Abstract
RNA modifications affect many biological processes and physiological diseases. The 5-methylcytosine (m5C) modification regulates the progression of multiple tumors. However, its characteristics and functions in hepatocellular carcinoma (HCC) remain largely unknown. Here, we found that HCC tissues had a higher m5C methylation level than the adjacent normal tissues. Transcriptome analysis revealed that the hypermethylated genes mainly participated in the phosphokinase signaling pathways, such as the Ras and PI3K-Akt pathways. The m5C methyltransferase NSUN2 was highly expressed in HCC tissues. Interestingly, the expression of many genes was positively correlated with the expression of NSUN2, including GRB2, RNF115, AATF, ADAM15, RTN3, and HDGF. Real-time PCR assays further revealed that the expression of the mRNAs of GRB2, RNF115, and AATF decreased significantly with the down-regulation of NSUN2 expression in HCC cells. Furthermore, NSUN2 could regulate the cellular sensitivity of HCC cells to sorafenib via modulating the Ras signaling pathway. Moreover, knocking down NSUN2 caused cell cycle arrest. Taken together, our study demonstrates the vital role of NSUN2 in the progression of HCC.
Keywords: 5-methylcytosine, Hepatocellular carcinoma, NSUN2, Ras pathway, Sorafenib
Introduction
Liver cancer accounts for the sixth most common cancer and the third leading cause of cancer mortality worldwide [1]. The main type of liver cancer is hepatocellular carcinoma (HCC), which is a primary malignant tumor originating from the liver epithelial tissue or mesenchymal tissue [2]. Various kinase-related signaling pathways are aberrantly activated in HCC, such as the Ras/Raf/MAPK/Erk pathway (Ras pathway) and the PI3K/Pten/Akt/mTOR pathway (PI3K-Akt pathway) [3], [4]. The Ras pathway regulates the proliferation, apoptosis, and differentiation of HCC cells [5]. This kinase pathway recruits the GRB2/SHC/SOS complex and promotes the phosphorylation of Ras and Raf when the membrane surface receptor of the epidermal growth factor receptor (EGFR) receives the stimulation signal. Then, a high level of phosphorylated Erk (p-Erk), as an activation marker, translocates into the nucleus and combines with other transcription initiation factors to promote oncogene expression [6]. As the first-line molecular-targeted drug for HCC, sorafenib can specifically inhibit Raf phosphorylation in the Ras pathway and plays an important role in inhibiting HCC cell proliferation and angiogenesis [7], [8].
The 5-methylcytosine (m5C) modification occurs in many types of RNAs, including mRNAs and non-coding RNAs. NOP2/Sun RNA methyltransferase (NSUN2) mainly catalyzes the formation of m5C as a writer protein [9], induces the differentiation of epidermal and neural stem cells [10], [11], and directly affects gene expression in viruses by regulating the splicing of HIV-1 RNA [12]. Modified RNAs are recognized by Y-box binding protein 1 (YBX1) and Aly/REF export factor (ALYREF). YBX1 and ALYREF promote mRNA stability [13] and nuclear translocation [14] as the readers of m5C. No m5C eraser has been identified yet, although some proteins are involved in m5C oxidation. For example, AlkB homolog 1 (ALKBH1) and ten-eleven translocation (TET) family proteins have been identified as dioxygenases that catalyze the conversion of m5C to hm5C, which regulates RNA degradation and mitochondrial activity [15], [16].
As a critical RNA m5C catalytic enzyme, the functions of NSUN2 have been described in multiple types of cancer. NSUN2 affects the mRNA stability of the heparin-binding growth factor (HDGF) by catalyzing m5C modification in its 3′-untranslated region (3′-UTR), which promotes the pathogenesis of bladder cancer [12]. Additionally, NSUN2 is overexpressed in breast cancer (BRCA) and hypopharyngeal squamous cell carcinoma (HPSCC) [17], [18]. Pan-cancer analysis showed that NSUN2 is positively correlated with DNA copy number and mRNA expression, which are associated with poor prognosis [18], [19]. NSUN2 can regulate the m5C modification of H19 lncRNA and promote the occurrence and development of HCC by recruiting G3BP stress granule assembly factor 1 (G3BP1) [20]. The m5C profiles of circular RNA and mRNA were discovered in HCC [21], [22]. However, the biological significance of NSUN2 and the characteristics of the m5C modification in HCC have not been fully investigated.
In this study, we analyzed the characteristics of the mRNA m5C modification in HCC tissues compared to those in the adjacent tissues at the single-nucleotide resolution. The mechanism by which NSUN2 regulates the expression of multiple target genes was determined at the bioinformatic and experimental levels. We examined the effect of NSUN2 on regulating HCC cell sensitivity to sorafenib by affecting the activity of the Ras pathway. Additionally, the down-regulation of NSUN2 in HCC cells arrested the cell cycle. The mechanisms of m5C regulated by NSUN2 were involved in the progression of HCC.
Results
mRNAs are frequently m5C-hypermethylated in HCC tissues
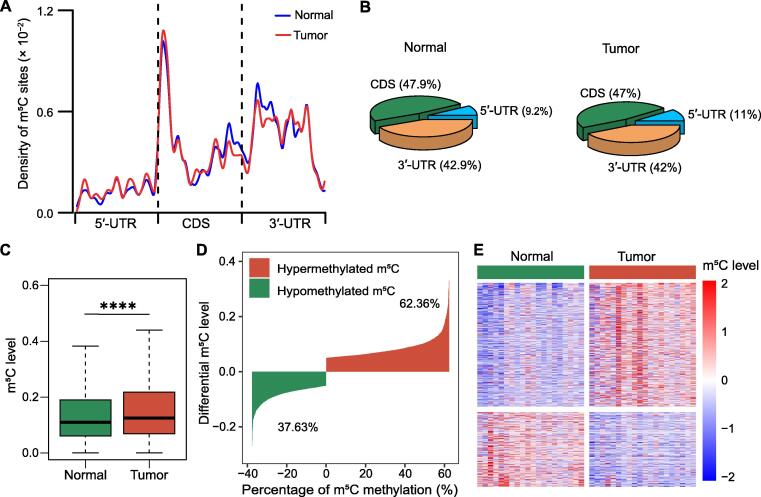
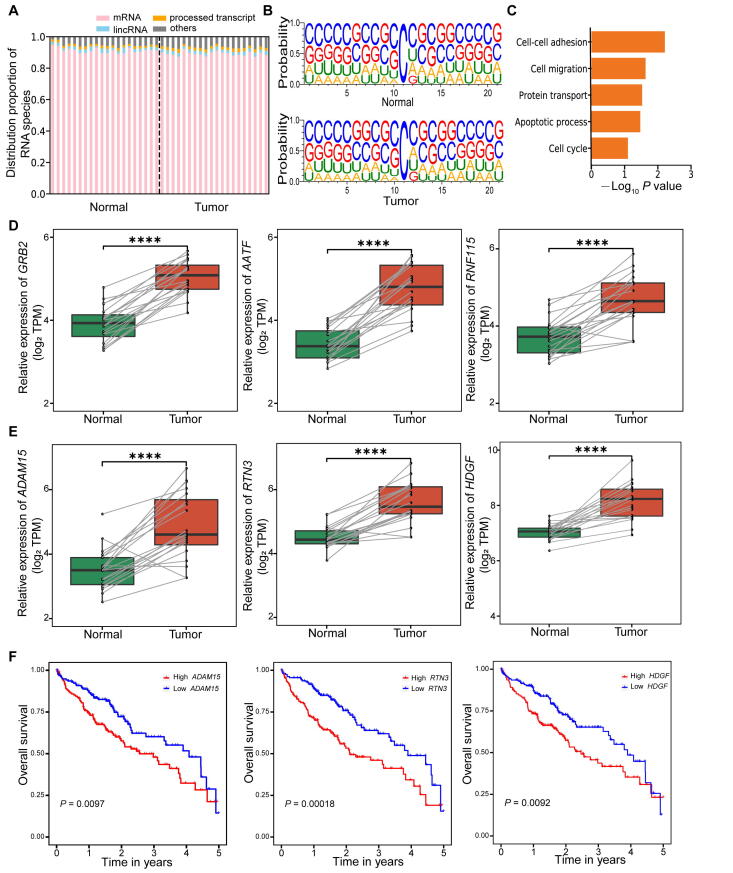
To reveal the m5C modification features in HCC, we collected 20 pairs of HCC tumor samples and analyzed the transcriptome (RNA sequencing; RNA-seq) data and RNA bisulfite sequencing (RNA-BisSeq) data. The m5C sites were enriched in mRNAs in the HCC tumor tissues and the adjacent tissues (Figure S1A). The distribution characteristics of the m5C modifications in HCC mRNAs were found to be enriched downstream of the translation initiation site in the mRNA coding sequence (CDS) region (Figure 1A). The distribution pattern was consistent with other mammalian cells previously reported [13]. The proportion of the m5C modification in different regions of the mRNA was statistically analyzed. The m5C sites covered in the 3′-UTR, CDS, and 5′-UTR were similar in cancer tissues and adjacent tissues, and the CDS region contained the highest number of m5C sites (Figure 1B). A sequence frequency logo displayed an embedding feature of m5C sites in CG-rich environments (Figure S1B). Our RNA-BisSeq data identified 2482 m5C sites in mRNAs with differential methylation levels (as shown in Table S1). Additionally, we found that mRNA m5C modification in HCC tissues was significantly higher than that in the adjacent tissues for the overall methylation level (Figure 1C). We found 1548 and 934 sites for hypermethylated and hypomethylated, respectively. The ratio of hypermethylated sites in tumor tissues was 62.36%, and the ratio of hypomethylated sites was 37.63% relative to that in the normal tissues (Figure 1D). The heatmap analysis showed the differential methylation level of the m5C sites between the adjacent tissues and HCC tissues (Figure 1E). In summary, mRNAs are frequently m5C-hypermethylated in HCC.
Figure 1.
mRNAs are frequentlym5C-hypermethylated in HCC tissuesA. Distribution pattern of the m5C sites on mRNAs in the HCC tissues (tumor) and the adjacent tissues (normal). B. Different proportions of m5C modifications in regions of mRNA between the HCC tissues and the adjacent tissues. C. The overall m5C modification level is higher in the HCC tissues than in the adjacent tissues, as determined by the BisSeq data analysis. Statistical significance was calculated by Wilcoxon test (****, P = 5.116E−09). D. Difference in the m5C modification levels between the HCC tissues and the adjacent tissues. E. Heatmap showing the differential m5C methylation levels between the HCC tissues and the adjacent tissues. HCC, hepatocellular carcinoma; m5C, 5-methylcytidine; CDS, coding sequence; UTR, untranslated region.
Multiple m5C-hypermethylated genes related to NSUN2 participate in oncogenic pathways
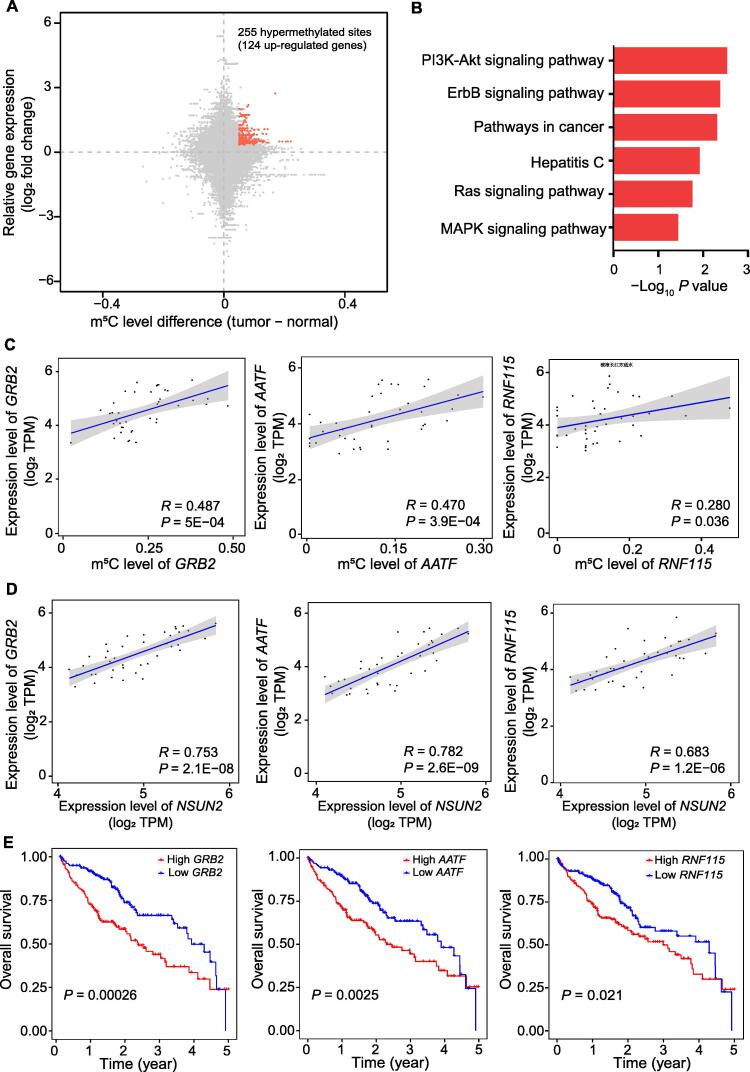
To further investigate the effect of m5C on the progress of HCC, we identified differentially expressed mRNAs with hypermethylated m5C sites in HCC tissues. We detected 255 hypermethylated sites, covering 124 genes with high mRNA expression (Figure 2A). Through the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of highly m5C-modified genes, several tumor-related pathways, including the PI3K-Akt, ErbB, and Ras signaling pathways, were found to be enriched in m5C modifications (Figure 2B). Moreover, these highly m5C-modified genes were found to be involved in the progression of cell migration, apoptosis, and cell cycle (Figure S1C). We investigated the genes (GRB2, AATF, RNF115, ADAM15, RTN3, and HDGF) that are highly expressed in HCC and modified by m5C (Figure S1D and E). To further determine whether the specific m5C-modified genes are regulated by NSUN2 in HCC, first, we analyzed the correlation between the mRNA expression level of target genes and their m5C modification level (Figure 2C). Then, we analyzed the correlation between the NSUN2 mRNA expression and the mRNA expression of the target genes (Figure 2D). The results showed that the mRNA expression of the target genes was related to their m5C modification level and also to the NSUN2 mRNA expression level (Figure 2C and D). Additionally, the results of the TCGA analysis indicated that higher expression levels of these target genes (GRB2, AATF, RNF115, ADAM15, RTN3, and HDGF) were associated with a poor prognosis of HCC (Figure 2E, Figure S1F). The aforementioned results suggest that multiple hypermethylated genes associated with NSUN2 participate in oncogenic pathways.
Figure 2.
Multiplem5C-hypermethylated genes related to NSUN2 participate in the oncogenic pathwaysA. The distribution of mRNAs with a significant change in the m5C methylation level and the gene expression level in HCC tissues and the adjacent tissues. B. The KEGG analysis showed that m5C-hypermethylated genes with high expression levels in the HCC tissues were enriched in oncogenic signaling pathways. C. A relation analysis showed that the expression levels of GRB2, RNF115, and AATF were positively correlated with their m5C modification levels. D. The expression levels of GRB2, RNF115, and AATF were positively correlated with the NSUN2 expression level. E. The overall survival analysis indicates the correlation of the mRNA expression of GRB2, RNF115, and AATF with poor prognosis in HCC patients. P values were calculated by Student’s t-test. KEGG, Kyoto Encyclopedia of Genes and Genomes; TPM, transcripts per kilobase of exon model per million mapped reads.
NSUN2 is highly expressed in HCC and regulates mRNA m5C modification
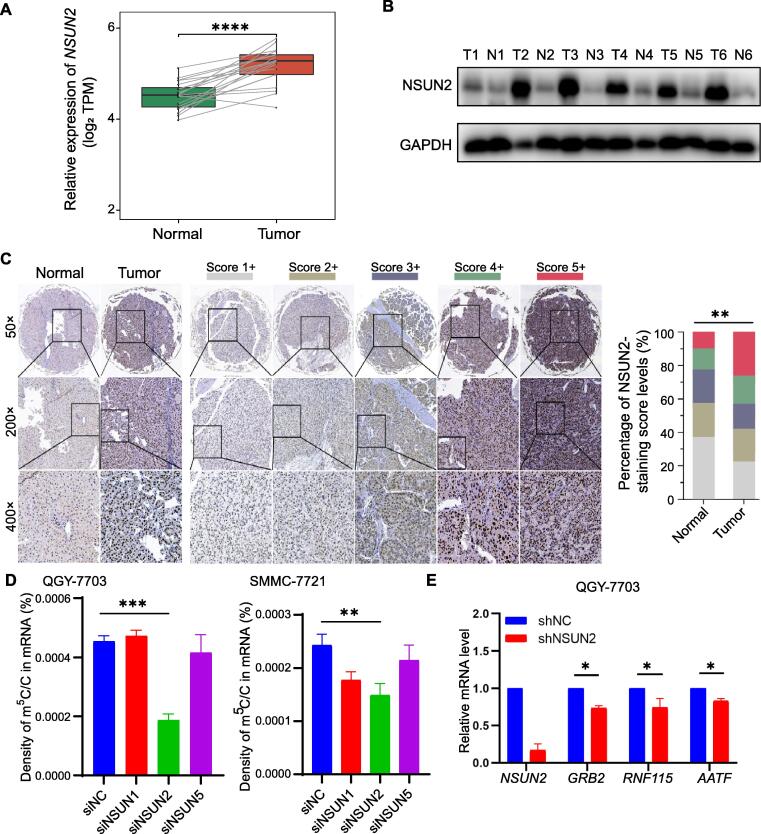
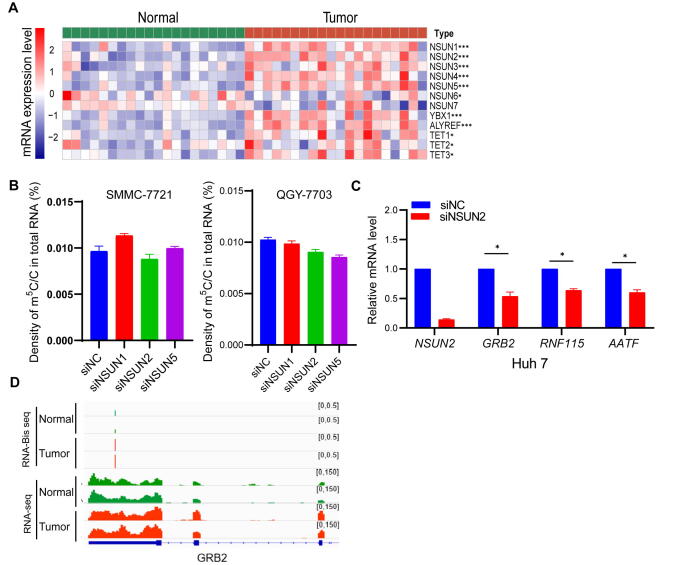
Transcriptome data analysis showed that m5C-related writer and reader proteins were highly expressed in HCC tissues (Figure S2A). Previous studies have shown that NSUN2 is involved in the regulation of m5C modification and affects tumor progression [20], [23]. Here we focused on the regulatory relationship between NSUN2 and m5C-modified target genes in the progression of HCC. Expression data displayed that NSUN2 mRNA was overexpressed in HCC tissues (Figure 3A). We confirmed the protein expression of NSUN2 in some of the HCC tissue cohorts (n = 6) through Western blot (Figure 3B). The results of the immunohistochemical analysis showed that NSUN2 had a higher expression in HCC tissues than that in the adjacent normal tissues (Figure 3C). Additionally, the m5C modification level of total RNA and mRNAs in the HCC cell lines (QGY-7703 and SMMC-7721) were analyzed by ultra-high performance liquid chromatography-mass spectrometry/mass spectrometry (UHPLC-MS/MS). We used siRNAs to knock down NSUN2 and its family members (NSUN1/5). NSUN2 was found to be an important methyltransferase for mRNAs in HCC cells (Figure 3D, Figure S2B). Real-time PCR revealed that the mRNA expression levels of GRB2, RNF115, and AATF were significantly decreased in the NSUN2-knockdown HCC cells (Figure 3E, Figure S2C). The integrative genomics viewer (IGV) tracks displayed the read coverage of the GRB2 mRNA in the RNA-seq and RNA-BisSeq data, and showed the up-regulation of m5C modifications and mRNA abundance in GRB2 in HCC tissues compared to that in the adjacent tissues (Figure S2D). We concluded that NSUN2 plays a critical role in regulating the m5C modification of the target genes (GRB2, RNF115, and AATF) in HCC.
Figure 3.
NSUN2 is highly expressed in HCC and regulates mRNA m5C modificationA. The expression of NSUN2 mRNA was higher in HCC tissues than in the adjacent tissues determined by transcriptome analysis. B. Western blot analysis showed the higher expression of NSUN2 in HCC tissues than in the adjacent tissues. GAPDH was used as a reference control. “T” indicates a tumor smaple, and “N” indicates the adjacent tissue. C. Immunohistochemical analysis showed the higher expression of NSUN2 in HCC tissues than in the adjacent tissues. D. In HCC cells, UHPLC-MS/MS analysis showed that the down-regulation of NSUN2 significantly decreased the density of m5C/C in mRNAs. E. The real-time PCR analysis showed that the mRNA expression of GRB2, RNF115, and AATF was significantly decreased when NSUN2 was silenced in QGY-7703 cells. Data were represented by mean ± SD. Statistical significance was determined by Student’s t-test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). UHPLC-MS/MS, ultra-high performance liquid chromatography-mass spectrometry/mass spectrometry.
NSUN2 affects the sensitivity of HCC cells to sorafenib by regulating the activity of the Ras pathway
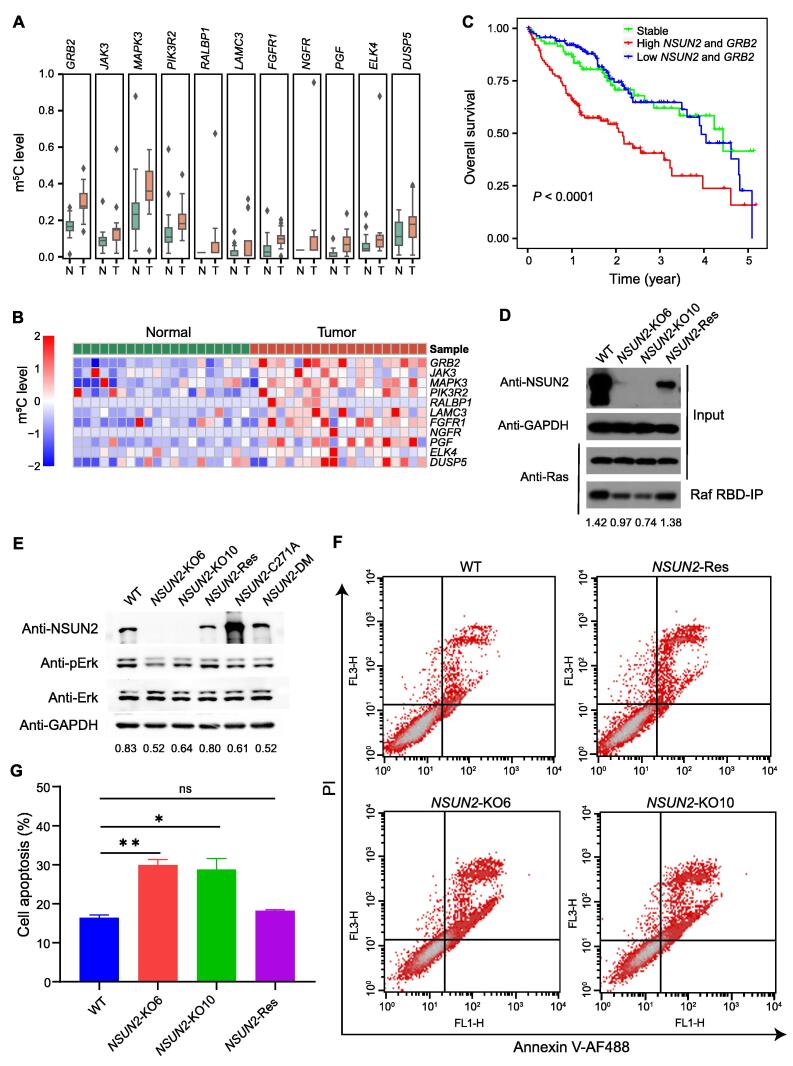
The activity of the Ras pathway is abnormally high in most HCC patients, which leads to a poor prognosis [24]. The phosphorylation of Raf is one of the crucial targets of sorafenib [25]. GRB2 is a critical upstream linker that promotes Raf phosphorylation that is regulated by NSUN2 in esophageal squamous cell carcinoma. A previous study has reported that GRB2 is a key upstream regulator of Raf phosphorylation and is regulated by NSUN2 [26]. To confirm the effect of the regulation of m5C by NSUN2 on the activity of the Ras pathway in HCC, the m5C modification levels of genes (such as GRB2, MAPK3, and PIK3R2) in the Ras pathway were analyzed. These genes were hypermethylated in HCC tissues (Figure 4A). A heatmap analysis showed that the m5C modification levels of these genes were increased in HCC tissues (Figure 4B). According to the results of the TCGA data analysis, HCC patients with higher expression of NSUN2 and GRB2 had the worst prognosis (Figure 4C).
Figure 4.
NSUN2 affects the sensitivity of HCC cells to sorafenib by regulating the activity of the Ras pathwayA. Box plots showing the mRNA m5C levels of the Ras pathway-related genes. B. Heatmap showing the differential mRNA m5C levels of the Ras pathway-related genes in HCC tissues and the adjacent tissues. C. The overall survival analysis indicated that the high expression of NSUN2 and GRB2 was correlated with the worst prognosis in HCC patients (****, P < 0.0001). D. Western blot showing the Ras activity detected in wild-type QGY-7703 cells (WT), NSUN2-knockout cells (NSUN2-KO6/KO10), and NSUN2-rescued cells (NSUN2-Res). E. Western blot of Erk and p-Erk in NSUN2-knockout cells (NSUN2-KO6/KO10) and NSUN2-rescued cells (NSUN2-Res, NSUN2-C271A, and NSUN2-DM). NSUN2-Res, NSUN2-C271A, and NSUN2-DM indicate wild-type rescued, binding site mutant rescued, and binding site and catalytic site double mutant rescued, respectively. GAPDH was used as a reference control. F. Sorafenib treatment and flow cytometry analysis of the apoptosis of QGY-7703 cells when NSUN2 was knockout or rescued. G. The statistical analysis of the apoptosis ratio shown in (F). Data were represented by mean ± SD. Statistical significance was determined by Student’s t-test (*, P < 0.05; **, P < 0.01; ns, not significant). Raf RBD, Ras-binding domain of Raf; IP, immunoprecipitation; p-Erk, phosphorylated-Erk; PI, propidium iodide.
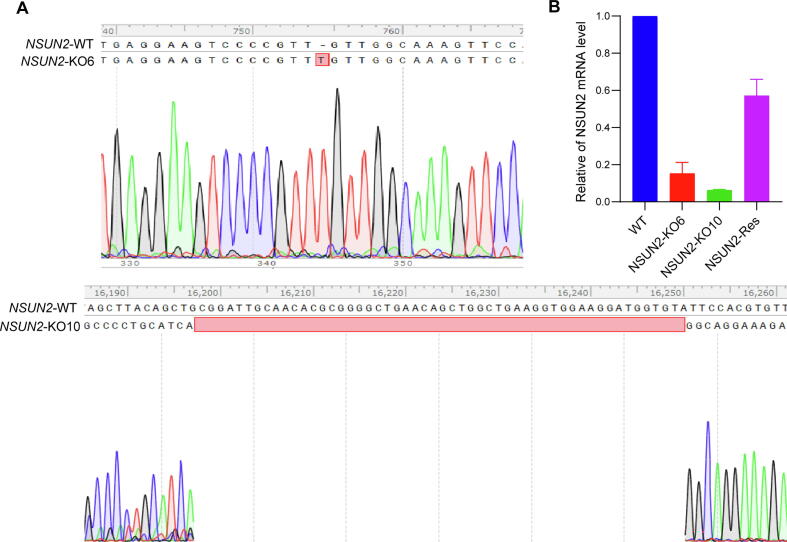
To further investigate the effect of NSUN2 on the level of active Ras, we constructed two NSUN2-konckout cell lines (NSUN2-KO6/KO10) and one NSUN2-rescued stable cell line (NSUN2-Res). The identification of NSUN2 knockout at the genome level and the mRNA expression level are shown in Figure S3. We found that the level of active Ras protein in NSUN2-knockout cells was significantly decreased, which could be rescued by wild-type NSUN2 (Figure 4D). The level of phosphorylated-Erk (p-Erk) is an important indicator of the activity of the Ras pathway. p-Erk decreased in HCC cells without changing the Erk protein level in NSUN2-knockout cells, and rescued by wild-type NSUN2. The changing of the p-Erk level cannot be rescued by mutant NSUN2 (Figure 4E).
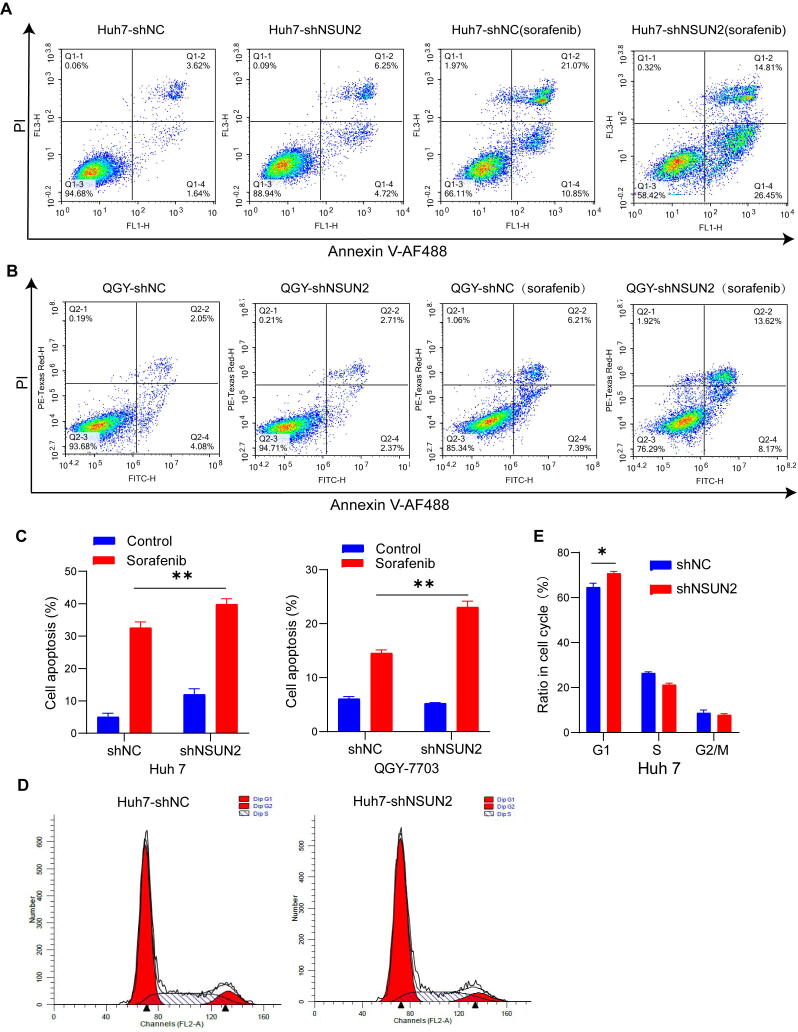
Sorafenib is a molecular inhibitor for the phosphorylation of Raf, and inhibits Ras activity, which is widely used in the systemic therapy of HCC. We investigated whether NSUN2 affects the sensitivity of sorafenib in HCC cell lines. Through flow cytometry analysis of the apoptotic HCC cells in the NSUN2-knockout group and the control group under sorafenib stress, the proportion of apoptotic HCC cells treated with sorafenib was significantly higher, compared to the proportion of apoptotic cells in the control group (Figure 4F). Data statistics are shown in Figure 4G. Similar results were obtained in the NSUN2-knockdown cells (Figure S4A and B). We observed that knockdown of NSUN2 did not increase the apoptotic rate of QGY-7703 cells but affected that of Huh7 cells. The increased sensitivity of these cell lines to sorafenib was consistent when NSUN2 is knocked down (Figure S4C). Additionally, we found that the down-regulation of NSUN2 was associated with cell cycle arrest (Figure S4D and E). In summary, NSUN2 affects the sensitivity of HCC cells to sorafenib by regulating the activity of the Ras pathway.
Discussion
In recent years, many RNA modifications have been identified [27]. As an essential epigenetic modification of RNA, m5C participates in different regulatory mechanisms and biological functions, especially in cancers [16], [17], [18], [19], [20], [21], [22]. In this study, the distribution characteristics of the m5C modification in HCC were studied. We discovered high levels of m5C modification and NSUN2 expression in HCC. The hypermethylated target genes (GRB2, AATF, and RNF115) participate in the carcinogenic pathways. NSUN2 affects the sensitivity of HCC cells to sorafenib by regulating the activity of the Ras pathway.
NSUN2 is highly expressed in multiple tumor types, such as gastric cancer and esophageal squamous cell carcinoma [18], [28], [29]. Here, we found that NSUN2 was overexpressed in HCC. Moreover, NSUN2 in HCC tissues was strongly correlated with the high methylation and expression of target genes, including GRB2, RNF115, AATF, ADAM15, RTN3, and HDGF. Li et al. found that NSUN2 coordinates with lin-28B, a novel m5C recognition protein, to catalyze the m5C modification of GRB2 and stabilize its mRNA expression. High levels of GRB2 promote the activation of the PI3K/Akt and Ras pathways in esophageal squamous cell carcinoma [30]. We demonstrated that NSUN2 inhibited the Ras activation and decreased the p-Erk level in HCC, which led to the increased sensitivity of HCC cells to sorafenib.
A previous study has reported that nascent RNA with m5C modification can regulate chromatin structures and recruit transcription factors. The m5C-mediated complex leads to 5-azacitidine resistance in leukemia cells, which provides new insights into the treatment of leukemia [31]. In our study, because of the critical role of NSUN2 in regulating the m5C modification and the expression of mRNAs related to the Ras pathway, the sensitivity of HCC cells to sorafenib was increased, which has great significance for the treatment of HCC patients. RNA epigenetics, especially m5C, can potentially regulate drug sensitivity.
Overall, we reveal the m5C landscape in HCC at a single-nucleotide resolution and verified the correlation between m5C-hypermethylated genes and HCC tumor characteristics. NSUN2 has been reported to be involved in various tumor-related cell processes, including affecting proliferation, apoptosis, and sorafenib sensitivity in HCC cells. Our study provides novel mechanisms for the effect of RNA epigenetic modification on HCC progression, which might help discover more effective HCC treatment targets and strategies.
Materials and methods
Cell lines and tissues
Human HCC cell lines (QGY-7703, Huh 7, and SMMC-7721) were cultured in Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in 5% CO2 at 37 °C. For sensitivity analysis of sorafenib, QGY-7703 cells were seeded in six-well plates and treated with 10 µM sorafenib for 24 h. The Huh7 cells were treated with 8 µM sorafenib for 24 h.
Plasmids, antibodies, and real-time PCR primers
PLKO.1-shcontrol (NC), pLKO.1-shNSUN2, psPAX2, and pMD2 were used for NSUN2 knockdown in HCC cells. The sequence of shNSUN2 was 5′-CCGGGCTGGCACAGGAGGGAATATACTCGAGTATATTCCCTCCTGTGCCAGCTTTTTG-3′. The sequence of siNSUN2 was: 5′-CACGUGUUCACUAAACCCUAUTT-3′.
The antibodies used in this study were anti-NSUN2 (Catalog No. 44056S, Cell Signaling Technology, Danvers, MA), anti-GAPDH (Catalog No. GB12002, Servicebio, Wuhan, China), anti-pErk (Catalog No. 4370s, Cell Signaling Technology), and anti-Erk (Catalog No. 4695s, Cell Signaling Technology). Real-time PCR primers for target gene quantification used in this study are as follows: RNF115 (forward 5′-CGGCAGTCGGATAGACAATAC-3′, reverse 5′-TGTCAGGACGAGAACTTCCTC-3′), GRB2 (forward 5′-CTGGGTGGTGAAGTTCAATTCT-3′, reverse 5′-GTTCTATGTCCCGCAGGAATATC-3′), ADAM15 (forward 5′-CCCTGAATGTACGAGTGGCAC-3′, reverse 5′-GGAGGAAGTTTTCGAGGGTGA-3′), AATF (forward 5′-TCAGCCTCCCTCTTGGACA-3′, reverse 5′-TCATCAGACGATCCTGGCAGA-3′), NSUN2 (forward 5′-GGTATCCTGAAGAACTTGCC-3′, reverse 5′-ATCTTATGATGAGGCCGCA-3′), and GAPDH (forward 5′-CGCTCTCTGCTCCTCCTGTTC-3′, reverse 5′-ATCCGTTGACTCCGACCTTCAC-3′).
RNA-BisSeq and RNA-seq for HCC tissues and adjacent tissues
Tissues were frozen in liquid nitrogen and broken using Qiagen tissue lyser II (Catalog No. 69982, Qiagen, Hilden, Germany). Then, 1 ml TRIzol was added to the broken tissues, and total RNA was extracted with chloroform-isopropyl alcohol. Next, the HCC mRNAs were enriched using the Dynabeads mRNA Purification Kit (Catalog No. 61006, Ambion, Waltham, MA), and samples were treated with DNase (Catalog No. AM22222, ThermoFisher Scientific, Waltham, MA) at 37 °C for 20 min to remove genomic DNA. After DNase treatment, mRNAs were fragmented by a fragmentation reagent (Catalog No. AM8740, Ambion), and then the alcohol method was used to precipitate the samples.
After alcohol precipitation, 10 ng of mRNA samples were taken for the transcription library, and 100–200 ng of mRNA samples were taken for bisulfite treatment, according to an earlier published method [32]. Finally, we used the KAPA stranded RNA-seq library preparation kit (Catalog No. KR1139, KAPA, Potters Bar, UK) for library construction. Sequencing was performed on an Illumina HiSeq PE150 sequencing system with a paired-end 150 bp read length.
UHPLC-MS/MS analysis
The UHPLC-MS/MS analysis was performed by a previously reported method [13]. Total RNA or mRNA (100–200 ng) was extracted from the QGY-7703 and SMMC-7721 cells, which were digested with 0.1 U nuclease P1 (Catalog No. M0660, New England Biolabs, Ipswich, MA) and 1.0 U calf intestinal alkaline phosphatase (Catalog No. 18009019, Invitrogen) at 37 °C overnight. Then, the mixture was filtered through a 3 K Omega membrane tube (Catalog No. OD010C35, PALL, New York, NY). Finally, we detected rm5C, rC, rU, rG, and rA using UHPLC-MS/MS.
Immunohistochemistry
The tissues were fixed with 5 ml of formaldehyde fixative solution. Then, they were dehydrated by adding molten paraffin wax at 58 °C. Tissues were cut into 15-µm sections using a rotary microtome, suspended in a water bath at 56 °C, and mounted onto gelatin-coated histological slides. The slides were dried overnight at room temperature. Then, we performed an immunohistochemistry analysis. The samples were incubated with anti-NSUN2 (1:100) overnight at 4 °C. Finally, the expression of NSUN2 in HCC tissues was visualized under a microscope using bright-field illumination.
Ras activation assay
RAS activity was analyzed with a Ras activation assay biochem kit (Catalog No. BK008, Cytoskeleton, Männedorf, Switzerland). The QGY-7703 cell lines containing the control group, the NSUN2-KO6 group, the NSUN2-KO10 group, and the NSUN2-Res group were prepared in advance, and equal concentrations of cells were collected and spread in a six-well plate. After 24 h, 500 µl of cell lysate was added to each well and centrifuged at 10,000 r/min at 4 °C for 2 min, and the supernatant protein was collected. The Bradford protein quantification kit (Catalog No. 23236, Invitrogen) was used to quantify the protein, and each group was diluted with cell lysate to equal volume and density. Then, 20 µl of whole-cell lysate was added to 5 µl of 5× sodium dodecyl sulfate (SDS) loading buffer, and the sample was boiled at 95 °C for 10 min as an input sample. The remaining samples were added with the same amount of Raf-RBD beads and rotated at 4 °C for 1 h. The beads were collected at 4 °C and centrifuged at 5000 g for 1 min. Then, 90% of the supernatant was removed, and the beads were cleaned three times with 500 µl of wash buffer. Finally, 1× SDS loading buffer was added, and the sample was boiled at 95 °C for 10 min as an immunoprecipitation (IP) sample. The samples were subjected to Western blot analysis, and the pan-RAS antibody was used to quantitatively identify the active Ras.
Flow cytometry analysis
The NC group and the shNSUN2 group cells were seeded in a six-well plate. The cell confluence reached 80% through overnight culture. Then, the cells were treated with sorafenib for 24 h. The cells were harvested and washed once with precooled phosphate buffer saline (PBS). According to the protocol of the dead cell apoptosis kit (Catalog No. V13241, Invitrogen), 5× annexin-binding buffer was diluted to 1× with deionized water, and the propidium iodide (PI) staining solution was diluted to 100 µg/ml. After the buffer was prepared, the cells were resuspended in 100 µl 1× annexin-binding buffer, 5 µl Alexa Fluor 488-annexin V, and 1 µl PI (100 µg/ml). The cells were incubated for 15 min at room temperature. Then, 400 µl of 1× annexin-binding buffer was added and gently mixed before flow cytometry analysis. All the experiments were repeated at least three times.
RNA-seq data analysis
The raw data were trimmed for adaptors by the Cutadapt software (v3.0), and low-quality bases were removed by the Trimmomatic software (v0.39) [33], [34]. The filtered clean reads were mapped to the hg19 genome with HISAT2 (v2.0) [35]. The HTSeq (v0.12.4) software was used to count reads mapped to each Ensembl gene [36]. Differentially expressed genes were calculated using DESeq2 (v1.30.1) [37]. The differential fold change cutoff was 1.2, and the false discovery rate (FDR) cutoff was 0.05.
RNA-BisSeq data analysis
The Cutadapt and Trimmomatic software were used to trim adaptors and remove low-quality bases [33], [34]. The clean reads were mapped to the hg19 genome by meRanGh from meRanTK (v1.2.0) [38].
The m5C sites were called by meRanCall from meRanTK. The luciferase spike-in conversion rates were evaluated to be over 99%. The sample-credible m5C sites satisfied coverage depth ≥ 30, methylated cytosine depth ≥ 5, and methylation level ≥ 0.1. The differential m5C methylation analysis criteria comprised coverage ≥ 10 for all samples and were used to compare methylation levels between tumor and normal samples. The differential m5C sites were defined as follows: mean m5C level difference ≥ 0.05 (tumor and normal samples) and P < 0.05 (Wilcoxon test). The m5C sites were annotated using bedtools (v2.26.0) intersectBed [39].
Pathway analysis
Hypermethylated and hypomethylated genes were used as input for DAVID (v6.8) (https://david.ncifcrf.gov/).
Statistical analysis
Data were analyzed using the Python and GraphPad Prism (v8) software. Two-way analysis of variance and Student’s t-test were performed to determine statistical significance. The error bars, when present, represent the mean ± SD. The experiments were repeated at least three times independently. Statistical significance was considered at P < 0.05.
Ethical statement
Human samples in this study were collected from the Biobank of the First Affiliated Hospital of Zhengzhou University, China. The study was approved by the Ethics Committee of Scientific Research and Clinical Trial of the First Affiliated Hospital of Zhengzhou University, China (Approval No. 2019-KY-0024-001). All participants provided written informed consent according to the institutional guidelines.
Data availability
The raw sequence data reported in this study have been deposited in the Genome Sequence Archive for Human [40] at the National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation (GSA-Human: HRA001101), and are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human/.
Competing interests
The authors declare no competing interests.
CRediT authorship contribution statement
Dan Song: Methodology, Validation, Investigation, Writing – original draft, Writing – review & editing. Ke An: Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Wenlong Zhai: Resources, Methodology, Investigation, Writing – review & editing. Luyao Feng: Validation, Investigation. Yingjie Xu: Validation, Investigation. Ran Sun: Validation. Yueqin Wang: Methodology, Investigation. Yun-Gui Yang: Methodology, Supervision, Project administration. Quancheng Kan: Conceptualization, Project administration. Xin Tian: Conceptualization, Supervision, Funding acquisition, Project administration, Writing – review & editing. All authors have read and approved the final manuscript.
Acknowledgments
We thank Prof. Jiaxue Wu from School of Life Sciences Fudan University for providing HCC cell lines. This study was financially supported by grants from the National Natural Science Foundation of China (Grant Nos. 32170594 and 31870809), the Province and Ministry Coconstruction Major Program of Medical Science and Technique Foundation of Henan Province (Grant No. SBGJ202001007), and the Special Fund for Young and Middle School Leaders of Henan Health Commission (Grant No. HNSWJW-2020017), China.
Handled by Jianhua Yang
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation and Genetics Society of China.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gpb.2022.09.007.
Contributor Information
Quancheng Kan, Email: kanqc@zzu.edu.cn.
Xin Tian, Email: tianx@zzu.edu.cn.
Supplementary material
The following are the Supplementary material to this article:
Supplementary Figure S1.
Distribution characteristics of m5C in HCC and expression pattern of target genes. A. The type and proportion of RNA in the sequencing library. B. The sequences are proximal to the mRNA m5C sites in HCC tissues and the adjacent tissues. C. The m5C hypermethylated and highly expressed genes in HCC tissues were involved in different cellular processes, determined by the GO functional analysis. D. and E. The expression of target genes, including GRB2, AATF, RNF115, ADAM15, RTN3, and HDGF, was higher in HCC tissues than that in the adjacent tissues, determined by transcriptome analysis, ****, P < 0.0001. F. The overall survival analysis showed that the higher expression of ADAM15, RTN3, and HDGF was correlated with a poor prognosis in HCC patients; ADAM15 (*, P = 0.0097), RTN3 (**, P = 0.00018), HDGF (*, P = 0.0092). TPM, transcripts per kilobase of exon model per million mapped reads; HCC, hepatocellular carcinoma; GO, gene ontology.
Supplementary Figure S2.
NSUN2 regulates downstream target gene expression, especially GRB2. A. The mRNA expression of m5C writers and readers in HCC tissues was higher than that in the adjacent tissues, determined by the transcriptomic analysis, ***, P < 0.001. B. In HCC cell lines, the UHPLC-MS/MS analysis showed that downregulation of NSUN2 did not change the density of m5C/C in total RNA. C. Real-time PCR analysis showed that the mRNA expression levels of GRB2, RNF115, and AATF were significantly lower in Huh7 NSUN2 knockdown cells, *, P < 0.05; **, P < 0.01. D. The m5C modification in the 3′ UTR and the expression of GRB2 mRNA were analyzed by IGV visualization in HCC tissues and the adjacent tissues. UHPLC-MS/MS, ultra-high performance liquid chromatography-mass spectrometry/mass spectrometry; UTR, untranslated region; IGV, itegrative genomics viewer.
Supplementary Figure S3.
Identification of NSUN2 knockout efficiency at the genome level and transcriptome level. A. NSUN2 knockout characteristics were identified by Sanger sequencing in the QGY-7703 cell genome. B. The mRNA expression levels of NSUN2 in NSUN2 knockout cells (KO6/KO10) and NSUN2 reconstituted cells were evaluated by real-time PCR. WT, wilg type ; KO, knock out ; Res, rescue.
Supplementary Figure S4.
Apoptosis and cycle analysis of NSUN2 on HCC cells. A. and B. After sorafenib treatment, flow cytometry analyses of the apoptosis of HCC cells rate when NSUN2 knockdown. The statistical analyses of the apoptosis ratio are shown in (C), **, P < 0.01. D. Flow cytometry analyses of the cell cycle of Huh 7 cells. These cells were arrested in the G1 phase when NSUN2 was knocked down. The statistical analyses of the arrest ratio are shown in (E), *, P < 0.05. Statistical significance was calculated by Student’s t-test, mean ± SD. shNC, nonsense control-shRNA.
The list of mRNA m5C sites in HCC.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Sia D., Villanueva A., Friedman S.L., Llovet J.M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka S., Arii S. Molecularly targeted therapy for hepatocellular carcinoma. Cancer Sci. 2009;100:1–8. doi: 10.1111/j.1349-7006.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun F., Wang J., Sun Q., Li F., Gao H., Xu L., et al. Interleukin-8 promotes integrin β3 upregulation and cell invasion through PI3K/Akt pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:449–459. doi: 10.1186/s13046-019-1455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akula S.M., Abrams S.L., Steelman L.S., Emma M.R., Augello G., Cusimano A., et al. RAS/RAF/MEK/ERK, PI3K/PTEN/AKT/mTORC1 and TP53 pathways and regulatory miRs as therapeutic targets in hepatocellular carcinoma. Expert Opin Ther Targets. 2019;23:915–929. doi: 10.1080/14728222.2019.1685501. [DOI] [PubMed] [Google Scholar]
- 6.Dimri M., Satyanarayana A. Molecular signaling pathways and therapeutic targets in hepatocellular carcinoma. Cancers (Basel) 2020;12:1–23. doi: 10.3390/cancers12020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordan J.D., Kennedy E.B., Abou-Alfa G.K., Beg M.S., Brower S.T., Gade T.P., et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38:4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 8.Wang C., Jin H., Gao D., Lieftink C., Evers B., Jin G., et al. Phospho-ERK is a biomarker of response to a synthetic lethal drug combination of sorafenib and MEK inhibition in liver cancer. J Hepatol. 2018;69:1057–1065. doi: 10.1016/j.jhep.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y.S., Yang W.L., Zhao Y.L., Yang Y.G. Dynamic transcriptomic m5C and its regulatory role in RNA processing. Wiley Interdiscip Rev RNA. 2021;12:e1639. doi: 10.1002/wrna.1639. [DOI] [PubMed] [Google Scholar]
- 10.Sajini A.A., Choudhury N.R., Wagner R.E., Bornelöv S., Selmi T., Spanos C., et al. Loss of 5-methylcytosine alters the biogenesis of vault-derived small RNAs to coordinate epidermal differentiation. Nat Commun. 2019;10:2550. doi: 10.1038/s41467-019-10020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores J.V., Cordero-Espinoza L., Oeztuerk-Winder F., Andersson-Rolf A., Selmi T., Blanco S., et al. Cytosine-5 RNA methylation regulates neural stem cell differentiation and motility. Stem Cell Reports. 2017;8:112–124. doi: 10.1016/j.stemcr.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X., Li A., Sun B.F., Yang Y., Han Y.N., Yuan X., et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell Biol. 2019;21:978–990. doi: 10.1038/s41556-019-0361-y. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X., Liu Z., Yi J., Tang H., Xing J., Yu M., et al. The tRNA methyltransferase NSun2 stabilizes p16INK4 mRNA by methylating the 3′-untranslated region of p16. Nat Commun. 2012;3:712. doi: 10.1038/ncomms1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Yang Y., Sun B.F., Chen Y.S., Xu J.W., Lai W.Y., et al. 5-methylcytosine promotes mRNA export – NSUN2 as the methyltransferase and ALYREF as an m5C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guallar D., Bi X., Pardavila J.A., Huang X., Saenz C., Shi X., et al. RNA-dependent chromatin targeting of TET2 for endogenous retrovirus control in pluripotent stem cells. Nat Genet. 2018;50:443–451. doi: 10.1038/s41588-018-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawarada L., Suzuki T., Ohira T., Hirata S., Miyauchi K., Suzuki T. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45:7401–7415. doi: 10.1093/nar/gkx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L., Ding J., Wang B., Chen X., Ying X., Yu Z., et al. RNA methyltransferase NSUN2 promotes hypopharyngeal squamous cell carcinoma proliferation and migration by enhancing TEAD1 expression in an m5C-dependent manner. Exp Cell Res. 2021;404 doi: 10.1016/j.yexcr.2021.112664. [DOI] [PubMed] [Google Scholar]
- 18.Frye M., Dragoni I., Chin S.F., Spiteri I., Kurowski A., Provenzano E., et al. Genomic gain of 5p15 leads to over-expression of Misu (NSUN2) in breast cancer. Cancer Lett. 2010;289:71–80. doi: 10.1016/j.canlet.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Manning M., Jiang Y., Wang R., Liu L., Rode S., Bonahoom M., et al. Pan-cancer analysis of RNA methyltransferases identifies FTSJ3 as a potential regulator of breast cancer progression. RNA Biol. 2020;17:474–486. doi: 10.1080/15476286.2019.1708549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Z., Xue S., Zhang M., Xu H., Hu X., Chen S., et al. Aberrant NSUN2-mediated m5C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma. Oncogene. 2020;39:6906–6919. doi: 10.1038/s41388-020-01475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y., Zhang Q., Zheng Q., Yu X., Guo W. Distinct 5-methylcytosine profiles of circular RNA in human hepatocellular carcinoma. Am J Transl Res. 2020;12:5719–5729. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q., Zheng Q., Yu X., He Y., Guo W. Overview of distinct 5-methylcytosine profiles of messenger RNA in human hepatocellular carcinoma and paired adjacent non-tumor tissues. J Transl Med. 2020;18:245. doi: 10.1186/s12967-020-02417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chellamuthu A., Gray S.G. The RNA methyltransferase NSUN2 and its potential roles in cancer. Cells. 2020;9:1758. doi: 10.3390/cells9081758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Lezana T., Lopez-Canovas J.L., Villanueva A. Signaling pathways in hepatocellular carcinoma. Adv Cancer Res. 2021;149:63–101. doi: 10.1016/bs.acr.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Llovet J.M., Montal R., Sia D., Finn R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PubMed] [Google Scholar]
- 26.Gale N.W., Kaplan S., Lowenstein E.J., Schlessinger J., Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993;363:88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- 27.Guo G., Pan K., Fang S., Ye L., Tong X., Wang Z., et al. Advances in mRNA 5-methylcytosine modifications: detection, effectors, biological functions, and clinical relevance. Mol Ther Nucleic Acids. 2021;26:575–593. doi: 10.1016/j.omtn.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Li J., Luo M., Zhou C., Shi X., Yang W., et al. Novel long noncoding RNA NMR promotes tumor progression via NSUN2 and BPTF in esophageal squamous cell carcinoma. Cancer Lett. 2018;430:57–66. doi: 10.1016/j.canlet.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Mei L., Shen C., Miao R., Wang J.Z., Cao M.D., Zhang Y.S., et al. RNA methyltransferase NSUN2 promotes gastric cancer cell proliferation by repressing p57Kip2 by an m5C-dependent manner. Cell Death Dis. 2020;11:270. doi: 10.1038/s41419-020-2487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su J., Wu G., Ye Y., Zhang J., Zeng L., Huang X., et al. NSUN2-mediated RNA 5-methylcytosine promotes esophageal squamous cell carcinoma progression via LIN28B-dependent GRB2 mRNA stabilization. Oncogene. 2021;40:5814–5828. doi: 10.1038/s41388-021-01978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng J.X., Chen L., Li Y., Cloe A., Yue M., Wei J., et al. RNA cytosine methylation and methyltransferases mediate chromatin organization and 5-azacytidine response and resistance in leukaemia. Nat Commun. 2018;9:1163. doi: 10.1038/s41467-018-03513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y.S., Ma H.L., Yang Y., Lai W.Y., Sun B.F., Yang Y.G. 5-methylcytosine analysis by RNA-BisSeq. Methods Mol Biol. 2019;1870:237–248. doi: 10.1007/978-1-4939-8808-2_18. [DOI] [PubMed] [Google Scholar]
- 33.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:1–3. [Google Scholar]
- 34.Bolger A.M., Marc L., Bjoern U. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D., Langmead B., Salzberg S.L. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anders S., Pyl P.T., Huber W. HTSeq — a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dietmar, Rieder, Thomas, et al. meRanTK: methylated RNA analysis ToolKit. Bioinformatics. 2016;32:782–785. doi: 10.1093/bioinformatics/btv647. [DOI] [PubMed] [Google Scholar]
- 39.Quinlan A.R., Hall I.M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen T., Chen X., Zhang S., Zhu J., Tang B., Wang A., et al. The Genome Sequence Archive Family: toward explosive data growth and diverse data types. Genomics Proteomics Bioinformatics. 2021;19:578–583. doi: 10.1016/j.gpb.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The list of mRNA m5C sites in HCC.
Data Availability Statement
The raw sequence data reported in this study have been deposited in the Genome Sequence Archive for Human [40] at the National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation (GSA-Human: HRA001101), and are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human/.