Abstract
Several immunodominant major proteins ranging from 23 to 30 kDa were identified in the outer membrane fractions of Ehrlichia chaffeensis and Ehrlichia canis. The N-terminal amino acid sequence of a 28-kDa protein of E. chaffeensis (one of the major proteins) was determined. The gene (p28), almost full length, encoding the 28-kDa protein was cloned by PCR with primers designed based on the N-terminal sequence of the E. chaffeensis 28-kDa protein and the consensus sequence between the C termini of the Cowdria ruminantium MAP-1 and Anaplasma marginale MSP-4 proteins. The p28 gene was overexpressed, and antibody to the recombinant protein was raised in a rabbit. The antibody and serum from a patient infected with E. chaffeensis reacted with the recombinant protein, three proteins (29, 28, and 25 kDa) of E. chaffeensis, and a 30-kDa protein of E. canis. Immunoelectron microscopy with the rabbit antibody revealed that the antigenic epitope of the 28-kDa protein was exposed on the surface of E. chaffeensis. Southern blot analysis with a 32P-labeled p28 gene probe revealed multiple copies of genes homologous to p28 in the E. chaffeensis genome. Six copies of the p28 gene were cloned and sequenced from the genomic DNA by using the same probe. The open reading frames of these gene copies were tandemly arranged with intergenic spaces. They were nonidentical genes and contained a semivariable region and three hypervariable regions in the predicted protein molecules. One of the gene copies encoded a protein with an internal amino acid sequence identical to the chemically determined N-terminal amino acid sequence of a 23-kDa protein of E. chaffeensis. Immunization with the recombinant P28 protein protected mice from infection with E. chaffeensis. These findings suggest that the 30-kDa-range proteins of E. chaffeensis represent a family of antigenically related homologous proteins encoded by a single gene family.
Ehrlichia chaffeensis, which causes human monocytic ehrlichiosis, is an obligate intracellular bacterium of monocytes and macrophages and belongs to the family Rickettsiaceae. Human ehrlichiosis is a tick-borne illness and was first reported in 1987 in the United States (21). Most patients have fever, chills, headache, arthralgia, myalgia, and hematologic abnormalities, including thrombocytopenia and leukopenia. Elevation of liver enzymes occurs in most patients. Since 1987, over 400 cases of human ehrlichiosis, detected primarily by serological means, have been reported in 30 states (3, 14, 16).
Recently, several protein antigens of E. chaffeensis were identified by Western blot analysis with naturally infected human sera, experimentally inoculated dog sera, or monoclonal antibodies (7–10, 13, 30, 35, 40–42). Two of these antigens, namely, a heat shock protein (HSP) 60 homolog (35) and a 120-kDa protein (41, 42), have been cloned, sequenced, and expressed. Two E. chaffeensis proteins ranging from 28 to 30 kDa were shown to be dominant antigens and were cross-reactive between two Ehrlichia spp.: E. chaffeensis and E. canis (7, 30). Studies with monoclonal antibodies (MAbs) against E. chaffeensis showed that two or three proteins of from 22 to 30 kDa react with three MAbs by Western blotting and that these antigens are exposed on the surface of the organism as determined by immunogold labeling of negatively staining ehrlichiae (8–10, 40). However, why multiple proteins of different molecular sizes react with the MAbs has not been answered. These E. chaffeensis antigens in the 30-kDa range have not been examined at the molecular level.
In this study, we demonstrated that a potentially immunoprotective 28-kDa protein (designated P28) located on the E. chaffeensis surface and antigenically cross-reactive proteins in the 30-kDa range are encoded by a multigene family.
MATERIALS AND METHODS
Organisms and purification.
The E. chaffeensis Arkansas strain and E. canis Oklahoma strain were cultivated in the DH82 dog macrophage cell line (30) and purified by Percoll density gradient centrifugation as described elsewhere (32, 38).
Preparation of the ehrlichial outer membrane fraction.
The procedure for Orientia tsutsugamushi was followed, with modifications (25). Briefly, purified ehrlichiae (100 μg) were suspended with 10 mM sodium phosphate buffer (pH 7.4) containing 0.1% sodium N-lauroyl sarcosine (Sarkosyl) (Sigma, St. Louis, Mo.), 50 μg (each) of DNase I (Sigma) and RNase A (Sigma) per ml, and 2.5 mM MgCl2. After incubation at 37°C for 30 min, the sample was separated by centrifugation at 10,000 × g for 1 h into the soluble supernatant and the insoluble precipitate. The insoluble pellet was resuspended two or three times with 0.1% Sarkosyl and centrifuged. The final pellet was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described elsewhere (31) and by electron microscopy. The pellet was used as the ehrlichial outer membrane fraction. To investigate contamination by the ehrlichial inner membrane, succinic dehydrogenase activity was examined as described elsewhere (11).
Analysis of the N-terminal amino acid sequences of outer membrane proteins in the 30-kDa range.
Proteins in the Sarkosyl-insoluble pellet prepared from 400 μg of purified E. chaffeensis were separated by reversed discontinuous SDS-PAGE (RdSDS-PAGE) (a 2.5-cm-long 17% gel on top of an 11-cm-long 12% gel) and electrophoretically transferred to a ProBlot membrane (Applied Biosystems, Foster City, Calif.) as described elsewhere (44). The portion of the membrane containing bound proteins was excised and analyzed with an Applied Biosystems protein sequencer (model 470).
Primer design for amplification of a gene (p28) encoding a 28-kDa major protein (P28) of E. chaffeensis.
The N-terminal amino acid sequence of P28 (one of the major proteins separated by RdSDS-PAGE as described above) was determined as DPAGSGINGNFYSGKYMP. We designed a forward primer, FECH1, based on amino acids 6 to 12 of this sequence: 5′-CGGGATCCGAATTCGG(A/T/G/C)AT(A/T/C)AA(T/C)GG(A/T/G/C)AA(T/C)TT(T/C)TA-3′. Amino acids at positions 1 to 5 of the N terminus of P28 were not included in this primer design to increase annealing efficiency, since Ser with six codons was present at position 5. For insertion into an expression vector, a 14-bp sequence (underlined) was added at the 5′ end of the primer to create an EcoRI site and a BamHI site.
A reverse primer was designed from two proteins which we found to be related to P28 based on N-terminal amino acid sequence comparison. One of the proteins was Cowdria ruminantium major antigen protein 1 (MAP-1). The C-terminal sequence of MAP-1 is as follows: (N terminus) … GGRFVF* (C terminus) (* is the termination codon) (36). The other protein was the Anaplasma marginale major surface protein 4 (MSP-4) (23), the entire amino acid sequence of which is homologous to that of C. ruminantium MAP-1 (36). The C-terminal sequence of MSP-4 is as follows: (N terminus) … GARFLFS* (C terminus). An oligonucleotide primer, RECH2, complementary to a DNA sequence corresponding to the amino acid sequence conserved between the C termini of MAP-1 and MSP-4, (N terminus) G(G/A)RF(V/L)F* (C terminus), was prepared, with the addition of a 9-bp sequence (underlined) including a NotI site at the 5′ end for ligation into an expression vector: 5′-AGCGGCCGCTTA(A/G)AA(T/C)A(C/G)(A/G)AA(C/T)CTT(C/G)CTCC-3′.
Cloning, sequencing, and expression of the p28 gene.
Genomic DNA of E. chaffeensis was isolated from purified organisms as described elsewhere (24). PCR amplification with FECH1 and RECH2 primers was performed with a Perkin-Elmer Cetus DNA Thermal Cycler (model 480). A 0.8-kb amplified product was cloned in the pCRII vector of a TA cloning kit, as described by the manufacturer (Invitrogen Co., San Diego, Calif.). The clone obtained was designated pCRIIp28. Both strands of the inserted DNA were sequenced by a dideoxy termination method with an Applied Biosystems 373A DNA sequencer.
The 0.8-kb p28 gene was excised from the clone pCRIIp28 by EcoRI-NotI double digestion, ligated into EcoRI-NotI sites of a pET 29a expression vector, and amplified in Escherichia coli BL21(DE3)pLysS (Novagen, Inc., Madison, Wis.). The clone (designated pET29p28) produced a fusion protein with a 35-amino-acid sequence carried from the vector at the N terminus.
Antisera and Western blot analysis.
Convalescent-phase serum from a patient with clinical signs of human ehrlichiosis was used as described previously (30). For preparation of the rabbit anti-recombinant P28 (anti-rP28) antibody, the gel band corresponding to rP28 in SDS-PAGE was excised without staining, minced in phosphate-buffered saline (PBS) (pH 7.4), and mixed with an equal volume of Freund’s incomplete adjuvant (Sigma). The mixture (1 mg of protein each time) was subcutaneously injected into a rabbit every 2 weeks for four times. Antibody titers of the patient serum and the rabbit anti-rP28 antibody against E. chaffeensis antigen were determined to be 1:2,560 and 1:1,280, respectively, by indirect immunofluorescence assay as described elsewhere (29).
Western blot analyses were performed with 1:1,000 dilutions of these sera by a procedure described elsewhere (31). The rabbit anti-rP28 antibody was preabsorbed twice with pET29a-transformed E. coli at 37°C for 1 h each at a 1:300 dilution prior to use. Alkaline phosphatase-conjugated affinity-purified anti-human or anti-rabbit immunoglobulin G (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) was used at a 1:1,000 or 1:2,000 dilution as a secondary antibody.
Immunoelectron microscopy.
E. chaffeensis-infected DH82 cells were sonicated and centrifuged at 400 × g for 10 min. The supernatant was then centrifuged at 10,000 × g for 10 min to obtain an ehrlichia-enriched pellet. The pellet was resuspended and incubated with rabbit anti-rP28 antibody or normal rabbit serum (1:100 dilution) at 37°C for 1 h in PBS containing 1% bovine serum albumin. After being washed, the ehrlichiae were incubated with gold-conjugated protein G (20 nm; Sigma) at a 1:30 dilution for 1 h at room temperature in PBS containing 1% bovine serum albumin. After being washed again, the specimen was fixed with 1.25% formaldehyde, 2.5% glutaraldehyde, and 0.03% trinitrophenol in 0.1 M cacodylate buffer (pH 7.4) for 24 h and postfixed in 1% osmium–1.5% potassium ferricyanide for 1 h (34). The section was then embedded in PolyBed 812 (Polysciences, Warrington, Pa.). The specimen was ultrathin sectioned at 60 nm, stained with uranyl acetate and lead citrate, and observed with a Philips 300 transmission electron microscope at 60 kV.
Southern blot analysis.
Genomic DNA extracted from the purified E. chaffeensis (200 ng) was digested with restriction endonucleases, electrophoresed, and transferred to a Hybond-N+ nylon membrane (Amersham, Arlington Heights, Ill.) by a standard method (33). The 0.8-kb p28 gene fragment from the clone pCRIIp28 was labeled with [α-32P]dATP by the random primer method by using a kit (Boehringer Mannheim, Indianapolis, Ind.), and the labeled fragment was used as a DNA probe. Hybridization was performed at 60°C in rapid-hybridization buffer (Amersham) for 20 h. The nylon sheet was washed in 0.1× SSC (1× SSC is 0.15 M sodium chloride and 0.015 M sodium citrate) with 1% SDS at 55°C, and the hybridized probes were exposed to Hyperfilm (Amersham) at −80°C.
Cloning and sequencing of genomic copies of the E. chaffeensis p28 gene.
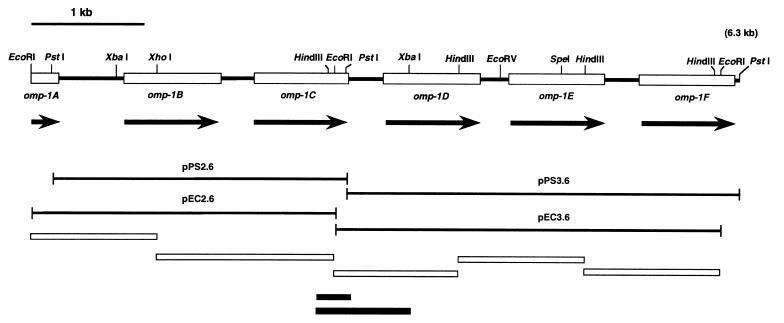
The EcoRI and PstI fragments of DNA, detected by genomic Southern blot analysis as described above, were inserted into pBluescript II KS(+) vectors, and the recombinant plasmids were introduced into E. coli DH5α. By using the colony hybridization method (33) with the 32P-labeled p28 gene probe, four positive clones were isolated from the transformant. The positive clones were designated pEC2.6, pEC3.6, pPS2.6, and pPS3.6. These contained the ehrlichial DNA fragments of 2.6 (EcoRI), 3.6 (EcoRI), 2.6 (PstI), and 3.6 (PstI) kb, respectively. The inserts of the clones pEC3.6 and pPS2.6 overlapped as shown in Fig. 6. The overlapping area was further confirmed by PCR of E. chaffeensis genomic DNA with two pairs of primer sets interposing the junctions of the four clones (see Fig. 6). The 1.1- to 1.6-kb HindIII-HindIII, HindIII-EcoRI, or XhoI-EcoRI DNA fragments in pEC2.6 and pEC3.6 were subcloned for sequencing. DNA sequencing was performed with suitable synthetic primers by the dideoxy termination method as described above.
FIG. 6.
Restriction map of 6.3 kb of E. chaffeensis genomic DNA including the omp-1 gene copies. The four DNA fragments pPS2.6, pPS3.6, pEC2.6, and pEC3.6 were cloned from the genomic DNA. Recombinant plasmid pPS2.6 has a sequence overlapping that of pEC3.6. The black boxes at the bottom show PCR-amplified fragments from the genomic DNA for confirmation of the overlapping area. Open boxes at the top indicate ORFs of omp-1 gene copies, with directions indicated by arrows. Open boxes at the bottom show DNA fragments subcloned for DNA sequencing.
Immunization of mice and E. chaffeensis challenge.
The rP28 band in SDS-PAGE was excised, minced, and mixed with an equal volume of Freund’s incomplete or complete adjuvant. Nine male BALB/c mice (6 weeks old) were divided into two groups. Five mice were intraperitoneally immunized a total of four times at 10-day intervals: twice with a mixture of the minced gel with rP28 (30 to 40 μg of protein per mouse each time) and incomplete adjuvant and twice with a mixture of the recombinant protein (the same amount as before) and complete adjuvant. Four mice were intraperitoneally injected with a mixture of the minced gel without protein and the respective adjuvants. For ehrlichia challenge, approximately 107 DH82 cells heavily infected with E. chaffeensis were disrupted by sonication in serum-free Dulbecco modified Eagle medium (GIBCO-BRL) and centrifuged at 200 × g for 5 min. The supernatant was diluted to a final volume of 5 ml, and 0.3 ml was inoculated intraperitoneally into each mouse 10 days after the last immunization.
Detection of E. chaffeensis 16S rDNA in Ehrlichia-challenged mice.
At day 5 postchallenge, approximately 1 ml of blood from each mouse was collected in an EDTA tube. Total DNA was prepared from 0.2 ml of the buffy coat from the blood with a QIAamp blood kit (Qiagen, Inc., Chatsworth, Calif.) and was used as the template for PCR detection of E. chaffeensis 16S ribosomal DNA (rDNA). PCR detection with primers HE1 (5′-CAATTGCTTATAACCTTTTGGTTATAAAT-3′) and HE3 (5′-TATAGGTACCGTCATTATCTTCCCTAT-3′), which yield a 389-bp fragment specific to E. chaffeensis 16S rDNA (4), was performed as described previously (39). The procedure allows detection from ≥10 pg of genomic DNA from purified E. chaffeensis.
Sequence analysis.
Nucleotide sequences were analyzed with the DNASIS program (Hitachi Software Engineering Co., Ltd., Yokohama, Japan). A homology search was carried out with the GenBank, Swiss Plot, PDB, and PIR databases by using the software basic local alignment search tool (2) in the BLAST network service (National Center for Biotechnology Information, Bethesda, Md.). Phylogenetic analysis was performed by using the PHYLIP software package (version 3.5) (17). An evolutionary distance matrix, generated by using the Kimura formula (17) in the PROTDIST, was used for construction of a phylogenetic tree by using unweighted pair-group method analysis (17). The data were also examined by using parsimony analysis (PROTPARS in PHYLIP). A bootstrap analysis was carried out to investigate the stability of randomly generated trees by using SEQBOOT and CONSENSE in the same package.
Nucleotide sequence accession numbers.
The nucleotide sequences of the p28 gene and its gene copies have been assigned GenBank accession numbers U72291 and AF021338, respectively.
RESULTS
Identification of major outer membrane proteins of E. chaffeensis.
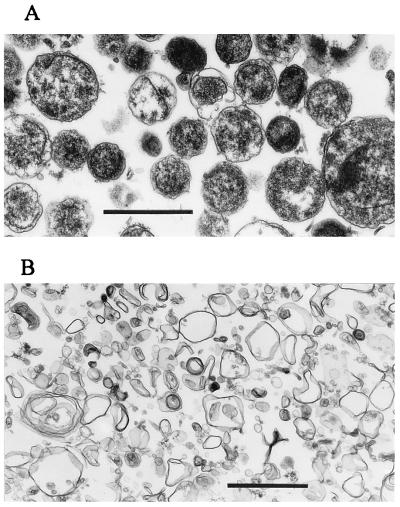
The ehrlichial outer membrane fraction was prepared from Percoll-purified E. chaffeensis by Sarkosyl treatment. Transmission electron microscopy revealed that the purified ehrlichial fraction consists of a mixture of small electron-dense and large light forms with slight disintegration of the inner membrane (Fig. 1A). The host inclusion membrane was not found with the purified ehrlichiae. Various sizes of membrane vesicles (<1 μm) without significant ribosomes or nuclear materials were observed in the Sarkosyl-insoluble fraction prepared from the purified organism (Fig. 1B). Succinic dehydrogenase (an inner membrane marker enzyme of gram-negative bacteria) activity was less than the detection limit (1 nmol/min/mg of protein) in the Sarkosyl-insoluble fraction, compared to approximately 10 nmol/min/mg of protein in the Percoll-purified organisms, suggesting that the insoluble fraction consisted primarily of the outer membrane of E. chaffeensis.
FIG. 1.
Transmission electron microscopy of Percoll-purified E. chaffeensis (A) and of the insoluble precipitate after 0.1% Sarkosyl treatment of the organism (B). Note outer membrane vesicles of various sizes in panel B. Bars, 1 μm.
Analysis of the Sarkosyl-soluble and insoluble fractions of E. chaffeensis by SDS-PAGE suggested that proteins in the 30-kDa range in the insoluble fraction represent the major outer membrane proteins of this organism (Fig. 2A). E. canis was antigenically cross-reactive with E. chaffeensis (7, 30). A similar result was obtained with E. canis by the same procedure (Fig. 2B). These findings indicate that the 30-kDa-range proteins represent the major outer membrane proteins of these two Ehrlichia spp. Since it was impossible to resolve overlapping protein bands in the 30-kDa range by conventional SDS-PAGE, RdSDS-PAGE was performed, and at least five proteins (P23, P25, P27, P28, and P29, designated based on the molecular sizes in Fig. 2C) of the outer membrane fraction of E. chaffeensis were resolved. The N-terminal amino acid sequences of all these proteins were chemically determined, and that of P28 was found to be homologous to that of C. ruminantium MAP-1 (36) by a BLAST search.
FIG. 2.
SDS-PAGE patterns of the insoluble precipitate and the soluble supernatant fraction after 0.1% Sarkosyl treatment of purified E. chaffeensis (A) and E. canis (B) and RdSDS-PAGE of major proteins in the 30-kDa range resolved from the Sarkosyl-insoluble pellet of E. chaffeensis (C). (A) Lanes: 1, Sarkosyl-soluble supernatant; 2, Sarkosyl-insoluble precipitate enriched with outer membrane; 3, Percoll gradient-purified E. chaffeensis. (B) Lanes: 1, Sarkosyl-soluble supernatant; 2, Sarkosyl-insoluble precipitate; 3, purified E. canis. Both gels were stained with Coomassie blue. Brackets indicate a 30-kDa cluster of major outer membrane proteins. (C) The separation gel consisted of a 17% gel on top of a 12% gel. The Sarkosyl-insoluble precipitate prepared from purified E. chaffeensis was blotted onto a ProBlot membrane and stained with amido black. The protein bands present in six lanes of the membrane were excised, and the N-terminal amino acid sequence of each protein was analyzed. Numbers on the right or left of panels indicate molecular masses in kilodaltons.
Cloning, sequencing, and expression of a gene (p28) encoding E. chaffeensis P28.
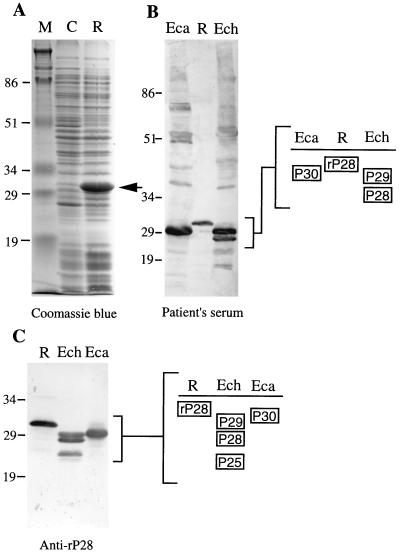
A 0.8-kb p28 gene, amplified by PCR, was cloned and sequenced as described in Materials and Methods. The 0.8-kb DNA fragment, cloned in pCRIIp28, had an open reading frame (ORF) of 756 bp encoding a 251-amino-acid protein (including both PCR primer regions) with a molecular mass of 27,685 Da. E. coli transformed with pET29p28 expressed a 31-kDa rP28 (Fig. 3A), which was larger than the native P28 because of the fusion protein. rP28 has an additional 35-amino-acid sequence including the S.Tag peptide (20) derived from a pET expression vector at the N terminus. The serum from a patient with clinical signs of human ehrlichiosis reacted strongly to rP28 (31 kDa) in E. coli, to P28 and P29 in E. chaffeensis, and also to P30 in E. canis (Fig. 3B). The rabbit anti-rP28 antibody recognized not only rP28 (31 kDa) and P28 but also P29 and P25 of E. chaffeensis and P30 of E. canis (Fig. 3C), indicating that P28 shares antigenic epitopes with these proteins.
FIG. 3.
Overexpression of the E. chaffeensis p28 gene (A) and Western blot analysis with convalescent-phase serum from a human ehrlichiosis patient (B) and with a rabbit anti-rP28 antibody (C). Lanes: M, molecular size markers; C, pET29a-transformed E. coli (negative control); R, pET29p28-transformed E. coli (recombinant) (arrowhead, rP28); Eca, purified E. canis; Ech, purified E. chaffeensis. Dominant protein antigens with the molecular masses of P25 to P30, and rP28 (31 kDa), are schematically shown. Numbers indicate molecular masses in kilodaltons.
Immunoelectron microscopy.
Transmission immunoelectron microscopy with colloidal gold-conjugated protein G and rabbit anti-rP28 antibody revealed gold particles bound to the E. chaffeensis surface (Fig. 4). The distribution of the particles was random and close to the surface, and they appeared as if almost embedded in the membrane, suggesting that the antigenic epitope only slightly protrudes on the surface. Nonetheless, the antigenic epitope was surface exposed and thus could be recognized by rabbit anti-rP28 antibody. No gold particles were observed on the host cytoplasmic membrane or E. chaffeensis incubated with normal rabbit serum.
FIG. 4.
Transmission electron microscopy of E. chaffeensis immunogold labeled with a rabbit anti-rP28 antibody. Protein G-gold particles (20 nm) are localized on the surface of the organism. Bar, 0.1 μm.
Identification and characterization of genomic copies of the E. chaffeensis p28 gene.
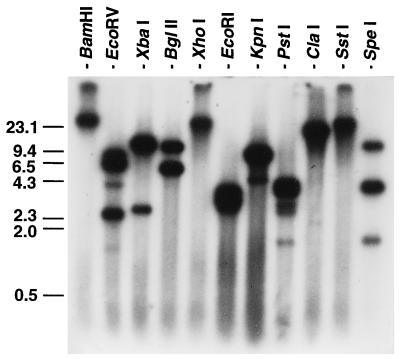
Genomic Southern blot analysis with several restriction enzymes resulted in one or more DNA fragments of E. chaffeensis which could hybridize to the 32P-labeled p28 gene probe (Fig. 5). The restriction enzymes used do not cut within the p28 gene portion of the pCRIIp28 insert, and therefore, this result indicates that multiple genes homologous to the p28 gene are present in the ehrlichial genome. XbaI, BglII, and KpnI produced two bands, SpeI generated three bands, and EcoRV and PstI produced multiple bands with different densities. EcoRI generated a broad band of 2.5 to 4 kb. These p28-homologous genes are designated the omp-1 (for outer membrane protein 1) family.
FIG. 5.
Genomic Southern blot analysis of E. chaffeensis with a 32P-labeled 0.8-kb p28 gene probe of the pCRIIp28 insert. Numbers indicate molecular sizes in kilobases.
Four DNA fragments from 2.6 to 3.6 kb were cloned from the EcoRI- and PstI-digested genomic DNA of E. chaffeensis by colony hybridization with the radiolabeled p28 gene probe. The DNA inserts of the two recombinant clones pEC3.6 and pPS2.6 overlapped as shown in Fig. 6. Sequencing revealed one 5′-truncated ORF of 243 bp (designated omp-1A) and five complete ORFs of 836 to 861 bp (designated omp-1B to omp-1F) that were tandemly arranged and homologous to the p28 gene, but not identical, in the ehrlichial genomic DNA of 6,292 bp. The intergenic spaces were 581 bp between omp-1A and omp-1B and 260 to 308 bp among the others. Putative promoter regions and ribosome-binding sites were identified in the noncoding regions upstream from the start codon of each gene.
Structures of proteins encoded by the genes of the E. chaffeensis omp-1 family.
Five complete omp-1 gene copies (omp-1B to omp-1F) encode 279- to 287-amino-acid proteins with molecular masses of 30,320 to 31,508 Da. omp-1A encodes an 82-amino-acid partial protein (9,243 Da) which lacks the N-terminal region. The 25-amino-acid sequence at the N termini of OMP-1B to OMP-1F (encoded by omp-1B to omp-1F, respectively) is predicted to be a signal peptide, because three carboxyl-terminal amino acids of the signal peptides (Ser-X-Ala in OMP-1B, Leu-X-Ser in OMP-C, and Ser-X-Ser in OMP-1D and OMP-1F) are among the preferred amino acid sequences of the signal peptidase at its processing site (26). The molecular masses of the mature OMP-1B to OMP-1F calculated based on the predicted amino acid sequences are 28,181 Da for OMP-1B, 27,581 Da for OMP-1C, 28,747 Da for OMP-1D, 27,776 Da for OMP-1E, and 27,933 Da for OMP-1F. The estimated isoelectric points of these proteins are 4.76 to 5.76.
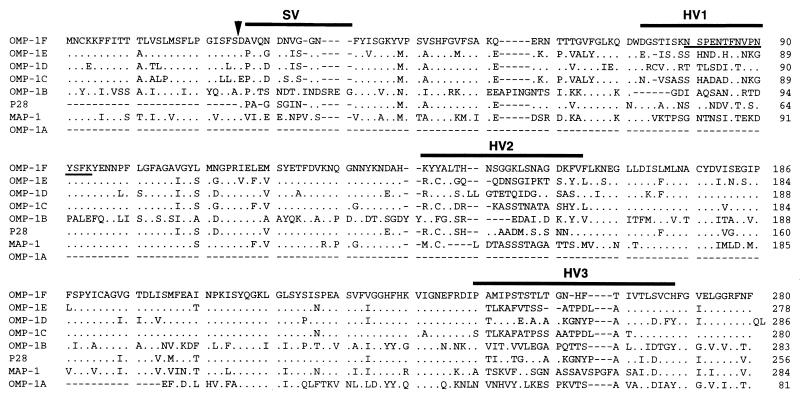
Alignment of predicted amino acid sequences of the E. chaffeensis OMP-1 proteins, along with that of C. ruminantium MAP-1 (36), which is related to the OMP-1 family, revealed substitutions or deletions of one or several contiguous amino acid residues throughout the molecules. The significant differences in sequences among the aligned proteins are seen in the regions designated semivariable (SV) and hypervariable (HV) in Fig. 7. Computer analysis for hydropathy revealed that protein molecules predicted for all omp-1 gene copies contain alternative hydrophilic and hydrophobic motifs which are characteristic of transmembrane proteins. HV1 and HV2 were found to be located in the hydrophilic regions (data not shown).
FIG. 7.
Amino acid sequence alignment of seven E. chaffeensis OMP-1 proteins and C. ruminantium MAP-1. Aligned positions of amino acids identical to those in OMP-1F are shown with dots. The sequence of C. ruminantium MAP-1 is from the report of Van Vliet et al. (36). Gaps (indicated by dashes) were introduced for optimal alignment of all proteins. Bars indicate a semivariable region (SV) and three hypervariable regions (HV1, HV2, and HV3). The chemically determined N-terminal amino acid sequence of E. chaffeensis P23, which was identical to the amino acid sequence of OMP-1F, is underlined. The arrowhead shows the putative cleavage site of the signal peptide.
An amino acid sequence in HV1 (underlined within OMP-1F in Fig. 7) was identical to the chemically determined N-terminal amino acid sequence (NSPENTFNVPNYSFK) of the E. chaffeensis native P23 protein, suggesting that P23 is derived from the omp-1F gene. Amino acid sequences identical to the N-terminal sequences of P25, P27, and P29 were not found among those from omp-1 gene copies cloned in this study (data not shown).
Similarities among amino acid sequences of the E. chaffeensis OMP-1 proteins.
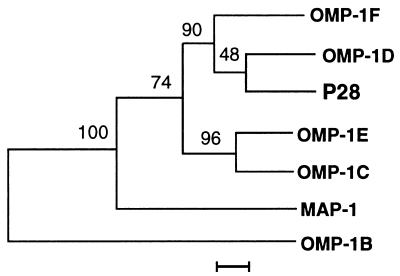
The amino acid sequences of five mature proteins without signal peptides (OMP-1C to OMP-1F and P28) were similar to one another (71 to 83%), but the sequence of OMP-1B was dissimilar to those of the five proteins (45 to 48%). The amino acid sequences of the five proteins showed an intermediate degree of similarity to that of C. ruminantium MAP-1 (59 to 63%), but the similarity between those of OMP-1B and C. ruminantium MAP-1 was low (45%). In Fig. 8, these relations are shown in a phylogenetic tree based on the amino acid sequence alignment. Three proteins (P28, OMP-1D, and OMP-1F) and two proteins (OMP-1C and OMP-1E) formed two separate clusters. OMP-1B was located distantly from these two clusters. C. ruminantium MAP-1 was positioned between OMP-1B and other members of the OMP-1 family.
FIG. 8.
Phylogenetic relationship among six members of the E. chaffeensis OMP-1 family and C. ruminantium MAP-1. The evolutionary distance values were determined by the method of Kimura (17), and the tree was constructed by unweighted pair-group method analysis. The scale bar shows 5% divergence in the amino acid sequences. The numbers at nodes are the proportions of 100 bootstrap resamplings that support the topology shown.
Protection against E. chaffeensis challenge in rP28-immunized mice.
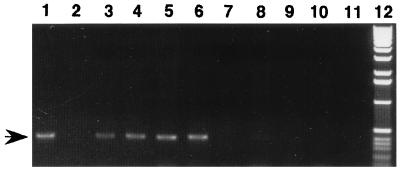
To investigate whether immunization with rP28 induces protection against E. chaffeensis infection, five mice were immunized with rP28 and four mice were inoculated with acrylamide gel without the recombinant protein (control). Before challenge, all five immunized mice had a titer of 1:160 against E. chaffeensis antigen by indirect immunofluorescence assay and all four nonimmunized mice were negative. Protection was assessed by PCR detection of E. chaffeensis 16S rDNA in the buffy coat of blood collected from the mice at 5 days postchallenge. E. chaffeensis can transiently establish infection in BALB/c mice. The infection is spontaneously cleared, as E. chaffeensis cannot be reisolated in cell culture at day 10 postinfection (28). Day 5 is the optimum time at which establishment of ehrlichial infection can be examined by PCR without the influence of residual DNA from the ehrlichiae used as the challenge before the spontaneous clearance of organisms takes place. The E. chaffeensis-specific DNA fragment was observed in all nonimmunized mice but not in any immunized mice, indicating that immunization with rP28 apparently protects mice from ehrlichial infection (Fig. 9) and suggesting that the P28 is a potential protective antigen.
FIG. 9.
PCR detection of E. chaffeensis 16S rDNA fragment in the blood of E. chaffeensis-challenged mice previously immunized with rP28 or nonimmunized. Template DNAs were prepared from blood buffy coats (0.2 ml) of all challenged mice. The arrow shows the E. chaffeensis-specific 16S rDNA fragment (389 bp) obtained by PCR amplification. Lanes: 1, positive control (with a total DNA from DH82 cells infected with E. chaffeensis as the template); 2, negative control (PCR without template); 3 to 6, nonimmunized mice; 7 to 11, immunized mice; 12, 1-kb DNA ladder marker (GIBCO).
DISCUSSION
The outer membrane is the site where the host-ehrlichia interaction takes place. So far, the outer membrane fraction has not been prepared from any Ehrlichia spp.; consequently, the protein composition of the outer membrane is unknown. Using a Sarkosyl method, we identified five major proteins (P23 to P29) in the insoluble fraction of E. chaffeensis. Three of the five (P25, P28, and P29) were found to be antigenically cross-reactive by using anti-rP28 antibody, and the antigenic epitopes were surface located in E. chaffeensis as demonstrated by transmission immunoelectron microscopy. These observations, in addition to results of analysis by transmission electron microscopy and examination of succinic dehydrogenase activity in the Sarkosyl-insoluble fraction, support the usefulness of the Sarkosyl procedure for preparation of a fraction enriched in the outer membrane of E. chaffeensis. Like for O. tsutsugamushi (25), the concentration of Sarkosyl required for E. chaffeensis was lower than those required for other facultative intracellular bacteria (6, 18, 37).
This is the first report in which the major outer membrane proteins of E. chaffeensis in the 30-kDa range are identified and characterized at the molecular genetic and protein sequence levels. We and other investigators previously reported protein antigens of E. chaffeensis ranging from 22 to 30 kDa (7–10, 13, 30, 40). The two dominant antigens, P28 and P29 in the current study, seem to correspond, respectively, to two proteins of 28 and 30 kDa reported by Rikihisa et al. (30) and to two proteins of 28 and 29 kDa reported by Chen et al. (7). In both previous studies, the antigens were recognized predominantly by convalescent-phase sera from human ehrlichiosis patients. P28 and P29 may also correspond, respectively, to proteins of 29 and 30 kDa reported by Chen et al. (8), both of which were recognized by the 7C1-B and 3C7 MAbs. The current study, using the anti-rP28 antibody, and the study of Chen et al. (8), using the MAbs, indicated that P28 (the current study) and the 29-kDa protein (8) share antigenic epitopes with P29 (the current study) and the 30-kDa protein (8), respectively. In the current study, P25, P28, and P29 were recognized by the anti-rP28 antibody. It is unknown whether E. chaffeensis P23, P25, and P27 (the current study) are identical to the three antigens of 22, 26, and 28 kDa recognized by MAb 1A9 (8). The E. canis 30-kDa protein was recognized by the antibody to rP28 of E. chaffeensis (the current study) and by the 7C1-B MAb to E. chaffeensis (8, 10). The 32-kDa MAP-1 of C. ruminantium (36) showed amino acid sequence similarity to all members of the E. chaffeensis OMP-1 family. C. ruminantium MAP-1 also was cross-reactive to a 27-kDa protein of E. canis (22), although it is unknown whether the 27-kDa protein is identical to P30 of E. canis in the current study. By 16S rDNA sequence comparison, E. chaffeensis, E. canis, and C. ruminantium are closely related (12). Consequently, the 30-kDa-range proteins in the OMP-1 family may be common antigens among the three species in the tribe Ehrlichieae.
By using the PCR-amplified p28 gene as a probe, six similar genes were identified in the E. chaffeensis genome. Genomic Southern blotting results suggest the presence of additional omp-1 gene copies. However, the precise number of copies cannot be determined, since restriction site polymorphism in the gene copies may result in the production of several bands from a single copy.
We think that P23 is generated from the OMP-1F by a specific processing, rather than by nonspecific degradation during the preparation of the outer membrane fraction, since there was no difference in protein profiles determined by SDS-PAGE among several batches of purified organisms or outer membrane fractions prepared in the presence or absence of proteinase inhibitors.
Recently, in A. marginale, which is related to E. chaffeensis as determined by 16S rDNA sequencing (12), two multigene families were found (1, 27). A family of msp-2 genes that encode a 36-kDa major surface protein constitute a minimum of 1% of the genome and are distributed widely throughout the chromosome. In addition, strain variations of the msp-2 copies were demonstrated (27). A family of msp-3 gene copies that encode a 63-kDa major surface protein are also distributed widely throughout the chromosome. msp-3-12 has a DNA sequence area homologous to that of msp-2 within the ORF of msp-3-12. msp-3-11 and msp-3-19 have a DNA sequence area homologous to that of msp-2 outside ORFs (1). The omp-1 gene family of E. chaffeensis is different from these gene families of A. marginale. First, the ORFs of omp-1 gene copies were tandemly arranged in the genome. Second, amino acid sequences among the omp-1 copies have a greater variation than the reported variations of msp-2 copies of Anaplasma. The similarities were 45 to 83% among six omp-1 copies, whereas the similarity is 95% between two msp-2 copies (15). Strain variability may also exist in E. chaffeensis, since the reactivities of protein antigens to MAb 7C1-B are different among three strains (8, 10).
In phylogenetic analysis, three proteins (P28, OMP-1D, and OMP-1F) belong to the same cluster. P23 (most likely derived from the omp-1F gene), which was identified in the E. chaffeensis outer membrane fraction, also belongs to this cluster. It is unknown whether omp-1D and other gene copies in different clusters are silent genes. These genes at least are not actively expressed in the population of E. chaffeensis from which our specimen was prepared, since the products from the omp-1 gene family, except for P23, P25, P28, and P29, were not recognized in the Sarkosyl-insoluble outer membrane fraction.
We demonstrated that rP28 protected mice from E. chaffeensis infection or accelerated the spontaneous clearance of E. chaffeensis, suggesting that this or other omp-1-related proteins may be a protective antigen. Further molecular genetic studies are required to elucidate the mechanisms of the antigenic polymorphism or possible antigenic variation, i.e., whether selective expression of the omp-1 gene copies is regulated at the transcriptional level or by recombination events (gene conversions) among the unique gene repertoire, such as in the cases of the pili of Neisseria gonorrhoeae (19), vmp of Borrelia hermsii (5), and vls of Borrelia burgdorferi (43).
ACKNOWLEDGMENTS
This work was supported by grant RO1 AI33123 from the National Institutes of Health. Norio Ohashi was a recipient of a 1995 postdoctoral fellowship from The Ohio State University, and Ning Zhi was a recipient of a 1995 graduate student fellowship from The Ohio State University.
We thank John Lowbridge for his assistance in N-terminal amino acid sequencing, Quin Lu for her assistance in DNA sequencing, Andrea Nicastro for her help in ehrlichial cultivation, and Jason Mott and Roy Barnewall for their editorial assistance.
REFERENCES
- 1.Alleman A R, Palmer G H, McGuire T C, McElwain T F, Perryman L E, Barbet A F. Anaplasma marginale major surface protein 3 is encoded by a polymorphic, multigene family. Infect Immun. 1997;65:156–163. doi: 10.1128/iai.65.1.156-163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Anderson B E, Dawson J E, Jones D C, Wilson K E. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson B E, Sumner J W, Dawson J E, Tzianabos T, Greene C R, Olson J G, Fishbein D B, Olsen-Rasmussen M, Holloway B P, George E H, Azad A F. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour A G. Linear DNA of Borrelia species and antigenic variation. Trends Microbiol. 1993;1:236–239. doi: 10.1016/0966-842x(93)90139-i. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S-M, Dumler J S, Feng H-M, Walker D H. Identification of the antigenic constituents of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1994;50:52–58. [PubMed] [Google Scholar]
- 8.Chen S-M, Popov V L, Feng H, Walker D H. Analysis and ultrastructural localization of Ehrlichia chaffeensis proteins with monoclonal antibodies. Am J Trop Med Hyg. 1996;54:405–412. doi: 10.4269/ajtmh.1996.54.405. [DOI] [PubMed] [Google Scholar]
- 9.Chen S-M, Popov V L, Westerman E L, Hamilton F G, Dumler J S, Feng H-M, Walker D H. Antigenic diversity among strains of Ehrlichia chaffeensis. In: Kasar J, Toman R, editors. Proceedings of the Vth International Symposium of Rickettsiae and Rickettsial Diseases. Bratislava, Slovak Republic: Slovak Academy of Sciences; 1996. pp. 329–334. [Google Scholar]
- 10.Chen S-M, Yu X-J, Popov V L, Westerman E L, Hamilton F G, Walker D H. Genetic and antigenic diversity of Ehrlichia chaffeensis: comparative analysis of a novel human strain from Oklahoma and previously isolated strains. J Infect Dis. 1997;175:856–863. doi: 10.1086/513982. [DOI] [PubMed] [Google Scholar]
- 11.Chopra I, Howe T G B, Ball P R. Lysozyme-promoted association of protein I molecules in the outer membrane of Escherichia coli. J Bacteriol. 1977;132:411–418. doi: 10.1128/jb.132.2.411-418.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dame J B, Mahan S M, Yowell C A. Phylogenetic relationship of Cowdria ruminantium, agent of heartwater, to Anaplasma marginale and other members of the order Rickettsiales determined on the basis of 16S rRNA. Int J Syst Bacteriol. 1992;42:270–274. doi: 10.1099/00207713-42-2-270. [DOI] [PubMed] [Google Scholar]
- 13.Dumler J S, Chen S-M, Asanovich K, Trigiani E, Popov V L, Walker D H. Isolation and characterization of a new strain of Ehrlichia chaffeensis from a patient with nearly fatal monocytic ehrlichiosis. J Clin Microbiol. 1995;33:1704–1711. doi: 10.1128/jcm.33.7.1704-1711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumler J S, Dawson J E, Walker D H. Human ehrlichiosis: hematopathology and immunohistologic detection of Ehrlichia chaffeensis. Hum Pathol. 1993;24:391–396. doi: 10.1016/0046-8177(93)90087-w. [DOI] [PubMed] [Google Scholar]
- 15.Eid G, French D M, Lundgren A M, Barbet A F, McElwain T F, Palmer G H. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect Immun. 1996;64:836–841. doi: 10.1128/iai.64.3.836-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eng T R, Harkess J R, Fishbein D B, Dawson J E, Greene C N, Redus M A, Satalowich E T. Epidemiologic, clinical, and laboratory findings of human ehrlichiosis in the United States, 1988. JAMA. 1990;264:2251–2258. [PubMed] [Google Scholar]
- 17.Felsenstein J. PHYLIP—phylogeny inference package (version 3.3) Cladistics. 1989;5:164–166. [Google Scholar]
- 18.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas R, Meyer T F. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986;44:107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- 20.Kim J S, Raines R T. Ribonuclease S-protein as a carrier in fusion proteins. Protein Sci. 1993;2:348–356. doi: 10.1002/pro.5560020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda K, Markowitz N, Hawley R C, Ristic M, Cox D, McDade J E. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 22.Mahan S M, Tebele N, Mukwedeya D, Semu S, Nyathi C B, Wassink L A, Kelly P J, Peter T, Barbet A F. An immunoblotting diagnostic assay for heartwater based on the immunodominant 32-kilodalton protein of Cowdria ruminantium detects false positives in field sera. J Clin Microbiol. 1993;31:2729–2737. doi: 10.1128/jcm.31.10.2729-2737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberle S M, Barbet A F. Derivation of the complete msp-4 gene sequence of Anaplasma marginale without molecular cloning. Gene. 1993;136:291–294. doi: 10.1016/0378-1119(93)90482-i. [DOI] [PubMed] [Google Scholar]
- 24.Ohashi N, Nashimoto H, Ikeda H, Tamura A. Diversity of immunodominant 56-kDa type-specific antigen (TSA) of Rickettsia tsutsugamushi: sequence and comparative analyses of the gene encoding TSA homologues from four antigenic variants. J Biol Chem. 1992;267:12728–12735. [PubMed] [Google Scholar]
- 25.Ohashi N, Tamura A, Ohta M, Hayashi K. Purification and partial characterization of a type-specific antigen of Rickettsia tsutsugamushi. Infect Immun. 1989;57:1427–1431. doi: 10.1128/iai.57.5.1427-1431.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- 27.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez M, Rikihisa Y, Wen B. Antigenic and genetic characterization of an Ehrlichia canis-like agent isolated from a human in Venezuela. J Clin Microbiol. 1996;34:2133–2139. doi: 10.1128/jcm.34.9.2133-2139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pretzman C I, Rikihisa Y, Ralph D, Gordon J C, Bech-Nielsen S. Enzyme-linked immunosorbent assay for Potomac horse fever disease. J Clin Microbiol. 1987;25:31–36. doi: 10.1128/jcm.25.1.31-36.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rikihisa Y, Ewing S A, Fox J C. Western blot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infection of dogs and humans. J Clin Microbiol. 1994;32:2107–2112. doi: 10.1128/jcm.32.9.2107-2112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rikihisa Y, Ewing S A, Fox J C, Siregar A G, Pasariba F H, Malole M B. Enzyme-linked immunosorbent assay and Western immunoblot analysis of Ehrlichia canis and canine granulocytic ehrlichiae. J Clin Microbiol. 1992;30:143–148. doi: 10.1128/jcm.30.1.143-148.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rikihisa Y, Zhang Y, Park J. Inhibition of infection of macrophages with Ehrlichia risticii by cytochalasins, monodansylcadaverine, and taxol. Infect Immun. 1994;62:5126–5132. doi: 10.1128/iai.62.11.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 34.Su H, Watkins N G, Zhang Y-X, Caldwell H D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990;58:1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumner J W, Sims K G, Jones D C, Anderson B E. Ehrlichia chaffeensis expresses an immunoreactive protein homologous to the Escherichia coli GroEL protein. Infect Immun. 1993;61:3536–3539. doi: 10.1128/iai.61.8.3536-3539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Vliet A H M, Jongejan F, van Kleef M, van der Zeijst B A M. Molecular cloning, sequence analysis, and expression of the gene encoding the immunodominant 32-kilodalton protein of Cowdria ruminantium. Infect Immun. 1994;62:1451–1456. doi: 10.1128/iai.62.4.1451-1456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verstreate D R, Creasy M T, Caveney N, Baldwin C L, Blab M W, Winter A J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982;35:979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss E, Williams J C, Dasch G A, Kang Y-H. Energy metabolism of monocytic Ehrlichia. Proc Natl Acad Sci USA. 1989;86:1674–1678. doi: 10.1073/pnas.86.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen B, Rikihisa Y, Fuerst P A, Chaichanasiriwithaya W. Analysis of 16S rRNA genes of ehrlichial organisms isolated from horses with clinical signs of Potomac horse fever. Int J Syst Bacteriol. 1995;45:315–318. doi: 10.1099/00207713-45-2-315. [DOI] [PubMed] [Google Scholar]
- 40.Yu X, Brouqui P, Dumler J S, Raoult D. Detection of Ehrlichia chaffeensis in human tissue by using a species-specific monoclonal antibody. J Clin Microbiol. 1993;31:3284–3288. doi: 10.1128/jcm.31.12.3284-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu X-J, Crocquet-Valdes P, Cullman L C, Piesman J F, Olson J G, Walker D H. Genetic divergence of a 120-kDa immunodominant protein of Ehrlichia chaffeensis: a potential recombinant diagnostic tool. In: Kasar J, Toman R, editors. Proceedings of the Vth International Symposium of Rickettsiae and Rickettsial Diseases. Batislava, Slovak Republic: Slovak Academy of Sciences; 1996. pp. 324–328. [Google Scholar]
- 42.Yu X-J, Crocquet-Valdes P, Walker D H. Cloning and sequencing of the gene for a 120-kDa immunodominant protein of Ehrlichia chaffeensis. Gene. 1997;184:149–154. doi: 10.1016/s0378-1119(96)00586-0. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J-R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of vmp-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Ohashi N, Lee E H, Tamura A, Rikihisa Y. Ehrlichia sennetsu groEL operon and antigenic properties of the GroEL homolog. FEMS Immunol Med Microbiol. 1997;18:39–46. doi: 10.1111/j.1574-695X.1997.tb01025.x. [DOI] [PubMed] [Google Scholar]