Abstract
Introduction
The coronavirus disease 2019 (COVID-19) pandemic became superimposed on the pre-existing obesity and diabetes mellitus (DM) pandemics. Since COVID-19 infection alters the metabolic equilibrium, it may induce pathophysiologic mechanisms that potentiate new-onset DM, and we evaluated this issue.
Method
A systematic review of the literature published from the 1 January 2020 until the 20 July 2023 was performed (PROSPERO registration number CRD42022341638). We included only full-text articles of both human clinical and randomized controlled trials published in English and enrolling adults (age > 18 years old) with ongoing or preceding COVID-19 in whom hyperglycemia was detected. The search was based on the following criteria: “(new-onset diabetes mellitus OR new-onset DM) AND (COVID-19) AND adults”.
Results
Articles on MEDLINE (n = 70) and the Web of Science database (n = 16) were included and analyzed by two researchers who selected 20 relevant articles. We found evidence of a bidirectional relationship between COVID-19 and DM.
Conclusions
This link operates as a pathophysiological mechanism supported by epidemiological data and also by the clinical and biological findings obtained from the affected individuals. The COVID-19 pandemic raised the incidence of DM through different pathophysiological and psychosocial factors.
Keywords: Diabetes mellitus, Hyperglycemia, Prediabetes, New-onset diabetes, New-onset hyperglycemia, Newly diagnosed diabetes, Severe acute respiratory syndrome coronavirus-2 infection, Coronavirus disease 2019
Key Summary Points
| The coronavirus disease of 2019 (COVID-19) pandemic became superimposed on the pre-existing obesity and diabetes mellitus (DM) pandemics. Since COVID-19 infection alters the metabolic equilibrium, it may induce pathophysiologic mechanisms that potentiate new-onset DM. |
| A systematic review of the literature was performed based on the following criteria: "(new-onset diabetes mellitus OR new-onset DM) AND (COVID-19) AND adults". Articles on MEDLINE (n = 67) and the Web of Science database (n = 16) were included and analyzed by two researchers who selected 17 relevant articles. |
| We found evidence of a bidirectional relationship between COVID-19 and DM. This link operates as a pathophysiological mechanism supported by epidemiological data and also by the clinical and biological findings obtained from the affected individuals. |
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus precipitated a new infectious diseases pandemic, with > 650 million cases and > 6.6 million deaths worldwide [1]. This new pandemic overlaps the current metabolic pandemics represented by diabetes mellitus (DM) and obesity. COVID-19 has been described as worsening the metabolic parameters of DM, while, on the other hand, the presence of DM, especially poor glycemic control, can lead to the development of more severe forms of COVID-19 with increased mortality rates [2, 3].
Patients with DM may have impaired immune responses, since hyperglycemia can favor hypoxia and maintain a heightened systemic inflammatory tone [4]. Some immune cells such as natural killer (NK) cells have been described as having a significant role in the atherosclerosis process [5] besides their well-studied protective role in SARS-CoV-2 infection in the last 3 years [6]. Researchers discovered that NK cells undergo important alterations in obesity, T2DM, and cardiovascular atherosclerotic disease (CAD) [5], with all of these effects leading to serious immunomodulation in these patients in the case of COVID-19. DM has been associated with more severe forms of SARS-CoV-2 infection, and patients with obesity and DM had higher mortality rates than persons without these conditions [7].
Using different mechanisms, the SARS-CoV-2 virus triggers the new onset of both type 1 DM (T1DM) and type 2 DM (T2DM). Viral infections are well-known triggers for both T1DM and T2DM; namely, Epstein–Barr virus or coxsackievirus are triggers for autoimmune T1DM [8] and hepatitis C virus is a trigger for T2DM [9].
Several mechanisms by which the SARS-CoV-2 virus can lead to hyperglycemia have been described, including (i) direct damage to pancreatic β-cells (secondary to SARS-CoV-2 binding to angiotensin-converting enzyme 2 (ACE2) receptors present on the surface of the pancreatic islets), (ii) the stress response promotes the increased release of catecholamines and glucocorticoids, and (iii) increased inflammatory activity, which intensifies insulin resistance [10]. Moreover, COVID-19 treatment can also cause hyperglycemia, considering that in moderate and severe forms of the infection, patients may benefit from corticosteroid therapy. This corticosteroid metabolic side effect may act as an add-on factor that leads to hyperglycemia.
There is also an indirect effect of the COVID-19 pandemic represented by the lockdown period, which led to the delay of medical appointments and inappropriate care of patients with chronic diseases like diabetes and ultimately increased death rates [11]. Furthermore, the isolation measures resulted in loneliness, social isolation, depression, anxiety, reduced physical activity, and unhealthy eating patterns with excess alcohol consumption and weight gain, which, taken together, increased the risk of DM significantly and delayed the detection of pre-existing hyperglycemia [12, 13]. Finally, all these factors, combined with the direct effects of SARS-CoV-2 infection itself, led to an increased incidence of diabetic ketoacidosis (DKA) among newly diagnosed and pre-existing T2DM, while, at the same time, data for patients with T1DM are less clear [14].
The significance of new-onset DM among patients with COVID-19 is reflected by their more significant metabolic burden, including dyslipidemia, high blood pressure (HBP), obesity, coronary artery disease, or chronic kidney disease, all of which contribute to poorer cardiometabolic outcomes from SARS-CoV-2 infection [15]. Beyond the metabolic point of view, a patient with COVID-19 infection and T2DM has a poorer prognosis and is more likely to develop severe forms of COVID-19 infection and die from them [4].
Aim of the Study
This systematic review aimed to identify/establish the possible connection between new-onset DM and SARS-CoV-2 infection in terms of epidemiologic data, acute DM complications (e.g., DKA), and pathogenic, inflammatory, or immunological mechanisms for a better understanding of this new pathology. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Methods
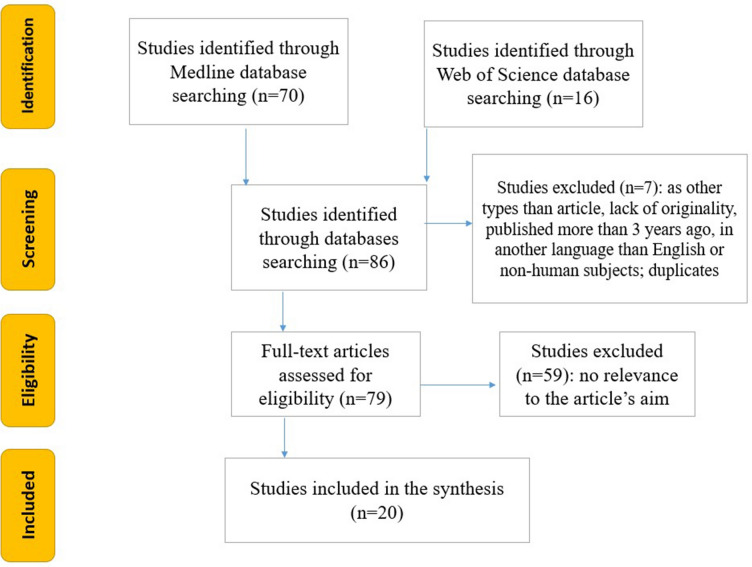
We developed an easily reproducible protocol for our study by following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for the systematic review protocol checklist. Furthermore, we used the Population, Intervention, Comparison, Outcome and Study Design (PICOS) strategy to guide our study rationale and to carry out a clear, useful, and systematic literature search. A systematic review of the literature published from 1 January 2020 until 20 July 2023 was performed using PROSPERO registration number CRD42022341638. We included only full-text articles of human clinical and randomized controlled trials published in English, enrolling adults (age > 18 years old) with ongoing or preceding COVID-19 in whom hyperglycemia was detected. The search was based on the following criteria: “(new-onset diabetes mellitus OR new-onset DM) AND (COVID-19) AND adults”. Seventy articles on MEDLINE and 16 on the Web of Science database were included and analyzed by two researchers, who selected 20 relevant ones (Fig. 1). Two researchers, TS and ICB, individually performed the screening in order to find articles relevant to our theme of interest, and any disagreements that occurred in the selection process were settled by a third reviewer (APS). Studies that were only full-text original articles published in English in the last 3 years and were only on the adult human population (age over 18 years old) were selected for a full-text review. This systematic review included 20 studies. We used the Newcastle–Ottawa Quality Assessment Scale to evaluate the quality of the trials, and we only included studies that scored intermediate (4–6 points) or high (7–9 points) on this scale. Additionally, we used the Cochrane risk-of-bias tool (RoB2 version) to assess the quality of RCTs, and we only included low-risk bias studies. In terms of data extraction, TS and ICB primarily searched the studies for the incidence of DM, COVID-19 severity metabolic consequences, effects of COVID-19 treatment on DM incidence, and linked pathophysiologic mechanisms in DM and COVID-19, and then compared their results to minimize bias. We extracted the following data: authors, publication year, study design, number of patients meeting the inclusion criteria, number of patients with COVID-19, number of controls, main outcome (odds ratio/hazard ratio and 95% confidence interval), and follow-up time. The studies found present both clinical and methodological heterogeneity in a manner that did not permit statistical analysis.
Fig. 1 .
Flowchart of the study selection process according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) recommendations
Results
New-Onset DM and DKA in COVID-19 Illness
The high prevalence of DKA and hyperosmolarity, with a need for higher insulin doses in patients with COVID‐19, raised the question of a possible new type of DM [16]. Ghash et al. reported a case of new-onset DM with DKA and DM-specific symptoms that underline their association's importance for a correct diagnosis [17]. Gentile et al. emphasize that attention should be paid to several categories of patients with new‐onset DM—(i) impaired fasting glucose and impaired glucose tolerance with persistently normal glycated hemoglobin (HbA1c) levels, (ii) temporary hyperglycemia in any acute or severe inflammatory disease, or (iii) symptoms and signs of ketoacidosis specific to DM—because there are frequent cases of misdiagnosing DKA in events with high blood glucose levels accompanied by respiratory acidosis and malnutrition‐driven ketosis or other conditions that were not sufficiently investigated [16]. However, it is important to emphasize that the newly diagnosed DM may also represent the unmasking of existing DM driven by the sudden metabolic disturbances caused by COVID‐19 illnesses [17].
COVID-19, ACE2 Receptors, and New-Onset DM
The hypothesis that the binding of SARS‐CoV‐2 to ACE2 receptors increases the risk of developing DKA has some conceptual support [16]. First, ACE2 receptors serve as the entry point for SARS-CoV-2, and its expression is downregulated after endocytosis of the virus complex, so the entry of SARS-CoV-2 into pancreatic islet cells may directly cause β-cell injury but can also lead to unopposed angiotensin II action, which may directly impede insulin secretion and the insulin signaling pathway [18–20]. In addition, the binding of SARS-CoV-2 to ACE-2 receptors provokes a decrease in glucose uptake into musculoskeletal cells, an increase in lipolysis in adipocytes, and an increase in hepatic gluconeogenesis [17]. Aldosterone-stimulated secretion can also lead to hypokalemia, further impairing insulin secretion [19, 20]. Besides the aforementioned mechanisms, reported autoimmune mechanisms include direct cellular lytic effects from the viral replication process and host autoimmunity secondary to the inflammatory response of autoreactive lymphocytes [18, 20]. Islet cell destruction secondary to SARS-CoV-2 infection can be an autoimmune pathophysiological mechanism, though this infection determines hyperglycemia in patients with COVID-19.
However, pre-existing DM predisposed patients to develop acidosis and ketoacidosis in cases of hospitalization for confirmed COVID‐19, since ketosis or ketonuria was present in 6.4% of a 658-patient cohort study [16]. Data from a smaller analysis (102 patients) reported negative ketone urinary tests in patients with newly diagnosed diabetes and SARS-CoV-2 infection and marked hyperglycemia (148–321 mg/dl) [21].
The Inflammatory Aspects
It is still unclear if the inflammatory cascades occurring in DKA and severe COVID‐19 act synergistically, given that elevated interleukin‐6 levels play an essential part in a maladaptive immune response to the SARS‐CoV‐2 virus [16] leading to new-onset T1DM with more severe DKA cases (44.3% in 2020 vs. 36% in 2019 in Germany) [20].
Gupta et al. reported that ketosis-prone diabetes (KPD), characterized by new-onset DM associated with ketosis/ketoacidosis in the presence of negative glutamic acid decarboxylase (GAD65), insulinoma-associated-2 (IA2), and zinc transporter 8 (ZnT8) autoantibodies (A −), and with β-cell secretion (A− β+ DM), is initially characterized by more severe DKA, worse glycemic control, and insulin dependence [10]. GAD65, IA2, and ZnT8 have been described as autoimmune biomarkers in patients with islet cell destruction in type 1 DM; they are present from stage 1 of this condition when hyperglycemia has not yet occurred [22]. Meanwhile, this DM phenotype may associate subsequent complete β-cell function recovery (β+) with a significant improvement in glycemic control without a high insulin dose demand or even in patients not dependent on insulin therapy administration at follow-up (Table 1) [10].
Table 1.
Results of the included studies (clinical trials and RCTs)
| Author | Study type | Number of patients meeting the inclusion criteria | Median follow-up time | Type of intervention | Main outcome |
|---|---|---|---|---|---|
| Ghosh et al. [2] | Observational study | 555 | NA | NOD compared to NOD COVID-19 patients |
NOD vs NOD COVID-19 Fasting blood glucose: 163 ± 66 vs 203 ± 97 mg/dL; p < 0.001; Postprandial blood glucose: 238 ± 97 vs 303 ± 127 mg/dL; p < 0.001; Glycated hemoglobin: 9.2 ± 2.4 vs 10.1 ± 2.5%; p < 0.001 C-peptide fasting: 1.9 ± 1.7 vs 2.5 ± 3.07 pmol/ml; p = 0.254 |
| Sathish et al. [21] | Retrospective cohort study | 102 | None | Admitted patients with mild-to-moderate COVID-19 at RT-PCR |
21 patients with NDD on admission 4 patients with marked hyperglycemia and random blood glucose between 148 and 321 mg/dl, negative urinary ketones test, without signs or symptoms of DKA |
| Gupta et al. [10] | Prospective cohort study | 42 | 10 months |
Patients with DKA who tested positive for COVID-19 at RT-PCR and with no previous history of DM or DKA were divided into two groups: Group 1, ketosis-prone diabetes (KPD) = absence of autoantibodies (A−) and tendency for β-cell secretory function to recover over time (β+); Group 2 = type 1A DM patients (A− β−) |
HbA1c 12.3 ± 2.4 vs 9.8 ± 1.3% in group 1 vs group 2, p = 0.02 DKA by arterial pH 7.14 ± 0.08 vs 7.26 ± 0.1, p = 0.01, serum bicarbonate 8.4 ± 4 vs 12.2 ± 3.2 mmol/L, p = 0.01; in group 1 vs group 2 After 10 months of follow-up from group 1, 4 patients still needed insulin therapy while 15 were independent of insulin Basal C-peptide was 0.65 ± 0.13 ng/mL for patients who needed insulin vs 2.1 ± 0.42 ng/mL for those independent from insulin Stimulated C-peptide 0.81 ± 0.14 vs 4.61 ± 1.32 ng/mL, p < 0.005 patients who needed insulin vs those independent from insulin |
| Cariou et al. [23] | Retrospective study | 243 | 28 days | Hospitalized SARS-CoV-2-positive patients with established DM and with NDD |
NDD (n = 67) vs established T2DM (n = 167): Mean age 61.9 ± 11.3 vs 64.1 ± 10.7 years, p = 0.1711 Time (days) between the onset of COVID-19 symptoms and hospitalization: 6 vs 5, OR: 1.08, CI 1.01–1.16, p = 0.0185; CRP levels: 108 vs 95 mg/L OR = 1.37, CI 1.00–1.88, p = 0.0492; Plasma glucose on admission: 170 vs 173 mg/dL OR = 1.25, CI 0.91–1.71, p = 0.1623 Discharge within seven days: 10.6 vs 22.9%, OR = 0.39, CI 0.17–0.96, p < 0.05 Discharge within 28 days: 47.0 vs 56.6%, OR = 0.69, CI 0.37–1.31, p > 0.05 |
| Qeadan et al. [24] | Retrospective study | 55.359 | 2 months | COVID-19-positive (SARS-CoV-2) patients without pre-existing T1DM versus COVID-19-negative patients with pre-existing or without T1DM | COVID-19 diagnosis has a 42% increased risk for new-onset T1DM (OR = 1.42, 95% CI 1.38–1.46) compared with patients without COVID-19 |
| Sane et al. [25] | Retrospective cross-sectional study | 244 | NA | 244 admitted patients with confirmed COVID-19 who were evaluated for NODM |
Male (aOR 2.9, 95% CI 1.2–7.1, p = 0.018), obesity (aOR 3.1, 95% CI 1.01–8.90, p = 0.048), higher potassium levels at admission (aOR 9.3, 95% CI 1.8–47.3, p = 0.007) and a family history of hypertension (aOR 3.7, 95% CI 1.3–10.5, p = 0.012) are risk factors for NODM after COVID-19 Presence of pulmonary embolism is considered a protective factor for NODM cases (aOR 0.15, 95% CI 0.06–0.40, p < 0.001) |
| Keerthi et al. [26] | Prospective observational study | 100 | 3 months | Confirmed COVID-19 (rapid antigen test or RT-PCR) evaluated for NODM |
NODM was associated with a family history of DM, higher BMI, oxygen or steroid duration (p < 0.001 for all), severity at admission (p = 0.006), and DKA (p = 0.0275) 10.3% of patients without DM developed NOPD and 13.8% developed NODM 16.6% of patients with prediabetes advanced to NODM |
| Alkhemeiri et al. [27] | Prospective single-center cohort study | 1204 | 3 months | COVID-19 infection and admitted to a COVID-19-designated hospital, tested for NODM or NOPD |
NODM was detected in 9.8% of patients (97.7% diagnosed with T2DM and 1.3% with T1DM), NOPD was detected in 2.3% of total patients Hypertension (65.4%) and obesity (44.2%) were the more commonly associated comorbidities in patients with DM |
| Naveed et al. [28] | Cohort study | 629,935 | A median of 257 days (102–356) | Patients tested for SARS-CoV-2, with positive versus negative tests |
Positive SARS-CoV-2 RT-PCR tests (HR = 1.17; 95% CI,1.06–1.28) and male gender (aHR 1.22; 95% CI 1.06–1.40) were associated with higher risks of incident DM The severity of COVID-19 was associated with a greater risk of DM in patients admitted to ICU (HR 3.29; 95% CI 1.98–5.48) or hospital (HR 2.42; 95% CI 1.87–3.15) compared to patients without SARS-CoV-2 infection The fraction of incident DM cases attributable to SARS-CoV-2 infection was 3.41% (95% CI 1.20–5.61%) overall and 4.75% (95% CI 1.30–8.20%) among males |
| Choi et al. [29] | Cohort study | 1,392,720 | 11.1 months (7.8–15.7) in the COVID-19 group and 11.2 months (7.8–15.7) in the group without COVID-19 | COVID-19 patients versus non-COVID-19 patients |
Patients with COVID-19 had an increased risk (HR 1.42; 95% CI 1.39–1.46) of newly diagnosed T2DM compared to those without COVID-19 The risk for newly diagnosed DM was associated with the severity of COVID-19: non-hospitalized (aHR 1.14; 95% CI 1.08–1.19), hospitalized (aHR 1.34; 95% CI 1.30–1.38), and ICU (aHR 1.78; 95% CI 1.59–1.99) The risk for newly diagnosed DM was slightly higher for the GC-treated patients compared to the non-GC-treated in the COVID-19(+) group (aHR 1.43; 95% CI 1.30–1.57 for < 60 mg group, aHR 1.37; 95% CI 1.27–1.47 for 60–419 mg, and aHR 1.34; 95% CI 1.21–1.48 for ≥ 420 mg vs aHR 1.29; 95% CI 1.25–1.32 for non-GC, p < 0.001 for all), and this increase was not dose dependent |
| Birabaharan et al. [30] | Cohort study | 600,055 | 1 year in the COVID-19 group (20 January 2020 to 20 January 2021) and 3 years (20 January 2018 to 20 January 2021) in the influenza group | COVID-19 patients versus patients with influenza |
New-onset DM RR 1.54 (95% CI 1.46–1.62) for mild COVID-19 versus mild influenza controls New-onset DM RR 1.46 (95% CI 1.26–1.69) for moderate/severe COVID-19 compared with moderate/severe influenza New-onset DM RR 1.42 [95% CI 1.13–1.80]) for moderate/severe COVID-19 compared to moderate/severe influenza when excluding steroid use |
| Sharma et al. [31] | Cohort study | 81,093 | 14 months (between March 2020 and May 2021) in both study groups | COVID(+) patients versus COVID(−) patients |
Higher risk of T2D onset in COVID-19(+) patients (N = 326/2,433) compared with COVID-19(−) patients (N = 380/6,594) (OR 1.4, P < 0.001) No statistically significant difference in risk of developing DKA between COVID-19(+) and COVID-19(−) (HR 0.98 [95% CI 0.46–1.56], P = 0.53) in patients with T1DM No statistically significant difference in risk of developing DKA between COVID-19(+) and COVID-19(−) (HR 1.38 [95% CI 0.79–2.41], P = 0.26) in patients with T2DM |
| Xie et al. [32] | Cohort study | 181,280 | 17 months in the COVID group and 17 months in the control group | COVID(+) patients versus COVID(−) patients |
COVID-19(+) had an increased risk (HR 1·40, 95% CI 1·36–1·44) and excess burden (13·46, 95% CI 12·11–14·84, per 1000 people at 12 months) of incident diabetes; COVID-19(+) had an increased risk (1·85, 1·78–1·92) and excess burden (12·35, 11·36–13·38) of incident antihyperglycemic use |
| Mithal et al. [15] | Retrospective study | 405 | NR | Observational study | Of 401 patients, 5.2% had NODM and 47.1% had pre-existing DM; the latter had more severe COVID-19 infections and higher mortality |
| Gentile et al. [16] | Review | NR | NR | NR |
The authors emphasize the need for attention to be paid to three important aspects in a proper NODM diagnosis: Impaired fasting glucose and impaired glucose tolerance with normal HbA1c levels Temporary hyperglycemia in any acute or severe inflammatory disease Symptoms and signs of ketoacidosis specific to DM |
| Suwanwongse et al. [18] | Case report | 2 | NR | NR | The authors highlight the bidirectional causality relationship between DM and COVID-19 |
| Reddy et al. [19] | Review and case report | 2 | NR | NR | DKA can be precipitated by COVID-19, and it can be a method through which DM is diagnosed |
| Boddu et al. [20] | Review | NR | NR | NR | SARS-CoV-2 infection may precipitate DKA in patients without previous DM diagnosis; it can be a way to diagnose T1DM |
| Sathish et al. [34] | Review | NR | NR | NR | SARS-CoV-2 infection may determine NODM trough: β-cell destruction, impaired insulin signalling, or oxidative stress |
| Fadini et al. [36] | Retrospective study | 413 | 3 months | Observational | Of the 413 patients included, 107 (25.6%) had DM, with 21 (5.2%) NDD. NDD and hyperglycemia were associated with greater COVID-19 infection severity |
DM diabetes mellitus, DKA diabetic ketoacidosis, NOD new-onset diabetes mellitus cases before COVID-19, NOD COVID-19 new-onset diabetes mellitus cases during COVID-19, NDD newly diagnosed diabetes mellitus, T1DM type 1 diabetes mellitus, CRP C-reactive protein, COVID-19 coronavirus disease 2019, RT-PCR reverse transcription polymerase chain reaction, T2DM type 2 diabetes mellitus, NODM new-onset diabetes mellitus, NOPD new-onset prediabetes, ICU intensive care unit, GC glucocorticoid, NR not reported, COVID(+) positive tests for COVID-19, COVID(−) negative tests for COVID-19
New-Onset DM Epidemiology
Ghosh et al. reported a similar incidence of new-onset DM before COVID-19 or during COVID-19 illness, but the latter group presented higher levels of fasting blood glucose (203 ± 97 vs 163 ± 66 mg/dL, p < 0.001), postprandial blood glucose (303 ± 127 vs 238 ± 97 mg/dL, p < 0.001), HbA1c (10.1 ± 2.5 vs 9.2 ± 2.4%, p < 0.001), and fasting C-peptide (2.5 ± 3.07 vs 1.9 ± 1.7 pmol/ml; p = 0.254) [2] (Table 1).
Cariou et al. evaluated 67 patients with newly diagnosed DM along with 176 patients with established T2DM. The newly diagnosed DM patients presented at a younger age (61.9 ± 11.3 vs 64.1 ± 10.7 years, p = 0.171), had less HTN (41.5 vs 79.4%, p < 0.0001) and cardiovascular diseases (CVD) (13.8 vs 36.5%, p = 0.002), and were more unlikely to be on statins (10.4 vs 47.2%, p < 0.001). In addition, these patients had a longer duration between the onset of COVID-19 symptoms and hospitalization (6 vs. 5 days, p = 0.0185) and higher CRP levels (OR:1.37, 95% CI 1–1.88, p = 0.04)., The between-group plasma glucose levels on admission were not different (OR: 1.25, 95% CI 0.91–1.71, p = 0.16). The newly diagnosed patients with diabetes had a greater need for hospitalization at 7 days (OR: 0.39, 95% CI 0.17–0.96, p < 0.05); however, there was no significant difference at 28 days (OR: 0.69, 95% CI 0.37–1.31, p > 0.05) [23], as seen in Table 1.
Qeadan et al. reported that in the United States, patients with a COVID-19 diagnosis had a 42% increased risk for new-onset T1DM (OR: 1.42, 95% CI 1.38–1.46) compared with those without COVID-19, with a higher risk for males (OR: 1.49, 95% CI 1.42–1.55) than for females (OR: 1.36, 95% CI 1.30–1.42). As far as race/ethnicity is concerned, the observed risks were: American Indian/Alaskan Native (OR: 2.30, 95% CI 1.86–2.82), Asian/Pacific Islander (OR: 2.01, 95% CI 1.61–2.53), Black (OR: 1.59, 95% CI 1.47–1.71), Hispanic (OR: 1.52, 95% CI 1.41–1.63), and White (OR: 1.18, 95% CI 1.13–1.23). Additionally, individuals from the Northeast region with a COVID-19 diagnosis had the highest risk of developing T1DM (OR: 1.71, 95% CI 1.61–1.81). Marital status did not impact T1DM risk among patients diagnosed with COVID-19 [24].
A study conducted in Ethiopia reported that 31.1% of COVID-19 patients developed new-onset DM, with T2DM prevailing (83.6%). Researchers found that male sex (aOR = 2.9, 95% CI 1.2, 7.1, p = 0.018), obesity (aOR = 3.1, 95% CI 1.01, 8.90, p = 0.048), higher potassium levels at admission (aOR = 9.3, 95% CI 1.8, 47.3, p = 0.007), and a family history of HTN (aOR = 3.7, 95% CI 1.3, 10.5, p = 0.012) all increased the risk for new-onset DM after COVID-19 infection. In addition, they demonstrated that the presence of pulmonary embolism is associated with 85% fewer cases of new-onset DM (aOR = 0.15, 95% CI 0.06, 0.40, p < 0.001) [25].
Keerthi et al. evaluated 100 patients with COVID-19 and discovered that new-onset DM (NODM) was associated with a family history of DM (p < 0.001), higher BMI (p < 0.001), duration of need for supplemental oxygen or parenteral steroids (p < 0.001), the severity of infection at the time of admission (p = 0.006), and diabetic ketoacidosis (p = 0.0275). Moreover, 10.3% of patients without diabetes developed new-onset prediabetes (NOPD), and 13.8% developed NODM, while 16.6% of patients with prediabetes advanced to NODM [26].
A study from the United Arab Emirates reported comparable data on new-onset DM and new-onset prediabetes. Alkhemeiri et al. followed patients with COVID-19 from November 2020 to the end of April 2021 and discovered a rate of new-onset DM of 9.8%, with a majority of type 2 DM (97.7%) and a 2.3% prevalence of new-onset prediabetes. The most common associated conditions were HTN (65.4%), obesity (44.2%), and coronary artery disease (18.9%) [27].
A recent cohort study including 629.935 patients from 1 January 2020 to 31 December 2021 and using the British Columbia COVID-19 Cohort platform reported that the incidence of DM was higher in positive-SARS-CoV-2-tested patients than in patients with negative SARS-CoV-2 tests (672.2 per 100.000 patients-year vs. 508.7 per 100.000 patients-year, p < 0.001) [28].
Similar results came from a Korean cohort study including 348,180 patients diagnosed with COVID-19 from January 2020 to September 2021 [29]. Likewise, in Canadians [26], Choi et al. found that COVID-19 is associated with a greater risk for newly diagnosed DM (aHR 1.30; 95% CI 1.27–1.33), and this risk is in proportion with the COVID-19 severity, so patients admitted to the ICU presented the highest risk (aHR 1.78, 95% CI 1.59–1.99) [29].
A cohort study including 994,722 individuals evaluated patients diagnosed with COVID-19 using the International Classification of Diseases (ICD-10 U07.1) from 20 January 2020 to 20 January 2021 and compared them to patients diagnosed with influenza (ICD-10 J09-J11) from 20 January 2018 to 20 January 2021. Patients with mild COVID-19 presented a 1.54 (95% CI 1.46–1.62) times higher risk for new-onset DM compared to those with mild influenza (the control group). The same higher risk was present among patients with moderate/severe COVID-19 compared with controls with moderate/severe influenza (RR 1.46 [95% CI 1.26–1.69]) [30].
A cohort study that was based on the Cleveland Clinic COVID-19 registry and included 81,093 patients who tested positive for COVID-19 between March 2020 and May 2021 evaluated the influence of SAR-CoV-2 infection on glycemia and diabetic ketoacidosis. Patients with positive COVID-19 tests had a greater risk for T2DM diagnosis post-infection (N = 326/2,433) compared to patients who tested negative for COVID-19 (N = 380/6,594; OR 1.4, P < 0.001) [31].
A cohort study including 181,280 participants from the US Department of Veterans Affairs national databases evaluated the patients who tested positive for SARS-CoV-2 infection between March 2020 and September 2021. Researchers demonstrated an increased risk (HR 1.40, 95% CI 1.36–1.44) of incident diabetes compared to patients with no evidence of SARS-CoV-2 infection enrolled in the same period in addition to the period between March 2018 to September 2018 [32].
C-Peptide and Immunological Status in COVID-19 and DM
When comparing the newly diagnosed patients with DM during the COVID-19 pandemic, the available studies report no differences in risk factors or biochemical parameters regarding COVID-19 antibody status [2]. Although patients with new-onset DM during the COVID-19 pandemic had a worse glycemic status compared with those with new-onset DM before the COVID-19 pandemic, the researchers did not find a significant difference between C-peptide levels in new-onset DM vs new-onset DM COVID-19 patients (1.9 ± 1.7 vs 2.5 ± 3.07 pmol/ml; p = 0.254). These results are consistent with the idea that there are no differences in beta-cell damage between newly diagnosed DM during COVID-19 and newly diagnosed DM before COVID-19 [2].
New-Onset Hyperglycemia in COVID-19
Mithal et al. reported new-onset hyperglycemia in patients hospitalized for COVID-19. Patients in the DM group were older (59.8 ± 12.1 vs 47.7 ± 16.5; p < 0.001) and they had higher proportions with HTN (111 ± 58.7 vs 53 ± 25; p < 0.001), CKD (10 ± 5.3 vs 2 ± 0.9; p = 0.016), and coronary artery disease (26 ± 13.8 vs 9 ± 4.2; p = 0.001). Also, more patients in the DM group had glucocorticoid therapy compared with the control group (148 ± 78.3 vs 115 ± 54.2; p < 0.001) [15].
Unhealthy Lifestyle During the COVID-19 Pandemic
During the COVID-19 pandemic, people adopted unhealthy diet patterns, partly in the context of lifestyle changes during pandemic restrictions. Researchers proposed that lifestyle changes including smoking cessation, limitation of alcohol consumption, healthy food choices with balanced micro- and macronutrient proportions, and good sleep quality may improve health outcomes in patients with SARS-CoV-2 infection [33].
We synthesized the proposed mechanisms through which SARS-CoV-2 infection may lead to hyperglycemia in patients, as presented in the included studies (Fig. 2).
Fig. 2.
Different mechanisms proposed as possible causes of hyperglycemia in SARS-CoV-2 infection
Discussion
Different aspects of the relationship between DM and COVID-19 have been studied since the outbreak of the COVID-19 pandemic.
COVID-19 and New-Onset DM
One of the aspects is the difference in incidence and prevalence between newly diagnosed and pre-existing DM among COVID-19 patients. A systematic review and meta-analysis of eight studies on 3711 patients reported a prevalence of 14.4% for newly diagnosed DM and a prevalence of 14.8% for pre-existing DM among patients hospitalized for COVID-19, underlining the importance of this new entity [34].
The Coronavirus SARS-CoV-2 and Diabetes Outcomes (CORONADO) study reported a prevalence of 2.8% for newly diagnosed DM in patients hospitalized for COVID-19. In this study, new-onset DM patients did not have a worse COVID-19 prognosis than patients with pre-existing DM, since the first group did not share the same comorbidities as the latter (CVD, microvascular complications, HTN, dyslipidemia, etc.). This included > 60% male participants with a mean age of around 70 years and T2DM in > 80% of cases. The need for tracheal intubation for mechanical ventilation and death within 7 days of admission were associated with a higher body mass index (BMI), dyspnea, lymphopenia, and high alanine transaminase activity and CRP levels, but not with age, sex, long-term glucose control, and usage of different classes of medication [22]. In contrast, others have found a relationship between long-term glucose control and immune response in SARS-CoV-2 infection [35]. However, Fadini et al. reported that newly detected DM and hyperglycemia are associated with a worse COVID-19 prognosis compared with pre-existing DM. Higher glucose levels at admission were associated with COVID-19 severity, particularly in patients without pre-existing DM. Furthermore, admission glucose level correlated with most clinical severity indexes, and its relation to adverse COVID-19 outcomes was mainly mediated by a deteriorating respiratory function [36]. Katsiki et al. reported that higher rates of mortality are associated with pre-existing DM or uncontrolled hyperglycemia, while in-hospital mortality rates are increased in the case of patients with DM and DKA; also, in that case, it is important to obtain good glycemic control, reflected by a tight glycemic variation between lowest fasting blood glucose (BG) and 2 h postprandial BG [37]. Interestingly, some authors describe diabetic lung as a restrictive pulmonary disease with impairment of diffusion capacity and lung volumes attributed to the effects of DM on pulmonary function, which could, at least in part, explain why patients with DM had a worse COVID-19 prognosis [38].
The new-onset DM COVID India study [2] was conducted to better understand the differences between new-onset DM before and throughout the COVID-19 pandemic. The study reported that patients with new-onset DM during the COVID-19 pandemic had higher glycemic indices, HbA1c, and CRP levels than new-onset DM cases before the pandemic, emphasizing the effects of SARS-CoV-2 on glucose metabolism. However, researchers highlighted that there were no differences in glycemic profile or C-peptide level between new-onset DM patients during the pandemic and new-onset DM cases before the pandemic [2]. These results call attention to the implication of SARS-CoV-2 infection in metabolic parameters, leading the way to future research investigating the implied pathophysiological mechanisms.
A recent meta-analysis including 17 studies of 38,149 youths revealed a higher diabetes mellitus incidence rate (incidence rate ratio [IRR] 1.14; 95% CI 1.08–1.21) during the first year of the SARS-CoV-2 pandemic compared to the pre-pandemic period [39]. However, these data may be interpreted in the context of considerable heterogeneity, which highlights the need for future studies with homogeneous inclusion criteria, disease management, and outcome measurements.
A multicenter study conducted in the UK revealed an 80% increase in T1DM incidence between 24 March and 4 June 2020 compared to a before the COVID-19 pandemic struck. However, these results were seen in only two of five inpatient clinics and have to be interpreted in the context of a small sample size (30 patients) [40].
In comparison, a study conducted in Romania showed that the incidence of T1DM increased by 16.9% in 2020 compared to preceding years, suggesting that SARS-CoV-2 infection may represent a trigger for T1DM onset. Also, the authors took into account the seasonal distribution of new DM cases. They observed that, in 2020, the proportion of cases diagnosed between July and December (57.8%) was significantly higher (p < 0.0001) compared to the same period in previous years (51%) [41].
SARS-CoV-2 Infection: Immunological Aspects in DM Pathogenesis
Another topic that has been widely studied is the pathophysiological connection between SARS-CoV-2 infection and the type of DM. A pathway specific to T1DM is the autoimmune destruction of pancreatic β-cells, which could potentially be induced by SARS-CoV-2 [16–18]. For T2DM, different mechanisms can contribute to its development, including SARS-CoV-2 binding to ACE2 receptors present on the surface of the pancreatic islets, greater release of catecholamines and glucocorticoids, and an increased systemic inflammatory activity leading to greater insulin resistance [10].
Glucocorticoids are among the drugs most frequently used in hospitalized patients with COVID-19, especially those needing respiratory support [42]. Brooks et al. [42] conducted a systematic review to describe the management of glucocorticoid-induced hyperglycemia (GCIH). They described the triple insult in patients with severe COVID-19 treated with glucocorticoid therapy: COVID-19-induced insulin resistance, COVID-19-induced pancreatic damage, and glucocorticoid-induced hyperglycemia. However, Choi et al. [29] reported that there was even a high risk of developing DM in COVID-19 patients who were not treated with glucocorticoids, suggesting that this mechanism may act as an add-on system in the pathogenesis of new-onset DM but cannot necessarily induce DM by itself in patients with SARS-CoV-2 infection. On the other hand, it should not be forgotten that glucocorticoid therapy is used in moderate to severe COVID-19 cases, which presents a higher risk for new-onset DM [28, 29]. These results make the glucocorticoid therapy issue a multivalent factor in new-onset DM in patients diagnosed with COVID-19, highlighting the need for a more comprehensive DM diagnosis in individuals treated with glucocorticoids during SARS-CoV-2 infection. Although the prevalence of hyperglycemia is high, there are no precise guidelines for managing hyperglycemia in COVID-19 patients treated with dexamethasone. However, management should include intravenous insulin infusion for patients admitted to the intensive care unit or basal-bolus insulin therapy for patients who are not critically ill [43]. Although some non-insulin agents have been studied, they are still not recommended among inpatient-treated individuals during the acute phase of COVID-19 [42]. Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) was a randomized controlled trial studying dapagliflozin in terms of organ damage or death prevention and recovery by day 30. This trial did not demonstrate that dapagliflozin reduces the risk for organ failure or death or improves recovery, although therapy with dapagliflozin was well tolerated [43].
COVID-19 Vaccination and DM
The early development of DM symptoms and exhaustion of insulin secretion characterize some cases of new-onset T1DM after COVID-19 vaccination [44]. Sasaki et al. reported high titers of anti-GAD65 and insulin autoantibody (> 2000 U/ml and 581 NU/ml, respectively) after the second dose of Moderna COVID-19 vaccination in one patient previously diagnosed with DM but not tested for autoimmunity before vaccination [45]. Autoantibody presence in patients with disease‐susceptible haplotypes is another possible presentation, so further follow-up to establish the underlying mechanisms and careful glycemic screening are necessary in such cases [44, 45].
A study conducted in Kuwait found that patients with T2DM had lower titers of SARS-CoV-2 IgG antibodies (138 ± 59.4 BAU/ml) and neutralizing antibodies (79.7 ± 19.5%) when compared with individuals without DM (154 ± 49.1 BAU/ml for IgG antibodies, 87.1 ± 11.6% for neutralizing antibodies) after two doses of BNT162b2 (Pfizer-BioNTech, Mainz, Germany) COVID-19 mRNA vaccine. These data revealed a less profound immune response following immunization among T2DM patients [46]. In contrast, the Immune response to COVID‐19 vaccination in people with Diabetes Mellitus (COVAC-DM) study reported no difference in anti-SARS-CoV-2 antibodies between patients with T1DM, patients with T2DM, and patients without diabetes after vaccination [47]. On the other hand, age (r = − 0.45, p < 0.001) and glomerular filtration rate (r = 0.28, p = 0.001) were found to be significantly negatively associated with antibody response [47].
A scoping review including 11 studies reported consistent data with previous studies, supporting the idea that COVID-19 increased the risk for new-onset DM. Chourasia et al. suggest that the severity of SARS-CoV-2 infection may determine the risk of new-onset DM, so patients who have been vaccinated against COVID-19 can develop a milder disease form; consequently, they have a lower risk of progressing to DM [48]. Furthermore, Kwan et al. noted that even though diabetes risk was higher among unvaccinated (OR 1.78; 95% CI 1.35–2.37; P < 0.001) than vaccinated (OR 1.07; 95% CI 0.64–1.77; P = 0.80) COVID-19 patients, the interaction term between SARS-CoV-2 vaccination status and newly diagnosed DM was not statistically significant (OR 0.59; 95% CI 0.34–1.06; P = 0.08) [49]. These various data support the importance of COVID-19 vaccination in DM prevention while at the same time emphasizing the need for future investigation of the influence of SARS-CoV-2 vaccines on DM incidence.
A systematic review and meta-analysis which included over 47 million patients and eight cohort studies reported a 66% higher risk of DM in COVID-19 patients compared with the control group (without COVID-19) [50]. Despite the fact that this meta-analysis included heterogeneous studies and did not classify the type of DM (type 1 or type 2 DM) in patients with COVID-19, this study triggered a wake-up call regarding COVID-19 as a risk factor for new-onset DM [50].
COVID-19 and DM: Inflammatory Aspects
Rizvi et al. described the link between post-COVID-19 syndrome, inflammation, and DM. The authors emphasized the bidirectional relationship between COVID-19 and DM, with greater inflammation and a worse prognosis for COVID-19 observed in DM patients and poor metabolic control observed in patients with DM diagnosed with SARS-CoV-2 infection [51].
COVID-19 and Obesity: A Comprehensive Approach
In addition, a team of international experts [7] made an important statement related to the bidirectional relationship between obesity and COVID-19. The COVID-19 pandemic played a considerable role in the obesity pandemic from different points of view: it increased the incidence of psychosocial stressors and stress-related eating disorders, patients with chronic diseases had difficulties accessing healthcare services throughout the COVID-19 pandemic, restrictions made it challenging to carry out outdoor physical activity, and people had poor dietary habits while working from home [7].
COVID-19 has also been studied from the perspective of cardiometabolic health. A team of international experts and The Cardiometabolic Panel of International Experts on Syndemic COVID-19 (CAPISCO) published an extensive study on immune dysregulation in obesity and CVD, which takes place in SARS-CoV-2 infection. This paper brought to light underlying mechanisms that explain the clinical presentation of patients with COVID-19: inflammatory status, thrombosis, and adipokine dysregulation (leptin vs adiponectin) [52]. Moreover, there are data regarding vascular dysfunction that seems to be simultaneous to the acute phase of COVID-19 infection but seems to increase the global cardiovascular risk reflected by premature aging of the vasculature [53].
A team of researchers from South and East Europe, the Middle East, and Africa analyzed the management of DM and obesity during the COVID-19 pandemic. Raz et al. recommend that insulin therapy should be initiated early, especially in the acute inflammatory phase, while sodium glucose loop transporter 2 inhibitors or glucagon-like peptide 1 receptor agonists should be initiated in patients who would benefit from cardiovascular risk reduction and obesity amelioration [54]. This approach highlights the importance of a more intensive therapeutic toolbox for clinicians to properly attain disease control.
Because patients with CVD are more prone to COVID-19 infection and have a worse prognosis, lipid-lowering therapy and renin–angiotensin–aldosterone system inhibitors are important to take into consideration. Katsiki et al. emphasize that statins, by reducing serum cholesterol levels, might significantly suppress COVID-19 infection. In addition, because statins are known to stabilize atherosclerotic plaque, early initiation of statin therapy may prevent a virus-induced acute coronary syndrome [55]. A study conducted on 324 cardiac surgery patients demonstrated that early postoperative statin was associated with a lower incidence of acute kidney injury (AKI) (OR 0.32; 95% CI 0.14–0.72, p = 0.006) [56]. A combination of statins/angiotensin receptor blockers may prevent endothelial dysfunction by influencing the host response to infection, not the virus [55]. Considering the associated HTN, dyslipidemia, and obesity in patients with newly diagnosed DM during COVID-19 pandemic, it is beneficial to treat these cardiovascular risk factors with appropriate therapy, such as statins, angiotensin receptor blockers (ARB), or angiotensin-converting enzyme (ACE) inhibitors.
A mini-review evaluated the therapeutic effects of heparin and tissue-type plasminogen activator on thrombosis and hemostasis in COVID-19 patients. Mazilu et al. supported the importance of anticoagulation and fibrinolysis in patients with SARS-CoV-2 infection. This therapeutic approach would minimize the risk of acute respiratory distress syndrome and/or the necessity for ventilator support in patients admitted to intensive care units. There is still a need for a better understanding of the potential side effects of the co-administration of novel (nebulized heparin and N-acetylcysteine) and classic (molnupiravir, remdesivir) antiviral treatments for SARS-CoV-2 infection [57].
COVID-19 and DM: Future Perpectives
There is interest in a more careful understanding of new-onset diabetes after COVID-19 (NODAC), classified in addition to T1DM and T2DM, in order to search the pathophysiology, natural history, and optimal management of this condition in future studies [58].
One of the studied elements of SARS-CoV-2 infection in patients with T2DM is the angiotensin-converting enzyme 2 (ACE2) receptor [59], which binds to the spike protein of SARS-CoV-2 and is abundant in the pancreas [60]. The CAPISCO Expert Panel pointed to the latest data on the beneficial effects of SGLT-2i and GLP-1 RA in decreasing mortality in patients with T2DM and COVID-19 [61]. Researchers described some mechanisms (reducing lung inflammation, decreasing cytokine production, reducing endothelial inflammation) by which antidiabetic therapies such as metformin or GLP-1 RA can represent protective treatment choices in patients with COVID-19 and T2DM [62, 63].
Studies included in this review addressed different confounding factors such as age [25, 27, 28], sex [25, 27, 28], comorbidities such as HTN [27], and other factors that can influence the relationship between new-onset DM and COVID-19, such as glucocorticoid therapy [29, 30, 32] or the COVID-19 severity [28–30].
Limitations of our study include the heterogeneity of the included studies, in terms of the study duration, the outcome measure, and the underlying pathophysiological mechanisms studied. Also, some studies classified DM and COVID-19 diseases based on ICD-10 codes, which can be inaccurate in some cases.
Our study highlighted the high interest in this hot topic which is the link between COVID-19 and new-onset DM, along with the heterogeneity of research in this area. Future investigation should clarify the long-term effects of SARS-CoV-2 infection on metabolic control and how the epidemiology of DM would be affected. Therefore, we consider it of vital importance to establish a better knowledge of how SARS-CoV-2 may determine new-onset DM, keeping in mind the health and socio-economic burdens both diseases represent. This new COVID-19 pandemic has brought into view how vulnerable we are in the face of the unknown, but, at the same time, it has allowed us to expand our knowledge.
It remains to be established if SARS-CoV-2 infection is a trigger for DM development or if patients with hyperglycemia during SARS-CoV-2 infection were just undiagnosed cases of DM before the pandemic. For patients who survived SARS-CoV-2 infection, the management of DM may be more accessible outside of the critical situation of a pandemic with appropriate therapeutic tools and without the activation of the inflammatory cascade and the use of glucocorticoid therapy, which maintains hyperglycemia.
Conclusions
There is still much that we need to understand regarding how SARS-CoV-2 infection influences glucose metabolism. Furthermore, COVID-19 and DM share a bidirectional relationship, worsening each other’s progression.
Based on actual data, patients with SARS-CoV-2 infection and hyperglycemia should be monitored closely, considering that this category of patients is at high risk for death and long-term complications from infection.
The latest research demonstrated a higher incidence of DM after the COVID-19 pandemic, with more acute presentations, such as DKA. In addition, patients with new-onset DM present a greater hospitalization duration, including ICU admission.
These latest data support the advice that active monitoring in terms of glucose metabolism should be performed in patients who have survived SARS-CoV-2 infection.
The available data from the start of SARS-CoV-2 pandemic demonstrate that COVID-19 and DM share pathophysiologic elements, such as ACE2 receptors. Also, the chronic inflammation status present in DM may be an essential pillar of the SARS-CoV-2 inflammatory cascade, supporting the idea of a bidirectional pathophysiologic relationship between these two conditions.
Finally, COVID-19 has a role in the development of DM complications [61, 64]. First, COVID-19 can lead to acute complications like DKA in new-onset or known T1DM or even a hyperosmolar hyperglycemic state in known T2DM because of the hyperglycemic status and lack of access to the healthcare system during a critical situation. Secondly, the pandemic may aggravate the development of chronic complications of DM, taking into consideration the less frequent visits to attending physicians, the sedentary lifestyle, anxiety, and eating disorders associated with the COVID-19 pandemic. This is why we believe that a syndemic multi-disciplinary approach must be put in place [61] in order to reduce the burden of post-COVID syndrome and related complications, particularly for patients with chronic diseases such as diabetes.
Author Contributions
All authors contributed to the writing of the article and its critical revision and approved its final version. Conceptualization: Anca Pantea Stoian, Manfredi Rizzo, Wael Al Mahmeed, Khalid Al-Rasadi, Kamila Al-Alawi, Maciej Banach, Yajnavalka Banerjee, Antonio Ceriello, Mustafa Cesur, Francesco Cosentino, Alberto Firenze, Massimo Galia, Su-Yen Goh, Andrej Janez, Sanjay Kalra, Nitin Kapoor, Peter Kempler, Nader Lessan, Paulo Lotufo, Dimitri P. Mikhailidis, Luigi Nibali, Nikolaos Papanas, Tiffany Powell-Wiley, Ali A. Rizvi, Amirhossein Sahebkar, Raul D. Santos, Peter P. Toth, and Vijay Viswanathan. Wrote the manuscript: Ioana-Cristina Bica, Teodor Salmen, and Anca Pantea Stoian. Searched the literature; Ioana-Cristina Bica, Manfredi Rizzo, and Teodor Salmen. Illustrated the figures and tables: Teodor Salmen, Anca Pantea Stoian. Edited the manuscript; Ioana-Cristina Bica, Teodor Salmen, Anca Pantea Stoian. Designed and supervised the review: Anca Pantea Stoian and Manfredi Rizzo. All authors have read and agreed to the published version of the manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
All named authors confirm that they have no conflicts of interest to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Teodor Salmen made the same contribution as the first author.
The original online version of this article was revised due to update in author name.
Change history
11/15/2023
A Correction to this paper has been published: 10.1007/s13300-023-01494-2
References
- 1.Worldometer. COVID Live: coronavirus statistics. Available from: https://www.worldometers.info/coronavirus/. Cited 17 Dec 2022.
- 2.Ghosh A, Anjana RM, Shanthi Rani CS, Jeba Rani S, Gupta R, Jha A, et al. Glycemic parameters in patients with new-onset diabetes during COVID-19 pandemic are more severe than in patients with new-onset diabetes before the pandemic: NOD COVID India Study. Diabetes Metab Syndr. 2021;15:215–220. doi: 10.1016/j.dsx.2020.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salmen T, Pietroşel VA, Mihai BM, Bica IC, Teodorescu C, Păunescu H, Coman OA, Mihai DA, Pantea SA. Non-insulin novel antidiabetic drugs mechanisms in the pathogenesis of COVID-19. Biomedicines. 2022;10(10):2624. doi: 10.3390/biomedicines10102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palano MT, Cucchiara M, Gallazzi M, Riccio F, Mortara L, Gensini GF, Spinetti G, Ambrosio G, Bruno A. When a friend becomes your enemy: natural killer cells in atherosclerosis and atherosclerosis-associated risk factors. Front Immunol. 2022;12:798155. doi: 10.3389/fimmu.2021.798155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zafarani A, Razizadeh MH, Pashangzadeh S, Amirzargar MR, Taghavi-Farahabadi M, Mahmoudi M. Natural killer cells in COVID-19: from infection, to vaccination and therapy. Future Virol. 2023. 10.2217/fvl-2022-0040 [DOI] [PMC free article] [PubMed]
- 7.Kapoor N, Kalra S, Al Mahmeed W, et al. The dual pandemics of COVID-19 and obesity: bidirectional impact. Diabetes Therapy. 2022;13(10):1723–36. [DOI] [PMC free article] [PubMed]
- 8.Filippi CM, Von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008;57:2863–2871. doi: 10.2337/db07-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabiani S, Fallahi P, Ferrari SM, Miccoli M, Antonelli A. Hepatitis C virus infection and development of type 2 diabetes mellitus: systematic review and meta-analysis of the literature. Rev Endocr Metab Disord. 2018;19:405–420. doi: 10.1007/s11154-017-9440-1. [DOI] [PubMed] [Google Scholar]
- 10.Gupta RD, Atri A, Mondal S, Bhattacharjee A, Garai R, Hazra AK, et al. Characterizing progressive beta-cell recovery after new-onset DKA in COVID-19 provoked A-β+ KPD (ketosis-prone diabetes): a prospective study from Eastern India. J Diabetes Compl. 2022;36:108100. doi: 10.1016/j.jdiacomp.2021.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantea Stoian A, Pricop-Jeckstadt M, Pana A, Ileanu BV, Schitea R, Geanta M, Catrinoiu D, Suceveanu AI, Serafinceanu C, Pituru S, Poiana C, Timar B, Nitipir C, Parvu S, Arsene A, Mazilu L, Toma A, Hainarosie R, Ceriello A, Rizzo M, Jinga V. Death by SARS-CoV 2: a Romanian COVID-19 multi-centre comorbidity study. Sci Rep. 2020;10:21613. doi: 10.1038/s41598-020-78575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammar A, Brach M, Trabelsi K, Chtourou H, Boukhris O, Masmoudi L, et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 international online survey. Nutrients. 2020;12:1583. doi: 10.3390/nu12061583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zugravu C, Salmen T, Ducu I, Mihai BM, Dima V, Berceanu C, Bohiltea AT, Smaranda N, Bacalbasa N, Balescu I, Bohiltea RE. The influence of the COVID-19 pandemic on lifestyle—a pilot study. Romanian J Infect Dis. 2021;24(4):190–7.
- 14.Khunti K, Valabhji J, Misra S. Diabetes and the COVID-19 pandemic. Diabetologia. 2022;23:1–12. doi: 10.1007/s00125-022-05833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mithal A, Jevalikar G, Sharma R, Singh A, Farooqui KJ, Mahendru S, et al. High prevalence of diabetes and other comorbidities in hospitalized patients with COVID-19 in Delhi, India, and their association with outcomes. Diabetes Metab Syndr. 2021;15:169–175. doi: 10.1016/j.dsx.2020.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentile S, Strollo F, Mambro A, Ceriello A. COVID-19, ketoacidosis and new-onset diabetes: are there possible cause and effect relationships among them? Diabetes Obes Metab. 2020;22:2507–8. doi: 10.1111/dom.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghash A, Misra A. Marked hyperglycemia and ketosis in a non-obese patient with new-onset diabetes and very mild COVID-19 symptoms: a case report. Diabetes Metab Syndr. 2021;15:213–214. doi: 10.1016/j.dsx.2020.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suwanwongse K, Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: causality or coincidence? A report of three cases. J Med Virol. 2021;93:1150–1153. doi: 10.1002/jmv.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy PK, Kuchay MS, Mehta Y, Mishra SK. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of the literature. Diabetes Metab Syndr. 2020;14:1459–1462. doi: 10.1016/j.dsx.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr. 2020;14:2211–2217. doi: 10.1016/j.dsx.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sathish T, Chandrika Anton M. Newly diagnosed diabetes in patients with mild to moderate COVID-19. Diabetes Metab Syndr. 2021;15:569–571. doi: 10.1016/j.dsx.2021.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108(9):1247–1252. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cariou B, Pichelin M, Goronflot T, Gonfroy C, Marre M, Raffaitin-Cardin C, et al. Phenotypic characteristics and prognosis of newly diagnosed diabetes in hospitalized patients with COVID-19: results from the CORONADO study. Diabetes Res Clin Pract. 2021;175:108695. doi: 10.1016/j.diabres.2021.108695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qeadan F, Tingey B, Egbert J, Pezzolesi MG, Burge MR, Peterson KA, et al. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: a nationwide cohort from the US using the Cerner Real-World Data. PLoS ONE. 2022;17:e0266809. doi: 10.1371/journal.pone.0266809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sane AH, Mekonnen MS, Tsegaw MG, Zewde WC, Mesfin EG, Beyene HA, Ashine TM, Tiruneh KG, Mengistie MA. New onset of diabetes mellitus and associated factors among COVID-19 patients in COVID-19 care centers, Addis Ababa, Ethiopia 2022. J Diabetes Res. 2022;12(2022):9652940. 10.1155/2022/9652940. [DOI] [PMC free article] [PubMed]
- 26.Keerthi BY, Sushmita G, Khan EA, Thomas V, Cheryala V, Shah C, Kumar GR, Haritha V. New onset diabetes mellitus in post-COVID-19 patients. J Family Med Prim Care. 2022;11(10):5961–5968. doi: 10.4103/jfmpc.jfmpc_316_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alkhemeiri A, Al Zaabi S, Lakshmanan J, El-Khatib Z, Awofeso N. COVID-19 case management outcomes amongst diabetes and hypertensive patients in the United Arab Emirates: a prospective study. Int J Environ Res Public Health. 2022;19:15967. doi: 10.3390/ijerph192315967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naveed Z, Velásquez García HA, Wong S, et al. Association of COVID-19 infection with incident diabetes. JAMA Netw Open. 2023;6(4):e238866. doi: 10.1001/jamanetworkopen.2023.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JH, Kim KM, Song K, Seo GH. Risk for newly diagnosed type 2 diabetes mellitus after COVID-19 among Korean adults: a nationwide matched cohort study. Endocrinol Metab (Seoul). 2023;38(2):245–52. 10.3803/EnM.2023.1662. [DOI] [PMC free article] [PubMed]
- 30.Birabaharan M, Kaelber DC, Pettus JH, Smith DM. Risk of new-onset type 2 diabetes in 600 055 people after COVID-19: A cohort study. Diabetes Obes Metab. 2022;24(6):1176–9. 10.1111/dom.14659. [DOI] [PMC free article] [PubMed]
- 31.Sharma A, Misra-Hebert AD, Mariam A, Milinovich A, Onuzuruike A, Koomson W, Kattan MW, Pantalone KM, Rotroff DM. Impacts of COVID-19 on glycemia and risk of diabetic ketoacidosis. Diabetes. 2023;72(5):627–637. doi: 10.2337/db22-0264. [DOI] [PubMed] [Google Scholar]
- 32.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filip R, Anchidin-Norocel L, Gheorghita R, Savage WK, Dimian M. Changes in dietary patterns and clinical health outcomes in different countries during the SARS-CoV-2 pandemic. Nutrients. 2021;13(10):3612. doi: 10.3390/nu13103612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sathish T, Tapp RJ, Cooper ME, Zimmet P. Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes Metab. 2021;47(2):101204. doi: 10.1016/j.diabet.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulcsar KA, Coleman CM, Beck SE, Frieman MB. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4:e131774. doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fadini GP, Morieri ML, Boscari F, Fioretto P, Maran A, Busetto L, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020;168:108374. doi: 10.1016/j.diabres.2020.108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsiki N, Gómez-Huelgas R, Mikhailidis DP, Pérez-Martínez P. Narrative review on clinical considerations for patients with diabetes and COVID-19: More questions than answers. Int J Clin Pract. 2021;75(11):e14833. doi: 10.1111/ijcp.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuso L, Pitocco D, Antonelli-Incalzi R. Diabetic lung, an underrated complication from restrictive functional pattern to pulmonary hypertension. Diabetes Metab Res Rev. 2019;35:e3159. doi: 10.1002/dmrr.3159. [DOI] [PubMed] [Google Scholar]
- 39.D’Souza D, Empringham J, Pechlivanoglou P, Uleryk EM, Cohen E, Shulman R. Incidence of diabetes in children and adolescents during the COVID-19 pandemic: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(6):e2321281. 10.1001/jamanetworkopen.2023.21281. [DOI] [PMC free article] [PubMed]
- 40.Unsworth R, Wallace S, Oliver NS, Yeung S, Kshirsagar A, Naidu H, Kwong RMW, Kumar P, Logan KM. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43(11):e170–1. 10.2337/dc20-1551. [DOI] [PubMed]
- 41.Vlad A, Serban V, Timar R, Sima A, Botea V, Albai O, Timar B, Vlad M. Increased incidence of type 1 diabetes during the COVID-19 pandemic in Romanian children. Medicina (Kaunas). 2021;57(9):973. 10.3390/medicina5709097342. [DOI] [PMC free article] [PubMed]
- 42.Brooks D, Schulman-Rosenbaum R, Griff M, Lester J, Low Wang CC. Glucocorticoid-induced hyperglycemia including dexamethasone-associated hyperglycemia in COVID-19 infection: a systematic review. Endocr Pract. 2022;28:1166–1177. doi: 10.1016/j.eprac.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kosiborod MN, Esterline R, Furtado RHM, Oscarsson J, Gasparyan SB, Koch GG, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalized with COVID-19 (DARE-19): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9:586–594. doi: 10.1016/S2213-8587(21)00180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasaki H, Itoh A, Watanabe Y, Nakajima Y, Saisho Y, Irie J, et al. Newly developed type 1 diabetes after coronavirus disease 2019 vaccination: a case report. J Diabetes Investig. 2022;13:1105–8. doi: 10.1111/jdi.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki K, Morioka T, Okada N, Natsuki Y, Kakutani Y, Ochi A, et al. New-onset fulminant type 1 diabetes after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. J Diabetes Investig. 2022;13:1286–1946. doi: 10.1111/jdi.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali H, Alterki A, Sindhu S.Alahmad B, Hammad M, Al-Sabah S, et al. Robust antibody levels in both diabetic and non-diabetic individuals after BNT162b2 mRNA COVID-19 vaccination. Front Immunol. 2021;12:752233. [DOI] [PMC free article] [PubMed]
- 47.Sourij C, Tripolt NJ, Aziz F, Aberer F, Forstner P, Obermayer AM, et al. Humoral immune response to COVID-19 vaccination in diabetes is age-dependent but independent of type of diabetes and glycaemic control: the prospective COVAC-DM cohort study. Diabetes Obes Metab. 2022;24:849–858. doi: 10.1111/dom.14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chourasia P, Goyal L, Kansal D, Roy S, Singh R, Mahata I, Sheikh AB, Shekhar R. Risk of new-onset diabetes mellitus as a post-COVID-19 condition and possible mechanisms: a scoping review. J Clin Med. 2023;12:1159. [DOI] [PMC free article] [PubMed]
- 49.Kwan AC, Ebinger JE, Botting P, Navarrette J, Claggett B, Cheng S. Association of COVID-19 vaccination with risk for incident diabetes after COVID-19 infection. JAMA Netw Open. 2023;6(2):e2255965. doi: 10.1001/jamanetworkopen.2022.5596550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ssentongo P, Zhang Y, Witmer L, et al. Association of COVID-19 with diabetes: a systematic review and meta-analysis. Sci Rep. 2022;12:20191. doi: 10.1038/s41598-022-24185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizvi AA, Kathuria A, Al Mahmeed W, Al-Rasadi K, Al-Alawi K, Banach M, Banerjee Y, Ceriello A, Cesur M, Cosentino F, et al. CArdiometabolic Panel of International experts on Syndemic COvid-19 (CAPISCO). Post-COVID syndrome, inflammation, and diabetes. J Diabetes Complicat. 2022;36(11):108336. [DOI] [PMC free article] [PubMed]
- 52.Lo Presti E, Nuzzo D, Al Mahmeed W, et al. CArdiometabolic Panel of International experts on Syndemic COvid-19 (CAPISCO). Molecular and pro-inflammatory aspects of COVID-19: The impact on cardiometabolic health. Biochim Biophys Acta Mol Basis Dis. 2022;1868(12):166559. doi: 10.1016/j.bbadis.2022.166559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zanoli L, Gaudio A, Mikhailidis DP, Katsiki N, Castellino N, Lo Cicero L, Geraci G, Sessa C, Fiorito L, Marino F, et al. Methuselah Study Group. Vascular dysfunction of COVID-19 is partially reverted in the long-term. Circ Res. 2022;130(9):1276–1285. doi: 10.1161/CIRCRESAHA.121.320460. [DOI] [PubMed] [Google Scholar]
- 54.Giorgino F, Bhana S, Czupryniak L, Dagdelen S, Galstyan GR, Janež A, Lalić N, Nouri N, Rahelić D, Stoian AP, Raz I. Management of patients with diabetes and obesity in the COVID-19 era: Experiences and learnings from South and East Europe, the Middle East, and Africa. Diabetes Res Clin Pract. 2021;172:108617. [DOI] [PMC free article] [PubMed]
- 55.Katsiki N, Banach M, Mikhailidis DP. Lipid-lowering therapy and renin-angiotensin-aldosterone system inhibitors in the era of the COVID-19 pandemic. Arch Med Sci. 2020;16(3):485–489. doi: 10.5114/aoms.2020.94503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Billings FT, 4th, Pretorius M, Siew ED, Yu C, Brown NJ. Early postoperative statin therapy is associated with a lower incidence of acute kidney injury after cardiac surgery. J Cardiothorac Vasc Anesth. 2010;24(6):913–920. doi: 10.1053/j.jvca.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazilu L, Katsiki N, et al. Thrombosis and haemostasis challenges in COVID-19—therapeutic perspectives of heparin and tissue-type plasminogen activator and potential toxicological reactions—a mini review. Food Chem Toxicol. 2021;148: 111974. [DOI] [PMC free article] [PubMed]
- 58.Wihandani DM, Purwanta MLA, Mulyani WRW, Putra IWAS, Supadmanaba IGP. New-onset diabetes in COVID-19: The molecular pathogenesis. Biomedicine (Taipei) 2023;13(1):3–12. doi: 10.37796/2211-8039.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020;18(9):2128–2130. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al Mahmeed W, Al-Rasadi K, Banerjee Y, Ceriello A, Cosentino F, Galia M, Goh SY, Kempler P, Lessan N, Papanas N, Rizvi AA, Santos RD, Stoian AP, Toth PP, Rizzo M. CArdiometabolic Panel of International experts on Syndemic COVID-19 (CAPISCO). Promoting a syndemic approach for cardiometabolic disease management during COVID-19: The CAPISCO International Expert Panel. Front Cardiovasc Med. 2021;8:787761. [DOI] [PMC free article] [PubMed]
- 62.Popovic DS, Papanas N, Koufakis T, Kotsa K, Al Mahmeed W, Al-Rasadi K, et al. Glucometabolic perturbations in type 2 diabetes mellitus and coronavirus disease 2019: causes, consequences, and how to counter them using novel antidiabetic drugs. Exp Clin Endocrinol Diabetes. 2023;131(5):260–267. 10.1055/a-2019-1111. [DOI] [PubMed]
- 63.Kim SH, Arora I, Hsia DS, Knowler WC, LeBlanc E, Mylonakis E, Pratley R, Pittas AG. New-onset diabetes after COVID-19. J Clin Endocrinol Metab. 2023 doi: 10.1210/clinem/dgad284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papachristou S, Stamatiou I, Stoian AP, et al. New-onset diabetes in COVID-19: time to frame its fearful symmetry. Diabetes Ther. 2021;12:461–4. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.