Abstract
Fibrolamellar hepatocellular carcinoma (FLC) is a rare liver cancer caused by a dominant recurrent fusion of the heat shock protein (DNAJB1) and the catalytic subunit of protein kinase A (PRKACA). Current therapies such as chemotherapy and radiation have limited efficacy, and new treatment options are needed urgently. We have previously shown that FLC tumors are dependent on the fusion kinase DNAJB1::PRKACA, making the oncokinase an ideal drug target. mRNA degrading modalities such as antisense oligonucleotides or small interfering RNAs (siRNAs) provide an opportunity to specifically target the fusion junction. Here, we identify a potent and specific siRNA that inhibits DNAJB1::PRKACA expression. We found expression of the asialoglycoprotein receptor in FLC to be maintained at sufficient levels to effectively deliver siRNA conjugated to the GalNAc ligand. We observe productive uptake and siRNA activity in FLC patient-derived xenografts (PDX) models in vitro and in vivo. Knockdown of DNAJB1::PRKACA results in durable growth inhibition of FLC PDX in vivo with no detectable toxicities. Our results suggest that this approach could be a treatment option for FLC patients.
Keywords: FLC, ASGR, GalNAc conjugation, siRNA, DNAJB1::PRKACA, liver cancer, Protein Kinase A, antisense oligonucleotides
Graphical abstract

Simon and colleagues discovered that a small interfering RNA (siRNA) that inhibits the fusion kinase DNAJB1:PRKACA drives fibrolamellar hepatocellular carcinoma (FLC). Using the asialoglycoprotein receptor, siRNA could be effectively delivered to FLC cells, inhibiting tumor growth in patient-derived xenografts, demonstrating its potential as a treatment option for FLC patients.
Introduction
Fibrolamellar hepatocellular carcinoma (FLC) is a rare liver cancer that occurs predominantly in young adults with no history of chronic liver disease.1 There are currently no effective treatments for FLC, and patients often face poor outcomes due to the lack of targeted therapies and the limited efficacy of conventional treatments such as chemotherapy, radiation, and surgery. To date, surgery is the only curative option.2,3 FLC is characterized by a chromosomal rearrangement resulting in the fusion of the DNAJB1 and PRKACA genes, which leads to the expression of a chimeric protein with constitutive kinase activity.4
In recent years, RNAi has emerged as a promising therapeutic strategy for various diseases, including cancer.5 However, the clinical application of RNAi has been hindered by the difficulty in delivering small interfering RNAs (siRNAs) to target cells and tissues, because siRNAs are unable to cross the cell membrane. To address these challenges, various siRNA delivery systems, including nanoparticles, liposomes, and conjugates have been developed.6 Among these, conjugates of siRNAs with ligands that specifically bind to receptors on target cells have shown promise for the targeted delivery of siRNAs. Most prominently, siRNAs conjugated to the ligand N-acetylgalactosamine (GalNAc) bind to the asialoglycoprotein receptor (ASGR), which is highly expressed on liver cells. GalNAc-siRNA have been approved in several indications, such as hereditary transthyretin-mediated amyloidosis and atherosclerotic cardiovascular disease.7
We have previously demonstrated that the fusion gene DNAJB1::PRKACA is sufficient to trigger the formation of FLC in healthy mouse liver, and the inhibition of DNAJB1::PRKACA using a small hairpin RNA (shRNA) targeting the fusion junction resulted in cell death.8,9,10 This demonstrates that DNAJB1:PRKACA is the oncogenic driver, and thus a potential drug target. Because FLC is a liver cancer, we hypothesized that ASGR protein levels could be sufficiently maintained to deliver siRNA targeting DNAJB1::PRKACA to FLC cells, resulting in targeted gene silencing. Previous studies have indicated that ASGR mRNA and protein are reduced in hepatocellular carcinoma (HCC).11 However, even in cases in which the expression of ASGR is reduced by 50%, it is still sufficient to drive the pharmacology of GalNAc conjugates, achieving potent knockdown in vivo.12 This has been confirmed also in models of HCC with reduced ASGR levels, in which GalNAc-conjugated antisense oligonucleotides (ASOs) have shown improved pharmacology in vivo.13
Our results demonstrate that ASGR is sufficiently expressed in multiple FLC patient-derived xenograft (PDX) models to efficiently deliver GalNAc-conjugated siRNA that specifically silences the expression of the DNAJB1::PRKACA fusion oncogene in FLC cells in vitro with minimal effect on native DNAJB1 and PRKACA. In addition, GalNAc-siRNA induces robust gene silencing in FLC PDX models in vivo and inhibits tumor growth. More important, we observed no apparent toxicity in mice treated with siRNA. Taken together, our findings suggest that GalNAc-conjugated siRNA targeting DNAJB1::PRKACA is a promising therapeutic strategy for FLC and highlights the potential of targeted delivery of ligand-conjugated siRNAs as a viable strategy for cancer therapy.
Results
In this study, we designed siRNAs targeting the fusion junction of DNAJB1::PRKACA based on our previously described shRNA screen.10 To enhance circulation time and nuclease resistance we modified siRNAs with phosphorothioate backbones on 5′ and 3′ ends and 5′-vinylphosphonate to increase potency.6,14,15 In addition, siRNAs were conjugated to GalNAc on the 5′ end of the passenger strand (Figure 1A). We tested five siRNAs on Huh7 cells, an HCC cell line, expressing the DNAJB1::PRKACA fusion mRNA under the DNAJB1 promoter. Cells were transfected with siRNAs and after 48 h analyzed for expression levels (Figure 1B). siRNA 1784402 showed the most potent knockdown of the fusion mRNA with a half-maximal inhibitory concentration (IC50) of 1 pM (Figure 1C). Moreover, we found that 1784402 showed a roughly 30-fold higher specificity to DNAJB1::PRKACA compared to native PRKACA and no effect on DNAJB1 (Figures 1C and 1D).
Figure 1.
Potent and specific siRNA-mediated knockdown of DNAJB1::PRKACA
(A) Sequences of siRNAs targeting the fusion junction; red and blue letters denote DNAJB1 and PRKACA sequences, respectively. (B) qRT-PCR results of Huh7-DNAJB1:PRKACA cells transfected with siRNAs in a dose-response experiment. RNA was profiled after 48 h and normalized to β-actin. n = 3 for all conditions, mean ± SD. (C) qRT-PCR results in Huh7-DNAJB1:PRKACA of 1784402 transfection, demonstrating preferential activity on DNAJB1::PRKACA over native PRKACA and DNAJB1. n = 3 for all conditions, mean ± SD. (D) Western blot analysis of Huh7-DNAJB1:PRKACA transfected with 1784402 at various concentrations.
Liver-directed delivery of siRNA using the ASGR-GalNAc system has shown great success in genetic diseases, with multiple antisense drugs approved and in clinical trials using the GalNAc modality.7,16 Because FLC is a liver tumor, we hypothesized that expression of ASGR could be maintained at high enough levels to allow the delivery of GalNAc-conjugated siRNAs to tumors. Factors that would affect the efficacy of GalNAc-conjugated siRNA through ASGR include the levels of expression, the amount on the surface, and internalization and recycling rates. We evaluated levels of the ASGR1 transcript in multiple FLC patients and PDX samples, including primary tumor, recurrence, metastasis, and respective PDX (Figure 2A). We found that ASGR1 expression was lower in primary tumors, metastasis, and recurrences relative to the adjacent nontransformed liver, although it was increased in PDX. The decrease in the patient tumor may be due to a different tumor microenvironment, dedifferentiation of FLC, or infiltration of stromal cells that do not express ASGR1. Bulk sequencing may not accurately capture the expression level because immune and stromal cells, which are negative for ASGR1, could dilute signal from the FLC tumor. This could also explain why expression was found to be higher in PDX models because these are more homogeneous tissues. To assess protein levels in FLC cells, we performed immunofluorescence on two rare patient samples for which healthy liver, primary tumor, and metastasis were available. We observed that ASGR1 protein in primary tumors and metastasis was similar in localization and abundance compared to healthy hepatocytes (Figures 2B and 2C). More important, immunofluorescence allowed us to observe a clear demarcation of ASGR1 levels between tumor cells and stromal cells as seen in FLC7 primary tumor tissue (asterisk), highlighting that expression levels of ASGR1 based on bulk sequencing can be affected by ASGR1−stromal cells. Although bulk RNA sequencing (RNA-seq) in FLC7 indicated that primary and metastatic tumors had lower expression levels of ASGR1 than healthy liver, we found this to not be the case in the primary tumor when quantifying protein levels in FLC cells (Figure 2B). In FLC8, we found that in agreement with RNA-seq data, ASGR1 levels were increased in both primary and metastatic tumor tissue compared to matched liver tissue. However, in both cases we observed a greater heterogeneity of ASGR1 expression in FLC cells compared to liver (Figure 2C). This could be relevant for the potential therapeutic efficacy of GalNAc-siRNAs because ASGR-low cells may take up reduced amounts of siRNA and be more resistant therapy. To test this we selected two PDX models with different ASGR1 expression levels—FLC1 and FLC9—with high and low expression, respectively (Figures 2A and 2D). We note that the FLC9 ASGR1 signal is more perinuclear compared to more cytoplasmic localization in FLC1, which could represent reduced cycling of ASGR to the plasma membrane in FLC9.
Figure 2.
ASGR1 in FLC
(A) RNA expression analysis of ASGR1 across patient samples of normal liver, primary tumor, recurrence, metastasis, and PDX passage 1. (B) Immunofluorescence of ASGR1 in tumor-adjacent healthy liver, primary tumor, and metastasis of 2 patient samples, patient FLC7 and FLC8. Asterisk marks ASGR1− stromal cells. All fields are 210 × 210 μm. (C) Quantification of signal intensity per cell from multiple fields of view from immunofluorescence images of ASGR1 in tumor-adjacent healthy liver (FLC7: n = 42; FLC8: n = 59), primary tumor (FLC7: n = 64; FLC8: n = 73), and metastasis (FLC7: n = 48; FLC8: n = 55) of patient FLC7 and FLC8 tissue. Mean ± SD, ordinary 1-way ANOVA and Dunnett’s multiple comparison test for post hoc comparisons. Significance levels: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. (D) Immunofluorescence images of ASGR1 in PDX tissue derived from FLC1 and FLC9. All fields are 210 × 210 μm. Higher magnification of 2 cells of FLC1 and FLC9 at 20 × 20 μm.
To test for productive uptake via ASGR, we treated cells of FLC1 and FLC9 PDX models with 1784402 GalNAc conjugates or with 1526196-control GalNAc-conjugated siRNA targeting coagulation factor 12 (F12) for 48 h. The siRNAs were added to cells immediately upon plating in vitro, because GalNAc conjugates quickly lose activity. This is potentially due to reduced endocytic recycling of ASGR upon dissociation and plating in vitro (unpublished data). We observed IC50 values of 4 nM (FLC1) and 1.2 μM (FLC9) (Figures 3A and 3B). These values were higher than the 1 nM previously reported for primary mouse hepatocytes, but provided evidence that ASGR is present at sufficient levels to mediate delivery of siRNAs in FLC in both ASGR1 high and low cells.17 Reduced efficacy in FLC9 is likely due to both reduced expression and cycling of ASGR as seen in PDX tissue (Figure 2D). Next, we assessed the effect of the siRNA on viability in vitro, treating freshly dissociated cells of PDX models FLC1 and FLC9, with a 10-μM dose of 1784402. Medium was replaced after 48 h before the first assessment of viability. We observed a decrease of between 50% and 70% in viability after 5 days compared to cells treated with 1526196-control siRNA (Figures 3C and 3D). Together, these results provide evidence that ASGR-mediated delivery of siRNA is possible in FLC cells in vitro, resulting in knockdown of DNAJB1::PRKACA and cell death. More important, we did not observe any cytotoxic effect of 1784402 in Huh7 cells expressing DNAJB1::PRKACA at a high dose of 25 nM, indicating that cell death observed in FLC is due to specific knockdown and addiction to DNAJB1::PRKACA (Figure S1A).
Figure 3.
Pharmacologic activity of GalNAc-siRNA in vitro and in vivo
(A) qRT-PCR assessment of DNAJB1::PRKACA expression in FLC1 PDX cells treated with siRNAs in a dose-response experiment. n = 3 for all conditions, mean ± SD. (B) qRT-PCR assessment of DNAJB1::PRKACA expression in FLC9 PDX cells treated with siRNAs in a dose-response experiment. n = 3 for all conditions, mean ± SD. (C) Viability over multiple days of FLC1 PDX cells treated with siRNAs at 10 μM. n = 3 for all conditions, mean ± SD. We used a 2-way ANOVA test with condition and day as factors and Sidak’s multiple comparison test for post hoc comparisons at each day. (D) Viability over multiple days of FLC9 PDX cells treated with siRNAs at 10 μM. n = 3 for all conditions, mean ± SD. We used a 2-way ANOVA test with condition and day as factors and Sidak’s multiple comparison test for post hoc comparisons at each day. (E) qRT-PCR results of F12 knockdown in FLC PDX and mouse liver tissue after injection of F12-targeting GalNAc-siRNA 1526196. siRNA was injected 2 times per week for 2 weeks subcutaneously at 5 mg/kg. n = 3 with 2-tailed unpaired t test, mean ± SD. (F) qRT-PCR results of DNAJB1::PRKACA, PRKACA, and DNAJB1 knockdown in FLC PDX and mouse liver tissue after injection of 1784402. siRNA was injected 5 times per week for 2 weeks subcutaneously at 7.5 mg/kg. n = 3 with 2-tailed unpaired t test, mean ± SD. Significance levels for (C)−(F): ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Differences between FLC1 and FLC9 in cell death sensitivity after knockdown may be influenced by multiple factors such as expression level of antiapoptotic proteins that we previously showed affect viability differences between FLC lines.18 We next investigated the pharmacology of GalNAc-siRNA in vivo. To compare the productive uptake of GalNAc-siRNA in FLC tumors relative to the murine hepatocytes in the liver, we used an siRNA 1526196 that targeted the mouse and human F12. Mice were implanted subcutaneously with FLC1 cells, and tumors were allowed to engraft for 2 months. We initially focused on FLC1 due to its higher ASGR1 expression and lower IC50 compared to FLC9. Mice were injected subcutaneously twice per week with 5 mg/kg of 1526196. After 2 weeks we assessed knockdown in the liver of mice and tumors. As expected, we found a greater than 98% reduction in F12 mRNA in the mouse liver, due to the high expression and cycling of ASGR in hepatocytes (Figure 3E). In tumors we found robust knockdown of ∼70% compared to vehicle. The reduced knockdown efficiency in the human FLC tumor relative to the healthy mouse liver likely reflects a number of possible factors, including differences in the overall expression, amount of ASGR on the surface, the recycling rate of ASGR, and the rate of release of the siRNA from the endocytic system.
We reasoned that higher and more frequent dosing with 1784402 should achieve robust knockdown of DNAJB1::PRKACA in vivo in FLC1 PDX. To assess the activity of 1784402, we implanted FLC1 cells in the livers of mice and treated with 7.5 mg/kg of 1784402 for 2 weeks 5 times per week. We found a reduction of ∼60% of DNAJB1::PRKACA expression in tumors (Figure 3F). More important, in the same cells, we saw no effect on native human PRKACA or DNAJB1. These results indicated that knockdown of DNAJB1::PRKACA is feasible in FLC tumors with high specificity.
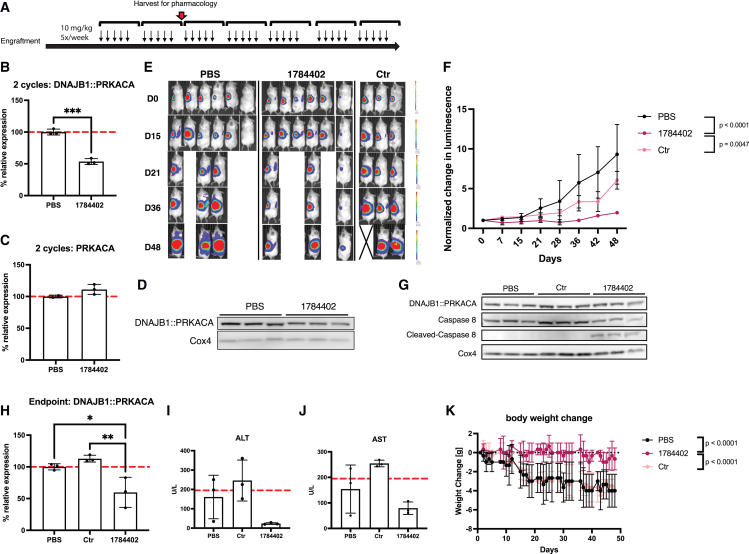
Next, we tested whether siRNA could achieve durable inhibition of tumor growth in vivo. Because most FLC patients die from metastasis rather than the primary liver tumor, we decided to implant FLC1 cells expressing luciferase in the spleen of mice and allowed cells to engraft for 2 months to mimic metastatic disease. Mice were randomized and treated with 1784402, 1526196-control siRNA, or PBS at 10 mg/kg 5 times per week by subcutaneous injection (Figure 4A). After 2 weeks, a subset of the tumors was harvested from mice treated with 1784402 or vehicle and profiled for mRNA and protein knockdown. Similar to hepatic tumors, we found knockdown of 50% on mRNA and protein level of DNAJB1::PRKACA with 1784402, without effect on native human PRKACA (Figures 4B–4D). The remaining mice were treated for an additional 5 weeks, and the tumor size was measured weekly by bioluminescence imaging. We observed a significant inhibition of tumor growth in mice treated with 1784402 compared to vehicle and control siRNA (Figures 4E and 4F). Furthermore, 1784402-treated tumors showed a marked increase in cleaved caspase-8, indicating cell death pathway activation even as DNAJB1::PRKACA knockdown was reduced on the protein level but not the RNA level, compared to the earlier time point (Figures 4G and 4H). We hypothesized that the decrease in knockdown and slight increase in tumor growth at the end of the study may be due to a downregulation of ASGR, thereby reducing siRNA uptake as a resistance mechanism. However, we observed increased ASGR1 expression in 1784402-treated tumors compared to control tumors, indicating persistent ASGR1 expression despite selection pressure (Figures S2A and S2B). A variety of other mechanisms, for instance, on the transcriptional level such as disregulation of AGO2 involved in the siRNA-mediated knockdown, could be responsible for reduced siRNA activity.19
Figure 4.
Tumor growth inhibition with 1784402 in FLC1 PDX without toxicity
(A) Schematic of the treatment. FLC1 PDXs were implanted in the spleen of mice. 1784402, 1526196 Ctr siRNAs, and PBS were injected 5 times per week for 7 weeks at 10 mg/kg subcutaneously. After 2 weeks of injections, 3 mice of PBS- and 1784402-treated animals were harvested for knockdown analysis. (B) qRT-PCR results. DNAJB1::PRKACA expression in FLC PDX tissue after 2 weeks of treatment. n = 3 with 2-tailed unpaired t test, mean ± SD. Significance levels: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. (C) qRT-PCR results. PRKACA expression in FLC PDX tissue after 2 weeks of treatment, mean ± SD. (D) Western blot of FLC1 PDX tissue after 2 weeks of treatment with 1784402. (E) Bioluminescence images of animals. Each day is shown at the same linear scale. (F) Normalized change of bioluminescence of animals treated with PBS, Ctr siRNA, or 1784402. Signal from animals taken out after 2 cycles was not included in graphing and statistical analysis, mean ± SD. Linear mixed-effects model with log tumor signal was used to analyze data across the study duration. Group: p = 0.0052; day: p < 0.0001; group × day: p < 0.0001. Significant post hoc pairwise comparisons of growth trajectories at Bonferroni-adjusted alpha level p < 0.025 (0.05/2) are indicated. (G) Western blot of FLC1 PDX tissue at the end of the study. (H) qRT-PCR results. DNAJB1::PRKACA expression in FLC PDX tissue at the end of the study. n = 3, mean ± SD with ordinary 1-way ANOVA and Sidak’s multiple comparison test for post hoc comparisons. Significance levels: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. (I) ALT enzyme levels in the serum of animals at the end of the study. n = 3, mean ± SD. Dotted line marks the upper limit of normal ALT levels in NSG mice. (J) AST enzyme levels in the serum of animals at the end of the study. n = 3, mean ± SD. Dotted line marks the upper limit of normal AST levels in NSG mice. (K) Normalized weight change of animals throughout the study. n = 3, mean ± SD. Linear mixed-effects model was used to analyze weight change across the study duration. Group: p = 0.0408; day: p < 0.0001; group × day: p < 0.0001. Significant post hoc pairwise comparisons at Bonferroni-adjusted alpha level <0.025 (0.05/2) are indicated.
More important, we did not detect any liver toxicity based on serum AST/ALT levels, indicating no toxic off-target effects and good tolerability of 1784402 (Figures 4I and 4J). In addition, 1784402-treated animals retained weight, whereas the weight of animals treated with vehicle deteriorated, a consequence of tumor growth. This supports the conclusion that 1784402 is well tolerated and reduces tumor burden (Figure 4K).
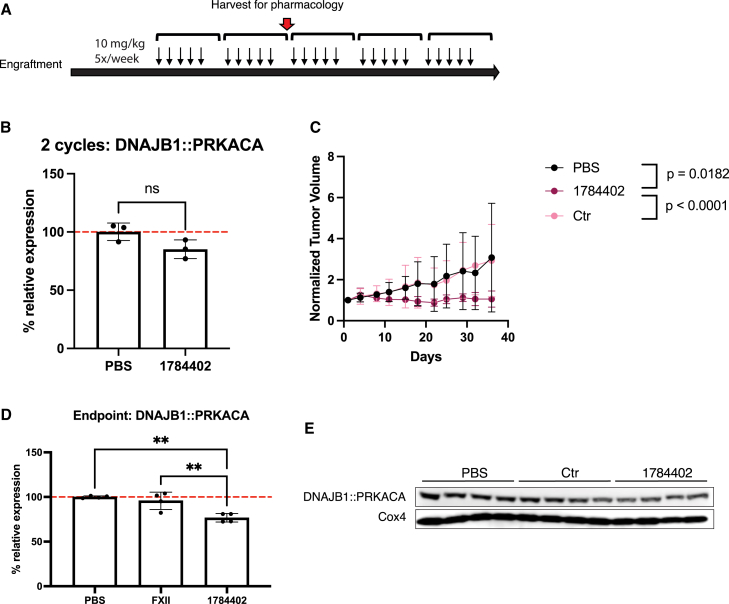
Next, we assessed in vivo activity of 1784402 in FLC9 to test for knockdown of DNAJB1::PRKACA in a PDX model with lower ASGR1 expression. We treated animals with subcutaneous tumors for a total of 5 weeks with 10 mg/kg 5 times per week (Figure 5A). After 2 weeks, 3 animals of the PBS and 1784402 groups were euthanized to assess knockdown in tumors. Compared to FLC1, we found that the knockdown at 2 weeks was only 15%, in line with the reduced ASGR levels and higher IC50 found in vitro (Figure 5B). However, we did observe significantly impaired tumor growth in animals treated with 1788402 compared to PBS and control siRNA-treated animals (Figure 5C). More important, we found that at the end of the study, knockdown had increased to 23% (Figures 5D and 5E).
Figure 5.
Tumor growth inhibition with 1784402 in an ASGR-low PDX
(A) Schematic of the treatment. FLC9 PDXs were implanted subcutaneously in mice. 1784402, 1526196 Ctr siRNAs, and PBS were injected 5 times per week for 5 weeks at 10 mg/kg subcutaneously. After 2 weeks of injections, 3 mice of PBS- and 1784402-treated animals were harvested for knockdown analysis. (B) qRT-PCR results. DNAJB1::PRKACA expression in FLC PDX tissue after 2 weeks of treatment. n = 3 with 2-tailed unpaired t test, mean ± SD. (C) Normalized tumor volume of animals injected with PBS, Ctr siRNA, or 1784402 siRNA. Animals taken out of the study after 2 weeks were not considered for growth analysis. n = 4, mean ± SD. Linear mixed-effects model with log tumor volume was used to analyze data across the study duration. Group: p = 0.2612; day: p < 0.0001; group × day: p = 0.0059. Significant post hoc pairwise comparisons at Bonferroni-adjusted alpha level <0.025 (0.05/2) are indicated. (D) qRT-PCR results. DNAJB1::PRKACA expression in FLC PDX tissue at the end of treatment. n = 4 with ordinary 1-way ANOVA and Sidak’s multiple comparison test for post hoc comparisons, mean ± SD. (E) Western blot of FLC9 tissue at the end of the study. Significance levels for (B)–(D): ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Finally, we tested the durability of 1784402 in a subcutaneous tumor model of FLC1. We treated mice with 1784402 at 10 mg/kg or vehicle for 3 weeks and then monitored tumor growth. We found that tumors started regressing after 14 days of treatment, continuing even without further injection of 1784402 until the end of the study, resulting in reduced tumor weight (Figures 6A and 6B). More important, markers of cell death as measured by TUNEL staining and cleaved caspase-8 remained elevated together with reduced cell proliferation even though we did not detect knockdown of DNAJB1:PRKACA in the remaining tumor tissue at the end of the study (Figures 6C–6E). We did not observe a difference in ASGR1 expression in the remaining tumor tissue, indicating that these cells did not downregulate ASGR1 expression as a resistance mechanism or that preexisting ASGR1-low cells could escape treatment and overgrow (Figure 6F). The durable activity is likely due to the high stability of modified siRNA and slow release from the endocytic system, as previously described.17 Together, these data indicate the potent and durable activity of 1784402.
Figure 6.
Durable tumor growth inhibition with 1784402 in FLC1 PDX
(A) Normalized tumor volume of animals injected 5 times per week for 3 weeks with 10 mg/kg subcutaneously with PBS or 1784402 siRNA. n = 3, mean ± SD. Linear mixed-effects model with log tumor volume was used to analyze data across the study duration. Group: p = 0.4630; day: p < 0.0001; group × day: p < 0.0001. (B) Tumor weight at the end of the study. n = 3 with 2-tailed unpaired student t-test, mean ± SD. (C) TUNEL index of tumor tissue at the end of the study. n = 3 with 2-tailed unpaired Student t test, mean ± SD. (D) Ki-67 index of tumor tissue at the end of the study. n = 3 with 2-tailed unpaired Student t test, mean ± SD. Significance levels for (B)–(D): ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. (E) Western blot of FLC PDX tissue at the end of the study. (F) RT-qPCR results. ASGR1 expression in FLC PDX tissue at the end of the study. n = 3, mean ± SD.
Discussion
FLC, a usually lethal liver tumor, is characterized by a unique fusion of two genes, DNAJB1 and PRKACA. This fusion is present in nearly all cases of FLC, making it a promising target for molecular therapies. In FLC, small-molecule inhibitors of protein kinase A, such as uprosertib, can inhibit the kinase activity of fusion DNAJB1::PRKACA but show no specificity over native kinase and rapidly distribute throughout the body, resulting in systemic side effects with limited efficacy in FLC.20 Thus, conventional small-molecule inhibitors have yet to demonstrate specific targeting of this fusion. Moreover, traditional small molecules often fail to effectively target the root cause of the disease, such as genetic alterations. In contrast, antisense therapies are highly programmable and can specifically inhibit expression of what are considered undruggable proteins.
In this study, we developed a therapeutic strategy for FLC using a GalNAc-conjugated siRNA that targets the fusion junction of the DNAJB1::PRKACA oncotranscript. We demonstrate promising activity in FLC PDX models in vitro and in vivo. The GalNAc-siRNAs are productively taken up by cells with both high and low expression of ASGR. Because there is greater target knockdown in a PDX model with higher ASGR expression, a theranostic biomarker for patients probing ASGR levels may be useful to stratify patients who may benefit more from this therapeutic approach. Compared to genetic diseases for which GalNAc-siRNA have been successfully used with biannual dosing, we have used an aggressive dosing scheme due to the more proliferative nature of cancer cells similar to other preclinical studies using ASOs.21,22 However, we did not detect apparent side effects, offering a wide therapeutic window. Our PDX model system is limited in that in human patients the liver may be a substantially bigger sink compared to the mouse liver due to the likely larger ratio of liver to tumor. Therefore, dosing in our mouse model would likely need to be adjusted to find both the optimal concentration and frequency for clinical translation.
To date, the effective use of siRNA therapeutics in oncology is hampered by challenges to achieve efficient uptake in the cancer cell. Various delivery methods have been developed to address these challenges. Conjugation of siRNAs to GalNAc has emerged as an effective strategy for liver-specific delivery, capitalizing on the high expression and fast recycling nature of ASGRs in hepatocytes. Our approach using GalNAc-conjugated siRNA confines distribution of the siRNA to the liver and tumor tissue, thereby efficiently concentrating the siRNA in the target tissue to achieve robust knockdown of DNAJB1-PRKACA with no apparent toxicity. In addition, our approach specifically targets the fusion junction of DNAJB1::PRKACA, without knockdown of PRKACA.
ASGR is a promising delivery receptor due to its high cycling rate, allowing enough siRNA to be internalized and “leak” into the cytoplasm to achieve knockdown.23 However, other approaches have been used for delivery in oncology. For instance, folate receptor-mediated delivery and lipid nanoparticles have been used to deliver RNA to tumor cells.24,25 MicroRNAs have been effectively targeted to breast and lung cancer models using folate conjugates. In FLC, the folate receptor is not overexpressed, but the basal expression may be sufficient for efficient delivery. Additional modalities are being developed for efficient delivery to extrahepatic tissues that could also be used to achieve uptake in tumor cells. For instance, lipid conjugates such as cholesterol have been used to deliver siRNA to tissues other than the liver.26,27 It remains to be seen whether this approach could also deliver siRNA to tumor cells, which could depend on the cell type of origin and tissue context. Recently, C16 conjugates have been used for safe and effective delivery of siRNA to the CNS, which could be used for gene silencing in brain tumors.28 These recent advances in targeted delivery have laid the foundation for siRNA therapies to address the need to target hard-to-drug proteins in oncology and translate to the clinic.
Chemical modifications in conjunction with efficient delivery systems such as GalNAc conjugation or lipid nanoparticles have transformed siRNAs from a powerful laboratory tool to a promising class of therapeutics.6 Our study highlights the potential of siRNA-based therapeutics for other cancer types that harbor fusion genes. The successful use of these strategies in the treatment of genetic diseases, along with the potential demonstrated in this study against FLC, underscores the transformative potential of siRNA therapeutics in precision medicine.
Materials and methods
siRNA synthesis
siRNAs were synthesiszed according to the reported methods.15,29 The siRNAs targeting DNAJB1::PRKACA contained several modifications:
-
•
5′[vpMOE]sFsMMMFMMMMMMMFMFMMMMMsMsM 3′
-
•
3′ MsMsMMMMMMMMFFFMFMMMMsMsM-3THAGN-5′
-
•
[vpMOE]: vinylphosphonate-2′-O-MOE T
-
•
3THAGN: triantennary GalNAc cluster
-
•
F: 2′-Fluoro
-
•
M: 2′-O-Me
-
•
s-phosphorothioate (all backbone linkages are phosphodiesters except for ones labeled with s)
Cell culture
Huh7-chimera cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). Cells dissociated from PDXs were cultured in RPMI and supplemented with 10% FBS and 2% penicillin/streptomycin or Kubota Medium (Phoenix Songs) with 2% penicillin/streptomycin. PDXs growing FLC tumors were previously generated directly from patient resections and validated based on histopathology and transcriptome.20
RNA extraction and qPCR analysis
Cultured cells were reverse transfected with siRNA with RNAiMAX in 96-well plates according to the manufacturer’s instructions (Thermo Fisher Scientific). For experiments in FLC PDX cells, siRNA was added directly to cells upon dissociation. To assess efficacy on RNA in vitro, cells were directly lysed in RLT buffer (Qiagen) supplemented with 1% 2-mercaptoethanol 48 h after the addition of siRNA. For RNA extraction from tumors grown in mice, a piece of tumor was homogenized in RLT buffer and centrifuged in QIAshredder (Qiagen) tubes at 21,000 × g for 3 min. Total mRNA was extracted with the RNeasy Kit (Qiagen) or with PureLink RNA extraction plates (Thermo Fisher Scientific) according to the manufacturer’s instructions. Gene expression was measured with the Luna Universal Probe OneStep RT-qPCR kit (New England Biolabs) normalizing to ACTB or B2M for human genes and Ppia for mouse genes. Sequences of primer-probe sets are as follows:
Human DNAJB1::PRKACA:
-
•
Probe: 56-FAM/AAGCGCGAG/ZEN/ATCTTCGACCGCTA/3IABkFQ
-
•
Forward primer: GAAGTTCAAGGAGATCGCTGAG
-
•
Reverse primer: GAGCGGGACTTTCCCATTT
Human PRKACA:
-
•
Forward primer: CAAGAAGGGCAGCGAGCA, reverse primer: CTGTGTTCTGAGCGGGACTT, probe: 56-FAM/AGAGCGTGA/ZEN/AAGAATTCTTAGCCAAAGCC/3IABkFQ
Human DNAJB1:
-
•
Forward primer: CAAGCGCGAGATCTTCGAC, reverse primer: GAACTCAGCAAACATGGCAT
-
•
Probe: 56-FAM/CCACTCCCC/ZEN/TTTAGGCCTTCCTC/3IABkFQ
Human ACTB:
-
•
Forward primer: CCGACTATGACTTAGTTGCGTTACA, reverse primer: GCCATGCCAATCTCATCTTGT
-
•
Probe: 5HEX/CCTTTCTTG/ZEN/ACAAAACCTAACTTGCGCAGA/3IABkFQ
Human B2M:
-
•
Forward primer: GGACTGGTCTTTCTATCTCTTGT
-
•
Reverse primer: ACCTCCATGATGCTGCTTAC
-
•
Probe: 5HEX/CCTGCCGTG/ZEN?TGAACCATGTGACT/3IABkFQ
Mouse PPIA:
-
•
Forward primer: TGGGTTAGAGAAGGCGTGTACTG, reverse primer: TCAGCGGCAACTGGGAAA
-
•
Probe: 5HEX/CGTTGGCAC/ZEN/GACACCTTCAGGGACT/3IABkFQ
Mouse F12:
-
•
Forward primer: CAAAGGAGGGACATGTATCAACAC, reverse primer: CTGGCAATGTTTCCCAGTGA
-
•
Probe: 56FAM/CCCAATGGG/ZEN/CCACACTGTCTCTGC/3IABkFQ
Human F12:
-
•
Forward primer: GTGCACGGATCCTCCATC, reverse primer: CAGCTTGGTCCTCACACAC
-
•
Probe: 56FAM/AATCACCCT/ZEN/GGCACGCATCG/3IABkFQ
Protein isolation and immunoblotting
Total protein from the cells in culture was extracted 72 h after treatment using radioimmunoprecipitation assay buffer (Sigma) supplemented with protease and phosphatase inhibitors (Complete EDTA-Free and PhosSTOP, Roche). Samples were vortexed and incubated at 4° for 20 min and then centrifuged at 21,000 × g for 15 min. Supernatants were collected, and protein concentrations were measured by a modified Lowry assay (DC protein assay, Bio-Rad). A total of 20 μg of protein per sample was diluted with 4× Nupage LDX sample buffer (Life Technologies) containing 10% β-mercaptoethanol and heated at 67°C for 10 min and loaded on 4%–12% Bis-Tris gels (Nupage, Invitrogen) and run in 3-(N-morpholino)propanesulfonic acid buffer for 50 min at 200 V. Transfer was performed using the iBlot (Life Technologies). Membranes were blocked for at least 1 h in 2.5%–5% milk (Carnation Powdered Milk) in Tris-buffered saline with 0.1% Tween 20 detergent (TBS-T) and probed with primary antibodies against PRKACA (PKA C-α (D38C6) Rabbit monoclonal antibody [mAb], Cell Signaling, 1:1,000 dilution), glyceraldehyde 3-phosphate dehydrogenase (GTX621408 mouse mAb, GeneTex, 1:5,000), DNAJB1 (Hsp40 (4868S), rabbit polyclonal, Cell Signaling, 1:1,000), Cox4 (3E11, rabbit monoclonal, Cell Signaling, 1:1,000), caspase-8 (1C12, mouse monoclonal, Cell Signaling, 1:1,000) in 2.5%–5% milk and incubated overnight with shaking at 4°C. After washing in TBS-T for 5 min, membranes were incubated with horseradish peroxidase–conjugated appropriate secondary antibodies (Sigma, A0545 goat anti-rabbit, A9917, goat anti-mouse, 1:10,000) in 5% milk in TBS-T for at least 1 h. Membranes were washed in TBS-T for 5 min 3 times and then incubated with Amersham ECL prime western blotting detection reagent (GE Healthcare) and imaged on a LiCor Blot Scanner (C-DiGit). When multiple proteins were probed, between antibodies the membranes were treated with Restore Plus Western Blot Stripping Buffer (46430, Thermo Scientific).
Cell viability assays
Cells were assayed for viability using PrestoBlue HS (Thermo Fisher Scientific) based on the manufacturer’s instructions. Briefly, cells were cultured in 96-well plates at a starting density of 30,000 cells per well. PrestoBlue was added at a 1:10 ratio and cells were incubated for 1–2 h before measurement at 560 nm excitation and 590 nm emission in a Spark Tecan instrument. Viability was calculated compared to cells treated with 20 μM chaetocin (positive, SelleckChem S8068) and untreated (negative) as percent survival = (positive − Treated)/(positive − negative) × 100.
Immunofluorescence
Tumor or normal adjacent liver was embedded in Optimal Cutting Temperature (Scigen) compound upon receiving the samples. Tissue slices were cut with a Leica CM3050 cryostat machine at a thickness of 30 μm and fixed on glass slides with 4% paraformaldehyde for 10 min. For each patient sample, adjacent normal liver, primary tumor, and metastasis slices were placed on the same glass slide and stained in parallel. Samples were washed 3 times in PBS and then incubated in blocking buffer (2.5% donkey serum, 2.5% goat serum, 1% BSA, 0.1% Triton X-100 in PBS) for 1 h at room temperature. For ASGR1 staining, slides were incubated overnight at 4°C in blocking buffer with primary antibody at a 1:400 dilution (ASGR1, PA5-52885, rabbit polyclonal, Thermo Fisher Scientific). Slides were washed with PBS 3 times before incubation with secondary antibodies (1:1,000, A-11011, Thermo Fisher Scientific) for 1 h at room temperature. Slides were washed 3 times with PBS and then incubated with DAPI (H3569, Thermo Fisher Scientific) for 1 min and washed again with PBS. Prolong gold antifade (P36934, Thermo Fisher Scientific) was added before glass cover slides were fixed. Samples were stored at 4°C until imaging with a confocal microscope (Olympus FluoViewFV3000; UPLXAPO 60× oil objective, numerical aperture 1.42).
Image quantification
Regions of interest (ROIs) were drawn around cell peripheries across several fields of view. The average fluorescence intensity of each ROI was quantified in CellSens (Olympus), with pixel offset substracted by measuring the average pixel intensity in a dark region.
RNA-seq
Through our Fibrolamellar Tissue Repository (https://fibrolamellar.rockefeller.edu/repository), we have been collecting samples of patients’ tumors and, when available, adjacent nontransformed tissue and metastases. Under supervision of our institutional review board approval (SSI 0797, SSI 0798) consent was obtained from patients scheduled for tumor resection. For each patient the diagnosis of FLC was confirmed both by the demonstration of the DNAJB1::PRKACA fusion transcript by RT-PCR and DNAJB1:PRKACA fusion protein by western blot.4 As part of our characterization the transcriptome was evaluated by RNA-seq.30 The expression levels of ASGR1 were obtained from these analyses.
Total RNA from patient tumors, adjacent nontransformed (“normal”) liver, and PDXs was extracted using the RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions. RNA concentration was measured in a Nanodrop 2000c (Thermo Fisher), assessing the quality by the 260/280 ratio. RNA integrity was estimated by the RNA integrity number (RIN), measured in a BioAnalyzer (Agilent). Samples with >100 ng/μL and a RIN >7 were sequenced. RNA-seq libraries were prepared with the TruSeq Stranded Total RNA Sample Prep Kit, using the Ribo-Zero Gold for ribosomal depletion (Illumina). Libraries were sequenced on an Illumina HiSEq 4000. Quality control of the reads was performed using FastQC version 0.11.9 and MultiQC version 1.15, and adapter trimming using BBDuk (in BBMap version 39.01). Then, reads were mapped to the human reference genome hg38 with the annotation GRCh38.103 using STAR, and the transcript expression was quantified using Salmon version 1.10.0. The bam files were indexed using Samtools version 1.10 and visualized in IGV version 2.16.0 to verify the presence of reads spanning the junction of the chimera DNAJB1:PRKACA. The normalized counts, transcripts per kilobase million, reads per kilobase of transcript per million reads mapped, and the differential gene expression analysis was performed in R using DESeq2.
Tumor dissociation
Tumor-bearing mice were euthanized in accordance with Institutional Animal Care and Use Committee approval (no. 20027-H). Tumors were harvested and cut into small pieces; connective tissue, blood vessels, and necrotic tissue were discarded and then transferred into 50-mL Falcon tubes with RPMI, 2% penicillin/streptomycin, collagenase 5 (Worthington, 1 mg/mL), neutral protease (Worthington, 0.5 U/mL), and DNAse (Roche, 1 μg/mL), and digested while rotating at 37°C until digestion was complete (Benchmark Scientific Roto-therm). The digested tissue was passed through a 200-μm (Pluriselect) strainer using a syringe plunger for the remaining pieces, and a 100-μm strainer (Fisher) was used thereafter. The cells were spun down at 321 × g for 5 min at 4°C. The pellet was depleted of red blood cells by a 10-s exposure to 1 mL of water followed by the addition of 49 mL of PBS. Cells were centrifuged, resuspended in RPMI medium, counted, subjected to mouse cell depletion according to the manufacturer’s instructions (Miltenyi Biotec, 130-104-694 and 130-122-729), and plated on collagen-coated plates for in vitro experiments.
Mice
For PDX studies, NSG mice were purchased from Jackson Laboratories and bred at The Rockefeller University animal facility specific pathogen-free immune-core. Mice were kept in 12-h light/dark cycles, fed an amoxicillin diet, and had ad libitum access to food and water. Both male and female mice were used for PDX passaging or in vivo studies. Mice were inspected at least twice per week for health and tumor growth. All of the experiments were conducted under animal-use protocols approved by The Rockefeller University in accordance with Institutional Animal Care and Use Committee approval.
In vivo tumor studies
Mice were anesthetized using isoflurane. For subcutaneous tumors, cells were injected subcutaneously with 200,000–500,000 cells in a 1:1 ratio mix or cells in RPMI and Matrigel (Corning). Tumors were allowed to engraft until the formation of palpable tumors. Depending on how quickly a particular tumor grew, the size was measured by a caliper one to three times per week.
For hepatic tumors, the liver was exposed and 250,000 cells were injected in a 1:1 ratio of RPMI and Matrigel. Fascia was sutured and skin was stapled. For splenic tumors, the spleen was exposed and 250,000 cells were injected suspended in RPMI. Fascia was sutured and skin was stapled.
Bioluminescence imaging
Animals were injected intraperitoneally with 150 mg/kg luciferin (PerkinElmer) and anesthetized in an isoflurane chamber. Imaging was started 10 min after injection, with exposure set first to Auto and then 30 s. The 30-s exposed images were used for display. Total flux was measured with the ROI drawn around the body of each mouse. For each day, all of the images were set to the same scale.
Toxicity analysis
Animals were terminally bled, and blood was allowed to coagulate in tubes at room temperature for at least 30 min. Tubes were centrifuged at 6,000 × g for 10 min at 4°C, and serum was collected from the upper fraction. Serum was analyzed for ALT and AST levels by the Laboratory of Comparative Pathology at Memorial Sloan Kettering Cancer Center.
Immunohistochemistry (IHC)
Subcutaneous tumors were extracted at the end of the study and fixed for 48 h in 10% formalin. IHC stainings for TUNEL and Ki-67 were performed at the Molecular Cytology Core Facility of Memorial Sloan Kettering Cancer Center.
All IHC slides were imaged on an Olympus IX83 microscope using a 10× objective, imaging the whole tissue section. Images were acquired using an Olympus DP26 camera and cellSens software (Olympus). We used ImageJ software (NIH) to calculate the percentage of positive cells for TUNEL and Ki-67 staining.
Statistical analysis
Most statistical analyses were performed using GraphPad Prism 9.0.1. No power calculation was performed. We used the following significance levels: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. We used a linear mixed-effects regression model with compound symmetry covariance structure to compare the growth trajectories between different experimental groups for tumor growth studies. The model included group, day (categorical), and group × day interaction as fixed effects. A significant group × day interaction indicated a difference in tumor growth over time between groups. Before analysis, due to a skewed tumor volume distribution, a log transformation was used for tumor volume. Residual plots were examined to evaluate the validity of model assumptions. Data analysis was performed using Proc Mixed in SAS Studio version 3.8. p <0.05 was considered statistically significant.
Data and code availability
Data were generated by the authors and are available on request from the corresponding author.
Acknowledgments
We thank all of the fibrolamellar patients and their caregivers through their contributions to The Fibrolamellar Registry, to our Fibrolamellar Tissue Repository, through work at the bench, and contributions in too many ways to enumerate. We also thank for their financial support critical seed funds from private foundations and the NIH/National Cancer Institute (NCI) R01CA248507 (to C.N. and S.M.S.); NIH/NCI P50CA210964 (to D.N. and S.M.S.); NIH/NCI U54CA243126 (to D.N. and S.M.S.); the Robertson Therapeutic Development Fund (to S.M.S.); the Center for Basic and Translational Research on Disorders of the Digestive System through the generosity of the Leona M. and Harry B. Helmsley Charitable Trust (to S.M.S.); The Sohn Conference Foundation (to S.M.S.); The Rally Foundation (to S.M.S.); The Bear Necessities (to S.M.S.); and The Truth365 (to S.M.S.).
Author contributions
Conceptualization: C.N., A.V., and S.M.S. Investigation: C.N., D.N., and A.V. Methodology: C.N., D.N., A.Q., R.V., and S.M.S. Data curation: C.N. and S.M.S. Formal analysis: C.N., D.N., D.R., C.S.J., A.Q., R.V., and S.M.S. Reagents: T.P.P. Writing – original draft: C.N. and S.M.S. Writing – review & editing: C.N., D.N., C.S.J., A.Q., R.V., T.P.P., A.R., and S.M.S. Visualization: C.N., D.N., and S.M.S. Resources: C.N. and S.M.S. Validation: C.N. and S.M.S. Funding acquisition: S.M.S. Supervision: R.V. and S.M.S.
Declaration of interests
T.P.P. and A.R. are employees at Ionis Pharmaceuticals.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.11.012.
Supplemental information
References
- 1.Torbenson M. Fibrolamellar carcinoma: 2012 update. Scientifica. 2012;2012 doi: 10.6064/2012/743790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lalazar G., Simon S.M. Fibrolamellar Carcinoma: Recent Advances and Unresolved Questions on the Molecular Mechanisms. Semin. Liver Dis. 2018;38:51–59. doi: 10.1055/s-0037-1621710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neill A.F., Church A.J., Perez-Atayde A.R., Shaikh R., Marcus K.J., Vakili K. Fibrolamellar carcinoma: An entity all its own. Curr. Probl. Cancer. 2021;45 doi: 10.1016/j.currproblcancer.2021.100770. [DOI] [PubMed] [Google Scholar]
- 4.Honeyman J.N., Simon E.P., Robine N., Chiaroni-Clarke R., Darcy D.G., Lim I.I.P., Gleason C.E., Murphy J.M., Rosenberg B.R., Teegan L., et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343:1010–1014. doi: 10.1126/science.1249484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLeod A.R., Crooke S.T. RNA Therapeutics in Oncology: Advances, Challenges, and Future Directions. J. Clin. Pharmacol. 2017;57:S43–S59. doi: 10.1002/jcph.957. [DOI] [PubMed] [Google Scholar]
- 6.Hu B., Zhong L., Weng Y., Peng L., Huang Y., Zhao Y., Liang X.J. Therapeutic siRNA: state of the art. Signal Transduct. Target. Ther. 2020;5:101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich M., Aigner A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs. 2022;36:549–571. doi: 10.1007/s40259-022-00549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kastenhuber E.R., Lalazar G., Houlihan S.L., Tschaharganeh D.F., Baslan T., Chen C.C., Requena D., Tian S., Bosbach B., Wilkinson J.E., et al. DNAJB1-PRKACA fusion kinase interacts with beta-catenin and the liver regenerative response to drive fibrolamellar hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA. 2017;114:13076–13084. doi: 10.1073/pnas.1716483114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelholm L.H., Riaz A., Serra D., Dagnæs-Hansen F., Johansen J.V., Santoni-Rugiu E., Hansen S.H., Niola F., Frödin M. CRISPR/Cas9 Engineering of Adult Mouse Liver Demonstrates That the Dnajb1-Prkaca Gene Fusion is Sufficient to Induce Tumors Resembling Fibrolamellar Hepatocellular Carcinoma. Gastroenterology. 2017;153:1662–1673.e10. doi: 10.1053/j.gastro.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumayer C., Ng D., Jiang C.S., Qureshi A., Lalazar G., Vaughan R., Simon S.M. Oncogenic Addiction of Fibrolamellar Hepatocellular Carcinoma to the Fusion Kinase DNAJB1-PRKACA. Clin. Cancer Res. 2023;29:271–278. doi: 10.1158/1078-0432.CCR-22-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witzigmann D., Quagliata L., Schenk S.H., Quintavalle C., Terracciano L.M., Huwyler J. Variable asialoglycoprotein receptor 1 expression in liver disease: Implications for therapeutic intervention. Hepatol. Res. 2016;46:686–696. doi: 10.1111/hepr.12599. [DOI] [PubMed] [Google Scholar]
- 12.Willoughby J.L.S., Chan A., Sehgal A., Butler J.S., Nair J.K., Racie T., Shulga-Morskaya S., Nguyen T., Qian K., Yucius K., et al. Evaluation of GalNAc-siRNA Conjugate Activity in Pre-clinical Animal Models with Reduced Asialoglycoprotein Receptor Expression. Mol. Ther. 2018;26:105–114. doi: 10.1016/j.ymthe.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim Y., Jo M., Schmidt J., Luo X., Prakash T.P., Zhou T., Klein S., Xiao X., Post N., Yin Z., MacLeod A.R. Enhanced Potency of GalNAc-Conjugated Antisense Oligonucleotides in Hepatocellular Cancer Models. Mol. Ther. 2019;27:1547–1557. doi: 10.1016/j.ymthe.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haraszti R.A., Roux L., Coles A.H., Turanov A.A., Alterman J.F., Echeverria D., Godinho B.M.D.C., Aronin N., Khvorova A. 5΄-Vinylphosphonate improves tissue accumulation and efficacy of conjugated siRNAs in vivo. Nucleic Acids Res. 2017;45:7581–7592. doi: 10.1093/nar/gkx507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakash T.P., Kinberger G.A., Murray H.M., Chappell A., Riney S., Graham M.J., Lima W.F., Swayze E.E., Seth P.P. Synergistic effect of phosphorothioate, 5'-vinylphosphonate and GalNAc modifications for enhancing activity of synthetic siRNA. Bioorg. Med. Chem. Lett. 2016;26:2817–2820. doi: 10.1016/j.bmcl.2016.04.063. [DOI] [PubMed] [Google Scholar]
- 16.Crooke S.T., Baker B.F., Crooke R.M., Liang X.H. Antisense technology: an overview and prospectus. Nat. Rev. Drug Discov. 2021;20:427–453. doi: 10.1038/s41573-021-00162-z. [DOI] [PubMed] [Google Scholar]
- 17.Brown C.R., Gupta S., Qin J., Racie T., He G., Lentini S., Malone R., Yu M., Matsuda S., Shulga-Morskaya S., et al. Investigating the pharmacodynamic durability of GalNAc-siRNA conjugates. Nucleic Acids Res. 2020;48:11827–11844. doi: 10.1093/nar/gkaa670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shebl B., Ng D., Lalazar G., Rosemore C., Finkelstein T.M., Migler R.D., Zheng G., Zhang P., Jiang C.S., Qureshi A., et al. Targeting BCL-XL in fibrolamellar hepatocellular carcinoma. JCI Insight. 2022;7 doi: 10.1172/jci.insight.161820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams B.D., Kasinski A.L., Slack F.J. Aberrant regulation and function of microRNAs in cancer. Curr. Biol. 2014;24:R762–R776. doi: 10.1016/j.cub.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalazar G., Requena D., Ramos-Espiritu L., Ng D., Bhola P.D., de Jong Y.P., Wang R., Narayan N.J.C., Shebl B., Levin S., et al. Identification of Novel Therapeutic Targets for Fibrolamellar Carcinoma Using Patient-Derived Xenografts and Direct-from-Patient Screening. Cancer Discov. 2021;11:2544–2563. doi: 10.1158/2159-8290.CD-20-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross S.J., Revenko A.S., Hanson L.L., Ellston R., Staniszewska A., Whalley N., Pandey S.K., Revill M., Rooney C., Buckett L.K., et al. Targeting KRAS-dependent tumors with AZD4785, a high-affinity therapeutic antisense oligonucleotide inhibitor of KRAS. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal5253. [DOI] [PubMed] [Google Scholar]
- 22.Ma W.K., Voss D.M., Scharner J., Costa A.S.H., Lin K.T., Jeon H.Y., Wilkinson J.E., Jackson M., Rigo F., Bennett C.F., Krainer A.R. ASO-Based PKM Splice-Switching Therapy Inhibits Hepatocellular Carcinoma Growth. Cancer Res. 2022;82:900–915. doi: 10.1158/0008-5472.CAN-20-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer A.D., Dowdy S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018;28:109–118. doi: 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orellana E.A., Tenneti S., Rangasamy L., Lyle L.T., Low P.S., Kasinski A.L. FolamiRs: Ligand-targeted, vehicle-free delivery of microRNAs for the treatment of cancer. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aam9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mainini F., Eccles M.R. Lipid and Polymer-Based Nanoparticle siRNA Delivery Systems for Cancer Therapy. Molecules. 2020;25 doi: 10.3390/molecules25112692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biscans A., Coles A., Haraszti R., Echeverria D., Hassler M., Osborn M., Khvorova A. Diverse lipid conjugates for functional extra-hepatic siRNA delivery in vivo. Nucleic Acids Res. 2019;47:1082–1096. doi: 10.1093/nar/gky1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osborn M.F., Khvorova A. Improving siRNA Delivery In Vivo Through Lipid Conjugation. Nucleic Acid Ther. 2018;28:128–136. doi: 10.1089/nat.2018.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown K.M., Nair J.K., Janas M.M., Anglero-Rodriguez Y.I., Dang L.T.H., Peng H., Theile C.S., Castellanos-Rizaldos E., Brown C., Foster D., et al. Expanding RNAi therapeutics to extrahepatic tissues with lipophilic conjugates. Nat. Biotechnol. 2022;40:1500–1508. doi: 10.1038/s41587-022-01334-x. [DOI] [PubMed] [Google Scholar]
- 29.Prakash T.P., Yu J., Migawa M.T., Kinberger G.A., Wan W.B., Østergaard M.E., Carty R.L., Vasquez G., Low A., Chappell A., et al. Comprehensive Structure-Activity Relationship of Triantennary N-Acetylgalactosamine Conjugated Antisense Oligonucleotides for Targeted Delivery to Hepatocytes. J. Med. Chem. 2016;59:2718–2733. doi: 10.1021/acs.jmedchem.5b01948. [DOI] [PubMed] [Google Scholar]
- 30.Simon E.P., Freije C.A., Farber B.A., Lalazar G., Darcy D.G., Honeyman J.N., Chiaroni-Clarke R., Dill B.D., Molina H., Bhanot U.K., et al. Transcriptomic characterization of fibrolamellar hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA. 2015;112:E5916–E5925. doi: 10.1073/pnas.1424894112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were generated by the authors and are available on request from the corresponding author.