Abstract
In this study, watermelon seeds (Citrullus lanatus) protein hydrolyzed (WSPH) was produced using microbial enzymes Alcalase and Protamex. Then, the effect of different concentrations of WSPH (0, 1, 2, and 3%) on the quality of the silver carp (Hypophthalmichthys molitrix) burger during refrigerated storage (4 ± 1 °C) was investigated. According to the results, WSPH by alcalase had significantly higher degree of hydrolysis and antioxidant activity (p < 0.05) and it was used for burger tests. The results showed that, the addition of WSPH was able to reduce the microbial, chemical spoilage and sensory score during 16 days compared to the control, and with increasing the concentration of WSPH, better results were observed (p < 0.05). According to the chemical, microbial and sensory indicators, WSPH at 3% could increase the shelf life of fish burgers up to 8 days compared to the control, and this treatment was within the permissible quality limit until the end of the refrigerated storage.
Graphical abstract
Keywords: Alcalase, Watermelon seed, Protomax, Lipid oxidation, Oxidative spoilage, Microbial spoilage
Introduction
Silver carp with the scientific name Hypophthalmichthys molitrix is one of the most important farmed fish in the world due to its rapid growth, the possibility of artificial reproduction and dense feeding and keeping, and its high resistance to physical and chemical factors of water. Also, this fish has a lower production cost than other farmed carp because it feeds on the primary links of the food chain (phytoplankton) (Valipour et al., 2017). The production of various fish meat products in many countries is increasing rapidly and their variety has increased a lot. Considering the rich reserves of aquatic animals, low price and high nutritional value, the production of various products from fish and aquatic animals in Iran has a high potential and a suitable position (Bahram et al., 2016; Fahim et al., 2017). A fish burger is similar to a hamburger except that fish meat is used instead of red meat. The basis of fish burger production is to somehow delay the spoilage of fish meat and create variety and produce a product that can be consumed quickly and easily. Since the dryness of the texture of fish burger will have a great impact on the attitude of consumers towards this product, the addition of improver materials will correct this defect to a large extent (Fahim et al., 2017; Hentati et al., 2019). Furthermore, due to the nutritional compounds found in fish and its products, these products are highly perishable. In addition to microbial spoilage and oxidation of lipids, mainly in processed products, increase the possibility of spoilage, which generally causes changes in the color, texture, taste and quality of food. In these products, hygiene measures should be carried out from the first stages to prevent its increase in spoilage, reduce the risk of quality loss (change in color, texture, taste and food quality) (Pezeshk et al., 2017; Shahosseini et al., 2021a). It seems required to use antioxidants and antimicrobial compounds to reduce the process of oxidation and microbial spoilage in order to increase the safety and sensory quality of burgers (Ghanbarinia et al., 2022; Sayas-Barberá et al., 2020). With the increasing awareness and willingness of consumers towards nutritional and health issues, the consumption of food containing artificial preservatives is decreasing. Therefore, it seems that in order to improve the burger texture and increase its shelf life, it is necessary to use bioactive peptides from plant sources in food formulation.
Bioactive peptides have various biological activities such as antioxidant, antimicrobial, anti-obesity, anti-diabetes and anti-blood pressure, in addition to these compounds; they also have good functional properties. Today, these proteins are used as protein supplements or substitutes in food formulations (Aydemir et al., 2022; Dorvaj et al., 2013; Elavarasan et al., 2014; Golpaigani et al., 2023; Mirzapour et al., 2022; Shahosseini et al., 2021b). Enzyme treatments are currently the most common method to modify proteins. There are different types of commercial enzymes that have been successfully used for the hydrolysis of food proteins. Despite the many industrial uses of enzymes, one of the main problems in using these compounds is the high cost of enzyme processes, and therefore cheaper proteolytic enzymes are preferred (Ovissipour et al., 2013; Shahosseini et al., 2022). Alcalase and Protamax are important enzymes that have been used in various researches (Aydemir et al., 2022; Golpaigani et al., 2023; Nemati et al., 2012, 2019; Shahosseini et al., 2022).
Watermelon (Citrullus lanatus) is a globally cultivated fruit, valued for its sweet flavor, high water content, and low calorific value (Maoto et al., 2019). In 2018, global watermelon usage stood at 166 million tons (FAOSTAT, 2020). The seed obtained from it is economically very important and is widely used in various sectors of the food industry. Therefore, watermelon seed (WS) is one of the most valuable side parts of watermelon; they are a very rich source of fat and protein (Khajavi et al., 2018; Petchsomrit et al., 2020). WS can be used as a protein source in the formulation of various foods because it is rich in protein. WSs can be used as a raw material for the production of high quality protein products in the formulation of food supplements and as functional components (Gadalkar & Rathod, 2019). WS proteins contain a variety of essential amino acids, mainly including arginine, glutamate, aspartic acid, and leucine, which can meet the needs of FAO for dietary proteins (Zhang et al., 2022). According to studies, WSs have high functional properties, antimicrobial and antioxidant activity. The use of such food products can increase the economic value of food products, especially in developing countries where protein consumption is less than optimal (Gadalkar & Rathod, 2019; Dash and Ghosh, 2017; Zhang et al., 2022).
However, based on our research, the use of hydrolyzed protein of WS (WSPH) in the formulation of fishery products has not been reported. Therefore, the purpose of this study is to investigate the effect of adding different concentrations of WSPH in silver carp fish burger formulation to improve quality and increase shelf life.
Materials and methods
Preparation of raw materials
WSs were purchased from Akhwan seed factory located in Tehran province and transported to the laboratory. Alcalase enzyme (extracted from Bacillus licheniformis) and Protamex (extracted from Bacillus subtilis) were obtained from Novozymes (Bagsvaerd, Denmark). All chemicals used in the experiment were prepared from Merck (Darmstadt, Germany), and all of them had laboratory grades.
Preparation of protein isolate from watermelon seeds
WSs were completely crushed by the mill and turned into powder. Then by adding hexane at a ratio of 10 to 1 (volume-weight), oil extraction was done. The degreasing process continued until the remaining oil was reduced below 1%. Next, the remaining solvent of meal was separated from it by a vacuum oven at a temperature of 40 °C for 24 h. The oil-free sample was then dispersed in distilled water at a ratio of 1 to 10 (weight-volume). In order to open the structure of the proteins, the pH of the mentioned solution was brought to 10 using a pH meter and 0.1 N sodium hydroxide. The solution was stirred at the specified pH and laboratory temperature for one hour. The solution was placed in a refrigerated centrifuge (Z36 HK, Labnet, Germany) for 20 min at a speed of 5000 g and a temperature of 4 °C. Then, by removing the sediment phase and bringing the pH of the solution phase to 5 using 0.1 N sodium hydroxide, the centrifugation process was repeated for 20 min at a speed of 5000 × g and a temperature of 4 °C. At this stage, the precipitate was collected and dried using an oven under vacuum at a temperature of 45 °C for 4 to 5 h. The dried protein isolate was collected in a container with a lid and kept in a dry and cool environment (Nia et al., 2018).
Hydrolyzed protein isolate of WS
For the purpose of enzymatic hydrolysis, the protein isolate was prepared (pH was adjusted using 5 mM phosphate buffer at the optimal pH value of enzyme activity for Alcalase 8.5, Protamex 7), then the enzyme (1% of the amount of protein in the isolate) WS protein was added and enzymatic hydrolysis was performed at 55 °C for 30 min in a shaker incubator (Comecta, Spain) at 200 × g. Sampling was done at time intervals of zero, 10, 20 and 30 min. Then the sample was heated at 85 °C for 15 min to inactivate the enzyme. Then, in order to remove possible sediments, the sample was centrifuged at a speed of 5000 × g for 15 min) and kept at refrigerator temperature (Ovissipour et al., 2013).
Measuring the amount of protein in WSs protein hydrolyzed (WSPH)
Based on the kjeldahl method, the samples were digested and then the total amount of precipitated protein in the aqueous phase was calculated by titration (6.25 × N) (AOAC, 2005).
Degree of hydrolysis
The amount of hydrolysis was measured with the help of trichloroacetic acid (TCA). The basis of this method was measuring the ratio of proteins soluble in 10% trichloroacetic acid to all proteins in the sample. For this purpose, 5 mL of the sample was mixed with 5 mL of 20% trichloroacetic acid and then centrifuged at 6700 × g for 10 min. Then, the amount of protein in the solution phase was measured and the amount of hydrolysis was calculated using the following formula (Nemati et al., 2019).
| 1 |
Determination of antioxidant activity
500 µL of each sample in different concentrations were mixed with 500 µl of 99.5% ethanol and 25 µl of 0.02% DPPH in 99.5% ethanol. The mixture was kept for 60 min at room temperature and in the dark, and DPPH radical scavenging was evaluated at a wavelength of 517 nm by a spectrophotometer. DPPH radical reducing activity was calculated according to Eq. (2). The control sample was prepared in a similar way, with the difference that distilled water was used instead of the sample (Bougatef et al. 2009).
| 2 |
The power of the hydrolyzed protein in the reducing of iron III was evaluated. In this test, one mL of the solution of each of the samples in different concentrations was mixed with 2.5 mL of 0.2 M phosphate buffer (pH = 6.6) and 2.5 mLs of potassium ferricyanide solution, 1%. The resulting mixture was kept in a greenhouse at 50 °C for 30 min and then 2.5 ml of 10% (TCA w/v) solution was added to it. The resulting mixture was centrifuged at 1650 g for 10 min. Finally, 2.5 mL of the resulting solution in the upper phase of the solution was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% iron chloride (w/v) solution. After 10 min, the absorption reaction of the resulting solution was read at 700 nm (Bougatef et al., 2009).
Amino acid analysis
After completing the test steps, in order to determine the profile of amino acids, the hydrolyzed protein powder was completely hydrolyzed for 24 h at 110 °C using 6 N sodium hydroxide. Then the digestion tubes were placed in the oven at 110 °C for 24 h. In this case, the volume inside the tubes is brought to 25 mL with distilled water, and then they were filtered using a 0.45-micron filter. Using o- o-phthalaldehyde (OPA) was used to derivative amino acids. The amount of total amino acids was determined using a smart line HPLC device (Germany) and using a C18 column with a fluorescent detector (RF-530) (Nemati et al., 2012).
Preparation of fish burger
Silver carp was prepared from carp breeding ponds in the suburbs of Amol city and transported to the National Aquatic Processing Research Center. The fish was caught early in the morning and transported to the Research Center with ice at a ratio of 1 to 1 at about 8 am. After washing the fish with cold water, first the head and tail, then the guts and intestines were separated and the fish was taken to the production hall for meat cutting. The fillets were turned into boneless minced meat with a deboning device (Sepamatic deboner, Germany) with a cylindrical hole diameter of 2 mm. To prepare fish burgers, minced fish meat, WSPH, along with permitted additives (Table 1) was prepared. Then, the dough prepared on each level was shaped separately by a manual kneading machine and packed in polyethylene wrappers (Fahim et al., 2017). After preparing the different treatments of burger, they were placed in refrigerated (4 ± 1 °C), and evaluated for chemical, microbial and sensory properties for 16 days at 4-day intervals.
Table 1.
Fish burger production formulation: (different treatments)
| Row of components | Compounds | T1 (%) | T2 (%) | T3 (%) | T4 (%) |
|---|---|---|---|---|---|
| 1 | Fish minced meat | 75 | 74 | 73 | 72 |
| 2 | Toasted flour | 8 | 8 | 8 | 8 |
| 3 | Salt | 1.5 | 1.5 | 1.5 | 1.5 |
| 4 | Onion | 10 | 10 | 10 | 10 |
| 5 | Garlic | 1.4 | 1.4 | 1.4 | 1.4 |
| 6 | water | 3.5 | 3.5 | 3.5 | 3.5 |
| 7 | Spices (nutmeg, thyme, pepper, cinnamon) | 1 | 1 | 1 | 1 |
| 8 | WSPH | – | 1 | 2 | 3 |
Measurement of oxidation indices
Measurement of peroxide value
The oil sample extracted from the fish burger was carefully weighed in a 250 mL Erlenmeyer with a sanding head and about 25 mL of chloroform acetic acid solution (chloroform to acetic acid ratio 2:3) was added to the contents of the Erlenmeyer. Then, 0.5 mL of saturated potassium iodide solution, 30 mL of distilled water and 0.5 mL of 1% starch solution were added to the collection and the amount of released iodine was titrated with 1% N sodium thiosulfate solution (Shahosseini et al., 2021b).
Measurement of thiobarbituric acid (TBA)
TBA was measured by colorimetric method. 200 mg of fish burger sample was transferred to a 25 mL flask and then made up to volume with 1-butanol. 5 mL of the above mixture was transferred to dry tubes with lids and 5 mL of TBA reagent was added to it (TBA reagent is obtained by dissolving 200 mg of TBA in 100 mL of 1-butanol solvent after filtering). The tubes with lids were placed in a water bath with a temperature of 95 °C for 2 h and then they were cooled at ambient temperature. Then the absorbance value (As) at 530 nm was read against the distilled water control (Ab). The amount of TBA (mg of malondialdehyde per kg) was calculated based on the following equation (Bagheri et al., 2016).
Measurement of Total Volatile Bases Nitrogen (TVB-N)
10 g of fish burger along with 2 g of magnesium oxide and 300 mL of distilled water were poured into the kjeldahl balloon, and then some glass pearls were added along with normal octane (antifoam). Next, the balloon was connected to the device and heated from below. Inside a 250 mL Erlenmeyer flask, 25 mL of 2% boric acid solution (2 g of boric acid in 100 mL of distilled water) was placed along with a few drops of methyl red reagent (0.1 g of methyl red in 100 mL of ethanol). Methyl red is red in acidic environment and yellow in alkaline environment. Distillation continued until 30 min passed from the boiling of the material in the flask, or the collection of about 125 mL of liquid in the Erlenmeyer. The boric acid solution turned yellow as soon as it was alkalized by distilled volatile nitrogen bases. The titration of this solution with 0.1 N sulfuric acid continued until the boric acid turned red again. The amount of TVB-N was obtained as milligrams per hundred grams of fish burger meat according to the following equation (Javadian et al., 2016).
Microbial tests
First, 10 g of samples were taken from each treatment using sterilized scalpel and tweezers in the presence of an alcohol lamp and a beaker containing alcohol and it was placed in 90 mL of 0.85% sterile physiological serum and homogenized for 60 s in a laboratory blender. Plate Count Agar culture medium was used for total viable count (TVC) from the prepared samples. After preparing the culture medium, 0.1 mL of the prepared samples was spread on the culture medium with a micro sampler. If the number of bacteria in a plate was high, dilution of the samples (up to log 6) was done in physiological serum solution. Cultured plates related to TVC were counted after 48 h of incubation at 35 °C (Valipour et al., 2017). Plate Count Agar medium was used to count psychrotrophic bacteria (PTC) from the prepared samples. 0.1 mL of the prepared samples was spread on the culture medium. Plates of PTC were counted after 10 days of incubation at 4 °C (Valipour et al., 2017).
Sensory test
The prepared burgers were fried for 3 min in a frying pan using common frying oil (Bahar, Tehran, Iran). Evaluation of sensory characteristics of fish burger samples was done by 10 semi-trained evaluators (5 women and 5 men, ages: 25–35 years) in terms of color, smell, crispness and overall acceptance on the first and last day of storage and by Five-point hedonic test so that a score of 5 indicates that the sample was very good and a score of 1 indicates that the sample was very bad. Shelf life criteria assumed that rejection would occur when the sensory attributes declined below 4.0 (Bahram et al., 2016).
Statistical analysis
To analyze the data, according to the normality of the data and the homogeneity of the variance, the analysis of variance (ANOVA) method was used. To compare the average data, Duncan’s test was used at the 5% level. All data were reported as mean standard deviation and all microbial and antioxidant tests were performed in 3 replicates. The software (spss version 18) was used for data analysis and Microsoft Excel 2007 was used for drawing graphs.
Results and discussion
Protein amounts in different treatments
The amount of primary protein of WS was equal to 18.66 ± 0.64% and the amount of isolated protein of WS was equal to 50.94 ± 0.75%. Also, the amounts of protein of WSPH in different treatments were between 60.63 and 90.78% (Table 2). The higher amount of protein in WSPH compared to WS isolate is due to protein breakdown during the hydrolysis process. Furthermore, centrifugation during the hydrolysis process separates the non-protein parts of the sample (Ghanbarinia et al., 2022). Gadalkar and Rathod (2019) reported that, the primary protein values of WSs and the primary protein values of defatted WSs were announced 18.72% and 54.48% respectively. Their results were consistent with the results of the present study.
Table 2.
Effect of enzyme hydrolysis type and time of watermelon seeds protein hydrolyzed (WSPH) on degree of hydrolysis and protein content
| Enzyme | Protein content (%) | Degree of hydrolysis (%) | |
|---|---|---|---|
| Type | Time (min) | ||
| Alcalase | 10 | 71.43 ± 1.90d | 15.33 ± 0.29e |
| 20 | 80.56 ± 1.46c | 21.51 ± 0.50c | |
| 30 | 90.98 ± 2.45a | 31.16 ± 0.86a | |
| Protamex | 10 | 60.63 ± 1.40e | 11.79 ± 0.38f |
| 20 | 71.24 ± 0.98d | 17.71 ± 0.58d | |
| 30 | 84.57 ± 1.57b | 23.46 ± 0.59b | |
Averages with the different letters (in same columns) indicate that there is significant difference at the P < 0.05
Evaluation of the degree of hydrolysis
The results related to the degree of hydrolysis show that the type of enzyme and the time of the process have a direct effect on the increasing trend of the degree of hydrolysis so that it increased continuously by both enzymes (Table 2) (P < 0.05). The degree of hydrolysis is significantly affected by the process time (P < 0.05), and the length of the peptide chain is inversely proportional to the degree of hydrolysis. Therefore, increasing the degree of hydrolysis shortens the length of the peptide chain and decreases the molecular weight distribution, and as a result, breaks the peptide bands and increases the free amino acids (Shahi et al., 2020). WSPH with alcalase enzyme showed a higher degree of hydrolysis in all hydrolysis times (P < 0.05). The reason for this may be expressed in the function of two enzymes in the ability of alcalase to break peptides into smaller peptides and even produce free amino acids (Mirzapour et al., 2022; Shahosseini et al., 2022).
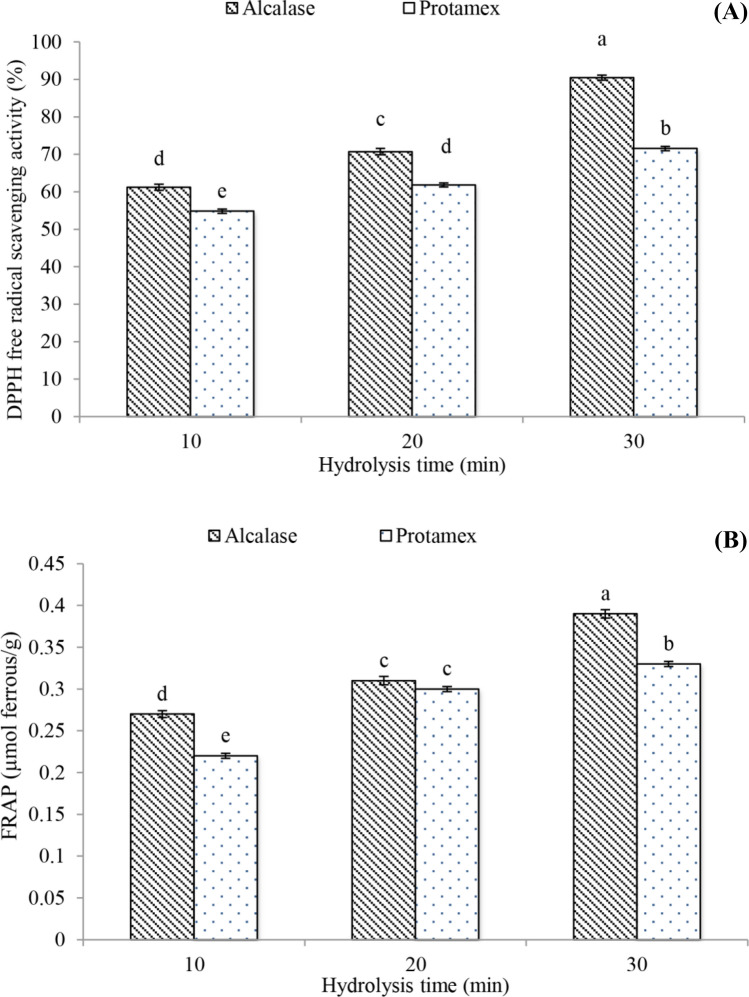
Antioxidant property
All the WSPH had a high ability to remove the DPPH free radical (Fig. 1a), and the antioxidant activity of the WSPH by the alcalase enzyme was significantly higher than that of the protamex enzyme in most times of the hydrolysis process (P < 0.05). This result may be because the WSPH by alcalase enzyme has more hydrophobic amino acid than Protamex, which helps to improve its antioxidant activity (Wen et al., 2019; Shahosseini et al., 2022), and alcalase enzyme has more hydrophobic amino acid than Protamex, which causes to improve its antioxidant activity (Wen et al., 2019; Shahosseini et al., 2022). DPPH free radical activity values increased with increasing hydrolysis time (P < 0.05) so that the WSPH by alcalase (at 30 min) had the highest antioxidant activity. Therefore, the antioxidant activity increases with the increase in the time of the hydrolysis process and following the increase in the degree of hydrolysis and the release of more active and hydrophobic peptides and amino acids, considering that the peptides and hydrophobic amino acids showed a faster reaction to DPPH radicals. Compared to hydrophilic amino acids, these compounds have a greater capacity to inhibit free radicals (Shahi et al., 2020).
Fig. 1.
Antioxidant activity of watermelon seeds protein hydrolyzed (WSPH) at different enzyme hydrolysis type and time
The ferric reducing power (Fig. 1b) of WSPH was significantly related to process parameters (enzyme type and hydrolysis time) (P < 0.05). The highest reducing power of ferric is related to a WSPH by alcalase (at 30 min), which had the highest antioxidant activity (0.39 μmol ferrous/g) (P < 0.05), while the lowest values were related to WSPH by Protamex enzyme (at time 10 min) (0.22 μmol ferrous/g) (P < 0.05). An increase in reducing power can be an indication of an increase in hydrogen or electron donation. Furthermore, with the passage of time and the increase in the degree of dehydration, the change in the size, structure, number of amino acids and peptides has an effect on the reduction of iron ions and all the mentioned factors may affect the property of metal ion reduction through hydrogen donation or electron donation. For example, hydrophobic amino acids such as histidine, proline, methionine, cysteine, tyrosine, and phenylalanine improve the antioxidant activity of peptides (Yeganeh et al., 2021; Shahosseini et al., 2022). Similar results were reported by Varedesara et al. (2021) regarding the grape seed protein hydrolyzade using alcalase enzyme.
According to the results of the degree of hydrolysis and antioxidant property, WSPH by alcalase enzyme at 30 min was used for the next tests.
Investigation of the amino acid profile
Amino acids are the building blocks of peptides and proteins, which provide basic characteristics due to the presence of carboxyl group (COOH–), properties of acids and amino group (NH2–) (Ibrahim et al., 2019). The amino acid profile of WSPH (Table 3) shows that this protein is rich in amino acids that can be presented as a useful nutritional source, and 17 amino acids out of 22 amino acids were present in it. Only the amino acid phenylalanine was present in a lower concentration compared to that reported by the FAO model (FAO, 1990). The results of the present study showed that the amount of free hydrophobic amino acids (HAA) is influenced by the type of protease. Thus, the amount of HAA in WSPH by alcalase was equal to 33.93% and for Protamex it was equal to 31.70%. In general, amino acids that have hydrophobic properties play an important role in the process of free radicals scavenging (Firmansyah & Abduh, 2019). Similar results have been reported by Mirzapour et al (2022) and Ghelich et al (2022) they demonstrated that HAA amont of canola meal and wheat germ protein hydrolysate (respectively) by alcalase was higher than other enzyme. The highest amounts of essential and non-essential amino acids for Alcalase and Protamex enzymes were leucine 7.84% and 7.05%, and glutamic acid 19.99% and 18.09%, respectively. Ibrahim et al., (2019) declared the essential amino acid values of WSs as 7.10% leucine and the highest non-essential amino acid values as 22.70% glutamic acid. Gadalkar and Rathod (2020) declared the values of essential amino acid of WSs as 7.52% leucine and the highest values of non-essential amino acid as 20.84% glutamic acid, the results of the mentioned studies were consistent with the results of our study.
Table 3.
Effect of enzyme hydrolysis type (at 30 min) of watermelon seeds protein hydrolyzed (WSPH) on amino acid composition
| Amino acid (g 100 g−1) | Alcalase | Protamex | FAO/WHO, 1990 |
|---|---|---|---|
| Threoninea | 4.70 ± 0.18a | 4.20 ± 0.06b | 3.4 |
| Valinea | 6.20 ± 0.31a | 5.40 ± 0.22b | 3.5 |
| Methioninea | 1.59 ± 0.11a | 1.05 ± 0.06b | |
| Isoleucine a | 5.70 ± 0.19a | 5.07 ± 0.09b | 2.8 |
| Leucinea | 7.84 ± 0.24a | 7.05 ± 0.21b | 6.6 |
| Phenyl alaninea | 5.60 ± 0.32a | 4.90 ± 0.11b | 6.3 |
| Histidinea | 4.88 ± 0.21a | 4.05 ± 0.09b | |
| Lysinea | 6.15 ± 0.09a | 5.87 ± 0.10b | 5.8 |
| Argininea | 10.25 ± 1.08b | 13.05 ± 1.24a | |
| Glycine | 5.99 ± 0.15a | 5.78 ± 0.12a | |
| Aspartic acid | 8.55 ± 0.09b | 9.11 ± 0.108a | |
| Glutamic acid | 19.99 ± 0.35a | 18.09 ± 0.22b | |
| Serine | 4.90 ± 0.22b | 5.78 ± 0.21a | |
| Alanine | 5.90 ± 0.09b | 6.35 ± 0.17a | |
| Tyrosine | 1.10 ± 0.14b | 1.80 ± 0.19a | 1.1 |
| Cystein | 0.1 ± 0.01a | 0.8 ± 0.02a | |
| Total amino acid | 99.44 | 98.35 | |
| HAAb | 33.93 | 31.70 |
aEssential amino acids
bCombined total of hydrophobic amino acids (HAA) = alanine, valine, isoleucine, leucine, tyrosine, phenylalanine, methionine and cysteine
Averages with the different letters (in same row) indicate that there is significant difference at the P < 0.05
According to the results, WSPH by alcalase had significantly higher degree of hydrolysis, HAA and antioxidant activity (p < 0.05) and it was used for burger tests.
Effect of WSPH on fish burger lipid oxidation
The peroxide index is one of the qualitative criteria for measuring the concentration of the primary oxidation products of fats (formation of monohydroperoxides) (Valipour et al., 2017). PV (Fig. 2a) increased significantly with increasing storage time in all samples (P < 0.05). The increase in PV in fish burgers with the increase in storage time indicates the entry of oxidative spoilage into the stage of chain reactions, which was predictable due to the composition of fish fatty acids and changes in the amount of long-chain polyunsaturated fatty acids (Hajhoseini et al., 2019). According to the obtained results, it was observed that the highest and lowest levels of fish burger PV were related to the control and the treatment containing 3% WSPH, respectively (P < 0.05). The reason for this is the antioxidant property of WSPH. Hydrolyzed proteins have peptides that are electron donors and can react with free radicals and convert them into more stable compounds. The result of this function will be to stop the oxidation chain reactions. The mechanism of peptides action as antioxidants is not clearly known, but HAA amino acids play an important role in the antioxidant activity of peptides and hydrolyzed protein (Ghanbarinia et al., 2022; Pezeshk et al., 2017). These results are consistent with the results of Ghanbarinia et al. (2022) regarding the effect of hydrolyzed protein of sesame meal on the PV of burger.
Fig. 2.
Lipid oxidation of fish burger enriched with watermelon seeds protein hydrolyzed (WSPH) during storage period
The limiting amount of fish meat for consumption in terms of PV index is 5 milliequivalents/ kg fat (Yanar, 2007). The fish burger containing 3% WSPH was healthy until the end of the storage period.
Another indicator of the progress of the oxidative reaction in protein products containing high oil is the TBA index. By measuring this index, the concentration of secondary metabolites of fat oxidation that results from the decomposition of monohydroperoxides can be measured and this reaction causes the rancidity of fats and has a direct effect on the taste of the food product (Shahosseini et al., 2021b). However, during the secondary phase of the autoxidation process other aldehydes (alkanals, 2-alkenals, dienals) are formed which react with TBA, and they are responsible for off-flavors (Guillén-Sans & Guzmán-Chozas, 1998). The values of TBA (Fig. 2b) increased significantly with increasing storage time in all samples (P < 0.05). According to the obtained results, it was observed that the highest and lowest amount of TBA of burger fish was related to the control and the treatment containing 3% WSPH, respectively (P < 0.05). The reason for this is the antioxidant property of hydrolyzed protein. HAA amino acids prevent the creation of free radicals and the propagation of free radical reactions, and this action is performed by trapping metal ions (such as iron) (Ghanbarinia et al., 2022). Similar results were observed by Mirzapour et al. (2022), they stated that the use of hydrolyzed protein of canola meal, delayed the rate of TBA in chicken nugget during the storage period.
The limiting amount of fish meat for consumption in terms of the TBA index is 2 mg of malondialdehyde/gr (Campo et al., 2006). The fish burger containing 3% WSPH was healthy until the end of the storage period.
Investigating the values of TVB-N
TVB-N is the nitrogen of protein compounds which is released as a result of the activity of proteolytic enzymes and the breakdown of protein structure. The formation of volatile nitrogen indicates the beginning of spoilage and its increase is a sign of the progress of spoilage of proteins in feed or food (Shahosseini et al., 2021b). The values of TVB-N (Fig. 3a) increased significantly with increasing storage time in all samples (P < 0.05). According to the obtained results, it was observed that the highest and lowest amount of TVB-N in burger fish were related to the control and the treatment containing 3% WSPH, respectively (P < 0.05). The creation of TVB-N compounds is mainly due to the activities of proteolytic bacteria in the product. The lower amount of TVB-N in burgers containing WSPH is probably due to the antimicrobial property of them, which affects the microbial population and the growth of bacteria. (Dash and Ghosh, 2017; Da Rocha et al., 2018). These results are consistent with the results of Ghanbarinia et al. (2022) regarding the effect of hydrolyzed protein of sesame meal on the TVB-N of burger.
Fig. 3.
Chemical and microbial parameters of fish burger enriched with watermelon seeds protein hydrolyzed (WSPH) during storage period
El-Lahamy et al. (2018) reported that the amount of TVB-N bases in very good, good and spoiled fish burgers is equivalent to 25, 30 and maximum 35 mg/g, respectively. The fish burger containing 3% WSPH was healthy until the end of the storage period.
Changes in microbiological properties
The results related to the amounts of TVC (Fig. 3b) and PTC (Fig. 3c) were consistent and increased significantly in all treatments during the 16-day storage period (P < 0.05). According to the obtained results, it was observed that the highest and lowest amount of mentioned bacteria in the fish burger was related to the control and the treatment containing 3% WSPH respectively (P < 0.05), which indicates the antimicrobial activity of the peptides. Antimicrobial activity of peptides and hydrolyzed proteins depends on the structure, length of peptides, sequence and characteristics of amino acids (Pane et al., 2017; Ghanbarinia et al., 2022). The mechanism of action of antimicrobial peptides is mainly based on the electrostatic interaction of peptides with the cell membrane of microorganisms, which many antimicrobial peptides stimulate the membrane by increasing permeability. They can enter the membrane and lead to its disruption. Furthermore, Kumar et al. (2018) stated that the antimicrobial activity of bioactive peptides may be caused by iron chelating activity.
The allowable amount of PTC and TVC is recommended for 7 log CFU/g (ICMSF, 2005). Accordingly, treatments with 3% WSPH remained healthy until the end of the storage period.
Sensory evaluation during storage
According to the Fig. 4, the addition of WSPH in fish burgers caused a decrease in the sensory scores (P < 0.05), the lowest values of the sensory score in containing 3% WSPH and the highest values of the control were observed (P < 0.05). Moreover, by increasing storage period, the sensory evaluation decreased significantly in all treatments, at the end of the storage period, the treatments containing 2 and 3% WSPH were approved by the evaluators. The improvement of sensory properties in treatments containing WSPH can be due to the antioxidant property of hydrolyzed protein, which acts as a strong anti-oxidation and reduces the oxidation of lipids, and as a result, can improve taste, texture, color and overall acceptability (Saad et al., 2021). These results are consistent with the results of Ghanbarnia et al. (2022) regarding the effect of hydrolyzed protein of sesame meal on the sensory evaluation of burger.
Fig. 4.
Sensory score of fish burger enriched with watermelon seeds protein hydrolyzed (WSPH) during storage period
All in all, the results of this research showed that the hydrolyzed protein of WSs by alcalase enzyme had significant antioxidant properties and the highest antioxidant activity was observed at the time of hydrolysis of 30 min. Furthermore, the results related to chemical and microbial indexes in the quality of fish burger showed, these indexes in the WSPH were lower than the control sample, and the best results were observed in the treatment containing 3% WSPH. All the measured factors were within the permissible range. Therefore, the WSPH can be used as a natural anti-microbial and antioxidant substance to increase the shelf life of fish burgers, so that it increases its shelf life for about 8 days under conditions of storage at refrigerator temperature. In addition, it is possible to increase the per capita consumption of meat products and improve the health of the society by producing various products with desirable and functional sensory properties (containing hydrolyzed protein).
Data availability
Data available on request from the authors.
Declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References:
- Aydemir LY, Diblan S, Aktas H, Cakitli G. Changes in bioactive properties of dry bean extracts during enzymatic hydrolysis and in vitro digestion steps. Food Measure. 2022;16:3682–3698. [Google Scholar]
- Bagheri R, Izadi Amoli R, Tabari Shahndash N, Shahosseini SR. Comparing the effect of encapsulated and unencapsulated fennel extracts on the shelf life of minced common kilka (Clupeonella cultriventris caspia) and Pseudomonas aeruginosa inoculated in the mince. Food Science and Nutrition. 2016;4(2):216–222. doi: 10.1002/fsn3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahram S, Rezaei M, Soltani M, Kamali A, Abdollahi M, Khezri Ahmadabad M, Nemati M. Effect of Whey Protein concentrate coating cinnamon oil on quality and shelf life of refrigerator beluga sturgeon (Huso huso) Journal of Food Quality. 2016;39:743–749. [Google Scholar]
- Bougatef A, Hajji M, Balti R, Lassoued I, Triki-Ellouz Y, Nasri M. Antioxidant & free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chemistry. 2009;114:1198–1205. [Google Scholar]
- Campo MM, Nute GR, Hughes SI, Enser M, Wood JD, Richardson RI. Flavour perception of oxidation in beef. Meat Science. 2006;72:303–311. doi: 10.1016/j.meatsci.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Dash P, Ghosh G. Fractionation, amino acid profiles, antimicrobial and free, radical scavenging activities of Citrullus lanatus seed protein. Natural Product Research. 2017;31(24):2945–2947. doi: 10.1080/14786419.2017.1305385. [DOI] [PubMed] [Google Scholar]
- Da Rocha M, Alemán A, Romani VP, López-Caballero ME, Gómez-Guillén MC, Montero P, Prentice C. Effects of agar films incorporated with fish protein hydrolysate or clove essential oil on flounder (Paralichthys orbignyanus) fillets shelf-life. Food Hydrocolloids. 2018;81:351–363. [Google Scholar]
- Dorvaj Z, Javadian SR, Oveissipour M, Nemati M. Use of Protein Hydrolysates From Caspian Sea Sprat (Clupeonella Cultiventris) As A Nitrogen Source For Bacteria Growth Media (Vibrio Anguillarum, Bacillus Licheniformis, Bacillus Subtilis) Journal of Aquatic Animals & Fisheries. 2013;4(15):11–18. [Google Scholar]
- El-Lahamy AA, Khalil KI, El-Sherif SA, Mahmud AA. Changes in quality attributes of sand smelt (Atherinahepsetus) fish burger and finger during frozen storage. Fisheries Research. 2018;2(2):6–11. [Google Scholar]
- Elavarasan K, Naveen Kumar V, Shamasundar BA. Antioxidant and Functional Properties of Fish Protein Hydrolysates from Fresh Water Carp (Catla catla) as Influenced by the Nature of Enzyme. Journal of Food Processing and Preservation. 2014;38(3):1207–1214. [Google Scholar]
- Fahim A, Khanipour A, Zare Gashti G, Amiri Sendsi A. Improved sensory properties and Shelf life of burger made from silver carp (Hypophthalmicthys molitrix) with using pectin. Journal of Fisheries Science and Technology. 2017;6(3):123–131. [Google Scholar]
- FAOSTAT. Statistical database. Food and Agriculture Organization of the United Nations. Available from http:// www.fao.org/faostat/en/#data/QC (last consult: 2020/25/06). (2020)
- FAO/WHO. Energy and protein requirements. Report of joint FAO/ WHO/UNU Expert Consultation Technical Report. FAO/WHO and United Nations University, Geneva, Series No. 724 (1990)
- Firmansyah M, Abduh MY. Production of protein hydrolysate containing antioxidant activity from Hermetia illucens. Heliyon. 2019;5(6):e02005. doi: 10.1016/j.heliyon.2019.e02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalkar SM, Rathod VK. Extraction of watermelon seed proteins with enhanced functional properties using ultrasound. Preparative Biochemistry & Biotechnology. 1-8 (2019) [DOI] [PubMed]
- Ghanbarinia SH, Ariaii P, Safari R, Najafian L. The effect of hydrolyzed sesame meal protein on the quality and shelf life of hamburgers during refrigerated storage. Animal Science Journal. 2022;93(1):e13729. doi: 10.1111/asj.13729. [DOI] [PubMed] [Google Scholar]
- Ghelich S, Ariaii P, Ahmadi M. Evaluation of functional properties of wheat germ protein hydrolysates and its effect on physicochemical properties of frozen yogurt. International Journal of Peptide Research and Therapeutics. 2022;28:69. [Google Scholar]
- Golpaigani MH, Ariaii P, Ahmadi, M, Safari R. Preservation effect of protein hydrolysate of rainbow trout roe with a composite coating on the quality of fresh meat during storage at 4 ± 1 °C. Food Measure. 17: 2416–2428 (2023)
- Guillén-Sans R, Guzmán-Chozas M. The Thiobarbituric Acid (TBA) reaction in foods: a review. Critical Reviews in Food Science and Nutrition. 1998;38(4):315–350. doi: 10.1080/10408699891274228. [DOI] [PubMed] [Google Scholar]
- Hajhoseini M, Hoseini S, Hoseini S. Effects of adding farm-raised beluga sturgeon by-products on some physicochemical properties, sensory and shelf life of instant soup. Journal of Food Research. 2019;29(2):121–136. [Google Scholar]
- Hentati F, Barkallah M, Atitallah AB, Dammak M, Louati I, Pierre G, Fendri I, Attia H, Michaud PH, Abdelkafi S. Quality Characteristics and Functional and Antioxidant capacities of Algae-Fortified Fish Burgers Prepared from Common Barbel (Barbus barbus) BioMed Research International. 2019;1:1–14. doi: 10.1155/2019/2907542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SE, Sulieman AME, Ali NA, Bothaina, Hakeem SA, Amin HB, Abdelmuhsin AA, Veettil VN. Amino acid profile of the watermelon, Citrullus vulgaris and detection of its antimicrobial activity. Bioscience Biotechnology Research Communications. 12(4) (2019)
- ICMSF . Microorganisms in foods 6: microbial ecology of food commodities, 2nd edn (1st edn published 1998) New York: Kluwer Academic/Plenum Publishers; 2005. [Google Scholar]
- Javadian SR, Shahoseini SR, Ariaii P. The effects of liposomal encapsulated thyme extract on the quality of fish mince and Escherichia coli O157: H7 inhibition during refrigerated storage. Journal of Aquatic Food Product Technology. 2016;26(1):115–123. [Google Scholar]
- Zhang Jixian, Wen Chaoting, Duan Yuqing, Zhang Haihui, Ma Haile. Structure and functional properties of watermelon seed protein-glucose conjugates prepared by different methods. LWT Food Science and Technology. 2022;155:113004. [Google Scholar]
- Khajavi R, Shams abadi HA, Asghari A. Evaluation some of quality characteristics during drying of watermelon seeds. Innovative Food Technologies. 2018;5(2):203–218. doi: 10.22104/jift.2017.511. [DOI] [Google Scholar]
- Kumar P, Kizhakkedathu JN, Straus SK. Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules. 2018;8(1):4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoto MM, Beswa D, Jideani AIO. Watermelon as a potential fruit snack. International Journal of Food Properties. 2019;22(1):355–370. [Google Scholar]
- Mirzapour Z, Ariaii P, Safari R, Ahmadi M. Evaluation the effect hydrolyzed Canola meal protein with composite coating on physicochemical and sensory properties of chicken nugget. International Journal of Peptide Research and Therapeutics. 2022;28:97. [Google Scholar]
- Nemati M, Javadian SR, Keshavarz M. Production of protein hydrolysates from Caspian shad (Alosa caspia) by-products using Alcalase enzyme. Journal of Marine Biology. 2019;11(43):87–95. [Google Scholar]
- Nemati M, Javadian SR, Ovissipour M, Keshavarz M. A study on the properties of alosa (Alosa caspia) by-products protein hydrolysates using commercial enzymes. World Applied Sciences Journal. 2012;18(7):950–956. [Google Scholar]
- Nia AP, Mortazavi SA, Mahoonk AS, Rad AHA, Armin M. Evaluation of the functional properties of hydrolysed protein of watermelon seeds (Citrullus lanatus) by pepsin enzyme. Journal of Innovation in Food Science and Technology. 2018;10(3):41–53. [Google Scholar]
- Ovissipour M, Rasco B, Shiroodi SG, Modanlow M, Gholami S, Nemati M. Antioxidant activity of protein hydrolysates from whole anchovy sprat (Clupeonella engrauliformis) prepared using endogenous enzymes and commercial proteases. Journal of the Science of Food and Agriculture. 2013;93:1718–1726. doi: 10.1002/jsfa.5957. [DOI] [PubMed] [Google Scholar]
- Pane K, Durante L, Crescenzi O, Cafaro V, Pizzo E, Zanfardino V, Izzo V, Donato A, Donato E. Antimicrobial potency of cationic antimicrobial peptides can be predicted from their amino acid composition: Application to the detection of “cryptic” antimicrobial peptides. Journal of Theoretical Biology. 2017;419:254–265. doi: 10.1016/j.jtbi.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Pezeshk S, Ojagh S, Rezaei M, Shabanpour B. Antioxidant and antibacterial effect of Protein Hydrolysis of Yellowfin Tuna Waste on Flesh Quality Parameters of Minced Silver Carp. Journal of Genetic Resources. 2017;3(2):103–112. [Google Scholar]
- Petchsomrit A, McDermott MI, Chanroj S, Choksawangkarn W. Watermelon seeds and peels: fatty acid composition and cosmeceutical potential. OCL. 2020;27:54. [Google Scholar]
- Rai M, Pandit R, Gaikwad S, Kövics G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. Journal of Food Science & Technology. 2016;53(9):3381–3394. doi: 10.1007/s13197-016-2318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad AM, Sitohy MZ, Ahmed AI, Rabie NA, Amin SA, Aboelenin SM, Soliman MM, El-Saadony MT. Biochemical and Functional Characterization of Kidney Bean Protein Alcalase-Hydrolysates and Their Preservative Action on Stored Chicken Meat. Molecules. 2021;26:4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalkar Sagar M, Rathod Virendra K. Extraction of watermelon seed proteins with enhanced functional properties using ultrasound. Preparative Biochemistry & Biotechnology. 2020;50:133–140. doi: 10.1080/10826068.2019.1679173. [DOI] [PubMed] [Google Scholar]
- Sayas-Barberá E, Martín-Sánchez AM, Cherif S, Ben-Abda J, Pérez-Álvarez JÁ. Effect of date (Phoenix dactylifera L) pits on the shelf life of beef burgers. Foods. 2020;9:102. doi: 10.3390/foods9010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahi Z, Sayyed-Alangi SZ, Najafian L. Effects of enzyme type and process time on hydrolysis degree, electrophoresis bands and antioxidant properties of hydrolyzed proteins derived from defatted Bunium persicum Bioss press cake. Heliyon. 2020;6:e03365. doi: 10.1016/j.heliyon.2020.e03365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahosseini SR, Javadian SR, Safari R. Evaluation of antibacterial and antioxidant activities of Liza abu viscera protein hydrolysate. Journal of Innovation in Food Science and Technology. 2021;30:123–146. [Google Scholar]
- Shahosseini SR, Safari R, Javadian SR. Evaluation antioxidant effects of Pullulan edible coating with watercress extract (Nasturtiumn officinale) on the chemical corruption of fresh beluga sturgeon fillet during storage in a refrigerator. Iranian Scientific Fisheries Journal. 2021;30(2):123–146. doi: 10.22092/ISFJ.2021.124553. [DOI] [Google Scholar]
- Shahosseini SR, Javadian SR, Safari R. Effects of molecular Weights-assisted enzymatic hydrolysis on antioxidant and anticancer activities of Liza abu muscle protein hydrolysates. International Journal for Peptide Research & Therapeutics. 2022;28:72. [Google Scholar]
- Tkaczewska J, Borawska-Dziadkiewicz J, Kulawik P, Duda I, Morawska M, Mickowska B. The effects of hydrolysis condition on the antioxidant activity of protein hydrolysate from Cyprinus carpio skin gelatin. LWT Food Science and Technology. 2020;117:108616. [Google Scholar]
- Valipour F, Ariaii P, Khademi D, Nemati M. Effect of chitosan edible coating enriched with eucalyptus essential oil and α-tocopherol on silver carp fillets quality during refrigerated storage. Journal of Food Safety. 2017;37:e12295. [Google Scholar]
- Varedesara MS, Ariaii P, Hesari J. The effect of grape seed protein hydrolysate on the properties of stirred yogurt and viability of Lactobacillus casei in it. Food Science and Nutrition. 2021;9:2180–2190. doi: 10.1002/fsn3.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C, Zhang J, Zhang H, Duan Y, Ma H. Effects of divergent ultrasound pretreatment on the structure of watermelon seed protein and the antioxidant activity of its hydrolysates. Food Chemistry. 2019;299(30):125165. doi: 10.1016/j.foodchem.2019.125165. [DOI] [PubMed] [Google Scholar]
- Yanar Y. Quality changes of hot Smoked Catfish (Clarias Gariepinus) during refrigerated storage. Journal of Muscle Foods. 2007;18:391–400. [Google Scholar]
- Yeganeh S, Esmaeili Kharyeki M, Ahmadi H. Effect of hydrolysis time on the antioxidant activity of Common carp (Cyprinus carpio) head protein hydrolysate. Iranian Scientific Fisheries Journal. 2021;29:29–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.