Significance
This study provides experimental evidence identifying oxazolones as the key intermediates in prebiotic peptide synthesis. These compounds yield the dipeptides upon reaction with water and generate tripeptides in the presence of other amino acids. These key steps in protein formation occur in pure water droplets. Amino acid chirality is preserved in forming the oxazolone and the addition of amino acids during peptide chain extension shows a strong chiral preference, viz. the aqueous droplet chemistry represents a simple route to chirally pure polypeptides. A direct connection between this intermediate and the dipeptide isomer, oxazolidinone, is demonstrated by simple hydration/dehydration. The oxazolone/oxazolidinone-mediated mechanism also occurs in macroscopic wet–dry cycling, establishing a strong connection between macroscopic and microscopic peptide synthesis.
Keywords: prebiotic chemistry, origin of life, origin of homochirality, peptide formation, microdroplet chemistry
Abstract
Peptide formation from amino acids is thermodynamically unfavorable but a recent study provided evidence that the reaction occurs at the air/solution interfaces of aqueous microdroplets. Here, we show that i) the suggested amino acid complex in microdroplets undergoes dehydration to form oxazolone; ii) addition of water to oxazolone forms the dipeptide; and iii) reaction of oxazolone with other amino acids forms tripeptides. Furthermore, the chirality of the reacting amino acids is preserved in the oxazolone product, and strong chiral selectivity is observed when converting the oxazolone to tripeptide. This last fact ensures that optically impure amino acids will undergo chain extension to generate pure homochiral peptides. Peptide formation in bulk by wet-dry cycling shares a common pathway with the microdroplet reaction, both involving the oxazolone intermediate.
Exploration of the origin of life, previously chiefly of intellectual interest, is acquiring practical value with increased efforts to explore and return samples from elsewhere in the solar system (1–3). Origin of life chemistry centers on condensation reactions that create biopolymers (e.g., peptides and nucleosides) from monomers (e.g., amino acids and nucleobases); these are crucial steps in prebiotic synthesis. The aqueous environment, often accepted as the locale for this chemistry, poses a thermodynamic barrier known as the “water paradox” (4–6). One suggested resolution (7, 8) is found in the unique chemical environment at the water/air interface of microdroplets including aerosols (9–11). A significant feature of microdroplet chemistry is reaction acceleration (12–14). Although acceleration mechanisms are still being investigated (15), two major factors are known to contribute. They are i) partial solvation of the reactants at the interface which reduces the activation energy (16), and ii) strong interfacial electric fields (17–19), which catalyze chemical reactions by generating highly reactive species (20–23) and by stabilizing the transition states (24).
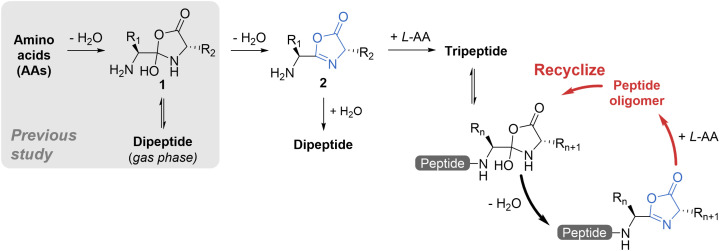
Examples have been reported of abiotic condensation of elementary building blocks (e.g., monosaccharides, nucleotides, phosphoric acids, and amino acids) to form more complex biomolecules (e.g., DNA, RNA, peptides) at the air–water interface of microdroplets (25–27) or in macroscopic solutions (28–30). A recent study demonstrated dipeptide formation from amino acids in water microdroplets without the need for heat, catalyst, or any other reagent (31). It was suggested that i) the extreme acidity of the droplet surface (32–34) allows protonation of the carboxylic acid group in the first amino acid to generate a reactive electrophile on carbon followed by ii) nucleophilic attack by the amine of the second amino acid accompanied by dehydration to generate the dipeptide isomer, oxazolidinone (1), after which iii) 1 undergoes isomerization to form the dipeptide (Scheme 1). The “dry surface” of water microdroplets resolves the water paradox. Tandem mass spectrometry (MS/MS) and ion mobility spectrometry were used in the structural elucidation of the gas phase ion [1+H]+.
Scheme 1.
Proposed mechanism for peptide formation mediated by oxazolidinone/oxazolone intermediates. All reactions, except as noted, occur in microdroplets. Free amino acids condense to form oxazolidinone 1, which undergoes isomerization, resulting in the generation of dipeptide (investigated in ref. 31). Through simple dehydration, oxazolidinone 1 converts to oxazolone 2, the key intermediate involved in peptide formation. It reacts with water or with a third amino acid to form dipeptide and tripeptide, respectively. The resulting di- or tripeptide undergoes a similar process, involving oxazolidinone/oxazolone formation, to further extend the peptide chain. The strong preference for L-amino acid during chain extension enforces the L-homochirality of peptides.
The phenomenon of homochirality, or the presence of single-handedness of chiral molecules that constitute biopolymers, remains an unresolved question in origin of life studies (35–38). In particular, the fact that native amino acids in proteins are exclusively left-handed poses a puzzle. An extraterrestrial origin of amino acid homochirality is unlikely although an initial chiral preference is likely. Reports of the chirality of pristine meteoritic amino acids indicate excess L-amino acids in some cases while reliable evidence for majority D-amino acids is absent (39–42). Therefore, understanding how the initial bias for one enantiomer is enriched from low excess enantiomer (ee) to high ee, as well as how the preferred chirality is transmitted to another chemical species, is needed to unravel the origin of homochirality. There is no generally accepted mechanism for this even though several chemical and physical effects have been established, e.g., asymmetric autocatalysis (43), chiral preference during phase transitions (44–48), and enantiospecific binding with self-assembled complexes, including serine octamers (49) and lipid membranes (37). Significantly, the air/water interface has been demonstrated to amplify chirality (50–52), implying a potential role for microdroplets in homochirality.
Peptide formation in microdroplets appears to involve a reaction intermediate, the oxazolone (2), formed by dehydration of oxazolidinone 1. A detailed discussion of the connection between dipeptide, oxazolidinone, and oxazolone is provided in SI Appendix, section I. The suggested importance of heterocyclic C5 compounds in origin of life chemistry (53) is bolstered by experimental evidence showing that intermediate 2 can be converted to a dipeptide or tripeptide upon reaction with water or a third amino acid, respectively (Scheme 1). That is, peptide chain extension follows a reaction sequence from condensation to give the oxazolone intermediate followed by amino acid addition. Furthermore, the oxazolone, with its intrinsic chiral center, can ensure a high chiral preference for L-amino acids during chain extension provided it is formed with and reacts with high chiral selectivity. The reactive intermediate oxazolone can mediate peptide chain extension in prebiotic environments and ensure the homochirality of L-peptides observed in nature, even if the amino acids themselves are not homochiral.
A significant question concerns the possible connection between bulk wet–dry cycling (54–57) as a route to peptide formation and the microdroplet mechanism. We addressed this by performing experiments on an intermediate (microliter) scale. The reaction intermediate in peptide formation via wet–dry cycling (101 μL scale) was found to show the same spectroscopic features (MS/MS spectra) as did microdroplet reactions (10−9 μL scale), suggesting these two processes share the oxazolidinone/oxazolone-mediated reaction pathway, thus establishing a strong connection between macroscopic and microscopic peptide syntheses.
Results and Discussion
Synthesis and Characterization of Oxazolones.
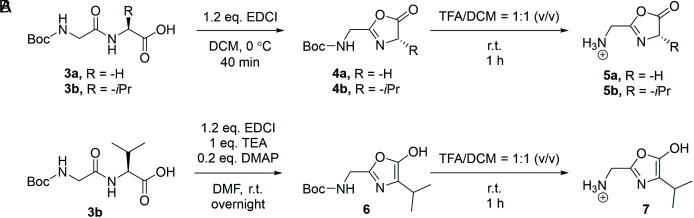
We synthesized the oxazolones 5 (Scheme 2A), through mild 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI)-mediated intramolecular condensation (58) to give the tert-butyloxycarbonyl (Boc)-protected oxazolones 4. Then, trifluoroacetic acid (TFA)-deprotection provided the protonated oxazolones (trifluoroacetate as counter ion) as the final products. Proton NMR (1H-NMR) and MS/MS analysis were used to confirm these assignments (SI Appendix, section VI).
Scheme 2.
Bulk synthesis of intermediates of peptide chain extension, oxazolones. A and B illustrate the synthetic routes to oxazolone 5 and enol isomer, oxazole 7, respectively.
A possible concern is keto-enol tautomerization (59), which would lead to epimerization of the chiral oxazolone 5b (Scheme 2B). Accordingly, the oxazolone enol tautomer 7 was prepared separately using a basic condensation reaction in the first step. The diagnostic neutral loss of CO (mass 28 Da) in the fragmentation of ions 5a and 5b is indicative of their unepimerized oxazolone structures (SI Appendix, Fig. S24 A and B) (60). By contrast, loss of CH2NH (mass 29 Da) and H2O (mass 18 Da) are observed from the precursor ion 7, which fragments to form stable fragment ions at m/z 128 and m/z 139 (SI Appendix, Fig. S24C). Suggested structures of those fragments are illustrated in SI Appendix, Fig. S26.
Major interest lies in the connection between the synthesized oxazolone 2 (or the protonated form, 5) and the proposed intermediate 1. To investigate this, we produced the corresponding oxazolidinone from glycine and then dehydrated and mass-selected the gas-phase ion (SI Appendix, Fig. S1). The major product ions of the resulting dehydrated oxazolidinone perfectly match the fragments of 5a (again from glycine), indicating that the oxazolone 5a and the dehydrated intermediate share the same structure. This result implies that the previous findings on oxazolidinone chemistry (31), including dipeptide and higher-order peptide formation, extend also to the synthesized oxazolone intermediate. To be precise, the oxazolone can function as an electrophile, facilitating dipeptide and tripeptide formation through reactions with water or other amino acids. To test this hypothesis, we conducted a series of experiments, and this chemistry is discussed below. It is notable that an isomer of oxazolone, diketopiperazine, might also act as the intermediate in microdroplets. Therefore, the corresponding control experiments were carried out and they ruled out this possibility (see SI Appendix, section II for details).
Dipeptide Formation from Oxazolones.
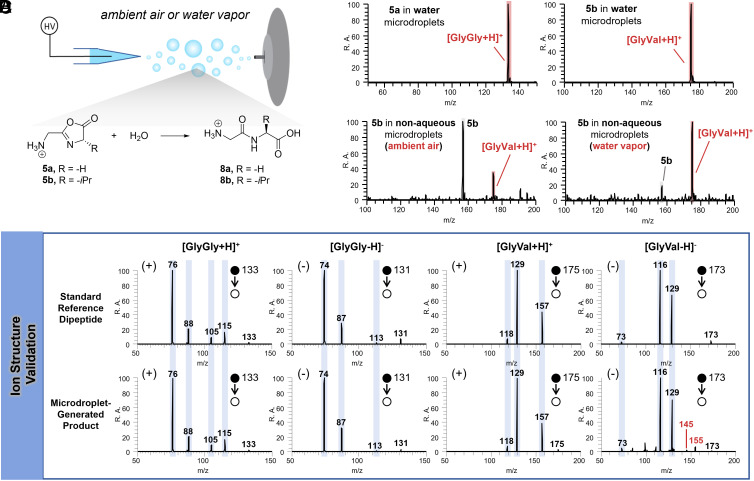
We sought to investigate the reaction between the oxazolones and H2O in aqueous microdroplets. The synthesized oxazolones 5a and 5b were dissolved in water separately, and then their chemistry in microdroplets was examined by nanoelectrospray ionization-MS (nESI-MS) (Fig. 1A). As expected, the corresponding dipeptides 8a (H-Gly-Gly-OH) and 8b (H-Gly-Val-OH) were produced from the highly reactive oxazolones 5a and 5b, respectively. The near-quantitative conversions seen in both cases (Fig. 1B) indicate the high reactivity of the intermediate and, meanwhile, raise the question of whether this transformation in fact occurred in the microdroplets, since solution reactions may also contribute during dissolution of 5 in water. To confirm that the conversion occurs in microdroplets, we carefully chose the more hydrophobic oxazolone, 5b, and dissolved it in nonaqueous solvents for subsequent microdroplet reactions. A clear increase in the transformation from 5b to 8b was seen when the gaseous environment surrounding the microdroplets was replaced with water vapor (Fig. 1C), providing strong evidence for the microdroplet-based conversion. In addition, the identical MS/MS fragmentation profiles of the generated products (8a and 8b) to those of the corresponding authentic dipeptides confirmed the successful dipeptide formation from oxazolone (Fig. 1D). It’s notable that under these conditions, the dipeptides 8a and 8b rather than their oxazolidinone isomers were produced and compared to the previous study (31); no external collisional heating was required to form the peptides.
Fig. 1.
Dipeptide formation through oxazolone hydration in microdroplets. (A) Illustration of microdroplet production by nESI; online microdroplet reaction and follow-up MS detection. Conversion from oxazolone to dipeptide by reaction with H2O occurs in microdroplets. (B) Mass spectra in the positive mode, showing the conversion from 5a and 5b to 8a and 8b respectively, in water microdroplets in ambient air. (C) Mass spectra in the positive mode, showing the online conversion from 5b to 8b in ACN/DCM (v/v = 1:1) microdroplets in ambient air and with added water vapor. (D) Comparison of MS/MS spectra obtained for the standard dipeptide (Top row) and the product generated in microdroplets (Bottom row) in both positive and negative modes; the fragments annotated with blue columns represent the diagnostic peaks of authentic dipeptides.
Tripeptide Formation via Oxazolones.
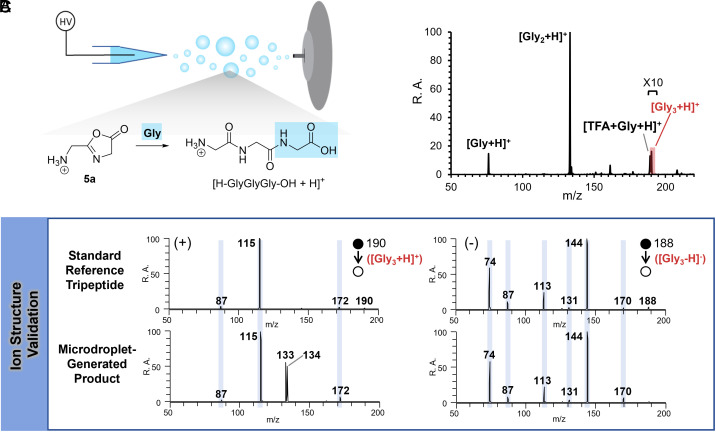
Higher-order peptides, e.g., Gly3, were observed previously through collisions of droplets containing amino acids (31). To confirm that the oxazolone structure serves as an intermediate in this peptide chain extension process, we mixed substrate 5a with a pure glycine solution and performed microdroplet reactions (Fig. 2A). As expected, the reaction produced the tripeptide, with the dipeptide still being the base peak (Fig. 2B). A mixture of products results because the shared electrophile 5a is involved in two competitive processes: i) reaction with water to form the dipeptide and ii) reaction with glycine to form the tripeptide. To enhance the generation of Gly3, we used more concentrated glycine solutions, successfully increasing tripeptide yield (SI Appendix, Fig. S31). The structures of the products formed in microdroplets were confirmed using MS/MS fragmentation and by comparison to the mass spectra of an authentic tripeptide (Fig. 2C).
Fig. 2.
Peptide chain extension reaction from the oxazolone intermediate. (A) Conversion from oxazolone to tripeptide by reacting with glycine in microdroplets. (B) Mass spectrum in the positive mode, showing the conversion from 5a to both dipeptide and tripeptide in water microdroplets containing glycine. (C) Comparison of MS/MS spectra obtained for the standard tripeptide (Top row) to the product generated in microdroplets (Bottom row) in both positive and negative modes; the fragments annotated in blue columns represent the diagnostic peaks of the authentic tripeptide.
Chiral Preference in Peptide Chain Extension via Chiral Oxazolone.
The homochirality of L-amino acids in peptides is of longstanding interest. Given the evidence presented above for prebiotic peptide chain extension via oxazolone intermediates, these questions arise i) to what extent is the original amino acid chirality preserved during oxazolone formation ii) to what extent is the chirality of the oxazolone preserved under the conditions of the chain extension reaction and iii) how chirally selective is the chain extension reaction? The answer to question i) is that original amino acid chirality is unimportant because the peptide chain extension is strongly homochiral as we show below. In answer to the second question, we found that the chiral oxazolone 5b did not undergo racemization during the tripeptide formation (SI Appendix, Fig. S6); note that even if keto-enol tautomerization had occurred (5b converted to 7), the much lower reactivity of aromatic oxazole 7 would prevent its reaction with other nucleophiles, like water (SI Appendix, Fig. S7) and amino acids (SI Appendix, Fig. S8). The preservation of the chiral center in the reactive oxazolone makes the third question highly important and we explored its chiral preference during tripeptide formation.
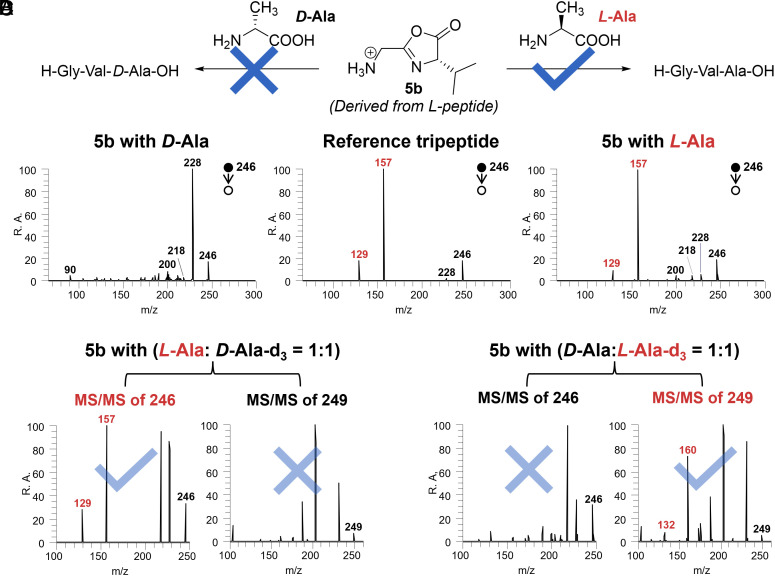
We first used the chiral oxazolone 5b (containing the L-isopropyl amino acid) and reacted it separately with L-Ala and D-Ala (Fig. 3 A and B). The diagnostic fragments of the authentic tripeptide (H-Gly-Val-Ala-OH) were observed only in the reaction involving L-Ala, indicating a strong chiral preference for the L-amino acid during oxazolone-mediated peptide chain extension. Notably, the sequence isomer (H-Ala-Gly-Val-OH) was not generated in this scenario, a finding which was validated using the distinctive MS/MS spectrum of this authentic tripeptide (SI Appendix, Fig. S9).
Fig. 3.
Chiral preference during the process of peptide chain extension. (A) Chiral oxazolone-directed diastereoselective tripeptide formation occurs due to preferential L-Alanine reaction. (B) MS/MS spectra of generated tripeptide or isomers when 5b was mixed with D-Ala (Left) and L-Ala (Right). The spectrum from the reference tripeptide, H-Gly-Val-Ala-OH is displayed in the Middle panel for comparison. Note that its diastereomer, H-Gly-Val-D-Ala-OH presents an identical MS2 spectrum (SI Appendix, Fig. S10). (C) Reaction between 5b and racemic alanine with D-Ala labeled by deuterium, with the results of MS/MS spectra of the ions at m/z 246 derived from L-isomer (Left) and m/z 249 derived from D-isomer (Right). (D) Reaction between 5b and racemic alanine with L-Ala labeled by deuterium, with the results of MS/MS spectra of the ions at m/z 246 derived from D-isomer (Left) and m/z 249 derived from L-isomer (Right).
To further confirm this chiral preference, we reacted 5b with racemic alanine solutions made up of deuterium-labeled L- and then with deuterium-labeled D-Ala. The relative abundances of the generated tripeptide ions (m/z 246 or 249) shown in single-stage MS (SI Appendix, Fig. S32) were low, likely because of steric hindrance of the bulky isopropyl group of 5b. To discriminate the signals of tripeptide from other possible interferences, MS/MS analysis was performed. As expected, the characteristic fragments of the tripeptide (m/z 129/132 and 157/160) were only observed in reactions involving L-Ala (Fig. 3 C, Left; Fig. 3 D, Right). This provides evidence for strong chiral selectivity in the peptide chain extension reaction.
An additional finding is a constant 10:9 ratio of the complex [D-Ala+5b+H2O]+ to [L-Ala+5b+H2O]+ (SI Appendix, Fig. S32; the complex structure is discussed in SI Appendix, Fig. S13. It is worth noting, parenthetically, that the adduct of alanine and oxazolidinone is formally equivalent to [Ala+5b+H2O], so involving oxazolidinone in the reaction). This result shows that complex formation is essentially nonselective and that the chiral preference must be associated with stereoselective transformation of the complex into the tripeptide, viz. only the correct complex originating from L-oxazolone and L-Ala is able to undergo dehydration to form a tripeptide.
The fact that chiral oxazolone selectively reacts with an amino acid of the same chirality was further confirmed by the experiment using the corresponding D-oxazolone, where the D-Ala was selected (SI Appendix, Fig. S11) to form the authentic tripeptide. The proposed mechanism depicted in SI Appendix, Fig. S12 i) shows the absence of racemization or conversion of racemized reactant to product and ii) demonstrates stereoselective interaction between the chiral oxazolone and amino acids of the same chirality.
To further support the observed chiral preference and to explore its generality, the L-oxazolone 5b was subject to microdroplet reactions with the various L- or D-amino acids, including phenylalanine, proline, leucine, and isoleucine. The results (SI Appendix, Figs. S14 and S17) clearly demonstrate the strong chiral preference: L-oxazolone reacts selectively with L-amino acids during peptide chain extension. It is also noteworthy that sequence isomers are generated to some extent in the cases of phenylalanine, leucine, and isoleucine, presumably due to its bulky side chain (refer to SI Appendix, section III for details).
Two significant findings emerge from this work: i) peptide chain extension occurs via an oxazolone intermediate and ii) chiral preference for L-amino acids is based on the L-oxazolone intermediate, so establishing a homochiral self-replication system. Similar to the general model of Frank and later authors (61–63), the L-oxazolone structure, generated at the C-terminus of the peptide by isomerization (to form oxazolidinone) and then dehydration, serves as a chiral template, selectively matching L-amino acids from a racemic pool (and disallowing addition of D-amino acids) to form the chain-extended peptide. This extended peptide undergoes repeated chiral selection during further chain growth, leading to the eventual biopolymer homochirality.
Shared Mechanism of Peptide Formation in Microdroplets and Bulk Wet-Dry Cycling.
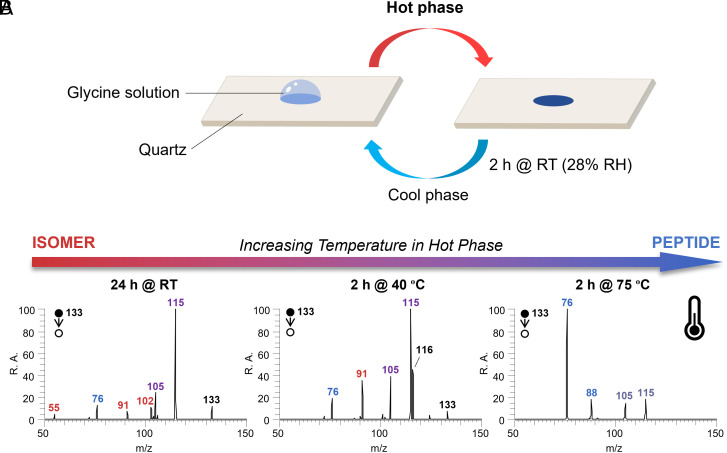
A widely considered driving force for prebiotic amino acid condensation is wet-dry cycling (54–57). This involves water evaporation (less water) at high temperatures and water adsorption (more water) at low temperatures, providing an alternative solution to overcome the water paradox. Interestingly, regulation of water amount might also occur within water microdroplets, which display a dry surface and wet interior as noted in the Introduction. The similarity between these two systems suggests that they might share the same mechanism, with both being mediated by oxazolone. To investigate this possibility, we performed wet-dry cycling of glycine aqueous solutions on the 15 μL scale at different temperatures and collected the resulting product mixtures for MS/MS analysis (Fig. 4A). Notably, we observed a clear trend: as the temperature in the hot phase increased, more dipeptide fragments were produced and fewer signals from isomer 1 were observed, suggesting facilitated conversion of the isomer to the authentic peptide with external heating. This result agrees with the reported transformation of oxazolidinone to dipeptide upon collisional heating in the microdroplet phase, as seen particularly when comparing MS/MS data at room temperature (RT) for 24 h (Fig. 4B) with the microdroplet result (figures 2E and 3A in the ref. 31). This result demonstrates that dipeptide formation in wet-dry cycling and microdroplets share the same reaction intermediates. It is noteworthy that the interface between solid (quartz) and water could potentially play a role in the formation of peptides (64, 65) although nothing in our results demands a reactive solid interface.
Fig. 4.
Wet-dry cycling to synthesize Gly2. (A) Illustration showing the bulk cycling process, including a hot phase and a cool phase. Glycine solution (15 μL, 10 mM) was used as starting material. After one cycle, the residue from the aqueous amino acid mixture was redissolved in 0.1% acetic acid aqueous solution for nESI-MS/MS analysis. (B) MS/MS spectra of Gly2 and its isomer at m/z 133 generated by wet–dry cycles with the hot phase at RT for 24 h (Left), 40 °C for 2 h (Middle), and 75 °C for 2 h (Right). The diagnostic fragments from the Gly2 and its oxazolidinone isomer (1) are annotated in blue and red, respectively. Peaks annotated in purple are shared fragments.
Conclusion and Implications.
Peptides (and proteins) are essential components in biosynthesis of complex biomolecular assemblies. Understanding how peptides might have been created under primitive earth conditions could shed light on the key processes in the origin of life (66). The experimental results presented here strongly suggest a role for oxazolones in prebiotic peptide synthesis. This study i) identifies the oxazole intermediate formed through dehydration of an initial oxazolidinone generated during peptide formation from amino acids in aqueous microdroplets ii) demonstrates that this intermediate facilitates peptide chain extension, iii) shows that this occurs in a chirally selective fashion which is reasonably ascribed to steric interactions, and iv) establishes a mechanistic connection between macroscopic (wet/dry cycling) and microscopic (microdroplet) scale peptide synthesis.
The most notable implications of these findings are the following: i) Chirally homogeneous peptides are expected to result from repeated chain extension reactions even when using diastereomeric or chirally impure amino acids as reagents, ii) homochirality is enforced in the growing peptide chain because the reactive intermediate oxazolone contains the previously added amino acid residue in proximity to the site of nucleophilic attack by the amine of the next amino acid, so establishing a steric barrier that selects the chirality of the added residue, iii) addition of each amino acid gives an oxazolidinone complex which, like its homolog 1, is only isolable as a gas-phase ion but undergoes dehydration to give the oxazolone intermediate, iv) the chirality of peptide chain extension is founded on the particular organic reactions involved, and similar considerations might well extend to other types of biopolymers formed by condensation reactions, and finally v) a microscopic form of wet/dry cycling between the interface and interior of individual droplets deserves further scrutiny as a route to prebiotic peptide formation. Given that meteorites, the best-known prebiotic sources of amino acids, have either diastereomeric or moderate to low excesses of the L-enantiomers (39–42), the chiral preferences established for chain extension will result in all L- or all D-residue peptides with the former predominating and subsequent evolutionary forces selecting for this homochiral form. The possibility of deviations in homochirality occurring upon the addition of achiral glycine (where the steric factor is absent) may be selected against by the same forces. The phenomena observed in oxazolone chemistry, including chirality preservation and transmission, are suggested to be linked to the development of homochirality in nature, at least as far as proteins are concerned. We suggest that the ability of microdroplets to speed up chemical reactions facilitates the synthesis of peptides and that this should also apply to other biopolymers.
Materials and Methods
Chemicals and Materials.
All chemicals and solvents were purchased from Sigma-Aldrich (St. Louis, MO) and Fisher Scientific (Hampton, NH) unless otherwise noted. Pure water was prepared using Direct-Q ® 3 UV Water Purification System from Merck KGaA (Darmstadt, Germany). The tripeptide standards, H-Gly-Val-Ala-OH and H-Ala-Gly-Val-OH, were synthesized by RS Synthesis (Louisville, KY). Information on the synthetic procedures for compounds 4 to 7 is given in SI Appendix, section IV. The general procedure for tripeptide formation in solution is given in SI Appendix, section V. The characterization of all synthesized products, including the H-NMR and MS/MS spectra, is given in SI Appendix, section VI. The quartz microscope slides (50.8 × 25.4 × 1.0 mm) used in wet-dry cycling were purchased from Fisher Scientific. The nESI emitters were made using Sutter Instrument micropipette tip puller (Novato, CA) and borosilicate glass capillaries (1.5 mm O.D.; 0.86 mm I.D.; 10 cm length).
MS and Microdroplets.
A Finnigan LTQ linear ion trap mass spectrometer (ThermoFisher Scientific, San Jose, CA) was used to perform nESI-MS and MS/MS analysis. All microdroplets were generated through nESI-MS (using a 5-μm internal diameter nESI emitter). Spray voltage was +2 kV or −2 kV, depending on the chosen polarity; the distance between the spray tip to inlet was 10 mm unless otherwise noted. Instrumental parameters were as follows: Capillary temperature 275 °C; capillary voltage 15 V; tube lens 65 V. The solution concentrations for microdroplet reactions were kept at 10 mM unless mentioned otherwise.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We acknowledge valuable discussions with Dylan T. Holden and Nicolás M. Morato. We also acknowledge the financial support from the Multi-University Research Initiative of the Air Force Office of Scientific Research (FA9550-21-1-0170) via Stanford University (sub-award 62741613-204669) and also support from the NSF (grant CHE-1905087).
Author contributions
L.Q. and R.G.C. designed research; L.Q. performed research; L.Q. and R.G.C. analyzed data; and L.Q. and R.G.C. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: N.K.D., University of California, San Diego; R.A.O., University of Melbourne; and V.V., University of Colorado Boulder.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Cronin J. R. R., Pizzarello S., Amino acids in meteorites. Adv. Sp. Res. 3, 5–18 (1983). [DOI] [PubMed] [Google Scholar]
- 2.Brack A., From interstellar amino acids to prebiotic catalytic peptides: A review. Chem. Biodivers. 4, 665–679 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Pizzarello S., Cooper G. W., Flynn G. J., “The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles” in Meteorites and the Early Solar System II, D. S. Lauretta, H. Y. McSween, Eds. (University of Arizona Press, 2021), pp. 625–652. [Google Scholar]
- 4.Deal A. M., Rapf R. J., Vaida V., Water-air interfaces as environments to address the water paradox in prebiotic chemistry: A physical chemistry perspective. J. Phys. Chem. A 125, 4929–4942 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Sutherland J. D., Opinion: Studies on the origin of life-the end of the beginning. Nat. Rev. Chem. 1, 1–8 (2017). [Google Scholar]
- 6.Ruiz-Lopez M. F., Francisco J. S., Martins-Costa M. T. C., Anglada J. M., Molecular reactions at aqueous interfaces. Nat. Rev. Chem. 4, 459–475 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Yan X., Bain R. M., Cooks R. G., Organic reactions in microdroplets: Reaction acceleration revealed by mass spectrometry. Angew. Chem. Int. Ed. Engl. 55, 12960–12972 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Wei Z., Li Y., Cooks R. G., Yan X., Accelerated reaction kinetics in microdroplets: Overview and recent developments. Annu. Rev. Phys. Chem. 71, 31–51 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Dobson C. M., Ellison G. B., Tuck A. F., Vaida V., Atmospheric aerosols as prebiotic chemical reactors. Proc. Natl. Acad. Sci. U.S.A. 97, 11864–11868 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuck A., The role of atmospheric aerosols in the origin of life. Surv. Geophys. 23, 379–409 (2002). [Google Scholar]
- 11.Donaldson D. J., Tervahattu H., Tuck A. F., Vaida V., Organic aerosols and the origin of life: An hypothesis. Orig. Life Evol. Biosph. 34, 57–67 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Bain R. M. M., Pulliam C. J. J., Cooks R. G. G., Accelerated Hantzsch electrospray synthesis with temporal control of reaction intermediates. Chem. Sci. 6, 397–401 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsh B. M., Iyer K., Cooks R. G., Reaction acceleration in electrospray droplets: Size, distance, and surfactant effects. J. Am. Soc. Mass Spectrom. 30, 2022–2030 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Li Y., Mehari T. F., Wei Z., Liu Y., Cooks R. G., Reaction acceleration at air-solution interfaces: Anisotropic rate constants for Katritzky transamination. J. Mass Spectrom. 56, e4585 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Chen C. J., Williams E. R., The role of analyte concentration in accelerated reaction rates in evaporating droplets. Chem. Sci. 14, 4704–4713 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu L., Wei Z., Nie H., Cooks R. G., Reaction acceleration promoted by partial solvation at the gas/solution interface. Chempluschem 86, 1362–1365 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Xiong H., Lee J. K., Zare R. N., Min W., Strong electric field observed at the interface of aqueous microdroplets. J. Phys. Chem. Lett. 11, 7423–7428 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Chamberlayne C. F., Zare R. N., Simple model for the electric field and spatial distribution of ions in a microdroplet. J. Chem. Phys. 152, 184702 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao H., Leven I., Head-Gordon T., Can electric fields drive chemistry for an aqueous microdroplet? Nat. Commun. 13, 280 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J. K., et al. , Spontaneous generation of hydrogen peroxide from aqueous microdroplets. Proc. Natl. Acad. Sci. U.S.A. 116, 19294–19298 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J. K., Samanta D., Nam H. G., Zare R. N., Micrometer-sized water droplets induce spontaneous reduction. J. Am. Chem. Soc. 141, 10585–10589 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Gong C., et al. , Spontaneous reduction-induced degradation of viologen compounds in water microdroplets and its inhibition by host-guest complexation. J. Am. Chem. Soc. 144, 3510–3516 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Qiu L., Cooks R. G., Simultaneous and spontaneous oxidation and reduction in microdroplets by the water radical cation/anion pair. Angew. Chem. Int. Ed. Engl. 61, e202210765 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaik S., Ramanan R., Danovich D., Mandal D., Structure and reactivity/selectivity control by oriented-external electric fields. Chem. Soc. Rev. 47, 5125–5145 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Nam I., Nam H. G., Zare R. N., Abiotic synthesis of purine and pyrimidine ribonucleosides in aqueous microdroplets. Proc. Natl. Acad. Sci. U.S.A. 115, 36–40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nam I., et al. , Abiotic production of sugar phosphates and uridine ribonucleoside in aqueous microdroplets. Proc. Natl. Acad. Sci. U.S.A. 114, 12396–12400 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju Y., et al. , Aqueous-microdroplet-driven abiotic synthesis of ribonucleotides. J. Phys. Chem. Lett. 13, 567–573 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Griffith E. C., Vaida V., In situ observation of peptide bond formation at the water-air interface. Proc. Natl. Acad. Sci. U.S.A. 109, 15697–15701 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar J. K., Oliver J. S., Proximity effects in monolayer films: Kinetic analysis of amide bond formation at the air−water interface using 1H NMR spectroscopy. J. Am. Chem. Soc. 124, 11307–11314 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Morasch M., et al. , Heated gas bubbles enrich, crystallize, dry, phosphorylate and encapsulate prebiotic molecules. Nat. Chem. 11, 779–788 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Holden D. T., Morato N. M., Cooks R. G., Aqueous microdroplets enable abiotic synthesis and chain extension of unique peptide isomers from free amino acids. Proc. Natl. Acad. Sci. U.S.A. 119, e2212642119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buch V., Milet A., Vácha R., Jungwirth P., Devlin J. P., Water surface is acidic. Proc. Natl. Acad. Sci. U.S.A. 104, 7342–7347 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang K.-H., Wei Z., Cooks R. G., Accelerated reactions of amines with carbon dioxide driven by superacid at the microdroplet interface. Chem. Sci. 12, 2242–2250 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vácha R., Buch V., Milet A., Devlin J. P., Jungwirth P., Autoionization at the surface of neat water: Is the top layer pH neutral, basic, or acidic? Phys. Chem. Chem. Phys. 9, 4736 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Blackmond D. G., The origin of biological homochirality. Cold Spring Harb. Perspect. Biol. 1, a032540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cline D. B., On the physical origin of the homochirality of life. Eur. Rev. 13, 49–59 (2005). [Google Scholar]
- 37.Martin H. S., Podolsky K. A., Devaraj N. K., Probing the role of chirality in phospholipid membranes. ChemBioChem 22, 3148–3157 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Sallembien Q., Bouteiller L., Crassous J., Raynal M., Possible chemical and physical scenarios towards biological homochirality. Chem. Soc. Rev. 51, 3436–3476 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Cronin J. R., Pizzarello S., Enantiomeric excesses in meteoritic amino acids. Science 275, 951–955 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Pizzarello S., Huang Y., Alexandre M. R., Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc. Natl. Acad. Sci. U.S.A. 105, 3700–3704 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glavin D. P., Dworkin J. P., Enrichment of the amino acid L-isovaline by aqueous alteration on Cl and CM meteorite parent bodies. Proc. Natl. Acad. Sci. U.S.A. 106, 5487–5492 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glavin D. P., et al. , The search for chiral asymmetry as a potential biosignature in our solar system. Chem. Rev. 120, 4660–4689 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto A., et al. , Achiral amino acid glycine acts as an origin of homochirality in asymmetric autocatalysis. Org. Biomol. Chem. 17, 4200–4203 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Breslow R., Levine M. S., Amplification of enantiomeric concentrations under credible prebiotic conditions. Proc. Natl. Acad. Sci. U.S.A. 103, 12979–12980 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cintas P., Chirality of living systems: A helping hand from crystals and oligopeptides. Angew. Chem. Int. Ed. Engl. 41, 1139–1145 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Levine M., Kenesky C. S., Mazori D., Breslow R., Enantioselective synthesis and enantiomeric amplification of amino acids under prebiotic conditions. Org. Lett. 10, 2433–2436 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Hein J. E., Blackmond D. G., On the origin of single chirality of amino acids and sugars in biogenesis. Acc. Chem. Res. 45, 2045–2054 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Nanita S. C., Cooks R. G., Serine octamers: Cluster formation, reactions, and implications for biomolecule homochirality. Angew. Chem. Int. Ed. Engl. 45, 554–569 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Yang P., Xu R., Nanita S. C., Cooks R. G., Thermal formation of homochiral serine clusters and implications for the origin of homochirality. J. Am. Chem. Soc. 128, 17074–17086 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Weissbuch I., Addadi L., Leiserowitz L., Lahav M., Total asymmetric transformations at interfaces with centrosymmetric crystals: Role of hydrophobic and kinetic effects in the crystallization of the system glycine/.alpha.-amino acids. J. Am. Chem. Soc. 110, 561–567 (1988). [Google Scholar]
- 51.Andelman D., Chiral discrimination and phase transitions in langmuir monolayers. J. Am. Chem. Soc. 111, 6536–6544 (1989). [Google Scholar]
- 52.Alonso C., et al. , Guest intercalation at corrugated surface of host monolayer crystal on water: Cholesteryl-L-glutamate and water-soluble amino acids. J. Am. Chem. Soc. 123, 10105–10106 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Lundberg R. D., Doty P., Polypeptides. XVII. A study of the kinetics of the primary amine-initiated polymerization of N-carboxy-anhydrides with special reference to configurational and stereochemical effects. J. Am. Chem. Soc. 79, 3961–3972 (1957). [Google Scholar]
- 54.Damer B., Deamer D., The hot spring hypothesis for an origin of life. Astrobiology 20, 429–452 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell T. D., et al. , Prebiotic condensation through wet–dry cycling regulated by deliquescence. Nat. Commun. 10, 4508 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forsythe J. G., et al. , Ester-mediated amide bond formation driven by wet-dry cycles: A possible path to polypeptides on the prebiotic Earth. Angew. Chem. Int. Ed. Engl. 54, 9871–9875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin C., et al. , Water-based dynamic depsipeptide chemistry: Building block recycling and oligomer distribution control using hydration-dehydration cycles. JACS Au 2, 1395–1404 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen F. M. F., Kuroda K., Benoiton N. L., A simple preparation of 5-oxo-4,5-dihydro-1,3-oxazoles (oxazolones). Synthesis 1979, 230–232 (1979). [Google Scholar]
- 59.de Castro P. P., Batista G. M. F., dos Santos H. F., Amarante G. W., Theoretical study on the epimerization of azlactone rings: Keto-Enol tautomerism or base-mediated racemization? ACS Omega 3, 3507–3512 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farrugia J. M., O’Hair R. A. J., Reid G. E., Do all b2 ions have oxazolone structures? Multistage mass spectrometry and ab initio studies on protonated N-acyl amino acid methyl ester model systems. Int. J. Mass Spectrom. 210–211, 71–87 (2001). [Google Scholar]
- 61.Frank F. C., On spontaneous asymmetric synthesis. Biochim. Biophys. Acta 11, 459–463 (1953). [DOI] [PubMed] [Google Scholar]
- 62.Wu M., Walker S. I., Higgs P. G., Autocatalytic replication and homochirality in biopolymers: Is homochirality a requirement of life or a result of it? Astrobiology 12, 818–829 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Laurent G., Lacoste D., Gaspard P., Emergence of homochirality in large molecular systems. Proc. Natl. Acad. Sci. U.S.A. 118, 1–6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brigiano F. S., Gierada M., Tielens F., Pietrucci F., Mechanism and free-energy landscape of peptide bond formation at the silica-water interface. ACS Catal. 12, 2821–2830 (2022). [Google Scholar]
- 65.Bujdák J., Faybíková K., Eder A., Yongyai Y., Rode B. M., Peptide chain elongation: A possible role of montmorillonite in prebiotic synthesis of protein precursors. Orig. Life Evol. Biosph. 25, 431–441 (1995). [DOI] [PubMed] [Google Scholar]
- 66.Frenkel-Pinter M., Samanta M., Ashkenasy G., Leman L. J., Prebiotic peptides: Molecular hubs in the origin of life. Chem. Rev. 120, 4707–4765 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.