Abstract
Anthraquinone (anthracene-9,10-dione) is a multifaceted chemical used in the paper industry, in the production of synthetic dyes, in crop protection against birds and is released from fossil fuels. Additionally, the anthraquinone scaffold, when substituted with sugars and hydroxyl groups is found in plants as metabolites. Because of these multiple applications, it is produced on a large scale worldwide. However, its toxicological aspects have gained interest, due to the low limits in the foods defined by legislation. Worrying levels of anthracene-9,10-dione have been detected in wastewater, atmospheric air, soil, food packaging and more recently, in actual foodstuffs. Recent investigations aiming to identify the anthracene-9,10-dione contamination sources in teas highlighted the packaging, leaves processing, anthracene metabolism, reactions between tea constituents and deposition from the environment. In this context, this review seeks to highlight the uses, sources, biological effects, analytical and regulatory aspects of anthracene-9,10-dione.
Graphical Abstract

Keywords: Anthraquinone; anthracene-9,10-dione; Contaminant; Oxygenated polycyclic aromatic hydrocarbons; Toxicity
Introduction
According to European Food Safety Authority, an emerging risk results from a newly identified hazard to which significant exposure may occur, or from unexpected new or increased significant exposure and/or susceptibility to a known hazard [1]. Today, many compounds are being investigated as emerging contaminants, such as drugs, agrochemicals and substances with wide technological uses [2] and the monitoring of air, soil and water quality has become a topic of global interest [3–6].
Over the years, very little research has been done on the oxygenated polycyclic aromatic hydrocarbon, 9,10-anthraquinone (anthracene-9,10-dione, CAS: 84-65-1) present in the environment. However, more recently, its presence as a new contaminant in food has been detected, causing concern in regulatory agencies [7–11]. It is difficult to define the source of specific contaminants which accidentally emerge in foodstuffs and therefore, investigations are needed to verify such information.
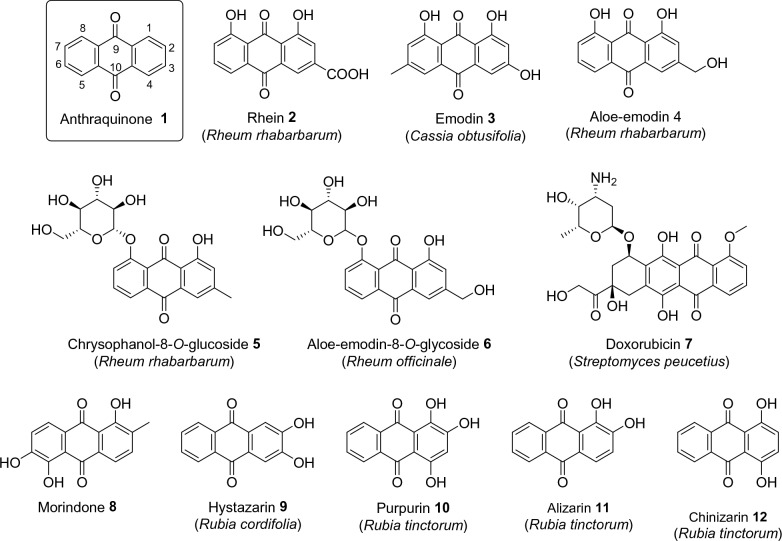
The chemical compound, anthracene-9,10-dione 1, is a rigid, planar and aromatic scaffold that has yet to be found in nature despite some of its derivatives being widely distributed among certain known plant families, such as Cassia obtusifolia and Rheum ruibarbum. In this particular group of plant metabolites, a wide structural diversity is found, so that hydroxyls and sugars are found in different positions on the aromatic rings, as showed in Fig. 1, structures 2–6 [12, 13]. Anthracene metabolites found in plants are used therapeutically as laxatives and have been extensively investigated due to their potential pharmacological effects [14, 15]. In plants, the role of anthraquinones and its glycosides include protection against insects and allelopathic effects [16]. Doxorubicin 7, which also has an anthraquinone nucleus, is an anticancer drug, produced by bacteria of the Streptomyces genus and known to bind to DNA-associated enzymes and intercalate with DNA base pairs [17]. Furthermore, Morindone 8, a natural product from Morinda citrifolia, presented an attractive starting point for potential antiproliferative agents against colorectal cancer [18].
Fig. 1.
Structure of anthracene-9,10-dione and natural anthraquinones found in plants, drugs and dyes
At the same time, as compounds with anthraquinone scaffold are found in natural products with pharmacological effects, the chemical compound 9,10-anthraquinone has also important uses. This is why it is present in the environment and in certain materials which are in direct contact with humans. In this context, the current review approaches anthracene-9,10-dione for the first time: the process whereby it is obtained, its various uses and toxicological, analytical, and regulatory aspects linked to it.
Materials and methods
All scientific publications involving the chemical compound 9,10-anthraquinone on Web of Science database were included in this review. The search was performed using the terms 9,10-anthraquinone, anthraquinone, oxygenated polycyclic aromatic hydrocarbons, oxy-PAH, oxygenated-PAH and ox-PAH. After the initial search, the articles about anthraquinones as secondary metabolites in vegetables and synthetic derivatives of the anthraquinone scaffold were excluded.
Results and discussion
Industrial production
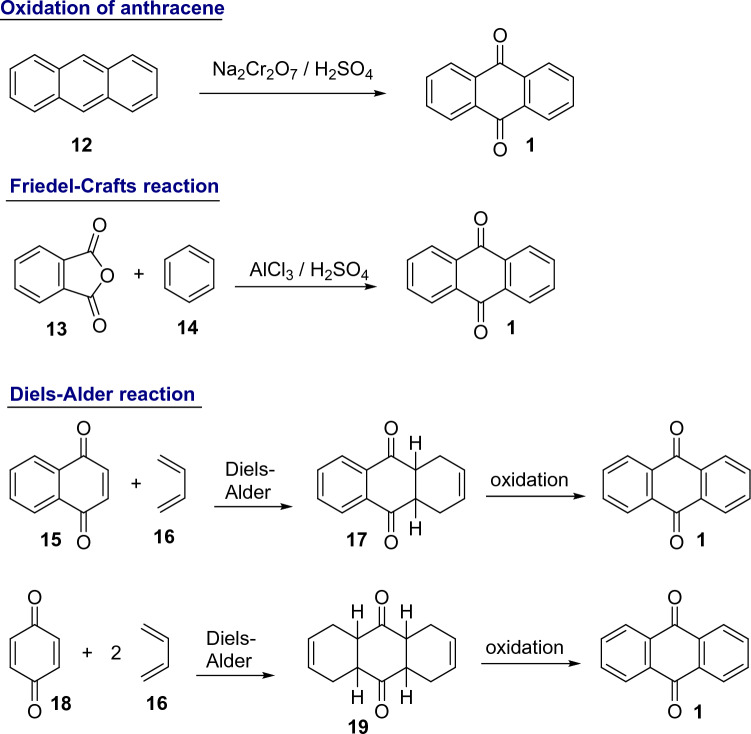
Due to its extensive use in crop protection, production of synthetic dyes and paper industry, anthracene-9,10-dione needs to be synthetically produced on a large scale. It is estimated that its production worldwide is almost 34 tons, of which more than half is produced in Western Europe [19]. The three most important synthetic approaches for anthracene-9,10-dione production are: (i) oxidation of anthracene obtained from coal tar 12, (ii) Friedel-Crafts reaction between phthalic anhydride 13 and benzene 14 to produce obenzoylbenzoic acid, which is then cyclized to anthracene-9,10-dione; and (iii) Diels-Alder reaction using naphthoquinone 15 or benzoquinone 18 as dienophile and 1,3-butadiene 16 as diene. These routes are represented in Fig. 2 [19, 20].
Fig. 2.
Industrial methods of anthracene-9,10-dione production
Anthracene-9,10-dione synthesis from anthracene was the first route, discovered in 1835 and widely explored [19]. Anthracene produced from coal and used as the starting material for anthracene-9,10-dione production, may contain toxic contaminants, particularly the mutagenic isomers of nitroanthracene. This toxicological aspect of anthracene-9,10-dione is worrying. While anthracene-9,10-dione produced by the Friedel-Crafts and Diels-Alder processes is relatively free of these polycyclic aromatic hydrocarbons and nitroanthracene contaminants, they may still be found in anthracene-9,10-dione produced using the oxidative process [21, 22].
Technological applications
Due to its use in crop protection, dye production and alkaline processing of cellulose, there is significant human exposure to anthracene-9,10-dione. Substituted derivatives of anthraquinone 9–12 (Fig. 1) obtained from natural sources or synthetic methods are used in the industrial processing of dyes. This is the second-largest class of dyes after azo dyes [23, 24]. A large proportion of the anthraquinone dyes are prepared using anthraquinonesulfonic acids and nitroanthraquinones, compounds obtained from electrophilic aromatic substitution reactions in anthraquinone scaffold [25]. In addition to anthracene-9,10-dione itself, its dye derivatives represent an important group of pollutants founds in wastewater [26].
Also, because of the limited number of chemical bird repellents available in the market, anthracene-9,10-dione is used as the active ingredient in formulations produced for this purpose. It is available in liquid form and can be used to spray on plants. One of its biological properties is its ability to repel birds, which makes it active substance usually used in biocides and plant protection products [27, 28]. Moreover, anthracene-9,10-dione is also cost effective [29]. Many countries use it in crop and seed treatments to discourage birds from eating their corn, rice, sunflower, wheat and soy cultures. Anthracene-9,10-dione is still used on plants whose aerial part is directly consumed by humans, such as lettuce and fruit crops [30]. Its efficacy as bird repellant was investigated on corn seeds against the attack of Canadian geese and Pheasants, and on sunflowers, against Red-winged Blackbirds. The concentrations used were 1.764 ppm, 9.000 ppm and 1.994 ppm respectively, leading to a repellency of 80% [28]. The treatment of maize seeds with 1% of anthracene-9,10-dione decreased its ingestion by sparrows by more than a half (14.92 ± 2.51 g for the experimental group and 35.26 ± 4.81 g for the control group). The same concentration applied to maize seedlings also decreased their ingestion: 15.58 ± 2.52 plants for the treated group and 24.31 ± 1.75 plants for the control group [31]. In addition to being non-lethal, anthracene-9,10-dione is attractive for crop protection because its concentrations as repellent cause no negative effect on seed germination [27]. It is also used in the management of birds at airports [32].
The repellent activity of anthracene-9,10-dione was also studied in small mammals, such as deer mice, ground squirrels, Californian voles, and cottontail rabbits. The results showed that anthracene-9,10-dione used on whole oats repelled feeding in these animals [33]. Anthracene-9,10-dione was used with efficacy for the protection of citrus plants against voles in California. Girdling damage to trees decreased significantly—by as much as 90–100% following a single application of anthracene-9,10-dione during two trials in summer and spring, respectively [34]. Despite extensive experimental evidence of anthracene-9,10-dione use in pest management, there are no reported studies as to the physiological mechanisms linked with its repellent effectiveness in vertebrates.
Since the 1970s, anthracene-9,10-dione has also had an important role the paper industry, where it is used in the alkaline processing of cellulose due to its capacity to improve cellulose delignification rate. In this application, anthracene-9,10-dione is added to a strong alkaline solution of sodium hydroxide and sodium sulfide and then used to separate cellulose and hemicellulose fibers, thereby degrading lignin [19, 35]. By the end of this process, the paper may contain small quantities of anthracene-9,10-dione, detectable in food packaging [36–38].
Toxicology of anthracene-9,10-dione
Despite the extensive growth in the number of chemical compounds discovered and incorporated into human life, the knowledge about their toxicity has not always grown at the same rate. As a result, there is lack of information in the literature as to the toxicodynamic aspects of anthracene-9,10-dione. Apart from a few published articles on its toxicity in vertebrates, the data available in the literature about its safety are controversial. Anthracene-9,10-dione is classified by the International Agency for Research on Cancer (IARC) as a possible carcinogen in humans (2B group). This is based on significant carcinogenic evidence in animals but only limited confirmation in humans [39].
The toxicity effect of certain quinones is well documented in the literature. Quinones 1,2-benzoquinone and 1,4-benzoquinone are metabolites originating from benzene oxidation by peroxidases in bone marrow and can cause myelosuppression [40]. Another reported toxic effect of quinones is linked with their activity in redox cycles, resulting in semiquinone radicals and leading to the formation of a reactive oxygen species [41–44]. Quinones with unsubstituted β carbons may act as Michael acceptors and cellular damage results from alkylation of cellular proteins and DNA [42, 45]. Some compounds with anthraquinone core are able to intercalate DNA due to their planarity [43]. This biological propriety has been investigated in the naturally occurring anthraquinone, morindone [46] and has been used in cancer treatment [17].
In relation to the toxicity of anthracene-9,10-dione in vertebrates, there is only one such investigation, published by the US National Program of Toxicology in 2005, that explores the chronic effect of anthracene-9,10-dione in rats. This study analyzed the exposure of rats to diary doses of up to 3.750 ppm for 2 years and identified an increase in the prevalence of urinary tract, liver and kidney tumors [47]. However, the study was flawed, since the anthracene-9,10-dione sample used in the investigation contained 9-nitroanthracene [21, 22], a compound which, as previously described, exists as an impurity of anthracene-9,10-dione industrial synthesis from anthracene oxidation.
In order to provide additional information to help interpret the results of this study, an analytical investigation was performed on samples used in biological assays. The concentration of 9-nitroanthracene present in samples used was 1.200 ppm and they still contained a phenanthrene (polycyclic aromatic hydrocarbon) level of 200 ppm. Chromatographic analysis showed absence of these compounds, in both samples purified using ethanol recrystallization and anthracene-9,10-dione samples obtained from Friedel-Crafts and Diels-Alder reactions [21].
Further research discovered that anthracene-9,10-dione obtained from anthracene oxidation without previous purification was mutagenic in a dose-dependent manner in E. coli Ames testing on strains TA98, TA100 and TA1537. In contrast, no such effect was observed in the purified sample. The same researchers tested anthracene-9,10-dione produced by the Friedel-Crafts and Diels-Alder processes and found that these samples were negative for mutagenicity [21]. Further investigations by the same research group reported a mutagenic effect of 9-nitroanthracene on tester strains, TA98 and TA100 without S9 microsomal activation, with the lowest observed effect level being 0.30 and 10 µg/plate, respectively. The 9-nitroanthracene also exhibited mutagenic activity in the L5178Y mouse lymphoma cell assay in the presence of S9, beginning with doses as low as 5 µg/mL [22]. These findings support those of the National Program of Toxicology’s anthracene-9,10-dione sample whose mutagenic tendency was said to be attributable to 9-nitroanthracene, the major contaminant found in the sample, according to chromatographic analysis.
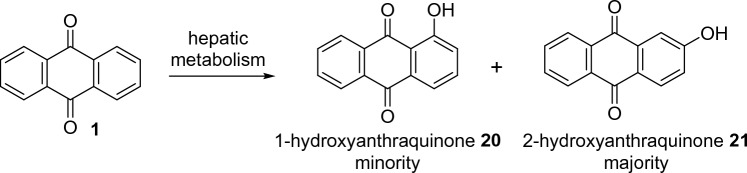
It should be noted that even if anthracene-9,10-dione is proven to be harmless, its metabolism requires attention. Anthracene-9,10-dione metabolism includes hepatic phase I oxidation reactions involving hydroxylation on positions C1 and C2, yielding 1-hidroxyanthraquinone 20 and 2-hidroxy anthraquinone 21 as its major urinary metabolites, as depicted in Fig. 3 [47]. These two metabolites exhibited only weak mutagenic activity in strains of tester bacteria and required S9 microsomal activation (1-hidroxyanthraquinone was a weak mutagen in the TA1537 strain, while 2-hidroxyanthraquinone presented a weak mutagenic response in TA100 and TA1537 strains) [22]. Another source of compound 20 in environment may be the result of a reaction between anthracene-9,10-dione, present as a contaminant in atmospheric air with hydroxyl radicals [48].
Fig. 3.
Anthracene-9,10-dione hepatic metabolism
Regarding the phase 2 reactions involved with anthracene-9,10-dione metabolism, there are not available data in the literature. Phase 2 reactions of glucuronidation, are one of the main metabolic reactions of anthraquinones isolated from vegetal sources [49]. Another possible metabolism for anthracene-9,10-dione, is the opening quinone ring reaction, yielding phthalates, which were reported in plants [50] and fungi [51].
Another investigation assessed the occupational exposure to anthracene-9,10-dione by workers subjected to road traffic pollution. Exposure to anthracene-9,10-dione and polycyclic aromatic hydrocarbons originating from traffic emissions increased the urinary excretion of 8-hydroxy-2’-deoxyguanosine in study participants [52]. The 8-hydroxy-2’-deoxyguanosine has been widely used as a biomarker for oxidative stress and carcinogenesis and its production is associated with increased oxidative damage in DNA [53].
Considering the effect of anthracene-9,10-dione on other organisms, an investigation highlighted the absence of toxicity on red swamp crayfish (Procambarus clarkia). The crayfish species is often associated with rice cultivation in the USA. Rice seeds treated with 1.76% wt did not result in acute toxicity (96 h) of anthracene-9,10-dione in juvenile crayfish through their ingestion. A 96-h aquatic acute toxicity test showed that the LC50 of anthracene-9,10-dione in juvenile crayfish was > 85 µg·L−1, a value above the water solubility limit for anthracene-9,10-dione. This finding suggests that the use of anthracene-9,10-dione in rice seeds produces low environmental impact on investigated organisms [54]. Anthracene-9,10-dione was also one of the less toxic oxygenated polycyclic aromatic hydrocarbons on Daphnia magna with an EC50 value of 1.108 nM, compared with their derivative 1,4-dihydroxyanthraquinone, that presented 122 nM as EC50 value [55]. Anthracene-9,10-dione was identified as the major photomodification product of anthracene and showed IC50 of 750 µg/L (3.60 µM) on green alga Scenedesmus vacuolatus [56].
Sources of anthracene-9,10-dione in the environment
Due to the potential risk to health, monitoring and measuring anthracene-9,10-dione levels are of crucial importance for ensuring the safety of the environment and various products. Investigations showed the presence of anthracene-9,10-dione as contaminant in stream water [57], coastal water [58], river sediments [59], soil [60, 61], atmospheric air [62–66] and food packaging [36, 37, 67]. While its presence in waste may be associated with effluents of industrial activities and its use in agriculture, the presence of anthracene-9,10-dione in atmospheric air is most likely linked to pollution caused by diesel and gasoline vehicles [62]. Anthracene-9,10-dione was identified as the most abundant oxygenated polycyclic aromatic hydrocarbon in a road tunnel in China [65] and amongst the most abundant in the atmospheric air of urban regions in Central Europe [68].
Anthracene-9,10-dione and numerous oxygenated polycyclic aromatic hydrocarbons have also been shown to form and accumulate after remediation of soils contaminated by polycyclic aromatic hydrocarbons. This chemical transformation produces contaminants with higher polarity, which are more likely to be transported in an aqueous phase [42]. An investigation highlighted that anthracene metabolism by living organisms found in soil is a source of anthracene-9,10-dione [60]. It is also found in river sediment where there is high correlation (r = 0.9166) between its concentration and the level of anthracene [59]. A high correlation was also reported between oxygenated polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbons in soils [61].
Sources of anthracene-9,10-dione in foods
Anthracene-9,10-dione in foods may be considered a new contaminant with poorly defined sources. In addition to its presence in the environment, anthracene-9,10-dione has also been identified in certain types of food packaging [36, 37, 67], another potential source of human exposure. Packaging products may contain traces of anthracene-9,10-dione through cellulose treatment [35], where the foods are affected by the migration of contaminants from the packaging materials [69]. Considering this possibility, the presence of anthracene-9,10-dione in food packaging is worrying, since about 1/3 of all industrialized foods are initially packaged in paper [70]. Investigations assessed anthracene-9,10-dione levels in pizza and chicken boxes and also tea bags [37] as well as a wide variety of packing materials [36, 67].
This emerging problem has promoted the appearing of theories about anthracene-9,10-dione source in foods. Recent investigations have attempted to identify anthracene-9,10-dione in teas, mainly from Camellia sinensis [7–11]. In relation to the origins of anthracene-9,10-dione in camellia teas, this contaminant was not found in non-processed plant material and only accumulated during leaf processing, suggesting that smoke exposure from the processing equipment may be the contamination source [9].
An investigation identified high uptake of anthracene from hydroponic solutions to Camellia sinensis roots with low translocation to stems and leaves. The same investigation found that anthracene main metabolites are anthracene-9,10-dione and anthrone. This study monitored the anthracene uptake, translocation and metabolism during 30 days, however the authors considered that continuous exposure to anthracene for long periods of time may be a source of anthracene-9,10-dione in tea leaves because tea plants were perennial crops [50].
Another plant used in the beverages preparation in which this contaminant was found is yerba-mate (unpublished data). Industrial yerba-mate processing involves different phases, including roasting, drying and trituration, in which smoke exposure is also present. During roasting, yerba-mate leaves are directly exposed to high temperatures (500 ºC) and smoke produced by wood combustion. In this step the contamination of the leaves with polycyclic aromatics hydrocarbons may occur, which may produce anthracene-9,10-dione when their oxidation occurs. The effect of yerba-mate leaves processing on chemical composition of beverages is a well-defined fact in literature [71–74]; however studies are necessary to identify the processing effect on anthracene-9,10-dione levels.
Another theory considers that endogenous anthracene-9,10-dione formation in tea plants form the Diels-Alder reaction between crotonaldehyde with benzoquinone (obtained from hydroquinone oxidation) precursors that can be present in teas. According to this model, anthracene-9,10-dione is a process-induced toxicant in tea. The same investigation showed that when commercial teas were heated at 60 °C for 72 h, they significantly increased the amount of anthracene-9,10-dione [75].
In a field-controlled experiment, Camellia sinensis plants were treated with anthracene-9,10-dione by pulverization. The two main findings of this study are the half-life of anthracene-9,10-dione permanency in the shoots of 3.7 days, and a decrease in its concentration during tea processing steps [7].
Analytical aspects
Knowledge gaps concerning the occurrence of anthracene-9,10-dione in the environment arise due to limited methodologies for its measurement. The available analytical methods for the measuring of anthracene-9,10-dione are mostly by gas chromatography coupled with mass spectrophotometry, as may be observed in Table 1. A small number of investigations used liquid chromatography methods such as HPLC-UV [8, 38, 76] or UHPLC-MS/MS [77] for anthracene-9,10-dione quantification.
Table 1.
Recent analytical determinations of anthracene-9,10-dione in different samples
| Samples | Range | Methods | References | |
|---|---|---|---|---|
| Food packing | Chicken and pizza package | 0.17–0.23 µg·g−1 | GC-MS | [37] |
| Tea bags | 0.15–0.18 µg·kg−1 | CG-MS | [37] | |
| Food package | 0.015–26.5 mg·kg−1 | GC-MS | [67] | |
| Food contact paper | 0.062 -1.2 mg·kg−1 | GC-MS | [36] | |
| Paper and cardboard | 1.07–66 mg·kg−1 | HPLC-MS | [38] | |
| Foods | Mussel tissue | 180.8 µg·kg−1 | GC-MS | [62] |
| Black tea | 0–0.44 µg·L−1 | HPLC-UV | [8] | |
| Black tea | 0.03 mg·kg−1 | GC-MS | [9] | |
| Green tea | 0.05 mg·kg−1 | GC-MS | [9] | |
| Black tea | 0–199.0 µg·kg−1 | GC-MS | [11] | |
| Tea (camelia and others) | 5.1–18.8 µg·kg−1 | GC-MS | [10] | |
| Lettuce | < 0.055 µg·g−1 | HPLC-UV | [76] | |
| Water air and soil | Stream water (EUA) | 0–0.066 µg·L−1 | GC-MS | [57] |
| Coastal waters (Japan) | 3.9–200 ng·L−1 | GC-MS | [58] | |
| River sediment (Czech Republic) | 7.9–149.1 ng·g−1 | GC-MS | [59] | |
| Soil 0–10 cm depth (Uzbekistan) | 15–959 ng·g−1 | GC-MS | [61] | |
| Soil 10–20 cm depth (Uzbekistan) | 12–315 ng·g−1 | GC-MS | [61] | |
| Air particulate phase (Czech Republic) | 4.3–37 ng·m−3 | GC-MS | [68] | |
| Air gas phase (Czech Republic) | 3.2–14 pg·m−3 | GC-MS | [68] | |
| Air particulate phase (China) | ||||
| Road tunnel entrance (day) | 1.63 ± 0.96 ng·m−3 | GC–MS | [65] | |
| Road tunnel exit (day) | 4.26 ± 1.99 ng·m−3 | GC–MS | [65] | |
| Road tunnel entrance (night) | 1.53 ± 1.12 ng·m−3 | GC–MS | [65] | |
| Road tunnel exit (night) | 3.18 ± 2.11 ng·m−3 | GC–MS | [65] | |
| Air particulate phase (China) | ||||
| Without traffic restriction | 0.92–1,92 pg·m−3 | GC–MS | [66] | |
| With traffic restriction | 0.25–1.38 pg·m−3 | GC–MS | [66] | |
| Air particulate phase (French) | 0.15–9.93 ng·m−3 | GC-MS | [63] | |
| Air gas phase (French) | 0.00–0.06 ng·m−3 | GC-MS | [63] | |
| Air particulate phase | 0.09–4.11 ng·m−3 | GC-MS | [64] | |
| Urban dust | 1.60 mg·kg−1 | GC-MS | [62] | |
| Diesel particulate | 47.70 mg·kg−1 | GC-MS | [62] | |
| Diesel extract | 5.23 mg·kg−1 | GC-MS | [62] | |
Regulatory aspects
The presence of anthracene-9,10-dione, which was previously neglected, is gaining attention. Despite this fact, according to the classification by the International Agency for Research on Cancer, anthracene-9,10-dione belongs to group 2B, defined as possibly carcinogenic to humans due to the existence of limited evidence as to its carcinogenicity [39].
The German Federal Institute for Risk Assessment defined the value of 0.01 mg·kg−1 as the maximum limit for anthracene-9,10-dione in products exported to Germany [78], while European Union Legislation defined the limit range from 0.01 to 0.02 mg·kg−1, see Table 2 [79]. These maximum values have caused restrictions on yerba-mate exported from South America to European countries, such as Germany. Another current issue was the detection, by European laboratories in 2011, of anthracene-9,10-dione in black tea imported from China, India, and Sri Lanka [8]. Due to its detection, the European Union has set a maximum residue limit of 0.02 mg·kg−1 for tea in Europe [79]. It is important to note that currently no Codex maximum residue limits are available for this active substance [80].
Table 2.
Anthracene-9,10-dione maximum limits defined by European Union
| Grups | Products | mg·kg−1 |
|---|---|---|
| Fruit fresh or frozen nuts | Citrus fruit, pome fruit, stone fruit, berries and small fruit, miscellaneous fruit, | 0.01 |
| Tree nuts | 0.02 | |
| Vegetables fresh or frozen | Root and tuber vegetables, bulb vegetables, fruiting vegetables, brassica vegetables, legume vegetables (fresh), stem vegetables (fresh), fungi, sea weeds | 0.01 |
| Leaf vegetables and fresh herbs | 0.01–0.02 | |
| Pulses, dry | Beans, lentils, peas, lupins and others | 0.01 |
| Oilseeds and oilfruits | Oilseeds, oilfruits | 0.02 |
| Cereals | Barley, buckwheat, maize, millet, oats, rice, rye, sorghum, wheat and others | 0.01 |
| Tea, coffee, herbal infusions and cocoa | Tea, coffee, herbal infusions (dried), cocoabeans (fermented or dried), carob (st johns bread), | 0.02 |
| Hops | Hops (dried), | 0.02 |
| Spices | Seeds, fruits and berries, bark, roots or rhizome, buds, flower stigma, aril | 0.02 |
| Sugar plants | Sugar beet (root), sugar cane, chicory roots and others | 0.01 |
| Products of animal origin-terrestrial animals | Swine tissue, bovine, sheep, goat, horses, asses, mules or hinnies, poultry-chicken, geese, duck, turkey and guinea fowl-, ostrich, pigeon and others farm animals, milk, bird eggs, amphibians and reptiles (frog legs, crocodiles), snails and other terrestrial animal products | 0.01 |
| Honey (royal jelly, pollen, honey comb with honey) | 0.02 |
Final remarks
Anthracene-9,10-dione is obtained in ton scale due to its use in the paper industry, in the production of synthetic dyes, in crop protection against birds. Its presence is ubiquitous as contaminant in air, water, food packaging and, more recently, its presence in foodstuffs has gained attention. Its occurrence in the environment may be linked to numerous sources: paper processing, crop protection and dye production as well as its release from fossil fuels. Though anthracene-9,10-dione occurred globally in foods and in the environment, the origin of contamination in foods remains unclearcurrently.
GC-MS-based techniques are still the most widely used for anthracene-9,10-dione quantification in foods and in the environment. Despite its increasing contact with human life, toxicological investigations on the effects of anthracene-9,10-dione on health are absent. Hence, due to the existence of numerous routes of exposure to anthracene-9,10-dione and the absence of available toxicological information as to its effects on human health, there is an increasing need to monitor its level in both the environment and various food products. Currently, more investigations are necessary, mainly about the contamination sources of anthracene-9,10-dione in foods, considering that its presence may be linked with chemical changes during the processing or external contamination. The questionable results about toxicological investigations of anthracene-9,10-dione in mammalians are another query to be searched.
Acknowledgements
The authors thanks URI – Erechim, National Council for Scientific and Technological Development (CNPq - grant number 421630/2022-1), Coordination of Superior Level Staff Improvement (CAPES – grant number 88881.710370/2022-01) and Foundation for the Support of Research in the State of Rio Grande do Sul (FAPERGS), all from Brazil, for their financial support.
Funding
The authors have not disclosed any funding.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alice Teresa Valduga and Itamar Luís Gonçalves contributed equally to this work.
Contributor Information
Alice Teresa Valduga, Email: valice@uricer.edu.br.
Itamar Luís Gonçalves, Email: itamar3141@yahoo.com.br.
References
- 1.EUROPEAN FOOD Safety Authority. Emerging risks. https://www.efsa.europa.eu/en/topics/topic/emerging-risks. Accessed 4 Sept 2023
- 2.Sauvé S, Desrosiers M. A review of what is an emerging contaminant. Chem Cent J. 2014;8:15–15. doi: 10.1186/1752-153x-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsunousi AAM, Sevik H, Cetin M, Ozel HB, Ozel HU. Periodical and regional change of particulate matter and CO2 concentration in misurata. Environ Monit Assess. 2021;193:707. doi: 10.1007/s10661-021-09478-0. [DOI] [PubMed] [Google Scholar]
- 4.Cetin M, Onac AK, Sevik H, Sen B. Temporal and regional change of some air pollution parameters in bursa. Air Qual Atmos Health. 2019;12:311–316. doi: 10.1007/s11869-018-00657-6. [DOI] [Google Scholar]
- 5.Cetin M, Aljama AMO, Alrabiti OBM, Adiguzel F, Sevik H. Zeren Cetin, I. determination and mapping of regional change of pb and cr pollution in ankara city center. Water Air Soil Pollut. 2022;233:163. doi: 10.1007/s11270-022-05638-1. [DOI] [Google Scholar]
- 6.Cetin M. The effect of urban planning on urban formations determining bioclimatic comfort area’s effect using satellitia imagines on air quality: a case study of bursa city. Air Qual Atmos Health. 2019;12:1237–1249. doi: 10.1007/s11869-019-00742-4. [DOI] [Google Scholar]
- 7.Wang X, Zhou L, Luo F, Zhang X, Sun H, Yang M, Lou Z, Chen Z. 9,10-anthraquinone deposit in tea plantation might be one of the reasons for contamination in tea. Food Chem. 2018;244:254–259. doi: 10.1016/j.foodchem.2017.09.123. [DOI] [PubMed] [Google Scholar]
- 8.Yusiasih R, Pitoi MM, Ariyani M, Koesmawati TA, Maulana H. Anthraquinone in indonesian infusion tea: analysis by hplc–uv and risk assessment. Chem Biol Technol Agric. 2019;6:19. doi: 10.1186/s40538-019-0155-2. [DOI] [Google Scholar]
- 9.Anggraini T, Neswati; Nanda RF, Syukri D. Identification of 9,10-anthraquinone contamination during black and green tea processing in indonesia. Food Chem. 2020;327:1–5. doi: 10.1016/j.foodchem.2020.127092. [DOI] [PubMed] [Google Scholar]
- 10.Díaz-Galiano FJ, Murcia-Morales M, Gómez-Ramos MdM, Ferrer C, Fernández-Alba AR. Presence of anthraquinone in coffee and tea samples. An improved methodology based on mass spectrometry and a pilot monitoring programme. Anal Methods. 2021;13:99–109. doi: 10.1039/d0ay01962c. [DOI] [PubMed] [Google Scholar]
- 11.Kartasasmita RE, Kurniawan F, Amelia T, Dewi CM, Harmoko H, Pratama Y. Determination of anthraquinone in some indonesian black tea and its predicted risk characterization. ACS Omega. 2020;5:20162–20169. doi: 10.1021/acsomega.0c01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans WC. Trease and evans’ pharmacognosy e-book. Elsevier Health Sciences. 2009 doi: 10.1016/b978-0-7020-2933-2.00055-1. [DOI] [Google Scholar]
- 13.El-Kashak WA, Elshamy AI, Mohamed TA, El Gendy AE-NG, Saleh IA, Umeyama A. Rumpictuside a: unusual 9,10-anthraquinone glucoside from rumex pictus forssk. Carbohydr Res. 2017;448:74–78. doi: 10.1016/j.carres.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Li P, Lu Q, Jiang W, Pei X, Sun Y, Hao H, Hao K. Pharmacokinetics and pharmacodynamics of rhubarb anthraquinones extract in normal and disease rats. Biomed Pharmacother. 2017;91:425–435. doi: 10.1016/j.biopha.2017.04.109. [DOI] [PubMed] [Google Scholar]
- 15.Duval J, Pecher V, Poujol M, Lesellier E. Research advances for the extraction, analysis and uses of anthraquinones: a review. Ind Crops Prod. 2016;94:812–833. doi: 10.1016/j.indcrop.2016.09.056. [DOI] [Google Scholar]
- 16.Cheng F, Cheng Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front Plant Sci. 2015;6:1020–1020. doi: 10.3389/fpls.2015.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI, Doxorubicin The good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 18.Chee CW, Zamakshshari NH, Lee VS, Abdullah I, Othman R, Lee YK, Mohd Hashim N. Nor Rashid, N. Morindone from morinda citrifolia as a potential antiproliferative agent against colorectal cancer cell lines. PLoS ONE. 2022;17:e0270970. doi: 10.1371/journal.pone.0270970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel A (2012) Anthraquinone. In: Elvers B (ed) Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, pp 503–5011
- 20.Cofrancesco AJ (2000) Anthraquinone. In: Kirk-othmer encyclopedia of chemical technology. John Wiley & Sons, Inc. 10.1002/0471238961.0114200803150618.a01
- 21.Butterworth BE, Mathre OB, Ballinger K. The preparation of anthraquinone used in the national toxicology program cancer bioassay was contaminated with the mutagen 9-nitroanthracene. Mutagenesis. 2001;16:169–177. doi: 10.1093/mutage/16.2.169. [DOI] [PubMed] [Google Scholar]
- 22.Butterworth BE, Mathre OB, Ballinger KE, Adalsteinsson O. Contamination is a frequent confounding factor in toxicology studies with anthraquinone and related compounds. Int J Toxicol. 2004;23:335–344. doi: 10.1080/10915810490517072. [DOI] [PubMed] [Google Scholar]
- 23.Walker G, Weatherley L. Biodegradation and biosorption of acid anthraquinone dye. Environ Pollut. 2000;108:219–223. doi: 10.1016/s0269-7491(99)00187-6. [DOI] [PubMed] [Google Scholar]
- 24.Li H-h, Wang Y-t, Wang Y, Wang H-x, Sun K-k, Lu Z. -m. bacterial degradation of anthraquinone dyes. J Zhejiang University-SCIENCE B. 2019;20:528–540. doi: 10.1631/jzus.b1900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bien HS, Stawitz J, Wunderlich K (2000) Anthraquinone dyes and intermediates. In: Elvers B (ed) Ullmann’s encyclopedia of industrial chemistry. John Wiley & Sons, Inc. 10.1002/14356007.a02_355
- 26.Berkessa YW, Yan B, Li T, Jegatheesan V, Zhang Y. Treatment of anthraquinone dye textile wastewater using anaerobic dynamic membrane bioreactor: performance and microbial dynamics. Chemosphere. 2020;238:124539. doi: 10.1016/j.chemosphere.2019.124539. [DOI] [PubMed] [Google Scholar]
- 27.Werner SJ, Linz GM, Carlson JC, Pettit SE, Tupper SK, Santer MM. Anthraquinone-based bird repellent for sunflower crops. Appl Anim Behav Sci. 2011;129:162–169. doi: 10.1016/j.applanim.2010.11.010. [DOI] [Google Scholar]
- 28.Werner SJ, Carlson JC, Tupper SK, Santer MM, Linz GM. Threshold concentrations of an anthraquinone-based repellent for canada geese, red-winged blackbirds, and ring-necked pheasants. Appl Anim Behav Sci. 2009;121:190–196. doi: 10.1016/j.applanim.2009.09.016. [DOI] [Google Scholar]
- 29.Curtis PD, Wise KL, Cummings J, Gabriel AD, Ganoe K, Miller JJ, Hunter ME, O’Neil KA, Lawrence JR, Cerosaletti PE, et al. Field evaluation of anthraquinone treatment to reduce corn seedling damage by birds. Crop Prot. 2019;123:59–62. doi: 10.1016/j.cropro.2019.05.021. [DOI] [Google Scholar]
- 30.DeLiberto ST, Werner SJ. Review of anthraquinone applications for pest management and agricultural crop protection. Pest Manag Sci. 2016;72:1813–1825. doi: 10.1002/ps.4330. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad S, Saleem Z, Jabeen F, Hussain B, Sultana T, Sultana S, Al-Ghanim KA, Al-Mulhim NMA, Mahboob S. Potential of natural repellents methylanthranilate and anthraquinone applied on maize seeds and seedlings against house sparrow (passer domesticus) in captivity. Brazilian J Biology. 2018;78:667–672. doi: 10.1590/1519-6984.171686. [DOI] [PubMed] [Google Scholar]
- 32.Clark L, Avery ML (2013) Effectiveness of chemical repellents in managing birds at airports. In: DeVault TL, Blackwell BF, Belant JL (eds) Wildlife in airport environments: preventing animal-aircraft collisions through science-based management. Johns Hopkins University Press & The Wildlife Society, Baltimore, pp 25–35. 10.1002/jwmg.735
- 33.Werner SJ, DeLiberto ST, Baldwin RA, Witmer GW. Repellent application strategy for wild rodents and cottontail rabbits. Appl Anim Behav Sci. 2016;185:95–102. doi: 10.1016/j.applanim.2016.10.008. [DOI] [Google Scholar]
- 34.Baldwin RA, Meinerz R, Witmer GW, Werner SJ. The elusive search for an effective repellent against voles: an assessment of anthraquinone for citrus crops. J Pest Sci. 2018;91:1107–1113. doi: 10.1007/s10340-018-0979-8. [DOI] [Google Scholar]
- 35.Hart PW, Rudie AW (2014) Anthraquinone-a review of the rise and fall of a pulping catalyst. Tappi J 13:23–31. 10.32964/tj13.10.23
- 36.Vavrouš A, Vápenka L, Sosnovcová J, Kejlová K, Vrbík K, Jírová D. Method for analysis of 68 organic contaminants in food contact paper using gas and liquid chromatography coupled with tandem mass spectrometry. Food Control. 2016;60:221–229. doi: 10.1016/j.foodcont.2015.07.043. [DOI] [Google Scholar]
- 37.Yang K-R, Seo HS, Lee YS, Choi MH, Hong J. A ht column gc/ms method for the determination of anthraquinone and its toxic impurities in paper products. Anal Methods. 2015;7:6060–6065. doi: 10.1039/c5ay01106j. [DOI] [Google Scholar]
- 38.Amosov AS, Ul’yanovskii NV, Kosyakov DS. Simultaneous determination of anthraquinone and bisphenol a in pulp and paper products by high performance liquid chromatography–tandem mass spectrometry. J Anal Chem. 2019;74:1089–1095. doi: 10.1134/s1061934819110029. [DOI] [Google Scholar]
- 39.International Agency for Research on Cancer (2011) Anthraquinone IARC monographs 41–70. 10.1093/occmed/kqr127
- 40.Han W, Wang S, Li M, Jiang L, Wang X, Xie K. The protective effect of diallyl trisulfide on cytopenia induced by benzene through modulating benzene metabolism. Food Chem Toxicol. 2018;112:393–399. doi: 10.1016/j.fct.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 41.Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 42.Lundstedt S, White PA, Lemieux CL, Lynes KD, Lambert IB, Oberg L, Haglund P, Tysklind M. Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (pahs) at pah-contaminated sites. Ambio. 2007;36:475–485. doi: 10.1579/0044-7447(2007)36[475:sfatho]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Doi AM, Irwin RD, Bucher JR. Influence of functional group substitutions on the carcinogenicity of anthraquinone in rats and mice: analysis of long-term bioassays by the national cancer institute and the national toxicology program. J Toxicol Environ Health - Part B. 2005;8:109–126. doi: 10.1080/10937400590909077. [DOI] [PubMed] [Google Scholar]
- 44.Malik EM, Müller CE. Anthraquinones as pharmacological tools and drugs. Med Res Rev. 2016;36:705–748. doi: 10.1002/med.21391. [DOI] [PubMed] [Google Scholar]
- 45.Bruins JJ, Albada B, van Delft F. Ortho-quinones and analogues thereof: highly reactive intermediates for fast and selective biofunctionalization. Chem—A Eur J. 2018;24:4749–4756. doi: 10.1002/chem.201703919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhakta D, Siva R. Morindone, an anthraquinone, intercalates DNA sans toxicity: a spectroscopic and molecular modeling perspective. Appl Biochem Biotechnol. 2012;167:885–896. doi: 10.1007/s12010-012-9744-2. [DOI] [PubMed] [Google Scholar]
- 47.National Toxicology Program NTP technical report on the toxicology and carcinogenesis studies of anthraquinone (cas no. 84–65-1) in f344/n rats and b6c3f1 mice (feed studies) National Toxicology Program Technical Report Series. 2005;494:1–358. [PubMed] [Google Scholar]
- 48.Miet K, Albinet A, Budzinski H, Villenave E. Atmospheric reactions of 9,10-anthraquinone. Chemosphere. 2014;107:1–6. doi: 10.1016/j.chemosphere.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 49.Wang D, Wang XH, Yu X, Cao F, Cai X, Chen P, Li M, Feng Y, Li H, Wang X. Pharmacokinetics of anthraquinones from medicinal plants. Front Pharmacol. 2021;12:638993. doi: 10.3389/fphar.2021.638993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang M, Luo F, Zhang X, Wang X, Sun H, Lou Z, Zhou L, Chen Z. Uptake, translocation, and metabolism of anthracene in tea plants. Sci Total Environ. 2022;821:152905. doi: 10.1016/j.scitotenv.2021.152905. [DOI] [PubMed] [Google Scholar]
- 51.Hammel KE, Green B, Gai WZ. Ring fission of anthracene by a eukaryote. Proc Natl Acad Sci USA. 1991;88:10605–10608. doi: 10.1073/pnas.88.23.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei Y, Han I-K, Hu M, Shao M, Zhang JJ, Tang X. Personal exposure to particulate pahs and anthraquinone and oxidative DNA damages in humans. Chemosphere. 2010;81:1280–1285. doi: 10.1016/j.chemosphere.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 53.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’ -deoxyguanosine (8-ohdg): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health Part C: Environ Carcinog Ecotoxicol. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 54.Barbee GC, Santer MM, McClain WR. Lack of acute toxicity of an anthraquinone bird repellent to non-target crayfish (procambarus clarkii) associated with rice-crayfish crop rotations. Crop Prot. 2010;29:506–508. doi: 10.1016/j.cropro.2009.10.013. [DOI] [Google Scholar]
- 55.Lampi MA, Gurska J, McDonald KIC, Xie F, Huang X-D, Dixon DG, Greenberg BM. Photoinduced toxicity of polycyclic aromatic hydrocarbons to daphnia magna: Ultraviolet-mediated effects and the toxicity of polycyclic aromatic hydrocarbon photoproducts. Environ Toxicol. 2006;25:1079–1087. doi: 10.1897/05-276r.1. [DOI] [PubMed] [Google Scholar]
- 56.Brack W, Altenburger R, Küster E, Meissner B, Wenzel K-D, Schüürmann G. Identification of toxic products of anthracene photomodification in simulated sunlight. Environ Toxicol Chem: An Int J. 2003;22:2228–2237. doi: 10.1897/02-450. [DOI] [PubMed] [Google Scholar]
- 57.Kolpin DW, Skopec M, Meyer MT, Furlong ET, Zaugg SD. Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Sci Total Environ. 2004;328:119–130. doi: 10.1016/j.scitotenv.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 58.Kurihara R, Shiraishi F, Tanaka N, Hashimoto S. Presence and estrogenicity of anthracene derivatives in coastal japanese waters. Environ Toxicol Chem. 2005;24:1984–1993. doi: 10.1897/04-310r.1. [DOI] [PubMed] [Google Scholar]
- 59.Machala M, Ciganek M, Bláha L, Minksová K, Vondráčk J. Aryl hydrocarbon receptor-mediated and estrogenic activities of oxygenated polycyclic aromatic hydrocarbons and azaarenes originally identified in extracts of river sediments. Environ Toxicol Chem. 2001;20:2736–2743. doi: 10.1002/etc.5620201212. [DOI] [PubMed] [Google Scholar]
- 60.Coutiño-González E, Hernández-Carlos B, Gutiérrez-Ortiz R, Dendooven L. The earthworm eisenia fetida accelerates the removal of anthracene and 9, 10-anthraquinone, the most abundant degradation product, in soil. Int Biodeterior Biodegrad. 2010;64:525–529. doi: 10.1016/j.ibiod.2010.05.002. [DOI] [Google Scholar]
- 61.Musa Bandowe BA, Shukurov N, Kersten M, Wilcke W. Polycyclic aromatic hydrocarbons (pahs) and their oxygen-containing derivatives (opahs) in soils from the angren industrial area, uzbekistan. Environ Pollut. 2010;158:2888–2899. doi: 10.1016/j.envpol.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Layshock JA, Wilson G, Anderson KA. Ketone and quinone-substituted polycyclic aromatic hydrocarbons in mussel tissue, sediment, urban dust, and diesel particulate matrices. Environ Toxicol Chem. 2010;29:2450–2460. doi: 10.1002/etc.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albinet A, Leoz-Garziandia E, Budzinski H, ViIlenave E. Simultaneous analysis of oxygenated and nitrated polycyclic aromatic hydrocarbons on standard reference material 1649a (urban dust) and on natural ambient air samples by gas chromatography–mass spectrometry with negative ion chemical ionisation. J Chromatogr A. 2006;1121:106–113. doi: 10.1016/j.chroma.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 64.Sienra MR. Oxygenated polycyclic aromatic hydrocarbons in urban air particulate matter. Atmos Environ. 2006;40:2374–2384. doi: 10.1016/j.atmosenv.2005.12.009. [DOI] [Google Scholar]
- 65.Zhao T, Yang L, Huang Q, Zhang Y, Bie S, Li J, Zhang W, Duan S, Gao H, Wang W. Pm2.5-bound polycyclic aromatic hydrocarbons (pahs) and their derivatives (nitrated-pahs and oxygenated-pahs) in a road tunnel located in qingdao, china: characteristics, sources and emission factors. Sci Total Environ. 2020;720:137521. doi: 10.1016/j.scitotenv.2020.137521. [DOI] [PubMed] [Google Scholar]
- 66.Zhao J, Zhang J, Sun L, Liu Y, Lin Y, Li Y, Wang T, Mao H. Characterization of pm2.5-bound nitrated and oxygenated polycyclic aromatic hydrocarbons in ambient air of langfang during periods with and without traffic restriction. Atmos Res. 2018;213:302–308. doi: 10.1016/j.atmosres.2018.06.015. [DOI] [Google Scholar]
- 67.Vapenka L, Vavrous A, Votavova L, Kejlova K, Dobias J, Sosnovcova J. Contaminants in the paper-based food packaging materials used in the czech republic. J Food Nutr Res. 2016 doi: 10.1080/02652030903225765. [DOI] [Google Scholar]
- 68.Lammel G, Kitanovski Z, Kukučka P, Novák J, Arangio AM, Codling GP, Filippi A, Hovorka J, Kuta J, Leoni C, et al. Oxygenated and nitrated polycyclic aromatic hydrocarbons in ambient air—levels, phase partitioning, mass size distributions, and inhalation bioaccessibility. Environ Sci Technol. 2020;54:2615–2625. doi: 10.1021/acs.est.9b06820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhunia K, Sablani SS, Tang J, Rasco B. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Compr Rev Food Sci Food Saf. 2013;12:523–545. doi: 10.1111/1541-4337.12028. [DOI] [PubMed] [Google Scholar]
- 70.Food Packaging Forum Foundation. Food packaging materials. http://www.foodpackagingforum.org/Food-Packaging-Health/Food-Packaging-Materials. Accessed 4 Sept 2023.
- 71.Molin RF, Dartora N, Borges ACP, Gonçalves IL, Di Luccio M, Valduga AT. Total phenolic contents and antioxidant activity in oxidized leaves of mate (ilex paraguariensis st. Hil) Brazilian Archives of Biology and Technology. 2014;57:997–1003. doi: 10.1590/s1516-8913201402305. [DOI] [Google Scholar]
- 72.Gonçalves IL, Dartora N, Piovezan Borges AC, Picolo AP, Dallago RM, de Mera L, Valduga AT. Accelerated maturation of processed yerba-mate under the controlled conditions of temperature and humidity. Nutr Food Sci. 2015;45:564–573. doi: 10.1108/nfs-12-2014-0105. [DOI] [Google Scholar]
- 73.Lewinski CS, Gonçalves IL, Piovezan Borges AC, Dartora N, de Mera L, Valduga AT. Effects of uv light on the physic-chemical properties of yerba-mate. Nutr Food Sci. 2015;45:221–228. doi: 10.1108/nfs-07-2014-0065. [DOI] [Google Scholar]
- 74.Valduga AT, Gonçalves IL, Magri E, Delalibera Finzer JR. Chemistry, pharmacology and new trends in traditional functional and medicinal beverages. Food Res Int. 2019;120:478–503. doi: 10.1016/j.foodres.2018.10.091. [DOI] [PubMed] [Google Scholar]
- 75.Zamora R, Hidalgo FJ. Formation of naphthoquinones and anthraquinones by carbonyl-hydroquinone/benzoquinone reactions: a potential route for the origin of 9,10-anthraquinone in tea. Food Chem. 2021;354:129530. doi: 10.1016/j.foodchem.2021.129530. [DOI] [PubMed] [Google Scholar]
- 76.Mauldin RE, Primus TM, Volz SA, Kimball BA, Johnston JJ, Cummings JL, York DL. Determination of anthraquinone in technical material, formulations, and lettuce by high performance liquid chromatography. J Agric Food Chem. 2002;50:3632–3636. doi: 10.1021/jf0113878. [DOI] [PubMed] [Google Scholar]
- 77.Guiñez M, Bazan C, Martinez LD, Cerutti S. Determination of nitrated and oxygenated polycyclic aromatic hydrocarbons in water samples by a liquid–liquid phase microextraction procedure based on the solidification of a floating organic drop followed by solvent assisted back-extraction and liquid chromatography–tandem mass spectrometry. Microchem J. 2018;139:164–173. doi: 10.1016/j.microc.2018.02.027. [DOI] [Google Scholar]
- 78.Federal Institute for Risk Assessment. BfR removes anthraquinone from its list of recommendations for food packaging. http://www.bfr.bund.de/cm/349/bfr-removes-anthraquinone-from-its-list-of-recommandations-for-food-packaging.pdf
- 79.Union European (2014) Commission regulation (E.U) no 1146/2014. Off J Eur Union. 10.5040/9781509909568.0013
- 80.Codex Alimentarius International Food Standards . Maximum residue limits (mrls) and risk management recommendations (rmrs) for residues of veterinary drugs in foods. Italy: FAO publication; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.