Abstract
Background:
Kidney function and its association with outcomes in patients with advanced heart failure (HF) has not been well defined.
Methods and Results:
We conducted a retrospective cohort study comprising all adult residents of Olmsted County, Minnesota, with HF who developed advanced HF from 2007–2017. Patients were grouped by estimated glomerular filtration rate (eGFR) at advanced HF diagnosis using the 2021 CKD-Epi equation. A linear mixed effects model was fitted to assess the relationship between development of advanced HF and longitudinal eGFR trajectory. A total of 936 patients with advanced HF (mean age 77 years, 56% male, 93.7% white) were included. 22% of patients had eGFR <30 at advanced HF diagnosis, 22% had eGFR 30–44, 23% had eGFR 45–59, and 32% had eGFR 60+ ml/min/1.73 m2. eGFR declined faster after advanced HF (7.6% vs. 10.9% annual decline before vs. after advanced HF), with greater declines after advanced HF in those with diabetes and preserved ejection fraction. eGFR <30 was associated with worse survival after advanced HF compared with eGFR 60+ mL/min/1.73m2 (adjusted HR 1.30, 95% CI 1.07–1.57). Conclusions: eGFR deteriorated faster after patients developed advanced HF. An eGFR <30 mL/min/1.73m2 at advanced HF diagnosis was associated with higher mortality.
Keywords: advanced heart failure, kidney function, kidney disease, outcomes, mortality
Graphical Abstract
INTRODUCTION
A subset of patients diagnosed with heart failure (HF) progress to advanced HF, which is characterized by symptoms of HF that interfere with daily life and are refractory to optimized medical management.1 While survival is limited for many patients with advanced HF,2 the prognostic impact of comorbidities is still being clarified. Substantial work has demonstrated the impact of comorbidities on outcomes in the general (stage C) HF population,3 but these relationships might differ once patients develop advanced HF. For example, a recent study found that diabetes mellitus was not associated with increased mortality in patients with advanced HF,4 despite this condition’s strong relationship with adverse outcomes in earlier stages of HF.5
Chronic kidney disease (CKD) and HF often coexist and share bidirectional interactions that can lead to acute or chronic worsening of the other condition.6 In patients with HF, the presence of CKD is associated with worse outcomes, including higher resource utilization and increased risks of hospitalization and mortality.7–9 Furthermore, CKD may reduce patient tolerance and efficacy of key guideline-directed HF medications.10 Structural heart disease is common among those with end-stage kidney failure, and the presence of reduced left ventricular ejection fraction (EF) increases risk of death.11 Kidney function declines more rapidly in HF than the general population, with associated adverse outcomes.12 It is also negatively impacted by acute exacerbations,13 and individuals requiring kidney replacement therapy (KRT) are less likely to recover kidney function in the setting of HF.14 However, the impact of the development of advanced HF on kidney function and associated outcomes has not been examined, and very few population-level studies of patients with advanced heart failure exist generally.
Leveraging a community cohort of patients with advanced HF, we describe the distribution of kidney function at advanced HF diagnosis and use of KRT in patients with advanced HF. We then evaluate the hypothesis that kidney function declines more rapidly after advanced HF by comparing the trajectory of kidney function before and after advanced HF diagnosis. Finally, we evaluate the associations of kidney function at advanced HF diagnosis with mortality and hospitalization risk.
METHODS
Study Population
This is a retrospective cohort study of adult residents in Olmsted County, Minnesota, with HF who developed advanced HF from 2007–2017. The Rochester Epidemiology Project links records from all medical providers, longitudinally capturing information about health care of the county’s residents.15 Potential patients declining the Minnesota Research Authorization (1.2%) were omitted from analysis. The Mayo Clinic and Olmsted Medical Center Institutional Review Boards approved this study.
The methods for identifying patients with advanced HF have been previously described.2,4 In brief, billing codes for HF and medical record review were used to develop a cohort of adult Olmsted County residents with prevalent HF between January 1, 2007, and December 31, 2017. Date of cohort entry was defined as the date of newly diagnosed (incident) HF for those that developed HF while living in the county; for individuals who moved to the county after their diagnosis of HF was established, the date of first clinical diagnosis of HF in the medical record was used as the date of cohort entry. Using a combination of electronic health record data and manual record review, the European Society of Cardiology definition of advanced HF1 was applied to this cohort to identify those with advanced HF in the study period. To qualify as having advanced HF, patients must meet all of the following criteria in a 12-month period: 1) episodes of congestion, low output, or malignant arrythmias requiring hospitalization; 2) evidence of severe cardiac dysfunction, including severely reduced EF, isolated right ventricular failure, nonoperable severe valvular heart disease, nonoperable severe congenital heart disease, or severe diastolic dysfunction or left ventricular structural abnormalities and preserved or mid-range EF; 3) severe impairment of exercise; and 4) severe and persistent symptoms of HF (New York Heart Association Functional Class III advanced or IV symptoms). These four criteria had to persist despite attempts to optimize guideline-directed medical therapy (Table S1). The advanced HF index date was defined as the date of first qualifying hospitalization. Patients were considered to have advanced HF from the index date until death or last follow-up.
Patient Characteristics
Patient information was obtained from the electronic medical record. Patients were assigned to have a comorbidity if it was listed as a clinical diagnosis at least 2 times within the 5 years prior to advanced HF; the comorbidity burden was summarized using the Charlson Comorbidity Index. All serum creatinine values for each patient were collected from time of cohort entry to last follow-up. Multiple creatinine measurements from the same date were averaged into a single value. Estimated glomerular filtration rate (eGFR) was calculated using the 2021 CKD-Epi equation.16 All patients receiving dialysis were considered to have an eGFR <30 mL/min/1.73 m2. Additional baseline laboratory values were collected from the date closest to advanced HF diagnosis, within +/− one year. Use of medications were as documented in the medical record at time of advanced HF. Echocardiographic data were obtained from the transthoracic echocardiogram closest to date of advanced HF, also always within one year thereof. EF closest to advanced HF diagnosis was used to stratify patients into advanced HF with mildly reduced or reduced EF (HFrEF, <50%), or preserved EF (HFpEF, ≥50%). Nephrology care in the year preceding advanced HF was recorded, as were the visit dates, KRT type (hemodialysis, peritoneal dialysis, or continuous renal replacement therapy [CRRT]), and setting (outpatient, hospital, or both) of any previous or ongoing dialysis treatment.
Outcomes
Data on all-cause hospitalizations in Olmsted County or at other Mayo Clinic sites after advanced HF diagnosis were obtained using Rochester Epidemiology Project resources. HF hospitalization was defined by primary ICD-9 or ICD-10 codes for HF as shown in Table S1. Advanced HF index hospitalization was not included. In-hospital or inter-hospital transfers were considered a single hospitalization. Surveillance for death occurs via several methods. The Mayo Clinic registration office records deaths noted in clinical care and local obituaries. Death data are also obtained quarterly from the State of Minnesota Department of Vital and Health Statistics. Cardiovascular (CV) mortality was defined by death certificate cause of death ICD-10 codes I00-I99.
Statistical Analysis
Patient were stratified by eGFR at the time of advanced HF diagnosis (<30, 30–44, 45–59, 60+ mL/min/1.73 m2), and baseline characteristics were summarized with mean (SD) or N (%) as appropriate. Individuals undergoing dialysis were grouped in the eGFR <30 mL/min/1.73 m2 category. Differences in characteristics by kidney function group were compared by ANOVA F-test, chi-square test, or Fisher’s exact test. Patients were censored from all outcomes analyses at time of heart transplant or LVAD placement.
A linear mixed effects model was fitted to assess the relationship between development of advanced HF and the longitudinal trajectory of eGFR over time. All eGFRs recorded after cohort entry (diagnosis of HF) through death or last follow-up were included. The dependent variable, eGFR, was log-transformed in the regression model prior to analysis so that residuals would be more normally distributed. The primary explanatory variables of interest included (i) time in years since HF diagnosis and (ii) time in years after advanced HF diagnosis, which is equal to zero before advanced HF diagnosis. In this model, time since HF diagnosis reflects the slope of log eGFR over time (average annualized change in log eGFR per year prior to advanced HF diagnosis). Time after advanced HF reflects the change in slope following advanced HF diagnosis. Results are also interpreted after transformation to the original eGFR scale by exponentiating the coefficient and assuming homogenous variance, interpreted as the annual (per year) multiplicative change in eGFR. Patients who received kidney transplant before advanced HF diagnosis were excluded from the kidney function trajectory model. If kidney transplant occurred after advanced HF diagnosis, they were censored at the time of kidney transplant.
Finally, the associations of kidney function with patient survival and hospitalizations after advanced HF diagnosis were evaluated. Kaplan-Meier survival curves were generated stratified by baseline eGFR groups at advanced HF. A Cox proportional hazard regression model evaluated the relationship of eGFR with all-cause mortality. The proportional hazards assumption was assessed using the scaled Schoenfeld residuals. The association of kidney function with CV versus non-CV mortality was assessed using cause-specific hazard regression analysis as the primary analysis and Fine-Gray subdistribution hazard modeling as a sensitivity analysis. Next, cumulative incidence curves were constructed for hospitalizations by kidney function groups. An Anderson-Gill model for recurrent events was used to examine the association of kidney function with hospitalization risk. Both mortality and hospitalization analyses were adjusted for factors associated with CKD severity including age, sex, EF, body mass index (BMI), systolic blood pressure, angiotensin converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB)/angiotensin receptor neprilysin inhibitor (ARNI) use, beta blocker (BB) use, mineralocorticoid receptor antagonist (MRA) use, hypertension, diabetes, peripheral vascular disease (PVD), and chronic obstructive pulmonary disease. These variables were complete except for BMI (n=9 missing). Interactions of kidney function with age, sex, EF, and diabetes were examined. A p value of <0.05 was used as the level of significance in all analyses. Analyses were performed using SAS Version 9.4.
RESULTS
A total of 6836 adult residents of Olmsted County had HF during the study period, of which 936 (13.7%) progressed to advanced HF. Among patients with advanced HF, 300 (32.1%) had an eGFR of 60+ mL/min/1.73 m2 at diagnosis, with the remaining patients roughly evenly distributed in eGFR groups of <30, 30–44, and 45–59 mL/min/1.73 m2 (22.1%, 22.4%, and 23.4%, respectively). The median (IQR) number of eGFR measurements per patient was 60 (35–100). Median follow-up time was 10.2 months overall and 6.9, 10.0, 10.4, and 13.5 months for respective eGFR subgroups of <30, 30–44, 45–59, and 60+ mL/min/1.73 m2. Patients with lower eGFR were, on average, older with a higher prevalence of several common comorbidities compared to those with higher eGFR. Laboratory trends were notable for lower hemoglobin and bicarbonate and higher phosphorus and potassium with lower eGFR. Use of ACEi/ARB/ARNI and BBs were similar between the eGFR groups. Use of MRAs was less common in patients with eGFR<30 mL/min/1.73 m2 (Table 1). Sodium glucose co-transporter 2 inhibitors (SGLT2i) were not used to treat HF in the study period. Most echocardiographic parameters, including EF, were similar in patients across eGFR categories, though patients with preserved kidney function had lower estimated right ventricular systolic pressure and less tricuspid regurgitation (Tables 1 and S2).
Table 1.
Characteristics of study population at advanced HF diagnosis.
| eGFR at advanced HF diagnosis (mL/min/1.73 m2) | Overall (N=936) | <30 (N=207) | 30–44 (N=210) | 45–59 (N=219) | 60+ (N=300) | p-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Demographics | ||||||
| Age, years, mean (SD) | 76.9 (14.6) | 78.1 (13.0) | 80.8 (11.4) | 77.8 (12.6) | 72.6 (17.6) | <0.001 |
| Female, n (%) | 417 (44.6) | 102 (49.3) | 89 (42.4) | 94 (42.9) | 132 (44.0) | 0.47 |
| Race | 0.18 | |||||
| White, n (%) | 876 (93.7) | 190 (91.8) | 201 (95.7) | 201 (91.8) | 284 (95.0) | |
| Other, n (%) | 59 (6.3) | 17 (8.2) | 9 (4.3) | 18 (8.2) | 15 (5.0) | |
| Hispanic ethnicity, n (%) | 15 (1.6) | 4 (1.9) | 4 (1.9) | 2 (0.9) | 5 (1.7) | 0.83 |
| Comorbidities | ||||||
| BMI, kg/m2, mean (SD) | 30.6 (8.4) | 30.4 (7.6) | 30.2 (7.7) | 31.3 (8.9) | 30.3 (9.0) | 0.56 |
| Hypertension, n (%) | 829 (88.6) | 194 (93.7) | 187 (89.1) | 201 (91.8) | 247 (82.3) | <0.001 |
| Hyperlipidemia, n (%) | 684 (73.1) | 156 (75.4) | 157 (74.8) | 164 (74.9) | 207 (69.0) | 0.29 |
| Coronary artery disease, n (%) | 661 (70.6) | 153 (73.9) | 160 (76.2) | 155 (70.8) | 193 (64.3) | 0.019 |
| Depression, n (%) | 257 (27.5) | 57 (27.5) | 54 (25.7) | 57 (26.0) | 89 (29.7) | 0.73 |
| Diabetes, n (%) | 415 (44.3) | 108 (52.2) | 94 (44.8) | 92 (42.0) | 121 (40.3) | 0.055 |
| COPD, n (%) | 537 (57.4) | 122 (58.9) | 112 (53.3) | 141 (64.4) | 162 (54.0) | 0.060 |
| Peripheral vascular disease, n (%) | 487 (52.0) | 129 (62.3) | 111 (52.9) | 118 (53.9) | 129 (43.0) | <0.001 |
| Cerebrovascular disease, n (%) | 217 (23.2) | 57 (27.5) | 46 (21.9) | 53 (24.2) | 61 (20.3) | 0.27 |
| Dementia, n (%) | 65 (6.9) | 15 (7.3) | 17 (8.1) | 11 (5.0) | 22 (7.3) | 0.62 |
| Charlson score, mean (SD) | 5.0 (2.5) | 6.2 (2.4) | 5.3 (2.3) | 4.9 (2.5) | 4.0 (2.2) | <0.001 |
| Smoking status, n (%) | 0.172 | |||||
| Never | 585 (63.6) | 134 (66.0) | 132 (63.8) | 136 (63.9) | 183 (61.6) | |
| Current | 73 (7.9) | 14 (6.9) | 10 (4.8) | 15 (7.0) | 34 (11.5) | |
| Former | 262 (28.5) | 55 (27.1) | 65 (31.4) | 62 (29.1) | 80 (26.9) | |
| EF | ||||||
| Mean (SD) | 43.5 (17.6) | 45.4 (16.1) | 43.6 (18.0) | 42.9 (17.5) | 42.6 (18.4) | 0.33 |
| <50%, n (%) | 530 (56.6) | 114 (55.1) | 115 (54.8) | 123 (56.2) | 178 (59.3) | |
| ≥50%, n (%) | 406 (43.4) | 93 (44.9) | 95 (45.2) | 96 (43.8) | 122 (40.7) | |
| Systolic BP, mean (SD) | 129.5 (26.1) | 133.3 (30.8) | 127.8 (25.8) | 125.5 (24.6) | 130.9 (23.3) | 0.011 |
| Labs | ||||||
| Creatinine, mg/dL, mean (SD) | 1.59 (0.97) | 2.86 (1.30) | 1.66 (0.29) | 1.27 (0.20) | 0.91 (0.20) | <0.001 |
| Hemoglobin, g/dL, mean (SD) | 11.4 (2.0) | 10.6 (1.9) | 11.1 (1.7) | 11.8 (2.0) | 11.8 (2.1) | <0.001 |
| NT-proBNP, pg/mL, mean (SD) | 10519 (11618) | 18274 (15211) | 11090 (10653) | 7679 (7203) | 6563 (8635) | <0.001 |
| Potassium, mmol/L, mean (SD) | 4.4 (0.6) | 4.6 (0.8) | 4.4 (0.6) | 4.4 (0.6) | 4.2 (0.5) | <0.001 |
| Bicarbonate, mmol/L, mean (SD) | 26.8 (5.0) | 24.5 (4.5) | 26.8 (4.8) | 27.8 (5.2) | 27.7 (4.8) | <0.001 |
| Phosphorus, mg/dL, mean (SD) | 4.0 (1.0) | 4.4 (1.2) | 4.0 (0.8) | 3.8 (0.9) | 3.6 (0.8) | <0.001 |
| Magnesium, mg/dL, mean (SD) | 2.1 (0.3) | 2.2 (0.4) | 2.1 (0.3) | 2.1 (0.3) | 2.0 (0.3) | <0.001 |
| Medications at advanced HF | ||||||
| ACE/ARB/ARNi, n (%) | 572 (61.1) | 122 (58.9) | 119 (56.7) | 137 (62.6) | 194 (64.7) | 0.27 |
| Beta blockers, n (%) | 699 (74.7) | 161 (77.8) | 155 (73.8) | 156 (71.2) | 227 (75.7) | 0.45 |
| MRA, n (%) | 146 (15.6) | 22 (10.6) | 25 (11.9) | 48 (21.9) | 51 (17.0) | 0.004 |
| Loop diuretics, n (%) | 744 (79.5) | 173 (83.6) | 169 (80.5) | 176 (80.4) | 226 (75.3) | 0.14 |
| Thiazide diuretic, n (%) | 171 (18.3) | 47 (22.7) | 37 (17.6) | 34 (15.5) | 53 (17.7) | 0.27 |
Missing data: 1 patient was missing race, 9 BMI, 16 smoking status, 184 NT-proBNP, 5 bicarbonate, 197 phosphorus, 65 magnesium.
ACE/ARB/ARNi: angiotensin converting enzyme/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitor, BMI: body mass index, COPD: chronic obstructive pulmonary disease, eGFR: estimated glomerular filtration rate, HF: heart failure, MRA: mineralocorticoid receptor antagonist, NT-proBNP: N-terminal pro-B-type natriuretic peptide, SD: standard deviation.
Patients with lower eGFR more often had received a kidney ultrasound or a consult with a nephrologist in the preceding year. As expected, the lowest eGFR group also had the highest proportion of patients who had ever been on dialysis. In total, 26 of 207 patients (12.6%) with eGFR<30 mL/min/1.73 m2 had started dialysis before advanced HF diagnosis. Another 39 of 207 (18.8%) started dialysis after advanced HF diagnosis. For patients with eGFR ≥30 mL/min/1.73 m2 at advanced HF diagnosis, a small number (n=7) had been on dialysis previously, with cessation of dialysis with kidney function improvement. In patients that required dialysis, the use of outpatient hemodialysis was most common. In patients with eGFR >60 mL/min/1.73 m2 at the time of advanced HF, however, dialysis often occurred with CRRT in the inpatient setting, suggesting acute kidney injury (Table S3). In total, 20 patients had kidney transplant; 11 before incident HF diagnosis (cohort entry), 4 after cohort entry but before advanced HF diagnosis, and 5 after advanced HF diagnosis.
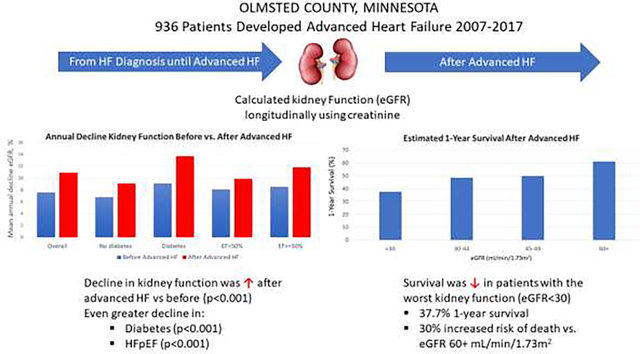
Average baseline eGFR declined over time after newly diagnosed HF, but it declined more quickly after patients developed advanced HF (Figure 1). Prior to advanced HF diagnosis, eGFR declined an average of 7.6% annually compared with 10.9% annually after advanced HF (p<0.001 for change in slope). As an example, in a typical patient starting at the average eGFR at cohort entry of 52.2 mL/min/1.73m2, the eGFR at advanced HF diagnosis (mean 5.1 years after cohort entry) would be 34.9 ml/min/m2. The mean eGFR decline in the year prior to advanced HF diagnosis would be 2.9 ml/min/m2 and would increase to 3.8 ml/min/m2 in the year after advanced HF diagnosis (Table S4). Decline in eGFR after advanced HF was greater for those with diabetes (9.1% before vs. 13.7% decline after advanced HF; absolute decline difference 4.6%) compared to patients without diabetes (6.8% before vs. 9.1% decline after advanced HF; absolute decline difference 2.2%, interaction for change in slope p<0.001) and in patients with EF ≥50% (8.5% before vs. 11.8% decline after advanced HF; absolute decline difference 3.4%) vs. <50% (8.1% before vs. 9.9% decline after advanced HF; absolute decline difference 1.8%, interaction for change in slope p<0.001).
Figure 1.

Estimated annual decrease in estimated glomerular filtration rate before and after advanced HF.
The estimated mean annual decline in estimated glomerular filtration rate (eGFR) before compared with after development of advanced heart failure (HF), overall, and by patient characteristics, is shown. The p value for the overall comparison is for the change in slope before vs. after advanced HF. The p values for female vs male, by diabetes status, and by ejection fraction are for interactions of change of slope by characteristic. EF: ejection fraction, eGFR: estimated glomerular filtration rate, HF: heart failure.
Median (IQR) survival after development of advanced HF was 12.9 (3.9–28.8) months overall and 6.9 (1.9–26.9), 11.0 (4.0–26.5), 11.5 (4.3–32.0), and 16.4 (5.5–31.0) months for those with eGFR <30, 30–44, 45–59, and 60+ mL/min/1.73 m2, respectively (p=0.021, Figure 2). Estimated 1-year survival was 37.7%, 48.5%, 49.8%, and 61.2% in patients with eGFR <30, 30–44, 45–59, and 60+ mL/min/1.73 m2, respectively. After adjustment for demographics, comorbidities, systolic blood pressure, and medications, survival was significantly worse for patients with eGFR <30 compared to patients with eGFR 60 mL/min/1.73 m2 or higher (hazard ratio, HR, 1.30, 95% CI 1.07–1.57, p= 0.039, Table 2). CV mortality was higher in patients with eGFR <30 mL/min/1.73 m2 compared to those with preserved kidney function in unadjusted analyses (HR 1.27, 95% CI 1.00–1.61). This association was no longer statistically significant after adjustment for potential confounders (HR 1.22, 95% CI 0.96–1.56). The adjusted associations of kidney function with CV (versus non-CV) mortality were similar when analyzed using a Fine-Gray modeling approach (Table S5).
Figure 2.
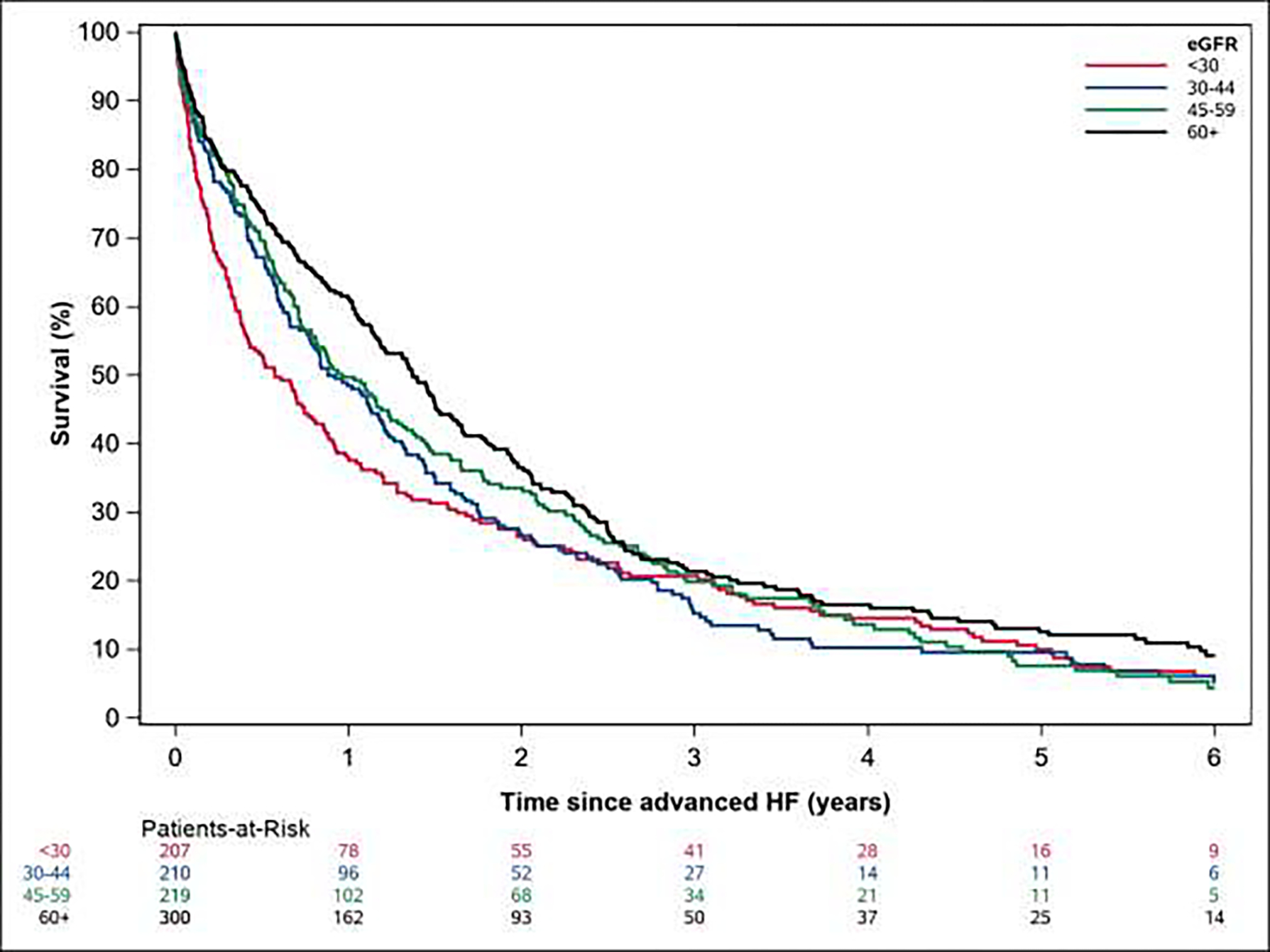
Kaplan-Meier survival curves according to baseline estimated glomerular filtration rate.
Kaplan-Meier estimated survival by estimated glomerular filtration rate at the time of advanced heart failure. eGFR: estimated glomerular filtration rate, HF: heart failure.
Table 2.
Survival and Hospitalization Risks by Estimated Glomerular Filtration Rate at Advanced Heart Failure.
| Estimated Glomerular Filtration Rate | P value | |||||
|---|---|---|---|---|---|---|
| <30 | 30–44 | 45–59 | ≥60 | |||
|
| ||||||
| Mortality | ||||||
| Unadjusted | 1.32 (1.10–1.60) | 1.25 (1.03–1.51) | 1.18 (0.98–1.43) | 1.00 (ref) | 0.022 | |
| Adjusteda | 1.30 (1.07–1.57) | 1.02 (0.84–1.25) | 1.07 (0.88–1.29) | 1.00 (ref) | 0.039 | |
| Cardiovascular Mortality | ||||||
| Unadjusted | 1.27 (1.00–1.61) | 1.42 (1.13–1.79) | 1.21 (0.96–1.54) | 1.00 (ref) | 0.026 | |
| Adjusteda | 1.22 (0.96–1.56) | 1.09 (0.86–1.38) | 1.11 (0.87–1.41) | 1.00 (ref) | 0.45 | |
| All-cause hospitalization | ||||||
| Unadjusted | 1.12 (0.89–1.40 | 0.94 (0.75–1.17) | 0.92 (0.76–1.12) | 1.00 (ref) | 0.28 | |
| Adjusteda | 1.09 (0.90–1.32) | 1.05 (0.88–1.26) | 0.96 (0.81–1.26) | 1.00 (ref) | 0.61 | |
| HF hospitalization | ||||||
| Unadjusted | 0.86 (0.65–1.14) | 0.89 (0.68–1.16) | 0.93 (0.71–1.22) | 1.00 (ref) | 0.75 | |
| Adjusteda | 0.86 (0.66–1.13) | 0.99 (0.77–1.26) | 0.97 (0.76–1.23) | 1.00 (ref) | 0.70 | |
Adjusted for age, sex, ejection fraction, body mass index, systolic blood pressure, diabetes, peripheral vascular disease, hypertension, chronic obstructive pulmonary disease, angiotensin converting enzyme inhibitor/ angiotensin receptor blocker/ angiotensin receptor neprilysin inhibitor, beta blocker, mineralocorticoid receptor antagonist.
In total, 3256 hospitalizations occurred in the study period. Estimated 2-year mean cumulative hospitalizations (95% CI) were 5.5 (4.6–6.5), 4.6 (3.9–5.4), 4.5 (3.9–5.2), and 4.9 (4.3–5.6) for those with eGFR<30, 30–44, 45–59, and 60+ mL/min/1.73 m2, respectively. There was no significant associations of kidney function with overall or HF-specific hospitalization risk among advanced HF patients (Table 2). While the rate of hospitalization per year of follow-up was numerically highest for patients with eGFR <30 mL/min/1.73 m2 (2.57 per person-year of follow-up), there was no significant difference compared to patients with eGFR 60 mL/min/1.73 m2 or higher (2.30 per person year of follow-up, p=0.28, Table S6).
DISCUSSION
In this population-based study, we elucidate important findings about kidney function in patients with advanced HF. First, advanced kidney disease is common in patients with advanced HF. Differences in comorbidity burden and laboratory values are largely consistent with expected trends in patients with worsening kidney function, including non-traditional cardiovascular risk factors prevalent in CKD such as hyperphosphatemia, anemia, and hyperkalemia. Importantly, the development of advanced HF accelerates kidney function decline in patients with HF. eGFR declined more quickly after advanced HF than before this diagnosis, and this decline was greater for patients with diabetes as well as those with HFpEF. Finally, while kidney function was not associated with hospitalization risk, an eGFR <30 was associated with worse survival compared to patients with an eGFR of 60+ mL/min/1.73 m2.
Limited information is available about kidney function in epidemiologic cohorts of patients with advanced HF. One previous registry reported the mean creatinine values for 1268 patients with stage D (advanced) HF to be 1.8 mg/dL. A total of 26% of patients had a creatinine >2.0 mg/dL.17 By comparison, the mean creatinine was slightly lower in our cohort at 1.59 mg/dL, although this difference may reflect similar kidney function in patients of different ages. Our population was older (mean age 76.9 vs. 69.6 years), and age-associated reduction in muscle mass and therefore creatinine could contribute to observed differences.18 Distribution of eGFR in our population can be compared to multiple cohorts of patients with stage C HF. In the Swedish HF Registry, 10.6% of 47,716 patients with clinician-judged HF had an eGFR <30 mL/min/1.73 m2 by the CKD-Epi 2012 equation.19 Elsewhere, McAlister and colleagues found that 16% of 754 community patients with HF had eGFR <30 mL/min/1.73 m2 by Cockcroft-Gault.20 We found that 22.1% of patients with advanced HF had eGFR <30 mL/min/1.73 m2, demonstrating that patients with advanced HF have a higher prevalence of stage 4–5 CKD compared with the general HF population. The proportion of patients with eGFR <30 mL/min/1.73 m2 in the present study is more comparable to patients hospitalized with acute decompensated HF, where the prevalence has been reported as 18.7%9 and 20.1%21 in two large hospitalized HF registries. Given that hospitalization is a hallmark of advanced HF and that creatinine often increases in patients with acute HF exacerbations,13 we would expect substantial overlap in advanced HF and hospitalized HF populations. Our patients did tend to have higher absolute rates of common comorbidities than patients enrolled in HF hospitalization registries (e.g., 57.2% vs. 70.6% with coronary artery disease, ADHERE21 vs. present investigation). In general, our population’s kidney function and comorbidity burden are commensurate with what might be expected in patients with advanced HF compared with patients in earlier stages of HF.
We found that kidney function as assessed by eGFR deteriorates more rapidly after a diagnosis of advanced HF. While an intuitive relationship, this result provides further support that the development of advanced HF is a notable clinical event. One study estimates median eGFR change in the stage C HF population at −2.57 mL/min/1.73 m2 per year,12 which is higher than rates of −0.5 to −1.0 mL/min/1.73 m2 per year in the general adult population.22 Our mean eGFR decline was 7.6% per year before and 10.9% per year after advanced HF diagnosis, which correlates to an eGFR decline of 2.9 mL/min/1.73 m2 in the year prior to advanced HF and 3.8 mL/min/1.73 m2 in the year after advanced HF in a typical patient. A decline in eGFR in the short or long term has been demonstrated to be associated with poor prognosis in HF. For example, one study noted survival differences with only a week of rising creatinine/decreasing eGFR after a HF hospitalization.13 A decline in kidney function over months or years has an even stronger association with adverse events.12 One study reported an annual decline in eGFR of >−10 mL/min/1.73 m2 per year was associated with a 2–3 fold increase in risk of CV death or hospitalization regardless of baseline eGFR.23 We found that patients with diabetes and/or HFpEF had an even greater eGFR decline compared with other patients. Recent advances in HF therapy including SGLT2i24 and sacubitril/valsartan25 have demonstrated kidney benefits for HF patients of all EFs, and these positive effects may prove especially advantageous for the HFpEF population. It would be of interest to re-evaluate trajectories of kidney function in a similar patient cohort once more widespread adoption of these therapies occurs.
Overall survival is poor once patients develop advanced HF, and we found that it is even worse in patients with the lowest eGFR. A stepwise association between decreasing eGFR and adverse outcomes is typical in stage C HF,26 but our results demonstrate that kidney function is only associated with adverse outcomes in advanced HF once it is severely reduced. CKD-associated drivers of cardiac dysfunction would include inflammation, vascular calcification, oxidative stress, proteinuria, and uremic toxin exposure as well as underlying systemic disease processes such as diabetes and aging. The lack of significant association of kidney function and hospitalization risk may support these multiple mediators underlying a multifactorial decline in health. Our cohort’s estimated 1-year survival was approximately 50%, which is slightly worse than an estimated 1-year survival of 72% observed in an advanced HF registry,17 but better than LVAD-eligible patients with advanced HF treated with medical management.27 Investigations of innovations such as destination mechanical interventions continue,28 but it remains a priority to optimize guideline directed therapies in earlier stages of HF and incorporate goals-of-care discussion throughout the HF trajectory.29 Our finding of an estimated 23.5% lower 1-year survival in patients with eGFR <30 compared with >60 mL/min/1.73 m2 suggests that discussions of goals and values are of particular importance in those patients with severely reduced kidney function.
This study has limitations. Our study population, while representative of the demographics of patients in the Upper Midwest United States, is more racially and ethnically homogenous than the overall population at risk for advanced HF.30 Estimating eGFR based on creatinine measured at time of advanced HF diagnosis may not fully represent patients’ overall kidney function. However, the incorporation of longitudinal creatinine/eGFR values into our mixed effects models enables better capture of the kidney function trajectory. There are many additional factors, such as contrast and other nephrotoxin exposure, that could impact kidney function trajectory in some individuals. This was not evaluated as a part of the present analysis but would be of interest to explore in the future. Strengths of the study include longitudinal capture of kidney function and outcomes in a population-based cohort which extends known mortality observations about CKD in HF to a community population with advanced HF.
These data provide important insights into the relationship between kidney function and advanced HF. In a large community population with advanced HF, advanced CKD was common. eGFR deteriorated faster after patients developed advanced HF. While survival was poor overall, an eGFR <30 mL/min/1.73 m2 was associated with even higher mortality in advanced HF.
Supplementary Material
Highlights/ Lay Summary.
Severe kidney disease often coexists with advanced HF (22% of patients had eGFR <30 mL/min/1.73 m2)
In patients with HF, kidney function deteriorates more rapidly after they develop advanced HF, with greater declines in patients with diabetes and HFpEF
Patients with advanced HF and severe kidney dysfunction (eGFR <30 mL/min/1.73 m2) have worse prognosis than patients with preserved kidney function; with a 1-year survival of 37.7% vs. 61.2%.
Lay summary: This study analyzed the kidney function of 936 Minnesota residents who developed the most severe stage of heart failure (“advanced” heart failure) between 2007 and 2017.When they were diagnosed with advanced heart failure, 22% of patients also had severe kidney disease. Kidney function deteriorated over time, but this progression accelerated significantly after patients developed advanced heart failure. Deterioration in kidney function was even worse for patients with diabetes and heart failure with preserved ejection fraction. Patient with advanced heart failure who had severe kidney dysfunction had the worst survival.
Acknowledgements:
We would like to thank Ellen Koepsell RN, Janet Gatzke RN, and Christina Stenzel for their assistance with data collection.
Sources of Funding:
This study was funded by the National Institutes of Health (R01 HL144529, PI: Dunlay. This study used the resources of the Rochester Epidemiology Project (REP) medical records-linkage system, which is supported by the National Institute on Aging (NIA; AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the Mayo Clinic.
Non-standard abbreviations and acronyms:
- CRRT
continuous renal replacement therapy
- KRT
kidney replacement therapy
Biography

Footnotes
Conflicts of Interest: The authors have no relationships with industry to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge-Caballero E, De Jonge N, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure. 2018;20:1505–1535. doi: 10.1002/ejhf.1236 [DOI] [PubMed] [Google Scholar]
- 2.Dunlay SM, Roger VL, Killian JM, Weston SA, Schulte PJ, Subramaniam AV, Blecker SB, Redfield MM. Advanced Heart Failure Epidemiology and Outcomes: A Population-Based Study. JACC: Heart Failure. 2021;9:722–732. doi: 10.1016/j.jchf.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nature Reviews Cardiology. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlay SM, Killian JM, McCoy RG, Redfield MM. Diabetes Mellitus in Advanced Heart Failure. Journal of Cardiac Failure. 2022;28:503–508. doi: 10.1016/j.cardfail.2021.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, Deswal A, Dickson VV, Kosiborod MN, Lekavich CL, et al. Type 2 Diabetes Mellitus and Heart Failure, A Scientific Statement From the American Heart Association and Heart Failure Society of America. Journal of Cardiac Failure. 2019;25:584–619. doi: 10.1016/j.cardfail.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 6.Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Löfman I, Szummer K, Dahlström U, Jernberg T, Lund LH. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. European Journal of Heart Failure. 2017;19:1606–1614. doi: 10.1002/ejhf.821 [DOI] [PubMed] [Google Scholar]
- 8.Damman K, Valente MAE, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. European Heart Journal. 2014;35:455–469. doi: 10.1093/eurheartj/eht386 [DOI] [PubMed] [Google Scholar]
- 9.Patel RB, Fonarow GC, Greene SJ, Zhang S, Alhanti B, DeVore AD, Butler J, Heidenreich PA, Huang JC, Kittleson MM, et al. Kidney Function and Outcomes in Patients Hospitalized With Heart Failure. Journal of the American College of Cardiology. 2021;78:330–343. doi: 10.1016/j.jacc.2021.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, Kasiske BL, Deswal A, deFilippi CR, Cleland JGF, et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney International. 2019;95:1304–1317. doi: 10.1016/j.kint.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 11.Hickson LJ, Negrotto SM, Onuigbo M, Scott CG, Rule AD, Norby SM, Albright RC, Casey ET, Dillon JJ, Pellikka PA, et al. Echocardiography Criteria for Structural Heart Disease in Patients With End-Stage Renal Disease Initiating Hemodialysis. Journal of the American College of Cardiology. 2016;67:1173–1182. doi: 10.1016/j.jacc.2015.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damman K, Masson S, Lucci D, Gorini M, Urso R, Maggioni AP, Tavazzi L, Tarantini L, Tognoni G, Voors A, et al. Progression of Renal Impairment and Chronic Kidney Disease in Chronic Heart Failure: An Analysis From GISSI-HF. Journal of Cardiac Failure. 2017;23:2–9. doi: 10.1016/j.cardfail.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 13.Givertz MM, Postmus D, Hillege HL, Mansoor GA, Massie BM, Davison BA, Ponikowski P, Metra M, Teerlink JR, Cleland JGF, et al. Renal Function Trajectories and Clinical Outcomes in Acute Heart Failure. Circulation: Heart Failure. 2014;7:59–67. doi: 10.1161/CIRCHEARTFAILURE.113.000556 [DOI] [PubMed] [Google Scholar]
- 14.Hickson LJ, Chaudhary S, Williams AW, Dillon JJ, Norby SM, Gregoire JR, Albright RC, McCarthy JT, Thorsteinsdottir B, Rule AD. Predictors of Outpatient Kidney Function Recovery Among Patients Who Initiate Hemodialysis in the Hospital. American Journal of Kidney Diseases. 2015;65:592–602. doi: 10.1053/j.ajkd.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocca WA, Yawn BP, St. Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clinic Proceedings. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. New England Journal of Medicine. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costanzo MR, Mills RM, Wynne J. Characteristics of “Stage D” heart failure: Insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM). American Heart Journal. 2008;155:339–347. doi: 10.1016/j.ahj.2007.10.020 [DOI] [PubMed] [Google Scholar]
- 18.Montesanto A, De Rango F, Berardelli M, Mari V, Lattanzio F, Passarino G, Corsonello A. Glomerular filtration rate in the elderly and in the oldest old: correlation with frailty and mortality. AGE. 2014;36:9641. doi: 10.1007/s11357-014-9641-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Löfman I, Szummer K, Hagerman I, Dahlström U, Lund LH, Jernberg T. Prevalence and prognostic impact of kidney disease on heart failure patients. Open Heart. 2016;3:e000324. doi: 10.1136/openhrt-2015-000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal Insufficiency and Heart Failure. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9 [DOI] [PubMed] [Google Scholar]
- 21.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High Prevalence of Renal Dysfunction and Its Impact on Outcome in 118,465 Patients Hospitalized With Acute Decompensated Heart Failure: A Report From the ADHERE Database. Journal of Cardiac Failure. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 22.Halbesma N, Brantsma AH, Bakker SJL, Jansen DF, Stolk RP, De Zeeuw D, De Jong PE, Gansevoort RT, for the Psg. Gender differences in predictors of the decline of renal function in the general population. Kidney International. 2008;74:505–512. doi: 10.1038/ki.2008.200 [DOI] [PubMed] [Google Scholar]
- 23.Fujisawa T, Suzuki S, Arita T, Yagi N, Otsuka T, Kano H, Matsuno S, Semba H, Kato Y, Uejima T, et al. Decline in eGFR over time and incidence of cardiovascular events: Shinken database analysis. Journal of Cardiology. 2021;77:626–633. doi: 10.1016/j.jjcc.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 24.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. New England Journal of Medicine. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 25.Spannella F, Giulietti F, Filipponi A, Sarzani R. Effect of sacubitril/valsartan on renal function: a systematic review and meta-analysis of randomized controlled trials. ESC Heart Failure. 2020;7:3487–3496. doi: 10.1002/ehf2.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ, on behalf of the Ci. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH). European Journal of Heart Failure. 2009;11:847–854. doi: 10.1093/eurjhf/hfp108 [DOI] [PubMed] [Google Scholar]
- 27.Rogers JG, Butler J, Lansman SL, Gass A, Portner PM, Pasque MK, Pierson RN. Chronic Mechanical Circulatory Support for Inotrope-Dependent Heart Failure Patients Who Are Not Transplant Candidates: Results of the INTrEPID Trial. Journal of the American College of Cardiology. 2007;50:741–747. doi: 10.1016/j.jacc.2007.03.063 [DOI] [PubMed] [Google Scholar]
- 28.Saku K, Yokota S, Nishikawa T, Kinugawa K. Interventional heart failure therapy: A new concept fighting against heart failure. Journal of Cardiology. 2022;80:101–109. doi: 10.1016/j.jjcc.2021.11.018 [DOI] [PubMed] [Google Scholar]
- 29.Truby LK, Rogers JG. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC: Heart Failure. 2020;8:523–536. doi: 10.1016/j.jchf.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 30.St. Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, Rocca WA. Generalizability of Epidemiological Findings and Public Health Decisions: An Illustration From the Rochester Epidemiology Project. Mayo Clinic Proceedings. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


