Significance
Interleukin (IL)-6, an essential indicator of cytokine release syndromes (CRS), regulates vascular homeostasis and inflammation. Inhibition of IL-6 receptor (IL-6R) signaling is beneficial for various CRS; however, it is limited by adverse effects related to poor understanding of mechanisms involved. Here, we discovered that hypoxia-inducible factor (HIF1) α signaling is activated by IL-6R trans-signaling in endothelial cells, which promotes vascular inflammatory responses and endothelial permeability by glycolysis. Short-term inhibition of IL-6R–HIF1α signaling attenuated proinflammatory cytokines and coagulation cascade activation, and it prevented vascular damage by preserving endothelial glycocalyx during sepsis and burn injury-induced CRS. Endothelial IL-6R–HIF1α signaling has crucial roles in progression of CRS, suggesting novel therapeutic strategies for cytokine storm-related disease by relieving adverse effects of anti-IL-6R antibody treatment.
Keywords: IL-6, anti-IL-6 receptor antibody, endothelial cell, cytokine release syndrome, burn injury
Abstract
Protection against endothelial damage is recognized as a frontline approach to preventing the progression of cytokine release syndrome (CRS). Accumulating evidence has demonstrated that interleukin-6 (IL-6) promotes vascular endothelial damage during CRS, although the molecular mechanisms remain to be fully elucidated. Targeting IL-6 receptor signaling delays CRS progression; however, current options are limited by persistent inhibition of the immune system. Here, we show that endothelial IL-6 trans-signaling promoted vascular damage and inflammatory responses via hypoxia-inducible factor-1α (HIF1α)–induced glycolysis. Using pharmacological inhibitors targeting HIF1α activity or mice with the genetic ablation of gp130 in the endothelium, we found that inhibition of IL-6R (IL-6 receptor)–HIF1α signaling in endothelial cells protected against vascular injury caused by septic damage and provided survival benefit in a mouse model of sepsis. In addition, we developed a short half-life anti-IL-6R antibody (silent anti-IL-6R antibody) and found that it was highly effective at augmenting survival for sepsis and severe burn by strengthening the endothelial glycocalyx and reducing cytokine storm, and vascular leakage. Together, our data advance the role of endothelial IL-6 trans-signaling in the progression of CRS and indicate a potential therapeutic approach for burns and sepsis.
Cytokine release syndrome (CRS) is characterized as an acute systemic inflammatory disease induced by infections such as sepsis and acute respiratory distress syndrome (ARDS), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19), or burns, trauma, ischemia, or immunotherapies including chimeric antigen receptor (CAR-T) cell therapy (1). The pathology of CRS is a complicated network of various critical cytokines [interleukin (IL)-1, IL-6, IL-18, interferon-γ, and tumor necrosis factor (TNF)-α] and pivotal driver immune cells (2). Despite accumulating information about the pathophysiology of CRS, it still causes severe and life-threatening complications (3). Recently, there has been great interest in the role of endothelial cells in CRS. Severe CRS caused by sepsis or CAR T-cell therapy often causes vascular damage, capillary leakage, and coagulation activation. Disruption of the endothelial cell barrier junction causes multiple organ failure and mortality in sepsis (4), suggesting that endothelial cells have critical roles in the control of CRS progression (5–7). Therefore, therapies improving vascular homeostasis might provide an alternative approach to CRS treatment.
IL-6 is a proinflammatory cytokine that has multiple functions mediated via several binding patterns (8, 9). Although elevated serum IL-6 levels are a hallmark of CRS and a useful diagnostic indicator of disease progression (9–12), the inhibition of IL-6 or IL-6 receptor (IL-6R) as a CRS treatment is limited because IL-6 has an important function in host defense against infections. Thus, the effects of IL-6 neutralization in several animal sepsis models are controversial (13–16). We previously demonstrated that endothelial IL-6R trans-signaling contributed to CRS progression and that the blockade of IL-6R signaling had beneficial clinical effects in severe COVID-19 patients by reducing serum plasminogen activator inhibitor-1 (PAI-1) levels (8). In addition, the efficacy of an anti-IL-6R antibody, tocilizumab, has been approved by the Food and Drug Administration for infectious disease, COVID-19 (9). These promising findings support the prominent therapeutic concept that the inhibition of IL-6 signaling can be used to treat infectious diseases, ARDS, and burns. However, targeting of IL-6R signaling has several limitations: 1) the precise molecular mechanisms involved in IL-6R trans-signaling in endothelial cells during inflammatory responses are unknown, and 2) the persistent inhibition of IL-6 signaling increases susceptibility to severe complications and has poor outcomes (17–19). In this context, inhibiting IL-6 signaling for therapeutic purposes requires further study.
Under hypoxia, the expression levels of hypoxia-induced factor (HIF)-1α and its transcriptional activity are increased. Among the HIF1α target genes are several that control blood vessel growth and survival, including vascular endothelial growth factor (VEGF)-A, VEGF-C, angiopoietin-like factor 4 (ANGPTL4), as well as genes related to glucose metabolism (20, 21). However, recent studies of HIF1α functions during inflammation indicated that factors other than hypoxia also induced HIF1α expression (22, 23). Cytokines including IL-1, TNF-α, IL-4, and transforming growth factor-β induced HIF1α in several cell types under normoxic conditions, but the mechanism controlling this induction may differ from that during hypoxia (24–26).
Here, we demonstrated that endothelial gp130 signaling activated vascular inflammatory responses by HIF1α-induced glycolysis, resulting in increased vascular permeability and cytokine storm during sepsis. By administering anti-IL-6R antibodies to several types of CRS models for therapeutic purposes, we found that the short-term inhibition of IL-6R signaling protected the vasculature from injury during CRS progression caused by sepsis and severe burns. Thus, our findings provide a rationale for the clinical testing of a short half-life anti-IL-6R antibody to treat patients with CRS induced by trauma or infection.
Results
IL-6R Trans-Signaling Activates HIF1α Activity to Amplify the Inflammatory Response of Endothelial Cells.
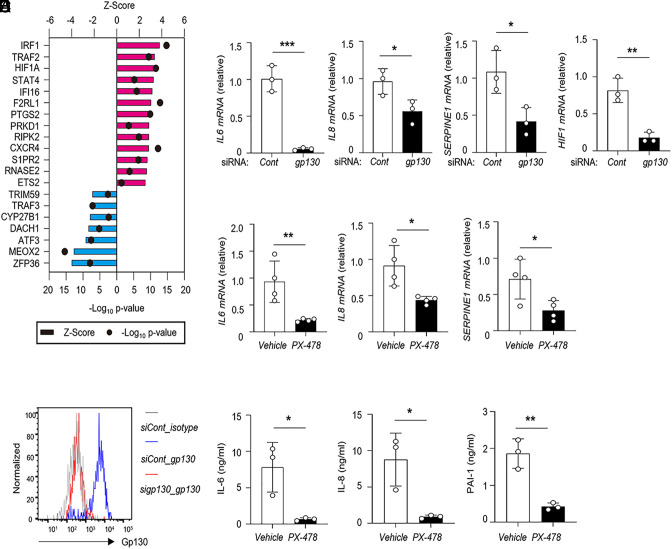
A previous study reported that IL-6R trans-signaling induced the production of IL-6, IL-8, monocyte chemoattractant protein (MCP)-1, and PAI-1 in vascular endothelial cells and that anti-IL-6R antibody treatment inhibited endothelial damage (8). Toll-like receptor-4 (TLR4) signaling is a strong inducer of IL-6 production in the endothelium. During inflammation, increasing serum levels of soluble IL-6R (sIL-6R) synergistically trigger inflammatory responses in endothelial cells via IL-6 trans-signaling. To determine how endothelial gp130 signaling regulates inflammatory responses, we performed RNA sequencing analysis (RNA-seq) to compare gene expressions between nontreated and LPS (lipopolysaccharide) plus sIL-6R-treated human umbilical vein endothelial cells (HUVECs). The RNA-seq datasets were analyzed by Ingenuity Pathway Analysis software, and differentially expressed genes based on RNA-seq data under IL-6R signaling-activated HUVECs revealed that inflammatory response-related pathways were substantially activated in IL-6R-mediated endothelial cells (GSE241139). Next, the set of genes up-regulated by activation induced by LPS plus sIL-6R was subjected to upstream regulator analysis. We found that HIF1α was an upstream regulator of many genes up-regulated in IL-6R-activated HUVECs (Fig. 1A). These analyses suggested HIF1α is a major downstream regulator of the IL-6R pathway during endothelial inflammation. Therefore, we investigated the role of gp130 signaling-mediated HIF1α signaling on the inflammatory response of endothelial cells using HUVECs. We performed the genetic ablation of IL6ST (which encodes gp130) by short interfering RNA (siRNA) in HUVECs (Fig. 1B). IL6ST knockdown substantially reduced the expressions of IL6, IL8, SERPINE1, and HIF1 in the presence of LPS plus sIL-6R, as determined by qRT-PCR (Fig. 1C). Next, we tested whether the activation of HIF1α mediated the inflammatory response in endothelial cells. Treatment with a pharmacological HIF1α inhibitor, PX478, suppressed the expression of proinflammatory cytokines and coagulation factors (Fig. 1D) and reduced the secretion of IL-6, IL-8, and PAI-1 (Fig. 1E) under LPS plus sIL-6R. To delineate the IL-6R-mediated HIF1α signaling, we added PX478 to HUVECs in the presence of IL-6 plus sIL-6R. The inhibition of HIF1α activation suppressed IL-6, IL-8, and PAI-1 production at the mRNA and protein levels (SI Appendix, Fig. S1 A and B). These data suggest that HIF1α activity is important for IL-6R trans-signaling-mediated inflammatory responses in endothelial cells.
Fig. 1.
Endothelial gp130–HIF1α signaling mediates proinflammatory responses and PAI-1 production. (A) Gene expression of HUVECs induced by LPS+sIL-6R stimulation assessed by RNA-seq. Upstream regulator analysis of genes upregulated in HUVECs under LPS+sIL-6R stimulation. All datasets were examined by Ingenuity Pathway Analysis (Qiagen Bioinformatics). (B) Flow cytometry analysis of gp130 expression after siRNA transfection. (C) Cells were treated with si-IL6ST for 48 h following combined stimulation, and the indicated gene expressions were analyzed by quantitative real-time PCR (qRT-PCR). (D and E) Cells were pretreated with PX-478 (80 μM) for 4 h followed by LPS+sIL-6R stimulation for 24 h, and IL-6, IL-8, and PAI-1 expression levels were analyzed by qRT-PCR (D) and ELISA (E). *P < 0.05, **P < 0.01, ***P < 0.005. P-values were determined using unpaired, two-tailed Student’s t tests. Data are representative of three (B, D, and E) independent experimental replicates and are presented as means ± SD.
Gp130–HIF1α Signaling Promotes Endothelial Inflammatory Responses via Glycolysis.
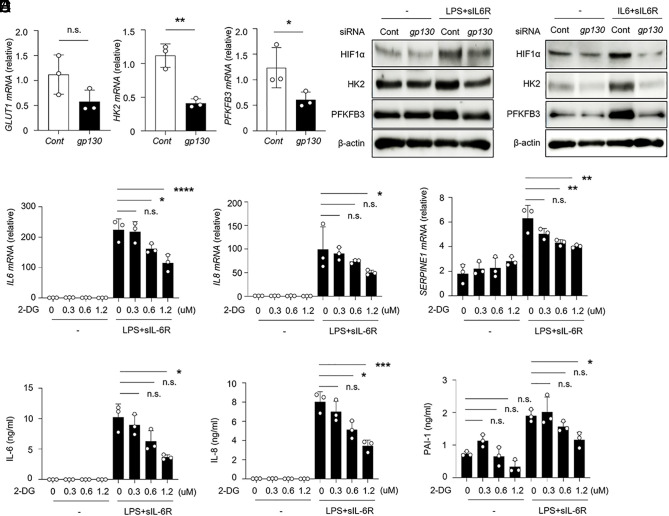
Previous studies demonstrated that HIF1α signaling promoted glycolytic metabolism in endothelial cells, which was linked to their proliferation and angiogenesis (27). Therefore, we examined whether HIF1α activity influenced glycolytic metabolism by IL-6R signaling. The levels of mRNA encoding glycolytic enzymes including hexokinase 2 (HK2) and phosphofructokinase-2/fructose-2,6-bisphosphatase-3 (PFKFB3) but not glucose transporter (GLUT1) were significantly decreased in IL6ST knockdown HUVECs in the presence of LPS plus sIL-6R (Fig. 2 A and B). In agreement with these data, the expression levels of glycolysis pathway-related proteins were increased depending on IL-6R trans-signaling in endothelial cells (Fig. 2C and SI Appendix, Fig. S2A). These data indicate that IL-6R trans-signaling promotes glycolysis via the activation of HIF1α. Next, to address whether gp130–HIF1α axis–induced glycolysis contributed to endothelial inflammatory responses, glycolytic activity was inhibited by 2-deoxy-D-glucose (2-DG), a glucose analog, in HUVECs under conditions of inflammation, and cytokines and PAI-1 levels were analyzed. Treatment with 2-DG abrogated the increase of IL6, IL8, and SERPINE1 at the mRNA and protein levels in a dose-dependent manner (Fig. 2 D and E). Accordingly, we also found consistent results when using IL-6 plus sIL-6R stimulation (SI Appendix, Fig. S2 B and C). Activating TLR4 signaling in endothelial cells augmented inflammatory responses and apoptosis (28). Thus, we tested whether TLR4 signaling contributed to glycolysis-mediated inflammatory responses in endothelial cells. Although LPS-stimulated HUVECs showed increased levels of IL-6, IL-8, and PAI-1 to a lower extent, treatment with 2-DG did not inhibit these inflammatory mediators in HUVECs (SI Appendix, Fig. S3 A and B). Collectively, these findings suggest that an increased level of glycolysis is critical for the activation of the inflammatory response promoted by gp130–HIF1α signaling in endothelial cells.
Fig. 2.
Endothelial gp130–HIF1α signaling promotes inflammatory responses via glycolysis. (A) Relative mRNA levels of the indicated genes were determined by qRT-PCR in HUVECs treated with siRNA against IL6ST under LPS+sIL-6R for 24 h. (B and C) Levels of glycolysis pathway-related proteins, HIF1α, HK2, and PFKFB3 were determined in HUVECs treated with siRNA against IL6ST under LPS+sIL-6R (B) or IL-6+sIL-6R (C) for 24 or 48 h, respectively. (D and E) HUVECs were pretreated with the indicated concentrations of 2-DG for 16 h followed by LPS+sIL-6R stimulation for 24 h, and IL-6, IL-8, and PAI-1 expression levels were analyzed by qRT-PCR (D) and ELISA (E). *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, n.s., not significant. P-values were determined using unpaired, two-tailed Student’s t tests (A) and one-way ANOVA (D and E). Data are representative of three (A, D, and E) independent experimental replicates and are presented as means ± SD.
Inhibition of gp130–HIF1α Signaling Ameliorates Cytokine Storm.
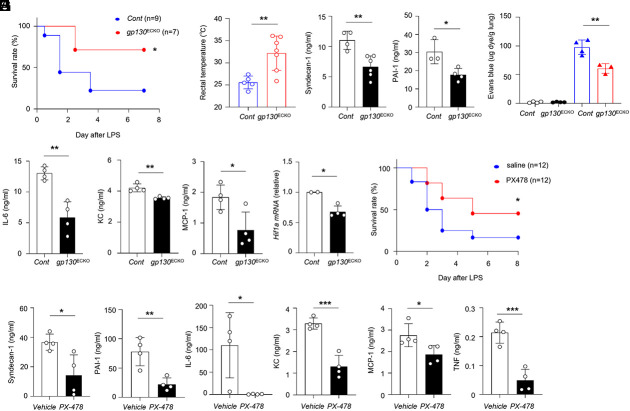
The postnatal deletion of gp130 in mice caused abnormal bone marrow functions (29). To examine the role of gp130 signaling in endothelial cells during CRS in vivo, we generated mice with a selective loss of gp130 in endothelial cells by crossing VE-cadherin (BAC)-CreERT2 transgenic mice and gp130flox/flox mice (referred to as gp130ECKO mice). The treatment of these mice with tamoxifen markedly reduced gp130 expression in the endothelial cells of adult mice. To delineate the involvement of gp130–HIF1α signaling in vascular inflammation, we established a mouse sepsis model using gp130ECKO mice. We investigated the physiological and vascular inflammatory responses by which the deletion of endothelial gp130 expression protected against LPS-induced mortality. After being subjected to a lethal dose of LPS (20 mg/kg), gp130ECKO mice showed a significant improvement in the survival rate compared with the control group (Fig. 3A). Compared with control mice, gp130ECKO mice were protected against LPS-induced hypothermia (Fig. 3B). In addition, we observed that gp130ECKO mice displayed markedly reduced serum PAI-1 levels, an indicator of coagulation cascade activation, and syndecan-1, which indicates glycocalyx degradation (Fig. 3C) as well as lung permeability (Fig. 3D). LPS-treated gp130ECKO mice had decreased levels of circulating proinflammatory cytokines and chemokines including IL-6, MCP-1, and KC, compared with control mice (Fig. 3E). Consistent with previous data, we found a significant reduction in Hif1a expression in primary lung endothelial cells from LPS-treated gp130ECKO mice (Fig. 3F). Thus, endothelial gp130 signaling promotes vascular injury and aggravates inflammatory responses via HIF1α activation. To evaluate the effects of HIF1α activity on systemic inflammatory responses further, we examined whether the administration of PX-478, an HIF1α inhibitor, attenuated LPS-induced endotoxic shock. PX-478 treatment improved the survival rate of LPS-injected mice (Fig. 3G). PX-478 treated mice showed markedly reduced serum levels of PAI-1 and syndecan-1 and serum levels of inflammatory cytokines including IL-6, TNF, KC, and MCP-1 (Fig. 3 H and I). Thus, the inhibition of HIF1α activity attenuated LPS-induced inflammatory responses. Collectively, our data demonstrate that endothelial gp130–HIF1α signaling induces vascular injuries and promotes the progression of cytokine storm.
Fig. 3.
Deficiency of gp130 signaling in endothelium ameliorates septic shock. (A) The survival rate of control (gp130flox/flox, cont) or gp130ECKO mice was monitored after LPS injection (20 mg/kg) (P = 0.0399). (B) Rectal temperatures of cont or gp130ECKO mice 48 h after LPS injection. (C–E) Mice were administrated LPS (2.4 mg/kg). (C) Levels of Syndecan-1 and PAI-1 were measured in the serum of cont and gp130ECKO mice. (D) Quantitative analysis of vascular permeability in the lungs of cont or gp130ECKO mice. (E) Levels of IL-6, KC, and MCP-1 were measured in the serum of cont and gp130ECKO mice. (F) Hif1a mRNA expression was detected by qRT-PCR in lung CD31+ cells from LPS-injected mice. (G and H) Survival rate (P = 0.0487) (G) or serum levels (H and I) from vehicle or PX-478 (10 mg/kg) treated wild-type mice following an intraperitoneal injection of LPS (10 mg/kg) for 24 h. *P < 0.05, **P < 0.01, ***P < 0.005. P-values were determined using unpaired, two-tailed Student’s t tests. Data are representative of three independent experimental replicates and are presented as means ± SD.
A Short Half-Life Anti-IL-6R Antibody with H435A Inhibits IL-6R Signaling and Improves Septic Shock Survival.
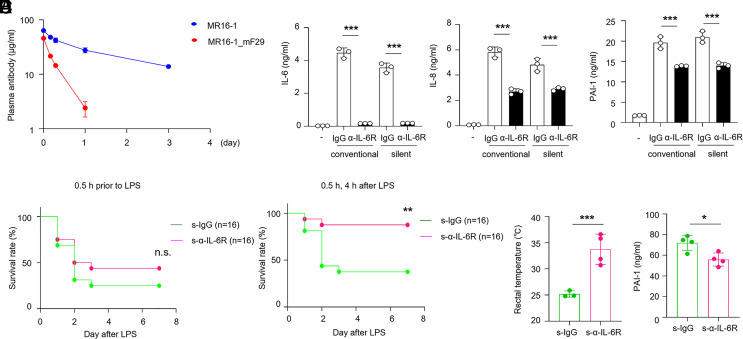
Although the blockade of IL-6R signaling is a beneficial therapy for cytokine storm (9, 30), adverse consequences such as secondary infection occur frequently (17). Thus, inhibition therapy for infectious diseases via IL-6 signaling should be considered carefully. We generated several variants of short half-life anti-human or mouse IL-6R monoclonal antibodies, which had an altered half-life in plasma (31). Mutation of immunoglobulin G (IgG) ablated binding to the neonatal Fc receptor (FcRn) (32) (SI Appendix, Fig. S4 A and B). Compared with human or mouse conventional antibodies (Tocilizumab, MR16-1, respectively), these variants (Tocilizumab_H435A, MR16-1_mF29) or corresponding isotype IgG1 variants did not bind to FcRn (SI Appendix, Fig. S5 A–D). To delineate the half-life of the anti-IL-6R antibody (referred to as silent anti-IL-6R antibody), we performed a pharmacokinetic study in mice. We found that variant MR16-1_mF29 had a rapid monoexponential disposition after a single administration (within 1 d), whereas the conventional antibody (MR16-1) was present in the serum for 3 d (Fig. 4A). Moreover, the variants showed comparable inhibitory activity with conventional antibodies as assessed by the reduction of the phosphorylation of STAT3 and cytokine production in HUVECs upon IL-6R trans-signaling (Fig. 4B and SI Appendix, Fig. S5E).
Fig. 4.
A short half-life anti-IL-6R antibody inhibits vascular inflammatory responses in vitro and attenuates endotoxemia in vivo. (A) Pharmacokinetic profiles of conventional (MR16-1) and silent anti-IL-6R (MR16-1_mF29) antibodies were determined. Blood samples were taken at the indicated time points and the plasma concentration of each agent was measured by ELISA. Values are the mean ± SEM (n = 5). (B) HUVECs were treated in the presence of conventional (isotype_IgG1, Tocilizumab) and silent antibodies (isotype_H435A, Tocilizumab_H435A). Levels of IL-6, IL-8, and PAI-1 in the culture supernatants after 48 h are shown. (C) Survival rates of wild-type mice treated intravenously with silent antibodies (s-isotype, s-α-IL-6R) injected 0.5 h before LPS injection (P = 0.3030). (D–F) Silent antibodies were intravenously administered to mice at 0.5 and 4 h after LPS challenge (13.3 mg/kg). Survival rates were monitored for 1 wk (P = 0.005) (D), and the rectal temperature of mice was measured at 48 h (E). (F) Serum PAI-1 levels were detected in mice after LPS challenge by ELISA. *P < 0.05, **P < 0.01, ***P < 0.005. (B, E, and F). P-values were determined using unpaired, two-tailed Student’s t tests. (B–F) Data are representative of three independent experimental replicates and are presented as means ± SD.
To examine whether silent anti-IL-6R antibody treatment had a beneficial survival effect against sepsis, we examined the survival rate of anti-IL-6R antibody treatment in a mouse endotoxemia model. In a preventive setting (single administration of antibody 1 h before LPS injection, designated as “pre-treatment”), anti-IL-6R antibody treatment did not significantly improve the survival rate (Fig. 4C). We also investigated the survival benefit of the double administration of anti-IL-6R antibody (56 mg/kg; at 0.5 and 4 h after challenge). In the setting of post-treatment, the blockade of IL-6R signaling by silent anti-IL-6R antibody increased the survival rate compared with isotype control treatment (Fig. 4D). Silent anti-IL-6R antibody treatment protected against hypothermia and vascular injury by inhibiting PAI-1 production after LPS challenge (Fig. 4 E and F). Thus, treatment with anti-IL-6R antibody attenuated endotoxin shock by inhibiting vascular injury.
The Short-Term Blockade of IL-6R Signaling Has Protective Effects in a Murine Burn Injury Model by Protecting Endothelial Integrity.
Cases of severe burn injury are at higher risk of developing infectious complications leading to sepsis. Host defense compromised by disruption of the skin barrier leads to increased susceptibility to burn wound infection and the subsequent development of cytokine storm. In addition to the sepsis model, we investigated the role of endothelial HIF1α signaling using a burn injury–induced cytokine storm model. We developed a murine dorsal thermal injury model in which animals received a 30 to 35% total body surface area injury (SI Appendix, Fig. S6A) (33). Intriguingly, PX-478 treatment did not improve the survival rate or serum levels of proinflammatory cytokines, chemokines, and PAI-1, suggesting that unlike the effects of HIF1α in the sepsis model, inhibition of HIF1α has insufficient to mitigate severe burn injury (SI Appendix, Fig. S6 B and C).
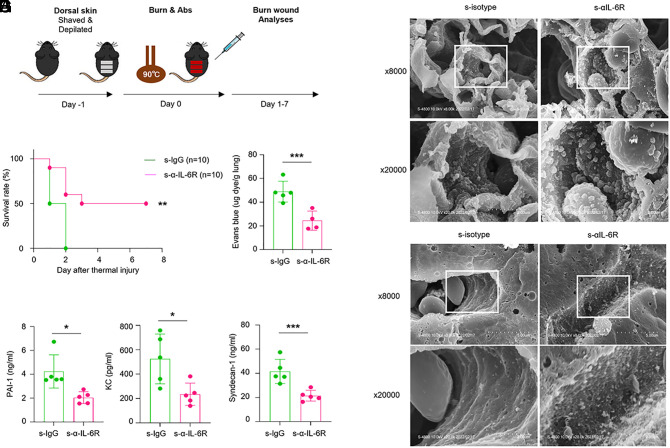
Global inhibition of IL-6 signaling might cause severe adverse effects. Therefore, we examine whether short-term IL-6R inhibition ameliorates severity of burn injury (Fig. 5A). We compared the survival rate of burn injury model groups treated with silent type isotype or anti-IL-6R antibodies. Notably, mice receiving silent anti-IL-6R antibodies had an improved survival rate compared with those receiving control antibodies in the severe burn injury model (Fig. 5B). To characterize how silent anti-IL-6R antibody treatment improved overall survival, we evaluated the levels of cytokines and chemokines in the serum of mice at 12 h after the burn injury. We observed a markedly lower level of KC in the silent anti-IL-6R antibody treatment group compared with the corresponding isotype treatment group (Fig. 5C). Given that cutaneous thermal injury causes a significant disruption of pulmonary endothelial barriers, we also evaluated the effect of silent anti-IL-6R antibodies on vascular injury. Consistent with the effects on sepsis, silent anti-IL-6R antibody treatment resulted in a significant reduction of serum PAI-1 levels after thermal injury (Fig. 5C). Furthermore, Evans blue dye leakage analysis revealed that the silent anti-IL-6R antibody effectively counteracted vascular leakage. Accordingly, treatment with silent anti-IL-6R antibodies profoundly reduced lung vascular injury related to burn injury (Fig. 5D).
Fig. 5.
Short-term inhibition of IL-6R signaling protects mice from thermal injury by preserving the endothelial glycocalyx. (A) Schematic diagram showing the severe burn injury model. (B) The survival rate of wild-type mice that received silent antibodies after burn injury was monitored for 1 wk (P = 0.003). (C) Serum levels of PAI-1 and KC were determined by ELISA 8 h after burn injury. (D) Vascular permeability of the lung 8 h after burn injury was measured by the extravascular leakage of Evans Blue. (E) Serum levels of syndecan-1 were determined by ELISA 6 h after burn injury. (F and G) Glycocalyx on the endothelial cell surface layer of pulmonary (F) and liver capillaries (G) was determined 8 h after burn injury. The original magnifications were ×8,000 and ×20,000. The moss-like structures on the surface of the vascular endothelium are the glycocalyx. *P < 0.05, ***P < 0.005. (C–E) P-values were determined using unpaired, two-tailed Student’s t tests. (B–G) Data are representative of three independent experimental replicates and are presented as means ± SD.
The vascular endothelial glycocalyx comprises of heparan sulfate and proteoglycans such as syndecan-1 and has a critical role as a barrier to prevent the adhesion of inflammatory immune cells onto the endothelial surface (34), which is destroyed during the early phase of inflammation by heparinase and TNF (35). However, little is known about the relationship between glycocalyx homeostasis and burn injury pathogenesis. Therefore, we investigated whether posttreatment with silent IL-6R antibody would preserve the endothelial glycocalyx in several organs of burn injury-induced mice. We quantified glycocalyx degradation by measuring serum syndecan-1 levels in mice 6 h after antibody treatment. We found a significant decrease in the release of syndecan-1 in the silent anti-IL-6R antibody treatment group compared with the isotype control (Fig. 5E). Furthermore, we estimated the endothelial capillary wall thickness in the lung and liver using scanning electron microscopic images with lanthanum nitrate staining. The endothelial glycocalyx area in the pulmonary and liver capillary was significantly preserved in the silent anti-IL-6R antibody treatment group compared with the isotype control group (Fig. 5 F and G). Collectively, these results demonstrated that the short-term inhibition of IL-6R signaling protected against cytokine storm and reduced burn injury-induced abnormal vascular permeability and glycocalyx integrity.
Discussion
The present study demonstrated vascular protection by the short-term inhibition of IL-6R-HIF1α signaling is a promising therapeutic strategy for cytokine storm. We found that the prevention of IL-6R–HIF1α signaling activation had a critical role in ameliorating sepsis and burn, mainly by preserving the endothelial glycocalyx and vascular integrity of vital organs, and inhibiting vascular injury and cytokine storm.
Previous studies showed that the augmentation of HIF1α expression boosted glycolysis in cancer cells by inducing glycolytic enzyme expression through the activation of transcription activity under hypoxic conditions (36, 37). Similarly, enhanced HIF1α expression induced glycolysis metabolism, which is essential for the activation of inflammatory macrophages, under normoxic conditions (38, 39). In the present study, we demonstrated that IL-6R trans-signaling induced the expression of glycolysis-related genes in vascular endothelial cells, suggesting IL-6 signaling-induced metabolic alterations toward glycolysis in endothelial cells might be initiated by transcriptional modification via HIF1α transcriptional activation.
Several studies demonstrated that IL-6 promoted tumor invasion. IL-6 signaling and hypoxia induced the epithelial-mesenchymal transition phenotype via the IL-6/STAT3/HIF1α feedback loop via an unknown mechanism (40, 41). Moreover, it was reported that smooth muscle cells act with IL-6 as an amplification loop to provide a concerted response of the vascular system (42). Accordingly, our study showed that the pharmacological inhibition of HIF1α or glycolysis in endothelial cells under IL-6R stimulation decreased IL-6 expression, suggesting that IL-6/STAT3/HIF1α positive feedback circuits are present in endothelial cells during inflammatory responses. Many studies have investigated the efficacy of HIF1α inhibition for burn injuries, but these have been controversial. In our study, the inhibition of HIF1α activity had beneficial effects in the sepsis model but not the burn injury model. Although HIF1α inhibition alleviated cytokine production, this activity is required for wound healing by promoting angiogenesis (43, 44).
Hypoxemia is a defining feature of ARDS, and a complication of pulmonary or systemic inflammation, which results in tissue hypoxia (45). The current study revealed that HIF1α expression in primary lung endothelial cells was decreased in gp130 ECKO mice during endotoxemia and was accompanied by the substantially reduced expression of inflammatory mediators. These findings indicate that cross-communication between IL-6R signaling and hypoxia may involve biological processes related to endothelial inflammatory responses; however, the link between IL-6R signaling and hypoxia requires further investigation.
The importance of IL-6 in the pathogenesis of sepsis or severe burn injury is underlined by data showing that increasing serum IL-6 levels are associated with disease severity (11, 46); however, IL-6 exerts protective roles as part of the immune defense system (47). In the sepsis model, LPS injection induced robust cytokine production in TLR4-expressing cells, leading to endotoxemia-induced cytokine storm. Our data indicate that endotoxin shock can be limited by the blockade of IL-6R signaling by reducing vascular damage. In a murine polymicrobial sepsis model, global IL-6 activity inhibition did not have beneficial effects, whereas selective IL-6 trans-signaling inhibition improved the survival rate, suggesting the importance of IL-6 activity in bacterial clearance during sepsis (48). Severe burn injury is at risk of invasive burn wound infection leading to sepsis. Furthermore, high levels of IL-6 strongly correlated with severity in burn patients (11). Short-term inhibition of IL-6R signaling improved the survival rate and reduced cytokine storm symptoms in the severe burn injury model. Regarding the prevalence of infection risk, this warrants further studies and supports the potential clinical evaluation of short half-life anti-IL-6R antibodies for the treatment of severe burns. Further, studies to test the efficacy of selective IL-6 trans-signaling in a severe burn model should be initiated.
Growing evidence has demonstrated the therapeutic efficacy of IL-6R signaling inhibition in CRS such as CAR-T cell therapy-induced cytokine storm and severe COVID-19 by preventing proinflammatory cytokine production and the inhibition of macrophage activation (49, 50). However, the long-term inhibition of IL-6R signaling has several functional limitations, which include off-target effects on the innate immune system (17). Thus, it might be difficult to use anti-IL-6R antibodies for treatment while avoiding the induction of adverse effects. Our study indicates that only the short-term inhibition of IL-6R signaling showed improved mortality rates and preserved the vasculature after burn injury, indicating a complex process during cytokine storm. Thus, we speculate that a therapeutic agent that can prevent the long-lasting inhibition of IL-6R signaling can provide substantial benefit to a broad spectrum of human CRS.
In summary, we demonstrated that the short-term inhibition of IL-6R signaling was highly effective at strengthening endothelial integrity and preserving the endothelial glycocalyx, blunting vascular leakage, and alleviating inflammation in response to sepsis- and trauma-induced cytokine storm. The approach described here may yield a unique therapeutic strategy against sepsis or severe trauma.
Materials and Methods
Detailed information on the materials, methods, and associated references can be found in SI Appendix, Supporting Materials and Methods.
Agents.
Recombinant human IL-6 (R&D Systems, Minneapolis, MN, USA), sIL-6R (IL-6R; R&D Systems), Ultrapure LPS (InvivoGen, Osaka, Japan), PX-478 (MCE), and 2-DG (Sigma-Aldrich) were used during HUVEC cultivation.
Cell Culture and Transfection.
HUVECs (Lonza) were maintained in EGM-2 medium supplemented with 2% FBS (Lonza) and cultured in humidified incubators at 37 °C and 5% CO2. HUVECs (30,000 cells/well) were pretreated with PX-478 or 2-DG at different concentrations for 4 or 16 h, respectively in RPMI medium (Nacalai Tesque) containing 10% FBS, and then stimulated with LPS (100 ng/mL) or IL-6 (50 ng/mL) with sIL-6R (100 ng/mL). For IL6ST knockdown experiments, HUVECs (30,000 cells per well) were added to 12-well plates, and 12 h later, cells were transfected with 40 nM of human IL6ST siRNA (Ambion, Thermo Scientific, MA, USA) or non-targeting control (Thermo Scientific) using Lipofectamine RNAiMACS (Life Technology/Invitrogen), according to the manufacturer’s instructions.
RNA Isolation and Quantitative RT-PCR.
Total RNA was isolated from HUVECs using an RNeasy Mini Kit (Qiagen K.K., Tokyo, Japan). cDNA was synthesized using a PrimeScript™ RT reagent kit (Takara Bio). A Quant Studio three Real-Time PCR instrument (Applied Biosystems, Foster City, CA, USA) was used for quantitative PCR with 2×PCR Master Mix (Thermo Scientific) and TaqMan primers specific for human IL6 (Hs00174131_m1), IL8 (Hs00174103_m1), SERPINE1 (Hs00167155_m1), PFKFB3 (Hs00998698_m1), and mouse Hif1a (Mm00468869_m1), and for quantitative PCR with SYBR Green PCR Master Mix (Thermo Scientific) and primers specific for human GLUT1, HK2, and HIF1 (Takara Bio) were used according to the manufacturer’s instructions. Quantitative PCR was performed at 95 °C for 15 s followed by 60 °C for 1 min for 40 cycles. mRNA levels of each gene were normalized to the level of β-actin mRNA using the ΔΔ Ct method.
Measurement of Cytokines, PAI-1, and Syndecan-1.
Human IL-6 and IL-8 levels in the supernatant of HUVECs cultures, and mouse IL-6, IL-8, MCP-1, IL-10, and TNF-α levels in sera from mice or appropriately diluted supernatants or sera were measured using a cytometric bead array kit (BD Biosciences, Franklin Lakes, NJ, USA) using a Lyrics flow cytometer (BD Biosciences). The serum or supernatant levels of syndecan-1 and mouse or human PAI-1 were measured using a quantitative ELISA kit (Abcam and R&D Systems, respectively).
Statistics.
Values are presented as the mean ± SD. Statistical differences between means were determined by an unpaired two-tailed Student’s t test or one-way ANOVA followed by Sidak’s multiple comparisons test, as appropriate. Kaplan–Meier survival plots and log-rank tests were used to compare the survival results between treatment groups.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank T. Tanaka for advice in preparing the manuscript and N. Matsuda for secretarial assistance. This work was supported by research grants from the Japan Society for the Promotion of Science KAKENHI grant JP22H02891 to S.K., the Naito Foundation, the Uehara Memorial Foundation, the Takeda Science Foundation, the Mochida Memorial Foundation for Medical and Pharmaceutical Research to S.K., and the Kishimoto Foundation. This work was conducted as part of “The Nippon Foundation—Osaka University Project for Infectious Disease Prevention.” We thank J. Ludovic Croxford, PhD, from Edanz for editing a draft of this manuscript.
Author contributions
S.K. and T.K. designed research; S.K., S.O., Z.L., H.I., Y.Z., H.C., H.Z., T.W., and H.M. performed research; S.K., D.O., and H.T. contributed new reagents/analytic tools; S.K., Z.L., and D.O. analyzed data; J.O. contributed to the conceptualization of this study; and S.K. and T.K. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: Y.C., Karolinska Institutet; C.H.J., University of Pennsylvania; and S.R.-J., Christian Albrechts Universität.
Contributor Information
Sujin Kang, Email: kang@ifrec.osaka-u.ac.jp.
Tadamitsu Kishimoto, Email: kishimoto@ifrec.osaka-u.ac.jp.
Data, Materials, and Software Availability
The raw data for RNA have been deposited in the NCBI Gene Expression Omnibus database (GSE241139) (51). All other data are included in the manuscript and/or SI Appendix.
Supporting Information
References
- 1.Lord J. M., et al. , The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 384, 1455–1465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fajgenbaum D. C., June C. H., Cytokine storm. N. Engl. J. Med. 383, 2255–2273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angus D. C., et al. , Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Joffre J., Hellman J., Ince C., Ait-Oufella H., Endothelial responses in sepsis. Am. J. Respir. Crit. Care Med. 202, 361–370 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg N. M., Steinberg B. E., Slutsky A. S., Lee W. L., Broken barriers: A new take on sepsis pathogenesis. Sci. Transl. Med. 3, 88ps25 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Kang S., Kishimoto T., Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 53, 1116–1123 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., et al. , Coagulation disorders after chimeric antigen receptor T cell therapy: Analysis of 100 patients with relapsed and refractory hematologic malignancies. Biol. Blood Marrow Transplant. 26, 865–875 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Kang S., et al. , IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. U.S.A. 117, 22351–22356 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishimoto T., Kang S., IL-6 revisited: From rheumatoid arthritis to CAR T cell therapy and COVID-19. Annu. Rev. Immunol. 40, 323–348 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto H., et al. , The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 8, 13995 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuura H., et al. , Clinical importance of a cytokine network in major burns. Shock 51, 185–193 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Moore J. B., June C. H., Cytokine release syndrome in severe COVID-19. Science 368, 473–474 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Remick D. G., Bolgos G., Copeland S., Siddiqui J., Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect. Immun. 73, 2751–2757 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedemann N. C., et al. , Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J. Immunol. 170, 503–507 (2003). [DOI] [PubMed] [Google Scholar]
- 15.van der Poll T., et al. , Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J. Exp. Med. 179, 1253–1259 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing Z., et al. , IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest. 101, 311–320 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimmig L. M., et al. , IL-6 Inhibition in critically Ill COVID-19 patients is associated with increased secondary infections. Front. Med. (Lausanne) 7, 583897 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopf M., et al. , Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Lang V. R., et al. , Risk of infections in rheumatoid arthritis patients treated with tocilizumab. Rheumatology (Oxford) 51, 852–857 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Nilsson I., Shibuya M., Wennstrom S., Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp. Cell Res. 299, 476–485 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Semenza G. L., Hypoxia-inducible factor 1: Control of oxygen homeostasis in health and disease. Pediatr. Res. 49, 614–617 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Haddad J. J., Harb H. L., Cytokines and the regulation of hypoxia-inducible factor (HIF)-1alpha. Int. Immunopharmacol. 5, 461–483 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Hellwig-Burgel T., Stiehl D. P., Wagner A. E., Metzen E., Jelkmann W., Review: Hypoxia-inducible factor-1 (HIF-1): A novel transcription factor in immune reactions. J. Interferon Cytokine Res. 25, 297–310 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Scharte M., Han X., Bertges D. J., Fink M. P., Delude R. L., Cytokines induce HIF-1 DNA binding and the expression of HIF-1-dependent genes in cultured rat enterocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G373–384 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Scharte M., et al. , IL-4 enhances hypoxia induced HIF-1alpha protein levels in human transformed intestinal cells. FEBS Lett. 580, 6399–6404 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Stiehl D. P., Jelkmann W., Wenger R. H., Hellwig-Burgel T., Normoxic induction of the hypoxia-inducible factor 1alpha by insulin and interleukin-1beta involves the phosphatidylinositol 3-kinase pathway. FEBS Lett. 512, 157–162 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Cao Y., Langer R., Ferrara N., Targeting angiogenesis in oncology, ophthalmology and beyond. Nat. Rev. Drug Discov. 22, 476–495 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Wang W., et al. , TLR4 activation induces nontolerant inflammatory response in endothelial cells. Inflammation 34, 509–518 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Yao L., Yokota T., Xia L., Kincade P. W., McEver R. P., Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood 106, 4093–4101 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang S., Tanaka T., Narazaki M., Kishimoto T., Targeting interleukin-6 signaling in clinic. Immunity 50, 1007–1023 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Igawa T., et al. , Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat. Biotechnol. 28, 1203–1207 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Roopenian D. C., Akilesh S., FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Fujimi S., et al. , Murine dendritic cell antigen-presenting cell function is not altered by burn injury. J. Leukoc. Biol. 85, 862–870 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinbaum S., Tarbell J. M., Damiano E. R., The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 9, 121–167 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Schmidt E. P., et al. , The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 18, 1217–1223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathupala S. P., Rempel A., Pedersen P. L., Glucose catabolism in cancer cells: Identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J. Biol. Chem. 276, 43407–43412 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Semenza G. L., Roth P. H., Fang H. M., Wang G. L., Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23763 (1994). [PubMed] [Google Scholar]
- 38.Kelly B., O’Neill L. A., Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Neill L. A., Pearce E. J., Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213, 15–23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gyamfi J., Lee Y. H., Eom M., Choi J., Interleukin-6/STAT3 signalling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells. Sci. Rep. 8, 8859 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang T., et al. , Interleukin-6 and hypoxia synergistically promote EMT-mediated invasion in epithelial ovarian cancer via the IL-6/STAT3/HIF-1alpha feedback loop. Anal. Cell Pathol. (Amst.) 2023, 8334881 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klouche M., Bhakdi S., Hemmes M., Rose-John S., Novel path to activation of vascular smooth muscle cells: Up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J. Immunol. 163, 4583–4589 (1999). [PubMed] [Google Scholar]
- 43.Liu Z., Liu L., Cheng X., Gao L., Expression and predictive value of HIF-1alpha and VEGF in patients with burns following treatment. Exp. Ther. Med. 20, 141 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X., et al. , Impaired angiogenesis and mobilization of circulating angiogenic cells in HIF-1alpha heterozygous-null mice after burn wounding. Wound Repair Regen. 18, 193–201 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirchandani A. S., et al. , Hypoxia shapes the immune landscape in lung injury and promotes the persistence of inflammation. Nat. Immunol. 23, 927–939 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giannoudis P. V., et al. , Correlation between IL-6 levels and the systemic inflammatory response score: Can an IL-6 cutoff predict a SIRS state? J. Trauma 65, 646–652 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Barton B. E., Jackson J. V., Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect. Immun. 61, 1496–1499 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barkhausen T., et al. , Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit. Care Med. 39, 1407–1413 (2011). [DOI] [PubMed] [Google Scholar]
- 49.June C. H., Sadelain M., Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salama C., Mohan S. V., Tocilizumab in patients hospitalized with Covid-19 pneumonia. Reply. N. Engl. J. Med. 384, 1473–1474 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Kang S., et al. , Inhibition of IL-6 signaling attenuates vascular damage caused by HIF1α activation. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE241139. Deposited 18 August 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
The raw data for RNA have been deposited in the NCBI Gene Expression Omnibus database (GSE241139) (51). All other data are included in the manuscript and/or SI Appendix.