ABSTRACT
This review gives an overview of the protective role of CD8+ T cells in SARS-CoV-2 infection. The cross-reactive responses intermediated by CD8+ T cells in unexposed cohorts are described. Additionally, the relevance of resident CD8+ T cells in the upper and lower airway during infection and CD8+ T-cell responses following vaccination are discussed, including recent worrisome breakthrough infections and variants of concerns (VOCs). Lastly, we explain the correlation between CD8+ T cells and COVID-19 severity. This review aids in a deeper comprehension of the association between CD8+ T cells and SARS-CoV-2 and broadens a vision for future exploration.
KEYWORDS: COVID-19, SARS-CoV-2, T-Lymphocytes, CD8-Positive T-Lymphocytes, immunopathology, viral infection, T-cell response
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) in China was initiated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).[1] Although COVID-19 is no longer considered a public health emergency of international concern, more than thirteen billion vaccine doses have been administered as of October 2023 and some drug treatment alternatives are currently in existence, there continues to be significant research interest in COVID-19 as an ongoing health event. [1–3] It is crucial to acknowledge that breakthrough infections continue to evolve, with several mutation variants evading natural infection or vaccine-induced neutralization. Moreover, the molecular mechanism underlying the progress to a severe COVID-19 state remains mostly elusive, and there is a need for additional methods to support the immunocompromised population and developing integrated care pathways. [3]
To date, a considerable amount of researches has concentrated on the SARS-CoV-2-specific humoral response in the public discourse, but several studies highlight the critical role of T cells, specifically CD8+ T cells, in the control of SARS-CoV-2 infection. [4, 5] A comprehensive investigation into T-cell subpopulation immunity in COVID-19 could provide a more holistic understanding of the immune landscape, which could shed light on public health policies and interventions consequentially. [6]
Here, we aim to discuss our current knowledge concerning the characterization of CD8+ T-cell responses in COVID-19 and suggest potential strategies that enhance their protective role, while preventing adverse effects. Several relevant reviews cover these topics in detail. In this review, we gathered and integrated information about the participation of CD8+ T cells in COVID-19, addressing existing disagreements in this context and providing plausible explanations for them.
An overview of the responses of CD8+ T cells in COVID-19
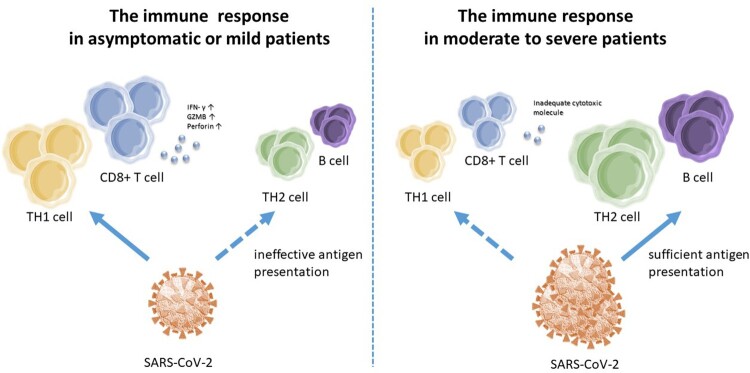
Up to now, evidence shows that CD8+ T cells might play a crucial role in determining the outcome of SARS-CoV-2 infection. It is confirmed that CD8+ T-cell responses inversely correlate with virologic outcomes. [7] While asymptomatic or mild patients are unable to generate robust germinal centre (GC) B-cell response specific to SARS-CoV-2, however, achieving potent, strong and variable virus-specific T helper type 1 (TH1) and CD8+ T cell response without systemic inflammation, [8–10] patients with moderate to severe illness show robust GC B-cell responses specific to SARS-CoV-2 and rare response of TH1 and CD8+ T cells. [7, 11] Taking the function of CD8+ T cells into account, more than half of CD8+ T cells in asymptomatic or mild donors simultaneously generated Granzyme B and perforin, but only 3% to 12% of CD8+ T cells in moderate to severe individuals were able to create both pyrolytic molecules. [11] (Figure 1) Moreover, the frequencies of interferon (IFN)-γ+CD8+ T cells and IL-4+CD8+ T cells responding to SARS-CoV-2 have a positive correlation with viral loads, while the dominance of IL-10+ T-cell responses is linked to minor illness. [9, 10, 12] The facts above suggest roles of CD8+ T cells in protective immunity in COVID-19.
Figure 1.
The different immune profiles in COVID-19 patients. SARS-CoV-2 induced a TH1 profile of immune responses, including virus-specific IFN-γ-producing CD8+ T cells with increasing production of Granzyme B(GZMB) and perforin in asymptomatic or mild patients, but was unable to initiate robust B cell and TH2 cell responses, which may be a failure of inefficient viral antigen production. In moderate to severe patients, sufficient and long-lasting antigen presentation was able to induce robust GC B cell responses specific to SARS-CoV-2, but a scarce response of TH1 and CD8+ T cells with inadequate cytotoxic molecules.
It is noticed that the decreased inflammation over time is linked to gradual development of effective CD8+ T-cell response specific to SARS-CoV-2 with continuous and profitable antibody neutralization activity in symptomatic patients. [13] In mild COVID-19 cases, the CD8+ T-cell subsets show a switch to T effector memory (TEM) surface phenotype, which indicates that they have effector functions with features of highly differentiated cells. [12] Moreover, the CD8+ T cells had greater levels of activated state and Ki-67 expression, compared to CD4+ T cells, suggesting the important function against viral infection. [12] One theory is that the SARS-CoV-2 replication may be successfully suppressed by the quick and strong TH1 and CD8+ T-cell responses at the first stage. This would result in ineffective viral antigen synthesis and so limit the GC reaction, which significantly relies on sufficient and long-lasting antigen presentation. [11] Supportively, according to an exploratory study about Intensive Care Unit (ICU) COVID-positive patients, the B/T8 rate (B cells to CD8+ T cells) was substantially lower in the survivors’ cohort, showing that some patients recovered before producing enough antibodies, which indicates that early cellular response might operate in defining the severity and duration of initial SARS-CoV-2 infection. [14, 15]
In terms of the targets of CD8+ T-cell responses towards SARS-CoV-2, recent studies indicate that CD8+ T-cell responses are broad and target the whole-virus proteome, with a great portion of which hitting the internal and/or non-structural viral protein epitopes. [8, 10, 13] In addition, studies have shown that M and N peptides induced a TH1 response with IFN-γ production by CD4+ T cells and degranulation by CD8+ T cells, which was predominant in mild patients. The S peptides caused a biased TH2 response with IL-4 production, which was predominant in hospitalized groups. [9, 16] These findings suggest that a narrower CD8+ T-cell response might be created by the COVID-19 vaccines against the spike protein, compared with CD8+ T-cell responses induced by natural infection in asymptomatic or mild patients. Additional class I epitopes generate from the M, nsp6, ORF3a, and/or N genes will be required for an ideal vaccine that is able to generate a robust CD8+ T-cell response. [10, 16] Nevertheless, assessing the whole SARS-CoV-2 ORFeome of epitopes, specific CD8+ T-cell responses remain inaccessible in about 30% of recovered individuals. [17, 18] One explanation might be that they may exhibit CD8+ T-cell responses to novel epitopes. The other proposal is that the main parts of SARS-CoV-2-specific CD8+ T-cell responses are tissue-resident memory T cells that are discovered in the tissues of airways and are not detectable in the bloodstream. [19, 20]
From a long-term perspective, virus-specific CD8+ T cells stayed quite steady with hallmarks of long-lived cells that were seen a maximum of 12 months following infection, despite the fact that antibody titres decreased progressively. [8, 21–23] Early investigations about SARS-CoV-1 and Middle East Respiratory Syndrome coronavirus (MERS-CoV) infection suggest that cellular responses persist for several years. [24–26] It is yet unknown if vaccination promoters can enhance cellular immunity and how long SARS-CoV-2-specific CD8+ T cells will stay viable.
Numerous studies have consistently demonstrated the autoimmune disease after SARS-CoV-2 infection or vaccination. [27, 28] These mechanisms include molecular mimicry, autoantibody formation, and immune system dysregulation. [29, 30] By analysing T-cell repertoires accumulated during the COVID-19 pandemic, researchers discovered with an increase of COVID-19-specific T cells, the percentages of ankylosing spondylitis-specific T cells considerably rose, suggesting T cell cross-reactivity that balances immunological defence and autoimmune risk. [31] Another study revealed a higher frequency of type II pneumocyte-associated antigen-specific autoreactive T cells in the blood of non-severe COVID-19 patients than those in severe cohorts, indicating a potential protective role of these cells in SARS-CoV-2 infection by promoting the elimination of infected type II pneumocyte and viral clearance. [32] However, there is currently no systematic and widely applicable explanation of auto-reactivity specifically within subpopulations of T cells, such as CD8+ T cells.
Cross-reactive CD8+ T cells in unexposed cohorts
Recent studies show that cross-reactive CD8+ T-cell responses are probably fundamental in determining the protective purpose of the immune system in SARS-CoV-2 infection. [33] These cross-reactive CD8+ T cells are usually reactive against more than one antigen, including RNA polymerase and other components of the viral replication and transcription complex of SARS-CoV-2 (polymerase cofactor NSP7, helicase NSP13). [20, 34–36] Many reports have found that a fraction of pre-existing SARS-CoV-2 cross-reactive CD8+ T cells in unexposed cohorts are probably produced following contact with human endemic coronavirus (HCoV). [20, 36–38] Other research found that different SARS-CoV-2 antigen responses in unexposed cohorts were connected with those targeted against EBV (Epstein-Barr virus) and CMV (Cytomegalovirus) positively. [20] These data indicate that previous exposure to other viruses drives the induction of anti-SARS-CoV-2 CD8+ T-cell responses. As T cell epitopes are conservative in current SARS-CoV-2 variants, if we can use these cross-reactive epitopes for a pan-coronavirus vaccine, it will be vital for prophylactic value.
Some experiments demonstrated that the cross-reactive CD8+ T cells in unexposed cohorts were killer cells with the expression of CD107a, as it is regarded that the expression of CD107a on CD8+ T cells has a close connection with lytic function. [38] More research is needed to determine whether these T-cell lines could have a function of lysing the infected cells. Through comparing the cross-reactive CD8+ T cells pre- and post-SARS-CoV-2 infection in the same donors, researchers showed the anti-viral functionality of the pre-existing CD8+ T cells. [33] Furthermore, the cross-reactive CD8+ T cells were found more abundant in COVID-19 patients than the unexposed individuals, and shared several T cell receptor (TCR) motifs between the unexposed ones and the exposed ones, indicating the protective purpose of the immune system in SARS-CoV-2 infection. [5, 39] However, this kind of protection is limited and supported by low tolerance for mutational variation and very little efficient reactivity. [8, 34, 35, 40] With the possibility of subsequent mutant strains and the current reduction in unexposed populations, the function of cross-reactive CD8+ T cells needs to be further explored.
Some studies indicate that bronchoalveolar lavage samples taken from healthy donors before the COVID-19 outbreak showed airway-resident SARS-CoV-2-cross-reactive CD8+ T cells with elevation of tumour necrosis factor (TNF) and IFN-γ. [36] Furthermore, SARS-CoV-2–specific memory CD8+ T cells in unexposed oropharyngeal lymphoid tissue have been discovered. [20] Since samples taken from interstitial tissue-resident memory T cells (TRM) are limited, which generally include more T cells capable of cytotoxicity, it is possible that the magnitude of TRM cells with cross-reactive capability as opposed to SARS-CoV-2 was underestimated. [20] Now, more research is required to ascertain whether the early viral containment may be caused by these pre-existing CD8+ TRM cells.
Although CD8+ T cell cross-reactivity does exist, it appears to be less common than CD4+ T cells. In comparison with SARS-CoV-2-cross-reactive CD4+ T cells in unexposed cases, a lesser degree of attacking pathogen-specific antigens has been explored in CD8+ T cells, both in the bloodstream and tissues. However, the frequency of CD4+ and CD8+ T cells have a correlation with the same donors. [10, 34, 36, 40] A cytotoxic component of the response is elicited by airway CD4+ T cells, and they may induce the generation of CXCR3 chemokines attracting migrating dendritic cells and increase the CD8+ T-cell response for completing elimination towards infected cells as well. [36] Others may suggest the disparity in the counts of CD4+ vs. CD8+cross-reactive memory T cells may be fundamental distinctions between different T cell types in antigen recognition. [40, 41] A deeper comparison between CD4+vs. CD8+cross-reactive T cells may help us understand more about the mechanisms of T-cell responses against SARS-CoV-2.
Airway-resident CD8+ T cells
It is commonly acknowledged that retaining sufficient pathogen-specific CD8+ T cells at the location of pathogen invasion is critical for the defensive role of immune surveillance offered by TRMs in many tissues. Similarly, there is a clear relationship between a better illness prognosis and an increased number of airway-resident CD8+ T cells in COVID-19 cases. [9] The bulk of the resident CD8+ T cells were discovered as terminally differentiated effector-memory cells, while very rare naïve T cells were observed. [42] Also, it was discovered that the CD8+ T cells in the airway were more capable of producing multiple cytokines than those in the circulation, indicating that SARS-CoV-2 infection may lead to the production of multifunctional memory CD8+ T cells in the mucous membranes of the respiratory tract. [37] In the long term, lung TRM is routinely identified in convalescent patients even 10 months following the first infection, despite the fact that contemporary circulation does not show tissue-resident patterns. [9] The nasal-resident T-cell responses lasted for around 140 days without significantly diminishing. [42] These results indicate the durability of CD8+ T-cell responses in the mucous site.
Unlike what has been observed in the blood, nasal T cell subdividing revealed a dominant CD8+ T cell subset. Of note, there were more CD8+ T cells (∼90%) expressing tissue-residency symbols, CD69+ CD103+, than CD4+ T cells (∼40%). [42] Though nearly all patients had detectable virus-specific TRM responses, with the exception of the multifunctional response discovered in tissue against N peptides, compatible models were not found in individuals regarding virus-targeted proteins and roles between peripheral circulation and lung tissues. [9, 16] Along with discoveries about TRMs in the airway tract, previous data on SARS-CoV-2 peptide-reactive CD8+ T cells in blood suggests that the responses in the periphery represent only a cluster of their more defensive mucous resident groups, which either have not yet cultivated tissue resident ability or are referred to as “ex-TRM cells’. [36] If we can identify the specific subgroup of “ex-TRM cells’ and use a certain model to trigger the TRM-cells expansion by next-generation vaccine, the airway-resident CD8+ T cell may play a crucial role in the first line of defencing against SARS-CoV-2 infection.
Currently, there is no research comparing the CD8+ TRM responses between the upper and the lower airways. The very first barrier to resist air-borne virus is comprised of lymphatic structures in the nasal cavity and in the upper respiratory tract (pharyngeal, lingual, and palatine tonsils). Given that some airborne viruses may be able to bypass the upper airway’s immune defences, the significance of the lower respiratory tract T cells is further highlighted. More information is thus required regarding the role of CD8+ TRMs at different sites of the airway.
CD8+ T-cell responses after vaccine
As of March 2023, there were at least 199 COVID-19 vaccines in pre-clinical progress and 183 vaccines in clinical research. [43] All existing COVID-19 vaccines are well tolerated and have good effectiveness against the original strain and variations of concern. [44] It is important to use COVID-19 research as a jumping-off point for learning more about the underlying process behind the long-lasting CD8+ T-cell responses induced by vaccines.
Experiments have confirmed that current popular vaccines, including mRNA vaccines, adenovirus-vector vaccines, and recombinant vaccines, can induce TH1-skewed T-cell immune responses with SARS-CoV-2-specific CD8+ T-cell augmentation. This has a potent negative correlation with the virologic endpoint, whereas CD8+ T cells with strong TH2 responses expand the danger of vaccine-associated enhanced respiratory disease (VAERD). [25, 45–47] Thus, it is widely assumed the best kind of self-protective immunity for SARS-CoV-2 exposure should consist of high titre neutralizing antibodies accompanied by a potent cellular and TH1-biased CD4+ effector response.
According to recent studies, mRNA vaccines induce robust acute and memory responses of IFN-γ-producing CD8+ T cells, a majority of which co-express Granzyme B. [46, 48, 49] When combinations of cytokines were assessed, the proportion of multifunctional (T cells with the ability to secrete > two cytokines) spike-specific CD8+ T cells was larger among the vaccinated cohort in contrast with placebo recipients. [25] Also, the robust CD8+ T-cell responses have a pronounced presence of the cytotoxic CD8+ T-cell marker, CD107a, [49] and display a TEM surface phenotype, holding the possibility to react quickly to invasion. [48, 50] Additional studies are necessary in order to comprehend the differentiation process of various T cell subsets completely, as well as how they develop post-vaccination in tandem with clinical outcomes. Surprisingly, heterologous immunization with an inactivated vaccine followed by an mRNA enhancer significantly increased RBD (Receptor binding domain)-specific memory B-cell responses and S1-specific T-cell responses against the Omicron variant compared with two or three doses of homologous inactivated vaccine. [51] It suggests that the use of mRNA boosters may bring additional benefits in the presence of emerging mutants.
The vaccine-induced CD8+ T-cell responses resemble those acquired upon natural infection, but are substantially higher than those observed in COVID-19 recovered cohorts. [45, 46, 50] The breadth and poly-specificity of vaccine-induced CD8+ T cells approve it further. Vaccines-induced CD8+ T cells target multiple peptides from various viral proteins, making them less sensible to the secondary conformation of single amino acid variants to alleviate the incidence of immune escape from mutated strains. [45, 50] According to the observation from live yellow fever virus vaccine, there is a distinct distribution of the early memory CD8+ T cells compared to natural infection, which may impact long-term maintenance features. [52] This disparity could be caused by distinctions in antigen contact time and location, as well as distinct inflammatory alteration following vaccination versus natural infection, such as decreased CD38 expression on memory CD8+ T cells following COVID-19 inoculation versus natural infection. [33, 46]
Multiple subsets of airway-resident CD8+ T cells increase in frequency following mRNA vaccination, many of which are SARS-CoV-2-specific. [53] One of the subtypes is TRM CD8+ T cells expressing CD69 and CD103, though CD69+CD103+CD8+ T cells in the peripheral circulation decline after vaccination. [53] One explanation for this is that these cells may migrate from the bloodstream into the tissues. Based on these results, aerosol vaccination is promising for the delivery of COVID-19 vaccines. Compared to intramuscular vaccination, intranasal vaccination exhibits the greater prospect of inducing multifunctional CD8+ T cells with cytotoxic potential and the tissue-resident memory marker, targeting a broad range of antigens, within the respiratory tract. [47, 54] Another route of vaccination, intradermal injection, has also raised concerns. The effectiveness of T-cell response of intradermal and partial vaccination in previously immunized populations has been confirmed by a study utilizing a 1/5 dose of intradermal booster (BNT162b2 mRNA vaccine) in healthy individuals who had finished two doses of inactivated SARS-CoV-2 vaccine. [55] These new immunization routes may open new perspectives to the immunized population.
It has been confirmed that vaccine-induced multifunctional memory CD8+ T-cells patrol the blood for 30∼180 days with a contraction about day 29, although this is only detectable in 60-67% of the subjects. [45, 48, 56] The memory CD8+ T cells’ estimated t1/2 at six months after COVID-19 vaccination was larger than 1 year. Furthermore, antibodies significantly decreased after mRNA vaccinations, while memory T and B cells remained mostly stable. [48] More research is needed to confirm how long these cells can defend against reinfection.
Results have shown that the number of vaccine-induced CD8+ T cells was almost the same in some participants within the tested dose range (1-50 μg mRNA) after two doses of BNT162b1, suggesting that the intensity of the T-cell response was not dose-dependent. [46] However, comparing the spike-specific CD8+ T-cell response after BNT162b2(30 μg of mRNA) immunization to which following the first dose of mRNA-1273(100 μg of mRNA), the higher concentration contributes to a stronger profile of CD8+ T-cell responses in frequency and multifunctionality, suggesting a positive correlation between the T-cell response and the dose concentration. [48, 57] The variance in delay time between the first and second vaccination also needs to be taken into account. As memory T cells need several weeks to achieve a potent state following the initial vaccination, a second immunization before the maximum development of memory T cells might lead to inadequate T-cell memory subsequently. [48]
CD8+ T-cell responses of SARS-CoV-2 variants and breakthrough infections
Nowadays, the onset of SARS-CoV-2 mutations may threaten the worldwide situation of widespread immunization programmes. With a significant overall decrease discovered of memory B cells and neutralizing antibodies in vaccinated people, given Omicron's consequential escape from antibodies, memory T cells may be crucial as a second layer of protection. [58] Recent studies show that over 95% of CD8+ T cell epitopes have little impact by mutations in most variants, which contradict the theory that mutations gained in Omicron are as a consequence of population-level T cell immunological pressure and show that variant evolution is not triggered by T cell evasion. At the same time, a total of 7% of previously identified CD4+epitopes are affected by varied VOC mutations. [45, 47, 49, 58] Since HLA class I binding peptides are shorter (typically 9–10 amino acids) than class II binding peptides (13-17 amino acids), CD8+ T cell epitopes are apparently better protected. However, this effect is offset by the fact that CD8+ T cells are often more vulnerable to amino acid changes than CD4+ T cells. [59] In the case of Omicron, however, individuals decline or lack of such reactions were observed in 25% or less, implying that certain HLA class profiles may be more vulnerable to Omicron. [58]
Noteworthy, at an interim follow-up, there was a significant rate of symptomatic breakthrough infection (BTI) cases resulting from alpha and delta strains, but immunity induced by the vaccine only diminished modestly in comparison with those without BTI. [60] According to previous reports, almost all individuals who have anti-SARS-CoV-2 CD8+ T-cell responses can defend against Omicron, indicating that there is no significant T-cell escape mutation in SARS-CoV-2 at present. In 15% of the cohort, the mutations of Omicron seem to derecognize CD8+ T cells. [22, 61, 62] This decrease in cross-reactive CD8+ T-cell responses may have pathogenic outcomes for many patients. [22] More research is needed to identify particular HLA class I epitopes connected to T-cell response reduction.
Historically, the phrase “hybrid immunity” has been used to describe the state of immunity obtained through a combination of SARS-CoV-2 infection and corresponding vaccine-induced changes. [63] It has been observed that SARS-CoV-2 infection leads to the recruitment of a significant population of T lymphocytes, specific to various SARS-CoV-2 proteins, into the nasal mucosa in addition to immunization. This finding aligns with the presence of spike-specific T lymphocytes in the bronchoalveolar lavage (BAL) and nasal mucosa of COVID-19-recovered patients. [42] Comparatively, cohorts with BTI experiences demonstrated more robust cellular responses targeting the spike protein than non-vaccinated controls, emphasizing the benefits of prophylactic vaccination. [64] Importantly, nasal cells in convalescent vaccine recipients exhibited distinct responses to SARS-CoV-2 peptide reservoirs, targeting other structural and non-structural proteins in addition to spike. Consequently, infection leads to the emergence of virus-specific T-cell responses that are not solely reliant on spike-protein-specific T cells induced by vaccination in the peripheral circulation. [42] In various mouse models of SARS-CoV-2 infection, antigen presentation by multiple viral protein-specific T cells rapidly induces the production of IFN and triggers a cascade of innate and adaptive immunological responses that impede viral replication. [42, 65] These findings underscore the need to consider other proteins, in addition to spike, in the development of effective vaccines.
CD8+ T-cell responses in immunocompromised people
It is of great importance to explore virus-specific CD8+ T-cell responses in immunocompromised people, as they might be more vulnerable to the virus infection. It was discovered that a group of HLA-I-typed HIV- 1-infected patients on anti-retroviral therapy had larger SARS-CoV-2-reactive T-cell responses than HIV-1-negative patients. It has been assumed that the examined HIV-1-infected participants’ peripheral blood mononuclear cells (PBMCs) might have an averagely larger percentage of CD8+ T cells. The second assumption is that a number of antiretroviral medications, including the protease inhibitor lopinavir, possess an antiviral impact on SARS-CoV-2 in vitro. [38] People living with HIV (PWH) who were immunized by two doses of mRNA vaccination, however, had a reduced T cell response targeting Delta and Omicron strains. This inadequate immune reconstitution is linked to breakthrough SARS-CoV-2 infections, suggesting that suboptimal T-cell responses have been created in certain PWH. [62] Moreover, in an investigation of immunological responses following two doses of immunization, researchers found a substantial reduction of IFN-producing cells against VOCs, such as Alpha, Kappa, Delta, and B1f.617.3, in a section of PWH with a prior T cell response compared to wild type. [66]
It has been confirmed that, in patients with chronic myeloid leukaemia (CML), mRNA vaccination against SARS-CoV-2 can generate neutralizing antibodies and multifunctional T-cell responses. [67] Tyrosine Kinase Inhibitor (TKI) therapy is very common in CML patients, which causes unpredictable immune deficits. TKIs suppress Src family kinases and induce severe T cell malfunction as well, because of the essential function that these kinases have in signalling downstream from the T cell receptor. However, the patients with CML displayed both monofunctional and multifunctional T-cell cytokine production to SARS-CoV-2 and showed a T-cell response alike to healthy cohorts evaluated in earlier studies of mRNA vaccine, indicating the efficacy of current immunization in CML patients. [67] In vaccinated patients with multiple myeloma (MM), there may be a quantitative rather than a qualitative difference in the absence of detectable spike-specific CD8+ T lymphocytes. According to recent research, the majority of patients with MM after 3 doses of SARS-CoV-2 mRNA vaccines cannot produce enough VOCs neutralizing antibody (almost 60%) and spike-specific CD8+ T cells (over 80%), suggesting they are still vulnerable to the latest SARS-CoV-2 Omicron sub-variant. [68] Given that new variations are anticipated to arise consistently, new techniques to induce virus-specific CD8+ T cells are required for these immunocompromised patients, especially, in populations with hematologic malignancies that should be given priority for this sort of vaccine.
CD8+ T cells and severe COVID-19
One of the concerning problems is that the COVID-19 patients who are elderly adults, pregnant women, people with common comorbidities, or with immune deficiency are at the biggest risk for progression into severe state. Lymphopenia is observed in many severe COVID-19 patients, which is partially related to the enhanced apoptosis of T cells during severe infection. [69] In activation-induced cell death, apoptosis mediated by caspase is a significant factor in immunological homeostasis and may help reduce an excessive cytokine response caused by SARS-CoV-2 infection. [70] Further research is needed on how to balance cytokine storm and lymphopenia that may result from it to help the severe patients surviving the dangerous period.
Focusing on the elderly people (≥ 65 years old), an overall loss in coordination of the CD4+ and CD8+ T cell responses was noted compared to younger patients, as evidenced by the elderly individuals having considerably greater CD4+/CD8+ T cell ratios. [71] Another fact is that beyond the age of 45, acutely infected individuals had considerably higher numbers of regulatory CD8+ T cells [69], which is linked to CD4+ and CD8+ T cell dysregulation, suggesting that various subgroups of Tregs(Regulatory T cells) may play diverse roles in SARS-CoV-2 infection and may contribute to the vulnerability of elderly individuals. Furthermore, the researchers found that an age-related reduction in naive CD8+ T cells was related to the risk of severe COVID-19, which may be the result of an impaired immune system of elderly patients to induce the differentiation of naïve precursors into effector CD8+ T cells defined by the expression of IFN-γ and the transcription factor T-bet. [72] These studies might help us to develop more efficient strategies to protect the elderly from the severe COVID-19.
Severe COVID-19 is featured by a hyperactivated/exhausted Pan-T cell phenotype. As opposed to healthy cohorts, CD4+ and CD8+ T cells in COVID-19 cases excessively expressed CD69 (an activation marker) and TIM-3 (a negative modulator of immune cell activity), which is also noted in cases where severe COVID-19 deceased vs. those who survived. [73] It has also been revealed that CD8+ T cells have enhanced PD1 and CTLA4 expression, which are linked to exhaustive phenotypes. Moreover, the strong activation status is related to an elevated effector phase and impaired terminal differentiation, suggesting that the severity of the illness may be caused by an interruption of the immune response's contractile process. [12] The use of checkpoint inhibitors to unlock their antiviral ability could be envisioned, as TIM-3 and PD-1 are druggable targets that have been proven to leverage lymphocyte responses in malignancies. [74] To prevent exacerbating the over-inflammatory condition that characterizes severe SARS-CoV-2 diseases, this should be meticulously scheduled.
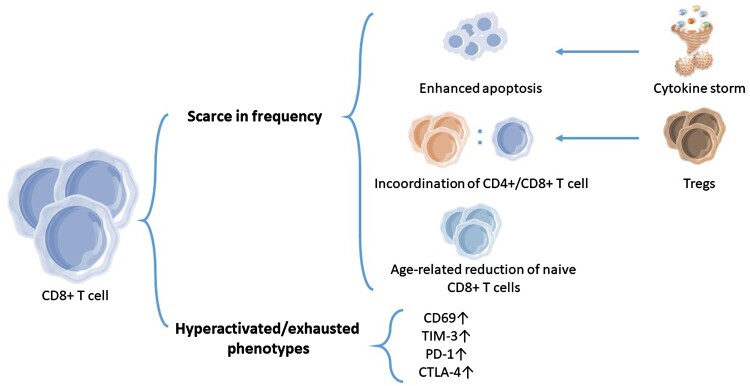
As the irreversible scarce but hyperactivated CD8+ T cells are correlated with the risk of severe COVID-19, [71, 75] the mechanism of their formation is of great importance. In addition to the T-cell apoptosis, a loss in coordination between the CD4 + and CD8+ T cell responses, the age-related reduction of naïve CD8+ T cells, or a hyperactivated/exhausted Pan-T cell phenotype, we still need more studies to explain the causes of reduced CD8+ T cell in severe COVID-19, thus providing patients with more targeted treatment. (Figure 2)
Figure 2.
The characterization of CD8+ T-cell responses in severe patients. In many severe COVID-19 patients, lymphopenia has been observed, which is partially caused by enhanced apoptosis of CD8+ T cells during infection. This might be beneficial to minimizing an excessive cytokine storm. In elderly severe patients, higher numbers of regulatory CD8+ T cells may contribute to the dysregulation of CD4+ and CD8+ T cells, thus resulting in scarce CD8+ T cells partially. An age-related reduction in naive CD8+ T cells was also related to the risk of severe COVID-19, as a consequence of an impaired immune system. The other characteristic of severe COVID-19 is a Pan-CD8+ T cell hyperactivated/exhausted phenotype, with an elevated expression of CD69, TIM-3, PD-1, and CTLA-4 and an increased production of effector molecules of CD8+ T cells.
Recently studies have concentrated on exploring the TCR repertoire as a biomarker for immune response to COVID-19. Variations in the SARS-CoV-2-specific repertoire have been observed in relation to disease severity, especially in CD8+ T cell subsets. [76, 77] Subsequent studies have revealed that specific subpopulations of CD8+ T cells may be utilized to predict the severity and progression of COVID-19. [76, 78] A reduced percentage of CD8+ TEM cells with TCRs containing COVID-19-associated amino acid sequences were found in COVID-19 patients with more severe state. [77] The presence of CD38+HLA-DR+KLRG1-CD8+ T cells during COVID-19 have been identified as a potential indicator of disease severity and clinical progression. [79] By combining single-cell RNA and TCR sequencing with CITE-seq antibodies, investigators have demonstrated significant differences in the expression of NK cell receptors and connected function of CD16+CD8+ cells between mild and severe disease. [80] Furthermore, the utilization of TCR beta repertoire data to develop a machine learning approach for dividing individuals based on prior SARS-CoV-2 infection has shown promising results. [78] However, further exploration is required to evaluate the applicability of such predictions in larger cohorts and over longer durations.
Discussion and outlook
Numerous explorations have been managed to learn the characterization of CD8+ T-cell responses and cellular responses. Several dominant problems still need more investigations, such as 1) whether the SARS-CoV-2-specific memory CD8+ T cells are durable and functional, 2) the discrepancy of the CD8+ T-cell response evolution among various immunity states, including only vaccination, hybrid immunity, natural infection, and immunocompromised condition, and 3) the function CD8+ T cells play in severe COVID-19 and new available drug targets for severe patients.
Current studies have confirmed that vaccination provides effective protection against the original and new variant strains of SARS-CoV-2, inducing reliable CD8+ T-cell responses in most people. However, with emerging breakthrough infections, we need new approaches. Next-generation vaccination is centred on inducing effective mucosal immunity, especially robust CD8+ T-cell responses specific to SARS-CoV-2. Different CD8+ T cell subsets which may contain various potentials causing disease are present in the blood and airway after SARS-CoV-2 infection. As a result, it is critical to induce the appropriate fraction of CD8+ T cells presenting in distinct compartments during possible mucosal vaccination or/and intramuscular vaccination to utilize the effects of CD8+ T cells in protective immunity while avoiding undesired pathology.
Acknowledgements
I confirm that anyone listed under the Acknowledgements section of the manuscript has been informed of their inclusion and approved this.
Funding Statement
This work was supported by R&D Program of Guangzhou Laboratory (SRPG22-006), and HUST Academic Frontier Youth Team (2018QYTD10).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.WHO Coronavirus (COVID-19) Dashboard . Available from: https://covid19.who.int/?mapFilter=vaccinations.
- 2.Sanyaolu A, Okorie C, Marinkovic A, et al. Current advancements and future prospects of COVID-19 vaccines and therapeutics: a narrative review. Ther Adv Vaccines Immunotherapy. 2022;10:251513552210975, doi: 10.1177/25151355221097559. PubMed PMID: 35664358; PubMed Central PMCID: PMCPMC9160920. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Statement on the fifteenth meeting of the IHR . (2005). Emergency committee on the COVID-19 pandemic. Available from: https://www.who.int/zh/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic.
- 4.Mortezaee K, Majidpoor J.. Cellular immune states in SARS-CoV-2-induced disease. Front Immunol. 2022;13:1016304, doi: 10.3389/fimmu.2022.1016304. PubMed PMID: 36505442; PubMed Central PMCID: PMCPMC9726761. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallajosyula V, Ganjavi C, Chakraborty S, et al. CD8(+) T cells specific for conserved coronavirus epitopes correlate with milder disease in COVID-19 patients. Sci Immunol. 2021;6(61). doi: 10.1126/sciimmunol.abg5669. PubMed PMID: 34210785; PubMed Central PMCID: PMCPMC8975171. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vardhana S, Baldo L, Morice WG, et al. Understanding T cell responses to COVID-19 is essential for informing public health strategies. Sci Immunol. 2022;7(71):eabo1303, doi: 10.1126/sciimmunol.abo1303. PubMed PMID: 35324269; PubMed Central PMCID: PMCPMC10344642. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergamaschi L, Mescia F, Turner L, et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity. 2021;54(6):1257–1275.e8. doi: 10.1016/j.immuni.2021.05.010. PubMed PMID: 34051148; PubMed Central PMCID: PMCPMC8125900. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner KI, Mateyka LM, Jarosch S, et al. Recruitment of highly cytotoxic CD8(+) T cell receptors in mild SARS-CoV-2 infection. Cell Rep. 2022;38(2):110214, doi: 10.1016/j.celrep.2021.110214. PubMed PMID: 34968416; PubMed Central PMCID: PMCPMC8677487. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grau-Expósito J, Sánchez-Gaona N, Massana N, et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat Commun. 2021;12(1):3010, doi: 10.1038/s41467-021-23333-3. PubMed PMID: 34021148; PubMed Central PMCID: PMCPMC8140108. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. PubMed PMID: 32473127; PubMed Central PMCID: PMCPMC7237901. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L, Zhou J, Yang S, et al. The dichotomous and incomplete adaptive immunity in COVID-19 patients with different disease severity. Sign Transduction Targeted Ther. 2021;6(1):113, doi: 10.1038/s41392-021-00525-3. PubMed PMID: 33686064; PubMed Central PMCID: PMCPMC7938043. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spoerl S, Kremer AN, Aigner M, et al. Upregulation of CCR4 in activated CD8(+) T cells indicates enhanced lung homing in patients with severe acute SARS-CoV-2 infection. Eur J Immunol 2021;51(6):1436–1448. doi: 10.1002/eji.202049135. PubMed PMID: 33784417; PubMed Central PMCID: PMCPMC8250120. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kared H, Redd AD, Bloch EM, et al. SARS-CoV-2-specific CD8+ T cell responses in convalescent COVID-19 individuals. J Clin Invest. 2021;131(5). doi: 10.1172/jci145476. PubMed PMID: 33427749; PubMed Central PMCID: PMCPMC7919723. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kos I, Balensiefer B, Lesan V, et al. Increased B-cell activity with consumption of activated monocytes in severe COVID-19 patients. Eur J Immunol 2021;51(6):1449–1460. doi: 10.1002/eji.202049163. PubMed PMID: 33788264; PubMed Central PMCID: PMCPMC8250224. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Guan F, Candotti F, et al. The role of B cells in COVID-19 infection and vaccination. Front Immunol. 2022;13:988536, doi: 10.3389/fimmu.2022.988536. PubMed PMID: 36110861; PubMed Central PMCID: PMCPMC9468879. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu H, Guan F, Miller H, et al. The role of SARS-CoV-2 nucleocapsid protein in antiviral immunity and vaccine development. Emerg Microbes Infect. 2023;12(1):e2164219, doi: 10.1080/22221751.2022.2164219. PubMed PMID: 36583642; PubMed Central PMCID: PMCPMC9980416. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa PR, Correia CA, Marmorato MP, et al. Humoral and cellular immune responses to CoronaVac up to one year after vaccination. Front Immunol. 2022;13:1032411, doi: 10.3389/fimmu.2022.1032411. PubMed PMID: 36341425; PubMed Central PMCID: PMCPMC9634255. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sette A, Crotty S.. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev 2022;310(1):27–46. doi: 10.1111/imr.13089. PubMed PMID: 35733376; PubMed Central PMCID: PMCPMC9349657. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poon MML, Rybkina K, Kato Y, et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci Immunol. 2021;6(65):eabl9105, doi: 10.1126/sciimmunol.abl9105. PubMed PMID: 34618554; PubMed Central PMCID: PMCPMC8626868. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niessl J, Sekine T, Lange J, et al. Identification of resident memory CD8(+) T cells with functional specificity for SARS-CoV-2 in unexposed oropharyngeal lymphoid tissue. Sci Immunol. 2021;6(64):eabk0894, doi: 10.1126/sciimmunol.abk0894. PubMed PMID: 34519539; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol 2020;21(11):1336–1345. doi: 10.1038/s41590-020-0782-6. PubMed PMID: 32887977; PubMed Central PMCID: PMCPMC7611020. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603(7901):488–492. doi: 10.1038/s41586-022-04460-3. PubMed PMID: 35102311; PubMed Central PMCID: PMCPMC8930768. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamo S, Michler J, Zurbuchen Y, et al. Signature of long-lived memory CD8(+) T cells in acute SARS-CoV-2 infection. Nature. 2022;602(7895):148–155. doi: 10.1038/s41586-021-04280-x. PubMed PMID: 34875673; PubMed Central PMCID: PMCPMC8810382. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J, Alshukairi AN, Baharoon SA, et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017;2(14). doi: 10.1126/sciimmunol.aan5393. PubMed PMID: 28778905; PubMed Central PMCID: PMCPMC5576145. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maaske J, Sproule S, Falsey AR, et al. Robust humoral and cellular recall responses to AZD1222 attenuate breakthrough SARS-CoV-2 infection compared to unvaccinated. Front Immunol. 2022;13:1062067, doi: 10.3389/fimmu.2022.1062067. PubMed PMID: 36713413; PubMed Central PMCID: PMCPMC9881590. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. PubMed PMID: 32668444; eng. [DOI] [PubMed] [Google Scholar]
- 27.Peng K, Li X, Yang D, et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study. EClinicalMedicine. 2023;63:102154, doi: 10.1016/j.eclinm.2023.102154. PubMed PMID: 37637754; PubMed Central PMCID: PMCPMC10458663. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe N, Bohgaki M, Kasahara H.. SARS-CoV-2 mRNA vaccination-induced autoimmune polyarthritis-like rheumatoid arthritis. Mayo Clin Proc 2022;97(8):1574–1575. doi: 10.1016/j.mayocp.2022.06.001. PubMed PMID: 35933141; PubMed Central PMCID: PMCPMC9174152. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sher EK, Ćosović A, Džidić-Krivić A, et al. COVID-19 a triggering factor of autoimmune and multi-inflammatory diseases. Life Sci 2023;319:121531, doi: 10.1016/j.lfs.2023.121531. PubMed PMID: 36858313; PubMed Central PMCID: PMCPMC9969758. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammadi B, Dua K, Saghafi M, et al. COVID-19-induced autoimmune thyroiditis: exploring molecular mechanisms. J Med Virol 2023;95(8):e29001, doi: 10.1002/jmv.29001. PubMed PMID: 37515444; eng. [DOI] [PubMed] [Google Scholar]
- 31.Zheng M. Autoreactive T cells of ankylosing spondylitis elicited by COVID-19 infection: a snapshot of immunological host defense and autoimmune imprinting. Autoimmun Rev. 2023;22(9):103392, doi: 10.1016/j.autrev.2023.103392. PubMed PMID: 37455010; eng. [DOI] [PubMed] [Google Scholar]
- 32.Lichtensteiger C, Koblischke M, Berner F, et al. Autoreactive T cells targeting type II pneumocyte antigens in COVID-19 convalescent patients. J Autoimmun 2023;140:103118, doi: 10.1016/j.jaut.2023.103118. PubMed PMID: 37826919; eng. [DOI] [PubMed] [Google Scholar]
- 33.Schulien I, Kemming J, Oberhardt V, et al. Characterization of pre-existing and induced SARS-CoV-2-specific CD8(+) T cells. Nat Med 2021;27(1):78–85. doi: 10.1038/s41591-020-01143-2. PubMed PMID: 33184509; eng. [DOI] [PubMed] [Google Scholar]
- 34.Swadling L, Diniz MO, Schmidt NM, et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. 2022;601(7891):110–117. doi: 10.1038/s41586-021-04186-8. PubMed PMID: 34758478; PubMed Central PMCID: PMCPMC8732273. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kundu R, Narean JS, Wang L, et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat Commun. 2022;13(1):80, doi: 10.1038/s41467-021-27674-x. PubMed PMID: 35013199; PubMed Central PMCID: PMCPMC8748880. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diniz MO, Mitsi E, Swadling L, et al. Airway-resident T cells from unexposed individuals cross-recognize SARS-CoV-2. Nat Immunol 2022;23(9):1324–1329. doi: 10.1038/s41590-022-01292-1. PubMed PMID: 36038709; PubMed Central PMCID: PMCPMC9477726. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheon IS, Li C, Son YM, et al. Immune signatures underlying post-acute COVID-19 lung sequelae. Sci Immunol. 2021;6(65):eabk1741, doi: 10.1126/sciimmunol.abk1741. PubMed PMID: 34591653; PubMed Central PMCID: PMCPMC8763087. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt KG, Nganou-Makamdop K, Tenbusch M, et al. SARS-CoV-2-Seronegative subjects target CTL epitopes in the SARS-CoV-2 nucleoprotein cross-reactive to common cold coronaviruses. Front Immunol. 2021;12:627568, doi: 10.3389/fimmu.2021.627568. PubMed PMID: 33995351; PubMed Central PMCID: PMCPMC8113865. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lineburg KE, Grant EJ, Swaminathan S, et al. CD8(+) T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity. 2021;54(5):1055–1065.e5. doi: 10.1016/j.immuni.2021.04.006. PubMed PMID: 33945786; PubMed Central PMCID: PMCPMC8043652. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipsitch M, Grad YH, Sette A, et al. Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat Rev Immunol. 2020;20(11):709–713. doi: 10.1038/s41577-020-00460-4. PubMed PMID: 33024281; PubMed Central PMCID: PMCPMC7537578. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grifoni A, Voic H, Dhanda SK, et al. T cell responses induced by attenuated flavivirus vaccination are specific and show limited cross-reactivity with other flavivirus species. J Virol. 2020;94(10). doi: 10.1128/jvi.00089-20. PubMed PMID: 32132233; PubMed Central PMCID: PMCPMC7199411. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim JME, Tan AT, Le Bert N, et al. SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8 + and CD4+ T cells of broad specificity. J Exp Med 2022;219(10). doi: 10.1084/jem.20220780. PubMed PMID: 35972472; PubMed Central PMCID: PMCPMC9386509. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.COVID-19 vaccine tracker and landscape: World Health Organization,; [updated 30 March 2023] . Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 44.Fiolet T, Kherabi Y, MacDonald CJ, et al. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202–221. doi: 10.1016/j.cmi.2021.10.005. PubMed PMID: 34715347; PubMed Central PMCID: PMCPMC8548286. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heitmann JS, Bilich T, Tandler C, et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature. 2022;601(7894):617–622. doi: 10.1038/s41586-021-04232-5. PubMed PMID: 34814158; PubMed Central PMCID: PMCPMC8791831. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. PubMed PMID: 32998157; eng. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Wang P, Yuan L, et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci Bull. 2022;67(13):1372–1387. doi: 10.1016/j.scib.2022.05.018. PubMed PMID: 35637645; PubMed Central PMCID: PMCPMC9134758. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Mateus J, Coelho CH, et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell. 2022;185(14):2434–2451.e17. doi: 10.1016/j.cell.2022.05.022. PubMed PMID: 35764089; PubMed Central PMCID: PMCPMC9135677. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang CY, Hwang KP, Kuo HK, et al. A multitope SARS-CoV-2 vaccine provides long-lasting B cell and T cell immunity against Delta and Omicron variants. J Clin Invest. 2022;132(10). doi: 10.1172/jci157707. PubMed PMID: 35316221; PubMed Central PMCID: PMCPMC9106357. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–577. doi: 10.1038/s41586-021-03653-6. PubMed PMID: 34044428; eng. [DOI] [PubMed] [Google Scholar]
- 51.Zuo F, Abolhassani H, Du L, et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat Commun. 2022;13(1):2670, doi: 10.1038/s41467-022-30340-5. PubMed PMID: 35562366; PubMed Central PMCID: PMCPMC9106736. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akondy RS, Fitch M, Edupuganti S, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017;552(7685):362–367. doi: 10.1038/nature24633. PubMed PMID: 29236685; PubMed Central PMCID: PMCPMC6037316. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ssemaganda A, Nguyen HM, Nuhu F, et al. Expansion of cytotoxic tissue-resident CD8(+) T cells and CCR6(+)CD161(+) CD4(+) T cells in the nasal mucosa following mRNA COVID-19 vaccination. Nat Commun. 2023;14(1):3357, doi: 10.1038/s41467-023-38887-7. PubMed PMID: 35688805; PubMed Central PMCID: PMCPMC9186487. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Afkhami S, D'Agostino MR, Zhang A, et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. 2022;185(5):896–915.e19. doi: 10.1016/j.cell.2022.02.005. PubMed PMID: 35180381; PubMed Central PMCID: PMCPMC8825346. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sophonmanee R, Ongarj J, Seeyankem B, et al. T-Cell responses induced by an intradermal BNT162b2 mRNA vaccine booster following primary vaccination with inactivated SARS-CoV-2 vaccine. Vaccines (Basel). 2022;10(9). doi: 10.3390/vaccines10091494. PubMed PMID: 36146571; PubMed Central PMCID: PMCPMC9501140. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oberhardt V, Luxenburger H, Kemming J, et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature. 2021;597(7875):268–273. doi: 10.1038/s41586-021-03841-4. PubMed PMID: 34320609; PubMed Central PMCID: PMCPMC8426185. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet (London, England). 2020;395(10240):1845–1854. doi: 10.1016/s0140-6736(20)31208-3. PubMed PMID: 32450106; PubMed Central PMCID: PMCPMC7255193. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185(5):847–859.e11. doi: 10.1016/j.cell.2022.01.015. PubMed PMID: 35139340; PubMed Central PMCID: PMCPMC8784649. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarke A, Sidney J, Methot N, et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2(7):100355, doi: 10.1016/j.xcrm.2021.100355. PubMed PMID: 34230917; PubMed Central PMCID: PMCPMC8249675. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calcoen B, Callewaert N, Vandenbulcke A, et al. High incidence of SARS-CoV-2 variant of concern breakthrough infections despite residual humoral and cellular immunity induced by BNT162b2 vaccination in healthcare workers: a long-term follow-up study in Belgium. Viruses. 2022;14(6). doi: 10.3390/v14061257. PubMed PMID: 35746728; PubMed Central PMCID: PMCPMC9228150. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Redd AD, Nardin A, Kared H, et al. Minimal crossover between mutations associated with Omicron variant of SARS-CoV-2 and CD8(+) T-Cell epitopes identified in COVID-19 convalescent individuals. mBio. 2022;13(2):e0361721, doi: 10.1128/mbio.03617-21. PubMed PMID: 35229637; PubMed Central PMCID: PMCPMC8941890. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casado JL, Vizcarra P, Martín-Colmenarejo S, et al. Lower T cell response against SARS-CoV-2 variants of concern after mRNA vaccine and risk of breakthrough infections in people living with HIV. AIDS (London, England). 2023. doi: 10.1097/qad.0000000000003504. PubMed PMID: 36779501; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bobrovitz N, Ware H, Ma X, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23(5):556–567. doi: 10.1016/s1473-3099(22)00801-5. PubMed PMID: 36681084; PubMed Central PMCID: PMCPMC10014083. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed MIM, Diepers P, Janke C, et al. Enhanced spike-specific, but attenuated nucleocapsid-specific T cell responses upon SARS-CoV-2 breakthrough versus non-breakthrough infections. Front Immunol. 2022;13:1026473, doi: 10.3389/fimmu.2022.1026473. PubMed PMID: 36582222; PubMed Central PMCID: PMCPMC9792977. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ishii H, Nomura T, Yamamoto H, et al. Neutralizing-antibody-independent SARS-CoV-2 control correlated with intranasal-vaccine-induced CD8(+) T cell responses. Cell Reports Medicine. 2022;3(2):100520, doi: 10.1016/j.xcrm.2022.100520. PubMed PMID: 35233545; PubMed Central PMCID: PMCPMC8768424. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benet S, Blanch-Lombarte O, Ainsua-Enrich E, et al. Limited humoral and specific T-cell responses after SARS-CoV-2 vaccination in PWH with poor immune reconstitution. J Infect Dis 2022;226(11):1913–1923. doi: 10.1093/infdis/jiac406. PubMed PMID: 36200261; PubMed Central PMCID: PMCPMC9619620. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrington P, Doores KJ, Radia D, et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukaemia. Br J Haematol 2021;194(6):999–1006. doi: 10.1111/bjh.17568. PubMed PMID: 34085278; PubMed Central PMCID: PMCPMC8239833. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azeem MI, Nooka AK, Shanmugasundaram U, et al. Impaired SARS-CoV-2 variant neutralization and CD8+ T-cell responses following 3 doses of mRNA vaccines in myeloma: correlation with breakthrough infections. Blood Cancer Discovery. 2023;4(2):106–117. doi: 10.1158/2643-3230.BCD-22-0173. PubMed PMID: 36511813; PubMed Central PMCID: PMCPMC9975771. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao M, Liu Y, Guo M, et al. Regulatory CD4(+) and CD8(+) T cells are negatively correlated with CD4(+) /CD8(+) T cell ratios in patients acutely infected with SARS-CoV-2. J Leukoc Biol 2021;109(1):91–97. doi: 10.1002/JLB.5COVA0720-421RR. PubMed PMID: 32930458; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lakhani S, Flavell RA.. Caspases and T lymphocytes: a flip of the coin? Immunol Rev. 2003;193:22–30. doi: 10.1034/j.1600-065x.2003.00046.x. PubMed PMID: 12752667; eng. [DOI] [PubMed] [Google Scholar]
- 71.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038. PubMed PMID: 33010815; PubMed Central PMCID: PMCPMC7494270. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Proietto D, Dallan B, Gallerani E, et al. Ageing curtails the diversity and functionality of nascent CD8(+) T cell responses against SARS-CoV-2. Vaccines (Basel). 2023;11(1). doi: 10.3390/vaccines11010154. PubMed PMID: 36679999; PubMed Central PMCID: PMCPMC9867380. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Varchetta S, Mele D, Oliviero B, et al. Unique immunological profile in patients with COVID-19. Cell Mol Immunol 2021;18(3):604–612. doi: 10.1038/s41423-020-00557-9. PubMed PMID: 33060840; PubMed Central PMCID: PMCPMC7557230. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Demaria O, Cornen S, Daëron M, et al. Harnessing innate immunity in cancer therapy. Nature. 2019;574(7776):45–56. doi: 10.1038/s41586-019-1593-5. PubMed PMID: 31578484; eng. [DOI] [PubMed] [Google Scholar]
- 75.Qin L, Duan X, Dong JZ, et al. The unreversible reduced but persistent activated NK and CD8(+) T cells in severe/critical COVID-19 during omicron pandemic in China. Emerg Microbes Infect. 2023;12(1):2208679, doi: 10.1080/22221751.2023.2179357. PubMed PMID: 37102227; PubMed Central PMCID: PMCPMC10187090. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schultheiß C, Paschold L, Simnica D, et al. Next-generation sequencing of T and B cell receptor repertoires from COVID-19 patients showed signatures associated with severity of disease. Immunity. 2020;53(2):442–455.e4. doi: 10.1016/j.immuni.2020.06.024. PubMed PMID: 32668194; PubMed Central PMCID: PMCPMC7324317. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahern DJ, Ai Z, Ainsworth M, et al. A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell. 2022;185(5):916–938.e58. doi: 10.1016/j.cell.2022.01.012. PubMed PMID: 35216673; PubMed Central PMCID: PMCPMC8776501. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shoukat MS, Foers AD, Woodmansey S, et al. Use of machine learning to identify a T cell response to SARS-CoV-2. Cell Rep Med. 2021;2(2):100192, doi: 10.1016/j.xcrm.2021.100192. PubMed PMID: 33495756; PubMed Central PMCID: PMCPMC7816879. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang B, Upadhyay R, Hao Y, et al. Multimodal single-cell datasets characterize antigen-specific CD8(+) T cells across SARS-CoV-2 vaccination and infection. Nat Immunol 2012;13(10):1725–1734. doi: 10.1038/ni.2403. PubMed PMID: 37735591; PubMed Central PMCID: PMCPMC10522491. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schreibing F, Hannani MT, Kim H, et al. Dissecting CD8+ T cell pathology of severe SARS-CoV-2 infection by single-cell immunoprofiling. Front Immunol. 2022;13:1066176, doi: 10.3389/fimmu.2022.1066176. PubMed PMID: 36591270; PubMed Central PMCID: PMCPMC9800604. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]