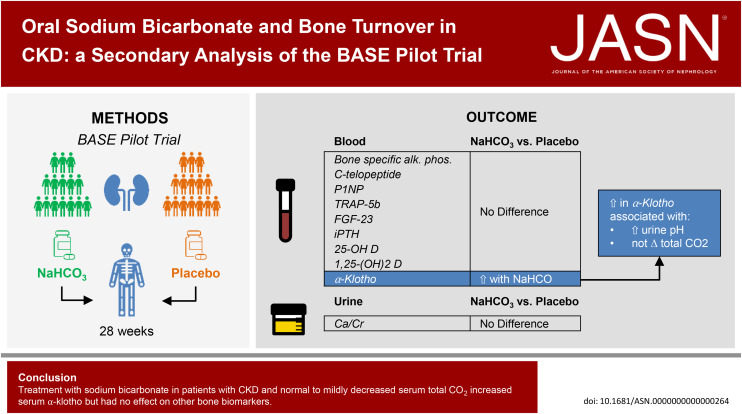
Visual Abstract
Keywords: Acidosis; CKD; bones, stones, and mineral metabolism
Abstract
Significance Statement
In CKD, metabolic acidosis is commonly treated with alkali in the hope that it will improve bone health. In a post hoc analysis of the Bicarbonate Administration to Stabilize eGFR Pilot Trial, we investigated whether sodium bicarbonate affects serum levels of bone turnover markers and other hormones related to bone health in individuals with CKD who have normal to slightly reduced total CO2 (20–28 mEq/L). Sodium bicarbonate increased serum levels of α-klotho but had no significant effect on other bone health markers, including intact fibroblast growth factor-23 (iFGF-23), intact parathyroid hormone (iPTH), and bone-specific alkaline phosphatase (B-SAP). Further study is needed to determine the effect of bicarbonate administration on clinical aspects of bone health.
Background
Treatment with alkali has been hypothesized to improve bone health in CKD by mitigating adverse effects of acid on bone mineral. We investigated the effect of treatment with sodium bicarbonate on bone turnover markers and other factors related to bone metabolism in CKD.
Methods
This is a post hoc analysis of the Bicarbonate Administration to Stabilize eGFR Pilot Trial in which 194 individuals with CKD and serum total CO2 20–28 mEq/L were randomly assigned to placebo or one of two doses of sodium bicarbonate (0.5 or 0.8 mEq/kg lean body weight per day) for 28 weeks. The following serum measurements were performed at baseline, week 12, and week 28: B-SAP, c-telopeptide, procollagen type I intact N-terminal propeptide, iPTH, iFGF-23, soluble klotho, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and tartrate-resistant acid phosphatase 5b. The difference (sodium bicarbonate versus placebo) in mean change of each bone biomarker from baseline was determined using linear mixed models.
Results
One hundred sixty-eight participants submitted samples for post hoc investigations. Mean eGFR was 37±10 ml/min per 1.73 m2 and mean total CO2 was 24±3 mEq/L at baseline. Sodium bicarbonate induced a dose-dependent increase in soluble klotho levels compared with placebo. There was no significant effect of treatment with either dose of sodium bicarbonate on any of the other bone biomarkers, including iFGF-23, iPTH, and B-SAP. Effects on bone biomarkers were similar in those with baseline serum total CO2 <24 mEq/L compared with those with total CO2 ≥24 mEq/L.
Conclusions
In this pilot trial of individuals with CKD and total CO2 20–28 mEq/L, sodium bicarbonate treatment increased serum klotho levels but did not affect other bone health markers over 28 weeks.
Clinical Trial registry name and registration number
ClinicalTrials.gov, NCT02521181.
Introduction
A major function of the kidney is to excrete the daily nonvolatile acid load to keep systemic pH and bicarbonate levels in the normal range. As kidney function declines, kidney acid excretion decreases, leading to acid retention and metabolic acidosis.1,2 Metabolic acidosis in CKD is associated with a number of adverse consequences, including CKD progression and skeletal muscle catabolism.3–7 Prior studies demonstrated that the bone can buffer excess acid; however, this leads to hypercalciuria and loss of bone mineral.8–10 Other studies have demonstrated that extracellular acidification increases activity of osteoclasts and inhibits the activity of osteoblasts, thereby affecting bone remodeling.11 Consequently, clinical practice guidelines recommend treating metabolic acidosis with alkali in patients with CKD in part to mitigate adverse effects of metabolic acidosis on bone health.12
Because the body staunchly defends against metabolic acidosis, it is possible that bone buffering helps offset impaired kidney acid excretion to maintain systemic pH and bicarbonate levels in the normal range. Hence, acid-mediated bone demineralization may be occurring even in individuals with CKD who have normal serum total CO2, which might be manifested by an increase in bone turnover rates. We hypothesize that alkali therapy preserves bone health in patients with CKD who also have normal serum total CO2 concentration. To investigate this hypothesis, we measured serum levels of turnover markers associated with bone formation (bone-specific alkaline phosphatase [B-SAP] and procollagen type I intact N-terminal propeptide [P1NP]) and bone resorption (C-terminal telopeptide of type I collagen [CTX-1] and tartrate-resistant acid phosphatase 5b [TRAP5b]) from stored samples in participants in the Bicarbonate Administration to Stabilize eGFR (BASE) Pilot Trial, a placebo-controlled trial designed to determine which of the two doses of sodium bicarbonate might be best to study in a subsequent phase 3 trial.13 In addition, we determined the effect of sodium bicarbonate treatment on hormones related to bone metabolism, including serum intact parathyroid hormone (iPTH), intact fibroblast growth factor-23 (iFGF-23), soluble α-klotho, and vitamin D levels.
Methods
Study Design and Population
This is a secondary analysis of the BASE Pilot Trial (NCT02521181, first posted on August 13, 2015), the details of which have been previously published.13 Briefly, 194 individuals with CKD at ten clinical sites in the United States were randomly assigned to receive higher dose sodium bicarbonate (HD-NaHCO3, 0.8 mEq/kg lean body weight [LBW], per day, n=90), lower dose sodium bicarbonate (LD-NaHCO3, 0.5 mEq/kg LBW per day, n=52), or placebo (n=52) for 28 weeks. Key inclusion criteria were age older than 18 years; serum total CO2 20–28 mEq/L; moderate-to-severe CKD, defined as eGFR 20 to <45 ml/min per 1.73 m2 with no specific urinary albumin/creatinine (ACR) requirements or eGFR 45 to <60 ml/min per 1.73 m2 with random ACR ≥50 mg/g; BP <160/100 mm Hg; and LBW 37.5–96.0 kg. Key exclusion criteria were current use of oral alkali, use of ≥5 antihypertensive and/or diuretic medications, serum potassium <3.3 or ≥5.5 mEq/L, severe congestive heart failure, and organ transplantation. Demographic information obtained included age, sex, and self-reported race and ethnicity and were obtained as required by the funding agency. Participants were advised to limit intake of sodium chloride; otherwise, no specific dietary recommendations were suggested. Of the 194 randomized participants, 168 (87%) consented to submit serum samples and were included in this post hoc analysis. Samples were collected from participants at the time of the visit, and fasting was not required. Only random urine samples were saved for post hoc analyses. The study was approved by institutional review boards at each recruitment site, and participants provided written informed consent. The BASE Pilot Trial was performed under the principles embodied in the Declaration of Helsinki.
Measurements
Serum samples were collected from participants at baseline (n=168) and weeks 12 (n=157) and 28 (n=152) after randomization. Measurements were performed by the Department of Laboratory Medicine at University of Washington. The following serum measurements were performed: B-SAP; CTX-1, P1NP, iPTH, iFGF-23, soluble α-klotho, 25-hydroxyvitamin D, 1, 25-dihydroxyvitamin D, and TRAP5b. B-SAP and iPTH concentrations were measured using a Beckman Coulter automated immunoassay analyzer (DxI 800). The concentrations of intact-P1NP and CTX-1 (Serum CrossLaps EIA) were quantified using the Immunodiagnostic Systems iSYS automated platform. The concentration of α-klotho was quantified by the commercially available IBL ELISA kit. The concentration of TRAP5b was determined using an ELISA from Immunodiagnostic Systems. iFGF-23 was measured using the Kainos two-site ELISA kit. Vitamin D metabolites were quantified using immunoaffinity enrichment and liquid chromatography-tandem mass spectrometry, as previously described.14,15 Random urine calcium concentration was also quantified using a Beckman Coulter AU5812 automated chemistry analyzer after adjusting the pH to 1 using concentrated hydrochloric acid (verified with pH paper) and indexed to random urine creatinine concentration.
Statistical Analyses
Descriptive statistics were used to compare participant characteristics in each of the randomization groups. Spearman correlations were used to examine associations of the bone markers with each other and with eGFR and serum total CO2.
Linear mixed models, with random intercept and random slope to account for within-subject correlation and individual differences, were used to evaluate changes in biomarker levels from baseline within and between groups. Our primary evaluation for each bone biomarker consisted of the slope of change from baseline in both sodium bicarbonate dose groups combined compared with placebo. We also evaluated the slope of change from baseline comparing LD-NaHCO3 and HD-NaHCO3 with placebo to investigate potential dose-response effects. We evaluated changes in biomarkers stratified by baseline total CO2 <24 and ≥24 mEq/L to examine whether any potential treatment effect might be more pronounced in individuals with lower total CO2 because acid-mediated bone damage is believed to be most pronounced with overt metabolic acidosis, but most BASE participants had normal serum total CO2 at baseline. Analyses were performed with SPSS version 26.0 (IBM Corp.) and Stata version 16 (StataCorp LLC, College Station, TX). All P values were two-tailed. P values < 0.05 were considered statistically significant. We also applied a Bonferroni correction to adjust for multiple comparisons.
Results
Baseline Characteristics
Table 1 shows baseline characteristics of the 168 individuals included in this study. The proportion of individuals who had samples were >80% in each assigned treatment group (placebo, 88%; LD-NaHCO3, 90%; HD-NaHCO3, 83%). Baseline characteristics were similar among the groups. Serum levels of bone turnover and other bone-related biomarkers were well balanced across groups at baseline. The mean (SD) eGFR was 37 (10) ml/min per 1.73 m2 and mean (SD) total CO2 was 24 (3) mEq/L at baseline. Sixty-seven participants (40%) had total CO2 <24 mEq/L. Median (interquartile range) iPTH was 66 (40–105) pg/ml, median (interquartile range) iFGF-23 was 99 (68–142) pg/ml, and mean (SD) serum 25(OH) vitamin D was 37 (13) ng/ml. Typical reference ranges for the bone turnover markers and serum klotho levels are shown in Supplemental Table 1. The mean (SD) dose of sodium bicarbonate was 4.19 (0.82) g/d for those in HD-NaHCO3 and 2.60 (0.54) g/d for those in LD-NaHCO3.
Table 1.
Baseline characteristics of participants in the study
| Variable | All | Placebo | Lower Dose NaHCO3 | Higher Dose NaHCO3 |
|---|---|---|---|---|
| N | 168 | 46 | 47 | 75 |
| Age, yr | 67 (12) | 66 (11) | 67 (14) | 68 (11) |
| Female, no. (%) | 47 (28) | 10 (22) | 16 (34) | 21 (28) |
| Race, no. (%) | ||||
| Black | 54 (32) | 20 (44) | 14 (30) | 20 (27) |
| White | 97 (58) | 23 (50) | 26 (55) | 48 (64) |
| Other | 14 (8) | 3 (7) | 5 (11) | 6 (8) |
| Unknown or not reported | 3 (2) | 0 (0) | 2 (4) | 1 (1) |
| Hispanic ethnicity, no. (%) | 15 (9) | 3 (7) | 6 (13) | 6 (8) |
| Diabetes mellitus, no. (%) | 89 (53) | 22 (48) | 25 (53) | 42 (56) |
| History of heart disease, no. (%) | 27 (16) | 7 (15) | 9 (19) | 11 (15) |
| CHF, no. (%) | 18 (11) | 6 (13) | 4 (9) | 8 (11) |
| Systolic BP, mm Hg | 127 (15) | 126 (16) | 130 (11) | 126 (16) |
| eGFR, ml/min per 1.73 m2 | 37 (10) | 36 (12) | 38 (10) | 37 (9) |
| Serum measurements | ||||
| Total CO2, mEq/L | 24 (3) | 24 (3) | 24 (3) | 25 (3) |
| B-SAP, μg/L | 12.8 (5.6) | 13.0 (6.4) | 12.6 (5.0) | 12.8 (5.5) |
| TRAP5b, U/L | 1.25 (0.63) | 1.24 (0.74) | 1.27 (0.62) | 1.24 (0.56) |
| P1NP, μg/L | 57 (31) | 55 (31) | 59 (33) | 57 (30) |
| CTX-1, ng/ml | 0.36 (0.38) | 0.36 (0.39) | 0.33 (0.28) | 0.37 (0.42) |
| iFGF-23, pg/mla | 99 (68–142) | 104 (56–162) | 101 (69–146) | 95 (69–130) |
| iPTH, pg/mla | 66 (40–105) | 69 (47–112) | 59 (33–97) | 64 (42–103) |
| Soluble klotho, pg/mla | 733 (605–927) | 759 (594–1001) | 676 (547–914) | 737 (628–928) |
| 25(OH) vitamin D, ng/ml | 37 (13) | 40 (13) | 36 (14) | 36 (12) |
| 1,25(OH)2 vitamin D, pg/ml | 37 (13) | 38 (14) | 35 (14) | 37 (12) |
| Phosphate, mg/dl | 3.51 (0.62) | 3.52 (0.63) | 3.44 (0.58) | 3.54 (0.65) |
| Urine measurements | ||||
| Albumin/Cr, mg/ga | 195 (22–731) | 139 (10–740) | 236 (45–702) | 200 (26–866) |
| Calcium/Cr, mg/g | 1.5 (0.7–3.4) | 1.3 (0.7–2.8) | 1.4 (0.8–3.0) | 1.6 (0.7–4.4) |
Continuous variables shown as mean (SD) except where indicated. B-SAP, bone-specific alkaline phosphatase; CHF, congestive heart failure; Cr, creatinine; CTX-1, C-terminal telopeptide of type I collagen; iFGF-23, intact fibroblast growth factor-23; OH, hydroxy; P1NP, procollagen type I intact N-terminal propeptide; iPTH, intact parathyroid hormone; TRAP5b, tartrate-resistant acid phosphatase 5b.
Indicates median (interquartile range).
Table 2 shows unadjusted Spearman correlations between baseline values of serum total CO2 and eGFR and the bone biomarkers investigated. There were no significant correlations between serum total CO2 and any of the serum bone biomarkers; however, total CO2 was weakly and directly correlated with urine calcium excretion (r=0.2). eGFR was inversely correlated with iFGF-23, iPTH, and CTX-1. Among the bone turnover biomarkers, the strongest correlations were observed between B-SAP and P1NP and between serum CTX-1 and P1NP (r values ≥0.6).
Table 2.
Spearman correlations between baseline eGFR, serum total CO2, and bone health markers
| Measurement | TCO2 | FGF-23 | PTH | Klotho | 25-D | 1,25-D | U Ca/Cr | B-SAP | CTX-1 | P1NP | TRAP5b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFRa | 0.13 | −0.43a | −0.25a | 0.07 | −0.02 | 0.13 | 0.01 | 0.06 | −0.28a | −0.07 | −0.03 |
| TCO2a | −0.04 | 0.03 | 0.07 | 0.07 | 0.12 | 0.20a | −0.13 | −0.02 | −0.02 | 0.08 | |
| FGF-23a | 0.30a | −0.05 | 0.02 | −0.35a | 0.13 | −0.09 | 0.14 | 0.02 | 0.05 | ||
| PTHa | 0.09 | −0.18a | 0.13 | −0.25 | 0.25a | 0.45a | 0.29 | 0.24 | |||
| Klothoa | −0.02a | 0.06 | −0.03 | 0.23a | −0.04 | 0.05 | 0.05 | ||||
| 25-Da | 0.14 | 0.17a | −0.03 | −0.14 | −0.19a | 0.001 | |||||
| 1,25-Da | 0.01a | 0.12a | −0.01 | −0.06 | 0.09 | ||||||
| Ca/Cra | −0.03 | −0.05 | 0.05 | 0.03 | |||||||
| BSAPa | 0.43a | 0.60a | 0.23a | ||||||||
| CTX-1a | 0.64a | 0.23a | |||||||||
| P1NPa | 0.19a |
1,25-D; 1,25-dihydroxyvitamin D; 25-D, 25-hydroxyvitamin D; B-SAP, bone-specific alkaline phosphatase; Ca/Cr, urine calcium/creatinine; CTX-1, C-terminal telopeptide of type I collagen; FGF-23, fibroblast growth factor-23; P1NP, procollagen type I intact N-terminal propeptide; PTH, parathyroid hormone; TCO2, total CO2; TRAP5b, tartrate-resistant acid phosphatase 5b.
P < 0.05.
Effect of Sodium Bicarbonate on Bone Biomarkers
Supplemental Figure 1 shows that sodium bicarbonate decreased urinary ammonium and increased urinary pH and serum total CO2 in the study participants. Serum klotho levels were significantly higher in LD-NaHCO3 and in HD-NaHCO3 compared with placebo during follow-up, and there was a suggestion of a dose-response effect on klotho levels. Those in LD-NaHCO3 (but not HD-NaHCO3) had higher iPTH compared with placebo during follow-up. Otherwise, treatment with either dose of sodium bicarbonate individually did not significantly affect other bone biomarkers compared with placebo (Table 3). The results were similar when both dose groups were analyzed in aggregate and compared with placebo, wherein treatment with either dose of sodium bicarbonate associated with increases in serum klotho, but no significant changes in any other parameter of bone health.
Table 3.
Effect of sodium bicarbonate on bone biomarkers
| Variable | Treatment | % Mean Change (95% CI) | P Value for % Mean Change Compared with Placebo | P Value Adjusted for Multiple Comparison |
|---|---|---|---|---|
| iFGF-23 | Placebo | 6.5 (0.9 to 12.1) | — | |
| Lower dose | 8.1 (2.5 to 13.7) | 0.697 | 0.867 | |
| Higher dose | 1.7 (−2.8 to 6.3) | 0.193 | 0.603 | |
| Both doses | 4.3 (0.8 to 7.8) | 0.511 | 0.712 | |
| iPTH | Placebo | −0.5 (−7.2 to 6.3) | — | |
| Lower dose | 9.6 (3.0 to 16.4) | 0.036 | 0.270 | |
| Higher dose | 4.1 (−1.3 to 9.4) | 0.301 | 0.658 | |
| Both doses | 6.3 (2.1 to 10.4) | 0.096 | 0.576 | |
| Soluble klotho | Placebo | −3.3 (−5.8 to −0.9) | — | |
| Lower dose | 0.7 (−1.7 to 3.2) | 0.023 | 0.230 | |
| Higher dose | 2.7 (0.7 to 4.7) | <0.001 | 0.006 | |
| Both doses | 1.9 (0.3 to 3.5) | <0.001 | 0.008 | |
| 25(OH) vitamin D | Placebo | 0.2 (−3.3 to 3.8) | — | |
| Lower dose | 2.5 (−1.0 to 6.0) | 0.376 | 0.705 | |
| Higher dose | 0.2 (−2.7 to 3.0) | 0.981 | 0.993 | |
| Both doses | 1.1 (−1.1 to 3.3) | 0.685 | 0.867 | |
| 1,25(OH)2 vitamin D | Placebo | −2.0 (−6.5 to 2.5) | — | |
| Lower dose | 1.7 (−2.7 to 6.1) | 0.247 | 0.658 | |
| Higher dose | 1.3 (−2.3 to 4.9) | 0.264 | 0.658 | |
| Both doses | 1.4 (−1.3 to 4.2) | 0.201 | 0.603 | |
| Urine calcium/Cr | Placebo | 7.3 (−8.3 to 22.8) | — | |
| Lower dose | −1.7 (−17.2 to 13.7) | 0.421 | 0.707 | |
| Higher dose | 0.8 (−11.5 to 13.1) | 0.522 | 0.712 | |
| Both doses | −0.2 (−9.8 to 9.4) | 0.424 | 0.707 | |
| B-SAP | Placebo | 0.1 (−2.2 to 2.5) | — | |
| Lower dose | −2.1 (−4.4 to 0.2) | 0.184 | 0.603 | |
| Higher dose | −1.4 (−3.3 to 0.4) | 0.310 | 0.658 | |
| Both doses | −1.7 (−3.1 to −0.3) | 0.197 | 0.603 | |
| CTX-1 | Placebo | 0.06 (−7.2 to 7.3) | — | |
| Lower dose | 7.1 (−0.2 to 14.4) | 0.183 | 0.603 | |
| Higher dose | −4.6 (−10.6 to 1.3) | 0.329 | 0.658 | |
| Both doses | 0 (−4.6 to 4.7) | 0.993 | 0.993 | |
| P1NP | Placebo | −1.1 (−5.2 to 2.9) | — | |
| Lower dose | −3.0 (−7.0 to 0.1) | 0.516 | 0.712 | |
| Higher dose | 0.8 (−2.5 to 4.1) | 0.461 | 0.712 | |
| Both doses | −0.7 (−3.3 to 1.8) | 0.870 | 0.932 | |
| TRAP5b | Placebo | 0.3 (−7.5 to 8.0) | — | |
| Lower dose | −1.5 (−9.2 to 6.2) | 0.752 | 0.867 | |
| Higher dose | −1.1 (−7.4 to 5.1) | 0.780 | 0.867 | |
| Both doses | −1.3 (−6.1 to 3.5) | 0.738 | 0.867 |
B-SAP, bone-specific alkaline phosphatase; CI, confidence interval; Cr, creatinine; CTX-1, C-terminal telopeptide of type I collagen; iFGF23, intact fibroblast growth factor-23; OH, hydroxy; P1NP, procollagen type I intact N-terminal propeptide; iPTH, intact parathyroid hormone; TRAP5b, tartrate-resistant acid phosphatase 5b.
We applied a Bonferroni correction to see whether the statistically significant findings for the effect of sodium bicarbonate randomization on changes in serum klotho might simply be due to multiple comparisons. After adjusting for multiple comparisons, klotho levels were still significantly higher when both dose groups were analyzed in aggregate and in HD-NaHCO3 alone, but not in LD-NaHCO3 alone (Table 3). We also compared change in klotho relative to change in total CO2 and urine pH after adjusting for randomization arm. There was no significant association between change in total CO2 and change in klotho (P = 0.926); however, there was a significant association between change in urine pH and change in klotho (P = 0.009) such that each 10% increase in urine pH correlated with a 3.5% (95% confidence interval, 0.9% to 6.1%) increase in soluble klotho.
Changes in all biomarkers in response to treatment with sodium bicarbonate were similar in those with total CO2 <24 mEq/L and ≥24 mEq/L at baseline (Figure 1). We also examined potential age and sex interactions in response to treatment on the principal bone turnover markers (B-SAP, CTX, P1NP, and TRAP5b) and soluble α-klotho. We found no evidence of an interaction by age (P ≥ 0.12) or sex (P ≥ 0.08) in these analyses.
Figure 1.
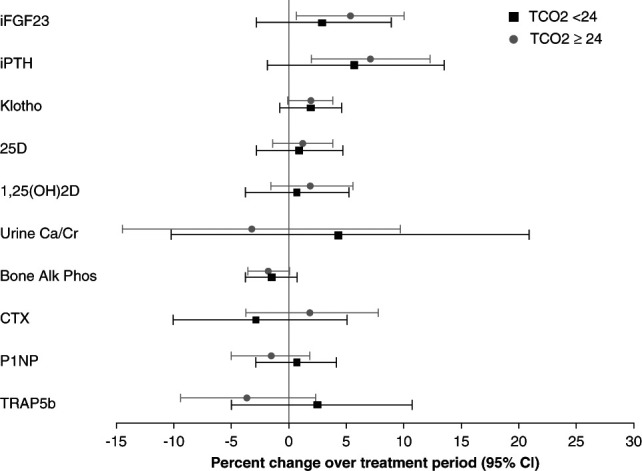
Difference in percent change in bone biomarkers for those treated with sodium bicarbonate as compared with those who received placebo during the study, stratified by serum total CO2 concentration (≥24 or <24 mEq/L). Squares and circles represent the mean percent change, and error bars represent the 95% confidence limits. 1,25OH(2)D; 1,25-dihydroxyvitamin D; 25D, 25-hydroxyvitamin D; Bone Alk Phos, bone-specific alkaline phosphatase; Ca/Cr, calcium/creatinine; CTX, C-terminal telopeptide of type I collagen; iFGF-23, intact fibroblast growth factor-23; P1NP, procollagen type I intact N-terminal propeptide; iPTH, intact parathyroid hormone; TCO2, serum total CO2; TRAP5b, tartrate-resistant acid phosphatase 5b.
Discussion
In this post hoc analysis of the BASE Pilot Trial, which enrolled individuals with moderate-to-severe CKD and serum total CO2 20–28 mEq/L, treatment with sodium bicarbonate over 28 weeks caused a statistically significant and dose-dependent increase in serum soluble α-klotho levels. Otherwise, treatment with sodium bicarbonate did not meaningfully change serum levels of biomarkers of bone health, including iPTH, iFGF-23, and a panel of biomarkers reflecting bone turnover. Overall, this study does not support a marked effect of sodium bicarbonate supplementation in modulating bone turnover in patients with mild-to-moderate CKD and serum total CO2 concentrations between 20 and 28 mEq/L and provides a new potential method to increase serum klotho levels in patients with CKD.
Landmark studies established that metabolic acidosis induces loss of bone architecture,8,11,16 and metabolic acidosis is a well-recognized contributor to bone disease in CKD, ESKD, and kidney transplant recipients.17–20 However, studies of the effect of alkali supplementation on bone in individuals with kidney disease are limited. In one study, treatment of metabolic acidosis with sodium bicarbonate for 3 months mildly attenuated increases in iPTH levels.21 In another study of individuals with metabolic acidosis, sodium bicarbonate had no significant effect on iPTH, CTX-1, or P1NP, but increased serum iFGF-23 levels.22 In kidney transplant recipients with metabolic acidosis, treatment with potassium citrate improved bone histomorphometry and preserved B-SAP, CTX-1, and P1NP levels but had no effect on iPTH, vitamin D metabolite levels, or bone mineral density (BMD) over 1 year.23 These trials were conducted in individuals with CKD and overt metabolic acidosis. By contrast, we studied individuals with CKD who, for the most part, had normal serum total CO2 levels and did not observe consistent effects on bone health markers overall (except soluble klotho). We also performed stratified analyses to determine whether there was a treatment effect for those with lower total CO2 (20–24 mEq/L); however, there was no suggestion that sodium bicarbonate treatment might be more impactful for those with total CO2 in this range. To our knowledge, the Alkali Therapy in CKD Trial is the only other study in patients with CKD that evaluated effects of sodium bicarbonate on bone in individuals with normal serum total CO2 and sodium bicarbonate did not have an effect on BMD relative to placebo.24
Most evidence supporting a beneficial effect of alkali therapy on bone are derived from studies in postmenopausal women and used potassium-based alkali. In a study of 18 postmenopausal women, potassium bicarbonate (60–120 mEq daily) reduced urinary calcium and phosphorus excretion over 18 days, and increased serum osteocalcin and decreased urinary hydroxyproline, suggesting net bone formation.25 Another study evaluated potassium bicarbonate in 170 postmenopausal women and found a dose-dependent reduction of urinary calcium excretion over 3 years.26 In a randomized controlled trial of 161 postmenopausal women with osteopenia, treatment with potassium citrate resulted in an increased BMD at the spine, femoral neck, and total hip over 12 months.27 Finally, in a study of 42 middle-aged adults, 4 weeks of treatment with potassium bicarbonate reduced plasma CTX-1 and urine calcium excretion.28 In sum, these findings show that potassium-based alkali diminishes urinary calcium loss and preserves BMD in people who largely have normal kidney function.
It is noteworthy that appreciable effects on bone turnover are primarily reported in studies evaluating potassium-based alkali, whereas studies, including this study, evaluating sodium-based alkali have found either marginal or no effects. Whether this is due to differences in the type of alkali, the accompanying cation, or differences in kidney function requires future study. We did not observe an increase in urinary calcium excretion in either sodium bicarbonate dose group, which may alleviate the concern that sodium bicarbonate induces negative calcium balance in CKD. Provision of potassium to persons with CKD requires caution, however, given risk of reduced kidney potassium excretion and resultant hyperkalemia.29,30
The one serum biomarker that changed in response to sodium bicarbonate in this study was α-klotho. α-klotho was selected as a biomarker because we previously demonstrated that higher α-klotho is a marker of low bone turnover confirmed by bone biopsy in CKD.31 α-klotho is primarily produced by the kidney, and metabolic acidosis has been associated with lower levels in CKD.32 Furthermor, in a pre–post study of 20 individuals with CKD and metabolic acidosis, sodium bicarbonate increased urinary, but not serum, α-klotho levels over 4 weeks.33 In this randomized, placebo-controlled trial with 28 weeks of sodium bicarbonate intervention, we identified an effect on serum α-klotho levels in individuals with CKD who mostly had normal serum total CO2 levels at baseline. This finding remained statistically significant after accounting for multiple comparisons. While this is consistent in direction with a possible reduction in bone turnover, no effect was seen across the broad panel of other turnover markers, including fibroblast growth factor-23 and iPTH. It is conceivable that the increase in serum α-klotho levels by alkalinization may be unrelated to bone turnover. Yoon et al. reported a mechanism that we believe accounts for the isolated change in serum α-klotho levels. They identified that increasing urine pH induces cleavage of membrane-bound klotho by a disintegrin and metalloproteinase 10 (ADAM10) in the kidney distal convoluted tubule through activation of the calcium-sensing receptor.34 Consistent with this biological hypothesis, we noted strong correlations of the observed change in urine pH with changes in serum klotho levels, but not with changes in serum total CO2. Hence, we think that the increase in serum α-klotho levels in response to treatment with sodium bicarbonate is due to an increase in urine pH rather than effects on bone.
The absence of bone histomorphometry data is the main limitation of this study. We also lack bone imaging for mineral content; however, we view this as a minor limitation because conventional bone imaging cannot assess bone turnover. Prandial status and diurnal variability can affect bone biomarkers.35 However, our study visits were scheduled when convenient for participants and fasting was not required, which may have affected the findings. The sample size was modest, and not all participants chose to submit samples for future analyses. Hence, the study may have been underpowered to detect small differences in markers. Given the 28-week intervention period, it is also conceivable that the study was too short to see meaningful effects on markers; however, this seems unlikely given positive results using potassium citrate in a prior study in kidney transplant recipients.23
Strengths of the study include the randomized, double-blind, placebo-controlled design; the evaluation of two doses of sodium bicarbonate allowing assessments of dose-response relationships; and the enrollment of individuals from multiple clinical sites. This is also the largest study evaluating the effect of sodium bicarbonate treatment on bone health markers in CKD. Finally, the examination of a number of different biomarkers represents the most comprehensive evaluation of the effect of sodium bicarbonate on bone turnover markers to date.
In this pilot trial of individuals with CKD and total CO2 20–28 mEq/L, sodium bicarbonate treatment did not have consistent effects on a variety of bone health markers over 28 weeks. However, sodium bicarbonate significantly increased serum soluble α-klotho concentrations, a finding that persisted even after accounting for multiple comparisons, and was strongly correlated with the degree of change in urine pH. We believe the increase in serum α-klotho levels is related to changes in urine pH rather than effects on bone. Overall, the findings were similar with low or high-dose sodium bicarbonate therapy and among those with total CO2 concentrations <24 mEq/L at baseline. Sodium bicarbonate therapy has been recommended by international practice guidelines for patients with CKD with metabolic acidosis,12 in part due to purported benefits on bone. In view of the current findings, additional large-scale trials with longer follow-up duration to assess changes in bone quality and fracture risk are urgently needed.
Supplementary Material
Acknowledgments
The investigators wish to thank the BASE Pilot Trial participants and study coordinators in all study sites.
Disclosures
A.K. Cheung reports Consultancy: 3D Communications, Boehringer-Ingelheim, and CSL Behring; Ownership Interest: Merck; and Patents or Royalties: UpToDate. L.F. Fried reports Consultancy: CSL Behring, Data Safety Monitoring boards, and Novo Nordisk; Ownership Interest: Stock ownership: Amgen, Archer Daniels Midland, ATT, Dow, Kroger, Procter & Gamble; and Research Funding: AstraZeneca. A. Hoofnagle reports Consultancy: Kilpatrick Townsend & Stockton LLP; Ownership Interest: Seattle Genetics; Research Funding: Waters; Patents or Royalties: SISCAPA Assay Technologies; Advisory or Leadership Role: Clinical Chemistry (Associate Editor); and Other Interests or Relationships: Expert witness for Kilpatrick, Townsend, and Stockton, LLC. T. Isakova reports Consultancy: Blueprint Partnership Manchester Ltd.; and Advisory or Leadership Role: Associate Editor, American Journal of Kidney Diseases. J.H. Ix reports Consultancy: Akebia, AstraZeneca, Bayer, Cincor, and Sanifit; Research Funding: Baxter International and Juvenile Diabetes Research Foundation; Honoraria: Akebia, AstraZeneca, Bayer, Cincor, and Sanifit; Advisory or Leadership Role: AlphaYoung; and Other Interests or Relationships: Executive Board for Kidney Disease: Improving Global Outcomes (KDIGO). R. Katz reports Consultancy: UCSD and UPenn; and Advisory or Leadership Role: CJASN Editorial Board. D.S. Raj reports Consultancy: Novo Nordics; Research Funding: NIH; Honoraria: Novo Nordics; Advisory or Leadership Role: NHLBI, NIDDK, and Novo Nordics; and Other Interests or Relationships: American Association of Kidney Patients. S. Sprague reports Consultancy: Amgen, Ardelyx, Bayer, Fresenius, Horizon, Litholink Corp, OPKO, Shire, and Vifor; Ownership Interest: Individually owned stocks; Apple, Baxter, Bristol Myers, Coca Cola, First Australia Fund, lBM, and Walgreens; Research Funding: Amgen, Amylot, Ardelyx, OPKO, Reata, and Takeda; Honoraria: Amgen, Ardelyx, Bayer, Fresenius, OPKO, and Vifor; Advisory or Leadership Role: American Association of Endocrine Surgeons, American Journal of Nephrology, International Federation of Clinical Chemistry and Laboratory Medicine-Work Group for Parathyroid Hormone, and National Kidney Foundation of Illinois; and Speakers Bureau: Amgen, Bayer, Fresenius, and OPKO. M. Wolf reports Consultancy: Akebia, Alexion, Amgen, Bayer, Enyo, Jnana, Pfizer, Pharmacosmos, Reata, and Torii; Ownership Interest: Akebia, Unicycive, and Walden; Research Funding: CSL Behring; Honoraria: Akebia, Alexion, Amgen, Bayer, Enyo, Jnana, Pfizer, Pharmacosmos, Reata, and Torii; and Advisory or Leadership Role: Akebia, Unicycive, and Walden. All remaining authors have nothing to disclose.
Funding
The BASE Pilot trial was sponsored by the National Institutes of Diabetes and Digestive and Kidney Diseases Pilot Clinical Trials consortium (U01DK097093, U01DK099877, U01DK099924, U01DK099930, U01DK099933). This work was also supported by the National Institutes of Diabetes and Digestive and Kidney Diseases (R01DK119528 to Dr. Ix and R01DK132823 to Dr. Raphael).
Author Contributions
Conceptualization: Joachim H. Ix, Kalani L. Raphael.
Data curation: Cynthia Kendrick, Brett Larive.
Formal analysis: Ronit Katz.
Investigation: Kalani L. Raphael.
Methodology: Andy Hoofnagle, Joachim H. Ix, Kalani L. Raphael.
Project administration: Jennifer Gassman, Joachim H. Ix, Kalani L. Raphael.
Supervision: Kalani L. Raphael.
Writing – original draft: Joachim H. Ix, Kalani L. Raphael.
Writing – review & editing: Alfred K. Cheung, Linda F. Fried, Jennifer Gassman, Andy Hoofnagle, Tamara Isakova, Joachim H. Ix, Ronit Katz, Cynthia Kendrick, Brett Larive, Dominic S. Raj, Kalani L. Raphael, Stuart Sprague, Myles Wolf.
Data Sharing Statement
Data from the BASE Pilot Trial are available through the NIDDK Repository. Data from the Bicarbonate Administration to Stabilize eGFR (https://doi.org/10.58020/agds-b607) reported here are available for request at the NIDDK Central Repository (NIDDK-CR) website, Resources for Research (R4R), https://repository.niddk.nih.gov/.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E549.
Supplemental Figure 1. Effect of sodium bicarbonate on urinary pH and ammonium and serum total CO2 in the subset of BASE Pilot Trial participants who were studied here.
Supplemental Table 1. Examples of normal values for bone turnover markers and klotho.
References
- 1.Welbourne T, Weber M, Bank N. The effect of glutamine administration on urinary ammonium excretion in normal subjects and patients with renal disease. J Clin Invest. 1972;51(7):1852–1860. doi: 10.1172/JCI106987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallet M Metzger M Haymann JP, et al.; NephroTest Cohort Study group. NephroTest Cohort Study g: urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int. 2015;88(1):137–145. doi: 10.1038/ki.2015.52 [DOI] [PubMed] [Google Scholar]
- 3.Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest. 1996;97(6):1447–1453. doi: 10.1172/JCI118566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest. 1986;77(2):614–621. doi: 10.1172/JCI112344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.May RC, Kelly RA, Mitch WE. Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J Clin Invest. 1987;79(4):1099–1103. doi: 10.1172/JCI112924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011;79(3):356–362. doi: 10.1038/ki.2010.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis. 2009;54(2):270–277. doi: 10.1053/j.ajkd.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushinsky DA, Chabala JM, Gavrilov KL, Levi-Setti R. Effects of in vivo metabolic acidosis on midcortical bone ion composition. Am J Physiol. 1999;277(5):F813–F819. doi: 10.1152/ajprenal.1999.277.5.F813 [DOI] [PubMed] [Google Scholar]
- 9.Lemann J, Jr., Litzow JR, Lennon EJ. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest. 1966;45(10):1608–1614. doi: 10.1172/JCI105467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemann J, Litzow JR, Lennon EJ. Studies of the mechanism by which chronic metabolic acidosis augments urinary calcium excretion in man. J Clin Invest. 1967;46(8):1318–1328. doi: 10.1172/JCI105624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krieger NS, Sessler NE, Bushinsky DA. Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Physiol. 1992;262(3 Pt 2):F442–F448. doi: 10.1152/ajprenal.1992.262.3.F442 [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. doi: 10.1038/kisup.2012.73 [DOI] [Google Scholar]
- 13.Raphael KL Isakova T Ix JH, et al. A randomized trial comparing the safety, adherence, and pharmacodynamics profiles of two doses of sodium bicarbonate in CKD: the BASE pilot trial. J Am Soc Nephrol. 2020;31(1):161–174. doi: 10.1681/ASN.2019030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laha TJ, Strathmann FG, Wang Z, de Boer IH, Thummel KE, Hoofnagle AN. Characterizing antibody cross-reactivity for immunoaffinity purification of analytes prior to multiplexed liquid chromatography-tandem mass spectrometry. Clin Chem. 2012;58(12):1711–1716. doi: 10.1373/clinchem.2012.185827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strathmann FG, Laha TJ, Hoofnagle AN. Quantification of 1α,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57(9):1279–1285. doi: 10.1373/clinchem.2010.161174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ro HK, Tembe V, Krug T, Yang PY, Bushinsky DA, Favus MJ. Acidosis inhibits 1,25-(OH)2D3 but not cAMP production in response to parathyroid hormone in the rat. J Bone Miner Res. 1990;5(3):273–278. doi: 10.1002/jbmr.5650050311 [DOI] [PubMed] [Google Scholar]
- 17.Cochran M, Wilkinson R. Effect of correction of metabolic acidosis on bone mineralisation rates in patients with renal osteomalacia. Nephron. 1975;15(2):98–110. doi: 10.1159/000180501 [DOI] [PubMed] [Google Scholar]
- 18.Heaf J, Tvedegaard E, Kanstrup IL, Fogh-Andersen N. Bone loss after renal transplantation: role of hyperparathyroidism, acidosis, cyclosporine and systemic disease. Clin Transplant. 2000;14(5):457–463. doi: 10.1034/j.1399-0012.2000.140503.x [DOI] [PubMed] [Google Scholar]
- 19.Lefebvre A, de Vernejoul MC, Gueris J, Goldfarb B, Graulet AM, Morieux C. Optimal correction of acidosis changes progression of dialysis osteodystrophy. Kidney Int. 1989;36(6):1112–1118. doi: 10.1038/ki.1989.309 [DOI] [PubMed] [Google Scholar]
- 20.Litzow JR, Lemann J, Jr., Lennon EJ. The effect of treatment of acidosis on calcium balance in patients with chronic azotemic renal disease. J Clin Invest. 1967;46(2):280–286. doi: 10.1172/JCI105530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur RP, Dash SC, Gupta N, Prakash S, Saxena S, Bhowmik D. Effects of correction of metabolic acidosis on blood urea and bone metabolism in patients with mild to moderate chronic kidney disease: a prospective randomized single blind controlled trial. Ren Fail. 2006;28(1):1–5. doi: 10.1080/08860220500461187 [DOI] [PubMed] [Google Scholar]
- 22.Kendrick J Shah P Andrews E, et al. Effect of treatment of metabolic acidosis on vascular endothelial function in patients with CKD: a pilot randomized cross-over study. Clin J Am Soc Nephrol. 2018;13(10):1463–1470. doi: 10.2215/CJN.00380118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starke A Corsenca A Kohler T, et al. Correction of metabolic acidosis with potassium citrate in renal transplant patients and its effect on bone quality. Clin J Am Soc Nephrol. 2012;7(9):1461–1472. doi: 10.2215/CJN.01100112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melamed ML Horwitz EJ Dobre MA, et al. Effects of sodium bicarbonate in CKD stages 3 and 4: a randomized, placebo-controlled, multicenter clinical trial. Am J Kidney Dis. 2020;75(2):225–234. doi: 10.1053/j.ajkd.2019.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris RC, Jr. Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. New Engl J Med. 1994;330(25):1776–1781. doi: 10.1056/NEJM199406233302502 [DOI] [PubMed] [Google Scholar]
- 26.Frassetto L, Morris RC, Jr., Sebastian A. Long-term persistence of the urine calcium-lowering effect of potassium bicarbonate in postmenopausal women. J Clin Endocrinol Metab. 2005;90(2):831–834. doi: 10.1210/jc.2004-1350 [DOI] [PubMed] [Google Scholar]
- 27.Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol. 2006;17(11):3213–3222. doi: 10.1681/ASN.2006030233 [DOI] [PubMed] [Google Scholar]
- 28.He FJ Marciniak M Carney C, et al. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension. 2010;55(3):681–688. doi: 10.1161/HYPERTENSIONAHA.109.147488 [DOI] [PubMed] [Google Scholar]
- 29.Turban S Juraschek SP Miller ER III, et al. Randomized trial on the effects of dietary potassium on blood pressure and serum potassium levels in adults with chronic kidney disease. Nutrients. 2021;13(8):2678. doi: 10.3390/nu13082678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung SMH Gritter M Wouda RD, et al. Short-Term effects of potassium chloride supplementation on fibroblast growth factor 23 and phosphate in CKD. Clin J Am Soc Nephrol. 2023;18(1):99–101. doi: 10.2215/CJN.09340822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes-Austin JM Katz R Semba RD, et al. Biomarkers of bone turnover identify subsets of chronic kidney disease patients at higher risk for fracture. J Clin Endocrinol Metab. 2020;105(8):e2903–e2911. doi: 10.1210/clinem/dgaa317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hage V Pelletier S Dubourg L, et al. In chronic kidney disease, serum α-Klotho is related to serum bicarbonate and proteinuria. J Ren Nutr. 2014;24(6):390–394. doi: 10.1053/j.jrn.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 33.Hage V, Villain C, Pelletier S, Laville M, Drai J, Fouque D. Bicarbonate supplement restores urinary klotho excretion in chronic kidney disease: a pilot study. J Ren Nutr. 2019;29(4):285–288. doi: 10.1053/j.jrn.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 34.Yoon J Liu Z Lee E, et al. Physiologic regulation of systemic klotho levels by renal CaSR signaling in response to CaSR ligands and pH o. J Am Soc Nephrol. 2021;32(12):3051–3065. doi: 10.1681/ASN.2021020276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain S, Camacho P. Use of bone turnover markers in the management of osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2018;25(6):366–372. doi: 10.1097/MED.0000000000000446 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the BASE Pilot Trial are available through the NIDDK Repository. Data from the Bicarbonate Administration to Stabilize eGFR (https://doi.org/10.58020/agds-b607) reported here are available for request at the NIDDK Central Repository (NIDDK-CR) website, Resources for Research (R4R), https://repository.niddk.nih.gov/.