Abstract
Adherence of enteropathogenic Escherichia coli (EPEC) to epithelial cells is dependent on a type IV fimbria, termed the bundle-forming pilus (BFP). A cluster of 14 genes is required for expression of BFP. The eighth gene in the cluster, bfpF, encodes a putative nucleotide-binding protein which resembles the PilT protein of Pseudomonas aeruginosa. It has been proposed that PilT is required for the retraction of the P. aeruginosa pilus, which results in twitching motility. To test the role of BfpF in BFP function and EPEC pathogenesis, two different mutations were constructed in the bfpF gene, one in the cloned gene cluster in a laboratory E. coli strain and one in wild-type EPEC. Neither mutation affected prepilin synthesis, leader sequence processing, or pilus biogenesis. However, both mutations resulted in increased localized adherence. In addition, the EPEC bfpF mutant displayed increased aggregation. The EPEC bfpF mutant was not deficient in attaching and effacing activity or invasion capacity. These results suggest that BfpF decreases aggregation and adherence by EPEC but that subsequent steps in EPEC pathogenesis do not require this protein.
Enteropathogenic Escherichia coli (EPEC) strains are a leading cause of infantile diarrhea in developing countries (10). EPEC adhere to epithelial cells in vitro in a pattern termed localized adherence (8, 31). This adherence is dependent on the bundle-forming pilus (BFP), a type IV fimbria of EPEC (12, 18), and is considered the first stage of EPEC pathogenesis (14). The remaining two stages of EPEC pathogenesis, collectively known as attaching and effacing, consist of the activation of host cell signal transduction pathways and intimate attachment of the bacteria to the epithelial cell (14). Following these stages, a small percentage of the bacteria are internalized by the host cell in a process called invasion, a step of unknown significance in EPEC pathogenesis.
Type IV fimbria are expressed by many pathogenic organisms, including Pseudomonas aeruginosa, Vibrio cholerae, and Neisseria gonorrhoeae (19, 34). Although very little is known about the biogenesis and function of type IV pili, several proteins similar to those involved in type IV fimbrial biogenesis are involved in protein secretion and DNA transfer systems in various organisms, suggesting that the assembly mechanisms in these systems are similar (19, 34). In addition to mediating adherence to epithelial cells, some type IV fimbriae also serve as receptors for bacteriophages (5, 37). Bradley described a phage-resistant mutant of P. aeruginosa that had increased piliation and bound bacteriophage throughout its length rather than at the junction of the pilus and the cell surface. This observation, and the results of experiments using pilus-specific antibodies, led him to suggest that wild-type P. aeruginosa strains are able to retract the pili from the surface of the bacteria and that the mutant is deficient in pilus retraction (5). The same mutant reported to be defective in pilus retraction is also incapable of a form of surface translocation known as twitching motility, suggesting that twitching motility is also dependent on pilus retraction (6). The mutation responsible for these phenotypes was later localized to a gene called pilT (40). Because the sequence of pilT revealed that it could encode a protein with putative nucleotide-binding domains, it is possible that PilT provides the energy for type IV pilus retraction in P. aeruginosa (19). Since the description of the pilT mutant, numerous genes required for twitching motility have been described (1–3, 9, 24, 29, 41) and PilT orthologs have been identified in other species that produce type IV pili (7).
Localized adherence by EPEC is dependent on the presence of a large (90-kb) plasmid, termed the EPEC adherence factor (EAF) plasmid (4), which contains a cluster of 14 genes necessary and sufficient for the expression of BFP (32, 33). This cluster has been cloned as plasmid pKDS135 (33) and, in combination with another fragment of the EAF plasmid, cloned as pJPN14, is sufficient to confer pilus biogenesis and the localized-adherence phenotype when transformed into laboratory strains of E. coli (33). The first gene of the cluster, bfpA, encodes bundlin, the major structural subunit of BFP (12). Bundlin is expressed as a preprotein that is cleaved to its mature form by a prepilin peptidase that is encoded by the bfpP gene (43). Several of the remaining 12 genes of the cluster have orthologs in other type IV fimbria or similar systems (33).
The sixth and eighth genes in the bfp cluster, bfpD and bfpF, encode putative nucleotide-binding proteins (33). The predicted amino acid sequence of the BfpF protein has 32% identity over 248 amino acids to that of the PilT protein of P. aeruginosa (33, 40), while BfpD is more closely related to PilB (27, 32). Previous experiments in our laboratory have shown that the BfpF protein is produced in a T7 expression system (33). The similarity between BfpF and PilT led us to hypothesize that BfpF is involved in the retraction of BFP. We predicted that bfpF mutants would adhere to epithelial cells in greater numbers than wild-type EPEC, since the number and length of pili in these bacteria would be greater than in wild-type EPEC. Because BFP have also been implicated in the autoaggregation of EPEC strains (36), we predicted that bfpF mutants would form larger clusters than wild-type EPEC. In addition, we predicted that EPEC with nonretractable pili would perform attaching and effacing and invasion less efficiently than wild-type EPEC, since pilus retraction could draw adherent bacteria closer to epithelial cells and increase their efficiency of intimate attachment to the cells. To test these hypotheses, we constructed two mutations in bfpF, one in the cloned gene cluster in a laboratory E. coli strain and one in the wild-type EPEC background.
MATERIALS AND METHODS
Strains and plasmids.
Bacterial strains and plasmids used in these experiments are listed in Table 1. Strains were grown on Luria-Bertani (LB) agar or in LB broth except where indicated. Antibiotics, when necessary, were added at the following concentrations: ampicillin, 200 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 20 μg/ml; and naladixic acid, 50 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype and/or feature(s) | Source or reference |
|---|---|---|
| Strains | ||
| E2348/69 | Prototype O127:H6 EPEC strain | 23 |
| JPN15 | E2348/69 cured of EAF plasmid | 21 |
| UMD901 | E2348/69 bfpA, with S substituted for C at position 129 | 42 |
| CVD206 | E2348/69 Δeae | 13 |
| UMD207 | E2348/69 Δeae, cured of the EAF plasmid | 16 |
| UMD916 | E2348/69 bfpF::aphA3 | This study |
| DH5α | supE44 ΔlacU169(φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 30 |
| HB101 | supE44 hsdSD20 (rB− mB−) recA1 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 30 |
| DH5αλpir | DH5α(λpir) | 25 |
| BL21(DE3) | F−omp [lon] hsdSB (rB− mB−; and E. coli B strain) with DE3, a λ prophage carrying the T7 RNA polymerase gene | 35 |
| Plasmids | ||
| pBluescript | High-copy-number cloning vector | Stratagene |
| pCVD442 | Positive-selection suicide vector | 13 |
| pDN19PBΔ | 1.3-kb PstI-BamHI fragment containing bfpP with a 100-bp deletion cloned into pDN19 | 43 |
| pKDS135 | bfp gene cluster cloned into pBR322 | 33 |
| pKDS134 | pKDS135 lacking bfpL | 33 |
| pJPN14 | Fragment of EAF plasmid, required for expression of bfp genes, cloned into pACYC184 | 26 |
| pRPA5 | 3-kb SacI-EcoRI fragment containing bfpF cloned in pBluescript | This study |
| pRPA6 | pRPA5 with a 72-bp KpnI fragment deleted | This study |
| pRPA102 | bfpF cloned into low-copy-number vector pWKS30 | This study |
| pUC18K | aphA3 gene cloned into pUC18, creating a nonpolar kanamycin cassette | 25 |
| pWKS30 | Low-copy-number cloning vector | 39 |
| pRPAF1 | pKDS135 with 72-bp KpnI fragment deleted | This study |
| pKDS8.1 | Asp700 fragment containing bfpF::aphA3 cloned into pBluescript | This study |
| pKDS8.2 | Asp700 fragment containing bfpF::aphA3 cloned into pCVD442 | This study |
| pUMD916 | bfpF::aphA3 EAF plasmid from UMD916 | This study |
| pMSD205 | bfpA gene cloned into pCR1000 under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter | 12 |
DNA cloning.
DNA restriction digests, electrophoresis, and ligations were performed by standard procedures (30). Plasmids were introduced into strains by CaCl2 transformation or electroporation. Electroporations were carried out in 10% glycerol in a 0.1-cm cuvette, using an E. coli pulser (Bio-Rad) set at 1.8 kV. To construct pRPAF1, pBluescript II SK (Stratagene, La Jolla, Calif.) was modified by cutting with KpnI, blunt ended by treatment with the large fragment of DNA polymerase I, and self-ligated, thus destroying the KpnI site in the multiple cloning region. A 3-kb SacI-EcoRI fragment from pKDS135 containing bfpF was then cloned into this modified pBluescript to form pRPA5. A 72-bp KpnI fragment was excised from pRPA5, which was self-ligated to form pRPA6. A 0.8-kb SacI-DraIII fragment from pRPA6 containing the deletion was used to replace the SacI-DraIII fragment of pKDS135, forming pRPAF1.
To introduce a bfpF mutation into wild-type EPEC, a 1.1-kb Asp700 fragment from pKDS135 was cloned into pBluescript II SK. A previously described nonpolar kanamycin cassette (25) was inserted into the unique BbrPI site of bfpF, resulting in pKDS8.1. The essential feature of this cassette is that it provides for reinitiation of the translation of the disrupted gene without affecting transcription. The insert was excised from pBluescript by using the SacI and SalI sites in the multiple cloning region and was ligated into the SacI and SalI sites of the positive-selection suicide vector pCVD442 (13) to form pKDS8.2. Plasmid pKDS8.2 was mobilized into wild-type EPEC by triparental conjugation, and allelic exchange was carried out as previously described (17). Potential mutant colonies were screened by PCR. PCR primers Donne-38 (5′-CTG TCA AGA TGG ACC GGA AGG TTG C-3′) and Donne-39 (5′-CTC TGT AAG TGG ATA CCG AAC GGG AAC-3′) were used to amplify the bfpF gene. Other primers used were Donne-28 (5′-CGC GGA TCC ATG GTT TCT AAA ATC ATG AAT-3′) and Donne-29 (5′-GCG AAG CTT TTA CTT CAT AAA ATA TGT AAC-3′) for amplification of bfpA and Donne-110 (5′-GCG GGG ATC CGT AAG TAA AAA TTA TGG TTC G-3′) and Donne-111 (5′ GCG GAA GCT TAA AGG CCG CTT TCT TTT C-3′) for amplification of bfpI. PCR was performed on fresh colonies, using Taq DNA polymerase (Boehringer Mannheim) in 50-μl samples. The reactions were run for 30 cycles of denaturation (94°C for 1 min), annealing (55°C for 1 min), and extension (72°C for 1 min). The bfpF mutant EPEC strain was named UMD916.
To complement the bfpF mutation in UMD916, the bfpF gene, along with its predicted ribosome-binding sequence, was amplified from wild-type EPEC by using primers Donne-235 (5′-GGG AAT TCC TGA TTC GGT GTG ATA TCA TG-3′) and Donne-236 (5′-GGG GAT CCT GCA TAA TAT TTT AGC TAA TCA GGT T-3′), which introduce an EcoRI site at the 5′ end and a BamHI site at the 3′ end of the gene. PCR was performed with DeepVent DNA polymerase (New England BioLabs) in 50-μl samples. The reactions were run for 30 cycles of denaturation (94°C for 1 min), annealing (55°C for 30 s), and extension (72°C for 1 min). The restriction sites added were used to clone the bfpF gene into the low-copy-number vector pWKS30 to make plasmid pRPA102. The construct was verified by restriction analysis and DNA sequencing and then electroporated into strain UMD916, producing strain UMD916(pRPA102). As a control strain for complementation, pWKS30 was electroporated into UMD916, producing strain UMD916(pWKS30).
Aggregation assay.
Bacterial strains were grown overnight in L broth at 37°C, with appropriate antibiotics being added when necessary. Overnight cultures were diluted 1:25 in Dulbecco’s modified Eagle’s medium (DMEM) and incubated for 4 h at 37°C in an atmosphere of 95% air–5% CO2. A drop of culture was placed on a microscope slide, and each culture was examined by phase-contrast microscopy for aggregation. Aliquots of each sample were also removed to measure the A600 of each culture. After each measurement, the aliquot was vortexed for 30 s while still in the cuvette and the A600 was measured again. The percent increase in A600 after vortexing was recorded as a quantitative aggregation index. Four separate experiments were performed, each with quadruplicate samples.
Tissue culture and adherence assay.
Qualitative adherence assays were performed with HEp-2 cells (ATCC CCL 23) in eight-well chamber slides (Nunc, Naperville, Ill.) as previously described (15). The number of bacteria adherent to 100 consecutively examined cells per sample was recorded without knowledge of strain identity. Since clusters containing 50 or more bacteria were recorded as having 50, this method tended to underestimate the difference between very adherent strains.
For quantitative assays of wild-type and isogenic mutant strains, 1 ml of HEp-2 cells was plated in each well of a 24-well plate at a concentration of 4 × 105 cells/ml and incubated overnight. Overnight bacterial cultures were diluted 1:100 in DMEM lacking cysteine and methionine, incubated for 4 h at 37°C in an atmosphere of 95% air–5% CO2, and then incubated for 15 min with 10 μCi of [35S]cysteine-[35S]methionine per ml at 37°C. The labeled bacteria were then centrifuged for 10 min at 900 × g and resuspended in fresh DMEM lacking cysteine and methionine. HEp-2 cells were washed with phosphate-buffered saline (PBS) and incubated with Eagle’s minimal essential medium containing cycloheximide (50 μg/ml) 30 min prior to infection. Cells were then washed again and infected with 1 ml of labeled bacteria for 1 h at 37°C in the presence of cycloheximide. After incubation, the cells were washed with PBS, lysed with 1 ml of 0.5% sodium dodecyl sulfate (SDS), and subjected to scintillation counting. Labeled bacteria were subjected to scintillation counting and enumerated by plate dilution to determine the number of CFU in the inoculum, so that the number of counts from each cell lysate could be converted to CFU. The calculated CFU for each sample was divided by the mean CFU for the wild type from the same experiment to yield a relative adherence value.
Invasion assay.
The gentamicin protection assay was performed as previously described (11). All strains used were inhibited by <6 μg of gentamicin per ml.
Immunoblotting.
Overnight bacterial cultures were centrifuged (10,000 × g, 5 min), and the cells were resuspended in water. An equal volume of 2× SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer (0.06 M Tris-HCl, pH 6.8; 2% SDS; 10% [vol/vol] glycerol) was added to each sample. Protein concentrations of bacterial lysates were determined by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.) in a multiplate reader with bovine serum albumin as a standard. Bromphenol blue (0.025%) and 2-mercaptoethanol (5%) were then added to the remainder of the lysates. Samples were denatured by boiling for 10 min in the SDS-PAGE buffer, and 5 μg of total protein per lane was loaded on 15% polyacrylamide gels and separated by electrophoresis. Samples were transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, Mass.). The blots were blocked with PBS containing 0.5% (vol/vol) Tween 20, reacted with a rabbit antibundlin antiserum (42) at a 1:5,000 dilution, incubated with a goat anti-rabbit antiserum conjugated with alkaline phosphatase (Boehringer Mannheim, Indianapolis, Ind.), and developed with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (Sigma, St. Louis, Mo.).
Transmission electron microscopy.
For viewing BFP, overnight bacterial cultures were diluted 1:100 in LB and grown until turbid. The bacteria were then centrifuged and resuspended in Eagle’s minimal essential medium. A 10-μl aliquot of the culture was spotted on Formvar-carbon-coated copper grids and incubated for 3 h at 37°C in an atmosphere of 95% air–5% CO2. The grids were coded to blind the investigator to the identities of the bacterial strains. The grids were then blotted, stained with phosphotungstic acid in PBS, and examined in a Jeol JEM-1200 EXII transmission electron microscope.
For measuring the distance between HEp-2 cells and adherent bacteria and for quantitative analysis of attaching and effacing efficiencies, 0.3-ml aliquots of HEp-2 cells at a concentration of 4 × 105/ml were incubated in Transwell filter units (Costar, Cambridge, Mass.) in 24-well plates. The cells were incubated for 3 days and then infected for 3 h with 10 μl of overnight bacterial cultures that had been coded to blind the investigator to their identity. The filter units were then washed and fixed with 2% formaldehyde–1% glutaraldehyde. Thin sections of the cells were cut, stained, and mounted on grids for viewing with a Jeol JEM-1200 EX transmission electron microscope. For grids that contained bacteria engaged in attaching and effacing lesions, the number of bacteria intimately attached to cells with the host cell membrane forming a concave surface conforming to the shape of the bacteria (attaching and effacing bacteria) was recorded. The number of bacteria not engaged in such an association with host cells was also recorded (nonattaching and noneffacing bacteria). For grids that contained no bacteria engaged in attaching and effacing lesions, photomicrographs of all fields that had five or more bacteria were taken at a magnification of ×10,000. The shortest distance between each bacterium and the closest cell was measured from photomicrographs. When more than one layer of bacteria was present, only the distances between those bacteria in the layer closest to the cell were recorded.
Competitive reverse transcription-PCR (RT-PCR).
Total RNA was isolated from wild-type EPEC and strain UMD916 by using the Perfect RNA Total RNA Isolation Kit (5 Prime→3 Prime Inc.). A 5-μg quantity of each RNA sample was treated with DNase, and bfpP gene cDNA was synthesized using reverse transcriptase and primer Donne-62 (5′-GCG AAG CTT TTA ATG ATA AAC TAA ACA TAT-3′). Negative controls were prepared by boiling the RNA samples for 15 min immediately after adding the reverse transcriptase. The cDNA samples were then treated with RNase, and 2 μg of each sample was used in a competitive PCR. Primers Donne-62 and Donne-63 (5′-CGC GGA TCC ATG CAA GAA AGT ATA TTT CTA-3′) were used to amplify bfpP. The competing DNA used was plasmid pDN19PBΔ, which contains the bfpP gene with a 100-bp deletion. PCRs were performed by adding equal amounts of cDNA and progressive twofold dilutions of pDN19PBΔ. Reactions were performed with Taq DNA polymerase in 50-μl samples and were run for 30 cycles of denaturation (94°C for 1 min), annealing (45°C for 30 s), and extension (72°C for 1 min). Reactions were performed with RNA extracted on three separate occasions.
RESULTS
Construction of two bfpF mutants.
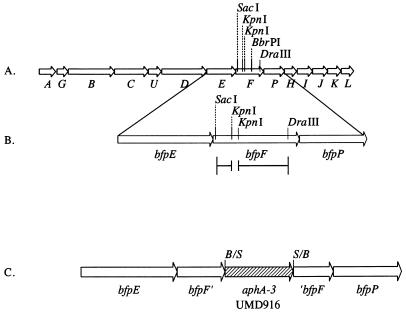
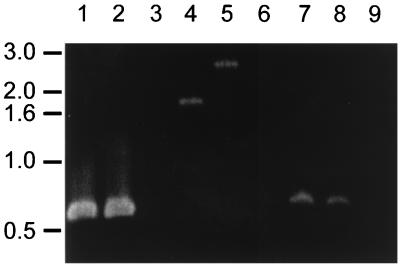
To study the role of BfpF in EPEC pathogenesis, mutations in the bfpF gene were made in the cloned bfp gene cluster in a laboratory E. coli background and in the wild-type EAF plasmid in an EPEC background. The location of each of these mutations in the bfpF gene is shown in Fig. 1. A 72-bp deletion was engineered in pKDS135, creating plasmid pRPAF1, and confirmed by restriction endonuclease digestion (data not shown). pRPAF1 was transformed into E. coli HB101 containing pJPN14 to study the role of BfpF in the recombinant E. coli system. In addition, plasmid pKDS8.2, containing bfpF with a nonpolar kanamycin cassette (25) inserted in the suicide vector pCVD442 (13), was constructed. This plasmid was mobilized into wild-type EPEC by triparental conjugation. The sacB gene on the suicide vector was employed to select for allelic exchange of the mutant copy of bfpF for wild-type bfpF, creating strain UMD916. PCR analysis of UMD916 and wild-type EPEC confirmed the presence of the additional 850 bp, corresponding to the size of the kanamycin cassette, in the bfpF gene of UMD916, with no changes in the mobility of PCR products of nearby loci (Fig. 2).
FIG. 1.
Map of the bfp gene cluster illustrating construction of bfpF mutants. (A) The entire bfp gene cluster is shown. Arrows represent bfp genes. Selected restriction enzyme sites shown in bfpF were used for cloning. (B) The bfpEFP genes are magnified to illustrate the construction of pRPAF1. A 72-bp KpnI site was deleted from a subcloned fragment containing bfpF as shown in the line below the map. The SacI-DraIII fragment from this construct was then used to replace the SacI-DraIII fragment of pKDS135 to generate pRPAF1. (C) The bfpEFP genes are magnified to illustrate the construction of strain UMD916. A nonpolar kanamycin resistance cassette containing the aphA3 gene was inserted via its flanking SmaI sites into the BbrPI site in a subcloned fragment containing bfpF. B/S and S/B represent the junctions of ligated fragments that had BbrPI and SmaI sites. A fragment containing this insert was cloned into positive-selection suicide vector pCVD442 for allelic exchange with the wild-type strain.
FIG. 2.
Analysis of the bfpF mutant and wild-type EPEC strains by PCR. Fragments of bfpA (lanes 1 to 3), bfpF (lanes 4 to 6), and bfpI (lanes 7 to 9) genes were amplified by PCR from wild-type EPEC strain E2348/69 (lanes 1, 4, and 7), bfpF mutant strain UMD916 (lanes 2, 5, and 8), and EAF plasmid-cured strain JPN15 (lanes 3, 6, and 9). PCR products were separated by agarose gel electrophoresis, stained with ethidium bromide, and photographed. The positions of molecular size markers (in kilobases) are indicated on the left.
The bfpF::aphA3 mutation is not polar on transcription of bfpP.
Competitive RT-PCR was performed on total RNA isolated from wild-type EPEC and UMD916 (Fig. 3). The results show that the level of transcription of bfpP, the gene directly downstream of bfpF, is equivalent in wild-type EPEC and UMD916. Therefore, the bfpF mutation in UMD916 does not affect the transcription of bfpP.
FIG. 3.
Competitive RT-PCR on the bfpP transcripts from wild-type EPEC and the bfpF mutant strain. Competitive RT-PCR on bfpP was performed on total RNA from wild-type EPEC and bfpF mutant strain UMD916. Plasmid pDN19PBΔ, containing bfpP with a 100-bp deletion, was used as the competitor DNA in the PCRs. Positive controls include PCR products of reactions using cDNA from wild-type EPEC (lane 1) and strain UMD916 (lane 2) and 0.2 μg of pDN19PBΔ DNA (lane 3) as a control for the competitor fragment. RNA from wild-type EPEC (lane 4) and strain UMD916 (lane 5) which was boiled immediately after addition of reverse transcriptase for cDNA synthesis were run as negative controls. The remaining lanes consisted of either wild-type EPEC cDNA (lanes 6 to 12) or strain UMD916 cDNA (lanes 13 to 19), with the following amount of pDN19PBΔ DNA added: 2 μg (lanes 6 to 13), 0.2 μg (lane 7 and 14), 0.1 μg (lanes 8 and 15), 0.05 μg (lanes 9 and 16), 0.025 μg (lanes 10 and 17), 0.0125 μg (lanes 11 and 18), or 4 × 10−6 μg (lanes 12 and 19). The positions of molecular size markers of 1,018 bp and 507 bp are indicated on the left.
BfpF is not required for expression or processing of bundlin.
The first gene in the bfp cluster, bfpA, encodes bundlin, the major structural subunit of BFP (12). Bundlin is encoded as a preprotein which is cleaved by the product of the bfpP gene (43), located directly downstream of bfpF in the bfp cluster (Fig. 1). To determine if either mutation affected bundlin expression or BfpP function, immunoblotting for bundlin was performed on both mutant strains (Fig. 4). Bundlin was expressed and processed by mutant strains in a manner indistinguishable from that of wild-type EPEC. In contrast, bundlin was not seen in bfpA mutant UMD901 and prebundlin was not fully processed in strain BL21(DE3) with plasmid pMSD205, which contains only the bfpA gene under the control of an inducible promoter. This result indicates that neither bfpF mutation affects expression of prebundlin or processing to bundlin and indicates that neither mutation disrupts expression of the bfpP locus immediately downstream of bfpF.
FIG. 4.
Expression and processing of bundlin in EPEC and in HB101 strains containing the cloned gene cluster. Whole-cell lysates of strains grown in LB were prepared, separated by SDS-PAGE on a 15% polyacrylamide gel, and analyzed by immunoblotting with an antibundlin antiserum. Lane 1, bfpA mutant strain UMD901; lane 2, wild-type strain E2348/69; lane 3, bfpF mutant UMD916; lane 4, BL21(DE3) with plasmid pMSD205, which contains bfpA alone; lane 5, HB101 with plasmids pKDS135 and pJPN14; lane 6, HB101 with plasmids pRPAF1 (bfpF) and pJPN14. The positions of molecular weight markers (in thousands) are indicated on the left.
BfpF is not required for expression of BFP.
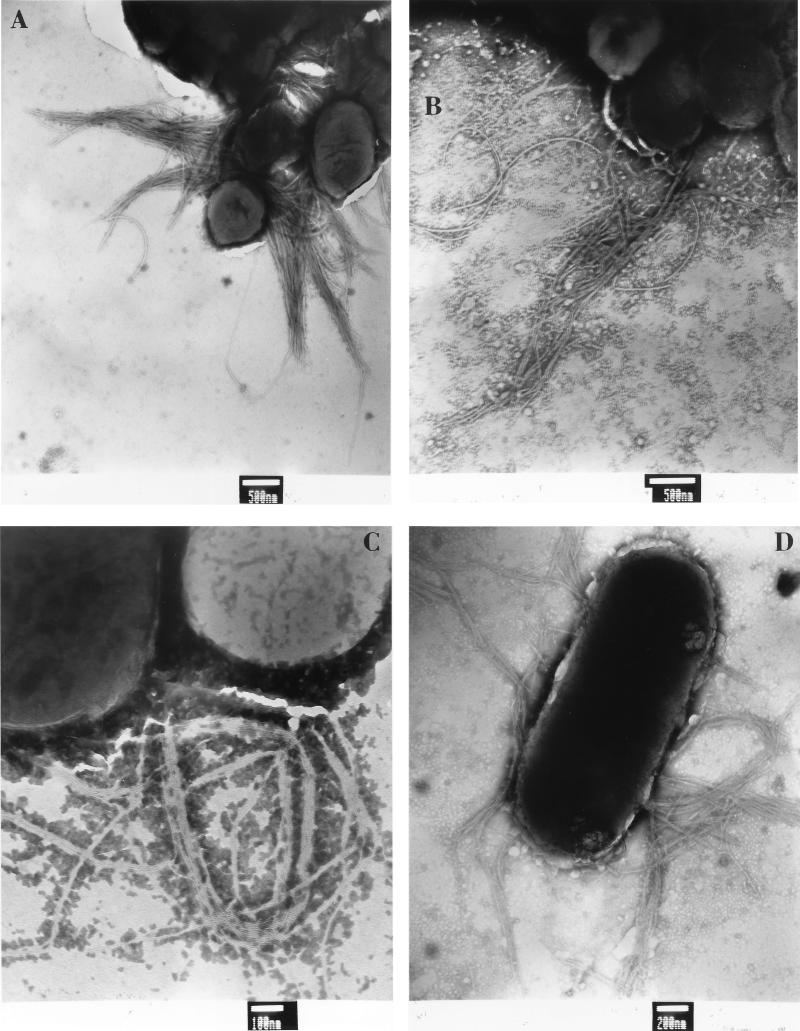
To examine the effect of BfpF on pilus biogenesis, both mutant strains were examined by negative staining and transmission electron microscopy (Fig. 5). BFP were observed both in the laboratory strain containing the mutated bfpF within the cloned bfp gene cluster and in the EPEC background strain UMD916 containing the bfpF::aphA3 mutation. In contrast, BFP were never observed in a laboratory strain containing the cloned bfp gene cluster lacking bfpL, the last gene in the cluster, or in the EAF plasmid-cured EPEC strain JPN15 (data not shown). We detected no dramatic differences between the wild-type and mutant strains in the number or length of the pili. However, because BFP were found in only a small percentage of bacteria, and since the pili aggregate to form bundles, it was impossible to exclude such differences.
FIG. 5.
Expression of BFP by EPEC and by E. coli HB101 strains containing the cloned bfp gene cluster. Strains were grown directly on Formvar-copper-coated grids, stained with phosphotungstic acid, and analyzed by electron microscopy. (A) Wild-type EPEC strain E2348/69. (B) bfpF mutant strain UMD916. (C) E. coli HB101 containing the complete bfp gene cluster in plasmid pKDS135 and plasmid pJPN14, which is also required for BFP expression. (D) HB101 containing pRPAF1, which is identical to pKDS135 except for a 72-bp bfpF deletion, and pJPN14. Bundles of flexible fimbriae are apparent in each frame. Bars indicate scale, as marked.
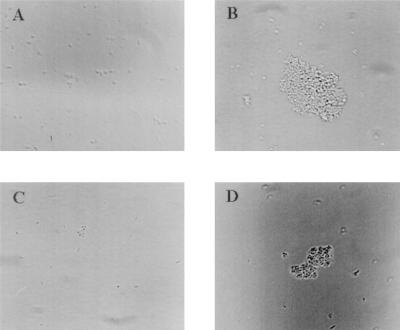
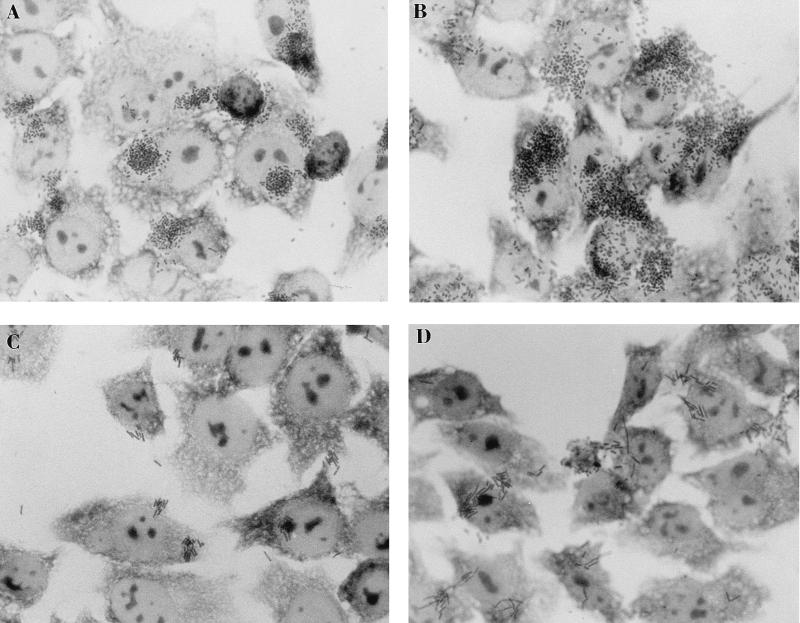
BfpF mutants aggregate to form larger clusters than wild-type EPEC.
When observed by phase-contrast microscopy, strain UMD916 was found to form much larger clusters than wild-type EPEC (Fig. 6). Strain UMD916(pRPA102), containing the bfpF gene cloned in the low-copy-number vector pWKS30, formed clusters similar in size to those formed by wild-type EPEC. Control strain UMD916(pWKS30), containing the cloning vector only, formed clusters similar in size to those formed by UMD916. To quantify the ability of the EPEC strains to aggregate, the A600 of a 4-h culture of each strain was measured before and after vortexing, and the percentage increase in A600 after vortexing was calculated and expressed as an aggregation index. Disaggregation of the bacteria by vortexing was verified by microscopy. The mean percent increase in the A600 ± the standard error of the mean for the wild-type strain after vortexing was 10.0% ± 0.91%, while that for UMD916 was 15.5% ± 1.34%. The aggregation index of the mutant was significantly higher than that of the wild type (P < 0.001, Student’s t test). Complementation of UMD916 with plasmid pRPA102 restored the aggregation index to 11.4% ± 1.25% (P = 0.17 versus the wild type; P = 0.02 versus UMD916), while the aggregation index of UMD916 containing the vector alone remained at 15.9% ± 1.16% (P < 0.001 versus the wild type; P = 0.41 versus UMD916). These values verify observations made by microscopy that strains UMD916 and UMD916(pWKS30) form larger clusters than wild-type EPEC and strain UMD916(pRPA102). These results indicate that BfpF inhibits the autoaggregation of EPEC.
FIG. 6.
Autoaggregation of EPEC strains. Overnight cultures of EPEC strains were diluted 1:25 in DMEM and incubated for 4 h. Samples were spotted on microscope slides and examined by phase-contrast microscopy. (A) Wild-type EPEC strain E2348/69. (B) bfpF mutant strain UMD916. (C) Strain UMD916 containing complementing plasmid pRPA102. (D) Strain UMD916 containing control vector pWKS30.
BfpF mutants adhere in greater numbers than do wild-type strains.
When the ability to perform localized adherence was assayed, each mutant strain appeared to adhere in greater numbers than its wild-type counterpart (Fig. 7). Adherence of HB101(pRPAF1, pJPN14) was confirmed to be greater than that of HB101(pKDS135, pJPN14) by direct counting. The mean numbers of adherent bacteria per 100 cells ± the standard deviations for four samples was 206 ± 128 for HB101(pKDS135, pJPN14) and 2,487 ± 240 for HB101(pRPAF1, pJPN14) (P < 0.001, Student’s t test). Adherence of wild-type EPEC and UMD916 was quantified by radioactively labeling bacteria and counting adherent bacteria by scintillation counting, since the number of adherent bacteria was too large to be counted visually. The results of these experiments confirmed that UMD916 adhered in much larger numbers to HEp-2 cells than did wild-type EPEC (Table 2). In contrast, bfpA mutant strain UMD901 adhered in much smaller numbers to HEp-2 cells than did the wild type. Attempts to complement UMD916 to reduce adherence to wild-type levels yielded uninterpretable results. Whereas introduction of bfpF into UMD916 in plasmid pRPA102 reduced adherence to the level of the wild type, the control vector pWKS30 had the same effect (data not shown). Thus, it appears that the presence of the pWKS30 vector in EPEC strains results in reduced localized adherence, rendering it impossible to assess the effect of the cloned bfpF gene on adherence.
FIG. 7.
Localized adherence of EPEC and of E. coli HB101 strains containing the cloned bfp gene cluster to HEp-2 cells. HEp-2 cells were incubated for 3 h with bacteria, fixed, and examined by light microscopy. (A) Wild-type EPEC strain E2348/69. (B) bfpF mutant strain UMD916. (C) E. coli HB101 with pKDS135, which contains the complete bfp gene cluster, and plasmid pJPN14, which is also required for BFP expression. (D) HB101 containing pRPAF1, which is identical to pKDS135 except for a 72-bp bfpF deletion, and pJPN14.
TABLE 2.
Adherence and invasive ability of bfpF mutant strain UMD916 in comparison to those of wild-type strain E2348/69 and bfpA mutant strain UMD901
| Strain | Relevant genotype | Mean % adherence ± SEa | Geometric mean % inoculum recoveredb |
|---|---|---|---|
| E2348/69 | Wild type | 100% ± 11% | 0.107% (0.072%, 0.160%) |
| UMD916 | bfpF | 836% ± 206% (P = 0.007) | 0.070% (0.050%, 0.090%) (P = 0.258) |
| UMD901 | bfpA | 0.13% ± 0.04% (P < 0.001) | 0.006% (0.003%, 0.009%) (P < 0.001) |
Mean percentages of wild-type adherence were determined with radiolabeled bacteria. Data are from three experiments performed in triplicate. Data from a fourth experiment were discarded because of an outlier value for the number of CFU from one strain. Statistical comparisons (versus the wild type) were made with Student’s paired t test.
Geometric mean percentages of inoculum recovered after gentamicin treatment were determined as a measure of invasiveness. Data are from five experiments performed in triplicate. The numbers in parenthesis are geometric means minus and plus the standard errors. Statistical comparisons (versus the wild type) were made by analysis of variance.
BfpF does not decrease the distance between bacteria and host cells and does not enhance attaching and effacing efficiency.
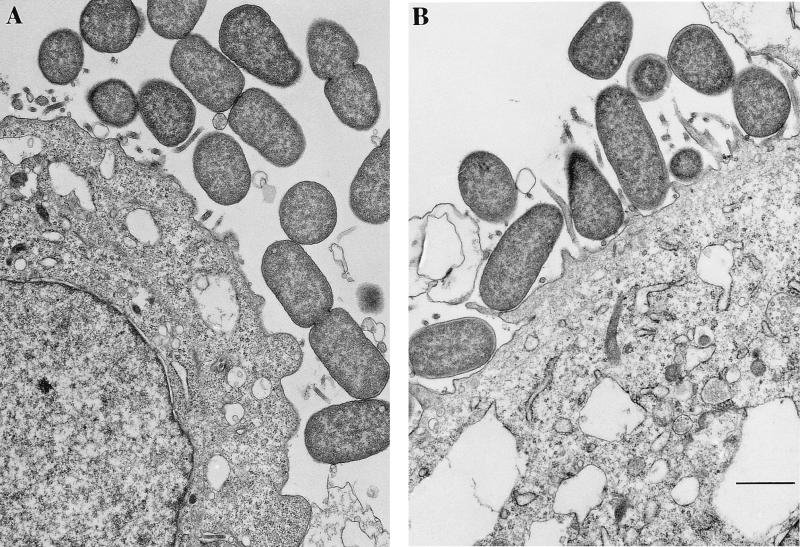
To determine whether a mutated bfpF gene would result in increased pilus length and thus increase the distance between bacteria and host cells, the mutated EAF plasmid from UMD916 was electroporated into strain UMD207 to create strain UMD207(pUMD916). UMD207 is an EPEC strain that has been cured of the EAF plasmid and has a mutation in the eae gene, which encodes intimin (20). Since intimin is required for intimate attachment of EPEC to epithelial cells (21), we chose this strain so that we could measure the distance between adherent bacteria and host cells in the absence of this intimate adhesin. Strain CVD206 (13), which has an eae mutation but a wild-type EAF plasmid, served as an isogenic control for UMD207(pUMD916), so that we could test the effect of BfpF on adherence. We predicted from our hypothesis that a strain with a mutated bfpF gene would have longer pili than wild-type EPEC and adhere at a greater distance from host cells. Bacteria were incubated with HEp-2 cells, and the distance between adherent bacteria and host cells was measured (Fig. 8). Surprisingly, the median distance of strain CVD206 from the epithelial cells (249 nm; interquartile range, 72 to 428 nm) was greater than that of UMD207(pUMD916) (72 nm; interquartile range, 17 to 249 nm). Thus, the mutation in bfpF appears to decrease rather than increase the distance of the bacteria from the host cells.
FIG. 8.
Transmission electron micrographs of isogenic EPEC strains adhering to HEp-2 cells. HEp-2 cells grown on Transwell filters were infected with eae mutant EPEC strain CVD206 (A) or eae mutant strain CVD207 (pUMD916) (B), which also carries the bfpF::aphA-3 mutation. Thin sections were cut and processed for electron microscopy. The distance between the cells and the bacteria in the layer closest to the cells was measured in these and similar photomicrographs. Bar, 500 nm.
To directly examine the effect of the bfpF mutation on attaching and effacing efficiency, the proportions of adherent wild-type EPEC and bfpF mutant bacteria that were engaged in attaching and effacing lesions were compared by transmission electron microscopy. Twenty-two percent of 225 wild-type EPEC strain E2348/69 bacteria and 27% of 209 bfpF mutant EPEC strain UMD916 bacteria were involved in attaching and effacing lesions (P = 0.030, χ2 test). Contrary to our hypothesis, this result indicates that BfpF decreases rather than increases the efficiency of attaching and effacing by EPEC.
BfpF is not required for invasion.
To determine if BfpF is required for invasion of epithelial cells by EPEC, gentamicin protection assays were performed on wild-type EPEC, UMD916, and UMD901. The results showed that UMD916 invades epithelial cells with the same efficiency as wild-type EPEC (Table 2). Contrary to our hypothesis, this result suggests that BfpF is not required for invasion.
DISCUSSION
In this study, we explored the role of BfpF in localized adherence and subsequent steps of EPEC pathogenesis. The deduced size and amino acid sequence of BfpF are similar to those of the PilT protein of P. aeruginosa. Based on this similarity, we predicted that BfpF would play a role analogous to that proposed for PilT in type IV pilus function, i.e., that bfpF would provide the energy required for the retraction of BFP. According to this hypothesis, pili would be longer and more numerous in the absence of BfpF. Therefore, since BFP is proposed to mediate aggregation and localized adherence, bfpF mutants would aggregate into larger clusters and would be hyperadherent compared to wild-type EPEC. However, we predicted that BfpF and pilus retraction would be required for optimal efficiency of attaching and effacing and invasion, the subsequent steps of EPEC pathogenesis.
To test these hypotheses, two bfpF mutants were created, one with a mutation in the cloned bfp gene cluster in a recombinant E. coli background and the other with a mutated gene in the native EAF plasmid in the wild-type EPEC background. To ensure that the only gene affected in each mutant strain was bfpF, we designed precise mutations that minimized the risk of nonpolar effects. We constructed an in-frame deletion of bfpF in the recombinant E. coli background and used a nonpolar aphA3 cassette to disrupt bfpF in the native EAF plasmid. This cassette was designed to have no effect on downstream genes (25). The cassette has no transcription attenuators or promoters, and it contains a consensus ribosome-binding site and an ATG start codon immediately following the aphA3 stop codon. Thus, transcription continues through the cassette into the 3′ end of the disrupted gene, and translation of the 3′ end of this gene is reinitiated. The results of competitive RT-PCR showed that transcription of bfpP in the EAF plasmid was not affected by the presence of the cassette. Western blotting demonstrated that bundlin was processed in each mutant in a manner identical to that of the wild type, showing that BfpP was expressed in both mutants. Finally, complementation studies showed that addition of a wild-type copy of bfpF reduced the autoaggregation of bfpF mutant strain UMD916 to that of wild-type EPEC, indicating that BfpF alone was responsible for this reduction. These results show that, as intended, the bfpF mutations are nonpolar.
Although we were unable to detect increased piliation in the bfpF mutants, the results of aggregation and adherence studies were consistent with our hypothesis. Thus, we found that the bfpF mutant strain aggregated to form larger clusters than wild-type EPEC and that the bfpF mutant adhered to epithelial cells in greater numbers than wild-type EPEC. Similarly, E. coli HB101 containing the bfp gene cluster with the bfpF mutation adhered in greater numbers than HB101 containing the wild-type gene cluster. Since increased aggregation could lead to increased adherence, these phenotypes are probably not independent.
Based on its homology with PilT and the proposed role of PilT in pilus retraction, we hypothesized that BfpF facilitates subsequent steps in EPEC pathogenesis by catalyzing the retraction of BFP, which could bring the adherent bacteria closer to the surface of the host cell to allow signal transduction and intimate attachment to take place with greater efficiency. To test this hypothesis, we measured the distance between HEp-2 cells and bacteria. To ensure that the adherence was not due to intimin, which mediates intimate adherence between EPEC and host cells, we constructed isogenic strains with wild-type and mutated bfpF alleles in an eae (intimin-deficient) background. Surprisingly, we found that the strain containing the bfpF mutation adhered closer to the cells than did the stain containing the wild-type bfpF allele. This result is the opposite of what we would expect if the mutant was incapable of retracting BFP. To directly test whether the bfpF mutant is deficient in attaching and effacing and invasion, we measured its ability to form attaching and effacing lesions and its ability to invade host cells. We found no defect in either phenotype. Considering that the bfpF mutant is hyperadherent, one might interpret the lack of increased invasion to represent a relative defect in the process. However, we are reluctant to draw conclusions based on data from different assays and prefer a more conservative interpretation of the results. Thus, while clearly demonstrating a role for BfpF in autoaggregation and localized adherence, we could not demonstrate, directly or indirectly, a role for BfpF in pilus retraction.
Considering the sequence similarities between BfpF and PilT, how can we explain the lack of evidence of a role for BfpF in bringing the bacteria closer to the host cell to facilitate attaching and effacing and invasion activity? One possibility is that although BfpF and PilT are similar, they are not true homologs. Thus, BfpF may not play a role analogous to that of PilT in pilus function. This suggestion is supported by differences in the sequences of PilT and BfpF. PilT contains two Walker boxes, which are motifs common to nucleotide-binding proteins (38). BfpF, however, contains only one Walker box. In addition, PilT contains two complete aspartate boxes, which are motifs common to nucleotide-binding proteins in type IV and similar systems (28), while BfpF lacks the aspartate residue in the second such box. These differences may reflect functional differences between the two proteins. Indeed, we were unable to complement a P. aeruginosa pilT mutant with the EPEC bfpF gene to restore twitching motility (data not shown). In contrast, we had no difficulty complementing a P. aeruginosa pilD (prepilin peptidase gene) mutant with bfpP (43).
Another possible explanation for the lack of BfpF involvement in attaching and effacing and invasion reflects differences between BFP and P. aeruginosa pili. Whereas BFP are very flexible and form bundles, P. aeruginosa pili are more rigid and do not aggregate (5). Also, while the receptor binding domain of the P. aeruginosa pilus has been localized at the tip (22), BFP have not been proven to bind to host cells. Therefore, even if bfpF mutants have longer pili, such mutants would not necessarily adhere at an increased distance from cells. Finally, it should be noted that there is no direct evidence demonstrating pilus retraction, even in P. aeruginosa. Therefore, it remains possible that BfpF is indeed a PilT homolog but that the proposed role for PilT in the retraction of the P. aeruginosa type IV pilus is incorrect.
In conclusion, we demonstrated that BfpF affects the function of BFP by reducing the autoaggregation and localized-adherence phenotypes associated with the pili. However, we are unable to provide evidence of a role for BfpF in BFP retraction, in promoting the close approximation of EPEC and host cells, in facilitating attaching and effacing lesion formation, or in enhancing invasion. The precise function of BfpF, if any, in interactions with the host remains to be elucidated.
ACKNOWLEDGMENTS
We thank Rebecca Wade for technical assistance and Stanley Kushner for providing pWKS30. We also thank the other members of the laboratory for their support and helpful suggestions over the course of these experiments.
This study was supported by Public Health Service award AI37606 from the National Institutes of Health.
REFERENCES
- 1.Alm R A, Bodero A J, Free P D, Mattick J S. Identification of a novel gene, pilZ, essential for type 4 fimbrial biogenesis in Pseudomonas aeruginosa. J Bacteriol. 1996;178:46–53. doi: 10.1128/jb.178.1.46-53.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm R A, Hallinan J P, Watson A A, Mattick J S. Fimbrial biogenesis genes of Pseudomonas aeruginosa: pilW and pilX increase the similarity of type 4 fimbriae to the GSP protein-secretion systems and pilY1 encodes a gonococcal PilC homologue. Mol Microbiol. 1996;22:161–173. doi: 10.1111/j.1365-2958.1996.tb02665.x. [DOI] [PubMed] [Google Scholar]
- 3.Alm R A, Mattick J S. Identification of a gene, pilV, required for type 4 fimbrial biogenesis in Pseudomonas aeruginosa, whose product possesses a pre-pilin-like leader sequence. Mol Microbiol. 1995;16:485–496. doi: 10.1111/j.1365-2958.1995.tb02413.x. [DOI] [PubMed] [Google Scholar]
- 4.Baldini M M, Kaper J B, Levine M M, Candy D C, Moon H W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2:534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- 5.Bradley D E. The adsorption of Pseudomonas aeruginosa pilus-dependent bacteriophages to a host mutant with nonretractile pili. Virology. 1974;58:149–163. doi: 10.1016/0042-6822(74)90150-0. [DOI] [PubMed] [Google Scholar]
- 6.Bradley D E. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can J Microbiol. 1980;26:146–154. doi: 10.1139/m80-022. [DOI] [PubMed] [Google Scholar]
- 7.Brossay L, Paradis G, Fox R, Koomey M, Hébert J. Identification, localization, and distribution of the PilT protein in Neisseria gonorrhoeae. Infect Immun. 1994;62:2302–2308. doi: 10.1128/iai.62.6.2302-2308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cravioto A, Gross R J, Scotland S M, Rowe B. An adhesive factor found in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Curr Microbiol. 1979;3:95–99. [Google Scholar]
- 9.Darzins A. Characterization of a Pseudomonas aeruginosa gene cluster involved in pilus biosynthesis and twitching motility: sequence similarity to the chemotaxis proteins of enterics and the gliding bacterium Myxococcus xanthus. Mol Microbiol. 1994;11:137–153. doi: 10.1111/j.1365-2958.1994.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 10.Donnenberg M S. Enteropathogenic Escherichia coli. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1995. pp. 709–726. [Google Scholar]
- 11.Donnenberg M S, Donohue-Rolfe A, Keusch G T. Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989;160:452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- 12.Donnenberg M S, Girón J A, Nataro J P, Kaper J B. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol Microbiol. 1992;6:3427–3437. doi: 10.1111/j.1365-2958.1992.tb02210.x. [DOI] [PubMed] [Google Scholar]
- 13.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli. Infect Immun. 1992;60:3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. . (Minireview.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnenberg M S, Nataro J P. Methods for studying adhesion of diarrheagenic Escherichia coli. Methods Enzymol. 1995;253:324–336. doi: 10.1016/s0076-6879(95)53028-2. [DOI] [PubMed] [Google Scholar]
- 16.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. The role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Invest. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girón J A, Ho A S Y, Schoolnik G K. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs M, Mattick J S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 20.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee K K, Sheth H B, Wong W Y, Sherburne R, Paranchych W, Hodges R S, Lingwood C A, Krivan H, Irvin R T. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol Microbiol. 1994;11:705–713. doi: 10.1111/j.1365-2958.1994.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 23.Levine M M, Nataro J P, Karch H, Baldini M M, Kaper J B, Black R E, Clements M L, O’Brien A D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 24.Martin P R, Watson A A, McCaul T F, Mattick J S. Characterization of a five-gene cluster required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol Microbiol. 1995;16:497–508. doi: 10.1111/j.1365-2958.1995.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 25.Ménard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataro J P, Maher K O, Mackie P, Kaper J B. Characterization of plasmids encoding the adherence factor of enteropathogenic Escherichia coli. Infect Immun. 1987;55:2370–2377. doi: 10.1128/iai.55.10.2370-2377.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunn D, Bergman S, Lory S. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J Bacteriol. 1990;172:2911–2919. doi: 10.1128/jb.172.6.2911-2919.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Possot O, Pugsley A P. Molecular characterization of PulE, a protein required for pullulanase secretion. Mol Microbiol. 1994;12:287–299. doi: 10.1111/j.1365-2958.1994.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 29.Russell M A, Darzins A. The pilE gene product of Pseudomonas aeruginosa, required for pilus biogenesis, shares amino acid sequence identity with the N-termini of type 4 prepilin proteins. Mol Microbiol. 1994;13:973–985. doi: 10.1111/j.1365-2958.1994.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Scaletsky I C A, Silva M L M, Trabulsi L R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohel I, Puente J L, Ramer S W, Bieber D, Wu C-Y, Schoolnik G K. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J Bacteriol. 1996;178:2613–2628. doi: 10.1128/jb.178.9.2613-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone K D, Zhang H-Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 34.Strom M S, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- 35.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 36.Vuopio-Varkila J, Schoolnik G K. Localized adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J Exp Med. 1991;174:1167–1177. doi: 10.1084/jem.174.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 38.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 40.Whitchurch C B, Hobbs M, Livingston S P, Krishnapillai V, Mattick J S. Characterisation of a Pseudomonas aeruginosa twitching motility gene and evidence for a specialised protein export system widespread in eubacteria. Gene. 1991;101:33–44. doi: 10.1016/0378-1119(91)90221-v. [DOI] [PubMed] [Google Scholar]
- 41.Whitchurch C B, Mattick J S. Characterization of a gene, pilU, required for twitching motility but not phage sensitivity in Pseudomonas aeruginosa. Mol Microbiol. 1994;13:1079–1091. doi: 10.1111/j.1365-2958.1994.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H-Z, Donnenberg M S. DsbA is required for stability of the type IV pilin of enteropathogenic Escherichia coli. Mol Microbiol. 1996;21:787–797. doi: 10.1046/j.1365-2958.1996.431403.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H-Z, Lory S, Donnenberg M S. A plasmid-encoded prepilin peptidase gene from enteropathogenic Escherichia coli. J Bacteriol. 1994;176:6885–6891. doi: 10.1128/jb.176.22.6885-6891.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]