Abstract
Interleukin-15 (IL-15) is a newly described cytokine that shares biological activities with IL-2. We report here results demonstrating the ability of IL-15 to enhance superoxide production and antifungal activity of human monocytes. After 18 and 48 h of treatment with IL-15, human elutriated monocytes manifested enhanced superoxide production in response to either phorbol myristate acetate or opsonized Candida albicans blastoconidia. Similar results were obtained when monocytes were treated with IL-2, but to a lesser extent. Combination studies with IL-15 and IL-2 showed no additive or synergistic effects. Following incubation of monocytes with IL-15 for 18 h, there was no significant increase in mRNA transcripts for components of the NADPH oxidase complex, p40-phox, p47-phox, and gp91-phox, suggesting a posttranscriptional modulation of enhanced superoxide production. Antibodies against the γ chain of the IL-2 receptor and, to a lesser extent, against the β chain partially abrogated the IL-15-mediated enhanced superoxide production. Additionally, human monocytes showed enhanced killing activity against C. albicans after 18 h of incubation with IL-15 or IL-2, but this treatment did not enhance the ability of these cells to phagocytose the organism. In addition, the enhanced fungicidal activity seen after 18 h of treatment was no longer detectable after 48 h of cytokine treatment. Culture supernatants from the IL-15-treated monocytes were assayed for the presence of other proinflammatory cytokines. IL-15 treatment did not induce the release of detectable levels of tumor necrosis factor alpha, IL-1β, or IL-12. Our results indicate that IL-15 upregulates the microbicidal activity of human monocytes against C. albicans.
Interleukin-15 (IL-15) is a recently identified cytokine of the four-helix bundle family that was independently described by two separate laboratories (6, 17). It shares functional attributes with IL-2, including enhanced proliferation of phytohemagglutinin-activated human T cells, generation of cytotoxic T lymphocytes, and activation of human NK cells (7, 14, 17). In addition, IL-15 can induce differentiation and proliferation of B lymphocytes (2) and is chemotactic for human T lymphocytes but not monocytes, polymorphonuclear leukocytes, or B lymphocytes (45). Northern blot analysis has indicated high-level expression of IL-15 message in monocytes/macrophages, placenta, and skeletal muscle and expression at lower levels in heart, lung, kidney, liver, and epithelial cells (17). However, unlike the extensively studied lymphokine IL-2, with which it shares many of the same bioactivities, IL-15 is not produced by activated peripheral blood T cells.
IL-15 utilizes the β and γ subunits of the IL-2 receptor (IL-2R) complex for binding and signal transduction (15). Anti-IL-2R-β-chain but not anti-IL-2R-α-chain antibodies have been shown to abrogate some IL-15 activities (2, 7, 15, 17, 45). Furthermore, B lymphocytes isolated from patients with X-linked severe combined immunodeficiency that lack the γ chain of the IL-2R cannot proliferate or differentiate in response to IL-15 (29). However, some cell types express one or more IL-15-specific receptor components that are distinctly different from known components of the IL-2R (16, 41).
Invasive fungal infections are an ever-increasing problem in the management of immunocompromised patients. With the poor prognosis associated with mycoses in this population, there has been an increased focus on potential means for augmenting the immune system. A number of cytokines, including the colony-stimulating factors (CSFs) granulocyte CSF, granulocyte-macrophage CSF (GM-CSF), and macrophage CSF (M-CSF), the interferons (IFNs) gamma IFN (IFN-γ) and IFN-α, and the interleukins IL-2 and IL-12, have been evaluated for their role in augmenting the host response to fungal pathogens (25, 37, 46).
Little information is available on the role of IL-15 in the host response to infection. IL-15 augments T-cell-mediated immunity against Toxoplasma gondii (21) and Salmonella cholaraesuis (33) and may be important in the host response to human immunodeficiency virus (HIV) infection (10, 39). To assess the potential role for IL-15 in augmenting the phagocytic host response, we examined its effects on functional responses of human monocytes and compared these responses to those seen with IL-2. Specifically, we examined the effects of IL-15 on human peripheral monocytes as measured by superoxide production, NADPH oxidase gene expression, fungicidal activity against and phagocytosis of Candida albicans, and the release of other cytokines.
MATERIALS AND METHODS
Reagents.
Phorbol myristate acetate (PMA) (Sigma Chemical Co., St. Louis, Mo.) was stored at −70°C in a stock solution of 2 mg/ml in dimethyl sulfoxide. Working solutions were prepared fresh daily by diluting PMA in Hanks balanced salt solution (HBSS) containing calcium and magnesium to a final concentration of 500 ng/ml. Horse heart cytochrome c (Sigma) was used at a final concentration of 50 μM. Mouse monoclonal anti-IL-2R-β-chain antibodies (R&D Systems, Minneapolis, Minn.), rabbit polyclonal anti-IL-2R-γ-chain antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), and mouse monoclonal anti-penicillin type I isotype-matched control antibodies (Calbiochem, San Diego, Calif.) were used in neutralization studies. Human recombinant IL-2 (4 × 106 U/mg) and human recombinant IL-15 (2 × 106 U/mg) were obtained from Genzyme (Boston, Mass.) and R&D Systems, respectively. Complete culture medium consisted of RPMI 1640 (Mediatech, Washington, D.C.), supplemented with 10% fetal calf serum (GIBCO Life Technologies, Gaithersburg, Md.) and l-glutamine and penicillin-streptomycin (Sigma).
Organism.
A clinical isolate of C. albicans (strain 86-21), originally isolated from a neutropenic patient, was used throughout these experiments. Prior to use, the organism was streaked from a frozen stock onto Sabouraud dextrose agar and incubated at 37°C for 24 h. Well-isolated colonies were subsequently inoculated into Emmon’s modification of Sabouraud glucose broth, incubated in a gyratory water bath at 37°C for 18 h, centrifuged, and washed twice with 0.154 M NaCl. Organisms were resuspended in HBSS, and the concentration was adjusted by hemacytometer counts. The blastoconidia were opsonized in 50% pooled human AB-positive serum for 30 min at 37°C, washed once, and resuspended in HBSS to a final concentration of 107 organisms/ml. Organisms were maintained on ice until use.
Isolation of peripheral blood monocytes.
Peripheral blood monocytes were isolated from healthy human donors by a two-step procedure consisting of automated leukophoresis and counterflow elutriation (model J-6M centrifuge; Beckman Instruments, Fullerton, Calif.) (42). Cell viability was ≥95% as determined by trypan blue exclusion, and the cell population isolated was ≥95% monocytes as determined by morphology and nonspecific esterase staining. Following isolation, the monocytes were washed twice, resuspended in HBSS without calcium and magnesium, and kept on ice until use.
Respiratory burst assay.
Superoxide anion (O2−) production was measured spectrophotometrically in a discontinuous assay for superoxide dismutase-inhibitable cytochrome c reduction (20). Monocytes (107) were incubated for 4, 18, or 48 h at 37°C with increasing concentrations of IL-2 or IL-15 in 1 ml of complete medium. Control cells were incubated in medium alone. Following cytokine treatment, the cells were washed once and used at a final concentration of 106 cells/ml in HBSS. PMA (500 ng/ml) or opsonized C. albicans blastoconidia (106) were added as stimuli, and the reaction mixtures were incubated on a rotator for 30 min at 37°C. Reference tubes contained the above-described constituents as well as 20 μg of superoxide dismutase per ml or contained stimuli without cells. Cells and organisms were removed by centrifugation at 4°C, and the absorbance at 550 nm was determined spectrophotometrically for each sample supernatant. Superoxide production was calculated by using the millimolar extinction coefficient for reduced cytochrome c and expressed as nanomoles of O2− per 106 cells per 30 min (28).
Fungicidal assay.
Monocytes were incubated for 18 h at 37°C with increasing concentrations of either IL-2 or IL-15 or with medium alone. Following cytokine treatment, the cells were washed once and their fungicidal activity was determined by a CFU assay. Serum-opsonized C. albicans blastoconidia were mixed with monocytes at a final effector-to-target cell ratio of 10:1 or 1:1 in HBSS containing 0.1% bovine serum albumin. Samples were incubated on a rotator at 37°C, and aliquots were collected at 30 and 90 min. Phagocytes were lysed in sterile water; samples were serially diluted and plated on Sabouraud dextrose agar plates. CFU were determined following a 24-h incubation at 37°C. The percent killing was calculated as follows: percent killing = 1 − sample CFU/control CFU × 100.
Phagocytosis assay.
Suspensions of 106 monocytes in complete medium were placed on 16-mm sterile circular glass cover slides in 12-well flat-bottomed plates (Costar, Cambridge, Mass.) and were incubated at 37°C in 5% CO2 for 1 h. The nonadherent cells were removed by washing the glass cover slides once with warm HBSS. A 1-ml suspension of 106 C. albicans blastoconidia suspended in prewarmed complete medium was then added and incubated with the cells for 1 h at 37°C in 5% CO2. Cover slides were washed three times with warm HBSS to remove extracellular organisms, and cells were fixed and stained with modified Wright-Giemsa stain. The percent phagocytosis was calculated by determining microscopically the percent monocytes containing organisms.
Neutralization studies.
Neutralization experiments were performed with antibodies against the β chain (mouse monoclonal immunoglobulin G1) and γ chain (affinity-purified rabbit polyclonal antibody) of the IL-2R. Monocytes were preincubated for 1 h at 37°C with gentle agitation with either anti-β or anti-γ IL-2R antibody in complete medium without antibiotics. IL-15 or IL-2 (100 ng/ml) was added, and the monocytes were incubated for an additional 18 h. Mouse anti-penicillin monoclonal antibodies and rabbit anti-chicken immunoglobulin G1, respectively, were used as isotype-matched controls. Superoxide anion release was measured as described above.
Northern blot analysis.
Total mRNA was isolated from cytokine-treated monocytes by the RNAzol method (TEL-TEST, Inc., Friendwood, Tex.) according to the instructions of the manufacturer. Twenty micrograms of mRNA per well was loaded and electrophoresed on a 1% agarose-formaldehyde gel. The mRNA was transferred to Hybond-N+ (Amersham, Arlington Heights, Il.) overnight and UV cross-linked. The membranes were prehybridized overnight at 42°C in a solution containing 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5× Denhardt’s solution, 50% formamide, 0.25% sodium dodecyl sulfate, and 20 μg of single-stranded DNA. Overnight hybridization with a random-primed radiolabelled cDNA probe was performed (38). Three separate full-length cDNA probes, p40-phox, p47-phox, and gp91-phox, were used consecutively. Washing conditions were as follows: 2×, 1×, and 0.5× SSC, each containing 0.1% sodium dodecyl sulfate, at 42°C for 20 min each. Autoradiography was performed at −70°C with X-ray film (X-OMAT-A-R-Kodak; Eastman Kodak Co., Rochester, N.Y.) and an intensifying screen. Beta-actin was used as a standard for quantitation of the amount of mRNA loaded.
Proinflammatory cytokine levels.
Levels of tumor necrosis factor alpha (TNF-α), IL1-β, and IL-12 released from cytokine-treated monocytes were determined with commercially available enzyme-linked immunosorbent assays. Monocytes were treated with 100 ng of IL-15 or IL-2 per ml, or with medium alone, and incubated for 18 h at 37°C. Cells were removed by centrifugation, and supernatants were analyzed according to the procedure recommended by the manufacturer (R&D Systems).
Statistical analysis.
Statistical significance was determined by using an unpaired Student t test. All comparisons were two sided, and a P value of <0.05 was considered significant.
RESULTS
Superoxide anion production.
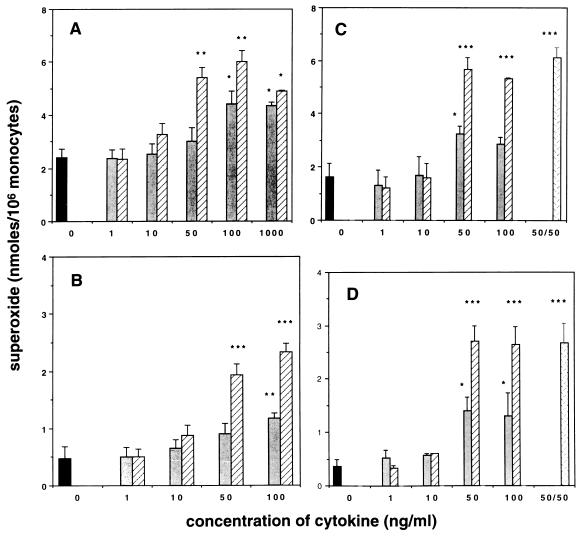
Human monocytes were treated with 1 to 1,000 ng of IL-15 or IL-2 per ml for 4, 18, or 48 h prior to measuring the superoxide dismutase-inhibitable superoxide anion release. Figure 1 shows the results obtained when monocytes were incubated for 18 h with IL-15 or IL-2 and then stimulated with PMA (Fig. 1A) or opsonized C. albicans blastoconidia (Fig. 1B). The effect was concentration dependent, with significant increases in measurable O2− from cells treated with ≥50 ng of IL-15 per ml or ≥100 ng of IL-2 per ml. Following PMA stimulation, IL-15-treated monocytes generated two- to threefold more O2− compared to untreated control monocytes (6.0 ± 0.4 versus 2.4 ± 0.3 nmol, respectively), with a maximal effect being seen with 100 ng/ml (Fig. 1A). Stimulation of IL-15-treated human monocytes with opsonized C. albicans also resulted in a significant increase in the generation of O2− compared with that for untreated control cells (2.3 ± 0.1 versus 0.5 ± 0.2 nmol of O2−, respectively) (Fig. 1B). IL-2 at 100 ng/ml induced an increase in the production of O2− to a lesser extent. In response to PMA and opsonized C. albicans, IL-2-treated monocytes released 4.4 ± 0.5 and 0.9 ± 0.2 nmol of O2−, respectively. Neither IL-15 nor IL-2 was able to directly induce O2− release from monocytes (data not shown).
FIG. 1.
Enhanced O2− production by IL-15- and IL-2-treated monocytes. Human elutriated monocytes were treated for 18 h (A and B) or 48 h (C and D) with increasing concentrations of IL-15 (▨) or IL-2 ( ). Untreated control cells were simultaneously incubated in medium alone (▪). Cells treated for 48 h also were incubated with a combination of IL-2 and IL-15 at 50 ng of each per ml (▩). Superoxide anion release was measured in response to PMA (A and C) or opsonized C. albicans blastoconidia (B and D), as described in Materials and Methods. Data were collected from four to six experiments, performed in duplicate with different donors, and are expressed as means ± standard errors of the means. There was a significant enhancement in O2− release from cells treated with ≥50 ng of IL-15 per ml (∗∗, P < 0.01; ∗∗∗, P < 0.001) or ≥100 ng of IL-2 per ml (∗, P ≤ 0.05; ∗∗, P < 0.01) in response to both PMA and C. albicans blastoconidia.
Similar results were obtained when monocytes were incubated for 48 h with either IL-15 or IL-2 and stimulated with PMA (Fig. 1C) or opsonized C. albicans blastoconidia (Fig. 1D). The amount of superoxide released following 48 h of preincubation with IL-15 was comparable to that released from monocytes treated for 18 h. Incubation of monocytes for 4 h with either IL-15 or IL-2 did not augment O2− production regardless of the stimuli used to trigger the respiratory burst (data not shown).
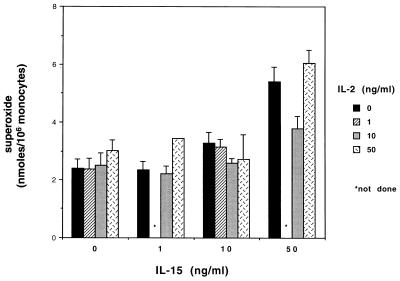
Effect of combinations of IL-2 and IL-15 on superoxide production.
Optimal release of O2− was observed when cells were preincubated with IL-2 and IL-15 at concentrations of ≥100 and 50 ng/ml, respectively. Therefore, combinations of suboptimal concentrations of each cytokine were analyzed to determine if a synergistic or additive effect could be observed. Figure 2 illustrates the results obtained when monocytes were treated with combinations of increasing concentrations of IL-15 and IL-2. The combinations of these cytokines did not show any additive or synergistic effect on the production of O2− following PMA stimulation. The effect of IL-15 appeared to be dominant, since the amount of O2− released with combinations of IL-15 and IL-2 was equivalent to the levels attained with IL-15 alone.
FIG. 2.
Effect of combinations of escalating concentrations of IL-15 and IL-2 on monocyte O2− release in response to PMA. Human elutriated monocytes were treated for 18 h with dose-escalating combinations of IL-15 and IL-2 or with increasing concentrations of each cytokine alone. Untreated control cells were simultaneously incubated in medium only. Superoxide anion release in response to PMA was measured as described in Materials and Methods. Data represent the means ± standard errors of the means from at least three separate experiments run in duplicate. There was no significant enhancement in O2− release with any combination of IL-15 and IL-2 compared with IL-15 alone.
Neutralization of IL-15-induced enhancement in monocyte superoxide production.
As the β and γ chains of the IL-2R have been shown to participate in binding and signaling of IL-15, studies were performed with anti-IL-2R-β- and anti-IL-2R-γ-chain antibodies to inhibit IL-15 activity. As shown in Table 1, antibodies against the γ chain of the IL-2R and, to a lesser extent, anti-β-chain antibodies were effective in partially abrogating IL-15 activity. There was 77 and 92% inhibition of the enhanced O2− release when the anti-γ-chain antibody was used at 5 and 10 μg/ml, respectively. There was no neutralization of the IL-15-induced enhancement when isotype-matched control antibodies were used.
TABLE 1.
Neutralization of IL-15-enhanced superoxide release from human monocytes
| Antibody (μg/ml) | Superoxide releasea | % Inhibitionb |
|---|---|---|
| None | 3.79 ± 0.40 | |
| Anti-IL2R-β (5) | 3.64 ± 0.31 | 10.25 ± 6.39 |
| Anti-IL2R-β (10) | 3.41 ± 0.22 | 25.17 ± 7.97 |
| Anti-IL2R-γ (5) | 0.75 ± 0.28 | 77.04 ± 13.8 |
| Anti-IL2R-γ (10) | 0.37 ± 0.13 | 92.75 ± 3.35 |
All cells were treated with IL-15 as described in Materials and Methods. Data represent the amounts of superoxide generated above that by untreated control cells and are expressed as nanomoles of O2− per 106 cells. Data represent means ± standard error of the means from at least three separate experiments performed in duplicate.
Percent inhibition of the IL-15-enhanced O2− release compared with that of cells treated with cytokine alone. There was no suppression in the IL-15-induced enhancement when isotype-matched control antibodies were used.
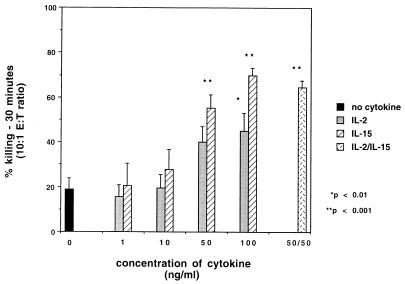
Antifungal activity and phagocytosis.
Figure 3 illustrates the enhanced fungicidal capability of monocytes treated with IL-15 for 18 h. There was 55.2 ± 6.0 and 69.7 ± 3.6% killing of C. albicans by monocytes treated with 50 and 100 ng of IL-15 per ml, respectively; with untreated control monocytes, there was 19.0 ± 4.7% killing. Treatment with IL-2 (100 ng/ml) also was effective in enhancing the fungicidal capability of human monocytes but to a lesser extent than IL-15 (45.0 ± 7.9% versus 69.7 ± 3.6%; P = 0.02). However, treatment with neither IL-15 nor IL-2 resulted in enhanced phagocytosis of the blastoconidia (data not shown). Since monocytes were washed prior to incubation with C. albicans, neither IL-2 nor IL-15 was present during the incubation of cells with organisms. Therefore, the decrease in CFU of C. albicans per milliliter was not due to a direct effect of the cytokine on the fungus. Monocytes incubated for 48 h with either IL-2 or IL-15 did not show enhanced killing activity against C. albicans despite the increase in O2− release (data not shown).
FIG. 3.
Fungicidal activity of monocytes against C. albicans following treatment with either IL-15 or IL-2 or a combination of IL-15 and IL-2. Monocytes were incubated with 1 to 100 ng of IL-15 or IL-2 per ml or with a combination of 50 ng of both cytokines per ml for 18 h prior to testing fungicidal activity against C. albicans. Untreated control cells were simultaneously incubated in medium alone. The figure depicts the 30-min time point at an effector-to-target cell (E:T) ratio of 10:1. Data were collected from three experiments performed in duplicate, using different donors, and are expressed as means ± standard errors of the means. There was a significant enhancement in fungicidal activity with cells treated with ≥50 ng of IL-15 per ml, 100 ng of IL-2 per ml, and the combination of these cytokines.
Northern blot analysis of IL-15-treated monocytes.
Northern blot analysis of mRNA extracted from untreated monocytes and from monocytes treated for 18 h with 100 ng of IL-15 per ml was performed to determine if enhanced O2− production was due to an upregulation in gene expression of components of the NADPH oxidase. With this method, there was no significant increase detected in mRNA for p40-phox, p47-phox, or gp91-phox (Table 2). Monocytes treated with 100 U of IFN-γ per ml as a positive control demonstrated an increase in mRNA expression for both gp91-phox and p47-phox.
TABLE 2.
Effect of IL-15 on mRNA expression for components of the phagocyte NADPH oxidase
| mRNA | Increase in expression
witha:
|
|
|---|---|---|
| IL-15 | IFN-γ | |
| gp91-phox | 1.28 | 2.20 |
| 1.40 | 1.60 | |
| p47-phox | 1.08 | 6.99 |
| 1.30 | 3.80 | |
| p40-phox | 1.05 | 1.10 |
| 1.10 | 1.40 | |
Expressed as fold increase over expression by resting cells (= 1). Total mRNA was isolated from monocytes that were treated with IL-15 (100 ng/ml), IFN-γ (100 U/ml), or medium alone for 18 h. Levels were determined by imaging densitometry after correction for total mRNA levels against a β-actin control. The data presented are results obtained from two separate experiments.
Release of proinflammatory cytokines.
In order to determine if the enhancement of superoxide release and fungicidal activity of IL-15-treated monocytes was due to the release of other proinflammatory cytokines, supernatants harvested from treated and nontreated cells were analyzed. As shown in Table 3, IL-15 treatment did not induce release of detectable levels of TNF-α, IL-1β, or IL-12 from these monocytes.
TABLE 3.
IL-15 does not induce the release of IL-1β, TNF-α, or IL-12a
| Treatment | Amt (pg/ml)b of:
|
||
|---|---|---|---|
| IL-1β | TNF-α | IL-12 | |
| None | <15.6 | <3.9 | <7.8 |
| IL-15 | <15.6 | <3.9 | <7.8 |
| IL-2 | 28 | <3.9 | <7.8 |
| LPS | 2,267 | 480 | NDc |
Supernatants harvested from monocytes cultured for 18 h with 100 ng of IL-15, IL-2, or LPS per ml or with medium alone were analyzed by enzyme-linked immunosorbent assay according to the manufacturer’s (R&D Systems) recommended procedures in order to determine levels of the indicated cytokines.
The values are the calculated means of results obtained from supernatants which were run in duplicate and collected from at least two separate experiments.
ND, not determined.
DISCUSSION
Invasive fungal infections, especially disseminated candidiasis, are major medical challenges in the management of immunocompromised patients (35). Since phagocytic cells play a critical role in normal host defenses, enhancing their function through administration of recombinant cytokines may be of potential clinical utility. Accordingly, we examined the ability of IL-15 to modulate normal human phagocyte function. We found that pretreatment of normal human monocytes with IL-15 enhanced O2− release in response to both soluble and particulate stimuli, i.e., PMA and opsonized C. albicans blastoconidia, respectively. The enhanced oxidative burst was observed following 18 or 48 h of treatment with IL-15. Furthermore, antibodies against the γ chain of the IL-2R were able to neutralize the IL-15 enhancement of O2− production. Treatment of monocytes with combinations of IL-15 and IL-2 did not result in additive or synergistic effects on O2− release. In addition, IL-15-treated (18 h) monocytes showed an increase in fungicidal activity against C. albicans but not an enhanced ability to phagocytose the organism. However, monocytes treated with IL-15 for 48 h did not retain the enhanced candidacidal activity.
IL-2 has been reported to modulate monocyte function by enhancing microbicidal and tumoricidal activity as well as cytokine and H2O2 release (12, 26, 43). Wahl et al. showed that IL-2 enhanced O2− production in monocytes that were preincubated with suboptimal concentrations of either lipopolysaccharide (LPS) or LPS plus IFN-γ (43). To our knowledge, there are no reports showing that IL-2 or IL-15 alone can augment candidacidal activity or O2− production in human monocytes. In our studies, we demonstrated that IL-15 alone can enhance the oxidative response from human monocytes. We observed similar results when monocytes were treated with IL-2, albeit to a lesser extent.
The effects of GM-CSF, M-CSF, IL-3, and IFN-γ on the respiratory burst and/or microbicidal activity of monocytes have been reported previously (5, 13, 27, 31, 40). Increased candidacidal activity of GM-CSF-treated monocytes was shown to correlate with an increase in O2− production (40). With human monocytes collected from sarcoma patients with severe neutropenia, increased O2− production following continuous infusion of recombinant GM-CSF has been reported (34). In a recent study, M-CSF has been shown to augment microbicidal activity of human monocytes collected from patients with metastatic cancer following M-CSF therapy (36). In our studies, IL-15 was not capable of prolonging the enhanced candidacidal activity of monocytes despite the continued enhancement of O2− production. It is possible that the physiologic changes that occur in these cells during culture affect their ability to maintain the enhanced candidacidal activity induced by IL-15. Similar results were reported by Wang et al., who showed that monocytes cultured in IFN-γ progressively lost their Candida-killing activity after 2 days in culture (44).
At this point, the mechanism by which IL-15 modulates monocyte function is not clear. Enhanced oxidative burst activity following cytokine treatment may be a result of its action on the phagocyte NADPH oxidase complex. Levels of protein and message for gp91-phox increase in response to IFN-γ (1, 18, 32). The effects of IFN-γ on p47-phox message and protein levels are more complex, with some authors reporting an increase and others reporting a decrease, depending on the types of phagocytic cells studied (1, 9, 18, 19, 24). However, the increase in O2− production following monocyte treatment with IL-15 did not correlate with an increase in mRNA for gp91-phox, p47-phox, or p40-phox. Thus, it is likely that the increased O2− release is not directly related to an upregulation of gene expression of NADPH oxidase components. Our findings suggest that the enhanced respiratory burst activity is due to one or more of the following: a posttranslational effect involving increased protein expression and/or assembly of the NADPH oxidase complex or a prostimulatory effect on signal transduction pathways critical for activation of the NADPH oxidase. This increase in O2− production without a significant increase in mRNA for NADPH oxidase components is similar to findings reported for monocytic cells treated with M-CSF or IFN-γ (9, 36).
Another potential mechanism responsible for the IL-15-induced enhancement of superoxide release could be its ability to stimulate the release of other proinflammatory cytokines. Those which previously have been shown to augment functional activity of phagocytic cells include IL-1β, TNF-α, and IL-12 (22, 23, 37). However, supernatants collected from IL-15-treated monocytes did not have detectable levels of any of these cytokines. These findings are similar to those recently reported by others (4, 30). However, Badolato et al. did report an increase in release of monocyte chemotactic protein 1 following IL-15 treatment (4), which also could be responsible for the upregulation in release of superoxide anions (3). It is possible that IL-15 treatment induces the release of still other inflammatory cytokines that could have a role in regulation of monocyte function, and this thus warrants further investigation.
There is a paucity of data available on the role of IL-15 in response to infectious agents or its effect on monocyte function. Recent studies suggest that IL-15 may have a potent immunoregulatory role during HIV infection by enhancing the proliferation of peripheral blood mononuclear cells in response to HIV-specific antigens (39) and by restoring the deficient production of IL-12 by peripheral blood mononuclear cells from HIV-positive patients (10). With murine models, it has recently been reported that IL-15 may play a protective role against Toxoplasma and Salmonella infections (21, 33). Here we provide evidence that IL-15 may play a role in modulating human monocyte antimicrobial activity against Candida.
The production of IL-15 by LPS-activated human monocytes and its role in NK cell activation have been reported (8). In addition, pretreatment of murine bone marrow-derived macrophages with IFN-γ followed by stimulation with BCG results in enhanced production of IL-15 mRNA (11). These data, along with data presented in this report, suggest that IL-15 may be an important protein in activating antimicrobial pathways of both phagocytic and nonphagocytic cells. Further investigation is warranted to determine the utility of IL-15 as a single agent and in combination with other immunomodulators for augmenting host defenses against opportunistic infections.
ACKNOWLEDGMENTS
We thank Robert Seder and Louis Rosenthal for their support and helpful suggestions.
REFERENCES
- 1.Abramson S L, Lomax K J, Malech H L, Gallin J I. Recombinant human interferon-γ and interleukin-4 regulate gene expression of several phagocyte oxidase components. Clin Res. 1990;38:2367. . (Abstract.) [Google Scholar]
- 2.Armitage R J, Macduff B M, Eisenman J, Paxton R, Grabstein K H. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–490. [PubMed] [Google Scholar]
- 3.Azuma E K, Yuo A, Matsushima T, Kasahara T, Mizoguchi H, Saito M, Takaku F, Kitagawa S. Activation and priming of human monocytes by monocyte chemotactic activating factor: cooperation with other inflammatory cytokines and close association between an increase in cytoplasmic free Ca2+ and intracellular acidification. Exp Hematol. 1996;24:169–175. [PubMed] [Google Scholar]
- 4.Badolato R, Negro Ponzi A, Millesimo M, Notarangelo L D, Musso T. Interleukin-15 (IL-15) induces IL-8 and monocyte chemotactic protein 1 production in human monocytes. Blood. 1997;90:2804–2809. [PubMed] [Google Scholar]
- 5.Bober L A, Grace M J, Pugliese-Sivo G, Rojas-Triana A, Sullivan L M, Narula S K. The effects of colony stimulating factors on human monocyte-cell function. Int J Immunopharmacol. 1995;17:385–392. doi: 10.1016/0192-0561(95)00025-w. [DOI] [PubMed] [Google Scholar]
- 6.Burton J D, Bamford R N, Peters C, Grant A J, Kurys G, Goldman C K, Brennan J, Roessler E, Waldmann T A. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carson W E, Giri J G, Lindenman M J, Linnet M L, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri M A. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson W E, Ross M E, Baiocchi R A, Marien M J, Boiani N, Grabstein K, Caligiuri M A. Endogenous production of Interleukin 15 by activated human monocytes is critical for optimal production of interferon-γ by natural killer cells in vitro. J Clin Invest. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassatella M A, Bazzoni F, Flynn R M, Dusi S, Trinchieri G, Rossi F. Molecular basis of interferon-γ and lipopolysaccharide enhancement of phagocyte respiratory burst capability: studies on the gene expression of several NADPH oxidase components. J Biol Chem. 1990;265:20241–20246. [PubMed] [Google Scholar]
- 10.Chehimi J, Marshall J D, Salvucci O, Frank I, Chehimi S, Kawecki S, Bacheller D, Rifat S, Chouaib S. IL-15 enhances immune functions during HIV infection. J Immunol. 1997;158:5978–5987. [PubMed] [Google Scholar]
- 11.Doherty T M, Seder R A, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- 12.Espinoza-Delgado I, Bosco M C, Musso T, Gusella G L, Longo D L, Varesio L. Interleukin-2 and human monocyte activation. J Leukocyte Biol. 1995;57:13–19. doi: 10.1002/jlb.57.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Fabian I, Shapira E, Gadish M, Kletter Y, Naggler A, Flidel O, Slavin S. Effects of human interleukin 3, macrophage and granulocyte-macrophage colony-stimulating factor on monocyte function following autologous bone marrow transplantation. Leukemia Res. 1992;16:703–709. doi: 10.1016/0145-2126(92)90021-x. [DOI] [PubMed] [Google Scholar]
- 14.Flamand L, Stefanescu I, Menezes J. Human herpesvirus-6 enhances natural killer cell cytotoxicity via IL-15. J Clin Invest. 1996;97:1373–1381. doi: 10.1172/JCI118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giri J G, Ahdieh M, Eisenman J, Shanebeck K, Kumaki S, Namen A, Park L S, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giri J G, Kumaki S, Ahdieh M, Friend D J, Loomis A, Shanebeck K, DuBose R, Cosman D, Park L S, Anderson D M. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn M A, Ahdieh M, Johnson L, Alderson M R, Watson J D, Anderson D M, Giri J G. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 18.Gupta J W, Kubin M, Hartman L, Cassatella M, Trinchieri G. Induction of expression of genes encoding components of the respiratory burst oxidase during differentiation of human myeloid cell lines induced by tumor necrosis factor and γ-interferon. Cancer Res. 1992;52:2530–2537. [PubMed] [Google Scholar]
- 19.Jendrossek V, Damm U, Liese J, Belohradsky B H, Gahr M. Is the IFN-γ-induced enhancement of superoxide production in CGD-phagocytes caused by increased expression of the p47-phox cytosolic protein? Immunodeficiency. 1993;4:187–190. [PubMed] [Google Scholar]
- 20.Johnston R B, Jr, Kee B B, Jr, Misra H P, Lehmeyer J E, Webb L S, Baehner R L, Rajagopalan K V. The role of superoxide generation in phagocytic bactericidal activity: studies with normal and chronic granulomatous disease neutrophils. J Clin Invest. 1975;55:1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan I A, Kasper L H. IL-15 augments CD8+ T cell mediated immunity against Toxoplasma gondiiinfection in mice. J Immunol. 1996;157:2103–2108. [PubMed] [Google Scholar]
- 22.Kharazmi A, Nielsen H, Bendtzen K. Recombinant interleukin 1 alpha and beta prime human monocyte superoxide production but have no effect on chemotaxis and oxidative burst response of neutrophils. Immunobiology. 1988;177:32–39. doi: 10.1016/s0171-2985(88)80089-5. [DOI] [PubMed] [Google Scholar]
- 23.Kitagawa S, Yuo A, Yagisawa M, Azuma E, Yoshida M, Furukawa Y, Takahashi M, Masuyama J, Takaku F. Activation of human monocyte functions by tumor necrosis factor: rapid priming for enhanced release of superoxide and erythrophagocytosis, but no direct triggering of superoxide release. Exp Hematol. 1996;24:559–567. [PubMed] [Google Scholar]
- 24.Kuga S, Takeshi O, Niiro H, Nunoi H, Nemoto Y, Nakano T, Ogo T, Umei T, Niho Y. Suppression of superoxide anion production by interleukin-10 is accompanied by a downregulation of the genes for subunit proteins of NADPH oxidase. Exp Hematol. 1996;24:151–157. [PubMed] [Google Scholar]
- 25.Levitz S M. Activation of human peripheral blood mononuclear cells by interleukin-2 and granulocyte-macrophage colony-stimulating factor to inhibit Cryptococcus neoformans. Infect Immun. 1991;59:3393–3397. doi: 10.1128/iai.59.10.3393-3397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malkovsky M, Loveland B, North M, Asherson L, Gao L, Ward P, Fiers W. Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes. Nature. 1987;325:262–265. doi: 10.1038/325262a0. [DOI] [PubMed] [Google Scholar]
- 27.Marodi L, Johnston R B., Jr Enhancement of macrophage candidacidal activity by interferon-gamma. Immunodeficiency. 1993;4:181–185. [PubMed] [Google Scholar]
- 28.Massey V. The microestimation of succinate and the extinction coefficient of cytochrome C. Biochim Biophys Acta. 1959;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- 29.Matthews D J, Clark P A, Herbert J, Morgan G, Armitage R J, Kinnon C, Minty A, Grabstein K H, Caput D, Ferrara P, Callard R. Function of the interleukin-2 (IL-2) receptor γ-chain in biologic responses of X-linked severe combined immunodeficient B cells to IL-2, IL-4, IL-13, and IL-15. Blood. 1995;85:38–42. [PubMed] [Google Scholar]
- 30.McInnes I B, Leung B P, Sturrock R D, Field M, Liew F Y. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 31.Nathan C F, Murray H W, Wiebe M E, Rubin B Y. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newburger P E, Ezekowitz R A B, Whitney C, Wright J, Orkin S H. Induction of phagocyte cytochrome b heavy chain gene expression by interferon-γ Proc. Natl Acad Sci USA. 1988;85:5215–5219. doi: 10.1073/pnas.85.14.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura H, Hiromatsu K, Kobayashi N, Grabstein K H, Paxton R, Sugamura K, Bluestone J A, Yoshikai Y. IL-15 is a novel growth factor for murine gamma delta T cells induced by Salmonellainfection. J Immunol. 1996;156:663–669. [PubMed] [Google Scholar]
- 34.Perkins R C, Vadham-Raj S, Scheule R K, Hamilton R, Holian A. Effects of continuous high dose rh-GM-CSF infusion on human monocyte activity. Am J Hematol. 1993;43:279–285. doi: 10.1002/ajh.2830430410. [DOI] [PubMed] [Google Scholar]
- 35.Pizzo P A, Rubin M, Freifeld A, Walsh T J. The child with cancer and infection. II. Nonbacterial infections. J Pediatr. 1991;119:845–857. doi: 10.1016/s0022-3476(05)83032-x. [DOI] [PubMed] [Google Scholar]
- 36.Roilides E, Lyman C A, Mertins S D, Cole D J, Venzon D, Pizzo P A, Chanock S J, Walsh T J. Ex vivoeffects of macrophage colony-stimulating factor on human monocyte activity against fungal and bacterial pathogens. Cytokine. 1996;8:42–48. doi: 10.1006/cyto.1996.0006. [DOI] [PubMed] [Google Scholar]
- 37.Roilides E, Pizzo P A. Modulation of host defenses by cytokines: evolving adjuncts in prevention and treatment of serious infections in immunocompromised hosts. Clin Infect Dis. 1992;15:508–524. doi: 10.1093/clind/15.3.508. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 746–752. [Google Scholar]
- 39.Seder R A, Grabstein K H, Berzofsky J A, McDyer J F. Cytokine interactions in human immunodeficiency virus-infected individuals: roles of interleukin (IL)-2 and IL-15. J Exp Med. 1995;182:1067–1077. doi: 10.1084/jem.182.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith P D, Lamerson C L, Banks S M, Saini S S, Wahl L M, Calderone R A, Wahl S M. Granulocyte-macrophage colony-stimulating factor augments human monocyte fungicidal activity for Candida albicans. J Infect Dis. 1990;161:999–1005. doi: 10.1093/infdis/161.5.999. [DOI] [PubMed] [Google Scholar]
- 41.Tagaya Y, Burton J D, Waldmann T A. Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO J. 1996;15:4928–4939. [PMC free article] [PubMed] [Google Scholar]
- 42.Wahl L M, Katona I M, Wilder R L, Winter C C, Haraqui B, Scher I, Wahl S M. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984;85:373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- 43.Wahl S M, McCartney-Francis N, Hunt D A, Smith P D, Wahl L M, Katona I M. Monocyte interleukin-2 receptor gene expression and interleukin-2 augmentation of microbicidal activity. J Immunol. 1987;139:1342–1347. [PubMed] [Google Scholar]
- 44.Wang M, Friedman H, Djeu J Y. Enhancement of human monocyte function against Candida albicansby the colony-stimulating factors (CSF), IL-3, granulocyte-macrophage-CSF and macrophage-CSF. J Immunol. 1989;143:671–677. [PubMed] [Google Scholar]
- 45.Wilkinson P C, Liew F Y. Chemoattraction of human blood T lymphocytes by IL-15. J Exp Med. 1995;181:1255–1259. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou P, Sieve M C, Bennett J, Kwon-Chung K J, Tewari R P, Gazzinelli R T, Sher A, Seder R A. IL-12 prevents mortality in mice infected with Histoplasma capsulatumthrough induction of IFN-γ. J Immunol. 1995;155:785–795. [PubMed] [Google Scholar]