Abstract
Myocardial injury is one of the most common comorbidity in SARS-CoV-2 infected patients, and has poor prognosis. However, the incidence of myocardial injury in patients with SARS-CoV-2 infection has not been sufficiently investigated during the Omicron wave. We conducted a retrospective study of 2690 patients with confirmed SARS-CoV-2 Omicron infection from Tongji Hospital. The results indicated that the myocardial injury accounted for 30.8% of the total patients with SARS-CoV-2 infection and was associated with higher in-hospital mortality than those without injury before and after propensity score matching (PSM) [adjusted hazard ratio (HR), 10.61; 95% confidence interval (CI), 7.76–14.51; P < 0.001; adjusted HR, 2.70; 95% CI, 1.86–3.93; P < 0.001; respectively]. Further, the levels of cytokines (IL-1β, IL-6, IL-10, and TNF-α) in patients with myocardial injury were higher than those without injury, and the higher levels of cytokines in the myocardial injury group were associated with increased mortality. Administration of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEI/ARB) could significantly reduce the mortality in patients with myocardial injury (adjusted HR, 0.52; 95% CI, 0.38–0.71; P < 0.001). Additionally, the level of angiotensin II increased in patients with SARS-CoV-2 infection was even higher in myocardial injury group compared to those without injury. Collectively, the study summarized the clinical characteristic and outcome of SARS-CoV-2 infected patients with myocardial injury during the Omicron wave in China, and validated the protective role of ACEI/ARB in improving the survival of those with myocardial injury.
Keywords: COVID-19, Omicron, Myocardial injury, In-hospital mortality, Cytokine storm
Highlights
-
•
Myocardial injury accounted for 30.8% of the patients with SARS-CoV-2 Omicron infection.
-
•
Higher mortality was observed in patients with myocardial injury compared to those without injury.
-
•
Incidence of myocardial injury and the level of myocardial inflammation were correlated.
-
•
The use of ACEIs/ARBs could reduce the mortality of Omicron infected patients with myocardial injury.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a highly contagious systemic disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has been evolving and sweeping the world over the past three years (Telenti et al., 2021; Del Rio et al., 2022). Confirmed cases of COVID-19 have exceeded 770 million September 27, 2023, including more than 6.9 million deaths. With gradual relaxation and related “zero-COVID-19” policies, a nationwide pandemic of the Omicron variant has been ongoing in China since November 2022 (Li et al., 2023; Lin et al., 2023). Although COVID-19 vaccines and oral antiviral drugs have been used successfully (Li et al., 2022a; Cao et al., 2023), a series of clinical problems caused by COVID-19 have not been completely resolved and continue to perplex clinicians and researchers (Koc et al., 2022).
Patients with COVID-19 can also have multi-organ injuries, among which myocardial injury is one of the most important and urgent concerns (Nishiga et al., 2020; Wang et al., 2020b; Ababneh et al., 2022). Myocardial injury is one of the core manifestations of cardiovascular involvement in COVID-19 patients (Li et al., 2021). Myocardial injury caused by COVID-19 is common in both adults and children (Wolfler et al., 2020; Melillo et al., 2022), particularly in critically ill patients (Jansson et al., 2022). The underlying mechanism of myocardial injury may be related to direct injury caused by SARS-CoV-2 infection and to indirect damage, such as that caused by myocardial inflammation, cytokine storm, and the hypercoagulable state (Siripanthong et al., 2022). Further, myocardial injury induced by SARS-CoV-2 is associated with an adverse prognosis and increased in-hospital mortality (Bonow et al., 2020; Lim, 2020). More than 20% of hospitalized patients have suffered from myocardial injury with high mortality since the Omicron epidemic (Henning, 2022; Case et al., 2023), suggesting that the effects on the myocardium did not fade with virus evolution.
To clarify the proportion and survival of patients with COVID-19 and myocardial injury during the Omicron epidemic in China, we conducted a retrospective cohort study including 2690 inpatients with COVID-19 (discharged or deceased) at Tongji Hospital during December 2022. In particular, we compared four cytokines in those Omicron infected patients with or without myocardial injury, explored the therapeutic effect of angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (ACEI/ARB) in patients with myocardial injury, and measured the concentration of angiotensin II (AngII).
2. Materials and methods
2.1. Study design and patient information
In this cohort study, we collected clinical information of patients with confirmed COVID-19 from Tongji hospital, China, since the Omicron pandemic in December 2022. SARS-CoV-2 infection was confirmed by two consecutive positive results from high-throughput sequencing or real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assays of nasal and pharyngeal swabs. Participants younger than 18-years of age and lack of basic relevant information, laboratory test results (especially troponin), treatment information, and results of RT-PCR for SARS-CoV-2 were excluded. All data were collected from the electronic medical record database of Tongji Hospital.
2.2. Data collection and endpoint definitions
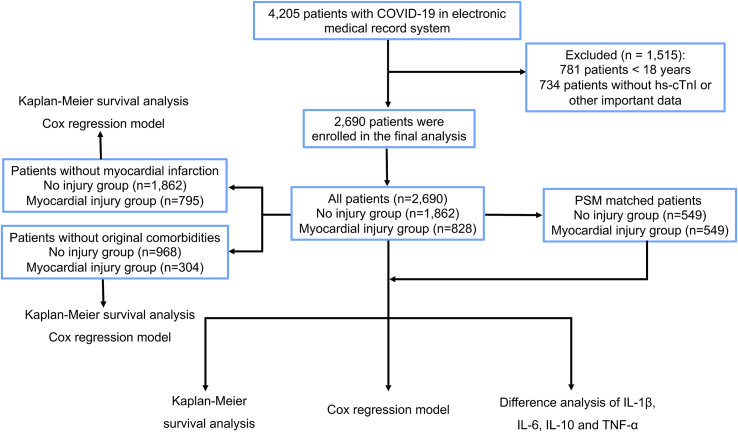
This study included 3424 patients older than 18 years of age, who were diagnosed with COVID-19 between December 2022 and February 2023. Of these, 2690 patients with clear hypersensitive cardiac troponin I (hs-cTnI, upper limit of normal, 26.2 pg/mL) data were included in the final clinical study (Fig. 1). Myocardial injury was defined as hs-cTnI > 26.2 pg/mL at least once during hospitalization. Patients were divided into myocardial injury and no injury groups.
Fig. 1.
The flowchart of study design.
The parameters used in the study included epidemiological, demographic, full medical history, hospitalization time, clinical, and laboratory data, along with treatment measures extracted from the electronic medical records at the time of admission. These records were independently examined by three researchers. The collected information included age, gender, comorbidities, exposure history, vital signs (temperature, respiratory rate, pulse rate, and systolic and diastolic blood pressures (SBP and DBP), blood laboratory tests (including routine blood tests, blood chemical variables, procalcitonin, coagulation function tests), therapeutic strategy during hospitalization, and clinical outcomes. In this cohort study, all-cause mortality during hospitalization was the primary endpoint of concern.
2.3. Level of AngII quantification
The concentration of AngII was quantified with a previously optimized method by ultra-performance liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) (Chen et al., 2021). Briefly, two kinds of solution were prepared before sample extraction. The enzyme inhibitor solution was prepared to contain phenylmethylsulfonyl fluoride (1 mmol/L) and soybean serine trypsin inhibitor (0.3 mg/mL) to inhibit the conversion and degradation of AngI to AngII. Another buffer solution was mixed with trihydroxymethyl aminomethane and bovine serum albumin by adjusting the pH to 6 with acetic acid.
The pooled quality controls were prepared when aliquoted the plasma samples. The 150 μL plasma or quality control samples were mixed with 33 μL of enzyme inhibitor solution, 7 μL formic acid, 200 μL deionized water, and 10 μL internal standard (13C6,15N4-AngII, 20 ng/mL). The 20 μL calibrator working solution was added to 130 μL buffer solution and mixed with the enzyme inhibitor solution and formic acid as well. The mixture was then centrifuged for 10 min and 350 μL supernatant was collected for the following solid-phase-extraction.
The Waters Oasis PRiME HLB 96-well uElution plate was active with 200 μL methanol and 200 μL of 1% formic acid. After sample loading, 500 μL of 20% methanol and 500 μL hexane acted as washing solution. Then the 40 μL methanol was used as an eluent to mix with 40 μL deionized water. The 10 μL solution was injected into LC-MS/MS system.
The liquid chromatographic separation was achieved with a Kinetex C18 column (100 mm × 2.1 mm × 2.6 μm, Phenomenex, USA) maintained at 40°. The Milli-Q water containing 0.03% ammonium fluoride (A) and methanol (B) was used as mobile phase following the gradient elution: 0−1 min 20% B, 1−1.5 min 30% B, 1.5−5.3 min 55% B, 5.3−5.35 min 98% B, 5.35−6.15 min 98% B, 6.20−7.0 min 20% B at a flow rate of 0.4 mL/min.
The AngII measurements were performed on AB Sciex 6500+ QqQ in electrospray ionization positive detection mode (AngII, m/z 523.8 → 784.4, 523.8 → 263.2, 13C6,15N4 AngII, m/z 527.8 → 791.5, 527.8 → 263.2). The quality control sample was acquired for every 10 samples to monitor the quality of measurement. The quantification was calculated against the area of its internal standard under the SCIEX OS environment. The results were expressed as pg/mL of plasma.
2.4. Statistical analysis and mapping
Statistical analyses and mapping were performed using SPSS (version 24.0; IBM, Armonk, NY, USA), R (version 4.1.1; R Foundation for Statistics Computing, Vienna, Austria), and GraphPad Prism (version 9.0; San Diego, CA, USA). To minimize the interference of confounding factors, we performed an accurate 1:1 propensity score matching (PSM) analysis of the observed baseline data and related treatments in the R environment. The matched variables included age, gender, disease history (e.g., hypertension, coronary artery disease, chronic heart failure, chronic kidney disease, and tumor), basic vital signs (temperature, respiratory rate, pulse rate, SBP, and DBP), clinical laboratory indicators (routine blood test results, blood biochemistry profile, and coagulation function), and other commonly used drugs (ACEI/ARB, diuretics, digitalis, calcium channel blockers, metformin, antiplatelet drugs, anticoagulant drugs, statins, and nitrates) in the myocardial injury and no injury groups. The optimal caliper width for PSM was set at 0.05.
The distribution and homoscedasticity of each dataset were tested using D'Agostino's and Pearson's omnibus normality tests. Continuous normally distributed data were reported as mean (deviation) and compared using Student's t-test. Continuous non-normally distributed data were reported as median [interquartile range (IQR)] and compared using the Wilcoxon rank-sum test. Categorical data were presented as n (percentage) and were compared using the Chi-square test, Fisher's exact test, and Cochran-Mantel-Haenszel test, as appropriate. Survival analysis methods, including Kaplan-Meier curves and Cox proportional hazard model analyses, were used to compare the time-of-endpoint events in different subgroups. Statistical significance was set at P < 0.05.
3. Results
3.1. Demographic data, clinical characteristics, and treatment
This study included 4205 patients with COVID-19 from Tongji Hospital. Of these, 1515 (36.0%) patients were excluded because they were < 18 years of age (18.6%) or lack of critical data (17.4%). Finally, 2690 patients were included in the final analysis, 60.6% of whom were male (Table 1). The median age of the patients was 68 years (IQR, 58–77 years). Hypertension (35.0%) was the most common comorbidity, followed by diabetes (20.4%). All patients were divided into the myocardial injury group (828, 30.8%) and the no injury group (1862, 69.2%). In the myocardial injury group, 33 patients (1.2%) had myocardial infarction confirmed by coronary angiography, and the remaining 795 patients (29.6%) had simple myocardial injury related to COVID-19 (Fig. 2). There was no significant difference in hospital stay between the two groups (10 days, IQR 7–14; 10 days IQR 7–15; respectively). The baseline characteristics of the myocardial injury and no injury groups after PSM are presented in Table 1. The median ages of the myocardial injury group (males: 64.8%) and the no injury group (male: 62.3%) were 72 (IQR, 60–82) and 71 years (IQR, 61–79), respectively. There were no significant differences in any baseline characteristics between the PSM groups [n = 549 for each group, all P > 0.05, standardized mean difference (SMD) < 0.1].
Table 1.
Baseline characteristics of COVID-19 patients in myocardial injury group and no injury group.
| All patients (n = 2690) | Unmatched |
PSM (1:1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No injury group (n = 1862) | Myocardial injury group (n = 828) | SMD | P | No injury group (n = 549) | Myocardial injury group (n = 549) | SMD | P | ||
| Gender: male (%) | 1630 (60.6) | 1068 (57.4) | 562 (67.9) | 0.215 | < 0.001 | 342 (62.3) | 356 (64.8) | 0.062 | 0.380 |
| Age, years | 68 (58–77) | 66 (56–75) | 74 (63–83) | 0.471 | < 0.001 | 71 (61–79) | 72 (60–82) | 0.004 | 0.219 |
| Age ≥ 65 years (%) | 1637 (60.9) | 1037 (55.7) | 600 (72.5) | 0.328 | < 0.001 | 379 (69.0) | 371 (67.6) | 0.031 | 0.604 |
| Age<65 years (%) | 1053 (39.1) | 825 (44.3) | 228 (27.5) | 0.328 | < 0.001 | 170 (31.0) | 178 (32.4) | 0.031 | 0.604 |
| Mortality (%) | 312 (11.6) | 47 (2.5) | 265 (32.0) | 0.920 | < 0.001 | 37 (6.7) | 109 (19.9) | 0.386 | < 0.001 |
| Smoking (%) | 480 (17.8) | 312 (16.8) | 168 (20.3) | 0.092 | 0.027 | 98 (17.9) | 107 (19.5) | 0.026 | 0.486 |
| Drinking (%) | 297 (11.0) | 197 (10.6) | 100 (12.1) | 0.048 | 0.253 | 58 (10.6) | 57 (10.4) | 0.033 | 0.921 |
| Original comorbidities (%) | |||||||||
| Hypertension (%) | 941 (35.0) | 617 (33.1) | 324 (39.1) | 0.126 | 0.003 | 212 (38.6) | 220 (40.1) | 0.020 | 0.621 |
| CHD (%) | 371 (13.8) | 220 (11.8) | 151 (18.2) | 0.186 | < 0.001 | 89 (16.2) | 90 (16.4) | < 0.001 | 0.935 |
| CHF (%) | 276 (10.3) | 112 (6.0) | 164 (19.8) | 0.454 | < 0.001 | 77 (14.0) | 85 (15.5) | 0.028 | 0.496 |
| T2D (%) | 550 (20.4) | 378 (20.3) | 172 (20.8) | 0.012 | 0.779 | 125 (22.8) | 119 (21.7) | 0.024 | 0.663 |
| CKD (%) | 436 (16.2) | 216 (11.6) | 220 (26.6) | 0.406 | < 0.001 | 116 (21.1) | 131 (23.9) | 0.072 | 0.278 |
| Tumor (%) | 297 (11.0) | 245 (13.2) | 52 (6.3) | 0.219 | < 0.001 | 45 (8.2) | 39 (7.1) | 0.038 | 0.496 |
| Vital signs | |||||||||
| Temperature (°C) | 36.5 (36.4–36.7) | 36.5 (36.3–36.7) | 36.5 (36.5–36.8) | 0.066 | < 0.001 | 36.5 (36.3–36.7) | 36.5 (36.4–36.8) | 0.047 | 0.114 |
| RR, breaths/min | 20 (20–20) | 20 (20–20) | 20 (20–20) | 0.178 | < 0.001 | 20 (20–20) | 20 (20–20) | 0.003 | 0.134 |
| Pulse, beats/min | 83 (78–92) | 82 (78–91) | 85 (78–96) | 0.823 | < 0.001 | 84 (78–94) | 83 (78–93) | 0.025 | 0.805 |
| SBP, mmHg | 126 (117–137) | 125 (116–135) | 129 (118–142) | 0.202 | < 0.001 | 129 (119–139) | 129 (118–142) | 0.001 | 0.849 |
| DBP, mmHg | 78 (70–84) | 78 (71–83) | 76 (69–85) | 0.062 | 0.124 | 78 (71–83) | 77 (70–86) | 0.011 | 0.679 |
Data were presented as n (%) or median with interquartile range (Q1–Q3).
PSM, propensity score matching; NYHA class, New York Heart Association functional classification; CHD, coronary heart disease; T2D, type 2 diabetes; CKD, chronic kidney disease; IQR, interquartile range; SMD, standardized mean difference; SBP, systolic blood pressure; DBL, diastolic blood pressure; RR, Respiratory rate.
Fig. 2.
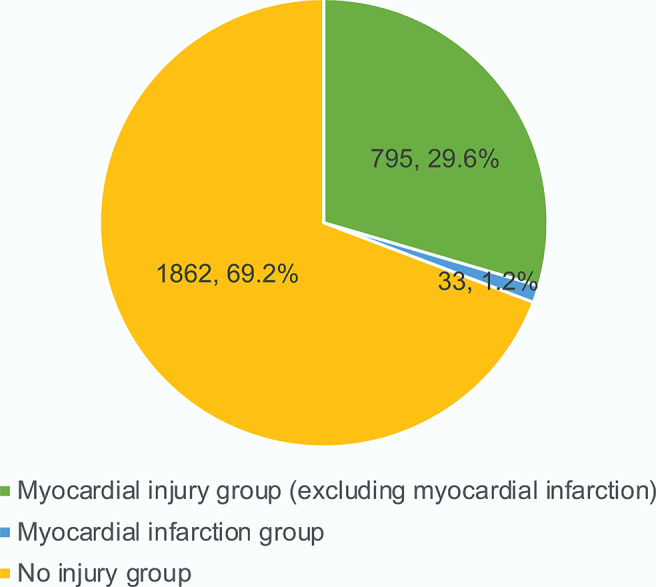
Incidence of myocardial injury in COVID-19 patients.
Laboratory results (routine blood test results, blood biochemistry profile, infection-related indicators, and coagulation function) before and after PSM in the myocardial injury and no injury groups are shown in Table 2. After PSM, there was no significant difference in the examination and test results between the groups (P < 0.05, SMD < 0.1, except for lactate dehydrogenase, cytokines, and cardiac injury-related indicators that were not involved in matching). Further, we matched some commonly used drugs (ACEI/ARB, diuretics, calcium channel blockers, β-blockers, statins, metformin, nitrates, anticoagulant drugs, and antiplatelet drugs) that might have potential effects (Table 3). All drug treatments were well-matched between the groups (P < 0.05, SMD < 0.1).
Table 2.
Laboratory results in myocardial injury group and no injury group.
| All patients (n = 2690) | Unmatched |
PSM (1:1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No injury group (n = 1862) | Myocardial injury group (n = 828) | SMD | P | No injury group (n = 549) | Myocardial injury group (n = 549) | SMD | P | ||
| hscTnI | 7.4 (2.8–23.2) | 4.6 (2.0–9.0) | 47.2 (26.1–153.9) | 0.331 | < 0.001 | 7.2 (3.6–13.3) | 38.6 (17.7–98.9) | 0.315 | < 0.001 |
| Glucose, mmol/L | 6.8 (5.7–8.8) | 6.5 (5.6–8.1) | 7.5 (6.0–10.6) | 0.435 | < 0.001 | 7.1 (5.9–9.3) | 7.2 (5.7–10.0) | 0.075 | 0.488 |
| HbA1c, % | 7.2 (6.7–7.9) | 7.2 (6.8–7.8) | 7.2 (6.7–8.3) | 0.200 | 0.011 | 7.2 (6.7–8.1) | 7.2 (6.7–8.1) | 0.064 | 0.900 |
| Routine blood test | |||||||||
| RBC count, × 1012/L | 3.98 (3.51–4.42) | 4.01 (3.59–4.42) | 3.90 (3.30–4.42) | 0.133 | 0.003 | 3.94 (3.44–4.39) | 3.87 (3.32–4.73) | 0.040 | 0.417 |
| Hemoglobin, g/L | 120 (103–133) | 121 (106–133) | 117 (95–133) | 0.087 | 0.007 | 119 (100–132) | 117 (96–132) | 0.051 | 0.358 |
| WBC count, × 109/L | 6.23 (4.46–7.10) | 5.88 (4.28–7.74) | 7.51 (5.01–11.15) | 0.511 | < 0.001 | 6.24 (4.53–8.81) | 6.67 (4.59–9.34) | 0.032 | 0.104 |
| Platelet count, × 109/L | 200 (141–268) | 211 (153–279) | 176 (124–242) | 0.362 | < 0.001 | 187 (135–252) | 190 (129–249) | 0.035 | 0.590 |
| Blood biochemistry | |||||||||
| ALT, U/L | 21 (14–34) | 20 (14–33) | 22 (14–37) | 0.163 | 0.042 | 20 (14–33) | 22 (14–36) | 0.091 | 0.428 |
| LDH, U/L | 254 (203–345) | 234 (194–293) | 336 (247–467) | 0.792 | < 0.001 | 268 (210–353) | 288 (221–392) | 0.015 | < 0.001 |
| Total bilirubin, μ mol/L | 8.6 (6.1–12.3) | 8.3 (6.0–11.8) | 9.3 (6.4–13.7) | 0.120 | < 0.001 | 8.6 (6.1–12.3) | 8.7 (6.2–13.1) | 0.041 | 0.408 |
| Total protein, g/L | 66.5 (62.1–70.7) | 67.1 (63.0–71.0) | 65.1 (59.9–69.2) | 0.334 | < 0.001 | 66.2 (61.9–70.0) | 65.4 (60.9–69.5) | 0.069 | 0.083 |
| Albumin/globulin | 1.08 (0.90–1.30) | 1.13 (0.95–1.35) | 0.97 (0.83–1.16) | 0.513 | < 0.001 | 1.03 (0.88–1.24) | 1.01 (0.86–1.21) | 0.069 | 0.220 |
| T-Ch, mmol/L | 3.68 (3.04–4.37) | 3.76 (3.18–4.44) | 3.43 (2.81–4.11) | 0.288 | < 0.001 | 3.61 (3.05–4.25) | 3.56 (2.92–4.16) | 0.068 | 0.168 |
| Triglyceride, mmol/L | 1.44 (1.13–1.84) | 1.43 (1.14–1.80) | 1.47 (1.13–1.93) | 0.064 | 0.106 | 1.42 (1.12–1.84) | 1.41 (1.10–1.86) | 0.027 | 0.935 |
| LDL, mmol/L | 2.12 (1.60–2.70) | 2.21 (1.71–2.77) | 1.86 (1.36–2.49) | 0.352 | < 0.001 | 2.08 (1.62–2.65) | 1.97 1.49–2.57) | 0.087 | 0.063 |
| HDL, mmol/L | 0.87 (0.72–1.02) | 0.90 (0.76–1.04) | 0.79 (0.64–0.97) | 0.395 | < 0.001 | 0.87 (0.72–1.00) | 0.82 (0.67–1.00) | 0.091 | 0.036 |
| Creatinine, μmol/L | 81 (64–112) | 75 (61–95) | 107 (74–204) | 0.566 | < 0.001 | 90 (70–129) | 92 (69–147) | 0.169 | 0.302 |
| eGFR, mL/min | 78.1 (51.0–93.4) | 84.5 (65.1–96.6) | 53.6 (25.2–81.0) | 0.848 | < 0.001 | 67.7 (41.8–87.9) | 64.9 (37.9–86.2) | 0.116 | 0.093 |
| C-RP, mg/L | 34.2 (9.2–79.1) | 24.0 (6.6–60.5) | 66.5 (26.2–130.1) | 0.702 | < 0.001 | 45.7 (12.9–89.3) | 46.5 (17.7–100.4) | 0.125 | 0.073 |
| Sodium, mmol/L | 137.9 (135.1–140.4) | 138.0 (135.3–140.1) | 137.5 (134.4–141.2) | 0.078 | 0.728 | 137.2 (134.4–139.6) | 137.4 (134.6–140.4) | 0.072 | 0.087 |
| Potassium, mmol/L | 4.12 (3.79–4.49) | 4.09 (3.79–4.41) | 4.21 (3.79–4.73) | 0.311 | < 0.001 | 4.20 (3.88–4.57) | 4.12 (3.73–4.63) | 0.042 | 0.154 |
| Lactic acid, mmol/L | 1.91 (1.71–2.19) | 1.88 (1.70–2.11) | 1.99 (1.74–2.42) | 0.467 | < 0.001 | 1.94 (1.74–2.21) | 1.92 (1.70–2.28) | 0.142 | 0.543 |
| Coagulation function | |||||||||
| APTT | 36.1 (32.8–40.3) | 35.9 (32.8–39.7) | 36.6 (32.6–42.0) | 0.193 | 0.003 | 36.5 (32.8–41.0) | 35.7 (31.9–40.4) | 0.010 | 0.147 |
| Prothrombin time, s | 13.3 (12.5–14.2) | 13.2 (12.5–13.9) | 13.7 (12.5–15.0) | 0.233 | < 0.001 | 13.4 (12.6–14.3) | 13.4 (12.4–14.6) | 0.002 | 0.722 |
| Thrombin time, s | 17.6 (16.3–18.8) | 17.6 (16.4–18.6) | 18.0 (16.1–19.5) | 0.220 | < 0.001 | 17.6 (16.3–18.8) | 17.6 (15.8–19.1) | 0.025 | 0.936 |
| International normalized ratio | 1.04 (0.97–1.13) | 1.02 (0.96–1.09) | 1.09 (1.00–1.21) | 0.230 | < 0.001 | 1.04 (0.98–1.13) | 1.05 (0.98–1.13) | 0.036 | 0.199 |
| Fibrinogen, mg/L | 4.32 (3.50–5.33) | 4.32 (3.46–5.29) | 4.34 (3.52–5.41) | 0.061 | 0.318 | 4.39 (3.65–5.23) | 4.27 (3.56–5.25) | 0.023 | 0.377 |
| D-dimer, mg/L | 0.99 (0.55–2.10) | 0.82 (0.46–1.51) | 1.80 (0.89–4.72) | 0.638 | < 0.001 | 1.17 (0.64–2.49) | 1.30 (0.73–2.37) | 0.006 | 0.118 |
Data were presented as medians and interquartile range (Q1–Q3).
PSM, propensity score matching; SMD, standardized mean difference; hsc-TnI, high-sensitivity-cardiac troponin I; HbA1c, glycated hemoglobin/hemoglobin A1c; LDL, low-density lipoprotein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; APTT, activated partial thromboplastin time. RBC, red blood cell; WBC, white blood cell; T-Ch, total cholesterol; ALT, Alanine aminotransferase; LDH, Lactate dehydrogenase.
Table 3.
Comparison of treatment of COVID-19 patients in myocardial injury group and no injury group.
| All patients (n = 2690) | Unmatched |
PSM (1:1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No injury group (n = 1862) | Myocardial injury group (n = 828) | SMD | P | No injury group (n = 549) | Myocardial injury group (n = 549) | SMD | P | ||
| ACEI/ARB | 537 (20.0) | 334 (17.9) | 203 (24.5) | 0.169 | < 0.001 | 121 (22.0) | 134 (24.4) | 0.047 | 0.353 |
| Diuretic | 714 (26.5) | 282 (15.1) | 432 (52.2) | 0.838 | < 0.001 | 198 (36.1) | 226 (41.2) | 0.093 | 0.083 |
| CCB | 826 (30.7) | 510 (27.4) | 316 (38.2) | 0.233 | < 0.001 | 202 (36.8) | 212 (38.6) | 0.041 | 0.533 |
| β-blocker | 520 (19.3) | 304 (16.3) | 216 (26.1) | 0.247 | < 0.001 | 121 (22.0) | 131 (23.9) | 0.048 | 0.473 |
| Statins | 620 (23.0) | 401 (21.5) | 219 (26.4) | 0.117 | 0.005 | 146 (26.6) | 141 (25.7) | 0.023 | 0.731 |
| Metformin | 176 (6.7) | 133 (7.1) | 46 (5.6) | 0.063 | 0.127 | 35 (6.4) | 34 (6.2) | < 0.001 | 0.901 |
| Nitrates | 248 (9.2) | 76 (4.1) | 172 (20.8) | 0.577 | < 0.001 | 53 (9.7) | 62 (11.3) | 0.033 | 0.375 |
| Anticoagulant drugs | 88 (3.3) | 52 (2.8) | 36 (4.3) | 0.088 | 0.036 | 24 (4.4) | 26 (4.7) | 0.048 | 0.772 |
| Antiplatelet drugs | 488 (18.1) | 302 (16.2) | 186 (22.5) | 0.162 | < 0.001 | 119 (21.7) | 118 (21.5) | 0.024 | 0.942 |
Data were presented as n (%).
PSM, propensity score matching; SMD, standardized mean difference; DPP4i, ipeptidyl peptidase 4 inhibitors; SGLT2i, sodium/glucose cotransporter-2 inhibitors; GLP1a, glucagon-like peptide-1 agonists; ARNI, angiotensin receptor-neprilysin inhibitors; CCB, Calcium channel blockers.
3.2. Clinical outcomes during hospitalization
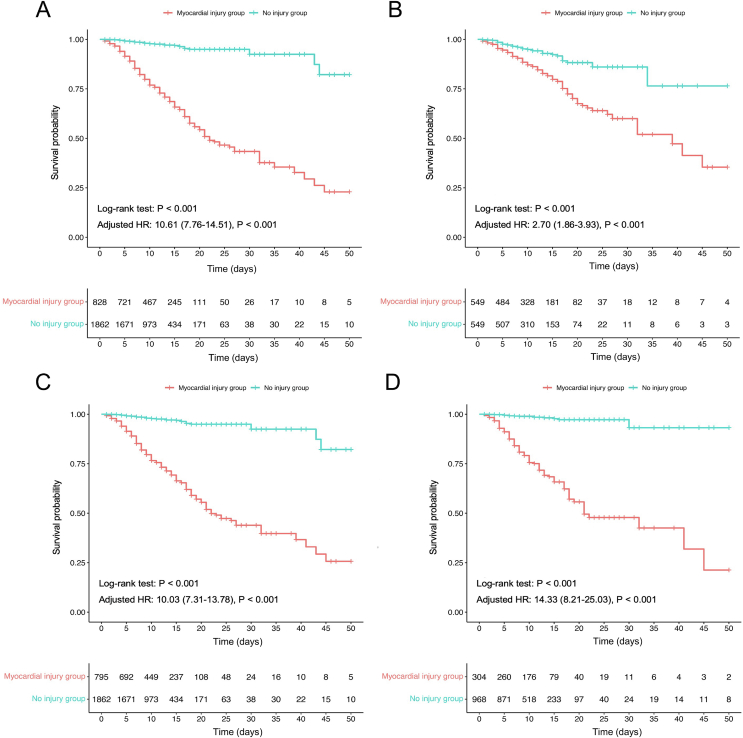
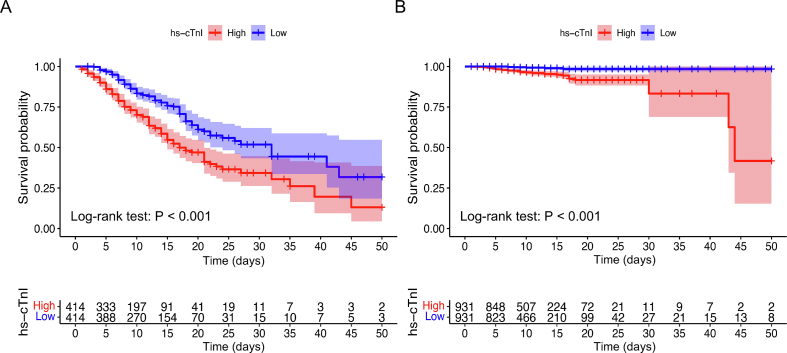
Among all patients enrolled in the cohort, 312 died during hospitalization (312/2690, 11.6%), 265 of whom were in the myocardial injury group (265/828, 32.0%) and 47 were in the no injury group (47/1862, 2.5%) (P < 0.001) (Table 1). The Kaplan-Meier survival curve and proportional Cox regression analyses (none of which violated the proportional hazard hypothesis) showed that mortality was significantly increased in the myocardial injury group [log-rank P < 0.001; crude hazard ratio (HR), 11.63; 95% confidence interval (CI), 8.52–15.86]; further, the results were consistent after PSM (log-rank P < 0.001; crude HR, 2.78; 95% CI, 1.92–4.05). When the multivariate Cox model was used to adjust for unbalanced confounding factors, the myocardial injury group still had a higher mortality before and after PSM (adjusted HR, 10.61; 95% CI, 7.76–14.51; P < 0.001; adjusted HR, 2.70; 95% CI, 1.86–3.93; P < 0.001; respectively) (Fig. 3A and B). We also compared the survival of the high and low hs-cTnI subgroups (50th percentile) in the myocardial injury and no injury groups. The results indicated that higher levels of hs-cTnI were associated with increased mortality in both the myocardial injury and no injury groups (both log-rank tests: P < 0.001) (Supplementary Fig. S1A and S1B).
Fig. 3.
The Kaplan-Meier survival curve of in-hospital mortality for COVID-19 patients in myocardial injury group and no injury group. A In all COVID-19 patients. B In COVID-19 patients after PSM. C In COVID-19 patients without myocardial infarction. D In COVID-19 patients without original comorbidities.
To rule out the effects of myocardial infarction and other important underlying diseases, we performed survival analyses after excluding myocardial infarction and other comorbidities (including coronary heart disease, hypertension, heart failure, atrial fibrillation, diabetes, and chronic kidney disease), which may cause an increase in hs-cTnI levels. The Kaplan-Meier survival curve and multivariate Cox model analyses showed that all-cause mortality was significantly higher in the myocardial injury group after excluding myocardial infarction (log-rank P < 0.001; adjusted HR, 10.03; 95% CI, 7.31–13.78; P < 0.001) and in the myocardial injury group after excluding other comorbidities (log-rank P < 0.001; adjusted HR, 14.33; 95% CI, 8.21–25.03; P < 0.001) than in the no injury group (Fig. 3C and D).
3.3. Comparison of cytokines between the myocardial injury and no injury groups
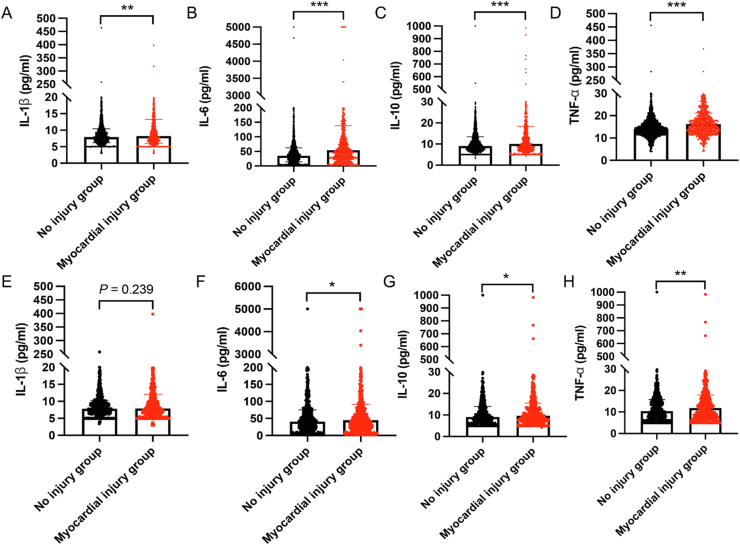
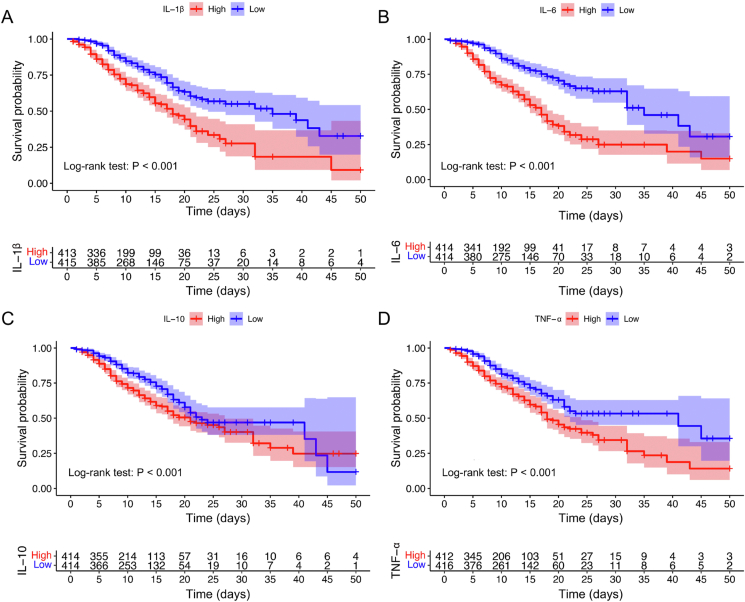
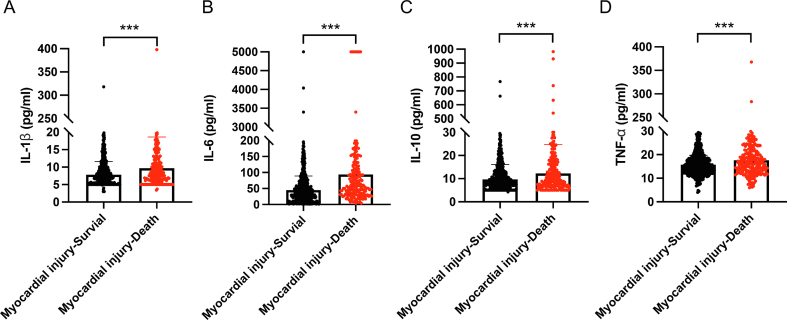
To further explore the potential causes of myocardial injury and its high mortality, we compared the inflammatory cytokine levels between the myocardial injury and no injury groups. The results showed that the levels of IL-1β, IL-6, IL-10, and TNF-α in the myocardial injury group were significantly higher than those in the no injury group (Fig. 4A–D). To minimize the effects of confounding factors, we compared the cytokines in the matched cohort and found that IL-6, IL-10, and TNF-α were still increased significantly in the myocardial injury group (Fig. 4E–H). These findings indicate that myocardial injury may be caused by the myocardial inflammation associated with SARS-CoV-2 infection. In addition, we compared the levels of cytokines between surviving and died patients in the myocardial injury group and the results showed that IL-1β, IL-6, IL-10, and TNF-α were significantly higher in died patients with myocardial injury (Supplementary Fig. S2). We also analyzed the survival of patients with high and low levels of the four cytokines in the myocardial injury group. The results indicated that patients with high levels of inflammatory cytokines (IL-1β, IL-6, IL-10, and TNF-α) were associated with higher mortality in the myocardial injury group (all log-rank P < 0.001) (Fig. 5A–D).
Fig. 4.
Comparison of cytokine levels in myocardial injury group and no injury group. A–D Comparison of IL-1β, IL-6, IL-10 and TNF-α levels in myocardial injury group and no injury group before PSM. E–H Comparison of IL-1β, IL-6, IL-10 and TNF-α levels in myocardial injury group and no injury group after PSM. Data are presented as the mean ± standard deviation, and statistical analyses were performed using a t-test. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. PSM, propensity score matching.
Fig. 5.
The Kaplan-Meier survival curve of in-hospital mortality for myocardial injury COVID-19 patients with different levels of cytokines. All cytokines have been binary classified by the 50th percentile as the cut-off value (IL-1β cut-off value: 8.21 pg/mL; IL-6 cut-off value: 53.08 pg/mL; IL-10 cut-off value: 10.04 pg/mL; TNF-α cut-off value: 16.16 pg/mL).
3.4. ACEI/ARB treatment-related survival analysis and the levels of AngII in COVID-19 patients
To clarify the effect of ACEI/ARB treatment on patients with myocardial injury, we divided all patients into four groups (myocardial injury group with/without ACEI/ARB and no injury group with/without ACEI/ARB) and analyzed their survival. The Kaplan-Meier survival curve and multivariate Cox model analyses showed that mortality was significantly lower in the myocardial injury group with ACEI/ARB than in the myocardial injury group without ACEI/ARB (log-rank P < 0.001; adjusted HR, 0.52; 95% CI, 0.38–0.71; P < 0.001), indicating that ACEI/ARB exerted a protective effect on COVID-19 patients with myocardial injury (Fig. 6A).
Fig. 6.
A The Kaplan-Meier survival curve of in-hospital mortality for COVID-19 patients with/without ACEI/ARB in myocardial injury group and no injury group. B Angiotensin II levels (ng/mL) on admission in COVID-19 patients (n = 34) and normal controls (NC, n = 30). C Angiotensin II (AngII) levels (ng/mL) on admission in myocardial injury group (n = 12) and no injury group (n = 22). Data are presented as the mean ± standard deviation, and statistical analyses were performed using a t-test. ∗∗∗∗P < 0.0001.
In addition, we also found that the level of AngII in patients with Omicron infection was significantly higher than that in normal controls (Fig. 6B, Supplementary Table S1). In patients with Omicron infection, the level of AngII in myocardial injury group was significantly higher than that in no injury group (Fig. 6C).
4. Discussion
In this retrospective cohort study, we analyzed the proportion and survival of COVID-19 patients with myocardial injury during the Omicron wave in China and compared four inflammatory cytokines between the myocardial injury and no injury groups. The results indicated that the myocardial injury group accounted for 30.8% of the COVID-19 patients and was associated with higher in-hospital mortality. We also found that the levels of inflammatory cytokines (IL-1β, IL-6, IL-10, and TNF-α) in the myocardial injury group were higher than those in the no injury group, and that the higher levels of cytokines in the myocardial injury group were associated with increased mortality. Analysis of infected patients treated with and without ACEI/ARB showed that the ACEI/ARB use in the myocardial injury group could significantly reduce the mortality of patients with COVID-19.
During the COVID-19 global pandemic, patients experienced a higher risk of death from cardiovascular and cerebrovascular diseases, and there are racial and ethnic differences in mortality. (Li et al., 2021; Wadhera et al., 2021; Yeo et al., 2023). Undoubtedly, myocardial injury is one of the core factors of all cardiovascular diseases and plays a significant role in the cardiovascular involvement of COVID-19 patients (Li et al., 2021). Although the exact global incidence rate of SARS-CoV-2-mediated myocardial injury remains uncertain, attention should be paid, as it seriously affects prognosis and mortality (Chen et al., 2020; Siripanthong et al., 2022). Early data indicated that myocardial injury accounted for 27.8% (52/187) of COVID-19 patients and was significantly related to fatal outcomes (Guo et al., 2020).
The Omicron variant has produced the largest number of SARS-CoV-2 associated hospitalizations since the beginning of the pandemic (Faust et al., 2022; Iuliano et al., 2022; Wang et al., 2023); further, our results showed that the proportion of cardiac muscle injuries was higher in patients infected with the Omicron variant (828/1862). SARS-CoV-2-mediated acute myocardial injury is more common in patients admitted to the intensive care unit (> 80%) (Jansson et al., 2022). In addition, a positive dose-response relationship has been shown between myocardial injury and the risk of short-term all-cause mortality (Li et al., 2022b). A large multicenter clinical study from Italy also indicated that patients with myocardial injury had a higher all-cause mortality than those without myocardial injury (40.0% vs. 9.1%, P = 0.001), similar to our results of patients infected with the Omicron variant (32.0% vs. 2.5%, P < 0.001). Further, myocardial injury may last until convalescence and affect cardiac function via multiple mechanisms (Kotecha et al., 2021; Artico et al., 2023). The adverse prognosis of patients with myocardial injury may be closely related to multisystem organ involvement and critical illness (Metkus et al., 2021).
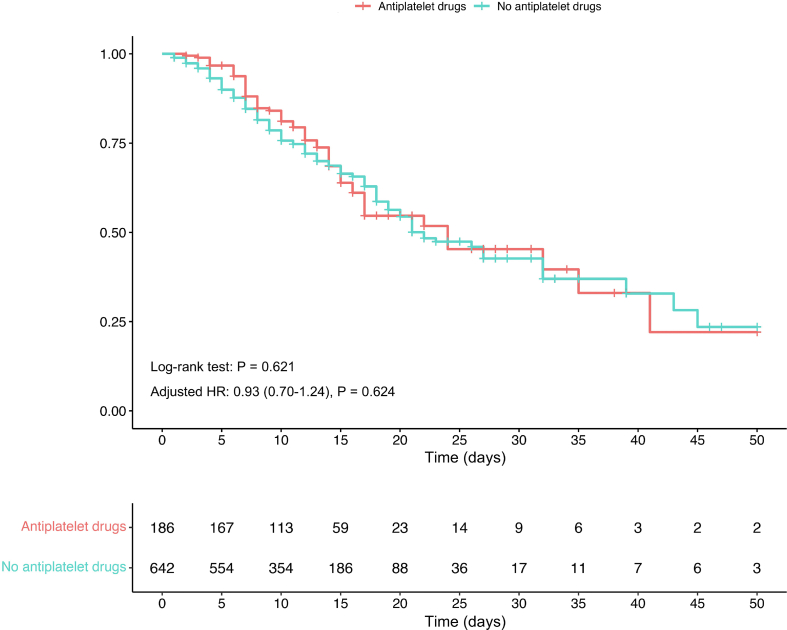
Myocardial injury may be associated with potential direct SARS-CoV-2 infection and indirect immune response mechanisms (Chung et al., 2021). Although a recent study showed that patients with myocardial injury had a certain degree of microinfarction and lower myocarditis in the convalescent period, our results showed that acute myocardial injury in COVID-19 inpatients, especially during the Omicron wave, was mainly related to myocardial inflammation, and not myocardial infarction or microthrombi. Our analysis indicated that antiplatelet drugs did not reduce the mortality of patients with COVID-19 (log-rank P = 0.621; adjusted HR, 0.93; 95% CI, 0.70–1.24; P = 0.624, Supplementary Fig. S3). Further, we have to emphasize that the sensitivity and specificity of cardiac MRI in the detection of intracardiac microthrombi are uncertain (Stuber and Baggish, 2023), and that the Lake Louise criteria cannot completely diagnose or rule out myocarditis (Ferreira et al., 2018). In fact, early studies and autopsies support the important role of myocardial inflammation in COVID-19-associated myocardial injury (Guo et al., 2020; Castiello et al., 2022; Hanson et al., 2022; Melillo et al., 2022). We also found a high proportion of myocardial inflammation in the first batch of patients with COVID-19 at Tongji Hospital and found that increased inflammatory cytokine levels can mediate cardiotoxicity and myocardial injury (Ammirati and Wang, 2020; Li et al., 2020). The core of myocarditis is mainly a cytokine storm, which may lead to potential chronic myocardial fibrosis and continue to affect cardiac function (Chen et al., 2020; Hartmann et al., 2021; Siripanthong et al., 2022). The spatial region-resolved proteome map also revealed the relationship between viral infection and a cytokine storm (Leng et al., 2022). Moreover, our results support the use of ACEI/ARB in patients infected with Omicron as well as myocardial injury, consistent with the main viewpoints and findings during the COVID-19 outbreak in 2020 (Zhang et al., 2020; Tajbakhsh et al., 2021). Although the use of ACEI/ARB in COVID-19 has been questioned, ACEI/ARB treatment has been shown to protect patients with COVID-19 patients, especially those with underlying diseases, through various protective mechanisms (Wang et al., 2020a). In particular, previous studies have explored the adverse effects of significantly increased AngII in patients with COVID-19 (Kundura et al., 2022; Wang et al., 2022). Our study further confirmed that the level of AngII in patients with Omicron infection was higher than that in normal controls, and further increased in myocardial injury group, which supported the important protective role of ACEI/ARB in patients with myocardial injury caused by Omicron infection.
This study has several limitations. First, this was a retrospective study and all data were obtained from a clinical database. Further, we could not obtain out-of-hospital survival data for patients with COVID-19. Furthermore, we were unable to assess the severity of complications, psychological factors, or compliance with medical prescriptions in patients with COVID-19. Finally, all patients were hospitalized at the Tongji Hospital. Our analysis did not compare data from other hospitals or foreign countries with those from Tongji Hospital.
5. Conclusions
In summary, our study showed that patients with COVID-19 and myocardial injury had a high proportion of mortality during the Omicron wave, and that there was a relationship between myocardial injury and myocardial inflammation. Further, ACEI/ARB treatment can reduce the mortality of patients with myocardial injury.
Data availability
The data underlying this article will be shared upon reasonable request by the corresponding author.
Ethics statement
Informed consent was obtained from each patient and the study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Research Ethics Committee of Tongji Medical College (no. TJ-IRB20210138). The study was conducted in accordance with the guidelines of the Declaration of Helsinki. Trial registration: ClinicalTrials.gov NCT05615792 (https://www.clinicaltrials.gov/ct2/show/NCT05615792).
Author contributions
Wu He: conceptualization, data curation, methodology, writing-original draft. Ke Xu: software, visualization, writing-original draft. Li Ni: supervision, data curation. Junfang Wu: investigation; methodology, resources. Yuxuan Zhang: investigation; methodology. Kun Miao: data curation, validation. Luyun Wang: data curation, validation. Dao Wen Wang: conceptualization, project administration, funding acquisition, writing-reviewing and editing.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
The authors would like to thank all patients, their families, and all investigators involved in this study. We thank all doctors and nurses who help care patients and provide clinical data. This study was supported in part by grant of National Key Research and Development Program of China (2022YFC3400700), National Natural Science Foundation of China (Nos. 82241034, 82370397, 31971358, U22A20266 and C-0052), Top-Notch Talent Program of Hubei Province and Tongji Hospital (No. 2021YBJRC005), Hubei Provincial Key Research and Developmental Program (2022BCA037), and Hubei Provincial Natural Science Foundation of China (2017CFB536).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2023.10.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
figs2.
figs3.
References
- Ababneh M.J., Al-Kasasbeh A., Jarrah M., Malkawi L., Sanduka O., Smadi A.M., Smadi M.M. Myocardial injury and its correlation to mortality in hospitalized COVID-19 patients: a retrospective cohort study. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.1039655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammirati E., Wang D.W. SARS-CoV-2 inflames the heart. The importance of awareness of myocardial injury in COVID-19 patients. Int. J. Cardiol. 2020;311:122–123. doi: 10.1016/j.ijcard.2020.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artico J., Shiwani H., Moon J.C., Gorecka M., McCann G.P., Roditi G., Morrow A., Mangion K., Lukaschuk E., Shanmuganathan M., Miller C.A., Chiribiri A., Prasad S.K., Adam R.D., Singh T., Bucciarelli-Ducci C., Dawson D., Knight D., Fontana M., Manisty C., Treibel T.A., Levelt E., Arnold R., Macfarlane P.W., Young R., McConnachie A., Neubauer S., Piechnik S.K., Davies R.H., Ferreira V.M., Dweck M.R., Berry C., Greenwood J.P. Myocardial involvement after hospitalization for COVID-19 complicated by troponin elevation: a prospective, multicenter, observational study. Circulation. 2023;147:364–374. doi: 10.1161/CIRCULATIONAHA.122.060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5:751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- Cao Z., Gao W., Bao H., Feng H., Mei S., Chen P., Gao Y., Cui Z., Zhang Q., Meng X., Gui H., Wang W., Jiang Y., Song Z., Shi Y., Sun J., Zhang Y., Xie Q., Xu Y., Ning G., Gao Y., Zhao R. Vv116 versus nirmatrelvir-ritonavir for oral treatment of COVID-19. N. Engl. J. Med. 2023;388:406–417. doi: 10.1056/NEJMoa2208822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case B.C., Shea C., Rappaport H., Cellamare M., Zhang C., Zhu M., Medranda G.A., Satler L.F., Ben-Dor I., Hashim H., Rogers T., Waksman R. The evolving impact of myocardial injury in patients with COVID-19 amid the omicron wave of the pandemic. Am. J. Cardiol. 2023;190:54–60. doi: 10.1016/j.amjcard.2022.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello T., Georgiopoulos G., Finocchiaro G., Claudia M., Gianatti A., Delialis D., Aimo A., Prasad S. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail. Rev. 2022;27:251–261. doi: 10.1007/s10741-021-10087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Li H., Hang W., Wang D.W. Cardiac injuries in coronavirus disease 2019 (COVID-19) J. Mol. Cell. Cardiol. 2020;145:25–29. doi: 10.1016/j.yjmcc.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Cheng Z., Peng Y., Wang Z., Huang C., Liu D., Wang B., Pan B., Guo W. A liquid chromatography-tandem mass spectrometry (lc-ms/ms)-based assay for simultaneous quantification of aldosterone, renin activity, and angiotensin ii in human plasma. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2021;1179 doi: 10.1016/j.jchromb.2021.122740. [DOI] [PubMed] [Google Scholar]
- Chung M.K., Zidar D.A., Bristow M.R., Cameron S.J., Chan T., Harding C.V., 3rd, Kwon D.H., Singh T., Tilton J.C., Tsai E.J., Tucker N.R., Barnard J., Loscalzo J. COVID-19 and cardiovascular disease: from bench to bedside. Circ. Res. 2021;128:1214–1236. doi: 10.1161/CIRCRESAHA.121.317997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio C., Omer S.B., Malani P.N. Winter of omicron-the evolving COVID-19 pandemic. JAMA. 2022;327:319–320. doi: 10.1001/jama.2021.24315. [DOI] [PubMed] [Google Scholar]
- Faust J.S., Du C., Liang C., Mayes K.D., Renton B., Panthagani K., Krumholz H.M. Excess mortality in Massachusetts during the delta and omicron waves of COVID-19. JAMA. 2022;328:74–76. doi: 10.1001/jama.2022.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., Kindermann I., Gutberlet M., Cooper L.T., Liu P., Friedrich M.G. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J. Am. Coll. Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P.J., Liu-Fei F., Ng C., Minato T.A., Lai C., Hossain A.R., Chan R., Grewal B., Singhera G., Rai H., Hirota J., Anderson D.R., Radio S.J., McManus B.M. Characterization of COVID-19-associated cardiac injury: evidence for a multifactorial disease in an autopsy cohort. Lab. Invest. 2022;102:814–825. doi: 10.1038/s41374-022-00783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C., Miggiolaro A., Motta J.D.S., Baena Carstens L., Busatta Vaz De Paula C., Fagundes Grobe S., Hermann de Souza Nunes L., Lenci Marques G., Libby P., Zytynski Moura L., de Noronha L., Pellegrino Baena C. The pathogenesis of COVID-19 myocardial injury: an immunohistochemical study of postmortem biopsies. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.748417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning R.J. Cardiovascular complications of COVID-19 severe acute respiratory syndrome. Am J Cardiovasc Dis. 2022;12:170–191. [PMC free article] [PubMed] [Google Scholar]
- Iuliano A.D., Brunkard J.M., Boehmer T.K., Peterson E., Adjei S., Binder A.M., Cobb S., Graff P., Hidalgo P., Panaggio M.J., Rainey J.J., Rao P., Soetebier K., Wacaster S., Ai C., Gupta V., Molinari N.M., Ritchey M.D. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, december 2020-january 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:146–152. doi: 10.15585/mmwr.mm7104e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson S., Blixt P.J., Didriksson H., Jonsson C., Andersson H., Hedström C., Engvall J., Aneq M., Chew M.S. Incidence of acute myocardial injury and its association with left and right ventricular systolic dysfunction in critically ill COVID-19 patients. Ann. Intensive Care. 2022;12:56. doi: 10.1186/s13613-022-01030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc H.C., Xiao J., Liu W., Li Y., Chen G. Long covid and its management. Int. J. Biol. Sci. 2022;18:4768–4780. doi: 10.7150/ijbs.75056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha T., Knight D.S., Razvi Y., Kumar K., Vimalesvaran K., Thornton G., Patel R., Chacko L., Brown J.T., Coyle C., Leith D., Shetye A., Ariff B., Bell R., Captur G., Coleman M., Goldring J., Gopalan D., Heightman M., Hillman T., Howard L., Jacobs M., Jeetley P.S., Kanagaratnam P., Kon O.M., Lamb L.E., Manisty C.H., Mathurdas P., Mayet J., Negus R., Patel N., Pierce I., Russell G., Wolff A., Xue H., Kellman P., Moon J.C., Treibel T.A., Cole G.D., Fontana M. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundura L., Gimenez S., Cezar R., André S., Younas M., Lin Y.L., Portalès P., Lozano C., Boulle C., Reynes J., Vincent T., Mettling C., Pasero P., Muller L., Lefrant J.Y., Roger C., Claret P.G., Duvnjak S., Loubet P., Sotto A., Tran T.A., Estaquier J., Corbeau P. Angiotensin ii induces reactive oxygen species, DNA damage, and t-cell apoptosis in severe COVID-19. J. Allergy Clin. Immunol. 2022;150:594–603.e592. doi: 10.1016/j.jaci.2022.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng L., Ma J., Zhang P.P., Xu S.C., Li X., Jin Y., Cai J., Tang R., Zhao L., He Z.C., Li M.S., Zhang H., Zhou L.R., Wu Z.H., Li T.R., Zhu Y.P., Wang Y.J., Wu H.B., Ping Y.F., Yao X.H., Zhu C.H., Guo H.T., Tan L.Y., Liang Z.Y., Bian X.W., Zhang S.Y. Spatial region-resolved proteome map reveals mechanism of COVID-19-associated heart injury. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang D.W., Zhao C. Cardiovascular involvement in patients with 2019 novel coronavirus disease. J Transl Int Med. 2021;9:152–160. doi: 10.2478/jtim-2021-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Jiang J., Wang F., Zhou N., Veronese G., Moslehi J.J., Ammirati E., Wang D.W. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J. Mol. Cell. Cardiol. 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Shi J., Liu X., Ward M.P., Wang Z., Liu R., Zhao Z., Yin Y., Liu Y., Hong J., Huang J., Chen X., Zhang Z. Early warning signals for omicron outbreaks in China: a retrospective study. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28341. [DOI] [PubMed] [Google Scholar]
- Li M., Wang H., Tian L., Pang Z., Yang Q., Huang T., Fan J., Song L., Tong Y., Fan H. COVID-19 vaccine development: milestones, lessons and prospects. Signal Transduct. Targeted Ther. 2022;7:146. doi: 10.1038/s41392-022-00996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Pei H., Zhou C., Lou Y. Myocardial injury predicts risk of short-term all-cause mortality in patients with COVID-19: a dose-response meta-analysis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.850447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G.B. Myocardial injury in patients with COVID-19. Nat. Rev. Cardiol. 2020;17:454. doi: 10.1038/s41569-020-0408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.F., Wu X., Li Y., Bian J., Li K., Jiang Y., Lu Z., Zhang B., Yang C., Sun C., Sun L., Zou H. Impact of combination preventative interventions on hospitalization and death under the pandemic of SARS-CoV-2 omicron variant in China. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28335. [DOI] [PubMed] [Google Scholar]
- Melillo F., Napolano A., Loffi M., Regazzoni V., Boccellino A., Danzi G.B., Cappelletti A.M., Rovere-Querini P., Landoni G., Ingallina G., Stella S., Ancona F., Dagna L., Scarpellini P., Ripa M., Castagna A., Tresoldi M., Zangrillo A., Ciceri F., Agricola E. Myocardial injury in patients with SARS-CoV-2 pneumonia: pivotal role of inflammation in COVID-19. Eur. J. Clin. Invest. 2022;52 doi: 10.1111/eci.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metkus T.S., Sokoll L.J., Barth A.S., Czarny M.J., Hays A.G., Lowenstein C.J., Michos E.D., Nolley E.P., Post W.S., Resar J.R., Thiemann D.R., Trost J.C., Hasan R.K. Myocardial injury in severe COVID-19 compared with non-COVID-19 acute respiratory distress syndrome. Circulation. 2021;143:553–565. doi: 10.1161/CIRCULATIONAHA.120.050543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siripanthong B., Asatryan B., Hanff T.C., Chatha S.R., Khanji M.Y., Ricci F., Muser D., Ferrari V.A., Nazarian S., Santangeli P., Deo R., Cooper L.T., Jr., Mohiddin S.A., Chahal C.A.A. The pathogenesis and long-term consequences of COVID-19 cardiac injury. JACC Basic Transl Sci. 2022;7:294–308. doi: 10.1016/j.jacbts.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber M., Baggish A.L. Acute myocardial injury in the covid-heart study: emphasizing scars while reassuring scares. Circulation. 2023;147:375–377. doi: 10.1161/CIRCULATIONAHA.122.062508. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh A., Gheibi Hayat S.M., Taghizadeh H., Akbari A., Inabadi M., Savardashtaki A., Johnston T.P., Sahebkar A. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti Infect. Ther. 2021;19:345–357. doi: 10.1080/14787210.2020.1822737. [DOI] [PubMed] [Google Scholar]
- Telenti A., Arvin A., Corey L., Corti D., Diamond M.S., García-Sastre A., Garry R.F., Holmes E.C., Pang P.S., Virgin H.W. After the pandemic: perspectives on the future trajectory of COVID-19. Nature. 2021;596:495–504. doi: 10.1038/s41586-021-03792-w. [DOI] [PubMed] [Google Scholar]
- Wadhera R.K., Figueroa J.F., Rodriguez F., Liu M., Tian W., Kazi D.S., Song Y., Yeh R.W., Joynt Maddox K.E. Racial and ethnic disparities in heart and cerebrovascular disease deaths during the COVID-19 pandemic in the United States. Circulation. 2021;143:2346–2354. doi: 10.1161/CIRCULATIONAHA.121.054378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.J., Edin M.L., Zeldin D.C., Li C., Wang D.W., Chen C. Good or bad: application of raas inhibitors in COVID-19 patients with cardiovascular comorbidities. Pharmacol. Ther. 2020;215 doi: 10.1016/j.pharmthera.2020.107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Gheblawi M., Nikhanj A., Munan M., MacIntyre E., O’Neil C., Poglitsch M., Colombo D., Del Nonno F., Kassiri Z., Sligl W., Oudit G.Y. Dysregulation of ace (angiotensin-converting enzyme)-2 and renin-angiotensin peptides in SARS-CoV-2 mediated mortality and end-organ injuries. Hypertension. 2022;79:365–378. doi: 10.1161/HYPERTENSIONAHA.121.18295. [DOI] [PubMed] [Google Scholar]
- Wang M., Liu Z., Wang Z., Li K., Tian Y., Lu W., Hong J., Peng X., Shi J., Zhang Z., Mei G. Clinical characteristics of 1139 mild cases of the SARS-CoV-2 omicron variant infected patients in shanghai. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Du Z., Zhu F., Cao Z., An Y., Gao Y., Jiang B. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395:e52. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfler A., Mannarino S., Giacomet V., Camporesi A., Zuccotti G. Acute myocardial injury: a novel clinical pattern in children with COVID-19. Lancet Child Adolesc Health. 2020;4:e26–e27. doi: 10.1016/S2352-4642(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo Y.H., Wang M., He X., Lv F., Zhang Y., Zu J., Li M., Jiao Y., Ebinger J.E., Patel J.K., Cheng S., Ji F. Excess risk for acute myocardial infarction mortality during the COVID-19 pandemic. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., Liu Y.M., Zhao Y.C., Huang X., Lin L., Xia M., Chen M.M., Cheng X., Zhang X., Guo D., Peng Y., Ji Y.X., Chen J., She Z.G., Wang Y., Xu Q., Tan R., Wang H., Lin J., Luo P., Fu S., Cai H., Ye P., Xiao B., Mao W., Liu L., Yan Y., Liu M., Chen M., Zhang X.J., Wang X., Touyz R.M., Xia J., Zhang B.H., Huang X., Yuan Y., Loomba R., Liu P.P., Li H. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request by the corresponding author.