Abstract
Objective
To evaluate the impact of age on oncological outcomes in a large contemporary cohort of patients treated with adequate Bacillus Calmette-Guerin (BCG).
Patients and Methods
We performed an IRB-approved retrospective study analyzing patients with NMIBC treated with adequate BCG at our institution from 2000 to 2020. Adequate BCG was defined as per US FDA guidelines as being receipt of at least five of six induction BCG instillations with a minimum of two of three planned maintenance or two sets of at least five of six re-induction BCG instillations within a span of 6 months. The study’s primary outcome was to determine if age >70 years was associated with progression to muscle invasive bladder cancer or distant metastasis. The cumulative incidence method and the competing-risk regression analyses were used to investigate the association of advanced age (>70 years) with progression, high-grade (HG) recurrence and cancer-specific mortality (CSM).
Results
Overall, data from 632 patients were analyzed: 355 patients (56.2%) were ≤70 years, and 277 (43.8%) were >70 years. Age >70 years did not adversely affect either cumulative incidence of progression or HG recurrence (p.=.0.067 and p.=.0.644, respectively). On multivariate competing-risk regression analyses, age >70 did not emerge as an independent predictor of progression or HG recurrence (Sub-standardized Hazard ratio [SHR] 1.57; 95% CI: 0.87–2.81, p=0.134 and SHR 1.05; 95% CI 0.77–1.44, p=0.749). Not unexpectedly, patients in the older group did have higher overall mortality (p<0.001) but not CSM (p=0.057)
Conclusion
Age>70 years was not associated with adverse oncological outcomes in a large contemporary cohort of patients receiving adequate intravesical BCG for NMIBC.
Keywords: Bacillus Calmette-Guerin, Progression, Non muscle invasive bladder cancer, Oncological outcomes, Age, competing risk analysis
1. Introduction
The standard treatment for non-muscle invasive bladder cancer (NMIBC) includes transurethral resection of the tumor (TURBT) followed by adjuvant intravesical treatment. According to guidelines, intravesical immunotherapy with Bacillus Calmette-Guerin (BCG) should be offered to intermediate- and high-risk patients (1,2). The anti-tumor activity of BCG is based on eliciting an immune response against bladder cancer cells (3).
In general, age is an important factor in the prognosis and treatment outcomes of many diseases, including cancer. As people age, the activity of the immune system and the ability to respond to treatments may be impacted, which can lead to decreased treatment efficacy and decreased overall health outcomes. On these bases, some have suggested that BCG may be less effective in older patients. Indeed, in older series, an association between advanced age and poorer outcomes in patients treated with BCG for NMIBC has been reported (4–6).
Age > 70 has been incorporated into various risk stratifications for recurrence and progression (7,8). Most recently, Sylvester et al. identified age >70 years as an independent predictor of progression to MIBC in a cohort of over 3000 NMIBC patients (8). Based on this data, the European Association of Urology (EAU) included age >70 among additional clinical risk factors in 2021 updated prognostic factor risk groups system (1). However, it should be noted that the analysis was conducted on a large cohort of patients who did not receive intravesical BCG therapy (8). Additionally, as we and others have recently reported in more contemporary series, BCG demonstrates higher efficacy than previously reported (9). Herein, we aim to evaluate the impact of age on oncological outcomes in a contemporary series of patients with NMIBC treated with adequate BCG.
2. Materials and methods
2.1. Study design and patients
This study was conducted with approval from our Institutional Review Board (IRB). We reviewed our institutional database of patients with NMIBC (cTa, cT1 and CIS) who were treated at our institution between January 2000 and April 2020 and identified patients who received ‘adequate’ BCG as defined by the United States Food and Drug Administration (FDA), International Bladder Cancer Group (IBCG) and European Association of Urology (EAU) (1,10,11). Adequate BCG was defined as receipt of at least five of six induction BCG instillations with a minimum of two of three planned maintenance or two sets of at least five of six re-induction BCG instillations within a span of 6 months.
All patients had pathology review by a specialized uro-pathologist at our institution. Patients were included for analysis if pathological information was available from their index TURBT specimen and at least one follow-up cystoscopy post-induction BCG was performed at our institution. BCG instillations and follow-up schedules were standardized among all the providers and were based on available NMIBC guidelines (1,2). For the purposes of analysis, patients were stratified into two groups: ≤ 70 years and >70 years according to EAU NMIBC guidelines, (1).
2.2. Study variables
Study variables in our dataset included patient demographics, tumor stage, grade, size, and multifocality, the presence of concurrent CIS, and primary or recurrent status. Additional variables included presence of variant histology and lymphovascular invasion (LVI). Tumor grade was scored according to the WHO 2004/2016 grading system.
2.3. Endpoints
The study’s main outcome was to determine if age >70 years was associated with progression to MIBC or distant metastasis. Secondary endpoints include high-grade (HG) recurrence, overall mortality (OM) and cancer-specific mortality (CSM) stratified according to age. The OM time was calculated as the number of months from the date of the first BCG instillation during the induction course to death from any cause or the last follow-up date. CSM time was defined as the number of months between the date of the first BCG instillation during the induction course and death attributed to bladder cancer. Time to HG-recurrence and time to progression to MIBC or metastatic disease were calculated from the date of the first BCG instillation during the induction course to the event date.
2.4. Statistical analysis
Statistical analysis was performed using Stata/SE version 17 (StataCorp, College Station, TX, USA). Statistical significance threshold was set at 0.05. Descriptive statistics were used to summarize the study cohort and by age. Categorical variables were analyzed using Pearson’s chi-square test or Fisher’s exact test, while Wilcoxon rank-sum tests were used to test continuous variables. The median follow-up of the study was determined by the reverse Kaplan-Meier method.
The Kaplan-Meier method was used to estimate the median OM, and the log-rank test was used to test the equality of the survivor functions. The cumulative incidence method was adopted to estimate CSM, HG recurrence, and progression after accounting for other causes of mortality (OCM) as competing risk events. The Pepe–Mori test was used to compare the cumulative incidence functions between groups.
The Fine-Gray method was used to model potential risk factors for the cumulative incidence of oncological outcomes, considering OCM as a competing event in a univariate fashion. We then included all factors with a p-value <0.25 in a multivariate model. In this model, age was investigated once as a dichotomous variable (≤ 70 and >70) and then as a continuous variable. A Cox regression multivariate model for progression was also calculated.
3. Results
Our initial query of the institutional database revealed 632 patients with NMIBC who met the inclusion criteria. Of the 632 patients, 355 patients (56.2%) were ≤70 years, and 277 (43.8%) were >70 years. Table 1 shows the clinicopathologic characteristic of the whole cohort stratified by age. There were no differences among age groups in tumor characteristics such as grade, initial tumor stage, concomitant CIS, size, recurrent tumor, presence of lymphovascular invasion (LVI) or variant histology. Receipt of peri-operative intravesical chemotherapy was similar between the two age groups (13.5% vs. 11.9%, p=0.5). Older patients were less likely to undergo reTUR (56.5% vs. 66.2%, p=0.013). The median number of BCG doses administered was higher in the ≤70 years (21 doses vs. 18 doses, p=0.008). BCG intolerance rate was higher among patients >70 years but not statistically significant (10.5% vs. 7.5%, p=0.2). The median follow-up was 66 months (IQR 35–101); 122 (19%) patients died from any cause, while 25 (4%) died from BC.
Table 1.
Patients’ demographics and tumor characteristics of NMIBC patients who underwent adequate BCG, stratified by age group.
| Overall Population (N=632) | Age≤70 (N=355) | Age>70 (N=277) | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | p-value |
| Gender | 0.3 | ||||||
| Male | 505 | 79.9 | 278 | 78.3 | 227 | 82.0 | |
| Smoker | 0.7 | ||||||
| Never | 203 | 32.2 | 116 | 32.8 | 87 | 31.4 | |
| Current/Past | 428 | 67.8 | 238 | 67.2 | 190 | 68.6 | |
| Grade | 0.13 | ||||||
| High | 574 | 90.8 | 317 | 89.3 | 257 | 92.8 | |
| Stage of entry TURBT | 0.6 | ||||||
| Ta | 306 | 48.4 | 168 | 47.3 | 138 | 49.8 | |
| Tis | 48 | 7.6 | 25 | 7.0 | 23 | 8.3 | |
| T1 | 278 | 44.0 | 162 | 45.6 | 116 | 41.9 | |
| Tumor | 0.11 | ||||||
| Recurrent | 209 | 33.1 | 108 | 30.4 | 101 | 36.5 | |
| Variant Histology | 0.6 | ||||||
| Yes | 27 | 4.3 | 14 | 4.0 | 13 | 4.7 | |
| Tumor Size>3 cm | 0.7 | ||||||
| Yes | 312 | 52.7 | 179 | 53.3 | 133 | 52.0 | |
| LVI | 0.7 | ||||||
| Yes | 7 | 1.1 | 3 | 0.9 | 4 | 1.5 | |
| Concomitant CIS | 0.07 | ||||||
| Yes | 202 | 32.0 | 103 | 29.0 | 99 | 35.7 | |
| Focality | 0.052 | ||||||
| Multiple | 312 | 49.6 | 163 | 46.2 | 149 | 54.0 | |
| Prior BCG | 0.4 | ||||||
| Yes | 51 | 8.1 | 26 | 7.3 | 25 | 9.0 | |
| Peri-operative Intravesical Chemotherapy | 0.5 | ||||||
| Yes | 79 | 12.8 | 47 | 13.5 | 32 | 11.9 | |
| Restaging TUR | 0.013 | ||||||
| Yes | 391 | 62.0 | 235 | 66.2 | 156 | 56.5 | |
TURBT: Transurethral resection of bladder; LVI: Lymphovascular invasion, CIS: carcinoma in situ. BCG: Bacillus Calmette-Guerin
Disease progression to muscle-invasive disease or metastasis was seen in 51 (8%) patients overall. No statistically significant difference emerged in cumulative incidence of progression between age groups (p=0.067; Fig.1A). On multivariate Fine-Gray competing-risk regression models, age >70 years was not associated with progression. (Sub-standardized Hazard ratio [SHR] SHR 1.57, 95% CI: 0.87–2.81, p=0.13).
Figure 1.
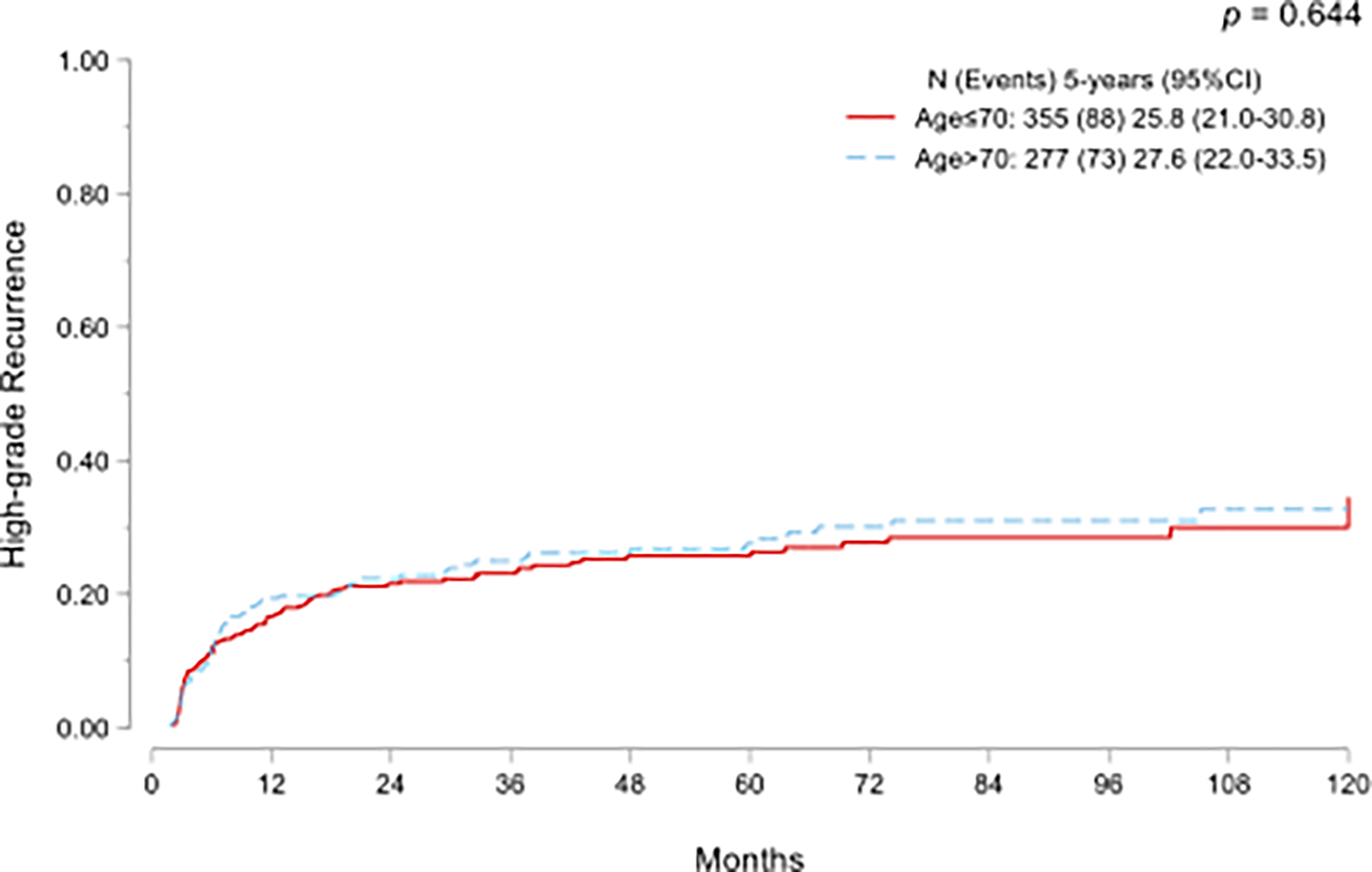
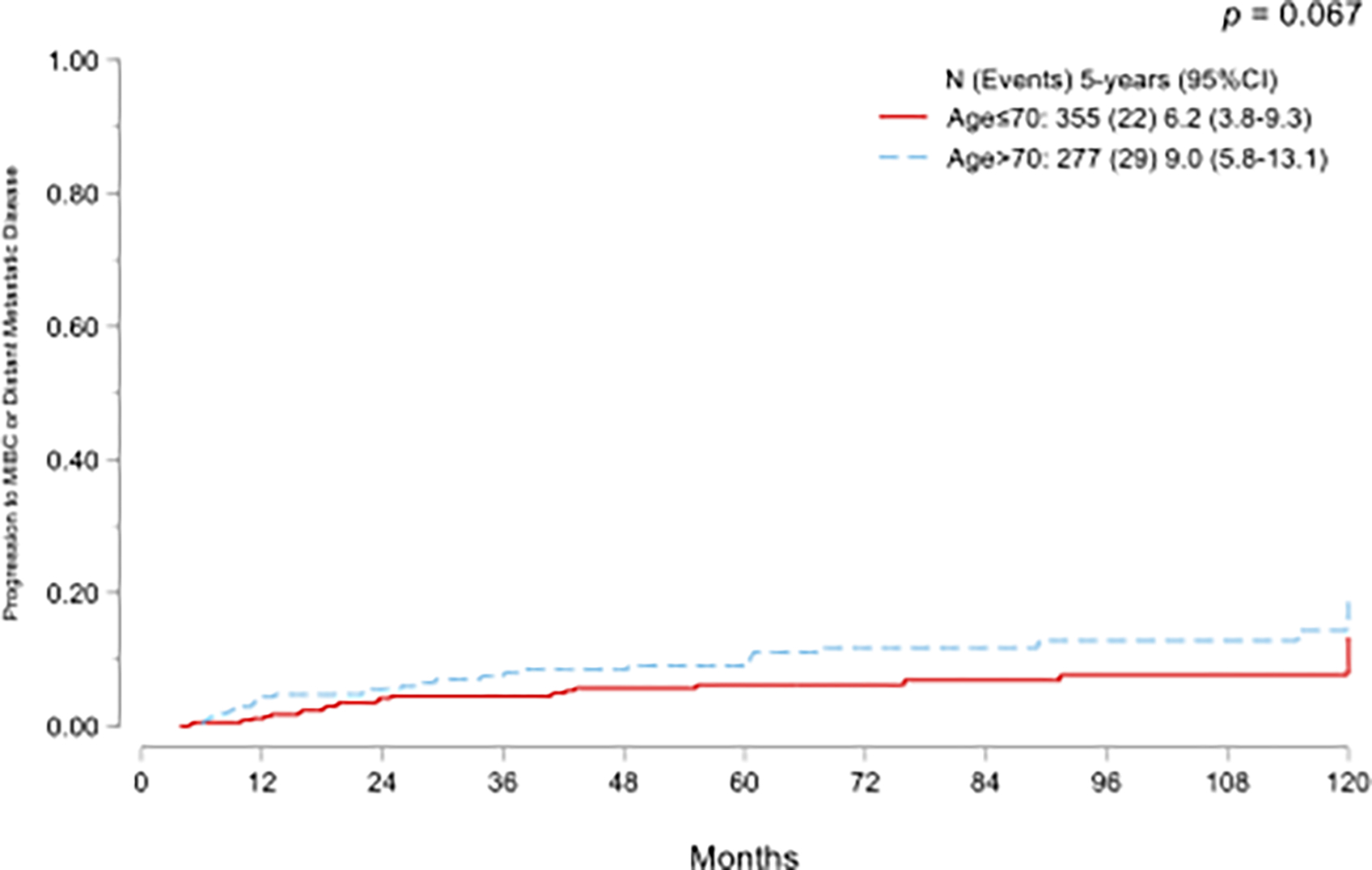
Cumulative incidence functions for progression to muscle-invasive bladder cancer or metastatic disease (A) and high-grade recurrence (B) in NMIBC treated with adequate BCG and stratified by age.
In addition, the association between age >70 years and progression was also analyzed using a multivariate Cox regression model, therefore, OCM was not considered a competing risk event. Our results revealed a higher HR (HR 1.76, 95% CI: 0.99–3.12, p=0.053) for age >70 years in predicting time to progression.
Similarly, there was no significant statistical difference in cumulative incidence of HG recurrence (p=0.644; Fig.1B). On multivariate Fine-Gray competing-risk regression models, age >70 years did not emerge as a predictor of HG recurrence (SHR 1.05, 95% CI 0.77–1.44, p=0.7; Table 2).
Table 2.
Univariate and Multivariate Fine-Gray regression model for HG recurrence and progression to MIBC or metastatic disease.
| High-Grade Recurrence | Progression to MIBC or Metastatic Disease | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| Characteristic | SHR | 95%CI | p-value | SHR | 95%CI | p-value | SHR | 95%CI | p-value | SHR | 95%CI | p-value |
| Age at diagnosis | ||||||||||||
| Age≤70 | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | ||||
| Age>70 | 1.07 | 0.78–1.45 | 0.7 | 1.05 | 0.77–1.44 | 0.7 | 1.75 | 1.01–3.04 | 0.047 | 1.57 | 0.87–2.81 | 0.13 |
| BMI | 1.01 | 0.98–1.03 | 0.6 | 0.94 | 0.91–0.97 | 0.001 | 0.94 | 0.91–0.98 | 0.002 | |||
| Gender | ||||||||||||
| Male | 1.00 | . | 1.00 | . | ||||||||
| Female | 1.19 | 0.82–1.73 | 0.3 | 0.82 | 0.40–1.69 | 0.6 | ||||||
| Smoker | ||||||||||||
| Never | 1.00 | . | 1.00 | . | 1.00 | . | ||||||
| Current/Past | 0.79 | 0.58–1.09 | 0.15 | 0.77 | 0.56–1.07 | 0.12 | 0.94 | 0.52–1.68 | 0.8 | |||
| Grade | ||||||||||||
| Low | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | ||||
| High | 8.26 | 2.02–33.77 | 0.003 | 13.49 | 1.86–97.78 | 0.01 | 4.88 | 0.68–34.99 | 0.11 | 3.36 | 0.45–24.96 | 0.23 |
| Stage of entry TURBT | ||||||||||||
| Ta | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | ||||
| Tis | 2.32 | 1.36–3.97 | 0.002 | 1.64 | 0.89–3.02 | 0.11 | 1.94 | 0.63–5.91 | 0.24 | 1.49 | 0.45–4.94 | 0.5 |
| T1 | 1.89 | 1.35–2.66 | <0.001 | 1.92 | 1.34–2.75 | <0.001 | 2.38 | 1.29–4.39 | 0.005 | 2.94 | 1.51–5.72 | 0.001 |
| Tumor | ||||||||||||
| Primary | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | ||||
| Recurrent | 1.32 | 0.96–1.82 | 0.08 | 1.73 | 1.23–2.43 | 0.001 | 1.58 | 0.99–2.74 | 0.1 | 2.40 | 1.34–4.30 | 0.003 |
| Variant Histology | ||||||||||||
| No | 1.00 | . | 1.00 | . | 1.00 | . | ||||||
| Yes | 0.51 | 0.20–1.29 | 0.15 | 0.50 | 0.19–1.31 | 0.16 | 1.06 | 0.25–4.41 | 0.9 | |||
| Size>3 cm | ||||||||||||
| No | 1.00 | . | 1.00 | . | ||||||||
| Yes | 1.09 | 0.79–1.51 | 0.6 | 0.77 | 0.43–1.39 | 0.4 | ||||||
| LVI | ||||||||||||
| No | 1.00 | . | 1.00 | . | 1.00 | . | ||||||
| Yes | 1.60 | 0.58–4.47 | 0.4 | 4.32 | 0.91–19.65 | 0.065 | 4.06 | 0.84–19.67 | 0.08 | |||
| CIS in entry tumor | ||||||||||||
| No | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | ||||
| Yes | 1.52 | 1.11–2.08 | 0.008 | 1.19 | 0.83–1.71 | 0.3 | 1.39 | 0.80–2.43 | 0.24 | 1.03 | 0.55–1.93 | 0.9 |
| Focality | ||||||||||||
| Single | 1.00 | . | 1.00 | . | 1.00 | . | 1.00 | . | ||||
| Multiple | 1.32 | 0.96–1.80 | 0.08 | 1.19 | 0.86–1.65 | 0.3 | 1.41 | 0.81–2.45 | 0.23 | 1.28 | 0.69–2.37 | 0.4 |
| Peri-operative Intravesical Chemotherapy | ||||||||||||
| No | 1.00 | . | 1.00 | . | ||||||||
| Yes | 0.95 | 0.58–1.54 | 0.8 | 0.87 | 0.36–2.09 | 0.8 | ||||||
MIBC: Muscle-invasive bladder cancer; SHR: Sub-standardized Hazard ratio; CI: confident interval; BMI: Body-mass index; TURBT: Transurethral resection of the bladder; LVI: Lymphovascular invasion, CIS: carcinoma in situ.
Furthermore, even when analyzed as a continuous variable, age was not associated with HG recurrence and progression (SHR: 1.01; 95% CI:0.99–1.02; p=0.16 and SHR: 1.02; 95%CI: 0.99–1.05; p=0.1; Supplementary Table 1).
As expected, a significant difference between groups was found in OM (p<0.001, Fig 2A) but there was no difference in CSM between the groups (p=0.057, Fig. 2B).
Figure 2.
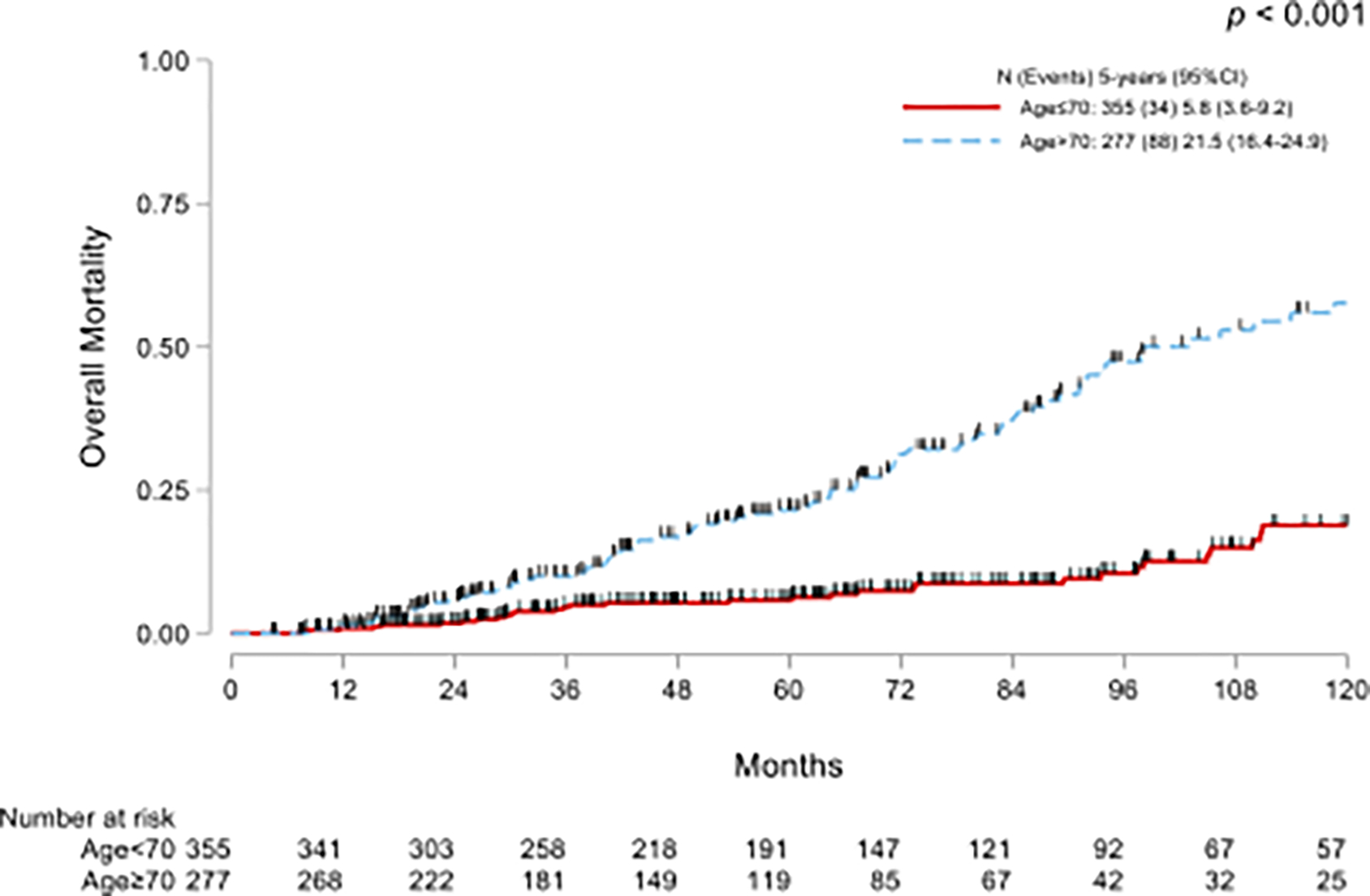
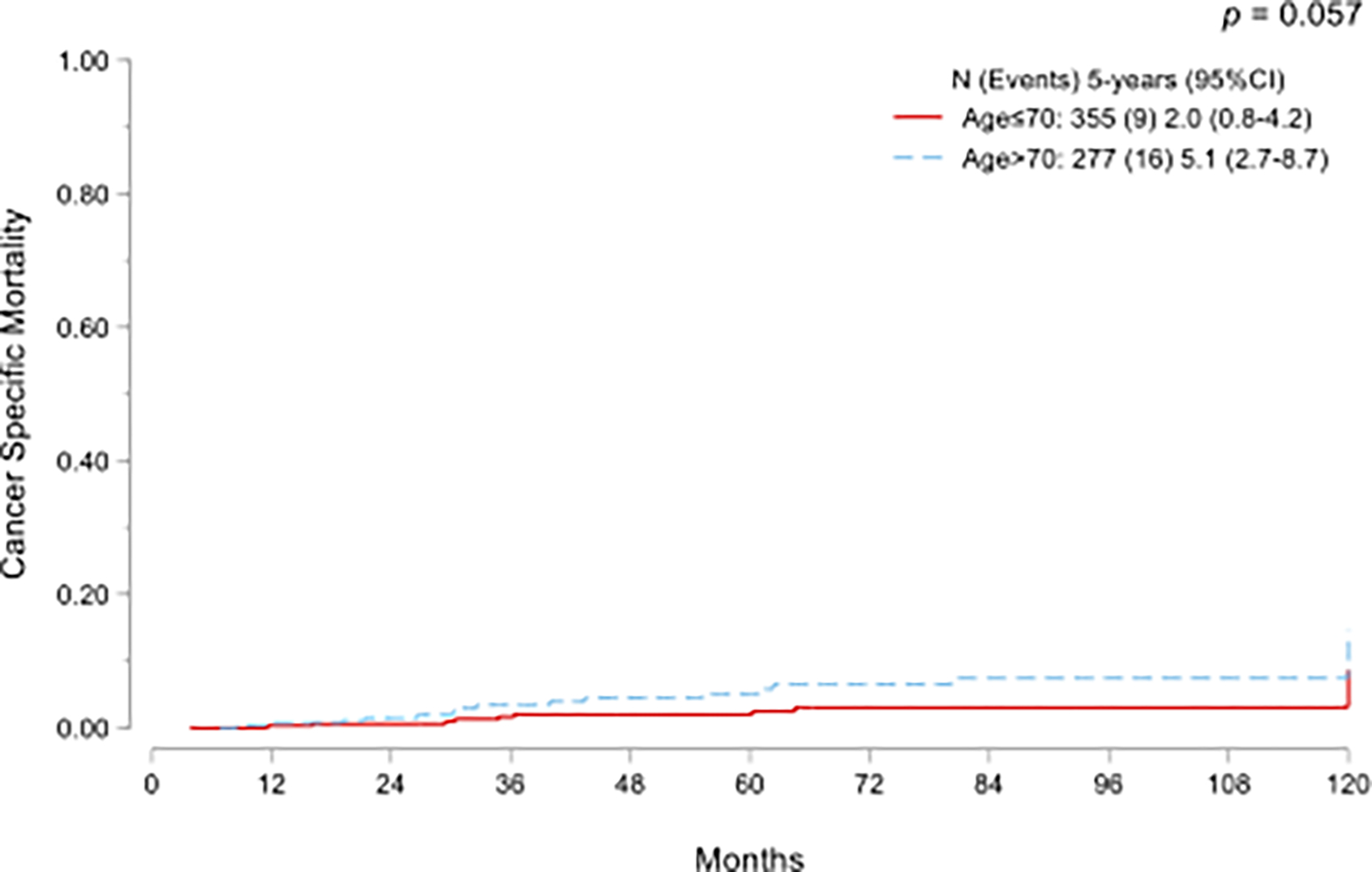
Kaplan-Meier curves for overall mortality (A) and cumulative incidence functions for cancer-specific mortality (B) in NMIBC treated with adequate BCG and stratified by age.
4. Discussion
In this contemporary cohort of patients with NMIBC treated with adequate BCG, age >70 years did not emerge as an independent risk factor for progression, HG recurrence, or CSM. However, as expected, age was associated with a significant difference in OM.
The incidence of BC is higher in the older population, with an average age at diagnosis of 70 years (12). Previous studies have proven that immune responses decrease with age; indeed, elderly patients are more susceptible to infections because the immune system decreases in robustness as one ages and impairs its ability to respond to infections and develop immunity (13). Although no standardized parameters have yet been defined, this condition is referred to as immunosenescence and characterized by a decrease in naive T-cells (14). Furthermore, several authors suggested that immunosenescence attenuates both adaptive and innate immune pathways (15).
It is widely accepted that BCG stimulates the immune system leading to antitumor activity driven by several cytokines and chemokines, which lead to the recruitment of immune system cells (granulocytes, CD4+ and CD8+ T cells, macrophages and natural killer) (16,17). Therefore, it has been hypothesized that immunology-based BCG antitumor activity is weaker in elderly patients.
In older series, this has been noted as well: Herr et al. reported a lower recurrence-free survival at five years (27% vs. 37%, p=0.005) in patients >70 years (4), while Oddens et al., with a median follow-up of 9.2 years found a negative correlation between age >70 years with PFS, CSS, and OS but not with RFS (5). Interestingly, the latter study showed that BCG is more effective than epirubicin chemotherapy, even in older patients (5). In a retrospective study of 2451 T1G3 patients treated with intravesical BCG, Gontero et al. showed, using a multivariate analysis, that age ≥70 was negatively associated with progression and OS and CSS (6). Recently, a large Japanese multicenter retrospective analysis, encompassing data from 31 centers, reported the impact of advanced age on over 2000 patients treated with BCG, and reported that advanced age >75 years was an independent predictor of both RFS and PFS in the T1 stage population (but not among patients with CIS). It should be noted that, after propensity score-matched analysis, advanced age was solely associated with recurrence (18).
Age was not identified as a prognostic risk factor in the 2006 European Organisation for Research and Treatment of Cancer (EORTC) NMIBC risk stratification, which was developed using patients treated with intravesical chemotherapy (19). In the updated 2016 EORTC nomogram, developed from trials where patients received 1–3 years of maintenance BCG, age was incorporated in the OS nomogram (20). Similarly, increasing age was associated with recurrence and progression in the Spanish Urology Association for Oncological Treatment (CUETO) scoring model, which was derived from patients treated with intravesical BCG (7).
On the other hand, Yuge et al. did not find an association between age and tumor recurrence in NMIBC treated with BCG (21). More recently, Calò et al. reported no difference in oncological outcomes among 123 T1 HG patients younger and older than 70 years (22). Additionally, Krajewski et al. showed that age >70 years was not associated with oncological outcomes in a cohort of 637 T1HG patients treated with BCG (23).
Recent work from our group has shown that patients with advanced age are more likely to have UROMOL transcriptomic class 2a and 2b tumours(24). Although these high-risk transcriptomic classes are associated with worse outcomes, their tumor biology may render them more sensitive to immunotherapies such as BCG (25). In particular, class 2a tumors are characterized by a high RNA-derived mutational load, resulting in an elevated neoantigen burden, and these patients may therefore benefit from immunotherapy (25). This may provide a potential explanation as to why age >70 was not a predictor of poor outomes in our series.
With so many conflicting reports, it is reasonable to ask: why yet another analysis of age? Our statistical approach represents a strength of our current study. In our series, as expected, the proportion of non-cancer-related death was higher in older patients (26% vs. 7%). Hence, we performed a competing-risk analysis considering OCM as a competing event when analyzing the risk of recurrence, progression, and cancer-specific mortality. This methodology is more appropriate for evaluating time-to-event outcomes than traditional survival analysis methods, such as Kaplan-Meier methods, when competing events could potentially affect the results. The Kaplan-Meier method assumes that individuals are censored if they do not experience the event of interest. If the proportion of deaths from other causes is non-negligible, the Kaplan-Meier method could lead to overestimating the event rate. Ignoring competing risk events affects the analysis in older patients because of the potential risk of non-cancer mortality. Under these circumstances, a high censoring rate due to competing events might lead to inaccurate estimates (26). For these reasons, we chose to account for the occurrence of competing events in our study. Notably, on Cox regression, we found that age was on the threshold for statistical significance for predicting progression. Our findings highlight that, when accounting for competing events, age does not emerge as a predictor of worse outcomes.
Our study is not devoid of limitations, mainly related to its retrospective design, which may lead to case selection bias. However, the patients in both cohorts were evenly matched. Older patients who are more co-morbid would be more likely to be offered intravesical treatment rather than early radical cystectomy, which might be offered to younger patients with similar tumor characteristics. While we attempted to adjust for this using a multivariable model, we acknowledge that undefined confounding factors would exist. Finally, the results concerning CSM should be considered taking into account the limited number of events in the entire sample (N=25, 4%).
5. Conclusion
In our contemporary cohort of patients with NMIBC treated with adequate BCG, age >70 years did not emerge as an independent risk factor for progression to MIBC or metastatic disease, nor of HG recurrence or CSM.
Supplementary Material
Funding/Support and role of the sponsor:
This research was supported by the Wayne B. Duddlesten Professorship in Cancer Research, the Raymond and Maria Floyd Bladder Cancer Research Foundation Grant to Ashish M. Kamat, and National Institute of Health/National Cancer Institute UTMD Anderson SPORE in Genitourinary Cancer (Bladder; P50CA091846) to Colin P. Dinney. Graciela M. Nogueras-Gonzalez was supported by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Financial disclosures:
Ashish M. Kamat certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Ashish M. Kamat is a consultant or advisory board member for Abbott Molecular, Arquer Diagnostics, ArTara Therapeutics, Asieris Pharmaceuticals, AstraZeneca, BioClin Therapeutics, Bristol Myers Squibb, Cepheid, Cold Genesys, Eisai, Engene, Ferring Pharmaceuticals, FerGene, Imagine Pharma, Janssen, MDxHealth, Medac, Merck, Pfizer, Photocure, ProTara Therapeutics, Roviant Sciences, Seattle Genetics, Sessen Bio, Theralase Technologies, TMC Innovation, and US Biotest; has received grants and/or research support from Adolor Corporation, Bristol Myers Squibb, FKD Industries, Heat Biologics, Merck, Photocure, SWOG/NIH, Specialized Programs of Research Excellence (SPORE), and AIBCCR; and holds the patent for Cytokine Predictors of Response to Intravesical Therapy (CyPRIT) jointly with UT MD Anderson Cancer Center. The other authors declare no competing interests.
6. References
- 1.Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022. Jan 1;81(1):75–94. [DOI] [PubMed] [Google Scholar]
- 2.Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016. Oct;196(4):1021–9. [DOI] [PubMed] [Google Scholar]
- 3.Lobo N, Brooks NA, Zlotta AR, Cirillo JD, Boorjian S, Black PC, et al. 100 years of Bacillus Calmette-Guérin immunotherapy: from cattle to COVID-19. Nat Rev Urol. 2021. Oct;18(10):611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herr HW. Age and Outcome of Superficial Bladder Cancer Treated with Bacille Calmette-Guérin Therapy. Urology. 2007. Jul 1;70(1):65–8. [DOI] [PubMed] [Google Scholar]
- 5.Oddens JR, Sylvester RJ, Brausi MA, Kirkels WJ, van de Beek C, van Andel G, et al. The effect of age on the efficacy of maintenance bacillus Calmette-Guérin relative to maintenance epirubicin in patients with stage Ta T1 urothelial bladder cancer: results from EORTC genito-urinary group study 30911. Eur Urol. 2014. Oct;66(4):694–701. [DOI] [PubMed] [Google Scholar]
- 6.Gontero P, Sylvester R, Pisano F, Joniau S, Vander Eeckt K, Serretta V, et al. Prognostic Factors and Risk Groups in T1G3 Non–Muscle-invasive Bladder Cancer Patients Initially Treated with Bacillus Calmette-Guérin: Results of a Retrospective Multicenter Study of 2451 Patients. Eur Urol. 2015. Jan 1;67(1):74–82. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez -Gomez Jesus, Madero R, Solsona E, Unda M, Martinez -Piñeiro Luis, Gonzalez M, et al. Predicting Nonmuscle Invasive Bladder Cancer Recurrence and Progression in Patients Treated With Bacillus Calmette-Guerin: The CUETO Scoring Model. J Urol. 2009. Nov;182(5):2195–203. [DOI] [PubMed] [Google Scholar]
- 8.Sylvester RJ, Rodríguez O, Hernández V, Turturica D, Bauerová L, Bruins HM, et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non–muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur Urol. 2021. Apr 1;79(4):480–8. [DOI] [PubMed] [Google Scholar]
- 9.Matulay JT, Li R, Hensley PJ, Brooks NA, Narayan VM, Grossman HB, et al. Contemporary Outcomes of Patients with Nonmuscle-Invasive Bladder Cancer Treated with bacillus Calmette-Guérin: Implications for Clinical Trial Design. J Urol. 2021. Jun;205(6):1612–21. [DOI] [PubMed] [Google Scholar]
- 10.U.S Department of Health and Human Services Food and Drug Administration. BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment Guidance for Industry. 2018. [URL: https://www.fda.gov/media/101468/download#:~:text=For%20the%20purposes%20of%20this,completion%20of%20adequate%20BCG%20therapy]. :13.
- 11.Kamat AM, Sylvester RJ, Böhle A, Palou J, Lamm DL, Brausi M, et al. Definitions, End Points, and Clinical Trial Designs for Non–Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol. 2016. Jun;34(16):1935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz PI, Meryn S, Bochner BH. The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int. 2010;105(3):300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawelec G. Immunosenescence and cancer. Biogerontology. 2017. Aug 1;18(4):717–21. [DOI] [PubMed] [Google Scholar]
- 15.Fulop T, Kotb R, Fortin CF, Pawelec G, de Angelis F, Larbi A. Potential role of immunosenescence in cancer development. Ann N Y Acad Sci. 2010;1197(1):158–65. [DOI] [PubMed] [Google Scholar]
- 16.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat Rev Urol. 2014. Mar;11(3):153–62. [DOI] [PubMed] [Google Scholar]
- 17.Jiang S, Redelman-Sidi G. BCG in Bladder Cancer Immunotherapy. Cancers. 2022. Jan;14(13):3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue T, Miyake M, Nishimura N, Onozawa M, Kashima S, Numakura K, et al. Association of Increased Age With Decreased Response to Intravesical Instillation of Bacille Calmette-Guérin in Patients With High-Risk Non-Muscle Invasive Bladder Cancer: Retrospective Multi-Institute Results From the Japanese Urological Oncology Research Group JUOG-UC-1901-BCG. Urology. 2022. Sep 1;167:158–64. [DOI] [PubMed] [Google Scholar]
- 19.Sylvester RJ, van der Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Eur Urol. 2006. Mar 1;49(3):466–77. [DOI] [PubMed] [Google Scholar]
- 20.Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non–Muscle-invasive Stage Ta–T1 Urothelial Bladder Cancer Patients Treated with 1–3 Years of Maintenance Bacillus Calmette-Guérin. Eur Urol. 2016. Jan 1;69(1):60–9. [DOI] [PubMed] [Google Scholar]
- 21.Yuge K, Kikuchi E, Matsumoto K, Takeda T, Miyajima A, Oya M. Could Patient Age Influence Tumor Recurrence Rate in Non-muscle-invasive Bladder Cancer Patients Treated with BCG Immunotherapy? Jpn J Clin Oncol. 2011. Apr 1;41(4):565–70. [DOI] [PubMed] [Google Scholar]
- 22.Calò B, Sanguedolce F, Fortunato F, Stallone G, d’Altilia N, Chirico M, et al. The impact of age on intravesical instillation of Bacille Calmette–Guerin treatment in patients with high-grade T1 bladder cancer. Medicine (Baltimore). 2019. Aug;98(31):e16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krajewski W, Rodríguez Faba O, Breda A, Pisano F, Poletajew S, Tukiendorf A, et al. Analysis of age influence on oncological results and toxicity of BCG immunotherapy in non-muscle invasive bladder cancer. World J Urol. 2020. Dec 1;38(12):3177–82. [DOI] [PubMed] [Google Scholar]
- 24.Lobo N, Sood A, Duan Z, Tan WS, Grajales V, Lindskrog SV, et al. Pd29–05 association of age with non-muscle invasive bladder cancer: a biological basis for epidemiological disparities? J Urol. 2023. Apr;209(Supplement 4):e826. [DOI] [PubMed] [Google Scholar]
- 25.Lindskrog SV, Prip F, Lamy P, Taber A, Groeneveld CS, Birkenkamp-Demtröder K, et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat Commun. 2021. Apr 16;12(1):2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004. Oct;91(7):1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


