Abstract
Background
Infections during pregnancy confers increased risk of maternal and perinatal morbidity and mortality. However, the case for advocating Haemophilus influenzae type B (Hib) and viral Influenza vaccinations in pregnancy is still debatable.
Objectives
To assess the impact of Hib and viral Influenza vaccinations during pregnancy on maternal, neonatal and infant health outcomes compared to placebo/control.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (29 January 2015) and reference lists of retrieved studies.
Selection criteria
All randomised controlled clinical trials (including cluster‐randomised trials) and quasi‐randomised trials evaluating Hib or viral influenza vaccination during pregnancy compared with no vaccination or placebo.
Data collection and analysis
Two review authors independently assessed trials for inclusion, risk of bias and extracted data. Data were checked for accuracy.
Main results
Two trials were included this review. One (involving 213 women and 213 neonates) evaluated the impact of Hib vaccination during pregnancy and the other study (involving 2116 women and 2049 neonates) evaluated the impact of viral influenza vaccination during pregnancy. Overall, the HiB vaccination trial was judged to be at 'high risk of bias' due to inadequate randomisation while the other trial was judged to be at 'low risk of bias'.
Hib vaccination during pregnancy versus placebo
One trial involving 213 women and 213 neonates evaluating the impact of Hib vaccination during pregnancy was included under this comparison. The study did not report on any of this review's prespecified primary outcomes (including mortality, respiratory tract infection and sepsis) or secondary outcomes (including adverse events) except preterm delivery. There was no clear difference between the Hib vaccination and placebo control groups in terms of preterm delivery (risk ratio (RR) 1.28, 95% confidence interval (CI) 0.12 to 13.86, one study, 213 participants), fetal distress (RR 1.23, 95% CI 0.67 to 2.26, one study, 213 infants), intubation (RR 1.03, 95% CI 0.55 to 1.95, one study, 213 infants) and neonatal jaundice (RR 1.01, 95% CI 0.52 to 1.97, one study, 213 infants). We could not grade the evidence for quality due to lack of outcome data.
Viral influenza vaccination during pregnancy versus placebo
One trial involving 2116 women and 2049 infants evaluating the impact of trivalent inactivated influenza vaccine (IIV3) during pregnancy was included under this comparison.
There was no clear difference between the viral influenza and placebo control group in terms of most of this review's primary outcomes: maternal death (RR 4.96, 95% CI 0.24 to 103.24, moderate quality evidence), infant death up to 175 days after birth (RR 0.71, 95% CI 0.37 to 1.37, moderate quality evidence), perinatal death (stillbirth and death in the first week of life) (RR 1.32, 95% CI 0.73 to 2.38, moderate quality evidence), influenza‐like illness in women (RR 0.96, 95% CI 0.79 to 1.16) or their babies (RR 1.02, 95% CI 0.94 to 1.09), any respiratory illness in women (RR 0.97, 95% CI 0.91 to 1.04, high quality evidence) or their babies (RR 1.01, 95% CI 0.95 to 1.07, high quality evidence). There were also no clear differences between vaccination and placebo control groups in terms of maternal hospitalisation for any infection (RR 2.27, 95% CI 0.94 to 5.49; 2116 women, moderate quality evidence), and neonatal hospitalisation for sepsis (RR 1.60, 95% CI 0.73 to 3.50; 2049 infants, moderate quality evidence). However, viral influenza vaccination during pregnancy was associated with a reduction in reverse‐transcriptase–polymerase‐chain‐reaction (RT‐PCR) confirmed influenza among infants (RR 0.51, 95% CI 0.30 to 0.88, one study, 2049 infants) and women (RR 0.50, 95% CI 0.29 to 0.86, one study, 2116 women).
In terms of this review's secondary outcomes, there were no clear differences in terms of the impact on pregnancy outcomes (miscarriage, preterm labour and stillbirth), hospitalisation for respiratory infection among women and infants. Similarly, there was no difference between the viral influenza vaccine and placebo control groups in terms of any adverse systemic reactions.
Authors' conclusions
There is limited evidence (from one small trial at a high risk of bias) on the effectiveness on Hib during pregnancy for improving maternal, neonatal and infant health outcomes.
Evidence from one large high quality trial on the effectiveness of viral influenza vaccine during pregnancy suggests reduced RT‐PCR confirmed influenza among women and their babies, suggesting the potential of this strategy for scale up but further evidence from varying contexts is required.
Further trials for both Hib and viral influenza vaccines with appropriate study designs and suitable comparison groups are required. There are currently two 'ongoing' studies ‐ these will be incorporated into the review in future updates.
Plain language summary
Haemophilus influenzae type B and viral influenza vaccinations during pregnancy for improving maternal, neonatal and infant health outcomes
Maternal immunisation with Haemophilus influenzae type B (Hib) and viral influenza vaccines may reduce the risk of infections in mothers and infants, however, this is an area of controversy. Both infections can cause severe pneumonia and deaths among children under five years of age, particularly in developing countries. Rates of influenza‐associated complications and consequent hospitalisations are substantially higher among pregnant women, infants and newborns. Pregnant women who are vaccinated against influenza have protective levels of anti‐influenza antibodies, which can be passively transferred to the infant to improve their health outcomes. Infants of immune mothers usually have influenza symptoms that are delayed in onset and of shorter duration. This review investigated whether vaccinating pregnant women with Hib and viral influenza vaccinations during pregnancy could reduce the risk of infection among mothers and babies and improve health outcomes for both.
Two trials were included this review. One trial (considered to be at a high risk of bias) evaluated the impact of Hib vaccination during pregnancy and the other trial (judged to be at a low risk of bias) evaluated the impact of viral influenza vaccination during pregnancy.
In one small study (involving 213 women, mainly Hispanic and with low income, and 213 neonates, conducted in the US), women were given either Hib vaccination or a placebo control at between 34 to 36 weeks gestation. This trial did not report on any of this review's primary outcomes, including: mortality, respiratory tract infection or sepsis among the women or their babies. Nor did the study report on any of this review's other secondary outcomes apart from preterm birth and there were no clear differences between the vaccination and placebo groups.
In one large trial (involving 2116 women and 2049 infants, conducted in Soweto, South Africa) pregnant women received either inactivated viral influenza vaccination or a placebo control. Viral influenza vaccination was associated with a reduction in confirmed influenza among women and their babies. However, there was no clear difference between groups in terms of pregnancy outcomes (miscarriage, preterm labour and stillbirth), influenza‐like illness in women or their babies (high quality evidence), any respiratory illness, hospitalisation for respiratory infections and deaths among women (moderate quality evidence) and their babies (moderate quality evidence), neonatal hospitalisation for sepsis (moderate quality evidence), or maternal hospitalisation for any infection (moderate quality evidence). Similarly, there was no clear difference in any adverse systemic reactions between the vaccine and placebo groups. Evidence from one large high quality trial on the effectiveness of viral influenza vaccine during pregnancy suggests reduced reverse‐transcriptase–polymerase‐chain‐reaction (RT‐PCR) ) confirmed influenza among women and their babies, suggesting the potential of this strategy for scale up but further evidence from varying contexts is required.
Further trials for both Hib and viral influenza vaccines with appropriate study designs and suitable comparison groups are required.
There are currently two ongoing studies ‐ these will be incorporated into this review in future updates.
Summary of findings
Summary of findings for the main comparison. Viral influenza vaccine compared with control in pregnancy for improving maternal, neonatal and infant health outcomes.
| Viral influenza vaccine compared with control in pregnancy for improving maternal, neonatal and infant health outcomes | ||||||
| Patient or population: Pregnant women aged 18 to 38 years and an estimated gestation of 20 to 36 weeks. Settings: South Africca. (All data from a single trial, Madhi 2014). Intervention: Viral influenza vaccine Comparison: Placebo vaccine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo vaccine | Viral influenza vaccine | |||||
| Maternal death | Study population | RR 4.96 (0.24 to 103.24) | 2116 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Infant death (up to 175 days) | Study population | RR 0.71 (0.37 to 1.37) | 2049 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | ||
| 21 per 1000 | 15 per 1000 (8 to 28) | |||||
| Perinatal death (stillbirth + death in first week of life) | Study population | RR 1.32 (0.73 to 2.38) | 2083 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | ||
| 18 per 1000 | 24 per 1000 (13 to 43) | |||||
| Any respiratory illness in women | Study population | RR 0.97 (0.91 to 1.04) | 2116 (1 RCT) | ⊕⊕⊕⊕ HIGH | ||
| 652 per 1000 | 632 per 1000 (593 to 678) | |||||
| Any respiratory illness in infants | Study population | RR 1.01 (0.95 to 1.07) | 2049 (1 RCT) | ⊕⊕⊕⊕ HIGH | ||
| 681 per 1000 | 688 per 1000 (647 to 729) | |||||
| Maternal hospitalisation for any infection | Study population | RR 2.27 (0.94 to 5.49) | 2116 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 7 per 1000 | 15 per 1000 (6 to 36) | |||||
| Neonatal hospitalisation due to sepsis within 28 days of birth | Study population | RR 1.60 (0.73 to 3.50) | 2049 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 10 per 1000 | 16 per 1000 (7 to 34) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Wide confidence interval crossing the line of no effect (‐1). We have not downgraded for few events or small sample size.
2Wide confidence interval crossing the line of no effect (‐1).
Background
Description of the condition
Infections such as tetanus have been controlled through maternal immunisation strategies thus reducing morbidity and mortality among women and children. However, the case for advocating Haemophilus influenzae type B (Hib) and viral Influenza vaccinations in pregnancy is still debatable.
Hib bacterium is responsible for around 600,000 episodes of severe pneumonia and an estimated 200,000 deaths among children under five years of age, chiefly through meningitis and pneumonia (Walker 2013; WHO 2005). Developed countries, with a vaccine coverage for Hib of around 92%, have virtually eliminated the disease (WHO 2005) however, the developing countries still bear the burden. The estimated incidence of clinical pneumonia in children under five years of age in developing countries is 0.28 episodes per child‐year compared to 0.05 episodes per child‐year in the developed countries (Rudan 2004; Rudan 2008)
Viral Influenza causes seasonal/annual epidemics with varying degrees of severity and mortality risks globally. It is responsible for around 1 million severe episodes of pneumonia and an estimated 140,000 deaths among children under five years of age (Walker 2013). The influenza viruses are classified into types A, B, and C on the basis of their core proteins. Influenza A viruses can be further broken down into different strains. Current subtypes of influenza A viruses found in people are influenza A (H1N1) and influenza A (H3N2) viruses. The virus causes morbidity in all age groups; however, rates of influenza‐associated complications and consequent hospitalisations are substantially higher among pregnant women (Hartert 2003; Lindsay 2006; Neuzil 1998), and infants and neonates (Glezen 1997; Izurieta 2000; Munoz 2003). Among pregnant women, influenza is found to be associated with serious complications, frequent hospitalisations and its associations with fetal malformations have also been explored (Acs 2005; Gilbert‐Barness 2010; Shi 2005). In neonates and infants, influenza infections predispose them to complications such as pneumonia and otitis media.
Description of the intervention
Influenza vaccines and antiviral therapy are not licensed in the United States for use in infants under the age of six months and one year respectively (Bridges 2013). However, evidence exists that pregnant women vaccinated against influenza have protective levels of anti‐influenza antibodies (Munoz 2005; Sumaya 1979). Higher levels of these antibodies have been reported in umbilical cord as compared to the serum levels of vaccinated women (Englund 1993). Existing evidence for passive transfer of antibodies and its effectiveness in improving child health outcomes have been controversial. A retrospective, clinic‐based study documented a non‐significant trend toward fewer respiratory infections among vaccinated pregnant women compared with unvaccinated pregnant women (Munoz 2005). However, another retrospective study that used clinical records data did not indicate a reduction in infections among vaccinated pregnant women or their infants (Black 2004). In another study, facility visits for respiratory illness among the infants were not substantially reduced (France 2006). A randomised controlled trial conducted in Bangladesh reported a significant reduction in respiratory illness among vaccinated pregnant women and their infants (Zaman 2008).
How the intervention might work
Young infants and pregnant women are at increased risk of serious consequences of influenza infection. Maternal immunisation is a strategy with substantial benefits for both mothers and infants. Infants of immune mothers usually have influenza symptoms that are delayed in onset, and of shorter mean duration compared with infants of non‐immune mothers (Reuman 1987). This is primarily due to the transfer of passive immunity through the passage of antibodies from the placenta to the fetus, providing protection to the infant (Sumaya 1979). Inactivated influenza vaccine is known to reduce proven influenza illness by 63% in infants up to six months of age and avert approximately a third of all febrile respiratory illnesses in mothers and young infants (Zaman 2008). Maternal vaccination thus works by decreasing the onset and severity of influenza in both mothers and their infants (Reuman 1987).
Why it is important to do this review
The role of vaccination for Hib and viral influenza during pregnancy is debatable. This subject is an area of controversial evidence and varied interpretations. All the evidence consists of non‐systematic findings reported from single studies leading to different interpretations. Due to the diversity of evidence and the lack of evidence‐based conclusions, there is a need to undertake a systematic review to derive substantial conclusions regarding the impact of maternal vaccination on maternal, neonatal and infant health outcomes.
Objectives
To assess the impact of Hib vaccinations during pregnancy on maternal, neonatal and infant health outcomes.
To assess the impact of Influenza vaccinations during pregnancy on maternal, neonatal and infant health outcomes.
Our review did not include maternal immunisation for pneumococcal disease as it is covered in a separate Cochrane review (Chaithongwongwatthana 2012).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials (including cluster‐randomised trials) and quasi‐randomised trials of Hib or viral influenza vaccinations among pregnant women versus no vaccines or placebo were eligible for inclusion. Studies presented in abstract form were also eligible for inclusion in the review if they provided sufficient information.
Types of participants
Healthy pregnant women with single or multiple gestation pregnancies.
Types of interventions
Hib vaccination (compared with no vaccination or placebo) during pregnancy.
Influenza virus vaccination (compared with no vaccination or placebo) during pregnancy.
Types of outcome measures
Primary outcomes
Mortality (perinatal, neonatal, infant, maternal).
Any infection of the respiratory tract (upper or lower tract) among women, neonates and infants.
Sepsis or other reported infections among women, neonates and infants.
Secondary outcomes
Frequency of hospitalisations of mother in both inpatient and emergency departments due to respiratory illnesses.
Frequency of outpatient facility visits by mother due to respiratory illnesses.
Frequency of hospitalisations of infant in both inpatient and emergency departments due to respiratory illnesses.
Frequency of outpatient facility visits by infant due to respiratory illnesses.
Pregnancy outcomes (stillbirth, low birthweight, abortion, preterm birth).
Adverse events*
Neonatal morbidities including fetal distress, neonatal intubation and jaundice*
*Outcomes not prespecified in the published protocol, see Differences between protocol and review.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (29 January 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors, Rehana A Salam (RAS) and Jai K Das (JKD), independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion and, if required, we consulted a third review author, Zulfiqar Bhutta (ZAB). Trials were scrutinised according to year, location of trial, and interventions to ensure that multiple publications from the same trial were included only once.
Data extraction and management
We designed a standardised form to extract data. For eligible studies, data extraction was carried out independently by two review authors (RAS, CDS) using the agreed form. Discrepancies were resolved through discussion or, if required, in consultation with a third review author (ZAB). Data were entered by review authors (RAS, JKD, ZSL) into Review Manager software (RevMan 2014) and checked for accuracy. When information regarding any of the outcomes was unclear, or there were missing or incomplete data, we attempted to contact authors of the original reports to clarify the issues.
Assessment of risk of bias in included studies
Two review authors (RAS, CDS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third review author (ZAB).
We were not blinded to trial authors, publication status or other study characteristics. Each domain was assigned a low or high risk of bias, using the definitions provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When there was insufficient information about the process the domain was assigned an unclear risk of bias.
If disagreements arose regarding extracted data, they were resolved through discussion. If no consensus was reached, then we contacted the trial authors in order to clarify the issue. In the event of missing or incomplete data, we contacted one of the trial’s authors to ask for the missing data. All relevant information was reported in the ’Risk of bias’ tables.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook(Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses.
The quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison.
Mortality (perinatal, neonatal, infant, maternal).
Any respiratory tract infection (upper or lower tract, among women, neonates and infants).
Sepsis or other reported infections among women, neonates and infants.
GRADEprofiler (GRADE 2014) was used to import data from Review Manager (RevMan 2014) in order to create a 'Summary of findings' table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Count data
In future updates, count data will be analysed using methods for dichotomous data. To consider the count data outcome as a dichotomous outcome, we will determine the number of participants in each intervention group, and the number of participants in each intervention group who experience at least one event.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trial for inclusion but in future updates, if we identify any cluster‐randomised trails for inclusion, we will adjust their standard errors using the methods described in the Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we will synthesise the relevant information and consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were not included since we did not expect many trials with cross‐over design and the possibility of important differences from the parallel designs.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). Data were not combined in meta‐analysis in this review. In future updates, we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
In future updates of this review, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses for the primary outcomes (where possible).
Age group among females (adolescent females versus older females).
Age group among children (neonates, infants and one to five year old children).
Type of intervention (haemophilus only, Influenza only, or both).
Geographical setting to rule out differences in outcomes among populations (lower‐ to middle‐income countries versus high‐income countries).
Trial quality (randomised controlled trials versus quasi‐randomised trials).
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
Planned sensitivity analysis were not necessary due to insufficient data. In future updates we will carry out planned sensitivity analysis, as necessary. Sensitivity analysis will be based on the randomisation process, with quasi‐randomised trials being excluded. Moreover, sensitivity analyses will be performed based on excluding cluster‐randomised controlled trials or where different ICCs were are used. We will also perform sensitivity analyses to assess the presence of adequate sequence generation and allocation concealment in the primary outcomes.
Results
Description of studies
Results of the search
As outlined in the scope of our protocol (Salam 2012), our analysis was limited to comparing the effectiveness of Hib and influenza vaccination versus no treatment or placebo.
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved 32 reports (relating to 25 trials). See Figure 1. We included two trials (three reports) (Glezen 1992; Madhi 2014) and two studies are ongoing (Garcia 2012; Tapia 2012). We excluded 21 studies (27 reports) (Englund 1995; Henkle 2010; Holmlund 2011; Jackson 2011; Madhi 2011a; Moniz 2010; Mulholland 1996; Munoz 2001; NIAID 2009a; NIAID 2009b; NIAID 2009c; NIAID 2010; Omer 2013; Quiambao 2003; Quiambao 2007; Schlaudecker 2013; Stockwell 2014; Tarrant 2013; Wong 2014; Wootten 2010; Zaman 2008).
1.

Study flow diagram.
Included studies
There are two included studies. One quasi‐randomised trial (involving 213 pregnant women and 213 neonates) evaluated the impact of Hib vaccine during pregnancy (Glezen 1992) and one randomised controlled trial (involving 2116 women and 2049 infants) evaluated the impact of viral influenza vaccine during pregnancy (Madhi 2014).
Glezen 1992 was conducted in Texas with pregnant women vaccinated between 34 to 36 weeks' gestation. The pregnant women (who were mostly Hispanic and of low‐income) received capsular polysaccharide (PRP) vaccine or placebo/saline. The trial excluded pregnant women who were expected to have a complicated pregnancy or delivery. Glezen 1992, did not report on any of the prespecified primary or secondary outcomes of this review, apart from preterm delivery (secondary outcome). Consequently, the outcomes reported by Glezen 1992 are reported in this review as additional secondary outcomes that were not prespecified in our published protocol (Salam 2012).
Madhi 2014, is comprised of two trials conducted in Soweto, South Africa among cohorts of pregnant women with and without HIV. The enrolment of HIV‐uninfected pregnant women was initiated at four antenatal clinics before the onset of the 2011 influenza season (March 3 through August 4) and the 2012 season (March 6 through July 2). Pregnant women with an age of 18 to 38 years and an estimated gestation of 20 to 36 weeks were enrolled. Pregnant women received trivalent inactivated influenza vaccine (IIV3) or placebo/saline. Participants were followed through to 24 weeks postpartum.
Please refer to the Characteristics of included studies table for more details.
Excluded studies
A total of 21 studies were excluded from the review as they did not satisfy the inclusion criteria: Henkle 2010 and Quiambao 2003 were not trials; Holmlund 2011 vaccinated infants with pneumococcal and Hib vaccines; Jackson 2011; NIAID 2009a; NIAID 2009c compared vaccinations with two different doses of influenza vaccine; Quiambao 2007 examined pneumococcal vaccines; Omer 2013 evaluated the combined effect of vaccinating women with influenza vaccine and infants with pneumococcal conjugate vaccine; Madhi 2011a evaluated trivalent influenza vaccine among HIV infected women; Moniz 2010; Stockwell 2014; Tarrant 2013; Wootten 2010 and Wong 2014 evaluated vaccine uptake; Englund 1995 did not have concurrent control group while Mulholland 1996; Munoz 2001; Schlaudecker 2013; NIAID 2009b; NIAID 2010 and Zaman 2008 did not have a placebo/control group.
Please refer to Characteristics of excluded studies table for more details.
Risk of bias in included studies
A graphical summary of the results of the 'Risk of bias' assessment for the included studies is provided in Figure 2 and Figure 3.
2.
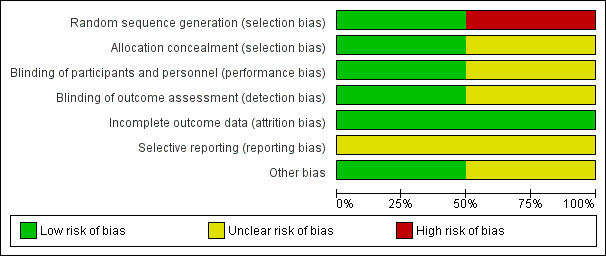
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Glezen 1992 was quasi‐randomised trial. Randomisation was inadequate in Glezen 1992 as women were randomised to the intervention or control group according to the eighth digit of their hospital district number by random number table. Hence, this study was judged to be at 'high risk of bias' for selection bias. Allocation concealment was a not clearly reported in Glezen 1992 and the study was judged to be at 'unclear' risk of bias.
Madhi 2014 was a randomised controlled trial. Madhi 2014 was judged to be at 'low risk of bias' for selection bias as randomisation was carried out by the study's statistician who used computer‐generated assignments with adequate allocation concealment.
Blinding
Blinding was 'unclear' in Glezen 1992 since the study report mentioned that it was double blinded however, no further details were provided on how it was done.
Madhi 2014 was judged to be at 'low risk of bias' for performance and detection bias. With the exception of the statistician and the pharmacist, study personnel and study participants were blinded to the group assignments in Madhi 2014.
Incomplete outcome data
Both included studies provided details on exclusion and attrition rates, along with the reasons for exclusion and thus were judged to be at 'low risk of bias' for attrition bias.
Selective reporting
Published protocols were not available for both of the included studies thus limiting us from making any judgment on selective reporting ('unclear' risk of bias). However, all the outcomes mentioned in the methods section were reported in results by Glezen 1992 while Madhi 2014 reported Important outcome data that were provided in a supplement published with the primary report.
Other potential sources of bias
Participants in the Glezen 1992 study were mostly Hispanic and of low‐income, suggesting possible selection bias. No other bias was identified, but we had insufficient information to allow us to fully assess this ’Risk of bias’ domain ('unclear' risk of bias).
No other potential sources of bias were detected for Madhi 2014 and we assessed this study as having a 'low' risk of bias.
Effects of interventions
See: Table 1
Comparison 1 ‐ Haemophilus influenzae type B (Hib) versus placebo
Primary outcomes
The included study (Glezen 1992) did not report on any of our prespecified primary outcomes.
Mortality (perinatal, neonatal, infant, maternal)
Any infection of the respiratory tract (upper or lower tract) among women, neonates and infants
Sepsis or other reported infections among women, neonates and infants
Secondary outcomes
Pregnancy outcomes ‐ (stillbirth, low birthweight, abortion, preterm birth)
Glezen 1992 reported the outcome of preterm birth. There was no clear difference in the incidence of preterm birth between the vaccination and placebo groups (risk ratio (RR) 1.28, 95% confidence interval (CI) 0.12 to 13.86, one study, 213 participants; Analysis 1.1). The study did not report any other pregnancy outcomes: stillbirth, low birthweight and abortion.
1.1. Analysis.
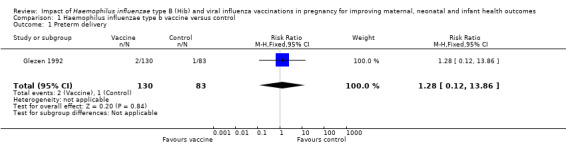
Comparison 1 Haemophilus influenzae type b vaccine versus control, Outcome 1 Preterm delivery.
The included study did not report on any other secondary outcomes of this review:
Frequency of hospitalisations of mother in both inpatient and emergency department due to respiratory illnesses
Frequency of outpatient facility visits by mother due to respiratory illnesses
Frequency of hospitalisations of infant in both inpatient and emergency department due to respiratory illnesses
Frequency of outpatient facility visits by infant due to respiratory illnesses
Non‐prespecified outcomes
Neonatal morbidity
Glezen 1992 reported neonatal morbidities including fetal distress, intubation and jaundice. There was a non‐significant impact on fetal distress (RR 1.23, 95% CI 0.67 to 2.26, one study, 213 infants; Analysis 1.2), neonatal jaundice (RR 1.01, 95% CI 0.52 to 1.97, one study, 213 infants; Analysis 1.3) and neonatal intubation (RR 1.03, 95% CI 0.55 to 1.95, one study, 213 infants; Analysis 1.4).
1.2. Analysis.

Comparison 1 Haemophilus influenzae type b vaccine versus control, Outcome 2 Fetal distress.
1.3. Analysis.
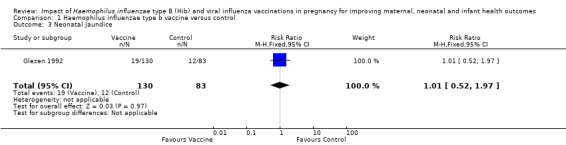
Comparison 1 Haemophilus influenzae type b vaccine versus control, Outcome 3 Neonatal jaundice.
1.4. Analysis.

Comparison 1 Haemophilus influenzae type b vaccine versus control, Outcome 4 Intubation.
Glezen 1992 did not report on maternal and infant hospitalisation for any infection or adverse events.
Comparison 2 ‐ Viral influenza versus placebo
Primary outcomes
Mortality (perinatal, neonatal, infant, maternal)
One study (Madhi 2014) reported maternal, infant and perinatal (stillbirth + death in first week of life) mortality. There was no clear difference between the vaccine and placebo group in terms of maternal mortality (RR 4.96, 95% CI 0.24 to 103.24; one study, 2116 women, moderate quality evidence), infant death up to 175 days (RR 0.71, 95% CI 0.37 to 1.37; one study, 2049 infants, moderate quality evidence) and perinatal mortality (stillbirth and death in the first week of life (RR 1.32, 95% CI 0.73 to 2.38; one study, 2083 infants, moderate quality evidence) (see Analysis 2.1; Analysis 2.2; Analysis 2.3).
2.1. Analysis.

Comparison 2 Viral influenza vaccine versus control, Outcome 1 Maternal death.
2.2. Analysis.

Comparison 2 Viral influenza vaccine versus control, Outcome 2 Infant death (up to 175 days).
2.3. Analysis.
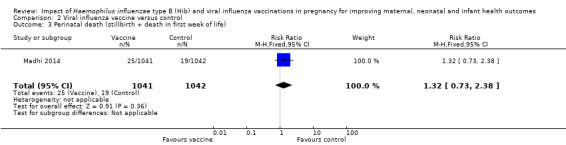
Comparison 2 Viral influenza vaccine versus control, Outcome 3 Perinatal death (stillbirth + death in first week of life).
Any infection of the respiratory tract (upper or lower tract) among women, neonates and infants
One study (Madhi 2014) reported reverse‐transcriptase–polymerase‐chain‐reaction (RT‐PCR) confirmed influenza, influenza‐like illness and any respiratory illness in women and infants. Vaccination was associated with a reduction in RT‐PCR confirmed influenza among infants (RR 0.51, 95% CI 0.30 to 0.88, one study, 2049 infants (Analysis 2.4)) and women (RR 0.50, 95% CI 0.29 to 0.86, one study, 2116 women (Analysis 2.5)).
2.4. Analysis.

Comparison 2 Viral influenza vaccine versus control, Outcome 4 RT‐PCR confirmed influenza in infants.
2.5. Analysis.

Comparison 2 Viral influenza vaccine versus control, Outcome 5 RT‐PCR confirmed influenza in women.
There was no clear difference between vaccination and placebo control in terms of influenza‐like illness among women (RR 0.96, 95% CI 0.79 to 1.16 (Analysis 2.9)) and infants (RR 1.02, 95% CI 0.94 to 1.09 (Analysis 2.6)) or any respiratory illness among infants (RR 1.01, 95% CI 0.95 to 1.07, high quality evidence (Analysis 2.8)) and women (RR 0.97, 95% CI 0.91 to 1.04, high quality evidence (Analysis 2.7)).
2.9. Analysis.

Comparison 2 Viral influenza vaccine versus control, Outcome 9 Influenza‐like illness in women.
2.6. Analysis.
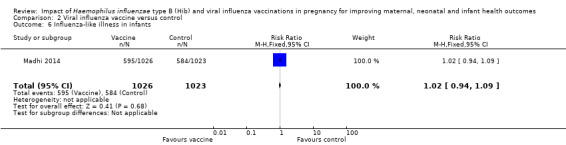
Comparison 2 Viral influenza vaccine versus control, Outcome 6 Influenza‐like illness in infants.
2.8. Analysis.

Comparison 2 Viral influenza vaccine versus control, Outcome 8 Any respiratory illness in infants.
2.7. Analysis.

Comparison 2 Viral influenza vaccine versus control, Outcome 7 Any respiratory illness in women.
Sepsis or other reported infections among women, neonates and infants.
The included study (Madhi 2014) also reported no clear difference between vaccine and placebo control groups in terms of maternal hospitalisation for any infection (RR 2.27, 95% CI 0.94 to 5.49, moderate quality evidence (Analysis 2.16)) or neonatal hospitalisation due to sepsis within 28 days of birth (RR 1.60, 95% CI 0.73 to 3.50, moderate quality evidence (Analysis 2.17)).
2.16. Analysis.
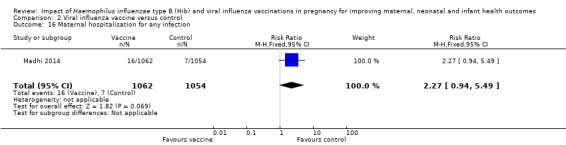
Comparison 2 Viral influenza vaccine versus control, Outcome 16 Maternal hospitalisation for any infection.
2.17. Analysis.
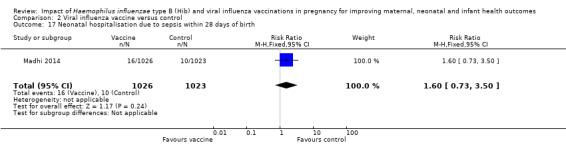
Comparison 2 Viral influenza vaccine versus control, Outcome 17 Neonatal hospitalisation due to sepsis within 28 days of birth.
Secondary outcomes
Frequency of hospitalisations of mother in both inpatient and emergency departments due to respiratory illnesses
There were no clear differences between vaccine and placebo control groups in terms of maternal hospitalisation for respiratory infection (Analysis 2.10).
2.10. Analysis.

Comparison 2 Viral influenza vaccine versus control, Outcome 10 Maternal hospitalisation for respiratory infection.
Frequency of outpatient facility visits by mother due to respiratory illnesses
This outcome was not reported by Madhi 2014.
Frequency of hospitalisations of infant in both inpatient and emergency departments due to respiratory illnesses
There was no clear difference between vaccine and placebo control group in terms of neonatal hospitalisation for respiratory infection (RR 0.85, 95% CI 0.52 to 1.39 (Analysis 2.11)).
2.11. Analysis.
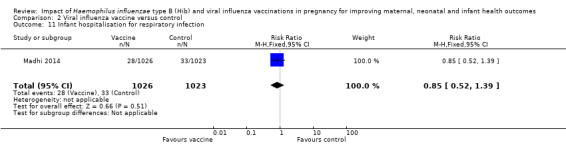
Comparison 2 Viral influenza vaccine versus control, Outcome 11 Infant hospitalisation for respiratory infection.
Frequency of outpatient facility visits by infant due to respiratory illnesses
This outcome was not reported by Madhi 2014.
Pregnancy outcomes ‐ (stillbirth, low birthweight, abortion, preterm birth)
There were no clear differences between vaccination and the placebo control group in terms of pregnancy outcomes: preterm labour (RR 0.92, 95% CI 0.53 to 1.59 (Analysis 2.12)) miscarriage (RR 0.60, 95% CI 0.14 to 2.49 (Analysis 2.13)) or stillbirth (RR 1.65, 95% CI 0.73 to 3.76 (Analysis 2.14)).
2.12. Analysis.

Comparison 2 Viral influenza vaccine versus control, Outcome 12 Preterm labour.
2.13. Analysis.
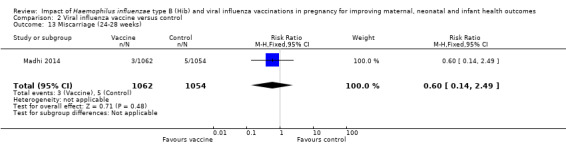
Comparison 2 Viral influenza vaccine versus control, Outcome 13 Miscarriage (24‐28 weeks).
2.14. Analysis.

Comparison 2 Viral influenza vaccine versus control, Outcome 14 Stillbirth.
Non‐prespecified outcomes
Adverse events
There was no difference in adverse events (at least one systemic reaction) between the vaccination and placebo control group (RR 1.06, 95% CI 0.87 to 1.30 (Analysis 2.15)).
2.15. Analysis.
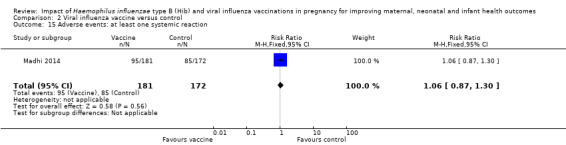
Comparison 2 Viral influenza vaccine versus control, Outcome 15 Adverse events: at least one systemic reaction.
Neonatal morbidities including fetal distress, neonatal intubation and jaundice
This outcome was not reported by Madhi 2014.
Discussion
Two trials were included this review. One evaluated the impact of Hib vaccination during pregnancy and the other evaluated the impact of viral influenza vaccination during pregnancy. Data were not combined in meta‐analysis.
Summary of main results
The Glezen 1992 study was a quasi‐randomised trial (involving 213 women and 213 babies) examining the effect of Hib vaccination in pregnancy but it did not report on any of this review’s primary outcomes (mortality, respiratory tract infections and sepsis among women, neonates and infants). There was no clear difference in the incidence of preterm birth between the vaccination and placebo control groups. None of this review's other secondary outcomes were reported in this study, including adverse events. However, the study reported no significant impact of maternal Hib vaccine on fetal distress, intubation and neonatal jaundice (outcomes which were not prespecified in the protocol for this review).
Madhi 2014 was a large randomised controlled trial (RCT) (involving 2116 women and 2049 infants) investigating the impact of viral influenza vaccination in pregnancy (compared with a placebo control). The study reported on this review's primary outcomes: mortality (perinatal, infant, maternal) and any infection of the respiratory tract (upper or lower) among women, neonates and infants, and sepsis or other reported infections among women, neonates and infants. A reduced incidence of reverse‐transcriptase–polymerase‐chain‐reaction (RT‐PCR) influenza was observed both in the women who received viral influenza immunisation and their infants. In contrast, there was no clear differences between the viral influenza and placebo control groups in respect of the incidence of influenza‐like illness or any respiratory illness in either the mothers or their babies. There was no clear difference between the vaccine and the control groups for maternal, infant and perinatal mortality. Among the secondary outcomes, Madhi 2014 reported on maternal and infant hospitalisation for any infection, maternal and infant hospitalisation for respiratory infection, pregnancy outcomes and adverse events. There was no difference between the vaccine and control groups for any of the secondary outcomes reported.
Overall completeness and applicability of evidence
We found insufficient evidence (from one small quasi‐randomised trial involving 213 women and their babies) to evaluate the effectiveness of Hib vaccinations during pregnancy for improving maternal, neonatal and infant health outcomes. The study did not report on any of this review's primary or secondary outcomes (with the exception preterm birth ‐ a secondary outcome).
We found evidence from one large trial (involving 2116 women and 2049 babies) on the effectiveness of viral influenza vaccine during pregnancy for improving maternal, neonatal and infant health outcomes. The trial reported on our three primary outcomes but did not report on three of our secondary outcomes.
Only one study Madhi 2014 reported data relating to adverse events.
The comparisons in this review each contain data from one study. However, there are two ongoing studies on the effectiveness of vaccinating pregnant women with viral influenza vaccination (see Characteristics of studies awaiting classification; Characteristics of ongoing studies). In future updates, data from these studies will be incorporated into this review to provide further evidence about viral influenza vaccine in pregnancy for improving maternal, neonatal and infant health outcomes. Further trials are needed to evaluate the effectiveness of Hib vaccine during pregnancy for improving maternal, neonatal and infant health outcomes.
Quality of the evidence
Glezen 1992 was judged to be at 'high risk of bias' as there was no true randomisation and poor compliance for the reporting of allocation concealment and blinding. Also there was no description on any of the aspects of blinding measures. However, rates of attrition were clearly reported, along with the reasons for losses to follow‐up.
Madhi 2014 was a large trial and was judged to be at 'low risk of bias' with adequate randomisation, allocation concealment, blinding and rates of attrition.
GRADE software was used to assess evidence for important outcomes separately for two comparisons. Please see the GRADE Table 1.
1. Hib vaccination during pregnancy versus placebo
We could not grade the evidence for quality due to lack of outcome data.
2. Viral influenza vaccination during pregnancy versus placebo
Five outcomes were graded to be of moderate quality: maternal death, infant death (up to 175 days), perinatal death (stillbirth + death in first week of life), maternal hospitalisation for any infection, and neonatal hospitalisation for sepsis. Two additional outcomes were graded as of high quality: any respiratory illness in women and any respiratory illness in infants.
Potential biases in the review process
We undertook a systematic, thorough search of the literature to identify all studies meeting the inclusion criteria and we are confident that the included trials met the set criteria. Study selection and data extraction were done in duplicate and independently and we reached consensus by discussing any discrepancies. A protocol was published for this review (Salam 2012). We could not carry out the a priori specified analysis for the effectiveness of Hib vaccines as the study did not report the prespecified primary and secondary outcomes listed in our protocol (Salam 2012).
Agreements and disagreements with other studies or reviews
For maternal Hib vaccination, data from other studies suggest that Hib conjugate vaccine given to women before pregnancy significantly increases the proportion of infants who had protective Hib antibody levels at birth and two months of age (Santosham 2001). It is hypothesised that maternal Hib‐specific antibody might be protective for the infant and lead to consequent decrease in disease during infancy (Amstey 1985; Anderson 1977). Our review found insufficient data to evaluate the effectiveness of Hib vaccination in pregnancy. We eagerly await the results of the completed and ongoing trials ‐ these will be incorporated in this review when it is updated and help to further evaluate the effectiveness of Hib vaccination in pregnancy on maternal, neonatal and infant health outcomes.
Our review findings from one large trial (Madhi 2014) shows that viral influenza vaccine during pregnancy can reduce confirmed influenza cases among women and infants. Previous data from observational and retrospective studies have reported mixed findings for viral influenza vaccinations. Observational studies have shown transplacental transfer of influenza‐specific antibodies and consequent protection to infants from naturally acquired maternal influenza infection however, there was no evidence to suggest reduction in the rate of infection (Puck 1980; Reuman 1987). Two retrospective data reviews did not find any difference in the incidence of medically attended acute respiratory illness in immunised mothers or their infants (Black 2004; France 2006). However studies have suggested vaccine safety during pregnancy (Deinard 1981; Sumaya 1979). The findings from a narrative review (Mak 2008) on the evidence of influenza vaccination suggest that the data on influenza vaccine safety in pregnancy are inadequate, however, it supports vaccinating healthy pregnant women in the second or third trimester and vaccinating in any trimester during a pandemic. We await the results of the completed and ongoing trials in the update to comment further on the effectiveness of viral influenza vaccination in pregnancy on maternal, neonatal and infant health outcomes.
National policies on viral influenza vaccination vary from country to country. The UK and Germany do not routinely vaccinate in pregnancy, whereas the USA and Canada recommend vaccinating healthy pregnant women regardless of trimester. Australia offers vaccination to healthy pregnant women in any trimester who will be in the second or third trimester during the influenza season. The World Health Organization recommends that all pregnant women should be immunised during the influenza season (WHO 2005).
Authors' conclusions
Implications for practice.
The evidence for effectiveness of Hib vaccine during pregnancy comes from one small quasi‐randomised trial and did not report on any of the review’s primary outcomes of interest, so further evidence is required. One large trial of good quality on viral influenza vaccine reported a reduction in confirmed influenza among immunised women and their babies, suggesting the potential of this strategy for scale up but further evidence from varying contexts is required.
There are further two studies that are ongoing ‐ these studies will be incorporated into this review in future updates.
Implications for research.
More well‐designed, large‐scale randomised controlled trials are needed with appropriate controls to establish the benefit of maternal Hib and viral influenza vaccination during pregnancy. Future trials should focus on reporting major outcomes including mortality (perinatal, neonatal, infant, maternal), respiratory tract infections and sepsis among women, neonates and infants. Researchers of future trials should undertake long‐term follow‐up of the women and their children in order to study the long‐term effects of maternal vaccination in respect of neonatal and infant health outcomes. Risk of bias should also be reduced by ensuring adequate randomisation and allocation concealment of the assignment and by achieving blinding of the participants, providers and the outcome assessors in order to produce trials of good methodological quality.
Acknowledgements
Nancy Medley (NM) for her support in the creation of the 'Summary of findings' table for this review. NM's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIIHR, NHS or the Department of Health.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Haemophilus influenzae type b vaccine versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm delivery | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.12, 13.86] |
| 2 Fetal distress | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.67, 2.26] |
| 3 Neonatal jaundice | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.52, 1.97] |
| 4 Intubation | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.55, 1.95] |
Comparison 2. Viral influenza vaccine versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 1 | 2116 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.96 [0.24, 103.24] |
| 2 Infant death (up to 175 days) | 1 | 2049 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.37, 1.37] |
| 3 Perinatal death (stillbirth + death in first week of life) | 1 | 2083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.73, 2.38] |
| 4 RT‐PCR confirmed influenza in infants | 1 | 2049 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.30, 0.88] |
| 5 RT‐PCR confirmed influenza in women | 1 | 2116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.29, 0.86] |
| 6 Influenza‐like illness in infants | 1 | 2049 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.09] |
| 7 Any respiratory illness in women | 1 | 2116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.04] |
| 8 Any respiratory illness in infants | 1 | 2049 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 9 Influenza‐like illness in women | 1 | 2116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.16] |
| 10 Maternal hospitalisation for respiratory infection | 1 | 2116 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.93 [0.48, 165.70] |
| 11 Infant hospitalisation for respiratory infection | 1 | 2049 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.39] |
| 12 Preterm labour | 1 | 2116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.59] |
| 13 Miscarriage (24‐28 weeks) | 1 | 2116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.14, 2.49] |
| 14 Stillbirth | 1 | 2116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.73, 3.76] |
| 15 Adverse events: at least one systemic reaction | 1 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.30] |
| 16 Maternal hospitalisation for any infection | 1 | 2116 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.94, 5.49] |
| 17 Neonatal hospitalisation due to sepsis within 28 days of birth | 1 | 2049 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.73, 3.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Glezen 1992.
| Methods | Pregnant women attending the Baylor Midwifery Clinic in Texas were randomised into the vaccine or placebo group depending on their hospital district number. Vaccine or placebo was administered during the prenatal visit at 34‐36 gestational weeks. Follow‐up consisted of the delivery and birth record of those delivered at Jefferson Davis Hospital. Cord blood samples were also obtained at delivery. | |
| Participants | Pregnant women of 30‐38 gestational weeks attending the Baylor Midwifery Clinic were enrolled (n = 237), and those delivering at Jefferson Davis Hospital were followed up (n = 213). | |
| Interventions | Group 1: capsular polysaccharide vaccine (n = 130). Group 2: placebo/saline (n = 83). |
|
| Outcomes | Preterm delivery, neonatal jaundice, fetal distress, neonatal intubation, measurements of cord sera antibodies. | |
| Notes | Participants were mostly Hispanic and of low‐income. Exclusions: pregnant women expected to have a complicated pregnancy course or delivery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Women were randomised to receive PRP or placebo (saline for injection) according to the eighth digit of their hospital district number by random number table". Comment: this seems to be a non‐random process and hence a quasi‐randomised trial. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study states that it is double‐blinded, but does not specify further. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study states that it is double‐blinded, but does not specify further. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition (213/237 participants were successfully followed up) was mentioned along with their reasons. |
| Selective reporting (reporting bias) | Unclear risk | Published protocol was not available but all the outcomes mentioned in the methods section were reported in the results. |
| Other bias | Unclear risk | Quote: "Participants were mostly Hispanic and of low‐income". Comment: possible selection bias. |
Madhi 2014.
| Methods | 2 randomised, double‐blind, placebo‐controlled trials were conducted in Soweto, South Africa among cohorts of pregnant women with and without HIV. Participants were followed through to 24 weeks postpartum. | |
| Participants | Pregnant women age of 18 to 38 years and an estimated gestation of 20 to 36 weeks were enrolled to receive inactivated influenza vaccine or placebo. Singld and multiple gestations were included. | |
| Interventions | We have only included data for women who did not have HIV. Group 1: 0.5 mL of Influenza vaccine containing 15 μg each of A/California/7/2009 (A/[H1N1]pdm09), A/Victoria/210/2009 (A/H3N2), and a B/Brisbane/60/2008–like virus (B/Victoria). N = 1062 Group 2: 0.9% normal saline solution. N = 1054. |
|
| Outcomes | Vaccine immunogenicity, vaccine efficacy and vaccine safety. Maternal and infant mortality, maternal and infant morbidity, causes and numbers of hospitalisations and side effects of vaccinations. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by the study statistician with the use of computer‐generated assignments". Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by the study statistician with the use of computer‐generated assignment". Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "With the exception of the statistician and the pharmacist, study personnel were unaware of the group assignments, as were the study participants". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "With the exception of the statistician and the pharmacist, study personnel were unaware of the group assignments, as were the study participants". Comment: adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group: mothers ‐ 86/1062 = 8% lost to follow‐up, infants ‐ 51/1026 = 5% lost to follow‐up. Control group: mothers ‐ 78/1054 = 7% lost to follow‐up, infants ‐ 52/1023 = 5% lost to follow‐up. Attrition rate similar across the 2 groups. Reasons for attrition included lost to follow‐up, withdrawal of consent, relocation and death. We have added deaths back into the analysis using the intention‐to‐treat numbers as denominators above. |
| Selective reporting (reporting bias) | Unclear risk | Published protocol not available. Important outcome data are provided in an 88‐page supplement published with the primary report. |
| Other bias | Low risk | None. |
PRP: capsular polysaccharide
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Englund 1995 | Study does not have concurrent control group. |
| Henkle 2010 | Study was done to assess the incidence of influenza virus infection in the non‐vaccinated study arm of a previously conducted trial. |
| Holmlund 2011 | Study vaccinated infants with pneumococcal and Haemophilus influenzae type B (Hib) vaccines along with the maternal immunisation and was done primarily to assess the impact of pneumococcal vaccine. |
| Jackson 2011 | Study compared vaccinations with 2 different doses of influenza vaccine. |
| Madhi 2011a | Study evaluates the safety and immunogenicity of trivalent influenza vaccine in HIV‐infected pregnant women. |
| Moniz 2010 | Study does not evaluate the outcome of interest that is maternal, newborn and child health outcomes but rather focuses on vaccine knowledge and uptake with text messaging. |
| Mulholland 1996 | Study did not have a suitable placebo/control group. |
| Munoz 2001 | Study did not have a suitable placebo/control group. |
| NIAID 2009a | Study compares 2 strengths of the H1N1 influenza vaccine. |
| NIAID 2009b | Study compares two different types of Flu vaccines (Fluzone® or Fluarix®) |
| NIAID 2009c | Study compares different strengths of the vaccine. |
| NIAID 2010 | Study compares three different types of flu vaccines. |
| Omer 2013 | Study evaluated the combined effects of antenatal influenza vaccination by mothers and pneumococcal conjugate vaccine receipt by infants. |
| Quiambao 2003 | Study was done primarily to study pneumococcal vaccine through a cross‐sectional survey. |
| Quiambao 2007 | Study was done primarily to study pneumococcal vaccine. |
| Schlaudecker 2013 | Study did not have a suitable placebo/control group. |
| Stockwell 2014 | Study evaluated the impact of influenza vaccine text message reminders in a low‐income obstetric population |
| Tarrant 2013 | Study does not evaluate the outcome of interest that is maternal, newborn and child health outcomes but rather focuses on vaccine knowledge and uptake after a brief education session. |
| Wong 2014 | Study is designed to assess the effectiveness of a brief educational intervention in improving the uptake of seasonal influenza vaccine among pregnant women in Hong Kong. |
| Wootten 2010 | Study compares methods for consenting on influenza vaccine uptake rate in pregnant women. |
| Zaman 2008 | Study did not have a suitable placebo/control group. |
Characteristics of ongoing studies [ordered by study ID]
Garcia 2012.
| Trial name or title | Clinical trial to evaluate the immunogenicity and safety of the 2011‐2012 vaccine against seasonal influenza on pregnant women |
| Methods | Open label non‐randomised study proposing that the 2011‐2012 seasonal influenza vaccine (including H3N2 and H1N1 subtypes of serotype A strain over the serotype B) administered to 15 ug (without adjuvant) via intramuscular in pregnant women will be safe and immunogenic. |
| Participants | Pregnant women from 14 to 34 weeks of gestation aged 18 to 39 years and had made at least 1 prenatal visit to confirm the pregnancy and available for follow‐up time. |
| Interventions | 2011‐2012 seasonal trivalent inactivated influenza vaccine (include A/California/7/2009 (H1N1)‐like, A/Perth/16/2009 (H3N2)‐like, and B/Brisbane/60/2008‐like antigens) administered to 15 ug without adjuvant, via intramuscular in pregnant and nonpregnant women. It is recommended that vaccines for use in the 2012‐2013 influenza season (northern hemisphere winter) contain the following:
|
| Outcomes | Immunogenecity and pregnancy outcomes. |
| Starting date | April 2012. |
| Contact information | Ma. de Lourdes Garcia Garcia, Instituto Nacional de Salud Publica, Mexico. freyes.ld@gmail.com |
| Notes | This study is not yet open for participant recruitment. |
Tapia 2012.
| Trial name or title | Prospective, randomized, controlled, observer‐blind trial to measure the efficacy, safety and immunogenicity of trivalent inactivated influenza vaccine and the safety and immunogenicity of quadrivalent meningococcal polysaccharide diphtheria conjugate vaccine in pregnant Malian women and their infants up to 6 months of age |
| Methods | This is a prospective, randomised, controlled, observer‐blind trial. Pregnant women in their 3rd trimester will be randomised to receive Vaxigrip or Menactra. Follow‐up of the women's and infants' (under 5 years of age) health (including monitoring of influenza‐like illness) will include: 5 visits to the clinic and weekly visits to the home. |
| Participants | Pregnant women in their 3rd trimester (n = 6000). |
| Interventions | Group 1: inactivated influenza vaccine trivalent types A and B (Vaxigrip) (Assumed n = 3000). Group 2 (control): meningococcal polysaccharide‐diphtheria toxoid conjugate vaccine (Menactra) (Assumed n = 3000). |
| Outcomes | Infants with influenza (n = 6000). |
| Starting date | September 2011. |
| Contact information | Milagritos D Tapia, MD 410‐706‐5332 mtapia@medicine.umaryland.edu |
| Notes | Study not yet open for participant recruitment. |
Differences between protocol and review
We have edited the secondary outcome 'Pregnancy outcomes (stillbirth, low birthweight, abortion)' to clarify that it includes preterm birth. The outcome now reads 'Pregnancy outcomes (stillbirth, low birthweight, abortion, preterm birth)'.
We have included additional secondary outcomes that were not listed in our published protocol (Salam 2012).
Neonatal morbidities including fetal distress, neonatal intubation and jaundice
Adverse events
We have clarified in 'Method/Types of studies' that studies published in abstract form only are eligible for inclusion in this review provided that sufficient information is available.
Contributions of authors
The draft protocol was written by Rehana A Salam (RAS) and Jai K Das (JKD). JKD and RAS carried out the screening. RAS and Chesarahmia Dojo Soeandy (CDS) extracted the data. RAS, JKD and Zohra S Lassi (ZSL) entered the data, created the comparisons and did the analysis. JKD, ZSL and CSD wrote the text of the review. Zulfiqar A Bhutta (ZAB) and RAS critically reviewed and modified the draft.
Sources of support
Internal sources
The Aga Khan University, Pakistan.
External sources
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
New
References
References to studies included in this review
Glezen 1992 {published data only}
- Glezen WP, Englund JA, Siber GR, Six HR, Turner C, Shriver D, et al. Maternal immunization with the capsular polysaccharide vaccine for haemophilus influenzae type b. Journal of Infectious Diseases 1992;165:134‐6. [DOI] [PubMed] [Google Scholar]
Madhi 2014 {published data only}
- Madhi SA. Vaccination of HIV‐uninfected pregnant women With trivalent influenza vaccine in the prevention of influenza illness during early infancy and in mothers: randomized controlled phase III trial evaluating safety, immunogenicity and efficacy. http://clinicaltrials.gov/ct2/show/record/NCT01306669 (accessed 29 June 2012).
- Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, et al. Influenza vaccination of pregnant women and protection of their infants. New England Journal of Medicine 2014;371(10):918‐31. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Englund 1995 {published data only}
- Englund JA, Glezen WP, Turner C, Harvey J, Thompson C, Siber GR. Transplacental antibody transfer following maternal immunization with polysaccharide and conjugate haemophilus influenzae type b vaccines. Journal of Infectious Diseases 1995;171(1):99‐105. [DOI] [PubMed] [Google Scholar]
Henkle 2010 {published data only}
- Henkle E, Steinhoff MC, Omer SB, Roy OE, Arifeen SE, Raqib R, et al. Incidence of influenza infection in early infancy in Southern Asia. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
Holmlund 2011 {published data only}
- Holmlund E, Nohynek H, Quiambao B, Ollgren J, Kayhty H. Mother‐infant vaccination with pneumococcal polysaccharide vaccine: Persistence of maternal antibodies and responses of infants to vaccination. Vaccine 2011;29(28):4565‐75. [DOI] [PubMed] [Google Scholar]
Jackson 2011 {published data only}
- Jackson LA, Patel SM, Swamy GK, Frey SE, Creech CB, Munoz FM, et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. Journal of Infectious Diseases. 2011;204(6):854‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madhi 2011a {published data only}
- Madhi SA. Trivalent influenza vaccine in HIV‐infected pregnant women and kinetics of transplacental anti‐influenza antibody transfer and persistence in young infants: a randomized controlled phase II trial evaluating safety and immunogenicity. http://clinicaltrials.gov/show/NCT01306682 (accessed 29 June 2012).
Moniz 2010 {published data only}
- Moniz M. Text messaging for preventative health during pregnancy; improving influenza vaccination rates in pregnancy: a randomized controlled trial of text messaging to increase vaccine uptake. http://clinicaltrials.gov/show/NCT01248520 (accessed 2012).
Mulholland 1996 {published data only}
- Mulholland K, Suara RO, Siber G, Roberton D, Jaffar S, N'Jie J. Maternal immunization with haemophilus influenzae type b polysaccharide‐tetanus protein conjugate vaccine in the Gambia. International Journal of Gynecology & Obstetrics 1996;55:89‐90. [PubMed] [Google Scholar]
- Mulholland K, Suara RO, Siber G, Roberton D, Jaffar S, N'Jie J, et al. Maternal immunization with haemophilus influenzae type b polysaccharide‐tetanus protein conjugate vaccine in the Gambia. JAMA 1996;275(15):1182‐8. [PubMed] [Google Scholar]
Munoz 2001 {published data only}
- Munoz FM, Englund JA, Cheesman CC, Maccato ML, Pinell PM, Nahm MH, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine 2001;20(6):826‐37. [DOI] [PubMed] [Google Scholar]
NIAID 2009a {published data only}
- National Institute of Allergy and Infectious Diseases (NIAID). A phase II study in pregnant women to assess the safety and immunogenicity of an unadjuvanted Sanofi Pasteur H1N1 inactivated influenza vaccine administered at two dose levels. http://clinicaltrials.gov/ct2/show/record/NCT00963430 (accessed 2 July 2012).
NIAID 2009b {published data only}
- Johnson R. Influenza vaccine in pregnant women. http://www.clinicaltrials.gov/ct2/show/NCT00905125 (accessed 10 March 2011).
- National Institute of Allergy and Infectious Diseases (NIAID). A randomized, double‐blind trial on the safety and immunogenicity of inactivated trivalent influenza vaccine in pregnant women. http://clinicaltrials.gov/ct2/show/record/NCT00905125 (accessed 2 July 2012).
NIAID 2009c {published data only}
- National Institute of Allergy and Infectious Diseases (NIAID). A phase II study in pregnant women to assess the safety and immunogenicity of an unadjuvanted Novartis H1N1 inactivated influenza vaccine administered at two dose levels. http://clinicaltrials.gov/show/NCT00992719 (accessed 2 July 2012).
NIAID 2010 {published data only}
- National Institute of Allergy and Infectious Diseases (NIAID). 2010‐2011 trivalent influenza vaccine (TIV) in pregnant women. http://clinicaltrials.gov/show/NCT01173211 (accessed 10 March 2011).
Omer 2013 {published data only}
- Omer SB, Zaman K, Roy E, Arifeen SE, Raqib R, Noory L, et al. Combined effects of antenatal receipt of influenza vaccine by mothers and pneumococcal conjugate vaccine receipt by infants: Results from a randomized, blinded, controlled trial. Journal of Infectious Diseases 2013;207(7):1144‐7. [DOI] [PubMed] [Google Scholar]
Quiambao 2003 {published data only}
- Quiambao BP, Nohynek H, Kayhty H, Ollgren J, Gozum L, Gepanayao CP, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the Philippines. Vaccine 2003;21(24):3451‐4. [DOI] [PubMed] [Google Scholar]
Quiambao 2007 {published data only}
- Quiambao BP, Nohynek HM, Kayhty H, Ollgren JP, Gozum LS, Gepanayao CP, et al. Immunogenicity and reactogenicity of 23‐valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine 2007;25(22):4470‐7. [DOI] [PubMed] [Google Scholar]
Schlaudecker 2013 {published data only}
- Schlaudecker EP, Steinhoff MC, Omer SB, McNeal MM, Roy E, Arifeen SE, et al. IgA and neutralizing antibodies to influenza a virus in human milk: a randomized trial of antenatal influenza immunization. PLoS ONE [Electronic Resource] 2013;8(8):e70867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockwell 2014 {published data only}
- Stockwell MS, Westhoff C, Kharbanda EO, Vargas CY, Camargo S, Vawdrey DK, et al. Influenza vaccine text message reminders for urban, low‐income pregnant women: a randomized controlled trial. American Journal of Public Health 2014;104(S1):e7‐e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarrant 2013 {published data only}
- Tarrant M. A randomized controlled trial of a brief educational intervention to increase uptake of influenza vaccine among pregnant women. http://clinicaltrials.gov/ct2/show/NCT01772901 (accessed 21 May 2013).
Wong 2014 {published data only}
- Wong VW, Fong DY, Tarrant M. Brief education to increase uptake of influenza vaccine among pregnant women: a study protocol for a randomized controlled trial. BMC Pregnancy and Childbirth 2014;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wootten 2010 {published data only}
- Wootten SH. Opting in vs opting out: impact on influenza vaccination in pregnant women. http://clinicaltrials.gov/ct2/show/record/ 2012.
Zaman 2008 {published data only}
- Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Altaye M, et al. Influenza immunization in pregnancy and birth weights: observations from a randomised controlled trial. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Dodd C, et al. Neonatal outcomes after influenza immunization during pregnancy: A randomised controlled trial. CMAJ: Canadian Medical Association journal 2012;184(6):645‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff MC, Schlaudecker EP, Omer SB, Roy E, Altaye M, Zaman K. Antibody persistence in young adults one year after pneumococcal immunization in pregnancy. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011.
- Steinhoff MC, Schlaudecker EP, Omer SB, Roy E, Zaman K. Influenza IgA antibody in human milk: a randomised trial of maternal influenza immunization. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011.
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. New England Journal of Medicine 2008;359(15):1555‐64. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Garcia 2012 {published data only}
- Garcia LG. Clinical trial to evaluate the immunogenicity and safety of the 2011‐2012 vaccine against seasonal influenza on pregnant women. http://clinicaltrials.gov/ct2/show/record/NCT01577316 (accessed 29 June 2012).
Tapia 2012 {published data only}
- Tapia MD. Prospective, randomized, controlled, observer‐blind trial to measure the efficacy, safety and immunogenicity of trivalent inactivated influenza vaccine and the safety and immunogenicity of quadrivalent meningococcal polysaccharide diphtheria conjugate vaccine in pregnant Malian women and their infants up to 6 months of age. http://clinicaltrials.gov/show/NCT01430689 (accessed 29 June 2012).
Additional references
Acs 2005
- Acs N, Banhidy F, Puho E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Research. Part A, Clinical and Molecular Teratology 2005;73:989‐96. [DOI] [PubMed] [Google Scholar]
Amstey 1985
- Amstey MS, Insel R, Munoz J, Pichichero M. Fetal‐neonatal passive immunization against Hemophilus influenzae, type b. American Journal of Obstetrics and Gynecology 1985;153(6):607‐11. [DOI] [PubMed] [Google Scholar]
Anderson 1977
- Anderson P, Smith DH, Ingram DL, Wilkins J, Wehrle F, Howie VM. Antibody to polyribophosphate of Haemophilus influenzae type b in infants and children: effect of immunization with polyribophosphate. Journal of Infectious Diseases 1977;136:S57‐S62. [DOI] [PubMed] [Google Scholar]
Black 2004
- Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D, et al. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. American Journal of Perinatology 2004;21(6):333‐9. [DOI] [PubMed] [Google Scholar]
Bridges 2013
- Bridges CB, Woods MDL, Coyne‐Beasley T. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older‐United States, 2013. Morbidity and Mortality Weekly Report. Surveillance Summaries : MMWR 2013;62(Suppl 1):9‐19. [PubMed] [Google Scholar]
Chaithongwongwatthana 2012
- Chaithongwongwatthana S, Yamasmit W, Limpongsanurak S, Lumbiganon P, DeSimone JA, Baxter JK, et al. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD004903.pub2] [DOI] [PubMed] [Google Scholar]
Deinard 1981
- Deinard AS, Ogburn P Jr. A/NJ/8/76 influenza vaccination program: effects on maternal health and pregnancy outcome. American Journal of Obstetrics and Gynecology 1981;140:240–5. [DOI] [PubMed] [Google Scholar]
Englund 1993
- Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. Journal of Infectious Diseases 1993;168(3):647‐56. [DOI] [PubMed] [Google Scholar]
France 2006
- France EK, Smith‐Ray R, McClure D, Hambidge S, Xu S, Yamasaki K, et al. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Archives of Pediatrics and Adolescent Medicine 2006;160(12):1277‐83. [DOI] [PubMed] [Google Scholar]
Gilbert‐Barness 2010
- Gilbert‐Barness E. Teratogenic causes of malformations. Annals of Clinical & Laboratory Science 2010;40:99‐114. [PubMed] [Google Scholar]
Glezen 1997
- Glezen WP, Taber LH, Frank AL, Gruber WC, Piedra PA. Influenza virus infections in infants. Pediatric Infectious Disease Journal 1997;16(11):1065‐8. [DOI] [PubMed] [Google Scholar]
GRADE 2014 [Computer program]
- McMaster University. GRADEpro [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Hartert 2003
- Hartert TV, Neuzil KM, Shintani AK, Mitchel EF Jr, Snowden MS, Wood LB, et al. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. American Journal of Obstetrics and Gynecology 2003;189(6):1705‐12. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Izurieta 2000
- Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. New England Journal of Medicine 2000;342(4):232‐9. [DOI] [PubMed] [Google Scholar]
Lindsay 2006
- Lindsay L, Jackson LA, Savitz DA, Weber DJ, Koch GG, Kong L, et al. Community influenza activity and risk of acute influenza‐like illness episodes among healthy unvaccinated pregnant and postpartum women. American Journal of Epidemiology 2006;163(9):838‐48. [DOI] [PubMed] [Google Scholar]
Mak 2008
- Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infectious Diseases 2008;8(1):44‐52. [DOI] [PubMed] [Google Scholar]
Munoz 2003
- Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Influenza virus infection in infancy and early childhood. Paediatric Respiratory Reviews 2003;4(2):99‐104. [PubMed] [Google Scholar]
Munoz 2005
- Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Safety of influenza vaccination during pregnancy. American Journal of Obstetrics and Gynecology 2005;192(4):1098‐106. [DOI] [PubMed] [Google Scholar]
Neuzil 1998
- Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. American Journal of Epidemiology 1998;148(11):1094‐102. [DOI] [PubMed] [Google Scholar]
Puck 1980
- Puck JM, Glezen WP, Frank AL, Six HR. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. Journal of Infectious Diseases 1980;142(6):844‐9. [DOI] [PubMed] [Google Scholar]
Reuman 1987
- Reuman PD, Ayoub EM, Small PA. Effect of passive maternal antibody on influenza illness in children: a prospective study of influenza A in mother‐infant pairs. Pediatric Infectious Disease Journal 1987;6(4):398‐403. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rudan 2004
- Rudan I, Tomaskovic L, Boschi‐Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bulletin of the World Health Organization 2004;82:895‐903. [PMC free article] [PubMed] [Google Scholar]
Rudan 2008
- Rudan I, Boschi‐Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization 2008;86:408‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salam 2012
- Salam RA, Das JK, Bhutta ZA. Impact of haemophilus influenza type B (Hib) and viral influenza vaccinations in pregnancy for improving maternal, neonatal and infant health outcomes. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009982] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santosham 2001
- Santosham M, Englund JA, McInnes P, Croll J, Thompson CM, Croll L, et al. Safety and antibody persistence following Haemophilus influenzae type b conjugate or pneumococcal polysaccharide vaccines given before pregnancy in women of childbearing age and their infants. Pediatric Infectious Disease Journal 2001;20(10):931‐40. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Shi 2005
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. International Journal of Developmental Neuroscience 2005;23:299‐305. [DOI] [PubMed] [Google Scholar]
Sumaya 1979
- Sumaya CV, Gibbs RS. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: reactogenicity and immunogenicity in mother and infant. Journal of Infectious Diseases 1979;140(2):141‐6. [DOI] [PubMed] [Google Scholar]
Walker 2013
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013;381(9875):1405‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2005
- WHO. Haemophilus influenzae type B (HiB). http://www.who.int/mediacentre/factsheets/fs294/en/index.html (accessed 2012) 2005.


