Highlights
-
•
Establishment of 3D cardiac organoids composed of cardiomyocyte and endothelial layers.
-
•
SARS-CoV-2 infection causes multi-lineage cardiac injuries.
-
•
Cardiovascular health should be greatly concerned in COVID-19 patients.
Dear editor,
The pandemic of coronavirus disease-19 (COVID-19) has lasted for more than three years, causing lots of extra death and posing a serious threat to public health. This pandemic swept the last global safety island in a matter of months, coinciding with the switch of zero-COVID policy of China. The causative agent, SARS-CoV-2, spreads globally and then lurks in the population with sporadic outbreaks in local areas. Meanwhile, the sequelae of convalescent COVID-19 patients receded slowly. The pan-tissue tropism of SARS-CoV-2 results in a wide range of symptoms in patients, including cardiovascular disorders. Cardiac complications occur in 20%–44% of hospitalized patients, which is an independent risk factor for COVID-19 mortality (Ma et al., 2022; Patone et al., 2022). Survivors of acute COVID-19 also have a substantial risk of cardiovascular disease burden lasting up to one year (Xie et al., 2022). Understanding the mechanisms of the viral effects on the cardiovascular system could contribute to therapeutic strategy development aimed at reducing the mortality and morbidity associated with SARS-CoV-2 infection. Previously studies utilizing two-dimensional (2D) differentiation model based on human-induced pluripotent stem cell (hiPSC) showed that SARS-CoV-2 can directly infect cardiomyocytes, but not endothelial or fibroblast cells (Bojkova et al., 2020; Bailey et al., 2021). In contrast, endothelium infection by SARS-CoV-2 was reported in autopsy and vascular organoids (Varga et al., 2020). It might be possible that 2D cell culture fails to mimic the complexity of the host organisms thus exhibits a different phenotype from natural tissues. An advanced model with higher similarity to in vivo human tissues/organs in physiological and pathological characteristics is expected to study SARS-CoV-2 infection and consequent pathophysiology of injured organs. The combination of 3D cardiac organoids and viral infection would provide a new tool for advancing research on cardiac pathogenesis and drug discovery (Clevers, 2020; Yang et al., 2023).
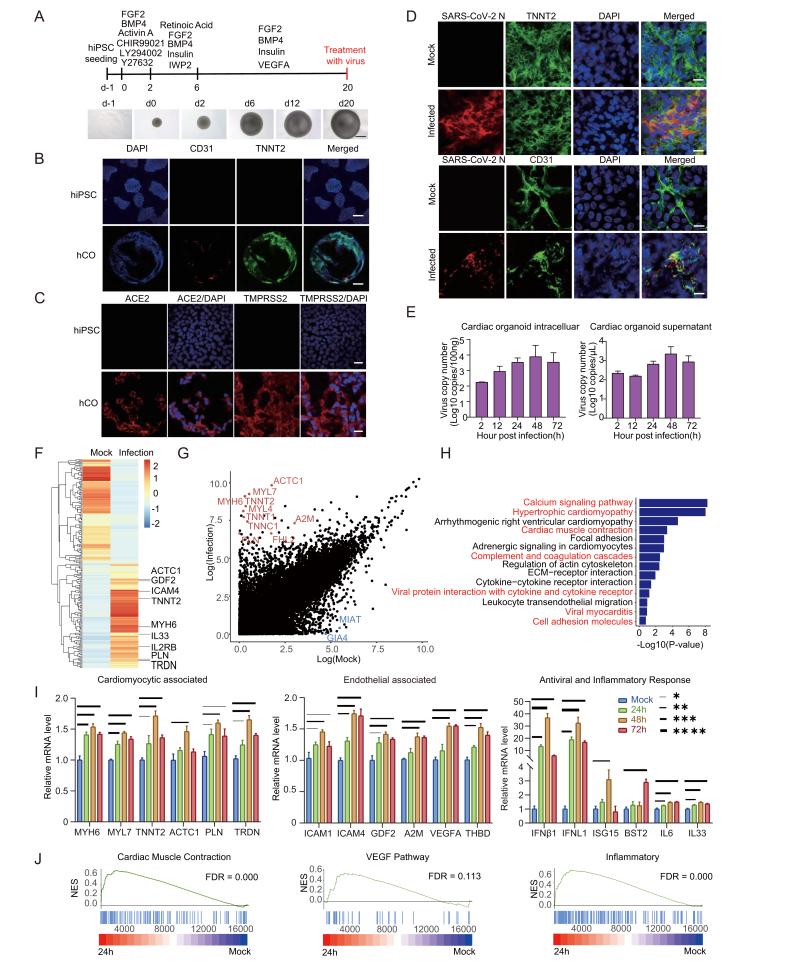
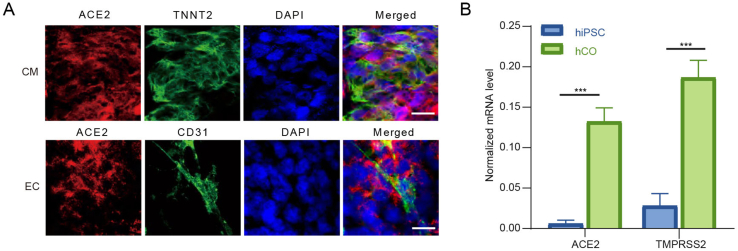
To establish human cardiac organoids (hCOs), the hiPSC were cultured and induced to assemble spontaneously by sequential addition of indicated growth factors and small molecules regulating heart development at different differentiation stages (Fig. 1A) (Lewis-Israeli et al., 2021). All experiment details were shown in Supplementary materials. Beating hCOs were observed at day 12 post differentiation, then all the organoids acquired robust and regular beating by day 20 post differentiation (Supplementary Video S1). After hCOs fixation, the myocardial and endocardial lineage were identified by immunofluorescence assay (IFA) using cell-specific markers Troponin T2 (TNNT2) and CD31 (also known as platelet endothelial cell adhesion molecule-1, PECAM-1), respectively (Fig. 1B). Further, we tested whether the hCOs express angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) which play a role in SARS-CoV-2 entry. The IFA and qRT-PCR results showed the expression of ACE2 and TMPRSS2 in hCOs, suggesting them potentially susceptible to SARS-CoV-2 infection (Fig. 1C and Supplementary Fig. S1).
Fig. 1.
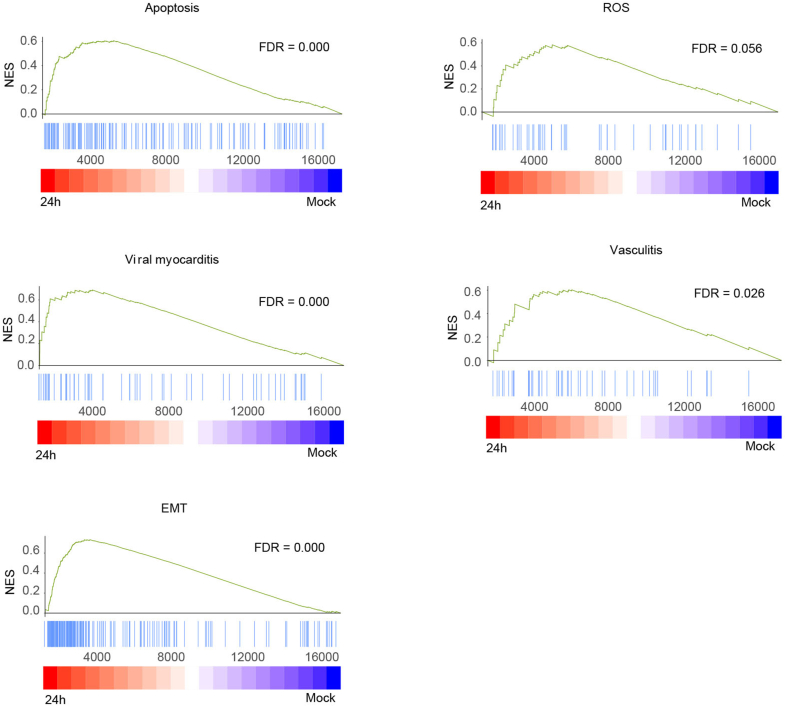
Overview of experimental protocol and results of 3D cardiac organoids culture. A Cardiac organoids were generated by step-wise differentiation of human-induced pluripotent stem cells. Scale bar: 200 μm. B Cell composition and distribution of cardiac organoids were assessed using immunofluorescence whole-mount microscopy. Scale bar: 100 μm. C Immunofluorescence staining for ACE2 and TMPRSS2 expression in hCOs and hiPSCs. Scale bar: 20 μm. D Immunofluorescence staining for SARS-CoV-2 N protein and cardiomyocyte marker TNNT2, as well as SARS-CoV-2 N protein and endothelial cell marker CD31. The hCOs were fixed by paraformaldehyde at 2 days post infection. Scale bar: 10 μm. E Viral RNA copies were quantified in SARS-CoV-2 infected cardiac organoids and the supernatant. F Heatmap showing differentially expressed genes (DEGs) in SARS-CoV-2-infected organoids (24 h) versus mock. G Differential normalized log2 expression in SARS-CoV-2-infected organoids (24 h) versus the mock infected organoids. H KEGG analysis of DEGs in SARS-CoV-2-infected organoids (24 h) versus mock infected organoids. I the cardiac organoids after SARS-CoV-2 infection were harvested to examine the expression of indicated genes using qRT-PCR. GAPDH was used as an internal control. Data were presented as mean ± SD. ∗ indicates P < 0.05; ∗∗ indicates P < 0.01; ∗∗∗ indicates P < 0.001. ∗∗∗∗ indicates P < 0.0001. J GSEA enrichment analysis of SARS-CoV-2-infected organoids (24 h) versus mock-infected organoids. Please see the Supplementary data Section for full experimental details.
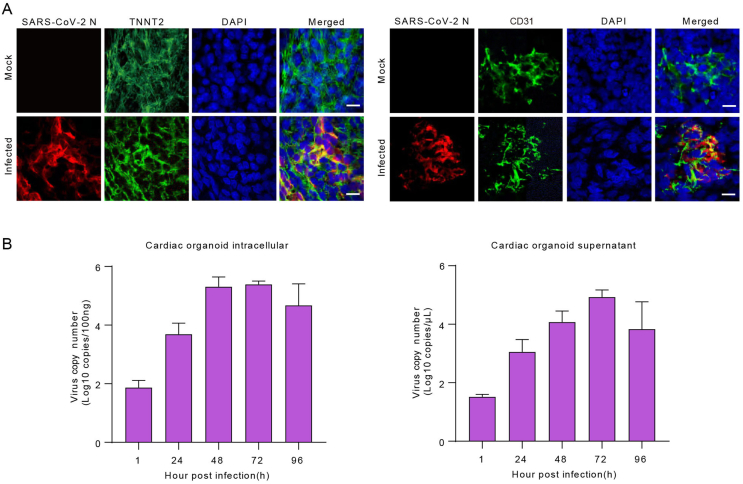
We then infected the hCOs with the SARS-CoV-2 prototype (Wuhan-Hu-1) and Omicron BA.5. All work involving infectious SARS-CoV-2 was performed in a Biosafety level 3 laboratory. The SARS-CoV-2 strains were cultured and titrated on Vero E6 cell line (ATCC CRL-1586). Then the hCOs were co-incubated with SARS-CoV-2 with multiplicity of infection (MOI) = 5. After washing twice with culture medium, the hCOs were maintained under 37 °C with 5% CO2 in U bottom ultra-low attachment 96-well plates. Viral infections were verified by co-localization of viral nucleocapsid protein (NP) with myocardial and endocardial lineage markers by immunofluorescence staining (Fig. 1D and Supplementary Fig. S2A). The hCOs and culture medium were collected for viral RNA quantification by qRT-PCR at planned time points. We observed a substantially increase in the BA.5 viral RNA load in the cells (more than 3 log units) and the culture medium (1 log unit) (Fig. 1E), and the prototype viral RNA load in the cells (more than 4 log units) and the culture medium (4 log units) at 48 h post infection (h.p.i.) (Supplementary Fig. S2B). The IFA and qPCR results together demonstrated that SARS-CoV-2 could infect and replicate in both cardiomyocytes and endothelial cells.
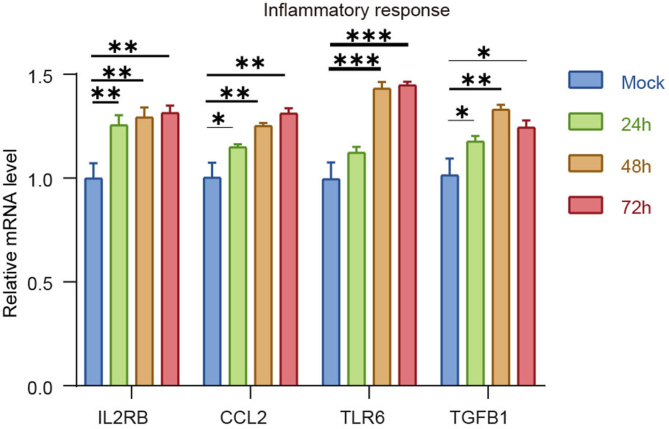
We performed transcriptome analysis to examine the host response to SARS-CoV-2 infection. hCOs infected with Omicron BA.5 were harvested at 0 h and 24 h post infection. RNA sequencing revealed a pronounced transcriptional response to cardiac injury. A total of 3,339 host genes were differentially expressed during SARS-CoV-2 infection, represented by the enrichment of ACTC1, MYL7, MYH6, MIAT, GIA4 etc. (Fig. 1F and G). Gene ontology (GO) function analysis of the enriched genes showed the correlation with various pathways, including the “calcium signaling pathway”, “hypertrophic cardiomyopathy”, “cardiac muscle contraction”, “viral myocarditis”, and “cell adhesion molecules” (Fig. 1H). We further determined whether SARS-CoV-2 infection and consequent myocardial/endothelial injuries and inflammatory responses would affect biological processes. We observed up-regulated expression of genes related to myocardial structure and function (TNNT2, PLN, ACTC1, TRDN, MYH6, MYL7), endothelial damage (ICAM1, ICAM4, VEGFA, THBD, GDF2, A2M), and antiviral and inflammatory response (IFN-β1, IFNL1, ISG15, BST2, IL6, IL33, etc.) (Fig. 1I and Supplementary Fig. S3). In addition, Gene Set Enrichment Analysis (GSEA) showed an enrichment of genes associated with myocardial contraction, endothelial regeneration, and inflammatory response within the organ class (Fig. 1J and Supplementary Fig. S4). These data indicated that SARS-CoV-2 infection impaired the contractile function of myocardial cells and the barrier function of endothelial cells by regulating the expression of genes involved in myocardial contraction, endothelial damage, and pro-inflammatory cytokines. Therefore, our study supports the view that cardiac injury in COVID-19 patients might be caused by direct damage to myocardial and endocardial lineages, and subsequent defects in myocardial contractile function.
Heart damage is a common feature in COVID-19 patients. Heart is a complex organ which composed of various cell types, including cardiomyocytes, fibroblasts, mural cells, endothelium cells, and immune cells (Litviňuková et al., 2020). More cell lineages and more abundant physiological functions in 3D hCOs play an important role in the study of heart diseases in vitro. In our study, we constructed hCOs that spontaneously assemble to 3D architecture with myocardial and endocardial co-development. Furthermore, both of the two cell types expressed ACE2 and TMPRSS2, which is consistent with previous reports (Nicin et al., 2020). Our results showed that the virus nucleocapsid protein was detected in both cardiomyocytes and endothelial cells in SARS-CoV-2-infected cardiac organoids. And there was a significant increase in viral RNA with prolonged infection periods. Combining the IFA and qPCR results, we demonstrated that SARS-CoV-2 directly infected both myocardial and endocardial lineages in hCOs.
Previous studies investigated the susceptibility of hiPSC-derived cardiomyocytes, endothelial cells, and cardiac fibroblasts to SARS-CoV-2, and found that SARS-CoV-2 only infected human cardiomyocytes but not endothelium and fibroblasts (Bailey et al., 2021; Perez-Bermejo et al., 2021). In our study, we showed that both SARS-CoV-2 prototype and Omicron BA.5 infected cardiac endothelial cells directly. The cardiac endothelial cells might exhibit a distinct gene expression pattern thus be susceptible in 3D hCOs with the presence of other lineages, compared to 2D culture. Meanwhile, the autopsy reports showed that SARS-CoV-2 was detected in both the endothelial cells and the cardiomyocytes (Bearse et al., 2021; Bräuninger et al., 2022; Schurink et al., 2020). This further illustrated that the 3D cardiac organoid model with multilineage co-development recapitulates in vivo features than the 2D cell culture. Our results provides a infection model for future studies aimed at elucidating the mechanisms of SARS-CoV-2 infection in endothelial cells, especially the sequence of infection in cardiomyocytes and endothelial cells and their potential interactions.
Studies have explored the influence of SARS-CoV-2 infection to hiPSC-derived cardiomyocytes. Our findings was consistent with the results that the enrichment of genes involved in myocardial function and the decrease of contraction frequency (Bojkova et al., 2020; Mills et al., 2021; Perez-Bermejo et al., 2021). Futhermore, our transcriptome sequencing results showed that the gene expression related with cardiomyocytes damage, endothelial cells damage, antiviral response and proinflammatory were upregulated after SARS-CoV-2 infection in hCOs. These results were consistent with the transcriptome sequencing results from clinical samples (Brauninger et al., 2022). These further validate the reliability of the cardiac organoid results, showing cardiac organoid was a valid infection model in vitro that could recapitulate the main features of the cardiac heart in vivo more naturally.
In summary, we investigated the heart damage caused by SARS-CoV-2 infection using hCOs as an in vitro model. Our results showed that 3D COs more naturally recapitulated the pathogenesis compared to 2D cell cultures or animal models, making them a valid in vitro model for advancing the development of preventative and therapeutic strategies.
Footnotes
Footnotes
We thank Dr. Changwen Ke from Guangdong Provincial Center for Disease Control and Prevention for providing the SARS-CoV-2 prototype strain. This work was supported by funds from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0490000), National Natural Science Foundation of China (32070180, 32022022 and 82061138006) and the Shanghai Pilot Program for Basic Research (Fudan University 21TQ1400100-21TQ003). The authors declare no competing interests.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2023.09.005.
Contributor Information
Pinfang Kang, Email: kangpinfang.1016@163.com.
Bing Zhao, Email: bingzhao@fudan.edu.cn.
Xinglou Yang, Email: yangxinglou@mail.kiz.ac.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
FigS1.
FigS2.
FigS3.
FigS4.
References
- Bailey A.L., Dmytrenko O., Greenberg L., Bredemeyer A.L., Ma P., Liu J., Penna V., Winkler E.S., Sviben S., Brooks E., Nair A.P., Heck K.A., Rali A.S., Simpson L., Saririan M., Hobohm D., Stump W.T., Fitzpatrick J.A., Xie X., Zhang X., Shi P.Y., Hinson J.T., Gi W.T., Schmidt C., Leuschner F., Lin C.Y., Diamond M.S., Greenberg M.J., Lavine K.J. SARS-CoV-2 infects human engineered heart tissues and models COVID-19 myocarditis. JACC Basic Transl Sci. 2021;6:331–345. doi: 10.1016/j.jacbts.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearse M., Hung Y.P., Krauson A.J., Bonanno L., Boyraz B., Harris C.K., Helland T.L., Hilburn C.F., Hutchison B., Jobbagy S., Marshall M.S., Shepherd D.J., Villalba J.A., Delfino I., Mendez-Pena J., Chebib I., Newton-Cheh C., Stone J.R. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Mod. Pathol. 2021;34:1345–1357. doi: 10.1038/s41379-021-00790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojkova D., Wagner J.U.G., Shumliakivska M., Aslan G.S., Saleem U., Hansen A., Luxán G., Günther S., Pham M.D., Krishnan J., Harter P.N., Ermel U.H., Frangakis A.S., Milting H., Zeiher A.M., Klingel K., Cinatl J., Dendorfer A., Eschenhagen T., Tschöpe C., Ciesek S., Dimmeler S. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc. Res. 2020;116:2207–2215. doi: 10.1093/cvr/cvaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuninger H., Stoffers B., Fitzek A.D.E., Meißner K., Aleshcheva G., Schweizer M., Weimann J., Rotter B., Warnke S., Edler C., Braun F., Roedl K., Scherschel K., Escher F., Kluge S., Huber T.B., Ondruschka B., Schultheiss H.P., Kirchhof P., Blankenberg S., Püschel K., Westermann D., Lindner D. Cardiac SARS-CoV-2 infection is associated with pro-inflammatory transcriptomic alterations within the heart. Cardiovasc. Res. 2022;118:542–555. doi: 10.1093/cvr/cvab322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. COVID-19: organoids go viral. Nat. Rev. Mol. Cell Biol. 2020;21:355–356. doi: 10.1038/s41580-020-0258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Israeli Y.R., Wasserman A.H., Gabalski M.A., Volmert B.D., Ming Y., Ball K.A., Yang W., Zou J., Ni G., Pajares N., Chatzistavrou X., Li W., Zhou C., Aguirre A. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 2021;12:5142. doi: 10.1038/s41467-021-25329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litviňuková M., Talavera-López C., Maatz H., Reichart D., Worth C.L., Lindberg E.L., Kanda M., Polanski K., Heinig M., Lee M., Nadelmann E.R., Roberts K., Tuck L., Fasouli E.S., DeLaughter D.M., McDonough B., Wakimoto H., Gorham J.M., Samari S., Mahbubani K.T., Saeb-Parsy K., Patone G., Boyle J.J., Zhang H.B., Zhang H., Viveiros A., Oudit G.Y., Bayraktar O.A., Seidman J.G., Seidman C.E., Noseda M., Hubner N., Teichmann S.A. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Ma W.J., Song T.Z., Wu Z.B., Liu Z.Y., Hu Z.X., Han J.B., Xu L., Zeng B., Wang B.S., Sun Y.N., Yu D.D., Wu Q., Yao Y.G., Zheng Y.T., Wang X.Q. Single-nucleus transcriptomic profiling of multiple organs in a rhesus macaque model of SARS-CoV-2 infection. Zool. Res. 2022;43:1041–1062. doi: 10.24272/j.issn.2095-8137.2022.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills R.J., Humphrey S.J., Fortuna P.R.J., Lor M., Foster S.R., Quaife-Ryan G.A., Johnston R.L., Dumenil T., Bishop C., Rudraraju R., Rawle D.J., Le T., Zhao W., Lee L., Mackenzie-Kludas C., Mehdiabadi N.R., Halliday C., Gilham D., Fu L., Nicholls S.J., Johansson J., Sweeney M., Wong N.C.W., Kulikowski E., Sokolowski K.A., Tse B.W.C., Devilée L., Voges H.K., Reynolds L.T., Krumeich S., Mathieson E., Abu-Bonsrah D., Karavendzas K., Griffen B., Titmarsh D., Elliott D.A., McMahon J., Suhrbier A., Subbarao K., Porrello E.R., Smyth M.J., Engwerda C.R., MacDonald K.P.A., Bald T., James D.E., Hudson J.E. BET inhibition blocks inflammation-induced cardiac dysfunction and SARS-CoV-2 infection. Cell. 2021;184:2167–2182.e22. doi: 10.1016/j.cell.2021.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicin L., Abplanalp W.T., Mellentin H., Kattih B., Tombor L., John D., Schmitto J.D., Heineke J., Emrich F., Arsalan M., Holubec T., Walther T., Zeiher A.M., Dimmeler S. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur. Heart J. 2020;41:1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., Watkinson P., Khunti K., Harnden A., Coupland C.A.C., Channon K.M., Mills N.L., Sheikh A., Hippisley-Cox J. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022;28:410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Bermejo J.A., Kang S., Rockwood S.J., Simoneau C.R., Joy D.A., Silva A.C., Ramadoss G.N., Flanigan W.R., Fozouni P., Li H.H., Chen P.Y., Nakamura K., Whitman J.D., Hanson P.J., McManus B.M., Ott M., Conklin B.R., McDevitt T.C. SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abf7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurink B., Roos E., Radonic T., Barbe E., Bouman C.S.C., de Boer H.H., de Bree G.J., Bulle E.B., Aronica E.M., Florquin S., Fronczek J., Heunks L.M.A., de Jong M.D., Guo L., du Long R., Lutter R., Molenaar P.C.G., Neefjes-Borst E.A., Niessen H.W.M., van Noesel C.J.M., Roelofs J.J.T.H., Snijder E.J., Soer E.C., Verheij J., Vlaar A.P.J., Vos W., van der Wel N.N., van der Wal A.C., van der Valk P., Bugiani M. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet. Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Zhang C., Wu Y.H., Liu L.B., Zhen Z.D., Fan D.Y., Song Z.R., Chang J.T., Wang P.G., An J. Mice 3D testicular organoid system as a novel tool to study Zika virus pathogenesis. Virol. Sin. 2023;38:66–74. doi: 10.1016/j.virs.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.