Abstract
Little attention has been paid to neglected tropical diseases (NTDs) in high-income countries and no literature provides an overview of NTDs in Japan. This scoping review aims to synthesize the latest evidence and information to understand epidemiology of and public health response to NTDs in Japan. Using three academic databases, we retrieved articles that mentioned NTDs in Japan, written in English or Japanese, and published between 2010 and 2020. Websites of key public health institutions and medical societies were also explored. From these sources of information, we extracted data that were relevant to answering our research questions. Our findings revealed the transmission of alveolar echinococcosis, Buruli ulcer, Chagas disease, dengue, foodborne trematodiases, mycetoma, scabies, and soil-transmitted helminthiasis as well as occurrence of snakebites within Japan. Other NTDs, such as chikungunya, cystic echinococcosis, cysticercosis, leishmaniasis, leprosy, lymphatic filariasis, rabies, and schistosomiasis, have been imported into the country. Government agencies tend to organize surveillance and control programs only for the NTDs targeted by the Infectious Disease Control Law, namely, echinococcosis, rabies, dengue, and chikungunya. At least one laboratory offers diagnostic testing for each NTD except for dracunculiasis, human African trypanosomiasis, onchocerciasis, and yaws. No medicine is approved for treatment of Chagas disease and fascioliasis and only off-label use drugs are available for cysticercosis, opisthorchiasis, human African trypanosomiasis, onchocerciasis, schistosomiasis, and yaws. Based on these findings, we developed disease-specific recommendations. In addition, three policy issues are discussed, such as lack of legal frameworks to organize responses to some NTDs, overreliance on researchers to procure some NTD products, and unaffordability of unapproved NTD medicines. Japan should recognize the presence of NTDs within the country and need to address them as a national effort. The implications of our findings extend beyond Japan, emphasizing the need to study, recognize, and address NTDs even in high-income countries.
Author summary
Neglected tropical diseases (NTDs) are an umbrella term for 20 diseases and disease groups recognized by the World Health Organization. Although NTDs are usually considered to be a public health problem in low- and middle-income countries, people living in high-income countries could also be affected. In this scoping review, we aimed to summarize the latest evidence and information to understand the epidemiology of and public health response to NTDs in Japan. Japan officially reported a yearly average of 340 NTD cases between 2014 and 2017; however, we found that it does not accurately reflect the current status of NTDs in the country. Our review suggests that 15 of 20 NTDs can be considered to be important public health concerns. While echinococcosis, chikungunya, dengue, and rabies are under control by law, many other NTDs are largely neglected by Japan’s health systems and solely addressed by a limited number of physicians and researchers. We recommend that the Government of Japan should assume responsibility for enhancing surveillance, expanding access to diagnostics and medicines, and ensuring affordability of unapproved NTD drugs.
Introduction
The World Health Organization (WHO) recognizes 20 diseases and disease groups as neglected tropical diseases (NTDs), which mainly affect people living in poverty [1]. NTDs accounted for approximately one percent of the global disease burden in 2012 [2] and at least 1.74 billion people require interventions against NTDs worldwide [1]. The United Nations’ Sustainable Development Goals (SDGs) include ending the epidemics of NTDs by 2030 as one of the global priorities [3].
Although NTDs are usually regarded as a public health problem in low- and middle-income countries, people living in high-income countries could be affected as well [4]. Hotez (2013) developed a concept of “blue marble health,” meaning that there is a paradoxical burden of NTDs among the poor living in wealthy countries [5]. Some underlying factors, including human migration [6] and climate change [7], are driving changes in the global distribution of NTDs. Researchers have investigated NTDs in high-income countries, for example in Italy [8, 9] and the United States [10]. High-income countries are no longer free from NTDs; Japan is no exception.
Little is known about the current status of NTDs in Japan. According to the Japan SDGs Action Platform run by the Ministry of Foreign Affairs of Japan, a yearly average of 340 NTD cases was reported between 2014 and 2017, which included cases of Buruli ulcer, cysticercosis, dengue, echinococcosis, foodborne trematodiases, leprosy, scabies, snakebite envenoming, and soil-transmitted helminthiases [11]. However, this is not a comprehensive case number because not all NTDs are reportable in Japan. To our knowledge, no literature has provided an overall situation of NTDs in Japan to date.
This scoping review aims to synthesize the latest evidence and information to understand epidemiology of and public health response to NTDs in Japan. To achieve this aim, the following guiding questions were addressed for each NTD: 1) What is the prevalence or incidence?; 2) How many cases are reported to the government bodies?; 3) Is the disease transmitted within Japan?; 4) Is any imported human case to Japan reported?; 5) Is there any human case surveillance implemented by the government bodies?; 6) Is there any control program implemented by government bodies?; 7) Is there any laboratory offering testing services for diagnosis?; and 8) Is there any vaccine or medicine available? Based on the findings, we also intend to develop recommendations to advance policy and research agenda for NTDs in Japan.
Methods
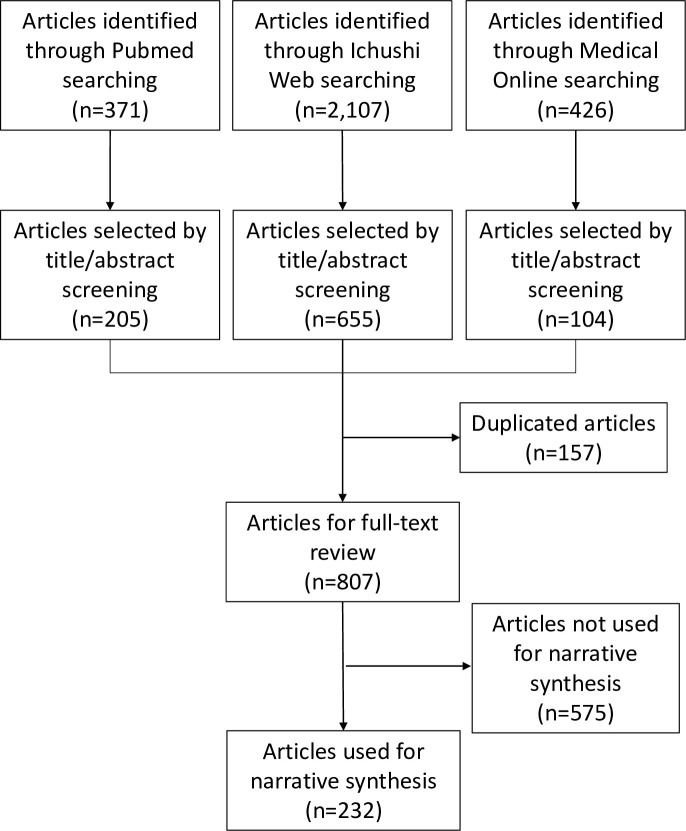
Our paper takes the form of scoping review. A scoping review is a useful research tool when the purpose of the review is to identify the types of available evidence or to scope a body of literature in a given field [12]. Our aim of synthesizing knowledge and information regarding NTDs in Japan justifies our choice of performing a scoping review. In reporting this study, we followed the recommendations of PRISMA Extension for Scoping Reviews (PRISMA ScR) [13]. We described the PRISMA ScR Flowchart in Fig 1.
Fig 1. PRISMA ScR Flowchart.
We developed a draft search protocol together including all authors of the Japan NTD Study Group. Two researchers independently tested the protocol by applying it to leishmaniasis and the other two researchers to scabies. We had a group discussion to compare the search results and revised the protocol until we reached a consensus. Once the protocol was established, one author was assigned to each NTD to collect data from the literature and websites. The same researcher synthesized the search results into a narrative. The narrative of each NTD was finally reviewed and revised by all authors. The following paragraphs describe our protocol in detail.
For our literature search, we used three online databases, namely, PubMed, Ichushi-Web, and Medical*Online. Ichushi-Web and Medical*Online are commonly used to search medical articles written in Japanese. Using the search strategy shown in S1 Document, we retrieved publications that met all of our inclusion criteria: 1) original research articles, clinical case reports, reviews, expert opinions, and commentaries that provide information about NTDs in Japan, 2) published in English or Japanese, and 3) published between January 1st, 2010 and December 31th, 2020. Then, we manually scanned the titles and abstracts of the retrieved articles and excluded articles that met any of our exclusion criteria: 1) an article focusing on the biology of pathogens, 2) an article focusing on product development, and 3) an article focusing on NTDs in countries other than Japan. Zotero (Corporation for Digital Scholarship, VA, USA) was used to store selected articles. As a data charting process, each researcher assessed the relevance of evidence in terms of our eight guiding questions. If the source of evidence included any information relevant to answering the guiding questions, we extracted that data for synthesis.
For the website search, we first browsed the Japanese government’s portal site [14] to identify laws framing responses to any NTDs, such as the Act on the Prevention of Infectious Diseases and Medical Care for Patients with Infectious Diseases (hereafter, the Infectious Diseases Control Law). When a disease is designated by the Infectious Diseases Control Law, that disease is subject to the national surveillance system. Second, we explored the Infectious Diseases Weekly Report [15], issued by the National Institute of Infectious Diseases (NIID) to identify notifications of NTDs between 2010 to 2020. Lastly, we collected information about the availability of vaccines, diagnostic testing, and medicines for NTDs in Japan. For vaccines, we searched the database of the Pharmaceuticals and Medical Devices Agency (PMDA) [16], a regulatory agency in Japan, to identify vaccine products approved in Japan. For diagnosis, the availability of diagnostic testing of any NTDs was searched based on the NIID’s Manual for the Detection of Pathogen [17] and an on-line directory named “Infectious Diseases Map for Advanced Progressive Laboratory Tests (IDMA)” [18], which is co-provided by the Japanese Association for Infectious Diseases and the Japanese Society for Clinical Microbiology. For medicines, we identified generic names of NTD medicines recommended by WHO using its Model List of Essential Medicine [19] or Fact Sheets [20], and then, checked whether that medicine has been approved by PMDA. We also looked into the website of the Research Group of Tropical Diseases and Parasitic Diseases [21] as this research group supplies some unapproved medicines for treatment of tropical diseases. In writing the Result section, we omitted citations to these websites to avoid redundancy.
For synthesis, each researcher used Table 1 to check whether she or he could collect data to answer the guiding questions. Then, the researcher summarized the data by constructing a narrative about epidemiology of and public health response to each NTD. All researchers read every narrative and revised it until we reached a consensus. The final narratives are presented in the Result section.
Table 1. Summary of epidemiology of and response to neglected tropical diseases in Japan between 2010 and 2020.
| NTD (WHO category) | NTD (sub-category) | Epidemiology | Response | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimation of incidence or prevalence | Number of cases reported to NIID (2010–2020) [15] |
Any evidence of domestic transmission? | Any evidence of imported cases? | Any surveillance of human cases by government bodies? [14] |
Any control program by government bodies? [14] |
Any laboratory offering confirmatory testing for diagnosis? [17, 18] |
At least one medicine approved by PMDA for treatment? [16] |
||
| Buruli ulcer | n/a | 52a | Yes [22, 23] |
No | Yes | No | Yes | Yes (rifampicin, clarithromycinb, and levofloxacinb) | |
| Chagas disease | 3,000–4,500 prevalent cases [24] | - | Yes [24, 25] |
Yes [26–31] |
No | No | Yes | No | |
| Cysticercosis | n/a | - | No | Yes | No | No | Yes | Yes (albendazoleb and praziquantelb) | |
| Dengue/Chikungunya | Dengue | 200–300 imported cases per year (More than 4,000 imported asymptomatic cases are expected.) [32] |
2,755 | Yes [33, 34] |
Yes [35, 36] |
Yes | Yes | Yes | - |
| Chikungunya | n/a | 142c | No | Yes [37, 38] |
Yes | Yes | Yes | - | |
| Dracunculiasis | n/ad | - | No | No | No | No | No | - | |
| Echinococcosis | Alveolar echinococcosis | n/a | 254 | Yes [39–45] |
Yes [46] |
Yes | Yes | Yes | Yes (albendazole) |
| Cystic echinococcosis | n/a | 10 | No | Yes [47–54] |
Yes | Yes | Yes | Yes (albendazole) | |
| Foodborne trematodiases | Clonorchiasis, Paragonimiasis | n/a | - | Yes [55–58] |
Yes [56–60] |
No | No | Yes | Yes (praziquantel) |
| Fascioliasis | n/a | - | Yes [61] |
Yes [62, 63] |
No | No | Yes | No | |
| Opisthorchiasis |
n/ad | - | No | No | No | No | Yes | Yes (praziquantelb) | |
| Human African trypanosomiasis | n/ad | - | No | No | No | No | No | Yes (pentamidineb) | |
| Leishmaniasis | n/a | - | No | Yes [64–70] |
No | No | Yes | Yes (amphotericin B and paromomycin sulfateb) | |
| Leprosy | Nearly zero new patients (among Japanese) [71] |
41 | No | Yes [71–75] |
Yes | No | Yes | Yes (rifampicin, dapsone, and clofazimine) | |
| Lymphatic filariasis | n/a | - | No | Yes [76, 77] |
No | No | Yes | Yes (ivermectinb and albendazoleb) | |
| Mycetoma | Mycetoma | n/a | - | Yes [78, 79] |
No | No | No | Yes | Yes (itraconazloleb) |
| Chromoblastomycosis | n/a | - | Yes [80–83] |
Yes [81] |
No | No | Yes | Yes (itraconazole) | |
| Sporotrichosis | n/a | - | Yes [84–87] |
No | No | No | Yes | Yes (itraconazole, amphotericin Bb, and potassium iodide) | |
| Paracoccidioidomycosis | n/a | - | No | Yes [88] |
No | No | Yes | Yes (itraconazloleb) | |
| Onchocerciasis | n/ad | - | No | No | No | No | No | Yes (ivermectinb) | |
| Rabies | Zero incidencee [89] |
1 | No | Yes [89] |
Yes | Yes | Yes | -f | |
| Scabies |
|
80,000–150,000 incident cases per year [90] |
- | Yes [91–98] |
Yes [94] |
No | No | Yes | Yes (ivermectin) |
| Schistosomiasis | n/a | - | No | Yes [99–107] |
No | Yes | Yes | Yes (praziquantel b) | |
| Snakebites | 1,000–3,000 incident cases per year [108–110] |
- | Yes [111–127] |
No | Yesg | No | - | Yes (Freeze-dried antivenomsh) | |
| Soil-transmitted helminthiases | Ascariasisi | n/a | - | Noj | Noj | No | No | Yes | Yes (pyrantel and albendazoleb) |
| Trichuriasis | n/a | - | No | Yes [128] |
No | No | Yes | Yes (mebendazole and albendazoleb) | |
| Necatoriasis | n/a | - | Noj | Noj |
No | No | Yes | Yes (pyrantel and albendazoleb) | |
| Strongyloidiasis | At least 15 cases found per year [129] |
- | Yes [130] |
Yes [131] |
No | No | Yes | Yes (ivermectin and albendazoleb) | |
| Trachoma | n/ad | - | No | No | No | No | Yes | Yes (azithromycin and tetracycline) | |
| Yaws | n/ad | - | No | No | No | No | No | Yes (azithromycinb and benzathine penicillinb) | |
Abbreviations
NIID: National Institute of Infectious Diseases
PMDA: Pharmaceuticals and Medical Devices Agency
Legend
-: Not applicable.
n/a: Data not available.
Notes
a Data not available for 2018–2020.
b For off-label use only.
c Data not available for 2010.
d The incidence and prevalence are probably zero since no evidence suggests either domestic transmission or importation.
e Rabies is eliminated in Japan, but one imported case was exceptionally found in 2020.
f Vaccines are approved by PMDA for pre- and post-exposure prophylaxis but rabies immune globulins are unapproved.
g Surveillance of habu bites in Kagoshima and Okinawa only.
h Yamakagashi antivenom is not approved by PMDA.
i Not including Ascaris suum infections.
Results
As Fig 1 shows, a total of 807 articles across 20 NTDs met our inclusion and exclusion criteria (see the numbers of selected articles for each disease in S1 Document). A full list of these 807 articles can be found in S2 Document. Among them, we used 232 articles for the narrative synthesis. In this Result section, we provided key findings from our review by disease area, following our guiding questions. Key findings are summarized in Table 1. A map of Japan showing the locations of prefectures mentioned in this review can be found in S3 Document.
Buruli ulcer
Epidemiology
Buruli ulcer is an infectious disease caused by Mycobacterium ulcerans. The route of infection is still unknown [134] and human-to-human transmission has not been reported globally [135]. In Japan, the first case was found in 1979 in which a 19-year-old female with no travel history was diagnosed with the infection of M. ulcerans subsp. shinshuense (Mikoshiba et al. (1982) cited by [22]). Since then, a total of 75 cases have been reported to the Leprosy Research Center (LRC) at NIID by the end of 2020, with an average of one to three cases per year (Table 2). A concentration of cases has been observed in the central western region, especially in Okayama Prefecture. As with the first case, all reported cases have been ulcerated cases caused by M. ulcerans subsp. shinshuense [136, 137]. This subspecies differs from those identified in West Africa and Australia where the disease is also reported and is a domestic strain to Japan or possibly to some parts of Asia [135]. Cases diagnosed in Japan from 1980 to 2010 showed no evidence of patient contact with an aquatic environment [22] and the route of transmission remains unclear in Japan, as also the case in other parts of the world. Further investigation is needed to understand the route of transmission of M. ulcerans in Japan.
Table 2. Number of NTD cases reported to the National Institute of Infectious Diseases between 1999 and 2020.
| Year | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buruli ulcer | 1 | 1 | 1 | 3 | 2 | 5 | 9 | 10 | 4 | 10 | 7 | 4 | 2 | 6 | ||||||||
| Chikungunya | 10 | 10 | 14 | 16 | 17 | 14 | 5 | 4 | 49 | 3 | ||||||||||||
| Dengue | 9 | 18 | 50 | 52 | 32 | 49 | 74 | 58 | 89 | 104 | 93 | 244 | 113 | 221 | 249 | 341 | 293 | 342 | 245 | 201 | 461 | 45 |
| Echinococcosis, alveolar | 6 | 20 | 13 | 8 | 20 | 25 | 18 | 19 | 23 | 23 | 26 | 19 | 22 | 15 | 19 | 28 | 27 | 27 | 29 | 18 | 27 | 23 |
| Echinococcosis, cystic | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 0 | 3 | 2 | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Leprosy | 19 | 14 | 13 | 16 | 8 | 12 | 6 | 7 | 12 | 7 | 2 | 4 | 5 | 3 | 4 | 5 | 7 | 3 | 2 | 3 | 5 | 4 |
| Rabies | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Source: National Institute of Infectious Diseases. We used the following webpages: https://www.niid.go.jp/niid/ja/ydata/10067-report-ja2019-20.html (for chikungunya, dengue, echinococcosis, and rabies), https://www.niid.go.jp/niid/ja/leprosy-m/1841-lrc/1707-expert.html (for leprosy), and https://www.niid.go.jp/niid/ja/bu-m/1842-lrc/1692-buruli.html (for Buruli ulcer). Exceptionally, data for the year 2020 were retrieved from NIID’s annual report (https://www.niid.go.jp/niid/images/idsc/idwr/IDWR2020/idwr2020-52-53.pdf). Since this 2020 report made no distinction between alveolar and cystic echinococcosis, we assumed that all cases were alveolar echinococcosis.
Public health response
No legal framework exists to respond to Buruli ulcer. Surveillance of Buruli ulcer is led by the LRC/ NIID, while there is no mandate issued by the government for the conduct. There could be under-reporting as the reporting of the disease is not mandatory, and the reporting only happens when attending physicians are aware of the reporting system. LRC compiles data and reports to WHO.
In Japan, the diagnosis of Buruli ulcer is based on the following criteria: 1) cutaneous manifestation compatible with Buruli ulcer, such as deep ulceration, necrotic tissue, presence of undermining, 2) a histopathological examination of the skin showing necrosis with limited inflammatory cells and/or presence of acid-fast bacilli, and 3) a positive result of a polymerase chain reaction (PCR) test detecting the M. ulcerans-specific IS2404 gene. PCR test is available at LRC as an administrative diagnostic test, and they receive samples nationwide. Mycobacterial culture is also recommended. However, it may take months to culture M. ulcerans and the results are often unavailable at the time of making treatment decisions; it is sometimes useful in examining the drug susceptibility.
Among two medicines recommended by WHO, rifampicin is approved in Japan for treatment of Buruli ulcer. The other medicine, clarithromycin, is also approved by PMDA but is available only for off-label use. In addition, levofloxacin is often added to the regimen, which is also available only for off-label use.
Chagas disease
Epidemiology
Chagas disease is caused by a parasite, Trypanosoma cruzi. In Japan, Chagas disease is mainly found among immigrants from endemic countries, such as Brazil, Bolivia, and Peru. The number of Latino immigrants living in Japan has increased after Latin Americans of Japanese origin were allowed to work and stay in Japan in 1990 [138]. Nearly 230,000 Latin Americans nowadays reside in Japan [25], and it is estimated that 3,000–4,500 people are infected with T. cruzi [24].
Triatoma rubrofasciata, a potential vector of T. cruzi, is distributed among the Nansei Islands in Okinawa and Kagoshima Prefectures, but the risk of T. cruzi transmission by this vector species is considered low [139, 140]. The parasite can be spread via congenital transmission, blood transfusion, and organ transplantation [141–143], which are the potential transmission route for Chagas disease in Japan.
Several cases of T. cruzi infection have been reported among Latino immigrants living in Japan [26, 27], including two cases in which patients were treated with benznidazole [28–31], one case in which a pacemaker was implanted for Chagas cardiomyopathy [144], and two cases of death from Chagas disease [145, 146]. It is noteworthy that two cases of congenital transmission were also reported in Japan [24, 25] and both cases were treated with benznidazole [26, 31, 147, 148].
The Japanese Red Cross Society revealed that 3 out of 18,076 (0.017%) at-risk blood donors, defined as those who were born or raised in Latin America, those whose mothers were born or raised in Latin America, or those with a travel history to Latin America, were positive for T. cruzi antibody [149]. The first T. cruzi-infected blood donor was found in 2013 [138, 150]. Another T. cruzi-infected donor found in 2014 had previously provided blood products to 11 recipients but the transfusional transmission of T. cruzi has not been confirmed to date in Japan [25, 149, 151, 152]. No case of transmission by organ transplantation has been reported to date [24].
Public health response
There is no legal framework for responding to Chagas disease. The Japanese Red Cross Society currently runs a screening program targeting all at-risk blood donors [140]. In addition, a non-profit organization provides weekly screening at the Consulate-General of Brazil in Tokyo [25]. Laboratory testing for the diagnosis of Chagas disease is available at Saitama Medical University (https://ccidr-saitama.com/en/clinical-research-2/). None of the therapeutics, benznidazole nor nifurtimox, is approved by PMDA. Both therapeutics can be obtained from WHO, according to the Research Group of Tropical Diseases and Parasitic Diseases’ website.
Dengue and chikungunya
Epidemiology
Dengue and chikungunya are febrile diseases caused by viruses that are transmitted to humans mainly by two mosquito species: Aedes aegypti and Aedes albopictus. A. aegypti has not established its life cycle in Japan, but there is a risk that this mosquito species can be imported into Japan [153]. A. albopictus is the main vector in Japan and its habitat has spread into the northern prefectures, probably due to global warming [154].
In Japan, more than 100 dengue cases have been reported to NIID each year since 2010, with the highest number of 461 in 2019 (Table 2). As only symptomatic cases are detected and reported to NIID, the actual number of infections, including asymptomatic cases, was estimated to be 20 times higher than the number of reported cases [32]. Dengue is largely an imported disease in Japan, as shown by one fatal case in 2016 with a person who returned from the Philippines [35]. From 1945 to 2013, no domestic transmission was confirmed [36], except for a case in which a nurse was infected by a needlestick injury in 1992 [155]. In 2013, a case of possible domestic transmission was reported, in which a German traveler was diagnosed with dengue fever after she visited Japan [156, 157]. In 2014, an outbreak of dengue fever occurred in Tokyo [33]. This outbreak resulted in the confirmation of 162 cases among visitors to public parks, such as Yoyogi Park in Tokyo [34]. Serotype 1 dengue virus was detected from mosquitoes collected in Yoyogi Park as well as from patients who had visited the park [158–161]. The virus was considered to have originated from Southeast Asia or China [162].
Between 2011 and 2020, a total of 142 chikungunya cases have been reported to NIID (Table 2). To date, all reported cases were imported from outside Japan, such as Sri Lanka, Malaysia, Angola, Indonesia, the Philippines, Tonga, the Commonwealth of Dominica, Colombia, and Cuba [37, 38]. Case reports described how chikungunya was imported to Japan, including two cases of Japanese siblings who visited the Cook Islands in 2015 [163], a case of a traveler from Angola to Japan in 2016 [164], a case of a Japanese returning traveler from India [165], a case of a Japanese woman who worked in the Commonwealth of Dominica in 2014 [166], and a case of a Japanese returning traveler from Jamaica [167]. The Narita Airport quarantine station also reported three cases among travelers from Southeast Asia in 2013 [168].
Public health response
Dengue and chikungunya are covered by the Infectious Disease Control Law and any confirmed cases should be immediately reported to the NIID. The Quarantine Law requires quarantine stations at ports and airports to offer medical examinations and blood tests for the detection of dengue and chikungunya to travelers who present symptoms and risks of having been bitten by mosquitoes. The Ministry of Health, Labor, and Welfare issued a guideline in 2015 for the prevention of mosquito-borne infections [169]. This guideline defines the roles of stakeholders, including the national and prefectural governments. The NIID also published a manual for local governments to conduct epidemiological surveys and mosquito control [169]. When a case of domestic dengue transmission was confirmed in Okinawa Prefecture in 2019, the prefectural government responded immediately following these guidelines and successfully prevented further transmission [170].
NIID has published laboratory procedure manuals for both diseases and laboratory tests are available at the NIID and Prefectural Institutes of Public Health [171, 172]. In addition, diagnosis of dengue is available at medical facilities listed by the Japanese Society of Travel and Health (http://jstah.umin.jp/03posttravel/index.htm). Dokkyo Medical University’s Saitama Medical Center also offers testing for chikungunya.
Dracunculiasis
Epidemiology
Dracunculiasis, or Guinea-worm disease, is caused by the parasitic worm, Dracunculus medinensis. In recent years, human cases have been reported only from some African countries. Our review found no evidence of dracunculiasis in Japan.
Public health response
No legal framework covers dracunculiasis. Laboratory services to support diagnosis of dracunculiasis are not available in Japan.
Echinococcosis
Epidemiology
Human echinococcosis involves two diseases: cystic echinococcosis caused by Echinococcus granulosus and alveolar echinococcosis caused by Echinococcus multilocularis. Between 2010 and 2019, a total of 10 and 231 cases were reported to NIID for the infection of E. granulosus and E. multilocularis, respectively. The reporting rates are relatively stable over the last 20 years for both infections (Table 2).
In Japan, cystic echinococcosis is considered to be an imported disease [47–49]. The infection of E. granulosus was confirmed in a Japanese female who might have been infected in the United Kingdom [50] and immigrants from Afghanistan [51], China [47], Nepal [52, 53], and Peru [54]. While E. granulosus seems to be continuously being introduced into Japan by imported cattle [173], there is no evidence that this parasite has established a transmission cycle infecting humans within Japan.
In contrast, E. multilocularis infection is endemic to the northern island of Hokkaido Prefecture. Between 1924–1926, foxes infected with E. multilocularis were artificially introduced to Hokkaido for rodent control by mankind [174]. Since the first human case was found in 1936, the infection has spread over Hokkaido by the 1980s [175]. The majority of human cases have occurred in Hokkaido, with some exceptions found in other prefectures [39]. Several clinical cases are well documented, including Japanese adults living in Hokkaido [40–42], a pediatric case in Hokkaido in which the patient died of liver failure [43], adult cases found in Chiba and Kanagawa Prefectures who had traveled to Hokkaido [44, 45], and a Bolivia-born female with a history of traveling to Hokkaido who was co-infected by E. granulosus and E. multilocularis [46]. In addition to foxes, the animal infection has also been reported in rats [176], horses [177, 178], pigs [39], dogs [179–181], a zoo-raised Diana monkey [182], and a flying squirrel [183]. The further spread of E. multilocularis from Hokkaido to other areas due to the travel of infected humans and animals is a significant concern in Japan. NIID considers that E. multilocularis has established its life cycle among wild dogs in some areas of Aichi Prefecture, but no human case has been yet reported [184].
Public health response
Echinococcosis is covered by the Infectious Diseases Control Law and any infected cases should be immediately reported to NIID. The Hokkaido local government has implemented an echinococcus control program, combining health education, screening for human echinococcosis, vector control, and improvement of clean water supply, which is considered to be key to preventing an outbreak of human infections in Hokkaido Prefecture [175]. NIID published a manual of the diagnostic procedure to detect and report echinococcus infections [185]. According to this manual, differential diagnosis is available at the NIID and the Hokkaido Institute of Public Health. For treatment, the WHO-recommended albendazole is approved by PMDA.
Foodborne trematodiases
Epidemiology
Foodborne trematodiases are caused by intakes of fish, crabs, or vegetables contaminated by the larvae of trematode. WHO recognizes four foodborne trematodiases: clonorchiasis, opisthorchiasis, fascioliasis, and paragonimiasis. Of these, clonorchiasis, fascioliasis, and paragonimiasis are endemic in Japan. Imported cases of opisthorchiasis have been reported in the past, but we found no literature for this review.
Clonorchiasis is caused by Clonorchis sinensis parasitizing the bile ducts in the liver. In Japan, C. sinensis used to be widely distributed all over the country except in Hokkaido Prefecture. The number of cases has fallen drastically after the 1970s as the population of the intermediate host, Parafossarulus snails, declined; however, the domestic transmission is not completely contained [55]. Sporadic imported cases and a few domestic cases are reported to date in Japan [56, 57].
Fascioliasis is caused by Fasciola hepatica and Fasciola gigantica. Fasciola spp. is primarily found in the ungulates, such as cattle, sika deer, and wild boars. The prevalence of fascioliasis in beef cattle has gradually decreased from 18.6% in 1964 to 0.06% in 2010; however, Fasciola spp. is still detected in cattle produced all over Japan [186]. A few cases of human fascioliasis have been reported, and there are risks of domestic transmission among small-scale cattle ranchers and their neighbors. The consumption of vegetables contaminated with metacercaria could be a source of Fasciola spp. infections in Japan [61]. In addition, some imported cases of human fascioliasis from Vietnam and China have been reported [62, 63].
Paragonimiasis is caused by an infection with Paragonimus spp. in the human lungs. P. westermani and P. miyazakii are widely distributed in all regions of Japan except in Hokkaido Prefecture [187]. About 50 cases of paragonimiasis continue to be reported annually [188]. Paragonimus spp. is transmitted by the consumption of inadequately cooked freshwater crabs [187, 189] or meat of wild boar and sika deer [190–193]. Some basins in Chiba Prefecture are identified with a high risk of domestic transmission as more than 90% of freshwater crabs were found with P. westermani [194]. In contrast to the decreasing number of cases among Japanese nationals, the number of cases among migrants living in Japan is increasing [58–60].
Public health response
Under the Food Sanitation Act, foodborne trematodiases are reportable to health centers as food poisoning events, but the food poisoning statistics do not count them as separate diseases. Confirmatory diagnosis is made by morphological identification of eggs but in Japan, diagnosis is based on a combination of imaging with X-rays and MRI, parasite-specific antibody tests, and molecular confirmation. Serological tests of foodborne trematodiases are available at Miyazaki University and Aichi Medical University. The Department of Parasitology of NIID offers both serological and molecular tests. Praziquantel is approved by PMDA for treatment of clonorchiasis and paragonimiasis. It can also be used for treatment of opisthorchiasis as an off-label drug. Triclabendazole is recommended by WHO for treatment of fascioliasis and paragonimiasis but is not approved by PMDA. Triclabendazole is stored by the Research Group of Tropical Diseases and Parasitic Diseases for clinical trials.
Human african trypanosomiasis
Epidemiology
Human African trypanosomiasis, or sleeping sickness, is caused by the parasite Trypanosoma brucei. Transmitted by tsetse flies, this disease is endemic in sub-Saharan African countries. Our review found no evidence of human African trypanosomiasis in Japan. Even if people infected with T. brucei come to Japan from the endemic regions, the risk of transmission is considered low because no tsetse flies have been found in the country [195].
Public health response
No legal framework is available for human African trypanosomiasis. The Japanese Red Cross Society does not take blood donations from those who have lived in Africa and have a history of the disease. No institution offers diagnostic testing. Among six WHO-recommended drugs, namely, pentamidine, suramin, melarsoprol, eflornithine, nifurtimox, and fexinidazole, only pentamidine is approved by PMDA for off-label use. Suramin, melarsoprol, and eflornithine can be obtained from WHO, according to the Research Group of Tropical Diseases and Parasitic Diseases.
Leishmaniasis
Epidemiology
Leishmaniasis is caused by infection with Leishmania parasites, which are transmitted by sandfly species. In Japan, a small number of imported cases of cutaneous and mucocutaneous leishmaniasis have been documented. These cases have been found in three groups, including Japanese nationals who came back from overseas, such as Venezuela [64], Burkina Faso [65], and Brazil [66]; Japanese descendants who had lived overseas, such as in Bolivia [67] and Brazil [68]; and non-Japanese nationals who came to Japan, such as a student from Sri Lanka [69], a child from Syria [64], and an adult from Ethiopia [70]. In 2006 and 2007, two cases of canine leishmaniasis were found in dogs that used to live in Italy [196]. One of the vector species, Phlebotomus squamirostris, has been found in Japan [197]. Despite the continuous importation of the parasites and the presence of vectors within the country, there is no evidence of domestic transmission of Leishmania parasites to humans.
Public health response
No legal framework responds to leishmaniasis. Diagnosis and treatment of leishmaniasis have occurred occasionally, only when experienced physicians could suspect the infection, particularly seeing immigrants or returnees with skin lesions. Aichi Medical University Hospital offers parasitological tests for leishmaniasis. Among five medicines listed by the WHO, namely, amphotericin B, meglumine antimoniate, miltefosine, paromomycin, and sodium stibogluconate, only amphotericin B is approved by PMDA for treatment of leishmaniasis. Paromomycin sulfate is also approved by PMDA but is available for off-label use only. Miltefosine and sodium stibogluconate can be privately imported [21].
Leprosy
Epidemiology
Leprosy, caused by Mycobacterium leprae, is nearly eliminated in Japan. Since 2008, the annual number of newly diagnosed cases has stayed below 10 (Table 2). While we found no evidence of recent domestic transmission, cases of leprosy continue to be reported. LRC reported seven new cases among Japanese nationals from 2010 to 2020 [71]. More than half of the new Japanese cases are reported from Okinawa Prefecture [198], where a few relapsed cases are also found every year [199]. Case reports describe conjugal infection among a couple [200], relapsed leprosy developing drug resistance [201], and leprous neuropathy [202]. Sequelae of leprosy are also documented, such as skin cancers formed at chronic ulcers [203, 204], intractable ulcers complicated by diabetes and the sequelae of leprosy [205], visual dysfunction [206, 207], and dysmasesis and dysphagia due to facial paralysis [208].
Alongside the domestic cases, there are also imported cases from endemic countries [72]. LRC reported a total of 38 new cases of foreign nationals from 2010 to 2020, which is 84% of the total number of new cases reported during the same period [71]. For example, three foreign nationals, including a Filipino, a Paraguayan, and a United State soldier born in Micronesia, were found with leprosy in Japan [73–75]. A Brazilian who had been treated for leprosy in Brazil 16 years ago was diagnosed with relapsed leprosy in Japan [209].
Public health response
Leprosy control in Japan has a history of over 100 years. The Leprosy Prevention Law was first established in 1907, then revised twice, and repealed in 1996 [210, 211]. The main approach of this law was to isolate patients in national leprosy sanatoria to break the chain of transmission, while the current scientific evidence tells us that this was not necessary, especially after the introduction of antibiotic treatment in the early 1950s. Between 1909 and 2010, a cumulative total of 56,575 patients were admitted to the 13 national sanatoria [212]. Although the law became a dead letter around 1970 [210], 1,718 persons still live in sanatoria as of 2016 [213]. The long-lasting isolation policy severely affected patients’ dignity and raison d’etre as human beings [214]. Even after their reintegration into society, ex-patients often face stigma and discrimination, and therefore many preferred to remain in sanatoria [215–217]. The patient associations continue to fight for the recovery of deprived human rights [213].
Currently, there is no legal framework for leprosy control, in terms of newly diagnosed cases. There are two existing laws aiming to restore the honor of those affected by the isolation policy and discrimination. LRC functions as a referral center for newly diagnosed cases and compiles data and reports to WHO. The LRC also provides administrative laboratory testing for the diagnosis of leprosy. Three WHO-recommended medicines, namely, rifampicin, dapsone, and clofazimine (multi-drug therapy; MDT), are approved by PMDA. The Japanese Leprosy Association publishes guidelines for diagnosis and treatment [218, 219]. Abolishment of the Leprosy Prevention Law in 1996 allowed any dermatologist to provide medical care to leprosy patients [220]; however, diagnosis of leprosy-related conditions tends to delay because of the lack of knowledge about leprosy among healthcare providers [72], no follow-up system for ex-patients, or patients’ hesitation to consult local physicians [204]. Current challenges include providing care to the small number of new, relapsed, or imported cases, as well as, addressing sequelae or complications among ex-patients [221].
Lymphatic filariasis
Epidemiology
Lymphatic filariasis is a mosquito-borne disease caused by Wuchereria bancrofti, Brugia malayi, and Brugia timori. This disease was endemic in the Kyushu Region, Okinawa Archipelago, and Ogasawara Islands of Tokyo but was eliminated in the 1970s by large-scale community screening, awareness campaigns, chemotherapy of the infected people with diethylcarbamazine (DEC), and vector control [222]. This set of interventions was a new strategy at the time, which is now adapted by endemic countries worldwide. In recent years, we only experience, on a yearly average, one or two imported cases among migrants or travelers [76, 77].
Public health response
No legal framework responds to lymphatic filariasis. Serological and molecular testing is available at Aichi Medical University. One of the three WHO-recommended medicines, DEC, is approved by PMDA for treatment of lymphatic filariasis. The other two WHO-recommended medicines; ivermectin and albendazole, are approved by PMDA but are available for off-label use only.
Mycetoma, chromoblastomycosis and other deep mycoses
In line with the WHO’s NTD road map [1], this section addresses four diseases: mycetoma, chromoblastomycosis, sporotrichosis, and paracoccidioidomycosis.
Epidemiology
Mycetoma is a chronic, granulomatous infectious disease and can be etiologically classified into two subgroups: eumycetoma caused by fungus and actinomycetoma caused by bacteria. About 20 pathogens are known to cause mycetoma [223]. Our review identified two cases of mycetoma which were caused by bacteria belonging to the Nocardia genra [78, 79]. Nocardia brasiliensis is the most common causative agent of cutaneous nocardiosis [79]. In Japan, mycetoma cases tend to be reported as bacterial or fungal infections rather than defining them as mycetoma because diagnosis of mycetoma largely relies on the identification of causative pathogens by molecular methods. Indeed, among 88 cases of N. brasiliensis infection found from 1995 to 2013, only 16 cases (18%) were reported as mycetoma [79]. This reporting practice seems to obscure the epidemiological understanding of mycetoma in Japan. Imported cases have not been reported for the study period.
Chromoblastomycosis is a fungal infectious disease often triggered by trauma. In Japan, Fonsecaea monophora is considered to be the most common causative agent [224]. Our review found case reports of F. monophora infection [80–82] as well as F. nubica infection [83]. While chromoblastomycosis is largely transmitted within Japan, an imported case from the Philippines was also found in 2008 [81]. Recently, 10 to 20 cases of chromoblastomycosis or phaeohyphomycosis have been reported every year [224].
Sporotrichosis is known to be associated with farming and gardening since its causative fungus can be found in soil or plants. Causative pathogens are morphologically similar fungi, including Sporothrix schenckii, S. globosa, and other S. spp. In Japan, a molecular study revealed that most of the clinical strains (291/300) previously identified as S. schenckii were actually S. globosa [225]. The guideline for treatment of fungus infections, published by the Japanese Dermatological Association, describes S. globosa as the main causative agent of sporotrichosis [224]. Our literature search identified 16 domestically transmitted cases [84–86]. Geographically, most of the cases were found in the river basins of the Kanto and Kyushu Regions [87]. This disease is commonly observed on the upper extremities in adults and on the face in children, although the number of pediatric cases is decreasing [224]. The number of newly diagnosed cases peaked in the 1980s and has gradually declined due to urbanization and a decrease in the number of farmers [226], with an average of approximately 10 cases per year since 2010 [224].
Paracoccidioidomycosis is caused by Paracoccidioides brasiliensis. The number of paracoccidioidomycosis cases increased rapidly in the 1990s, mainly among Brazilians coming to Japan, but has decreased since 2000 [227]. Between 1961 and 2015, 21 cases were reported and this disease is recognized as one of the imported fungal infections in Japan [88].
Public health response
No legal framework responds to mycetoma, chromoblastomycosis, sporotrichosis, and paracoccidioidomycosis. At the moment of this writing, IDMA provides a list of 17 laboratories. Among them, the Medical Mycology Research Center at Chiba University is known as a specialized diagnostic facility (http://www.pf.chiba-u.ac.jp/). Treatment of mycetoma depends on the causative pathogens. WHO does not specify any drug but itraconazole, which is described as treatment of eumycetoma in the WHO’s NTD road map [1]. Itraconazole is also included in the WHO Model Lists of Essential Medicines for treatment of chromoblastomycosis, sporotrichosis, and paracoccidioidomycosis. In Japan, itraconazole is approved by PMDA for treatment of chromoblastomycosis and sporotrichosis. In addition, amphotericin B and potassium iodide are recommended by the WHO Model Lists for treatment of sporotrichosis. Both medicines are approved by PMDA while amphotericin B is available for off-label use only. The Japanese Dermatological Association has a guideline for treatment of fungal infections published in 2019 [224].
Onchocerciasis
Epidemiology
Onchocerciasis, also commonly known as river blindness, is caused by a parasite called Onchocerca volvulus. O. volvulus is transmitted by repeated bites from black flies (Simulium spp.). In Japan, no cases of onchocerciasis caused by O. volvulus, including imported cases, have been reported to date. In contrast, several human cases of zoonotic onchocerciasis, caused by Onchocerca dewittei japonica, have been reported in the last 20 years [228–232]. The terminal hosts of O. dewittei japonica are domestic animals, such as cattle and horses, as well as wild animals, such as wild boar and Japanese deer.
Public health response
No legal framework responds to onchocerciasis. No hospitals and laboratories offer diagnostic testing. Ivermectin, a medicine recommended by WHO for treatment of onchocerciasis, is approved by PMDA but for off-label use only.
Rabies
Epidemiology
In Japan, there were large epidemics of rabies before the 1940s [89]. Rabies was successfully eliminated by 1957 and, as Table 2 shows, only a few cases have been reported in recent years: one imported case in 1970 and two cases in 2006 with travel histories abroad [233]. In 2020, one Filipino worker developed rabies in Japan, who was previously bitten by a dog back in the Philippines [234].
One study showed that the incidence of animal bites among Japanese expatriates and travelers in Thailand was 1.7 and 43.1 per 1,000 person-months, respectively [235]. Certain numbers of Japanese travelers are treated in Japan for possible rabies exposure abroad [236, 237]. The majority of exposures occurred in Asia due to dog or cat bites. One study showed that only 13.0% - 16.7% of travelers bitten by animals abroad received rabies immunoglobulin (RIG) before they returned to Japan [238].
Public health response
Three laws, the Rabies Prevention Law, the Domestic Animal Infectious Diseases Control Law, and the Infectious Diseases Control Law, frame prevention and control of human and animal rabies, such as registration and vaccination of domestic dogs, required quarantine of susceptible imported animals, and planning of science-based national actions. Notification of human rabies is mandatory. The government published a guideline for rabies control in 2001 and revised it in 2013 [239]. More about government rabies control is described elsewhere [240]. Laboratory diagnostic tests for rabies confirmation are available at NIID, Oita University, and Kagoshima University. RIG is not available in Japan. Two human rabies vaccines are approved by PMDA, namely, Rabipur (GSK) and Inactivated Tissue Culture Rabies Vaccine (KM Biologics CO., Ltd.).
There have been ongoing discussions about the mandatory annual vaccination of domestic dogs. The current vaccination schedule can provide dogs with protective antibody levels [241]. Although the official data report coverage of over 70%, the true coverage could be lower because of unregistered and unvaccinated dogs. While a web-based survey suggested a high prevalence of immunity among domestic dogs [242], two small-scale studies suggested low protection levels in impounded dogs [243] and juvenile dogs [244]. Furthermore, some researchers argue for the discontinuation of the mandatory vaccination of domestic dogs based on several pieces of evidence. The risk of the re-introduction of a rabid dog to Japan is remarkably low [245, 246]. Even when a rabid dog enters Japan, it is unlikely to cause large outbreaks [247], partly because the probability for a dog to have contact with other dogs is low in Japan [248]. In that situation, the implementation of mandatory vaccination was shown to be economically inefficient [249, 250].
Scabies
Epidemiology
Scabies is a skin infestation caused by a scabies mite or Sarcoptes scabiei var. hominis. In Japan, scabies outbreaks have been regularly reported. Most outbreaks occur in hospitals, nurseries, and care homes [91–98]. There is no nationwide epidemiological data, but a report estimated the annual number of cases to be between 80,000 and 150,000 [90].
Public health response
No legal framework responds to scabies. No mandatory reporting system has been established but hospitals and facilities are encouraged to report outbreaks to local health authorities. The Japanese Dermatological Association published the latest guideline for the diagnosis and treatment of scabies in 2015 [251]. The accuracy of diagnosis depends on dermatologists’ ability to identify S. scabiei using dermoscopy or microscopy. Among WHO-recommended medicines, only ivermectin is approved by PMDA for treatment of scabies. Benzyl benzoate and permethrin are not approved by PMDA. Benzyl benzoate has been used for a long time for treatment of scabies in Japan, although this unapproved medicine is not listed as the first-line topical treatment [251]. Besides, phenothrin lotion and topical sulfur preparation are approved by PMDA in Japan.
Schistosomiasis
Epidemiology
Schistosoma japonicum, one of the causative agents of schistosomiasis, was once endemic to Japan but its transmission to humans has been considered to be interrupted since 1996 [252]. In recent years, some obsolete cases have been reported with egg embolism and old granuloma, as a consequence of past infections [253–255]. Imported cases of schistosomiasis have been reported mainly among Asian immigrants and Japanese travelers infected in endemic countries, such as China [99], the Philippines [100–104], and some African countries [105–107]. Schistosomes are continuously brought to Japan; however, the risk of domestic schistosome transmission is considerably low because the vector snail of S. japonicum, Oncomelania hupensis nosophora, is distributed only in Kofu Basin and Obitsu River Basin [252, 256–258] and vectors of other schistosomes have not been found in Japan.
Public health response
No legal framework responds to schistosomiasis. Serological testing for diagnosis of S. japonicum is available at Miyazaki University upon request from physicians (http://www.med.miyazaki-u.ac.jp/parasitology/detail.htm). The WHO-recommended first-line drug, praziquantel, is approved by PMDA, but it is available for off-label use only.
Snakebites
Epidemiology
Among approximately 20 venomous snakes inhabiting Japan [259, 260], Gloydius blomhoffii (or mamushi) is the most epidemiologically important viper. Annually, 1,000–3,000 cases of mamushi bites are reported, including approximately 10 fatal cases [108–110]. The mortality rate of mamushi bites is estimated to be 0.07–0.20% [110, 261]. Rhabdophis tigrinus (or yamakagashi) is also widely distributed in Japan. While less than one case of yamakagashi bites is annually recorded on average, the in-hospital mortality rate can be 10–11% [262, 263]. Gloydius tsushimaensis only inhabits Tsushima Island in Nagasaki Prefecture, where 72 cases of snake bites by this species were reported between 2005 and 2018, including one death [264]. In the Nansei Islands in Okinawa and Kagoshima Prefectures, five species belonging to the Protobothrops genus (or habu) are responsible for snake bites. Okinawa Prefecture alone recorded around 100 cases of habu bites annually between 2012 and 2014, but no fatal case was reported [265–267]. A rare case of snake bite by an illegally-kept eastern green mamba (Dendroaspis angusticeps) was also reported [268].
Case reports describe various clinical conditions caused by mamushi bites, including death [111], acute renal failure [112–114], cerebral infarction [115], osteomyelitis [116], thrombocytopenia [117], compartment syndrome [118], diplosia [119], and limited range of motion in hand fingers [120]. Some pediatric cases of mamushi bites are also reported [121–125]. Other case reports describe yamakagashi bites causing disseminated intravascular coagulation [126] and habu bites inducing acute compartment syndrome [127]. Snakebites are a serious public health concern in Japan.
Public health response
No legal framework responds to snakebites. There is no national surveillance system, except for Okinawa and Kagoshima Prefectures reporting the number of habu bites. In Japan, no diagnostic kits are available for clinicians to confirm snake-venom poisoning, requiring clinicians to make a clinical diagnosis including the identification of snake species [269]. Japan Snake Center (https://www.snake-center.com/diagnosis) offers consultations to health care providers. For treatment, no snake antivenom is specified by WHO. Freeze-dried antivenoms for mamushi and habu bites are approved by PMDA. Yamakagashi antivenom is available only for clinicians participating in a clinical study [270]. Many local governments publish lists of hospitals that store mamushi antivenom. Despite the availability of antivenoms, whether and when to administer antivenoms has not been standardized in Japan [271]. After a review article concluded that antivenom is the definitive therapy for severe cases [269], some agree that antivenoms should be administered to severe cases [117] or as early as possible [272], but others argue that it is difficult to establish a consistent treatment approach [273]. In addition, several case reports suggest that traditional herbal medicines were effective in some local lesions [274–277]. There is no consensus among physicians about treatment of snakebites in Japan.
Soil-transmitted helminthiases
Epidemiology
Soil-transmitted helminthiases are a group of chronic intestinal diseases caused by several species of helminths. This group includes ascariasis, ancylostomiasis, necatoriasis, trichuriasis, and strongyloidiasis. The annual cases of soil-transmitted helminthiases have decreased in the last decades, except for strongyloidiasis, which has been constantly found in Japan [129, 278].
Strongyloidiasis is mainly caused by Strongyloides stercoralis, which is transmitted from the contaminated soil to humans through the skin. Distinctive characteristics of this parasite are autoinfection that can persist and replicate within a host for decades. It potentially leads to life-threatening events such as hyperinfection syndrome and disseminated strongyloidiasis, particularly in immunocompromised patients. In Japan, strongyloidiasis cases are reported nationwide but are more likely to be found in people over 50 years of age, especially in subtropical areas of Okinawa and Kagoshima Prefectures [129]. The prevalence in endemic areas is estimated to reach 18.7% [130]. At least 15 strongyloidiasis cases were estimated to be diagnosed per year in Japan [129].
Strongyloidiasis has been reported particularly among patients with other diseases and conditions, including adult human T-cell lymphotropic virus type 1 infection, meningitis, solid organ malignancy, rheumatic diseases, type 2 diabetes mellitus, steroid use, and organ or bone marrow transplant [279–285]. These cases suggest that the use of immunosuppressive drugs in asymptomatic carriers of S. stercoralis may be associated with hyperinfection syndrome and disseminated strongyloidiasis. An imported case from Cambodia is documented in which the patient was co-infected with strongyloidiasis and extrapulmonary tuberculosis [131].
Ascariasis, ancylostomiasis, necatoriasis, and trichuriasis, were endemic to Japan but the transmission of these helminths to human beings was considered to be almost eliminated by 1975 [286]. Nowadays, infections with these helminths are sporadically reported, including presumably imported cases [128] and a case of multiple infections with Ascaris lumbricoides, Trichuris trichiura, and Necator americanus in an 84-year-old woman [132]. A clinical testing facility continues to detect a few ascariasis cases between 2000 and 2018 [133], but it is unknown whether these cases indicate recent Ascaris transmission. In the late 2010s, a school-based study detected no infection in five prefectures, suggesting that transmission has become rare even in the areas where high prevalence was previously reported [278], but it is not clear whether transmission of A. lumbricoides, Ancylostoma duodenal, N. americanus, and T. trichiura is eliminated in Japan.
Public health response
No legal framework responds to soil-transmitted helminthiases. The Tokyo Health Service Association (https://www.yobouigaku-tokyo.or.jp/) has offered parasite screening tests to long-term overseas residents upon their return to Japan since 1987 [287]. Fecal examinations for the diagnosis of A. lumbricoides, T. trichiura, A. duodenale, and N. americanus infections are widely accessible at local hospitals and clinics, particularly through commercial laboratories. As for WHO-recommended medicines, pyrantel is approved by PMDA for treatment of ascariasis, ancylostomiasis, and necatoriasis. Mebendazole and ivermectin are also approved for treatment of trichuriasis and strongyloidiasis, respectively. Albendazole is approved but available for off-label use only. Levamisole is not approved in Japan.
Taeniasis and cysticercosis
Epidemiology
Taeniasis is an intestinal infection caused by three species of zoonotic cestode: Taenia solium, Taenia saginata, and Taenia asiatica. Only T. solium causes serious conditions known as neurocysticercosis. This review focused on cysticercosis caused by T. solium. T. solium is not considered to be transmitted to humans in Japan because most of the cysticercosis cases are imported or related to travel histories to endemic areas including Nepal, India, and Cambodia [288–293]. However, a literature review [294] identified 39 cysticercosis cases that were found between 1994 and 2010, including seven Japanese nationals without oversea travel histories. This evidence suggests that T. solium domestic transmission cannot be ruled out. The possibility of T. solium domestic transmission is further supported by the outbreak of human taeniasis due to T. asiatica in Tokyo in 2010, as T. solium and T. asiatica share the same transmission pathways, such as raw pork meat consumption [295].
Public health response
T. solium infection is reportable as food poisoning under the Food Sanitation Act. Cysticercosis is not covered by the Infectious Diseases Control Law. In Japan, all domestic animals, such as pigs and cows going into the food chain are mandatorily inspected before harvesting (carcass inspection) under the Slaughterhouse Act and the Act on Domestic Animal Infectious Diseases Control. Molecular confirmation of histopathologic specimens is highly useful for the diagnosis of cysticercosis and is available in NIID. Neurocysticercosis is usually suspected due to neurological symptoms, abnormal findings in brain imaging, and the travel history to endemic areas. Serological tests are available at Miyazaki University. The WHO-recommended two anthelmintics, namely, albendazole and praziquantel, are approved by PMDA but available for off-label use only. Niclosamide is not available in Japan.
Trachoma
Epidemiology
Trachoma is a disease of the eye caused by infection with the bacteria, Chlamydia trachomatis. No case report was found in our review. Trachoma was frequently reported in the 1950s and 1960s and, to our best knowledge, the last case was reported in 1984 [296]. Meanwhile, other forms of infection with C. trachomatis, such as chlamydial conjunctivitis, chlamydial infection of the genital tract, and chlamydia pneumonia are commonly reported in Japan. For instance, a sentinel survey reported 144−887 cases of genital infections by C. trachomatis between 2010 and 2018 [297].
Public health response
No legal framework responds to trachoma. Laboratory tests for diagnosing infection with C. trachomatis, such as PCR or antigen tests, are widely available in Japan. The WHO-recommended antibiotics, azithromycin, and tetracycline are approved by PMDA.
Yaws
Epidemiology
Yaws is a chronic bacterial disease caused by infection with Treponema pallidum susbp. pertenue. Our review found no evidence of yaws in Japan.
Public health response
No legal framework responds to yaws. No hospitals and laboratories offer to test for yaws. However, serological testing of yaws can be substituted by testing for syphilis, and this is widely available in Japan. Azithromycin and benzathine penicillin are recommended by WHO for treatment and are approved by PMDA but available for off-label use only.
Discussion
To our knowledge, this is the first study that summarized the current epidemiology of and public health responses to the 20 NTDs in Japan. In recent years, NTDs are barely regarded as contemporary public health problems in Japan because we experience only a limited number of cases and/or in limited settings as shown in this study. For several NTDs, such as lymphatic filariasis, rabies, schistosomiasis, leprosy, trachoma, and some soil-transmitted helminthiases, this has been largely a result of our successful disease control programs during the 20th century [240, 298–303]. In addition, Japan has undergone substantial economic development and social changes during this time, which in part, may have contributed to controlling NTDs within the country. However, our review showed evidence that we still have cases of NTDs and the burden from this set of diseases in Japan. For instance, alveolar echinococcosis, Chagas disease, chromoblastomycosis, dengue, foodborne trematodiases (excluding opisthorchiasis), scabies, and strongyloidiasis have been transmitted within Japan as well as imported from other countries recently (Table 1).
In the context of global health, high-income countries such as Japan are expected to play the role of donor countries to combat NTDs in low- and middle-income countries [304, 305]. Japan and other high-income countries should recognize the need to address NTDs within their own countries in addition to their global roles. With globalization and the rapid movement of people, cases of NTDs can be seen anywhere, and thus, it is indeed becoming more and more of a global problem than ever before. NTD patients in high-income countries, whether a locally acquired or an imported case, should not be left behind in the global efforts to end NTDs.
Hotez pointed out that NTDs affect the poor living in wealthy countries [5]. While we did not find any study analyzing the distribution of NTD burdens by income levels in Japan, we found that some diseases mainly affect immigrants, who tend to live with economic vulnerabilities. For example, Chagas disease almost exclusively affects Latino communities in Japan. Similarly, cystic echinococcosis, cysticercosis, leishmaniasis, leprosy, lymphatic filariasis, and schistosomiasis are largely observed among immigrants. These findings provide the critical implication that Japan should pay additional attention to imported NTDs. As we embrace globalization in Japan, we are seeing a growing number of foreign residents in the country. Just in the past decade, it increased from 2.0 million to 2.7 million between 2012 and 2021 [306]. Addressing the health needs of immigrants is important to build an equitable health system since immigrants in Japan tend to experience barriers to health care and poorer health outcomes [307, 308].
Not surprisingly, we found a paucity of epidemiological data, even for the NTDs that are domestically transmitted in Japan, such as foodborne trematodiases and mycetoma (Table 1). With the currently available data, it is not possible to understand the accurate burden of NTDs. The government reported that the average number of NTD patients in Japan was 340 per year through the Japan SDG Action Platform [11]. However, our study showed that this number seems to be grossly underestimated. There were at least 80,000 people who required treatment for scabies alone each year [90]. More population-level surveillance data along with clinical case reports are needed to better understand the epidemiology of NTDs in Japan. Furthermore, surveillance of NTDs in a high-income country like Japan may help improve the understanding of the situation of NTDs in other countries. For example, while laboratory testing for anti-microbial resistance is unavailable in many leprosy endemic countries, a recent epidemiological study in Japan could report cases of anti-microbial resistant strains of M. leprae coming from the Philippines [303]. Likewise, a case of Buruli ulcer with the same strain as that of Japan (M. ulcerans subsp. shinshuense) was reported from the Netherlands in an immigrant from China [309]. M. ulcerans subsp. shinshuense is currently known as a strain domestic to Japan, but this case report presented the possibility that it may be a strain affecting more countries in Asia. These examples underpin the importance and the need for more global surveillance of NTDs, involving high-income countries.
The levels of public health response to NTDs highly varied by disease in Japan (Table 1). For surveillance, mandatory reporting is established only for echinococcosis, rabies, dengue, and chikungunya under the Act on the Prevention of Infectious Diseases. Two prefectures have set regulations to count snakebites by habu. There is no law or regulation for the other NTDs to be reported to government agencies. For control programs by government bodies, national schemes exist for rabies, dengue, and chikungunya. Echinococcosis is controlled by the Hokkaido Prefectural Government. For diagnosis, we could identify at least one laboratory that offers confirmatory testing for all NTDs except for dracunculiasis, human African trypanosomiasis, onchocerciasis, and yaws. Despite the availability of laboratories, physicians’ capacities to suspect NTDs and send samples to laboratories would require strengthening. In terms of the availability of medicines, none of the WHO-recommended medicines is approved in Japan for Chagas disease and fascioliasis. For other diseases, at least one of the WHO-recommended medicines is approved; however, drugs for cysticercosis, opisthorchiasis, human African trypanosomiasis, onchocerciasis, schistosomiasis, and yaws are available only for off-label use and patients can be required to pay all costs of treatment and related services.
Based on the findings from this review, we developed some recommendations on a way forward to advance the research and control agenda for each NTD (Table 3). A group discussion was used to discuss and reach a consensus. The discussion did not involve any assessment of the ethical, economic, and operational aspects of these recommendations, and thus, we do not intend to provide instruction about which recommendations should be prioritized. In addition to these disease-specific recommendations, we also identified three policy issues across disease areas as discussed below.
Table 3. Authors’ recommendations to advance research or control agenda for NTDs in Japan.
| NTD | Recommendations |
|---|---|
| Buruli ulcer | ♦ Educate dermatologists and other physicians from relevant fields to promote early diagnosis and treatment. Diagnosis of Buruli ulcer is available in Japan but requires physicians’ ability to suspect the disease from the skin conditions. ♦ Expand a physician-researcher network to improve reporting of Buruli ulcer. The current reporting system is managed by the Leprosy Research Center of the NIID and the expert group but is based on voluntary reporting from physicians leading to under-reporting. |
| Chagas disease | ♦ Investigate access to diagnosis and treatment in Japan. Two unapproved drugs, benznidazole and nifurtimox, can be obtainable from WHO, but whether patients have access to them is not clearly understood. ♦ Establish screening programs to detect infected persons before they develop symptoms. For instance, serological testing can be integrated into workplace medical checkups or prenatal checkups in municipalities with large Latino populations. |
| Cysticercosis | ♦ Educate physicians to suspect cysticercosis when immigrants from endemic areas present central nervous system infections. Domestic transmission can be triggered by infected international travelers or agricultural workers. ♦ Maintain the mandatory inspection of meat. |
| Dengue and chikungunya | ♦ Monitor outbreaks of dengue and a new case of domestic transmission of chikungunya. ♦ Pay attention to the trend of imported cases from overseas and continue to monitor mosquitoes in Japan to prevent new outbreaks. |
| Echinococcosis | ♦ Investigate why transmission of E. multilocularis to humans is evidenced only in Hokkaido. The risk of its spread into the main islands was investigated in 2003 [310] but needs to be updated. |
| Foodborne trematodiases | ♦ Establish a monitoring system to detect hot spots of clonorchiasis, fascioliasis, and paragonimiasis, for instance, by regular stool tests for school children in areas known to be endemic in the past. Since the transmission patterns of these diseases have varied due to increased immigration, changes in dietary habits, and heavy rains/flooding, it is necessary to monitor possible epidemic areas to understand the risks. |
| Leishmaniasis | ♦ Educate physicians to address sporadically imported cases. ♦ Consider adopting topical paromomycin for treatment of cutaneous leishmaniasis [311] to expand treatment options. |
| Leprosy | ♦ Educate and organize dermatologists to manage leprosy patients. The repeal of the Leprosy Prevention Law in 1996 allowed patients to be managed at general hospitals or clinics, but dermatologists in general are not experienced in managing leprosy patients. ♦ Surveillance of leprosy is continued at the LRC/NIID and the expert group, without any legal basis. The LRC can consider establishing integrated surveillance of skin NTDs that exist in Japan, to ensure the sustainability of the leprosy surveillance program. |
| Lymphatic filariasis | ♦ Investigate whether patients found in Japan have access to treatment. |
| Mycetoma, chromoblastomycosis, and other deep mycoses | ♦ Educate dermatologists to promote timely diagnosis. ♦ Build a consensus among clinical researchers to report mycetoma, chromoblastomycosis, sporotrichosis, and paracoccidioidomycosis following international definitions of these diseases. |
| Rabies | ♦ Consider revising the mandatory annual vaccination of domestic dogs, as this strategy is suggested to be no more cost beneficial. The risk of having rabies introduced to and spread in Japan is considerably low. ♦ Secure access to rabies immune globulins (RIG). Since RIG is not approved in Japan, it should probably be provided through a research group. |
| Scabies | ♦ Establish outbreak surveillance and control systems, since outbreaks of scabies have been regularly observed in Japan. ♦ Evaluate the efficacy and safety of crotamiton and benzyl benzoate for treatment of scabies and consider obtaining PMDA approval. |
| Schistosomiasis | ♦ Raise awareness and educate physicians to promote diagnosis of schistosomiasis, since obsolete cases and imported cases have been regularly found in Japan. ♦ Consider obtaining PMDA approval of praziquantel for treatment of schistosomiasis, as this drug is only available for off-label use. |
| Snakebites | ♦ Accumulate clinical cases to get the currently proposed treatment guideline updated and accepted by the medical community. Although mamushi and habu antivenoms are approved by PMDA, there is no consensus in the medical community on whether and when to administer antivenoms. ♦ Guarantee government support for the production of yamakagashi antivenom. Yamakagashi antivenoms are voluntarily produced by a research group at the Japan Snake Center. ♦ Establish information systems at all prefectural governments to collect and publish the number of mamushi bites, just as Okinawa and Kagoshima Prefectures do for habu bites. Although mamushi bites are frequently reported from all over Japan, there is no national surveillance system. ♦ Collect data about the use of mamushi antivenoms to understand the demand for sustainable production and storage of antivenoms. |
| Soil-transmitted helminthiases | ♦ Consider testing for Strongyloides infections to avoid hyper-infection syndrome and disseminated strongyloidiasis, particularly in patients over 50 years of age with a history of residence in Kagoshima and Okinawa Prefectures who will be treated with immunosuppressive drugs, anticancer drugs, or steroids. |
Note: We have no specific recommendations for dracunculiasis, human African trypanosomiasis, onchocerciasis, trachoma, and yaws. We consider that these diseases are not Japan’s priority because we did not find any evidence of domestic transmission nor imported cases.
First, we argue that some NTDs require a legal basis for better public health response. Our review suggests that the government provides organized responses to the NTDs that are already covered by the Infectious Disease Control Law (Table 1). This law classifies infectious diseases into five categories based on each disease’s infectivity and severity. It then defines the roles of stakeholders to organize reporting, prevention, and healthcare provision. In general, diseases that are considered highly infective or severe, such as Ebola virus disease, tuberculosis, and cholera, are classified into Class I, II, or III. Other infectious diseases whose infectivity or severity are not considered so problematic are classified into Class IV or V (Table 4). Although NTDs are not highly infective or severe diseases, we argue that some NTDs are yet eligible for Class IV or V, as Table 4 explains. Establishing a legal basis for these NTDs can be a critical step to advancing their surveillance and control in Japan. In addition, snakebite envenoming would need similar legal support outside of the Infectious Disease Control Law.
Table 4. Infectious Disease Control Law and NTDs that can be designated by this law.
| How are diseases classified by the law? | Which NTD can fit the law’s classification? | ||||
|---|---|---|---|---|---|
| Class | Definition of the class by the law1 | Examples of diseases already designated by the law | Interpretation of classification by the Ministry of Health, Labour, and Welfare2 | NTDs to be designated | Rationale |
| Class IV | [A]ny known infectious disease [. . .] which is transmissible to human beings through animals or animal corpses, food or drink, clothing, bedding, or other physical items and which is likely to affect the health of citizens (Article 6- [5]-(xi)) | Hepatitis E, hepatitis A, yellow fever, Q fever, rabies, anthrax, avian influenza, botulism, malaria, and tularemia | Diseases that are not classified into Class I, II, or III [i.e., infectivity and severity of illness is relatively low] and that are mainly transmitted to humans via animals and other items. | Scabies | Mites are transmissible to human beings through clothing and bedding. Scabies is widely spread in Japan and its outbreaks are regularly reported. More than 80,000 incident cases per year are estimated. The government should standardize and guide response to scabies outbreaks, such as mite control. |
| Class V | [A]ny known infectious disease (excluding Class IV Infectious Diseases) [. . .] which is likely to affect the health of citizens (Article 6- [6]-(ix)) | Influenza, viral hepatitis, cryptosporidiosis, acquired immunodeficiency syndrome, genital chlamydia infection, syphilis, measles, and methicillin-resistant Staphylococcus aureus infection | Diseases whose surveillance data need to be provided to the public and healthcare workers. | Buruli ulcer | It is transmitted within Japan, but its surveillance is voluntarily conducted by NIID. Dermatologists should be informed for better case findings. |
| Chagas disease | 3,000–4,500 infected people are estimated to live in Japan, with a few cases of mother-to-child transmission. No surveillance is in place. The public and healthcare workers should be informed, particularly in Latino communities. | ||||
| Clonorchiasis | Imported and domestic cases are confirmed in Japan but no surveillance is in place. The public and healthcare workers should be informed, particularly in areas where domestic transmission has been found. | ||||
| Cysticercosis | Cases are reported among immigrants and Japanese travelers. Domestic transmission cannot be ruled out. No surveillance is active for this disease. The public and healthcare workers should be informed for better case findings. | ||||
| Fascioliasis | This disease is endemic in Japan with some imported cases found. No surveillance is in place. The public and healthcare workers should be informed, particularly in areas where domestic transmission has been found. | ||||
| Leprosy | Imported cases and cases from past infections are being found in Japan. Its surveillance is voluntarily conducted by NIID. Dermatologists should be informed for better case findings. | ||||
| Mycetoma, chromoblastomycosis, and sporotrichosis | These diseases are endemic in Japan. No surveillance is in place. The public and healthcare workers should be informed for better case findings, particularly in epidemic areas. | ||||
| Paragonimiasis | This disease is endemic in Japan with some imported cases found. No surveillance is in place. The public and healthcare workers should be informed, particularly in areas where domestic transmission has been found. | ||||
| Strongyloidiasis | Imported and domestic cases are confirmed in Japan. At least 15 cases are found per year, but no surveillance is in place. Healthcare workers, particularly those who attend to immunosuppressed patients, should be informed about better treatment. | ||||
1. English translation of the articles is extracted from the Japanese Law Translation database (http://www.japaneselawtranslation.go.jp/) provided by the Ministry of Justice, Japan.
2. Reference material presented at the third meeting of the Health Sciences Council (Taskforce for Infectious Diseases) held on March 14, 2014. Available from https://www.mhlw.go.jp/file/05-Shingikai-10601000-Daijinkanboukouseikagakuka-Kouseikagakuka/0000040509.pdf
Next, we propose that the government should be more responsible for securing the provision of NTD-related health products that are not commercially available in Japan. For instance, diagnostics for Chagas disease, foodborne trematodiases, lymphatic filariasis, and schistosomiasis are produced or purchased by researchers doing investigations on each disease. Similarly, the yamakagashi antivenom is produced only by a research group at the Japanese Snake Center. In addition, medicines for Chagas disease and fascioliasis are not approved in Japan, although they are listed as essential medicines by WHO. Currently, the Research Group of Tropical Diseases and Parasitic Diseases stores triclabendazole for fascioliasis. These NTD products can become unavailable when research funds are discontinued. The government can allocate a budget for securing NTD products, or more preferably, can create a new office to address this problem within public institutions, such as the National Institute of Infectious Diseases or the National Center of Global Health and Medicine.
Finally, we emphasize the importance of addressing the unaffordability of treatment using unapproved drugs. In Japan, treatment of Chagas disease and fascioliasis requires the use of unapproved drugs (Table 1). Unapproved drugs are not covered by health insurance and, in the worst case, patients can be required to fully pay for all health services received. To ensure patient affordability, obtaining PMDA approval is the standard way so that these drugs can be covered by insurance. However, this option can be possible only when manufacturers make decisions to apply for PMDA approval. Alternatively, patients can receive unapproved drugs for free when they are enrolled in clinical trials. Although the main purpose of a clinical trial is not to protect patients from financial risks, this seems to be the only realistic way to ensure the affordability of unapproved medicines. At the time of this writing, a clinical trial of triclabendazole for fascioliasis is recruiting patients [312]. Launching clinical trials would also be recommended for benznidazole and nifurtimox.
Our review involves several limitations. First, our search strategy may have been unable to find some relevant publications, particularly when the Japanese databases register papers using different disease names than the controlled terms that we adopted as search terms. Second, NTD cases are likely to be underdiagnosed in Japan, which leads to an underestimation of the burden of NTDs. Even if diagnosed, cases usually remain undocumented when medications are not available. Third, our review may involve our own bias. Each author had more knowledge and expertise in one or a few disease areas, which could influence the literature search and writing processes. To minimize this potential bias, we developed the search protocol together. Each author read through the manuscript and any disagreements were resolved through group discussion. Lastly, our discussions of public health response assessed the availability of laboratory testing and medicines, but we could not fully assess whether they are accessible to patients. Further research on access to NTD health technologies in Japan is required.
In conclusion, our review found that 15 out of 20 NTDs can be considered important public health concerns in Japan. Among these 15 NTDs, some are transmitted within Japan and others are imported from endemic countries. Japan’s health systems address only a portion of 15 NTDs, particularly five diseases that are targeted by the Infectious Disease Control Law. NTD control often requires support from the public sector. Strengthening government support for nationwide surveillance of NTDs as well as for ensuring the availability and affordability of NTD-related health technologies, can be a critical step to advancing NTD control in Japan. Our illustration of NTDs in Japan also implies that even high-income countries should study, recognize, and address NTDs to reduce health disparities within their own countries.
Supporting information
(XLSX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
KY received funding from the School of Tropical Medicine and Global Health, Nagasaki University, for publication of this article. The funder did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021–2030 [Internet]. Geneva; 2020. [cited 2021 Mar 22]. Available from: https://www.who.int/publications-detail-redirect/9789240010352. [Google Scholar]
- 2.Fitzpatrick C, Nwankwo U, Lenk E, de Vlas SJ, Bundy DAP. An Investment Case for Ending Neglected Tropical Diseases. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major Infectious Diseases [Internet]. 3rd ed. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2017 [cited 2021 Mar 22]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK525199/. [PubMed]
- 3.Fitzpatrick C, Engels D. Leaving no one behind: a neglected tropical disease indicator and tracers for the Sustainable Development Goals. Int Health. 2016. Mar;8 Suppl 1:i15–18. doi: 10.1093/inthealth/ihw002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotez PJ. Ten Global “Hotspots” for the Neglected Tropical Diseases. PLoS Negl Trop Dis [Internet]. 2014 May 29 [cited 2021 Mar 22];8(5). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4038631/. [DOI] [PMC free article] [PubMed]
- 5.Hotez PJ. NTDs V.2.0: “Blue Marble Health”—Neglected Tropical Disease Control and Elimination in a Shifting Health Policy Landscape. PLoS Negl Trop Dis. 2013 Nov 21;7(11):e2570. [DOI] [PMC free article] [PubMed]
- 6.Abbas M, Aloudat T, Bartolomei J, Carballo M, Durieux-Paillard S, Gabus L, et al. Migrant and refugee populations: a public health and policy perspective on a continuing global crisis. Antimicrob Resist Infect Control. 2018;7:113. doi: 10.1186/s13756-018-0403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth M. Climate Change and the Neglected Tropical Diseases. Adv Parasitol. 2018;100:39–126. doi: 10.1016/bs.apar.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilli M, Botta A, Bartoloni A, Corti G, Zammarchi L. Hospitalization for Chagas disease, dengue, filariasis, leishmaniasis, schistosomiasis, strongyloidiasis, and Taenia solium taeniasis/cysticercosis, Italy, 2011–2016. Infection. 2020. Oct;48(5):695–713. doi: 10.1007/s15010-020-01443-2 [DOI] [PubMed] [Google Scholar]
- 9.Zammarchi L, Gobbi F, Angheben A, Spinicci M, Buonfrate D, Calleri G, et al. Schistosomiasis, strongyloidiasis and Chagas disease: the leading imported neglected tropical diseases in Italy. J Travel Med. 2020. Feb 3;27(1). doi: 10.1093/jtm/taz100 [DOI] [PubMed] [Google Scholar]
- 10.Hotez PJ. The rise of neglected tropical diseases in the ‘new Texas’. PLoS Negl Trop Dis. 2018. Jan;12(1):e0005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Foreign Affairs of Japan. JAPAN SDGs Action Platform ‐ SDG Indicators [Internet]. [cited 2021 Mar 23]. Available from: https://www.mofa.go.jp/mofaj/gaiko/oda/sdgs/statistics/goal3.html.
- 12.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018. Nov 19;18(1):143. doi: 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018. Oct 2;169(7):467–73. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 14.Agency Digital, Government of Japan. e-Gov Law Search [Internet]. Available from: https://elaws.e-gov.go.jp/. [Google Scholar]
- 15.National Institute of Infectious Diseases, Japan. Infectious Diseases Weekly Report [Internet]. Available from: https://www.niid.go.jp/niid/ja/idwr.html.
- 16.Pharmaceuticals and Medical Devices Agency. Pharmaceuticals Information Search [Internet]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuSearch/.
- 17.National Institute of Infectious Diseases, Japan. Laboratory Manuals for Pathogen Detection [Internet]. Available from: https://www.niid.go.jp/niid/ja/labo-manual.html.
- 18.Japanese Association for Infectious Diseases, Japanese Society for Clinical Microbiology. Infectious Diseases Map for Advanced Progressive Laboratory Tests:IDMAP [Internet]. Available from: https://www.kansensho.or.jp/modules/idmap/idmap.html.
- 19.World Health Organization. Electronic Essential Medicines List [Internet]. Available from: https://list.essentialmeds.org/.
- 20.World Health Organization. Fact sheets [Internet]. Available from: https://www.who.int/news-room/fact-sheets.
- 21.Research Group of Tropical Diseases and Parasitic Diseases. Medicines stored by our research group [Internet]. [cited 2020 Oct 16]. Available from: https://www.nettai.org/%E4%BF%9D%E7%AE%A1%E8%96%AC%E5%89%A4/.
- 22.Nakanaga K, Hoshino Y, Yotsu RR, Makino M, Ishii N. Nineteen cases of Buruli ulcer diagnosed in Japan from 1980 to 2010. J Clin Microbiol. 2011. Nov;49(11):3829–36. doi: 10.1128/JCM.00783-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohtsuka M, Kikuchi N, Yamamoto T, Suzutani T, Nakanaga K, Suzuki K, et al. Buruli ulcer caused by Mycobacterium ulcerans subsp shinshuense: a rare case of familial concurrent occurrence and detection of insertion sequence 2404 in Japan. JAMA Dermatol. 2014. Jan;150(1):64–7. doi: 10.1001/jamadermatol.2013.6816 [DOI] [PubMed] [Google Scholar]
- 24.Imai K. Chagas disease [in Japanese]. Rinsho Biseibutsu Clin Microbiol. 2014;41(4):353–7. [Google Scholar]
- 25.Miura S. Infectious diseases ‐ Chagas disease and its current situation in Japan [in Japanese]. Nihon Iji Shimpo Jpn Med J. 2018;(4928):55–6. [Google Scholar]
- 26.Imai K, Misawa K, Osa M, Tarumoto N, Sakai J, Mikita K, et al. Chagas disease: a report of 17 suspected cases in Japan, 2012–2017. Vol. 47, Tropical Medicine and Health. 2019. p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ono T, Miyahira Y. Current parasitic, insect and animal-borne diseases: 1- imported parasitic diseases among children [in Japanese]. Shonika Rinsho Jpn J Pediatr. 2012;65(3):360–8. [Google Scholar]
- 28.Ikeno Y, Miura S, Sayama Y, Fukushima F, Sakio H, et al. A case of Chagas disease patient who was treated with benznidazole [in Japanese]. Vol. 26, Nihon Rinsyo Kiseichu Gakkaishi (Clinical Parasitology). 2015. p. 90–2. [Google Scholar]
- 29.Ikeno Y, Miuara S, Maeda T, Imai K, Sayama Y, et al. A case of chronic Chagas disease patient who was treated with benznidazole and kept remission for five years [in Japanese]. Vol. 30, Nihon Rinsyo Kiseichu Gakkaishi (Clinical Parasitology). 2019. p. 62–4. [Google Scholar]
- 30.Imai K, Maeda T, Sayama Y, Osa M, Mikita K, Kurane I, et al. Chronic Chagas disease with advanced cardiac complications in Japan: Case report and literature review. Parasitol Int. 2015. Oct;64(5):240–2. doi: 10.1016/j.parint.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 31.Maeda T, Nanun M, Sayama Y, Misawa K, Imai K, et al. Two cases of Chagas disease with benznidazole treatment [in Japanese]. Vol. 24, Rinsho To Biseibutsu (Clinical Microbiology). 2013. p. 33–6. [Google Scholar]
- 32.Yuan B, Nishiura H. Estimating the actual importation risk of dengue virus infection among Japanese travelers. PloS One. 2018;13(6):e0198734. doi: 10.1371/journal.pone.0198734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutsuna S, Kato Y, Moi ML, Kotaki A, Ota M, Shinohara K, et al. Autochthonous dengue fever, Tokyo, Japan, 2014. Emerg Infect Dis. 2015. Mar;21(3):517–20. doi: 10.3201/eid2103/141662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kori M, Awano N, Inomata M, Kuse N, Tone M, Yoshimura H, et al. The 2014 autochthonous dengue fever outbreak in Tokyo: A case series study and assessment of the causes and preventive measures. Respir Med Case Rep. 2020;31:101246. doi: 10.1016/j.rmcr.2020.101246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ujiie M. Deaths due to dengue in Japanese travellers. J Travel Med. 2021. Jul 7;28(5). doi: 10.1093/jtm/taab076 [DOI] [PubMed] [Google Scholar]
- 36.Ishikawa H, Shimogawara R. Risk Assessment of Dengue Autochthonous Infections in Tokyo during Summer, Especially in the Period of the 2020 Olympic Games. Jpn J Infect Dis. 2019. Nov 21;72(6):399–406. doi: 10.7883/yoken.JJID.2019.094 [DOI] [PubMed] [Google Scholar]
- 37.Kutsuna S, Kato Y, Katanami Y, Yamamoto K, Takeshita N, Hayakawa K, et al. A Retrospective Single-center Analysis of 16 Cases of Imported Chikungunya Fever in Japan. Intern Med. 2018;57(3):325–8. doi: 10.2169/internalmedicine.8196-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama E, Tajima S, Kotaki A, Shibasaki K, Itokawa K, Kato K, et al. A summary of the imported cases of Chikungunya fever in Japan from 2006 to June 2016. J Travel Med [Internet]. 2018. Jan 1 [cited 2021 Feb 25];25(1). Available from: https://academic.oup.com/jtm/article/doi/10.1093/jtm/tax072/4763690. doi: 10.1093/jtm/tax072 [DOI] [PubMed] [Google Scholar]
- 39.Kimura M, Toukairin A, Tatezaki H, Tanaka S, Harada K, Araiyama J, et al. Echinococcus multilocularis detected in slaughtered pigs in Aomori, the northernmost prefecture of mainland Japan. Jpn J Infect Dis. 2010. Jan;63(1):80–1. [PubMed] [Google Scholar]
- 40.Chida K, Kamiyama T, Kakisaka T, Yokoo H, Hatanaka K, Taketomi A. A Case of Hepatic Alveolar Echinococcosis with Complete Obstruction of the Inferior Vena Cava [in Japanese]. Nihon Rinsho Geka Gakkai Zasshi J Jpn Surg Assoc. 2015;76(5):1124–9. [Google Scholar]
- 41.Ojima H, Kanai Y. Alveolar echinococcosis ‐ hint for diagnosis [in Japanese]. In: Byori To Rinsho (Pathology and Clinical Medicine) [Internet]. Bunkodo; 2019. [cited 2021 Jan 15]. p. 203–5. Available from: http://mol.medicalonline.jp/library/journal/abstract?GoodsID=ag9brysh/2019/0037s1/058&name=0203-0205j&UserID=1100001448-00. [Google Scholar]
- 42.Sunahara M, Kamiyama T, Sato N, Kurauchi N, Suzuki S, Kimura J. A Case of Hepatic Alveolar Echinococcosis with Peritoneal Dissemination. Nihon Rinsho Geka Gakkai Zasshi J Jpn Surg Assoc. 2013;74(10):2857–62. [Google Scholar]
- 43.Yoshida T, Kamiyama T, Okada T, Nakanishi K, Yokoo H, Kamachi H, et al. Alveolar echinococcosis of the liver in children. Vol. 17, Journal of Hepato-Biliary-Pancreatic Sciences. 2010. p. 152–7. doi: 10.1007/s00534-009-0114-6 [DOI] [PubMed] [Google Scholar]
- 44.Matsudaira S, Ishizaki Y, Yoshimoto J, Imamura H, Fukumura Y, Kawasaki S. A Case of Hepatic Alveolar Echinococcosis Treated Radically by Hepatectomy 29 Years after Infection by Echinococcus multilocularis. Nihon Rinsho Geka Gakkai Zasshi J Jpn Surg Assoc. 2018;79(10):2145–9. [Google Scholar]
- 45.Osawa E, Tsuchida T, Numada K, Yoneyama K, Kasahara A, Murakami A, et al. A case of alveolar echinococcosis of the liver in a resident of Kanagawa Prefecture which was difficult to diagnose [in Japanese]. Vol. 63, Yokohama Igaku (Yokohama Medical Journal). 2012. p. 605–9. [Google Scholar]
- 46.Kataoka R, Kojima K. A case of alveolar echinococcosis of the liver presented without associated history [in Japanese]. Vol. 62, Rinsho Houshasen (Japanese Journal of Clinical Radiology). 2017. p. 571–4. [Google Scholar]
- 47.Hata R, Toyama M, Ichimura Y, Sawada Y, Kaneko M. A case of echinococcosis of the liver involving parent and child [in Japanese]. Jpn J Med Technol. 2014;63(1):69–73. [Google Scholar]
- 48.Okada F, Kumai Y, Sato H, Ohbayashi H, Ono A, Ando Y, et al. Image diagnosis of infectious diseases ‐ image findings of parasitic diseases in lung [in Japanese]. Vol. 73, Nihon Kyobu Rinsho (The Japanese Journal of Chest Diseases). 2014. p. 1451–9. [Google Scholar]
- 49.Oku Y. Echinococcosis [in Japanese]. Vol. 33, Kagaku Ryoho no Ryoiki (Antibiotics & Chemotherapy). 2017. p. 428–35. [Google Scholar]
- 50.Nakamura K, Ito A, Yara S, Haranaga S, Hibiya K, Hirayasu T, et al. A case of pulmonary and hepatic cystic Echinococcosis of CE1 stage in a healthy Japanese female that was suspected to have been acquired during her stay in the United Kingdom. Am J Trop Med Hyg. 2011. Sep;85(3):456–9. doi: 10.4269/ajtmh.2011.11-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuchikata T. A case of 13-year-old girl with look-alike hepatophyma [in Japanese]. Vol. 77, Shonika Shinryo (The Journal of Pediatric Practice). 2014. p. 553–6. [Google Scholar]
- 52.Morishima Y, Ichimura S, Yamazaki H, Sugiyama H. Cystic echinococcosis in a Napalese person ‐ infection of heart with Echinococcus ortleppi [in Japanese]. Vol. 25, Clinical Parasitology. 2014. p. 99–101. [Google Scholar]
- 53.Tanaka T, Hirata T, Aragaki S, Higashiarakawa M, Kishimoto K, et al. A case of cystic echinococcosis in an international student from Nepal [in Japanese]. Vol. 25, Clinical Parasitology. 2014. p. 95–8. [Google Scholar]
- 54.Yamashita N, Hirai T, Ito T. A case of liver cystic echinococcosis. J Gastroenterol Cancer Screen. 2010;48(1):61–6. [Google Scholar]
- 55.Yoshida Y. Clonorchiasis—a historical review of contributions of Japanese parasitologists. Parasitol Int. 2012. Mar;61(1):5–9. doi: 10.1016/j.parint.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 56.Matsubayashi J, Taira K, Yogo A, Kito Y, Ura K, Toyota E, et al. Intrahepatic Bile Duct Carcinoma with Clonorchiasis [in Japanese]. Jpn J Gastroenterol Surg. 2015;48(4):328–36. [Google Scholar]
- 57.Kominami Y, Aikata H, Hiramatsu K, Tanaka M, Naeshiro N. A case of clonorchiasis complicated with the expansion of liver cyst [in Japanese]. J Jpn Soc Gastroenterol. 2013;110(3):456–64. [PubMed] [Google Scholar]
- 58.Ikehara M, Komase Y, Morita A, Yamaguchi H, Yamamoto T. Paragonimiasis in a person whose symptoms were shown 22 years after emigrating to Japan from Laos. J Infect Chemother Off J Jpn Soc Chemother. 2010. Feb;16(1):49–52. [DOI] [PubMed] [Google Scholar]
- 59.Hoshina T, Tamura K, Kawano S, Kato T, Sato F, Horino T, et al. Two cases of Paragonimiasis westermani in a Chinese family diagnosed with the Ouchterlony double diffusion method. Kansenshogaku Zasshi. 2014. Nov;88(6):866–70. doi: 10.11150/kansenshogakuzasshi.88.866 [DOI] [PubMed] [Google Scholar]
- 60.Okamoto H, Uramoto H, Mimura Y, Hoshida Y. Two cases of paragonimiasis verified by the presence of eggs in sputum cytology specimens. J Jpn Soc Clin Cytol. 2014;53(4):292–7. [Google Scholar]
- 61.Sakurai T, Watanabe Y, Inui A, Oshima H, Isonuma H, Dambara T, et al. A case of human fascioliasis infected from eating watercress in Japan. Jpn J Hosp Gen Med. 2013;5(2):62–6. [Google Scholar]
- 62.Sato Y, Takasaka Y. A Case of Fascioliasis Suspected of being a Malignant Tumor. Nihon Gekakei Rengo Gakkaishi J Jpn Coll Surg. 2011;36(5):850–5. [Google Scholar]
- 63.Yamanaka Y, Takeuchi M. A Case of fascioliasis [in Japanese]. Kanzo. 2020;61:607–12. [Google Scholar]
- 64.Imai K, Tarumoto N, Amo K, Takahashi M, Sakamoto N, Kosaka A, et al. Non-invasive diagnosis of cutaneous leishmaniasis by the direct boil loop-mediated isothermal amplification method and MinION nanopore sequencing. Parasitol Int. 2018;67(1):34–7. [DOI] [PubMed] [Google Scholar]
- 65.Ono M, Takahashi K, Taira K, Uezato H, Takamura S, Izaki S. Cutaneous leishmaniasis in a Japanese returnee from West Africa successfully treated with liposomal amphotericin B. J Dermatol. 2011;38(11):1062–5. doi: 10.1111/j.1346-8138.2011.01270.x [DOI] [PubMed] [Google Scholar]
- 66.Tsujimoto K, Nakagawa T, Uno K, Yamada Y, Ogawa T, Kasahara K, et al. A case of a cutaneous leishmaniasis patient who developed symptoms in Brazil [in Japanese]. Vol. 23, Clinical Parasitology. 2012. p. 34–6. [Google Scholar]
- 67.Okumura Y, Yamauchi A, Nagano I, Itoh M, Hagiwara K, Takahashi K, et al. A case of mucocutaneous leishmaniasis diagnosed by serology. J Dermatol. 2014;41(8):739–42. doi: 10.1111/1346-8138.12564 [DOI] [PubMed] [Google Scholar]
- 68.Matsuura H, Utsumi D, Fujimoto W, Mitekura H, Urishihara K, Uezato H. A case of a cutaneous leishmaniasis patient who was infected in Brazil [in Japanese]. Vol. 72, Nishi Nihon Hifuka (The Nishinihon Journal of Dermatology). 2010. p. 116–20. [Google Scholar]
- 69.Ito K, Takahara M, Ito M, Oshiro M, Takahashi K, Uezato H, et al. An imported case of cutaneous leishmaniasis caused by Leishmania (Leishmania) donovani in Japan. J Dermatol. 2014;41(10):926–8. doi: 10.1111/1346-8138.12609 [DOI] [PubMed] [Google Scholar]
- 70.Tokoro M, Nakamoto K, Arai T, Kaneko Y, Hayashi N, Ueda M. A case of a visceral leishmaniasis patient coinfected with HIV [in Japanese]. Vol. 20, Clinical Parasitology. 2010. p. 96–8. [Google Scholar]
- 71.National Institute of Infectious Diseases. Leprosy: information for the public [Internet]. 2021 [cited 2022 Mar 9]. Available from: https://www.niid.go.jp/niid/ja/leprosy-m/1841-lrc/1693-general.html.
- 72.Yotsu R. Tropical diseases in Japan ‐ bacteria ‐ leprosy [in Japanese]. Nihon Iji Shimpo (Japan Medical Journal). 2019. p. 36–7. [Google Scholar]
- 73.Nakamura I, Yamaguchi S, Kariya Y, Matori S, Taira K, Yamamoto Y, et al. Hansen’s Disease of Lepromatous Type Occurring in a Micronesian US Soldier [in Japanese]. Nishi Nihon Hifuka. 2013;75(4):326–30. [Google Scholar]
- 74.Namisato M. Leukoderma acquisitum centrifugum (halo nevus) developed following type 1 leprosy reaction [in Japanese]. Jpn J Lepr. 2018;86(3):175–9. [Google Scholar]
- 75.Ukai Y, Sugimoto T, Kawauchi Y, Ohi T, Fujii N, Nakanishi T, et al. Case reports: Filipino BL type Hansen’s disease that occurred in Japan [in Japanese]. Vol. 59, Hifuka no Rinsho. 2017. p. 675–8. [Google Scholar]
- 76.Nagaoka F, Itoh M, Kadozaka T, Takagi H. Cases of suspected filarial infection reported to our laboratory in the past five years (Japanese). Nihon Rinsho Kiseichu Gakkaishi Clin Parasitol. 2015;26(1):68–70. [Google Scholar]
- 77.Ohtake S, Kurita H, Fujikawa N, Osada H, Nakashima Y, Itoh M, et al. A case of scrotal edema associated with filarial infection. Jpn J Urol Surg. 2016;29(2):165–8. [Google Scholar]
- 78.Ichinomiya A, Nishimura K, Takenaka M, Utani A, Nishimoto K. Mycetoma caused by Nocardia transvalensis with repeated local recurrences for 25 years without dissemination to viscera. J Dermatol. 2014. Jun;41(6):556–7. doi: 10.1111/1346-8138.12496 [DOI] [PubMed] [Google Scholar]
- 79.Suzuki C, Shimoyama A, Itsunoi T, Sei Y. Diseases of fascia and subcutaneous tissue] Clinical case: Primary cutaneous nocardiosis (mycetoma type) on the buttocks [in Japanese]. Vol. 38, Hifubyo Shinryou (Practical Dermatology). 2016. p. 29–32. [Google Scholar]
- 80.Mitomo T, Ushigome Y, Fukuda T, Karino Y, Shiohara T. Two cases of chromomycosis identified as Fonsecaea monophora by molecular phylogenetic analysis [in Japanese]. Vol. 57, Medical Mycology Journal. 2016. p. J133–9. [DOI] [PubMed] [Google Scholar]
- 81.Sugiyama Y, Suzuki Y, Sugaya K, Tokura Y, Yaguchi T, Kamei K, et al. Chromoblastomycosis caused by Fonsecaea monophora. Med Mycol J. 2011;52(3):255–60. doi: 10.3314/mmj.52.255 [DOI] [PubMed] [Google Scholar]
- 82.Taniguchi T, Tsuji M, Takei K, Takahara M, Anzai K, Matsuda T, et al. A case of chromoblastomycosis due to Fonsecaea monophora treated with surgical excision [in Japanese]. Vol. 82, Nishi Nihon Hifuka. 2020. p. 289–93. [Google Scholar]
- 83.Yanagihara S, Kobayashi H, Kamo R, Hirata C, Hiruma M, Nishimura K, et al. Chromoblastomycosis caused by Fonsecaea nubica: First report from Japan. J Dermatol. 2015;42(8):833–4. doi: 10.1111/1346-8138.12898 [DOI] [PubMed] [Google Scholar]
- 84.Tsubomitsu T, Sawada M, Dekio G, Ninomiya A, Ishizaki J, Tanaka M, et al. A case of lymphangitic sporotrichosis in which the mechanism of spontaneous resolution was considered during the course of the disease [in Japanese]. Vol. 35, Nihon Rinho Hifuka Ikai Zasshi (Journal of the Japan Organization of Clinical Dermatologists). 2018. p. 508–13. [Google Scholar]
- 85.Kurihara Y, Matsumoto N, Hata Y, Doki T, Miyakawa S. A case of sporotrichosis with white colonies arising from a rat bite [in Japanese]. Vol. 31, Nihon Rinho Hifuka Ikai Zasshi (Journal of the Japan Organization of Clinical Dermatologists). 2014. p. 477–80. [Google Scholar]
- 86.Sakamoto T, Ushigami T, Anzai K, Fujii T, Abe S, Mochizuki T, et al. A case of sporotrichosis rapidly diagnosed by Direct PCR [in Japanese]. Vol. 9, Hifu No Kagaku (Skin Research). 2010. p. 254–9. [Google Scholar]
- 87.Naka W. Educational Series Superficial mycosis Sporotrichosis [in Japanese]. Vol. 53, Medical Mycology Journal. 2012. p. 163–7. [DOI] [PubMed] [Google Scholar]
- 88.Watanabe T, Kamei K. Import fungal disease [in Japanese]. Vol. 31, Kokyu. 2012. p. 1106–10. [Google Scholar]
- 89.Kurosawa A, Tojinbara K, Kadowaki H, Hampson K, Yamada A, Makita K. The rise and fall of rabies in Japan: A quantitative history of rabies epidemics in Osaka Prefecture, 1914–1933. PLoS Negl Trop Dis. 2017;11(3):e0005435. doi: 10.1371/journal.pntd.0005435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.National Institute of Infectious Diseases. Scabies [in Japanese] [Internet]. 2015. Available from: https://www.niid.go.jp/niid/ja/kansennohanashi/380-itch-intro.html.
- 91.Amada D. A case of patient with autism spectrum disorder and crusted (Norwegian) scabies [in Japanese]. Vol. 37, Nihon Primary case Rengou Gakkaishi. 2014. p. 340–1. [Google Scholar]
- 92.Kasamatsu Y, Shirano M, Goto S. A case of AIDS with exacerbation of generalized scabies throughout the body by IRIS [in Japanese]. Vol. 25, Clinical Parasitology. 2014. p. 68–70. [Google Scholar]
- 93.Takai T, Kawata Y, Nakamura K, Murata Y. Scabies Infestation within the Horny Layer of a Facial Hyperkeratotic Nodule [in Japanese]. Vol. 124, Nihon Hifuka Gakkaizasshi. 2014. p. 2299–303. [Google Scholar]
- 94.Ichinomiya A, Nishimoto K. Case of scabies at Nagasaki Ekisaikai Hospital during the two years from September 2013 to August 2015 [in Japanese]. Vol. 16, Nagasaki-ekisaikai-byouinkaihou. 2015. p. 1–5. [Google Scholar]
- 95.Hattori M, Kanazawa N. Two epidemic outbreaks of scabies [in Japanese]. Vol. 33, Nihon Rinsho Hifukaikai Zasshi. 2016. p. 383–8. [Google Scholar]
- 96.Mashima E, Haruyama S, Yoshioka H, Yamada Y, Nakamura M. Case report of scabies among five siblings treated as intractable eczema [in Japanese]. Vol. 35, Nihon Shounihifuka gakkaizassi. 2016. p. 51–5. [Google Scholar]
- 97.Shiroyama R, Igarashi R, Honda Y, Yoshida T, Oshida K, Higuchi S, et al. A Case of Infantile Scabies Difficult to Differentiate from Multisystem Langerhans Cell Histiocytosis [in Japanese]. Vol. 122, Nihon Shounika Gakkai Zasshi (The journal of the Japan Pediatric Society). 2018. p. 760–6. [Google Scholar]
- 98.Aragaki A, Shirono M. Review of the measures against outbreaks of scabies in the psychiatric ward in Japan [in Japanese]. Vol. 61, Nihon Seishinka Kango Gakujyutusyuukaishi. 2020. p. 17–21. [Google Scholar]
- 99.Machida K, Yamada T, Shimura E, Umemura M, Onoue S, Kaneko M, et al. Schistosomiasis japonica in a patient who emigrated from China: a case report [in Japanese]. Vol. 115, Nihon Shokakibyo Gakkai Zasshi. 2018. p. 1094–100. [DOI] [PubMed] [Google Scholar]
- 100.Hosho K, Ikebuchi Y, Ueki M, Nakamura K, Yashima K, Maeda N, et al. Schistosoma Japonica identified by laparoscopic and colonoscopic examination. Vol. 22, Digestive Endoscopy. 2010. p. 133–6. [DOI] [PubMed] [Google Scholar]
- 101.Yanagisawa S, Yuasa T, Tanaka T. Clinical Diagnosis of Schistosoma japonicum Infection Complicating Infective Endocarditis and Liver Cirrhosis. Vol. 49, Internal Medicine. 2010. p. 1001–5. doi: 10.2169/internalmedicine.49.3121 [DOI] [PubMed] [Google Scholar]
- 102.Ema A, Imai S, Nakagawa M, Kumamoto Y, Nagase T, Kanai T. A case of acute appendicitis with ova of Schistoso [in Japanese]. Vol. 72, The Journal of the Japan Surgical Association. 2011. p. 1773–7. [Google Scholar]
- 103.Mori T, Matsumoto S, Imoto Y, Shinomiya H, Wada S. Schistosoma japonicum found in an area not endemic [in Japanese]. Vol. 55, Acta hepatologica Japonica. 2014. p. 254–8. [Google Scholar]
- 104.Ishihara R, Hashimoto A, Saiki R, Ikenoyama Y, Tahara Y. A case of Schistosomiasis japonica with a history of living in the Philippines [in Japanese]. Vol. 26, Clinical Parasitology. 2015. p. 52–4. [Google Scholar]
- 105.Murayama S, Nomura S, Kaneko M, Honma Y, Takemura T, Tomita K. Infection with schistosomal parasites in Africa [in Japanese]. Vol. 66, Japanese journal of clinical urology. 2012. p. 319–22. [Google Scholar]
- 106.Hasegawa Y, Nishiii M, Masui S, Yoshio Y, Kanda H, Kanai M, et al. Urinary schistosomiasis: report of a case [in Japanese]. Hinyokika Kiyo. 2014. Feb;60(2):91–4. [PubMed] [Google Scholar]
- 107.Mizuno Y, Hayashi N, Chigusa Y. A case of bilharziasis with calcification of the seminal vesicle [in Japanese]. Vol. 26, Clinical Parasitology. 2015. p. 49–51. [Google Scholar]
- 108.Miike T, Sakamoto Y. Perfect cure of diseases caused by summer animals ‐ snakebites [in Japanese]. Derma. 2018. p. 66–76. [Google Scholar]
- 109.Tajika M. Main points of diagnosis and treatment of snakebites [in Japanese]. Thromb Med. 2014;4(3):286–90. [Google Scholar]
- 110.Yoneda M, Nakamura T, Mori S, Kurai M, Yoshino A, Ito M, et al. Five cases of mamushi bites and their treatment [in Japanese]. Nihon Shounikaikai Kaiho (The Journal of the Japan Pediatric Association). 2017. p. 197–201. [Google Scholar]
- 111.Okamoto O, Nakashima R, Yamamoto S, Hashimoto T, Takasaki T, Tokuda H, et al. A lethal case of mamushi (Gloydius blomhoffii) bite: severe bowel symptoms as a lethal sign. Acute Med Surg. 2017. Jan;4(1):135–9. doi: 10.1002/ams2.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kawamoto K, Miyamoto K, Harada N, Yamashita N. A case of acute renal failure due to viper bite and clinical characteristics of 64 cases over 8 years [in Japanese]. Vol. 1, Journal of Japan Society for Blood Purification in Critical Care. 2010. p. 141–5. [Google Scholar]
- 113.Nakamura K, Ideno N, Murakami M, Ogawa Y, Momii S. A case of acute renal and respiratory failure due to mamushi bite [in Japanese]. Nihon Kyukyu Igakukai Zasshi. 2010;21(10):843–8. [Google Scholar]
- 114.Tamehiro K, Shima H, Taki K. A survived case of serious viper bite complicated with acute renal failure [in Japanese]. Vol. 15, Nihon Rinsho Kyukyu Igakukai Zasshi (Journal of Japanese Society for Emergency Medicine). 2012. p. 546–9. [Google Scholar]
- 115.Kawamura Y, Nagai M, Kuraishi N, Tamura M. A case of cerebral infarction associated with Japanese mamushi (Gloydius blomhoffii blomhoffii) bite [in Japanese]. J Jpn Assoc Rural Med. 2014;63(1):57–60. [Google Scholar]
- 116.Kotake M, Suzuki S. Two cases that developed purulent osteomyelitis after mamushi bites [in Japanese]. Vol. 35, Nihon Syugekagakkai Zasshi (The journal of Japanese Society for Surgery of the Hand). 2019. p. 1244–8. [Google Scholar]
- 117.Yoshimine S, Seyama A, Suga A, Murakami M, Hayashi M, Inoue T, et al. Clinical study of 67 cases of Japanese mamushi viper (Gloydius blomhoffii) bite [in Japanese]. J Jpn Assoc Rural Med. 2019;68(4):468–74. [Google Scholar]
- 118.Ishikawa T, Soejima O, Nakagawa H, Kitamura T, Naito M. Five cases that developed compartment syndrome in hands and arms after mamushi bites [in Japanese]. Vol. 26, Nihon Syugekagakkai Zasshi (The Journal of Japanese Society for Surgery of the Hand). 2010. p. 453–5. [Google Scholar]
- 119.Kimura N, Okabe N, Futamura-Nakagawa R, Koda F, Izumo A, Furue M. Clinical study of 81 cases of “mamushi” viper bite during the past 11 years [in Japanese]. Nishi Nihon Hifuka. 2015;77(6):584–8. [Google Scholar]
- 120.Kushibe I, Kawai H, Takano D. Experience of hand therapy in a case of mamushi bite [in Japanese]. Vol. 9, Nihon Hand Therapy Gakkaishi (The Journal of Japanese Hand Therapy Society). 2017. p. 96–100. [Google Scholar]
- 121.Fujii K, Harada D, Kujurai D, Kase K, Miyatake S. A case of a child bitten by mamushi and treated with antivenom [in Japanese]. Vol. 38, Nihon Kyukyu Igakukai Kanto Chihokai Zasshi (KANTO Journal of Japanese Association for Acute Medicine). 2017. p. 322–4. [Google Scholar]
- 122.Iguchi A, Kitazawa K, Nishimura T, Tanese S, Toube Y, Kobayashi H, et al. Eight pediatric cases of mamushi bite with administration of antivenom [in Japanese]. Vol. 17, Nihon Shouni Kyukyu Igakukai Zasshi (Journal of Japanese Society of Emergency Pediatrics). 2018. p. 401–7. [Google Scholar]
- 123.Shimamoto T, Miyachi M, Kuwahara Y, Ogawa H, Nishimura A, Imai M. A case of pit viper bite complicated by wound infection. Kyotofuritsu Yosanoumi Byoinshi. 2012;9(1):59–63. [Google Scholar]
- 124.Terashita S, Murakami M, Saito Y, Hashimoto I, Igarashi N, Tsuhata S. Clinical analysis of childhood pit viper bite in Toyama area [in Japanese]. Vol. 70, Shonika Rinsho (Japanese Journal of Pediatrics). 2017. p. 1308–11. [Google Scholar]
- 125.Tsubokura Y, Kume N, Kobe N, Yamazoe T, Masuda N, Yamauchi S, et al. A pediatric case of mamushi bite diagnosed five days after the injury [in Japanese]. Vol. 36, Nihon Shouni Hifuka Gakkai Zasshi. 2017. p. 65–9. [Google Scholar]
- 126.Hifumi T, Murakawa M, Sakai A, Ginnaga A, Yamamoto A, Ato M, et al. Potentially fatal coagulopathy secondary to yamakagashi (Rhabdophis tigrinus) bites that completely recovered with antivenom treatment. Acute Med Surg. 2014. Aug 8;2(2):123–6. doi: 10.1002/ams2.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Matori S, Miyagi T, Hanashiro H, Sonosaki T, Hayashi K, Awasawa T, et al. Early onset of acute compartment syndrome diagnosed by a simple needle manometer technique [in Japanese]. Nishi Nihon Hifuka. 2013;76(5):454–8. [Google Scholar]
- 128.Narasaka T, Suzuki H, Mizokami H, Miura M. A case of whipworm disease complicated by tuberculous peritonitis and detected by colonoscopy [in Japanese]. Clin Parasitol. 2012;23(1):29–31. [Google Scholar]
- 129.Ikuno H, Ishikawa T, Norose K. Status of Strongyloidiasis in Japan, 2000–2017. Am J Trop Med Hyg. 2020. Aug;103(2):727–34. doi: 10.4269/ajtmh.19-0969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis. 2013;7(7):e2288. doi: 10.1371/journal.pntd.0002288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yamanashi H, Kanbara S, Murase K, Maeda T. Eosinophilia, a marker of asymptomatic Strongyloides infection, in a young patient with extrapulmonary tuberculosis. BMJ Case Rep. 2018. Jan 4;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Inoue K, Ozaka S, Okamoto K, Ogawa R, Mizukami K, Okimoto T, et al. Multiple infections with helminths–whipworm, hookworm, and roundworm. Endoscopy. 2014. Mar 27;46(S 01):E117–8. doi: 10.1055/s-0034-1364887 [DOI] [PubMed] [Google Scholar]
- 133.Sugiyama H, Morishima Y, Kagawa C, Araki J, Iwaki T, Ikuno H, et al. Current Incidence and Contamination Sources of Ascariasis in Japan [in Japanese]. Shokuhin Eiseigaku Zasshi J Food Hyg Soc Jpn. 2020;61(4):103–8. [DOI] [PubMed] [Google Scholar]
- 134.WHO. World Health Organization. 2021 [cited 2021 Nov 5]. Buruli ulcer (Mycobacterium ulcerans infection). Available from: https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- 135.Yotsu RR, Nakanaga K, Hoshino Y, Suzuki K, Ishii N. Buruli ulcer and current situation in Japan: a new emerging cutaneous Mycobacterium infection. J Dermatol. 2012. Jul;39(7):587–93. doi: 10.1111/j.1346-8138.2012.01543.x [DOI] [PubMed] [Google Scholar]
- 136.National Institute of Infectious Diseases. National Institute of Infectious Diseases Infectious Agents Surveillance Report. 2018. Infectious Agents Surveillance Report. Available from: https://www.niid.go.jp/niid/ja/kansennohNoanashi/2913-bu-intro.html.
- 137.WHO. The Global Health Observatory, World Health Organization. 2021. Number of new reported cases of Buruli ulcer. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/number-of-new-reported-cases-of-buruli-ulcer.
- 138.Yasukawa K. Blood donor screening for Trypanosoma cruzi infection in Japan. Transfusion (Paris). 2014;54(3):745–6. doi: 10.1111/trf.12505 [DOI] [PubMed] [Google Scholar]
- 139.Nara T. Chagas disease [in Japanese]. Igaku No Ayumi J Clin Exp Med. 2015;253(1):119–23. [Google Scholar]
- 140.Nara T. Globalization, global warming, and infectious disease control ‐ vector-borne disease, Chagas disease [in Japanese]. Vol. 70, Shonika Rinsho (Japanese Journal of Pediatrics). 2017. p. 2267–71. [Google Scholar]
- 141.Coura JR. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions—a comprehensive review. Mem Inst Oswaldo Cruz. 2015;110(3):277–82. doi: 10.1590/0074-0276140362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010. Aug;115(1–2):22–7. doi: 10.1016/j.actatropica.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 143.Gascon J, Vilasanjuan R, Lucas A. The need for global collaboration to tackle hidden public health crisis of Chagas disease. Expert Rev Anti Infect Ther. 2014;12(4):393–5. doi: 10.1586/14787210.2014.896194 [DOI] [PubMed] [Google Scholar]
- 144.Miyanaga D, Tama N, Amaya N, Sarasawa K, Ikeda H, Fukuoka Y, et al. A case of Chagas cardiomyopathy with severe heart failure and ventricular tachycardia [in Japanese]. Shinzo Heart. 2019;51(9):932–8. [Google Scholar]
- 145.Satoh F, Tachibana H, Hasegawa I, Osawa M. Sudden death caused by chronic Chagas disease in a non-endemic country: Autopsy report. Pathol Int. 2010. Mar;60(3):235–40. doi: 10.1111/j.1440-1827.2009.02503.x [DOI] [PubMed] [Google Scholar]
- 146.Shintaku M, Takeda S, Miura S, Yutani C, Tsutsumi Y. Chronic Chagastic cardiomyopathy associated with membranoproliferative glomerulonephritis: Report of an autopsy case. Pathol Int. 2020;70(1):47–52. doi: 10.1111/pin.12883 [DOI] [PubMed] [Google Scholar]
- 147.Imai K, Maeda T, Sayama Y, Mikita K, Fujikura Y, Misawa K, et al. Mother-to-child transmission of congenital Chagas disease, Japan. Emerg Infect Dis. 2014. Jan;20(1):146–8. doi: 10.3201/eid2001.131071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nagumo M, Maeda T, Imai K, Misawa K, Kei M, Fujikura Y, et al. A case report of congenital Chagas’ disease in Japan [in Japanese]. J Natl Def Med Coll. 2013;38(3):225–30. [Google Scholar]
- 149.Sayama Y, Furui Y, Takakura A, Ishinoda M, Matsumoto C, Taira R, et al. Seroprevalence of Trypanosoma cruzi infection among at-risk blood donors in Japan. Transfusion (Paris). 2019. Jan;59(1):287–94. doi: 10.1111/trf.14999 [DOI] [PubMed] [Google Scholar]
- 150.Momose S. Safety measures for blood products [in Japanese]. Vol. 44, Kansensyo (The Infection). 2014. p. 97–102,91–92. [Google Scholar]
- 151.Hirayama K. Preparedness for Chagas disease [in Japanese]. Nihon Iji Shimpo Jpn Med J. 2014;(4723):47. [Google Scholar]
- 152.Momose S. A case of blood donation by a person positive to Chagas disease [in Japanese]. Rinsho Kensagaku Zasshi Med Technol. 2014;42(8):766–9. [Google Scholar]
- 153.Sukehiro N, Kida N, Umezawa M, Murakami T, Arai N, Jinnai T, et al. First Report on Invasion of Yellow Fever Mosquito, Aedes aegypti, at Narita International Airport, Japan in August 2012. Jpn J Infect Dis. 2013;66(3):189–94. doi: 10.7883/yoken.66.189 [DOI] [PubMed] [Google Scholar]
- 154.Mogi M, Tuno N. Impact of climate change on the distribution of Aedes albopictus (Diptera: Culicidae) in northern Japan: retrospective analyses. J Med Entomol. 2014;51(3):572–9. doi: 10.1603/me13178 [DOI] [PubMed] [Google Scholar]
- 155.Ohnishi K. Needle-stick dengue virus infection in a health-care worker at a Japanese hospital. J Occup Health. 2015;57(5):482–3. doi: 10.1539/joh.14-0224-CS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schmidt-Chanasit J, Emmerich P, Tappe D, Gunther S, Schmidt S, Wolff D, et al. Autochthonous dengue virus infection in Japan imported into Germany, September 2013. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2014. Jan 23;19(3). doi: 10.2807/1560-7917.es2014.19.3.20681 [DOI] [PubMed] [Google Scholar]
- 157.Ueno R, Nishiura H. Letter to the editor: diagnosis of a single imported dengue case who had traveled to Japan ‐ how serious is it for travelers? Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2014. Feb 27;19(8):20715. [DOI] [PubMed] [Google Scholar]
- 158.Kobayashi D, Murota K, Fujita R, Itokawa K, Kotaki A, Moi ML, et al. Dengue Virus Infection in Aedes albopictus during the 2014 Autochthonous Dengue Outbreak in Tokyo Metropolis, Japan. Am J Trop Med Hyg. 2018. May;98(5):1460–8. doi: 10.4269/ajtmh.17-0954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nakayama E, Kotaki A, Tajima S, Kawada M, Miura K, Gemma A, et al. Two different dengue virus strains in the Japanese epidemics of 2014. Virus Genes. 2016. Oct;52(5):722–6. doi: 10.1007/s11262-016-1356-4 [DOI] [PubMed] [Google Scholar]
- 160.Seki N, Iwashita Y, Moto R, Kamiya N, Kurita M, Tahara N, et al. An autochthonous outbreak of dengue type 1 in Tokyo, Japan 2014. Nihon Koshu Eisei Zasshi Jpn J Public Health. 2015;62(5):238–50. doi: 10.11236/jph.62.5_238 [DOI] [PubMed] [Google Scholar]
- 161.Tajima S, Nakayama E, Kotaki A, Moi ML, Ikeda M, Yagasaki K, et al. Whole Genome Sequencing-Based Molecular Epidemiologic Analysis of Autochthonous Dengue Virus Type 1 Strains Circulating in Japan in 2014. Jpn J Infect Dis. 2017. Jan 24;70(1):45–9. doi: 10.7883/yoken.JJID.2016.086 [DOI] [PubMed] [Google Scholar]
- 162.Quam MB, Sessions O, Kamaraj US, Rocklöv J, Wilder-Smith A. Dissecting Japan’s Dengue Outbreak in 2014. Am J Trop Med Hyg. 2016. Feb;94(2):409–12. doi: 10.4269/ajtmh.15-0468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kondo M, Akachi S, Ando K, Nomura T, Yamanaka K, Mizutani H. Two Japanese siblings affected with Chikungunya fever with different clinical courses: Imported infections from the Cook Islands. J Dermatol. 2016. Jun;43(6):697–700. doi: 10.1111/1346-8138.13253 [DOI] [PubMed] [Google Scholar]
- 164.Takaya S, Kutsuna S, Nakayama E, Taniguchi S, Tajima S, Katanami Y, et al. Chikungunya Fever in Traveler from Angola to Japan, 2016. Emerg Infect Dis. 2017. Jan;23(1):156–8. doi: 10.3201/eid2301.161395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eimoto A, Nakata T, Aoki M, Oiwa T, Adachi M, Takeuchi M. A case of Chikungunya fever after traveling to India [in Japanese]. Vol. 13, Nihon Kyukyu Igakukai Chubu Chihokaishi (Chuubu Journal of Acute Medicine). 2017. p. 22–4. [Google Scholar]
- 166.Imai K, Nakayama E, Maeda T, Mikita K, Kobayashi Y, Mitarai A, et al. Chikungunya Fever in Japan Imported from the Caribbean Islands. Jpn J Infect Dis. 2016;69(2):151–3. doi: 10.7883/yoken.JJID.2015.063 [DOI] [PubMed] [Google Scholar]
- 167.Sasaki Y, Manda S, Sato T, Maeda T, Miyazaki T, Nakanishi K, et al. Chikungunya Virus Infection Presenting with Persistent Arthralgia without Fever. J Gen Fam Med. 2015. Oct;16(3):204–7. [Google Scholar]
- 168.Furuichi M, Makie T, Honma Y, Isoda T, Miyake S. Laboratory-Confirmed Dengue Fever and Chikungunya Fever Cases at the Narita Airport Quarantine Station in 2013. Jpn J Infect Dis. 2015;68(2):142–4. doi: 10.7883/yoken.JJID.2014.242 [DOI] [PubMed] [Google Scholar]
- 169.National Institute of Infectious Diseases. Guide to Response and Control of Dengue Fever, Chikungunya Fever, and Other Mosquito-borne Infectious Diseases For local governments [Internet]. 2017. Available from: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000163947.pdf.
- 170.Naha Public Health Center. Progress Report on Response to Suspected Dengue Infection in Naha City [Internet]. 2019. Available from: https://www.city.naha.okinawa.jp/nahahokenjyo/kansensyou/kansensyoubetu/denguefever.files/1.pdf.
- 171.National Institute of Infectious Diseases. Diagnostic Manual for Chikungunya Virus infection [Internet]. 2013. Available from: https://www.niid.go.jp/niid/images/lab-manual/CHIKV.v1.1.pdf.
- 172.National Institute of Infectious Diseases. Diagnostic Manual for Dengue Virus Infection [Internet]. 2014. Available from: https://www.niid.go.jp/niid/images/lab-manual/Dengue2014.pdf.
- 173.Guo ZH, Kubo M, Kudo M, Nibe K, Horii Y, Nonaka N. Growth and genotypes of Echinococcus granulosus found in cattle imported from Australia and fattened in Japan. Parasitol Int. 2011. Dec;60(4):498–502. doi: 10.1016/j.parint.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 174.Vuitton DA, Wang Q, Zhou HX, Raoul F, Knapp J, Bresson-Hadni S, et al. A historical view of alveolar echinococcosis, 160 years after the discovery of the first case in humans: part 1. What have we learnt on the distribution of the disease and on its parasitic agent? Chin Med J (Engl). 2011. Sep;124(18):2943–53. [PubMed] [Google Scholar]
- 175.Yagi K. The Control Measures of Alveolar Echinococcosis Conducted by Hokkaido Local Government [in Japanese]. Report of the Hokkaido Institute of Public Health. 2017. p. 1–7. [Google Scholar]
- 176.Fukumoto SI, Yamada S, Fushikida M, Toyada S, Nishikawa T, Higuchi H, et al. Natural larval Echinococcus multilocularis infection in a Norway rat, Rattus norvegicus, captured indoors in Hokkaido, Japan. J Vet Med Sci. 2017. Nov;79(11):1857–60. doi: 10.1292/jvms.17-0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Goto Y, Sato K, Yahagi K, Komatsu O, Hoshina H, Abiko C, et al. Frequent isolation of Echinococcus multilocularis from the livers of racehorses slaughtered in Yamagata, Japan. Jpn J Infect Dis. 2010;63(6):449–51. [PubMed] [Google Scholar]
- 178.Hifumi T, Ikeda K, Etoh Y, Ikawa K, Nishimura K, Ogawa T, et al. The Relationship between Hepatic Grayish White Solid Nodules Found in Horses Brought to a Slaughterhouse in Fukuoka Prefecture and Larval Echinococcus multilocularis Infection. J Jpn Vet Med Assoc. 2015;68(4):253–7. [Google Scholar]
- 179.Irie T, Mukai T, Yagi K. Echinococcus multilocularis Surveillance Using Copro-DNA and Egg Examination of Shelter Dogs from an Endemic Area in Hokkaido, Japan. Vector Borne Zoonotic Dis Larchmt N. 2018;18(7):390–2. doi: 10.1089/vbz.2017.2245 [DOI] [PubMed] [Google Scholar]
- 180.Irie T, Yamada K, Morishima Y, Yagi K. High probability of pet dogs encountering the sylvatic cycle of Echinococcus multilocularis in a rural area in Hokkaido, Japan. J Vet Med Sci. 2019. Nov;81(11):1606–8. doi: 10.1292/jvms.19-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Morishima Y, Tomaru Y, Fukumoto SI, Sugiyama H, Yamasaki H, Hashimoto C, et al. Canine Echinococcosis Due to Echinococcus multilocularis: a Second Notifiable Case from Mainland Japan. Jpn J Infect Dis. 2016. Sep 21;69(5):448–9. doi: 10.7883/yoken.JJID.2015.573 [DOI] [PubMed] [Google Scholar]
- 182.Yamano K, Kouguchi H, Uraguchi K, Mukai T, Shibata C, Yamamoto H, et al. First detection of Echinococcus multilocularis infection in two species of nonhuman primates raised in a zoo: a fatal case in Cercopithecus diana and a strongly suspected case of spontaneous recovery in Macaca nigra. Parasitol Int. 2014. Aug;63(4):621–6. doi: 10.1016/j.parint.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 183.Oikawa E, Shimura R, Nishimura M, Furuoka H. First Case of Echinococcus multilocularis Infection in a Zoo-Housed Flying Squirrel (Pteromys volans orii). Vol. 75, The Journal of Veterinary Medical Science. 2013. p. 659–61. doi: 10.1292/jvms.12-0455 [DOI] [PubMed] [Google Scholar]
- 184.Morishima Y, Sugiyama H, Yamazaki H, Kon M, Hasegawa A, Doi R. Introduction of Echinococcus into nonendemic areas by infected domestic animals. 2019;40–2. [Google Scholar]
- 185.Yamano K, Yagi K, Morishima Y, Sugiyama H, Yamasaki H. Basics of Laboratory Examinations of Echinococcosis and Intestinal Helminthiasis [in Japanese] [Internet]. National Institute of Infectious Diseases; 2017. Available from: https://www.niid.go.jp/niid/images/lab-manual/Echinococcus2014Nov.pdf. [Google Scholar]
- 186.Okajima J, Shibata K, Takahashi E, Nagafuchi T, Okajima K, Nonaka N. Current status and its epidemiological consideration of Fasciola and Eurytrema infections in beef cattle of Japan. J Vet Med Sci. 2016. Jun 1;78(5):785–90. doi: 10.1292/jvms.15-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sugiyama H, SHIBATA K, MORISHIMA Y, MUTO M, YAMASAKI H, KAWAKAMI Y. Current Status of Lung Fluke Metacercarial Infection in Freshwater Crabs in the Kawane Area of Shizuoka Prefecture, Japan. J Vet Med Sci. 2013;75(3):249–53. doi: 10.1292/jvms.12-0325 [DOI] [PubMed] [Google Scholar]
- 188.Yoshida A. Paragonimiasis. Jpn J Med Pharm Sci. 2017. Nov;74(11):1393–8. [Google Scholar]
- 189.Ohari Y, Suzuki Y, Shibahara T, Itagaki T. First report of Paragonimus skrjabini miyazakii metacercariae in Geothelphusa dehaani (Sawagani) occurring in Iwate Prefecture, Japan. J Vet Med Sci. 2019. Aug;81(8):1109–12. doi: 10.1292/jvms.19-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Matsuo K, Moribe J, Takashima Y, Kasuya S, Yoshida A, Abe N, et al. Possibility of Paragonimiasis Due to Consumption of Raw Deer Meat. J Jpn Vet Med Assoc. 2018;71(8):449–53. [Google Scholar]
- 191.Sugiyama H, Shibata K, Kawakami Y, Arakawa K, Morishima Y, Yamasaki H, et al. Paragonimiasis Due to the Consumption of Wild Boar Meat in Japan: Contamination Levels of Lung Fluke Larvae in Muscle Samples of Wild Boars Caught in Kagoshima Prefecture. Jpn J Infect Dis. 2015;68(6):536–7. doi: 10.7883/yoken.JJID.2015.280 [DOI] [PubMed] [Google Scholar]
- 192.Yatera K, Hanaka M, Hanaka T, Yamasaki K, Nishida C, Kawanami T, et al. A rare case of paragonimiasis miyazakii with lung involvement diagnosed 7 years after infection: A case report and literature review. Parasitol Int. 2015;64(5):274–80. doi: 10.1016/j.parint.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 193.Ohuchi M, Inoue S, Ozaki Y, Fujita T, Ueda K, Hanaoka J. A case of paragonimiasis westermani infection caused by eating raw deer meat. J Jpn Assoc Chest Surg. 2014;28(2):170–6. [Google Scholar]
- 194.Banzai A, Sasaki T, Sugiyama H, Kawakami Y. Infection status of Paragonimus westermani metacercariae in the Japanese freshwater crab Geothelphusa dehaani from the Kobado area of Chiba Prefecture, Japan. Med Entomol Zool. 2018;69(1):1–5. [Google Scholar]
- 195.Kita K. African sleeping sickness. Jpn J Pediatr. 2017;70:2283–8. [Google Scholar]
- 196.Kawamura Y, Yoshikawa I, Katakura K. Imported leishmaniasis in dogs, US military bases, Japan. Emerg Infect Dis. 2010;16(12):2017–9. doi: 10.3201/eid1612.100389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sanjoba C, Yusuf O, Asada M, Sumbuu G, Osada Y, Miyagi I, et al. Sandflies of the genus Phlebotomus endemic to Japan. Eisei Dobutsu Med Entomol Zool. 2010;61(2):159. [Google Scholar]
- 198.Koba A, Mori S, Ishii N. Survey of newly diagnosed Japanese leprosy patients [in Japanese]. Nihon Hansenbyo Gakkai Zasshi. 2011. Feb;80(1):11–6. [DOI] [PubMed] [Google Scholar]
- 199.Takahashi K. Current situations of diverse skin diseases in Okinawa and understanding of disease contexts [in Japanese]. Vol. 88, Nihon Hansenbyo Gakkai Zasshi (Japanese Journal of Leprosy). 2019. p. 39–41. [Google Scholar]
- 200.Ozaki M, Tomoda M. Conjugal leprosy infection in Japan—case report and review [in Japanese]. Nihon Hansenbyo Gakkai Zasshi. 2012. Apr;81(1–2):135–43. [DOI] [PubMed] [Google Scholar]
- 201.Namisato M, Fujiwara T. Two cases of leprosy who had drug resistance related mutations to diaphenylsulfone, rifampicin and new quinolone [in Japanese]. Nihon Hansenbyo Gakkai Zasshi. 2018;86(3):181–7. [Google Scholar]
- 202.Koike H, Hashimoto R, Tomita M, Kawagashira Y, Iijima M, Nagamatsu M, et al. The Wide Range of Clinical Manifestations in Leprous Neuropathy: Two Case Reports. Vol. 50, Internal Medicine. 2011. p. 2223–6. doi: 10.2169/internalmedicine.50.5604 [DOI] [PubMed] [Google Scholar]
- 203.Fukai K, Komatsu K, Matsuo Y, Hayashi K, Kariya Y, Miyagi T, et al. Two cases of squamous cell carcinoma arising on refractory skin ulcers in patients with a history of Hansen’s disease [in Japanese]. Nishi Nihon Hifuka. 2019;81(2):115–9. [Google Scholar]
- 204.Ishida Y, Inoue T, Tsuchiya I, Maeda M, Hirano A. Two cases of leprosy related conditions and relevant issues on medical services for ex-leprosy patients who live in communities in Japan [in Japanese]. Nihon Hansenbyo Gakkai Zasshi. 2010. Feb;79(1):3–10. [DOI] [PubMed] [Google Scholar]
- 205.Baba M, Nurse and Care Staff in Handicapped Person’s Ward, NST Committee, Bedsore Committee. Successful team medicine to preserve foot function in intractable ulcer complicated by diabetes and the sequelae of Hansen’s disease [in Japanese]. Jpn J Foot Care. 2017;15(3):135–8. [Google Scholar]
- 206.Joko S, Horie D. A case of a patient with a history of leprosy-related uveitis to whom cataract surgery was performed after phototherapeutic keratectomy [in Japanese]. Vol. 24, Ganka Syuzyutsu (Japanese Journal of Ophthalmic Surgery). 2011. p. 199–203. [Google Scholar]
- 207.Shigeyasu C, Nakashima T, Keino H, Ikeda K, Yamane みお, Horie D, et al. Clinical Science: State of Ocular Sequelae and Visual Dysfunction in Individuals with Hansen’s Disease [in Japanese]. Vol. 123, J Jpn Ophthalmol Soc. 2019. p. 51–7. [Google Scholar]
- 208.Ishizaki T, Ogura S, Yamada M, Shiroi T, Takamori H. Experience of implant treatment in a leprosy sanatorium [in Japanese]. Vol. 10, Japanese Journal of Maxillo Facial Implants. 2011. p. 231–5. [Google Scholar]
- 209.Iwata M, Matsumura Y, Ozaki M, Nakanaga K, Hoshino Y, Ishii N, et al. Relapsed Leprosy with Infiltrative Ring-shaped Erythematous Plaques Seen in a Brazilian 16 Years after Multi-drug Therapy [in Japanese]. Hifu No Kagaku. 2014;13(6):426–30. [Google Scholar]
- 210.Inaba T. Historical Overview of Leprosy Control in Japan [in Japanese]. Kokuritsu Hansenbyo Shiryokan Kenkyu Kiyo (Research Bulletin, National Hansen’s Disease Museum). 2019. p. 1–15. [Google Scholar]
- 211.Makino M. Learning from the history of leprosy—looking back at one hundred years of medicine at the leprosaria [in Japanese]. Nihon Hansenbyo Gakkai Zasshi. 2010. Feb;79(1):25–36. [DOI] [PubMed] [Google Scholar]
- 212.Mori S, Ato M, Ishii N. A Study on the Entering and Out-going Trends at Japan’s National Hansen’s Disease Sanatoriums [in Japanese]. Nihon Hansenbyo Gakkai Zasshi. 2019;88(2):53–75. [PubMed] [Google Scholar]
- 213.Fuzisaki M. What does psychiatry learn from the fight of leprosy patients? [in Japanese]. Vol. 59, Byoin Chiiki Seishin Igaku (Jpn. J. Hospital & Community Psychi). 2016. p. 22–4. [Google Scholar]
- 214.Masubuchi Y, Takayama H, Isogai R. How did people living in the leprosy sanatoria satisfy their needs? [in Japanese]. Nihon Kango Gakkai Ronbunsyu: Seizin Kango II. 2010. p. 251–3. [Google Scholar]
- 215.Yokose R. The suffering of female ex-patients: from the narratives of women who have recovered from hansen’s disease [in Japanese]. Bioethics. 2013;23(1):54–62. [Google Scholar]
- 216.Yokose R. The continued suffering of recovered Hansen’s Disease patients with regards to their future despite having achieved their rehabilitation: Narratives by those who left Hansen’s disease sanatoriums with regards to their later abode [in Japanese]. Bioethics. 2014;24(1):126–35. [Google Scholar]
- 217.Yokota Y. Leprosy and human rights: trends in japan and in the world [in Japanese]. Nihon Hansenbyo Gakkai Zasshi. 2014;83(3):125–9. [PubMed] [Google Scholar]
- 218.Goto M, Nogam R, Okano Y, Gidoh M, Yotsu R, Ishida Y, et al. Guidelines for the treatment of Hansen’s disease in Japan (third edition) [in Japanese]. Nihon Hansen Gakkai Zasshi Jpn J Lepr Off Organ Jpn Lepr Assoc. 2013. Dec;82(3):143–84. [DOI] [PubMed] [Google Scholar]
- 219.Yotsu R, Suzuki K, Mori S, Ishii N. Diagnosis of Hansen’s disease [in Japanese]. Nihon Hansenbyo Gakkai Zasshi. 2011;80(1):59–70. [DOI] [PubMed] [Google Scholar]
- 220.Ishii N. Hansen’s disease and dermatology [in Japanese]. Vol. 81, Nishi Nihon Hifuka (The Nishinihon Journal of Dermatology). 2019. p. 461–4. [Google Scholar]
- 221.Ozaki M. Challenges of medical services for leprosy: reflection on the transition of medicines for treatment [in Japanese]. Nihon Hansenbyo Gakkai Zasshi. 2018;87(2):71–2. [Google Scholar]
- 222.Kobayashi T. The Control of Lymphatic Filariasis in Japan. Jpn J Hist Pharm. 2019;54(2):83–8. [Google Scholar]
- 223.Yotsu R. New dermatology seminarium ‐ Skin infectious diseases to be aware of zoonosis and tropical skin diseases [in Japanese]. Vol. 130, Nihon Hifuka Gakkai Zasshi (The Japanese Journal of Dermatology). 2020. p. 2355–60. [Google Scholar]
- 224.Mochizuki T, Tsuboi Y, Igarashi K, Ishizaki J, Ushigami T, Ogawa H, et al. Japanese Society of Dermatology Guidelines for the treatment of dermatomycosis 2019 [in Japanese]. Vol. 129, Nihon Hifuka Gakkai Zasshi (The Japanese Journal of Dermatology). 2019. p. 2639–73. [Google Scholar]
- 225.Suzuki R, Yikelamu A, Tanaka R, Igawa K, Yokozeki H, Yaguchi T. Studies in Phylogeny, Development of Rapid Identification Methods, Antifungal Susceptibility, and Growth Rates of Clinical Strains of Sporothrix schenckii Complex in Japan. Med Mycol J. 2016;57(3):E47–57. [DOI] [PubMed] [Google Scholar]
- 226.Kusuhara M. Skin diseases common in Kyushu Region sporotrichosis and chromomycosis in Kyushu and Okinawa Region [in Japanese]. Vol. 29, Nihon Rinho Hifuka Ikai Zasshi (Journal of the Japan Organization of Clinical Dermatologists). 2012. p. 773–7. [Google Scholar]
- 227.Watanabe T, Kamei K. Current status of imported fungal infections and countermeasures [in Japanese]. Nihon Naika Gakkai Zasshi (The Journal of the Japanese Society of Internal Medicine). 2014. p. 2074–679. [DOI] [PubMed] [Google Scholar]
- 228.Fukuda M, Uni S, Otsuka Y, Eshita Y, Nakatani J, Ihara K, et al. A new case of zoonotic onchocercosis in northern Kyushu, Japan. Parasitol Int. 2015. Dec;64(6):519–21. [DOI] [PubMed] [Google Scholar]
- 229.Fukuda M, Otsuka Y, Uni S, Boda T, Daisaku H, Hasegawa H, et al. Zoonotic onchocerciasis in Hiroshima, Japan, and molecular analysis of a paraffin section of the agent for a reliable identification. Parasite Paris Fr. 2011. May;18(2):185–8. doi: 10.1051/parasite/2011182185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fukuda M, Otsuka Y, Uni S, Bain O, Takaoka H. Genetic evidence for the presence of two species of Onchocerca from the wild boar in Japan. Parasite Paris Fr. 2010. Mar;17(1):33–7. doi: 10.1051/parasite/2010171033 [DOI] [PubMed] [Google Scholar]
- 231.Uni S, Fukuda M, Ogawa K, Lim YAL, Agatsuma T, Bunchom N, et al. Zoonotic infection with Onchocerca dewittei japonica in an 11-year-old boy in Kansai Region, Western Honshu, Japan. Parasitol Int. 2017. Oct;66(5):593–5. doi: 10.1016/j.parint.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 232.Uni S, Fukuda M, Otsuka Y, Hiramatsu N, Yokobayashi K, Takahashi H, et al. New zoonotic cases of Onchocerca dewittei japonica (Nematoda: Onchocercidae) in Honshu, Japan. Parasit Vectors. 2015. Jan 27;8:59. doi: 10.1186/s13071-015-0655-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Malerczyk C, Detora L, Gniel D. Imported human rabies cases in europe, the United States, and Japan, 1990 to 2010. J Travel Med. 2011. Dec;18(6):402–7. doi: 10.1111/j.1708-8305.2011.00557.x [DOI] [PubMed] [Google Scholar]
- 234.Nosaki Y, Maeda K, Watanabe M, Yokoi T, Iwai K, Noguchi A, et al. Fourth imported rabies case since the eradication of rabies in Japan in 1957. J Travel Med. 2021. Sep 20;taab151. doi: 10.1093/jtm/taab151 [DOI] [PubMed] [Google Scholar]
- 235.Kashino W, Piyaphanee W, Kittitrakul C, Tangpukdee N, Sibunruang S, Lawpoolsri S, et al. Incidence of potential rabies exposure among Japanese expatriates and travelers in Thailand. J Travel Med. 2014. Aug;21(4):240–7. doi: 10.1111/jtm.12124 [DOI] [PubMed] [Google Scholar]
- 236.Miyazu M, Kikuchi H, Goto Y, Yamamoto E. Rabies Postexposure prophylaxis [in Japanese]. Vol. 7, Nihon Tokouigakkaishi (Journal of Japanese Society of travel and Health). 2014. p. 35–9. [Google Scholar]
- 237.Yuhji Kuroda. Importance of Vaccines from the Viewpoint of Imported Infectious Diseases About Rabies [in Japanese]. Nihonijishinpo Jpn Med J. 2016;(4789):27–33. [Google Scholar]
- 238.Takayama N, Suganuma A, Yanagisawa N, Nakayama E. Trends of patients seeking post-exposure prophylaxis at our hospital: exposure and consultations abroad [in Japanese]. Vol. 143, Nihon Ishikai Zashi. 2014. p. 1529–33. [Google Scholar]
- 239.Inoue S. Rabies Guideline in Japan 2013 [in Japanese]. Vol. 67, Jyuui Chikusan Shinpou. 2014. p. 171–5. [Google Scholar]
- 240.Takahashi-Omoe H, Omoe K, Okabe N. Regulatory systems for prevention and control of rabies, Japan. Emerg Infect Dis. 2008. Sep;14(9):1368–74. doi: 10.3201/eid1409.070845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Watanabe I, Yamada K, Aso A, Suda O, Matsumoto T, Yahiro T, et al. Relationship between virus-neutralizing antibody levels and the number of rabies vaccinations: a prospective study of dogs in Japan. Jpn J Infect Dis. 2013;66(1):17–21. doi: 10.7883/yoken.66.17 [DOI] [PubMed] [Google Scholar]
- 242.Hidano A, Hayama Y, Tsutsui T. Prevalence of immunity presumed using rabies vaccination history and household factors associated with vaccination status among domestic dogs in Japan. Jpn J Infect Dis. 2012;65(5):396–402. doi: 10.7883/yoken.65.396 [DOI] [PubMed] [Google Scholar]
- 243.Matsumoto N, Ida M. Research on rabies virus antibody level in dogs [in Japanese]. Tottori ken eisei Kankyo kenkyushohou. 2010. p. 5–7. [Google Scholar]
- 244.Saeki J, Yamamoto S, Yabe M, Nagasaki J, Hayashi K. Survey of rabies antibody prevalence in young dogs younger than 91 days [in Japanese]. Vol. 68, Nihon Jyuuisikai zassi. 2015. p. 135–40. [Google Scholar]
- 245.Kwan NCL, Ogawa H, Yamada A, Sugiura K. Quantitative risk assessment of the introduction of rabies into Japan through the illegal landing of dogs from Russian fishing boats in the ports of Hokkaido, Japan. Prev Vet Med. 2016. Jun 1;128:112–23. doi: 10.1016/j.prevetmed.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 246.Kwan NCL, Sugiura K, Hosoi Y, Yamada A, Snary EL. Quantitative risk assessment of the introduction of rabies into Japan through the importation of dogs and cats worldwide. Epidemiol Infect. 2017;145(6):1168–82. doi: 10.1017/S0950268816002995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kadowaki H, Hampson K, Tojinbara K, Yamada A, Makita K. The risk of rabies spread in Japan: a mathematical modelling assessment. Epidemiol Infect. 2018. Jul;146(10):1245–52. doi: 10.1017/S0950268818001267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kwan NCL, Inoue M, Yamada A, Sugiura K. Evaluating the contact rate between companion dogs during dog walking and the practices towards potential cases of rabies among dog owners in Japan. Zoonoses Public Health. 2019. Jun;66(4):393–400. doi: 10.1111/zph.12573 [DOI] [PubMed] [Google Scholar]
- 249.Kwan NCL, Yamada A, Sugiura K. Benefit-cost analysis of the policy of mandatory annual rabies vaccination of domestic dogs in rabies-free Japan. PloS One. 2018;13(12):e0206717. doi: 10.1371/journal.pone.0206717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yamada A, Makita K, Kadowaki H, Ito N, Sugiyama M, Kwan NCL, et al. A Comparative Review of Prevention of Rabies Incursion between Japan and Other Rabies-Free Countries or Regions. Jpn J Infect Dis. 2019. Jul 24;72(4):203–10. doi: 10.7883/yoken.JJID.2018.431 [DOI] [PubMed] [Google Scholar]
- 251.Executive Committee of Guideline for the Diagnosis and Treatment of Scabies. Guideline for the diagnosis and treatment of scabies in Japan (third edition): Executive Committee of Guideline for the Diagnosis and Treatment of Scabies. J Dermatol. 2017 Sep;44(9):991–1014. [DOI] [PubMed]
- 252.Tanaka H, Tsuji M. From discovery to eradication of schistosomiasis in Japan: 1847–1996. Int J Parasitol. 1997. Dec;27(12):1465–80. doi: 10.1016/s0020-7519(97)00183-5 [DOI] [PubMed] [Google Scholar]
- 253.Kirinoki M, Hayashi N, Chigusa Y. Schistosomiasis [in Japanese]. Vol. 42, Dokkyo Journal of Medical Sciences. 2015. p. 233–7. [Google Scholar]
- 254.Sakai Y, Iwamoto K, Tanaka M, Yamada K. A case of rectal cancer with ova of Schistosoma Japonicum. Gastroenterol Endosc. 2007;49(9):2504–10. [Google Scholar]
- 255.Imai J, Ichikawa H, Mizukami H, Suzuki T, Watanabe N, Mine T, et al. Colonic High-grade Tubular Adenomas Associated with Schistosoma japonicum [in Japanese]. Vol. 41, The Tokai Journal of Experimental and Clinical Medicine. 2016. p. 22–3. [PubMed] [Google Scholar]
- 256.Nihei N. Focused on distribution and monitoring of Oncomelania nosophora in Japan [in Japanese]. Vol. 63, Medical Entomology and Zoology. 2012. p. 249–56. [Google Scholar]
- 257.Minai M. Oncomelania nosophora in Kofu basin,Yamanashi Prefecture, Japan [in Japanese]. Vol. 63, Medical Entomology and Zoology. 2012. p. 257–62. [Google Scholar]
- 258.Nihei N, Kirinoki M, Koen H, Tsuyuguchi T, Saito Y, Taira K, et al. Changes in the habitat of Oncomelania hupensis nosophora, the intermediate host snail of Schistosoma japonicum, along the Obitsu River basin, Chiba Prefecture, Japan [in Japanese]. Vol. 69, Medical Entomology and Zoology. 2018. p. 19–29. [Google Scholar]
- 259.Sakai A. Snakes and snake venom [in Japanese]. Vol. 74, Koushueisei (The Journal of Public Health Practice). 2010. p. 377–81. [Google Scholar]
- 260.Sakai A. Diagnosis and treatment of snakebite by Mamushi and Yamakagashi [in Japanese]. Chudoku Kenkyu Jpn J Toxicol. 2013. Sep;26(3):193–9. [PubMed] [Google Scholar]
- 261.Yasunaga H, Horiguchi H, Kuwabara K, Hashimoto H, Matsuda S. Short report: venomous snake bites in Japan. Am J Trop Med Hyg. 2011. Jan;84(1):135–6. doi: 10.4269/ajtmh.2011.10-0403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hifumi T, Sakai A, Yamamoto A, Murakawa M, Ato M, Shibayama K, et al. Clinical characteristics of yamakagashi (Rhabdophis tigrinus) bites: a national survey in Japan, 2000–2013. J Intensive Care. 2014;2(1):19. doi: 10.1186/2052-0492-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hifumi T, Sakai A, Yamamoto A, Murakawa M, Ato M, Shibayama K, et al. Effect of antivenom therapy of Rhabdophis tigrinus (Yamakagashi snake) bites. J Intensive Care. 2014;2(1):44. doi: 10.1186/s40560-014-0044-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yokoi H, Sakai A, Kodama T, Magome S, Nagayasu T, Tawara M, et al. Severe hypofibrinogenemia in patients bitten by Gloydius tsushimaensis in Tsushima Island, Nagasaki, Japan, and treatment strategy. Toxicon. 2020. Dec;188:142–9. doi: 10.1016/j.toxicon.2020.10.027 [DOI] [PubMed] [Google Scholar]
- 265.Izumi Y, Terada K, Morine N, Kudaka J. Epidemiology of venomous snakebite in Okinawa prefecture in 2014 [in Japanese]. Okinawaken Eiseikankyo Kenkyuzyoho (Annual Report of Okinawa Prefectural Institute of Health and Environment). 2015. p. 81–3. [Google Scholar]
- 266.Uezu Y, Mahoe Y, Terada T, Morine N, Kudaka J. Epidemiology of venomous snakebite in Okinawa Prefecture in 2012 [in Japanese]. Okinawaken Eiseikankyo Kenkyuzyoho (Annual Report of Okinawa Prefectural Institute of Health and Environment). 2013. p. 79–81. [Google Scholar]
- 267.Uezu Y, Terada K, Morine N, Kudaka J. Epidemiology of venomous snakebite in Okinawa Prefecture in 2013 [in Japanese]. Okinawaken Eiseikankyo Kenkyuzyoho (Annual Report of Okinawa Prefectural Institute of Health and Environment). 2014. p. 75–7. [Google Scholar]
- 268.Shirokawa M, Seki K, Nakajima Y, Koyama S, Mitsusada M. Patient without neurotoxic symptom after being bitten by an eastern green Mamba (Dendroaspis angusticeps). Nihon Kyukyu Igakukai Zasshi. 2011;22(9):777–81. [Google Scholar]
- 269.Hifumi T, Sakai A, Kondo Y, Yamamoto A, Morine N, Ato M, et al. Venomous snake bites: clinical diagnosis and treatment. J Intensive Care. 2015. Apr 1;3(1):16. doi: 10.1186/s40560-015-0081-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hifumi T. Clinical serum therapy [in Japanese]. Vol. 25, Nihon Syuchu Chiryo Igakukai Zasshi (Journal of the Japanese Society of Intensive Care Medicine). 2018. p. 235–42. [Google Scholar]
- 271.Taki K, Ariyoshi K, Sakai A, Ishikawa H, Nakashima K, Endoh Y. Analysis of viper bites by the national survey [in Japanese]. J Jpn Soc Emerg Med. 2014;17(6):753–60. [Google Scholar]
- 272.Mogi M, Murate W, Sakai R, Suzuki K, Matsunaga K. Clinical study of 22 viper bite cases at our hospital [in Japanese]. Hifu No Kagaku. 2017;16(2):113–9. [Google Scholar]
- 273.Tsujimoto T, Chishiro T, Kotani Y, Kamei J, Yamada Y. Analysis of 38 Mamushi pit viper bite cases where patients did not receive serum antitoxin [in Japanese]. Vol. 28, Journal of Japanese Association for Acute Medicine. 2017. p. 48–54. [Google Scholar]
- 274.Imamura T, Takemoto M, Kouno H, Ito T. Case report: a case of edema of the right upper extremity after a Mamushi bite that was treated using Goreisan [in Japanese]. Vol. 64, Bouei Eisei (National Defense Medical Journal). 2017. p. 275–9. [Google Scholar]
- 275.Masui D, Fukahori S, Kurahachi T, Sakamoto S, Higashidate N, Naoki H, et al. A case of focal lesion after a viper bite successfully treated with herbal medicine. Nihon Shouni Kyukyu Igakukai Zasshi J Jpn Soc Emerg Pediatr. 2020;19(2):190–4. [Google Scholar]
- 276.Nakae H. Two cases of viper bite envenomation treated with Saireito [in Japanese]. Kampo Med. 2013;64(4):216–21. [Google Scholar]
- 277.Nakae H, Igarashi T. A case of lymphedema of the arm after viper venom successfully treated with traditional Japanese medicines [in Japanese]. Vol. 61, Japanese Society of Occupational Medicine and Traumatology. 2013. p. 204–7. [Google Scholar]
- 278.Hasegawa M, Pilotte N, Kikuchi M, Means AR, Papaiakovou M, Gonzalez AM, et al. What does soil-transmitted helminth elimination look like? Results from a targeted molecular detection survey in Japan. Parasit Vectors. 2020. Jan 8;13(1):6. doi: 10.1186/s13071-019-3875-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sasaki Y, Taniguchi T, Kinjo M, McGill RL, McGill AT, Tsuha S, et al. Meningitis associated with strongyloidiasis in an area endemic for strongyloidiasis and human T-lymphotropic virus-1: a single-center experience in Japan between 1990 and 2010. Infection. 2013. Dec;41(6):1189–93. doi: 10.1007/s15010-013-0483-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nishi Y, Fukushima T, Nomura S, Tomoyose T, Nakachi S, Morichika K, et al. Characterization of patients with aggressive adult T-cell leukemia-lymphoma in Okinawa, Japan: a retrospective analysis of a large cohort. Int J Hematol. 2016. Oct;104(4):468–75. doi: 10.1007/s12185-016-2042-y [DOI] [PubMed] [Google Scholar]
- 281.Tanaka T, Hirata T, Parrott G, Higashiarakawa M, Kinjo T, Kinjo T, et al. Relationship Among Strongyloides stercoralis Infection, Human T-Cell Lymphotropic Virus Type 1 Infection, and Cancer: A 24-Year Cohort Inpatient Study in Okinawa, Japan. Am J Trop Med Hyg. 2016. Feb;94(2):365–70. doi: 10.4269/ajtmh.15-0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ishikawa S, Maeda T, Hattori K, Watanabe T, Kuramoto T, Ueno S, et al. A case of adenocarcinoma developed in the small intestine with chronic strongyloidiasis. Clin J Gastroenterol. 2017. Dec;10(6):519–23. doi: 10.1007/s12328-017-0783-7 [DOI] [PubMed] [Google Scholar]
- 283.Nabeya D, Haranaga S, Parrott GL, Kinjo T, Nahar S, Tanaka T, et al. Pulmonary strongyloidiasis: assessment between manifestation and radiological findings in 16 severe strongyloidiasis cases. BMC Infect Dis. 2017. May 2;17(1):320. doi: 10.1186/s12879-017-2430-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hosoda T, Sakamoto M, Orikasa H, Kubomura A, Misaki T, Okabe N. Septic Meningitis and Liver Abscess due to Hypermucoviscous Klebsiella pneumoniae Complicated with Chronic Strongyloidiasis in a Human T-lymphotropic Virus 1 Carrier. Intern Med Tokyo Jpn. 2020. Jan 1;59(1):129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Mukaigawara M, Narita M, Shiiki S, Takayama Y, Takakura S, Kishaba T. Clinical Characteristics of Disseminated Strongyloidiasis, Japan, 1975–2017. Emerg Infect Dis. 2020. Mar;26(3):401–8. doi: 10.3201/eid2603.190571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kobayashi A, Hara T, Kajima J. Historical aspects for the control of soil-transmitted helminthiases. Parasitol Int. 2006;55:S289–91. doi: 10.1016/j.parint.2005.11.042 [DOI] [PubMed] [Google Scholar]
- 287.The Tokyo Health Service Association, 2018. Available from: https://www.yobouigaku-tokyo.or.jp/nenpo/pdf/2018/10.pdf.
- 288.Hara W, Ohji S, Mitsui T, Fukahori H, Nomura K. A nepalese case in Japan: neurocysticercosis with a solitary nodular brain lesion that had difficulty distinguishing it from cerebral tuberculoma [in Japanese]. Vol. 30, Shinkeichiryogaku. 2013. p. 45–50. [Google Scholar]
- 289.Kawajiri S, Yamauchi T, Kitai R, Arai T, Higashino Y, Tsunetoshi K, et al. A case of cysticercosis showing peculiar MRI image changes [in Japanese]. Vol. 41, CIkenkyu. 2019. p. 37–40. [Google Scholar]
- 290.Sato A, Nakamura I, Fujita H, Fukushima S, Mizuno Y, Fujii T, et al. Neurocysticercosis with Diplopia Responds Well to Albendazole. Vol. 55, Internal Medicine. 2016. p. 1219–22. doi: 10.2169/internalmedicine.55.6176 [DOI] [PubMed] [Google Scholar]
- 291.Shoji H, Hirai T, Ishikura T, Takuma T, Okino T, Wakatsuki Y, et al. A case of cysticercosis with multiple lesions in the brain and femoral muscles [in Japanese]. Vol. 87, Kansenshogaku Zasshi. The Journal of the Japanese Association for Infectious Diseases. 2013. p. 608–12. [DOI] [PubMed] [Google Scholar]
- 292.Yamasaki H, Sugiyama H, Morishima Y, Ohmae H, Shiinogi S, Okuyama K, et al. A case of racemose neurocisticercosis Racemose [in Japanese]. Vol. 21, Clinical Parasitology. 2011. p. 29–32. [Google Scholar]
- 293.Yokota K, Furukawa K. A Case of Cysticercosis with Multiple Intracerebral and Intramuscular Nodular Lesions [in Japanese]. Vol. 86, Kansenshogaku Zasshi. The Journal of the Japanese Association for Infectious Diseases. 2012. p. 27–30. [DOI] [PubMed] [Google Scholar]
- 294.Yanagida T, Sako Y, Nakao M, Nakaya K, Ito A. Taeniasis and cysticercosis due to Taenia solium in Japan. Parasit Vectors. 2012. Jan 17;5:18. doi: 10.1186/1756-3305-5-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Braae UC, Hung NM, Satrija F, Khieu V, Zhou XN, Willingham AL. Porcine cysticercosis (Taenia solium and Taenia asiatica): mapping occurrence and areas potentially at risk in East and Southeast Asia. Parasit Vectors. 2018. Nov 29;11(1):613. doi: 10.1186/s13071-018-3203-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Terauchi M. A recent case report of acute trachoma [in Japanese]. Ibarakiken Rinshoigaku Zasshi. 1984. p. 207. [Google Scholar]
- 297.National Institute of Infectious Diseases, Japan. National Institute of Infectious Diseases Infectious Agents Surveillance Report [in Japanese] [Internet]. 2019 [cited 2021 Jan 25]. Available from: https://www.niid.go.jp/niid/ja/ydata/9005-report-jb2018.html.
- 298.Iijima W, Inoue H, Ichikawa T. Introducing activities of the Archives of Infectious Diseases History (AIDH) project: Historical epidemiology. Trop Med Health. 2021. Jan 27;49(1):9. doi: 10.1186/s41182-020-00296-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kikuchi I. Hansen’s disease in Japan: a brief history. Int J Dermatol. 1997. Aug;36(8):629–33. doi: 10.1046/j.1365-4362.1997.00230.x [DOI] [PubMed] [Google Scholar]
- 300.Koba A, Ishii N, Mori S, Fine PEM. The decline of leprosy in Japan: patterns and trends 1964–2008. Lepr Rev. 2009 Dec;80(4):432–40. [PubMed] [Google Scholar]
- 301.Miyashita. Legal and social measures against Trachoma in Japan. Br J Ophthalmol. 1935 Jun;19(6):323–5. [DOI] [PMC free article] [PubMed]
- 302.Tada I. Parasite controls in Japan with emphasis on special characteristics. Trop Med Health. 2008;3(Supple 2008):49–68. [Google Scholar]
- 303.Yotsu RR, Miyamoto Y, Mori S, Ato M, Sugawara-Mikami M, Yamaguchi S, et al. Hansen’s disease (leprosy) in Japan, 1947–2020: an epidemiologic study during the declining phase to elimination. Int J Infect Dis. 2022. Dec 1;125:265–74. doi: 10.1016/j.ijid.2022.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Molyneux DH, Hotez PJ, Fenwick A. ‘Rapid-impact interventions’: how a policy of integrated control for Africa’s neglected tropical diseases could benefit the poor. PLoS Med. 2005. Nov;2(11):e336. doi: 10.1371/journal.pmed.0020336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Molyneux DH, Asamoa-Bah A, Fenwick A, Savioli L, Hotez P. The history of the neglected tropical disease movement. Trans R Soc Trop Med Hyg. 2021. Jan 28;115(2):169–75. doi: 10.1093/trstmh/trab015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.National Statistics Center. Portal Site of Official Statistics of Japan. [cited 2022 Dec 15]. Statistics of Foreign Residents in Japan. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00250012&tstat=000001018034&cycle=1&tclass1=000001060399&tclass2val=0.
- 307.Higuchi M, Endo M, Yoshino A. Factors associated with access to health care among foreign residents living in Aichi Prefecture, Japan: secondary data analysis. Int J Equity Health. 2021. Jun 10;20(1):135. doi: 10.1186/s12939-021-01465-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kobori E, Maeda Y, Yamamoto T. Mortality rates of foreign national residents in Japan: comparison with the Japanese population and a possible healthy migrant effect [in Japanese]. Nihon Koshu Eisei Zasshi Jpn J Public Health. 2017;64(12):707–17. [DOI] [PubMed] [Google Scholar]
- 309.Faber WR, Arias-Bouda LM, Zeegelaar JE, Kolk AH, Fonteyne PA, Toonstra J, et al. First reported case of Mycobacterium ulcerans infection in a patient from China. Trans R Soc Trop Med Hyg. 2000;94(3):277–9. doi: 10.1016/s0035-9203(00)90320-1 [DOI] [PubMed] [Google Scholar]
- 310.Doi R, Matsuda H, Uchida A, Kanda E, Kamiya H, Konno K, et al. Possibility of invasion of Echinococcus into Honshu with pet dogs from Hokkaido and overseas [in Japanese]. Nihon Koshu Eisei Zasshi Jpn J Public Health. 2003;50(7):639–49. [PubMed] [Google Scholar]
- 311.Ben Salah A, Ben Messaoud N, Guedri E, Zaatour A, Ben Alaya N, Bettaieb J, et al. Topical Paromomycin with or without Gentamicin for Cutaneous Leishmaniasis. N Engl J Med. 2013. Feb 7;368(6):524–32. doi: 10.1056/NEJMoa1202657 [DOI] [PubMed] [Google Scholar]
- 312.National Institute of Public Health of Japan. Japan Registry of Clinical Trials. 2019 [cited 2021 Dec 9]. Efficacy and safety of triclabendazole for the treatment of fasciolosis. Available from: https://jrct.niph.go.jp/en-latest-detail/jRCTs071190020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.