Abstract
IMPORTANCE:
Delirium is a common postoperative complication for older patients in the ICU. Ketamine, used primarily as an analgesic, has been thought to prevent delirium.
OBJECTIVE:
Determine the prevalence and association of delirium with low-dose ketamine use in ICU patients after abdominal surgery.
DESIGN:
Single-center, retrospective, propensity-matched cohort study.
SETTING:
Eight hospital academic medical center.
PATIENTS:
Cohort comprising 1836 patients admitted to the ICU after abdominal surgery between June 23, 2018 and September 1, 2022.
MAIN OUTCOMES AND MEASURES:
Propensity score matching (PSM) with a 3:1 ratio between no-ketamine use and ketamine use was performed through a greedy algorithm (caliper of 0.005). Outcomes of interest included: delirium (assessed by Confusion Assessment Method—ICU), mean pain score (Numeric Pain Scale or Critical Care Pain Observation Tool score as available), mean opioid consumption (morphine milligram equivalents), length of stay (d), and mortality.
RESULTS:
Prevalence of delirium was 47.71% (95% CI, 45.41–50.03%) in the cohort. Of 1836 patients, 120 (6.54%) used low-dose ketamine infusion. After PSM, the prevalence of delirium was 56.02% (95% CI, 51.05–60.91%) in all abdominal surgery patients. The ketamine group had 41% less odds of delirium (odds ratio [OR] = 0.59; 95% CI, 0.37–0.94; p = 0.026) than patients with no-ketamine use. Patients with ketamine use had higher mean pain scores (3.57 ± 2.86 vs. 2.21 ± 2.09, p < 0.001). In the subgroup analysis, patients in the ketamine-use group 60 years old or younger had 64% less odds of delirium (OR = 0.36; 95% CI, 0.13–0.95; p = 0.039). The mean pain scores were higher in the ketamine group for patients 60 years old or older. There was no significant difference in mortality and opioid consumption.
CONCLUSIONS AND RELEVANCE:
Low-dose ketamine infusion was associated with lower prevalence of delirium in ICU patients following abdominal surgery. Prospective studies should further evaluate ketamine use and delirium.
Keywords: delirium, critical care, surgical procedures, ketamine, outcomes assessment, healthcare delivery
KEY POINTS
Question: Following abdominal surgery in ICU patients, what is the prevalence of delirium, and the association of low-dose ketamine use with delirium?
Findings: In this retrospective, propensity-matched cohort study, the prevalence of delirium was 47.71% in the cohort. After propensity score matching, the prevalence of delirium was 56.02% among ICU patients following abdominal surgery. We found that ketamine use was associated with 41% less odds of delirium in ICU patients than in the no-ketamine use group following abdominal surgery.
Meaning: The role of ketamine use in association with delirium needs further evaluation in abdominal surgery patients with prospective studies or randomized clinical trials.
Delirium is a common, serious, and highly burdensome neuropsychiatric condition characterized by reduced attention, awareness, and cognition changes (1). The reported incidence of delirium varies, ranging from 10% to 70% of all surgical patients older than 60 years (2). Among patients admitted to the ICU undergoing mechanical ventilation, an incidence rate of up to 80% has been reported (3). The recently estimated annual cumulative healthcare costs attributable to delirium per patient were $44,291 (95% CI, $34,554–$56,673), implying that the national burden of delirium on the healthcare system is estimated at $32.9 billion (95% CI, $25.7 billion–$42.2 billion) (4). Although causal relationships have not been established, delirium is associated with increased morbidity and mortality, prolonged hospital and ICU stay, and functional and cognitive decline with nursing home or long-term care facility placement (5).
Critically ill surgical patients are more likely to develop delirium during their ICU stay due to pain, sepsis, and polypharmacy (6). Strategies for preventing delirium aim at the reduction of contributing factors, treatment of comorbid disease, and pharmacologic management to decrease the duration of delirium, such as haloperidol and dexmedetomidine (7). However, no standardized treatment exists to prevent delirium in high-risk surgical patients.
Ketamine is an N-methyl-d-aspartate receptor antagonist and a dissociate anesthetic with a half-life of about 4 hours and has been posited as an analgo-sedative and neuroprotective agent (8, 9). Around 80% of ketamine is excreted in the urine as conjugates of hydroxylated ketamine metabolites with glucuronic acid (8). Ketamine’s strong analgesic effect at subanesthetic doses allows it to be used during postoperative care for patients receiving mechanical ventilation (10). In adult ICU patients, ketamine use for the maintenance of sedation has been shown to have favorable hemodynamic and respiratory effects (10). Ketamine has also been posited to inhibit HCN1 receptors, which mediate the hyperpolarization-activated cation current. Such inhibition is pertinent to delirium because HCN1 channels are essential for regulating states of consciousness and are upregulated by inflammation (6). In addition, ketamine has been shown to reduce pain and opioid consumption, which are well-known risk factors for the development of delirium (11). Current consensus guidelines from the American Academy of Pain Medicine support using ketamine for acute pain in certain populations, although specific recommendations are lacking (12).
There is conflicting evidence on the association of ketamine with delirium and postoperative neurocognitive disorders. The study by Perbet et al (13) single-center, double-masked randomized trial reported that the addition of low doses of ketamine reduces the occurrence rate and duration of delirium in medico-surgical ICU patients. However, a meta-analysis of six randomized trials stated an inconclusive effect of ketamine on postoperative delirium (14). Another study concluded that perioperative ketamine does not prevent postoperative delirium or postoperative neurocognitive disorders (15). Furthermore, a multicenter randomized clinical trial found that intraoperative ketamine does not reduce delirium and may cause more hallucinations and nightmares with increasing doses compared with placebo in adults older than 60 years undergoing major cardiac and noncardiac surgery under general anesthesia (16).
Furthermore, there is a lack of data on the association of ketamine with delirium in abdominal surgery patients. A randomized clinical trial in elective abdominal surgery patients found more delirium in the low-dose ketamine group (0.125 mg/kg/hr) than in the minimal-dose ketamine group (0.015 mg/kg/hr) or the placebo group (17). Therefore, the primary aim of this study was to determine the prevalence of delirium in ICU patients following abdominal surgery. The secondary aim of this study was to determine the association between low-dose ketamine infusion (0.1–0.35 mg/kg/hr) with delirium and other outcomes, including pain score, opioid consumption, postoperative length of stay, and mortality.
MATERIALS AND METHODS
Data Source and Cohort Selection
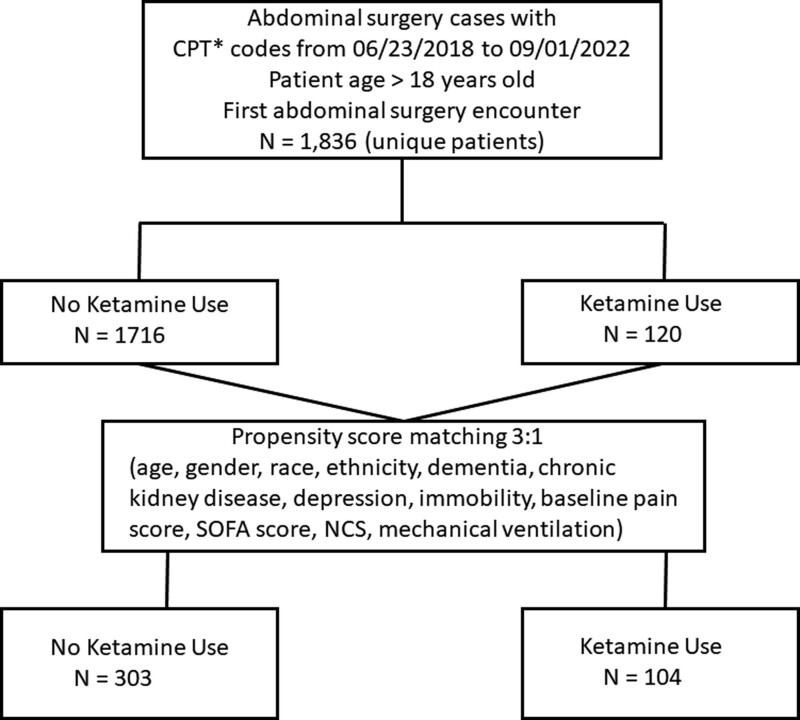
We conducted a single-center, retrospective cohort study using data from the electronic health record (EHR; Epic Clarity Database, Epic Systems Corporation, Verona, WI) at an academic medical center—the Houston Methodist Hospital System, comprising eight hospitals. Health records of abdominal surgery patients aged more than 18 years from June 23, 2018, to September 1, 2022 (Fig. 1), were extracted by current procedural terminology codes: 43622, 43631, 43632, 43633, 43840, definition 44005, 44125, 44130, 44143, 44145, 44160, 45113, 47361, 47701, 47760, 48150, 48153, 48155, 49000, 49002, and 48548. The Houston Methodist Research Institute Institutional Review Board (IRB) approved this study with an exemption from obtaining informed consent because this was a retrospective review (IRB approval ID number Pro00031398; approval date: July 2, 2021; study title: Clinical Outcomes in Surgery). Our article adheres to the ethical standards of the Helsinki Declaration of 1975 as most recently amended.
Figure 1.
Flow diagram of abdominal surgical cases with ketamine use in the ICU. CPT = current procedural terminology, NCS = NarxCare Score, SOFA = Sequential Organ Failure Assessment. *CPT to include surgeries: “43622,” “43631,” “43632,” “43633,” “43840,” “44005,” “44125,” “44130,” “44143,” “44145,” “44160,” “45113,” “47361,” “47701,” “47760,” “48150,” “48153,” “48155,” “49000,” “49002,” ‘4854.
Based on the inclusion criteria, 1836 unique patients were identified, as shown in the flowchart (Fig. 1). EHR data of abdominal surgery patients admitted to the ICU were extracted. We obtained data on demographics (age, gender, race, and ethnicity); baseline comorbidities (dementia, chronic kidney disease [CKD], depression, and immobility); ICU admission records (mechanical ventilation and the Confusion Assessment Method-ICU [CAM-ICU] tool score (18). The EHR data of Sequential Organ Failure Assessment (SOFA) score (19) was also calculated and recorded within 24-hour postoperative period, comprising six organ systems, including respiratory, cardiovascular, hepatic, coagulation, renal, and neurologic systems. The SOFA score was calculated as the sum of its six elements. The variable baseline pain score was calculated as the mean of the numeric pain scale (NPS) recorded before abdominal surgery. The baseline NarxCare Score (NCS) (20) data, which quantitatively assesses patient-specific prescription drug use (sedatives, stimulants, and opioids combined), available in the EHR, was also collected before ketamine administration. The patients were categorized as ketamine use or no-ketamine use groups.
At our institute, low-dose ketamine infusion for postoperative pain management is restricted to ICU patients only. Since 2018, critical care physicians of our institute have been using the low-dose ketamine infusion for acute pain management at their discretion, based on the evidence published in the literature (21). Subsequently, a standardized protocol of low-dose ketamine infusion (0.1–0.35 mg/kg/hr) was approved on June 1, 2019 (policy and procedure system_PCPS210 low-dose ketamine for pain management) and used as an adjunct for acute pain management. The protocol was developed based on the consensus guidelines published as the “Use of IV Ketamine Infusions for Acute Pain Management” by the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists (12). Under our institution’s policy, a physician (anesthesiologist, intensivist, or pain management) can order the low-dose ketamine infusion. The clinical protocol or pathway takes time to approve at our institute. Therefore, in this study, we included all 21 patients who received low-dose ketamine infusion before approval of the standardized ketamine infusion protocol of Houston Methodist Hospital.
Outcomes Measures
We obtained data on delirium using the CAM-ICU tool. In the no-ketamine use group, delirium was defined as any positive CAM-ICU, that is, one episode within 72 hours after admission to the ICU. In the ketamine use group, delirium was defined as any positive CAM-ICU from the day of ketamine administration to day 3 or discontinuation of ketamine infusion, whichever came first. At our institute, delirium screening is performed every 12 hours using a validated CAM-ICU tool (18) on all patients admitted to the ICU.
In the no-ketamine use group, mean pain score was calculated as the mean of the NPS of postoperative days 1–3. In the ketamine-use group, the mean pain score was calculated as the mean of the NPS from the day of ketamine administration to day 3 or discontinuation of ketamine infusion, whichever came first. The Critical Care Pain Observation Tool (CPOT) score (22) was used for pain assessment if the NPS was not documented in the EHR. At our institute, the CPOT is used as a behavioral assessment of pain scale for patients who cannot express or verbalize pain and is used for both intubated and nonintubated patients. The CPOT score was collected from 16 patients in the no-ketamine use group and in 1 patient in the ketamine use group.
We also obtained the data on opioid consumption using mean morphine milligram equivalents (MMEs), length of stay (d), and mortality.
Statistical Analysis
Means ± sd were presented for the continuous variables and frequencies and percentages for the categorical variables. We used the t-test or Mann-Whitney U test for the continuous variables and the Chi-square test or Fisher exact test for categorical variables as appropriate to compare patients experiencing the presence or absence of delirium. Propensity score matching (PSM) with a 3:1 ratio between no-ketamine and ketamine use was performed through a greedy algorithm based on a caliper of 0.005, which was obtained as having a maximum width of 0.2 sds of the logit of the estimated propensity score (23). A logistic regression model was fitted to ketamine use as the dependent variable, whereas the covariates used were age, gender, race, ethnicity, dementia, CKD, depression, immobility, SOFA score, NCS, mechanical ventilation, and baseline pain score. The standardized mean difference (SMD) was used to assess the balance between the two groups before and after PSM. An absolute SMD value of less than 0.1 was used as the cutoff for sufficient balance between the two groups. Conditional logistic regression for binary outcomes and linear regression with clustering for continuous outcomes were used to account for the clustering created by matching as appropriate. Odds ratios (ORs) for binary outcomes and coefficients for the continuous outcomes with their 95% CIs were reported as a measure of association between ketamine use and each outcome of interest. The five outcomes of interest were delirium, mortality, opioid (MME) use, mean pain score, length of stay, and mortality after surgery after matching. The subgroup analysis for patients 60 years old or older and 60 years younger was done using the same statistical analysis methods. A two-sided p value of 0.05 was considered statistically significant, and all the tests were performed using Stata V17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
RESULTS
Prevalence of Delirium
Of 1836 ICU patients, 876 (47.71%) had delirium after abdominal surgeries. The prevalence of delirium was 47.71% (95% CI, 45.41–50.03%). Table 1 presents the baseline characteristics of ICU patients by the presence or absence of delirium. The mean age of the patients with delirium was older (66.29 ± 14.35 yr) than those without delirium (63.68 ± 15.31 yr; p < 0.001). Similarly, the mean respective SOFA (2.17 ± 0.50) and NCS (137.66 ± 155.74) scores were higher in the patients experiencing delirium compared with the SOFA (1.95 ± 0.40) and NCS score (123.37 ± 151.88) of their counterpart (both p < 0.05). More men experienced delirium compared with women (52.51% vs. 46.67%, p = 0.012). Delirium was more prevalent in patients with preexisting dementia (9.82% vs. 4.58%), CKD (35.96% vs. 25.73%), immobility (11.19% vs. 6.15%), and mechanical ventilation (70.78% vs. 28.44%; all p < 0.001).
TABLE 1.
Baseline Characteristics by Presence or Absence of Delirium
| Delirium | p | |||
|---|---|---|---|---|
| Total, n = 1836 | Absent, n = 960 | Present, n = 876 | ||
| Age at surgery | 64.93 ± 14.91 | 63.68 ± 15.31 | 66.29 ± 14.35 | < 0.001 |
| Age groups | 0.004 | |||
| ≤ 60 | 589 (32.08) | 337 (35.10) | 252 (28.77) | |
| > 60 | 1247 (67.92) | 623 (64.90) | 624 (71.23) | |
| Gender | 0.012 | |||
| Female | 928 (50.54) | 512 (53.33) | 416 (47.49) | |
| Male | 908 (49.46) | 448 (46.67) | 460 (52.51) | |
| Race | 0.96 | |||
| Asian | 85 (4.73) | 46 (4.87) | 39 (4.57) | |
| Black | 354 (19.70) | 182 (19.28) | 172 (20.16) | |
| Other | 27 (1.50) | 14 (1.48) | 13 (1.52) | |
| White | 1,331 (74.07) | 702 (74.36) | 629 (73.74) | |
| Ethnicity | 0.49 | |||
| Hispanic or Latino | 316 (17.38) | 171 (17.96) | 145 (16.74) | |
| Not Hispanic or Latino | 1,502 (82.62) | 781 (82.04) | 721 (83.26) | |
| Dementia | < 0.001 | |||
| No | 1,706 (92.92) | 916 (95.42) | 790 (90.18) | |
| Yes | 130 (7.08) | 44 (4.58) | 86 (9.82) | |
| Chronic kidney disease | < 0.001 | |||
| No | 1,274 (69.39) | 713 (74.27) | 561 (64.04) | |
| Yes | 562 (30.61) | 247 (25.73) | 315 (35.96) | |
| Depression | 0.16 | |||
| No | 1,585 (86.33) | 839 (87.40) | 746 (85.16) | |
| Yes | 251 (13.67) | 121 (12.60) | 130 (14.84) | |
| Immobility | < 0.001 | |||
| No | 1,679 (91.45) | 901 (93.85) | 778 (88.81) | |
| Yes | 157 (8.55) | 59 (6.15) | 98 (11.19) | |
| Sequential Organ Failure Assessment score | 2.06 ± 0.46 | 1.95 ± 0.40 | 2.17 ± 0.50 | < 0.001 |
| NarxCare Score | 130.21 ± 153.86 | 123.37 ± 151.88 | 137.66 ± 155.74 | 0.048 |
| Baseline pain score | 3.16 ± 3.80 | 3.23 ± 3.77 | 3.08 ± 3.82 | 0.42 |
| Mechanical ventilation | < 0.001 | |||
| No | 943 (51.36) | 687 (71.56) | 256 (29.22) | |
| Yes | 893 (48.64) | 273 (28.44) | 620 (70.78) | |
Data were presented as mean ± sd for continuous variables and number (%) for categorical variables. Chi-square or Fisher exact test for categorical variables and t-test or Mann-Whitney U test for continuous variables were used to compare patients based on the presence or absence of delirium.
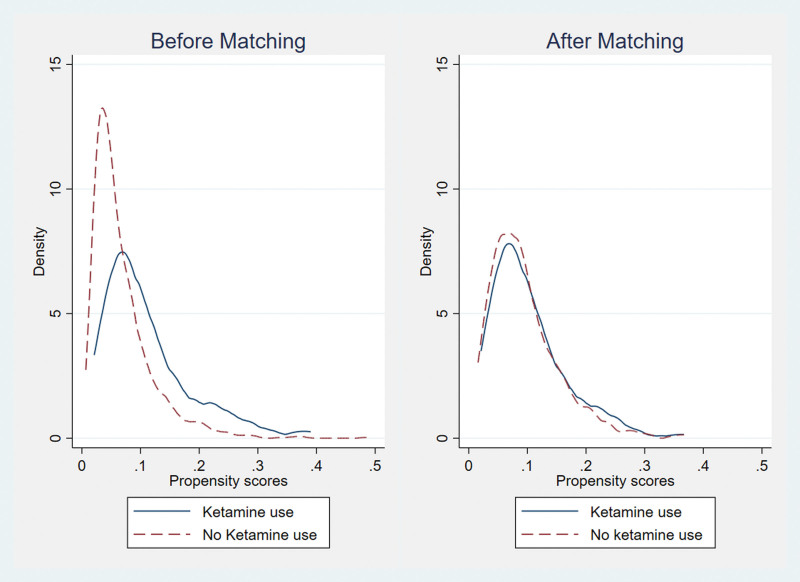
Table 2 depicts patient characteristics by ketamine before and after PSM. Before PSM, patients’ age at surgery, race, CKD, depression, SOFA score, NCS, and mechanical ventilation had an absolute SMD greater than 0.1. After the PSM, all characteristics were balanced between the two groups based on ketamine administration (all absolute SMD < 0.1). The propensity score distribution before and after PSM are presented in Figure 2. After PSM, the two distributions of the propensity score were the same.
TABLE 2.
Baseline Characteristics by Ketamine Use Before and After Propensity Score Matching
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Ketamine Use | SMD | Ketamine Use | SMD | |||
| No | Yes | No | Yes | |||
| n = 1716 | n = 120 | n = 303 | n = 104 | |||
| Age at surgery | 65.25 ± 14.79 | 60.30 ± 15.92 | 0.333 | 62.08 ± 15.25 | 61.31 ± 15.66 | 0.050 |
| Gender | –0.012 | 0.016 | ||||
| Female | 868 (50.58) | 60 (50.00) | 152 (50.17) | 52 (50.00) | ||
| Male | 848 (49.42) | 60 (50.00) | 151 (49.83) | 52 (50.00) | ||
| Race | –0.191 | 0.029 | ||||
| Asian | 84 (5.00) | 1 (0.86) | 4 (1.32) | 0 (0.00) | ||
| Black | 334 (19.87) | 20 (17.24) | 42 (13.86) | 18 (17.31) | ||
| Other | 26 (1.55) | 1 (0.86) | 8 (2.64) | 1 (0.96) | ||
| White | 1237 (73.59) | 94 (81.03) | 249 (82.18) | 85 (81.73) | ||
| Ethnicity | 0.074 | –0.003 | ||||
| Hispanic or Latino | 292 (17.20) | 24 (20.00) | 52 (17.16) | 19 (18.27) | ||
| Not Hispanic or Latino | 1406 (82.80) | 96 (80.00) | 251 (82.84) | 85 (81.73) | ||
| Dementia | –0.087 | –0.066 | ||||
| No | 1597 (93.07) | 109 (90.83) | 282 (93.07) | 95 (91.35) | ||
| Yes | 119 (6.93) | 11 (9.17) | 21 (6.93) | 9 (8.65) | ||
| Chronic kidney disease | –0.199 | 0.050 | ||||
| No | 1201 (69.99) | 73 (60.83) | 179 (59.08) | 64 (61.54) | ||
| Yes | 515 (30.01) | 47 (39.17) | 124 (40.92) | 40 (38.46) | ||
| Depression | –0.275 | –0.009 | ||||
| No | 1492 (86.95) | 93 (77.50) | 240 (79.21) | 82 (78.85) | ||
| Yes | 224 (13.05) | 27 (22.50) | 63 (20.79) | 22 (21.15) | ||
| Immobility | 0.008 | 0.042 | ||||
| No | 1569 (91.43) | 110 (91.67) | 273 (90.10) | 95 (91.35) | ||
| Yes | 147 (8.57) | 10 (8.33) | 30 (9.90) | 9 (8.65) | ||
| Sequential Organ Failure Assessment score | 2.05 ± 0.46 | 2.18 ± 0.52 | –0.275 | 2.14 ± 0.51 | 2.16 ± 0.52 | –0.027 |
| NarxCare Score | 125.54 ± 150.83 | 197.06 ± 179.98 | –0.468 | 179.96 ± 174.90 | 196.10 ± 177.58 | –0.092 |
| Baseline pain score | 3.15 ± 3.79 | 3.29 ± 3.90 | –0.036 | 3.26 ± 3.86 | 3.38 ± 3.92 | –0.029 |
| Mechanical ventilation | –0.424 | 0.043 | ||||
| No | 681 (73.30) | 262 (28.89) | 93 (30.69) | 34 (32.69) | ||
| Yes | 248 (26.70) | 645 (71.11) | 210 (69.31) | 70 (67.31) | ||
PSM = propensity score matching, SMD = standardized mean difference.
Data were presented as mean ± sd for continuous variables and number (%) for categorical variables. The propensity score matching was performed based on the logistic regression model. The model included ketamine as a binary dependent variable and all independent variables, including all variables listed in the table. Bold values indicate patient’s characteristics by ketamine before and after PSM. Before PSM, patients’ age at surgery, race, chronic kidney disease, depression, Sequential Organ Failure Assessment score, NarxCare Score, and mechanical ventilation had an absolute SMD greater than 0.1, which is represented by the boldface values.
Figure 2.
Distribution of propensity score before and after propensity score matching.
Association of Low-Dose Ketamine Use With Delirium, Pain, and Opioid Consumption After PSM
Table 3 shows the discrete outcomes in abdominal surgery ICU patients with low-dose ketamine and no-ketamine use groups. The median ketamine infusion start time was 38 hours (IQR: 10–129) after being admitted to the ICU. The results show that after the PSM, the prevalence of delirium in abdominal surgery patients was 56.02% (95% CI, 51.05–60.91%) in all abdominal surgery patients. Low-dose ketamine was associated with 41% less odds of delirium (OR = 0.59; 95% CI, 0.37–0.94; p = 0.026) than the no-ketamine use group. However, we failed to identify a significant association between the use of ketamine and mortality. Table 3 also shows the association between continuous outcomes in patients with ketamine use and no-ketamine use. Patients with ketamine use had 1.36 higher mean pain scores than no-ketamine use (coefficient: 1.36; 95% CI, 0.66–2.05; p < 0.001. No statistically significant difference was found for opioid consumption and postoperative length of stay. The results for subgroup analysis by age are presented in eTable 1 (http://links.lww.com/CCX/B287). In the subgroup analysis, patients 60 years old or younger in the ketamine use group were associated with 64% less odds of delirium (OR = 0.36; 95% CI, 0.13–0.95; p = 0.039) compared with no-ketamine use group. These associations were not seen in patients with age greater than 60. The mean pain scores were 1.83 higher in the ketamine group (coefficient: 1.84; 95% CI, 0.87–2.81; p < 0.001) for patients 60 years older but not for patients 60 years old or younger. Both groups failed to show any significant association with mortality, opioid consumption, and postoperative length of stay.
TABLE 3.
Association of Ketamine Use With the Outcomes After Propensity Score Matching
| Descriptive | Model (Ketamine Use Vs. No-Ketamine Use) | |||
|---|---|---|---|---|
| Outcomes | No-Ketamine use (n = 303) | Ketamine use (n = 104) | ORa (95% CI) | p |
| Delirium | 0.59 (0.37 to 0.94) | 0.026 | ||
| Absent | 124 (40.92) | 55 (52.88) | ||
| Present | 179 (59.08) | 49 (47.12) | ||
| Mortality | 1.26 (0.62 to 2.56) | 0.515 | ||
| No | 272 (89.77) | 91 (87.50) | ||
| Yes | 31 (10.23) | 13 (12.50) | ||
| Coefficientb (95% CI) | ||||
| Mean pain scales | n = 302 | n = 82 | ||
| 2.21 ± 2.09 | 3.57 ± 2.86 | 1.36 (0.66 to 2.05) | < 0.001 | |
| Mean opioid use (morphine milligram equivalents) | n = 250 | n = 57 | ||
| 12.04 ± 6.97 | 11.29 ± 7.27 | –0.20 (–2.25 to 1.86) | 0.850 | |
| Postoperative length of stay (d) | 7.53 ± 10.23 | 9.95 ± 14.65 | 2.60 (–0.15 to 5.34) | 0.064 |
OR = odds ratio.
Conditional logistic regression was used after propensity score matching.
Linear regression with clustering was used after propensity score matching.
Data presented as mean ± sd for continuous variables and number (%) for categorical variables below descriptive. Wilcoxon matched-pairs signed-rank test was used to compare pain score and opioid use before and after ketamine use. Bold values indicate statistically significant p values.
DISCUSSION
The evidence on the association of ketamine with delirium in abdominal surgery patients is lacking. In our single-center retrospective cohort study, the overall prevalence of delirium was 47.71% in ICU patients following abdominal surgery, similar to previous studies (24, 25). In the present study, delirium was prevalent in older male patients with multiple comorbidities, high SOFA scores, high NCS, and mechanical ventilation use. PSM created a balanced patient cohort based on ketamine administration, as many predisposing factors of delirium (26) were used as covariates for PSM, including age, dementia, depression, CKD, and mechanical ventilation use. After the PSM, the prevalence of delirium was 56.02 % (95% CI, 51.05–60.91%) among ICU patients following abdominal surgery. Low-dose ketamine use was associated with a 41% lower prevalence of delirium and 1.36 higher mean pain score. However, we failed to identify a significant association between low-dose ketamine use and opioid consumption, length of stay, and mortality.
No consensus exists regarding the efficacy of ketamine in the prevention of delirium. Still, one study reported an unclear effect (14) and another reported no superiority of ketamine to placebo for preventing postoperative brain dysfunction and delirium (27). A recent randomized clinical trial analyzed the impact of ketamine infusion in mechanically ventilated ICU patients and found that low doses of ketamine reduced the incidence and duration of delirium but did not decrease the consumption of opiates (13). Similarly, in the present study, low-dose ketamine use was associated with a 41% lower prevalence of delirium. Although a clear, dose-dependent relationship for psychomimetic effects has not yet been established for subanesthetic doses of ketamine, nearly all therapeutic and most adverse pharmacologic effects are dose-related. Therefore, additional prospective studies and randomized clinical trials comparing different dose ranges are required.
Previous observational studies (28, 29) and a recent meta-analysis (30) have reported that ketamine used as an adjunctive analgesic was associated with reduced opioid requirements, goal sedation scores, pain scale, CPOT scores, and minimal to no adverse events. In contrast, our present study failed to identify an association between ketamine use and opioid consumption but a higher pain score. However, higher pain scores in the low-dose ketamine use group may not necessarily be due to ketamine and can be attributed to other factors, such as abdominal surgery and the use of mechanical ventilation, which are well-known pertinent risk factors for postoperative pain.
In a randomized clinical trial by Avidian et al (16), the administration of a subanaesthetic dose of ketamine did not reduce the incidence of postoperative delirium, affect postoperative pain, or decrease postoperative opioid administration in patients 60 years old or older undergoing major surgery. However, a subgroup analysis in our study found that low-dose ketamine use was associated with 64% fewer odds of delirium and 1.83 higher mean pain scores in patients 60 years old or younger, but these associations were not seen in patients 60 years older.
Our study has several limitations worth noting. First, a retrospective design with the potential of missing or incomplete data can create a selection bias, which we tried to minimize by adjusting for confounders in our statistical analyses by PSM. Second, data on other potential risk factors, specifically medications such as anticholinergics and antihistamines, is missing. However, our clinical practice avoids anticholinergics and antihistamines for ICU patients unless absolutely indicated. Although we ensured most predisposing factors associated with delirium were included in PSM, there is a possibility that our matching criteria may still not be comprehensive enough to control for other possible confounders in our specific cohort, which may have impacted the results. Finally, owing to the variables used for the PSM, the sample size of the ketamine use group was small, which could limit generalizability to other settings, such as medical and cardiovascular ICUs. Thus, our results regarding the association of ketamine with delirium should be applied cautiously.
CONCLUSIONS
This study found an association between the use of low-dose ketamine infusion and a lower prevalence of delirium in ICU patients following abdominal surgery. In our clinical practice, we have observed patients experience significant pain improvement and reduced opioid consumption with low-dose ketamine. However, the present study did not translate our clinical experience for these specific outcomes, possibly due to multiple potential confounders and selection bias. Further prospective studies are warranted to gauge the association of ketamine with delirium after abdominal surgery in broader settings.
ACKNOWLEDGMENTS
The authors thank Dorothy Lewis, PhD, and Jacob M. Kolman, MA, ISMPP CMPP (Academic Affairs, Houston Methodist Academic Institute), and Richard Sucgang, PhD (Center for Health Data Science and Analytics, Houston Methodist Research Institute), for critical and linguistic review of a draft of this article.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflict of interest.
REFERENCES
- 1.Wilson JE, Mart MF, Cunningham C, et al. : Delirium. Nat Rev Dis Primers 2020; 6:90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasilevskis EE, Han JH, Hughes CG, et al. : Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol 2012; 26:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr J, Fraser GL, Puntillo K, et al. ; American College of Critical Care Medicine: Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41:263–306 [DOI] [PubMed] [Google Scholar]
- 4.Gou RY, Hshieh TT, Marcantonio ER, et al. : One-year medicare costs associated with delirium in older patients undergoing major elective surgery. JAMA Surg 2021; 156:462–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kukreja D, Günther U, Popp J: Delirium in the elderly: Current problems with increasing geriatric age. Indian J Med Res 2015; 142:655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Shu S, Bayliss DA: HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci 2009; 29:600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JM, Cho YJ, Ahn EJ, et al. : Pharmacological strategies to prevent postoperative delirium: A systematic review and network meta-analysis. Anesth Pain Med (Seoul) 2021; 16:28–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanos P, Moaddel R, Morris PJ, et al. : Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev 2018; 70:621–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheldon AL, Robinson MB: The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int 2007; 51:333–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amer M, Maghrabi K, Bawazeer M, et al. : Adjunctive ketamine for sedation in critically ill mechanically ventilated patients: An active-controlled, pilot, feasibility clinical trial. J Intensive Care 2021; 9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C, Chen C, Tsai P, et al. : Protective effects of dexmedetomidine-ketamine combination against ventilator-induced lung injury in endotoxemia rats. J Surg Res 2011; 167:273. [DOI] [PubMed] [Google Scholar]
- 12.Schwenk ES, Viscusi ER, Buvanendran A, et al. : Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med 2018; 43:456–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perbet S, Verdonk F, Godet T, et al. : Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: A randomised double-blind control trial. Anaesth Crit Care Pain Med 2018; 37:589–595 [DOI] [PubMed] [Google Scholar]
- 14.Hovaguimian F, Tschopp C, Beck-Schimmer B, et al. : Intraoperative ketamine administration to prevent delirium or postoperative cognitive dysfunction: A systematic review and meta-analysis. Acta Anaesthesiol Scand 2018; 62:1182–1193 [DOI] [PubMed] [Google Scholar]
- 15.Fellous S, Dubost B, Cambriel A, et al. : Perioperative ketamine administration to prevent delirium and neurocognitive disorders after surgery: A systematic review and meta-analysis. Int J Surg 2023; 109:3555–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avidan MS, Maybrier HR, Abdallah AB, et al. ; PODCAST Research Group: Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: An international, multicentre, double-blind, randomised clinical trial. Lancet (London, England) 2017; 390:267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bornemann-Cimenti H, Wejbora M, Michaeli K, et al. : The effects of minimal-dose versus low-dose S-ketamine on opioid consumption, hyperalgesia, and postoperative delirium: A triple-blinded, randomized, active- and placebo-controlled clinical trial. Minerva Anestesiol 2016; 82:1069–1076 [PubMed] [Google Scholar]
- 18.Inouye SK, van Dyck CH, Alessi CA, et al. : Clarifying confusion: The confusion assessment method—a new method for detection of delirium. Ann Intern Med 1990; 113:941–948 [DOI] [PubMed] [Google Scholar]
- 19.Ferreira FL, Bota DP, Bross A, et al. : Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286:1754–1758 [DOI] [PubMed] [Google Scholar]
- 20.Huizenga JE, Breneman BC, Patel VR, et al. : NARxCHECK® score as a predictor of unintentional overdose death. Appriss, Louisville, KY: 2016; [Google Scholar]
- 21.Patanwala AE, Martin JR, Erstad BL: Ketamine for analgosedation in the intensive care unit: A systematic review. J Intensive Care Med 2017; 32:387–395 [DOI] [PubMed] [Google Scholar]
- 22.Rafiei M, Ghadami A, Irajpour A, et al. : Validation of critical care pain observation tool in patients hospitalized in surgical wards. Iran J Nurs Midwifery Res 2016; 21:464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC: Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10:150–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohsen S, Moss SJ, Lucini F, et al. : Impact of family presence on delirium in critically ill patients: A retrospective cohort study. Crit Care Med 2022; 50:1628–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Pinto M, Jelacic J, Edwards WT: Very-low-dose ketamine for the management of pain and sedation in the ICU. J Opioid Manag 2008; 4:54–56 [DOI] [PubMed] [Google Scholar]
- 26.Wittmann M, Kirfel A, Jossen D, et al. : The impact of perioperative and predisposing risk factors on the development of postoperative delirium and a possible gender difference. Geriatrics 2022; 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollinger A, Rüst CA, Riegger H, et al. : Ketamine vs haloperidol for prevention of cognitive dysfunction and postoperative delirium: A phase IV multicentre randomised placebo-controlled double-blind clinical trial. J Clin Anesth 2021; 68:110099. [DOI] [PubMed] [Google Scholar]
- 28.Buchheit JL, Yeh DD, Eikermann M, et al. : Impact of low-dose ketamine on the usage of continuous opioid infusion for the treatment of pain in adult mechanically ventilated patients in surgical intensive care units. J Intensive Care Med 2019; 34:646–651 [DOI] [PubMed] [Google Scholar]
- 29.Groth CM, Droege CA, Connor KA, et al. : Multicenter retrospective review of ketamine use in the ICU. Crit Care Explor 2022; 4:e0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheeler KE, Grilli R, Centofanti JE, et al. : Adjuvant analgesic use in the critically ill: A systematic review and meta-analysis. Crit Care Explor 2020; 2:e0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.