposited in the repository of the National Center for Biotechnology Information and can be accessed using BioProject PRJNA892734 (M.tuberculosis) (76) and PRJNA892458 (Mus musculus) (77).
Supporting Information
References
- 1.Ahn K., Kornberg A., Polyphosphate kinase from. Purification and demonstration of a phosphoenzyme intermediate. J. Biol. Chem. 265, 11734–11739 (1990). [PubMed] [Google Scholar]
- 2.Zhang H., Ishige K., Kornberg A., A polyphosphate kinase (PPK2) widely conserved in bacteria. Proc. Natl. Acad. Sci. U.S.A. 99, 16678–16683 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishige K., Zhang H., Kornberg A., Polyphosphate kinase (PPK2), a potent, polyphosphate-driven generator of GTP. Proc. Natl. Acad. Sci. U.S.A. 99, 16684–16688 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nocek B., et al. , Polyphosphate-dependent synthesis of ATP and ADP by the family-2 polyphosphate kinases in bacteria. Proc. Natl. Acad. Sci. U.S.A. 105, 17730–17735 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sureka K., Sanyal S., Basu J., Kundu M., Polyphosphate kinase 2: A modulator of nucleoside diphosphate kinase activity in mycobacteria. Mol. Microbiol. 74, 1187–1197 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Kumble K. D., Kornberg A., Endopolyphosphatases for long chain inorganic polyphosphate in yeast and mammals. J. Biol. Chem. 271, 27146–27151 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Sethuraman A., Rao N. N., Kornberg A., The endopolyphosphatase gene: Essential in. Proc. Natl. Acad. Sci. U.S.A. 98, 8542–8547 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh R., et al. , Polyphosphate deficiency in is associated with enhanced drug susceptibility and impaired growth in guinea pigs. J. Bacteriol. 195, 2839–2851 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sureka K., et al. , Polyphosphate kinase is involved in stress-induced mprAB-sigE-rel signalling in mycobacteria. Mol. Microbiol. 65, 261–276 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Chuang Y. M., et al. , Deficiency of the novel exopolyphosphatase Rv1026/PPX2 leads to metabolic downshift and altered cell wall permeability in. mBio 6, e02428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rijal R., Cadena L. A., Smith M. R., Carr J. F., Gomer R. H., Polyphosphate is an extracellular signal that can facilitate bacterial survival in eukaryotic cells. Proc. Natl. Acad. Sci. U.S.A. 117, 31923–31934 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole S. T., et al. , Deciphering the biology of from the complete genome sequence. Nature 393, 537–544 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Chuang Y. M., Belchis D. A., Karakousis P. C., The polyphosphate kinase gene ppk2 is required for inorganic polyphosphate regulation and virulence. mBio 4, e00039-00013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh M., et al. , Establishing virulence associated polyphosphate kinase 2 as a drug target for Mycobacterium tuberculosis. Sci. Rep. 6, 26900 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi M. Y., et al. , The two PPX-GppA homologues from Mycobacterium tuberculosis have distinct biochemical activities. PLoS One 7, e42561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiwari P., et al. , Exopolyphosphatases PPX1 and PPX2 from Mycobacterium tuberculosis regulate dormancy response and pathogenesis. Microb. Pathog. 173, 105885 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., et al. , RbpA and sigma(B) association regulates polyphosphate levels to modulate mycobacterial isoniazid-tolerance. Mol. Microbiol. 108, 627–640 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Tong L., Structure and function of biotin-dependent carboxylases. Cell Mol. Life Sci. 70, 863–891 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronan J. E. Jr., Waldrop G. L., Multi-subunit acetyl-CoA carboxylases. Prog. Lipid. Res. 41, 407–435 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Szymona M., Widomski J., A kinetic study on inorganic polyphosphate glucokinase from Mycobacterium tuberculosis H37RA. Physiol. Chem. Phys. 6, 393–404 (1974). [PubMed] [Google Scholar]
- 21.Hsieh P. C., Kowalczyk T. H., Phillips N. F., Kinetic mechanisms of polyphosphate glucokinase from Mycobacterium tuberculosis. Biochemistry 35, 9772–9781 (1996). [DOI] [PubMed] [Google Scholar]
- 22.Shiba T., et al. , Inorganic polyphosphate and the induction of rpoS expression. Proc. Natl. Acad. Sci. U.S.A. 94, 11210–11215 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boshoff H. I., et al. , The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: Novel insights into drug mechanisms of action. J. Biol. Chem. 279, 40174–40184 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Augenstreich J., et al. , ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell Microbiol. 19, e12726 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Barczak A. K., et al. , Systematic, multiparametric analysis of Mycobacterium tuberculosis intracellular infection offers insight into coordinated virulence. PLoS Pathog. 13, e1006363 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh P. C., Shenoy B. C., Haase F. C., Jentoft J. E., Phillips N. F., Involvement of tryptophan(s) at the active site of polyphosphate/ATP glucokinase from Mycobacterium tuberculosis. Biochemistry 32, 6243–6249 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Hsieh P. C., Shenoy B. C., Jentoft J. E., Phillips N. F., Purification of polyphosphate and ATP glucose phosphotransferase from Mycobacterium tuberculosis H37Ra: Evidence that poly(P) and ATP glucokinase activities are catalyzed by the same enzyme. Protein Expr. Purif. 4, 76–84 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Umbarger H. E., Amino acid biosynthesis and its regulation. Annu. Rev. Biochem. 47, 532–606 (1978). [DOI] [PubMed] [Google Scholar]
- 29.Chuang Y. M., et al. , Stringent response factors PPX1 and PPK2 play an important role in Mycobacterium tuberculosis metabolism, biofilm formation, and sensitivity to isoniazid in vivo. Antimicrob. Agents Chemother. 60, 6460–6470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy H. N., et al. , The OtsAB pathway is essential for trehalose biosynthesis in Mycobacterium tuberculosis. J. Biol. Chem. 280, 14524–14529 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Ballicora M. A., Iglesias A. A., Preiss J., ADP-glucose pyrophosphorylase, a regulatory enzyme for bacterial glycogen synthesis. Microbiol. Mol. Biol. Rev. 67, 213–225, table of contents (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bornemann S., alpha-Glucan biosynthesis and the GlgE pathway in Mycobacterium tuberculosis. Biochem. Soc. Trans. 44, 68–73 (2016). [DOI] [PubMed] [Google Scholar]
- 33.De Smet K. A. L., Weston A., Brown I. N., Young D. B., Robertson B. D., Three pathways for trehalose biosynthesis in mycobacteria. Microbiology (Reading) 146, 199–208 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Goins C. M., Dajnowicz S., Smith M. D., Parks J. M., Ronning D. R., Mycolyltransferase from Mycobacterium tuberculosis in covalent complex with tetrahydrolipstatin provides insights into antigen 85 catalysis. J. Biol. Chem. 293, 3651–3662 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takayama K., Wang C., Besra G. S., Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 18, 81–101 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandra P., Grigsby S. J., Philips J. A., Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 20, 750–766 (2022), 10.1038/s41579-022-00763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNab F., Mayer-Barber K., Sher A., Wack A., O’Garra A., Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orrenius S., Zhivotovsky B., Nicotera P., Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 4, 552–565 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Festjens N., Vanden Berghe T., Vandenabeele P., Necrosis, a well-orchestrated form of cell demise: Signalling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta 1757, 1371–1387 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Corbett D., et al. , Two zinc uptake systems contribute to the full virulence of Listeria monocytogenes during growth in vitro and in vivo. Infect. Immun. 80, 14–21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehdiratta K., et al. , Kupyaphores are zinc homeostatic metallophores required for colonization of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 119, e2110293119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cambier C. J., O’Leary S. M., O’Sullivan M. P., Keane J., Ramakrishnan L., Phenolic glycolipid facilitates mycobacterial escape from microbicidal tissue-resident macrophages. Immunity 47, 552–565.e554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kugelberg E., Immune evasion: Mycobacteria hide from TLRs. Nat. Rev. Immunol. 14, 62–63 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Abebe F., Belay M., Legesse M., Mihret A., Franken K. S., Association of ESAT-6/CFP-10-induced IFN-gamma, TNF-alpha and IL-10 with clinical tuberculosis: Evidence from cohorts of pulmonary tuberculosis patients, household contacts and community controls in an endemic setting. Clin. Exp. Immunol. 189, 241–249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerrini V., et al. , Storage lipid studies in tuberculosis reveal that foam cell biogenesis is disease-specific. PLoS Pathog. 14, e1007223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shim D., Kim H., Shin S. J., Mycobacterium tuberculosis infection-driven foamy macrophages and their implications in tuberculosis control as targets for host-directed therapy. Front. Immunol. 11, 910 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffin J. E., et al. , Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem. Biol. 19, 218–227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaisinghani N., et al. , Necrosis driven triglyceride synthesis primes macrophages for inflammation during Mycobacterium tuberculosis infection. Front. Immunol. 9, 1490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knight M., Braverman J., Asfaha K., Gronert K., Stanley S., Lipid droplet formation in Mycobacterium tuberculosis infected macrophages requires IFN-gamma/HIF-1alpha signaling and supports host defense. PLoS Pathog. 14, e1006874 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell D. G., Cardona P. J., Kim M. J., Allain S., Altare F., Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 10, 943–948 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowlin M. Q., Gray M. J., Inorganic polyphosphate in host and microbe biology. Trends Microbiol. 29, 1013–1023 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao N. N., Kornberg A., Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli. J. Bacteriol. 178, 1394–1400 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Advani M. J., Siddiqui I., Sharma P., Reddy H., Activity of trifluoperazine against replicating, non-replicating and drug resistant M. tuberculosis. PLoS One 7, e44245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warman A. J., et al. , Antitubercular pharmacodynamics of phenothiazines. J. Antimicrob. Chemother. 68, 869–880 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shubin H., Sherson J., Pennes E., Glaskin A., Sokmensuer A., Prochlorperazine (compazine) as an aid in the treatment of pulmonary tuberculosis. Antibiotic Med. Clin. Ther. (New York) 5, 305–309 (1958). [PubMed] [Google Scholar]
- 56.Brown M. R., Kornberg A., The long and short of it - polyphosphate, PPK and bacterial survival. Trends Biochem. Sci. 33, 284–290 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Neville N., et al. , A dual-specificity inhibitor targets polyphosphate kinase 1 and 2 enzymes to attenuate virulence of pseudomonas aeruginosa. mBio 12, e0059221 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dahl J. U., et al. , The anti-inflammatory drug mesalamine targets bacterial polyphosphate accumulation. Nat. Microbiol. 2, 16267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mittal P., Karthikeyan S., Chakraborti P. K., Amino acids involved in polyphosphate synthesis and its mobilization are distinct in polyphosphate kinase-1 from Mycobacterium tuberculosis. PLoS One 6, e27398 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ehebauer M. T., et al. , Characterization of the mycobacterial acyl-CoA carboxylase holo complexes reveals their functional expansion into amino acid catabolism. PLoS Pathog. 11, e1004623 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gande R., et al. , Acyl-CoA carboxylases (accD2 and accD3), together with a unique polyketide synthase (Cg-pks), are key to mycolic acid biosynthesis in Corynebacterianeae such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J. Biol. Chem. 279, 44847–44857 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Kuroda A., et al. , Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 293, 705–708 (2001). [DOI] [PubMed] [Google Scholar]
- 63.Bordes P., Genevaux P., Control of toxin-antitoxin systems by proteases in Mycobacterium tuberculosis. Front. Mol. Biosci. 8, 691399 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gray M. J., et al. , Polyphosphate is a primordial chaperone. Mol. Cell 53, 689–699 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hood M. I., Skaar E. P., Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 10, 525–537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siegrist M. S., et al. , Mycobacterial Esx-3 is required for mycobactin-mediated iron acquisition. Proc. Natl. Acad. Sci. U.S.A. 106, 18792–18797 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serafini A., Boldrin F., Palu G., Manganelli R., Characterization of a Mycobacterium tuberculosis ESX-3 conditional mutant: Essentiality and rescue by iron and zinc. J. Bacteriol. 191, 6340–6344 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peyron P., et al. , Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 4, e1000204 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clemmensen H. S., et al. , An attenuated Mycobacterium tuberculosis clinical strain with a defect in ESX-1 secretion induces minimal host immune responses and pathology. Sci. Rep. 7, 46666 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rao V., Fujiwara N., Porcelli S. A., Glickman M. S., Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J. Exp. Med. 201, 535–543 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito T., et al. , Inorganic polyphosphate potentiates lipopolysaccharide-induced macrophage inflammatory response. J. Biol. Chem. 295, 4014–4023 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee W. L., Chao H. T., Cheng M. H., Wang P. H., Rationale for using raloxifene to prevent both osteoporosis and breast cancer in postmenopausal women. Maturitas 60, 92–107 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Dutta N. K., et al. , Inhibiting the stringent response blocks Mycobacterium tuberculosis entry into quiescence and reduces persistence. Sci. Adv. 5, eaav2104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pethe K., et al. , Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat. Med. 19, 1157–1160 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Andries K., et al. , A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307, 223–227 (2005). [DOI] [PubMed] [Google Scholar]
- 76. chugh, et al. , RNA sequencing analysis of total RNA isolated from mid-log phase cultures of wild type and Δppk1 mutant strain of Mycobacterium tuberculosis H37Rv. NCBI. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA892734. Deposited 21 October 2021.
- 77. Chugh, et al. , Host RNA-seq analysis of mRNA isolated from naive and mice infected with either wild type or Δppk1 mutant strain at 28 days post-infection. NCBI. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA892458. Deposited 20 October 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Data Availability Statement
The RNA-seq data reported in this article has been obtained from mid-log phase cultures of Mycobacterium tuberculosis strains or lung tissues of uninfected and infected Mus musculus. The raw data sequence files have been deposited in the repository of the National Center for Biotechnology Information and can be accessed using BioProject PRJNA892734 (M.tuberculosis) (76) and PRJNA892458 (Mus musculus) (77).