Abstract
Mycobacterium tuberculosis readily activates both CD4+ and Vδ2+ γδ T cells. Despite similarity in function, these T-cell subsets differ in the antigens they recognize and the manners in which these antigens are presented by M. tuberculosis-infected monocytes. We investigated mechanisms of antigen processing of M. tuberculosis antigens to human CD4 and γδ T cells by monocytes. Initial uptake of M. tuberculosis bacilli and subsequent processing were required for efficient presentation not only to CD4 T cells but also to Vδ2+ γδ T cells. For γδ T cells, recognition of M. tuberculosis-infected monocytes was dependent on Vδ2+ T-cell-receptor expression. Recognition of M. tuberculosis antigens by CD4+ T cells was restricted by the class II major histocompatibility complex molecule HLA-DR. Processing of M. tuberculosis bacilli for Vδ2+ γδ T cells was inhibitable by Brefeldin A, whereas processing of soluble mycobacterial antigens for γδ T cells was not sensitive to Brefeldin A. Processing of M. tuberculosis bacilli for CD4+ T cells was unaffected by Brefeldin A. Lysosomotropic agents such as chloroquine and ammonium chloride did not affect the processing of M. tuberculosis bacilli for CD4+ and γδ T cells. In contrast, both inhibitors blocked processing of soluble mycobacterial antigens for CD4+ T cells. Chloroquine and ammonium chloride insensitivity of processing of M. tuberculosis bacilli was not dependent on the viability of the bacteria, since processing of both formaldehyde-fixed dead bacteria and mycobacterial antigens covalently coupled to latex beads was chloroquine insensitive. Thus, the manner in which mycobacterial antigens were taken up by monocytes (particulate versus soluble) influenced the antigen processing pathway for CD4+ and γδ T cells.
Mycobacterium tuberculosis, the etiologic agent of human tuberculosis, is spread readily from person to person by inhalation of aerosolized mycobacteria (8). A hallmark of M. tuberculosis infection is the ability of most healthy individuals to control the infection by mounting an acquired immune response, in which antigen-specific T cells and mononuclear phagocytes arrest the growth of M. tuberculosis bacilli and maintain control over dormant bacilli within granulomas (reviewed in reference 25). This protective cellular immune response results in conversion of the tuberculin skin test from negative to positive and probably in increased resistance to reinfection with tubercle bacilli.
CD4+ αβ-T-cell-receptor (αβ TCR)-bearing T cells (CD4+ T cells) are readily activated by mycobacterial antigens and have a dominant role in the protective immune response to M. tuberculosis in humans (2, 34). These CD4+ T cells not only secrete cytokines but also serve directly as cytotoxic effector cells against M. tuberculosis-infected macrophages (6). In addition to CD4+ T cells, M. tuberculosis antigens activate other human T-cell subsets such as γδ TCR+ T cells (γδ T cells) (15, 16, 18). Vδ2+ and Vγ9+ γδ T cells are particularly responsive to live M. tuberculosis (15). A role for both γδ and CD4+ T cells in protective immunity to acute M. tuberculosis infection has been demonstrated in murine models (20, 21, 26, 27). A recent study of humans suggests that Vγ9+ and Vδ2+ γδ T-cell numbers and function are reduced in tuberculosis patients (23).
Functional comparisons of human CD4+ and γδ T-cell responses of healthy tuberculin-positive persons demonstrate that both T-cell subsets have similar cytotoxic effector functions for M. tuberculosis-infected monocytes and produce large amounts of gamma interferon (IFN-γ), with γδ T cells being slightly more efficient producers of IFN-γ than CD4+ T cells (37). Despite similarities in function, these two T-cell subsets differ in the mycobacterial antigens recognized by their TCRs and the manners in which antigens are presented to them by M. tuberculosis-infected mononuclear phagocytes. CD4+ T cells recognize a wide diversity of mycobacterial peptides in the context of class II major histocompatibility complex (MHC) molecules, which include secreted as well as somatic antigens (6, 13, 33, 37). In contrast, Vγ9+ and Vδ2+ γδ T cells, the dominant γδ TCR subsets activated by M. tuberculosis, recognize mycobacterial antigens in a non-MHC-restricted manner and the repertoire of antigens includes small phosphate-containing antigens such as TUBag’s (5, 9, 19, 22, 29, 36).
Both blood monocytes and alveolar macrophages infected with M. tuberculosis are efficient antigen-presenting cells for mycobacterial antigen-specific CD4+ and γδ T cells (1, 5). However, little is known about how M. tuberculosis-infected mononuclear phagocytes process antigens for these two T-cell subsets. M. tuberculosis bacilli are taken up by mononuclear phagocytes through a variety of surface receptors, including complement receptor 4, mannose receptor, and complement receptor 3 (17, 31, 32). Within mononuclear phagocytes, the mycobacteria reside within phagosomes and modulate the phagosome by preventing fusion with acidic lysosomal compartments (7). Although the vacuolar membranes surrounding the phagosome acquire endosomal markers, the vesicular proton ATPase is actively excluded, resulting in an elevated pH of 6.3 to 6.5 compared to the normal lysosomal pH of 4.5 (7, 35). The elevated pH in the phagosome does not appear to inhibit the ability of mycobacterial antigens to be processed and presented to CD4+ and Vδ2+ γδ T cells. This study was undertaken to gain insight into the mechanisms used by monocytes infected with live M. tuberculosis bacilli to process mycobacterial antigens for presentation to both CD4+ and γδ T cells.
MATERIALS AND METHODS
Chemical reagents and monoclonal antibodies.
Chloroquine, ammonium chloride, cytochalasin D, and Brefeldin A were purchased from Sigma (St. Louis, Mo.). Chloroquine and ammonium chloride were dissolved in phosphate-buffered saline, and cytochalasin D and Brefeldin A were dissolved in dimethyl sulfoxide. Phycoerythrin (PE)-conjugated anti-interleukin 2 receptor alpha chain (IL-2Rα) (CD25-PE; Becton Dickinson, San Jose, Calif.), fluorescein isothiocyanate (FITC)-conjugated OKT-4 (CD4-FITC; Ortho Diagnostics, Raritan, N.J.), and FITC-conjugated TCRδ-1 (γδ-FITC; T Cell Sciences, Cambridge, Mass.) were purchased and used according to the manufacturers’ instructions with FITC- and PE-conjugated isotypic controls. Purified anti-human TCR-Vδ2 antibody C448.15D was a gift from Simon R. Carding, University of Pennsylvania, Philadelphia. Purified L243 (anti-HLA-DR) was a gift from Cliff Harding, (Case Western Reserve University, Cleveland, Ohio).
Bacteria and antigens.
M. tuberculosis H37Ra was cultured in Middlebrook 7H9 with ADC enrichment, and frozen stocks were prepared as described previously (5, 15). Bacterial counts and viability were performed by light microscopy and by counting CFU on 7H10 medium. M. tuberculosis-H37Ra stocks were tested periodically for viability and with an M. tuberculosis complex-specific DNA probe (AccuProbe; Gen-Probe, San Diego, Calif.) to ensure purity of the M. tuberculosis stocks. Before use in T-cell assays, mycobacteria were washed three times in RPMI 1640 and sonicated for 20 s to disrupt clumps. The viability was routinely more than 50%.
Formaldehyde-fixed M. tuberculosis was prepared by suspending bacilli in 1 ml of RPMI 1640 (109/ml) containing 1.5% (vol/vol) formaldehyde as previously described (12). In brief, mycobacteria were incubated at room temperature for 2 h with constant mixing, pelleted, and washed three times. The cells were resuspended in 1 ml of RPMI 1640 and kept at 4°C. Viability was less than 1 CFU/ml.
Cytosolic antigens of M. tuberculosis H37Ra were prepared as previously described (37). Cytosolic mycobacterial antigens were coupled covalently to carboxylated latex beads by carbodiimide linkage (Polysciences, Inc., Warrington, Pa.) as per the manufacturer’s instructions. Briefly, 0.5 ml of a 2.5% suspension of carboxylated beads was washed with carbonate buffer followed by phosphate buffer. To the suspension of beads in phosphate buffer, an equal volume of 2% solution of carbodiimide was added, and the mixture was incubated for 4 h. Beads were washed with borate buffer, before 500 μg of cytosolic mycobacterial antigens per ml was added, and incubated overnight on a rotary shaker. After incubation, beads were pelleted and resuspended in 0.1 M ethanolamine and then centrifuged and blocked in bovine serum albumin before being stored in phosphate-buffered saline. Beads were tested with peripheral blood mononuclear cells (PBMC) from healthy tuberculin skin test-positive donors to test their ability to stimulate T-cell proliferation, with equivalent levels of proliferation to soluble mycobacterial antigens being observed at 106 to 107 beads/ml.
Isolation of PBMC and monocytes.
PBMC were isolated by density centrifugation over sodium diatrizoate-Hypaque gradients, and monocytes were obtained by adherence from PBMC as previously described (37). Briefly, PBMC were incubated on plastic tissue culture dishes precoated with pooled human serum, nonadherent cells were removed, and plastic-adherent cells (≥90% monocytes by Wright’s, peroxidase, and nonspecific esterase staining) were collected by scraping the dishes with a plastic policeman. PBMC were isolated from healthy tuberculin-positive persons (18 to 45 years old). They were selected for consistency of resting γδ T-cell expansion (20 to 50% γδ TCR+ T cells) after stimulation with live M. tuberculosis.
Generation of M. tuberculosis-specific CD4+ and γδ T-cell lines.
CD4+ and γδ T-cell lines specific for M. tuberculosis were generated as described in previous studies (4, 6, 37). In brief, PBMC were stimulated with M. tuberculosis bacilli or with soluble mycobacterial antigens for 7 to 10 days. Then, CD4+ and γδ T-cell subsets were enriched by negative selection with magnetic beads coated with antibodies (Dynal, Great Neck, N.Y.). For γδ T-cell enrichment, cells were treated simultaneously with anti-CD4- and anti-CD8-coated beads. For CD4+ T-cell enrichment, cells were treated first with TCRδ-1 and then with goat anti-mouse immunoglobulin G-coated beads and anti-CD8-coated beads. Antibody-coated beads were used at a 10:1 bead-to-cell ratio, based on the estimated number of T cells from each T-cell subset (CD4+, CD8+, and γδ T cells) present after 7 to 9 days of stimulation with live M. tuberculosis. Generally, one cycle of treatment was sufficient for depletion of the T cells, although in some experiments two cycles were performed. Purity of selected T-cell populations was assessed by two-color cytometry.
CD4+ and γδ T cell lines were maintained with biweekly stimulation with mycobacterial antigens, irradiated PBMC, and recombinant IL-2. CD4+ T cell lines were maintained on autologous PBMC as APC, and generally γδ-T-cell lines were stimulated with HLA-mismatched PBMC. Mycobacterial antigen-specific T-cell lines stimulated in vitro two to three times and maintained for 8 weeks were considered short-term lines and were derived from seven donors (seven lines for CD4+ and four lines for γδ T cells). T-cell lines (n = 4 for CD4 T cells, n = 2 for γδ T cells) maintained for more than 12 weeks were considered long-term lines. Most experiments shown were performed with long-term T-cell lines; however, results were validated with both short-term and long-term lines. Purity of phenotype of T-cell lines was monitored by flow cytometry.
Proliferation assays.
CD4 and γδ T cells (2 × 104 to 2.5 × 104 per 200-μl well) were cocultured with 5 × 104 monocytes per well as APC for 72 h in 96-well plates. Cells were pulsed with 1 μCi of [3H]thymidine (ICN, Costa Mesa, Calif.) for 12 to 16 h before being harvested on glass fiber filters. [3H]thymidine incorporation was measured by liquid scintillation counting and expressed as counts per minute.
For antigen uptake and processing, monocytes were incubated with whole M. tuberculosis (5:1 or 10:1 bacterium-to-cell ratio) for 4 h. Then cells were washed and fixed with paraformaldehyde as previously described (3). Briefly, cells were washed and fixed with paraformaldehyde for 1 min, followed by neutralization with 0.15 M glycine (pH 7.2) for 20 min at room temperature and then washing (four times). Cells were incubated in tissue culture medium for 60 min to remove residual paraformaldehyde before use in proliferation assays. Before the cells were pulsed with 1 μCi of [3H]thymidine, 50 μl of supernatant was harvested to measure the levels of secreted IFN-γ. The levels of IFN-γ were measured by enzyme-linked immunosorbent assay (Endogen, Cambridge, Mass.).
For cytochalasin D treatment, monocytes were pulsed with cytochalasin D (10 μg/ml) for 30 min before addition of mycobacteria (5:1 bacterium-to-cell ratio) and incubated for 2 h. Monocytes then were washed five times to remove unphagocytosed bacteria and irradiated before addition to the proliferation assay with CD4 and γδ T cells.
For class II MHC blocking, irradiated monocytes were incubated with anti-HLA-DR (L243) monoclonal antibody (MAb) (10 μg/ml) for 120 min on ice before addition of CD4 T cells in the proliferation assay. For anti-Vδ2 antibody blocking experiments, γδ T cells were incubated with C448.D15 (2 μg/ml) for 60 min on ice before being added to irradiated monocytes in the proliferation assay.
Cytotoxicity assay.
Monocytes were incubated for either 2 to 4 h or 10 to 12 h with live M. tuberculosis (5:1 or 10:1 bacterium-to-monocyte ratio), soluble mycobacterial antigens (50 μg/ml), soluble antigens covalently coupled to latex beads (5:1 or 10:1 bead-to-monocyte ratio) or no antigen. After incubation, monocytes were washed and labeled for 1 h at 37°C with 100 μCi of 51Cr (New England Nuclear, Boston, Mass.) before being used as targets in 4-h cytotoxicity assays with CD4+ and γδ T cells as previously described (37).
For inhibition of ongoing antigen processing, monocytes were preincubated for 8 to 12 h with the antigens indicated in the figures before addition of chloroquine (400 μM) or Brefeldin A (5 μg/ml) according to published procedures (3, 14, 30). Antigen-pulsed monocytes were treated with inhibitors for 120 min, and inhibitors were removed by washing. Monocytes were then pulsed with 51Cr before being used as targets.
For inhibition of initial antigen uptake and processing, monocytes were incubated with chloroquine or ammonium chloride (NH4Cl, 10 and 50 mM) for 30 min before addition of M. tuberculosis bacilli, soluble mycobacterial antigens, or latex beads. Antigen and inhibitors were coincubated for 120 min. After coincubation, monocytes were washed and labeled with 51Cr. The inhibitors did not increase average spontaneous 51Cr release from the usual 15 to 20% for M. tuberculosis-infected monocytes.
Statistical analysis.
Statistical analysis was determined by paired Student’s t test, and a P of <0.05 was considered significant.
RESULTS
Requirement for phagocytosis and processing of M. tuberculosis bacilli by monocytes for CD4+ and γδ T cells.
In earlier studies, we demonstrated that live M. tuberculosis bacilli readily activate peripheral blood γδ T cells from healthy tuberculin-positive persons. Activation of T cells by live M. tuberculosis is not restricted to γδ T cells but also results in activation of CD4+ T cells. A representative experiment is shown in Fig. 1, in which IL-2Rα (CD25) expression on CD4+ and γδ T cells was measured after stimulation of PBMC with live M. tuberculosis bacilli. The activation of these two T-cell subsets by M. tuberculosis antigens is dependent on antigen-presenting cells, and blood monocytes are efficient APC for both CD4+ and γδ T cells. We have used the activation of peripheral blood CD4+ and γδ T cells by M. tuberculosis bacilli as a means to derive M. tuberculosis antigen-specific CD4+ and γδ T-cell lines for the antigen-processing experiments described below. Whereas αβ TCR usage and mycobacterial antigen recognition by CD4+ T cells are characterized by marked diversity, γδ T-cell activation by mycobacterial antigens is limited predominantly to T cells expressing TCR consisting of Vγ9 and Vδ2 chains. As shown in Fig. 2B, activation of γδ T cells was inhibited by MAb C448.15D, specific for the Vδ2 chain of γδ TCR, indicating that activation of γδ T cells by live-M. tuberculosis-infected monocytes was dependent on Vδ2 expression. Recognition of M. tuberculosis by CD4 T cells was blocked by anti-HLA-DR antibody (Fig. 2A); in contrast, recognition of M. tuberculosis by γδ T cells is not restricted by MHC molecules (5).
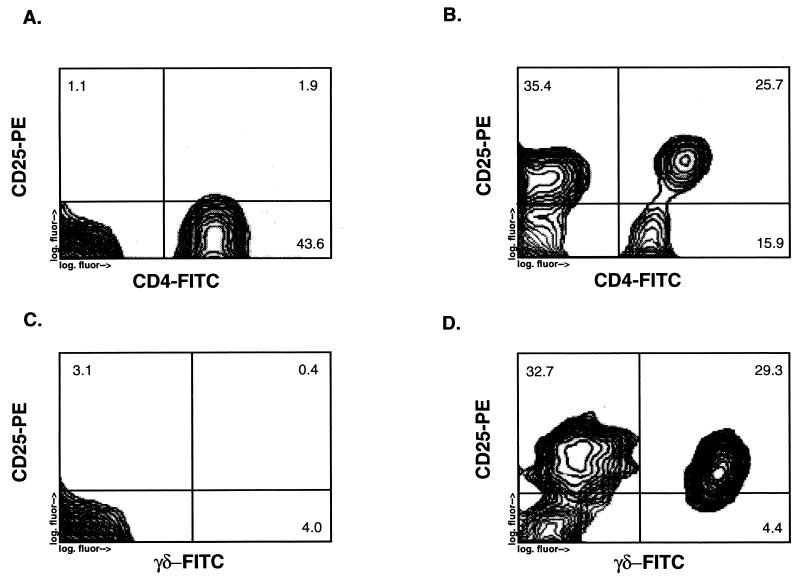
FIG. 1.
Upregulation of IL-2Rα (CD25) expression on peripheral blood CD4+ and γδ T cells by live M. tuberculosis. Shown are the results of an analysis by two-color flow cytometry of CD25 expression on CD4+ and γδ T cells from peripheral blood T cells stimulated for 7 days with either live M. tuberculosis (5 × 106 bacilli per ml) (B and D) or no antigen (A and C). The y axis represents PE fluorescence for CD25, and the x axis represents FITC fluorescence for either CD4+ or γδ T cells.
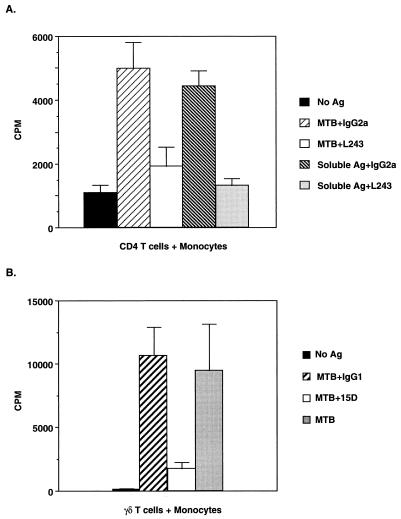
FIG. 2.
(A) Restriction of CD4 T cells by class II MHC (HLA-DR) expression on M. tuberculosis-infected monocytes. Monocytes were pretreated with either L243 (10 μg/ml) or isotypic control MAb (10 μg/ml) on ice for 120 min before the addition of CD4 T cells (2.5 × 104/well) from a long-term T-cell line with and without M. tuberculosis or soluble mycobacterial antigen in a proliferation assay. The results are the means and standard deviations of triplicate wells and are representative of four experiments. (B) Dependence on Vδ2 TCR expression by γδ T cells for activation by M. tuberculosis-infected monocytes. γδ T cells (2.5 × 104/well) were pretreated with either C448.15D (15D) or isotypic control MAb (2 μg/ml) on ice for 60 min before being added to irradiated heterologous monocytes (5 × 104/well) with and without M. tuberculosis (5 × 106/ml) in a proliferation assay. The results are the means and standard deviations of triplicate wells and are representative of three experiments. Ag, antigen; MTB, M. tuberculosis.
Next, we determined if phagocytosis and processing by monocytes of M. tuberculosis bacilli were required for presentation of antigens to CD4+ and γδ T-cell lines. Monocytes were pretreated with cytochalasin D, an inhibitor of phagocytosis by inhibiting actin polymerization, for 30 min before M. tuberculosis bacilli were added. After 2 h of incubation, infected monocytes were washed extensively to remove nonphagocytosed bacilli and cytochalasin D, before treated monocytes were added as APC to the T-cell proliferation assay. As shown in Fig. 3, pretreatment of monocytes with cytochalasin D inhibited the proliferation of both CD4+ and γδ T cells. Cytochalasin D pretreatment did not inhibit the ability of monocytes to present soluble mycobacterial antigens to both T-cell subsets, indicating that cytochalasin D treatment did not affect APC function of monocytes (41,921 cpm).
FIG. 3.
Requirement for phagocytosis by monocytes of M. tuberculosis bacilli for activation of CD4+ and γδ T cells. Monocytes (MO) were incubated with M. tuberculosis (MTB) in the presence (CCD-MO+MTB) or absence (MO+MTB) of cytochalasin D (CCD) for 120 min, after which they were washed and irradiated before coculture with CD4+ (A) and γδ (B) T-cell lines in a proliferation assay. Results are means and standard deviations of triplicate wells and are representative of five experiments. Monocyte targets were from the same donor as the CD4+ T-cell line, and the γδ T-cell line was derived from an unrelated donor.
Fixation of monocytes with paraformaldehyde before exposure to M. tuberculosis prevented processing and presentation of mycobacterial antigens to both CD4+ and γδ T cells, whereas monocytes fixed with paraformaldehyde after infection with M. tuberculosis were able to activate both T-cell subsets (Fig. 4). In addition, we measured IFN-γ release in response to fixed APC by CD4 and γδ T-cell lines. IFN-γ results were similar to proliferation results. Fixing the monocytes before pulsing them with bacteria did not stimulate CD4 and γδ T cells to make detectable levels of IFN-γ, while fixing the monocytes after infection with M. tuberculosis induced IFN-γ production by CD4 T cells (949 pg/ml) and γδ T cells (354 pg/ml). Consistent with the results of the proliferation experiments, IFN-γ levels in response to fixed APC were reduced 50% in comparison with those in response to unfixed APC.
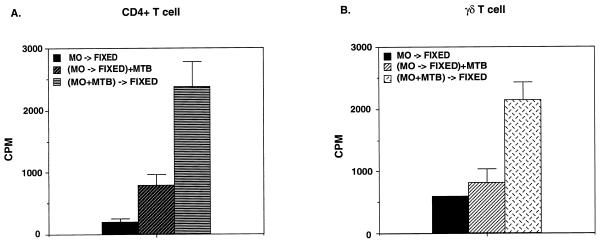
FIG. 4.
Requirement for uptake and processing of M. tuberculosis bacilli for presentation to both CD4+ and γδ T cells. Monocytes (MO) were fixed with paraformaldehyde either before (MO→FIXED) or after [(MO+MTB)→FIXED] a 120-min incubation with live M. tuberculosis (MTB). Fixed monocytes then were added to a proliferation assay with CD4+ (A) and γδ (B) T cells. Results are means and standard deviations of triplicate wells and are representative of four experiments.
Inhibition by Brefeldin A of mycobacterial antigen processing for γδ but not CD4+ T cells.
The above-described experiments demonstrated that initial uptake by phagocytosis and antigen processing were required to present antigens from M. tuberculosis bacilli by monocytes to CD4+ and γδ T cells. To further characterize the antigen-processing pathways of M. tuberculosis-infected monocytes, the effects of inhibitors of antigen processing were tested in cytotoxicity assays with CD4+ and γδ T cells as cytotoxic T lymphocytes (CTL). Processing of M. tuberculosis bacilli by monocytes was inhibited by Brefeldin A for γδ T cells. The results of two representative experiments of five are shown in Fig. 5A and C. Processing for CD4+ T cells (Fig. 5B) was not inhibited by Brefeldin A in the CTL assay (n = 4, P > 0.375). The same M. tuberculosis-infected monocytes were used in CTL assays with both CD4+ and γδ T cells (Fig. 5A and B), clearly establishing the differential effects of Brefeldin A on processing of M. tuberculosis bacilli for these two T-cell subsets (inhibition for γδ T cells was 53 to 60% in five experiments; P < 0.025). Processing for γδ T cells (and for CD4+ T cells [data not shown]) of soluble cytosolic antigens of M. tuberculosis by monocytes was not sensitive to Brefeldin A treatment (5 μg/ml), in contrast to the findings with intact bacilli (Fig. 5D) (n = 5, P > 0.4). In these experiments, monocytes were exposed first to either live M. tuberculosis or soluble mycobacterial antigens and second to treatment with Brefeldin A for 2 h and then washed extensively before being tested in the CTL assay. No further antigens were added during the CTL assay, indicating that the ligand(s) recognized by γδ T cells was stably expressed on the surfaces of antigen-pulsed monocytes. Increasing the concentration of Brefeldin A to 20 μg/ml did not change the differential effects on antigen processing for γδ and CD4+ T cells (data not shown). These results indicated that in M. tuberculosis-infected monocytes, Brefeldin A inhibited ongoing processing for γδ T cells of antigens originating from the bacilli but not for CD4+ T cells.
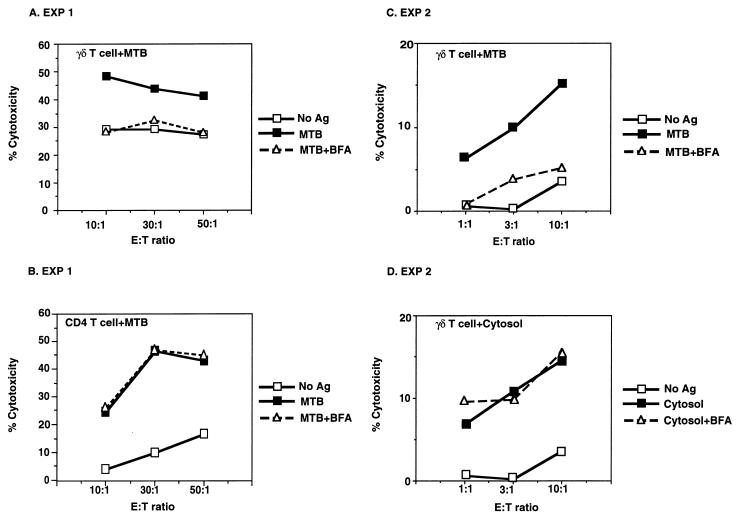
FIG. 5.
Differential effects of Brefeldin A on antigen processing of M. tuberculosis bacilli for γδ and CD4+ T cells. Monocytes incubated for 12 h with either M. tuberculosis (MTB) (A, B, and C) or cytosolic mycobacterial antigens (Ag) (D) were treated with Brefeldin A (BFA) for 120 min before serving as targets in a CTL assay with either γδ T-cell lines (A, C, and D) or CD4+ T cell lines (B). In experiment 1 (EXP 1), the same monocyte targets were used for both CD4+ and γδ T-cell lines, with the γδ T-cell lines being derived from an HLA-mismatched donor. Results are representative of four experiments. E:T ratio, effector-to-target ratio.
The Vγ9+ and Vδ2+ γδ T-cell lines in this study did respond to isopentenyl pyrophosphate (IPP; 25 μg/ml). However, in contrast to M. tuberculosis bacilli or soluble mycobacterial antigens, IPP could not be stably pulsed onto monocytes for use in CTL assays but needed to be added and present continuously during a 4-h CTL assay (data not shown).
Inability of lysosomotropic agents to inhibit processing of M. tuberculosis bacilli for CD4+ and γδ T cells.
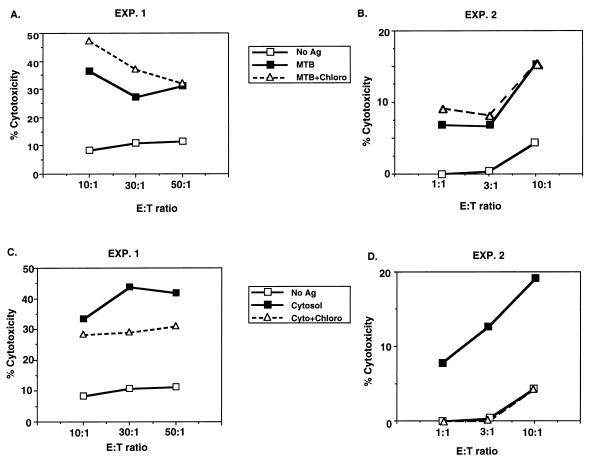
Since M. tuberculosis bacilli reside largely within the phagosomes of mononuclear phagocytes and are able to inhibit phagosomal acidification, the effects of lysomotropic agents on antigen processing of M. tuberculosis bacilli for γδ and CD4+ T cells were tested. As shown in Fig. 6A and C, treatment of M. tuberculosis-infected monocytes with chloroquine did not affect ongoing antigen processing for CD4+ T cells. In fact, in three of six experiments chloroquine appeared to enhance processing of M. tuberculosis, as was reflected in increased cytotoxicity (Fig. 6A and 6C) (n = 6, P > 0.375). Processing of M. tuberculosis bacilli for γδ T cells was not affected by chloroquine (data not shown).
FIG. 6.
Effect of an inhibitor of lysosomal acidification on antigen processing of M. tuberculosis bacilli by monocytes for CD4+ T-cell lines. Monocytes incubated for 12 h with either M. tuberculosis (MTB) (A and B) or cytosolic antigens (Ag) (C and D) of M. tuberculosis were treated with chloroquine (Chloro) for 120 min before serving as targets in a CTL assay with CD4+ T cells. The long-term CD4+ T-cell line used in experiment 1 (EXP. 1) was generated against total cytosolic proteins of M. tuberculosis, and the long-term CD4+ T-cell line used in experiment 2 was generated against purified 30-kDa (85B) antigen of M. tuberculosis. Results are representative of six experiments. E:T ratio, effector-to-target ratio.
The lack of effect of chloroquine on M. tuberculosis processing was observed for concentrations ranging from 40 to 2,000 μM, with 400 μM being used as the optimal concentration. The insensitivity to chloroquine was not dependent on the load of M. tuberculosis bacilli when M. tuberculosis-to-monocyte ratios were varied from 1:1 to 30:1 (data not shown). Antigen processing of soluble mycobacterial antigens for CD4+ T cells was inhibited by chloroquine, serving as a control for the effect of chloroquine (Fig. 6B and D) (n = 6, P < 0.025). The inability of chloroquine to inhibit processing of live M. tuberculosis for CD4+ T cells suggested that processing for class II MHC molecules of M. tuberculosis antigens from live bacilli differed from processing of soluble mycobacterial antigen. However, in these experiments chloroquine was added to monocytes already infected with M. tuberculosis or pulsed with soluble antigens, and thus the effect of chloroquine was on ongoing antigen processing. Thus, we determined the effect of chloroquine treatment on monocytes as they initiate the processing of mycobacterial antigens for CD4+ T cells. In these experiments, monocytes were treated with chloroquine during a 4-h exposure to M. tuberculosis bacilli. As shown in the results of a representative experiment in Fig. 7, chloroquine did not inhibit processing of antigens of live M. tuberculosis bacilli (n = 4, P > 0.375) but did inhibit processing of soluble antigens (data not shown). To ensure that insensitivity of processing of M. tuberculosis bacilli for CD4+ T cells and γδ T cells was not restricted to chloroquine, additional experiments with an alternative lysomotropic agent, ammonium chloride (NH4Cl), were performed. As shown in Fig. 8, treatment of monocytes with ammonium chloride at the time of initial uptake did not inhibit subsequent processing of M. tuberculosis bacilli for CD4+ T cells (Fig. 8A) (n = 3, P > 0.375) but did inhibit processing of soluble antigen (Fig. 8B) (n = 3, P < 0.05), indicating that processing of M. tuberculosis bacilli was resistant to two different lysomotropic agents.
FIG. 7.
Effect of chloroquine on the initial uptake and processing of M. tuberculosis bacilli for CD4+ T cells. Monocytes were preincubated with or without chloroquine (CHLORO) for 30 min, and then M. tuberculosis bacilli (MTB) were added for 120 min and chloroquine treatment was continued. Monocytes were then tested in a CTL assay with a CD4+ T-cell line. In this experiment, chloroquine treatment reduced soluble antigen (Ag) presentation by monocytes by 40%. The long-term CD4+ T-cell line used in this assay is reactive to total cytosolic proteins of M. tuberculosis. Results are representative of three experiments. E:T ratio, effector-to-target ratio.
FIG. 8.
Effect of ammonium chloride (NH4Cl) on the initial uptake and processing of M. tuberculosis bacilli for CD4+ T cells. Monocytes were preincubated with or without ammonium chloride for 30 min, and then M. tuberculosis bacilli (MTB) (A) or cytosolic mycobacterial antigens (CYTOSOL) (B) were added for 120 min on initial uptake and processing of M. tuberculosis bacilli for CD4+ T cells and ammonium chloride treatment was continued. Monocytes then were tested in a CTL assay with a CD4+ T-cell line. Results are representative of three experiments. Ag, antigen; E:T ratio, effector-to-target ratio.
Chloroquine insensitivity of processing of particulate mycobacterial antigens for CD4+ T cells.
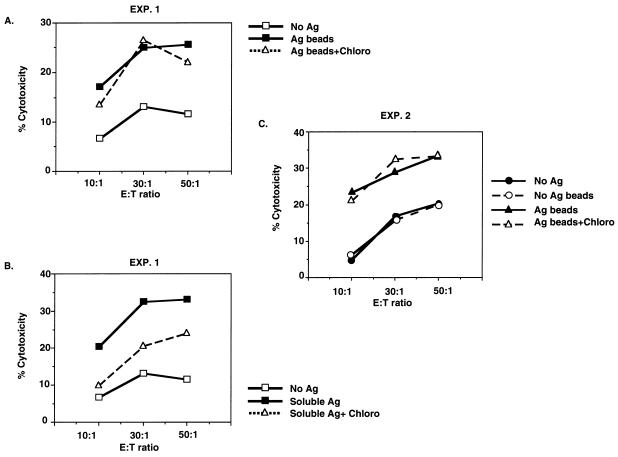
To determine if chloroquine insensitivity of antigen processing for CD4+ T cells was unique to live M. tuberculosis bacilli or due to the particulate nature of the antigen, experiments were performed with cytosolic antigens of M. tuberculosis covalently linked to latex beads. As shown in Fig. 9A and C, these particulate antigen preparations were also found to be chloroquine insensitive (n = 3; P > 0.3), whereas processing of soluble cytosolic antigens for CD4+ T cells remained inhibitable with chloroquine in the same experiment (Fig. 9B) (P < 0.005). When formaldehyde and dead M. tuberculosis bacilli were used, again no inhibition by chloroquine was observed, whereas soluble antigens were inhibited (data not shown). These findings suggested that it is the particulate nature of mycobacterial antigens and not the viability of the bacilli which determines the chloroquine insensitivity of antigen processing for CD4+ T cells by human monocytes.
FIG. 9.
Chloroquine insensitivity of antigen processing of cytosolic mycobacterial antigens coupled to latex microspheres for CD4+ T cells. Monocytes were incubated with latex beads coupled with cytosolic mycobacterial antigens (A and C) or with soluble mycobacterial antigens (B) and then treated with chloroquine (Chloro) before use in a CTL assay with a CD4+ T-cell line. Results of two of four representative experiments are shown. Ag, antigen; E:T ratio, effector-to-target ratio.
DISCUSSION
Monocytes infected with M. tuberculosis are efficient antigen-presenting cells for mycobacterial antigen-specific CD4+ and γδ T cells from healthy tuberculin skin test-positive persons (25). The results of our studies indicate that there are a number of unique features to the mechanisms used by human monocytes to process and present antigens originating from M. tuberculosis-containing phagosomes to both of these T-cells subsets. First, uptake of M. tuberculosis bacilli by phagocytosis and subsequent processing were required for antigen presentation to both CD4+ and γδ T cells. Second, the recognition of M. tuberculosis-infected monocytes by γδ T cells was dependent on Vδ2+ TCR and by CD4+ T cells on class II MHC molecules. Processing of antigens for Vδ2+ γδ T cells was inhibitable by Brefeldin A. In contrast, processing of M. tuberculosis for CD4+ T cells was unaffected by Brefeldin A. Third, lysomotropic agents such as chloroquine and ammonium chloride did not affect the processing of M. tuberculosis bacilli for CD4+ and γδ T cells. This was true both when inhibitors were added during initial uptake and short-term processing of M. tuberculosis bacilli and when lysomotropic agents were added to ongoing antigen processing. In contrast, both inhibitors were able to block the processing of soluble mycobacterial antigens for CD4+ T cells in both situations. Fourth, the chloroquine and ammonium chloride insensitivity of processing of M. tuberculosis bacilli was not dependent on the viability of the bacteria, since both formaldehyde-fixed dead bacteria and mycobacterial antigens covalently coupled to latex beads were chloroquine insensitive. Thus, the particulate nature of mycobacterial antigens may have a role in determining insensitivity to agents.
In the last few years, increasing evidence suggests that M. tuberculosis-activated Vδ2+ γδ T cells are predominantly activated by small phosphate-containing molecules. A number of these antigens have been identified and include four TUBag’s isolated from M. tuberculosis, which are small (±500 Da) phosphorylated molecules which also contain nucleotide (9). Others have described IPP and related prenyl phosphates as possible antigens for Vδ2+ T cells (36). These phosphate ligands are not secreted by mycobacteria belonging to the M. tuberculosis group. Instead, they remain associated with the bacterial cell mass (10). Studies with TUBag’s and synthetic IPP determined that these small antigens could be presented by fixed APC without a requirement for processing (22, 24). However, these small antigens were not stably associated with APC since antigen had to be present continuously during the assay and could not be pulsed onto APC (10). These studies also determined that T-cell–APC contact was required for activation of Vδ2+ γδ T cells.
In contrast, our studies with M. tuberculosis bacilli indicated that antigens for Vδ2+ γδ T cells are processed and remain stably associated on the surfaces of monocytes. This conclusion was supported by three lines of evidence. First, monocytes fixed before addition of M. tuberculosis bacilli were unable to activate Vδ2+ γδ T cells whereas those fixed after infection were able to present antigen. Second, monocytes infected with M. tuberculosis or pulsed with soluble mycobacterial antigens could be washed extensively and still present antigen to Vδ2+ γδ T cells, indicating that antigens for Vδ2+ γδ T cells remained stably associated with monocyte surfaces. The γδ-T-cell lines used in our study did react to synthetic small phosphate-containing antigens such as IPP. However, IPP was not stably associated on monocytes for recognition by γδ T cells and was readily removed by washing. In contrast, soluble mycobacterial antigen and live M. tuberculosis bacilli were stably pulsed onto monocytes and extensive washing did not remove antigen for Vδ2+ γδ T cells. Third, Brefeldin A could inhibit processing of M. tuberculosis antigens for Vδ2+ γδ T cells. Brefeldin A inhibits transport from the endoplasmic reticulum to the trans-Golgi network, thus suggesting either that in M. tuberculosis-infected monocytes, antigen(s) for γδ T cells becomes associated with molecules migrating from the endoplasmic reticulum to the trans-Golgi network or that M. tuberculosis antigens for γδ T cells require transport through the endoplasmic reticulum and trans-Golgi network to arrive on the surfaces of monocytes. These results are consistent with a model in which small phosphate antigens or epitopes of M. tuberculosis are associated with a carrier molecule which requires processing and allows phosphate antigens or epitopes to remain stably associated on the surfaces of APC. Our observation that M. tuberculosis bacilli contain a 10- to 14-kDa cytosolic antigen which stimulates Vδ2+ γδ T cells is consistent with this model (4). Further supporting evidence for a carrier molecule was provided by the ability of cytosolic antigens covalently linked to latex beads to stimulate Vδ2+ γδ T cells with monocytes as APC (data not shown).
In contrast to the poorly understood processing of antigens for γδ T cells, the cellular pathways for processing of soluble antigens for presentation by class II MHC molecules to CD4+ T cells are much better characterized (reviewed in reference 11). Antigens are internalized by endocytosis or phagocytosis and concentrated within endosomes. As endosomes mature and fuse with lysosomes, proteases break down protein antigens into peptide fragments and the pH progressively decreases. Class II MHC molecules are present in late endosomal and early lysosomal compartments, where antigen-derived peptides bind to class II MHC molecules. The processing of particulate antigens (e.g., bacteria) is less-well-understood, and the site where mycobacterial peptides are loaded on class II MHC molecules in macrophages is unknown. After phagocytosis, M. tuberculosis bacilli generally remain within phagosomes and class II MHC molecules can be found in these phagosomes (7). M. tuberculosis bacilli modulate the phagosome by preventing fusion with acidic lysosomal compartments and excluding the vesicular proton ATPase, resulting in an elevated pH of 6.3 to 6.5 compared to the normal lysosomal pH of 4.5 (35). Nevertheless, M. tuberculosis-infected monocytes readily process and present antigens to CD4+ T cells.
Our finding that processing of M. tuberculosis bacilli is resistant to lysomotropic agents is consistent with the possibility that mycobacterial antigens become associated with class II MHC molecules within phagosomes. Chloroquine-resistant processing of M. tuberculosis, however, is not unique to live bacteria, since processing of particulate mycobacterial antigens for CD4+ T cells also is chloroquine resistant. Thus, processing of particulate mycobacterial antigens by monocytes may not depend on acidification of the phago-lysosomal compartment. Our studies were performed with live M. tuberculosis, formaldehyde-fixed bacteria, and cytosolic antigens of M. tuberculosis linked to latex beads. These are complex antigen preparations and include not only a large number of different proteins but also nonprotein constituents such as lipoarabinomannan, phosphatidyl-myo-inositol mannosides and other phospholipids, and complex carbohydrates, which may influence antigen processing in endosomal compartments. Traffic of lipoarabinomannan out of phagosomes has been demonstrated, supporting the concept of dynamic interactions between M. tuberculosis phagosomes and endosomal compartments (38). Chloroquine-resistant processing of particulate mycobacterial antigens may be unique to mycobacteria, since the original studies of inhibition of antigen processing for class II MHC molecules by agents were performed with particulate heat-killed Listeria monocytogenes and murine peritoneal macrophages (39). Alternatively, it is possible that human monocytes differ from murine macrophages in how they process particulate antigens for class II MHC molecules. Based on studies with human macrophages chronically infected with M. bovis BCG, it has been suggested that mycobacteria can reside within a compartment outside the route of normal antigen processing for CD4+ T cells (28). This result is consistent with our findings that particulate mycobacterial antigens may have unique antigen-processing requirements.
The antigen-processing mechanisms used by M. tuberculosis-infected mononuclear phagocytes are critical in the recruitment of T-cell subsets and in the determination of the antigen repertoire recognized by CD4+ and γδ T cells. Our study indicates that there are distinct pathways for the processing by human monocytes of antigens emanating from M. tuberculosis-containing phagosomes for CD4+ and γδ T cells. In addition, the manner in which the antigen is present within monocytes (particulate versus soluble) influences the antigen-processing mechanisms. Further studies of the antigen processing of M. tuberculosis bacilli by macrophages is necessary to understand the regulation of T-cell-subset activation in the protective immune response to M. tuberculosis, an understanding of which is necessary for the design of improved vaccines and immunotherapies for tuberculosis.
ACKNOWLEDGMENTS
Special thanks go to Cliff Harding for many helpful suggestions for experiments with inhibitors of antigen processing and for critically reviewing the manuscript.
This work was supported by National Institutes of Health grant AI-27243.
REFERENCES
- 1.Balaji K N, Schwander S, Rich E A, Boom W H. Alveolar macrophages as accessory cells for human γδ T cells activated by M. tuberculosis. J Immunol. 1995;154:5959–5968. [PubMed] [Google Scholar]
- 2.Barnes P F, Mistry S D, Cooper C L, Pirmez C, Rea T H, Modlin R L. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142:1114–1119. [PubMed] [Google Scholar]
- 3.Bhardwaj V, Colston M J. The processing and presentation of mycobacterial antigens by human monocytes. Eur J Immunol. 1988;18:691–696. doi: 10.1002/eji.1830180506. [DOI] [PubMed] [Google Scholar]
- 4.Boom W H, Balaji K N, Nayak R, Tsukaguchi K, Chervenak K A. Characterization of a 10- to 14-kilodalton protease-sensitive Mycobacterium tuberculosis H37Ra antigen that stimulates human γδ T cells. Infect Immun. 1994;62:5511–5518. doi: 10.1128/iai.62.12.5511-5518.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boom W H, Chervenak K A, Mincek M A, Ellner J J. Role of the mononuclear phagocyte as an antigen-presenting cell for human gamma delta T cells activated by live Mycobacterium tuberculosis. Infect Immun. 1992;60:3480–3488. doi: 10.1128/iai.60.9.3480-3488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boom W H, Wallis R S, Chervenak K A. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991;59:2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemens D L, Horwitz M A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comstock G W. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15. doi: 10.1164/arrd.1982.125.3P2.8. [DOI] [PubMed] [Google Scholar]
- 9.Constant P, Davodeau F, Peyrat M A, Poquet Y, Puzo G, Bonneville M, Fournie J J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 10.Constant P, Poquet Y, Peyrat M-A, Davodeau F, Bonneville M, Fournie J-J. The antituberculous Mycobacterium bovis BCG vaccine is an attenuated mycobacterial producer of phosphorylated nonpeptidic antigens for human gamma delta T cells. Infect Immun. 1995;63:4628–4633. doi: 10.1128/iai.63.12.4628-4633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 12.Fulton S A, Johnson J M, Wolf S F, Sieburth D S, Boom W H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding C. Techniques for studying phagocytic processing of bacteria for class I or II MHC-restricted antigen recognition by T lymphocytes. Methods Cell Biol. 1994;45:307–320. doi: 10.1016/s0091-679x(08)61859-2. [DOI] [PubMed] [Google Scholar]
- 14.Harding C, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–4935. [PubMed] [Google Scholar]
- 15.Havlir D V, Ellner J J, Chervenak K A, Boom W H. Selective expansion of human gamma delta T cells by monocytes infected with live Mycobacterium tuberculosis. J Clin Invest. 1991;87:729–733. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havlir D V, Wallis R S, Boom W H, Daniel T M, Chervenak K, Ellner J J. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun. 1991;59:665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch C S, Ellner J J, Russell D G, Rich E A. Complement receptor-mediated uptake and tumor necrosis factor-alpha-mediated growth inhibition of M. tuberculosis by human alveolar macrophages. J Immunol. 1994;152:743–753. [PubMed] [Google Scholar]
- 18.Kabelitz D, Bender A, Schondelmaier S, Schoel B, Kaufmann S H. A large fraction of human peripheral blood gamma/delta T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med. 1990;171:667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabelitz D, Bender A, Prospero T, Wesselborg S, Janssen O, Pechhold K. The primary response of human gamma/delta + T cells to Mycobacterium tuberculosis is restricted to V gamma 9-bearing cells. J Exp Med. 1991;173:1331–1338. doi: 10.1084/jem.173.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladel C H, Flesch I E, Arnoldi J, Kaufmann S H. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J Immunol. 1994;153:3116–3122. . (Erratum, 154:4223.) [PubMed] [Google Scholar]
- 21.Ladel C H, Blum C, Dreher A, Reifenberg K, Kaufmann S H. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur J Immunol. 1995;25:2877–2881. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 22.Lang F, Peyrat M A, Constant P, Davodeau F, David-Ameline J, Poquet Y, Vie H, Fournie J J, Bonneville M. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–5994. [PubMed] [Google Scholar]
- 23.Li B, Rossman M D, Imir T, Fusun Oner-Eyuboglu A, Lee C W, Biancaniello R, Carding S R. Disease-specific changes in γδ T cells repertoire and function in patients with pulmonary tuberculosis. J Immunol. 1996;157:4222–4229. [PubMed] [Google Scholar]
- 24.Morita C T, Beckman E M, Bukowski J F, Tanaka Y, Band H, Bloom B R, Golan D E, Brenner M B. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 25.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 26.Orme I M, Collins F M. Protection against Mycobacterium tuberculosis infection by adoptive transfer. J Exp Med. 1983;158:74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orme I M, Miller E S, Roberts A D, Furney S K, Griffin J P, Dobos K M, Chi D, Rivoire B, Brennan P J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–196. [PubMed] [Google Scholar]
- 28.Pancholi P, Mirza A, Bhardwaj N, Steinman R M. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science. 1993;260:984–986. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer K, Schoel B, Gulle H, Kaufmann S H, Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990;20:1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- 30.Pfeifer J D, Wick M J, Roberts R L, Findlay K, Normark S J, Harding C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 31.Schlesinger L S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 32.Schlesinger L S, Bellinger K C, Payne N R, Horwitz M A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 33.Schoel B, Gulle H, Kaufmann S H. Heterogeneity of the repertoire of T cells of tuberculosis patients and healthy contacts to Mycobacterium tuberculosis antigens separated by high-resolution techniques. Infect Immun. 1992;60:1717–1720. doi: 10.1128/iai.60.4.1717-1720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selwyn P A, Sckell B M, Alcabes P, Friedland G H, Klein R S, Schoenbaum E E. High risk of active tuberculosis in HIV-infected drug users with cutaneous anergy. JAMA. 1992;268:504–509. [PubMed] [Google Scholar]
- 35.Sturgill-Koszycki S, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka Y, Morita C, Tanaka Y, Nieves E, Brenneer M B, Bloom B M. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 37.Tsukaguchi K, Balaji K N, Boom W H. CD4+ alpha-beta T cell and gamma-delta T cell responses to Mycobacterium tuberculosis: similarities and differences in antigen recognition, cytotoxic effector function, and cytokine production. J Immunol. 1995;154:1786–1796. [PubMed] [Google Scholar]
- 38.Xu S, Cooper A, Sturgill-Koszycki S, van-Heyningen T, Chatterjee D, Orme I, Allen P, Russell D G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]
- 39.Ziegler H K, Unanue E R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA. 1982;79:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]