Abstract
Recombinant adeno-associated virus (rAAV) vectors could be manufactured by plasmid transfection into human embryonic kidney 293 (HEK293) cells or baculovirus infection of Spodoptera frugiperda (Sf9) insect cells. However, systematic comparisons between these systems using large-scale, high-quality AAV vectors are lacking. rAAV from Sf9 cells (Sf9-rAAV) at 2–50 L and HEK293 cells (HEK-rAAV) at 2–200 L scales were characterized. HEK-rAAV had ∼40-fold lower yields but ∼10-fold more host cell DNA measured by droplet digital PCR and next-generation sequencing, respectively. The electron microscope observed a lower full/empty capsid ratio in HEK-rAAV (70.8%) than Sf9-rAAV (93.2%), while dynamic light scattering and high-performance liquid chromatography analysis showed that HEK-rAAV had more aggregation. Liquid chromatography tandem mass spectrometry identified different post-translational modification profiles between Sf9-rAAV and HEK-rAAV. Furthermore, Sf9-rAAV had a higher tissue culture infectious dose/viral genome than HEK-rAAV, indicating better infectivity. Additionally, Sf9-rAAV achieved higher in vitro transgene expression, as measured by ELISA. Finally, after intravitreal dosing into a mouse laser choroidal neovascularization model, Sf9-rAAV and HEK-rAAV achieved similar efficacy. Overall, this study detected notable differences in the physiochemical characteristics of HEK-rAAV and Sf9-rAAV. However, the in vitro and in vivo biological functions of the rAAV from these systems were highly comparable. Sf9-rAAV may be preferred over HEK293-rAAV for advantages in yields, full/empty ratio, scalability, and cost.
Keywords: adeno-associated virus, gene therapy, Sf9, HEK293, AAV packaging
Graphical abstract

Liu and colleagues reported notable differences in physiochemical characteristics but highly comparable biological activities and efficacy of rAAV vectors manufactured by large-scale HEK293 and Sf9 systems. The Sf9 system may be preferred for advantages in yields, scalability, and cost.
Introduction
Adeno-associated virus (AAV) is a small single-stranded DNA virus that infects humans and other animal species.1 Recombinant AAV (rAAV) has been developed for gene delivery due to its sustained expression, low immunogenicity, and reasonably good tropism to multiple tissues, including the brain.2 rAAV has been used for in vivo gene delivery in more than 255 clinical trials,3 and there are a total of 7 regulatory approvals, including 3 in between 2022 and 2023 alone. rAAV can be applied in the case of gene replacement,4,5 gene editing,6,7 and RNA editing technologies,8 enabling broad therapeutic impacts. Moreover, extensive efforts on AAV engineering for optimized delivery efficiency and specificity have been made,9 further empowering AAV for in vivo gene delivery.
rAAV can be manufactured by various systems, including plasmid transfection in human embryonic kidney 293 (HEK293) cells,10,11,12 baculovirus transduction into Sf9 insect cells,13,14,15,16 herpes simplex virus transduction into baby hamster kidney or HEK293 cells,17 and producer/packaging cell lines.18,19 The HEK293 system is the most widely used,3 due to its ease of setup, short timeline, and low technical requirements.20 The Sf9 system, as the second most popular system, has gained growing interest, due to the high yields, full/empty ratio, and lower cost. There has been a series of studies aiming to compare HEK293 and Sf9 systems for AAV packaging.21,22,23,24,25,26,27,28,29,30,31,32 However, many studies failed to apply the most up-to-date technologies for Sf9, HEK293, or both, as there have been optimizations of each system over the years. Moreover, a systematic comparison of protein purity, genome integrity, infectivity, post-translational modifications (PTMs), in vitro and in vivo transgene expression, and in vivo efficacy, using large-scale, high-quality AAV vectors is lacking. Therefore, this study aims to fill this gap by performing a head-to-head comparison of AAV vectors using state-of-the-art HEK293 and Sf9 systems through SDS-PAGE, next-generation sequencing (NGS), liquid chromatography tandem mass spectrometry (LC-MS/MS), tissue culture infectious dose (TCID50) assays, transgene expression measurement by ELISA, and immunofluorescence. The results presented here will provide valuable information for key decisions on AAV manufacturing systems for clinical development and application.
Results
Titer, purity, full/empty ratio, and aggregation profile of AAV vectors
AAV2.N54 (an engineered capsid generated by inserting a 10-amino acid peptide of LALGQTTKPA between amino acids 587 and 588 of the capsid protein) encoding aflibercept, a recombinant fusion protein that inhibits vascular endothelial growth factor (VEGF) and thus prevents choroidal neovascularization, was selected for this study. rAAV vectors were produced using Sf9 (2 and 50 L) and HEK293 (2, 10, 50, and 200 L) systems (designated as Sf9-rAAV and HEK-rAAV, respectively) following the purity and safety requirements for ocular applications. The final yields of each system at different scales were measured by qPCR. As shown in Table 1, Sf9-rAAV had significantly higher total viral genome (vg) numbers than HEK-rAAV across different scales, e.g., for 50 L, 4.41 × 1015 vg from Sf9-rAAV vs. 1.30 × 1014 vg from HEK-rAAV. Empty and full capsids were collected after CsCl ultracentrifugation and separately loaded in SDS-PAGE gels. As shown in Figures 1A and 1B, VP1, VP2, and VP3 proteins were observed in both Coomassie blue staining and silver staining. Based on the signals in the Coomassie blue staining gel images, the VP1:2:3 ratios were calculated: HEK-rAAV (15.1:16.7:52.3) vs. Sf9-rAAV (7.2:17.6:62.5) (Table 2). Sf9-rAAV had a lower VP1 ratio, but a more balanced VP1:2:3 ratio (closer to 1:1:10). Also, genomes of highly purified Sf9-rAAV and HEK-rAAV were heterogeneous as detected by silver staining (Figure 1B). These bands could be completely removed when Benzonase was included in the gel buffer (data not shown), supporting that they were DNA bands. As expected, DNA genomes could be observed in lanes loaded with full capsids, but not in lanes with empty capsids. Individual bands containing genomes were cut and subject to qPCR using primers targeting the genome of interest (GOI). There were several bands in lanes of HEK-rAAV identified by qPCR as non-GOI genomes, indicating a higher percentage of DNA impurities in HEK-rAAV. Further, the alkaline agarose gel patterns showed the heterogenicity of vector genomes in Sf9-rAAV and HEK-rAAV (Figure 1C): multiple fragments at various lengths were detected and further identification is needed. Notably, in addition to the expected band of ∼4.8 kb, there was a band of ∼2.5 kb in HEK-rAAV, but not in Sf9-rAAV, indicating a truncated genome sequence. In addition, the aggregation profile of AAV products was measured by dynamic light scattering (DLS) and high-performance liquid chromatography (HPLC). As shown in Table S1, both the hydrodynamic diameter and polydisperse index of Sf9-rAAV were smaller than HEK-rAAV, indicating less aggregation and higher stability when rAAV concentration was >1 × 1013 vg/mL. HPLC results also showed that Sf9-rAAV had less aggregation than HEK-rAAV (Figure 1D). In addition, electron microscopy was performed to assess full and empty capsids in Sf9-rAAV and HEK-rAAV (Figure 1E). The full/empty ratio of Sf9-rAAV (93.2%) was significantly higher than that of HEK-rAAV (70.8%). Notably, electron microscopy was performed with highly purified rAAV vectors after ultracentrifugation and thus reflects both the impact of packaging systems and the purification process on the full capsid ratio.
Table 1.
Yields of Sf9-rAAV and HEK-rAAV at various scales
| Scale | Total vg |
|
|---|---|---|
| HEK-rAAV | Sf9-rAAV | |
| 2L | 5.50E+13 | 8.48E+13 |
| 3L | 5.2E13 | N/A |
| 10L | 9.13E13 | N/A |
| 50L | 1.30E+14 | 2.98E+15 |
| 200L | 3.00E+15 | N/A |
N/A, not available.
Figure 1.
Purity, genome integrity, and full/empty ratio of Sf9-rAAV and HEK-rAAV
(A) Coomassie blue staining of Sf9-rAAV (2 L) and HEK-rAAV (10 L) and (B) silver staining of Sf9-rAAV (50 L) and HEK-rAAV (200L) to assess VP1, 2, and 3 proteins and genomic DNA. Empty and full vectors were separately loaded. Each silver-stained band was cut, and DNA was extracted and determined by qPCR.
(C) Agarose gel images of Sf9-rAAV (2 and 50 L) and HEK-rAAV (2, 10, 50, and 200 L) to evaluate genomic DNA. E, empty capsids; F, full capsids; M, maker.
(D) The aggregation profiles of Sf9-rAAV and HEK-rAAV were assessed by HPLC.
(E) Full and empty capsids were assessed by electron microscope.
Table 2.
VP1:2:3 ratio of Sf9-rAAV and HEK-rAAV
| VP | Apparent MW (Da) | Sf9 |
HEK293 |
||||
|---|---|---|---|---|---|---|---|
| Run 1 | Run 2 | Average | Run 1 | Run 2 | Average | ||
| VP1 | 87 | 7.2 | 7.2 | 7.2 | 14.7 | 15.4 | 15.1 |
| VP2 | 72 | 19.2 | 15.9 | 17.6 | 16.2 | 17.2 | 16.7 |
| VP3 | 62 | 73.7 | 51.3 | 62.5 | 60.2 | 44.3 | 52.3 |
The VP1:2:3 ratios of HEK-rAAV (10 L) and Sf9-rAAV (2 L) were calculated based on the signal strength from Coomassie blue staining gel images.
Genome identity by NGS and protein PTM profiling by LC-MS/MS
To identify the packaged genome sequences, NGS with Illumina and PacBio were initially performed with Sf9-rAAV. As shown in Table S2, PacBio and Illumina detected minor differences in the rep-cap plasmid (0.06% vs. 0.12%), plasmid backbone, and baculovirus (0.62% vs. 1.88%), host cell DNA (0.03% vs. 0.00%). Since PacBio had advantages in long-read sequencing, both HEK-rAAV and Sf9-rAAV were analyzed by PacBio for a direct comparison. As shown in Table 3, Sf9-rAAV contained slightly higher vector genome content and lower host cell DNA (HCD). Further, Sf9-rAAV had fewer chimeric events with HCD than HEK-rAAV (1 vs. 48). In addition, a large portion of AAV genomes were truncated. HEK-rAAV had a higher close-to-full fragment ratio than Sf-rAAV (33.7% vs. 21.9%). Interestingly, while Sf9-rAAV had more minor fragments, the major truncations occurred at both ITR ends. In contrast, HEK-rAAV had major truncations at the N terminus of the gene of interest (Figure S1). Truncated genomes would have an impact on transgene expression, which warrants further investigation.
Table 3.
The genomic DNA profile of Sf9-rAAV and HEK-rAAV was assessed by PacBio
| DNA source | Sf9-rAAV | HEK-rAAV |
|---|---|---|
| AAV vector genome | 99.29% | 98.84% |
| Rep-Cap plasmid | 0.06% | 0.40% |
| GOI plasmid backbone and pHelper plasmid or baculoviral sequence | 0.62% | 0.34% |
| HCD | 0.03% | 0.38% |
| Chimeric events with HCD | 0.00% | 0.04% |
Sf9-rAAV (2 L) and HEK-rAAV (50 L) were tested by PacBio NGS. The percentage was calculated by dividing the reads of each using the total unique mapped reads.
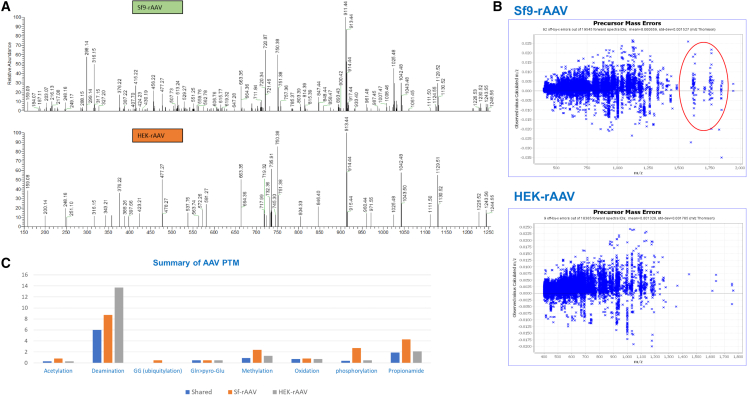
To assess the PTMs of AAV capsid proteins, LC-MS/MS analysis was performed using HEK-rAAV and Sf9-rAAV. As shown in Figure 2A, more PTMs were detected in Sf9-rAAV than in HEK-rAAV. Moreover, more high mass-to-charge ratio species were detected in Sf9-rAAV than in HEK-rAAV (Figure 2B), indicating that there were more ions with a higher molecular weight. These ions tend to carry a variety of modifications. Together these results supported that there were more PTMs in Sf9-rAAV than in HEK-rAAV. Detailed analysis showed that Gln to pyro-Glu occurred nearly equally in both Sf9-rAAV and HEK-rAAV, which is consistent with previous findings in bioreactors. In addition, other modifications such as phosphorylation, acetylation, methylation, ubiquitination, and glycosylation were found to occur more in Sf9-rAA than HEK-rAAV (Figure 2C). However, less deamination occurred in Sf9-rAAVs. AAV capsid protein PTMs have the potential to alter the stability, subcellular location, vector activity, and host cell interactions and may also be beneficial for process development and production.
Figure 2.
PTMs profile of Sf9-rAAV and HEK-rAAV assessed by LC-MS/MS
PTMs profile (A) and mass-to-charge ratio (M/Z) species (B) of Sf9-rAAV and HEK-rAAV. (C) Summary of specific PTMs identified.
In vitro infectivity, transgene expression, and biological activity
Since a previous study using rAAV vectors reported that Sf9-rAAV had lower infectivity/potency,25 the infectivity of HEK-rAAV and Sf9-rAAV was assessed by the TCID50 assay, a standard method to measure infectivity with HeLa RC32 indicator cell line. TCID50/mL (tissue culture infectious dose 50%/mL) is the concentration of infectious organisms in the inoculum determined from the dilution at which the inoculum infects 50% of the target cultures. Sf9-rAAV had a higher TCID50/vg than HEK-rAAV (Figure 3C and Table S3), indicating better infectivity.
Figure 3.
Highly comparable in vitro infectivity and in vivo efficacy of Sf9-rAAV and HEK-rAAV
FA representative images (A) and bar graph data based on images (B). The laser choroidal neovascularization mouse model (n = 11–15 each group) was intravitreally injected with sham (Sf9-rAAV or HEK-rAAV vectors encoding disrupted transgene sequence, standard of care (aflibercept), Sf9-rAAV, and HEK-rAAV encoding aflibercept at 4 × 108 (medium dose) or 1.6 × 1010 vg (high dose). Scale bar, 50 μm. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001; ns, not significant, Tukey’s multiple comparisons test. (C) Infectivity of Sf9-rAAV and HEK-rAAV measured by TCID50 assay.
To assess transgene expression in vitro, aflibercept expression was quantified by ELISA after transduction of both rAAV vectors into HEK293 cells and representative cell line ARPE19. The ELISA was established based on ligand-receptor binding where the recombinant VEGF-A165 was coated in the wells of a 96-well plate. The rAAV transduced cell culture supernatant was added to form VEGF-A165-aflibercept complex, which was then detected by horseradish peroxidase (HRP)-labeled goat anti-human IgG1 Fc conjugate antibody. In HEK293 cells, the expression of Sf9-rAAV was ∼2-fold higher than that of HEK-rAAV (13.6 ± 0.6 vs. 7.3 ± 0.3 μg/mL in HEK293 and 2.1 ± 0.2 vs. 1.2 ± 0.1 μg/mL in ARPE19) (Table 4). This was in accordance with the infectivity TCID50 results discussed above, even though the VP1 ratio in HEK-rAAV was higher than that in Sf9-rAAV. These results indicated that higher VP1 is not the only criterion for robust transgene expression. A balanced VP1:2:3 ratio closer to 1:1:10 may be more important than the absolute amount of each VP component.
Table 4.
In vitro expression of GOI detected by ligand-receptor binding ELISA
| AAV | Bioreactor scale | Aflibercept (μg/mL) |
|
|---|---|---|---|
| HEK293 | ARPE19 | ||
| HEK-rAAV | 200 L | 7.3 ± 0.3a | 1.2 ± 0.1a |
| Sf9-rAAV | 50 L | 13.6 ± 0.6a | 2.1 ± 0.2a |
mean +SD.
In addition, the transgene product, aflibercept, functions through binding VEGF-A165; thus, the binding affinity of aflibercept to VEGF is a strong indicator of therapeutic benefit and should be measured. Therefore, aflibercept proteins from transduced cells were purified. N-terminal protein sequencing was performed using the purified proteins to confirm that no cleavage occurred during the protein production. The binding affinity of purified proteins was then measured by BIACore or ELISA. The ELISA and BIACore results showed that purified proteins obtained by HEK293 cells transduced with SF9-rAAV and HEK-rAAV had similar binding affinity as the U.S. Food and Drug Administration-approved, commercially available protein drug aflibercept (Table S4).
In vivo aflibercept expression and efficacy
In the laser choroidal neovascularization mouse model, the lesions were induced by laser. rAAV2.N54-aflibercept, produced by HEK293 or Sf9 systems, was intravitreally injected into mice at 4 × 108 vg/eye (medium dose) or 1.6 × 1010 vg/eye (high dose). Aflibercept (a commercial product of aflibercept) was dosed at 40 μg/eye as a control. As shown in Table 5, Sf9-rAAV and HEK-rAAV had similar transgene expression at 28 days post-dosing. Serum samples showed no aflibercept expression in mice injected with AAV, while mice injected with aflibercept showed very high aflibercept concentration (∼45 ng/mL), similar to the previously published data.33 The leakage of aflibercept into the blood circulation is a phenomenon that should be avoided to reduce off-target toxicity risk.
Table 5.
In vivo expression of GOI detected by ligand-receptor binding ELISA
| AAV type | Bioreactor scale | Aflibercept (pg/eye) 28 days post@4E8 |
|---|---|---|
| Sf9-rAAV | 2 L | 118.1 ± 78 |
| HEK-rAAV | 10 L | 135.0 ± 49 |
At 28 days after intravitreal dosing of 4 × 108 vg, transgene expression in the whole eye was measured by ELISA.
In terms of efficacy, the extent of wound healing represented the efficacy of the treatment, which was assessed by measuring the fluorescence angiography (FA) as the lesion area. The lesion area would decrease or heal completely, leaving no lesion spots, if there were a treatment efficacy. Figure 3A shows that aflibercept and 4 × 108 vg/eye dose of Sf9-and HEK-rAAV achieved similar lesion area reduction and healing. The efficacy was dose-dependent as the animals injected with a higher dose of AAV (1.6 × 1010 vg/eye) showed more pronounced wound healing, and, in some cases, the lesion was completely healed. In summary, Sf9- and HEK293-rAAV had very similar efficacies. The lesion area was calculated as pixel2 to quantitatively reflect the treatment efficacy (Figure 3B). There was a significant negative correlation between the lesion area and the concentration of aflibercept in the ocular samples (Table S5). These results indicated that, as the aflibercept concentration increased, the lesion area decreased.
Discussion
The two major AAV production systems include transient plasmid transfection into mammalian HEK293 cells10,11,12 and infection of Sf9 insect cells using baculovirus.13,14,15,16 The HEK293 system is the most widely applied system due to the low technical bar and resources required and fast timeline; however, it is also limited by the high empty capsid ratio, low yields, and lack of scalability.20 In contrast, the key advantages of the Sf9 system are high yields (10-fold),28,29 full/empty capsid ratio,24,34,35 scalability,34,35 and less replication-competent AAV (rcAAV) risk, while the main disadvantages are related to the learning curve to master production of mammalian rAAV in insect cells, e.g., instability of AAV genome in baculovirus after several rounds of passages, optimal VP1:2:3 ratio, and the downstream process to remove residual baculovirus and insect cell impurities.20
There has been a long-lasting debate over which system is preferable for AAV manufacturing and subsequent clinical applications. Many studies attempted to address this issue by comparing the quality, in vitro, and in vivo activities of AAV vectors from these two systems. One study observed a greater degree of truncated and unresolved species in AAV genomes of the Sf9 system.24 Another study revealed different PTMs and genome methylation profiles between Sf9-rAAV and HEK-rAAV and also showed that HEK-rAAV had greater in vitro potency/infectivity.25 Yet, pieces of evidence in support of Sf9-rAAV also accumulated over the years. Kondratov et al.21 showed that the Sf9 system had lower DNA impurities, a more balanced VP1:2:3 ratio, and higher transgene expression in mice. These results were corroborated by other studies, providing evidence of less DNA impurity in Sf9-rAAV.22,26 In vivo results from another study showed that, although HEK-rAAV and Sf9-rAAV achieved comparable transgene expression when regular-size GOI packaged, transgene expression mediated by HEK-rAAV decreased over time when oversized GOI packaged. Further analysis indicated that the decrease was due to vector genome loss and epigenetic regulation may have played a role in Sf9-rAAV-mediated more durable transgene expression.23 Further, a meta-analysis of 255 AAV clinical trials observed similar durability and treatment-emergent serious adverse events risk between HEK-rAAV and Sf9-rAAV.3
However, many of these studies fail to use high-standard, large-scale AAV vectors manufactured with state-of-the-art technology from both systems to perform a de facto head-to-head comparison. The Sf9 system has been optimized over the years, with multiple generations—namely, ThreeBac,14 TwoBac,36 OneBac,13,22 and MonoBac.37 Each generation is different enough from the other in many aspects and, in turn, different from the HEK293 system, which may also partially explain the contradictory results from these comparison studies because different Sf9 systems were used. Moreover, it is relatively difficult to identify the optimal cell density and baculovirus-to-cell ratio to produce rAAV.38 In this study, we manufactured high-quality AAV vectors in large scales up to 200 L using state-of-the-art technology and conducted a systematic comparison. Similar to a previous study,21 our results of PacBio and Illumina sequencing showed that Sf9 had lower DNA impurities, which was also supported by SDS-PAGE and subsequent qPCR analysis. Although Sf9-rAAV had a lower VP1 ratio, as observed in other studies23,31 and ours, the infectivity, in vitro and in vivo transgene expression, the biological activity of transgene products, and in vivo efficacy were highly comparable between these two systems, corroborating the similar clinical performances as summarized in Shen et al.3 Similar to previous studies,25,26 different PTM profiles on protein levels were also observed; however, the implications of the differences warrant further investigation. In summary, the high-quality and large-scale Sf9-rAAV and HEK-rAAV are largely comparable in many key quality parameters, while Sf9-rAAV had less DNA impurity and aggregation. Considering the higher yields,28,29 full/empty capsid ratio,24,34,35 and lower rcAAV risk, the Sf9 system may be preferable for AAV manufacturing.
Materials and methods
Construction of plasmids
To construct AMI054-pFB-inCap2.N54-inRep-kozak-hr2 (N54), wildtype AAV2 (wtAAV2) was first digested with XbaI and BsiWI to isolate the 9113-bp backbone. N54 mutations were introduced into the capsid gene by two PCR fragments. The first PCR fragment of 763-bp was amplified with forward primer A855 (5′-TACTGACTCGGAGTACCAGCTCCCGTACGTCCTCGGCTCGGCGC-3′) and reverse primer A043 (5′-TGCAGGCTTTGTTGTCTGGCCGAGTGCTAGGTTGCCTCTCTGGAGGTTG-3′), and the cap2 gene as the template. The second PCR fragment of 496-bp was amplified with forward primer A044 (5′-CTAGCACTCGGCCAGACAACAAAGCCTGCAAGGCAAGCAGCTACCGCAG-3′) and reverse primer A038 (5′-CGAGACTGCAGGCTCTAGATTACAG-3′) and the cap2 gene as the template. These two fragments were cloned into the wtAAV2 to create the N54 plasmid through the NEBuilder HiFi DNA Assembly reaction (New England Biolabs, Ipswich, MA).
AMI120-pFB-scCMV-SV40-intron-kozak-aflibercept-GCRS(TCC) was constructed by inserting manually codon-optimized aflibercept gene that was synthesized by Twist Biosciences (South San Francisco, CA).
Cell culture
HEK293 and retinal pigmental cells (ARPE19) cells were cultured in DMEM + GlutaMax (DMEM+GlutMax; Thermo Fisher Scientific, Waltham, MA), supplemented with 10% fetal bovine serum (FBS; ATCC, Manassas, VA) and 1% antibiotic and antimycotic liquid (Thermo Fisher Scientific, Waltham, MA). The Sf9-V432AG cell line was derived from the Sf9 cell line (Expression Systems, Davis, CA) by stably transfected with baculovirus immediate-early gene under the control of cytomegalovirus (CMV) promoter. Sf9-V432AG cells were cultured in ESF AF medium (Expression Systems) containing 100 U/mL penicillin and 100 μg/mL streptomycin (Thermo Fisher Scientific, Pleasanton, CA) in Corning storage bottle with gentle shaking at 160 rpm and 28°C. Once cells grow to ∼1 × 107 cells/mL, they were split into a 1:4 ratio in fresh media into a new bottle and continuously cultured for maintenance purposes.
Generation of recombinant baculovirus
Recombinant baculoviruses (rBVs) were generated with the Bac-to-Bac Baculovirus Expression System (Invitrogen, Carlsbad, CA). Briefly, a pFastBac shuttle plasmid containing the target gene was transformed into the Δcath-DH10Bac competent bacteria (Virovek, Hayward, CA), and white colonies were selected. Recombinant bacmids prepared from these white colonies were transfected into the Sf9-V432AG cells for 3 to 4 days to generate rBV passage (P) 0 seed stocks according to the manufacturer’s protocol. The seed stocks were amplified generally in a 1:200 ratio into P1 working stocks and used for AAV production. The rBV titers were determined with the qPCR method using primers corresponding with the gentamicin gene (F-primer A246: 5′-TGGCTCAAGTATGGGCATCA-3′; R-primer A247: 5′-CCGCATGGATTTGACTTGGT-3′) and the SYBR Green reagent (Thermo Fisher Scientific) in the QuantStudio 7 Pro PCR system (Thermo Fisher Scientific).
rAAV production
AAV vectors were produced both in HEK293 and Sf9-V432AG cells for comparative studies. HEK293 cell-produced AAV vectors were obtained from Vigene Biosciences (VBI), a Contract Development and Manufacturing Organization. The plasmids containing the GOI and specific engineered capsid genes were cloned into VBI’s plasmid backbone and used for AAV manufacturing. Briefly, three plasmids, GOI, replication-capsid (Rep-Cap), and adenovirus helper, at equal ratios were mixed with transfection reagent polyethyleneimine for 15 min and then added to the HEK293 suspension culture. The AAV products were harvested 72 h post-transfection, lysed, and purified by AAVx resin chromatography and iodixanol gradient ultracentrifuge.
Insect cells isolated from Spodoptera frugiperda such as Sf9 can only be maintained for certain passages and the baculovirus and AAV productivity declines significantly after passage 30 or higher. It was reported that by incorporation of baculovirus transactivators IE1 and IE0 in the expression cassette, the cell viability, protein integrity, and productivity were all increased.39 Therefore, an Sf9-V432AG stable cell line was generated by transfection of V432-pcDNA-CMV-IE1-pIE1-NeoR plasmid (Figure S2) into Sf9 cells and subsequent G418 selection. To produce AAV vectors in Sf9 cells, the Sf9-V432AG cell line was used according to the following method described.16 Briefly, 0.1 multiplicity of infection (MOI) of each rBV-inCap-inRep and rBV-GOI were used to co-transfect the cells at a density of ∼5 × 106 cells/mL containing 50% fresh ESF AF media supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin for 3 days at 28°C and 180 rpm. At the end of infection, the whole culture (cells and supernatant) was adjusted to contain 50 mM Tris-HCl, pH8.0, 2 mM MgCl2, 1% Sarkosyl, 1% Triton X-100, and 25 U/mL Benzonase (Millipore Sigma, St. Louis, MO). Cellular nucleic acids were digested by incubating at 37°C for 60 min with vigorous shaking. The clear supernatant was prepared by depth filtration followed by 0.2 μm sterile filtration. The supernatant was loaded onto the AAVx column that was pre-equilibrated with equilibration buffer 20 mM Tris, 150 mM NaCl, and 1 mM EDTA, pH 7.3, and impurities were washed off with wash buffer 20 mM Tris, 2 M NaCl, and 1 mM EDTA, pH 7.3, and a no salt wash without 2 M NaCl. AAV vectors were eluted with elution buffer 100 mM glycine and 500 mM arginine pH 2.5 and neutralized with Tris-HCl pH 10. To remove empty capsids from the full AAV particles containing AAV genomes, the column-purified AAV vectors were added with CsCl powder to a final density of 1.34 g/mL and subjected to one round of ultracentrifugation at 65,000 rpm, 15°C for 20 h in a 70 Ti rotor (Beckman Coulter, Brea, CA) or 45,000 rpm for 30 h in 45Ti for scale larger than 50 L. The full AAV particles were collected and buffer exchanged with PD-10 desalting columns or G-25 Sepharose column chromatography for scales larger than 50 L (Cytiva, Marlborough, MA) in the final buffer containing 1× PBS and 150 mM NaCl with 0.001% Pluronic F-68 and sterilized with 0.2 μm filtration.
Droplet digital PCR and qPCR
AAV titer was determined with droplet digital PCR (ddPCR). The method referenced Bio-Rad ddPCR protocol of Bulletin 7407 (Measuring Adeno-Associated Virus (AAV) Vector Genome Titer Using Droplet Digital PCR Protocol). Briefly, AAV samples were treated with DNase 1, diluted, and denatured. The viral DNAs were mixed with a ddPCR mixture. The droplets of ddPCR were generated using a QX200 droplet generator and run in a C1000 Touch Thermal cycler with a 96-deep well ration module. The results were read using QX200 Droplet Reader. The concentrations were calculated according to the reading and dilution factors.
Identification of the silver stain bands was performed by qPCR. Briefly, the impurity bands on silver stain gels were cut out and broken into small pieces. The broken gels containing each band were resuspended in water and vortexed overnight to release the stained components in the gel. qPCR analyses using primers/probes targeted to GOI (aflibercept) were performed. Forward primer, 5′-CGGCCTGATGACCAAGAAGA-3′; reverse primer, 5′-AGGTGTGGGTCTTGTCCTTC-3′; Taqman probe, 5′-CACCTTCGTGAGGGTGCACGA3′.
SDS-PAGE and alkaline agarose gel
AAV vectors were mixed 1:1 ratio with 2× SDS-loading buffer and placed on a heating block at 95°C for 5 min. The AAV mixture of 10–20 μL/lane (∼1 × 1011 vg) was loaded on the 10% SDS-gel and electrophoresed at 100 V until the dye reached the bottom of the gel. The gel was stained and photographed according to the Manufacturer’s protocol (Thermo Fisher Scientific).
Alkaline agarose gel was prepared by adding 1 g agarose to 98 mL water and microwaving to dissolve. Two milliliters of 50× alkaline electrophoresis buffer (2.5 M NaOH, 50 mM EDTA) were added to the solution and mixed when the solution was warm to hand, and the gel was casted. Approximately 5 × 1010 vg of each AAV sample or 500 ng DNA ladder was diluted to 25 μL, mixed with 5 μL 6× alkaline sample loading buffer (Thermo Fisher Scientific), treated at 95°C for 3 min, and cooled on ice before loading on the gel. The gel was run at 2 V/cm with an ice bag put on top of the gel box to prevent overheating. Once the electrophoresis was completed, the gel was soaked in 0.1 M Tris-HCl pH 7.5 with gentle shaking for 1 h followed by staining with SYBR Gold (Invitrogen) in 0.1 M NaCl for 1 h.
DLS and HPLC
AAV aggregation was evaluated by DLS using an Anton Parr Litesizer 500. The particle size of each AAV sample was measured with three repetitions. Two particle size standards of 220 nm and 20 nm were measured as the controls. Then, AAV aggregation was also assessed by size exclusion HPLC using the Agilent HPLC-1100 system. The system was set up as follows: flow rate (0.5 mL/min), run time (35 min), detection wave (260 nm and 280 nm), column thermostat temperature (23°C), and injection volume for rAAV, assay control, and blank controls (10 μL). The column was equilibrated at initial conditions 1 h prior to the injection. All samples were analyzed in duplicate. The result was deemed valid when the following conditions were met: (1) blank controls at 260/280 nm showed no peak of rAAV was eluted; (2) standard control showed a single peak of rAAV at the retention time of 18.74–18.75 min; and (3) the value of assay control was within the range with the % coefficiency of variation of <20%.
Transmission electron microscopy
To observe full and empty capsids in AAV products, negative staining of the grids was performed first. Briefly, glow discharge was set as 300 mesh formvar/carbon copper grids from Ted Product No. 01753_F (carbon side up). Locking tweezers were used to pick up the grid at the edge, and 5 μL of the sample solution was applied to the freshly glowed grids, followed by 2 min of incubation. Water was removed by touching the torn edge of filter paper to the edge of the forceps jaw where it contacts the grid. One drop of aqueous 1% of uranyl acetate in water was added to the grid for 1 min. The grid was kept dry with ragged torn edges of filter paper, and samples were observed under the transmission electron microscope. The grids were imaged using a Tecnai 12 120kV TEM (FEI, Hillsboro, OR), and data were recorded using Gatan Rio 16 CMOS with Gatan Microscopy Suite software (Gatan Inc., Pleasanton, CA). Counting of empty and full capsids was performed using the Cole Parmer Counter Pen.
rAAV vector transduction and transgene expression quantification by ELISA
Expression profile of AAV2.aflibercept construct was checked in HEK293 and ARPE19 cells. Cells were plated at a seeding density of 1 × 106 cells/well in a 6-well plate. After 24 h, cells were transduced with the AAV2.aflibercept construct at 100,000 MOI. Since serum does not interfere in the transduction for AAV2, the experiment was performed in a complete medium containing 10% FBS. For other serotypes, serum-free medium was used for transduction followed by replacement with complete medium 24 h after transduction. The spent medium was collected on day 5 after transduction to perform aflibercept in-house ELISA.
Purification of aflibercept and dissociate constant measurement
The HEK293T cells were plated into a 15-cm Petri dish at 2 × 107 cells/dish and transduced at 105 vg/cells. The supernatant was harvested 72 h post-transduction and purified using Protein A affinity chromatography. For ELISA, 0.1 μg/mL VEGF (VEGF165, GenScript, Piscataway, NJ) was coated onto a 96-well Nunc Maxisorp flat-bottom plate (Thermo Fisher Scientific). After overnight incubation at 4°C, the plate was washed thrice with wash buffer (phosphate buffer saline with 0.01% tween 20). Blocking buffer (Casein in PBS, Thermo Fisher Scientific) was added to every well and incubated at 37°C for 2 h. Standards and samples were added to the plate and incubated at 37°C for 1 h. The plate was washed three times and a biotinylated anti-Fc detection antibody (Abcam, Cambridge, UK) was added to every well and incubated for 1 h at 37°C. The plate was again washed three times with PBST and streptavidin HRP was added to every well. After 1 h at 37°C, the plate was again washed thrice and developed using TMB. The plate was immediately read at 450 nm after stopping the reaction. The standard curve was fit using linear regression fit using Excel software.
For BIACore, aflibercept was immobilized on the chip and VEGF-A165 was sequentially injected and titrated from 0.5, 1, 2, 4, and 8 nM. The flow rate was kept constant at 30 μL/min using 60 s for each association and 900 s for the dissociation phase at the end.
NGS
NGS was performed by GeneWiz using Illumina HiSeq 2× 150PE. The data were analyzed by Azenta Life Sciences (Burlington, MA). Reference genomes containing the AAV insert genome between ITR-ITR, AAV plasmid backbone sequence, Rep-Cap plasmid sequence, baculoviral sequence, and SF9 host genome were concatenated. After trimming using Skewer, reads were mapped to the concatenated reference genome using BWA. The statistics are listed in the following table. Following the alignment step, variant calling was performed using LoFreq version 2.1.5. Probabilistic realignment of mapped reads was performed using the LoFreq Viterbi command to correct mapping errors. Insertion/deletion (INDEL) qualities were added into the bam files using LoFreq indelqual command. Finally, single nucleotide polymorphisms/INDELs were detected using the LoFreq call command with default settings. The results included the summary of NGS data, quality score distribution of the data, and distribution of GC content.
In addition, PacBio Hifi seq was also performed. Reference genomes that included the AAV genome (ITR-ITR), the AAV plasmid backbone, the Rep-Cap plasmid, the helper or baculoviral sequence, and packaging host genomes were concatenated. Before concatenation, all of the sequences in the other reference genomes that share high similarities with the AAV genome were hidden. The concatenated reference genome was used to map reads after trimming. Lengths of all reads mapping to the vector reference between ITR-ITR were determined and their distributions are plotted. After the alignment process, variant calling was carried out. Mapped readings were realigned probabilistically to fix any mapping faults. The bam files contain INDEL characteristics.
Median TCID50 assay
A TCID50 assay was used to determine the infectivity of AAV vectors according to the published protocol40 with modifications. HeLa RC32 cells were plated on a 96-well plate at 4 × 104 cells/well in 50 μL volume the day before usage. The next day each AAV sample was serial diluted from 1 × 1011 to 1 × 106 with a serum-free DMEM diluent containing 25 mM HEPES and 3.2 × 108 particles/mL of adenovirus type 5 (ATCC). The spending medium was replaced with 50 μL/well of the serial diluted AAV solutions. After incubation in a CO2 incubator for 2–4 h at 37°C, the cells were fed with an additional 50 μL/well of fresh DMEM containing 20% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin and cultured for 3 days at 37°C in the CO2 incubator. At the end of incubation, the cells were lysed by adding 85 μL/well of a lysis buffer and gently agitating by pipette. The lysates were transferred to a PCR plate and incubated at 37°C for 1 h, 55°C for 2 h, 95°C for 30 min, and stored at 4°C before usage. The lysates were diluted at a 1:5 ratio with ultrapure water and qPCR assay was run with the primers/probe mentioned above. Positive wells were scored and TCID50 was calculated according to the Spearman-Karber formula.
LC-MS/MS
Samples were lysed in 50 mM triethylammonium bicarbonate (TEAB) containing 5% SDS using a bead beater. Following lysis, samples were reduced in 10 mM DTT for 30 min at 55°C. Following reduction, proteins were alkylated in 30 mM acrylamide at room temperature for 30 min. Samples were then loaded onto S-trap devices (Protifi, LLC, Fairport, NY) and digestion was performed with 0.5 μg trypsin/LysC (Promega) at 37°C overnight. Following digestion, peptides were eluted using 35 μL 50 mM TEAB, 35 μL 0.2% formic acid in water, and two elutions of 80% acetonitrile with 0.2% formic acid in water. Eluted peptides were then dried in a speed-vac. Following drying, samples were reconstituted in a final reconstitution buffer (2% acetonitrile with 0.1% formic acid in water) for LC-MS/MS acquisition. Mass spectrometry experiments were performed using an Orbitrap Exploris 480 mass spectrometer (Thermo Scientific, San Jose, CA) attached to an Acquity M-Class UPLC system (Waters Corporation, Milford, MA) RRID:SCR022215. The UPLC system was set to a flow rate of 300 nL/min, where mobile phase A was 0.2% formic acid in water and mobile phase B was 0.2% formic acid in acetonitrile. The analytical column was prepared in-house with an I.D. of 100 microns pulled to a nanospray emitter using a P2000 laser puller (Sutter Instrument, Novato, CA). The column was packed with Dr. Maisch 1.8 micron C18 stationary phase to a length of approximately 25 cm. Peptides were directly injected into the column with a gradient of 2%–45% mobile phase B, followed by a high-B wash over a total of 80 min. The mass spectrometer was operated in a data-dependent mode using HCD fragmentation for MS/MS spectra generation. The raw data were analyzed using Byonic v4.4.1 (Protein Metrics, Cupertino, CA) to identify peptides and infer proteins. A concatenated FASTA file containing the AAV sequence, Homo sapiens sequences (for HEK293) or Spodoptera frugiperda sequences (for Sf9), and other likely contaminants and impurities was used to generate an in silico peptide library. Proteolysis with trypsin/LysC was assumed to be semi-specific allowing for N-ragged cleavage with up to two missed cleavage sites. Both precursor and fragment mass accuracies were held within 12 ppm. Cysteine modified with propionamide was set as a fixed modification in the search. Variable modifications included oxidation on methionine, histidine, and tryptophan, deoxidation on methionine and tryptophan, deamidation of glutamine and asparagine, acetylation and ubiquitination of lysine, methylation of lysine and arginine, and phosphorylation on serine, threonine, and tyrosine. SUMOylation and common glycosylation were also considered variable modifications. Proteins were held to a false discovery rate of 1% using the standard reverse-decoy technique.41
In vivo expression and efficacy
C57BL/6 mice were purchased from Jackson Laboratory. Mice were injected intravitreally with N54-aflibercept vector on day −28. The formulation buffer for Sf9-rAAV was 10 mM sodium phosphate with 150 mM NaCl and 0.01% Pluronic F68, and for HEK-rAAV was 10 mM sodium phosphate with 180 mM NaCl and 0.001% Pluronic F68. On day −3, vehicle and aflibercept were injected into the animals. On day 0, laser injury was induced by using a high-intensity laser to create 4 spots in the retina. On day 7, animals were weighed, FA was performed, blood was collected, and animals were euthanized. Ocular samples were collected and snap-frozen for ELISA. Serum for all the animals was collected by coagulating the blood drawn through cardiac puncture. FA analysis shows the lesion area on day 7 after laser induction. Ocular samples were homogenized in RIPA buffer in the presence of protease inhibitors. Samples were analyzed using the standardized aflibercept ELISA described above in the Methods. All mouse care and handling procedures were in compliance with institutional animal welfare protocols.
Statistical analysis
All statistical tests were performed using GraphPad Prism (La Jolla, CA). Data were presented as mean ± SD. For multiple group comparisons, Tukey’s multiple comparisons test was used. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Data and code availability
Original data will be available upon request.
Acknowledgments
Mass spectrometry measurements were performed at the Vincent Coates Foundation Mass Spectrometry Laboratory, Stanford University Mass Spectrometry (SUMS - RRID: SCR017801). Electron microscopy was performed by Reena Zalpuri and Danielle Jorgens from the Electron Microscope Lab at the University of California Berkley. This study was fully financed by Avirmax Biopharma Inc.
Author contributions
J.L., S.P., N.L., A.C., M.D., Y.L., K.L., F.L., Y.Y., R.L., D.H., and S. Li conducted the experiments; S. Liu designed the experiments; L.O. analyzed the data, performed a final review of results, and wrote the paper.
Declaration of interests
J.L., S.P., N.L., A.C., M.D., Y.L., K.L., D.H., S. Li, L.O., and S. Liu. are employees of Avirmax Biopharma Inc. Other authors declare no conflict of interest.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.11.022.
Supplemental information
References
- 1.Pupo A., Fernández A., Low S.H., François A., Suárez-Amarán L., Samulski R.J. AAV vectors: The Rubik's cube of human gene therapy. Mol. Ther. 2022;30:3515–3541. doi: 10.1016/j.ymthe.2022.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen W., Liu S., Ou L. rAAV immunogenicity, toxicity, and durability in 255 clinical trials: A meta-analysis. Front. Immunol. 2022;13:1001263. doi: 10.3389/fimmu.2022.1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathwani A.C., Tuddenham E.G.D., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C., et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flotte T.R., Cataltepe O., Puri A., Batista A.R., Moser R., McKenna-Yasek D., Douthwright C., Gernoux G., Blackwood M., Mueller C., et al. AAV gene therapy for Tay-Sachs disease. Nat. Med. 2022;28:251–259. doi: 10.1038/s41591-021-01664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ou L., Przybilla M.J., Tăbăran A.F., Overn P., O'Sullivan M.G., Jiang X., Sidhu R., Kell P.J., Ory D.S., Whitley C.B. A novel gene editing system to treat both Tay-Sachs and Sandhoff diseases. Gene Ther. 2020;27:226–236. doi: 10.1038/s41434-019-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ou L., DeKelver R.C., Rohde M., Tom S., Radeke R., St Martin S.J., Santiago Y., Sproul S., Przybilla M.J., Koniar B.L., et al. ZFN-mediated in vivo genome editing corrects murine Hurler syndrome. Mol. Ther. 2019;27:178–187. doi: 10.1016/j.ymthe.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi Z., Qu L., Tang H., Liu Z., Liu Y., Tian F., Wang C., Zhang X., Feng Z., Yu Y., et al. Engineered circular ADAR-recruiting RNAs increase the efficiency and fidelity of RNA editing in vitro and in vivo. Nat. Biotechnol. 2022;40:946–955. doi: 10.1038/s41587-021-01180-3. [DOI] [PubMed] [Google Scholar]
- 9.Ghauri M.S., Ou L. AAV Engineering for Improving Tropism to the Central Nervous System. Biology (Basel) 2023;12:186. doi: 10.3390/biology12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chahal P.S., Schulze E., Tran R., Montes J., Kamen A.A. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J. Virol. Methods. 2014;196:163–173. doi: 10.1016/j.jviromet.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grieger J.C., Soltys S.M., Samulski R.J. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol. Ther. 2016;24:287–297. doi: 10.1038/mt.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blessing D., Vachey G., Pythoud C., Rey M., Padrun V., Wurm F.M., Schneider B.L., Déglon N. Scalable production of AAV vectors in orbitally shaken HEK293 cells. Mol. Ther. Methods Clin. Dev. 2019;13:14–26. doi: 10.1016/j.omtm.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslanidi G., Lamb K., Zolotukhin S. An inducible system for highly efficient production of recombinant adeno-associated virus (rAAV) vectors in insect Sf9 cells. Proc. Natl. Acad. Sci. USA. 2009;106:5059–5064. doi: 10.1073/pnas.0810614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urabe M., Ding C., Kotin R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 15.Kotin R.M., Snyder R.O. Manufacturing clinical grade recombinant Adeno-associated virus using invertebrate cell lines. Hum. Gene Ther. 2017;28:350–360. doi: 10.1089/hum.2017.042. [DOI] [PubMed] [Google Scholar]
- 16.Chen H. Intron splicing-mediated expression of AAV Rep and Cap genes and production of AAV vectors in insect cells. Mol. Ther. 2008;16:924–930. doi: 10.1038/mt.2008.35. [DOI] [PubMed] [Google Scholar]
- 17.Feudner E., de Alwis M., Thrasher A.J., Ali R.R., Fauser S. Optimization of recombinant adeno-associated virus production using an herpes simplex virus amplicon system. J. Virol. Methods. 2001;96:97–105. doi: 10.1016/s0166-0934(01)00298-1. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Z., Qiao C., Hu P., Li J., Xiao X. A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Hum. Gene Ther. 2011;22:613–624. doi: 10.1089/hum.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin J., Frederick A., Luo Y., Jackson R., Joubert M., Sol B., Poulin F., Pastor E., Armentano D., Wadsworth S., Vincent K. Generation and characterization of adeno-associated virus producer cell lines for research and preclinical vector production. Hum. Gene Ther. Methods. 2013;24:253–269. doi: 10.1089/hgtb.2013.046. [DOI] [PubMed] [Google Scholar]
- 20.Clément N., Grieger J.C. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol. Ther. Methods Clin. Dev. 2016;3:16002. doi: 10.1038/mtm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondratov O., Marsic D., Crosson S.M., Mendez-Gomez H.R., Moskalenko O., Mietzsch M., Heilbronn R., Allison J.R., Green K.B., Agbandje-McKenna M., Zolotukhin S. Direct head-to-head evaluation of recombinant adeno-associated viral vectors manufactured in human versus insect cells. Mol. Ther. 2017;25:2661–2675. doi: 10.1016/j.ymthe.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mietzsch M., Hering H., Hammer E.M., Agbandje-McKenna M., Zolotukhin S., Heilbronn R. OneBac 2.0: Sf9 cell lines for production of AAV1, AAV2, and AAV8 vectors with minimal encapsidation of foreign DNA. Hum. Gene Ther. Methods. 2017;28:15–22. doi: 10.1089/hgtb.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handyside B., Ismail A.M., Zhang L., Yates B., Xie L., Sihn C.R., Murphy R., Bouwman T., Kim C.K., De Angelis R., et al. Vector genome loss and epigenetic modifications mediate decline in transgene expression of AAV5 vectors produced in mammalian and insect cells. Mol. Ther. 2022;30:3570–3586. doi: 10.1016/j.ymthe.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran N.T., Lecomte E., Saleun S., Namkung S., Robin C., Weber K., Devine E., Blouin V., Adjali O., Ayuso E., et al. Human and insect cell-produced recombinant adeno-associated viruses show differences in genome heterogeneity. Hum. Gene Ther. 2022;33:371–388. doi: 10.1089/hum.2022.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rumachik N.G., Malaker S.A., Poweleit N., Maynard L.H., Adams C.M., Leib R.D., Cirolia G., Thomas D., Stamnes S., Holt K., et al. Methods matter: standard production platforms for recombinant AAV produce chemically and functionally distinct vectors. Mol. Ther. Methods Clin. Dev. 2020;18:98–118. doi: 10.1016/j.omtm.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebberink E.H.T.M., Ruisinger A., Nuebel M., Thomann M., Heck A.J.R. Assessing production variability in empty and filled adeno-associated viruses by single molecule mass analyses. Mol. Ther. Methods Clin. Dev. 2022;27:491–501. doi: 10.1016/j.omtm.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao R., Farraha M., Logan G.J., Igoor S., Kok C.Y., Chong J.J.H., Alexander I.E., Kizana E. Performance of cardiotropic rAAV vectors is dependent on production method. Viruses. 2022;14:1623. doi: 10.3390/v14081623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang A., Wang Y., Wu S., Zuo W., Guo C., Hong W., Zhu S. Efficient production of an avian adeno-associated virus vector using insect cell/baculovirus expression system. J. Virol. Methods. 2017;240:26–31. doi: 10.1016/j.jviromet.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Deng X., Zou W., Yan Z., Qiu J. Establishment of a recombinant AAV2/hbov1 vector production system in insect cells. Genes. 2020;11:439. doi: 10.3390/genes11040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y., Jiang L., Geng H., Yang T., Han Z., He X., Lin K., Xu F. A recombinant baculovirus efficiently generates recombinant adeno-associated virus vectors in cultured insect cells and larvae. Mol. Ther. Methods Clin. Dev. 2018;10:38–47. doi: 10.1016/j.omtm.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohlbrenner E., Aslanidi G., Nash K., Shklyaev S., Campbell-Thompson M., Byrne B.J., Snyder R.O., Muzyczka N., Warrington K.H., Jr., Zolotukhin S. Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system. Mol. Ther. 2005;12:1217–1225. doi: 10.1016/j.ymthe.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giles A., Lock M., Chen S.J., Turner K., Wesolowski G., Prongay A., Petkov B.N., Olagbegi K., Yan H., Wilson J.M. Significant differences in capsid properties and potency between AAV vectors produced in Sf9 and HEK293 cells. Hum. Gene Ther. 2023;34:1003–1021. doi: 10.1089/hum.2022.116. [DOI] [PubMed] [Google Scholar]
- 33.Xin H., Biswas N., Li P., Zhong C., Chan T.C., Nudleman E., Ferrara N. Heparin-binding VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.1921252118. e1921252118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cecchini S., Virag T., Kotin R.M. Reproducible high yields of recombinant adeno-associated virus produced using invertebrate cells in 0.02- to 200-liter cultures. Hum. Gene Ther. 2011;22:1021–1030. doi: 10.1089/hum.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurasawa J.H., Park A., Sowers C.R., Halpin R.A., Tovchigrechko A., Dobson C.L., Schmelzer A.E., Gao C., Wilson S.D., Ikeda Y. Chemically Defined, High-Density Insect Cell-Based Expression System for Scalable AAV Vector Production. Mol. Ther. Methods Clin. Dev. 2020;19:330–340. doi: 10.1016/j.omtm.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith R.H., Levy J.R., Kotin R.M. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009;17:1888–1896. doi: 10.1038/mt.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galibert L., Jacob A., Savy A., Dickx Y., Bonnin D., Lecomte C., Rivollet L., Sanatine P., Boutin Fontaine M., Le Bec C., Merten O.W. Monobac System-A Single Baculovirus for the Production of rAAV. Microorganisms. 2021;9:1799. doi: 10.3390/microorganisms9091799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Destro F., Joseph J., Srinivasan P., Kanter J.M., Neufeld C., Wolfrum J.M., Barone P.W., Springs S.L., Sinskey A.J., Cecchini S., et al. Mechanistic Modeling Explains the Production Dynamics of Recombinant Adeno-Associated Virus with the Baculovirus Expression Vector System. Mol. Ther. Methods Clin. Dev. 2023;30:122–146. doi: 10.1016/j.omtm.2023.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gómez-Sebastián S., López-Vidal J., Escribano J.M. Significant productivity improvement of the baculovirus expression vector system by engineering a novel expression cassette. PloS one. 2014;9:e96562. doi: 10.1371/journal.pone.0096562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.François A., Bouzelha M., Lecomte E., Broucque F., Penaud-Budloo M., Adjali O., Moullier P., Blouin V., Ayuso E. Accurate titration of infectious AAV particles requires measurement of biologically active vector genomes and suitable controls. Mol. Ther. Methods Clin. Dev. 2018;10:223–236. doi: 10.1016/j.omtm.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elias J.E., Gygi S.P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data will be available upon request.