Abstract
The interaction between a surface protein antigen (PAc) of Streptococcus mutans and human salivary agglutinin was analyzed with a surface plasmon resonance biosensor. The major component sugars of the salivary agglutinin were galactose, fucose, mannose, N-acetylglucosamine, N-acetylgalactosamine, and N-acetylneuraminic acid. Binding of salivary agglutinin to PAc was calcium dependent and heat labile and required a pH greater than 5. Binding was significantly inhibited by N-acetylneuraminic acid and α2,6-linked sialic acid-specific lectin derived from Sambucus sieboldiana in a dose-dependent manner. Pretreatment of the salivary agglutinin with sialidase reduced the binding activity of the agglutinin to the PAc molecule. The agglutinin was dissociated into high-molecular-mass glycoprotein and secretory immunoglobulin A (sIgA) components by electrophoretic fractionation in the presence of 1% sodium dodecyl sulfate and 1% 2-mercaptoethanol. Neither of the components separated by electrophoretic fractionation, high-molecular-mass glycoprotein or sIgA, bound to the PAc molecule. Furthermore, the high-molecular-mass glycoprotein strongly inhibited the binding of the native salivary complex to PAc. These results suggest that the complex formed by the high-molecular-mass salivary glycoprotein and sIgA is essential for the binding reaction and that the sialic acid residues of the complex play an important role in the interaction between the agglutinin and PAc of S. mutans.
Streptococcus mutans has been implicated as a prime causative organism of dental caries, one of the most common diseases in humans. Colonization of the surface of a tooth by S. mutans is thought to be initiated by attachment of the organism to acquired pellicles on tooth surfaces (21). A 190-kDa surface protein antigen, which has been variously designated as antigen I/II, B, IF, P1, SR, MSL-1, and PAc (37), is known to be one of the factors which mediates the binding of the organism to the tooth surface (3, 21, 23).
Various salivary components, such as secretory immunoglobulin A (sIgA) (36), β2-microglobulin (12), histidine-rich polypeptides (32), a 60-kDa glycoprotein (1), lysozyme (39), lactoferrin (43), and high-molecular-mass glycoproteins (4, 7, 13, 20), have been reported to bind to S. mutans and/or to induce agglutination of the organism. Much attention has also been focused on the interaction between PAc and salivary components (5, 8, 21, 27–29). Russell and Mansson-Rahemtulla (38) reported that antigen I/II binds to several salivary components, including basic proline-rich proteins with molecular weights of 28,000 and 38,000, lysozyme, and α-amylase separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Much remains to be learned about the interaction between native salivary agglutinin and PAc of S. mutans.
In this study, using a surface plasmon resonance biosensor, we isolated and characterized a complex of high-molecular-mass salivary glycoprotein and sIgA which binds to PAc. The data presented in this report suggest that the complex of salivary glycoprotein and sIgA is an important component in the interaction between saliva and the PAc protein in a native condition and that sialic acid residues in the complex play a significant role in this interaction.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are listed in Table 1. Strains of S. mutans and Streptococcus gordonii were grown at 37°C for 18 h in brain heart infusion broth (Difco Laboratories, Detroit, Mich.). For transformants of S. mutans, erythromycin at a final concentration of 10 μg/ml was added.
TABLE 1.
Bacterial strains
| Strain | Description | Reference or source |
|---|---|---|
| S. mutans | ||
| MT8148 | Serotype c PAc+ | 21 |
| PAcEm-2 | Serotype c Emr PAc−; transformant of MT8148 with pPC12 Emr | 21 |
| PAcEm-3 | Serotype c Emr PAc−; transformant of MT8148 with pPC12 Emr | 21 |
| TK18 | Serotype c Emr PAc+; transformant of GS-5 with pSM1 | 21 |
| Xc | Serotype c PAc+ | 47 |
| Xc31 | Serotype c antigen− Emr PAc+; transformant of Xc carrying P15A replicon and Em inserted into gluA (a glucose-1-phosphate uridylyltransferase gene) | 51 |
| S. gordonii ATCC 10558 | Type strain | ATCC |
Saliva.
Unstimulated whole saliva was collected from a single donor (male, 39 years of age) in an ice-chilled plastic tube and clarified by centrifugation at 12,000 × g for 15 min.
Isolation of salivary agglutinin and sIgA.
Salivary components which bind to whole cells of S. mutans were isolated by a modification of the method of Rundegren and Arnold (36). Clarified saliva diluted 1/2 with aggregation buffer (1.5 mM KH2PO4, 6.5 mM Na2HPO4, 2.7 mM KCl, 137 mM NaCl [pH 7.2]) was incubated with an equal volume of a cell suspension of S. mutans MT8148 (5 × 109 cells/ml) at 37°C for 30 min. Cells were collected by centrifugation at 5,000 × g for 15 min and washed once with aggregation buffer, and adsorbed salivary components were eluted with the same buffer supplemented with 1 mM EDTA. The eluate was subsequently filtered (0.2-μm pore size), dialyzed against an aggregation buffer containing 0.02% NaN3, and subjected to gel filtration chromatography on Superdex 200 HR (Pharmacia, Uppsala, Sweden) equilibrated with aggregation buffer. The eluate at the void volume was collected and used as salivary agglutinin. In addition, salivary lysozyme was also eluted by gel filtration of S. mutans-binding salivary components on Superdex 200 HR. Fractions containing lysozyme were identified by Western blotting with rabbit anti-human lysozyme antibody, collected, and used as salivary lysozyme. An electrophoretic fractionation system (Prepcell NA-1800; Nippon Eido, Tokyo, Japan) was used to dissociate the components of salivary agglutinin. The sample was treated with 1% SDS and 1% 2-mercaptoethanol at 100°C for 5 min and subjected to Prepcell, which was composed of 8 ml of 3% stacking gel containing 0.1% SDS and 42 ml of 5% resolving gel containing 0.1% SDS. Prepcell was run at 50 mA for 18 h. Fractions (0.5 ml each) were subjected to SDS-PAGE followed by silver staining. Fractions containing salivary components were pooled, and SDS in the fractions was removed as a precipitate by adding potassium phosphate buffer (final concentration, 50 mM; pH 7.0). Pooled fractions were concentrated by 60% saturated ammonium sulfate precipitation and dialyzed against potassium phosphate-buffered saline (PBS; pH 7.0). Salivary sIgA was purified from clarified saliva in a native condition by using jacalin-agarose (Vector Laboratories, Burlingame, Calif.) according to the procedure of Hortin and Trimpe (17).
Chemical analysis of purified salivary components.
Component sugars in the purified salivary components were analyzed by high-performance liquid chromatography (HPLC) with fluorescence labeling (45). The salivary samples (1 μg) were hydrolyzed in 4 M trifluoroacetic acid at 100°C for 3 h and dried at 50°C. Free amino groups were acetylated by adding 50 μl of a mixture of methanol-pyridine-water (6:3:2) and 2 μl of acetic anhydride. The solution was incubated at room temperature for 30 min. After the solution was dried by evaporation at 50°C, a coupling reagent (10 μl) was added (0.67 g of 2-aminopyridine per ml in acetic acid) and the mixture was heated at 90°C for 20 min. After the excess reagents were removed by evaporation under a stream of nitrogen gas at 60°C for 20 min, a reducing reagent (10 μl) was added (60 mg of borane-dimethylamine complex per ml in acetic acid) and the mixture was heated at 90°C for 35 min and dried under a stream of nitrogen gas at 50°C for 10 min. The sample was dissolved in 1 ml of distilled water and analyzed by HPLC with a PALPAK Type A column (4.6 by 150 mm; Takara Shuzo Co., Ohtsu, Japan) at a flow rate of 0.3 ml per min at 65°C.
Sialic acids, N-acetylneuraminic acid, and N-glycolylneuraminic acid were analyzed by HPLC according to the method of Hara et al. (16). A 1-μg sample was hydrolyzed at 80°C for 1 h in 200 μl of 50 mM HCl. After the solution was cooled, 200 μl of DMB solution (7 mM 1,2-diamino-4,5-methylenedioxybenzene, 0.75 M 2-mercaptoethanol, and 18 mM sodium hydrosulfite) was added. The mixture was heated at 60°C for 2.5 h in the dark to develop fluorescence. The sample was analyzed by HPLC with a Nakanopac ODS-A column (6 by 150 mm; Ajinoki Co., Handa, Japan) at a flow rate of 1 ml per min at 40°C.
Protein content was determined according to the method of Lowry et al. (26), with bovine serum albumin as a standard.
rPAc.
Recombinant PAc (rPAc) was purified from the culture supernatants of transformant S. mutans TK18 by ammonium sulfate precipitation, chromatography on DEAE-cellulose, and subsequent gel filtration on Sepharose CL-6B (Pharmacia) (21).
Binding of salivary agglutinin to rPAc.
The binding of salivary agglutinin to rPAc was determined with a BIAcore 2000 (Pharmacia), which permits real-time interaction analysis of two interacting macromolecules (19). rPAc was immobilized on the carboxymethylated dextran-coated gold surface of a CM5 sensor chip by the primary amino groups according to the method of Johnsson et al. (18). Thirty-five microliters of rPAc (300 μg/ml) in 10 mM sodium acetate buffer (pH 4.5) was passed over the activated surface, and approximately 6.3 ng of rPAc per mm2 was bound to the surface. The flow of PBS (pH 7.0) was maintained at 5 μl per min throughout the immobilizing process. Salivary agglutinin in the running buffer was exposed to the rPAc-immobilized surface at a flow rate of 10 μl/min. To examine the effect of pH on the binding of salivary components to rPAc, 10 mM potassium phosphate buffer (pH 4 to 7) containing 0.15 M NaCl and 1 mM CaCl2 and 10 mM Tris-HCl buffer (pH 7 to 9) containing 0.15 M NaCl and 1 mM CaCl2 were used as running buffers. To examine the divalent cation specificity, CaCl2, MgCl2, or MnCl2 was added to PBS (pH 7.0) at a final concentration of 0 to 2.5 mM. The dissociation phase was followed by the injection of the same buffer at 10 μl per min. All binding experiments were performed at 25°C. The surface plasmon resonance signal in each binding cycle was expressed in resonance units (RU). A resonance of 1,000 RU corresponds to a shift of 0.1° in the resonance angle, which means a change in surface protein concentration of approximately 1 ng/mm2 (44).
Inhibition assay.
The effects of sugars and lectins on the binding of the salivary component to rPAc were examined. Either fucose, galactose, mannose, N-acetylgalactosamine, N-acetylglucosamine, or N-acetylneuraminic acid was added to the salivary agglutinin suspension (25 μg/ml) in PBS (pH 7.0) for the BIAcore assay, at a final concentration of 0.1 M. Varying amounts of Maackia amurensis agglutinin (MAM; Honen Corp., Tokyo, Japan), which recognizes α2,3-linked sialic acid residues (49), or Sambucus sieboldiana agglutinin (SSA; Honen Corp.), which recognizes α2,6-linked sialic acid residues (40), were added to the salivary agglutinin suspension for the BIAcore assay. The percent inhibition in the BIAcore assay was calculated as follows: percent inhibition = 100 × [(a − b)/a], where a is the mean RU without inhibitor and b is the mean RU with inhibitor.
Heat and sialidase treatments.
Salivary agglutinin was heated at 50 to 100°C for 15 min and subjected to the BIAcore assay. For sialidase treatment, the component was dissolved in 200 μl of 50 mM sodium acetate buffer (pH 4.5 for sialidase from Clostridium perfringens and pH 5.5 for sialidases from Arthrobacter ureafaciens and Vibrio cholerae). For sialidase from V. cholerae, 10 mM CaCl2 and 0.1 M NaCl were also added. Each mixture was incubated overnight at 37°C and dialyzed against PBS at 4°C.
Aggregation assay.
Whole streptococcal cells were suspended in aggregation buffer at an optical density at 550 nm (OD550) of approximately 1.5. Either clarified whole saliva (100 μl) or 10 μl of salivary agglutinin (0.5 mg/ml) was mixed with 1 ml of the cell suspension, and the total volume of the reaction mixture was adjusted to 1.5 ml with aggregation buffer. CaCl2 was also added to the mixture of salivary agglutinin at a final concentration of 1 mM. Bacterial aggregation was determined by monitoring the change of OD550 at 37°C for 60 min with a visible-UV recording spectrophotometer equipped with a CPS-240A cell positioner (UV-2200; Shimadzu Co., Kyoto, Japan).
PAGE, Western blotting, and lectin blotting.
Native PAGE and SDS-PAGE were performed in 3 to 15% gradient polyacrylamide slab gels according to the methods of Davis (9) and Laemmli (22), respectively. After electrophoresis, the gels were stained with silver. Electrophoresis calibration kits (Pharmacia) were used for molecular mass markers.
For Western blotting and lectin blotting, salivary samples were subjected to native PAGE or SDS-PAGE and transferred electrophoretically to nitrocellulose membranes according to the method of Burnette (6). After blocking with 1% bovine serum albumin in Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl [pH 7.5]) plus 1% Triton X-100 (TBS-Triton), the membranes were treated with rabbit antibody against human IgA(α) (Zymed Laboratories, South San Francisco, Calif.). After washing with TBS-Triton, the antibodies bound to proteins immobilized on the membrane were detected with horseradish peroxidase-conjugated goat anti-rabbit IgG (Zymed Laboratories). The membrane was also treated with a 4-μg/ml solution of biotinylated MAM or SSA (Honen Corp.) in TBS-Triton, and the lectins bound to glycoproteins immobilized on the membrane were detected with horseradish peroxidase-conjugated avidin D (Vector Laboratories).
Statistical analysis.
Differences between the control and the test sample in the binding of salivary components to rPAc were determined by Student’s t test.
RESULTS
Analysis of salivary agglutinin.
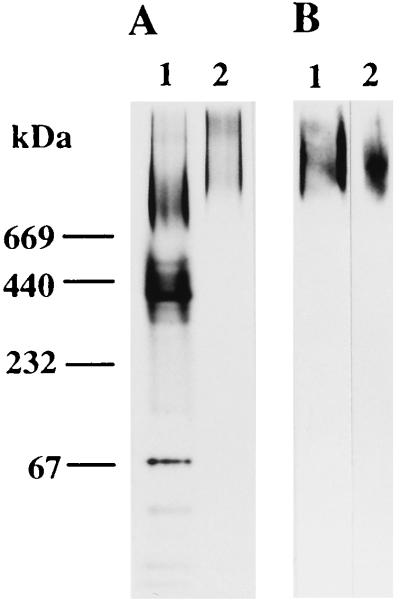
Salivary agglutinin, which bound to whole cells of S. mutans, was purified by gel filtration on Superdex 200 HR and analyzed by native PAGE and SDS-PAGE. Native PAGE analysis of salivary agglutinin bound to whole cells of S. mutans showed diffuse high-molecular-mass bands of more than 669 kDa (Fig. 1A, lane 2). The high-molecular-mass bands reacted with anti-human IgA(α) antibody in the Western blots (Fig. 1B, lane 2). SDS-PAGE analysis of salivary agglutinin showed diffuse 145- to 630-kDa bands (Fig. 2A, lane 2). Reducing treatment of the diffuse 145- to 630-kDa component with 2-mercaptoethanol resulted in its separation into diffuse 145- to 420-kDa bands, three bands corresponding to components of sIgA (25, 59, and 88 kDa), and a faint band of 80 kDa (Fig. 2A, lane 5). sIgA purified from clarified saliva by using jacalin-agarose was also subjected to SDS-PAGE. It showed three bands of 25, 59, and 88 kDa after reducing treatment (Fig. 2A, lane 6). Antibody to the heavy chain of human IgA (α chain) reacted with the nonreduced 145- to 630-kDa bands and reduced the 59-kDa band in Western blots (Fig. 2B, lanes 1 and 2). Salivary glycoprotein-sIgA complex was separated by SDS-PAGE in the reduced state and transferred to a nitrocellulose membrane. The pattern of sialylation of the salivary proteins was examined by using two types of lectin that recognize terminal sialic acid residues. High-molecular-mass salivary glycoprotein and two components of sIgA (59 and 88 kDa) were recognized by SSA but not by MAM (Fig. 2C, lanes 1 and 3). Salivary sIgA purified in a native condition by using jacalin-agarose was also recognized only by SSA (Fig. 2C, lanes 2 and 4).
FIG. 1.
Native PAGE (A) and Western blot (B) analyses of human salivary agglutinin. (A) Salivary samples were subjected to native PAGE (3 to 15% polyacrylamide), and the gel was stained with silver. The molecular mass markers used were bovine serum albumin (67 kDa), catalase (232 kDa), ferritin (440 kDa), and thyroglobulin (669 kDa). (B) Salivary components on the gel were electrophoretically transferred to a nitrocellulose membrane, and the membrane was reacted with rabbit antibody against human IgA(α). Lanes: 1, whole saliva (2 μg); 2, salivary agglutinin (1 μg).
FIG. 2.
SDS-PAGE (A), Western blotting (B), and lectin blotting (C) analyses of human salivary agglutinin. (A) Salivary samples were suspended in SDS-PAGE nonreducing (1% SDS) or reducing (1% SDS, 1% 2-mercaptoethanol) buffer and heated at 100°C for 3 min. Samples were then subjected to SDS-PAGE (3 to 15% polyacrylamide), and the gels were stained with silver. The molecular mass markers used were α-lactalbumin (14.4 kDa), carbonic anhydrase (30 kDa), ovalbumin (43 kDa), bovine serum albumin (67 kDa), β-galactosidase (116 kDa), and myosin (212 kDa). Lanes: 1, nonreduced whole saliva (2 μg); 2, nonreduced salivary agglutinin (1 μg); 3, nonreduced sIgA (0.5 μg); 4, reduced whole saliva (2 μg); 5, reduced salivary agglutinin (1 μg); 6, reduced sIgA (0.5 μg). (B) Salivary components on the gel were electrophoretically transferred to a nitrocellulose membrane, and the membrane was reacted with rabbit antibody against human IgA(α). Lanes: 1, nonreduced salivary agglutinin (1 μg); 2, reduced salivary agglutinin (1 μg). (C) Salivary components on the gel were electrophoretically transferred to nitrocellulose membranes, and the membranes were reacted with biotinylated SSA (lanes 1 and 2) or biotinylated MAM (lanes 3 and 4). Lanes: 1 and 3, reduced salivary agglutinin (50 μg); 2 and 4, reduced sIgA (5 μg).
Aggregation of streptococcal cells.
Typical strains of S. mutans serotype c (strains MT8148 and Xc), PAc-defective transformants of strain MT8148 (strains PAcEm-2 and PAcEm-3), a serotype antigen-defective transformant of strain Xc (strain Xc31), and S. gordonii ATCC 10558 were tested by spectrophotometric assay for the ability to aggregate in the presence of whole saliva or salivary agglutinin. Whole saliva induced strong aggregation of PAc-producing strains of S. mutans and S. gordonii ATCC 10558, while it could not induce aggregation of PAc-defective strains of S. mutans (Table 2). Salivary agglutinin induced strong aggregation of PAc-producing strains of S. mutans but did not induce aggregation of PAc-defective strains of S. mutans and S. gordonii ATCC 10558. Both whole saliva and salivary agglutinin induced aggregation of the serotype c antigen-defective transformant of strain Xc.
TABLE 2.
Aggregation of streptococcal cells induced by whole saliva and salivary agglutinina
| Strain | Aggregationb with:
|
||
|---|---|---|---|
| No additives | Whole saliva | Salivary agglutinin | |
| S. mutans | |||
| MT8148 | <0.01 | 0.43 ± 0.03 | 0.40 ± 0.02 |
| PAcEm-2 | <0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 |
| PAcEm-3 | <0.01 | 0.02 ± 0 | 0.02 ± 0 |
| Xc | <0.01 | 0.44 ± 0.05 | 0.45 ± 0.04 |
| Xc31 | <0.01 | 0.36 ± 0.02 | 0.37 ± 0.03 |
| S. gordonii ATCC 10558 | <0.01 | 0.47 ± 0.04 | 0.06 ± 0.01 |
Streptococcal cells grown in brain heart infusion broth were harvested and suspended in aggregation buffer. The suspensions were adjusted to an OD550 of approximately 1.5 with aggregation buffer. The cell suspensions (1 ml) were mixed with 100 μl of whole saliva or 10 μl of salivary agglutinin (0.5 mg/ml), and the total volume of the reaction mixture was adjusted to 1.5 ml.
Expressed as the reduction of OD550 for saliva-treated and control suspensions after 1 h. The values are the means ± standard deviations of triplicate assays.
Binding of salivary agglutinin to rPAc.
The interaction between salivary agglutinin and the rPAc molecule was analyzed by the BIAcore assay. The addition of CaCl2 to the running buffer enhanced the binding of salivary agglutinin to rPAc (Table 3). The maximum increase of RU was obtained when 1 mM CaCl2 was added to PBS. The other divalent cations, magnesium and manganese, were not effective. The binding capacity of salivary agglutinin to rPAc was stable up to 60°C, but it was lost when salivary agglutinin was heated at 80°C for 15 min (Fig. 3A). The optimal pH for binding of salivary agglutinin was 7.0, and binding did not occur below pH 5 (Fig. 3B). Based on these data, PBS (pH 7.0) supplemented with 1 mM CaCl2 was used as the running buffer for the BIAcore assay in subsequent experiments.
TABLE 3.
Effects of cations on the binding of salivary agglutinin to rPAca
| Cation | Binding of salivary agglutinin to rPAc at indicated concn (mM)b
|
|||
|---|---|---|---|---|
| 0 | 0.1 | 1.0 | 2.5 | |
| Calcium | 7 ± 1 | 242 ± 8 | 794 ± 17 | 726 ± 28 |
| Magnesium | 5 ± 1 | 6 ± 1 | 7 ± 0 | 10 ± 1 |
| Manganese | 6 ± 1 | 5 ± 1 | 5 ± 1 | 7 ± 1 |
Salivary agglutinin (25 μg/ml) in PBS (pH 7.0) containing various amounts of CaCl2, MgCl2, or MnCl2 was allowed to react with rPAc immobilized on a sensor chip.
Binding is expressed as RU determined by BIAcore assay. The values are the means ± standard deviations of triplicate assays.
FIG. 3.
Heat stability of salivary agglutinin (A) and the effect of pH on the binding of the agglutinin to rPAc (B). (A) After salivary agglutinin (25 μg/ml) was treated at 25 to 100°C for 15 min, the samples were subjected to BIAcore analysis. (B) Reactions were carried out with salivary agglutinin (25 μg/ml) in 10 mM potassium phosphate buffer (pH 4 to 7) containing 0.15 M NaCl and 1 mM CaCl2 (○) and 10 mM Tris-HCl buffer (pH 7 to 9) containing 0.15 M NaCl and 1 mM CaCl2 (•). The binding of salivary agglutinin to rPAc is expressed as RU determined by BIAcore assay. Values are given as the means ± standard deviations of triplicate assays.
Chemical composition of salivary components.
As mentioned above, the salivary agglutinin eluted from saliva-treated whole cells of S. mutans is composed mainly of high-molecular-mass glycoprotein and sIgA. We tried to dissociate these components by using electrophoretic fractionation in the presence of 1% SDS and 1% 2-mercaptoethanol. Fractions containing high-molecular-mass (145- to 420-kDa) glycoprotein and fractions containing sIgA components (25, 59, and 88 kDa) were pooled separately and used as the preparation of high-molecular-mass glycoprotein and that of sIgA components, respectively. The composition of the salivary agglutinin preparation was 74.8% protein, 4.0% fucose, 4.5% galactose, 0.7% mannose, 4.2% N-acetylgalactosamine, 5.5% N-acetylglucosamine, and 5.3% N-acetylneuraminic acid. The high-molecular-mass glycoprotein separated by electrophoretic fractionation was 73.0% protein, 4.4% fucose, 4.9% galactose, 0.8% mannose, 4.6% N-acetylgalactosamine, 5.8% N-acetylglucosamine, and 5.8% N-acetylneuraminic acid. The preparation of sIgA components separated by electrophoretic fractionation was 90.2% protein, 1.1% fucose, 2.0% galactose, 0.5% mannose, 0.5% N-acetylgalactosamine, 3.5% N-acetylglucosamine, and 1.5% N-acetylneuraminic acid.
Inhibition of binding of salivary agglutinin to rPAc.
The inhibitory effects of various sugars on the binding of salivary agglutinin to the rPAc molecule were examined. N-Acetylneuraminic acid, which is one of the component sugars of salivary agglutinin, caused the highest inhibition (98%), whereas fucose, galactose, and mannose only caused weak inhibition (Table 4). Other aminosugars, such as N-acetylgalactosamine and N-acetylglucosamine, had negligible inhibitory effect.
TABLE 4.
Effect of various sugars on the binding of salivary agglutinin to rPAca
| Sugar | Binding (RU)b | % Inhibitionc |
|---|---|---|
| Control | 775 ± 27 | |
| Fucose | 720 ± 25 | 7.1 |
| Galactose | 619 ± 20d | 20.1 |
| Mannose | 664 ± 22d | 14.3 |
| N-Acetylgalactosamine | 752 ± 28 | 3.0 |
| N-Acetylglucosamine | 781 ± 31 | 0 |
| N-Acetylneuraminic acid | 15 ± 1e | 98.1 |
Salivary agglutinin (25 μg/ml) in PBS (pH 7.0) containing a monosaccharide (0.1 M) and 1 mM CaCl2 was allowed to react with rPAc immobilized on a sensor chip.
Binding is expressed as RU determined by BIAcore assay. The values are the means ± standard deviations of triplicate assays.
Percent inhibition in the BIAcore assay was calculated as follows: percent inhibition = 100 × [(a − b)/a], where a is the mean RU without inhibitor (control) and b is the mean RU with inhibitor.
P < 0.01 compared with control.
P < 0.001 compared with control.
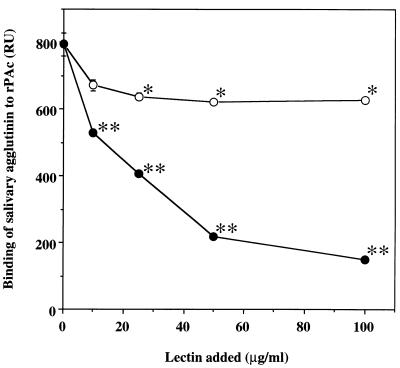
The effects on binding of two types of lectin, MAM and SSA, which recognize α2,3-linked and α2,6-linked sialic acid residues, respectively, were also examined. Binding of salivary agglutinin to rPAc was strongly inhibited in a dose-dependent manner by the addition of SSA, but the degree of inhibition by MAM was very low (Fig. 4). Treatment of salivary agglutinin with sialidases from A. ureafaciens and V. cholerae resulted in approximately 50% inhibition of binding to rPAc (Table 5). On the other hand, the treatment of salivary agglutinin with sialidase from C. perfringens showed a small inhibitory effect.
FIG. 4.
Dose-dependent inhibition of the binding of salivary agglutinin to rPAc by the sialic acid-specific lectins MAM (○) and SSA (•). Salivary agglutinin (25 μg/ml) was allowed to react with rPAc immobilized on a sensor chip in the presence of various amounts of lectin (0 to 100 μg/ml). The binding is expressed as RU determined by BIAcore assay. Values are given as the means ± standard deviations of triplicate assays. Single asterisk, P < 0.01; double asterisk, P < 0.001 (compared with control [no addition of lectin]).
TABLE 5.
Effect of sialidase treatment on the binding of salivary agglutinin to rPAca
| Origin of sialidase | Binding (RU)b | % Inhibitionc |
|---|---|---|
| Control | 810 ± 28 | |
| A. ureafaciens | 421 ± 19d | 48 |
| C. perfringens | 647 ± 7e | 20 |
| V. cholerae | 378 ± 20d | 53 |
Salivary agglutinin (25 μg/ml) was treated with each sialidase (115 mU/ml) and then allowed to react with rPAc immobilized on a sensor chip.
Binding is expressed as RU determined by BIAcore assay. The values are the means ± standard deviations of triplicate assays.
The percent inhibition was calculated as follows: percent inhibition = 100 × [(a − b)/a], where a is the mean RU induced by native salivary agglutinin (control) and b is the mean RU induced by sialidase-treated salivary agglutinin.
P < 0.001 compared with control.
P < 0.01 compared with control.
Binding of salivary components to rPAc.
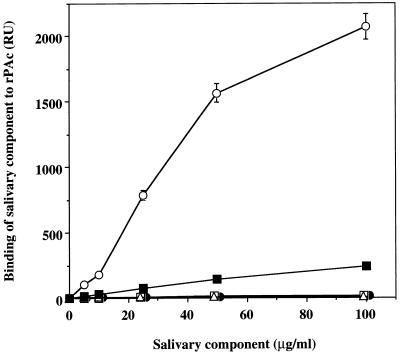
The binding to rPAc of high-molecular-mass glycoprotein and sIgA components separated from salivary agglutinin by electrophoretic fractionation, of sIgA purified by using jacalin-agarose, and of salivary lysozyme was examined by BIAcore assay. High-molecular-mass glycoprotein and sIgA components separated by electrophoretic fractionation did not bind to rPAc, but native sIgA purified by using jacalin-agarose bound weakly to rPAc (Fig. 5). In addition, salivary lysozyme separated by gel filtration in a native condition showed almost no binding activity to the rPAc protein in the BIAcore assay. The effects of salivary components separated by electrophoretic fractionation on the binding of salivary agglutinin to rPAc were also investigated. As shown in Fig. 6, the high-molecular-mass glycoprotein separated by electrophoretic fractionation had considerably greater inhibitory effect than the sIgA components.
FIG. 5.
Binding of salivary components to rPAc. The binding to rPAc of high-molecular-mass glycoprotein and sIgA components separated from salivary agglutinin by electrophoretic fractionation, sIgA purified by using jacalin-agarose, and salivary lysozyme was determined by BIAcore assay. Values are given as the means ± standard deviations of RU in triplicate assays. Symbols: ○, salivary agglutinin; •, high-molecular-mass glycoprotein separated by electrophoretic fractionation; □, sIgA components separated by electrophoretic fractionation; ▪, sIgA purified by using jacalin-agarose; ▵, salivary lysozyme.
FIG. 6.
Effects of salivary components on the binding of salivary agglutinin to rPAc. Salivary agglutinin (25 μg/ml) was allowed to react with rPAc immobilized on a sensor chip in the absence (control) or presence of various amounts of high-molecular-mass salivary glycoprotein separated by electrophoretic fractionation (•) or sIgA components separated by electrophoretic fractionation (□) (0 to 400 μg/ml). The binding is expressed as RU determined by BIAcore assay. Values are given as the means ± standard deviations of triplicate assays. Single asterisk, P < 0.01; double asterisk, P < 0.001 (compared with the control).
DISCUSSION
Several salivary components are known to bind to and/or agglutinate S. mutans. Among these components, a high-molecular-mass agglutinin is thought to bind to the PAc of S. mutans (5, 8, 15, 27, 28). On SDS-PAGE, salivary agglutinin that binds to S. mutans appeared as diffuse bands with molecular masses of about 400 kDa, suggesting that it may be a complex of several types of glycoprotein with different molecular masses. The variation in mass may depend on the length or number of carbohydrate chains covalently associated with each polypeptide. This study revealed that native PAc-binding salivary agglutinin is a complex of a high-molecular-mass glycoprotein, sIgA, and a minor component. We have recently shown that all of the whole-saliva preparations from 10 healthy subjects between 27 and 42 years old contained the agglutinin complex, which is bound to whole cells of S. mutans (31), suggesting that the agglutinin may be widely distributed in the human population.
N-Acetylneuraminic acid showed significant inhibitory effect on the binding of salivary agglutinin to the rPAc molecule. Lectin blotting revealed that both high-molecular-mass salivary glycoprotein and sIgA contain α2,6-linked sialic acid residues. In addition, lectin SSA, which recognizes α2,6-linked sialic acid residues, significantly inhibited the binding of salivary agglutinin to the rPAc molecule. These results suggest that the α2,6-linked sialic acid residues of salivary agglutinin are mainly responsible for its binding to the PAc molecule. Surface protein SSP-5 from S. gordonii M5 has the lectin-like property of binding to the α2,3-linked sialic acid residues of salivary agglutinin in the presence of calcium ions (11). In this study, whole saliva induced aggregation of S. gordonii ATCC 10558, but salivary agglutinin that binds to S. mutans did not induce aggregation of that strain (ATCC 10558). The sialic acid residues of salivary agglutinin that are recognized by the cell surface proteins of oral streptococci may differ with different species.
The binding of salivary agglutinin to rPAc was calcium dependent. Calcium ions are thought to be required as a bridge between negatively charged groups on the bacterial surface and similarly charged groups on the agglutinin (33), although some lectins require calcium ions as integral active-site constituents (10) or for specific conformational changes that are recognized by other molecules (14). Further studies are required to clarify the mechanism of calcium involvement in the interaction between salivary agglutinin and PAc.
In this study, nonreduced high-molecular-mass salivary agglutinin reacted with antibody against human IgA, suggesting that the agglutinin was a complex of glycoprotein and sIgA. Oligosaccharides of sIgA are known to be receptors for the lectin-mediated adhesion of oral bacteria, such as Actinomyces naeslundi and S. gordonii (35). To clarify the roles of salivary glycoprotein and sIgA, we tried to dissociate salivary agglutinin by using solutions such as 6 M guanidine-HCl, 8 M urea, and 1% SDS. All of these treatments failed to separate the two components. Salivary glycoprotein and sIgA were separated by the treatment of salivary agglutinin with 1% SDS and 1% 2-mercaptoethanol, suggesting that these components are linked by disulfide bonds. Once separated by electrophoretic fractionation, both of these components no longer bound to rPAc. Moreover, the addition of high-molecular-mass glycoprotein separated by electrophoretic fractionation significantly decreased the RU value in the binding of salivary agglutinin to rPAc in a dose-dependent manner in the BIAcore assay. These results suggest that high-molecular-mass salivary glycoprotein plays an important role in the interaction between the native salivary complex and the rPAc molecule.
Recently, Senpuku et al. (39) reported that lysozyme binds strongly to the rPAc molecule in the BIAcore assay. They observed an increase of approximately 40 RU when 20 μl of lysozyme (1.05 × 10−5 M) was injected onto the surface of a sensor chip. Although almost the same amount of lysozyme, purified in a native condition by gel filtration on Superdex 200 HR, was injected onto the surface of a sensor chip in this study, only a very small increase in RU (approximately 5 to 10 RU) was seen in the BIAcore assay. The increase in RU may be induced by a change in pH or salt concentration or by the presence of surfactant in the reaction mixture. The difference in RU seen in these two studies seems to be caused by such differences.
Human salivary mucins have been shown to interact with many oral bacteria and to protect against oral diseases (41, 46). Human submandibular and sublingual saliva contains both high- (more than 1,000 kDa) and low-molecular-mass (200 to 300 kDa) mucins (MG1 and MG2, respectively) (41). Highly glycosylated mucins can form homotypic and heterotypic complexes because of their negatively charged sialic acid and sulfate residues, as well as their hydrophobic domains (30, 50). The salivary agglutinin isolated in this study is similar to salivary mucins in molecular size and several other characteristics. Salivary agglutinin, however, contains fewer carbohydrates than salivary mucins, and the sialylation pattern involves α2,6 linkage, while salivary mucins have both α2,3 and α2,6 sialylation (41). Salivary agglutinin and mucins may be secreted by the same cells in salivary glands, but they may be hydrolyzed by different kinds of bacterial protease and glycosidase. The salivary agglutinin isolated in this study may be an assembly of cleaved mucins.
Human salivary mucins are known to make complexes with other salivary proteins, such as sIgA and lysozyme (24). Complexes of mucins with such protective proteins are believed to help concentrate these components at various tissue-environment interfaces. On the other hand, Brack and Reynolds (4) reported that rat salivary mucin complex loses agglutination activity with S. mutans after dissociation treatment with 6 M urea. Biesbrock et al. (2) also showed that mucin no longer binds to Pseudomonas aeruginosa and Staphylococcus aureus after sIgA is removed from the mucin-sIgA complex under dissociating conditions. Such dissociation treatments of mucins or complexes of mucin and other salivary proteins may destroy the interaction capability of mucins with bacteria. In this study, the individual components (high-molecular-mass glycoprotein and sIgA) of the salivary agglutinin complex did not interact as effectively with rPAc as did the intact complex itself. Furthermore, the interaction of the agglutinin complex with rPAc was heat labile. The binding of the agglutinin complex to rPAc was, however, inhibited by 98% in the presence of the monosaccharide N-acetylneuraminic acid, and the binding was significantly inhibited by treatment of the agglutinin complex with either sialidases or α2,6-linked sialic acid-specific lectin. Treatment of N-acetylneuraminic acid with heat (at 100°C for 5 min in the presence of 1% SDS and 1% 2-mercaptoethanol or at 80°C for 15 min) had no effect on the ability of the monosaccharide to inhibit the binding of the agglutinin complex to rPAc. Degradation of the agglutinin complex with endogenous salivary proteases (41) and/or conformational changes and denaturation of the polypeptide chain that could occur during the dissociation or heat treatment might hide or damage the N-acetylneuraminic acid residue-containing PAc-binding epitope on the agglutinin macromolecule. The possibility also exists that the naked nonglycosylated region as well as the glycosylated region may play a role in the interaction of the agglutinin macromolecule with PAc of S. mutans.
The serotype c-specific polysaccharide antigen of S. mutans is composed of a backbone structure of 1,2- and 1,3-linked rhamnosyl polymer with side chains consisting of a terminal α-linked glucose unit (25). We recently cloned four genes (rmlA, rmlB, rmlC, and rmlD) involved in dTDP–l-rhamnose synthesis and a gene (gluA) encoding glucose-1-phosphate uridylyltransferase from S. mutans serotype c and showed that these genes are involved in the biosynthesis of the serotype c antigen (47, 48, 51). We have insertionally inactivated the rml genes and the gluA gene of S. mutans Xc. All of the rml-inactivated mutant strains, but not the gluA mutant strain, were observed by the naked eye to clump in the absence of human whole saliva. Therefore, we examined whether human whole saliva and salivary agglutinin induce aggregation of the serotype c antigen-deficient transformant Xc31, in which the gluA gene was insertionally inactivated. Whole saliva and salivary agglutinin induced strong aggregation of the transformant, suggesting that the serotype c antigen does not interact with salivary agglutinin.
Rosan et al. (34) compared the saliva-mediated S. mutans-aggregating and adhesion-promoting activities in a group of caries-susceptible individuals and a group of caries-resistant individuals. They demonstrated that there is a significant increase in S. mutans-aggregating activity in saliva of the caries-resistant group versus that of the caries-susceptible group and a significant decrease in S. mutans adhesion-promoting activity in saliva of the caries-resistant group. Furthermore, Slomiany et al. (42) reported that the bacterial aggregating epitope of salivary mucins is expressed to a greater extent in caries-resistant individuals than in caries-susceptible individuals. These findings suggest that salivary components may play an important role in the resistance to dental caries. However, further work is needed to determine the role of the salivary agglutinin isolated in this study in oral health.
In conclusion, this study suggests that the high-molecular-mass salivary glycoprotein-sIgA complex binds to PAc of S. mutans via α2,6-linked sialic acid residues and that the complexing of glycoprotein and sIgA in a native condition is essential for the accomplishment of this function.
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-Aid for Developmental Scientific Research (A)07557134 (T.K.) and (B)09470474 (Y.Y.) from the Ministry of Education, Science, Sport and Culture of Japan and by the Uehara Memorial Foundation (T.K.).
REFERENCES
- 1.Babu J P, Beachey E H, Hasty D L, Simpson W A. Isolation and characterization of a 60-kilodalton salivary glycoprotein with agglutinating activity against strains of Streptococcus mutans. Infect Immun. 1986;51:405–413. doi: 10.1128/iai.51.2.405-413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biesbrock A R, Reddy M S, Levine M J. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect Immun. 1991;59:3492–3497. doi: 10.1128/iai.59.10.3492-3497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen W H, Schilling K, Giertsen E, Pearson S, Lee S F, Bleiweis A, Beeman D. Role of a cell surface-associated protein in adherence and dental caries. Infect Immun. 1991;59:4606–4609. doi: 10.1128/iai.59.12.4606-4609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brack C M, Reynolds E C. Characterization of a rat salivary sialoglycoprotein complex which agglutinates Streptococcus mutans. Infect Immun. 1987;55:1264–1273. doi: 10.1128/iai.55.5.1264-1273.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady L J, Piacentini D A, Crowley P J, Oyston P C F, Bleiweis A S. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect Immun. 1992;60:1008–1017. doi: 10.1128/iai.60.3.1008-1017.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnette W N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 7.Carlén A, Olsson J. Monoclonal antibodies against a high-molecular-weight agglutinin block adherence to experimental pellicles on hydroxyapatite and aggregation of Streptococcus mutans. J Dent Res. 1995;74:1040–1047. doi: 10.1177/00220345950740040301. [DOI] [PubMed] [Google Scholar]
- 8.Crowley P J, Brady L J, Piacentini D A, Bleiweis A S. Identification of a salivary agglutinin-binding domain within cell surface adhesin P1 of Streptococcus mutans. Infect Immun. 1993;61:1547–1552. doi: 10.1128/iai.61.4.1547-1552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis B J. Disc electrophoresis-II. Method and application to human serum proteins. Ann N Y Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 10.Derewenda Z, Yariv J, Helliwell J R, Kalb (Gilboa) A J, Dodson E J, Papiz M Z, Wan T, Campbell J. The structure of the saccharide-binding site of concanavalin A. EMBO J. 1989;8:2189–2193. doi: 10.1002/j.1460-2075.1989.tb08341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan Y, Fisher E, Malamud D, Golub E, Demuth D R. Calcium-binding properties of SSP-5, the Streptococcus gordonii M5 receptor for salivary agglutinin. Infect Immun. 1994;62:5220–5226. doi: 10.1128/iai.62.12.5220-5226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ericson D. Agglutination of Streptococcus mutans by low-molecular-weight salivary components: effect of β2-microglobulin. Infect Immun. 1984;46:526–530. doi: 10.1128/iai.46.2.526-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ericson T, Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983;133:255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- 14.Geng J-G, Moore K L, Johnson A E, McEver R P. Neutrophil recognition requires a Ca2+-induced conformational change in the lectin domain of GMP-140. J Biol Chem. 1991;266:22313–22318. [PubMed] [Google Scholar]
- 15.Hajishengallis G, Koga T, Russell M W. Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J Dent Res. 1994;73:1493–1502. doi: 10.1177/00220345940730090301. [DOI] [PubMed] [Google Scholar]
- 16.Hara S, Yamaguchi M, Takemori Y, Furuhata K, Ogura H, Nakamura M. Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Anal Biochem. 1989;179:162–166. doi: 10.1016/0003-2697(89)90218-2. [DOI] [PubMed] [Google Scholar]
- 17.Hortin G L, Trimpe B I. Lectin affinity chromatography of proteins bearing O-linked oligosaccharides: application of jacalin-agarose. Anal Biochem. 1990;188:271–277. doi: 10.1016/0003-2697(90)90605-9. [DOI] [PubMed] [Google Scholar]
- 18.Johnsson B, Löfås S, Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991;198:268–277. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- 19.Jönsson U, Fägerstam L, Ivarsson B, Johnsson B, Karlsson R, Lundh K, Löfås S, Persson B, Roos H, Rönnberg I, Sjölander S, Stenberg E, Ståhlberg R, Urbaniczky C, Östlin H, Malmqvist M. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. BioTechniques. 1991;11:620–627. [PubMed] [Google Scholar]
- 20.Kishimoto E, Hay D I, Gibbons R J. A human salivary protein which promotes adhesion of Streptococcus mutans serotype c strains to hydroxyapatite. Infect Immun. 1989;57:3702–3707. doi: 10.1128/iai.57.12.3702-3707.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koga T, Okahashi N, Takahashi I, Kanamoto T, Asakawa H, Iwaki M. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990;58:289–296. doi: 10.1128/iai.58.2.289-296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lee S F, Progulske-Fox A, Erdos G W, Piacentini D A, Ayakawa G Y, Crowley P J, Bleiweis A S. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II) Infect Immun. 1989;57:3306–3313. doi: 10.1128/iai.57.11.3306-3313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine M J, Reddy M S, Tabak L A, Loomis R E, Bergey E J, Jones P C, Cohen R E, Stinson M W, Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res. 1987;66:436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- 25.Linzer R, Reddy M S, Levine M J. Immunochemical aspects of serotype carbohydrate antigens of Streptococcus mutans. In: Hamada S, Michalek S M, Kiyono H, Menaker L, McGhee J R, editors. Molecular microbiology and immunology of Streptococcus mutans. Amsterdam, The Netherlands: Elsevier Science Publishers; 1986. pp. 29–38. [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Moisset A, Schatz N, Lepoivre Y, Amadio S, Wachsmann D, Schöller M, Klein J-P. Conservation of salivary glycoprotein-interacting and human immunoglobulin G-cross-reactive domains of antigen I/II in oral streptococci. Infect Immun. 1994;62:184–193. doi: 10.1128/iai.62.1.184-193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munro G H, Evans P, Todryk S, Buckett P, Kelly C G, Lehner T. A protein fragment of streptococcal cell surface antigen I/II which prevents adhesion of Streptococcus mutans. Infect Immun. 1993;61:4590–4598. doi: 10.1128/iai.61.11.4590-4598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakai M, Okahashi N, Ohta H, Koga T. Saliva-binding region of Streptococcus mutans surface protein antigen. Infect Immun. 1993;61:4344–4349. doi: 10.1128/iai.61.10.4344-4349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieuw Amerongen A V, Bolsher J G M, Veerman E C I. Salivary mucins: protective functions in relation to their diversity. Glycobiology. 1995;5:733–740. doi: 10.1093/glycob/5.8.733. [DOI] [PubMed] [Google Scholar]
- 31.Oho, T., and T. Koga, Unpublished data.
- 32.Payne J B, Iacono V J, Crawford I T, Lepre B M, Bernzweig E, Grossbard B L. Selective effects of histidine-rich polypeptides on the aggregation and viability of Streptococcus mutans and Streptococcus sanguis. Oral Microbiol Immunol. 1991;6:169–176. doi: 10.1111/j.1399-302x.1991.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 33.Rolla G, Bonesvoll P, Opermann R. Interactions between oral streptococci and salivary proteins. In: Kleinberg I, Ellison S A, Mandel I D, editors. Saliva and dental caries. Oxford, England: IRL Press; 1979. pp. 227–241. [Google Scholar]
- 34.Rosan B, Appelbaum B, Golub E, Malamud D, Mandel I D. Enhanced saliva-mediated bacterial aggregation and decreased bacterial adhesion in caries-resistant versus caries-susceptible individuals. Infect Immun. 1982;38:1056–1059. doi: 10.1128/iai.38.3.1056-1059.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruhl S, Sandberg A L, Cole M F, Cisar J O. Recognition of immunoglobulin A1 by oral actinomyces and streptococcal lectins. Infect Immun. 1996;64:5421–5424. doi: 10.1128/iai.64.12.5421-5424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rundegren J, Arnold R R. Differentiation and interaction of secretory immunoglobulin A and a calcium-dependent parotid agglutinin for several bacterial strains. Infect Immun. 1987;55:288–292. doi: 10.1128/iai.55.2.288-292.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell M W. Immunization against dental caries. Curr Opin Dent. 1992;2:72–80. [PubMed] [Google Scholar]
- 38.Russell M W, Mansson-Rahemtulla B. Interaction between surface protein antigens of Streptococcus mutans and human salivary components. Oral Microbiol Immunol. 1989;4:106–111. doi: 10.1111/j.1399-302x.1989.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 39.Senpuku H, Kato H, Todoroki M, Hanada N, Nishizawa T. Interaction of lysozyme with a surface protein antigen of Streptococcus mutans. FEMS Microbiol Lett. 1996;139:195–201. doi: 10.1111/j.1574-6968.1996.tb08202.x. [DOI] [PubMed] [Google Scholar]
- 40.Shibuya N, Tazaki K, Song Z, Tarr G E, Goldstein I J, Peumans W J. A comparative study of bark lectins from three elderberry (Sambucus) species. J Biochem. 1989;106:1098–1103. doi: 10.1093/oxfordjournals.jbchem.a122972. [DOI] [PubMed] [Google Scholar]
- 41.Slomiany B L, Murty V L N, Piotrowski J, Slomiany A. Salivary mucins in oral mucosal defense. Gen Pharmacol. 1996;27:761–771. doi: 10.1016/0306-3623(95)02050-0. [DOI] [PubMed] [Google Scholar]
- 42.Slomiany B L, Piotrowski J, Czajkowski A, Shovlin F E, Slomiany A. Differential expression of salivary mucin bacterial aggregating activity with caries status. Int J Biochem. 1993;25:935–940. doi: 10.1016/0020-711x(93)90250-i. [DOI] [PubMed] [Google Scholar]
- 43.Soukka T, Roger V, Söderling E, Tenovuo J. Binding of Streptococcus mutans, serotype c, to saliva-coated hydroxyapatite in the presence and absence of human lactoferrin. Microb Ecol Health Dis. 1994;7:139–144. [Google Scholar]
- 44.Stenberg E, Persson B, Roos H, Urbaniczky C. Quantitative determination of surface concentration of protein with surface plasmon resonance using radiolabeled proteins. J Colloid Interface Sci. 1991;143:513–526. [Google Scholar]
- 45.Suzuki J, Kondo A, Kato I, Hase S, Ikenaka T. Analysis by high-performance anion-exchange chromatography of component sugars as their fluorescent pyridylamino derivatives. Agric Biol Chem. 1991;55:283–284. [Google Scholar]
- 46.Tabak L A. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol. 1995;57:547–564. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 47.Tsukioka Y, Yamashita Y, Nakano Y, Oho T, Koga T. Identification of a fourth gene involved in dTDP-rhamnose synthesis in Streptococcus mutans. J Bacteriol. 1997;179:4411–4414. doi: 10.1128/jb.179.13.4411-4414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsukioka Y, Yamashita Y, Oho T, Nakano Y, Koga T. Biological function of the dTDP-rhamnose synthesis pathway in Streptococcus mutans. J Bacteriol. 1997;179:1126–1134. doi: 10.1128/jb.179.4.1126-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W-C, Cummings R D. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked α-2,3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–4585. [PubMed] [Google Scholar]
- 50.Wu A M, Csako G, Herp A. Structure, biosynthesis, and function of salivary mucins. Mol Cell Biochem. 1994;137:39–55. doi: 10.1007/BF00926038. [DOI] [PubMed] [Google Scholar]
- 51.Yamashita, Y., Y. Tsukioka, Y. Nakano, T. Oho, K. Tomihisa, and T. Koga. Unpublished data.