Abstract
Background and Objective
Sotrovimab 500 mg administered by a single intravenous (IV) infusion has been granted special approval for emergency use in Japan for treatment of SARS-CoV-2 infection in adults and children aged ≥ 12 years weighing ≥ 40 kg. This Phase 1, single-dose study investigated the pharmacokinetics, safety, and tolerability of IV or intramuscular (IM) sotrovimab 500 mg doses versus placebo in healthy Japanese and Caucasian volunteers.
Methods
This was a two-part, Phase 1, randomized, placebo-controlled, single-blind study. In Part 1, participants received a single sotrovimab 500 mg IV infusion or matching placebo on Day 1. In Part 2, participants received a single sotrovimab 500 mg IM dose or matching placebo on Day 1, administered as two 4 mL injections.
Results
There was no effect of ethnicity on the peak or total serum exposure of IV sotrovimab through Week 18; after adjusting for body weight, the point estimate and 90 % confidence interval for the ratio of total exposure between Japanese and Caucasian participants fell within conventional bioavailability bounds (80–125%). Geometric mean Cmax and AUClast following a single IM administration of sotrovimab were higher in Japanese participants compared with Caucasian participants, even after adjustment for body weight. Overall, a single IV or IM dose of sotrovimab was well tolerated by both Japanese and Caucasian participants.
Conclusions
After adjusting for body weight, exposures following a single IV dose of sotrovimab 500 mg were similar between Japanese and Caucasian participants, and higher in Japanese participants following IM administration. Higher exposures were not associated with any safety signals.
Trial Registration
ClinicalTrials.Gov: NCT04988152.
Key Points
| Sotrovimab exposures following intravenous (IV) administration were similar between Japanese and Caucasian participants and were higher in Japanese participants following intramuscular (IM) administration and after adjusting for body weight. |
| These higher exposures do not appear to be associated with any safety signals. |
| Single IV and IM doses of sotrovimab 500 mg were well tolerated by both Japanese and Caucasian participants. |
Introduction
Severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2) has caused a devasting pandemic, with enormous public health and social impacts [1, 2]. Those at greatest risk of severe coronavirus disease 2019 (COVID-19) include people with advanced age (> 55 years), males, and those with pre-existing medical conditions, such as asthma, chronic obstructive pulmonary disease, diabetes, obesity, chronic kidney disease, heart disease, and hypertension [3]. Globally, more than 628 million confirmed cases of COVID-19 and 6.5 million deaths had been reported to the WHO from January 3, 2020 to November 4, 2022 [4]. In Japan, 22.5 million confirmed cases and more than 46,000 deaths were reported during the same period [5].
Monoclonal antibodies (mAbs) directed against SARS-CoV-2 are available for both the treatment and prevention of COVID-19 [6]. Neutralizing mAbs given as early treatment in outpatients with mild-to-moderate COVID-19 disease can prevent the need for hospitalization due to disease progression in patients at risk for complications, such as respiratory distress, respiratory failure, or other organ failure. Additionally, an efficacious neutralizing mAb given early in the disease course has been shown to ameliorate the severity and duration of COVID-19 [7] and may potentially reduce transmission [8]. Several mAbs have, at least for a period, received emergency use authorization around the world, and are typically administered by intravenous (IV) infusion [6]. A mAb that can be administered via intramuscular (IM) injection may be of benefit in the outpatient setting where there are logistical challenges to IV administration.
Sotrovimab is an engineered, dual-action, human mAb, which was developed from a parental antibody isolated from a survivor of the SARS outbreak in 2003 [9–13]. It binds to a conserved epitope in the SARS-CoV-2 spike protein, which is distinct from the angiotensin-converting enzyme (ACE) 2 binding site [10]. Clinical efficacy and safety of a single sotrovimab 500 mg IV infusion was evaluated in patients with mild-to-moderate COVID-19 at high risk for disease progression in the Phase 2/3 COMET-ICE (NCT04545060) trial, which was conducted outside of Japan [7]. Final results for the primary endpoint of the COMET-ICE trial showed a 79 % (p < 0.001) relative-risk reduction in all-cause > 24-h hospitalization or death due to any cause in high-risk COVID-19 patients receiving sotrovimab, compared with placebo, during the 28 days following treatment administration. In addition, the pharmacokinetics (PK), efficacy, safety, and tolerability of a single dose of sotrovimab 500 mg IM versus sotrovimab 500 mg IV has been assessed in the Phase 3 COMET-TAIL trial (NCT04913675), which was also conducted outside of Japan [14]; sotrovimab 500 mg IM was found to be well tolerated and non-inferior to IV administration in the COMET-TAIL trial.
Sotrovimab 500 mg administered by a single IV infusion was granted special approval for emergency use in Japan for the treatment of SARS-CoV-2 infection in adults and children aged ≥ 12 years weighing ≥ 40 kg, based (in part) on the results of the COMET-ICE trial and preliminary data from the current study [15]. As sotrovimab is a human non-host-targeted mAb, it is expected to be metabolized like endogenous immunoglobulin G. However, the effect of ethnicity on drug PK, and the potential interaction between ethnicity and route of administration, had not yet been fully evaluated. In addition, for reasons of speed, given the urgency of the global pandemic, no Phase 1 (healthy volunteers) study of sotrovimab had been conducted. Here, we present final results of a Phase 1 single-dose study, which was designed to investigate the PK, safety, and tolerability of single IV and IM doses of sotrovimab 500 mg versus placebo in healthy Japanese and Caucasian volunteers.
Methods
This was a two-part, Phase 1, multicenter, randomized, placebo-controlled, single-blind study (ClinicalTrials.gov: NCT04988152; Fig. 1). In Part 1, healthy Japanese and Caucasian volunteers were randomized (ratio 4:1) to receive a single 500 mg IV 30-min infusion of sotrovimab or matching placebo on Day 1. Part 2 involved a different group of healthy volunteers to Part 1, and commenced after enrollment was completed for Part 1; healthy Japanese and Caucasian volunteers were randomized to receive a single 500 mg IM dose of sotrovimab or matching placebo (ratio 4:1) on Day 1, administered as two 4-mL injections (one in each dorsogluteal injection site).
Fig. 1.
Study design. EOS end of study, IM intramuscular, IV intravenous, PK pharmacokinetics
Participants
Enrollment occurred at two Phase 1 clinical units in the USA. Healthy volunteers could be included if they were capable of giving informed consent, were aged 18 to ≤ 65 years, and had a body mass index (BMI) between 18 and 29.9 kg/m2. Participants aged 55 years or older were required to have received all doses in a primary SARS-CoV-2 vaccine series at the time of signing informed consent. All participants were required to test negative for COVID-19, determined by two consecutive SARS-CoV-2 negative reverse transcriptase polymerase chain reaction tests. Female participants were required not to be pregnant or breastfeeding.
Japanese participants had to meet all of the following criteria: be of Japanese ancestry, defined as having been born in Japan and being descendants of four ethnically Japanese grandparents and two ethnically Japanese parents; hold a Japanese passport or identity papers; be able to speak Japanese; and have lived outside of Japan for fewer than 10 years at the time of screening. Caucasian participants had to be of Caucasian ancestry, as evidenced by appearance and verbal confirmation of familial heritage (two Caucasian parents and four Caucasian grandparents).
Participants were excluded from the study if they had a history or presence of cardiovascular, respiratory, hepatic, renal, gastrointestinal, endocrine, hematologic, or neurological disorders capable of significantly altering the absorption, metabolism, or elimination of drugs; or constituting a risk when taking the study intervention or interfering with the interpretation of data; lymphoma, leukemia, or any malignancy within the past 5 years, except for basal cell or squamous epithelial carcinomas of the skin that had been resected with no evidence of metastatic disease for 3 years, or breast cancer within the past 10 years; presence of hepatitis B surface antigen, a hepatitis C antibody test result, or a hepatitis C ribonucleic acid test result at screening or within 3 months prior to the first dose of study intervention, or a positive human immunodeficiency virus antibody test. Prohibited prior or concomitant therapy included treatment with biological agents (such as mAbs, including marketed drugs) within 3 months or five half-lives (whichever was longer) prior to dosing, convalescent plasma from a recovered COVID-19 patient, and an anti-SARS-CoV-2 mAb within the last 3 months.
Participants were admitted to the unit on Day − 1, following screening between Day − 1 and Day − 28. Dosing took place on Day 1, and participants remained at the unit for the first 3 days. Following discharge, they returned for follow-up safety assessments and blood sampling up to Week 18. Blood samples were collected on Days 1, 2, 3, 8, 15 (± 1 day), 29 (± 2 days), 43 (± 3 days), 57 (± 4 days), 85 (± 7 days), and Day 127 (± 7 days).
Objectives and Endpoints
The primary objectives in Part 1 were to assess the PK, safety, and tolerability of sotrovimab administered via IV infusion in healthy Japanese and Caucasian participants through Day 29 (in line with the pivotal COMET-ICE efficacy study [7]). The primary objectives in Part 2 were to assess the PK, safety, and tolerability of sotrovimab administered via IM injections in healthy Japanese and Caucasian participants through Day 29.
The primary PK endpoints were maximum observed serum concentration (Cmax), area under the serum concentration-time curve from Day 1 to Day 29 (AUCD1–29), time to Cmax (Tmax), and serum concentration at Day 29 (CD29) of sotrovimab were observed. Measurement of sotrovimab serum concentration was performed using a validated electrochemiluminescence immunoassay method (lower limit of quantification 0.1 μg/mL). The primary safety endpoints included occurrence of adverse events (AEs), serious AEs (SAEs), and AEs of special interest (AESI; infusion-related reactions, including hypersensitivity reactions, injection-site reactions, immunogenicity-related adverse drug reactions, and AEs potentially related to antibody-dependent disease enhancement) through Day 29. Severity of AEs was graded according to the Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, version 2.1 (July 2017). Clinically significant electrocardiogram (ECG) abnormalities, vital signs, and laboratory parameters were also evaluated.
Secondary objectives were to assess PK (Cmax, area under the plasma concentration-time curve from time zero to infinity [AUCinf], area under the concentration-time profile to the last measurable concentration [AUClast], Tmax, time of last analytically quantifiable concentration [Tlast], terminal half-life [t½], as data permit) and safety through Week 18. In addition, AUCinf following IV and IM administration (absolute bioavailability) was intended to be assessed; however, AUClast was used instead, due to the number of participants with the extrapolated portion of AUCinf as a percent (%AUCextrap) > 20 %.
Immunogenicity (the presence of antidrug antibodies [ADAs]) through Day 29 and Week 18 was an exploratory endpoint. Antibodies to sotrovimab were evaluated in serum samples collected from all participants. Antidrug antibodies were assessed using a validated, multi-tiered approach, consisting of screening, confirmation, and titration assays, to evaluate anti-sotrovimab antibodies.
Assays
Sotrovimab concentrations in serum were determined via a validated electro-chemiluminescent (ECL) method, which used anti-idiotypic antibodies specific to sotrovimab for capture and detection. Human serum samples were minimally diluted 25-fold with assay buffer (1 % bovine serum albumin in phosphate-buffered saline [PBS]) prior to analysis. The diluted serum samples were added to an Meso-scale discovery (MSD) High Bind plate (ECL capable) adsorbed with AbD34205-mu-IgG2a capture antibody and ruthenylated-AbD42688-rFab antibody to detect sotrovimab. A fixation step (freshly prepared 1 % formaldehyde in PBS) was applied, followed by MSD read buffer. The assay plate was then read using an MSD ECL plate reader. The quantifiable analytical range for the method was 100 to 10,000 ng/mL.
A three-tier approach (screen, confirm, titer) was used to detect any ADA to sotrovimab. Validated ECL bridging assays were used to analyze the samples. The cut point factors of 1.300 and 1.548 for the screen and titer assay, respectively, and the confirmatory cut point of 32.347 % were determined statistically using 50 lots of COVID-19-positive human serum. The screening and confirmatory sensitivity were established to be 22.970 and 31.812 ng/mL, respectively, using an anti-idiotype antibody to sotrovimab as positive control.
Statistical Analysis
No formal statistical technique was used to calculate the study sample size, and no hypothesis-testing was conducted. Sample size was based on sufficiency to adequately estimate the ratio of exposures between Japanese and Caucasian participants, and IV and IM administration routes.
The PK analysis set included all participants in the safety analysis set who had at least one PK observation available. The safety analysis set included all randomized participants who were exposed to the study treatment.
Pharmacokinetic parameters were calculated using standard non-compartmental methods. Pharmacokinetic comparisons through Day 29 between Japanese and Caucasian participants were made using an analysis of covariance (ANCOVA) model with loge-transformed Cmax or AUCD1–29 as dependent variables and adjusting for loge-transformed body weight (to incorporate allometry). The geometric least squares (LS) means ratio of Cmax and AUCD1–29 (Japanese and Caucasian participants) and associated 90 % confidence intervals (CIs) were calculated. Pharmacokinetic comparisons through Week 18 were made in a similar way, and geometric LS means ratio of AUClast (Japanese and Caucasian participants) and associated 90 % CIs were calculated.
An exploratory comparison of bioavailability (AUClast ratio) following a single IM dose of 500 mg sotrovimab (Part 2) relative to the single IV dose of 500 mg sotrovimab (Part 1) was performed using an ANCOVA model, with loge-transformed AUClast as a dependent variable and adjusting for ethnicity and body weight (loge-transformed), and included all participants.
Safety data were summarized using frequency and percentages.
Results
Participant Disposition
In Part 1, 39 volunteers were screened, and 24 were enrolled and randomized. Eleven volunteers failed screening (six did not meet the inclusion/exclusion criteria, two failed per physician’s decision, and three withdrew their consent) and four participants met the eligibility criteria but were no longer needed for enrollment. The PK analysis set included nine Japanese and nine Caucasian volunteers who received a single sotrovimab 500 mg IV infusion. The safety analysis set included 12 Japanese and 12 Caucasian participants who were randomized and exposed to study treatment.
In Part 2, 53 volunteers were screened, and 24 were enrolled and randomized. Eight volunteers failed screening (five did not meet the inclusion/exclusion criteria and three withdrew their consent) and 21 participants met eligibility criteria but were no longer needed for enrollment. The PK analysis set included 10 Japanese and 10 Caucasian volunteers who received a single sotrovimab 500 mg IM dose. The safety analysis set included 12 Japanese and 12 Caucasian participants who were randomized and exposed to study treatment.
The first participant was screened on July 6, 2021, and the last participant completed the Week 18 (end of study) visit on December 7, 2021. No participants in either Part withdrew from the study. There were no cases of SARS-CoV-2 infection and no visits were impacted by the COVID-19 pandemic throughout the study.
Baseline Demographics and Characteristics
Consistent with the well-established anthropometric measurements between populations in Japan and the West, in both parts, the volunteers in the Japanese cohorts were shorter and of lower body weight and BMI than those in the Caucasian cohort (Table 1). In Part 1, the Japanese cohort also had more females, which likely contributed to the lower body weight in the Japanese versus Caucasian cohort.
Table 1.
Participant demographics and baseline characteristics (safety analysis set)
| Part 1 | Part 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sotrovimab 500 mg IV dose | Placebo IV dose | Sotrovimab 500 mg IM dose | Placebo IM dose | |||||||||||
| Japanese (n = 9) | Caucasian (n = 9) | Total (N = 18) | Japanese (n = 3) | Caucasian (n = 3) | Total (N = 6) | Japanese (n = 10) | Caucasian (n = 10) | Total (N = 20) | Japanese (n = 2) | Caucasian (n = 2) | Total (N = 4) | |||
| Median age, years (range) | 50.0 (31–61) | 40.0 (27–49) | 43.5 (27–61) | 29.0 (24–38) | 35.0 (32–43) | 33.5 (24–43) | 52.0 (26–56) | 36.0 (19–51) | 44.0 (19–56) | 39.5 (27–52) | 44.5 (43–46) | 44.5 (27–52) | ||
| Female, n (%) | 8 (89) | 5 (56) | 13 (72) | 2 (67) | 1 (33) | 3 (50) | 7 (70) | 7 (70) | 14 (70) | 2 (100) | 1 (50) | 3 (75) | ||
| Mean (SD) weight, kg | 53.2 (5.0) | 73.3 (9.9) | 63.3 (12.9) | 60.0 (6.2) | 71.3 (21.1) | 65.7 (15.3) | 58.8 (7.2) | 72.3 (12.2) | 65.6 (11.9) | 60.3 (3.8) | 76.5 (20.8) | 68.4 (15.4) | ||
| Mean (SD) height, cm | 157.8 (6.4) | 168.3 (9.1) | 163.1 (9.3) | 165.3 (7.9) | 175.7 (12.9) | 170.5 (11.1) | 162.8 (6.0) | 168.9 (9.5) | 165.8 (8.3) | 162.9 (6.9) | 169.5 (9.2) | 166.2 (7.7) | ||
| Mean (SD) BMI, kg/m2 | 21.4 (1.6) | 25.9 (2.4) | 23.6 (3.1) | 22.1 (4.1) | 22.7 (4.4) | 22.4 (3.8) | 22.1 (1.6) | 25.2 (2.7) | 23.7 (2.7) | 22.7 (0.5) | 26.4 (4.4) | 24.5 (3.3) | ||
BMI body mass index, IM intramuscular, IV intravenous, SD standard deviation
Pharmacokinetics
In Part 1, the shape of the PK profile following a single IV dose of 500 mg sotrovimab was similar for Japanese and Caucasian participants (Fig. 2). Results for the primary PK endpoints (Day 29) are included in Table 2. The mean Cmax, AUCD1–29, Tmax, and CD29 (unadjusted for body weight) were all numerically higher in Japanese than in Caucasian participants. The geometric LS means ratio of Cmax and AUCD1–29 was similar between Japanese 1.07 (90 % CI 0.85–1.35) and Caucasian 1.05 (90 % CI 0.93–1.19) participants after adjusting for body weight (Table 2). Data for Part 1 secondary PK endpoints (Week 18) are shown in Table 3. At Week 18, the geometric LS means ratio for AUClast (Japanese vs Caucasian) was 1.07 (90 % CI 0.93–1.23), indicating no effect of ethnicity on peak or total serum exposure of IV sotrovimab through Week 18.
Fig. 2.
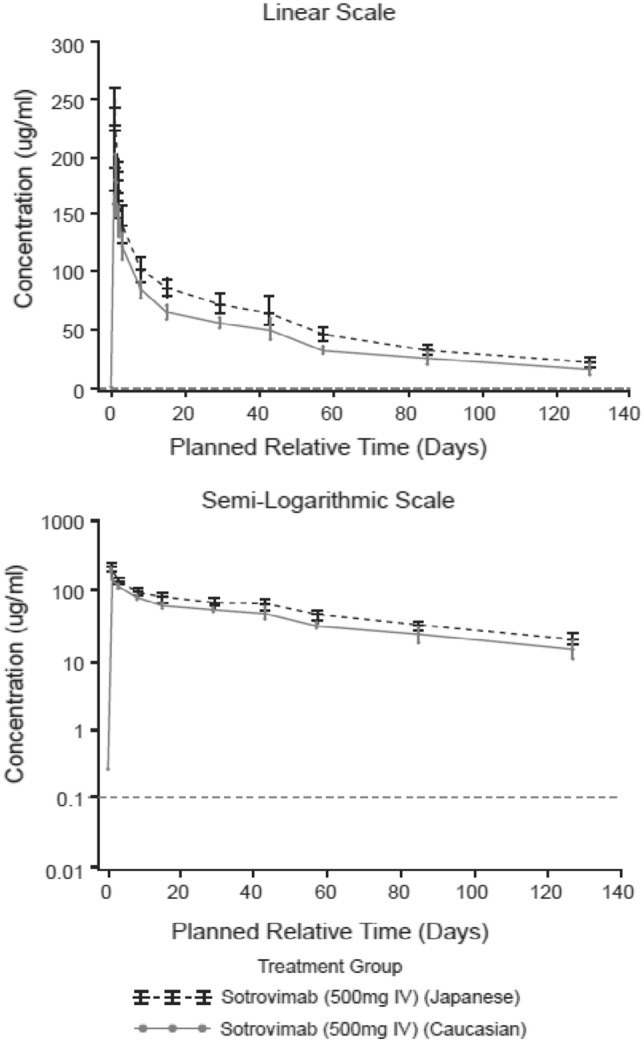
Part 1: geometric mean (95 % CI) sotrovimab serum concentration-time plots following sotrovimab 500 mg IV through Week 18 (PK analysis set). CI confidence interval, IV intravenous, PK pharmacokinetic. Horizontal dotted line is lower limit of quantification
Table 2.
Untransformed derived serum PK parameters through Day 29, and ethnicity comparison, in Part 1 and Part 2 (PK analysis set)
| Part 1: sotrovimab 500 mg IV dose | Part 2: sotrovimab 500 mg IM dose | |||
|---|---|---|---|---|
| Japanese (n = 9) | Caucasian (n = 9) | Japanese (n = 10) | Caucasian (n = 10) | |
| Mean (SD) Cmax, µg/mL | 242 (44) | 190 (28) | 63 (20) | 36 (16) |
| Mean (SD) CD29, µg/mL | 72.2 (10.1) | 55.8 (5.1) | 46.4 (14.4) | 32.3 (14.9) |
| Mean (SD) Tmax, h | 2.44 (1.6) | 1.02 (0.4)a | 199.9 (65.7) | 320.3 (248.3) |
| Mean (SD) AUCD1–29, day*µg/mL | 2715 (309) | 2163 (191) | 1428 (419) | 811 (353) |
| Geometric LS means ratio Cmax (Japanese and Caucasian participants)b | 1.07 (90 % CI 0.85–1.35) | 1.70 (90 % CI 1.15–2.51) | ||
| Geometric LS means ratio AUCD1–29 (Japanese and Caucasian participants)b | 1.05 (90 % CI 0.93–1.19) | 1.59 (90 % CI 1.12–2.24) | ||
ANCOVA analysis of covariance, AUCD1–29 area under the serum concentration-time curve from Day 1 to Day 29, CD29 serum concentration at Day 29, CI confidence interval, Cmax maximum observed serum concentration, IM intramuscular, IV intravenous, LS least squares, PK pharmacokinetic, SD standard deviation, Tmax time to Cmax
aOne Caucasian participant was excluded from the Tmax summary due to slow drug distribution following IV infusion
bThe geometric LS means ratio and 90 % CIs were obtained from an ANCOVA model with loge-transformed Cmax or AUCD1–29 as dependent variables, ethnicity as an exploratory variable, and loge-transformed body weight as covariate
Table 3.
Untransformed derived serum PK parameters through Week 18, and ethnicity comparison, in Part 1 and Part 2 (PK analysis set)
| Part 1: sotrovimab 500 mg IV dose | Part 2: sotrovimab 500 mg IM dose | |||
|---|---|---|---|---|
| Japanese (n = 9) | Caucasian (n = 9) | Japanese (n = 10) | Caucasian (n = 10) | |
| Mean (SD) AUClast,a day*µg/mL | 6632 (705) | 5130 (581) | 4135 (1103) | 2295 (880) |
| Mean (SD) CL, mL/day | 67.6 (14.6)b | 94.9 (28.1)b | 87.9b | 193.6 (103.4)b |
| Mean (SD) Lambda z, /day | 0.013 (0.018) | 0.012 (0.004) | 0.011 (0.002) | 0.011 (0.002) |
| Median (range) t½, day | 56.4 (40.9–65.7) | 58.9 (33.6–113.6) | 67.3 (53.2–85.5) | 67.4 (48.6–89.2)c |
| Median (range) Tlast, day | 126 (119–126) | 126 (125–127) | 128 (125–129) | 126 (119–132) |
| Mean (SD) Vss, mL | 4468.7 (420.2)b | 5303.3 (135.3)b | – | – |
| Mean (SD) Vz, mL | 4660.4 (519.5)b | 5557.6 (8.7)b | 6741.4b | 14085.1 (7975.1)b |
| Geometric LS means ratio AUClast (Japanese and Caucasian participants),d day*µg/mL | 1.07 (90 % CI 0.93–1.23) | 1.58 (90 % CI 1.18–2.11) | ||
%AUCextrap extrapolated portion of AUCinf as a percent, ANCOVA analysis of covariance, AUCinf area under the plasma concentration-time curve from time zero to infinity, AUClast area under the concentration-time profile to the last measurable concentration, CI confidence interval, CL clearance, IM intramuscular, IV intravenous, LS least squares; PK pharmacokinetic, SD standard deviation, t½, terminal half-life, Tlast time of last analytically quantifiable concentration, Vss volume of distribution at steady state, Vz apparent volume of distribution during terminal phase
aAUClast used instead of AUCinf due to the high number of participants with %AUCextrap > 20 %
bn ≤ 3
cOne participant was excluded from the t½ summary due to adjusted R2 < 0.8
dThe geometric LS means ratio and 90 % CIs were obtained from an ANCOVA model with loge-transformed AUClast as dependent variables, ethnicity as an exploratory variable, and loge-transformed body weight as covariates
In Part 2, the systemic exposure was higher for Japanese participants compared with Caucasian participants following a single IM dose of 500 mg sotrovimab; however, the shape of the PK profiles was similar for both groups when viewed on a semi-logarithmic scale (Fig. 3). The mean Cmax, AUCD1–29, and CD29 (unadjusted for body weight) were higher in Japanese than in Caucasian participants, while Tmax was shorter (Table 2). The geometric LS means ratio for Cmax and AUCD1–29 between Japanese and Caucasian participants after adjusting for body weight were 1.70 (90 % CI 1.15–2.51) and 1.59 (90 % CI 1.12–2.24), respectively (Table 2). At Week 18, the value for AUClast was higher in Japanese than in Caucasian participants, while values for median t½ and Tlast were similar between the groups. The geometric LS means ratio for AUClast (Japanese vs Caucasian) after adjusting for body weight was 1.58 (90 % CI 1.18–2.11; Table 3).
Fig. 3.
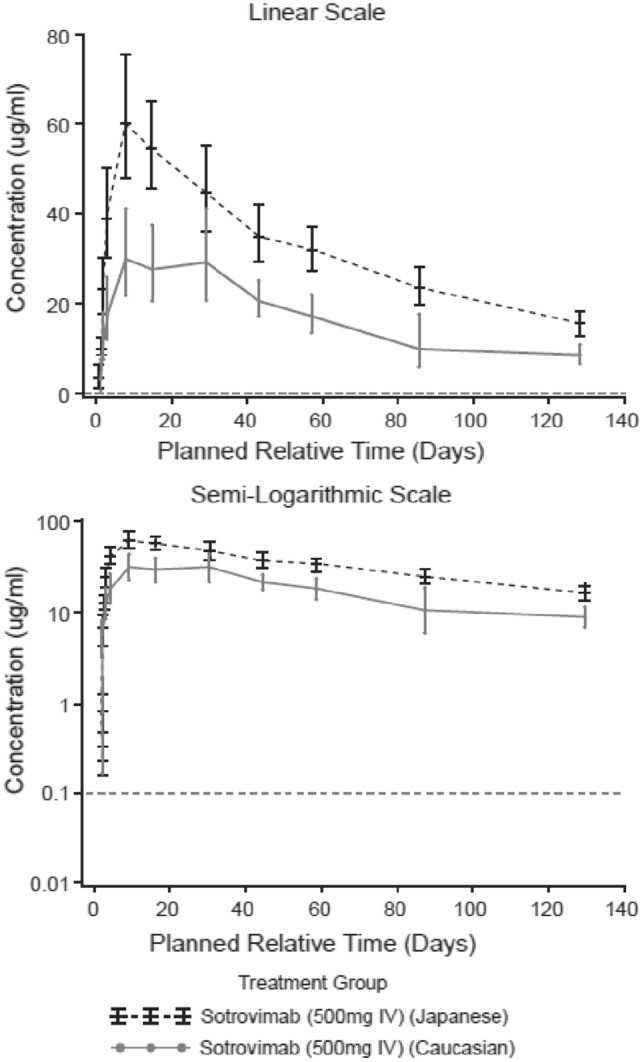
Part 2: geometric mean (95 % CI) sotrovimab serum concentration-time plots following sotrovimab 500 mg IM through Week 18 (PK analysis set). CI confidence interval, IM intramuscular, PK pharmacokinetic. Horizontal dotted line is lower limit of quantification
In the exploratory comparison of bioavailability (AUClast ratio) following a single IM dose of 500 mg sotrovimab (Part 2) relative to the single IV dose of 500 mg sotrovimab (Part 1), the geometric LS mean for AUClast was 2969.37 day*μg/mL for the IM dose and 5732.97 day*μg/mL for the IV dose. The geometric LS means ratio (IM:IV) was 0.52 (90 % CI 0.45–0.60). A post hoc subgroup analysis of the IM:IV ratio for AUClast at Week 18 was also performed, by adding an interaction term for route of administration by ethnicity in the model used for the pre-planned overall bioavailability analysis. A significant interaction of route of administration with ethnicity was identified (p = 0.00497). A further analysis for each ethnicity subgroup (Japanese and Caucasian) separately was therefore performed, adjusting for body weight (Table 4). The geometric LS mean for AUClast for Japanese participants was 4233.94 day*μg/mL for the IM dose and 6225.18 day*μg/mL for the IV dose. The geometric LS mean for AUClast for Caucasian participants was 2137.53 day*μg/mL for the IM dose and 5128.85 day*μg/mL for the IV dose. The geometric LS means ratio (IM:IV) was 0.68 (90 % CI 0.59–0.78) for Japanese participants and 0.42 (90 % CI 0.33–0.52) for Caucasian participants.
Table 4.
Post hoc subgroup comparison of AUClast IM:IV ratio at Week 18 by ethnicity (PK analysis set)
| Geometric LS mean | |||||
|---|---|---|---|---|---|
| IM (n = 10) | IV (n = 9) | Ratio (IM:IV)a | 90 % CI | ||
| Japanese AUClast, day*µg/mL | 4233.94 | 6225.18 | 0.68 | 0.59–0.78 | |
| Caucasian AUClast, day*µg/mL | 2137.53 | 5128.85 | 0.42 | 0.33–0.52 | |
ANCOVA analysis of covariance, AUClast area under the concentration-time profile to the last measurable concentration, CI confidence interval, IM intramuscular, IV intravenous, LS least squares, PK pharmacokinetic
aRatio estimates and 90 % CIs are obtained from an ANCOVA model, with AUClast at Week 18 as the dependent variable, route of administration (IV, IM) as explanatory variable, and body weight as covariate
Safety
No SAEs were reported in either study part. Table 5 shows the AEs in Part 1 (IV administration) through Week 18 by System Organ Class and Preferred Term. Three AEs in two participants (one Japanese and one Caucasian) were reported in the IV placebo group; one of these (Grade 1 decreased appetite in a Caucasian participant) was considered to be related to study treatment. In the IV sotrovimab group, three AEs were reported in three participants (two Japanese and one Caucasian). One AE in a Japanese participant, Grade 1 rash, was considered to be an AESI (AESI group: hypersensitivity Standardised MedDRA Queries [SMQ] narrow term).
Table 5.
Part 1 (IV): summary of AEs reported through Week 18 (safety analysis set)
| System Organ Class and Preferred Term | Placebo IV dose | Sotrovimab 500 mg IV dose | ||||
|---|---|---|---|---|---|---|
| Japanese (n = 3) | Caucasian (n = 3) | Total (N = 6) | Japanese (n = 9) | Caucasian (n = 9) | Total (N = 18) | |
| Any AE (%) | 1 (33) | 1 (33) | 2 (33) | 2 (22) | 1 (11) | 3 (17) |
| Gastrointestinal disorders (%) | ||||||
| Any event | 1 (33) | 0 | 1 (17) | 0 | 0 | 0 |
| Diarrhea | 1 (33) | 0 | 1 (17) | 0 | 0 | 0 |
| General disorders and administration-site conditions (%) | ||||||
| Any event | 0 | 0 | 0 | 0 | 1 (11) | 1 (6) |
| Fatigue | 0 | 0 | 0 | 0 | 1 (11) | 1 (6) |
| Infections and infestations (%) | ||||||
| Any event | 0 | 0 | 0 | 1 (11) | 0 | 1 (6) |
| Herpes zoster | 0 | 0 | 0 | 1 (11) | 0 | 1 (6) |
| Metabolism and nutrition disorders (%) | ||||||
| Any event | 0 | 1 (33) | 1 (17) | 0 | 0 | 0 |
| Decreased appetite | 0 | 1 (33)a | 1 (17) | 0 | 0 | 0 |
| Musculoskeletal and connective tissue disorders (%) | ||||||
| Any event | 0 | 1 (33) | 1 (17) | 0 | 0 | 0 |
| Pain in extremity | 0 | 1 (33) | 1 (17) | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders (%) | ||||||
| Any event | 0 | 0 | 0 | 1 (11) | 0 | 1 (6) |
| Rash | 0 | 0 | 0 | 1 (11) | 0 | 1 (6) |
AE adverse event, IV intravenous
aAEs considered to be related to study treatment
Table 6 shows the AEs in Part 2 (IM administration) through Week 18 by the System Organ Class and Preferred Term. One AE (headache, Grade 1), reported by a Caucasian participant who received IM placebo, was considered to be related to study treatment. Eighteen AEs (in seven participants, four Japanese and three Caucasian) were reported in the IM sotrovimab group. In the Japanese sotrovimab group, seven AEs (headache, n = 2; somnolence, n = 3; injection-site pain, n = 1; myalgia, n = 1; all Grade 1) were considered to be treatment related. Of the ten AEs reported in the Caucasian sotrovimab group, sleep paralysis (Grade 1), feeling hot (Grade 2), and nightmare (Grade 1) were considered by the investigators to be related to study treatment.
Table 6.
Part 2 (IM): summary of AEs reported through Week 18 (safety analysis set)
| System Organ Class and Preferred Term | Placebo IM dose | Sotrovimab 500 mg IM dose | |||||
|---|---|---|---|---|---|---|---|
| Japanese (n = 2) | Caucasian (n = 2) | Total (N = 4) | Japanese (n = 10) | Caucasian (n = 10) | Total (N = 20) | ||
| Any AE (%) | 0 | 1 (50) | 1 (25) | 4 (40) | 3 (30) | 7 (35) | |
| Nervous system disorders (%) | |||||||
| Any event | 0 | 1 (50) | 1 (25) | 3 (30) | 1 (10) | 4 (20) | |
| Headache | 0 | 1 (50)a | 1 (25) | 2 (20)a | 0 | 2 (10) | |
| Somnolence | 0 | 0 | 0 | 3 (30)a | 0 | 3 (15) | |
| Sleep paralysis | 0 | 0 | 0 | 0 | 1 (10)a | 1 (5) | |
| General disorders and administration-site conditions (%) | |||||||
| Any event | 0 | 0 | 0 | 2 (20) | 1 (10) | 3 (15) | |
| Feeling hot | 0 | 0 | 0 | 1 (10) | 1 (10)a | 2 (10) | |
| Injection-site pain | 0 | 0 | 0 | 1 (10)a | 0 | 1 (5) | |
| Injury, poisoning, and procedural complications (%) | |||||||
| Any event | 0 | 0 | 0 | 0 | 2 (20) | 2 (10) | |
| Arthropod bite | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
| Contusion | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
| Facial bones fracture | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
| Limb injury | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
| Musculoskeletal and connective tissue disorders (%) | |||||||
| Any event | 0 | 0 | 0 | 1 (10) | 0 | 1 (5) | |
| Myalgia | 0 | 0 | 0 | 1 (10)a | 0 | 1 (5) | |
| Psychiatric disorders (%) | |||||||
| Any event | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
| Nightmare | 0 | 0 | 0 | 0 | 1 (10)a | 1 (5) | |
| Reproductive system and breast disorders (%) | |||||||
| Any event | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
| Dysmenorrhea | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
| Respiratory, thoracic, and mediastinal disorders (%) | |||||||
| Any event | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
| Cough | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
| Vascular disorders (%) | |||||||
| Any event | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
| Vascular pain | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) | |
AE adverse event, IM intramuscular
aAEs considered to be related to study treatment
In Part 1, four participants treated with placebo (two Japanese and two Caucasian) and eight participants treated with sotrovimab (four Japanese and four Caucasian) with normal ECG at baseline, presented an abnormal ECG at least once post-baseline. None of the findings were clinically significant. One Japanese participant in the placebo group had a change from normal systolic blood pressure (SBP) at baseline to low SBP post-baseline, as did two participants in the sotrovimab group (one Japanese and one Caucasian). No participants in either the placebo or sotrovimab-treated groups showed any shifts from baseline Grade in vital signs. Baseline to post-baseline Grade shifts in clinical chemistry results were mainly from Grade 0 to Grade 1. In the sotrovimab IV group, Grade 0 to Grade 2 shifts were recorded for one Japanese (total bilirubin) and two Caucasian (glucose, aspartate aminotransferase) participants, and a Grade 0 to Grade 3 shift was recorded in one Japanese participant (direct bilirubin; this was the same participant who had the Grade 0 to Grade 2 shift in total bilirubin); all of these events were either resolved or resolving by Day 127 (Week 18).
In Part 2, one Japanese participant in the placebo group and nine in the sotrovimab group (three Japanese and six Caucasian participants) with a normal ECG at baseline, presented with an abnormal ECG at least once post-baseline. No findings were clinically significant. One Caucasian participant in the sotrovimab group had a documented change from normal SBP at baseline to a low SBP post-baseline (Day 3). There were no changes in pulse rate post-baseline relative to baseline. One Japanese participant in the sotrovimab group showed a shift from Grade 0 to Grade 1 in diastolic blood pressure at Week 18. Baseline to post-baseline Grade shifts in clinical chemistry results were mainly from Grade 0 to Grade 1. Grade 0 to Grade 2 shifts were recorded in two Japanese participants, one in the placebo group (creatinine) and one in the sotrovimab group (glucose).
Immunogenicity
There were no reports of treatment-emergent ADAs in Part 1 (IV) of the study. In Part 2 (IM), one Caucasian participant was confirmed as positive for treatment-emergent ADAs. Three other participants (one Japanese and two Caucasian) tested positive for anti-sotrovimab antibodies; all were treatment-unaffected (titers remained ≤ 4-fold baseline titer) and none of these four participants exhibited PK profiles indicative of effects of treatment-emergent ADA (the individual serum concentration-time profiles for the overall study population are provided as Supplemental Material [Fig. S1]). No participants with confirmed anti-sotrovimab antibodies had neutralizing antibodies against sotrovimab.
Discussion
This Phase 1, single-dose study assessed the PK, safety, and tolerability of sotrovimab 500 mg IV dose (Part 1) or IM dose (Part 2) versus placebo in healthy Japanese and Caucasian volunteers. Data from Part 1 through Day 29 have been reported previously [15] and supported approval of sotrovimab in Japan; a single 500 mg IV dose of sotrovimab was well tolerated in both ethnic groups, and there was no apparent effect of ethnicity on the PK exposure. It is important to note that the primary endpoint was set to be PK assessment up to Day 29 to match the timepoint for primary efficacy analyses in sotrovimab pivotal clinical trials. In COMET-ICE and COMET-TAIL clinical trials [7, 14], the primary efficacy outcome was hospitalization (for > 24 hours) for any cause or death within 29 days after randomization. Here, we extend these findings with data through Week 18 following a single IV dose (Part 1), and Day 29 and Week 18 following a single IM dose (Part 2). In Part 1, there was no effect of ethnicity on the peak or total serum exposure of IV sotrovimab through Week 18. Of note, after adjusting for body weight, the point estimate and 90 % CI for the ratio of total exposure between Japanese and Caucasian participants fell wholly within conventional bioavailability bounds (80–125%). The observation that differences in exposure were accounted for by differences in body weight is consistent with reports for other mAbs [16]. Furthermore, the estimates of half-life and Lambda z were similar between Caucasian and Japanese participants, indicating no effect of ethnicity on sotrovimab elimination. Sotrovimab clearance and volume of distribution were marginally higher in Caucasian compared with Japanese participants, reflecting the small and non-significant difference in exposures (7 %, with the CIs including unity) following IV administration.
In Part 2, geometric mean Cmax and AUClast following a single IM administration of sotrovimab were higher in Japanese participants compared with Caucasian participants, even after adjustment for body weight. These data indicate higher exposure in Japanese participants compared with Caucasian participants following IM administration of sotrovimab. The observed differences are most likely due to better absorption from the injection site in Japanese participants; this is supported by the higher Cmax and AUC following IM administration in Japanese participants but comparable exposures between Caucasian and Japanese participants following IV administration. It is also supported by the comparable Lambda z and half-life estimates between the two groups, indicating similar elimination following both IV and IM administration. Sotrovimab clearance and volume of distribution estimates were higher in Caucasian participants, reflecting the lower observed exposures compared with Japanese participants. However, these parameters were estimated in only a small number of participants (three or less), and the results should be interpreted with caution.
A post hoc subgroup analysis of the IM:IV ratio for AUClast through Week 18 indicated increased bioavailability in Japanese participants. Studies of other mAbs have reported slightly higher variability in PK properties following gluteal IM administration compared with IV administration and also IM administration into the thigh [17]. Several factors are known to affect exposure following IM administration, including BMI, sex, age, ethnicity, and adiposity around the injection site [18, 19]. Amongst these previously reported factors, it is possible that lower BMI and/or less adiposity around the gluteal injection site may contribute to the better absorption observed in Japanese participants following IM administration.
Although the present study showed higher exposures in Japanese participants compared with Caucasian participants following IM administration, the differences in exposure were not associated with any safety concerns. Overall, a single IV or IM dose of sotrovimab was well tolerated by both Japanese and Caucasian participants. In both groups, the AEs reported were generally consistent with those observed in a previous placebo-controlled study of IV sotrovimab in patients with mild-to-moderate COVID-19 [7], and in additional clinical studies incorporating IM dosing [14, 20]. No SAEs were reported in either study part. None of the reports of abnormal ECGs at least once post-baseline was considered to be clinically significant, and reported Grade shifts in clinical chemistry were mostly from Grade 0 to Grade 1. There were no signs of hepatotoxicity or drug-induced liver injury. Finally, the observed incidence of treatment-emergent ADAs was low, and no participants with confirmed anti-sotrovimab antibodies had neutralizing antibodies against sotrovimab.
The current study was designed in a single-blind, placebo-controlled fashion, although the sponsor remained blinded to treatment allocation during study conduct, as in a double-blind trial. A single-blind design is commonly used in randomized, controlled PK bridging studies, to minimize the risk of bias arising from study participants being aware they are receiving the study drug. In addition, the inclusion of a placebo arm allowed for evaluation of AEs attributable to sotrovimab.
The study design had a minimum sample size requirement of eight participants on active treatment and two on placebo (4:1 randomization ratio) for each ethnicity subgroup in each study part. A total of 24 participants were randomized in each part to achieve at least 20 evaluable subjects, accounting for potential dropouts. However, there were no dropouts in any of the study parts, therefore the observed randomization ratio was 3:1 in Part 1 and 5:1 in Part 2, with the minimum sample size requirements achieved for each ethnicity group in each part of the study.
The relatively small sample size constitutes a potential limitation of this study, in that it restricted the opportunity to evaluate the occurrence of rare events, such as infusion-related reactions or anaphylaxis, and also to identify factors that might influence the bioavailability of sotrovimab following IM administration. Adjustment for body weight using allometric principles in analysis of maximum and total exposures was used to mitigate, in part, this small sample size. There was also an imbalance in the number of females and males included in Part 1 of the study. Other potential limitations include no assessment of different IM injection sites and a duration of sampling insufficient to accurately estimate t½ and AUCinf (although the objective of this study was to investigate early PK properties). In addition, the study was designed using a parallel design (as opposed to the commonly used cross-over design for bioavailability studies) due to the long half-life of sotrovimab and the need for an excessively prolonged washout period between the different regimens.
In summary, sotrovimab exposures following IV administration were similar between Japanese and Caucasian participants and were higher in Japanese participants following IM administration and after adjusting for body weight. These higher exposures do not appear to be associated with any safety signals. Single IV and IM doses of sotrovimab 500 mg were well tolerated by both Japanese and Caucasian participants. No safety concerns that would impact the favorable risk/benefit profile for sotrovimab were identified in this study.
Acknowledgements
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Tony Reardon of Apollo, OPEN Health Communications, and was funded by GSK.
Declarations
Funding
This study was sponsored by Vir Biotechnology, Inc., in collaboration with GSK. The study was supported by a COVID-19 grant from the Ministry of Health, Labour and Welfare of Japan. Open access publication was supported by GSK.
Conflict of interest
AN, DB, SAH, MH, AML, AO, NO, SS, YS, AS, RW, and DA are employees of and/or hold stocks/shares in GSK. MO and IAH (at time of the study) were employees of and/or held stocks/shares in GSK. EA and JES are employees of, and/or hold stocks/shares in, Vir Biotechnology, Inc. AZH has nothing to disclose. EYY is an employee of Parexel; Parexel received funding from GSK to assist in the conduct of the study.
Availability of data and material
Manuscript data will not be deposited in a repository. Data can be made available on reasonable request to the corresponding author.
Ethics approval
This study was conducted in accordance with the principles of the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonisation Good Clinical Practice guidelines, and applicable laws and regulations.
Consent to participate
Written informed consent was provided by all participants prior to study entry.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed to manuscript development and approved the final version for submission. Study design: AN, EA, DB, SAH, IAH, AML, AO, MO, NO, JES, SS, YS, AS, and DA. Data collection, analysis, and interpretation: all authors.
References
- 1.Gebru AA, Birhanu T, Wendimu E, et al. Global burden of COVID-19: situational analysis and review. Hum Antibodies. 2021;29(2):139–148. doi: 10.3233/HAB-200420. [DOI] [PubMed] [Google Scholar]
- 2.Monteiro Pires S, Wyper GMA, Wengler A, et al. Burden of disease of COVID-19: strengthening the collaboration for national studies. Front Public Health. 2022;10:907012. doi: 10.3389/fpubh.2022.907012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control. Risk factors and risk groups. 2022. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/risk-factors-risk-groups. Accessed 11 May 2023.
- 4.World Health Organization. WHO Coronavirus (COVID-19) dashboard: global vaccination data. 2022. https://covid19.who.int. Accessed 11 May 2023.
- 5.World Health Organization. WHO Coronavirus (COVID-19) dashboard: Japan vaccination data. 2022. https://covid19.who.int/region/wpro/country/jp. Accessed 11 May 2023.
- 6.Hwang Y-C, Lu R-M, Su S-C, et al. Monoclonal antibodies for COVID-19 therapy and SARS-CoV-2 detection. J Biomed Sci. 2022;29(1):1. doi: 10.1186/s12929-021-00784-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327(13):1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch C, Park YS, Piechotta V, et al. SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19. Cochrane Database Syst Rev. 2022;6(6):CD014945. 10.1002/14651858.CD014945.pub2. [DOI] [PMC free article] [PubMed]
- 9.Pinto D, Park Y-J, Beltramello M, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 10.Cathcart AL, Havenar-Daughton C, Lempp FA, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. Preprint posted online April 1, 2022. 10.1101/2021.03.09.434607.
- 11.Ko S-Y, Pegu A, Rudicell RS, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514(7524):642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalevsky J, Chamberlain AK, Horton HM, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28(2):157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudinski MR, Coates EE, Houser KV, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018;15(1):e1002493. doi: 10.1371/journal.pmed.1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro AE, Sarkis E, Acloque J, et al. Intramuscular versus intravenous SARS-CoV-2 neutralizing antibody sotrovimab for treatment of COVID-19 (COMET-TAIL): a randomized non-inferiority clinical trial. Open Forum Infect Dis. 2023;10(8):ofad354. 10.1093/ofid/ofad354. [DOI] [PMC free article] [PubMed]
- 15.Okamasa A, Okour M, Austin D, et al. Safety and pharmacokinetic evaluation of anti-SARS-CoV-2 monoclonal antibody sotrovimab in Japanese/Caucasian healthy adults during intravenous infusion. Kansenshogaku zasshi. 2022;96(2):39–45. 10.11150/kansenshogakuzasshi.96.39(article in Japanese).
- 16.Chiba K, Yoshitsugu H, Kyosaka Y, et al. A comprehensive review of the pharmacokinetics of approved therapeutic monoclonal antibodies in Japan: are Japanese phase I studies still needed? J Clin Pharmacol. 2014;54(5):483–494. doi: 10.1002/jcph.231. [DOI] [PubMed] [Google Scholar]
- 17.Bender Ignacio RA, Wohl DA, Arends W, et al. Comparative pharmacokinetics of tixagevimab/cilgavimab (AZD7442) administered intravenously versus intramuscularly in symptomatic SARS-CoV-2 infection. Clin Pharmacol Ther. 2022;112(6):1207–1213. doi: 10.1002/cpt.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuidema J, Pieters FAJM, Duchateau GSMJE. Release and absorption rate aspects of intramuscularly injected pharmaceuticals. Int J Pharm. 1988;47(1–3):1–12. 10.1016/0378-5173(88)90209-8.
- 19.Larkin TA, Ashcroft E, Hickey BA, Elgellaie A. Influence of gender, BMI and body shape on theoretical injection outcome at the ventrogluteal and dorsogluteal sites. J Clin Nurs. 2018;27(1–2):e242–e250. doi: 10.1111/jocn.13923. [DOI] [PubMed] [Google Scholar]
- 20.Gupta AK, Perez-Rodríguez MT, Gonzalez-Rojas Y, et al. Safety, tolerability, and viral pharmacodynamics of the IgG monoclonal antibody sotrovimab administered via intramuscular injection for the treatment of early mild-to-moderate COVID-19. Open Forum Infect Dis. 2022;9(Suppl 2):ofac492.992. 10.1093/ofid/ofac492.992.