Summary
Background
Increases in invasive group A streptococcal disease (iGAS) have recently been reported in multiple countries in the northern hemisphere, occurring during, and outside of, typical spring peaks. We report the epidemiology of iGAS among children in Australia from 1 July 2018 to 31 December 2022.
Methods
The Paediatric Active Enhanced Disease Surveillance (PAEDS) Network prospectively collected iGAS patient notifications for children and young people aged less than 18 years admitted to five major Australian paediatric hospitals in Victoria, Queensland, Western Australia and the Northern Territory. Patients were eligible for inclusion if they had GAS isolated from a normally sterile body site, or met clinical criteria for streptococcal toxic shock syndrome or necrotising fasciitis with GAS isolated from a non-sterile site. We report patients’ clinical and demographic characteristics, and estimate minimum incidence rates.
Findings
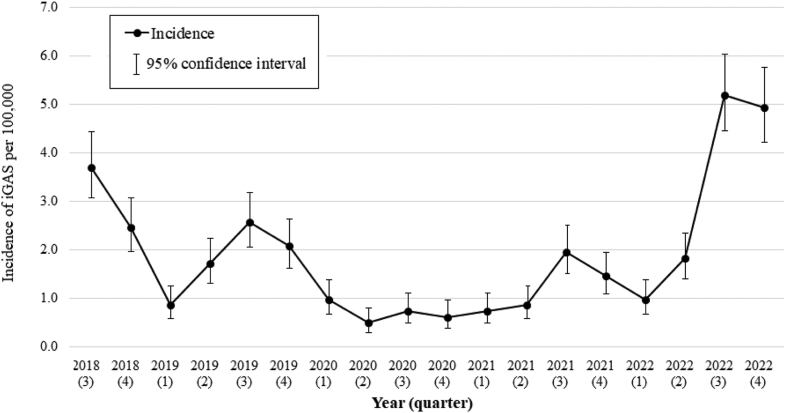
We identified 280 paediatric iGAS patients, median age 4.5 years (interquartile range 1.4–6.4). We observed a pre-pandemic peak annualised incidence of 3.7 per 100,000 (95% CI 3.1–4.4) in the 3rd quarter of 2018, followed by a decline to less than 1.0 per 100,000 per quarter from 2020 to mid-2021. The annualised incidence increased sharply from mid-2022, peaking at 5.2 per 100,000 (95% CI 4.4–6.0) in the 3rd quarter and persisting into the 4th quarter (4.9 per 100,000, 95% CI 4.2–5.7). There were 3 attributable deaths and 84 (32%) patients had severe disease (overall case fatality rate 1%, 95% CI 0.2–3.3). Respiratory virus co-infection, positive in 57 of 119 patients tested, was associated with severe disease (RR 1.9, 95% CI 1.2–3.0). The most common emm-type was emm-1 (60 of 163 isolates that underwent emm-typing, 37%), followed by emm-12 (18%).
Interpretation
Australia experienced an increase in the incidence of iGAS among children and young people in 2022 compared to pandemic years 2020–2021. This is similar to northern hemisphere observations, despite differences in seasons and circulating respiratory viruses. Outbreaks of iGAS continue to occur widely. This emphasises the unmet need for a vaccine to prevent significant morbidity associated with iGAS disease.
Funding
Murdoch Children's Research Institute funded open access publishing of this manuscript.
Keywords: Invasive group A streptococcus, Streptococcus pyogenes, Bacteraemia, Sepsis, Epidemiology, Viruses
Research in context.
Evidence before this study
On December 12, 2022, the World Health Organisation reported a surge in the incidence of invasive Group A streptococcus (iGAS) disease in multiple northern hemisphere countries, particularly affecting children and occurring during influenza and RSV seasons. We searched PubMed for clinical and laboratory studies published before March 15, 2023, using the search terms “invasive group A streptococcus” or “Streptococcus pyogenes” and “epidemiology” or “viruses∗” to identify studies describing recent and historical trends in iGAS incidence. The Australian PAEDS Network has been prospectively collecting data on iGAS hospital admissions for children and young people admitted to five tertiary hospitals in four of eight states and territories since 2016. In response to reports from the northern hemisphere, and anecdotal reports of increased incidence of iGAS in multiple states and territories in Australia, we conducted an epidemiological analysis of paediatric iGAS hospitalisations from July 2018 to December 2022.
Added value of this study
Our study provides the first report of an intense iGAS surge in the southern hemisphere contemporaneous to northern hemisphere surges, despite different seasonality and circulating respiratory virus context. Pneumonia complicated by empyema or bacteraemia with no focus together represented 50% of presentations in 2022, similar to pre-pandemic years. There was no association between year of presentation and risk of severe disease. emm-1 and emm-12 were most commonly represented.
Implications of all the available evidence
Our data lends weight to a phenomenon of increased iGAS reported in several countries since 2022. This increase is likely due to a combination of environmental, population and microbial virulence factors, and more research is needed to investigate whether new hypervirulent strains might be responsible. In addition to causing persistently high disease burden in low-resource settings, a year-on-year rise of iGAS incidence has been noted in the past decades in many high-income countries, excluding the pandemic years 2020–2021. These data add to estimates of the increasing burden of GAS disease, and emphasise the need for effective prevention, including through the development of safe and effective vaccines.
Introduction
Prior to the COVID-19 pandemic, several high-income regions reported increasing invasive group A streptococcal (iGAS) disease incidence as well as intermittent outbreaks.1, 2, 3, 4, 5 With the onset of the pandemic, several European countries and the United States observed a period of greatly reduced iGAS disease in 2020 and 2021.6 This reduction occurred in concert with non-pharmacologic interventions aimed at reducing transmission of SARS-CoV-2. Then, from early to mid-2022, countries in Europe (United Kingdom, Sweden, France, The Netherlands, Ireland) and some parts of the United States reported a marked increase in iGAS hospital admissions and deaths, especially among children aged less than 10 years.7, 8, 9, 10 As of early 2023, paediatric iGAS incidence reported in England is several times higher than pre-COVID-19 levels.11 This pattern resembles incidence changes observed for respiratory virus infections, with a substantial decline associated with 2020 and 2021 public health restrictions followed by unusually intense and unseasonal outbreaks during periods of eased restrictions.12,13 In mid-December 2022, the World Health Organization advised European countries to be vigilant for iGAS among children, to diagnose and treat cases promptly, and to consider launching public health campaigns to raise awareness of iGAS among clinicians and the public.14 Similar public health advice has been issued in the United States.15 While household contacts of cases may have an approximately 2000-times increased risk of secondary iGAS disease, the role for contact chemoprophylaxis is debated and recommendations vary internationally.16
We previously reported on iGAS burden in Australian children and young people (CYP) aged less than 18 years referred to seven tertiary paediatric centres across six (of eight total) states and territories (also known as jurisdictions) in Australia, with an estimated national incidence of 1.6 per 100,000 (95% confidence interval, CI, 1.1–2.3) between 1 July 2016 and 30 June 2018. The incidence among Aboriginal Australian and Torres Strait Islander (Indigenous) CYP was 2.1-fold higher than among Australian children overall (3.4/100,000 (95% CI, 2.1–55.7)).17 During this period, an increased incidence was observed in the third quarter of 2017 (July to September), which coincided with two months of winter and early-spring in regions with notifying hospitals, with the exception of the Northern Territory, which experiences a dry season at this time.17 Annual incidence peaks occurring in late-winter and early-spring have also been reported in the United States and Europe.4,18 This seasonal pattern is within the context of overall increasing population rates and periodic upsurges with historical time series documenting epidemic cycles of severe GAS manifestations.19
Australia closed international borders to non-Australian citizens on 20 March 2020, with staged international border reopening on 11 November 2021. Public health measures were instituted by each jurisdiction to varying degrees and included state border and school closures. Melbourne, the state capital of Victoria, experienced one of the world's longest aggregate periods in ‘lockdown’ to prevent SARS-COV-2 transmission during 2020 and 2021 (with a cumulative total 262 days in lockdown ending on October 21, 2021).20 In mid-2021, iGAS laboratory notifications became a national legal requirement in Australia, although there is no coordinated reporting on the disease burden (medical practitioners are not required to notify cases or clinical information) and the sensitivity and specificity of laboratory notification data has not been established.21 In May 2022, Northern Territory released a public health alert regarding increase in iGAS disease.22 Subsequently in late-2022, the state of Victoria released public health advice highlighting increased cases of iGAS, especially among children.23
The Paediatric Active Enhanced Disease Surveillance (PAEDS) Network collects prospective surveillance data on iGAS admissions across major paediatric hospitals in Australia, separate to any statutory notification requirements.24 To update our previous estimates of iGAS incidence17 and identify whether paediatric cases increased from mid-2018 to 2022, we described the clinical epidemiology of iGAS patients notified to the PAEDS Network.
Methods
Data source
iGAS admissions from 1 July 2018 to 31 December 2022 were collected at five tertiary/quaternary paediatric hospitals in four Australian jurisdictions: The Royal Children's Hospital (RCH) and Monash Children's Hospital (MCH; Victoria), Queensland Children's Hospital (QCH; Queensland), Royal Darwin Hospital (RDH; Northern Territory), Perth Children's Hospital (PCH; Western Australia) (Figure S1). This includes all tertiary and quaternary paediatric hospitals in the included jurisdictions, which cover an estimated 3,194,957 CYP aged 0–17 years (Table S1). New South Wales, which has an estimated CYP population size similar to Victoria (Table S1), and South Australia (which has an estimated CYP population size one quarter of Victoria) were previously included in the PAEDS Network surveillance from 2016 to June-2018.25 New South Wales and South Australia did not, however, have resourcing for study nurses to collect data so did not contribute to this analysis. Patients may have been diagnosed in a non-notifying hospital and transferred to a PAEDS Network hospital for management and were captured in the analysis; however, some CYP, particularly older children, may have been managed in peripheral general hospitals for their entire admission and therefore be omitted from this analysis.
Study procedures
Eligible patients were notified to study nurses via laboratory notifications, and study nurses also identified patients by actively screening admission lists.
Case definition and clinical data
CYP less than 18 years old admitted with iGAS during the study period were eligible for inclusion.24 We defined iGAS as GAS isolated from a normally sterile body site using standard diagnostic microbiological laboratory procedures (including culture or polymerase chain reaction (PCR)). Patients presenting with streptococcal toxic shock syndrome (STSS) and necrotising fasciitis were included if GAS was isolated from a sterile site, or a non-sterile site with compatible clinical criteria in the absence of an alternative causative organism.26 The final diagnosis was made by treating clinicians. ‘Severe disease’ was defined as admission to the intensive care unit (ICU), or requirement for (any of) vasoactive drugs, renal replacement therapy, mechanical ventilation, extracorporeal membrane oxygenation. Study nurses collected and entered data onto a RedCap®27 case report form, including episodes of medical care in the 7 days preceding iGAS hospitalisation, clinical presentation and course, laboratory data including emm-typing, management, outcome at hospital discharge (survival, morbidity or attributable death). Children dead on hospital arrival meeting the case definition of iGAS, were captured in this surveillance. In Queensland, Victoria and Western Australia, emm-gene typing was done for GAS isolates using standard laboratory protocols that did not include whole genome sequencing to identify further virulence factors or lineages.28 Whether contact prophylaxis was offered to household contacts <10 years old was also recorded; noting recommendations differ in each jurisdiction, as no national recommendations currently exist (Table S2).
In 2020, the PAEDS Network case report form was updated to collect respiratory virus co-infection data, defined as a positive respiratory virus PCR test taken at the time of admission from nasal swab, sputum, nasopharyngeal aspirate, endotracheal tube secretions or bronchoalvealar lavage fluid. Respiratory virus testing was performed according to local laboratory procedures (AusDiagnostics® Respiratory Pathogens 24-well assay (RDH), 16-well assay (RCH), 12-well assay (MCH); Biomerieux Biofire® Respiratory 2.1 Panel (PCH); Hologic Panther Fusion® SARS-CoV-2/Influenza A/B/RSV/Parainfluenza 1–4/Adenovirus/human metapneumovirus and rhinovirus assays (QCH)).29, 30, 31
Statistical analyses
Descriptive epidemiological analyses considered selected demographic and clinical features. We estimated the iGAS incidence for children aged 0–17 years using Australian Bureau of Statistics census population estimates each year quarter of the study period (Table S1). Denominator data were grouped according to age group and state/territory of notifying hospital. Individual iGAS cases were only included in the numerator once, regardless of the number of times they were admitted to hospital. The 95% CIs for the incidence were estimated using a Poisson distribution. Incidence estimates were adjusted by a factor of 4 to obtain the annualised incidence by quarter. Estimates were termed ‘minimum incidence rates’ because notifying hospitals' catchment populations did not necessarily cover the entire jurisdiction, and patients will have been hospitalised at non-tertiary/quaternary hospitals.
We calculated univariate risk ratios (RR) with 95% CIs to investigate whether selected demographic and clinical features were associated with iGAS admissions and to investigate the likelihood of patients’ household contacts being offered prophylactic antibiotics. When selecting reference groups, Victoria was selected as it had the highest number of cases, the middle age group was selected from which to compare younger and older groups, and females were selected to highlight the comparative higher risk for males. Binomial regression was used to calculate RR of severe disease against demographic, clinical and laboratory variables as well as syndrome. Clinical and laboratory variables were selected according to features commonly thought to be associated with severe iGAS.25,26 STATA 17 (Stata Corporation, College Station, TX) was used for all analyses.
Ethical considerations
Ethics approval was obtained from the Sydney Children's Hospital Network Human Research Ethics Committee (the coordinating site for all PAEDS Network studies), HREC/18/SCHN/72, with local governance approval at each site. A waiver of consent applied for collection of demographic and clinical data.
Role of the funding source
There is no funding support for this study.
Results
During the period 1 July 2018 to 31 December 2022, 292 paediatric iGAS patients were notified to the PAEDS Network, 280 of whom met the study case definition and were included in the analysis.
The mean annual incidence of iGAS across the study period was 1.8 per 100,000 children (95% CI 1.7–1.9). The annualised incidence declined to less than 1.0 per 100,000 children from 2020 to the second quarter of 2021. From the first quarter of 2022, the annualised incidence rose sharply to peak at 5.2 per 100,000 (95% CI 4.4–6.0) in the third quarter (i.e. mid-winter to mid-spring in Queensland, Victoria and Western Australia, dry season in the Northern Territory), continuing at high levels into the fourth quarter (mid-spring to mid-summer/end of dry into wet season; incidence 4.9 per 100,000, 95% CI 4.2–5.7) (Fig. 1). Quarterly incidence per jurisdiction is shown in Figure S1B.
Fig. 1.
Annualised minimum incidence of notified invasive group A streptococcal (iGAS) disease among children and young people aged less than 18 years, by yearly quarter, July 2018–December 2022ˆ. ˆQ3 (July to September) includes 2 months of winter and one month of spring in Victoria, Western Australia and Queesnland, and the dry season in the Northern Territory.
Median age at presentation was 4.5 years (interquartile range, IQR, 1.4–6.3). Incidence peaked among infants aged less than 1 year (7.9 per 100,000, 95% CI 4.3–13.2) and was lowest among those aged 15–17 years (1.0 per 100,000, 95% CI 0.3–2.3; Figure S2). Compared to children aged 5–9 years, the RR of admision for children aged less than 1 year was 4.1 (95% CI 2.0–8.3) and for children aged 1–4 years was 2.5 (95% CI 1.4–4.4). The incidence for Indigenous children was 1.8-times higher than for non-Indigenous children (95% CI 0.8–4.1), but this finding did not reach statistical significance (Table 1).
Table 1.
Key demographic characteristics of patients with iGAS notified to PAEDS network hospitals, Australia, 1 July 2018–31 December 2022.
| Number of cases | Proportion of total cases (%) | Minimum incidence per 100,000 children (95% CI) | Risk ratio (unadjusted) (95% CI) | |
|---|---|---|---|---|
| Jurisdiction | ||||
| Northern Territory | 21 | 7.5 | 8.1 (2.6–18.8) | 4.6 (1.8–11.9) |
| Western Australia | 65 | 23.2 | 2.2 (1.2–3.8) | 1.3 (0.7–2.4) |
| Queensland | 83 | 29.6 | 1.5 (0.9–2.4) | 0.9 (0.5–1.6) |
| Victoria | 111 | 39.6 | 1.8 (1.1–2.6) | Reference |
| Age Group (years) | ||||
| <1 | 45 | 16.1 | 7.9 (4.3–13.2) | 4.1 (2.0–8.3) |
| 1–4 | 135 | 48.2 | 4.8 (3.3–6.7) | 2.5 (1.4–4.42) |
| 5–9 | 68 | 24.3 | 1.2 (1.1–3.0) | Reference |
| 10–14 | 25 | 8.9 | 1.2 (0.6–2.1) | 0.6 (0.3–1.3) |
| 15–17 | 7 | 2.5 | 1.0 (0.3–2.2) | 0.5 (0.2–1.4) |
| Median age (range; IQR, years) | 4.5 (0.0–16.8; 1.4–6.4) | |||
| Indigenous | ||||
| Yes | 33 | 11.8 | 3.3 (1.3–6.8) | 1.9 (0.8–4.1) |
| No/Unknowna | 247 | 88.2 | 1.8 (1.3–2.3) | Reference |
| Gender | ||||
| Female | 108 | 38.6 | 1.5 (1.0–2.2) | Reference |
| Male | 172 | 61.4 | 2.3 (1.6–3.1) | 1.5 (0.9–2.5) |
| Main language spoken at home | ||||
| English | 213 | 76.1 | NA | NA |
| Other language | 21 | 7.5 | NA | NA |
| Unknown | 46 | 16.4 | NA | NA |
| Season of presentationb | ||||
| Summer | 54 | 20.8 | 0.4 (0.2–0.7) | Reference |
| Autumn | 32 | 12.4 | 0.2 (0.1–0.4) | 0.6 (0.2–1.5) |
| Winter | 73 | 28.2 | 0.5 (0.3–0.8) | 1.3 (0.6–2.8) |
| Spring | 100 | 38.6 | 0.7 (0.4–1.0) | 1.8 (0.9–3.7) |
| Total | 280 | 100.0 | 1.8 (1.7–1.9) | NA |
Reference group selection: Jurisdiction, Victoria has the highest number of cases; Age, middle risk group compared to other groups; Sex, highlights the elevated risk for males compared to females.
IQR; interquartile range.
Included 10 cases with unknown Indigenous status and 237 cases recorded as ‘No’.
Excludes patients notified at Royal Darwin Hospital as the region has a tropical climate with wet and dry seasons.
Clinical features of notified children
Clinical data were available for 264 patients. Pneumonia (68/264, 26%) and bacteraemia without focus (64/264, 24%) were the most common syndromes (Table 2, Figure S3). Fifty-nine cases (87%) of pneumonia were complicated by empyema. Non-necrotising soft tissue infections were the most common presentations in pandemic years 2020 and 2021 (27% and 24%, respectively). There was a similar proportion of pneumonia and empyema in 2022 compared to pre-pandemic years (I2 = 4.23, p = 0.12).
Table 2.
Syndrome of patients with iGAS notified to PAEDS Network hospitals, Australia, 1 July 2018 to 31 December 2022.
| Clinical syndrome (n, % of total cases) | Total | July–December 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|
| Pneumonia | 68 (25.8%) | 16 (37.2%) | 15 (25.9%) | 4 (18.2%) | 6 (15.9%) | 27 (26.2%) |
| Bacteraemia (no focus) | 64 (24.2%) | 13 (30.2%) | 16 (27.5%) | 1 (4.5%) | 9 (23.7%) | 25 (24.3%) |
| Empyema | 59 (22.3%) | 16 (37.2%) | 11 (19.0%) | 3 (13.6%) | 2 (5.3%) | 27 (26.2%) |
| STSS | 43 (16.3%) | 8 (18.6%) | 9 (15.5%) | 5 (22.7%) | 3 (7.9%) | 18 (17.5%) |
| Non-necrotising soft tissue | 38 (14.0%) | 4 (9.3%) | 3 (5.2%) | 6 (27.3%) | 9 (23.7%) | 16 (15.5%) |
| Osteomyelitis | 32 (12.1%) | 5 (11.6%) | 10 (17.2%) | 4 (18.2%) | 3 (7.9%) | 10 (9.7%) |
| Arthritis | 31 (11.7%) | 1 (2.3%) | 10 (17.2%) | 0 | 3 (7.9%) | 17 (16.5%) |
| Necrotising fasciitis | 6 (2.3%) | 0 | 0 | 1 (4.5%) | 3 (7.9%) | 2 (1.9%) |
| Meningitis | 5 (1.9%) | 0 | 0 | 3 (13.6%) | 1 (2.6%) | 1 (0.9%) |
| Peritonitis | 3 (1.1%) | 1 (2.3%) | 0 | 0 | 1 (2.6%) | 1 (0.9%) |
| Surgical site | 2 (0.7%) | 0 | 0 | 0 | 1 (2.6%) | 1 (0.9%) |
| Other | 33 (12.5%) | 5 (11.6%) | 9 (15.5%) | 6 (27.3%) | 4 (10.5%) | 9 (8.7%) |
| Total casesa | 264 | 43 | 58 | 22 | 38 | 103 |
Other: subdural empyema, appendicitis, sinusitis, myositis, central line infection, muscle abscess, mastoiditis, rhabdomyolysis, lymphadenitis, tonsillitis, mediastinitis, neck abscess, bursitis, epiglottitis.
At least one sterile site isolate was collected in 260 cases (304 total isolates). 156 cases had a positive blood culture, 35 of these had a second positive invasive site.
GAS was isolated from a non-invasive site in 74 cases (28%), usually from a skin swab (12 cases).
Cases may have more than one sydrome, as determined by the treating clinician. 42 cases were diagnosed with 2 different clinical syndromes and 1 with 3 clinical syndromes (soft tissue infection, osteomyelitis and peritonitis).
In the seven days prior to hospital admission, 187/264 patients (71%) consulted a doctor. Of these, 130 (70%) consulted a general practitioner, 118 (63%) an emergency department physician, and 61 (33%) both. Eighty-six patients (33%) consulted a doctor more than once. The diagnosis at this initial consultation was recorded for 136 patients; most commonly it was an unspecified viral illness or upper respiratory tract infection (44/136, 32%), followed by lower respiratory tract infection (12/136, 9%) or rash or skin infection (11/136, 8%). Overall, 147/187 children (79%) were sent home after this consultation without being hospitalised.
On the day of hospital admission, the most common presenting clinical feature was fever (234 patients, 89%) with a median duration of 3 days prior to admission (IQR 1–5). One hundred and seven patients (41%) had a rash, of whom 29 had petechiae or purpura (27%). Ninety-four patients (36%) had limb pain reported. In the month preceding iGAS hospitalisation, one patient had a varicella zoster virus infection.
Eighty-four patients (32%) had severe disease. Of those, forty-seven patients (56%) required mechanical ventilation, 47 (56%) required vasoactive drugs, 9 (10%) required extracorporeal membrane oxygenation, and 11 (13%) required renal replacement therapy. Twenty-one (25%) patients with severe disease were admitted to ICU for observation or non-invasive respiratory support without any other specific therapy listed above. Fifty-nine out of 84 patients with severe disease received clindamycin (70%), 35/84 (41%) received intravenous immunoglobulin. Demographic, clinical and laboratory characteristics and the RR of severe disease are outlined in Table 3. STSS, pneumonia and empyema were associated with an increased risk of severe disease. The proportion of patients with severe disease did not change by year of presentation (I2 = 4.25, p = 0.37) and disease severity was not associated with year of presentation.
Table 3.
Riska of severe diseaseb according to selected demographic, clinical and laboratory characteristics of patients with iGAS notified to PAEDS Network hospitals, Australia, 1 July 2018 to 31 December 2022.
| Characteristic | Severe disease n/N (%) | Non-severe disease n/N (%) | Risk ratio (95% CI) |
|---|---|---|---|
| Year of presentation | |||
| 2018 | 17/43 (40%) | 26/43 (60%) | Reference group |
| 2019 | 16/58 (28%) | 42/58 (72%) | 0.7 (0.4, 1.2) |
| 2020 | 7/22 (32%) | 15/22 (68%) | 0.8 (0.4, 1.6) |
| 2021 | 8/38 (21%) | 30/38 (79%) | 0.5 (0.3, 1.1) |
| 2022 | 36/102 (35%) | 66/102 (65%) | 0.9 (0.6, 1.4) |
| Indigenous | 7/32 (22%) | 25/32 (78%) | 0.7 (0.3, 1.3) |
| Age <4 years | 51/152 (34%) | 101/152 (66%) | 1.1 (0.8, 1.6) |
| Female | 35/106 (33%) | 71/106 (67%) | 1.1 (0.7,1.5) |
| Presenting feature | |||
| GP/ED visit in previous 7 days | 63/182 (35%) | 119/182 (65%) | 1.3 (0.9, 2.0) |
| NSAID previous 7 days | 33/86 (38%) | 53/86 (62%) | 1.4 (0.9, 2.1) |
| Rash (any) | 41/107 (38%) | 66/107 (62%) | 1.5 (1.0, 2.1) |
| Petechial or purpuric rash | 12/28 (43%) | 16/28 (57%) | 1.4 (0.9, 2.2) |
| Fever | 75/230 (33%) | 155/230 (67%) | 1.4 (0.7, 2.9) |
| Limb pain | 21/94 (22%) | 73/94 (78%) | 0.7 (0.4, 1.0) |
| Bolus given within 4 h | 48/85 (56%) | 37/85 (44%) | 3.0 (2.0, 4.3) |
| Syndrome | |||
| Bacteraemia with no focus | 18/64 (28%) | 46/64 (72%) | 0.9 (0.5, 1.3) |
| STSS | 38/43 (88%) | 5/43 (12%) | 4.4 (3.3, 5.8) |
| Pneumonia | 42/68 (62%) | 26/68 (38%) | 2.9 (2.1, 4.0) |
| Empyema | 39/59 (66%) | 20/59 (34%) | 3.01 (2.19, 4.13) |
| NF | 1/6 (17%) | 5/6 (83%) | 0.5 (0.1, 3.1) |
| Skin and soft tissue infection excluding NF | 7/38 (18%) | 31/38 (82%) | 0.5 (0.3, 1.1) |
| Arthritis | 3/31 (9.7%) | 28/31 (90%) | 0.3 (0.1, 0.8) |
| Osteomyelitis | 4/32 (12%) | 28/32 (88%) | 0.4 (0.1, 0.9) |
| Meningitis | 3/5 (60%) | 2/5 (40%) | 1.9 (0.9, 4.0) |
| Other | 10/33 (30%) | 23/33 (70%) | 1.0 (0.5, 1.6) |
| Laboratory feature | |||
| Renal impairment | 26/32 (81%) | 6/32 (19%) | 3.2 (2.4, 4.3) |
| Liver impairment | 28/46 (61%) | 18/46 (39%) | 2.3 (1.6, 3.2) |
| Coagulopathy | 49/68 (72%) | 19/68 (28%) | 4.0 (2.9, 5.6) |
| Neutropenia | 8/11 (73%) | 3/11 (27%) | 2.5 (1.6, 3.7) |
| Respiratory virus co-infection | 25/57 (44%) | 32/57 (56%) | 1.9 (1.2, 3.0) |
GP, general practitioner; ED, emergency department; NSAID, non-steroidal anti-inflammatory drug; NF, necrotising fasciitis; STSS, streptococcal toxic shock syndrome.
Bold denotes statistical significance.
Univariate risk ratio using binomial regression STATA 17 (Stata Corporation, College Station, TX).
Severe disease was defined as intensive care admission, or any of mechanical ventilation, inotropes, vasopressors or extracorporeal membrane oxygenation.
168 patients (64%) had a surgical procedure, including 54 who had two or more surgical procedures (maximum 13 procedures). One patient underwent an above knee amputation and another had multiple finger-tips amputated.
Three patients died, all in 2022 (case fatality rate 1%, 95% CI 0.2–3.3); two had STSS and one had bacteraemia with no focus (all had severe disease). Outcome at discharge was reported for 228 (86%) patients; 43 (16%) had ongoing morbidity reported, including 33 with physical impairment (82%), and 14 with fatigue (32%). Four patients had permanent disability (2 hemiplegia, 1 above knee amputation, 1 immobility and intellectual disability in a patient with meningitis).
One hundred and thirty-eight (49%) patients had one or more household contacts offered prophylaxis to prevent secondary disease (Table S3).
Respiratory virus co-infection
In addition to isolated SARS-CoV-2 tests that were performed on all hospitalised children, from 2020 to 2022, 119 of 163 (73%) patients had an extended molecular respiratory pathogen panel sample collected; 7/22 (32%) in 2020, 26/38 (68%) in 2021, and 86/107 (72%) in 2022. In total 57 of 119 (48%) patients tested had a respiratory virus identified (4/7 in 2020, 7/26 on 2021 and 46/86 in 2022, Figure S4). Only three of 163 iGAS patients (2%) had a SARS-CoV-2 co-infection detected. Influenza A or B was detected in 3/163 (2%) patients. In 2022, the most commonly identified viruses were rhinovirus or rhinovirus/enterovirus (20/46, 43%), human metapneumovirus (12/46, 26%) and adenovirus (10/46, 22%) (Figure S4). Respiratory viruses (2020–2022) were detected in 22 patients with pneumonia (35%) and 21 with pneumonia complicated by pleural empyema (37%). Patients with a respiratory virus detected were more likely to have severe disease (RR 1.9, CI 1.2–3, Table 3).
Molecular (emm-type) distribution
Molecular (emm-type) typing data were available for 163 patients (58%). A total of 25 unique emm-types were detected. The most common was emm-1, identified in 60 patients (37%) followed by emm-12 in 30 patients (18%; Fig. 2). emm-1 was most commonly associated with pneumonia (28/60, 47%); emm-12 was most commonly associated with pneumonia and bacteraemia without focus (9/30 each, 30%). Of patients with severe disease, emm-typing data were available for 60/84 (71%); 27/60 were emm-1 (45%) and 11/60 were emm-12 (18%, Figure S5). During the 2022 surge, isolates from 74/107 (69%) patients were available; 30/74 were emm-1 (41%) and 18/74 were emm-12 (24%).
Fig. 2.
emm-type distribution among 163 paediatric iGAS cases notified to the PAEDS Network from 1 July 2018 to 31 December 2022. ∗‘Other’ indicates 19 different emm-types: emm-2, -6, -9, -11, -22, -28, -36, 41, -44, -49, -59, -71, -76, -77, -82, -87, -92, -110. 1 isolate was non-typeable.
Discussion
We identified an intense and unseasonal surge in iGAS occurring from mid-2022 in a large cohort of Australian CYP, contemporaneous to the iGAS increase seen in countries in the northern hemisphere. We observed a peak annualised incidence of 5.2 per 100,000 in the third quarter of 2022 which persisted at similarly high levels into the fourth quarter. At this stage, it is unclear whether this high incidence of iGAS will persist or increase further in 2023.
Our findings are similar to those reported in the northern hemisphere, despite different seasons and circulating respiratory viruses.7,8 The iGAS increase in the UK has continued into the first quarter of 2023.11 Reports from the United States indicate that the unseasonal increase in paediatric iGAS has persisted in 2023 in some areas, merging with their usual peak period (December to April).10 Europe has also reported a similar pattern of high incidence among those aged 1–4 years.32, 33, 34, 35 We did not observe an increase in pneumonia complicated by empyema in 2022 compared to pre-pandemic years 2018–2019, in contrast to international reports.7,8
Our previous analysis of PAEDS data described a peak iGAS incidence of 3.4 per 100,000 (95% CI: 2.5–4.5) occurring among Australian children in the third quarter of 2017. This peak was associated with a severe influenza season.17 Both the pre-pandemic peak we described in the third quarter of 2018, and the peak occurring in the third quarter of 2022 exceed this incidence. The proportion of iGAS patients with severe disease, and the case fatality rate, was lower in our study than in our previous analysis, which included New South Wales and South Australia (32% versus 41% and 1% vs 3%, respectively) possibly due to inclusion of additional quaternary centres in the prior dataset.25 This case fatality rate is consistent with reports from other high-income countries (0% amongst children 1–5 years in Norway and 3% among children 0–5 years in USA).36
It is unclear why there has been a contemporaneous surge in paediatric iGAS reported in Australia, the US and Europe, but there are several possible explanations. First, reduced social contact through the pandemic due to non-pharmacological interventions such as school closures and mask wearing may have caused relatively lower dynamic population immunity to GAS through delayed initial pathogen exposures and removing the usual periodic boosting effect from seasonal exposures.37,38 This was reflected most clearly in Victoria which had the lowest incidence of iGAS coinciding with the longest period of public health restrictions including school closures and mask mandates. Western Australia, in contrast, had long periods of border closures but only short periods of school closures, and a higher incidence of iGAS compared to Victoria in 2020/2021.39 Second, changes in GAS strains or a highly virulent strain may be responsible, but diverse GAS emm-types have been associated with the recent surges, suggesting a single virulent clone is not a major driver.37,40,41 Although detailed genomic and microbiological studies are needed to answer that question, including to assess whether the M1UK strain was implicated in 2022 presentations,42 we also observe that the emm-types associated with the current outbreak is no different from those usually associated with iGAS in Australia.17,25 Third, resurgent circulating respiratory viruses have been proposed as a driver for the surge in iGAS. Temporal associations between influenza and iGAS have been previously described.17,43,44 The 2022 iGAS peak observed in our study did not occur during the peak influenza season (which occurred during quarter 2, 2022), unlike in the northern hemisphere.35,41,45 With the ceasing of restrictions in Australia, there was widespread infection of CYP with the Omicron variant of SARS-CoV-2; serosurveys in mid-2022 found that around two-thirds of children had evidence of previous infection.46,47 However, SARS-CoV-2 and iGAS co-infection was detected in just three patients, despite near-universal testing at most hospitals since 2020. RSV became nationally notifiable in Australia in 2022. Peak notifications occurred in the third quarter of 2022 in Victoria, Northern Territory and Western Australia when the peak iGAS incidence in our cohort was noted, and RSV activity peaked in the second quarter of 2022 in Queensland (which notified 30% of iGAS patients and where the peak incidence of iGAS occurred in the third quarter of 2022).48 A high proportion of RSV co-infection during this surge in iGAS incidence was not reflected in our data (5 patients in 2022 had RSV co-infection). Internationally, high rates of respiratory virus co-infection with iGAS have not been widely documented. In UK laboratory surveillance data from January 2018 to November 2022, respiratory virus co-infection occurred in 25.8% of patients during peak iGAS detections, similar to levels seen in past seasons.7 In 2020 and 2021 in Australia, iGAS surges did not follow the pattern of out-of-season RSV surges that occurred during breaks between lockdowns.49 Finally, varicella zoster virus vaccination is included in the Australian national immunisation schedule at 18 months of age, likely accounting for the minimal number of patients (1 of 264) with varicella infection in the month preceding iGAS hospitalisation, in contrast to reports from the Netherlands.8
There are limitations to this analysis. First, our dataset is not representative of Australian paediatric iGAS patients as our case detection was limited to five major hospitals located in four of the eight Australian jurisdictions. Despite this, all tertiary and quaternary hospitals in participating states and territories were included, so our reporting on severe cases in included jurisdictions is likely to be complete. While the PAEDS Network provides the largest longitudinal clinical and epidemiological data on paediatric iGAS currently available in Australia, this dataset is relatively small and the factors we have identified as being associated with severe disease should not be over-interpreted. Second, certain severe GAS infections where GAS is not isolated from a normally sterile site, such as retropharyngeal abscess or mastoiditis, were not consistently notified to the PAEDS Network and therefore were excluded from this analysis. Ensuring the use of standardised definitions for iGAS disease, including confirmed or probable case classifications, is important to enable incidence comparisons between studies.50 Third, emm-type data were not available for almost half of the included patients. National surveillance data will be important to provide a clearer picture of circulating emm-types in the future. The PAEDS Network's iGAS data is important for informing national control and prevention efforts, including vaccine development, particularly as this data source enables changes in iGAS presentations to be described while national reporting pathways remain in development.
Conclusions
This multicentre analysis suggests Australian CYP have been affected by an unseasonal increase in iGAS disease, occurring at the same time as increases in the northern hemisphere. There is an ongoing burden of iGAS disease in children, which is frequently severe, and appears to occur in surges. Our data add weight to calls to develop a vaccine to reduce the global burden of iGAS and other severe GAS syndromes including acute rheumatic fever and rheumatic heart disease.
Contributors
YN Abo and J Oliver are joint first authors and were responsible for the concept and design, data analysis and preparation of the initial draft manuscript. A McMinn, N Crawford and AC Steer were responsible for project administration and resources. N Crawford and AC Steer are joint supervising authors. All authors contributed to the data interpretation and review, editing, and manuscript revisions. All authors are responsible for reported content and approve the manuscript as submitted.
Declaration of interests
AC Steer acts as Co-Director of Australian Strep A Vaccine Initiative and Co-Chair of Strep A Vaccine Global Consortium. The rest of the authors declare no competing interest.
Acknowledgements
We would like to thank the PAEDS Coordinating Centre at The Children's Hospital at Westmead, Sydney, Australia; the PAEDS research nurses; Ms Kristy Azzopardi, Murdoch Children's Research Institute, Melbourne, Australia; Professor Jim Buttery, Murdoch Children's Research Institute, Melbourne, Australia; and all the microbiology laboratories for submitting isolate reports to the surveillance database as well as isolates to the reference laboratories for molecular typing.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100873.
Appendix A. Supplementary data
References
- 1.Tyrrell G.J., Fathima S., Kakulphimp J., et al. Increasing rates of invasive group A streptococcal disease in Alberta, Canada; 2003-2017. Open Forum Infect Dis. 2018;5(8) doi: 10.1093/ofid/ofy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver J., Wilmot M., Strachan J., et al. Recent trends in invasive group A Streptococcus disease in Victoria. Comm Dis Intell. 2019;43 doi: 10.33321/cdi.2019.43.8. [DOI] [PubMed] [Google Scholar]
- 3.Bronze M.S., Dale J.B. The reemergence of serious group A streptococcal infections and acute rheumatic fever. Am J Med Sci. 1996;311(1):41–54. doi: 10.1097/00000441-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Lamagni T.L., Darenberg J., Luca-Harari B., et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008;46(7):2359–2367. doi: 10.1128/jcm.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamagni T., Guy R., Chand M., et al. Resurgence of scarlet fever in England, 2014-16: a population-based surveillance study. Lancet Infect Dis. 2018;18(2):180–187. doi: 10.1016/s1473-3099(17)30693-x. [DOI] [PubMed] [Google Scholar]
- 6.Prasad N., Rhodes J., Deng L., et al. Changes in the incidence of invasive bacterial disease during the COVID-19 pandemic in the United States, 2014-2020. J Infect Dis. 2023 doi: 10.1093/infdis/jiad028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guy R., Henderson K.L., Coelho J., et al. Increase in invasive group A streptococcal infection notifications, England, 2022. Euro Surveill. 2023;28(1) doi: 10.2807/1560-7917.ES.2023.28.1.2200942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Kempen E.B., Bruijning-Verhagen P.C.J., Borensztajn D., et al. Increase in invasive group a streptococcal infections in children in the Netherlands, a survey among 7 hospitals in 2022. Pediatr Infect Dis J. 2023;42 doi: 10.1097/inf.0000000000003810. [DOI] [PubMed] [Google Scholar]
- 9.Bagcchi S. Surge of invasive group A streptococcus disease. Lancet Infect Dis. 2023;23(3):284. doi: 10.1016/s1473-3099(23)00043-9. [DOI] [PubMed] [Google Scholar]
- 10.Increase in invasive group A strep infections, 2022–2023. US Centres for Disease Control and Prevention; 2023. https://www.cdc.gov/groupastrep/igas-infections-investigation.html Available from: [Google Scholar]
- 11.UKHSA update on scarlet fever and invasive group A strep: United Kingdom health security agency. 2023. https://www.gov.uk/government/news/ukhsa-update-on-scarlet-fever-and-invasive-group-a-strep-1 Available from: [Google Scholar]
- 12.Taylor A., Whittaker E. The changing epidemiology of respiratory viruses in children during the COVID-19 pandemic: a canary in a COVID time. Pediatr Infect Dis J. 2022;41(2):e46–e48. doi: 10.1097/inf.0000000000003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billard M.N., van de Ven P.M., Baraldi B., et al. International changes in respiratory syncytial virus (RSV) epidemiology during the COVID-19 pandemic: association with school closures. Influenza Other Respir Viruses. 2022;16(5):926–936. doi: 10.1111/irv.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . 2022. Increase in invasive group A streptococcal infections among children in Europe, including fatalities Geneva, Switzerland.https://www.who.int/europe/news/item/12-12-2022-increase-in-invasive-group-a-streptococcal-infections-among-children-in-europe--including-fatalities Available from: [Google Scholar]
- 15.National Center for Immunization and Respiratory Diseases. Division of Bacterial Diseases. Centers for Disease Control and Prevention . United States Government; Atlanta, United States: 2022. Increase in invasive group A strep infections, 2022–2023.https://www.cdc.gov/groupastrep/igas-infections-investigation.html Available from: [Google Scholar]
- 16.Mearkle R., Saavedra-Campos M., Lamagni T., et al. Household transmission of invasive group A Streptococcus infections in England: a population-based study, 2009, 2011 to 2013. Euro Surveill. 2017;22(19) doi: 10.2807/1560-7917.Es.2017.22.19.30532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver J., Thielemans E., McMinn A., et al. Invasive group A Streptococcus disease in Australian children: 2016 to 2018—a descriptive cohort study. BMC Public Health. 2019;19(1):1750. doi: 10.1186/s12889-019-8085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson G.E., Pondo T., Toews K.A., et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478–486. doi: 10.1093/cid/ciw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Efstratiou A., Lamagni T. In: Streptococcus pyogenes: basic biology to clinical manifestations Oklahoma City. Ferretti J.J., Stevens D., Fischetti V.A., editors. University of Oklahoma Health Sciences Center; 2022. Chapter 19. Epidemiology of Streptococcus pyogenes. [PubMed] [Google Scholar]
- 20.Jose R. Reuters; 2021. Melbourne readies to exit world's longest COVID-19 lockdown. [Google Scholar]
- 21.Dow A., Cunningham M. The Age; 2023. Two children die amid 'marked increase' in invasive strep A infections. [Google Scholar]
- 22.Health alert: increase in group A streptococcal (GAS) diseases. Northern territory government CDC surveillance. https://health.nt.gov.au/__data/assets/pdf_file/0009/1111212/group-a-streptococcal-health-alert-220531.pdf Available from:
- 23.Victorian Government Department of Health . 2022. Health warning on invasive group A streptococcal disease Melbourne.https://www.health.vic.gov.au/health-advisories/health-warning-on-invasive-group-a-streptococcal-disease Australia. Available from: [Google Scholar]
- 24.The Australian Paediatric Surveillance Unit . National Centre for Immunisation Research and Surveillance; Sydney, Australia: 2022. Paediatric active enhaned disease surveillance.https://paeds.org.au/surveillance-and-research Available from: [Google Scholar]
- 25.Thielemans E., Oliver J., McMinn A., et al. Clinical description and outcomes of Australian children with invasive group A streptococcal disease. Pediatr Infect Dis J. 2020;39(5):379–384. doi: 10.1097/INF.0000000000002596. [DOI] [PubMed] [Google Scholar]
- 26.Steer A.C., Lamagni T., Curtis N., et al. Invasive group a streptococcal disease: epidemiology, pathogenesis and management. Drugs. 2012;72(9):1213–1227. doi: 10.2165/11634180-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frost H.R., Davies M.R., Velusamy S., et al. Updated emm-typing protocol for Streptococcus pyogenes. Clin Microbiol Infect. 2020;26(7):946.e5–946.e8. doi: 10.1016/j.cmi.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 29.AusDiagnostics respiratory pathogens 2023. 2023. https://www.ausdiagnostics.com/respiratory-pathogens/ Available from: [Google Scholar]
- 30.Biomeriux . In: Internet. Biomerieux, editor. 2022. BioFire® respiratory panel 2.1 (RP2.1) product information. [Google Scholar]
- 31.Panther fusion® respiratory assays. 2023. https://www.hologic.com/hologic-products/diagnostic-solutions/panther-fusion-respiratory-assays Available from: [Google Scholar]
- 32.Ladhani S.N., Guy R., Bhopal S.S., et al. Paediatric group A streptococcal disease in England from October to December, 2022. Lancet Child Adolesc Health. 2023;7(2):e2–e4. doi: 10.1016/S2352-4642(22)00374-1. [DOI] [PubMed] [Google Scholar]
- 33.Cobo-Vázquez E., Aguilera-Alonso D., Carrasco-Colom J., et al. Increasing incidence and severity of invasive Group A streptococcal disease in Spanish children in 2019-2022. Lancet Reg Health Eur. 2023;27 doi: 10.1016/j.lanepe.2023.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.HPSC update on group A streptococcus: health protection surveillance centre. Ireland. 2023. https://www.hpsc.ie/news/title-22663-en.html Available from: [Google Scholar]
- 35.de Gier B., Marchal N., de Beer-Schuurman I., et al. Increase in invasive group A streptococcal (Streptococcus pyogenes) infections (iGAS) in young children in the Netherlands, 2022. Euro Surveill. 2023;28(1) doi: 10.2807/1560-7917.Es.2023.28.1.2200941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherwood E., Vergnano S., Kakuchi I., et al. Invasive group A streptococcal disease in pregnant women and young children: a systematic review and meta-analysis. Lancet Infect Dis. 2022;22:1076. doi: 10.1016/S1473-3099(21)00672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ladhani S.N., Guy R., Bhopal S.S., et al. Paediatric group A streptococcal disease in England from October to December, 2022. Lancet Child Adolesc Health. 2022;7:e2. doi: 10.1016/S2352-4642(22)00374-1. [DOI] [PubMed] [Google Scholar]
- 38.Needle R.F., Russell R.S. Immunity debt, a gap in learning, or immune dysfunction? Viral Immunol. 2023;36(1):1–2. doi: 10.1089/vim.2022.0204. [DOI] [PubMed] [Google Scholar]
- 39.Moore H.C., Le H., Mace A., et al. Interrupted time-series analysis showed unintended consequences of non-pharmaceutical interventions on pediatric hospital admissions. J Clin Epidemiol. 2022;143:1–10. doi: 10.1016/j.jclinepi.2021.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Kempen E.B., Bruijning-Verhagen P.C., Borensztajn D., et al. Increase in invasive group a streptococcal infections in children in the Netherlands, a survey among 7 hospitals in 2022. Pediatr Infect Dis J. 2022;42:e122. doi: 10.1097/INF.0000000000003810. [DOI] [PubMed] [Google Scholar]
- 41.Barnes M., Youngkin E., Zipprich J., et al. Notes from the field: increase in pediatric invasive group A streptococcus infections–Colorado and Minnesota, October-December 2022. MMWR Morb Mortal Wkly Rep. 2023;72(10):265–267. doi: 10.15585/mmwr.mm7210a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies M.R., Keller N., Brouwer S., et al. Detection of Streptococcus pyogenes M1UK in Australia and characterization of the mutation driving enhanced expression of superantigen SpeA. Nat Commun. 2023;14(1):1051. doi: 10.1038/s41467-023-36717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darenberg J., Henriques-Normark B., Lepp T., et al. Increased incidence of invasive group A streptococcal infections in Sweden, January 2012-February 2013. Euro Surveill. 2013;18(14) doi: 10.2807/1560-7917.es2013.18.14.20443. [DOI] [PubMed] [Google Scholar]
- 44.Tasher D., Stein M., Simões E.A., et al. Invasive bacterial infections in relation to influenza outbreaks, 2006-2010. Clin Infect Dis. 2011;53(12):1199–1207. doi: 10.1093/cid/cir726. [DOI] [PubMed] [Google Scholar]
- 45.Department of Health and Aged Care . 2022. Australian influenza surveillance report–2022 national influenza season summary: commonwealth of Australia.https://www.health.gov.au/resources/publications/aisr-2022-national-influenza-season-summary?language=en Available from: [Google Scholar]
- 46.Paediatric SARS-CoV-2 serosurvey 2022, Australia summary report: paediatric active enhanced surveillance. 2022. [Google Scholar]
- 47.Cheng D.R., Schrader S., McMinn A., et al. Paediatric admissions with SARS-CoV-2 during the Delta and Omicron waves: an Australian single-centre retrospective study. BMJ Paediatr Open. 2023;7(1) doi: 10.1136/bmjpo-2023-001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National communicable disease surveillance dashboard–respiratory syncytial virus. Australian Government Department of Health and Aged Care; 2023. https://nindss.health.gov.au/pbi-dashboard/ Available from: [Google Scholar]
- 49.Abo Y.N., Clifford V., Lee L.Y., et al. COVID-19 public health measures and respiratory viruses in children in Melbourne. J Paediatr Child Health. 2021;57:1886. doi: 10.1111/jpc.15601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller K.M., Lamagni T., Cherian T., et al. Standardization of epidemiological surveillance of invasive group A streptococcal infections. Open Forum Infect Dis. 2022;9(Suppl 1):S31–S40. doi: 10.1093/ofid/ofac281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.